- 1Department of Clinical Research in Neurology, Center for Neurodegenerative Diseases and the Aging Brain, Pia Fondazione Cardinale G. Panico, University of Bari Aldo Moro, Bari, Italy
- 2Department of Neurosciences, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom
- 3Department of Basic Medicine, Neuroscience, and Sense Organs, University of Bari Aldo Moro, Bari, Italy
Delusions are part of the neuropsychiatric symptoms that patients suffering from neurodegenerative conditions frequently develop at some point of the disease course and are associated with an increased risk of cognitive and functional decline. Delirium is a syndrome characterized by acute onset of deficits in attention, awareness, and cognition that fluctuate in severity over a short time period. Delusions and delirium are frequently observed in the context of neurodegeneration, and their presence can easily mislead clinicians toward a misdiagnosis of psychiatric disorder further delaying the proper treatment. Risk factors for developing delusion and delirium in neurodegenerative conditions have been investigated separately while the possible interplay between these two conditions has not been explored so far. With this study, we aim to achieve a more comprehensive picture of the relationship between delusions and delirium in neurodegeneration by analyzing prevalence and subtypes of delusions in different neurodegenerative disorders; providing an overview of clinical tools to assess delusions in neurodegenerative patients and how delusions are covered by delirium assessment tools and discussing the possible common pathophysiology mechanisms between delusion and delirium in neurodegenerative patients. A more extensive characterization of the relationship between delusions and delirium may help to understand whether delusions may constitute a risk factor for delirium and may ameliorate the management of both conditions in patients with neurodegenerative disorders.
Introduction
The term delusion is widely used in scientific literature, however, its conceptualization is challenging and delusions remain among the most elusive concepts in psychiatry (1). The current diagnostic and statistical manual of mental disorders (DSM-V) defines delusions as fixed false beliefs that are not amenable to change in light of conflicting evidence (2). Delusions are considered a multidimensional phenomenon characterized along with a number of themes/delusional beliefs which may vary across the different psychiatric and neurological disorders (3). Delusions are frequently experienced by patients with neurodegenerative disorders during the course of their illness and (4), together with hallucinations, are symptoms that may mimic a psychiatric condition (psychosis mimics) and lead to a misdiagnosis between neurocognitive and psychiatric disorders (5). Moreover, delusions are part of the constellation of symptoms defining delirium, a syndrome characterized by acute onset of deficits in attention, awareness, and cognition that fluctuate in severity over a relatively short time span (typically days or weeks) (6, 7) and similarly may be easily mistaken for a psychiatric disorder. Delusions and delirium are frequently observed during the disease course of neurodegeneration (8, 9), and are associated with overall worse cognitive and functional outcomes (10, 11).
Notwithstanding the prevalence of delusions in patients with neurodegenerative disorders during episodes of delirium has been scarcely investigated, likely due to the fact that when assessing delirium neuropsychiatric symptoms, including delusions and hallucinations, are typically clustered together.
An early study by Lerner et al. that investigated delirium in a small cohort of AD patients showed that a history of hallucinations and paranoid delusions were more common in patients delirium (12). More recently a handful of studies have shown higher frequency and severity of delusions in patients with dementia and delirium compared with patients with dementia without delirium (13). The objective of this article is to achieve a more comprehensive picture of the possible interplay between delusions and delirium in patients with neurodegenerative disorders. To achieve this goal we firstly analyse the prevalence and subtypes of delusional beliefs in neurodegenerative disorders of different etiology (namely, Alzheimer's Disease, Parkinson's Disease, Dementia with Lewy bodies and Frontotemporal lobar degeneration); then, we provide an overview of clinical tools and rating scales to assess delusions in neurodegenerative patients and how delusions are explored by the most commonly used delirium assessment tools. Finally, we discuss the possible common pathophysiology mechanisms between delusion and delirium in neurodegenerative patients.
Delusions in Neurodegenerative Disorders
Alzheimer's Disease
Alzheimer's Disease (AD) is the most prevalent neurodegenerative disorder accounting for an estimated 60-80% of all cases of dementia (14). The underlying mechanisms and causes for AD are still not completely understood. However, it has been established that histopathological characteristics of AD include extracellular β-amyloid peptides deposition and formation of neurofibrillary tangles arising from the intracellular accumulation of hyperphosphorylated Tau proteins (15–17). Clinically, AD presents as a slow progressive disease with a long preclinical phase and disease progression which spans several stages and includes preclinical Alzheimer's disease, mild cognitive impairment (MCI) due to Alzheimer's disease, and Alzheimer's disease dementia (18, 19). More recently, it has been advanced the concept of AD as a continuum, spanning from an asymptomatic phase where pathophysiological changes are already evident, to the symptomatic phase where biomarkers changes continue and symptoms progressively worsen leading to the eventual loss of independence (20). Neuropsychiatric symptoms (NPS) such as aggression, depression, apathy, and psychosis are increasingly recognized to be significant features of Alzheimer's disease and are reported to be associated with faster cognitive decline (21–23). The concept of mild behavioral impairment (MBI) has been recently proposed as a diagnostic construct aimed to identify patients with NPS, who have an increased risk of developing dementia, but may not present cognitive symptoms (24). Delusions in AD have been described since Alois Alzheimer's index case of A. Deter, who presented initially with emotional distress and delusions of infidelity, followed by cognitive impairment (25).
In early observational studies, the prevalence of delusions in AD was reported to be between 10 and 73 % (26). Differences in assessment tools used along with the different definitions of delusions and the clinical population investigated may be responsible for the wide difference in prevalence across studies. A systematic review and meta-analysis that investigated the prevalence of neuropsychiatric symptoms in AD, reported an overall pooled delusions prevalence of 31% although with considerable heterogeneity across individual studies results (27). Indeed the authors highlighted that the prevalence was influenced by population origin and disease duration. Similarly, a previous study showed that a longer duration of illness was independently associated with the presence of psychosis and delusions (28), although this finding was not been confirmed in subsequent studies (29). By contrast, it has been shown that the severity of cognitive decline is predictive of delusions. The cumulative incidence rates for delusions in patients with probable AD in a study by Paulsen et al. were 20.1, 36.1, 49.5, and 51.3%, at 1-, 2-, 3-, and 4-year postbaseline evaluations, respectively. Another study by D'Onofrio et al. found that patients with AD who had delusions had higher grades of cognitive impairment and a more severe stage of dementia assessed by the Clinical Dementia Rating Scale (CDR) (30). Delusions have been associated with increased risk of cognitive and functional decline (31), and increased risk of mortality (30) although additional studies are required to confirm this association. Delusions appear to be consistently associated with older age whilst contrasting evidence are reported regarding sex, other demographic variables as well as the use of medications and medical comorbidities as possible risk factors for developing delusions (32, 33). Delusions are frequently associated with other psychological conditions (34–36) like depression and in turn, may increase the risk of developing other neuropsychiatric symptoms including agitation aggression, depressed mood, apathy, irritability, aberrant motor activity, sleep disturbances, eating disorders and hallucinations (30). Most notably, up to 44% of patients with AD may experience delusions associated with hallucinations. D'Onofrio et al. found that delusions of theft were the main delusion's subtype in patients with AD (50.4%) whilst delusions of abandonment, persecution and infidelity were found in 25.6, 15.7, and 8.3% of cases, respectively (30). Misidentification phenomena have also been reported in AD including phantom boarder delusion and the “one's house is not the one's home” delusion (37, 38). More rarely AD patients exhibit paranoid delusions. A study by Naasan et al. found paranoid delusions were reported in 9.1 % of autopsy-confirmed patients with AD, persecutory in 1.8 % and misidentification of people in 4.5 % (39).
Dementia With Lewy Bodies and Parkinson's Disease
Dementia with Lewy bodies (DLB) is the second most common degenerative dementia. It is characterized by visual hallucinations, cognitive fluctuation, parkinsonism and dementia. Parkinson's disease dementia (PDD) is defined as dementia that arises in the context of established Parkinson's disease (PD), and shares both clinical and neuropathological characteristics with DLB (40). It is believed that DLB and PDD represent a spectrum disorder with similar cortical and subcortical Lewy body pathology but different onset of cognitive and motor symptoms. The landscape of psychosis in Parkinson's disease (PD) has been redefined in 2007 by consensus recommendations from an international workgroup (41). In PD, psychosis may exist on a continuum in which minor phenomena progress from hallucinations with retained insight to hallucinations with loss of insight and delusions (42). Delusions in PD are less frequent than hallucinations. Prevalence estimates range from 3 to 16% (43–46) and are higher in PD dementia (PDD; 19–51%) (47–49). Delusions are most commonly paranoid and the most common theme is infidelity (44). Delusional misidentification syndromes, a specific subset of delusions characterized by pathological familiarity, including Capgras syndrome and reduplicative paramnesia have also been described in PD (50–52). By contrast, delusions are very common in DLB, occurring in up to 60 % of cases (53, 54), and have become one of the supportive features for the clinical diagnosis of DLB (55, 56). A study by Nagahama et al. showed that hallucinations, misidentification experiences and delusions were found, respectively, in 78, 56, and 25% of DLB cases (57). In particular, misidentifications of people (17%), of objects (28%), reduplication of people (10%) and phantom boarder (11%) were the most frequent misidentification symptoms whilst delusions of theft (14%) and persecution (11%) were the most common delusions. Furthermore, Naasan et al. have also shown that in patients with DLB and AD co-pathology, delusions were more frequent than in patients with AD and Frontotemporal lobar degeneration (FTLD) (39). In 20% of cases, they were characterized by more than one subtype and in 10% of cases, they occurred in the first 3 years of the disease.
In patients with DLB and comorbid AD pathology, the most frequent delusions were reported to be of paranoid content (19%) followed by misidentification of people (13.8%) and place (10.3%). The persecutory content and of grandeur occurred in 8.5% of cases and of jealousy in 5.2% (39).
Aarsland et al. investigated delusions in DLB, and PD with and without dementia. The authors found that delusions were significantly more common in DLB (57%) than PDD (29%) and PD without dementia (7%) (53). Phantom boarder phenomenon and paranoid ideation were the most common delusion in these disorders. Finally, it has been shown that patients with DLB who presented delusions have higher disease severity, more neuropsychiatric symptoms and are more cognitively impaired (58). Frequent and distressing delusions in DLB have been associated with higher caregiver burden and with worse patient's quality of life compared with patients with other neurodegenerative disorders (58).
Frontotemporal Lobar Degeneration
Frontotemporal lobar degeneration (FTLD) is a clinically and pathologically heterogeneous syndrome associated with degeneration of the frontal and anterior temporal lobes and clinically characterized by a progressive impairment of behavior and/or language. Clinical variants include behavioral FTD, mainly characterized by changes in personality and behavior, and FTLD- associated aphasic syndromes, namely primary progressive aphasia (PPA) which is divided into semantic, progressive non-fluent and logopenic variants (59–61). The majority of pathologies associated with FTLD clinical syndromes include either tau-positive (FTLD-TAU) or TAR DNA-binding protein 43 (TDP-43)-positive (FTLD-TDP) inclusion bodies whilst AD pathology has been found in more than 70% of logopenic PPA cases (61).
A study estimated delusion prevalence of 14% in FTD patients, with paranoid and somatic being the most more frequent types of delusions (62). More recently, Sha et al. found that delusions were the presenting neuropsychiatric symptom in 21% of patients with bvFTD with an expansion in C9orf72, in 18% of patients with FTD and motor neuron disease (FTD-MND) carrying the C9orf72 mutation, and in 10.5% of the FTD-MND non-carrier (63). Naasan et al. investigated psychotic symptoms in autopsy confirmed neurodegenerative pathology and found that patients with FTLD-TDP were significantly more likely to have delusions (34.8%) compared to patients with AD and FTLD-tau (16.2 and 10.5%, respectively). In FTLD-TDP, delusions tend to occur in the first 3 years of the disease and are characterized by bizarre content in 7.4%, paranoid content in 26.5% and persecutory content in 14.5% of cases. Furthermore, patients with FTLD-TDP had also frequently delusions of misidentification and grandiosity and were primarily seen in patients with FTLD-TDP-B pathology at a frequency of 9.4%. By contrast, In FTLD-tau delusions were 8.3% of paranoid content and 3.8% persecutory (39).
Delusions Assessment in Neurocognitive Disorders and Cognitive Impairment
Several assessment tools have been developed according to the DSM-V definition of delusions, mainly aimed at allowing clinicians to ascertain the presence and intensity of delusion in different clinical settings and to investigate the association between specific delusional beliefs and psychiatric disorders of different etiology. Nonetheless, instruments specifically developed to evaluate the presence and features of delusional beliefs in patients suffering neurocognitive disorders and mild cognitive impairment are lacking. Indeed, the general approach of studies on delusion in neurocognitive disorders consists in administering multidimensional scales that assess delusions within a wide range of behavioral disturbances. Therefore, information on sensitivity, specificity and accuracy of these tools in identifying the presence of delusions are not currently available.
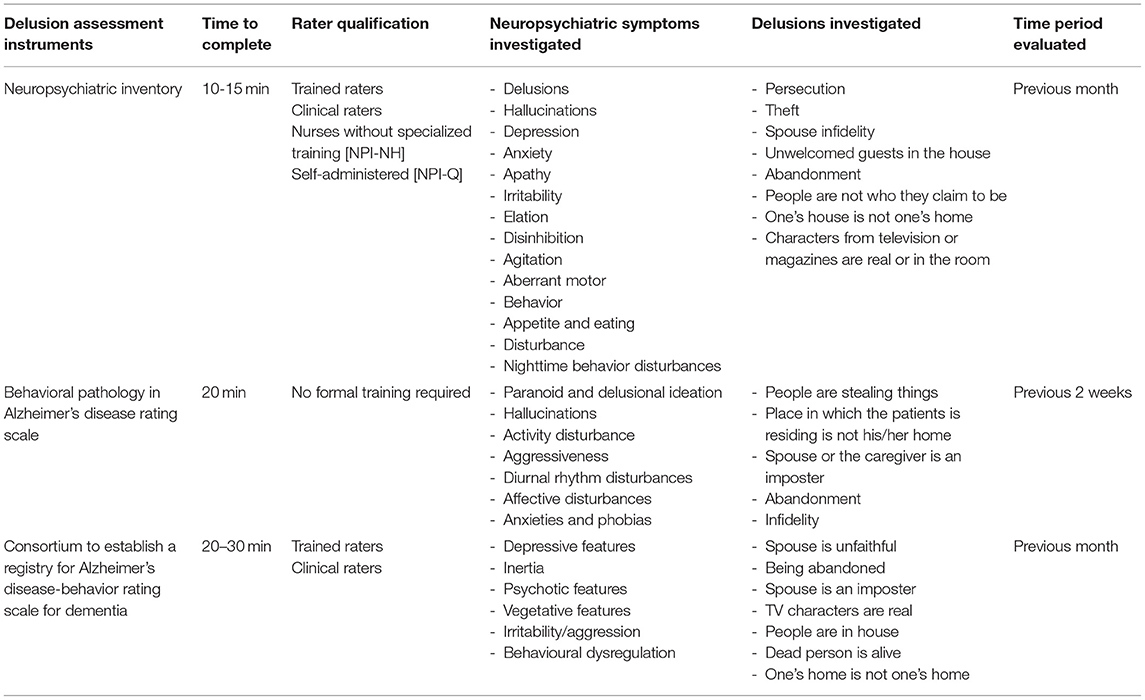
The Neuropsychiatric Inventory (NPI) and the Behavioral Pathology in Alzheimer's Disease Rating Scale (BEHAVE-AD) have been the two most widely used instruments for this purpose followed by the Consortium to Establish a Registry for Alzheimer's Disease-Behavior Rating Scale for Dementia (CERAD-BRSD) (Table 1) (64). The NPI has been by far the most commonly used instrument (65). The NPI is based on a structured interview conducted with a caregiver and/or patient's relative that takes ~10-15 min. The interview has to be administered to a caregiver sufficiently good at assessing the patient's behavior and requires a trained psychologist or clinician. The NPI assesses 12 neuropsychiatric disturbances, including delusions, experienced by the patient over the past month via screening questions (66). Each neuropsychiatric disturbance is rated for presence (range 0-4) and severity (range 0-3). NPI total score ranges between 0 and 144 with higher scores indicating greater behavioral disturbance. A domain score ≥ 4 is indicative of clinically significant symptoms.
If the delusions are present a scripted question is asked in order to assess their features and the caregiver is asked to rate delusions frequency, severity, and associated distress. More in detail the NPI assess the following delusional beliefs: delusions of persecution and theft (paranoid delusions), spouse infidelity (Othello syndrome), unwelcomed guests in the house (phantom boarder delusion), abandonment, people not being who they claim to be (Capgras syndrome), delusion that “one's house is not one's home” and delusion that characters from television or magazines are real or in the room.
The NPI exhibit excellent psychometric proprieties (67), has been translated into more than 40 languages (66), and is available in different versions including a version for unsupervised completion and a version for use in residential settings (68, 69).
The BEHAVE-AD is the second most commonly used instrument to assess delusional beliefs in AD and related dementia (70). The BEHAVE-AD is a clinical rating scale that evaluates changes in patient's behavior over the previous 2 weeks. It is based on an interview conducted with the caregiver or a patient's relative which takes ~20 min. The original version of the scale rated symptoms based on severity and provides separate measures for delusions and behavioral abnormalities. Each symptom is rated for presence (0-1) and severity (range 0-3), total BEHAVE-AD score ranges between 0 and 75.
Five delusional beliefs are investigated by the BEHAVE-AD: delusion that people are stealing things, delusion that the place in which the patient is residing is not his/her home, delusion that the spouse or the caregiver is an imposter, delusion of abandonment and delusion of infidelity. The BEHAVE-AD display good psychometric properties (71), has been revised to provide information on both severity and frequency and has been validated for telephone-based administration (72). Finally, the CERAD-BRSD is a rating scale developed to assess behavioral abnormalities in patients with cognitive impairment (73). The CERAD-BRSD is administered to the patient's caregiver or relative and requires a trained interviewer. The CERAD-BRSD consist of 48 items investigating presence, frequency and severity of specific behavioral abnormalities; total score ranges between 0 and 167. It assesses the following delusional beliefs with respect to their frequency over the past month: belief that spouse is unfaithful, belief of being abandoned, belief that spouse is an imposter, belief that TV characters are real, belief that people are in the house, belief that dead person is alive and belief that “one's home is not one's home.” The CERAD-BRSD exhibits excellent psychometric properties (74), and has been used as an outcome measure in numerous intervention studies (75), but is more time- and training-demanding for administration compared to the NPI and the BEHAVE-AD.
More recently the Etiological assessment of psychotic symptoms in dementia (EAPSID) has been proposed as a tool to evaluate delusions in dementia patients from an etiological perspective (76). EAPSID provides a functional analysis of delusional beliefs assessed through the NPI or the BEHAVE-AD by inquiring on type of delusion, content of delusion, context and frequency of occurrence, where does the delusion occur, reactions of others to the delusions as well as the patients' verbal expressions of the delusions. The EAPSID seems a promising tool for identifying guidance programs for caregivers/relatives to efficiently manage the delusional beliefs experienced by the patients with a potentially positive impact on quality of life (77).
Delusions Assessment in Neurocognitive Disorders and Cognitive Impairment
Regrettably, the domain of delusion is often overlooked by the instruments used to rate the presence and severity of delirium. A recent review of literature on delirium assessment tools reported that only four of the eleven most commonly used delirium assessment tools cover the domain of delusion (78). More importantly among the three most commonly used delirium assessment tools, i.e., the Confusion Assessment Method, the Delirium Rating Scale and the Memorial Delirium Assessment Scale only the two letters investigate the presence of delusion (79). Moreover, due to the intrinsic features of delirium (acute onset and rapid symptoms fluctuation), these instruments were developed to assess the presence of delusions over a very short time span (from a day to a few hours before the acute onset) thus precluding the possibility of assessing whether the presence of delusion could represent a risk factor for delirium.
Pathophysiology and Hypotheses
Various pathophysiological hypotheses have been proposed regarding the etiology of both delusions and delirium in neurodegenerative disorders (Figure 1). These hypotheses come from the observation that the development of these conditions is facilitated by the interplay of predisposing and precipitating factors (80, 81). Predisposing factors are older age, visual and hearing impairment, severe illness, cognitive impairment, depression, malnutrition, pressure ulcers, urinary incontinence, polypharmacy, which represent the baseline vulnerabilities of an older person (81, 82). Precipitating factors are the acute noxious insults or hospitalization-related factors that contribute to delirium, such as infection, metabolic imbalance, or surgery (83). Furthermore, different groups of drugs can trigger both delirium and delusions, particularly sedative-hypnotics with documented psychoactive effect, narcotics, and anticholinergic drugs (84, 85). Delirium could not be explained by a single etiological theorem. It is becoming increasingly clear that delirium may be the consequence of a neurotoxic event affecting a vulnerable brain, in the context of a neurotransmitter and inflammatory derangement.
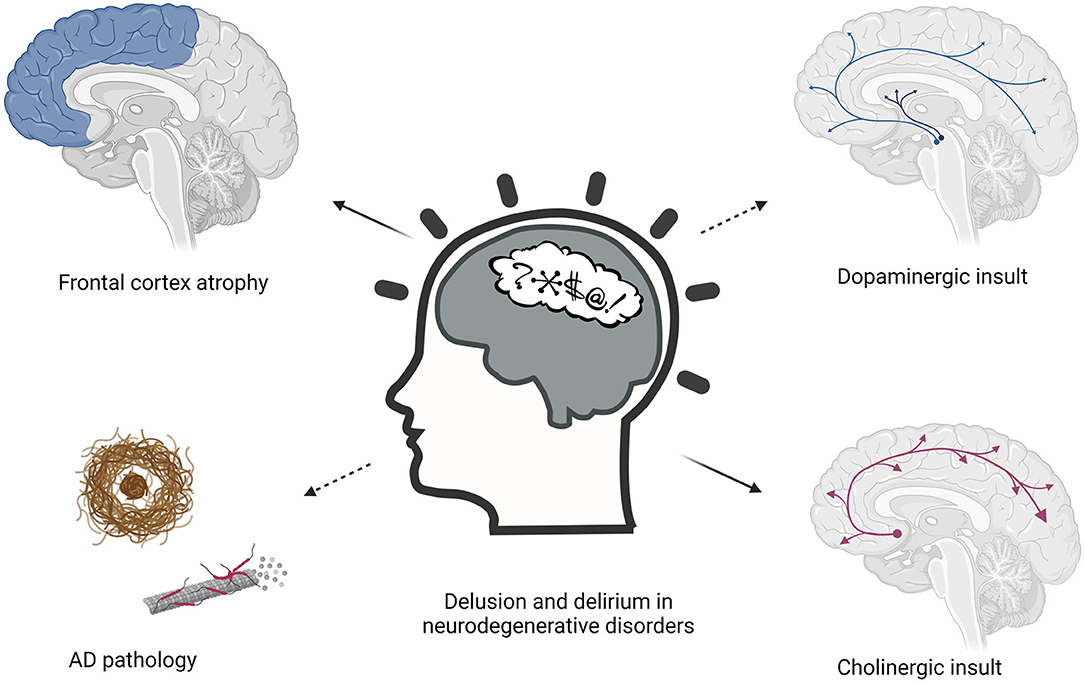
Figure 1. Pathophysiological hypothesis about the etiology of delusions and delirium in neurodegenerative disorders.
Some evidence suggested that delusion can be the result of dysfunction of frontal lobe circuitry. A study by Zubenko et al. showed an association between psychosis and frontal lobe hypometabolism, as well as density of senile plaques and neurofibrillary tangles in the midfrontal cortex of AD patients (86). Another study by Paulsen et al. demonstrated that AD patients with psychotic symptoms will exhibit the typical cognitive deficits of AD but with particularly severe deficits in fronto-subcortical functions compared to patients without psychotic symptoms, confirming the so-called hypofrontality model (87). Similarly, other studies revealed a significant relationship between delusional thought and metabolic rates over frontal regions in both AD and DLB patients (88, 89). Muscarinic acetylcholine receptor density was increased in the frontal cortex of patients with AD presenting psychotic symptoms compared to AD without psychosis (90).
Recently, a new technique called atrophy network mapping identified a delusions network that included regions in the bilateral ventrolateral frontal, orbitofrontal frontal, and superior frontal cortices. Noteworthy Frontal lobe dysfunction has been associated also with delirium. Choi et al. investigated resting-state functional connectivity in patients with delirium (91). They found that dorsolateral prefrontal cortex activity and posterior cingulate cortex activity were inversely correlated in comparison subjects while patients experiencing an episode of delirium shows increased functional connectivity between the two regions. Another study demonstrated that longer duration of delirium was associated with smaller superior frontal lobe in Intensive Care Unit survivors (92). Therefore, data have shown that both delirium and delusions can be associated with changes in frontal cortex.
Among neurotransmitters, acetylcholine and dopamine, are the most frequently associated with delirium and delusions (93, 94). Many neurodegenerative conditions, including AD and DLB have been associated with an extensive loss of basal forebrain projection neurons, which is considered to be the major cholinergic output of the central nervous system (95, 96). Cholinergic basal forebrain atrophy occurs early in both diseases and predicts cognitive decline (97, 98). Also in PD, basal forebrain atrophy has been associated with early cognitive decline (99, 100). Dysregulation of the cholinergic system has been suspected in the pathophysiology of primary psychotic disorders (101, 102) and can contribute to behavioral disturbances in patients with dementia (103).
Interestingly, a study found that cholinergic basal forebrain volume is associated with future psychotic symptoms in PD patients (104). Moreover, serum anticholinergic activity (SAA) levels have been found to increase during the acute phase of delirium, have been linked with a higher burden of delirium symptoms (105), and decline with the resolution of delirium (106). On the other hand, the use of anticholinergic drugs is closely related to the occurrence of delirium (107).
A recent study on postoperative delirium found that the changes in the preoperative activity of Acetylcholinesterase (AChE), Butyrylcholinesterase (BuChE) and Choline acetyltransferase (ChAT) in CSF were associated with the development of postoperative delirium in elderly patients, which may be related to central cholinergic degradation (108). Although the cholinergic deficiency hypothesis suggests treatment with acetylcholinesterase inhibitors may prevent and improve delirium two double-blind, randomized trials failed to demonstrate a statistically significant effect of cholinesterase inhibitors in the prevention and treatment of postoperative delirium (109, 110).
Dopamine has also been involved in the pathophysiology of delirium. Ramirez-Bermudez et al. found that psychotic symptoms in delirious patients were related to increased CSF homovanillic acid (HVA) levels (111). Another study conducted by the same group confirmed that the association between delirium and CSF HVA concentration was significant also in patients without exposure to antipsychotics (112). The dopaminergic activation of the nigrostriatal pathway is critical for the production of hallucinations and perceptual changes (113), which can be present during delirium. Interestingly, psychotic symptoms have been associated with striatal denervation assessed with 123I-FP-CIT SPECT, in both PD and DLB patients (114, 115). Dopaminergic circuitry and dopaminergic medication have also a clear relationship with psychotic symptoms in PD and DLB (116) although that cannot fully explain the phenomenon. A hypothesis that has been raised is that denervation hypersensitivity of mesolimbic and mesocortical dopaminergic receptors predisposes patients to a hypersensitivity response which may manifest as psychosis (41). The link between the susceptibility to develop delirium/delusion and the dopaminergic disruption seen in synucleinopathies and in other neurodegenerative diseases could represent an important area for future research.
Kim et al. studied psychosis symptoms in patients with severe Alzheimer's disease who are cognitively normal. They showed that neuritic plaque severity, and not the NFTs, is associated with a higher risk of psychosis in this population (117). Similarly, reduced CSF amyloid Aß1-42 have been found in de novo PD patients who will develop psychosis (118), suggesting that delusions can be linked to amyloid pathology also in PD. Similarly, preclinical AD brain pathology has been recently linked to the occurrence of delirium in non-demented patients. Idland and colleagues observed a reduction in CSF A42, indicating amyloidosis, in hip fracture patients without dementia developing delirium (119).
The neuroinflammatory hypothesis of delirium, postulate that an acute peripheral event, such as infection or surgery, triggers a systemic inflammatory response and activation of microglia in the central nervous system (120). Peripheral inflammation can induce changes in CNS by several routes, including proinflammatory cytokines (121), endothelial activation with disruption of the blood-brain barrier (122), and microglial activation (123). Interleukin 6 (IL-6) has been strongly associated with the duration of delirium in non-demented patients (124). A study by Macdonald et al. found that the level of C-reactive protein, an acute-phase protein released in response to increasing concentrations of IL-6, predicted the onset and recovery of delirium (125). Neurodegenerative diseases have been also inextricably related to neuroinflammation (126). Persistent inflammatory stimulation is induced by endogenous, such as genetic mutation and protein aggregation, or environmental, such as infection, trauma, and drugs factors (6, 7). The inflammatory responses involve microglia and astrocytes and can lead to neurodegenerative diseases (127). Although neuroinflammation can be initially a protective response in the brain, persistent inflammatory responses are detrimental and may promote the onset and progression of neurodegenerative diseases (128). Field et al. hypothesize that decreased cholinergic function confers increased susceptibility to acute inflammation-induced cognitive deficits and found that cholinergic depletion predisposes to the development of acute cognitive deficits upon subsequent systemic inflammatory insult (129). This hypothesis may explain why patients with neurodegenerative disorders affecting the cholinergic basal forebrain, are particularly susceptible to developing delirium. While neuroinflammation has been linked with primary psychiatric disorders (130), it should be determined if it can have a role in the pathophysiology of psychiatric disorders related to neurodegeneration.
Conclusions
Delusional beliefs and delirium are frequently observed during the course of dementia. Delusions and delirium when present in a neurodegenerative condition represent an additional disease burden significantly increasing the risk of institutionalization and posing relevant distress to the patients' caregiver and relatives. Furthermore, their characteristics can easily mislead clinicians toward a misdiagnosis of psychiatric disorder further delaying the proper treatment. The diagnosis of delirium may be even more challenging within the context of some types of neurodegenerative disorders, especially DLB and PD dementia, characterized by a “sensitivity” to minor insults, even subclinical, and recurrent complex visual hallucinations and delusions. There is not a clear indication so far on how to evaluate the presence of a pre-existing psychosis (including delusion) in patients with a neurocognitive disorder who develop delirium and the assessment or inclusion of pre-existing and long-established delusion is also not addressed by current standard tools. Therefore, the development of an instrument for accurate recognition of delusions in patients with neurodegenerative disorders who develop delirium is of the utmost importance. Delirium assessment instruments should consider the anticholinergic burden and should include a more extensive characterization of delusional beliefs specifically inquiring whether delusions are present in a time span (from week to months) typically not covered by the commonly used delirium rating scales. Such a tool may shed light on the relationship between delusions and delirium in neurodegeneration and may help to understand whether pre-existing delusion may constitute a risk factor for delirium and a prognostic factor for its clinical course. Further studies using multimodal neuroimaging are warranted to test the hypotheses of unbalance between different networks and neurotransmitter deficits in these conditions.
Author Contributions
DU, VG, MF, and GL: conception and design of work and drafting the manuscript. All authors were involved in critical revision of the manuscript for important intellectual content.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Feyaerts J, Henriksen MG, Vanheule S, Myin-Germeys I, Sass LA. Delusions beyond beliefs: a critical overview of diagnostic, aetiological, and therapeutic schizophrenia research from a clinical-phenomenological perspective. Lancet Psychiatry. (2021) 8:237–49. doi: 10.1016/S2215-0366(20)30460-0
2. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association (2013).
3. Appelbaum PS, Robbins PC, Roth LH. Dimensional approach to delusions: comparison across types and diagnoses. Am J Psychiatry. (1999) 156:1938–43.
4. Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. (2000) 157:708–14. doi: 10.1176/appi.ajp.157.5.708
5. McKee J, Brahm N. Medical mimics: differential diagnostic considerations for psychiatric symptoms. Ment Health Clin. (2016) 6:289–96. doi: 10.9740/mhc.2016.11.289
6. Thom RP, Levy-Carrick NC, Bui M, Silbersweig D. Delirium. Am J Psychiatry. (2019) 176:785–93. doi: 10.1176/appi.ajp.2018.18070893
7. Meagher D. Delirium: the role of psychiatry. Adv Psychiatr Treatment. (2001) 7:433–42. doi: 10.1192/apt.7.6.433
8. Ballard CG, Saad K, Patel A, Gahir M, Solis M, Coope B, Wilcock G. The prevalence and phenomenology of psychotic symptoms in dementia sufferers. Int J Geriatr Psychiatry. (1995) 10:477–85. doi: 10.1002/gps.930100607
9. Addesi D, Maio R, Smirne N, Laganà V, Altomari N, Puccio G, et al. Prevalence of delirium in a population of elderly outpatients with dementia: a retrospective study. J Alzheimers Dis. (2018) 61:251–7. doi: 10.3233/JAD-170339
10. Fischer CE, Ismail Z, Schweizer TA. Delusions increase functional impairment in Alzheimers disease. Dement Geriatr Cogn Disord. (2012) 33:393–9. doi: 10.1159/000339954
11. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. (2010) 304:443–51. doi: 10.1001/jama.2010.1013
12. Lerner AJ. Hedera P, Koss E, Stuckey J, Friedland RP. Delirium in Alzheimer disease. Alzheimer Dis Assoc Disord. (1997) 11:16–20. doi: 10.1097/00002093-199703000-00004
13. Hasegawa N, Hashimoto M, Yuuki S, Honda K, Yatabe Y, Araki K, et al. Prevalence of delirium among outpatients with dementia. Int Psychogeriatr. (2013) 25:1877–83. doi: 10.1017/S1041610213001191
14. Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer's disease. Lancet. (2021) 397:1577–90. doi: 10.1016/S0140-6736(20)32205-4
15. Raskin J. Cummings J, Hardy J, Schuh K, Dean RA. Neurobiology of Alzheimer's disease: integrated molecular, physiological, anatomical, biomarker, cognitive dimensions. Curr Alzheimer Res. (2015) 12:712–22. doi: 10.2174/1567205012666150701103107
16. DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer's disease. Mol Neurodegener. (2019) 14:32. doi: 10.1186/s13024-019-0333-5
17. Fan L, Mao C, Hu X, Zhang S, Yang Z, Hu Z, et al. New insights into the pathogenesis of Alzheimer's disease. Front Neurol. (2019) 10:1312. doi: 10.3389/fneur.2019.01312
18. Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer's disease: definition, natural history, diagnostic criteria. Alzheimers Dement. (2016) 12:292–323. doi: 10.1016/j.jalz.2016.02.002
19. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. (2011) 7:270–9. doi: 10.1016/j.jalz.2011.03.008
20. Aisen PS, Cummings J, Jack CR Jr, Morris JC, Sperling R, Frölich L, et al. On the path to 2025: understanding the Alzheimer's disease continuum. Alzheimers Res Ther. (2017) 9:60. doi: 10.1186/s13195-017-0283-5
21. Lee GJ, Lu PH, Hua X, Lee S, Wu S, Nguyen K, et al. Alzheimer's disease neuroimaging, depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer's disease-related regions. Biol Psychiatry. (2012) 71:814–21. doi: 10.1016/j.biopsych.2011.12.024
22. Benoit M, Berrut G, Doussaint J, Bakchine S, Bonin-Guillaume S, Frémont P, et al. Apathy and depression in mild Alzheimer's disease: a cross-sectional study using diagnostic criteria. J Alzheimers Dis. (2012) 31:325–34. doi: 10.3233/JAD-2012-112003
23. Bidzan L, Bidzan M, Pachalska M. Aggressive and impulsive behavior in Alzheimer's disease and progression of dementia. Med Sci Monit. (2012) 18:Cr182–9. doi: 10.12659/MSM.882523
24. Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. (2016) 12:195–202. doi: 10.1016/j.jalz.2015.05.017
25. Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, et al. Neuropsychiatric symptoms in Alzheimer's disease: past progress and anticipation of the future. Alzheimers Dement. (2013) 9:602–8. doi: 10.1016/j.jalz.2012.12.001
26. Rao V, Lyketsos CG. Delusions in Alzheimer's disease: a review. J Neuropsychiatry Clin Neurosci. (1998) 10:373–82. doi: 10.1176/jnp.10.4.373
27. Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: systematic review and meta-analysis. J Affect Disord. (2016) 190:264–71. doi: 10.1016/j.jad.2015.09.069
28. Hirono N, Mori E, Yasuda M, Ikejiri Y, Imamura T, Shimomura T, et al. Factors associated with psychotic symptoms in Alzheimer's disease. J Neurol Neurosurg Psychiatry. (1998) 64:648–52. doi: 10.1136/jnnp.64.5.648
29. Ikeda M, Shigenobu K, Fukuhara R, Hokoishi K, Nebu A, Maki N, et al. Delusions of Japanese patients with Alzheimer's disease. Int J Geriatr Psychiatry. (2003) 18:527–32. doi: 10.1002/gps.864
30. D'Onofrio G, Panza F, Sancarlo D, Paris FF, Cascavilla L, Mangiacotti A, et al. Delusions in patients with Alzheimer's Disease: a multidimensional approach. J Alzheimers Dis. (2016) 51:427–37. doi: 10.3233/JAD-150944
31. Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. (2005) 62:1601–8. doi: 10.1001/archneur.62.10.1601
32. Devanand DP, Miller L, Richards M, Marder K, Bell K, Mayeux R, et al. The Columbia University Scale for psychopathology in Alzheimer's disease. Arch Neurol. (1992) 49:371–6. doi: 10.1001/archneur.1992.00530280051022
33. Bassiony MM, Lyketsos CG. Delusions and hallucinations in Alzheimer's disease: review of the brain decade. Psychosomatics. (2003) 44:388–401. doi: 10.1176/appi.psy.44.5.388
34. Chemerinski E, Petracca G, Tesón A, Sabe L, Leiguarda R, Starkstein SE. Prevalence and correlates of aggressive behavior in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. (1998) 10:421–5. doi: 10.1176/jnp.10.4.421
35. Rapoport MJ, van Reekum R, Freedman M, Streiner D, Simard M, Clarke D, et al. Relationship of psychosis to aggression, apathy and function in dementia. Int J Geriatr Psychiatry. (2001) 16:123–30. doi: 10.1002/1099-1166(200102)16:2<123::AID-GPS260>3.0.CO;2-1
36. Lam LC, Tang WK, Leung V, Chiu HF. Behavioral profile of Alzheimer's disease in Chinese elderly–a validation study of the Chinese version of the Alzheimer's disease behavioral pathology rating scale. Int J Geriatr Psychiatry. (2001) 16:368–73. doi: 10.1002/gps.345
37. Tsai SJ, Hwang JP, Yang CH, Liu KM. Delusional jealousy in dementia. J Clin Psychiatry. (1997) 58:492–4. doi: 10.4088/JCP.v58n1105
38. Holt AEM, Albert ML. Cognitive neuroscience of delusions in aging. Neuropsychiatr Dis Treat. (2006) 2:181–9. doi: 10.2147/nedt.2006.2.2.181
39. Naasan G, Shdo SM, Rodriguez EM, Spina S, Grinberg L, Lopez L, et al. Psychosis in neurodegenerative disease: differential patterns of hallucination and delusion symptoms. Brain. (2021) 144:999–1012. doi: 10.1093/brain/awaa413
40. McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. (2005) 65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1
41. Ravina B, Marder K, Fernandez HH, Friedman JH, McDonald W, Murphy D, et al. Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS, NIMH work group. Movement Disord. (2007) 22:1061–8. doi: 10.1002/mds.21382
42. Ffytche DH, Creese B, Politis M, Chaudhuri KR, Weintraub D, Ballard C, et al. The psychosis spectrum in Parkinson disease. Nat Rev Neurol. (2017) 13:81–95. doi: 10.1038/nrneurol.2016.200
43. Factor SA, Scullin MK, Sollinger AB, Land JO, Wood-Siverio C, Zanders L, et al. Cognitive correlates of hallucinations and delusions in Parkinson's disease. J Neurol Sci. (2014) 347:316–21. doi: 10.1016/j.jns.2014.10.033
44. Marsh L, Williams JR, Rocco M, Grill S, Munro C, Dawson TM. Psychiatric comorbidities in patients with Parkinson disease and psychosis. Neurology. (2004) 63:293–300. doi: 10.1212/01.WNL.0000129843.15756.A3
45. Riedel O, Klotsche J, Spottke A, Deuschl G, Förstl H, Henn F, et al. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson's disease. J Neurol. (2010) 257:1073–82. doi: 10.1007/s00415-010-5465-z
46. Solla P, Cannas A, Ibba FC, Loi F, Corona M, Orofino G, et al. Gender differences in motor and non-motor symptoms among Sardinian patients with Parkinson's disease. J Neurol Sci. (2012) 323:33–9. doi: 10.1016/j.jns.2012.07.026
47. Naimark D, Jackson E, Rockwell E, Jeste DV. Psychotic symptoms in Parkinson's disease patients with dementia. J Am Geriatr Soc. (1996) 44:296–9. doi: 10.1111/j.1532-5415.1996.tb00918.x
48. Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ, Frades-Payo B, Agüera-Ortiz L, Weintraub D, Riesco A, Kurtis MM, Chaudhuri KR. Neuropsychiatric symptoms and caregiver's burden in Parkinson's disease. Parkinsonism Relat Disord. (2015) 21:629–34. doi: 10.1016/j.parkreldis.2015.03.024
49. Aarsland D, Brønnick K, Ehrt U, De Deyn PP, Tekin S, Emre M, et al. Neuropsychiatric symptoms in patients with Parkinson's disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. (2007) 78:36–42. doi: 10.1136/jnnp.2005.083113
50. Pagonabarraga J, Llebaria G, García-Sánchez C, Pascual-Sedano B, Gironell A, Kulisevsky J. A prospective study of delusional misidentification syndromes in Parkinson's disease with dementia. Mov Disord. (2008) 23:443–8. doi: 10.1002/mds.21864
51. Stewart JT. Frégoli syndrome associated with levodopa treatment. Mov Disord. (2008) 23:308–9. doi: 10.1002/mds.21843
52. Roane DM, Rogers JD, Robinson JH, Feinberg TE. Delusional misidentification in association with Parkinsonism. J Neuropsychiatry Clin Neurosci. (1998) 10:194–8. doi: 10.1176/jnp.10.2.194
53. Aarsland D, Ballard C, Larsen JP, McKeith I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson's disease with and without dementia. Int J Geriatr Psychiatry. (2001) 16:528–36. doi: 10.1002/gps.389
54. Engelborghs S, Maertens K, Nagels G, Vloeberghs E, Mariën P, Symons A, et al. Neuropsychiatric symptoms of dementia: cross-sectional analysis from a prospective, longitudinal Belgian study. Int J Geriatr Psychiatry. (2005) 20:1028–37. doi: 10.1002/gps.1395
55. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. (2017) 89:88–100. doi: 10.1212/WNL.0000000000004058
56. Yamada M, Komatsu J, Nakamura K, Sakai K, Samuraki-Yokohama M, Nakajima K, et al. Diagnostic criteria for dementia with lewy bodies: updates and future directions. J Mov Disord. (2020) 13:1–10. doi: 10.14802/jmd.19052
57. Nagahama Y, Okina T, Suzuki N, Matsuda M, Fukao K, Murai T. Classification of psychotic symptoms in dementia with Lewy bodies. Am J Geriatr Psychiatry. (2007) 15:961–7. doi: 10.1097/JGP.0b013e3180cc1fdf
58. Tzeng R-C, Tsai C-F, Wang C-T, Wang T-Y, Chiu P-Y. Delusions in patients with dementia with lewy bodies and the associated factors. Behav Neurol. (2018) 2018:6707291. doi: 10.1155/2018/6707291
59. Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. (1998) 51:1546–54. doi: 10.1212/WNL.51.6.1546
60. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. (2011) 76:1006–14. doi: 10.1212/WNL.0b013e31821103e6
61. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. (2011) 134:2456–77. doi: 10.1093/brain/awr179
62. Omar R, Sampson EL, Loy CT, Mummery CJ, Fox NC, Rossor MN, et al. Delusions in frontotemporal lobar degeneration. J Neurol. (2009) 256:600–7. doi: 10.1007/s00415-009-0128-7
63. Sha SJ, Takada LT, Rankin KP, Yokoyama JS, Rutherford NJ, Fong JC, et al. Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology. (2012) 79:1002–11. doi: 10.1212/WNL.0b013e318268452e
64. Jeon YH, Sansoni J, Low LF, Chenoweth L, Zapart S, Sansoni E, et al. Recommended measures for the assessment of behavioral disturbances associated with dementia. Am J Geriatr Psychiatry. (2011) 19:403–15. doi: 10.1097/JGP.0b013e3181ef7a0d
65. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. (1994) 44:2308–14. doi: 10.1212/WNL.44.12.2308
66. Cummings JL. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology. (1997) 48:S10–6. doi: 10.1212/WNL.48.5_Suppl_6.10S
67. Saari T, Koivisto A, Hintsa T, Hänninen T, Hallikainen I. Psychometric properties of the neuropsychiatric inventory: a review. J Alzheimers Dis. (2020) 1–15. doi: 10.31234/osf.io/n8pv3
68. Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. (2000) 12:233–9. doi: 10.1176/jnp.12.2.233
69. Wood S, Cummings JL, Hsu MA, Barclay T, Wheatley MV, Yarema KT, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry. (2000) 8:75–83. doi: 10.1097/00019442-200002000-00010
70. Monteiro IM, Boksay I, Auer SR, Torossian C, Ferris SH, Reisberg B. Addition of a frequency-weighted score to the Behavioral Pathology in Alzheimer's Disease Rating Scale: the BEHAVE-AD-FW: methodology and reliability. Eur Psychiatry. (2001) 16(Suppl 1):5s–24s. doi: 10.1016/S0924-9338(00)00524-1
71. Sclan SG, Saillon A, Franssen E, Hugonot-Diener L, Saillon A, Reisberg B. The behavior pathology in Alzheimer's disease rating scale (behave-ad): reliability and analysis of symptom category scores. Int J Geriatr Psychiatry. (1996) 11:819–30.
72. Monteiro IM, Boksay I, Auer SR, Torossian C, Sinaiko E, Reisberg B. Reliability of routine clinical instruments for the assessment of Alzheimer's disease administered by telephone. J Geriatr Psychiatry Neurol. (1998) 11:18–24. doi: 10.1177/089198879801100105
73. Tariot PN. CERAD behavior rating scale for dementia. Int Psychogeriatr. (1996) 8(Suppl 3):317–20; discussion 351-4. doi: 10.1017/S1041610297003542
74. Mack JL, Patterson MB, Tariot PN. Behavior rating scale for dementia: development of test scales and presentation of data for 555 individuals with Alzheimer's disease. J Geriatr Psychiatry Neurol. (1999) 12:211–23. doi: 10.1177/089198879901200408
75. Higgins M, Koch K, Hynan LS, Carr S, Byrnes K, Weiner MF. Impact of an activities-based adult dementia care program. Neuropsychiatr Dis Treat. (2005) 1:165–9. doi: 10.2147/nedt.1.2.165.61050
76. Cohen-Mansfield J, Golander H, Ben-Israel J, Garfinkel D. The meanings of delusions in dementia: a preliminary study. Psychiatry Res. (2011) 189:97–104. doi: 10.1016/j.psychres.2011.05.022
77. Cohen-Mansfield J, Golander H. Responses and interventions to delusions experienced by community-dwelling older persons with dementia. J Geriatr Psychiatry Neurol. (2021). doi: 10.1177/08919887211042937
78. Jones RN, Cizginer S, Pavlech L, Albuquerque A, Daiello LA, Dharmarajan K, et al. Assessment of instruments for measurement of delirium severity: a systematic review. JAMA Intern Med. (2019) 179:231–9. doi: 10.1001/jamainternmed.2018.6975
79. Grover S, Kate N. Assessment scales for delirium: a review. World J Psychiatry. (2012) 2:58–70. doi: 10.5498/wjp.v2.i4.58
80. Soysal P, Isik AT. Pathogenesis of delirium. In: Isik AT, Grossberg GT, editors. Delirium in Elderly Patients. Cham: Springer International Publishing (2018). p. 7–18.
81. Zahodne LB, Fernandez HH. Pathophysiology and treatment of psychosis in Parkinson's disease: a review. Drugs Aging. (2008) 25:665–82. doi: 10.2165/00002512-200825080-00004
82. Bulut EA, Isik AT. Approach to the elderly patient with delirium: geriatrician's perspective. In: Isik AT, Grossberg GT, editors. Delirium in Elderly Patients. Cham: Springer International Publishing (2018). p. 49–57.
83. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons: predictive model and interrelationship with baseline vulnerability. JAMA. (1996) 275:852–7. doi: 10.1001/jama.1996.03530350034031
84. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. (2004) 80:388. doi: 10.1136/pgmj.2003.017236
85. Sawada H, Oeda T, Yamamoto K, Umemura A, Tomita S, Hayashi R, et al. Trigger medications and patient-related risk factors for Parkinson disease psychosis requiring anti-psychotic drugs: a retrospective cohort study. BMC Neurol. (2013) 13:145. doi: 10.1186/1471-2377-13-145
86. Zubenko GS, Moossy J, Martinez AJ, Rao G, Claassen D, Rosen J, et al. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol. (1991) 48:619–24. doi: 10.1001/archneur.1991.00530180075020
87. Paulsen JS, Salmon DP, Thal LJ, Romero R, Weisstein–Jenkins C, Galasko D, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. (2000) 54:1965. doi: 10.1212/WNL.54.10.1965
88. Sultzer DL, Brown CV, Mandelkern MA, Mahler ME, Mendez MF, Chen ST, et al. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer's disease. Am J Psychiatry. (2003) 160:341–9. doi: 10.1176/appi.ajp.160.2.341
89. Nagahama Y, Okina T, Suzuki N, Matsuda M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain. (2010) 133:557–67. doi: 10.1093/brain/awp295
90. Lai MK, Lai OF, Keene J, Esiri MM, Francis PT, Hope T, et al. Psychosis of Alzheimer's disease is associated with elevated muscarinic M2 binding in the cortex. Neurology. (2001) 57:805–11. doi: 10.1212/WNL.57.5.805
91. Choi SH, Lee H, Chung TS, Park KM, Jung YC, Kim SI, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. (2012) 169:498–507. doi: 10.1176/appi.ajp.2012.11060976
92. Gunther ML, Morandi A, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med. (2012) 40:2022–32. doi: 10.1097/CCM.0b013e318250acc0
93. Young BK, Camicioli R, Ganzini L. Neuropsychiatric adverse effects of antiparkinsonian drugs. characteristics, evaluation and treatment. Drugs Aging. (1997) 10:367–83. doi: 10.2165/00002512-199710050-00005
94. Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. (2008) 63:764–72. doi: 10.1093/gerona/63.7.764
95. McKeith IG, Dementia with Lewy bodies. Br J Psychiatry. (2002) 180:144–7. doi: 10.1192/bjp.180.2.144
96. Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer's disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, treatment strategies. Prog Neurobiol. (2002) 68:209–45. doi: 10.1016/S0301-0082(02)00079-5
97. Brueggen K, Dyrba M, Barkhof F, Hausner L, Filippi M, Nestor PJ, et al. Basal forebrain and hippocampus as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment - a multicenter DTI and volumetry study. J Alzheimers Dis. (2015) 48:197–204. doi: 10.3233/JAD-150063
98. Grothe MJ, Schuster C, Bauer F, Heinsen H, Prudlo J, Teipel SJ. Atrophy of the cholinergic basal forebrain in dementia with Lewy bodies and Alzheimer's disease dementia. J Neurol. (2014) 261:1939–48. doi: 10.1007/s00415-014-7439-z
99. Ray NJ, Bradburn S, Murgatroyd C, Toseeb U, Mir P, Kountouriotis GK, et al. In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson's disease. Brain. (2018) 141:165–76. doi: 10.1093/brain/awx310
100. Schulz J, Pagano G, Fernández Bonfante JA, Wilson H, Politis M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson's disease. Brain. (2018) 141:1501–16. doi: 10.1093/brain/awy072
101. Caton M, Ochoa ELM, Barrantes FJ. The role of nicotinic cholinergic neurotransmission in delusional thinking. NPJ Schizophr. (2020) 6:16. doi: 10.1038/s41537-020-0105-9
102. Tandon R. Cholinergic aspects of schizophrenia. Br J Psychiatry. (1999) 174:7–11. doi: 10.1192/S0007125000293586
103. Minger SL, Esiri MM, McDonald B, Keene J, Carter J, Hope T, et al. Cholinergic deficits contribute to behavioral disturbance in patients with dementia. Neurology. (2000) 55:1460–7. doi: 10.1212/WNL.55.10.1460
104. Barrett MJ, Blair JC, Sperling SA, Smolkin ME, Druzgal TJ. Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology. (2018) 90:e1618–26. doi: 10.1212/WNL.0000000000005421
105. Flacker JM, Cummings V, Mach JR Jr, Bettin K, Kiely DK, Wei J. The association of serum anticholinergic activity with delirium in elderly medical patients. Am J Geriatr Psychiatry. (1998) 6:31–41. doi: 10.1097/00019442-199802000-00005
106. Flacker JM, Lipsitz LA. Serum anticholinergic activity changes with acute illness in elderly medical patients. J Gerontol A Biol Sci Med Sci. (1999) 54:M12–6. doi: 10.1093/gerona/54.1.M12
107. Egberts A, Moreno-Gonzalez R, Alan H, Ziere G, Mattace-Raso FUS. Anticholinergic drug burden and delirium: a systematic review. J Am Med Dir Assoc. (2021) 22:65–73.e4. doi: 10.1016/j.jamda.2020.04.019
108. Lin X, Tang J, Liu C, Li X, Cao X, Wang B, Dong R, et al. Cerebrospinal fluid cholinergic biomarkers are associated with postoperative delirium in elderly patients undergoing Total hip/knee replacement: a prospective cohort study. BMC Anesthesiol. (2020) 20:246. doi: 10.1186/s12871-020-01166-9
109. Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post-surgical delirium. Am J Geriatr Psychiatry. (2005) 13:1100–6. doi: 10.1097/00019442-200512000-00010
110. Sampson EL, Raven PR, Ndhlovu PN, Vallance A, Garlick N, Watts J, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. (2007) 22:343–9. doi: 10.1002/gps.1679
111. Ramirez-Bermudez J, Ruiz-Chow A, Perez-Neri I, Soto-Hernandez JL, Flores-Hernandez R, Nente F, et al. Cerebrospinal fluid homovanillic acid is correlated to psychotic features in neurological patients with delirium. Gen Hosp Psychiatry. (2008) 30:337–43. doi: 10.1016/j.genhosppsych.2008.01.007
112. Ramírez-Bermúdez J, Perez-Neri I, Montes S, Nente F, Ramirez-Abascal M, Carrillo-Mezo R, et al. Dopaminergic hyperactivity in neurological patients with delirium. Arch Med Res. (2019) 50:477–83. doi: 10.1016/j.arcmed.2019.11.002
113. Kuepper R, Skinbjerg M, Abi-Dargham A. “The dopamine dysfunction in schizophrenia revisited: new insights into topography and course,” in Current Antipsychotics. Handjournal of Experimental Pharmacology, vol 212, eds G. Gross and M. Geyer (Berlin: Springer). doi: 10.1007/978-3-642-25761-2_1
114. Jaakkola E, Joutsa J, Mäkinen E, Johansson J, Kaasinen V. Ventral striatal dopaminergic defect is associated with hallucinations in Parkinson's disease. Eur J Neurol. (2017) 24:1341–7. doi: 10.1111/ene.13390
115. Roselli F, Pisciotta NM, Perneczky R, Pennelli M, Aniello MS, De Caro MF, et al. Severity of neuropsychiatric symptoms and dopamine transporter levels in dementia with Lewy bodies: a 123I-FP-CIT SPECT study. Mov Disord. (2009) 24:2097–103. doi: 10.1002/mds.22702
116. Williams-Gray CH, Foltynie T, Lewis SJ, Barker RA. Cognitive deficits and psychosis in Parkinson's disease: a review of pathophysiology and therapeutic options. CNS Drugs. (2006) 20:477–505. doi: 10.2165/00023210-200620060-00004
117. Kim J, Schweizer TA, Fischer CE, Munoz DG. Psychosis in “cognitively asymptomatic” elderly subjects is associated with neuritic plaque load, not neurofibrillary tangles. Alzheimer Dis Assoc Disord. (2018) 32:185–9. doi: 10.1097/WAD.0000000000000250
118. Ffytche DH, Pereira JB, Ballard C, Chaudhuri KR, Weintraub D, Aarsland D. Risk factors for early psychosis in PD: insights from the Parkinson's Progression Markers Initiative. J Neurol Neurosurg Psychiatry. (2017) 88:325–31. doi: 10.1136/jnnp-2016-314832
119. Idland AV, Wyller TB, Støen R, Eri LM, Frihagen F, Ræder J, et al. Preclinical amyloid-β and axonal degeneration pathology in delirium. J Alzheimers Dis. (2017) 55:371–9. doi: 10.3233/JAD-160461
120. van Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, de Rooij SEJA. Neuroinflammation in delirium: a postmortem case-control study. Rejuven Res. (2011) 14:615–22. doi: 10.1089/rej.2011.1185
121. MacLullich AM, Edelshain BT, Hall RJ, de Vries A, Howie SE, Pearson A, et al. Cerebrospinal fluid interleukin-8 levels are higher in people with hip fracture with perioperative delirium than in controls. J Am Geriatr Soc. (2011) 59:1151–3. doi: 10.1111/j.1532-5415.2011.03428.x
122. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
123. Jalleh R, Koh K, Choi B, Liu E, Maddison J, Hutchinson MR. Role of microglia and toll-like receptor 4 in the pathophysiology of delirium. Med Hypotheses. (2012) 79:735–9. doi: 10.1016/j.mehy.2012.08.013
124. McNeil JB, Hughes CG, Girard T, Ware LB, Ely EW, Chandrasekhar R, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS ONE. (2019) 14:e0226412. doi: 10.1371/journal.pone.0226412
125. Macdonald A, Adamis D, Treloar A, Martin F. C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing. (2007) 36:222–5. doi: 10.1093/ageing/afl121
126. Kwon HS, Koh S-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegeneration. (2020) 9:42. doi: 10.1186/s40035-020-00221-2
127. Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, et al. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine. (2016) 1:1003.
128. Chen W-W, Zhang X, Huang W-J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep. (2016) 13:3391–6. doi: 10.3892/mmr.2016.4948
129. Field RH, Gossen A, Cunningham C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J Neurosci. (2012) 32:6288–94. doi: 10.1523/JNEUROSCI.4673-11.2012
Keywords: delirium, delusion, neurodegeneration, Alzheimer's disease, Dementia with Lewy bodies, frontotemporal dementia, neurodegenerative disease
Citation: Urso D, Gnoni V, Filardi M and Logroscino G (2022) Delusion and Delirium in Neurodegenerative Disorders: An Overlooked Relationship? Front. Psychiatry 12:808724. doi: 10.3389/fpsyt.2021.808724
Received: 03 November 2021; Accepted: 24 December 2021;
Published: 18 January 2022.
Edited by:
Vincenza Frisardi, Santa Maria Nuova Hospital, ItalyCopyright © 2022 Urso, Gnoni, Filardi and Logroscino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giancarlo Logroscino, Z2lhbmNhcmxvLmxvZ3Jvc2Npbm9AdW5pYmEuaXQ=
†These authors have contributed equally to this work and share first authorship
 Daniele Urso
Daniele Urso Valentina Gnoni
Valentina Gnoni Marco Filardi
Marco Filardi Giancarlo Logroscino
Giancarlo Logroscino