
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 13 February 2023
Sec. Mood Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.1016700
This article is part of the Research TopicData Mining Methods for Analyzing Cognitive and Affective Disorders Based on Multimodal Omics-Volume IIView all 6 articles
Background: Studies on the association of homocysteine level with poststroke depression (PSD) have yielded conflicting results. This systematic review and meta-analysis aimed to evaluate the elevated homocysteine level at the acute stage of ischemic stroke in predicting PSD.
Methods: Two authors systematically searched articles indexed in PubMed and Embase databases up to 31 January 2022. Studies evaluating the association of homocysteine level with the development of PSD in patients with acute ischemic stroke were selected.
Results: A total of 10 studies involving 2,907 patients were identified. The pooled adjusted odds ratio (OR) of PSD was 3.72 [95% confidence intervals (CI) 2.03–6.81] for the top vs. bottom homocysteine level. The value of elevated homocysteine level in predicting PSD was stronger in ≥6-month follow-up (OR 4.81; 95% CI 3.12–7.43) than those in ≤ 3-month follow-up subgroup (OR 3.20; 95% CI 1.29–7.91). Moreover, a per unit increase in homocysteine level conferred a 7% higher risk of PSD.
Conclusion: Elevated homocysteine level in the acute stage of ischemic stroke may be an independent predictor of PSD.
Stroke remains a global public health burden resulting in substantial mortality and morbidity (1). Neuropsychiatric disorders represent the common consequence associated with stroke (2). Poststroke depression is the most frequent neuropsychiatric disorder (3, 4). Approximately, over 30% of survivors suffered from poststroke depression at the early or late stage of stroke (5). Poststroke depression has been linked with cognitive impairments (6), functional independence (7), recurrent vascular events (8), and worse survival (9). Considering its negative impacts, the identification of effective biomarkers for predicting poststroke depression is of particular importance.
Homocysteine is an amino acid produced by chemically altering adenosine. The elevated blood level of homocysteine has been identified as an independent risk factor for stroke (10). Moreover, increased homocysteine levels can also be recognized as a predictor of adverse outcomes in patients with acute ischemic stroke (11). Interestingly, homocysteine level was higher in individuals with depression than in healthy controls (12). However, available studies regarding the association of homocysteine level with depression after stroke have yielded conflicting results (13–18). Nevertheless, the findings of these studies were established on a small number of patients.
Currently, no previous meta-analysis has yet investigated the association between homocysteine level and the development of poststroke depression. To address this knowledge gap, we conducted this systematic review and meta-analysis to evaluate the utility of elevated homocysteine levels at the acute stage in predicting poststroke depression among ischemic stroke survivors.
This systematic review and meta-analysis were conducted and reported according to the checklist of Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Two authors systematically searched articles indexed in PubMed and Embase databases up to 31 January 2022. Search keywords included the following terms in combination: “depression” OR “poststroke depression” OR “post-stroke depression” AND “stroke” AND “homocysteine” OR “hyperhomocysteinemia.” We also manually searched the reference lists of each related article to find any possible missing studies.
The inclusion criteria were as follow: (1) participants with a diagnosis of ischemic or hemorrhage stroke, (2) blood homocysteine level at the acute stage as exposure, (3) depression diagnosed by a valid instrument after stroke as an outcome measure, (4) provided the multivariate-adjusted relative risk of poststroke depression for the top vs. bottom homocysteine level or per unit increase in homocysteine level, and (5) study design: cohort study or post hoc analysis of clinical trials. The exclusion criteria included: (1) patients with a history of depression before stroke onset, (2) case-control as study design, and (3) poststroke depression was diagnosed <1 month.
The data collected from the eligible study included: the first author's name, publication year, year of publication, type of stroke, study design, patients' number, percentage of the male gender, age of patients, tool and time of depression assessment, the prevalence of poststroke depression, the fully adjusted relative risk of poststroke depression, and adjusted covariates. The risk of bias in included studies was evaluated using the Newcastle–Ottawa Scale (NOS) for the cohort studies (19). Studies with seven points or more were defined as low risk of bias. The discrepancy between the two authors was resolved by discussion.
Meta-analyses were carried out using STATA 12.0 (Stata Corp LP, College Station). The association of elevated homocysteine level with poststroke depression was expressed by pooling fully adjusted odds ratio (OR) with 95% confidence intervals (CI) for the top vs. bottom homocysteine level or per unit increase in homocysteine level. Heterogeneity across studies was measured using the I2 statistic (I2 > 50% indicating significance) and Cochran's Q-test (p < 0.10 indicating significance). The selection of a fixed-effect model or random-effect model was based on the without or with significant heterogeneity. Publication bias was evaluated by Begg's test (20) and Egger's test (21). Leave-one-out sensitivity analysis was performed to investigate the reliability of the pooling risk summary. Subgroup analyses were conducted according to the time of poststroke depression assessment (≤ 3 vs. ≥6 months).
Figure 1 shows the study selection process. Briefly, a total of 218 potentially relevant articles were identified through electronic and manual searches. After excluding duplicate publications and evaluating titles or abstracts, 24 articles were retrieved for full-text evaluation. In total, 14 articles were further removed after applying the inclusion and exclusion criteria. Thus, 10 studies (13–18, 22–25) were finally included in this meta-analysis.
Table 1 shows a summary of the main characteristics of eligible studies. All eligible studies were conducted in China and published between 2014 and 2020. One study (18) was a post hoc analysis of clinical trials and others were prospective cohort studies. The sample sizes of individual studies ranged from 191 to 408, with a total of 2,907 patients. The time of poststroke depression assessment was between 3 months and 1 year. Depression was determined by the Diagnostic and Statistical Manual of Mental Disorders (DSM) and Beck Depression Inventory Fast Screen (BDI-FS) criteria. The prevalence of poststroke depression ranged between 23.3 and 43.5%. According to the NOS, all the included studies were grouped as low risk of bias (NOS 6–8 points).
Five studies (15, 16, 18, 22, 23) reported the association of elevated homocysteine levels with poststroke depression by categorical analysis. As shown in Figure 2, significant heterogeneity (I2 = 82.5%, p < 0.001) across studies was found. The pooled adjusted OR of poststroke depression was 3.72 (95% CI 2.03–6.81) for the top vs. bottom homocysteine level. Egger's test (p = 0.026) but not Begg's test (p = 0.462) suggested evidence of publication bias. After imputing two potential missing studies, the pooled OR of poststroke depression (OR 2.41; 95% CI 1.04–5.58) remained statistically significant under a trim-and-fill analysis (Figure 3). Leave-one-out sensitivity analysis demonstrated the robustness of the pooling risk summary (all p-values < 0.05; Supplemental Table S1). Subgroup analysis showed that the value of elevated homocysteine level in predicting PSD was stronger in ≥6-month follow-up studies (OR 4.81;95% CI 3.12–7.43; Figure 2A) than those ≤ 3-month follow-up studies (OR 3.20;95% CI 1.29–7.91; Figure 2B).
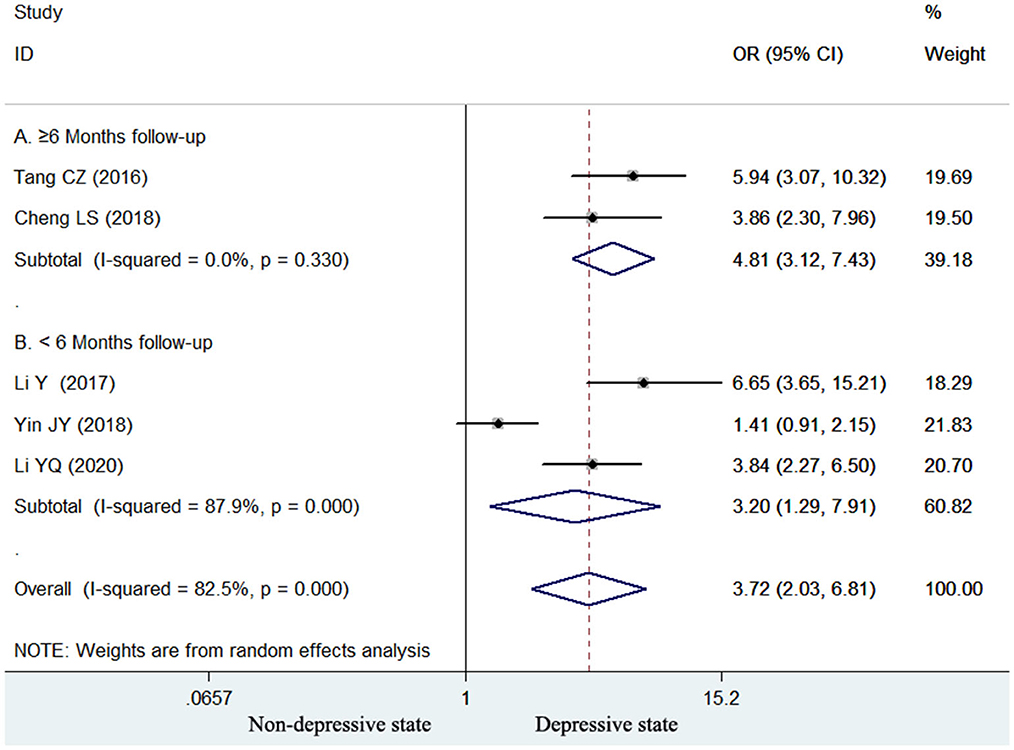
Figure 2. Forest plots showing the pooled OR with 95% CI of poststroke depression for the top vs. bottom homocysteine level in a random effect model.
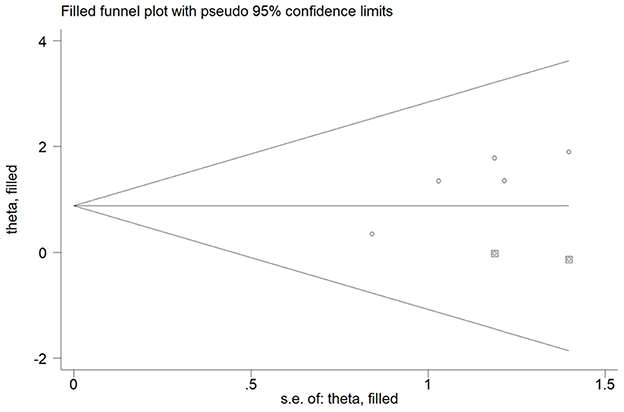
Figure 3. Funnel plot showing the impact of elevated homocysteine level on poststroke depression. The circles alone are real studies and the circles enclosed in boxes are “filled” studies.
Seven studies (13–17, 24, 25) reported the value of homocysteine level in predicting poststroke depression by continuous variable analysis. As shown in Figure 4, no significant heterogeneity (I2 = 27.5%, p = 0.219) across studies was found. The pooled adjusted OR of poststroke depression was 1.07 (95% CI 1.04–1.10) for per unit increase in homocysteine level. Begg's test (p = 0.548) and Egger's test (p = 0.114) suggested without evidence of publication bias. Leave-one-out sensitivity analysis confirmed the reliability of the pooling risk summary (all p-values < 0.05; Supplemental Table S2). Subgroup analysis showed that the value of elevated homocysteine level in predicting poststroke depression was stronger in ≥6-month follow-up studies (OR 1.13; 95% CI 1.06–1.21; Figure 4A) than those ≤ 3-month follow-up studies (OR 1.06; 95% CI 1.03–1.09; Figure 4B).
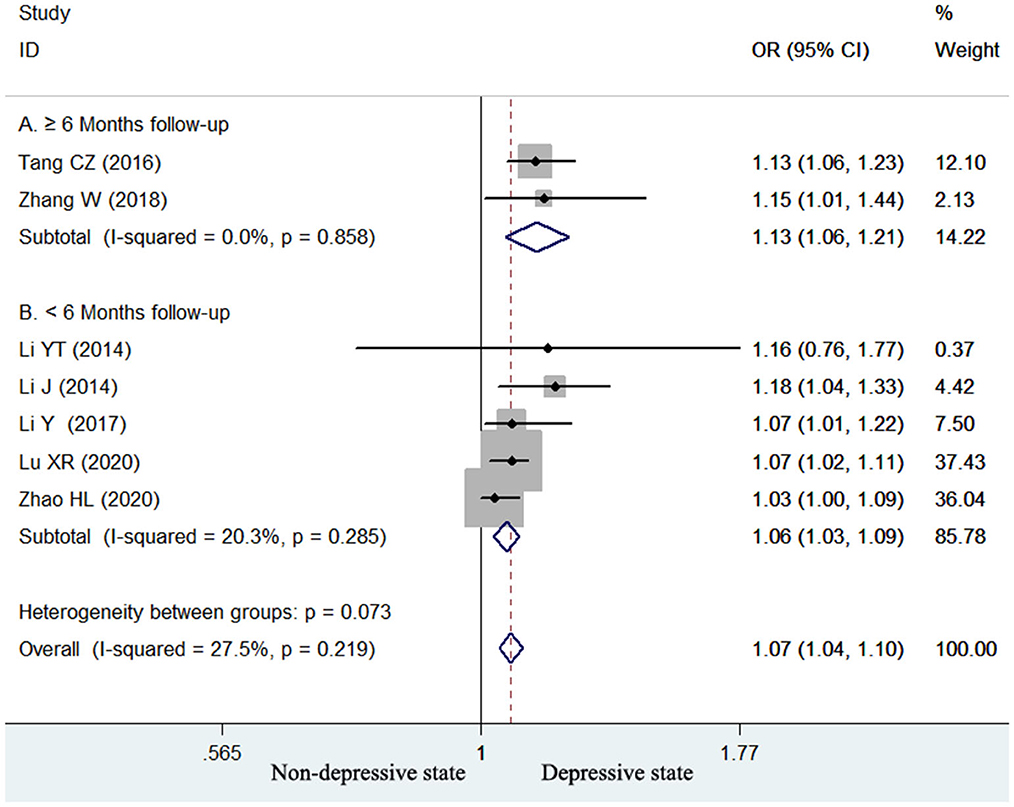
Figure 4. Forest plots showing the pooled OR with 95% CI of poststroke depression for per unit increase in homocysteine level in a fixed-effect model.
The current systematic review and meta-analysis analyzed the impact of homocysteine level at the acute stage of ischemic stroke on the development of depression. This meta-analysis demonstrated that acute ischemic stroke patients with elevated homocysteine levels had an increased risk of poststroke depression in stroke survivors. Ischemic stroke survivors with the top homocysteine level had a 3.72-fold higher risk of poststroke depression compared with those with bottom homocysteine levels. Furthermore, a per unit increase in homocysteine level conferred a 7% higher risk of poststroke depression. Blood homocysteine level at the acute stage of ischemic stroke may be an independent predictor of poststroke depression.
A previous meta-analysis (12) has demonstrated that hyperhomocysteinemia had a 34% higher risk of depression in the general population. Our meta-analysis specially focused on the impact of elevated homocysteine levels on depression in patients with acute ischemia. Compared with the general population, the presence of stroke was found to reinforce the predictive value of homocysteine level. A well-designed case-control study (26) showed that stroke survivors (at least 9 months) with major poststroke depression had higher levels of serum homocysteine than those with similar age and functional ability. Another Swedish cohort study (27) demonstrated that elevated homocysteine level was a significant predictor of depressive symptoms among stroke survivors even after accounting for age and gender. These studies further supported that homocysteine may involve in the development of poststroke depression.
The mechanisms underlying elevated homocysteine levels' relationship to the development of poststroke depression have not been fully elucidated. Plausible explanations underlying this association were as follows: (1) elevated homocysteine level can cause neurotransmitter deficiency and inhibit monoamine neurotransmitter metabolism (28); (2) hyperhomocysteinemia can increase the vulnerability of hippocampal neurons to neurotoxic (29, 30) and oxidative injury (31, 32); (3) homocysteine can exaggerate microglia activation and neuroinflammation (33); and (4) homocysteine can upregulate the N-methyl-D-aspartate receptors-mediated synaptic alterations (34).
Depression most frequently developed within the first year after a stroke (35). Subgroup analyses suggested that the value of homocysteine level in predicting poststroke depression appeared to be stronger in ≥6-month follow-up studies than those in ≤ 3-month follow-up studies in both categorical and continuous variable analyses. These findings revealed that the association between elevated homocysteine level and poststroke depression tended to be strengthened with the lengthening of follow-up. Therefore, ischemic stroke patients with elevated homocysteine levels should be early managed with homocysteine-lowering agents.
Depressive symptoms are common among stroke survivors (36). The reported prevalence of poststroke depression ranged between 23.3 and 43.5% in the included studies. Poststroke depression can further increase morbidity and mortality risk. Considering the positive correlation of hyperhomocysteinemia with poststroke depression, the determination of blood homocysteine level can provide early prognostic information. On the other hand, whether management of hyperhomocysteinemia can reduce depression risk among stroke survivors is another interesting issue. However, a randomized controlled trial showed that lowering homocysteine levels by supplementation with vitamin B12 and folic acid did not reduce depressive symptoms in older adults with hyperhomocysteinemia (37). Future clinical trials are warranted to investigate the impact of the homocysteine-lowering intervention on the development of poststroke depression.
Several limitations should be acknowledged in our study. First, blood homocysteine level was tested only at the acute stage of stroke rather than dynamic measurement. Single detection of homocysteine level at a time point may have led to selection bias. Second, various thresholds of elevated homocysteine levels were reported in the analyzed studies, thus preventing clinicians from discriminating against those in need of homocysteine-lowering intervention. Third, significant heterogeneity (I2 = 82.5%) was present in the pooling risk summary by categorial homocysteine analysis. Different thresholds of elevated homocysteine level, time of depression assessment, and methods of depression diagnosis may contribute to the heterogeneity. Fourth, the lack of adjusting several uncontrolled confounding factors such as folate, vitamin B6, or vitamin B12 level may confound the pooling risk estimate. Fifth, all included patients were from the Chinese population and generalization of the current results to other patients should be done with caution. Finally, this meta-analysis could not distinguish the impacts of elevated homocysteine levels on different types of depression and subtypes of ischemic stroke.
Elevated homocysteine level at the acute stage of ischemic stroke may be an independent predictor of poststroke depression in stroke survivors. Future studies are necessary to investigate whether homocysteine-lowering intervention can reduce the development of poststroke depression.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Study conception, design, and revising the article critically for important intellectual content: JZ. Literature search, acquisition of data, data extraction, and interpretation of data: YLia and XS. Data analysis: LC and YLi. Drafting the article: YLia. All authors have read the final approval of the version to be published.
This research was supported by the Foshan Science and Technology Innovation Project of Guangdong Province of China (Medical Technology Innovation Platform) (Grant No. FS0AA-KJ218-1301-0011) and Medical Scientific Research Project of Health Bureau of Foshan City of Guangdong Province of China (Grant No. 20230824A010505).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1016700/full#supplementary-material
1. Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Nemani K, Gurin L. Neuropsychiatric complications after stroke. Semin Neurol. (2021) 41:85–100. doi: 10.1055/s-0040-1722723
3. Alajbegovic A, Djelilovic-Vranic J, Nakicevic A, Todorovic L, Tiric-Campara M. Post stroke depression. Med Arch. (2014) 68:47–50. doi: 10.5455/medarh.2014.68.47-50
4. Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. (2014) 9:1017–25. doi: 10.1111/ijs.12357
5. Schottke H, Giabbiconi CM. Post-stroke depression and post-stroke anxiety: prevalence and predictors. Int Psychogeriatr. (2015) 27:1805–12. doi: 10.1017/S1041610215000988
6. Arauz A, Rodriguez-Agudelo Y, Sosa AL, Chavez M, Paz F, Gonzalez M, et al. Vascular cognitive disorders and depression after first-ever stroke: the Fogarty-Mexico Stroke Cohort. Cerebrovasc Dis. (2014) 38:284–9. doi: 10.1159/000366471
7. Ezema CI, Akusoba PC, Nweke MC, Uchewoke CU, Agono J, Usoro G. Influence of post-stroke depression on functional independence in activities of daily living. Ethiop J Health Sci. (2019) 29:841–6. doi: 10.4314/ejhs.v29i1.5
8. Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, et al. Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2017) 48:e30–43. doi: 10.1161/STR.0000000000000113
9. Cai W, Mueller C, Li YJ, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. (2019) 50:102–9. doi: 10.1016/j.arr.2019.01.013
10. Wu X, Zhou Q, Chen Q, Li Q, Guo C, Tian G, et al. Association of homocysteine level with risk of stroke: a dose-response meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. (2020) 30:1861–9. doi: 10.1016/j.numecd.2020.07.026
11. Zhang H, Huang J, Zhou Y, Fan Y. Association of homocysteine level with adverse outcomes in patients with acute ischemic stroke: a meta-analysis. Curr Med Chem. (2021) 28:7583–91. doi: 10.2174/0929867328666210419131016
12. Moradi F, Lotfi K, Armin M, Clark CCT, Askari G, Rouhani MH. The association between serum homocysteine and depression: a systematic review and meta-analysis of observational studies. Eur J Clin Invest. (2021) 51:e13486. doi: 10.1111/eci.13486
13. Li J, Zhao YD, Zeng JW, Chen XY, Wang RD, Cheng SY. Serum brain-derived neurotrophic factor levels in post-stroke depression. J Affect Disord. (2014) 168:373–9. doi: 10.1016/j.jad.2014.07.011
14. Li YT, Zhao Y, Zhang HJ, Zhao WL. The association between serum leptin and post stroke depression: results from a cohort study. PLoS ONE. (2014) 9:e103137. doi: 10.1371/journal.pone.0103137
15. Tang CZ, Zhang YL, Wang WS Li WG, Shi JP. Serum levels of high-sensitivity C-reactive protein at admission are more strongly associated with poststroke depression in acute ischemic stroke than homocysteine levels. Mol Neurobiol. (2016) 53:2152–60. doi: 10.1007/s12035-015-9186-2
16. Li Y, Cao LL, Liu L, Qi QD. Serum levels of homocysteine at admission are associated with post-stroke depression in acute ischemic stroke. Neurol Sci. (2017) 38:811–7. doi: 10.1007/s10072-017-2848-2
17. Zhang W, Wang W, Kuang L. The relation between insulin-like growth factor 1 levels and risk of depression in ischemic stroke. Int J Geriatr Psychiatry. (2018) 33:e228–e33. doi: 10.1002/gps.4774
18. Yin J, Zhong C, Zhu Z, Bu X, Xu T, Guo L, et al. Elevated circulating homocysteine and high-sensitivity C-reactive protein jointly predicts post-stroke depression among Chinese patients with acute ischemic stroke. Clin Chim Acta. (2018) 479:132–7. doi: 10.1016/j.cca.2018.01.011
19. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale. (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed March 6, 2022).
20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
22. Cheng LS, Tu WJ, Shen Y, Zhang LJ, Ji K. Combination of high-sensitivity c-reactive protein and homocysteine predicts the post-stroke depression in patients with ischemic stroke. Mol Neurobiol. (2018) 55:2952–8. doi: 10.1007/s12035-017-0549-8
23. Li YQ, Zhang M, Xue M, Li J, Liu PP, Chen G. Correlation of serum homocysteine and platelet-to-lymphocyte ratio with post-stroke depression. Chin J Stroke. (2020) 15:406–10.
24. Lu X, Duan J, Cheng Q, Lu J. The association between serum growth differentiation factor-15 and 3-month depression after acute ischemic stroke. J Affect Disord. (2020) 260:695–702. doi: 10.1016/j.jad.2019.09.037
25. Zhao H, Mo M, Miao C, Li L, Yang H, Liu Y, et al. Association of serum biomarker neurofilament light concentration with post-stroke depression: a preliminary study. Gen Hosp Psychiatry. (2020) 64:17–25. doi: 10.1016/j.genhosppsych.2020.01.006
26. Chatterjee K, Fall S, Barer D. Mood after stroke: a case control study of biochemical, neuro-imaging and socio-economic risk factors for major depression in stroke survivors. BMC Neurol. (2010) 10:125. doi: 10.1186/1471-2377-10-125
27. Pascoe MC, Crewther SG, Carey LM, Noonan K, Crewther DP, Linden T. Homocysteine as a potential biochemical marker for depression in elderly stroke survivors. Food Nutr Res. (2012) 56. doi: 10.3402/fnr.v56i0.14973
28. Gariballa S. Testing homocysteine-induced neurotransmitter deficiency, and depression of mood hypothesis in clinical practice. Age Ageing. (2011) 40:702–5. doi: 10.1093/ageing/afr086
29. Bhatia P, Singh N. Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundam Clin Pharmacol. (2015) 29:522–8. doi: 10.1111/fcp.12145
30. Kruman, II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, et al. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. (2000) 20:6920–6. doi: 10.1523/JNEUROSCI.20-18-06920.2000
31. Widner B, Fuchs D, Leblhuber F, Sperner-Unterweger B. Does disturbed homocysteine and folate metabolism in depression result from enhanced oxidative stress? J Neurol Neurosurg Psychiatry. (2001) 70:419. doi: 10.1136/jnnp.70.3.419
32. Nath N, Prasad HK, Kumar M. Cerebroprotective effects of hydrogen sulfide in homocysteine-induced neurovascular permeability: involvement of oxidative stress, arginase, and matrix metalloproteinase-9. J Cell Physiol. (2019) 234:3007–19. doi: 10.1002/jcp.27120
33. Chen S, Dong Z, Cheng M, Zhao Y, Wang M, Sai N, et al. Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J Neuroinflammation. (2017) 14:187. doi: 10.1186/s12974-017-0963-x
34. Wang M, Liang X, Zhang Q, Luo S, Liu H, Wang X, et al. Homocysteine can aggravate depressive like behaviors in a middle cerebral artery occlusion/reperfusion rat model: a possible role for NMDARs-mediated synaptic alterations. Nutr Neurosci. (2022) 1−13. doi: 10.1080/1028415X.2022.2060642
35. Zhang N, Wang CX, Wang AX, Bai Y, Zhou Y, Wang YL, et al. Time course of depression and one-year prognosis of patients with stroke in mainland China. CNS Neurosci Ther. (2012) 18:475–81. doi: 10.1111/j.1755-5949.2012.00312.x
36. Mitchell AJ, Sheth B, Gill J, Yadegarfar M, Stubbs B, Yadegarfar M, et al. Prevalence and predictors of post-stroke mood disorders: a meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen Hosp Psychiatry. (2017) 47:48–60. doi: 10.1016/j.genhosppsych.2017.04.001
37. de Koning EJ, van der Zwaluw NL, van Wijngaarden JP, Sohl E, Brouwer-Brolsma EM, van Marwijk HW, et al. Effects of two-year vitamin B12 and folic acid supplementation on depressive symptoms and quality of life in older adults with elevated homocysteine concentrations: additional results from the B-PROOF Study, an RCT. Nutrients. (2016) 8:748. doi: 10.3390/nu8110748
Keywords: poststroke depression, homocysteine, risk factor, meta-analysis, acute ischemic stroke
Citation: Liang Y, Shi X, Chen L, Li Y and Zhong J (2023) Homocysteine level at the acute stage of ischemic stroke as a biomarker of poststroke depression: A systematic review and meta-analysis. Front. Psychiatry 13:1016700. doi: 10.3389/fpsyt.2022.1016700
Received: 11 August 2022; Accepted: 05 December 2022;
Published: 13 February 2023.
Edited by:
Marcin Siwek, Jagiellonian University, Medical College, PolandReviewed by:
Alina Wilkowska, Medical University of Gdansk, PolandCopyright © 2023 Liang, Shi, Chen, Li and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Zhong,  emhvbmdqaWFucGluZzAxQG91dGxvb2suY29t
emhvbmdqaWFucGluZzAxQG91dGxvb2suY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.