
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 19 January 2023
Sec. Addictive Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.1014630
This article is part of the Research Topic Appropriateness and Safety of using Cannabinoid and Psychedelic Medicines as Treatments for Psychiatric Disorders View all 7 articles
Targeting the endocannabinoid system may have a role in the treatment of post-traumatic stress disorder (PTSD). However, few studies have examined the effectiveness of cannabis on symptoms of PTSD, and more research is needed to ascertain cannabis’ effectiveness. In this retrospective naturalistic study, we followed 14 relatively mature (32-68 years of age), treatment-resistant, chronic combat post-traumatic patients who remained severely symptomatic despite treatment with many lines of conventional treatment prior to receiving medicinal cannabis. Our findings show that total sleep score, subjective sleep quality, and sleep duration significantly improved (p < 0.01). Total PTSD symptom score and its subdomains (intrusiveness, avoidance, and alertness) showed improvement (p < 0.05). However, there was no improvement in the frequency of nightmares (p = 0.27). The mean follow-up time was 1.1 ± 0.8 years (range of 0.5 to 3 years).
Post-traumatic stress disorder (PTSD) is a mental disorder that can develop after a person is exposed to a traumatic event, such as threatened or actual death, serious injury, or sexual violence. Exposure can be direct, as a witness, as learning a traumatic event happened to a close person, or as repeated or extreme exposure to details of a traumatic event, such as during a line of work. The clinical presentation varies, and symptoms may include: Fear-based reexperiencing (such as in intrusive recollections, nightmares, or dissociative states); Intense physiological or psychological distress when exposed to triggering cues that remind of the traumatic events and persistent avoidance of such cues (internally or externally); Negative alterations in cognition or mood including difficulty in remembering important parts of the event, negative expectations about oneself, others or the future. Persistent negative mood states with decreased ability to feel positive feelings. Diminished interest in previously enjoyed activities and feelings of detachment or estrangement from others; Alterations in arousal and reactivity, including irritable behavior, hypervigilance, exaggerated startle response, and more (1). According to different guidelines for the treatment of post-traumatic stress disorder (PTSD), including the American Veterans Affairs (VA) and American Department of Defense (DoD) guidelines (2), and the International Society for Traumatic Stress Studies (ISTSS) (3), the first line treatment of PTSD includes psychotherapy and/or SSRIs or SNRIs. The most evidence-based and effective treatments are cognitive behavioral therapy (CBT), specifically prolonged exposure (PE), as well as eye-movement desensitization and reprocessing (EMDR) (4, 5). However, a large proportion of patients avoid psychological treatment, and the dropout rate among veterans is high (6). Remission rates with medication are only around 20 to 30%, and their side effects cause low compliance resulting in poor efficacy (7, 8). Considering the limitations of these treatments and the need for effective treatment for patients diagnosed with PTSD, an interest in medical cannabis for PTSD has risen in recent years.
In the past two decades, there has been growing literature implicating the involvement of the endocannabinoid system (eCS) in the etiology of PTSD (9, 10). The endocannabinoid system is a system of cannabinoids produced in our body and includes endogenous cannabinoids, such as N-arachidonoyl ethanolamine (anandamide) and 2-arachidonoyl glycerol (2-AG) and CB1 and CB2 receptors. CB1 receptors are located primarily in the brain and are widely located in the same areas in the brain that are involved in PTSD - the amygdala, hippocampus, and prefrontal cortex (11). CB2 receptors are located primarily in peripheral immunological tissue, although their presence in the central nervous system has also recently been documented. In fact, activating circuits and mechanisms involving CB receptors are similar to pathways involved in PTSD (12).
By activating CB1 receptors in the amygdala, cannabis can potentially reduce fear, anxiety, and aversive memories (13–18). By stimulating CB1 receptors in the prefrontal cortex, cannabis may increase serotonin levels, thus reducing depression, and improving mood, memory, and neurogenesis (17). Cannabis may also decrease hyperarousal and intrusive memories by activating CB1 receptors in the hippocampus and thus might help to reduce PTSD symptoms (13). Furthermore, studies demonstrate a lower concentration of endocannabinoids in patients with PTSD. For example, in a study by Hill et al., endocannabinoid levels were measured in 46 people (24 with PTSD and 22 without PTSD) who were exposed to the 9/11 terror attack. They found that 2-arachidonoylglycerol (2-AG) levels were significantly lower among those who developed PTSD (19).
Cannabinoids are a group of active compounds found in the cannabis plants. The most well-known cannabinoids are Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), which bind to the endocannabinoid receptors mentioned above. Both THC and CBD act on cannabinoid receptors CB1.CBD acts on CB2 too as well as other targets such as Fatty acid amide hydrolase (FAAH), serotonin 5-HT1A receptor and more (20). THC is the primary psychoactive ingredient in cannabis. It reduces anxiety (but can also increase anxiety), improves sleep, reduces nightmares, increases hunger, and helps in the extinction of fear memory (15, 21, 22). Cannabidiol (CBD) is a non-psychotomimetic cannabinoid that causes the least side effects while reducing the anxiety and psychoactive symptoms caused by THC (23). CBD significantly reduces the consolidation of aversive memories and has an anti-inflammatory effect with neuroprotective, analgesic, sedative, antiemetic, antispasmodic, anti-inflammatory, and anxiolytic properties (24). Cannabis use is not without its dangers. Cannabis use disorder afflicts about 22% of cannabis users, and 13% develop cannabis dependence (25). Cannabis use may increase the risk of psychosis and hinder its treatment (26, 27).
Literature reviews from the past two years that have examined the effectiveness of cannabis on PTSD symptoms count a small number of heterogeneous studies (open-label, longitudinal, and retrospective studies) with methodological problems and many limitations (28, 29). To date, there were only two randomized, controlled clinical trials for PTSD patients. The first used the synthetic cannabinoid nabilone (30), and the second used smoked cannabis but for only three weeks and was underpowered to detect significant differentiation from placebo (31). The conclusions of the few systematic reviews examining the effectiveness of cannabis on PTSD symptoms are that cannabis and synthetic cannabinoids may have a role in the treatment of PTSD, but there is currently limited evidence regarding their safety and efficacy (28, 29). Therefore, additional research is needed to better understand the effectiveness and safety of cannabis in the treatment of PTSD.
Israel has one of the longest-running medical cannabis programs in the world, starting in the 1990s (32). However, the use of medical cannabis for PTSD in Israel began only in 2014 (33). The use of cannabis for PTSD increased to almost 10% of total licenses by 2018, although Israel has a lower prevalence of PTSD than the USA (1.5% vs. 6.8%, respectively) (32). Medical cannabis is available as dried buds for inhalation or smoking and as an oil for sublingual ingestion. License for the medicinal use of cannabis for PTSD requires an application sent to the Medical Cannabis Unit at the Ministry of Health on behalf of a patient by the treating psychiatrist. Licenses are valid for a year and require that the psychiatrist request extending the permit annually (34). The initial dose is 20 grams per month, with possible increments of 10 grams at the treating physician’s request. We advised patients to start with a low-THC concentration strain. The cannabis is dispensed at specialized pharmacies given special permit by the Ministry of Health. According to the Ministry of Health guidelines at the time, medical cannabis may be prescribed to patients who are diagnosed with moderate PTSD and above, lasting at least three years, and characterized by great distress. Cannabis will only be given after at least two trials with different drugs and two psychological interventions have been attempted. Contraindications to treatment include a history of psychosis or drug abuse (33). In this study, we examined the effectiveness of medical cannabis among patients suffering from chronic combat-PTSD in a clinical setting in Israel.
The study is a retrospective naturalistic study that used data meticulously gathered in a real-life clinical setting during routine follow-up unrelated to the study. It was approved by the institutional review boards and was conducted following the International Conference on Harmonization guidelines and ethical principles of the Declaration of Helsinki (approval# 0118-19-COM1). Patients consented verbally for their anonymized data to be examined with no patient declining.
Since 2015, our specialized psychiatric trauma unit has offered medical cannabis to treatment-resistant combat-PTSD veterans who fit the MOH criteria. Patients complete questionnaires every six months as part of their treatment follow-up and before applying for a medical cannabis license. The two questionnaires used are the Hebrew versions of the PSQI (35) and PDS (36).
The Pittsburgh Sleep Quality Index Hebrew version (PSQI-H) is a self-administered ten-question questionnaire (Cronbach’s alpha = 0.72). A previous study had the original English questionnaire translated, back-translated, refined, and later validated by fluent speakers of both English and Hebrew. We used the following questions: (A) subjective quality of sleep rated between 0 (very good) to 3 (very bad), (B) duration of sleep in hours, (C) Inability to fall asleep within 30 minutes rated between 0 (not in the past month) to 3 (three or more times a week), and (D) total score calculated from all ten questions (higher scores indicate worse sleep quality). The Posttraumatic Diagnostic Scale (PDS) is a 49-item measure that assesses all the DSM-IV criteria for PTSD and measures symptom severity. For this study, we only used part three of the questionnaire, which includes 17 questions that measure the severity/frequency of PTSD symptoms in the past month, as the diagnosis was already well established. Questions range between 0 (never or once in the past two weeks) to 3 (at least five times a week). Symptoms can be separated into reexperiencing, avoidance, and arousal clusters. Although a formal validation of the Hebrew translation of the PDS has not been published, several theses have implemented similar translations. Weisblum (37) reported that her translation had a satisfactory internal consistency level (Cronbach’s Alpha = 0.90) in a study of parents whose children underwent heart catheterization or cardiac surgery. We used Weisblum’s translation as we found it most accurate and faithful to the original English questionnaire.
We extracted data from the questionnaires given to patients diagnosed with combat-PTSD who started treatment with cannabis at the trauma unit at the Brüll Mental Health Center, Tel Aviv, between 2015 and 2018. The questionnaires were completed as part of the patients’ standard assessment just before receiving medical cannabis and every six months afterward.
This study included all patients who were treated with cannabis for combat-PTSD and who completed PDS and PSQI questionnaires both before starting treatment with cannabis and at least once afterward (no less than six months after starting cannabis treatment). In addition, these patients had to fit the criteria for medical cannabis treatment laid out by the Ministry of Health: (1) PTSD lasting at least three years (2) Moderate or severe severity of PTSD (3) At least two previous medications were used for at least two months, including SSRIs or SNRIs (4) Two psychotherapeutic treatments. Excluded for treatment by the MOH criteria: any history of psychosis or current psychosis or any substance abuse (specifically, patients in our sample stated they had no previous cannabis use).
We compared each patient’s post- and pre-treatment questionnaires using SPSS with paired samples t-test for ratio and quasi-interval variables, Wilcoxon signed-rank test for ordinal variables, and Hodges-Lehmann estimator for confidence intervals for ordinal scale variables. We used the latest questionnaire when more than one post-treatment questionnaire was available. In addition, we used Mann-Whitney U Test to compare gender-specific differences in total PSQI and PDS symptom severity scores.
After screening all patients’ files between 2015-2018, we extracted data from the files of 14 men and women (12 men, 2 women) who met the study’s inclusion and exclusion criteria. No patient files were excluded. Patient’ mean age was 49.5 ± 13.1. Most patients (86%) were currently married and had children. All cases were chronic, with average years since trauma being 27.6 ± 12.3 (range 10-45) and years under treatment prior to being prescribed cannabis averaged 7.7 ± 5.2 (range 1-17). The mean follow-up was 1.1 ± 0.8 years (0.5-3). Comorbidities and previous treatments appear in Table 1.
After treatment with cannabis, total sleep score, subjective sleep quality, and sleep duration significantly improved (p < 0.01). However, improvement in difficulty falling asleep in under 30 minutes was statistically only marginally improved (p = 0.58). Total PTSD symptom score and its subdomains (intrusiveness, avoidance, and alertness) showed improvement (p < 0.05). However, there was no improvement in the frequency of nightmares (p = 0.27). The two women included displayed similar improvement to the 12 men included. Clinical results appear in Table 2 and Figures 1, 2.
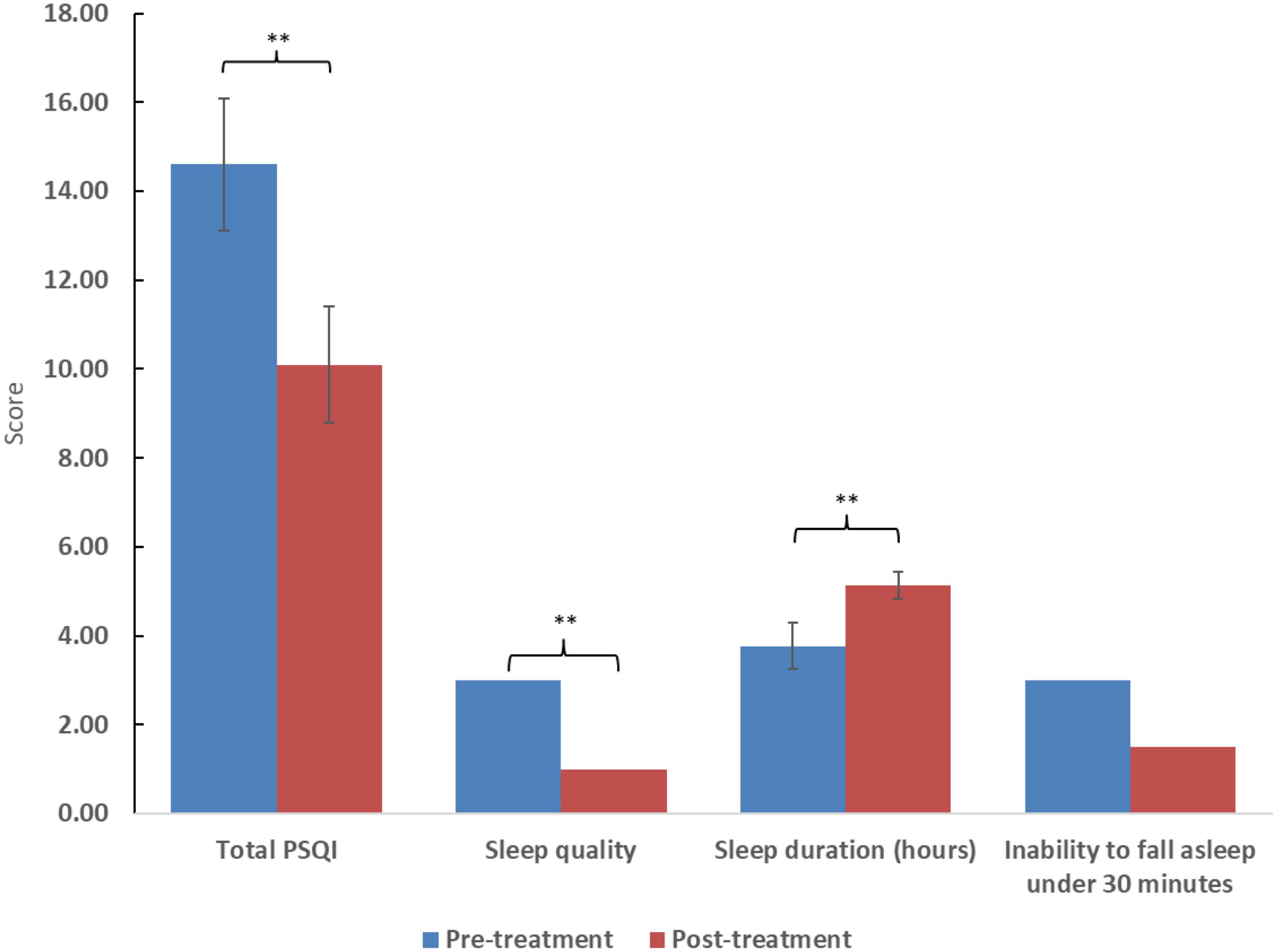
Figure 1. Scores of the posttraumatic diagnostic scale (PDS) and selected subdomains. Higher scores reflect worse symptom severity. Significance: *p < 0.05, **p < 0.01. For ratio and quasi-interval scales, means and standard errors are displayed, while medians are displayed for ordinal scales.
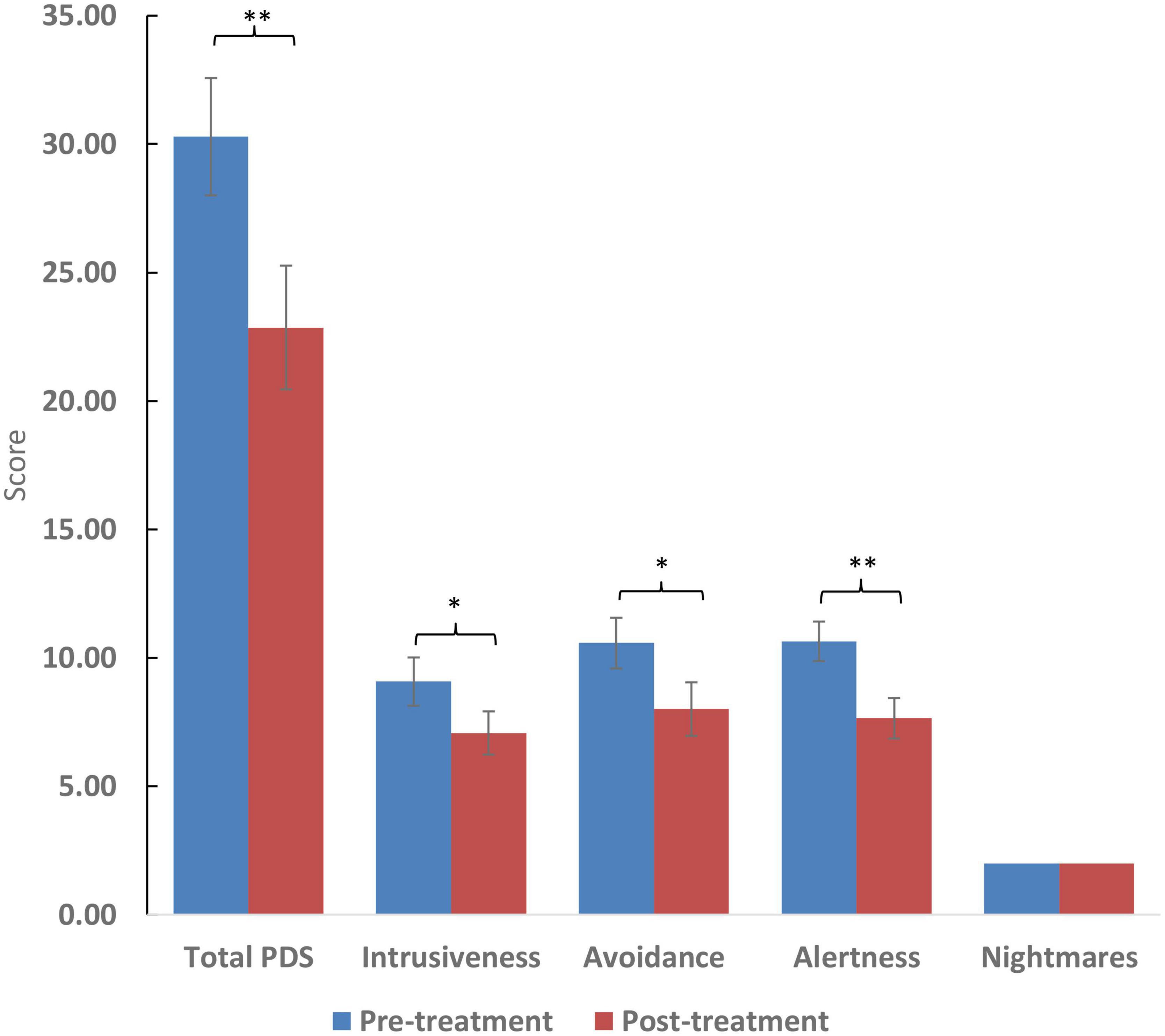
Figure 2. Scores of the pittsburgh sleep quality index (PSQI) and selected questions. Higher scores reflect worse symptom severity except in the case of sleep duration which is measured in hours. Significance: **p < 0.01. For ratio and quasi-interval scales, means and standard errors are displayed, while medians are displayed for ordinal scales.
Regarding gender, we found post-treatment PDS scores to be statistically significantly lower in women compared to men (7 vs. 23.7, p < 0.05) without significant difference pre-treatment (23.5 vs. 30, p = 0.44). In addition, PSQI total scores post-treatment were marginally statistically lower in women (3 vs. 9.9. p = 0.051), but pre-treatment was unavailable as one of the women did not complete the entire PSQI questionnaire correctly.
All patients reported only using cannabis before going to sleep, and no patient reported or displayed any sign of cannabis misuse, abuse, or side effects in repeated psychiatric interviews during follow-ups. No patient stopped using cannabis during the follow-up period. The amount of cannabis did not exceed 20 grams per month, but the minimal amount used could not be discerned.
Many studies have found a possible role for medical cannabis in the treatment of PTSD with no definite conclusion as of yet due to the paucity of studies and methodological limitations (38). We aimed to add to the building body of knowledge by demonstrating the possible usefulness of cannabis for at least a subset of patients with specific properties. In this retrospective naturalistic study, we used the files of patients diagnosed with chronic combat-PTSD who were treated with cannabis after exhausting all other treatment options. Our study is unique primarily due to the nature of the participants. All participants were relatively mature (mean age was 50 years old), treatment-resistant, chronic combat-PTSD patients who had undergone several pharmacological treatment lines and prolonged exposure therapy prior to receiving treatment with cannabis, and were treated in the same clinic by the same expert psychiatrist in the field of PTSD (NN). Prior to treatment with cannabis, patients continued to suffer from moderate to high-level PTSD symptoms despite being in treatment for an average of 7 years. Cannabis was only prescribed after receiving the standard treatment and not as primary treatment, with none of the patients having a background of substance abuse, and all of them had never used cannabis before the study. In addition, the study’s uniqueness is also in the long follow-up of patients, averaging over a year (range 0.5-3 years), enabling the assessment of the effect of cannabis on PTSD symptoms, possible side effects, and the possibility of addiction.
The study’s findings show an overall improvement in sleep quality and duration, as well as a decrease in PTSD symptoms. According to the PDS questionnaire, there was a reduction of at least 20% in PTSD symptoms in over 65% of patients, with nearly 80% showing improvement. Surprisingly, unlike other studies (28), the decrease in nightmares was observed but was not significant, maybe due to the small number of participants.
The widespread use of cannabis today in patients with PTSD, whether legally or otherwise, is often problematic due to the onset of use at a young age and the potential for addiction and dependence (39). There is also a problem with patients preferring cannabis treatment as their first treatment over evidence-based pharmacological and psychological treatments (40). In our study, the pre-selection process created a mostly homogeneous group of relatively mature patients without prior use of cannabis who were treatment-resistant to several evidence-based therapies. By selecting this group, we have shown that cannabis may be beneficial and alleviate some suffering among patients resistant to evidence-based treatments.
It is known that subjects suffering from PTSD tend to use cannabis as a form of self-medication more widely than the general population (41, 42), thus suffering from high rates of substance-related disorders. Our long-term follow-up allowed us to see that none of the patients suffered from side effects and symptoms of addiction. It is possible that the characteristics of our participants (mature, in treatment for many years by the same physician, mostly married with children) led to a more responsible use (just before bedtime, in a fixed amount, without recreational use) which played a role in the good outcome.
The limitations of the study should be noted. First, the study is retrospective, and does not include placebo or control groups. However, it may be argued that as patients were stable in their symptoms prior to cannabis treatment, they were their own controls. Second, while we ensured that the dose of cannabis did not exceed 20 grams per month, the type of compounds, the ratio of CBD to THC, the route of administration, timing of usage, and the actual amount used were not controlled as patients are mainly free to try different strains without notifying their psychiatrist. Third, formal validation of the Hebrew translation of the PDS has not been published as of yet, though several translations were used in various theses and studies. Therefore, we used an unpublished translation that we found most accurate and faithful to the original. Lastly, the sample is relatively small, consisting of only 14 patients, and the majority (86%) were men. Although it seemed that women benefited more from cannabis treatment, the sample size was too small (a total of two women) to draw any conclusions. The gender difference may warrant further study.
To the best of our knowledge, this is the first published study examining long-term cannabis efficacy in chronic combat treatment-resistant PTSD patients. The study we conducted is consistent with existing literature which indicates a decrease in PTSD symptoms under medical cannabis treatment. There are still very few studies examining the effectiveness of medical cannabis for PTSD patients. One needs to remember that the endocannabinoid system works in different ways, even in the same disorder and among different genders and ages, which may also affect outcomes. Our results suggest the importance of the selection process in terms of patients receiving medical cannabis. Future research should clarify the long-term effects of cannabis on different groups of patients suffering from PTSD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study involving human participants was reviewed and approved by the Institutional Review Board of Clalit Health Services and was conducted following the International Conference on Harmonization guidelines and ethical principles of the Declaration of Helsinki (approval# 0118-19-COM1). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
NN: study design, data collection, and writing. CA: data collection, statistical analysis and interpretation, and writing and editing. PT: supervision and writing review and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing (2022). doi: 10.1176/appi.books.9780890425787
2. Department of Veteran Affairs. Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. Washington, DC: Department of Veteran Affairs (2017).
3. International Society for Traumatic Stress Studies. Posttraumatic Stress Disorder Prevention and Treatment Guidelines: Methodology and Recommendations. Chicago, IL: International Society for Traumatic Stress Studies (2019).
4. Forbes D, Creamer M, Bisson J, Cohen J, Crow B, Foa E, et al. A guide to guidelines for the treatment of PTSD and related conditions. J Trauma Stress. (2010) 23:537–52. doi: 10.1002/jts.20565
5. Schnurr P, Friedman M, Engel C, Foa E, Shea M, Chow B, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. (2007) 297:820–30. doi: 10.1001/jama.297.8.820
6. Cook J, O’Donnell C, Dinnen S, Bernardy N, Rosenheck R, Hoff R. A formative evaluation of two evidence-based psychotherapies for PTSD in VA residential treatment programs. J Trauma Stress. (2013) 26:56–63. doi: 10.1002/jts.21769
7. Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle L, Marmar C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuro Psychopharmacol Biol Psychiatry. (2009) 33:169–80. doi: 10.1016/j.pnpbp.2008.12.004
8. Cipriani A, Williams T, Nikolakopoulou A, Salanti G, Chaimani A, Ipser J, et al. Comparative efficacy and acceptability of pharmacological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol Med. (2018) 48:1975–84. doi: 10.1017/S003329171700349X
9. Neumeister A, Seidel J, Ragen B, Pietrzak R. Translational evidence for a role of endocannabinoids in the etiology and treatment of posttraumatic stress disorder. Psychoneuroendocrinology. (2015) 51:577–84. doi: 10.1016/j.psyneuen.2014.10.012
10. Hill M, Campolongo P, Yehuda R, Patel S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. (2018) 43:80–102. doi: 10.1038/npp.2017.162
11. McDonald A, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. (2001) 107:641–52. doi: 10.1016/s0306-4522(01)00380-3
12. Das R, Kamboj S, Ramadas M, Yogan K, Gupta V, Redman E, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology. (2013) 226:781–92. doi: 10.1007/s00213-012-2955-y
13. Atsak P, Hauer D, Campolongo P, Schelling G, McGaugh J, Roozendaal B. Glucocorticoids interact with the hippocampal endocannabinoid system in impairing retrieval of contextual fear memory. Proc Natl Acad Sci U.S.A. (2012) 109:3504–9. doi: 10.1073/pnas.1200742109
14. Bitencourt R, Pamplona F, Takahashi R. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. (2008) 18:849–59. doi: 10.1016/j.euroneuro.2008.07.001
15. Do Monte F, Souza R, Bitencourt R, Kroon J, Takahashi R. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav Brain Res. (2013) 250:23–7. doi: 10.1016/j.bbr.2013.04.045
16. Gomes FV, Reis D, Alves F, Corrêa F, Guimarães F, Resstel L. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT 1A receptors. J Psychopharmacol. (2012) 26:104–13. doi: 10.1177/0269881110389095
17. Lemos J, Resstel L, Guimarães F. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res. (2010) 207:105–11. doi: 10.1016/j.bbr.2009.09.045
18. Pamplona F, Prediger R, Pandolfo P, Takahashi R. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology. (2006) 188:641–9. doi: 10.1007/s00213-006-0514-0
19. Hill M, Bierer L, Makotkine I, Golier J, Galea S, McEwen B, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. (2013) 38:2952–61. doi: 10.1016/j.psyneuen.2013.08.004
20. Almeida D, Devi L. Diversity of molecular targets and signaling pathways for CBD. Pharmacol Res Perspect. (2020) 8:e00682. doi: 10.1002/prp2.682
21. Fraser G. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. (2009) 15:84–8. doi: 10.1111/j.1755-5949.2008.00071.x
22. Buckner J, Jeffries E, Crosby R, Zvolensky M, Cavanaugh C, Wonderlich S. The impact of PTSD clusters on Cannabis use in a racially diverse trauma-exposed sample: an analysis from ecological momentary assessment. Am J Drug Alcohol Abuse. (2018) 44:532–42. doi: 10.1080/00952990.2018.1430149
23. Niesink R, van Laar M. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry. (2013) 4:130. doi: 10.3389/fpsyt.2013.00130
24. Orsolini L, Chiappini S, Volpe U, De Berardis D, Latini R, Papanti G, et al. Use of medicinal Cannabis and synthetic cannabinoids in post-traumatic stress disorder (PTSD): a systematic review. Medicina. (2019) 55:1–14. doi: 10.3390/medicina55090525
25. Leung J, Chan G, Hides L, Hall W. What is the prevalence and risk of Cannabis use disorders among people who use Cannabis? A systematic review and meta-analysis. Addict Behav. (2020) 109:106479. doi: 10.1016/j.addbeh.2020.106479
26. Martinotti G, Di Iorio G, Sepede G, De Berardis D, De Risio L, Di Giannantonio M. Cannabis use and psychosis: theme introduction. Curr Pharm Des. (2012) 18:4991–8. doi: 10.2174/138161212802884627
27. Ricci V, Martinotti G, Ceci F, Chiappini S, Di Carlo F, Burkauskas J, et al. Duration of untreated disorder and Cannabis use: an observational study on a cohort of young Italian patients experiencing psychotic experiences and dissociative symptoms. Int J Environ Res Public Health. (2021) 18:12632. doi: 10.3390/ijerph182312632
28. Rehman Y, Saini A, Huang S, Sood E, Gill R, Yanikomeroglu S. Cannabis in the management of PTSD: a systematic review. AIMS Neurosci. (2021) 8:414–34. doi: 10.3934/Neuroscience.2021022
29. Hindocha C, Cousijn J, Rall M, Bloomfield M. The effectiveness of cannabinoids in the treatment of posttraumatic stress disorder (PTSD): a systematic review. J Dual Diagn. (2020) 16:120–39. doi: 10.1080/15504263.2019.1652380
30. Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. (2015) 51:585–8. doi: 10.1016/j.psyneuen.2014.11.002
31. Bonn-Miller M, Sisley S, Riggs P, Yazar-Klosinski B, Wang J, Loflin M, et al. The short-term impact of 3 smoked Cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. (2021) 16:e0246990. doi: 10.1371/journal.pone.0246990
32. Sznitman S. Trends in medical Cannabis licensure, Israel, 2013–2018. Drug Alcohol Rev. (2020) 39:763–7. doi: 10.1111/dar.13116
33. Medical Cannabis Unit. Procedure 106 – License for Cannabis. Jerusalem: Ministry of health (2022).
34. Medical Cannabis Unit. Procedure 154 – Medical Cannabis: Information and Medical Guide. Jerusalem: Ministry of health (2019).
35. Shochat T, Tzischinsky O, Oksenberg A, Peled R. Validation of the Pittsburgh Sleep Quality Index Hebrew Translation (PSQI-H) in a sleep clinic sample. Isr Med Assoc J. (2007) 9:853–6.
36. Foa E, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: the posttraumatic diagnostic scale. Psychol Assess. (1997) 9:445–51. doi: 10.1037/1040-3590.9.4.445
37. Weisblum R. Contribution of Attachment System and Sense of Coherence to the Long Term Coping of Parents to Children with Congenital Heart Disease ASD (Atrial Septic Defect) That Corrected With Cardiac Surgery or Catheterization. Ramat Gan: Bar-Ilan University (2011).
38. Bedard-Gilligan M, Lehinger E, Cornell-Maier S, Holloway A, Zoellner L. Effects of Cannabis on PTSD recovery: review of the literature and clinical insights. Curr Addict Rep. (2022) 9:203–16. doi: 10.1007/s40429-022-00414-x
39. Millar S, Mongan D, Smyth B, Perry I, Galvin B. Relationships between age at first substance use and persistence of Cannabis use and Cannabis use disorder. BMC Public Health. (2021) 21:997. doi: 10.1186/s12889-021-11023-0
40. Kruger D, Kruger J. Medical Cannabis users’ comparisons between medical Cannabis and mainstream medicine. J Psychoactive Drugs. (2019) 51:31–6.
41. Boden M, Babson K, Vujanovic A, Short N, Bonn-Miller M. Posttraumatic stress disorder and Cannabis use characteristics among military veterans with Cannabis dependence. Am J Addict. (2013) 22:277–84. doi: 10.1111/j.1521-0391.2012.12018.x
Keywords: cannabis, post-traumatic stress disorder (PTSD), treatment-resistant, combat PTSD, posttraumatic diagnostic scale (PDS), pittsburgh sleep quality index (PSQI), sleep quality
Citation: Nacasch N, Avni C and Toren P (2023) Medical cannabis for treatment-resistant combat PTSD. Front. Psychiatry 13:1014630. doi: 10.3389/fpsyt.2022.1014630
Received: 08 August 2022; Accepted: 26 December 2022;
Published: 19 January 2023.
Edited by:
Ben H. Amit, Reuth Medical & Rehabilitation Center, IsraelReviewed by:
Domenico De Berardis, ASL 4, ItalyCopyright © 2023 Nacasch, Avni and Toren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Avni,  Y2hlbi5hdm5pQGNsYWxpdC5vcmcuaWw=
Y2hlbi5hdm5pQGNsYWxpdC5vcmcuaWw=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.