- 1Department of Orthopedics, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, China
- 2Department of Thyroid and Breast Surgery, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, China
Background: The chronic pain and functional limitations in osteoarthritis (OA) patients can increase risk of psychiatric disorders, e.g., major depression disorder (MDD), which may further aggravate the clinical symptoms of OA. Early detection of MDD is essential in the clinical practice of OA.
Materials and methods: Two hundred and fifteen participants with knee OA were recruited, including 134 MDD patients (i.e., MDD group) and 81 ones without MDD (i.e., control group). Among them, 81 OA participants in the control group received a 3-year follow-up and were divided into trans-MDD group (who transforming into MDD; N = 39) and non-MDD group (who keeping non-MDD; N = 42) at the end of the follow-up. The 17-item Hamilton Depression Scale (HAMD-17), Self-Rating Depression Scale (SDS), and Visual Analogue Scale (VAS) were performed. Furthermore, serum levels of brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), S100B, and IGF-1 were detected.
Results: (1) Compared with OA participants without MDD, there were significant decrease in serum BDNF and significant increase in serum VEGF and S100B and VAS scores in OA participants with MDD. (2) A mediation of the association was found between the VAS scores and the HAMD-17 scores through the BDNF as mediator in OA participants with MDD. (3) Significantly lower baseline BDNF levels and higher baseline S100B levels were detected in OA participants who transforming to MDD after a 3-year follow-up when compared with those who keeping non-MDD. (4) In the trans-MDD group, significant associations of the change of serum BDNF levels with rate of change of HAMD-17 scores were found, and baseline serum S100B levels positively correlated with the HAMD-17 scores at the end of the follow-up. (5) In OA participants, the composite indicator of BDNF, VEGF, and S100B differentiated MDD patients from controls with the area under the curve (AUC) value of 0.806, and the combined indicator of baseline BDNF and S100B distinguished trans-MDD participants from non-MDD ones with an AUC value of 0.806.
Conclusion: Serum BDNF, VEGF, and S100B may be potential biomarkers to identify MDD in OA patients. Meanwhile, serum BDNF and S100B shows great potential to predict the risk of MDD for OA.
Introduction
Osteoarthritis (OA) is a common cause of disability and source of societal cost in elders, with 250 million persons being currently affected in the world (1). As a whole joint disease, the complex pathogenesis of OA involving in mechanical, inflammatory, and metabolic factors, can induce structural destruction of the synovial joint (2). Pain is the dominant symptom of OA, and in particular, the pain in knee OA is considered as typically transitioning from intermittent weight-bearing pain to a chronic pain (3). Due to the current clinical therapies are difficult to cure OA completely, the chronic pain and functional limitations can increase risk of negative psychological outcomes, such as major depression disorder (MDD), which will further diminishes quality of life for OA patients (4–6). Furthermore, individuals with MDD exhibit more significant anhedonia and greater daily negative affect compared to non-depressed ones (7), which may aggravate the feelings of pain in OA. A previous study indicated that duloxetine, an antidepressant, was effective to alleviate not only MDD but also pain in OA patients (8). Therefore, early identification of MDD may be crucial for improving the outcomes of patients with OA.
Although the coexistence of the chronic pain and MDD can tend to further aggravate the severity of both disorders (9–11), the underlying association between them is unclear. A review revealed that the common neuroplasticity mechanism changes may be an important route for the occurrence and development of the chronic pain and MDD (12). The neurotrophin hypothesis indicates that numerous neurotrophins are responsible for controlling the neuronal plasticity (13), as a result, the detection of blood neurotrophins may be a potential approach to identify MDD in OA. Recently, Shi et al. performed a comprehensive meta-analysis on the blood neurotrophins for the clinical application of MDD and revealed that significantly reduced brain-derived neurotrophic factor (BDNF) and significantly increased insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), and S100B protein may be potential diagnostic biomarkers for MDD (14). Meanwhile, Stefani et al. also found that abnormal serum levels of BDNF and S100B were associated to the chronic pain (15). Thus, it is essential to assess the clinical value of these neurotrophins in OA.
The present study aimed to investigate whether the BDNF, VEGF, S100B, and IGF-1 in serum levels can be helpful to identify MDD in knee OA patients and to evaluate the potential relationship of these neurotrophins with depression and pain assessments. Furthermore, a 3-year follow-up was conducted in the present study, and the dynamic characteristics of these serum indicators and the symptoms of depression and pain in knee OA patients were also assessed.
Materials and methods
Participants
A total of 215 participants with knee OA presenting to the outpatient department were recruited from the Xiangyang Central Hospital, and among them, 134 patients with a history of depression (defined as the MDD group) and 81 ones without a depressive behavior (defined as the control group). The present study was approved by the Ethics Committee of Xiangyang Central Hospital (approval No. XYCH2017-023), and all participants provided signed informed consent.
A diagnosis of knee OA was made by an orthopedic surgeon based on the American College of Rheumatology clinical criteria (16). The inclusion criteria included (1) between 45 and 80 years of age; (2) knee pain of duration ≥ 6 months; (3) ≥2 scores of the Kellgren and Lawrence osteoarthritis scale (17); (4) being able to stand up independently from a chair and lay prone; and (5) body mass index ≤ 30. Furthermore, MDD was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. MDD patients satisfied the 17-item Hamilton Depression Scale (HAMD-17) score of ≥8 and treatment-naïve, and each patient had no family history of a mental disorder. For all participants, the following exclusion criteria were applied: (1) any neurological illnesses; (2) lower limb fractures, post traumatic knee osteoarthritis, or head trauma; (3) total knee arthroplasty; (4) unexplained radiating leg pain; (5) secondary mental disorders; (6) alcohol or drug abuse; (7) active tumors; (8) history of significant physical disorder (e.g., endocrine disease, autoimmune disease or liver or kidney dysfunction); and (9) pregnancy and breastfeeding.
A 3-year observational follow-up was conducted for all participants of the control group, and among 81 OA participants, 39 participants transformed into depression (defined as the trans-MDD group) and others kept non-depression (defined as the non-MDD group).
Additionally, no specific intervention (i.e., surgical treatment) was conducted for each OA participant, and non-surgical treatment that included non-pharmacological and pharmacological treatment were recorded (please see Table 1). Non-pharmacological treatment consisted of education, weight management, and strength training exercises, and pharmacological treatment comprised paracetamol and oral non-steroidal anti-inflammatory drugs. As an observational study, no new treatment was assigned to participants during the follow-up, and for each OA participants, the treatment type was not changed until the end of follow-up.
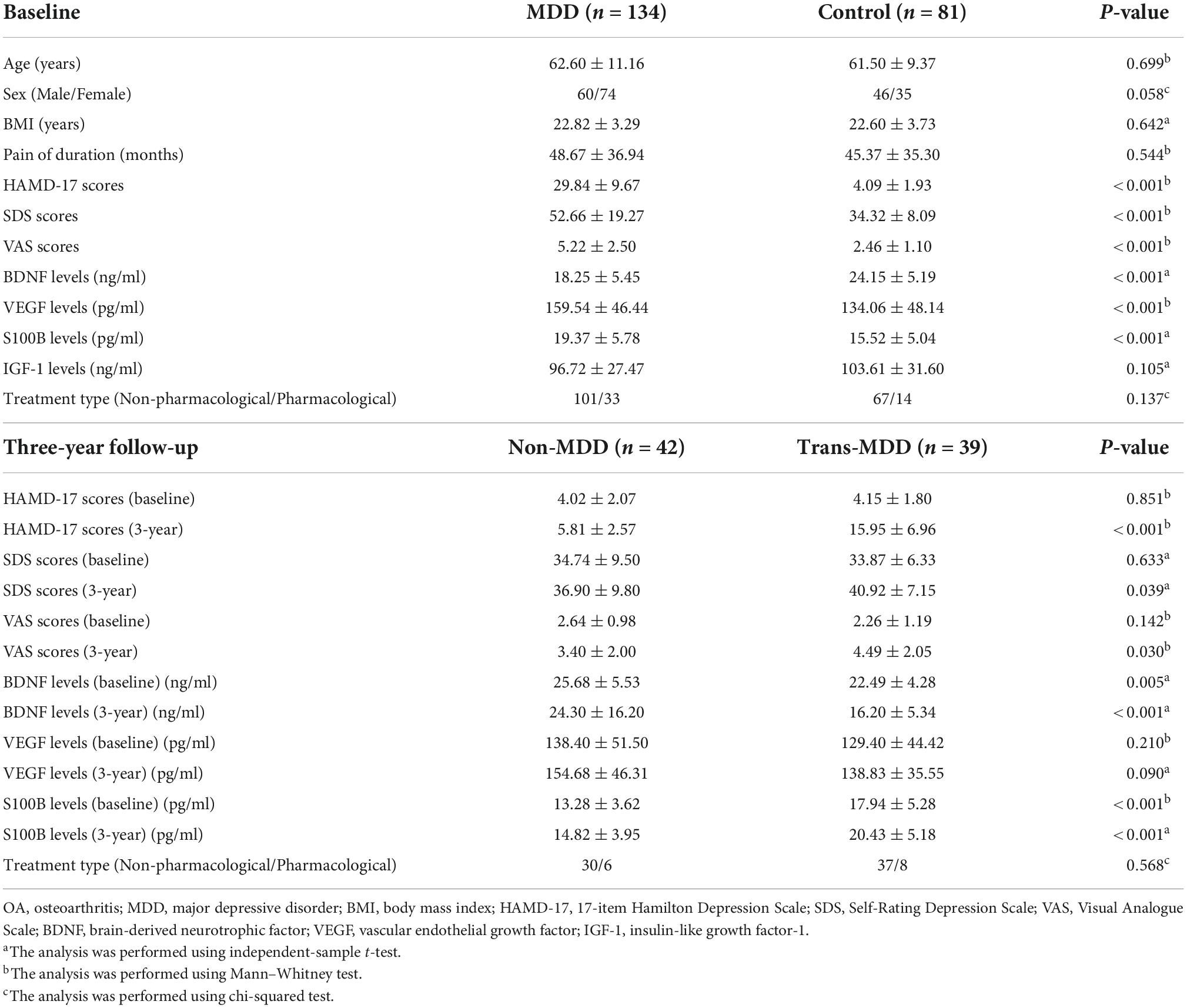
Table 1. The clinical features and serum levels of neurotrophins in knee OA patients at baseline and after 3-year follow-up.
Assessment tools
The HAMD-17 as a primary test tool, was used to assess depressive symptoms, and the Self-Rating Depression Scale (SDS) were also used as the second test tool to assess the severity of depression. Meanwhile, the Visual Analogue Scale (VAS) was used to measure pain intensity in knee pain. The higher the score of these scales, the more severe of symptoms.
Sample collection
Venous blood was collected from each OA participant after overnight fasting into a vacutainer tube (without anticoagulant). Within 30 min of collection, the coagulated blood was centrifuged at 3500 rpm for 10 min at 4°C to obtain serum. The serum samples were aliquoted and stored at −80°C. Blood was obtained from the 81 OA participant in the control group at baseline and the end of 3-year observational follow-up, respectively. For participants in the MDD group, samples were collected once at baseline.
Enzyme-linked immunosorbent assay analyses
The concentrations of serum neurotrophins (BDNF, VEGF, S100B, and IGF-1) were measured using the commercial ELISA kits (R&D Systems, Minneapolis, MN, USA; BDNF: catalog No.DBD000; VEGF: catalog No. DVE00; S100B: catalog No. DY1820-05; IGF-1: catalog No. DG100B) in accordance with the manufacturer’s protocols. The concentration in each plate was determined using a microplate reader (Thermo Scientific™, Shanghai, China). Each sample was measured in triplicate, and the inter- and intra-assay coefficients of variation were <4%.
Statistical analysis
Statistical analyses were performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). The normal distribution of the data was assessed using the Kolmogorov-Smirnov test, and the Levene’s homogeneity of variance test was also performed. Categorical variable (i.e., sex) was analyzed using the chi-squared test, and continuous variables (i.e., age, pain of duration, body mass index [BMI], emotion and pain assessments, and serum neurotrophins’ levels) were analyzed using the independent-sample t-test or Mann–Whitney test. The paired t-test was used to determine the difference of variables before and after follow-up. Partial correlation analysis was used to determine a possible association of emotion or pain assessments with serum neurotrophins’ levels, controlling age, sex, pain of duration, BMI, and treatment types. Notably, the change value of serum neurotrophins level was calculated with “Δ = baseline − the end of follow-up,” and the change rate of depression or pain assessment was calculated with “%Δ = (baseline − the end of follow-up)/baseline.” P < 0.05 was considered as statistical significance (two tailed).
As described in our previous study (18), the mediation analysis was utilized to determine whether serum neurotrophins could mediate the association between depressive behaviors and knee pain, which is based on a standard three-variable mediation model (19). Covariates were age, sex, pain of duration, BMI, and treatment types.
The area under the curve (AUC) of the receiver operating characteristic curve (ROC) was calculated to determine the distinguishing ability of serum neurotrophins which exhibited significant difference between two groups. The Youden index (20) was used to obtain optimal values of sensitivity and specificity. In addition, the binary logistic regression (21) (dependent variable: MDD/control; independent variable: serum neurotrophins) was used to calculate a predicted value of each patient that can be regarded as the combined indicator based on these serum neurotrophins.
Results
Characteristic of participants at baseline
In the Table 1, 134 OA participants showed significantly increased the HAMD-17 and SDS scores when compared with the other 81 OA participants. Therefore, these OA participants were divided into the MDD group (N = 134) and the control group (N = 81).
The MDD group showed significantly higher VAS scores and serum levels of VEGF and S100B than the control group (Table 1). Meanwhile, the serum levels of BDNF was significantly reduced in the MDD group as compared to the control group (Table 1). However, the serum IGF-1 levels had no significant difference between MDD and control groups (Table 1). Furthermore, the clinical characteristics (e.g., age, sex, BMI, pain of duration, and treatment types) were non-significantly different between two groups (Table 1).
Relationship of serum neurotrophins with emotion and pain assessments in the major depression disorder group at baseline
Correlation analyses in 134 OA participants with MDD demonstrated that there were negative correlations between serum BDNF levels and the HAMD-17, SDS, and VAS scores (Figures 1A–C). Besides, mediation analysis indicated that serum levels of BDNF significantly mediated the effect of the VAS scores on the HAMD-17 scores (Figure 1D) where covariates were controlled for age, sex, pain of duration, BMI, and treatment types.
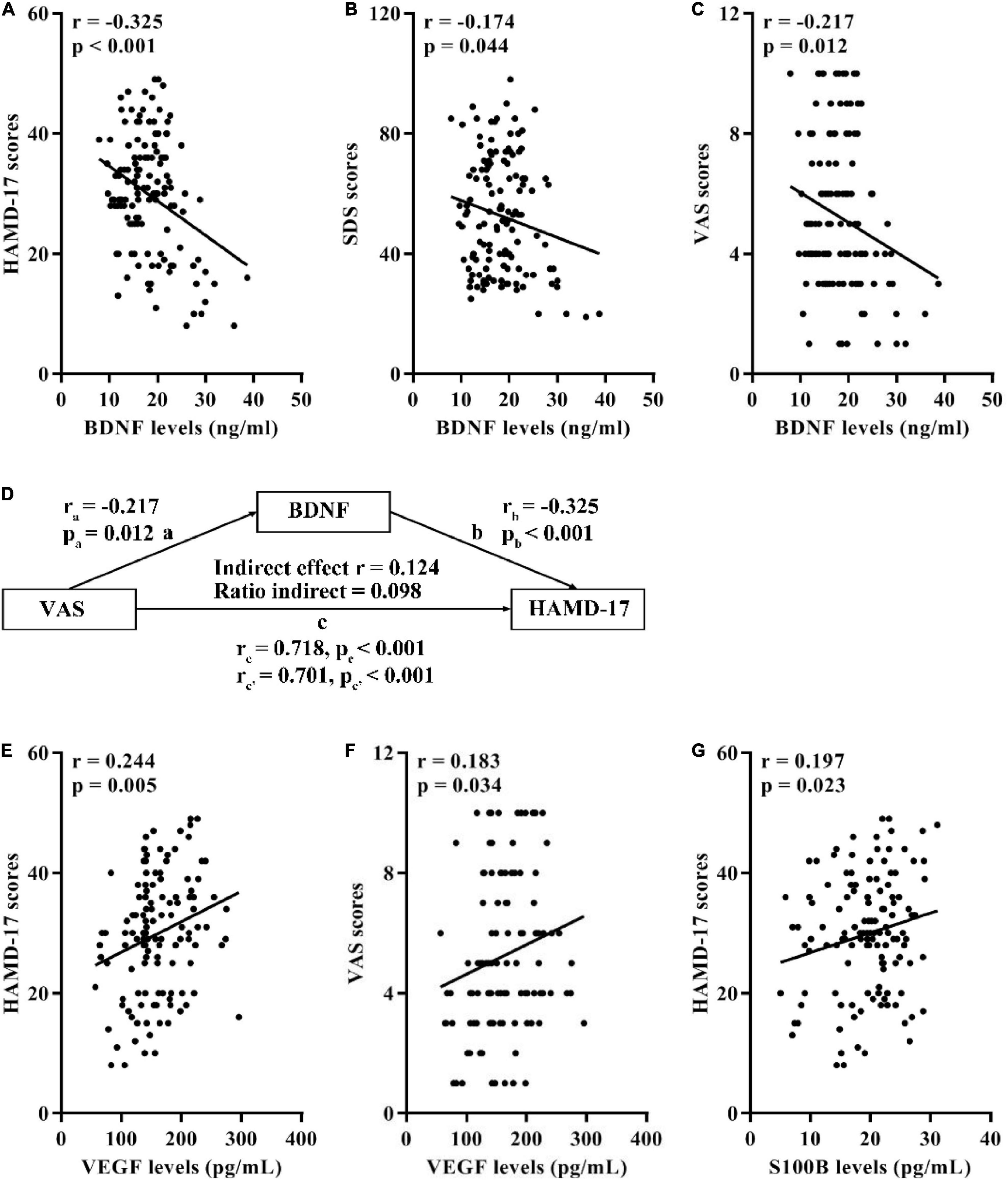
Figure 1. Associations of serum BDNF, VEGF, and S100B with the HAMD-17, SDS, and VAS scores in 134 OA participants with MDD. (A) Correlation between BDNF levels and the HAMD-17 scores. (B) Correlation between BDNF levels and the SDS scores. (C) Correlation between BDNF levels and the VAS scores. (D) Mediation effects of serum BDNF on the association between the HAMD-17 and VAS scores. (E) Correlation between VEGF levels and the HAMD-17 scores. (F) Correlation between VEGF levels and the VAS scores. (G) Correlation between S100B levels and the HAMD-17 scores. BDNF, brain-derived neurotrophic factor; VEGF, vascular endothelial growth factor; HAMD-17, 17-item Hamilton Depression Scale; SDS, Self-Rating Depression Scale; VAS, Visual Analogue Scale; OA, osteoarthritis; MDD, major depressive disorder.
Furthermore, the serum levels of VEGF was positively correlated with the HAMD-17 and VAS scores (Figures 1E,F) in MDD patients. Moreover, there was a positive correlation between serum S100B levels and the HAMD-17 scores (Figure 1G).
Characteristic of osteoarthritis participants in the control group after 3-year follow-up
At the end of 3-year follow-up, 39 OA participants showed depressive behaviors, who were assigned to the trans-MDD group, and 42 OA participants as the non-MDD group, didn’t display any depressive disorders.
As shown in Table 1, baseline HAMD-17, SDS, and VAS scores, baseline serum VEGF levels, and treatment types were not significantly different between the trans-MDD and non-MDD groups. Meanwhile, baseline serum levels of BDNF and S100B had significant difference between two groups (Table 1).
After the follow-up, 39 OA participants in the trans-MDD group showed significantly lower serum BDNF levels and higher serum S100B levels than 42 ones in the non-MDD group (Table 1). However, serum levels of VEGF after the follow-up exhibited no significant difference between two groups (Table 1).
In addition, baseline serum BDNF and S100B levels changed significantly after the follow-up in the trans-MDD group, however, there was no significant difference in in the non-MDD group (Figures 2A–D). For serum VEGF levels, neither the trans-MDD group nor the non-MDD group had significant difference between baseline and the end of follow-up (Figures 2E,F).
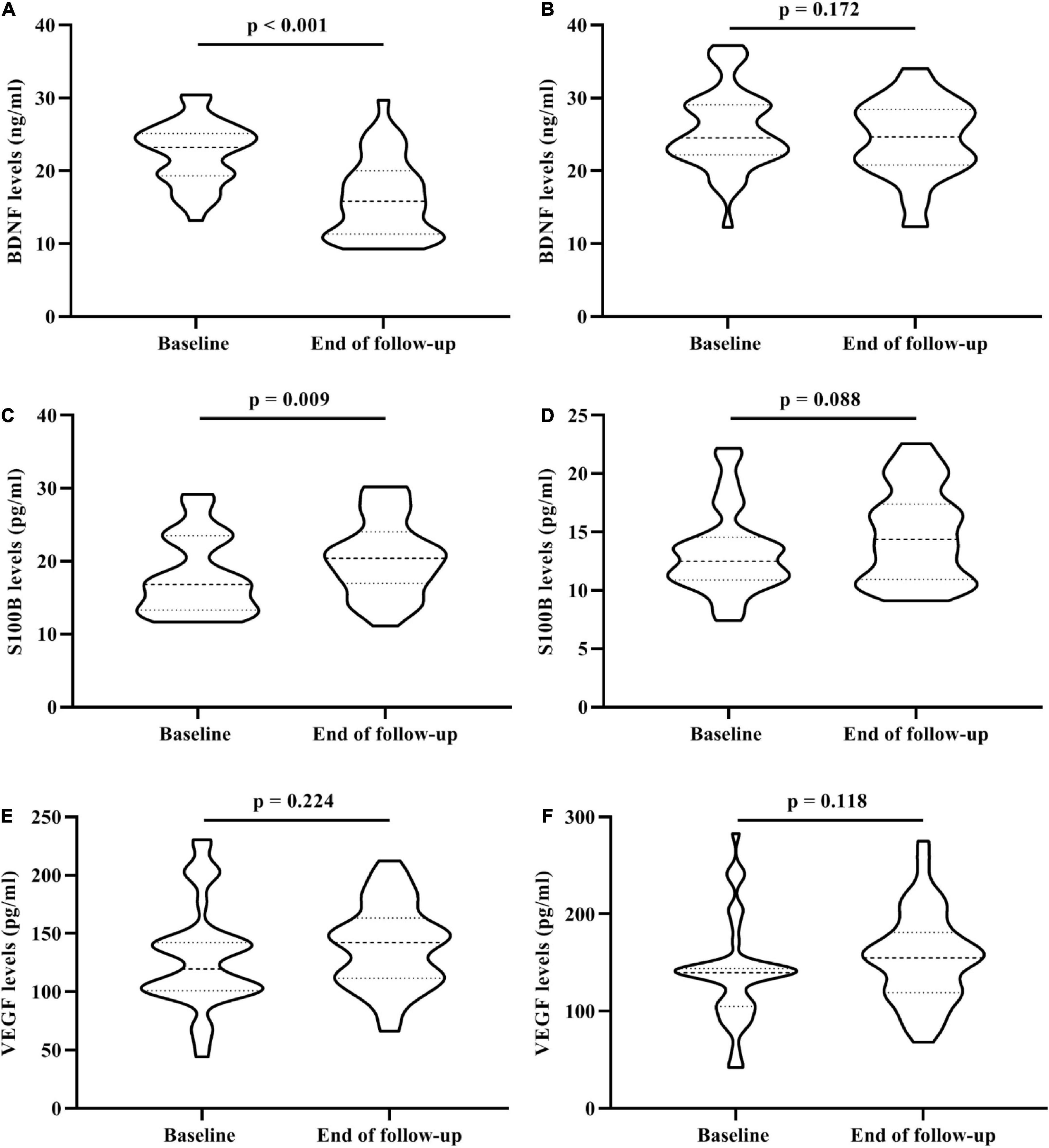
Figure 2. The change of serum BDNF, VEGF, and S100B levels in 81 OA participants who underwent the 3-year follow-up. (A) The change of serum BDNF levels between baseline and the end of follow-up in trans-MDD group. (B) The change of serum BDNF levels between baseline and the end of follow-up in non-MDD group. (C) The change of serum S100B levels between baseline and the end of follow-up in trans-MDD group. (D) The change of serum S100B levels between baseline and the end of follow-up in non-MDD group. (E) The change of serum VEGF levels between baseline and the end of follow-up in trans-MDD group. (F) The change of serum VEGF levels between baseline and the end of follow-up in non-MDD group. BDNF, brain-derived neurotrophic factor; VEGF, vascular endothelial growth factor; OA, osteoarthritis; MDD, major depressive disorder.
Correlation analysis in the trans-major depression disorder group after the 3-year follow-up
At the conclusion of the 3-year follow-up in the trans-MDD group, a significant negative correlation between the change of serum BDNF levels and rate of change of HAMD-17 scores was observed (Figure 3A), with the change of serum BDNF levels positively associated with the VAS scores (Figure 3B).
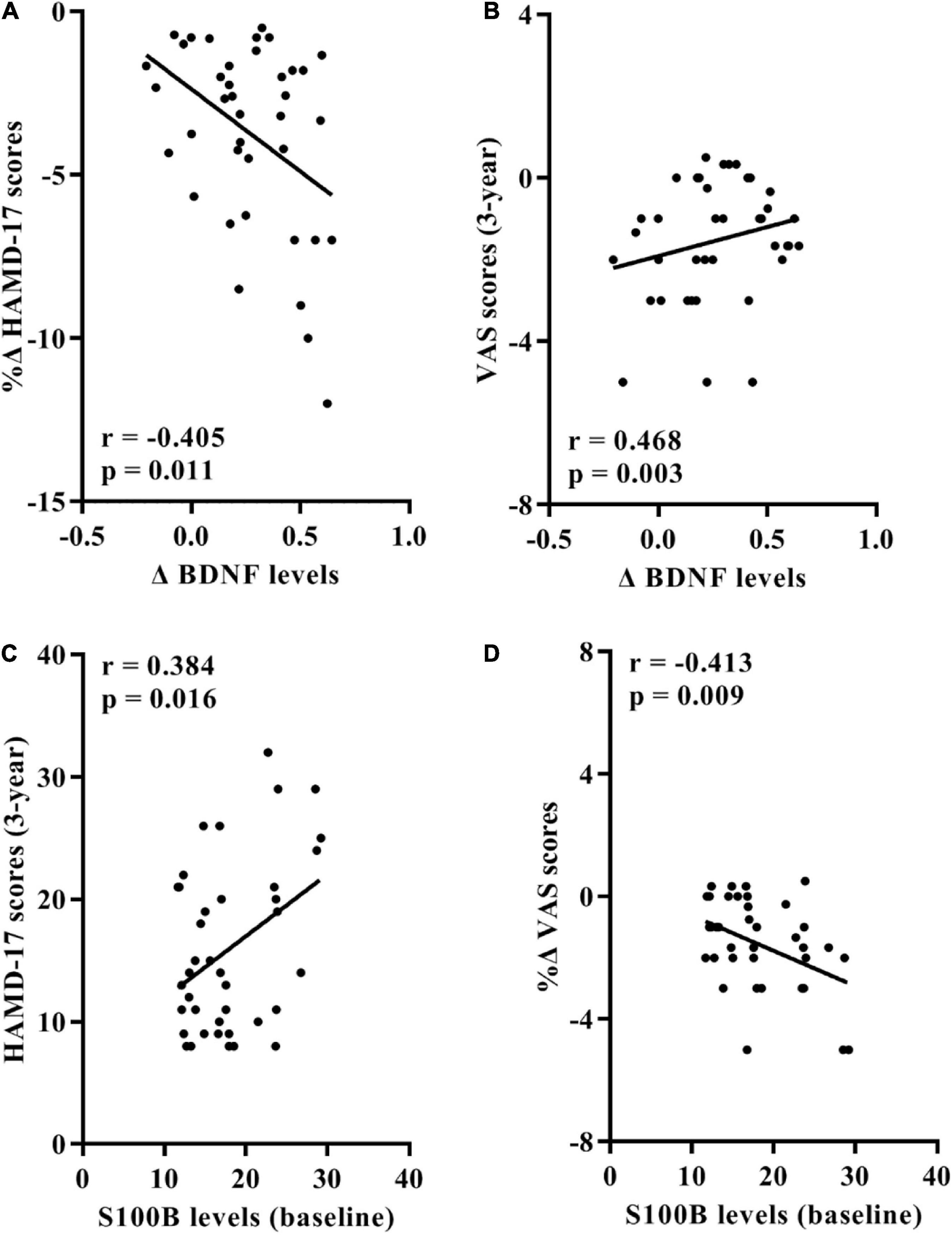
Figure 3. Associations between serum BDNF/S100B levels and the HAMD-24 and VAS scores in 39 participants of trans-MDD group after the 3-year follow-up. (A) Correlation between rates of change of HAMD-17 scores and the change value of BDNF levels. (B) Correlation between the change value of BDNF levels and the VAS scores at the conclusion of the 3-year follow-up. (C) Correlation between the HAMD-17 scores at the end of the 3-year follow-up and serum S100B levels at baseline. (D) Correlation between rates of change of VAS scores and serum S100B levels at baseline. BDNF, brain-derived neurotrophic factor; HAMD-17, 17-item Hamilton Depression Scale; VAS, Visual Analogue Scale; OA, osteoarthritis; MDD, major depressive disorder.
Furthermore, baseline serum S100B levels positively correlated with the HAMD-17 scores at the end of follow-up and negatively correlated with the change rate of VAS scores (Figures 3C,D).
Receiver operating characteristic curve analyses
Figure 4A displays the AUC value of BDNF, VEGF, and S100B at 0.799 (95% CI: 0.739∼0.850), 0.668 (CI: 0.600∼0.730), and 0.706 (95% CI: 0.640∼0.766), respectively, for distinguishing MDD patients from controls in all OA participants. However, the combination of BDNF, VEGF, and S100B provided greater diagnostic power with an AUC value of 0.834 (95% CI: 0.778∼0.881), corresponding to a specificity of 88.89% and a sensitivity of 62.69% for identifying MDD in OA participants (Figure 4A).
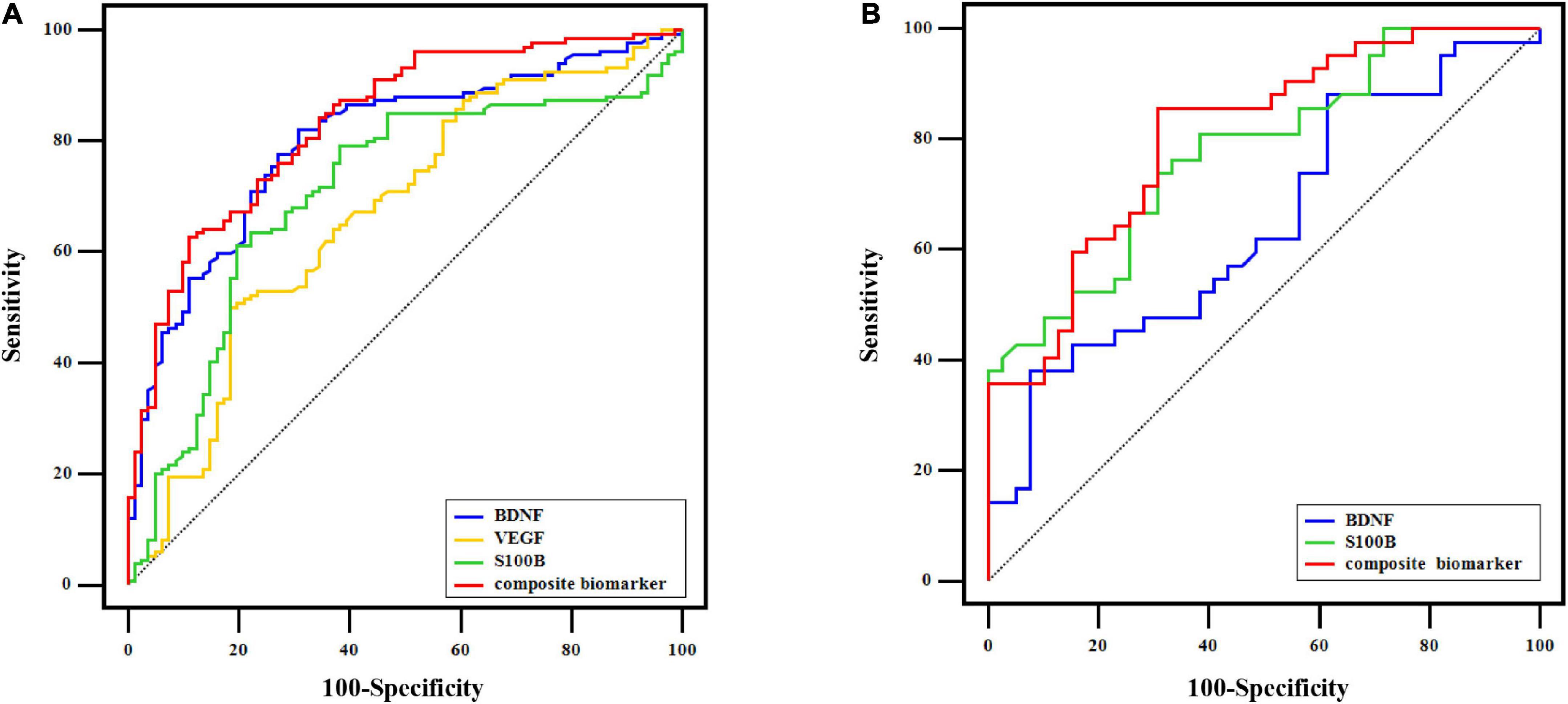
Figure 4. ROC curve analysis. (A) In 215 OA participants, ROC curves of BDNF, VEGF, S100B, and the composite biomarker of their combination for distinguishing MDD from control at baseline. (B) ROC curves of baseline BDNF, baseline S100B, and the combination of the two indicators to distinguish OA participants who transformed into MDD from those with non-MDD after the follow-up.
As shown in Figure 4B, the combined indicator of baseline BDNF and S100B was able to differentiate OA participants in the trans-MDD group from the non-MDD group with the AUC value of 0.806 (95% CI: 0.703∼0.885; specificity: 69.23%; sensitivity: 85.71%), which was better than single baseline BDNF (AUC = 0.644; 95% CI: 0.530∼0.747) or baseline S100B (AUC = 0.777; 95% CI: 0.670∼0.862).
Discussion
The present study firstly determined the feature of serum neurotrophins in knee OA patients with MDD and the potential association of serum neurotrophins with depression and pain symptoms. The main findings in the present study are as follow: (1) in comparison with OA participants without depressive behaviors, a significant decrease in serum BDNF and significant increase in serum VEGF and S100B and VAS scores in OA participants with MDD were observed; (2) in OA participants, there were significant correlations between serum levels of BDNF, VEGF, and S100B and depression and pain assessment scores in MDD patients; (3) particularly, a mediation of the association was found between the VAS scores and the HAMD-17 scores through the BDNF as mediator in OA participants with MDD; (4) significantly lower baseline BDNF levels and higher baseline S100B levels were detected in OA participants who transforming to MDD after a 3-year follow-up when compared with those who showed normal behaviors; (5) at the end of follow-up, significant associations of serum BDNF and S100B with the performance of depression and pain were found in the trans-MDD group; (6) in OA participants, the composite indicator of BDNF, VEGF, and S100B had optimal power to differentiate MDD patients from controls, and the combination of BDNF and S100B could effectively predict the transformation of MDD after the 3-year follow-up. Taken together, serum BDNF, VEGF, and S100B may be a potential diagnostic biomarker of MDD in knee OA, with serum BDNF and S100B in particular being able to predict the occurrence of MDD in the development of OA.
Several previous studies had found that in various body fluids (e.g., plasma, synovia), OA patients showed significant higher BDNF levels, and BDNF/TrkB signaling contributes to developing chronic OA pain (22–24). As a driving force behind neuroplasticity, BDNF-related signal pathway not only influences the sensitization of pain pathways but also restrains the negative effect of MDD (25, 26). In the present study, significantly increased BDNF in serum levels were found in OA participants with MDD, and OA participants who transformed into MDD after the follow-up also showed significantly increased serum BDNF levels at baseline and the end of follow-up, which suggested that serum BDNF may be used as a potential diagnostic and predictive biomarker to identify MDD in OA. Furthermore, in OA participants with MDD, the negative correlation between BDNF levels and the HAMD-17, SDS, and VAS scores suggests that the decrease in serum levels of BDNF may reflect the severity of depression and pain. Interestingly, a mediator model further found that pain can affect a depressive state through a change in serum BDNF levels, strongly indicating that BDNF may play an important role in mediating the association of the pain with MDD. Recently, Dimmek et al. detected that in OA-related chronic widespread pain patients, reduced serum BDNF levels may induce the anxiety and depression via affecting the function of immune cells (27), which supported the present findings on relationships of BDNF with depression and pain. Additionally, previous studies showed serum/plasma BDNF levels can elevated in OA patients after the continuous non-surgical treatment, with improving the pain symptom (28, 29), which suggested that BDNF levels can be affected by the severity of OA. Similarly, the present study observed that in the development of OA, lower serum BDNF levels reflected the more serious of pain and predicted the high risk of MDD. Although low serum BDNF levels were also found and antidepressants could increase BDNF levels in depressed patients (30), a meta-analysis provided an overall increased trend of BDNF in MDD patients and higher serum BDNF levels may play an important role in the pathophysiology of MDD (14). Consequently, serum BDNF as a valuable indicator may contribute to early identifying MDD in OA patients.
S100B is a calcium-binding protein produced predominantly by astrocytes, and is able to enhance neuroplasticity (31). Previous studies showed that S100B levels were significantly increased in serum, synovial fluid, and knee synovial tissue of OA patients and had an effect on the repair of cartilage damage (15, 32, 33). Meanwhile, S100B was also involved in the pathology of MDD and showed significant increase in peripheral blood and autopsy brain tissues individuals with MDD (14, 34, 35). The present study displayed that in OA participants, patient with MDD showed significantly elevated serum S100B levels and positive correlation between S100B levels and the HAMD-17 scores. Importantly, our study also found that increased serum S100B levels in OA patients would aggravate the chronic pain and lead to the occurrence of MDD with the development of OA. Although the role and mechanism of S100B in OA is unclear, the clinically diagnostic and predictive value of S100B for MDD had been determined in OA, which is helpful to conduct the early antidepressive strategies for OA.
Vascular endothelial growth factor is expressed in articular cartilage and increases in serum levels have been associated with the progression of OA (36, 37). Previous studies showed that inhibition of VEGF-related signaling pathway (e.g., PIM2/VEGF signaling, VEGF-A/VEGFR2 signaling) can attenuate the early development of OA, and several therapies targeting VEGF and its receptors had been investigated for the treatment of OA (38–40). Furthermore, VEGF is a vascular permeability factor, and when VEGF levels are pathologically elevated, blood brain barrier integrity may be impaired, with affecting central nervous system homeostasis, which may lead to the dysfunction of brain and the emergence of MDD (41, 42). In the present study, OA participants with MDD showed significantly higher serum VEGF levels than those with non-MDD, and the significantly positive associations of VEGF levels with the HAMD-17 and VAS scores were also observed in OA participants with MDD, which suggested that VEGF may be an important indicator to reflect the severe of OA, especially psychosomatic symptoms. Although in OA participants, the abnormal change of serum VEGF levels was not observed between trans-MDD and non-MDD groups after the 3-year follow-up, VEGF is still a vital target for the treatment of OA accompanying MDD.
Some previous studies reported that higher serum IGF-1 concentration presented a high risk of knee OA (43, 44), however, lower serum IGF-1 levels were also found in other studies (45, 46). Although IGF-1 is involved in the proliferation and differentiation of chondrocyte and the synthesis of collagen and glycosaminoglycan (45, 47, 48), the role of IGF-1 in OA pathogenesis is unknown. In the present study, no difference in serum IGF-1 levels between MDD and control groups may be associated with the inconsistent changes of IGF-1 levels in OA patients (43–46). Further investigation is necessary to evaluate the clinical value of IGF-1 in OA with MDD.
In the present study, our findings provide evidence for the diagnosis of MDD in OA patients using serum neurotrophins. Compared with the single indicator, a panel of BDNF, VEGF, and S100B is recommended for optimal differential diagnosis of MDD. In addition, we also found that the collective contribution of BDNF and S100B is an optimal combined biomarker for predicting the high risk of MDD in OA patients.
However, several limitations in the present study need be considered. Firstly, OA participants in the present study did not restricted the treatment type, and during the follow-up, individual treatment of each OA patient was also uncontrolled. Although confounding factors were adjusted in the data analysis, different treatment types may have an effect on the serum neurotrophins levels. Treatment-free OA patients will be focused on in the future study. Secondly, magnetic resonance imaging is the important means for whole-joint evaluation in OA study and brain function evaluation in MDD research, however, analysis of imaging data was lacking in the present study. We will further improve the imaging analysis in the subsequent study. Thirdly, the larger sample size is essential to further assess serum neurotrophins in OA patients with different degrees of MDD. Lastly, commercially available BDNF ELISA kit can recognize both BDNF (mature) and its precursor proBDNF in the human blood, thus, the present levels of BDNF are the total BDNF levels including BDNF (mature) and proBDNF (49, 50).
Conclusion
The present study demonstrated that OA patients with MDD showed significantly changed serum BDNF, VEGF, and S100B and significant associations of these neurotrophins with depression and pain assessments, which suggested that these indicators may be potential biomarkers to identify MDD in OA patients. Furthermore, findings of the follow-up study further found that serum BDNF and S100B were potential biomarkers to predict the risk of MDD for OA.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Xiangyang Central Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XL and GC designed the study, contributed to the discussion, and revised the manuscript. PZ drafted the manuscript. BW, YZ, and ZW recruited the participants, completed the assessments, and collected the blood samples. YX and JS conducted the ELISA research. PZ and CL contributed to the data analyses and discussion. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank all participants in the present study for their cooperation and psychiatrists for their assistance in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9
2. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Primers. (2016) 2:16072. doi: 10.1038/nrdp.2016.72
3. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. (2013) 21:1145–53. doi: 10.1016/j.joca.2013.03.018
4. Parmelee PA, Behrens EA, Costlow Hill K, Cox BS, Decaro JA, Keefe FJ, et al. Momentary associations of osteoarthritis pain and affect: depression as moderator. J Gerontol B Psychol Sci Soc Sci. (2021) 77:1240–9. doi: 10.1093/geronb/gbab221
5. Zheng S, Tu L, Cicuttini F, Zhu Z, Han W, Antony B, et al. Depression in patients with knee osteoarthritis: risk factors and associations with joint symptoms. BMC Musculoskelet Disord. (2021) 22:40. doi: 10.1186/s12891-020-03875-1
6. Wang ST, Ni GX. Depression in osteoarthritis: current understanding. Neuropsychiatr Dis Treat. (2022) 18:375–89. doi: 10.2147/NDT.S346183
7. Lamers F, Swendsen J, Cui L, Husky M, Johns J, Zipunnikov V, et al. Mood reactivity and affective dynamics in mood and anxiety disorders. J Abnormal Psychol. (2018) 127:659–69. doi: 10.1037/abn0000378
8. Jesus C, Jesus I, Agius M. Treatment of Depression in patients with Osteoarthritis: the importance of an early diagnosis and the role of Duloxetine. Psychiatr Danub. (2016) 28(Suppl–1):149–53.
9. Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. (1997) 13:116–37. doi: 10.1097/00002508-199706000-00006
10. Kostov S, Schug SA. Depression and chronic pain. Saudi J Anaesth. (2018) 12:377–8. doi: 10.4103/sja.SJA_69_18
11. Smesam HN, Qazmooz HA, Khayoon SQ, Almulla AF, Maes M, Al-Hakeim HK. Pathway phenotypes underpinning depression, anxiety, and chronic fatigue symptoms due to acute rheumatoid arthritis: a precision nomothetic psychiatry analysis. J Pers Med. (2022) 12:476. doi: 10.3390/jpm12030476
12. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plastic. (2017) 2017:9724371. doi: 10.1155/2017/9724371
13. Thoenen H. Neurotrophins and neuronal plasticity. Science. (1995) 270:593–8. doi: 10.1126/science.270.5236.593
14. Shi Y, Luan D, Song R, Zhang Z. Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: a systematic review and meta-analysis. Eur Neuropsychopharmacol. (2020) 41:40–51. doi: 10.1016/j.euroneuro.2020.09.633
15. Stefani LC, Leite FM, Da Graça LTM, Zanette SA, De Souza A, Castro SM, et al. BDNF and serum S100B levels according the spectrum of structural pathology in chronic pain patients. Neurosci Lett. (2019) 706:105–9. doi: 10.1016/j.neulet.2019.05.021
16. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. (1986) 29:1039–49. doi: 10.1002/art.1780290816
17. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. (1957) 16:494–502. doi: 10.1136/ard.16.4.494
18. Zhang P, Zhou Y, Chen G, Li J, Wang B, Lu X. Potential association of bone mineral density loss with cognitive impairment and central and peripheral amyloid-β changes: a cross-sectional study. BMC Musculoskelet Disord. (2022) 23:626. doi: 10.1186/s12891-022-05580-7
19. Ma Y, Li B, Wang C, Shi Z, Sun Y, Sheng F, et al. 5-HTTLPR polymorphism modulates neural mechanisms of negative self-reflection. Cereb Cortex. (2014) 24:2421–9. doi: 10.1093/cercor/bht099
20. Youden WJ. Index for rating diagnostic tests. Cancer. (1950) 3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3
21. Shi Y, Song R, Wang Z, Zhang H, Zhu J, Yue Y, et al. Potential clinical value of circular RNAs as peripheral biomarkers for the diagnosis and treatment of major depressive disorder. EBioMedicine. (2021) 66:103337. doi: 10.1016/j.ebiom.2021.103337
22. Simão AP, Mendonça VA, De Oliveira Almeida TM, Santos SA, Gomes WF, Coimbra CC, et al. Involvement of BDNF in knee osteoarthritis: the relationship with inflammation and clinical parameters. Rheumatol Int. (2014) 34:1153–7. doi: 10.1007/s00296-013-2943-5
23. Gowler PRW, Li L, Woodhams SG, Bennett AJ, Suzuki R, Walsh DA, et al. Peripheral brain-derived neurotrophic factor contributes to chronic osteoarthritis joint pain. Pain. (2020) 161:61–73. doi: 10.1097/j.pain.0000000000001694
24. Sorkpor SK, Galle K, Teixeira AL, Colpo GD, Ahn B, Jackson N, et al. The relationship between plasma BDNF and pain in older adults with knee osteoarthritis. Biol Res Nurs. (2021) 23:629–36. doi: 10.1177/10998004211012479
25. Nijs J, Meeus M, Versijpt J, Moens M, Bos I, Knaepen K, et al. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin Therap Targets. (2015) 19:565–76. doi: 10.1517/14728222.2014.994506
26. Castrén E, Monteggia LM. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry. (2021) 90:128–36. doi: 10.1016/j.biopsych.2021.05.008
27. Dimmek DJ, Korallus C, Buyny S, Christoph G, Lichtinghagen R, Jacobs R, et al. Brain-derived neurotrophic factor and immune cells in osteoarthritis, chronic low back pain, and chronic widespread pain patients: association with anxiety and depression. Medicina. (2021) 57:327. doi: 10.3390/medicina57040327
28. Da Graca-Tarragó M, Deitos A, Patrícia Brietzke A, Torres IL, Cadore Stefani L, Fregni F, et al. Electrical intramuscular stimulation in osteoarthritis enhances the inhibitory systems in pain processing at cortical and cortical spinal system. Pain Med. (2016) 17:877–91. doi: 10.1111/pme.12930
29. Suchting R, Teixeira AL, Ahn B, Colpo GD, Park J, Ahn H. Changes in brain-derived neurotrophic factor from active and sham transcranial direct current stimulation in older adults with knee osteoarthritis. Clin J Pain. (2021) 37:898–903. doi: 10.1097/AJP.0000000000000987
30. Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. (2003) 54:70–5. doi: 10.1016/S0006-3223(03)00181-1
31. Luo KR, Hong CJ, Liou YJ, Hou SJ, Huang YH, Tsai SJ. Differential regulation of neurotrophin S100B and BDNF in two rat models of depression. Prog Neuropsychopharmacol Biol Psychiatry. (2010) 34:1433–9. doi: 10.1016/j.pnpbp.2010.07.033
32. Zhu L, Weng Z, Shen P, Zhou J, Zeng J, Weng F, et al. S100B regulates inflammatory response during osteoarthritis via fibroblast growth factor receptor 1 signaling. Mol Med Rep. (2018) 18:4855–64. doi: 10.3892/mmr.2018.9523
33. Zhu L, Zhou J, Zeng J, Zhang X, Shen P, Weng F. [The role and mechanism of S100 calcium binding protein B in osteoarthritis cartilage damage repair]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2018) 32:1429–34.
34. Tural U, Irvin MK, Iosifescu DV. Correlation between S100B and severity of depression in MDD: a meta-analysis. World J Biol Psychiatry. (2021) [Online ahead of print].. doi: 10.1080/15622975.2021.2013042
35. Zhang L, Verwer RWH, Zhao J, Huitinga I, Lucassen PJ, Swaab DF. Changes in glial gene expression in the prefrontal cortex in relation to major depressive disorder, suicide and psychotic features. J Affect Disord. (2021) 295:893–903. doi: 10.1016/j.jad.2021.08.098
36. Ballara S, Taylor PC, Reusch P, Marmé D, Feldmann M, Maini RN, et al. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. (2001) 44:2055–64. doi: 10.1002/1529-0131(200109)44:9<2055::AID-ART355>3.0.CO;2-2
37. Song JL, Li L, Fang H, Cai DZ. Intraperitoneal injection of thalidomide alleviates early osteoarthritis development by suppressing vascular endothelial growth factor expression in mice. Mol Med Rep. (2018) 18:571–9. doi: 10.3892/mmr.2018.8980
38. Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im HJ. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J Bone Mineral Res. (2016) 31:911–24. doi: 10.1002/jbmr.2828
39. Qian JJ, Xu Q, Xu WM, Cai R, Huang GC. Expression of VEGF-a signaling pathway in cartilage of ACLT-induced osteoarthritis mouse model. J Orthopaedic Surg Res. (2021) 16:379. doi: 10.1186/s13018-021-02528-w
40. Meng Y, Yin D, Qiu S, Zhang X. Abrine promotes cell proliferation and inhibits apoptosis of interleukin-1β-stimulated chondrocytes via PIM2/VEGF signalling in osteoarthritis. Phytomedicine. (2022) 96:153906. doi: 10.1016/j.phymed.2021.153906
41. Licht T, Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell Mol Life Sci. (2013) 70:1727–37. doi: 10.1007/s00018-013-1280-x
42. Zheng W, Gu LM, Zhou YL, Wang CY, Lan XF, Zhang B, et al. Plasma VEGF concentrations and ketamine’s effects on suicidal ideation in depression with suicidal ideation. Front Psychiatry. (2022) 13:855995. doi: 10.3389/fpsyt.2022.855995
43. Hartley A, Sanderson E, Paternoster L, Teumer A, Kaplan RC, Tobias JH, et al. Mendelian randomization provides evidence for a causal effect of higher serum IGF-1 concentration on risk of hip and knee osteoarthritis. Rheumatology. (2021) 60:1676–86. doi: 10.1093/rheumatology/keaa597
44. Pelsma ICM, Claessen K, Slagboom PE, Van Heemst D, Pereira AM, Kroon HM, et al. Variants of FOXO3 and RPA3 genes affecting IGF-1 levels alter the risk of development of primary osteoarthritis. Eur J Endocrinol Eur. (2021) 184:29–39. doi: 10.1530/EJE-20-0904
45. Hochberg MC, Lethbridge-Cejku M, Scott WW Jr., Reichle R, Plato CC, Tobin JD. Serum levels of insulin-like growth factor in subjects with osteoarthritis of the knee. Data from the Baltimore Longitudinal Study of Aging. Arthritis Rheum. (1994) 37:1177–80. doi: 10.1002/art.1780370811
46. Lis K. [Insulin-like growth factor 1 (IGF-1) and growth hormone (hGH) as the markers of osteoarthritis]. Chirurgia Narzadow Ruchu i Ortopedia Polska. (2008) 73:49–52.
47. Guenther HL, Guenther HE, Froesch ER, Fleisch H. Effect of insulin-like growth factor on collagen and glycosaminoglycan synthesis by rabbit articular chondrocytes in culture. Experientia. (1982) 38:979–81. doi: 10.1007/BF01953688
48. Heilig J, Paulsson M, Zaucke F. Insulin-like growth factor 1 receptor (IGF1R) signaling regulates osterix expression and cartilage matrix mineralization during endochondral ossification. Bone. (2016) 83:48–57. doi: 10.1016/j.bone.2015.10.007
49. Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, et al. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One. (2012) 7:e42676. doi: 10.1371/journal.pone.0042676
Keywords: osteoarthritis, major depression disorder, brain-derived neurotrophic factor, vascular endothelial growth factor, S100B, follow-up
Citation: Zhang P, Xiong Y, Wang B, Zhou Y, Wang Z, Shi J, Li C, Lu X and Chen G (2022) Potential value of serum brain-derived neurotrophic factor, vascular endothelial growth factor, and S100B for identifying major depressive disorder in knee osteoarthritis patients. Front. Psychiatry 13:1019367. doi: 10.3389/fpsyt.2022.1019367
Received: 15 August 2022; Accepted: 10 October 2022;
Published: 25 October 2022.
Edited by:
Dan Song, Shenzhen Shekou People’s Hospital, ChinaReviewed by:
Hussein Kadhem Al-Hakeim, University of Kufa, IraqKenji Hashimoto, Chiba University, Japan
Copyright © 2022 Zhang, Xiong, Wang, Zhou, Wang, Shi, Li, Lu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyan Lu, ZWR1Y2F0aW9ud2IxQDEyNi5jb20=; Gang Chen, bXh5aDE1OTc1M0AxMjYuY29t
†These authors have contributed equally to this work and share senior authorship
 Peng Zhang1
Peng Zhang1 Gang Chen
Gang Chen