- 1Department of Psychiatry, Trinity College, Dublin, Ireland
- 2Department of Psychiatry, Tallaght University Hospital, Dublin, Ireland
- 3Trinity College Institute of Neuroscience, Trinity College, Dublin, Ireland
- 4School of Psychology, Trinity College, Dublin, Ireland
- 5Global Brain Health Institute, Trinity College, Dublin, Ireland
- 6Transpharmation Ireland Ltd, Institute of Neuroscience, Trinity College, Dublin, Ireland
- 7Discipline of Physiology, School of Medicine, Trinity College, Dublin, Ireland
- 8School of Pharmacy and Pharmaceutical Sciences, Trinity College, Dublin, Ireland
- 9Department of Psychiatry and Neurobehavioral Science, University College Cork, Cork, Ireland
- 10APC Microbiome Ireland, University College Cork, Cork, Ireland
Accumulating clinical evidence shows that psychedelic therapy, by synergistically combining psychopharmacology and psychological support, offers a promising transdiagnostic treatment strategy for a range of disorders with restricted and/or maladaptive habitual patterns of emotion, cognition and behavior, notably, depression (MDD), treatment resistant depression (TRD) and addiction disorders, but perhaps also anxiety disorders, obsessive-compulsive disorder (OCD), Post-Traumatic Stress Disorder (PTSD) and eating disorders. Despite the emergent transdiagnostic evidence, the specific clinical dimensions that psychedelics are efficacious for, and associated underlying neurobiological pathways, remain to be well-characterized. To this end, this review focuses on pre-clinical and clinical evidence of the acute and sustained therapeutic potential of psychedelic therapy in the context of a transdiagnostic dimensional systems framework. Focusing on the Research Domain Criteria (RDoC) as a template, we will describe the multimodal mechanisms underlying the transdiagnostic therapeutic effects of psychedelic therapy, traversing molecular, cellular and network levels. These levels will be mapped to the RDoC constructs of negative and positive valence systems, arousal regulation, social processing, cognitive and sensorimotor systems. In summarizing this literature and framing it transdiagnostically, we hope we can assist the field in moving toward a mechanistic understanding of how psychedelics work for patients and eventually toward a precise-personalized psychedelic therapy paradigm.
Introduction
Translational Psychedelic science is evolving rapidly (1–3). Initial data suggests that the dose dependent, transient, altered state of information processing induced by psychedelics can be harnessed by the psychotherapeutic process to lead to clinical benefits across a range of disorders. Accumulating preliminary clinical studies have shown that this synergistic combination of psychopharmacology and psychotherapy may improve outcomes in depression (4, 5), treatment resistant depression (TRD) (6–8) and addiction disorders (9, 10).
While results from ongoing well-powered double-blind randomized controlled trials (RCTs) will determine whether psychedelic therapy translates into clinical benefits for non-psychotic disorders in clinical psychiatry (11, 12), it has been proposed that psychedelic therapy may have broad therapeutic benefits via the attenuation of overly-restricted and maladaptive patterns of cognition and behavior (13, 14). Exploratory studies suggest potential benefits of psilocybin therapy in OCD (15), eating disorders (16) and migraine suppression (17), with ongoing RCTs of psilocybin therapy in MDD, bipolar disorder type II depression, alcohol use disorder, smoking cessation, cocaine addiction, opioid addiction, anorexia nervosa, depression in Mild Cognitive Impairment, OCD and various types of headaches (18).
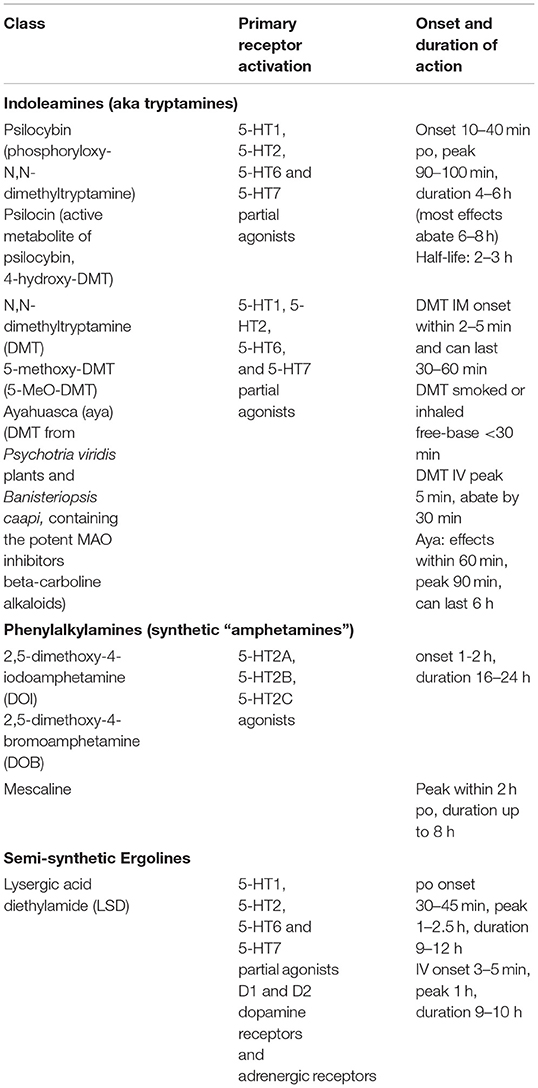
A precise mechanistic understanding of psychedelics is challenging because of the synergistic action of pharmacotherapy and psychotherapy, together with the induction of a wide range of complex subjective experiences with marked individual variation (19). The primary initial pharmacological target of the classical psychedelics appears to be activation of 5-HT2A receptors (Box 1) particularly in cortical layer 5 pyramidal cells (20–27). A contemporary explanatory model—the Relaxed Beliefs under Psychedelics and the Anarchic Brain (REBUS)—proposes that psychedelics via action at 5-HT2A receptors in higher-order cortical regions (27) relax the typical constraints that higher order brain systems impose on emotions, cognitions, and sensory perceptions. This amounts to a decrease in the weight on (or precision of) prior beliefs, which in some disorders may be pathological (e.g., negative self-evaluations). This model proposes that psychedelics may facilitate an increase of information flow from bottom up signaling systems, opening the individual to information that they are otherwise biased to ignore or discount (13).
Box 1. Classical psychedelics.
The belief-recalibration process proposed by the REBUS model illustrates one mechanism through which psychedelic therapy may operate as a transdiagnostic therapeutic option for a broad range of disorders, particularly those with overly constrained beliefs or behaviors, such as major depression, anxiety and addiction disorders (13, 28). This model provides a framework for understanding their lack of efficacy in conditions such as psychosis spectrum disorders, where some have hypothesized there is insufficient constraint imposed on lower-level perceptions and cognitions. It follows that these disorders are exacerbated by psychedelics (29–31). Other overlapping models, focus on 5-HT2A receptor induced altered thalamic gating in cortico-striato-thalamo-cortical (CSTC) feedback loops (32–34).
As we accumulate more knowledge about the precise mechanisms of action, and how this might vary across individuals, we can begin to refine personalized treatment strategies. Currently available strategies to refine therapeutic outcomes include dose (and interval) optimization, modification of psychological interventions (perhaps dependent on the level of complexity or severity) and optimization of environmental ambiances/cues (setting) (35–38). Precise-personalized-predictive psychobiological markers are at an early stage of development, with exploratory clinical studies suggesting baseline Autonomic Nervous System activity (39), functional connectivity patterns (40–42) and cingulate cortical thickness (43), together with psychological factors such as absorption and openness (44–46) and language analysis (47) as potentially useful predictors of therapeutic outcomes. This research is reflective of a much broader advance toward individualized treatment approaches across all aspects of psychiatry, where the mantra is to move beyond one-size-fits-all toward more personalized care plans. In order to develop and build on these precision medicine approaches, there is growing consensus that research needs to traverse multiple levels of analysis.
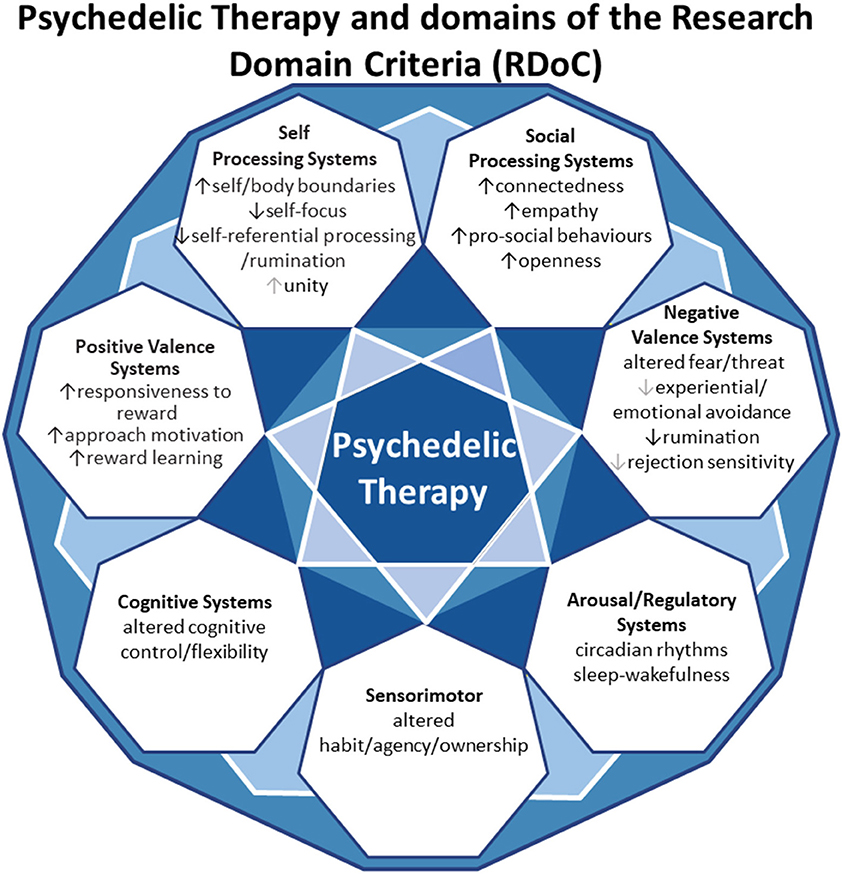
In this review, we aim to anchor the accumulation of basic and applied research in psychedelics to the National Institute of Mental Health's Research Domain Criteria (RDoC), thereby adding structure to a fast-growing field. The transdiagnostic dimensional RDoC constructs are negative and positive valence systems, arousal regulation, social processing, cognitive and sensorimotor systems (Figure 1). In each section we will discuss, where available, research that spans multiple levels of analysis from genes, molecules, proteins, cells, circuits, physiology, behavior, self-report, and paradigms (Figure 2) (48–50). This review complements existing meta-analyses on the effects of psychedelic therapy (51–55) and recent reviews on the topic (18, 33). But in contrast, by framing and organizing the empirical data on psychedelics around the RDoC criteria, we aim to advance the field specifically toward a systems based precise-personalized psychedelic therapy paradigm.
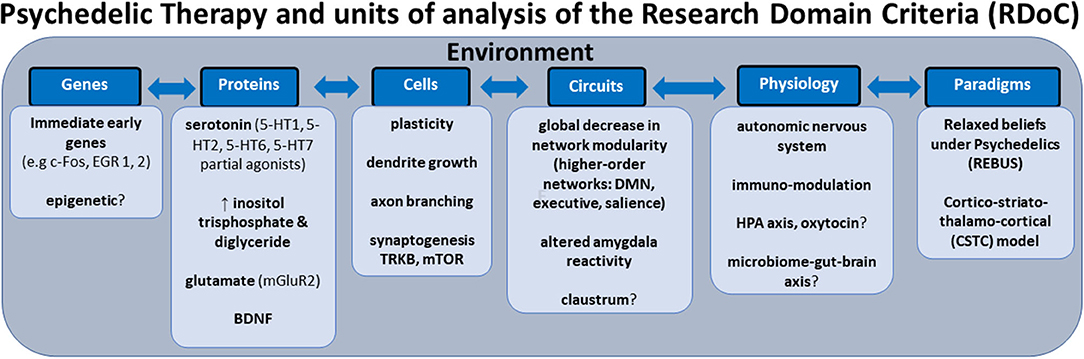
Figure 2. Transdiagnostic psychedelic therapy and units of analysis of the research domain criteria (RDoC).
Integrating Psychedelic Therapy and the Research Domain Criteria
Personalized-precision psychiatry is impeded by two major issues that are partially related— (i) the reliance on categorical diagnostic systems and high levels of comorbidity and heterogeneity (56–60) and (ii) an over-reliance on small scale studies that cannot capture the complexity of mental health and illness, and as a result have failed to generate robust prediction/decision models needed for personalization. To the former point, there is broad consensus that categorical diagnostic labels, while necessary for pragmatic treatments in clinical settings, do not signify unitary, biologically credible, or informative markers of mental health and for example the overlap of previously presumed distinct psychiatric diagnoses, in terms of genes and brain networks, have been demonstrated by large neuroimaging (60–63) and genetic data sets (64–66). To the latter, there is increasing awareness that effect sizes in mental health science are generally small, regardless of whether variables are biological (67) or psychosocial (68). Thus, for personalization to occur, studies must move toward integrating multiple variables that have individually low predictive power—such approaches require large samples for accurate model development (69). Absent large datasets, a transdiagnostic and dimensional approach (compared to a categorical one) may do something to resolve both issues; if we can more accurately, validly and reliably capture mental health phenomena and the underlying biosignatures, then the effect sizes we observe will increase (59).
There are emerging signals that deconstructing categorical diagnoses into dimensional constructs may facilitate enhanced treatment precision. A recent clinical trial adopting an RDoC approach to the investigation of a selective κ-opioid receptor blocker for anhedonia across mood and anxiety disorders showed that this compound increased fMRI ventral striatum activation during reward anticipation compared to placebo (70). A study dividing MDD disorder symptoms into positive valence symptoms (impaired motivation, impaired energy, and anhedonia) and negative valence symptoms (anxiety and interpersonal sensitivity) showed that antidepressants were more effective for positive valence symptoms (71).
The evolving neuroscientific framework of the RDoC aims to integrate developmental processes and environmental inputs over the trajectory of the life course to determine the mechanisms underlying normal-range functioning and then how disruptions correspond to psychopathology. It is anticipated that the identification of targetable biosignatures that either cut across traditional disorder categories or that are unique to specific clinical phenomenon will improve outcomes for people with mental health disorders.
In the sections that follow, we will consider if and how psychedelic therapy operates across the RDoC domains in the hope that harnessing an integrative neuroscience systems model, encompassing environmental information exchange processes, may add the precision we need to transition to personalized psychedelic therapy practices that are transdiagnostic and evidence based. Although well-powered longitudinal clinical studies will be required to determine whether transdiagnostic dimensional biotypes or psycho-biotypes will optimize therapeutic response rates to psychedelic therapy (40, 41, 72), it is hoped that this review will lay a foundation for future research.
Modulation of Negative Valence Systems
NVS are primarily responsible for responses to aversive (threat) situations or context, such as fear, anxiety, and loss (73, 74). Specifically, RDoC breaks NVS into acute threat (fear), potential threat (anxiety), sustained threat, loss and frustrative non-reward constructs. As we will outline in the next sections, psychedelic therapy may recalibrate NVS hyper-responsivity and positive valence systems (PVS) deficits across a range of psychiatric disorders.
Loss
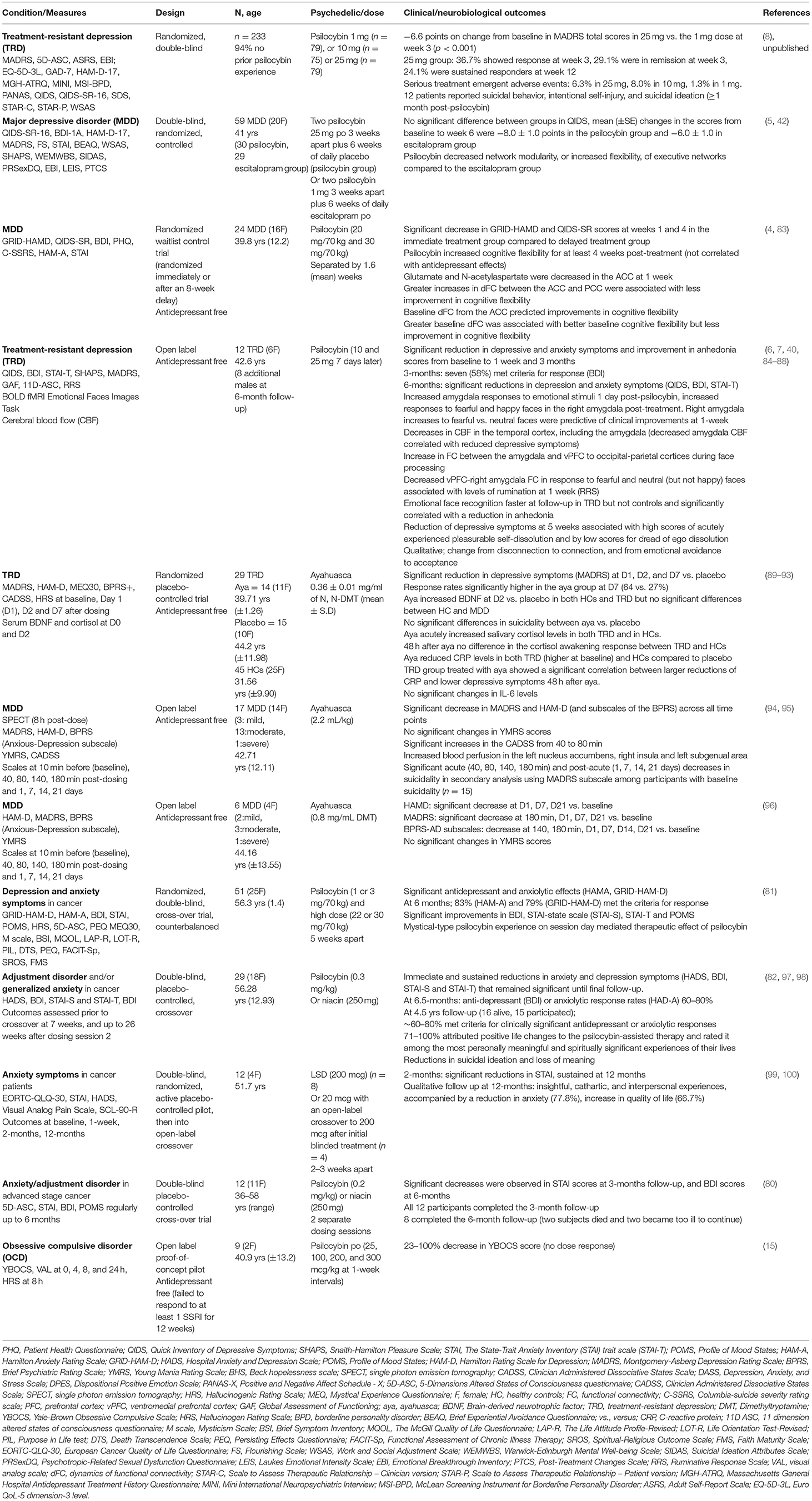
At the behavioral unit of analysis, the loss construct includes attentional biases to negative information, loss of motivation/drive, sadness, shame and rumination and is a component of several disorders but shares most features with depressive disorders (75). Some of the most important evidence for the operation of psychedelics on the NVS unsurprisingly comes from studies in depression. Pre-modern studies conducted during the 1950-60's first indicated a role of psychedelic therapy for depression and anxiety symptoms (76), which aligns with modern-era studies (77–79). The initial double-blind, randomized, placebo-controlled clinical studies in the modern-era of psychedelic therapy (psilocybin) showed an immediate and sustained antidepressant and anxiolytic effect in people with depressive symptoms associated with life-threatening cancer (80–82) (Table 1). In subgroups, these antidepressant and anxiolytic effects were sustained for several years (97), as were reductions in suicidal ideation and loss of meaning (98). Similarly, recent data suggest efficacy for another group with high levels of loss, those who survived Acquired immunodeficiency syndrome (AIDS) (101).
An open-label feasibility study of psilocybin therapy (10 mg) then 7 days later 25 mg, of 12 people diagnosed with treatment-resistant depression (TRD) showed that 67% of participants had significantly reduced depression symptoms (measured by MADRS) at 1 week, with 40% of participants showing a sustained response at 3 months post-dose (6). Measures of anhedonia, which overlap with reward dysfunction (see below) and anxiety, which overlap with threat processing (as discussed above) also improved (Table 1). Furthermore, in some participants these antidepressant and anxiolytic effects were sustained at 6 month follow up (7).
A randomized, waiting list-controlled clinical trial, though still without a placebo control, confirmed the immediate and sustained antidepressant effects of psilocybin therapy in (non-treatment resistant) MDD (4). This study also comprised two psilocybin sessions but at higher doses (20 mg/70 kg and 30 mg/70 kg) than the previous study. This study showed that 16 participants (67%) at week 1 and 17 (71%) at week 4 had a clinically significant response (GRID-HAMD), whereas 14 participants (58%) at week 1 and 13 participants (54%) at week 4 were in remission (4). A phase 2, double-blind, randomized, controlled trial (n = 59) showed that psilocybin therapy was at least as effective as escitalopram in reducing depressive symptoms in MDD (5). Preliminary data from a phase 2b TRD trial (n = 233) demonstrated that psilocybin 25 mg resulted in a statistically significant treatment difference of −6.6 points on change from baseline in MADRS total scores compared to 1mg dose at week 3 (8) (Table 1). Whereas exploratory studies are underway to determine the safety and efficacy of psilocybin therapy in conjunction with SSRI's (102). Interestingly, a recent double-blind, placebo-controlled, cross-over study in 23 in healthy controls (HCs) who received 14 days of escitalopram or placebo prior to psilocybin (25 mg), suggested that escitalopram had minimal effects on subjective, pharmacokinetic, or physiological readouts (103).
It is established that the limbic system and specifically the amygdala (104, 105) are important transdiagnostic nodes in the therapeutic modulation of negative-positive valence systems. Hyper-reactivity of the amygdala is associated with negative processing/affectivity and an attentional bias to negative valenced information, which can occur across a range of stress related disorders, such as depression and various anxiety disorders (106–109). Increased access to information flow from the limbic system during psychedelic therapy is one of the mechanisms thought to underlie therapeutic change (13). In keeping with a recalibration of NVS and PVS responsivity, several studies in HCs have demonstrated attenuation of amygdala reactivity, associated with predilection toward positive compared to negative stimuli in the acute phase post-psilocybin (110–112). This effect may be sustained for up to 1 month (113). Overlapping effects have also been demonstrated for LSD in HCs, which impaired the recognition of sad and fearful faces (114) and reduced reactivity of the left amygdala and the right medial prefrontal cortex (mPFC) relative to placebo during the presentation of fearful faces (115). Very low dose LSD (13 mcg) decreased amygdala connectivity with the left and right postcentral gyrus and the superior temporal gyrus, and increased amygdala seed-based connectivity with the right angular gyrus, right middle frontal gyrus, and the cerebellum in 20 young HCs, though there were “weak and variable effects on mood” (116). While not investigating the amygdala, a recent pilot randomized trial in HCs, perhaps limited by a small sample size of 22, did not show acute or protracted alterations in the recognition of emotional facial expressions after a single dose of ayahuasca (117).
In contrast to the above studies in HCs, which generally show decreases in amygdala reactivity, an open label study of 19 antidepressant free TRD subjects, found increased amygdala responses to emotional faces 1 day after psilocybin (84). In the same cohort of TRD participants, decreased cerebral blood flow in the amygdala correlated with reduced depressive symptoms 1-day post-psilocybin (40). While the loss construct encompasses several transdiagnostic components, rumination and increased self-focus may be particularly important transdiagnostic psychedelic therapy targets. Rumination refers to recursive self-focused negative thinking and is a component of a variety of disorders across mood, anxiety, addiction, and some personality disorders (118–120). The aforementioned TRD study showed that decreased ventromedial prefrontal cortex-right amygdala functional connectivity during face processing was associated with reduced ruminative thinking at 1 week (85).
The corticolimbic system and the immuno-endocrine system are intrinsically linked. However, at this point limited conclusions can be drawn about the loss construct and immuno-endocrine mechanisms. An 8-week social isolation model in juvenile marmosets, resulted in decreased fecal cortisol levels in both ayahuasca and saline treated groups, though in the male animals, ayahuasca reduced scratching behavior and increased feeding (121). In humans, a single dose of ayahuasca acutely increased salivary cortisol levels in both TRD patients and in HCs in a parallel arm, randomized double-blinded placebo-controlled trial (92). Before ayahuasca the TRD group had a blunted salivary cortisol awakening response and hypocortisolemia compared to HCs, though 48 h after ayahuasca there were no differences in the cortisol awakening response or plasma cortisol levels between the groups (92). In the same cohort ayahuasca reduced C-reactive protein (CRP) levels in both TRD (which were higher at baseline) and HCs compared to placebo, though this may be related to the increases in cortisol (89, 93). The TRD group treated with ayahuasca showed a significant correlation between larger reductions of CRP and lower depressive symptoms 48 h after ayahuasca (93). However, there were no significant changes in IL-6 levels (93).
A non-controlled study of 11 HCs, that analyzed salivary cortisol and immune markers 30 min before after 90 min after inhaled 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) found a significant increase in cortisol levels and decrease in IL-6 concentrations, whereas there were no changes in CRP and IL-1β (122). Although this was an exploratory study, neither the cortisol nor the immune markers correlated with subjective experiences (122). The precise impact of psychedelic induced acute cortisol activation and whether this is a therapeutic component is not fully clear, nor is the predictive implications of baseline cortisol or hormonal levels on the response to psychedelic therapy or on sustained effects. Similarly, the clinical relevance of the immune-modulatory effects is not yet clear.
Fear and Threat Systems
When threat systems become excessively or repeated activated, which then exceeds an organism's ability to meet the demands (allostatic overload), psychopathology may ensue (123, 124). Psychedelics modulate acute and sustained fear/threat responses. A study in mice injected with low doses of psilocybin resulted in extinguished cued fear conditioning significantly more rapidly than high-dose psilocybin or saline-treated mice (125). A previous study in rats showed that N,N-DMT initially resulted in anxiogenic responses, but the long-lasting effects tended to reduce anxiety by facilitating the extinction of cued fear memory (126). Similarly, chronic, intermittent, low doses of DMT produced enhanced fear extinction learning without impacting working memory or social interaction and exhibited an antidepressant-like effect in the forced swim test (FST) in rats (127).
A recent study in male mice using the relatively selective 5-HT2A/2C receptor agonist DOI (1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane) showed that it accelerated fear extinction, reduced immobility time in the FST, increased the density of transitional dendritic spines in the frontal cortex, and for the first time showed epigenetic changes in enhancer regions of genes involved in synaptic assembly which lasted for 7 days, in conjunction with more transient transcriptomic changes (128). The clinical relevance of putative epigenetic changes in humans are not yet clear (129).
From the neuroendocrine mechanistic perspective, a study of psilocybin treatment in male mice, showed that psilocybin acutely increased plasma corticosterone and anxiety like behaviors in the open field test (OFT) (130). The acute anxiogenic effects correlated with the post-acute anxiolytic effects and chronic corticosterone administration suppressed the psilocybin induced acute corticosterone and behavioral changes (130). The authors postulated that psilocybin may act as an initial stressor that provides resilience to subsequent stress (130). Indeed, this transient acute anxiety and subsequent attenuation of anxiety can occur in some individuals who undergo psychedelic therapy. It is important to note that not all pre-clinical studies are consistent, in part due to strain and model effects. The aforementioned study did not find significant changes in the sucrose preference test or the FST following psilocybin in C57BL/6J male mice (130), echoing a previous study which did not show effects of psilocin or psilocybin on the FST or in the OFT in Flinders Sensitive Line rats (131).
Another rodent study comparing psilocybin to the N-methyl-D-aspartate receptor antagonist—ketamine—showed that rats that received psilocybin and 5-min weekly arena exposure for the first 3 weeks exhibited significantly less anxiety-like behavior in the elevated plus-maze (EPM) compared to controls, whereas rats that received the ketamine and weekly arena exposure did not display a significant decrease in anxiety in the EPM (132). Rats that received psilocybin or ketamine and no arena exposure did not display a significant decrease in anxiety in the EPM (132). The authors postulated that psilocybin facilitates a period of “behavioral flexibility” in which exploration of a non-home-cage environment reduces their anxiety during future exploration of a novel environment (132). In the same study, psilocybin decreased immobility in the FST for up to 5 weeks after administration compared to control rats, whereas ketamine injected rats displayed decreased immobility up to 2 weeks, suggesting a longer lasting therapeutic effect of psilocybin over ketamine (132). It will be intriguing to see if clinical trials comparing psilocybin to ketamine reproduce the putative longer lasting therapeutic effect of psilocybin (NCT03380442).
In humans, dysregulated fear and threat responses underlie a range of psychiatric disorders and psychedelic therapy may revise dysregulated or maladaptive fear/threat responses. A review of 20 human studies of psychedelics in ICD-10 anxiety disorders from 1940 to 2000, albeit of sub-optimal methodological rigor (e.g., lack of control groups, blinding and standardization), indicated improvements in anxiety levels (133). The subsequent clinical trials in people diagnosed with cancer (80–82, 134) and the studies in depression (4, 6) also suggest anxiolytic effects of psychedelic therapy.
One of the notable conditions associated with dysregulated fear conditioning (and avoidance of conditioned contextual cues), together with emotional regulation, and dysfunctional neural activity in cortico-amygdala circuits, involving exaggerated amygdala and attenuated mPFC activity, is Post-Traumatic Stress Disorder (PTSD) (109, 135–139). Other anxiety disorders share overlapping neurobiological pathways linked to fear/threat circuitry and attentional bias of negative valenced information, though there is variability in the fear evoking stimuli (106, 140, 141).
While PTSD overlaps with other conditions in the domains of hypervigilance, avoidance and altered emotional valance, the vivid re-experiencing of the trauma is perhaps a point of divergence from many other conditions. Memory reconsolidation dysregulation is a cardinal clinical feature of PTSD and memories can be strengthened or weakened according to new experiences. Classical psychedelics have the capacity to acutely enhance the vividness and recall of autobiographical memories (142) which in the context of psychedelic therapy requires great care and attention. These autobiographical memories are highly influenced by environmental inputs such as music (143), which is linked to increased parahippocampal cortex-visual cortex enhanced visual imagery, including imagery of an autobiographical nature (144). In terms of therapeutic utility, it is noteworthy that psilocybin leads to more vivid and visual recollections, associated with enhanced activation of visual and sensory cortical regions after viewing positive autobiographical memory cues (145). In terms of advancing the mechanistic understanding, undoubtedly future preclinical studies will delve into the impact of psychedelics on memory engram storage and retrieval (146, 147).
It is not known whether psychedelic therapy has the potential to augment therapies, such as cognitive processing therapy or prolonged exposure therapy in PTSD or indeed in any other anxiety disorder. However, there are preliminary indicators that psychedelic therapy may be useful in PTSD (148, 149). A retrospective, self-report survey of Veterans 30 days before and 30 days after participation in a psychedelic clinical program utilizing ibogaine and 5-MeO-DMT reported significant reductions in symptoms of PTSD, depression, anxiety, suicidal ideation and cognitive impairment (148). Increases in psychological flexibility (discussed below) were associated with the improvements in self-reported PTSD symptoms, depression, and anxiety (148). It will be interesting to ascertain whether the same psychedelic therapy induced modulation of cortico-limbic circuits (as discussed in section Loss construct above) will underpin therapeutic changes in PTSD and other anxiety disorders. As with all these studies, future challenges include precisely disentangling the contribution of psychedelics from psychotherapy, with some suggesting that the only way to definitively achieve this would be via the rather challenging process of administering psychedelic compounds under general anesthesia or sleep (150).
Excessive fear/anxiety may lead to maladaptive patterns of avoidance. Some of the potential therapeutic subjective experiences induced by psychedelics involve the transition from experiential (151, 152) and emotional (88) avoidance to acceptance. Interestingly, attachment avoidance at baseline may be linked with psilocybin-related challenging experiences (153). Similarly, high neuroticism has been associated with unpleasant/anxious reactions in 3,4-ethylenedioxymethamphetamine (MDMA) therapy (154). This again highlights the vital importance of preparation sessions, particularly pertinent in those with marked threat sensitivity/anxiety.
Frustrative Non-reward
The neural circuitry underling aggressive reactions (in the context of negative emotions) involve amygdala hyper-responsivity coupled with hypoactivity of prefrontal regions, which overlaps with threat processing circuitry (155, 156). The frustrative non-reward construct refers to “reactions elicited in response to withdrawal/prevention of reward, i.e., by the inability to obtain positive rewards following repeated or sustained efforts.” This could potentially be associated with some aspects of depression or aggression (157). Sensitivity to frustration, particularly in relation to interpersonal rejection and negative emotions focused on others (158) are components of emotionally unstable personality (disorder) (EUPD) (borderline personality disorder). It has been proposed that psychedelic therapy could assist with emotion regulation, mindfulness, and self-compassion in people with EUPD (159). There are tentative indicators of potential utility. For example, a non-controlled observational study of 45 HCs who participated in an ayahuasca session reported significant improvements in mindfulness capabilities and emotional regulation in the subgroup with borderline-personality traits (Table 1) (160). However, it is premature to draw any conclusions about the utility of psychedelic therapy in EUPD or other maladaptive personality traits/disorders (161).
In terms of other personality traits, data suggests that psychedelics may increase openness (44, 162–164). Moreover, higher baseline scores in the personality trait of absorption (focused attention) (45, 46) and openness may be useful predictors of a therapeutic psychedelic experience, reportedly linked to increases in brain entropy as measured by fMRI (and experiences of “ego-dissolution”) (165), though 5-HT2AR binding did not appear to correlate with variations in openness (166, 167), highlighting the individual variability in 5-HT2AR levels after psilocybin and the complex relationship with subjective changes.
Modulation of Neuroplasticity as a Transdiagnostic Mechanism
In terms of RDoC, structural and functional neuroplasticity broadly falls under molecular and cellular units of analysis and probably applies, at least some degree, to all domains. The ability of psychedelics to rapidly rewire neural circuitry by engaging plasticity mechanisms has given rise to the term—“psychoplastogens” (168–173). While, it is generally accepted that the quality of the subjective experience, dependent on the optimization of set and setting (context) is a critical component of the therapeutic mechanism of action of psychedelic therapy (87, 174), some propose that the subjective effects may not be necessary to produce long-lasting changes in mood and behavior (171).
The classical psychedelics may share glutamatergic activity-dependent neuroplastic effects with ketamine (175) and on a longer timescale, with some types of conventional antidepressants (176). A study in rats utilized fluorescence microscopy and electrophysiology techniques to show that changes in neuronal structure are accompanied by increased synapse number and function, and the structural changes in the PFC and increase in glutamate induced by serotonergic psychedelics appear to lead to BDNF secretion, neurotrophin receptor tyrosine kinase (TrkB) stimulation, and ultimately mammalian target of rapamycin (mTOR) activation (177). Furthermore, both LSD and ketamine activated cortical neuron growth mechanisms after <1 h, an effect which lasted for several days (178) and could be divided into an initial stimulation phase requiring TrkB activation and a growth period involving sustained mTOR and AMPA receptor activation (178).
In mice, a single dose of psilocybin resulted in a 10% increase in spine size and density in the medial frontal cortex, which occurred within 24 h and persisted for 1 month (179). In pigs, a single dose of psilocybin compared to saline resulted in 4% higher levels of hippocampal synaptic vesicle protein 2A (SV2A) and lowered hippocampal and PFC 5-HT2AR density (180). Seven days post-psilocybin, there was still significantly higher SV2A density in the hippocampus and the PFC, whereas there were no longer any differences in 5-HT2AR density (180). Previous studies showed psychedelics increase early response genes in the PFC (181, 182) and this was further confirmed by a rapid dose dependent preferential modulation of plasticity-related genes in the PFC compared to the hippocampus in rats (183).
A recent pre-clinical study compared ketamine to Tabernanthalog (TBG)—a water-soluble, non-hallucinogenic, non-toxic analog of ibogaine (184). Both TBG (50 mg/kg) and ketamine reduced immobility in mice in the FST, though the effects of ketamine were more durable and ketanserin blocked the effect of TBG (184). TBG promoted structural neural plasticity, produced antidepressant-like effects and in keeping with the transdiagnostic effects, also reduced alcohol and heroin-seeking behavior in rodents (184). A single lower dose of TBG (10 mg/kg) administered to mice after unpredictable mild stress, rescued deficits in anxiety like behavior and cognitive flexibility, associated with restoration of excitatory neuron dendritic spines (185), thus echoing the effects of ketamine (186), albeit via different primary pathways.
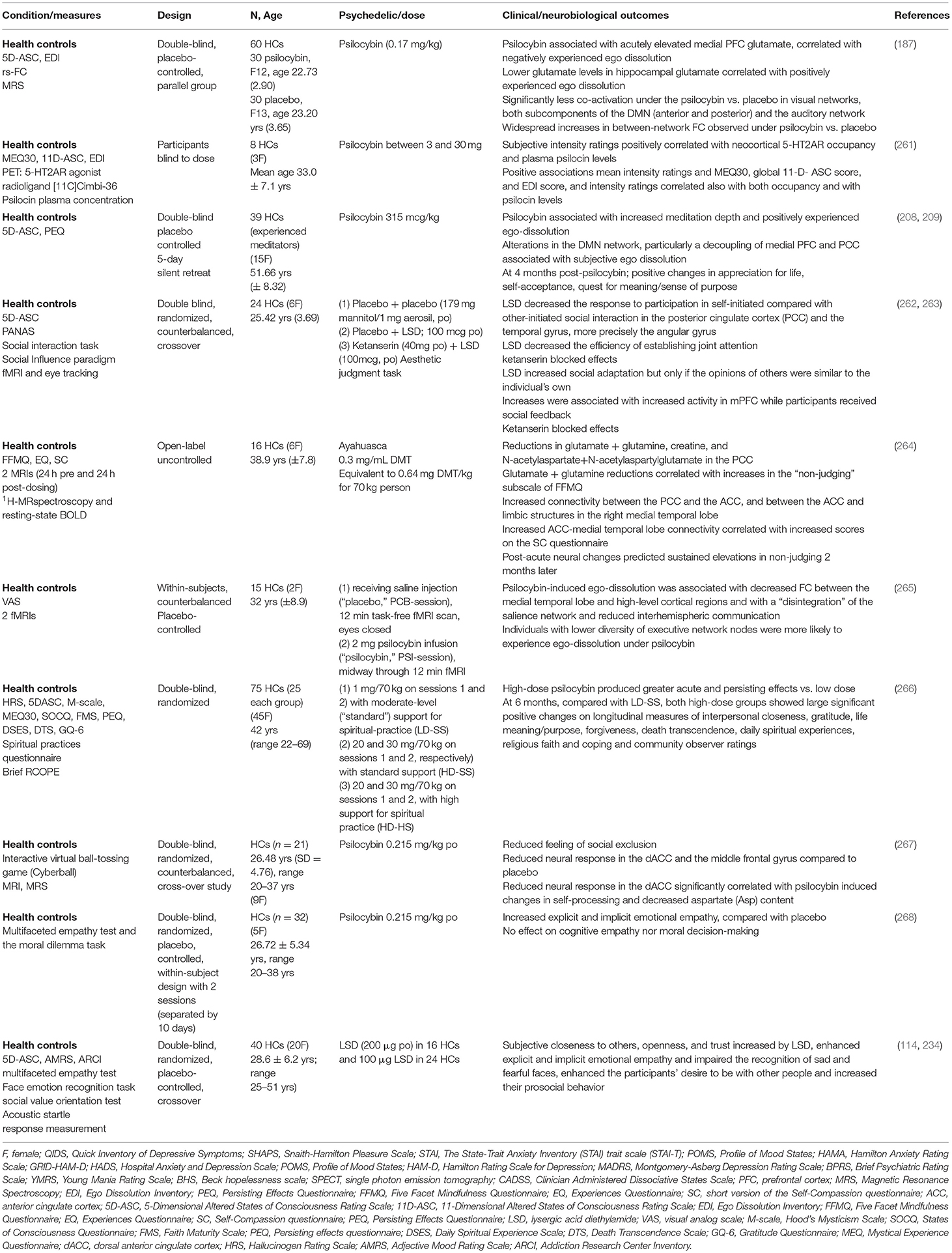
Notwithstanding the gap between animal and human studies in demonstrating molecular changes in plasticity, there are indicators of alignment with the pre-clinical data. For example, a magnetic resonance spectroscopy (MRS) imaging study in HCs showed psilocybin modulated glutamate levels in the medial PFC (187). In blood, one small preliminary clinical trial showed that 2 days after ayahuasca BDNF levels increased in both the TRD and the HC groups (90), whereas other studies in HCs showed that LSD increased blood BDNF levels (188, 189). However, BDNF levels did not increase in a recent randomized pilot study in 22 HCs after a single dose of ayahuasca (117).
Modulation of Positive Valence Systems
PVS are primarily responsible for responses to positive motivational situations or contexts, such as reward seeking, consummatory behavior, and reward/habit learning.
Reward System
Reward-pathway dysfunction is associated with a range of disorders (190, 191), including but not limited to mood (192, 193), anxiety (194, 195), addiction disorders (196, 197) and eating disorders (198, 199). Psychedelic therapy induced attenuation of maladaptive reward signaling, or a recalibration of reward/fear systems (PVS/NVS) may be useful targets across the various disorders. Psychedelics may alter maladaptive signaling in the mesolimbic reward circuitry, either indirectly via 5-HT signaling in the case of psilocybin or directly via activation of dopamine receptors (D1 and D2) like LSD (200, 201). A microdialysis study in awake rats found that intraperitoneal administration of psilocin significantly increased extracellular dopamine but not serotonin in the nucleus accumbens, increased serotonin and decreased dopamine in the mPFC, but neither were altered in the ventral tegmental area (202). An electrophysiological study in male mice showed that LSD altered neuronal activity in both the reticular and mediodorsal thalamus, partially mediated by the D2 receptor (34). Another recent study in chronically stressed male mice suggested that 5-HT2A independent mechanisms may be of importance in psilocybin induced anti-hedonic responses and associated cortico-mesolimbic reward circuit modulation (203).
The functional interaction between 5-HT and dopamine systems across molecular and neural networks was further expounded by a recent study in mice showing psilocybin increased FC between 5-HT-associated networks and resting-state networks of the murine DMN, thalamus, and midbrain, whereas it decreased FC within dopamine-associated striatal networks (204). It should be noted that this contrasts with the majority of human studies in HCs (as discussed below) that report acute decreases in DMN FC, thus highlighting the challenges of translation (32, 205–208).
In healthy humans, a structural MRI study showed a positive correlation between psilocybin induced feelings of unity, bliss, spiritual experience, and insightfulness subscales of the 5-Dimensional Altered States of Consciousness Rating Scale (5D-ASC) and right hemisphere rostral anterior cingulate thickness in HCs after controlling for sex and age (43). Whereas, a double-blind placebo-controlled study of 38 healthy experienced mediators that received psilocybin, reported positive changes in appreciation for life, self-acceptance, quest for meaning/sense of purpose at 4 months post-psilocybin (209). A pooled sample of HCs (n = 110) who had received between 1 and 4 oral doses of psilocybin (45–315 μg/kg) from eight double-blind placebo-controlled experimental studies (1999–2008), reported that the majority of subjects described the experience as pleasurable, enriching, and non-threatening (210).
A Positron emission tomography (PET) study in healthy humans showed that psilocybin increased striatal dopamine concentrations, and this increase correlated with euphoria and depersonalization phenomena (211), whereas the mixed 5-HT2/D2 antagonist risperidone attenuated the effects of psilocybin (212). This again re-enforces the divergence between the potential therapeutic benefit of psychedelic therapy in some reward dysregulated conditions, like depression, anxiety, and addiction, while exacerbating conditions like psychosis spectrum and manic disorders.
Addiction
The multi-layered complexities underlying addiction disorders are not only limited to reward and habit dysregulation but may include other constructs such as impulsivity and compulsivity (213). Compared to other recreational substances, psychedelics exhibit minimal reinforcing effects and are among the least harmful, with minor physiological side effects (24, 214, 215). Furthermore, preliminary clinical studies indicate a therapeutic use in alcohol use disorder, and for smoking cessation (216, 217). An open label pilot study of oral psilocybin in one or two supervised sessions in addition to Motivational Enhancement Therapy reduced alcohol consumption, which was maintained at 36 weeks, in a group of 10 participants with alcohol dependence disorder (10, 218). Although the mechanisms have yet to be fully elucidated, changes in alcohol consumption were associated with what is described as the “mystical” quality of the psilocybin experience (10).
Consistent with this, a subsequent online survey (n = 343) of people with prior alcohol use disorder, reported that insight, mystical-type effects, and personal meaning of experiences, together with higher psychedelic dose, were associated with a greater reduction in alcohol consumption (219). However, the potential mediating influence of negative and positive valence system modulation should also be acknowledged. Interestingly, neither psilocybin nor LSD administered in a high dosage regimen or chronic microdosing regime had long-lasting effects on relapse-like drinking in an alcohol deprivation effect rat model (220). Only sub-chronic treatment with psilocybin produced a short-lasting anti-relapse effect (220). A recent study showed that psilocybin restored alcohol dependence–induced metabotropic glutamate receptor (mGluR2) down-regulation and reduced alcohol-seeking behavior in rats (221). Interestingly, in a rodent food reward model, low dose psilocybin and ketamine failed to positively affect motivation or attention, though subtle improvements in attention and impulsive behavior were noted in “low performing” rats (222).
A pilot study of psilocybin and cognitive-behavioral therapy in people with tobacco addiction reported that 12 of 15 participants (80%) showed 7-day point prevalence abstinence at 6-month follow-up (9). Smoking cessation outcomes were significantly correlated with measures of mystical experience, of whom 9 of the 15 participants (60%) met criteria for “complete” mystical experience, defined as a score of ≥60% on each of the following subscales: unity, transcendence of time and space, ineffability, sacredness, noetic quality, and positive mood (223). A follow up qualitative study of participants (n = 12) reported vivid insights into self-identity, together with experiences of interconnectedness, awe, and curiosity which persisted beyond the duration of acute dosing (224). Clinical trials across a range of addiction disorders are currently underway to determine whether these promising preliminary studies progress to clinical utility (Table 3).
Depression
Reward hyposensitivity and decreased approach motivation is related to anhedonia, a cardinal feature of the Depression (192, 225). There are several psychological constructs by which psychedelic therapy may re-ignite reward deficits in states of anhedonia, including potential experiences of awe, curiosity, (explorative search), novelty, intrinsic motivation, psychological insight, and enhanced meaning/purpose (226). Conversely, reward hypersensitivity and elevated approach motivation is related to a subgroup of hypo/manic symptoms characterized by excessive approach motivation and psychomotor hyperactivation in the context of bipolar disorder (192). This reward hypo-hypersensitivity divergence maps onto the contra-indication of psychedelic therapy in bipolar type 1 disorders (BPAD I) (226, 227) and caution will be required in the treatment of the depressive phase of BPAD II (228). We await with interest the results of an open label safety and efficacy psilocybin (25 mg) therapy study in depressed participants with BPAD II and the future integration of dimensional approaches, such as reward-related reactivity assessments (Table 3).
Modulation of Arousal and Regulatory Systems
RDoC's Arousal/Regulatory Systems are responsible for generating activation of neural systems as appropriate for various contexts and providing appropriate homeostatic regulation of such systems as energy balance and sleep (74).
Arousal
Arousal is a continuum of sensitivity of the organism to stimuli, both external and internal. Several interacting systems are involved in arousal regulation, including but not limited to, the sympathomedullary and the immuno-endocrine system, which act as mediators to alter neural circuitry and function, particularly in the corticolimbic system. Psychedelics are highly context sensitive, “non-specific amplifiers” (229) of internal and/or external signals (immediate environment), in part due to the effects of 5-HT2AR signaling (230, 231). Psychedelics acutely modulate the Autonomic Nervous System (ANS) (39), neuroendocrine (232), and immune systems (233).
Psychedelics activate the sympathetic nervous system, including blood pressure, heart rate, body temperature, and pupillary dilation, probably via 5-HT2A and/or α1-adrenergic receptor-mediated mechanisms (114, 234–236). A recent randomized, placebo-controlled crossover trial in 25 HCs using electrocardiographic recordings showed that LSD increased sympathetic activity, which was positively associated with a range of subjective effects, measured by 5D-ASC (39). However, it should be noted that similar correlations were found for the placebo condition. In contrast, ketanserin increased parasympathetic tone and negatively associated with the subjective effects of LSD (39).
As discussed above, psychedelics also acutely stimulate the neuroendocrine system. In a seminal randomized placebo-controlled study of healthy experienced psychedelic users, IV DMT acutely and dose dependently increased blood cortisol, corticotropin, and other hormones such as prolactin and growth hormone (and ß-endorphin) (237). By 5 h post-dose, all endocrine markers returned to baseline values (237, 238). A double-blind, placebo-controlled study showed high dose psilocybin (315 μg/kg) acutely increased plasma ACTH and cortisol (and prolactin and thyroid stimulating hormone) in HCs (239). LSD (200 μg) increased plasma concentrations of the cortisol, cortisone, corticosterone, and 11-dehydrocorticosterone compared with placebo in 16 HCs using a randomized, double-blind, placebo-controlled cross-over study design (240). Other studies have also shown acutely increased plasma levels of cortisol, prolactin, oxytocin, and epinephrine due to LSD administration (234).
Psychedelics modulate the immune system via 5-HT1, 5-HT2, and sigma-1 receptor activity (18, 233, 241–248). Altered immune system function, mainly characterized by chronic low-grade inflammation is associated with a range of psychiatric disorders (57, 249–251) and it remains an open question whether the potential anti-inflammatory activity of psychedelics will play a role in autoimmune disorders (252) or chronic pain (253, 254).
Sleep-Wakefulness
Sleep interference is almost ubiquitous across psychiatric disorders (255). Psilocybin (0.26 mg/kg) increased REM sleep latency in a randomized, double-blind placebo controlled cross over study of 20 HCs (256). Psilocybin suppressed slow-wave activity in the first sleep cycle but did not affect NREM sleep, EEG power spectra in NREM or REM sleep across the whole night (256).
Modulation of Social Processing Systems
RDoC broadly defines systems for social processes as mediating responses in interpersonal settings of various types, including perception and interpretation of others' actions (74). The biologically encoded time-lagged personal narrative is constantly under the influence of bidirectional information exchange processes with the wider socio-environmental system. The multifaceted neural circuitry and molecular signaling pathways underlying social cognition, under the influence of environmental cues, are of fundamental importance to social species (257–259). A complex intertwined relationship exists between social isolation, disconnectedness, perceived disconnection, and poor mental health (158, 260). Psychedelic compounds alter social cognitive processes (Table 2) and studies in rodents are beginning to elucidate the underlying mechanistic pathways. A study in male mice showed that repeated doses of LSD (30 μg/kg, daily for 7 days), but not a single dose, resulted in more time interacting with a stranger mouse in the direct social interaction test, associated with potentiation of mPFC excitatory transmission via 5-HT2A and AMPA receptors and via an increasing phosphorylation of the mTORC1 protein (269). Moreover, the inactivation of mPFC glutamate neurotransmission impaired social behavior and negated the prosocial effects of LSD (269). Another study suggested that psilocybin attenuated some of the sociability deficits in a prenatal valproic acid mouse model of autism (270).
Affiliation and Attachment
Experiences of disconnection or exclusion are common across psychiatric disorders and can manifest as social withdrawal, apathy, and anhedonia (260). Using a paradigm designed to induce feelings of social exclusion, a double-blind, randomized, counterbalanced, cross-over study of healthy participants (n = 21) reported that psilocybin induced reduced feelings of social exclusion (267) (Table 2). A placebo-controlled, double-blind, random-order, crossover study conducted using LSD (100 μg) in 24 HCs and LSD (200 μg) in 16 HCs, enhanced the participants' desire to be with other people and increased their prosocial behavior on the Social Value Orientation test (114, 234). In addition to significant positive changes in gratitude, life meaning/purpose, forgiveness and death transcendence, a double-blind study comparing low and high dose psilocybin therapy in HCs reported sustained increases in experiences of interpersonal closeness at 6 month follow up, associated with mystical-type experiences (266). It is interesting to note that psychedelics can increase oxytocin plasma levels (234), though the therapeutic relevance is not yet clear.
In keeping with possible increases in openness (210) and connectedness (88, 271, 272), studies have shown that psychedelic use may be associated with increases in nature relatedness (273–275), pro-environmental behaviors (276) and more broadly experiences of personal meaning (81, 148, 209, 219, 277). Taken together, psychedelic therapy induced changes in social processing systems and specifically social reward processing and behavior and enhanced experiences of connectedness (88) has potential therapeutic implications not only for depressive, anxiety, addiction, some personality disorders, but perhaps for social deficits in subtypes of adult autism spectrum disorders.
Perception and Understanding of Others
There are preliminary indictors that classical psychedelics may enhance certain types of empathy (Table 2). LSD (114, 234) and psilocybin (268) acutely increased explicit and implicit emotional empathy, using the multifaceted empathy test and moral dilemma task in HCs, compared to placebo (268). Psilocybin did not affect the ability to take another person's perspective or affect the understanding of another person's mental state (cognitive empathy), nor did it affect moral decision-making (268). Using an aesthetic judgment task involving social feedback, LSD increased social adaptation to group opinions that were relatively similar to the individuals own opinions, associated with 5-HT2A activation and increased activity of the mPFC (263). Comparisons of psychedelic therapy delivered in individual settings compared to group settings offers an intriguing avenue to further explore how social processing domains and constructs such as perception and understanding of others may be shaped by the context in which the therapy is delivered. Non-controlled group studies have suggested that shared experiences, including acute relational experiences of perceived togetherness, may facilitate enhanced perception and understanding of others (272, 278). Controlled transdiagnostic studies directly comparing group to individual psychedelic therapy could decipher the relative therapeutic contribution of a group setting either before, during or after psychedelic administration.
Perception and Understanding of Self
Notwithstanding the challenges of disentangling self from self-as social agent, current thinking implicates altered self-processing as the primary mode of action of psychedelic therapy with downstream implications for social processing systems (33). However, elucidating the precise temporal dynamics of altered self and self-as social agent, whilst also considering the pervasive emotional background is challenging. Nonetheless, the experience of a transient attenuation of the demarcation between self and other/environment or “ego dissolution” appears to be a pivotal transdiagnostic therapeutic mechanism (Table 2). This is especially relevant for excessive self-referential processes, which often manifest with negative valence. For example, ruminative or obsessional thoughts, which are components across a range of disorders, such as depression, anxiety disorders, eating disorders, addiction disorders and some types of personality disorders.
In contrast to disorders of constrained “self-focus,” which may benefit from a “broader spectrum of thought patterns and emotions” induced by psychedelic therapy (13, 33, 279), psychosis spectrum disorders appear not to benefit. This may be due to baseline features which include aberrant stability between intrinsic and extrinsic self-processing networks (280), aberrant salience attribution (281) and a loosening of higher-level priors (13). Some of these experiences are attenuated by second generation antipsychotics (e.g., clozapine, olanzapine, quetiapine, and risperidone), which block 5-HT2A and dopamine receptors (282). A previous study in HCs showed that risperidone attenuated the effects of psilocybin (212).
The intensity of psilocybin induced subjective experiences, including ego dissolution are dose dependent and appear to correlate with cerebral 5-HT2ARs occupancy and plasma psilocin levels (261). While the molecular cascade initiated by 5-HT2AR activation and downstream cortical glutamate modulation (24, 177) are key neurobiological substrates of self-processing alterations, the full molecular pathways and how they map onto the self-concept have yet to be fully determined, and at least in this regard, only partial assistance can be derived from preclinical models. From the perspective of refining personalized-precision psychedelic therapy, a PET study in 16 HCs showed that lower neocortical 5-HT2AR binding before psilocybin was associated with longer peak effects, a more rapid decrease in subjective drug intensity effects and higher scores on the Mystical Experience Questionnaire (283).
An MRS study in HCs showed that psilocybin acutely elevated mPFC glutamate, which was associated with negatively experienced ego dissolution, whereas lower levels in hippocampal glutamate secondary to psilocybin, were associated with positively experienced ego dissolution (187). A previous MRS study of 16 HCs 1 day after consuming ayahuasca showed reductions in glutamate and glutamine in the posterior cingulate cortex (PCC), which correlated with increases in the “non-judging” subscale of the Five Facets Mindfulness Questionnaire (264). Similarly, one week after psilocybin therapy, glutamate and N-acetylaspartate concentrations were decreased in the Anterior Cingulate Cortex (ACC) in an open-label study of 24 patients with MDD (83). A double blind, randomized, counterbalanced, crossover study of 24 HCs utilizing MRI and eye tracking showed that LSD decreased the response to participation in self-initiated compared with other-initiated social interaction in the PCC and the temporal gyrus, more precisely the angular gyrus (262) (Table 2).
Neural Circuitry
One of the higher-order brain networks modulated by psychedelics that has gained attention in recent years is the DMN, associated with a range of experiences and conditions (284), including but not limited to self-reflection and rumination (13, 120, 265, 285, 286) and meta-cognitive processes (287). Alterations in DMN rsFC have been demonstrated across a range of disorders. However, a clear and consistent DMN signature specific to any disorder has yet to emerge, underscoring the complexities of mapping correlates of subjective experiences, but also the limitations of biosignature exploration utilizing categorical diagnoses.
Psychedelics reliably alter DMN circuitry and studies in HCs reported decreases in rsFC within the DMN induced by psilocybin (205), LSD (32, 207) and ayahuasca (206). In fifteen HCs intravenous psilocybin resulted in a significant decrease in the positive coupling between the mPFC and PCC (205). LSD (75 μg) 100 min after IV administration decreased connectivity between the parahippocampus and retrosplenial cortex and correlated strongly with ratings of ego-dissolution and altered meaning in 20 HCs (207). Notwithstanding the differences between experienced users who may be more receptive to psychedelic therapy compared to people with mental health disorders, ayahuasca resulted in a significant decrease in activity through most parts of the DMN, including the PCC and the medial mPFC in a group of ten experienced users (206). A decoupling of the mPFC and PCC was associated with positively experienced ego dissolution in a psilocybin double-blind placebo controlled study of 38 healthy experienced mediators (208). Furthermore, the meditators in the psilocybin group reported increased meditation depth and positively experienced ego-dissolution, while at 4 months post-psilocybin they reported positive changes in appreciation for life, self-acceptance, quest for meaning and sense of purpose (209). Interestingly, alteration of the DMN is not limited to classical psychedelics. Oral administration of MDMA (125 mg) to 45 HCs in a randomized, placebo-controlled, double-blind, crossover design showed decreased connectivity within the DMN, two visual networks, and the sensorimotor network (288). Another recent placebo controlled study of 12 healthy males using vaporized salvinorin A, acutely attenuated the DMN during peak effects (first half of 20 min scan) (289), highlighting the overlap with classical psychedelics.
Unsurprisingly given the complex multi-modal nature of self-processing, a single neural correlate such as the DMN may not fully capture the complexities of the self-processing concept (33, 290). Psychedelics alter global brain connectivity, of which the DMN is but one. For example, increased global FC correlated with ego dissolution in an LSD study of 15 HCs (291) and more recently the subjective effects of LSD have been shown to be non-uniform in time, depending on the particular state of the brain at a given point in time (290, 292), with multi-modal imaging techniques (fMRI, diffusion MRI, PET) highlighting the importance of 5-HT2A receptors (27). Previous studies in HCs showed that psilocybin (2 mg) IV destabilized a frontoparietal subsystem (293), whereas IV LSD (75 μg) and IV psilocybin increased the fractal dimension of bold blood oxygen level dependent (BOLD) time-series from regions assigned to the dorsal-attention network (294). Furthermore, a recent rsFC fMRI study in 10 healthy volunteers showed that the executive control network was decreased at 1-week, which was associated with increased mindfulness at 3 months, but there were no other significant changes in other networks (295).
From a personalized point of view, a study suggested that baseline brain connectivity may be a useful predictive marker (41). This double-blind, placebo controlled, randomized, cross-over study of 23 HCs who received oral psilocybin (0.2 mg/kg) and underwent resting-state functional connectivity fMRI scans at three time points (41) showed that psilocybin reduced associative, and concurrently increased sensory brain-wide connectivity over time from administration to peak-effects (41). Furthermore, the participants who had the lowest values in hyper-connected areas and had the highest values in hypo-connected regions displayed the strongest psilocybin induced changes in global brain connectivity (41).
In contrast to the aforementioned psychedelic induced acute decreases in DMN integrity in HCs, an open-labeled study in TRD (n = 20) reported an increase in DMN rsFC 1-day post-psilocybin (40). The reduction of depressive symptoms at 5 weeks was predicted by high scores of acutely experienced pleasurable self-dissolution and by low scores for dread of ego dissolution (87). Furthermore, the increased ventromedial prefrontal cortex-bilateral inferior lateral parietal cortex rsFC, 1-day post-dose, predicted treatment response at 5 weeks post-dose (40). Data from this study (n = 16) (40) combined with the psilocybin therapy vs. escitalopram study (n = 43) indicated that psilocybin was associated with a global decrease in network modularity, indicative of enhanced flexibility (as high modularity scores indicate a greater degree of separation between brain networks) (42). This decrease in modularity was associated with improvements in depression scores at 6-weeks as measured by the Beck Depression inventory (42). In contrast, no changes in modularity were observed with escitalopram, suggesting a tentative biomarker of response to psilocybin therapy.
Modulation of Cognitive Systems
The RDoC organizes cognitive systems into attention, working memory, perception, memory (declarative), language, and cognitive control constructs.
Cognitive Control
Cognitive control refers to a “system that modulates the operation of other cognitive and emotional systems, in the service of goal-directed behavior, when prepotent modes of responding are not adequate to meet the demands of the current context. Additionally, control processes are engaged in the case of novel contexts, where appropriate responses need to be selected from among competing alternatives” (74). This collection of executive control processes include goal-selection, maintenance, updating, as well as response selection and inhibition denotes the ability to switch between different mental sets, tasks, or strategies and plays a vital role in an individual's ability to adapt to environmental changes (296). The underlying neural circuitry involves the default mode, salience, and executive networks, with 5-HT2ARs playing an important role (297–299).
Psychedelics transiently impair certain aspects of cognition in a dose-dependent manner (142, 300–302). For example, a study in HCs showed that LSD (100 μg) compared to placebo acutely impaired executive functions, cognitive flexibility, and working memory on the Intra/Extra-Dimensional shift task, and Spatial Working Memory task, but did not influence the quality of decision-making and risk taking on the Cambridge Gambling Task (302). Similarly, psilocybin decreased attentional tracking ability in HCs, which the authors speculated was due to the inability to inhibit distracting stimuli (303). More recently, re-treatment with ketanserin (40 mg) normalized all LSD-induced cognitive deficits (302). Psychedelic induced impairment of aspects of cognitive flexibility was also observed in a probabilistic reversal learning paradigm in 19 HCs who received IV LSD (75μg) or placebo at two sessions, two weeks apart (Kanen 2021). In this study LSD resulted in more perseverative responding, though the reward learning rate and to a lesser degree the punishment learning rate were enhanced (304).
The complex relationship between cognitive flexibility, neural flexibility, and emotion has recently been highlighted by an open-label study of 24 patients with MDD (83). This study showed that psilocybin therapy decreased perseverative errors in a set-shifting task but did not impact response inhibition, selective attention, or abstract reasoning (83). The improvements in selective aspects of cognitive flexibility did not correlate with improvements in depression. Unexpectedly, greater increases in neural flexibility as measured by dynamics of FC (dFC) between the ACC and PCC, and greater baseline dFC from the ACC were associated with less improvement in cognitive flexibility (83). The practical inferences for the precise-personalized psychedelic therapy paradigm are not fully clear.
A retrospective survey self-report survey of U.S. Veterans in a psychedelic clinical program, reported significant reductions in cognitive impairment as measured by the Medical Outcomes Study—Cognitive Functioning subscale (148). However, changes in the negative valence domain may have led to secondary subjective improvements in the self-reported cognitive domains in this study. Similarly, limited conclusions can be drawn from a non-controlled study in self-selected HCs showing improvements in Cognitive flexibility and the Wisconsin Picture Card Sorting Task 24 h after ayahuasca, which the authors acknowledge could be attributed to practice effects (305).
The acute impairment in some executive domains induced by psychedelic compounds is especially relevant to neurodevelopmental disorders such as schizophrenia, which notwithstanding the inter-individual variability are associated with deficits in cognitive flexibility (306). The further acute impairment in cognitive control induced by psychedelics may in part explain the detrimental negative effects of these substances in psychosis or in those with predispositions to psychosis. Indeed, LSD induced “cognitive bizarreness” associated with loss of self-boundaries and cognitive control as measured by the 5D-ASC in 25 HCs (307) and “mind-wandering” (308) may be counterproductive for those at risk of developing psychosis.
A recent study focused on the claustrum, a thin sheet of gray matter, embedded in the white matter of the cerebral hemispheres and situated between the putamen and the insular cortex, with a rich supply of 5-HT2A receptors and glutamatergic connectivity to the cerebral cortex. The claustrum is thought to be associated with cognitive task switching (309, 310) and salience processing (311), known to be dysfunctional in psychosis (312). Psilocybin acutely reduced claustrum activity and altered its connectivity with the DMN and frontoparietal task control network (FPTC) in a study involving 15 HCs, thus implicating this region as a potential mediator in psilocybin therapy (310).
Obsessive Compulsive Disorder
OCD, frequently comorbid with anxiety and depression, involves deficits in cognitive control, goal-directed planning habit, reward processing (313–315) and negative valence system dysregulation, including abnormal fear extinction (316). Rodent studies have shown that psilocybin reduced digging in the marble burying test—a surrogate for compulsive behavior (317, 318). However, a recent rodent study showed that blockade of 5-HT2A or 5-HT2CRs did not attenuate the effect of psilocybin on digging, suggesting that a different mechanism dominates this effect (318). A psilocybin therapy proof of concept study of antidepressant free people diagnosed with OCD (n = 9) that had failed to respond to at least one SSRI, reported a 23–100% decrease in the Yale-Brown Obsessive Compulsive Scale in the 24 h following ingestion (YBOCS) (15) (Table 1). Limited conclusions can be drawn from this study due to lack of a control group and lack of a clear dose-response relationship to changes in the YBOCS. Results from ongoing clinical trials in OCD will give a clearer picture and it will be interesting to parse potential therapeutic effects of psychedelic therapy according to cognitive control, and negative and positive valence processing systems (Table 3).
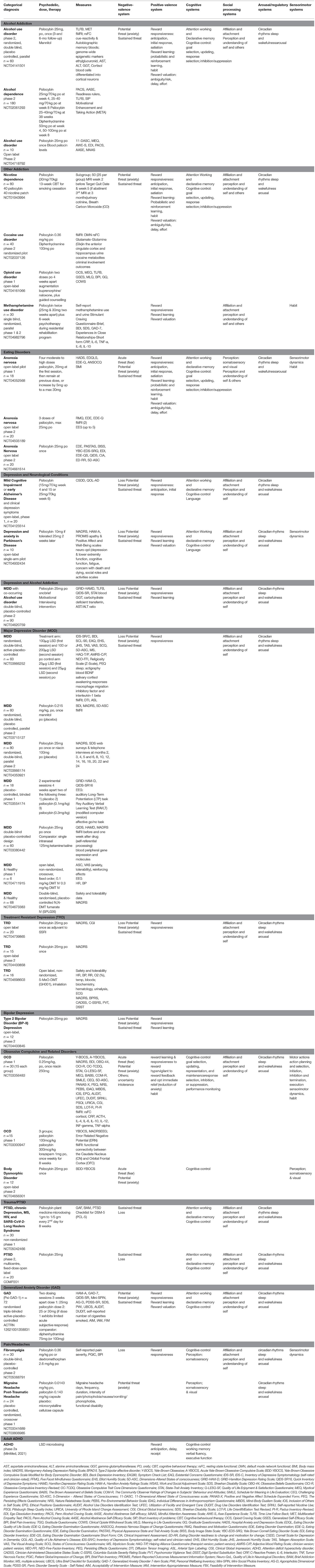
Table 3. Currently registered clinical trials with psychedelics: potential for future integration of outcomes with RDoC.
Eating Disorders
Eating disorders also involve elements of altered cognitive control/reward processing (319, 320), together with aberrant fear/threat encoding processes or threat sensitivity associated with body/food/weight gain/body perception. Enhanced psychological flexibility induced by psychedelic therapy has been proposed as a potential therapeutic mechanism of psychedelic therapy in eating disorders (321). While a preliminary study suggested a benefit of psychedelic therapy in improving depression and well-being scores in people with a self-reported lifetime diagnosis of an eating disorder (16), we await results from ongoing clinical trials (Table 3) to determine whether psychedelic therapy will lead to clinically meaningful benefits in those with eating disorders (322). It is worth noting the possibility that psychedelic therapy may be of utility for disorders related to compulsive overeating, perhaps better categorized as food addiction.
Psychological Flexibility
The “psychological flexibility” concept lacks precise definition, but broadly refers to the ability to recognize and adapt to various situational demands and shift mind-sets/behavioral repertoires (323). It is associated with divergent thinking (DT), a spontaneous and free-flowing pattern where many solutions are possible, with the prospect of novel idea generation. Convergent thinking (CT), in contrast, focuses on the delivery of a single solution. Deficits in psychological flexibility underlie a broad spectrum of psychopathologies. Excessively constrained thought may occur in depression, PTSD/anxiety, OCD, addiction and eating disorders, whereas excessively variable thought may occur in ADHD or some personality disorders (324) and unconstrained thought may occur in psychosis (31). Psychological flexibility has been proposed as a potential transdiagnostic mediator of psychedelic therapy (148, 325, 326). However, the precise impact of psychedelics on psychological flexibility or on DT and CT are not fully clear. For example, a recent double blind, placebo-controlled study of sixty HCs, all of whom had previous psychedelic experiences, found that psilocybin (0.17 mg/kg) acutely decreased CT, which remained decreased for 7 days, whereas measures of DT including fluency and originality decreased, and scores of novelty increased compared to placebo, which were associated with alterations in the DMN (187, 327).
Attention/Working Memory and Memory (Declarative)
Psychedelics acutely and dose dependently impair attention (328, 329), memory task performance (142, 300, 302) and spatial working memory (212). On the other hand, it appears that other domains such as the recall and vividness of autobiographical memory may be accentuated (142–145).
Language and Perception
A computational analysis of semantic and non-semantic language in HCs who received IV LSD (75 μg) and placebo reported that LSD was associated with unconstrained speech (increased verbosity and a reduced lexicon) which was noted to be similar to speech changes during manic psychoses (330). Automated natural language processing methods (331, 332) or digital text analysis (333) may have the potential to improve prediction of psychosis outcomes and there are early indicators that quantitative descriptions of psychedelic experiences derived using Natural Language Processing may play a role in predicting therapeutic outcomes or trajectory in psychedelic therapy (47).
Psychedelics may induce visual imagery (334–336), distortions in the perception of time and space (337, 338) and synaesthesia (339, 340). Auditory and tactile perceptual changes occur less frequently but can occur at higher doses (210, 341). The implications of alterations in these systems for personalized psychedelic therapy are not fully clear, though it is interesting to note the recent proof of concept study showing a role for psilocybin therapy in migraine suppression (17), a condition known to be associated with aberrant connections from the somatosensory cortex to the frontal lobe (342).
Psychedelics over-engage primary sensory cortices and mostly encompasses visual hallucinations (often geometric) with preserved insight monitoring whereas hallucinations in psychosis, are mostly related to overactivation of associative networks, mainly include auditory hallucinations and poor reality monitoring (341). Electrophysiological correlates of IV DMT induced complex visionary experiences during “breakthrough” periods in 13 HCs were associated with a delta/theta rhythmicity (343). A further analysis of the same EEG data with eyes closed reported an EEG wave signal similar to those observed during eyes-open visual stimulation (344). The changes in resting state EEG, which included decreased spectral power in the alpha/beta bands, accompanied by widespread increases in signal diversity, were not specific to the visual system, but also correlated with somatic and metacognitive/affective domains (343, 344). Interestingly, a recent EEG study in freely moving rats showed some overlap with human studies, with a time-dependent global decrease and desynchronization of EEG activity, particularly in the frontal and sensorimotor cortex (345).
Similar to the previously discussed vulnerability to adverse effects of psychedelics in people with incoherent self-concept/aberrant salience in the context of psychosis spectrum disorder, baseline dysfunction in the some of the perceptual systems may increase the risk of adverse events in psychedelic therapy. For example, there is limited high-quality data on the rare condition—Hallucinogen Persisting Perception Disorder (HPPD) (346–348), which in most cases is due to a “subtle over-activation of predominantly neural visual pathways that worsens anxiety after ingestion of arousal-altering drugs, including non-hallucinogenic substances” (347). The authors note that a personal or family history of anxiety and pre-drug use complaints of tinnitus, eye floaters, and concentration problems may predict vulnerability for HPPD (347).
Sensorimotor Systems
Sensorimotor systems are primarily responsible for the control and execution of motor behaviors, and their refinement during learning and development (74). The Sensorimotor Dynamics subconstruct: “processes involved in the specification or parameterization of an action plan and program based on integration of internal or external information, such as sensations and urges and modeling of body dynamics. This process is continuously and iteratively refined via sensory information and reward-reinforced information.”
The highly complex Functional neurological disorders (FNDs), previously known as conversion disorders, involve not only sensorimotor, but also salience, central executive, and limbic networks (349–351). There are no modern era clinical studies of psychedelic therapy in FNDs and systematic reviews of studies from several decades ago are not able to draw firm conclusions due to small numbers of low-quality studies, often lacking control groups and valid outcome measures (352, 353). It is also worth noting that LSD (100 μg) increased sensory-somatomotor brain-wide and thalamic connectivity in 24 HCs, while concurrently reducing associative networks (32). Using a Roving Somatosensory Oddball Task and simultaneous EEG/fMRI in 15 HCs, the same researcher showed that psilocybin (0.2 mg/kg) disrupted tactile prediction error processing in the mPFC, associated with increased salience attribution to non-salient stimuli (354). It remains an open question whether the complex and disrupted sensorimotor modeling of body dynamics and the accompanying emotional processing in conditions such as FNDs (or indeed eating disorders) can be therapeutically modulated by psychedelic therapy (Table 3).
Psilocybiome—An Additional Unit of Analysis?
In keeping with an interconnected systems based psychiatry paradigm that conceptualizes the individual as a complex composite of interacting systems across all levels of organization, it has been proposed that the microbial ecosystem (microbiome) may serve as an additional transdiagnostic unit of analysis in the RDoC framework (355, 356). At the interface between the individual and the environment, the microbiome is intrinsically linked to human health and may play a contributory physiological role in some psychiatric disorders (357–359). This microbial signaling system communicates with the brain through the gut-brain axis via the immune system (360), tryptophan metabolism (361), the HPA axis (362), the vagus nerve (363) and by the production of microbial metabolites, such as short chain fatty acids (SCFA's) (364). The microbiota-gut-brain (MGB) signaling system operates throughout life but is particularly important during early development when it influences the development of the neural circuitry underlying social, cognitive, and emotional brain domains (365, 366). Preclinical research has revealed that neurotransmission, neurogenesis, myelination, dendrite formation and blood brain barrier organization are partially under the influence of this MGB axis signaling system (367–371). At the behavioral level, MGB axis signaling modulates cognitive function and patterns related to social interaction, locomotor activity and stress management (362, 372, 373). The gut microbiome also modulates psychotropic drug metabolism and absorption, which in turn modifies gut microbiota composition (374–376). Thus, the gut microbiome is an unconscious processing system that contributes to emotional, cognitive, and behavioral regulation (377). Acute and sustained psychedelic responses are influenced by bidirectional biofeedback information signals from the periphery and the environment. Consequently, the interaction of the classical psychedelics and the microbiome and mycobiome (fungal community) and associated signaling pathways, together with the potential mediating influence of the microbiome on the interaction between psychedelic therapy and acute and sustained dietary behavioral patterns may have implications for the optimization of precise-personalized-systems based psychedelic therapy (378).
Personalized Psychedelic Therapy
The precise-personalized transdiagnostic paradigm is not without critics and major challenges. As yet, it has not delivered discernible translational benefits to patients (379). Regrettably, there are no psychobiological signatures to guide clinical practice, which still involves clinical assessment and trial and error treatment approaches (380). It is not yet clear whether a transdiagnostic paradigm will add translatable precision to clinical psychiatry, which comprises the severe end of the dimensions (381, 382). Some argue that the RDoC's utility may be limited for the most serious of mental disorders, including dementia, autism, schizophrenia, and bipolar disorder, and may be more useful for depression, anxiety disorders (including PTSD/OCD) and some personality disorders (383, 384).
However, the precise-personalized integrative neuroscience framework is at an early evolutionary stage (385) and the divergence between therapeutic utility for some disorders and exacerbation of others, indicates a role for the RDoC constructs and associated underlying units of analysis, to enhance the understanding and application of psychedelic therapy. While transdiagnostic treatments are not unique to psychedelic compounds, the potential for psychedelics to induce profound transient changes in emotion, thought and perception with marked inter-individual variation, together with the potential to exacerbate underlying pre-dispositions to psychosis and mania (30, 226, 227) compels a greater emphasis on a precise-personalized paradigm. Echoing the general lack of personalized precision in clinical psychiatry, comprehensive clinical assessments are the only available method to identify and exclude participants with disorders that may be exacerbated by psychedelic therapy.
Notwithstanding the reliance on clinical measures, currently available strategies to optimize therapeutic outcomes involve refinement of pharmacotherapy and psychotherapy schedules, though the precise ratio has yet to be determined. It appears that body weight adjusted dosing, albeit over a narrow dosing range of 20–30 mg, may have limited impact on the subjective effects of psilocybin (386) and it remains to be seen whether potential pharmacological modulators such as 5-HT2A receptor gene polymorphisms influence therapeutic response. Moreover, the precise interaction of other psychotropics (SSRI, SNRI, antipsychotics, and mood stabilizers) (387) and psychedelic therapy has yet to be determined. From the psychotherapeutic angle, a high-quality systematized foundation is a vital (388), though there is major scope for the advance of personalization/individualization in the context of an RDoC framework. It will also be interesting to consider the implications of psychedelic therapy for the Neuroscience-based Nomenclature project, developed to progress a more precise neuroscience based psychopharmacological nomenclature (389).
There are preliminary indicators that the advances in the mechanistic understanding of psychedelics may translate into more precise-personalized approaches (41, 42). As translational psychedelic science advances, a complete understanding of the molecular cascades and bidirectional information exchange processes between internal and environmental systems will require analysis across genome, transcriptome, proteome, metabolome, microbiome, epigenome, connectome, physiome and exposome (environmental) levels (390). Deciphering the precise interaction between these systems may advance treatment personalization algorithms, perhaps assisted by advances in technology, such as virtual reality (391, 392), smartphones (393) and biosensors/biofeedback (394). Yet, it should be noted that even if the whole endeavor reduces down to an elaborate set of multi-layered fluctuating ones and zeros or some superposition thereof, or special molecular configurations and information processing pathways yet to be discovered, it is the relationship between the complex configurations underlying our experiences and the empathetic sharing and compassionate understanding of those experiences with others and the environment that is the matter of meaning and the potential of psychedelic therapy.
Conclusions and Perspectives
Psychedelic science and its translational corollary psychedelic therapy are evolving rapidly. Advances in the mechanistic understanding of the underlying pathways, which involve multiple interacting systems may also prompt the development of novel compounds lacking undesirable properties. Several large scale RCTs will determine whether psychedelic therapy translates into the psychiatric clinic for a range of non-psychotic spectrum disorders. Given the translatable transdiagnostic antidepressant, anxiolytic, and anti-addictive therapeutic potential of psychedelic therapy, deconstructing categorical psychiatric diagnoses according to dimensional systems and constructs that align with neurobiological systems may advance more targeted applications, with the possibility of optimizing therapeutic outcomes. As such, integration of the RDoC transdiagnostic dimensional framework with psychedelic therapy as it advances toward the psychiatric clinic has potential to progress an interconnected systems based precise-personalized psychedelic therapy paradigm and narrow the translational gap between neuroscience and psychiatry.
Further insights can be gained from clinical studies in progress with psychedelic therapy although the extent to which they have been designed with this in mind may hamper efforts at integration. Additionally, evolution of multimodal prediction estimation algorithms based on dimensional psychobiological signatures may optimize the delivery of psychedelic therapy and ultimately augment clinical assessments. Apart from the vitally important context (as broadly defined), exploratory studies have proposed baseline functional connectivity patterns and cingulate cortical thickness, autonomic nervous system activity, together with psychological factors as therapeutic predictors. Further unraveling the complex and dynamic molecular cascades and information processing pathways across all levels of analysis from micro to macro, within and between psychiatric disorders and how they converge on the acute and sustained therapeutic subjective trajectory may enhance a more complete systems level understanding of psychedelic therapy and is an important objective for translational neuroscience.
Limitations
This is a narrative review which attempts to conceptualize psychedelic therapy in the context of an evolving RDoC framework and primarily focuses on the effects of psychedelics. The psychotherapy aspect as it relates to RDoC is underdeveloped. This review does not focus on a systematic analysis of the potential side-effects/risks of psychedelic therapy.
Author Contributions
JK wrote the manuscript. CG, GC, JP, AH, CK, and VO'K edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
VO'K was supported by the Health Research Board (HRB) through HRB Grant Code: 201651.12553 and the Meath Foundation, Tallaght University Hospital. VO'K was the Principal Investigator (PI) on the COMPASS trials (COMP001, 003, 004) in Ireland. JK is sub-PI on the COMPASS trials (COMP 001, 003, 004) in Ireland. GC was supported by the HRB through (HRA POR/2011/23 and HRA-POR-2-14-647) and supported by a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (Grant Number 20771). CG was supported by grant funding from MQ: Transforming mental health (MQ16IP13), the Global Brain Health Institute (18GPA02), and Science Foundation Ireland (19/FFP/6418).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Paul Quinlan who designed Figure 1. We sincerely thank all of our participants in the psilocybin studies, who in spite of the burden of their depression, participated in a generous spirit. We apologize to colleagues whose work we were unable to cite due to space constraints.
References
1. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. (2021) 78:121–2. doi: 10.1001/jamapsychiatry.2020.2171
2. Yaden DB, Yaden ME, Griffiths RR. Psychedelics in psychiatry—keeping the renaissance from going off the rails. JAMA Psychiatry. (2021) 78:469–70. doi: 10.1001/jamapsychiatry.2020.3672
3. Corrigan K, Haran M, Mccandliss C, Mcmanus R, Cleary S, Trant R, et al. Psychedelic perceptions: mental health service user attitudes to psilocybin therapy. Ir J Med Sci. (2021) 1–13. doi: 10.1007/s11845-021-02668-2
4. Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry. (2021) 78:481–9. doi: 10.1001/jamapsychiatry.2020.3285
5. Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. (2021) 384:1402–11. doi: 10.1056/NEJMoa2032994
6. Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. (2016) 3:619–27. doi: 10.1016/S2215-0366(16)30065-7
7. Carhart-Harris RL, Bolstridge M, Day CMJ, Rucker J, Watts R, Erritzoe DE, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacol (Berl). (2018) 235:399–408. doi: 10.1007/s00213-017-4771-x
8. Compass. COMP360 Psilocybin Therapy in Treatment-Resistant Depression: Phase IIb Results. (2021). Available online at: https://compasspathways.com/positive-topline-results/ (accessed November 30, 2021).
9. Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. (2014) 28:983–92. doi: 10.1177/0269881114548296
10. Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. (2015) 29:289–99. doi: 10.1177/0269881114565144
11. Rucker JJ, Young AH. Psilocybin: from serendipity to credibility? Front Psychiatry. (2021) 12:e659044. doi: 10.3389/fpsyt.2021.659044
12. Kelly JR, Baker A, Babiker M, Burke L, Brennan C, O'Keane V. The psychedelic renaissance: the next trip for psychiatry? Ir J Psychol Med. (2019) 1–5. doi: 10.1017/ipm.2019.39
13. Carhart-Harris RL, Friston KJ. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev. (2019) 71:316–44. doi: 10.1124/pr.118.017160
14. Teixeira PJ, Johnson MW, Timmermann C, Watts R, Erritzoe D, Douglass H, et al. Psychedelics and health behaviour change. J Psychopharmacol. (2021) 02698811211008554. doi: 10.1177/02698811211008554
15. Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. (2006) 67:1735–40. doi: 10.4088/JCP.v67n1110
16. Spriggs MJ, Kettner H, Carhart-Harris RL. Positive effects of psychedelics on depression and wellbeing scores in individuals reporting an eating disorder. Eating Weight Disord. (2020) 26:1265–70. doi: 10.1007/s40519-020-01000-8
17. Schindler EAD, Sewell RA, Gottschalk CH, Luddy C, Flynn LT, et al. Exploratory controlled study of the migraine-suppressing effects of psilocybin. Neurotherapeutics. (2020) 18:534–43. doi: 10.1007/s13311-020-00962-y
18. Inserra A, De Gregorio D, Gobbi G. Psychedelics in psychiatry: neuroplastic, immunomodulatory, neurotransmitter mechanisms. Pharmacol Rev. (2021) 73:202–77. doi: 10.1124/pharmrev.120.000056
19. Hirschfeld T, Schmidt TT. Dose-response relationships of psilocybin-induced subjective experiences in humans. J Psychopharmacol. (2021) 35:384–97. doi: 10.1177/0269881121992676
20. Glennon RA, Titeler M, Mckenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. (1984) 35:2505–11. doi: 10.1016/0024-3205(84)90436-3
21. González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. (2007) 53:439–52. doi: 10.1016/j.neuron.2007.01.008
22. Weber ET, Andrade R. Htr2a gene and 5-HT(2A) receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci. (2010) 4:36. doi: 10.3389/fnins.2010.00036
23. Andrade R. Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology. (2011) 61:382–6. doi: 10.1016/j.neuropharm.2011.01.015
25. Kim K, Che T, Panova O, Diberto JF, Lyu J, Krumm BE, et al. Structure of a hallucinogen-activated Gq-Coupled 5-HT2A serotonin receptor. Cell. (2020) 182:1574–88.e1519. doi: 10.1016/j.cell.2020.08.024
26. Burt JB, Preller KH, Demirtas M, Ji JL, Krystal JH, Vollenweider FX, et al. Transcriptomics-informed large-scale cortical model captures topography of pharmacological neuroimaging effects of LSD. Elife. (2021) 10:e69320. doi: 10.7554/eLife.69320
27. Singleton SP, Luppi AI, Carhart-Harris RL, Cruzat J, Roseman L, Deco G, et al. Psychedelics Flatten the brain's energy landscape: evidence from receptor-informed network control theory. bioRxiv. (2021) 2021.05.14.44419.
28. Petri G, Expert P, Turkheimer F, Carhart-Harris R, Nutt D, Hellyer PJ, et al. Homological scaffolds of brain functional networks. J. R. Soc. Interface. (2014) 11:20140873. doi: 10.1098/rsif.2014.0873
29. González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. (2008) 452:93–7. doi: 10.1038/nature06612
30. Dos Santos RG, Bouso JC, Hallak JEC. Ayahuasca, dimethyltryptamine, and psychosis: a systematic review of human studies. Ther Adv Psychopharmacol. (2017) 7:141–57. doi: 10.1177/2045125316689030
31. Swanson LR. Unifying theories of psychedelic drug effects. Front. Pharmacol. (2018) 9:e00172. doi: 10.3389/fphar.2018.00172
32. Preller KH, Burt JB, Ji JL, Schleifer CH, Adkinson BD, Stämpfli P, et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife. (2018) 7:e35082. doi: 10.7554/eLife.35082
33. Vollenweider FX, Preller KH. Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci. (2020) 21:611–24. doi: 10.1038/s41583-020-0367-2
34. Inserra A, De Gregorio D, Rezai T, Lopez-Canul MG, Comai S, Gobbi G. Lysergic acid diethylamide differentially modulates the reticular thalamus, mediodorsal thalamus, and infralimbic prefrontal cortex: an in vivo electrophysiology study in male mice. J Psychopharmacol. (2021) 35:469–82. doi: 10.1177/0269881121991569
35. Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol (Oxford, England). (2008) 22:603–20. doi: 10.1177/0269881108093587
36. Barrett FS, Preller KH, Kaelen M. Psychedelics and music: neuroscience and therapeutic implications. Int Rev Psychiatry. (2018) 30:350–62. doi: 10.1080/09540261.2018.1484342
37. Carhart-Harris RL, Roseman L, Haijen E, Erritzoe D, Watts R, Branchi I, et al. Psychedelics and the essential importance of context. J Psychopharmacol. (2018) 32:725–31. doi: 10.1177/0269881118754710
38. Strickland JC, Garcia-Romeu A, Johnson MW. Set and setting: a randomized study of different musical genres in supporting psychedelic therapy. ACS Pharmacol Transl Sci. (2020) 4:472–8. doi: 10.31234/osf.io/f5dmt
39. Olbrich S, Preller KH, Vollenweider FX. LSD and ketanserin and their impact on the human autonomic nervous system. Psychophysiology. (2021) 58:13822. doi: 10.1111/psyp.13822
40. Carhart-Harris RL, Roseman L, Bolstridge M, Demetriou L, Pannekoek JN, Wall MB, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep. (2017) 7:13187. doi: 10.1038/s41598-017-13282-7
41. Preller KH, Duerler P, Burt JB, Ji JL, Adkinson B, Stämpfli P, et al. Psilocybin induces time-dependent changes in global functional connectivity. Biol Psychiatry. (2020) 88:197–207. doi: 10.1016/j.biopsych.2019.12.027
42. Daws R, Timmerman C, Giribaldi B, Sexton J, Wall M, Erritzoe D, et al. Nature Portfolio. (2021).
43. Lewis CR, Preller KH, Braden BB, Riecken C, Vollenweider FX. Rostral anterior cingulate thickness predicts the emotional psilocybin experience. Biomedicines. (2020) 8:34. doi: 10.3390/biomedicines8020034
44. Maclean KA, Johnson MW, Griffiths RR. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol. (2011) 25:1453–61. doi: 10.1177/0269881111420188
45. Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of psilocybin response in healthy volunteers. PLoS ONE. (2012) 7:e30800. doi: 10.1371/journal.pone.0030800
46. Haijen E, Kaelen M, Roseman L, Timmermann C, Kettner H, Russ S, et al. Predicting responses to psychedelics: a prospective study. Front Pharmacol. (2018) 9:897. doi: 10.3389/fphar.2018.00897
47. Cox DJ, Garcia-Romeu A, Johnson MW. Predicting changes in substance use following psychedelic experiences: natural language processing of psychedelic session narratives. Am J Drug Alcohol Abuse. (2021) 47:444–54. doi: 10.1080/00952990.2021.1981357
48. Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. (2010) 167:748–51. doi: 10.1176/appi.ajp.2010.09091379
49. Carcone D, Ruocco AC. Six years of research on the national institute of mental health's research domain criteria (RDoC) initiative: a systematic review. Front Cell Neurosci. (2017) 11:e00046. doi: 10.3389/fncel.2017.00046
50. Clark LA, Cuthbert B, Lewis-Fernández R, Narrow WE, Reed GM. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health's Research Domain Criteria (RDoC). Psychol Sci Public Interest. (2017) 18:72–145. doi: 10.1177/1529100617727266
51. Dos Santos RG, Bouso JC, Alcázar-Córcoles M, Hallak JEC. Efficacy, tolerability, and safety of serotonergic psychedelics for the management of mood, anxiety, and substance-use disorders: a systematic review of systematic reviews. Expert Rev Clin Pharmacol. (2018) 11:889–902. doi: 10.1080/17512433.2018.1511424
52. Luoma JB, Chwyl C, Bathje GJ, Davis AK, Lancelotta R. A meta-analysis of placebo-controlled trials of psychedelic-assisted therapy. J Psychoactive Drugs. (2020) 52:289–99. doi: 10.1080/02791072.2020.1769878
53. Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. (2020) 177:391–410. doi: 10.1176/appi.ajp.2019.19010035
54. Romeo B, Karila L, Martelli C, Benyamina A. Efficacy of psychedelic treatments on depressive symptoms: a meta-analysis. J Psychopharmacol. (2020) 34:1079–85. doi: 10.1177/0269881120919957
55. Galvão-Coelho NL, Marx W, Gonzalez M, Sinclair J, De Manincor M, Perkins D, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology. (2021) 238:341–54. doi: 10.1007/s00213-020-05719-1
56. Krystal JH, Tolin DF, Sanacora G, Castner SA, Williams GV, Aikins DE, et al. Neuroplasticity as a target for the pharmacotherapy of anxiety disorders, mood disorders, and schizophrenia. Drug Discov Today. (2009) 14:690–7. doi: 10.1016/j.drudis.2009.05.002
57. Vogelzangs N, De Jonge P, Smit JH, Bahn S, Penninx BW. Cytokine production capacity in depression and anxiety. Transl Psychiatry. (2016) 6:e825. doi: 10.1038/tp.2016.92
58. Caspi A, Houts RM, Ambler A, Danese A, Elliott ML, Hariri A, et al. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the dunedin birth cohort study. JAMA Network Open. (2020) 3:e203221. doi: 10.1001/jamanetworkopen.2020.3221
59. Gillan CM, Seow TXF. Carving out new transdiagnostic dimensions for research in mental health. Biol Psychiatry Cogn Neurosci Neuroimaging. (2020) 5:932–4. doi: 10.1016/j.bpsc.2020.04.013
60. Janiri D, Moser DA, Doucet GE, Luber MJ, Rasgon A, Lee WH, et al. Shared neural phenotypes for mood and anxiety disorders: a meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry. (2020) 77:172–9. doi: 10.1001/jamapsychiatry.2019.3351
61. McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. (2017) 174:676–85. doi: 10.1176/appi.ajp.2017.16040400
62. Sha Z, Wager TD, Mechelli A, He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. (2019) 85:379–88. doi: 10.1016/j.biopsych.2018.11.011
63. Boedhoe PSW, Van Rooij D, Hoogman M, Twisk JWR, Schmaal L, Abe Y, et al. Subcortical brain volume, regional cortical thickness, and cortical surface area across disorders: findings from the ENIGMA ADHD. ASD, and OCD Working Groups. Am J Psychiatry. (2020) 177:834–43. doi: 10.1176/appi.ajp.2020.19030331
64. Smoller JW, Ripke S, Lee P, Neale B, Nurnberger J, Santangelo S, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. (2013) 381:1371–9. doi: 10.1016/S0140-6736(12)62129-1
65. Brainstorm C, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, et al. Analysis of shared heritability in common disorders of the brain. Science (New York, N.Y.). (2018) 360:eaap8757. doi: 10.1126/science.aap8757
66. Jaffe AE, Barry BK, Tao R, Tran MN, Page SC, Maynard KR, et al. Decoding shared versus divergent transcriptomic signatures across cortico-amygdala circuitry in PTSD and depressive disorders. bioRxiv. (2021). doi: 10.1101/2021.01.12.426438
67. Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Towards reproducible brain-wide association studies. bioRxiv. (2020). doi: 10.1101/2020.08.21.257758
68. Baldwin JR, Caspi A, Meehan AJ, Ambler A, Arseneault L, Fisher HL, et al. Population vs. individual prediction of poor health from results of adverse childhood experiences screening. JAMA Pediatr. (2021) 175:385–93. doi: 10.1001/jamapediatrics.2020.5602
69. Gillan CM, Whelan R. What big data can do for treatment in psychiatry. Curr Opin Behav Sci. (2017) 18:34–42. doi: 10.1016/j.cobeha.2017.07.003
70. Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J, Lisanby SH, et al. A randomized proof-of-mechanism trial applying the ‘fast-fail' approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat Med. (2020) 26:760–8. doi: 10.1038/s41591-020-0806-7
71. Medeiros GC, Rush AJ, Jha M, Carmody T, Furman JL, Czysz AH, et al. Positive and negative valence systems in major depression have distinct clinical features, response to antidepressants, and relationships with immunomarkers. Depress Anxiety. (2020) 37:771–83. doi: 10.1002/da.23006
72. Trivedi MH, Mcgrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, et al. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): rationale and design. J Psychiatr Res. (2016) 78:11–23. doi: 10.1016/j.jpsychires.2016.03.001
73. Nicholson JR, Sommer B. The research domain criteria framework in drug discovery for neuropsychiatric diseases: focus on negative valence. Brain Neurosci Adv. (2018) 2:2398212818804030. doi: 10.1177/2398212818804030
74. Nimh. Research Domain Criteria. (2021). Available online at: https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/index.shtml (accessed November 30, 2021).
75. Woody ML, Gibb BE. Integrating NIMH research domain criteria (RDoC) into depression research. Curr Opin Psychol. (2015) 4:6–12. doi: 10.1016/j.copsyc.2015.01.004
76. Rucker JJH, Jelen LA, Flynn S, Frowde KD, Young AH. Psychedelics in the treatment of unipolar mood disorders: a systematic review. J Psychopharmacol. (2016) 30:1220–9. doi: 10.1177/0269881116679368
77. Muttoni S, Ardissino M, John C. Classical psychedelics for the treatment of depression and anxiety: a systematic review. J Affect Disord. (2019) 258:11–24. doi: 10.1016/j.jad.2019.07.076
78. Goldberg SB, Shechet B, Nicholas CR, Ng CW, Deole G, Chen Z, et al. Post-acute psychological effects of classical serotonergic psychedelics: a systematic review and meta-analysis. Psychol Med. (2020) 50:2655–66. doi: 10.1017/S003329172000389X
79. Andersen KAA, Carhart-Harris R, Nutt DJ, Erritzoe D. Therapeutic effects of classic serotonergic psychedelics: a systematic review of modern-era clinical studies. Acta Psychiatr Scand. (2021) 143:101–18. doi: 10.1111/acps.13249
80. Grob CS, Danforth AL, Chopra GS, Hagerty M, Mckay CR, Halberstadt AL, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancerpsilocybin for anxiety in advanced-stage cancer. JAMA Psychiatry. (2011) 68:71–8. doi: 10.1001/archgenpsychiatry.2010.116
81. Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol (Oxford, England). (2016) 30:1181–97. doi: 10.1177/0269881116675513
82. Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol (Oxford, England). (2016) 30:1165–80. doi: 10.1177/0269881116675512
83. Doss MK, PovaŽan M, Rosenberg MD, Sepeda ND, Davis AK, Finan PH, et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl Psychiatry. (2021) 11:574. doi: 10.1038/s41398-021-01706-y
84. Roseman L, Demetriou L, Wall MB, Nutt DJ, Carhart-Harris RL. Increased amygdala responses to emotional faces after psilocybin for treatment-resistant depression. Neuropharmacology. (2018) 142:263–9. doi: 10.1016/j.neuropharm.2017.12.041
85. Mertens LJ, Wall MB, Roseman L, Demetriou L, Nutt DJ, Carhart-Harris RL. Therapeutic mechanisms of psilocybin: CHANGES in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J Psychopharmacol. (2020) 34:167–80. doi: 10.1177/0269881119895520
86. Stroud JB, Freeman TP, Leech R, Hindocha C, Lawn W, Nutt DJ, et al. Psilocybin with psychological support improves emotional face recognition in treatment-resistant depression. Psychopharmacology (Berl). (2018) 235:459–66. doi: 10.1007/s00213-017-4754-y
87. Roseman L, Nutt DJ, Carhart-Harris RL. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol. (2018) 8:e00974. doi: 10.3389/fphar.2017.00974
88. Watts R, Day C, Krzanowski J, Nutt D, Carhart-Harris R. Patients' accounts of increased “connectedness” and “acceptance” after psilocybin for treatment-resistant depression. J Human Psychol. (2017) 57:520–64. doi: 10.1177/0022167817709585
89. Palhano-Fontes F, Barreto D, Onias H, Andrade KC, Novaes MM, Pessoa JA, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. (2019) 49:655–63. doi: 10.1017/S0033291718001356
90. De Almeida RN, Galvão ACM, Da Silva FS, Silva E, Palhano-Fontes F, Maia-De-Oliveira JP, et al. Modulation of serum brain-derived neurotrophic factor by a single dose of ayahuasca: observation from a randomized controlled trial. Front Psychol. (2019) 10:1234. doi: 10.3389/fpsyg.2019.01234
91. Zeifman RJ, Palhano-Fontes F, Hallak J, Arcoverde E, Maia-Oliveira JP, Araujo DB. The impact of ayahuasca on suicidality: results from a randomized controlled trial. Front Pharmacol. (2019) 10:1325. doi: 10.3389/fphar.2019.01325
92. Galvão ACM, De Almeida RN, Silva E, Freire FAM, Palhano-Fontes F, Onias H, et al. Cortisol modulation by ayahuasca in patients with treatment resistant depression and healthy controls. Front Psychiatry. (2018) 9:185. doi: 10.3389/fpsyt.2018.00185
93. Galvão-Coelho NL, De Menezes Galvão AC, De Almeida RN, Palhano-Fontes F, Campos Braga I, Lobão Soares B, et al. Changes in inflammatory biomarkers are related to the antidepressant effects of Ayahuasca. J Psychopharmacol (Oxford, England). (2020) 34:1125–33. doi: 10.1177/0269881120936486
94. Sanches RF, de Lima Osório F, Dos Santos RG, Macedo LR, Maia-de-Oliveira JP, Wichert-Ana L, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol. (2016) 36:77–81. doi: 10.1097/JCP.0000000000000436
95. Zeifman RJ, Singhal N, dos Santos RG, Sanches RF, de Lima Osório F, Hallak JEC, et al. Rapid and sustained decreases in suicidality following a single dose of ayahuasca among individuals with recurrent major depressive disorder: results from an open-label trial. Psychopharmacology. (2021) 238:453–9. doi: 10.1007/s00213-020-05692-9
96. Osório FL, Sanches RF, Macedo LR, Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. (2015) 37:13–20. doi: 10.1590/1516-4446-2014-1496
97. Agin-Liebes GI, Malone T, Yalch MM, Mennenga SE, Ponté KL, Guss J, et al. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J Psychopharmacol. (2020) 34:155–66. doi: 10.1177/0269881119897615
98. Ross S, Agin-Liebes G, Lo S, Zeifman RJ, Ghazal L, Benville J, et al. Acute and sustained reductions in loss of meaning and suicidal ideation following psilocybin-assisted psychotherapy for psychiatric and existential distress in life-threatening cancer. ACS Pharmacol Transl Sci. (2021) 4:553–62. doi: 10.1021/acsptsci.1c00020
99. Gasser P, Kirchner K, Passie T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: a qualitative study of acute and sustained subjective effects. J Psychopharmacol. (2015) 29:57–68. doi: 10.1177/0269881114555249
100. Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. (2014) 202:513–20. doi: 10.1097/NMD.0000000000000113
101. Anderson BT, Danforth A, Daroff PR, Stauffer C, Ekman E, Agin-Liebes G, et al. Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: an open-label safety and feasibility pilot study. EClinicalMedicine. (2020) 27:538. doi: 10.1016/j.eclinm.2020.100538
102. Kelly JR, Crockett MT, Alexander L, Haran M, Baker A, Burke L, et al. Psychedelic science in post-COVID-19 psychiatry. Ir J Psychol Med. (2021) 38:93–8. doi: 10.1017/ipm.2020.94
103. Becker AM, Holze F, Grandinetti T, Klaiber A, Toedtli VE, Kolaczynska KE, et al. Acute effects of psilocybin after escitalopram or placebo pretreatment in a randomized, double-blind, placebo-controlled, cross-over study in healthy subjects. Clin Pharmacol Therap. (2021). doi: 10.1002/cpt.2487
104. Roddy D, Kelly JR, Farrell C, Doolin K, Roman E, Nasa A, et al. Amygdala substructure volumes in major depressive disorder. NeuroImage Clin. (2021) 31:102781. doi: 10.1016/j.nicl.2021.102781
105. Nasa A, Gaughan C, Mahmoud M, Kelly JR, Roman E, Levins KJ, et al. The human dorsal hippocampal commissure: Delineating connections across the midline using multi-modal neuroimaging in major depressive disorder. Neuroimage Rep. (2021) 1:100062. doi: 10.1016/j.ynirp.2021.100062
106. Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD social anxiety disorder, specific phobia. Am J Psychiatry. (2007) 164:1476–88. doi: 10.1176/appi.ajp.2007.07030504
107. Groenewold NA, Opmeer EM, De Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. (2013) 37:152–63. doi: 10.1016/j.neubiorev.2012.11.015
108. Stuart SA, Butler P. Munafò MR, Nutt DJ, Robinson ES. A translational rodent assay of affective biases in depression and antidepressant therapy. Neuropsychopharmacology. (2013) 38:1625–35. doi: 10.1038/npp.2013.69
109. Ben-Zion Z, Shany O, Admon R, Keynan NJ, Avisdris N, Balter SR, et al. Neural responsivity to reward versus punishment shortly after trauma predicts long-term development of post-traumatic stress symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. (2021). doi: 10.1016/j.bpsc.2021.09.001
110. Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. (2012) 72:898–906. doi: 10.1016/j.biopsych.2012.04.005
111. Kraehenmann R, Preller KH, Scheidegger M, Pokorny T, Bosch OG, Seifritz E, et al. Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol Psychiatry. (2015) 78:572–81. doi: 10.1016/j.biopsych.2014.04.010
112. Grimm O, Kraehenmann R, Preller KH, Seifritz E, Vollenweider FX. Psilocybin modulates functional connectivity of the amygdala during emotional face discrimination. Eur Neuropsychopharmacol. (2018) 28:691–700. doi: 10.1016/j.euroneuro.2018.03.016
113. Barrett FS, Doss MK, Sepeda ND, Pekar JJ, Griffiths RR. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci Rep. (2020) 10:2214. doi: 10.1038/s41598-020-59282-y
114. Dolder PC, Schmid Y, Müller F, Borgwardt S, Liechti ME. LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology. (2016) 41:2638–46. doi: 10.1038/npp.2016.82
115. Mueller F, Lenz C, Dolder PC, Harder S, Schmid Y, Lang UE, et al. Acute effects of LSD on amygdala activity during processing of fearful stimuli in healthy subjects. Transl Psychiatry. (2017) 7:e1084. doi: 10.1038/tp.2017.54
116. Bershad AK, Preller KH, Lee R, Keedy S, Wren-Jarvis J, Bremmer MP, et al. H. Preliminary report on the effects of a low dose of LSD on resting-state amygdala functional connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging. (2020) 5:461–7. doi: 10.1016/j.bpsc.2019.12.007
117. Rocha JM, Rossi GN, De Lima Osório F, Bouso JC, De Oliveira Silveira G, Yonamine M, et al. Effects of ayahuasca on the recognition of facial expressions of emotions in naive healthy volunteers: a pilot, proof-of-concept, randomized controlled trial. J Clin Psychopharmacol. (2021) 41:267–74. doi: 10.1097/JCP.0000000000001396
118. Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci. (2008) 3:400–24. doi: 10.1111/j.1745-6924.2008.00088.x
119. Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci. (2010) 10:470–8. doi: 10.3758/CABN.10.4.470
120. Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. (2015) 78:224–30. doi: 10.1016/j.biopsych.2015.02.020
121. Da Silva FS, Silva EAS, Sousa GM Jr., Maia-De-Oliveira JP, Soares-Rachetti VP, De Araujo DB, et al Acute effects of ayahuasca in a juvenile non-human primate model of depression. Braz J Psychiatry. (2019) 41:280–8. doi: 10.1590/1516-4446-2018-0140
122. Uthaug MV, Lancelotta R, Szabo A, Davis AK, Riba J, Ramaekers JG. Prospective examination of synthetic 5-methoxy-N,N-dimethyltryptamine inhalation: effects on salivary IL-6, cortisol levels, affect, and non-judgment. Psychopharmacology. (2020) 237:773–85. doi: 10.1007/s00213-019-05414-w
123. Mcewen BS. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatry. (2017) 74:551–2. doi: 10.1001/jamapsychiatry.2017.0270
124. Zorn JV, Schür RR, Boks MP, Kahn RS, Joëls M, Vinkers CH. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology. (2017) 77:25–36. doi: 10.1016/j.psyneuen.2016.11.036
125. Catlow BJ, Song S, Paredes DA, Kirstein CL, Sanchez-Ramos J. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res. (2013) 228:481–91. doi: 10.1007/s00221-013-3579-0
126. Cameron LP, Benson CJ, Dunlap LE, Olson DE. Effects of N, N-dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chem Neurosci. (2018) 9:1582–90. doi: 10.1021/acschemneuro.8b00134
127. Cameron LP, Benson CJ, Defelice BC, Fiehn O, Olson DE. Chronic, intermittent microdoses of the psychedelic NN-dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS Chem Neurosci. (2019) 10:3261–70. doi: 10.1021/acschemneuro.8b00692
128. De La Fuente Revenga M, Zhu B, Guevara CA, Naler LB, Saunders JM, Zhou Z, et al. Prolonged epigenetic and synaptic plasticity alterations following single exposure to a psychedelic in mice. bioRxiv. (2021). doi: 10.1101/2021.02.24.432725
129. Ruffell SGD, Netzband N, Tsang W, Davies M, Butler M, Rucker JJH, et al. Ceremonial ayahuasca in amazonian retreats—mental health and epigenetic outcomes from a six-month naturalistic study. Front Psychiatry. (2021) 12:e687615. doi: 10.3389/fpsyt.2021.687615
130. Jones NT, Zahid Z, Grady SM, Sultan ZW, Zheng Z, Banks MI, et al. (2020). Delayed Anxiolytic-Like Effects of Psilocybin in Male Mice Are Supported by Acute Glucocorticoid Release. bioRxiv. 2020.2008.2012.248229. doi: 10.1101/2020.08.12.248229
131. Jefsen O, Højgaard K, Christiansen SL, Elfving B, Nutt DJ, Wegener G, et al. Psilocybin lacks antidepressant-like effect in the Flinders Sensitive Line rat. Acta Neuropsychiatr. (2019) 31:213–9. doi: 10.1017/neu.2019.15
132. Hibicke M, Landry AN, Kramer HM, Talman ZK, Nichols CD. Psychedelics, but not ketamine, produce persistent antidepressant-like effects in a rodent experimental system for the study of depression. ACS Chem Neurosci. (2020) 11:864–71. doi: 10.1021/acschemneuro.9b00493
133. Weston NM, Gibbs D, Bird CIV, Daniel A, Jelen LA, Knight G, et al. Historic psychedelic drug trials and the treatment of anxiety disorders. Depress Anxiety. (2020) 37:1262–79. doi: 10.1002/da.23065
134. Reiche S, Hermle L, Gutwinski S, Jungaberle H, Gasser P. Majić T. Serotonergic hallucinogens in the treatment of anxiety and depression in patients suffering from a life-threatening disease: a systematic review. Prog Neuro Psychopharmacol Biol Psychiatry. (2018) 81:1–10. doi: 10.1016/j.pnpbp.2017.09.012
135. Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. (2013) 47:1469–78. doi: 10.1016/j.jpsychires.2013.05.031
136. Brown VM, Labar KS, Haswell CC, Gold AL, Mccarthy G, Morey RA. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. (2014) 39:351–9. doi: 10.1038/npp.2013.197
137. Ross DA, Arbuckle MR, Travis MJ, Dwyer JB, Van Schalkwyk GI, Ressler KJ. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. (2017) 74:407–15. doi: 10.1001/jamapsychiatry.2016.3325
138. Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol Psychiatry. (2017) 81:1023–9. doi: 10.1016/j.biopsych.2016.11.015
139. Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, et al. Amygdala and insula connectivity changes following psychotherapy for posttraumatic stress disorder: a randomized clinical trial. Biol Psychiatry. (2021) 89:857–67. doi: 10.1016/j.biopsych.2020.11.021
140. Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. (2006) 51:871–82. doi: 10.1016/j.neuron.2006.07.029
141. Mcteague LM, Laplante M-C, Bulls HW, Shumen JR, Lang PJ, Keil A. Face perception in social anxiety: visuocortical dynamics reveal propensities for hypervigilance or avoidance. Biol Psychiatry. (2018) 83:618–28. doi: 10.1016/j.biopsych.2017.10.004
142. Healy CJ. The acute effects of classic psychedelics on memory in humans. Psychopharmacology. (2021) 238:639–53. doi: 10.1007/s00213-020-05756-w
143. Kaelen M, Giribaldi B, Raine J, Evans L, Timmerman C, Rodriguez N, et al. The hidden therapist: evidence for a central role of music inpsychedelic therapy. Psychopharmacology. (2018) 235:505–19. doi: 10.1007/s00213-017-4820-5
144. Kaelen M, Roseman L, Kahan J, Santos-Ribeiro A, Orban C, Lorenz R, et al. LSD modulates music-induced imagery via changes in parahippocampal connectivity. Eur Neuropsychopharmacol. (2016) 26:1099–109. doi: 10.1016/j.euroneuro.2016.03.018
145. Carhart-Harris RL, Leech R, Williams TM, Erritzoe D, Abbasi N, Bargiotas T, et al. Implications for psychedelic-assisted psychotherapy: functional magnetic resonance imaging study with psilocybin. Br J Psychiatry. (2012) 200:238–44. doi: 10.1192/bjp.bp.111.103309
146. Tonegawa S, Pignatelli M, Roy DS, Ryan TJ. Memory engram storage and retrieval. Curr Opin Neurobiol. (2015) 35:101–9. doi: 10.1016/j.conb.2015.07.009
147. Ryan TJ, De San Luis CO, Pezzoli M, Sen S. Engram cell connectivity: an evolving substrate for information storage. Curr Opin Neurobiol. (2021) 67:215–27. doi: 10.1016/j.conb.2021.01.006
148. Davis AK, Averill LA, Sepeda ND, Barsuglia JP, Amoroso T. Psychedelic treatment for trauma-related psychological and cognitive impairment among us special operations forces veterans. Chronic Stress. (2020) 4:2470547020939564. doi: 10.1177/2470547020939564
149. Krediet E, Bostoen T, Breeksema J, Van Schagen A, Passie T, Vermetten E. Reviewing the potential of psychedelics for the treatment of PTSD. Int J Neuropsychopharmacol. (2020) 23:385–400. doi: 10.1093/ijnp/pyaa018
150. Nutt D, Erritzoe D, Carhart-Harris R. Psychedelic psychiatry's brave new world. Cell. (2020) 181:24–8. doi: 10.1016/j.cell.2020.03.020
151. Wolff M, Evens R, Mertens LJ, Koslowski M, Betzler F, Gründer G, et al. Learning to let go: a cognitive-behavioral model of how psychedelic therapy promotes acceptance. Front Psychiatry. (2020) 11:5. doi: 10.3389/fpsyt.2020.00005
152. Zeifman RJ, Wagner AC, Watts R, Kettner H, Mertens LJ, Carhart-Harris RL. Post-psychedelic reductions in experiential avoidance are associated with decreases in depression severity and suicidal ideation. Front Psychiatry. (2020) 11:e00782. doi: 10.3389/fpsyt.2020.00782
153. Stauffer CS, Anderson BT, Ortigo KM, Woolley J. Psilocybin-assisted group therapy and attachment: observed reduction in attachment anxiety and influences of attachment insecurity on the psilocybin experience. ACS Pharmacol Transl Sci. (2021) 4:526–32. doi: 10.1021/acsptsci.0c00169
154. Studerus E, Vizeli P, Harder S, Ley L, Liechti ME. Prediction of MDMA response in healthy humans: a pooled analysis of placebo-controlled studies. J Psychopharmacol. (2021) 35:556–65. doi: 10.1177/0269881121998322
155. New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, et al. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. (2007) 32:1629–40. doi: 10.1038/sj.npp.1301283
156. Soloff PH, Abraham K, Burgess A, Ramaseshan K, Chowdury A, Diwadkar VA. Impulsivity and aggression mediate regional brain responses in Borderline Personality Disorder: an fMRI study. Psychiatry Res Neuroimaging. (2017) 260:76–85. doi: 10.1016/j.pscychresns.2016.12.009
157. Michelini G, Palumbo IM, DeYoung CG, Latzman RD, Kotov R. Linking RDoC and HiTOP: A new interface for advancing psychiatric nosology and neuroscience. Clin Psychol Rev. (2021) 86:102025. doi: 10.1016/j.cpr.2021.102025
158. Seidl E, Padberg F, Bauriedl-Schmidt C, Albert A, Daltrozzo T, Hall J, et al. Response to ostracism in patients with chronic depression, episodic depression and borderline personality disorder a study using Cyberball. J Affect Disord. (2020) 260:254–62. doi: 10.1016/j.jad.2019.09.021
159. Zeifman RJ, Wagner AC. Exploring the case for research on incorporating psychedelics within interventions for borderline personality disorder. J Context Behav Sci. (2020) 15:1–11. doi: 10.1016/j.jcbs.2019.11.001
160. Domínguez-Clavé E, Soler J, Pascual JC, Elices M, Franquesa A, Valle M, et al. Ayahuasca improves emotion dysregulation in a community sample and in individuals with borderline-like traits. Psychopharmacology (Berl). (2019) 236:573–80. doi: 10.1007/s00213-018-5085-3
161. Van Mulukom V, Patterson RE, Van Elk M. Broadening Your Mind to Include Others: The relationship between serotonergic psychedelic experiences and maladaptive narcissism. Psychopharmacology (Berl). (2020) 237:2725–37. doi: 10.1007/s00213-020-05568-y
162. Carhart-Harris RL, Kaelen M, Bolstridge M, Williams TM, Williams LT, Underwood R, et al. The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol Med. (2016) 46:1379–90. doi: 10.1017/S0033291715002901
163. Bouso JC, Dos Santos RG, Alcázar-Córcoles MÁ, Hallak JEC. Serotonergic psychedelics and personality: a systematic review of contemporary research. Neurosci Biobehav Rev. (2018) 87:118–32. doi: 10.1016/j.neubiorev.2018.02.004
164. Erritzoe D, Smith J, Fisher PM, Carhart-Harris R, Frokjaer VG, Knudsen GM. Recreational use of psychedelics is associated with elevated personality trait openness: exploration of associations with brain serotonin markers. J Psychopharmacol. (2019) 33:1068–1075. doi: 10.1177/0269881119827891
165. Lebedev AV, Kaelen M, Lövdén M, Nilsson J, Feilding A, Nutt DJ, et al. LSD-induced entropic brain activity predicts subsequent personality change. Hum Brain Mapp. (2016) 37:3203–13. doi: 10.1002/hbm.23234
166. Stenbaek DS, Kristiansen S, Burmester D, Madsen MK, Frokjaer VG, Knudsen GM, et al. Trait OPENNESS and serotonin 2A receptors in healthy volunteers: a positron emission tomography study. Hum Brain Mapp. (2019) 40:2117–24. doi: 10.1002/hbm.24511
167. Madsen MK, Fisher PM, Stenbæk DS, Kristiansen S, Burmester D, Lehel S, et al. A single psilocybin dose is associated with long-term increased mindfulness, preceded by a proportional change in neocortical 5-HT2A receptor binding. Eur Neuropsychopharmacol. (2020) 33:71–80. doi: 10.1016/j.euroneuro.2020.02.001
168. Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. (1997) 17:2785–95. doi: 10.1523/JNEUROSCI.17-08-02785.1997
169. Morales-Garcia JA, De La Fuente Revenga M, Alonso-Gil S, Rodriguez-Franco MI, Feilding A, Perez-Castillo A, et al. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci Rep. (2017) 7:5309. doi: 10.1038/s41598-017-05407-9
170. Olson DE. Psychoplastogens: a promising class of plasticity-promoting neurotherapeutics. J Exp Neurosci. (2018) 12:1179069518800508. doi: 10.1177/1179069518800508
171. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. (2020) 4:563–7. doi: 10.1021/acsptsci.0c00192
172. De Vos CMH, Mason NL, Kuypers KPC. Psychedelics and neuroplasticity: a systematic review unraveling the biological underpinnings of psychedelics. Front Psychiatry. (2021) 12:1575. doi: 10.3389/fpsyt.2021.724606
173. Vargas MV, Meyer R, Avanes AA, Rus M, Olson DE. Psychedelics and other psychoplastogens for treating mental illness. Front Psychiatry. (2021) 12:727117. doi: 10.3389/fpsyt.2021.727117
174. Yaden DB, Griffiths RR. The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. (2020) 4:568–72. doi: 10.1021/acsptsci.0c00194
175. Kadriu B, Greenwald M, Henter ID, Gilbert JR, Kraus C, Park LT, et al. Ketamine and serotonergic psychedelics: common mechanisms underlying the effects of rapid-acting antidepressants. Int J Neuropsychopharmacol. (2021) 24:8–21. doi: 10.1093/ijnp/pyaa087
176. Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. (2008) 33:88–109. doi: 10.1038/sj.npp.1301574
177. Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. (2018) 23:3170–82. doi: 10.1016/j.celrep.2018.05.022
178. Ly C, Greb AC, Vargas MV, Duim WC, Grodzki ACG, Lein PJ, et al. Transient stimulation with psychoplastogens is sufficient to initiate neuronal growth. ACS Pharmacol Transl Sci. (2020). 4:452–60. doi: 10.1021/acsptsci.0c00065
179. Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. (2021) 109:2535–44.e4. doi: 10.1016/j.neuron.2021.06.008
180. Raval NR, Johansen A, Donovan LL, Ros NF, Ozenne B, Hansen HD, et al. A single dose of psilocybin increases synaptic density and decreases 5-HT(2A) receptor density in the pig brain. Int J Mol Sci. (2021) 22:835. doi: 10.3390/ijms22020835
181. Nichols CD, Sanders-Bush E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology. (2002) 26:634–42. doi: 10.1016/S0893-133X(01)00405-5
182. Martin DA, Marona-Lewicka D, Nichols DE, Nichols CD. Chronic LSD alters gene expression profiles in the mPFC relevant to schizophrenia. Neuropharmacology. (2014) 83:1–8. doi: 10.1016/j.neuropharm.2014.03.013
183. Jefsen OH, Elfving B, Wegener G, Müller HK. Transcriptional regulation in the rat prefrontal cortex and hippocampus after a single administration of psilocybin. J Psychopharmacol. (2021) 35:483–93. doi: 10.1177/0269881120959614
184. Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature. (2021) 589:474–9. doi: 10.1038/s41586-020-3008-z
185. Lu J, Tjia M, Mullen B, Cao B, Lukasiewicz K, Shah-Morales S, et al. An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress. Mol Psychiatry. (2021). doi: 10.1038/s41380-021-01159-1
186. Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. (2019) 364:eaat8078. doi: 10.1126/science.aat8078
187. Mason NL, Kuypers KPC, Müller F, Reckweg J, Tse DHY, Toennes SW, et al. Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology. (2020) 45:2003–11. doi: 10.1038/s41386-020-0718-8
188. Hutten N, Mason N, Dolder P, Theunissen E, Holze F, Liechti ME, et al. Low doses of LSD acutely increase BDNF blood plasma levels in healthy volunteers. ACS Pharmacol Transl Sci. (2021) 4:431–66. doi: 10.1021/acsptsci.0c00099
189. Holze F, Vizeli P, Ley L, Müller F, Dolder P, Stocker M, et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology. (2021) 46:537–44. doi: 10.1038/s41386-020-00883-6
190. Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. (2012) 4:19. doi: 10.1186/1866-1955-4-19
191. Hägele C, Schlagenhauf F, Rapp M, Sterzer P, Beck A, Bermpohl F-U, et al. Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology. (2015) 232:331–41. doi: 10.1007/s00213-014-3662-7
192. Nusslock R, Alloy LB. Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. J Affect Disord. (2017) 216:3–16. doi: 10.1016/j.jad.2017.02.001
193. Eckstrand KL, Forbes EE, Bertocci MA, Chase HW, Greenberg T, Lockovich J, et al. Trauma affects prospective relationships between reward-related ventral striatal and amygdala activation and 1-year future hypo/mania trajectories. Biol Psychiatry. (2021) 89:868–77. doi: 10.1016/j.biopsych.2020.11.017
194. Cremers HR, Veer IM, Spinhoven P, Rombouts SRB, Roelofs K. Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Front Behav Neurosci. 8:e00439. doi: 10.3389/fnbeh.2014.00439
195. Carlton CN, Sullivan-Toole H, Ghane M, Richey JA. Reward circuitry and motivational deficits in social anxiety disorder: what can be learned from mouse models? Front Neurosci. (2020) 14:154–154. doi: 10.3389/fnins.2020.00154
196. Baskin-Sommers AR, Foti D. Abnormal reward functioning across substance use disorders and major depressive disorder: considering reward as a transdiagnostic mechanism. Int J Psychophysiol. (2015) 98:227–39. doi: 10.1016/j.ijpsycho.2015.01.011
197. Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev. (2019) 99:2115–40. doi: 10.1152/physrev.00014.2018
198. Berner LA, Marsh R. Frontostriatal circuits and the development of bulimia nervosa. Front Behav Neurosci. (2014) 8:395. doi: 10.3389/fnbeh.2014.00395
199. Steinglass JE, Walsh BT. Neurobiological model of the persistence of anorexia nervosa. J Eat Disord. (2016) 4:19. doi: 10.1186/s40337-016-0106-2
200. Marona-Lewicka D, Thisted RA, Nichols DE. Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl). (2005) 180:427–35. doi: 10.1007/s00213-005-2183-9
201. De Gregorio D, Posa L, Ochoa-Sanchez R, Mclaughlin R, Maione S, Comai S, et al. The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT(1A), D(2) and TAAR(1) receptors. Pharmacol Res. (2016) 113:81–91. doi: 10.1016/j.phrs.2016.08.022
202. Sakashita Y, Abe K, Katagiri N, Kambe T, Saitoh T, Utsunomiya I, et al. Effect of psilocin on extracellular dopamine and serotonin levels in the mesoaccumbens and mesocortical pathway in awake rats. Biol Pharm Bull. (2015) 38:134–8. doi: 10.1248/bpb.b14-00315
203. Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Nat Acad Sci. (2021) 118:e2022489118. doi: 10.1073/pnas.2022489118
204. Grandjean J, Buehlmann D, Buerge M, Sigrist H, Seifritz E, Vollenweider FX, et al. Psilocybin exerts distinct effects on resting state networks associated with serotonin and dopamine in mice. Neuroimage. (2021) 225:117456. doi: 10.1016/j.neuroimage.2020.117456
205. Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Nat Acad Sci. (2012) 109:2138–43. doi: 10.1073/pnas.1119598109
206. Palhano-Fontes F, Andrade KC, Tofoli LF, Santos AC, Crippa JA, Hallak JE, Ribeiro S, et al. The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PLoS ONE. (2015) 10:e0118143. doi: 10.1371/journal.pone.0118143
207. Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Nat Acad Sci. (2016) 113:4853–8. doi: 10.1073/pnas.1518377113
208. Smigielski L, Scheidegger M, Kometer M, Vollenweider FX. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage. (2019) 196:207–15. doi: 10.1016/j.neuroimage.2019.04.009
209. Smigielski L, Kometer M, Scheidegger M, Krähenmann R, Huber T, Vollenweider FX. Characterization and prediction of acute and sustained response to psychedelic psilocybin in a mindfulness group retreat. Sci Rep. (2019) 9:14914. doi: 10.1038/s41598-019-50612-3
210. Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol. (2011) 25:1434–52. doi: 10.1177/0269881110382466
211. Vollenweider FX, Vontobel P, Hell D, Leenders KL. 5-HT Modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man—A PET study with [11C]raclopride. Neuropsychopharmacology. (1999) 20:424–33. doi: 10.1016/S0893-133X(98)00108-0
212. Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. (1998) 9:3897–902. doi: 10.1097/00001756-199812010-00024
213. Brooks SJ, Lochner C, Shoptaw S, Stein DJ. Using the research domain criteria (RDoC) to conceptualize impulsivity and compulsivity in relation to addiction. Prog Brain Res. (2017) 235:177–218. doi: 10.1016/bs.pbr.2017.08.002
214. Krebs TS. Johansen P-Ø. Psychedelics and mental health: a population study. PLOS ONE. (2013) 8:e63972. doi: 10.1371/journal.pone.0063972
215. Johnson MW, Griffiths RR, Hendricks PS, Henningfield JE. The abuse potential of medical psilocybin according to the 8 factors of the controlled substances act. Neuropharmacology. (2018) 142:143–66. doi: 10.1016/j.neuropharm.2018.05.012
216. Krebs TS, Johansen PO. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol. (2012) 26:994–1002. doi: 10.1177/0269881112439253
217. Divito AJ, Leger RF. Psychedelics as an emerging novel intervention in the treatment of substance use disorder: a review. Mol Biol Rep. (2020) 47:9791–9. doi: 10.1007/s11033-020-06009-x
218. Nielson EM, May DG, Forcehimes AA, Bogenschutz MP. The psychedelic debriefing in alcohol dependence treatment: illustrating key change phenomena through qualitative content analysis of clinical sessions. Front Pharmacol. (2018) 9:132. doi: 10.3389/fphar.2018.00132
219. Garcia-Romeu A, Davis AK, Erowid F, Erowid E, Griffiths RR, Johnson MW. Cessation and reduction in alcohol consumption and misuse after psychedelic use. J Psychopharmacol. (2019) 33:1088–101. doi: 10.1177/0269881119845793
220. Meinhardt MW, Güngör C, Skorodumov I, Mertens LJ, Spanagel R. Psilocybin and LSD have no long-lasting effects in an animal model of alcohol relapse. Neuropsychopharmacology. (2020) 45:1316–22. doi: 10.1038/s41386-020-0694-z
221. Meinhardt MW, Pfarr S, Fouquet G, Rohleder C, Meinhardt ML, Barroso-Flores J, et al. Psilocybin targets a common molecular mechanism for cognitive impairment and increased craving in alcoholism. Sci Adv. (2021) 7:eabh2399. doi: 10.1126/sciadv.abh2399
222. Higgins GA, Carroll NK, Brown M, Macmillan C, Silenieks LB, Thevarkunnel S, et al. Low doses of psilocybin and ketamine enhance motivation and attention in poor performing rats: evidence for an antidepressant property. Front. Pharmacol. (2021) 12:e640241. doi: 10.3389/fphar.2021.640241
223. Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev. (2014) 7:157–64. doi: 10.2174/1874473708666150107121331
224. Noorani T, Garcia-Romeu A, Swift TC, Griffiths RR, Johnson MW. Psychedelic therapy for smoking cessation: qualitative analysis of participant accounts. J Psychopharmacol. (2018) 32:756–69. doi: 10.1177/0269881118780612
225. Nusslock R, Walden K, Harmon-Jones E. Asymmetrical frontal cortical activity associated with differential risk for mood and anxiety disorder symptoms: an RDoC perspective. Int J Psychophysiol. (2015) 98:249–61. doi: 10.1016/j.ijpsycho.2015.06.004
226. Brown T, Shao W, Ayub S, Chong D. Cornelius C. A physician's attempt to self-medicate bipolar depression with NN-dimethyltryptamine (DMT). J Psychoactive Drugs. (2017) 49:294–6. doi: 10.1080/02791072.2017.1344898
227. Szmulewicz AG, Valerio MP, Smith JM. Switch to mania after ayahuasca consumption in a man with bipolar disorder: a case report. Int J Bipolar Disord. (2015) 3:4–4. doi: 10.1186/s40345-014-0020-y
228. Gard DE, Pleet MM, Bradley ER, Penn AD, Gallenstein ML, Riley LS, et al. Evaluating the risk of psilocybin for the treatment of bipolar depression: a review of the research literature and published case studies. J Affect Disord Rep. (2021) 6:100240. doi: 10.1016/j.jadr.2021.100240
229. Grof S. Realms of the Human Unconscious Observations From LSD Research. New York, NY: Viking Press (1975).
230. Carhart-Harris RL, Kaelen M, Whalley MG, Bolstridge M, Feilding A, Nutt DJ, et al. enhances suggestibility in healthy volunteers. Psychopharmacology (Berl). (2015) 232:785–94. doi: 10.1007/s00213-014-3714-z
231. Carhart-Harris RL, Nutt DJ. Serotonin and brain function: a tale of two receptors. J Psychopharmacol. (2017) 31:1091–120. doi: 10.1177/0269881117725915
232. Schindler EAD, Wallace RM, Sloshower JA, D'souza DC. Neuroendocrine associations underlying the persistent therapeutic effects of classic serotonergic psychedelics. Front Pharmacol. (2018) 9:177. doi: 10.3389/fphar.2018.00177
233. Szabo A. Psychedelics and Immunomodulation: novel approaches and therapeutic opportunities. Front Immunol. (2015) 6:e00358. doi: 10.3389/fimmu.2015.00358
234. Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. (2015) 78:544–53. doi: 10.1016/j.biopsych.2014.11.015
235. Dolder PC, Schmid Y, Steuer AE, Kraemer T, Rentsch KM, Hammann F, et al. Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin Pharmacokinet. (2017) 56:1219–30. doi: 10.1007/s40262-017-0513-9
236. Holze F, Vizeli P, Müller F, Ley L, Duerig R, Varghese N, et al. Distinct acute effects of LSD. MDMA, and d-amphetamine in healthy subjects. Neuropsychopharmacology. (2020) 45:462–71. doi: 10.1038/s41386-019-0569-3
237. Strassman RJ. Qualls CR. Dose-response study of NN-dimethyltryptamine in humans I Neuroendocrine, autonomic, cardiovascular effects. Arch Gen Psychiatry. (1994) 51:85–97. doi: 10.1001/archpsyc.1994.03950020009001
238. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of NN-dimethyltryptamine in humans. Biol Psychiatry. (1996) 39:784–95. doi: 10.1016/0006-3223(95)00200-6
239. Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl). (2004) 172:145–56. doi: 10.1007/s00213-003-1640-6
240. Strajhar P, Schmid Y, Liakoni E, Dolder PC, Rentsch KM, Kratschmar DV, et al. Acute effects of lysergic acid diethylamide on circulating steroid levels in healthy subjects. J Neuroendocrinol. (2016) 28:12374. doi: 10.1111/jne.12374
241. Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD. Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharmacol Exp Ther. (2008) 327:316–23. doi: 10.1124/jpet.108.143461
242. Nau F Jr., Yu B, Martin D, Nichols CD. Serotonin 5-HT2A receptor activation blocks TNF-α mediated inflammation in vivo. PLoS ONE. (2013) 8:e75426. doi: 10.1371/journal.pone.0075426
243. Dos Santos RG. Immunological effects of ayahuasca in humans. J Psychoactive Drugs. (2014) 46:383–8. doi: 10.1080/02791072.2014.960113
244. Szabo A, Kovacs A, Frecska E, Rajnavolgyi E. Psychedelic NN-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PLoS ONE. (2014) 9:e106533. doi: 10.1371/journal.pone.0106533
245. Szabo A, Kovacs A, Riba J, Djurovic S, Rajnavolgyi E, Frecska E. The endogenous hallucinogen and trace amine N.N-dimethyltryptamine (DMT) displays potent protective effects against hypoxia via sigma-1 receptor activation in human primary iPSC-derived cortical neurons and microglia-like immune cells. Front. Neurosci. (2016) 10:e00423. doi: 10.3389/fnins.2016.00423
246. Flanagan TW, Nichols CD. Psychedelics as anti-inflammatory agents. Int Rev Psychiatry. (2018) 30:363–75. doi: 10.1080/09540261.2018.1481827
247. Flanagan TW, Sebastian MN, Battaglia DM, Foster TP, Cormier SA, Nichols CD. 5-HT2 receptor activation alleviates airway inflammation and structural remodeling in a chronic mouse asthma model. Life Sci. (2019) 236:116790. doi: 10.1016/j.lfs.2019.116790
248. Flanagan TW, Sebastian MN, Battaglia DM, Foster TP, Maillet EL, Nichols CD. Activation of 5-HT(2) Receptors reduces inflammation in vascular tissue and cholesterol levels in high-fat diet-fed apolipoprotein e knockout mice. Sci Rep. (2019) 9:13444. doi: 10.1038/s41598-019-49987-0
249. Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J. Neuroimmune Pharmacol. (2013) 8:900–20. doi: 10.1007/s11481-013-9462-8
250. Vogelzangs N, Beekman A, De Jonge P, Penninx B. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. (2013) 3:e249. doi: 10.1038/tp.2013.27
251. Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. (2019) 49:1958–70. doi: 10.1017/S0033291719001454
252. Thompson C, Szabo A. Psychedelics as a novel approach to treating autoimmune conditions. Immunol Lett. (2020) 228:45–54. doi: 10.1016/j.imlet.2020.10.001
253. Castellanos JP, Woolley C, Bruno KA, Zeidan F, Halberstadt A, Furnish T. Chronic pain and psychedelics: a review and proposed mechanism of action. Regional Anesthesia andamp. Pain Med. (2020) 45:486–94. doi: 10.1136/rapm-2020-101273
254. Bornemann J, Close JB, Spriggs MJ, Carhart-Harris R, Roseman L. Self-medication for chronic pain using classic psychedelics: a qualitative investigation to inform future research. Front Psychiatry. (2021) 12:735427. doi: 10.3389/fpsyt.2021.735427
255. Freeman D, Sheaves B, Waite F, Harvey AG, Harrison PJ. Sleep disturbance and psychiatric disorders. Lancet Psychiatry. (2020) 7:628–37. doi: 10.1016/S2215-0366(20)30136-X
256. Dudysová D, Janku K, Šmotek M, Saifutdinova E, Koprivová J, Bušková J, et al. The effects of daytime psilocybin administration on sleep: implications for antidepressant action. Front Pharmacol. (2020) 11:e602590. doi: 10.3389/fphar.2020.602590
257. Dunbar RI. The social brain meets neuroimaging. Trends Cogn Sci. (2012) 16:101–2. doi: 10.1016/j.tics.2011.11.013
258. Sliwa J, Freiwald WA. A dedicated network for social interaction processing in the primate brain. Science. (2017) 356:745–9. doi: 10.1126/science.aam6383
259. Tomova L, Wang KL, Thompson T, Matthews GA, Takahashi A, Tye KM, et al. Acute social isolation evokes midbrain craving responses similar to hunger. Nat Neurosci. (2020) 23:1597–605. doi: 10.1038/s41593-020-00742-z
260. Santini ZI, Jose PE, York Cornwell E, Koyanagi A, Nielsen L, Hinrichsen C, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. (2020) 5:e62–70. doi: 10.1016/S2468-2667(19)30230-0
261. Madsen MK, Fisher PM, Burmester D, Dyssegaard A, Stenbaek DS, Kristiansen S, et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology. (2019) 44:1328–34. doi: 10.1038/s41386-019-0324-9
262. Preller KH, Schilbach L, Pokorny T, Flemming J, Seifritz E, Vollenweider FX. Role of the 5-HT2A receptor in self- and other-initiated social interaction in lysergic acid diethylamide-induced states: a pharmacological fMRI study. J Neurosci. (2018) 38:3603–11. doi: 10.1523/JNEUROSCI.1939-17.2018
263. Duerler P, Schilbach L, Stämpfli P, Vollenweider FX, Preller KH. LSD-induced increases in social adaptation to opinions similar to one's own are associated with stimulation of serotonin receptors. Sci Rep. (2020) 10:12181. doi: 10.1038/s41598-020-68899-y
264. Sampedro F, De La Fuente Revenga M, Valle M, Roberto N, Dominguez-Clave E, Elices M, et al. Assessing the psychedelic “after-glow” in ayahuasca users: post-acute neurometabolic and functional connectivity changes are associated with enhanced mindfulness capacities. Int J Neuropsychopharmacol. (2017) 20:698–711. doi: 10.1093/ijnp/pyx036
265. Lebedev AV, Lövdén M, Rosenthal G, Feilding A, Nutt DJ, Carhart-Harris RL. Finding the self by losing the self: neural correlates of ego-dissolution under psilocybin. Hum Brain Mapp. (2015) 36:3137–53. doi: 10.1002/hbm.22833
266. Griffiths RR, Johnson MW, Richards WA, Richards BD, Jesse R, Maclean KA, et al. Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol. (2018) 32:49–69. doi: 10.1177/0269881117731279
267. Preller KH, Pokorny T, Hock A, Kraehenmann R, Stämpfli P, Seifritz E, et al. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A. (2016) 113:5119–24. doi: 10.1073/pnas.1524187113
268. Pokorny T, Preller KH, Kometer M, Dziobek I, Vollenweider FX. Effect of psilocybin on empathy and moral decision-making. Int J Neuropsychopharmacol. (2017) 20:747–57. doi: 10.1093/ijnp/pyx047
269. De Gregorio D, Popic J, Enns JP, Inserra A, Skalecka A, Markopoulos A, et al. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc Nat Acad Sci. (2021) 118:e2020705118. doi: 10.1073/pnas.2020705118
270. Mollinedo-Gajate I, Song C, Sintes-Rodriguez M, Whelan T, Soula A, Selimbeyoglu A, et al. Psilocybin rescues sociability deficits in an animal model of autism. bioRxiv. (2020). doi: 10.1101/2020.09.09.289348
271. Carhart-Harris RL, Erritzoe D, Haijen E, Kaelen M, Watts R. Psychedelics and connectedness. Psychopharmacology (Berl). (2018) 235:547–50. doi: 10.1007/s00213-017-4701-y
272. Kettner H, Rosas FE, Timmermann C, Kärtner L, Charhart-Harris RL, Roseman L. Psychedelic communitas: intersubjective experience during psychedelic group sessions predicts enduring changes in psychological wellbeing and social connectedness. Front Pharmacol. (2021) 12:e623985. doi: 10.3389/fphar.2021.623985
273. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. (2018) 32:811–9. doi: 10.1177/0269881117748902
274. Kettner H, Gandy S, Haijen E, Carhart-Harris RL. From egoism to ecoism: psychedelics increase nature relatedness in a state-mediated and context-dependent manner. Int J Environ Res Public Health. (2019) 16:5147. doi: 10.3390/ijerph16245147
275. Gandy S, Forstmann M, Carhart-Harris RL, Timmermann C, Luke D, Watts R. The potential synergistic effects between psychedelic administration and nature contact for the improvement of mental health. Health Psychology Open. (2020) 7:2055102920978123. doi: 10.1177/2055102920978123
276. Forstmann M, Sagioglou C. Lifetime experience with (classic) psychedelics predicts pro-environmental behavior through an increase in nature relatedness. J Psychopharmacol. (2017) 31:975–88. doi: 10.1177/0269881117714049
277. Griffiths RR, Richards WA, Mccann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl). (2006) 187:268–83; discussion 284–92. doi: 10.1007/s00213-006-0457-5
278. Roseman L, Ron Y, Saca A, Ginsberg N, Luan L, Karkabi N, et al. Relational processes in ayahuasca groups of palestinians and israelis. Front Pharmacol. (2021) 12:e607529. doi: 10.3389/fphar.2021.607529
279. Breeksema JJ, Niemeijer AR, Krediet E, Vermetten E, Schoevers RA. Psychedelic treatments for psychiatric disorders: a systematic review and thematic synthesis of patient experiences in qualitative studies. CNS Drugs. (2020) 34:925–46. doi: 10.1007/s40263-020-00748-y
280. Ebisch SJH, Aleman A. The fragmented self: imbalance between intrinsic and extrinsic self-networks in psychotic disorders. Lancet Psychiatry. (2016) 3:784–90. doi: 10.1016/S2215-0366(16)00045-6
281. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. (2003) 160:13–23. doi: 10.1176/appi.ajp.160.1.13
282. Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. (2016) 16:3385–403. doi: 10.2174/1568026616666160608084834
283. Stenbæk DS, Madsen MK, Ozenne B, Kristiansen S, Burmester D, Erritzoe D, et al. Brain serotonin 2A receptor binding predicts subjective temporal and mystical effects of psilocybin in healthy humans. J Psychopharmacol. (2020) 35:459–68. doi: 10.1177/0269881120959609
284. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. (2008) 1124:1–38. doi: 10.1196/annals.1440.011
285. Carhart-Harris RL, Friston KJ. The default-mode, ego-functions and free-energy: a neurobiological account of Freudian ideas. Brain. (2010) 133:1265–83. doi: 10.1093/brain/awq010
286. Knyazev GG, Savostyanov AN, Bocharov AV, Levin EA, Rudych PD. Intrinsic connectivity networks in the self- and other-referential processing. Front Human Neurosci. (2020) 14:e579703. doi: 10.3389/fnhum.2020.579703
287. Soto D, Theodoraki M, Paz-Alonso PM. How the human brain introspects about one's own episodes of cognitive control. Cortex. (2018) 107:110–20. doi: 10.1016/j.cortex.2017.10.016
288. Müller F, Holze F, Dolder P, Ley L, Vizeli P, Soltermann A, et al. MDMA-induced changes in within-network connectivity contradict the specificity of these alterations for the effects of serotonergic hallucinogens. Neuropsychopharmacology. (2020) 46:545–53. doi: 10.1038/s41386-020-00906-2
289. Doss MK, May DG, Johnson MW, Clifton JM, Hedrick SL, Prisinzano TE, et al. The acute effects of the atypical dissociative hallucinogen salvinorin A on functional connectivity in the human brain. Sci Rep. (2020) 10:16392. doi: 10.1038/s41598-020-73216-8
290. Luppi AI, Carhart-Harris RL, Roseman L, Pappas I, Menon DK, Stamatakis EA, et al. alters dynamic integration and segregation in the human brain. Neuroimage. (2021) 227:117653. doi: 10.1016/j.neuroimage.2020.117653
291. Tagliazucchi E, Roseman L, Kaelen M, Orban C, Muthukumaraswamy SD, Murphy K, et al. Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr Biol. (2016) 26:1043–50. doi: 10.1016/j.cub.2016.02.010
292. Luppi AI, Craig MM, Pappas I, Finoia P, Williams GB, Allanson J, et al. Consciousness-specific dynamic interactions of brain integration and functional diversity. Nat Commun. (2019) 10:4616. doi: 10.1038/s41467-019-12658-9
293. Lord LD, Expert P, Atasoy S, Roseman L, Rapuano K, Lambiotte R, et al. Dynamical exploration of the repertoire of brain networks at rest is modulated by psilocybin. Neuroimage. (2019) 199:127–42. doi: 10.1016/j.neuroimage.2019.05.060
294. Varley TF, Carhart-Harris R, Roseman L, Menon DK, Stamatakis EA. Serotonergic psychedelics LSD and psilocybin increase the fractal dimension of cortical brain activity in spatial and temporal domains. Neuroimage. (2020) 220:117049. doi: 10.1016/j.neuroimage.2020.117049
295. Mcculloch DE, Madsen MK, Stenbæk DS, Kristiansen S, Ozenne B, Jensen PS, et al. Lasting effects of a single psilocybin dose on resting-state functional connectivity in healthy individuals. J Psychopharmacol. (2021) 2698811211026454. doi: 10.1177/02698811211026454
296. Uddin LQ. Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nature Reviews Neuroscience. (2021) 22:167–79. doi: 10.1038/s41583-021-00428-w
297. Nichols DE. Hallucinogens. Pharmacol Ther. (2004) 101:131–81. doi: 10.1016/j.pharmthera.2003.11.002
298. Kim C, Cilles SE, Johnson NF, Gold BT. Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum Brain Mapp. (2012) 33:130–42. doi: 10.1002/hbm.21199
299. Zhang G, Stackman RW. The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol. (2015) 6:e00225. doi: 10.3389/fphar.2015.00225
300. Barrett FS, Carbonaro TM, Hurwitz E, Johnson MW, Griffiths RR. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: effects on cognition. Psychopharmacology. (2018) 235:2915–27. doi: 10.1007/s00213-018-4981-x
301. Bayne T, Carter O. Dimensions of consciousness and the psychedelic state. Neurosci Consciousness. (2018) 2018:niy008. doi: 10.1093/nc/niy008
302. Pokorny T, Duerler P, Seifritz E, Vollenweider FX, Preller KH. LSD acutely impairs working memory, executive functions, cognitive flexibility. but not risk-based decision-making. Psychol Med. (2019) 50:2255–64. doi: 10.1101/532234
303. Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. (2005) 17:1497–508. doi: 10.1162/089892905774597191
304. Kanen JW, Luo Q, Kandroodi MR, Cardinal RN, Robbins TW, Carhart-Harris RL, et al. Effect of lysergic acid diethylamide (LSD) on reinforcement learning in humans. bioRxiv. (2021) 2020.12.04.412189.
305. Murphy-Beiner A, Soar K. Ayahuasca's ‘afterglow’: improved mindfulness and cognitive flexibility in ayahuasca drinkers. Psychopharmacology. (2020) 237:1161–9. doi: 10.1007/s00213-019-05445-3
306. Waltz JA. The neural underpinnings of cognitive flexibility and their disruption in psychotic illness. Neuroscience. (2017) 345:203–17. doi: 10.1016/j.neuroscience.2016.06.005
307. Kraehenmann R, Pokorny D, Vollenweider L, Preller KH, Pokorny T, Seifritz E, et al. Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl). (2017) 234:2031–46. doi: 10.1007/s00213-017-4610-0
308. Wießner I, Falchi M, Palhano-Fontes F, Oliveira Maia L, Feilding A, Ribeiro S, et al. Low-dose LSD and the stream of thought: increased discontinuity of mind, deep thoughts and abstract flow. Psychopharmacology. (2021). doi: 10.1007/s00213-021-06006-3
309. Krimmel SR, White MG, Panicker MH, Barrett FS, Mathur BN, Seminowicz DA. Resting state functional connectivity and cognitive task-related activation of the human claustrum. Neuroimage. (2019) 196:59–67. doi: 10.1016/j.neuroimage.2019.03.075
310. Barrett FS, Krimmel SR, Griffiths R, Seminowicz DA, Mathur BN. Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. Neuroimage. (2020) 218:116980. doi: 10.1016/j.neuroimage.2020.116980
311. Smith JB, Watson GDR, Liang Z, Liu Y, Zhang N, Alloway KD. A role for the claustrum in salience processing? Front Neuroanat. (2019) 13:e00064. doi: 10.3389/fnana.2019.00064
312. Mallikarjun PK, Lalousis PA, Dunne TF, Heinze K., Reniers RLEP, Broome MR, et al. Aberrant salience network functional connectivity in auditory verbal hallucinations: a first episode psychosis sample. Transl Psychiatry. (2018) 8:69. doi: 10.1038/s41398-018-0118-6
313. Fineberg NA, Menchon JM, Zohar J, Veltman DJ. Compulsivity—a new trans-diagnostic research domain for the roadmap for mental health research in Europe (ROAMER) and research domain criteria (RDoC) initiatives. Eur Neuropsychopharmacol. (2016) 26:797–9. doi: 10.1016/j.euroneuro.2016.04.001
314. Gillan CM, Robbins TW, Sahakian BJ, Van Den Heuvel OA, Van Wingen G. The role of habit in compulsivity. Eur Neuropsychopharmacol. (2016) 26:828–40. doi: 10.1016/j.euroneuro.2015.12.033
315. Gillan CM, Kalanthroff E, Evans M, Weingarden HM, Jacoby RJ, Gershkovich M, et al. Comparison of the association between goal-directed planning and self-reported compulsivity vs. obsessive-compulsive disorder diagnosis. JAMA Psychiatry. (2019) 77:1–10. doi: 10.1001/jamapsychiatry.2019.2998
316. Dougherty DD, Brennan BP, Stewart SE, Wilhelm S, Widge AS, Rauch SL. Neuroscientifically informed formulation and treatment planning for patients with obsessive-compulsive disorder: a review. JAMA Psychiatry. (2018) 75:1081–7. doi: 10.1001/jamapsychiatry.2018.0930
317. Matsushima Y, Shirota O, Kikura-Hanajiri R, Goda Y, Eguchi F. Effects of psilocybe argentipes on marble-burying behavior in mice. Biosci Biotechnol Biochem. (2009) 73:1866–8. doi: 10.1271/bbb.90095
318. Odland AU, Kristensen JL, Andreasen JT. Investigating the role of 5-HT2A and 5-HT2C receptor activation in the effects of psilocybin, DOI and citalopram on marble burying in mice. Behav Brain Res. (2021) 401:113093. doi: 10.1016/j.bbr.2020.113093
319. Ehrlich S, Geisler D, Ritschel F, King JA, Seidel M, Boehm I, et al. Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. J Psychiatry Neurosci. (2015) 40:307–15. doi: 10.1503/jpn.140249
320. Treasure J, Zipfel S, Micali N, Wade T, Stice E, Claudino A, et al. Anorexia nervosa. Nat Rev Disease Primers. (2015) 1:15074. doi: 10.1038/nrdp.2015.74
321. Foldi CJ, Liknaitzky P, Williams M, Oldfield BJ. Rethinking therapeutic strategies for anorexia nervosa: insights from psychedelic medicine and animal models. Front Neurosci. (2020) 14:e00043. doi: 10.3389/fnins.2020.00043
322. Spriggs MJ, Douglass HM, Park RJ, Read T, Danby JL, De Magalhães FJC, et al. (2021). Study Protocol for “Psilocybin as a Treatment for Anorexia Nervosa: A Pilot Study”. Frontiers in Psychiatry 12. doi: 10.3389/fpsyt.2021.735523
323. Cherry KM, Hoeven EV, Patterson TS, Lumley MN. Defining and measuring “psychological flexibility”: a narrative scoping review of diverse flexibility and rigidity constructs and perspectives. Clin Psychol Rev. (2021) 84:101973. doi: 10.1016/j.cpr.2021.101973
324. Christoff K, Irving ZC, Fox KCR, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: a dynamic framework. Nat Rev Neurosci. (2016) 17:718–31. doi: 10.1038/nrn.2016.113
325. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Context Behav Sci. (2020) 15:39–45. doi: 10.1016/j.jcbs.2019.11.004
326. Girn M, Mills C, Roseman L, Carhart-Harris RL, Christoff K. Updating the dynamic framework of thought: creativity and psychedelics. Neuroimage. (2020) 213:116726. doi: 10.1016/j.neuroimage.2020.116726
327. Mason NL, Kuypers KPC, Reckweg JT, Müller F, Tse DHY, Da Rios B, et al. Spontaneous and deliberate creative cognition during and after psilocybin exposure. Transl Psychiatry. (2021) 11:209. doi: 10.1038/s41398-021-01335-5
328. Heekeren K, Neukirch A, Daumann J, Stoll M, Obradovic M, Kovar KA, et al. Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and NN-dimethyltryptamine (DMT) models of psychosis. J Psychopharmacol. (2007) 21:312–20. doi: 10.1177/0269881107077734
329. Daumann J, Heekeren K, Neukirch A, Thiel CM, Möller-Hartmann W, Gouzoulis-Mayfrank E. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl). (2008) 200:573–83. doi: 10.1007/s00213-008-1237-1
330. Sanz C, Pallavicini C, Carrillo F, Zamberlan F, Sigman M, Mota N, et al. The entropic tongue: disorganization of natural language under LSConsciousness D. Cognition. (2021) 87:103070. doi: 10.1016/j.concog.2020.103070
331. Corcoran CM, Carrillo F, Fernández-Slezak D, Bedi G, Klim C, Javitt DC, et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. (2018) 17:67–75. doi: 10.1002/wps.20491
332. Low DM, Bentley KH, Ghosh SS. Automated assessment of psychiatric disorders using speech: a systematic review. Laryngosc Investig Otolaryngol. (2020) 5:96–116. doi: 10.1002/lio2.354
333. Birnbaum ML, Ernala SK, Rizvi AF, Arenare ER, Van Meter A, et al. Detecting relapse in youth with psychotic disorders utilizing patient-generated and patient-contributed digital data from Facebook npj. Schizophrenia. (2019) 5:17. doi: 10.1038/s41537-019-0085-9
334. Carbonaro TM, Johnson MW, Hurwitz E, Griffiths RR. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: similarities and differences in subjective experiences. Psychopharmacology (Berl). (2018) 235:521–34. doi: 10.1007/s00213-017-4769-4
335. Kometer M, Vollenweider FX. Serotonergic Hallucinogen-Induced Visual Perceptual Alterations. Curr Top Behav Neurosci. (2018) 36:257–82. doi: 10.1007/7854_2016_461
336. Schartner MM, Timmermann C. Neural network models for DMT-induced visual hallucinations. Neurosci Conscious. (2020) 2020:niaa024. doi: 10.1093/nc/niaa024
337. Wittmann M, Carter O, Hasler F, Cahn BR, Grimberg U, Spring P, et al. Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol. (2007) 21:50–64. doi: 10.1177/0269881106065859
338. Yanakieva S, Polychroni N, Family N, Williams LTJ, Luke DP, Terhune DB. The effects of microdose LSD on time perception: a randomised, double-blind, placebo-controlled trial. Psychopharmacology. (2019) 236:1159–70. doi: 10.1007/s00213-018-5119-x
339. Sinke C, Halpern JH, Zedler M, Neufeld J, Emrich HM, Passie T. Genuine and drug-induced synesthesia: a comparison. Conscious Cogn. (2012) 21:1419–34. doi: 10.1016/j.concog.2012.03.009
340. Luke D, Terhune D. The induction of synaesthesia with chemical agents: a systematic review. Front Psychol. (2013) 4:e00753. doi: 10.3389/fpsyg.2013.00753
341. Leptourgos P, Fortier-Davy M, Carhart-Harris R, Corlett PR, Dupuis D, Halberstadt AL, et al. Hallucinations under psychedelics and in the schizophrenia spectrum: an interdisciplinary and multiscale comparison. Schizophr Bull. (2020) 46:1396–408. doi: 10.1093/schbul/sbaa117
342. Ren J, Xiang J, Chen Y, Li F, Wu T, Shi J. Abnormal functional connectivity under somatosensory stimulation in migraine: a multi-frequency magnetoencephalography study. J Headache Pain. (2019) 20:3. doi: 10.1186/s10194-019-0958-3
343. Timmermann C, Roseman L, Schartner M, Milliere R, Williams LTJ, Erritzoe D, et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep. (2019) 9:16324. doi: 10.1038/s41598-019-51974-4
344. Alamia A, Timmermann C, Nutt DJ, Vanrullen R, Carhart-Harris RL. DMT alters cortical travelling waves. Elife. (2020) 9:e59784. doi: 10.7554/eLife.59784.sa2
345. Vejmola C, Tylš F, Piorecká V, Koudelka V, Kaderábek L, Novák T, et al. Psilocin, LSD mescaline, and DOB all induce broadband desynchronization of EEG and disconnection in rats with robust translational validity. Transl Psychiatry. (2021) 11:506. doi: 10.1038/s41398-021-01603-4
346. Orsolini L, Papanti GD, De Berardis D, Guirguis A, Corkery JM, Schifano F. The “Endless Trip” among the NPS Users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. (2017) 8:240. doi: 10.3389/fpsyt.2017.00240
347. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. (2018) 36:333–60. doi: 10.1007/7854_2016_457
348. Martinotti G, Santacroce R, Pettorruso M, Montemitro C, Spano MC, Lorusso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, therapeutic perspectives. Brain Sci. (2018) 8:47. doi: 10.3390/brainsci8030047
349. Edwards MJ, Adams RA, Brown H, Pareés I, Friston KJ. A Bayesian account of ‘hysteria’. Brain. (2012) 135:3495–512. doi: 10.1093/brain/aws129
350. Boeckle M, Liegl G, Jank R, Pieh C. Neural correlates of conversion disorder: overview and meta-analysis of neuroimaging studies on motor conversion disorder. BMC Psychiatry. (2016) 16:195. doi: 10.1186/s12888-016-0890-x
351. Pick S, Goldstein LH, Perez DL, Nicholson TR. Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry. (2019) 90:704–11. doi: 10.1136/jnnp-2018-319201
352. Butler M, Seynaeve M, Nicholson TR, Pick S, Kanaan RA, Lees A, et al. Psychedelic treatment of functional neurological disorder: a systematic review. Therap Adv Psychopharmacol. (2020) 10:2045125320912125. doi: 10.1177/2045125320912125
353. Stewart B, Dean JG, Koek A, Chua J, Wabl R, Martin K, et al. Psychedelic-assisted therapy for functional neurological disorders: a theoretical framework and review of prior reports. Pharmacol Res Perspect. (2020) 8:e00688. doi: 10.1002/prp2.688
354. Preller K. Altered prediction-error processing may underlie psilocybin-induced changes in self-processing. Biol Psychiatry. (2021) 89:S6. doi: 10.1016/j.biopsych.2021.02.036
355. Kelly JR, Clarke G, Cryan JF, Dinan TG. Dimensional thinking in psychiatry in the era of the research domain criteria (RDoC). Ir J Psychol Med. (2018) 35:89–94. doi: 10.1017/ipm.2017.7
356. Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. (2021) e21573. doi: 10.1001/jamapsychiatry.2021.2573
357. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. (2015) 9:392. doi: 10.3389/fncel.2015.00392
358. Cryan JF., O'riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
359. Kelly JR, Borre YCOB, Patterson E, El Aidy S, Deane J, Kennedy PJ, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
360. Erny D, Hrabe De Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
361. O'mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. (2015) 277:32–48. doi: 10.1016/j.bbr.2014.07.027
362. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. (2004) 558:263–75. doi: 10.1113/jphysiol.2004.063388
363. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. (2011) 108:16050–5. doi: 10.1073/pnas.1102999108
364. Stilling RM, Van De Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. (2016) 99:110–32. doi: 10.1016/j.neuint.2016.06.011
365. Codagnone MG, Spichak S, O'mahony SM, O'leary OF, Clarke G, Stanton C, et al. Programming bugs: microbiota and the developmental origins of brain health and disease. Biol Psychiatry. (2019) 85:150–63. doi: 10.1016/j.biopsych.2018.06.014
366. Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG. The role of the gut microbiome in the development of schizophrenia. Schizophr Res. (2020) 234:4–23. doi: 10.1016/j.schres.2020.02.010
367. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. (2011) 108:3047–52. doi: 10.1073/pnas.1010529108
368. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. (2014) 6:263ra158. doi: 10.1126/scitranslmed.3009759
369. Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF. O'leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry. (2015) 78:e7–9. doi: 10.1016/j.biopsych.2014.12.023
370. Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. (2016) 6:e774. doi: 10.1038/tp.2016.42
371. Luczynski P, Whelan SO, O'sullivan C, Clarke G, Shanahan F, Dinan TG, et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci. (2016) 44:2654–66. doi: 10.1111/ejn.13291
372. Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. (2014) 19:146–8. doi: 10.1038/mp.2013.65
373. Desbonnet L, Clarke G, Traplin A, O'sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. (2015) 48:165–73. doi: 10.1016/j.bbi.2015.04.004
374. Clarke G, Sandhu KV, Griffin BT, Dinan TG, Cryan JF, Hyland NP. Gut reactions: breaking down xenobiotic-microbiome interactions. Pharmacol Rev. (2019) 71:198–224. doi: 10.1124/pr.118.015768
375. Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. (2019) 570:462–7. doi: 10.1038/s41586-019-1291-3
376. Cussotto S, Walsh J, Golubeva AV, Zhdanov AV, Strain CR, Fouhy F, et al. The gut microbiome influences the bioavailability of olanzapine in rats. EBioMedicine. (2021) 66:103307. doi: 10.1016/j.ebiom.2021.103307
377. Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. (2015) 63:1–9. doi: 10.1016/j.jpsychires.2015.02.021
378. Kuypers KPC. Psychedelic medicine: the biology underlying the persisting psychedelic effects. Med Hypotheses. (2019) 125:21–4. doi: 10.1016/j.mehy.2019.02.029
379. Kelly BD. Psychiatry's future: biology, psychology, legislation, and “the fierce urgency of now”. Indian J Psychol Med. (2020) 42:189–92. doi: 10.4103/IJPSYM.IJPSYM_492_19
380. Insel TR. Star Neuroscientist Tom Insel Leaves the Google-Spawned Verily for a Startup. (2017). Available online at: https://www.wired.com/2017/05/star-neuroscientist-tom-insel-leaves-google-spawned-verily-startup/ (accessed November 30, 2021).
381. Patrick CJ, Hajcak G. RDoC: translating promise into progress. Psychophysiology. (2016) 53:415–24. doi: 10.1111/psyp.12612
382. Fusar-Poli P, Solmi M, Brondino N, Davies C, Chae C, Politi P, et al. Transdiagnostic psychiatry: a systematic review. World Psychiatry. (2019) 18:192–207. doi: 10.1002/wps.20631
383. Ross CA, Margolis RL. Research domain criteria: cutting edge neuroscience or galen's humors revisited? Complex Psychiatry. (2018) 4:158–63. doi: 10.1159/000493685
384. Ross CA, Margolis RL. Research domain criteria: strengths, weaknesses, and potential alternatives for future psychiatric research. Complex Psychiatry. (2019) 5:218–36. doi: 10.1159/000501797
385. Sanislow CA. RDoC at 10: changing the discourse for psychopathology. World Psychiatry. (2020) 19:311–2. doi: 10.1002/wps.20800
386. Garcia-Romeu A, Barrett FS, Carbonaro TM, Johnson MW, Griffiths RR. Optimal dosing for psilocybin pharmacotherapy: considering weight-adjusted and fixed dosing approaches. J Psychopharmacol. (2021) 35:353–61. doi: 10.1177/0269881121991822
387. Nayak S, Gukasyan N, Barrett FS, Erowid E, Erowid F, Griffiths RR. Classic psychedelic coadministration with lithium, but not lamotrigine, is associated with seizures: an analysis of online psychedelic experience reports. PsyArXiv. (2021) 54:240–5. doi: 10.1055/a-1524-2794
388. Tai SJ, Nielson EM, Lennard-Jones M, Johanna Ajantaival R-L, Winzer R, et al. Development and evaluation of a therapist training program for psilocybin therapy for treatment-resistant depression in clinical research. Front Psychiatry. (2021) 12:e586682. doi: 10.3389/fpsyt.2021.586682
389. Zohar J, Stahl S, Moller H-J, Blier P, Kupfer D, Yamawaki S, et al. A review of the current nomenclature for psychotropic agents and an introduction to the neuroscience-based nomenclature. Eur Neuropsychopharmacol. (2015) 25:2318–25. doi: 10.1016/j.euroneuro.2015.08.019
390. Topol EJ. Individualized medicine from prewomb to tomb. Cell. (2014) 157:241–53. doi: 10.1016/j.cell.2014.02.012
391. Park MJ, Kim DJ, Lee U, Na EJ, Jeon HJ. A literature overview of virtual reality (VR) in treatment of psychiatric disorders: recent advances and limitations. Front Psychiatry. (2019) 10:505. doi: 10.3389/fpsyt.2019.00505
392. Aday JS, Davoli CC, Bloesch EK. Psychedelics and virtual reality: parallels and applications. Therap Adv Psychopharmacol. (2020) 10:2045125320948356. doi: 10.1177/2045125320948356
393. Gillan CM, Rutledge RB. Smartphones and the neuroscience of mental health. Annu Rev Neurosci. (2021). 44:129–51. doi: 10.1146/annurev-neuro-101220-014053
Keywords: psychedelics, hallucinogens, psilocybin, research domain criteria (RDoC), lysergic acid diethylamide (LSD), dimethyltryptamine (DMT), psychiatry
Citation: Kelly JR, Gillan CM, Prenderville J, Kelly C, Harkin A, Clarke G and O'Keane V (2021) Psychedelic Therapy's Transdiagnostic Effects: A Research Domain Criteria (RDoC) Perspective. Front. Psychiatry 12:800072. doi: 10.3389/fpsyt.2021.800072
Received: 22 October 2021; Accepted: 19 November 2021;
Published: 17 December 2021.
Edited by:
Antonio Metastasio, Camden and Islington NHS Foundation Trust, United KingdomReviewed by:
Serdar M. Dursun, University of Alberta, CanadaPaul Glue, University of Otago, New Zealand
Copyright © 2021 Kelly, Gillan, Prenderville, Kelly, Harkin, Clarke and O'Keane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John R. Kelly, kellyjr@tcd.ie; orcid.org/0000-0002-9545-0615
 John R. Kelly
John R. Kelly Claire M. Gillan3,4,5
Claire M. Gillan3,4,5 Jack Prenderville
Jack Prenderville Andrew Harkin
Andrew Harkin Gerard Clarke
Gerard Clarke Veronica O'Keane
Veronica O'Keane