Abstract
Background: Face-to-face cognitive behavioral therapy (CBT) is one of the most widely used non-pharmacological treatment approaches for insomnia. The aim of this study is to assess the efficacy of face-to-face delivered CBT on health outcomes and to evaluate the effect of CBT components as subgroup variables to explain the efficacy of face-to-face delivered CBT on health outcomes in adults over 18 years old with insomnia.
Methods: Relevant randomized controlled trial studies published in the past 22 years were searched through the electronic databases. The Physiotherapy Evidence Database (PEDro) scale was used to assess the quality of the 31 included studies. The mean difference and standard deviation of outcome variables and subgroup variables were analyzed using random effect model, and the heterogeneity among the articles was assessed with the Q-test and I2. Egger regression analysis was used to assess publication bias.
Results: The meta-analysis showed a significant reduction in Insomnia Severity Index [standardized mean difference (SMD) = −2.56, 95% CI −3.81 to −1.30, p < 0.001], Pittsburgh Sleep Quality Index (SMD = −0.96, 95% CI −1.25 to −0.68, p < 0.001), sleep onset latency (SMD = −1.31, 95% CI −2.00 to −0.63, p < 0.001), wakening after sleep onset (SMD = −1.44, 95% CI −2.14 to −0.74, p < 0.001), number of awakenings (SMD = −1.18, 95% CI −2.10 to −0.26, p < 0.05), depression (SMD = −1.14, 95% CI −1.85 to −0.42, p < 0.01), and fatigue (SMD = −2.23, 95% CI −3.87 to −0.58, p < 0.01), and a significant increase in total sleep time (SMD = 0.63, 95% CI 0.28 to 0.98, p < 0.001), sleep efficiency (SMD = 1.61, 95% CI 0.92 to 2.29, p < 0.001), and physical health (SMD = 0.42, 95% CI 0.08 to 0.76, p < 0.05), in the CBT intervention group compared with the control group. There was no significant change in anxiety (SMD = −0.62, 95% CI −1.55 to 0.32, p > 0.05) and mental health (SMD = 1.09, 95% CI −0.59 to 2.77, p > 0.05) in CBT intervention group compared with control group. Group-delivered studies with larger number of intervention sessions and longer duration of single session provided a larger improvement in sleep quality.
Conclusion: Face-to-face delivered CBT is effective in increasing total sleep time, sleep efficiency, and physical health, and reducing Insomnia Severity Index scores, Pittsburgh Sleep Quality Index scores, sleep onset latency, wakening after sleep onset, number of awakenings, depression, anxiety, and fatigue in patients with insomnia. Face-to-face delivered CBT is more effective when delivered through a larger number of sessions with longer duration of each session, and when delivered in groups. Face-to-face CBT is recommended to provide treatment to patients with insomnia in clinical settings.
Systematic Review Registration:www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020200091, identifier: CRD4202020009.
Introduction
Insomnia is one type of psychiatric disease that influences the quality, timing, and amount of sleep, resulting in fatigue and mental distress (1). Insomnia has a high prevalence across the world (2). It was reported that the global prevalence of insomnia was up to 30% (3). Mentally, insomnia can impair daytime concentration and generate anxious or depressed feels that may lead to or aggravate other psychiatric diseases such as depression and anxiety (4). Physically, a chronic condition of insomnia can increase the risks of a great amount of chronic diseases such as diabetes, cardiovascular diseases, and cancer (5).
Currently, insomnia is treated with pharmacological and non-pharmacological treatments. Cognitive behavioral therapy (CBT) is one of the widely studied non-pharmacological treatment option for insomnia, with marked long-term and short-term effects (6). It is currently the first-line therapy in managing all kinds of psychiatric diseases including depression, anxiety, and insomnia (7). The CBT approach perceives the psychiatric symptoms are underpinned by distorted cognition and related behaviors, and these symptoms could be reduced if the distorted cognition and behaviors are corrected (8). Based on the CBT principles, multiple CBT strategies were developed to change the thinking and behavioral patterns, and different strategies were involved in CBT treatments (9). For patients with insomnia, previous studies indicated that it was common for these patients to have dysfunctional understandings about sleep and worries about falling asleep (10). These thoughts may lead to behavioral changes such as spending more time in bed trying to fall asleep and irregular sleep times, which may make falling asleep more difficult, further providing reinforcement of the dysfunctional understandings and creating a vicious loop that exacerbates the existing insomnia symptoms (10). Applying CBT to these patients can break this loop from multiple directions. Cognitive behavioral therapy has been commonly delivered in a face-to face format, in which patients has face-to-face consultations with the therapists. The consultations can take place between one patient and a therapist individually (individual delivered) or one or more therapists and multiple patients (group delivered). The face-to-face delivery of CBT is commonly used to patients with insomnia because the components of CBT such as alliances building can be easily achieved in the face-to-face therapy mode. Previous studies have shown that CBT provided a similar efficacy in treating insomnia compared with pharmacological treatments (11). Furthermore, it has been found CBT treatment has fewer side effects than sleep medication intake (6).
Existing meta-analysis studies also found that CBT had a significant overall effect on sleep outcomes in patients with insomnia (12, 13), but these studies focused on sleep outcomes only, and subgroup analysis of CBT components was not included. In addition to sleep outcomes (Insomnia Severity Index, Pittsburgh Sleep Quality Index, total sleep time, sleep efficiency, sleep onset latency, wakening after sleep onset, and number of awakenings), health outcomes were considered in this study, including psychiatric diseases (scores of depression and anxiety scales), fatigue (scores of fatigue scales), and quality-of-life related physical and mental health (scores of quality-of-life surveys). By assessing the effect of face-to-face CBT on these outcomes, our study will provide a comprehensive overview of the effect of face-to-face CBT on the health status of insomnia patients from a different perspective. We also conducted subgroup analysis to assess the effects of different CBT treatment designs and components on the sleep and psychiatric disease outcomes and the overall efficacy of face-to-face CBT to help develop a reasonable CBT treatment plan.
Methods
The procedure of this study was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines (14). The protocol of this study was registered at the International Prospective Register of Systematic Reviews (PROSPERO Registration ID: CRD42020200091, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020200091).
Search Strategy
The database search process was completed by two researchers (Xu and Sun) independently. Five electronic databases including PubMed, Scopus, EMBASE, Cochrane Library, and PsycINFO were searched for relevant studies by the two researchers. Gray literatures were acquired from ProQuest central: Dissertations and Thesis. The following Boolean approach for the keywords were used for the search: “insomnia” OR “sleep” AND “cognitive behavioral therapy” OR “cognitive behavior therapy” OR “CBT” AND “face-to-face delivered” OR “group delivered” OR “individual delivered” AND “randomized controlled trial” OR “randomized controlled trial” OR “RCT.” The search results from the defined five electronic databases were exported into the EndNote X9 software, and duplicates across databases were removed. Two researchers then examined the titles and abstracts of the articles and excluded articles that did not focus on insomnia or CBT. The full text of the rest of the articles were examined and selected based on the inclusion and exclusion criteria.
Study Selection
Full-text assessment of the articles was also completed by two researchers independently. In this study, PICO approach (15) was used to develop the inclusion and exclusion of articles. Only randomized controlled trial studies were included in this study. The studies must be published in English, and the publishing date should be from 1 January 1990 to 31 August 2021, with no restriction in sample size. Articles that were not peer reviewed or that scored 3 or less on the PEDro (16) scale were excluded from the study. If full text of the article was not available online, reasonable attempts were made, such as sending emails directly to the authors to request the full text of the articles.
Inclusion Criteria
P: The participants in the studies must be over 18 years in age and be self-reported or diagnosed with insomnia according to the International Classification of Sleep Disorders (ICSD), diagnostic and statistical manual of mental disorders fourth edition (DSM-IV) or fifth edition (DSM-V), or sleep questionnaires such as the Insomnia Severity Index (score > 7) (17), and Pittsburgh Sleep Quality Index (score > 4) (18). Participants should be randomly assigned to CBT intervention groups or control groups.
I: The face-to-face delivered CBT should be applied to the participants as interventions of insomnia. The CBT intervention must utilize at least two of the core components, including sleep restriction, stimulus control, cognitive therapy, and sleep hygiene. The number of other components in the intervention plan is not limited.
C: Participants in the control group in the studies received normal treatment, were placed in a waiting list (waiting list control, WLC), or received placebo treatments that did not provide significant effects on sleep.
O: The studies must report at least one of the primary outcomes required in this meta-analysis study. The primary outcomes include Insomnia Severity Index, Pittsburgh Sleep Quality Index, total sleep time, sleep efficiency, sleep onset latency, wake after sleep onset, and number of awakenings.
Exclusion Criteria
P. The participants in the study were diagnosed with other severe psychiatric diseases including schizophrenia, severe depression, and severe bipolar disorders.
I. The intervention group received mindfulness-based intervention or remote-delivered CBT intervention.
C. No proper control groups presented in the study or patients in the control groups received any forms of face-to-face delivered CBT intervention.
O. The study did not provide complete data for the primary outcomes.
Quality Assessment
Studies passing the full-text assessment underwent a further quality assessment process conducted by two researchers independently using the Physiotherapy Evidence Database (PEDro) scale (16). The PEDro scale defines the quality of the articles by giving scores ranging from 0 to 10. The marking criteria include random allocation of participants, concealed allocation of participants, similar baseline characteristics between intervention and control groups, blinding to all participants, blinding to all therapists in the study, blinding to assessors who assessed the key outcomes of the study, enough outcome measures obtained, participants received intervention or control condition as study designed, presence of between-group comparison analysis, and presence of both point measures and measures of variability. For each of the criteria, 1 point would be given if the study completely met the criteria; otherwise, no point would be given. Articles scoring 3 or less were defined as poor quality articles (19). Scores 4–5 indicated moderate quality studies, scores 6–8 indicated good quality studies, and scores 9–10 indicated excellent quality studies (19).
Research Outcomes
The primary outcomes in this study were sleep outcomes, including severity of insomnia measured by the Insomnia Severity Index (17), sleep quality measured by the Pittsburgh Sleep Quality Index (18), total sleep time, sleep efficiency, sleep onset latency, wakening after sleep onset, and number of awakenings.
The secondary outcomes in this study included psychiatric disease outcomes, fatigue, and quality-of-life related physical and mental health. The psychiatric disease outcomes included scores of depression scales (20–24) and anxiety scales (25–27). The fatigue outcome was measured by the scores of various fatigue scales (28, 29). The quality-of-life related health outcomes were measured by the scores of the physical health section and mental health section in the short-form quality-of-life surveys (SF-12 and SF-36) (30).
Data Extraction
The extraction of data was completed by two researchers independently. The third researcher was invited to confirm the data if disagreement occurred between the two researchers. If the article provided results for both post intervention and follow-up, the results in the longer intervention time for follow-up were extracted. Reasonable attempts, such as direct email requests to the authors, were also made to acquire the full datasets of the studies, if possible, when the published articles did not provide enough data.
Characteristics of the included studies and the PEDro scores are summarized in Table 1. The study characteristics include the location where the study was conducted (country), sample size, gender distribution (percentage of females), average age, type of disorder including any comorbid diseases, diagnosis criteria, drugs used in the study, intervention format, presence of manuals, number of intervention sessions, duration of a single session, total treatment time, type of therapist, percentage of participants that completed the treatment, type of control group, outcome variable names, and length of follow-up period.
Table 1
| References | Location | N | Female, n (%) | Age | PEDro score | Disorder | Diagnosis | Drugs | Intervention | Delivery format |
|---|---|---|---|---|---|---|---|---|---|---|
| Arnedt et al. (34) | USA | 17 | 6 (35%) | 46 | 6 | Insomnia, alcohol dependent | ISI ≥8, ICSD, DSM-IV | Not specified | CBT for insomnia for alcohol dependent | Individual |
| Ayabe et al. (35) | Japan | 51 | 15 (30%) | 60 | 8 | Pharmacotherapy-resistant insomnia | ISI ≥8, ICSD, DSM-IV | Benzodiazepine | Add-on cognitive behavioral treatment for insomnia | Individual |
| Bothelius et al. (36) | Sweden | 66 | 57 (86%) | 51 | 8 | Chronic insomnia | Self-report, daytime impairment | Hypnotics | Manual-guided CBT-I delivered by ordinary primary care personnel | Group |
| Carney et al. (37) | USA | 107 | 68 (73%) | 42 | 5 | Insomnia, depression | ISI ≥15, SE, TWT, SCID, HAMD-17 | Escitalopram | CBT for insomnia | Individual |
| Chen et al. (38) | Taiwan | 72 | 42 (58%) | 58 | 6 | Sleep disturbance, renal disease | PSQI >5, self-report | Not specified | CBT for insomnia | Group |
| Currie et al. (39) | Canada | 60 | 18 (30%) | 43 | 5 | Insomnia, alcohol dependent | Self-report, SCID | Hypnotics | Brief individual cognitive behavioral interventions for insomnia | Individual |
| Drake et al. (40) | USA | 154 | 154 (100%) | 56 | 5 | Chronic insomnia | DSM-5, ICSD | Not specified | CBT for insomnia | Individual |
| Ellis et al. (41) | UK | 40 | Not reported | 33 | 7 | Acute insomnia | DSM-5 | Not specified | “Single-shot” CBT for insomnia | Individual |
| Espie et al. (42) | UK | 201 | 137 (68%) | 54 | 7 | Persistent insomnia | DSM-IV, ICSD | Not specified | Nurse-administered small-group CBT | Group |
| Garland et al. (43) | USA | 45 | 43 (96%) | 56 | 6 | Insomnia | ICSD | Armodafinil | CBT for insomnia | Individual |
| Hou et al. (44) | China | 98 | 56 (57%) | 53 | 8 | Insomnia, renal disease | PSQI >7, SCL-90 | Not specified | Progressive muscle relaxation, sleep-related behavior modification | Group |
| Jacobs et al. (45) | USA | 30 | 27 (70%) | 47 | 6 | Insomnia | ICSD | Hypnotics | CBT + 1 telephone treatment session | Individual |
| Jansson-Fröjmark et al. (46) | Sweden | 32 | 20 (63%) | 56 | 5 | Insomnia, hearing impairment | ICSD | Not specified | CBT for insomnia | Individual |
| Jungquist et al. (47) | USA | 28 | 24 (86%) | 49 | 5 | Insomnia, chronic pain | ICSD | Drugs for pain | CBT for insomnia | Individual |
| Lovato et al. (48) | Australia | 118 | 63 (53%) | 64 | 6 | Insomnia | PSQI, self-report | Not specified | Brief 4-week group-administered CBT for insomnia | Group |
| Manber et al. (49) | USA | 30 | 17 (61%) | 35 | 7 | Insomnia, major depression | DSM-IV, HRSD-17, ICSD | Escitalopram | CBT for insomnia | Individual |
| McCrae et al. (50) | USA | 76 | 76 (100%) | 53 | 8 | Insomnia, chronic pain | ICSD | Antidepressants, drugs for pain | CBT for insomnia | Individual |
| Morin et al. (51) | USA | 78 | 50 (64%) | 65 | 5 | Insomnia | ICSD | Temazepam | CBT (stimulus control, sleep restriction, sleep hygiene, cognitive therapy) | Group |
| Norell-Clarke et al. (52) | Sweden | 64 | 25 (77%) | 52 | 8 | Insomnia, depressive symptom | DSISD, SCID, BDI | Not specified | Group CBT for insomnia | Group |
| Pigeon et al. (53) | USA | 21 | 14 (67%) | 51 | 5 | Insomnia, chronic pain | ICSD, apnea-hypopnea index <10 | Not specified | CBT for insomnia, CBT for pain | Individual |
| Sadler et al. (54) | Australia | 72 | 40 (56%) | 75 | 4 | Insomnia, depression | DSM-5 | Not specified | CBT for insomnia | Group |
| Savard et al. (55) | Canada | 58 | 58 (100%) | 54 | 8 | Insomnia secondary to cancer | IIS, ICSD | Hypnotics | Immediately delivered CBT | Group |
| Schiller et al. (56) | Sweden | 51 | 32 (63%) | 42 | 7 | Moderate insomnia | Self-report | Not specified | Workplace-based group CBT for insomnia | Group |
| Sivertsen et al. (57) | Norway | 30 | 16 (53%) | 61 | 5 | Chronic insomnia | DSM-IV | Not specified | CBT (sleep hygiene, sleep restriction, stimulus control, cognitive therapy, and relaxation) | Individual |
| Smith et al. (58) | USA | 100 | 79 (79%) | 59 | 5 | Insomnia, osteoarthritis | ICSD, self-report | Not specified | Standardized CBT for insomnia | Individual |
| Soeffing et al. (59) | USA | 47 | Not reported | 64 | 8 | Insomnia, hypnotic dependent | SCID, ICSD | Hypnotics | CBT for insomnia | Individual |
| Song et al. (60) | Korea | 40 | 26 (65%) | 56 | 6 | Insomnia, restless leg syndrome | ICSD-3 | Not specified | CBT for insomnia | Group |
| Talbot et al. (61) | USA | 45 | 31 (69%) | 37 | 5 | Insomnia, PTSD | DSM-IV | Not specified | CBT for insomnia | Individual |
| Taylor et al. (63) | USA | 100 | 85 (85%) | 32 | 5 | Insomnia | DSM-5, SE <85%, self-report | Not specified | CBT for insomnia | Group |
| Taylor et al. (62) | USA | 151 | 27 (18%) | 32 | 8 | Insomnia | DSM-5, SE <85%, self-report | Not specified | CBT for insomnia | Group |
| Vitiello et al. (64) | USA | 367 | 288 (79%) | 73 | 8 | Insomnia, osteoarthritis pain | Self-report | Not specified | CBT for insomnia | Group |
| References | Manual | Number of sessions (n) | Duration of sessions (min) | Duration of treatment (weeks) | Therapist type | Compliance (%) | Control | Outcome measurements | Follow up | |
| Arnedt et al. (34) | Yes | 8 | 15–60 | 8 w | 2 Authors with certificate | 59 | Behavioral placebo treatment | ISI, sleep diaries, BDI-II, STAI-T, MFI-20, SF-36 | N/A | |
| Ayabe et al. (35) | Yes | 5 | 50 | 10 w | Trained clinical psychologists | 96 | Treatment as usual | ISI, PSQI, sleep diaries, SDS | 4 w | |
| Bothelius et al. (36) | Yes | 5 | 60–90 | 9 w | 4 Primary health-care nurses and 1 social worker | 85 | Wait list control | ISI, sleep diaries | 18 m | |
| Carney et al. (37) | Yes | 4 | 60 | 8 w | Master's-level students | 63 | Sleep hygiene | ISI, Sleep diaries, HAMD-17 | 6 m | |
| Chen et al. (38) | No | 2 | 30 | 6 w | 2 Psychiatrists and 1 assistant psychologist | 100 | Sleep hygiene | PSQI, sleep diaries, BAI, BDI, FSS | N/A | |
| Currie et al. (39) | Yes | 5 | 60 | 7 w | 3 Mental health professionals | 78% | Wait list control | PSQI, sleep diaries, BDI | 6 m | |
| Drake et al. (40) | No | 6 | <60 | 6 w | 1 Registered nurse | 97 | Sleep hygiene | ISI, sleep diaries | 6 m | |
| Ellis et al. (41) | No | 1 | 60–70 | 1 w | 1 Practicing health psychologist and somnologist | 100 | Wait list control | ISI, sleep diaries | 1 m | |
| Espie et al. (42) | No | 5 | 60 | 5 w | 7 Primary care nurses | 89 | Treatment as usual | PSQI, sleep diaries, SF-36 | 6 m | |
| Garland et al. (43) | No | 7 | 15–60 | 7 w | Not reported | 83 | Medication only | Sleep diaries | N/A | |
| Hou et al. (44) | No | 36 | 20 | 3 m | Not reported | 100 | Treatment as usual | PSQI, SCL-90 | N/A | |
| Jacobs et al. (45) | No | 4 | 30 | 6 w | Not reported | 93 | Placebo treatment | Sleep diaries | 12 m | |
| Jansson-Fröjmark et al. (46) | Yes | 7 | <60 | 7 w | 3 Trained psychologists | 94 | Wait list control | ISI, sleep diaries, HADS | 3 m | |
| Jungquist et al. (47) | Yes | 8 | 30–60 | 8 w | 1 Masters prepared nurse therapist | 75 | Treatment as usual | ISI, sleep diaries, BDI | N/A | |
| Lovato et al. (48) | No | 4 | 60 | 4 w | 5 Trainee psychologists | 97 | Wait list control | ISI, sleep diaries, flinders fatigue scale, sleep anticipatory anxiety | 3 m | |
| Manber et al. (49) | No | 7 | 60 | 12 w | 2 Licensed clinical psychologists | 73 | Sleep hygiene | ISI, sleep diaries, HRSD-17 | N/A | |
| McCrae et al. (50) | Yes | 8 | 50 | 8 w | 3 Predoctoral students in clinical psychology | 72 | Wait list control | Sleep diaries, BDI, STAI-T | 6 m | |
| Morin et al. (51) | No | 8 | 90 | 8 w | 1 Licensed clinical psychologist or 1 postdoctoral | 95 | Placebo treatment | Sleep diaries | 24 m | |
| Norell-Clarke et al. (52) | Yes | 4 | 120 | 8 w | 1 Licensed psychologist | 83 | Relaxation training | ISI, sleep diaries, BDI-II | 6 m | |
| Pigeon et al. (53) | Yes | 10 | 30–60 | 10 w | 2 Experienced CBT psychologists | 100 | Wait list control | ISI, sleep diaries, CESD-R, MFI | N/A | |
| Sadler et al. (54) | Yes | 8 | 60–90 | 8 w | 2 Therapists | 96 | Psychoeducation | ISI, sleep diaries, GDS | 3 m | |
| Savard et al. (55) | Yes | 8 | 90 | 8 w | 1 Master-level psychologist | 93 | Wait list control | ISI, sleep diaries | 12 m | |
| Schiller et al. (56) | No | 5 | 120 | 3 m | 1 Trained, certified clinical psychologist | 100 | Wait list control | ISI, sleep diaries, HADS | 3 m | |
| Sivertsen et al. (57) | Yes | 6 | 50 | 6 w | 2 Clinical psychologists | 94 | Placebo treatment | Sleep diaries | 6 m | |
| Smith et al. (58) | Yes | 8 | 45 | 8 w | Postdoctoral clinical psychology fellows/faculty | 91 | Behavioral desensitization | ISI, sleep diaries | 6 m | |
| Soeffing et al. (59) | No | 8 | 60 | 8 w | Doctoral students in clinical psychology | 100 | sham biofeedback treatment | Sleep diaries | N/A | |
| Song et al. (60) | Yes | 4 | 60 | 4 w | Not reported | 63 | Sleep hygiene | ISI, sleep diaries, BDI, BAI | 3 m | |
| Talbot et al. (61) | Yes | 8 | 60 | 8 w | Licensed clinical psychologists, certified psychiatrist | 93 | Wait list control | ISI, PSQI, sleep diaries, BDI | 6m | |
| Taylor et al. (63) | No | 6 | 60 | 6 w | Licensed clinical psychologists, clinical psychology postdoctoral fellows, licensed clinical social worker | 86 | Minimal contact | ISI, sleep diaries | 6 m | |
| Taylor et al. (62) | No | 6 | 60 | 6 w | Licensed clinical psychologists, clinical psychology postdoctoral fellows, licensed clinical social worker | 87 | Minimal contact | ISI, sleep diaries, MFI, BDI, BAI | 6 m | |
| Vitiello et al. (64) | No | 6 | 90 | 6 w | 1 master's-level family counselor and 1 Ph.D. psychologist | 93 | Education only control | ISI, SE, GDS | 9 m |
Characteristics of included studies.
BAI, Beck anxiety inventory; BDI, Beck depression inventory; CBT, cognitive behavioral therapy; CESD-R: Center for Epidemiologic Studies Depression Scale-revised; DSISD, Duke structured clinical interview for insomnia; DSM-IV, diagnostic and statistical manual of mental disorders fourth edition; DSM-5, diagnostic and statistical manual of mental disorders fifth edition; FSS, fatigue severity scale; GDS, geriatric depression scale; HADS, hospital anxiety and depression scale; HAMD-17, Hamilton depression rating scale; HRSD-17, 17-item Hamilton rating scale for depression; ICSD, international classification of insomnia; IIS, insomnia interview schedule; ISI, insomnia severity index; MFI, multidimensional fatigue inventory; PSQI, Pittsburgh Sleep Quality Index; PTSD, posttraumatic stress disorder; PEDro, Physiotherapy Evidence Database; RT, relaxation training; SDS, self-rating depression scale; SCID, structured clinical interview for DSM-IV; SCL 90, symptom checklist 90; SE, sleep efficiency; SF-36: 36-item medical outcomes study short-form general health survey; SOL, sleep onset latency; STAI-T, state-trait anxiety inventory-trait subscale; TWT, total wake time; WASO, wake after sleep onset.
The mean and standard deviation for the primary and secondary outcomes were extracted directly from the results sections, tables, and figures in the studies for both the CBT intervention group and the control group before and after the intervention. The mean difference was calculated by subtracting the mean before intervention from the mean after intervention, and the total pooled mean difference was also calculated. The standardized mean difference (SMD) was calculated by dividing the mean difference by the pooled standard deviation (31).
Subgroup Data Extraction
The designs and groupings of the subgroup variables were based on the recommended CBT design from the CBT for insomnia guidelines (32) written by Perlis and colleagues. Subgroup information was collected mainly based on the characteristics of intervention delivery and CBT components in each study. The characteristics of intervention were collected from each study and coded into subgroup manually. The subgroups include participant dropout rate (0 for <20% drop out rate and 1 for ≥20% drop out rate), number of treatment sessions (0 for <6 sessions and 1 for ≥6 sessions), duration of treatment sessions (0 for <1 h and 1 for ≥1 h), duration of treatment (0 for <6 weeks and 1 for ≥6 weeks), form of delivery (0 for group-delivered and 1 for individual-delivered), drugs used in the study (0 for no drugs, 1 for hypnotics, and 2 for other drugs including antidepressants and pain drugs), and co-morbid diseases (0 for no co-morbid diseases, 1 for psychiatric diseases, and 2 for chronic diseases). The CBT module component subgroups included treatment rationale, sleep hygiene, relapse prevention, relaxation training, and basic sleep information. The presence of each component was coded as 1, and the absence of certain module was coded as 0.
Data Analysis
Because of expected heterogenicity, a random effects model, which provided a statistical parameter of the variations among the studies (33), was used to measure the mean difference and SMD of all outcome variables, and the results were presented as forest plots. A SMD between 0.2 and 0.5 indicated a small effect size, between 0.5 and 0.8 a medium effect size, and >0.8 a large effect size. In addition, the 95% confidence interval (CI) and p-value were determined for both mean difference and SMD. A p-value < 0.05 suggested that the result was statistically significant. The Q test and I2-values were used to assess the heterogenicity of the studies. The I2-value ranges from 0 to 100%. An I2-value larger than 50% indicates that the study has a high heterogenicity and a subgroup analysis is required to explore the possible causes. In addition, Egger regression analysis was used to assess the potential publication bias among the studies for each of the research outcomes, and the funnel plots were presented. A p-value >0.05 indicated that the publication bias for the research outcome was not significant. At the study level, sensitivity analysis was conducted by removing one study at a time vs. all the studies together to identify whether the overall publication bias results for the research outcome were related to any particular study or studies. The software used for data analysis was STATA 17.0.
Results
Study Selection
A total of 2,854 studies were identified through database search and other sources. After removing the duplicates, 2,297 studies were screened by titles and abstracts. After the screening of titles and abstracts, 2,088 studies were excluded, and the remaining 209 studies underwent full-text assessment. Another 178 studies were excluded after full-text assessment. Among the 178 excluded studies, 19 were meta-analysis or systematic reviews. Thirty-three studies were not related to insomnia. Forty-two studies did not provide sufficient data for analysis. Twenty-seven studies did not have control groups. Twenty-eight studies provided PEDro scores lower than 3 and were considered as low-quality studies. Thirteen studies were study protocols for clinical trials. Sixteen studies involved participants aged under 18, which did not meet the inclusion criteria. The final number of eligible studies included for meta-analysis was 31. The detailed process of study screening for meta-analysis was presented in Figure 1.
Figure 1
PRISMA flow chart for study selection.
Study Characteristics
A total number of 2,449 patients were included in the 31 studies (34–64), with 1,107 of which in the intervention group and 1,342 of which in the control group. A total of 12 studies (37, 39, 40, 46, 47, 51, 53, 54, 57, 58, 61, 63) received PEDro scores of 4–5, suggesting that they had moderate quality. The remaining 19 studies (34–36, 38, 41–45, 48–50, 52, 55, 56, 59, 60, 62, 64) received PEDro scores of 6–8, suggesting that they had good quality. The studies were carried out in nine different countries including USA (34, 37, 40, 43, 45, 47, 49–51, 53, 58, 59, 61–64), UK (41, 42), Canada (37, 39, 55), Australia (48, 54), China (38, 44), Japan (35), Sweden (36, 46, 52, 56), Norway (57), and Korea (60). The percentage of female taking part in the studies was 66.9%, and the average age of the participants was 54.6 years. All of the studies included patients with insomnia. Among of which, some of the studies included patients with different co-morbid diseases including alcohol dependence (34, 39), chronic pain (47, 50, 53, 58, 64), renal diseases (38, 44), hearing impairment (46), depression (37, 49, 52, 54), and posttraumatic stress disorder (61). The percentage of patients that reported co-morbid depression was 30.7% (34, 37–39, 46, 47, 49, 50, 52–55, 60–62), and the percentage of patients that reported co-morbid anxiety was 20.1% (34, 38, 46, 50, 54, 55, 60, 62). Thirteen studies (32, 34, 35, 40, 42, 43, 45–47, 49–51, 53, 55) used the ICSD as diagnosis criteria. Six studies (34, 35, 42, 49, 57, 61) diagnosed insomnia using the DSM-IV, while five studies (40, 41, 54, 62, 63) used the DSM-V. All of the included studies used either group-delivered or individual-delivered CBT in the intervention group, with 16 out of 31 studies (34–37, 39, 46, 47, 50, 52–55, 57, 58, 60, 61) using intervention manuals. Moreover, the total number of intervention sessions ranged from 1 to 36 and the time of a single session ranged from 15 to 120 min. The duration of the intervention ranged from 1 day (one single session) to 3 months. The CBT interventions were all delivered by trained professionals. In addition, an average of 88% of the patients completed the treatment across the 31 included studies. All of the included studies reported at least one of the sleep outcomes. The characteristics of studies included for meta-analysis were displayed in Table 1.
Overall Effect of Face-to-Face CBT on Sleep Outcomes
The results of meta-analysis were presented in Table 2. The forest plots were presented in Figures 2, 3.
Table 2
| Variables | Studies (n) | Participants (N) | Mean difference | Effect size | Publication bias | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference (95% CI) | Q-test | I2 (%) | Standardized mean difference (95% CI) | Q-test | I2 (%) | Egger's t (95% CI) | |||
| Insomnia Severity Index | 20 | 1,398 | −5.19*** (−6.30, −4.07) | 1952.01*** | 97.57*** | −2.56*** (−3.81, −1.30) | 516.28*** | 98.89*** | 1.05 (−0.89, 2.69) |
| Pittsburgh Sleep Quality Index | 5 | 462 | −3.13*** (−3.71, −2.56) | 2.93 | 0.00 | −0.96*** (−1.25, −0.68) | 7.43 | 47.62 | 0.20 (−3.99, 4.52) |
| Total sleep time | 25 | 1,523 | 23.21*** (14.56, 31.87) | 235.35*** | 83.31*** | 0.63*** (0.28, 0.98) | 215.91*** | 91.20*** | −0.18 (−1.19, 1.00) |
| Sleep efficiency | 24 | 1,698 | 8.53*** (6.41, 10.65) | 919.31*** | 96.67*** | 1.61*** (0.92, 2.29) | 474.64*** | 97.26*** | 1.51(−0.36, 2.27) |
| Sleep onset latency | 21 | 1,477 | −16.43*** (−21.04, −11.81) | 447.91*** | 93.75*** | −1.31*** (−2.00, −0.63) | 367.33*** | 97.09*** | −0.87 (−2.02, 0.84) |
| Wake after sleep onset | 20 | 1,387 | −25.74*** (−32.46, −19.02) | 217.21*** | 92.56*** | −1.44*** (−2.14, −0.74) | 360.23*** | 97.01*** | −0.42 (−1.76, 1.18) |
| Number of awakenings | 7 | 543 | −0.69*** (−0.91, −0.46) | 18.60** | 72.02** | −1.18* (−2.10, −0.26) | 142.09*** | 95.23*** | 0.98 (−1.22, 2.71) |
| Depression | 15 | 753 | −3.67*** (−5.66, −1.68) | 294.27*** | 97.58*** | −1.14** (−1.85, −0.42) | 135.21*** | 95.20*** | 0.04 (−1.82, 1.89) |
| Anxiety | 8 | 493 | −0.66 (−1.53, 0.22) | 73.20*** | 88.09*** | −0.62 (−1.55, 0.32) | 67.90*** | 95.83*** | −0.86 (−1.89, 0.90) |
| Fatigue | 6 | 426 | −4.74* (−9.34, −0.14) | 860.29*** | 99.94*** | −2.23** (−3.87, −0.58) | 183.37*** | 97.35*** | −1.77 (−5.01, 1.43) |
| Physical health | 3 | 369 | 1.90*** (1.02, 2.78) | 1.01 | 0.00 | 0.42* (0.08, 0.76) | 3.85 | 51.86 | 1.00 (−9.78, 11.45) |
| Mental health | 3 | 369 | 4.95* (1.01, 8.90) | 10.40** | 80.07** | 1.09 (−0.59, 2.77) | 86.20*** | 97.34*** | −3.18 (−14.87, 8.91) |
Total effects of CBT on all outcomes.
CI, confidence interval.
p < 0.05,
p < 0.01,
p < 0.001.
Figure 2
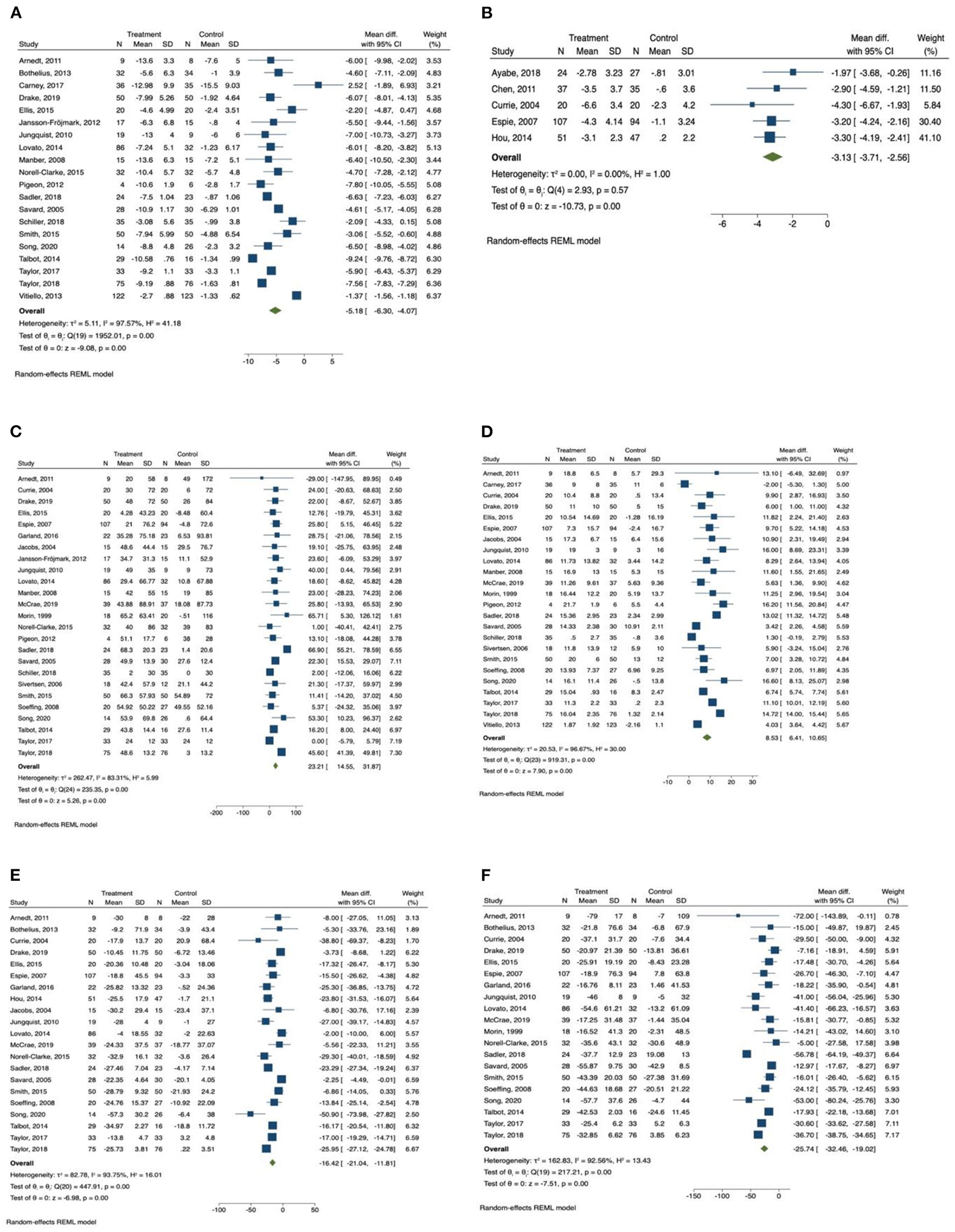
Forest plots of the effect of CBT for sleep outcomes. (A) Insomnia Severity Index, (B) Pittsburgh Sleep Quality Index, (C) total sleep time, (D) sleep efficiency, (E) sleep onset latency, (F) wake after sleep onset.
Figure 3
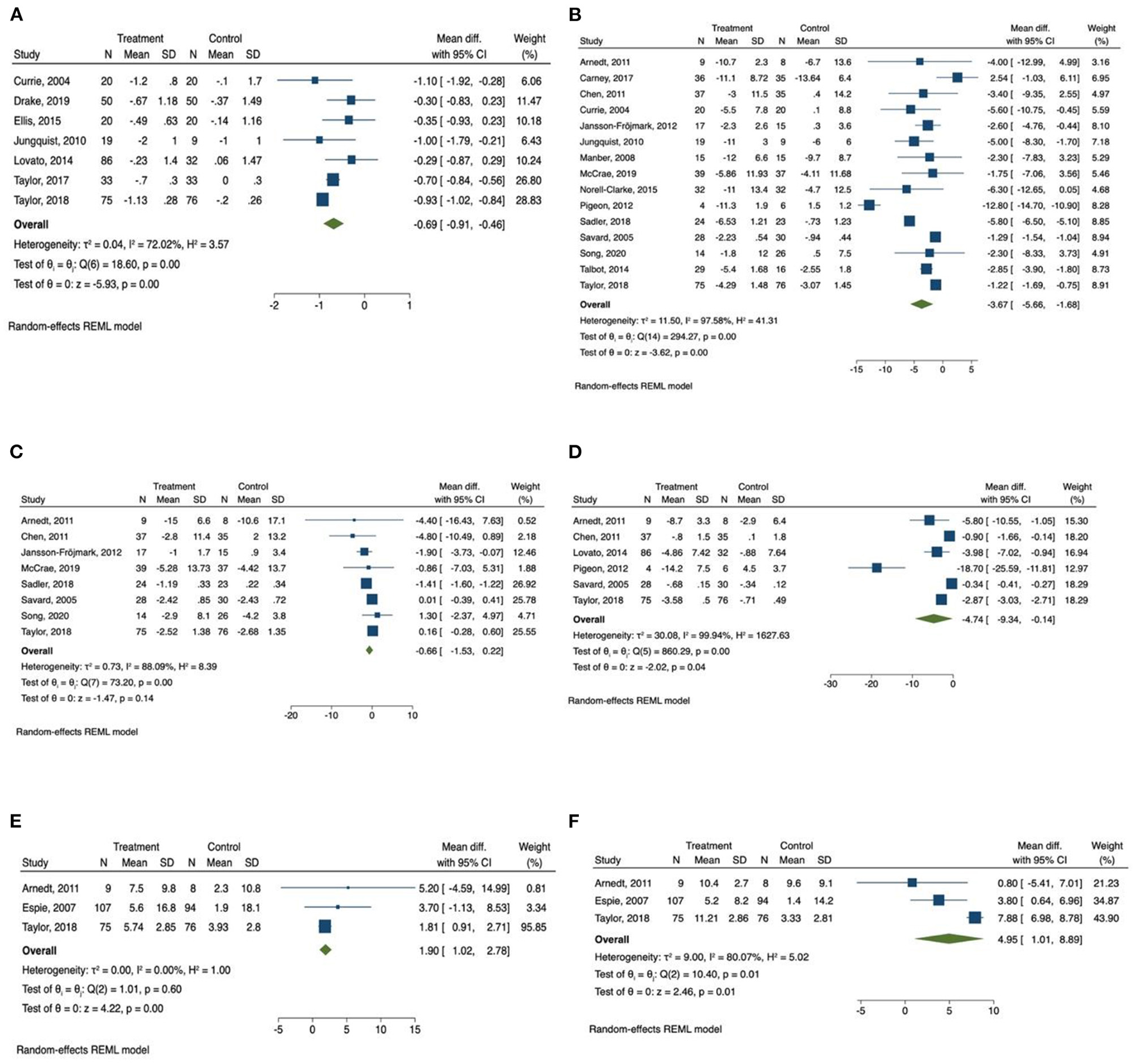
Forest plots of the effect of CBT for sleep and health outcomes. (A) Number of awakenings, (B) depression, (C) anxiety, (D) Fatigue, (E) physical health, (F) mental health.
Insomnia Severity Index was reported by 20 studies (34, 36, 37, 40, 41, 46–49, 52–56, 58, 60–64) as an outcome measurement. Patients in the CBT intervention group showed a significant reduction in insomnia severity as measured by Insomnia Severity Index (mean difference = −5.19, 95% CI −6.30 to −4.07, p < 0.001, I2 = 97.57) compared with the control group, with large effect size (SMD = −2.56, 95% CI −3.81 to −1.30, p < 0.001). Subgroup analysis was conducted for Insomnia Severity Index, and the results are presented in Table 3. We found that the subgroup variable that provided greatest effect in improving reducing insomnia severity was relapse prevention. Studies that included relapse prevention (mean difference = −6.765, 95% CI −8.117 to −5.413, p < 0.001, I2 = 84.05) reported a significantly greater improvement in insomnia severity scores than studies that did not include relapse prevention (mean difference = −6.042, 95% CI −7.106 to −4.978, p < 0.001, I2 = 90.56). Other effective subgroup variables included drop-out rate <20%, number of sessions ≥6, duration of one session <1 h, sleep hygiene, and relaxation training.
Table 3
| Subgroups | Insomnia Severity Index | Total sleep time | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (n) | Participant (n) | MD (95% CI) | SMD (95% CI) | MD I2 (%) | MD Q-test | p Between | Study (n) | Participant (n) | MD (95% CI) | SMD (95% CI) | MD I2 (%) | MD Q-test | p Between | |
| Dropout rate <20% ≥20% | 15 5 | 1,194 204 | −5.368*** (−6.608, −4.128) −4.422** (−7.449, −1.369) | −2.912*** (−4.529, −1.295) −1.535* (−2.956, −0.114) | 97.93*** 79.81* | 1937.33*** 12.81* | <0.001 | 19 6 | 1,274 249 | 22.87*** (12.66, 33.08) 22.76*** (16.31, 29.21) | 0.66** (0.23, 1.09) 0.55* (0.03, 1.07) | 86.26*** 0.00 | 231.55*** 1.50 | <0.001 |
| Number of sessions <6 sessions ≥6 sessions | 7 13 | 469 929 | −3.691*** (−5.616, −1.766) −5.908*** (−7.203, −4.612) | −0.718** (−1.152, −0.284) −3.581*** (−5.326, −1.836) | 73.67** 98.20*** | 20.53** 1931.33*** | <0.001 | 8 17 | 603 920 | 15.77** (4.12, 27.43) 25.52*** (14.51, 36.52) | 0.28** (0.11, 0.44) 0.80** (0.30, 1.30) | 23.97 88.75*** | 7.97 217.84*** | <0.001 |
| Duration of one session <1 h ≥1 h | 6 14 | 304 1,094 | −6.516*** (−8.456, −4.576) −4.668*** (−5.965, −3.372) | −2.995 (−6.007, 0.016) −2.399** (−3.777, −1.021) | 81.40** 97.94*** | 36.34*** 1549.47*** | <0.001 | 11 14 | 532 991 | 16.40*** (9.81, 22.99) 27.18*** (14.27, 40.08) | 0.32*** (0.15, 0.49) 0.85** (0.27, 1.44) | 0.00 91.65*** | 2.16 220.71*** | <0.001 |
| Length of intervention ≤ 6 weeks >6 weeks | 7 13 | 760 638 | −5.100*** (−6.910, −3.290) −5.223*** (−6.712, −3.733) | −2.898* (−5.143, −0.654) −2.368** (−3.937, −0.800) | 98.80*** 93.47*** | 1443.20*** 193.81*** | <0.001 | 9 16 | 776 747 | 23.47** (9.97, 36.96) 22.99*** (11.39, 34.59) | 0.67 (−0.02, 1.37) 0.60** (0.22, 0.98) | 85.62*** 76.93*** | 159.40*** 72.19*** | <0.001 |
| Form of delivery Group–delivered Individual–delivered | 10 10 | 925 473 | −5.021*** (−6.341, −3.702) −5.304*** (−7.311, −3.297) | −3.094** (−4.856, −1.332) −2.009* (−3.826, −0.192) | 98.18*** 86.56*** | 1535.76*** 83.10*** | <0.001 | 10 15 | 853 670 | 28.04** (12.02, 44.06) 17.10*** (10.84, 23.37) | 1.03* (0.23, 1.83) 0.33*** (0.18, 0.48) | 95.15*** 0.00 | 219.32*** 3.70 | <0.001 |
| Drug treatment No drug used Hypnotics Other | 16 1 3 | 1,203 66 129 | −5.384*** (−6.556, −4.212) −4.600*** (−7.111, −2.089) −3.707 (−9.669, 2.254) | −3.019*** (−4.527, −1.510) −0.874** (−1.374, −0.374) −0.707 (−1.760, 0.346) | 97.88*** N/A 84.43** | 1939.74*** 0.00 12.18** | <0.001 | 17 5 3 | 1,183 200 140 | 22.36*** (11.95, 32.76) 20.36* (1.50, 39.23) 30.64* (6.05, 55.24) | 0.75** (0.25, 1.25) 0.34* (0.06, 0.61) 0.38* (0.04, 0.73) | 89.58*** 0.00 0.00 | 231.11*** 3.28 0.36 | <0.001 |
| Comorbid disease None Psychiatric Chronic | 6 9 5 | 434 736 228 | −4.633*** (−6.215, −3.052) −5.276*** (−7.413, −3.139) −5.410*** (−7.142, 3.679) | −0.945*** (−1.277, −0.613) −3.924** (−6.448, −1.399) −2.082** (−3.585, −0.579) | 64.06* 99.32*** 66.78* | 13.86* 1922.02*** 10.65* | <0.001 | 10 8 7 | 714 460 349 | 17.44** (6.99, 27.90) 25.70* (5.37, 46.03) 21.99*** (15.92, 28.05) | 0.31*** (0.17, 0.46) 1.03** (0.01, 2.05) 0.59** (0.18, 1.01) | 22.46 95.89*** 0.00 | 10.90 207.94*** 1.89 | <0.001 |
| Treatment rationale No Yes | 17 3 | 1,231 167 | −5.480*** (−6.614, −4.345) −2.748 (−7.507, 2.011) | −2.929*** (−4.351, −1.507) −0.497 (−1.286, 0.293) | 97.69*** 80.93* | 1942.46*** 8.98* | <0.001 | 22 3 | 1,351 172 | 23.79*** (14.41, 33.17) 18.59 (−2.04, 39.21) | 0.68** (0.29, 1.07) 0.23 (−0.07, 0.53) | 85.86*** 0.00 | 233.72*** 0.93 | <0.001 |
| Sleep hygiene No Yes | 4 16 | 172 1,226 | −3.498** (−5.521, −1.476) −5.477*** (−6.708, −4.245) | −0.650*** (−0.957, −0.343) −3.030*** (−4.543, −1.517) | 41.70 98.06*** | 5.12 1944.75*** | <0.001 | 8 17 | 325 1,198 | 15.66* (2.42, 28.90) 24.37*** (13.82, 34.92) | 0.32** (0.10, 0.53) 0.77** (0.27, 1.27) | 18.16 88.83*** | 6.55 219.76*** | <0.001 |
| Relapse prevention No Yes | 12 8 | 1,076 322 | −4.292*** (−5.751, −2.833) −6,765*** (−8.117, −5.413) | −2.194** (−3.666, −0.722) −3.151** (−5.531, −0.771) | 98.25*** 84.05*** | 1464.78*** 57.67*** | <0.001 | 16 9 | 990 533 | 19.75*** (9.68, 29.81) 28.43*** (13.00, 43.87) | 0.56* (0.12, 1.00) 0.77* (0.161, 1.37) | 81.62*** 75.59*** | 181.15*** 52.82*** | <0.001 |
| Relaxation training No Yes | 11 9 | 816 582 | −4.472*** (−6.296, −2.647) −6.042*** (−7.106, −4.978) | −1.997* (−3.623, −0.371) −3.236** (−5.189, −1.282) | 97.50*** 90.56*** | 71.06 *** 58.29*** | <0.001 | 11 14 | 583 940 | 19.70*** (14.87, 24.53) 25.07*** (12.48, 37.65) | 0.49** (0.17, 0.81) 0.74* (0.17, 1.32) | 0.00 88.72*** | 6.66 215.32*** | <0.001 |
| Basic sleep information No Yes | 10 10 | 857 541 | −5.732*** (−7.259, −4.205) −4.511*** (−6.153, −2.868) | −4.080*** (−6.232, −1.928) −0.869*** (−1.267, −0.472) | 98.82*** 71.68*** | 1920.24*** 30.35*** | <0.001 | 13 12 | 764 759 | 24.91*** (12.01, 37.82) 17.93*** (8.12, 27.74) | 0.89** (0.25, 1.54) 0.31*** (0.17, 0.46) | 92.27*** 18.20 | 215.45*** 11.44 | <0.001 |
Subgroup analysis for Insomnia Severity Index and total sleep time.
MD, mean difference; SMD, standardized mean difference; CI, confidence interval.
p < 0.05,
p < 0.01,
p < 0.001.
Pittsburgh Sleep Quality Index was reported in only five studies (35, 38, 39, 42, 44), and there was an overall significant improvement in sleep quality measured by Pittsburgh Sleep Quality Index in the intervention group (mean difference = −3.13, 95% CI −3.71 to −2.56, p < 0.001, I2 = 0.00) in comparison with the control group, with large effect size (SMD = −0.96, 95% CI −1.25 to −0.68, p < 0.001).
Among the sleep quality outcomes, the intervention group reported a significant increase in total sleep time (mean difference = 23.21, 95% CI 14.56 to 31.87, p < 0.001, I2 = 83.31) compared with the control group, with medium effect size (SMD = 0.63, 95% CI 0.28 to 0.98, p < 0.001). The results of subgroup analysis for total sleep time are displayed in Table 3. The duration of intervention sessions showed greatest effect on total sleep time. In studies in which the duration of one session was longer than 60 min (mean difference = 27.18, 95% CI 14.27 to 40.08, p < 0.001, I2 = 91.65), a greater effect of CBT was observed than in studies providing sessions shorter than 60 min (mean difference = 16.40, 95% CI 9.81 to 22.99, p < 0.001, I2 = 0.00). Other effective CBT characteristics included number of sessions ≥6, group delivered, treatment with drugs, sleep hygiene, relaxation training, and relapse prevention.
We also found that there was a significant increase in sleep efficiency (mean difference = 8.53, 95% CI 6.41 to 10.65, p < 0.001, I2 = 96.67) in the CBT intervention group compared with the control group, with large effect size (SMD = 1.61, 95% CI 0.92 to 2.29, p < 0.001). Subgroup analysis was conducted for sleep efficiency, and the results are presented in Table 4. Both relapse prevention and relaxation training showed a significant effect in improving sleep efficiency. Studies that included relapse prevention (mean difference = 10.834, 95% CI 7.572 to 14.097, p < 0.001, I2 = 87.34) had a greater effect on sleep efficiency than studies that did not include relapse prevention (mean difference = 7.476, 95% CI 4.920 to 10.031, p < 0.001, I2 = 97.01). Likewise, studies that included relaxation training reported a more significant increase in sleep efficiency (mean difference = 9.779, 95% CI 7.087 to 12.472, p < 0.001, I2 = 93.96) than studies that did not include relaxation training (mean difference = 6.787, 95% CI 3.737 to 9.837, p < 0.001, I2 = 95.83). Sleep efficiency was also significantly improved when drop-out rate was <20%, number of sessions were ≥6, length of intervention was ≤ 6 weeks, and sessions were group delivered.
Table 4
| Subgroups | Sleep efficiency | Sleep onset latency | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (n) | Participant (n) | MD (95% CI) | SMD (95% CI) | MD I2 (%) | MD Q-test | p Between | Study (n) | Participant (n) | MD (95% CI) | SMD (95% CI) | MD I2 (%) | MD Q-test | p Between | |
| Dropout rate <20% ≥20% | 17 7 | 1,378 320 | 9.196*** (6.953, 11.438) 6.863** (1.908, 11.818) | 1.951*** (1.032, 2.871) 0.787** (0.286, 1.288) | 96.62*** 87.45*** | 850.68*** 29.50*** | <0.001 | 16 5 | 1,258 219 | −17.288*** (−22.149, −12.428) −13.494* (−25.890, −1.097) | −1.509*** (−2.379, −0.640) −0.634** (−1.101, −0.166) | 92.64*** 77.70*** | 177.91*** 20.90*** | <0.001 |
| Number of sessions <6 sessions ≥6 sessions | 8 16 | 610 1,088 | 7.431** (3.017, 11.845) 9.129*** (6.820, 11.438) | 0.585*** (0.281, 0.889) 2.128*** (1.180, 3.075) | 86.02*** 96.90*** | 45.70*** 830.25*** | <0.001 | 8 13 | 599 878 | −19.335*** (−30.200, −8.471) −15.535*** (−20.706, −10.365) | −0.643*** (−1.023, −0.263) −1.721*** (−2.766, −0.676) | 78.26*** 95.30*** | 29.72*** 415.05*** | <0.001 |
| Duration of one session <1 h ≥1 h | 9 15 | 455 1,243 | 8.072*** (5.567, 10.578) 8.815*** (5.841, 11.789) | 1.242** (0.406, 2.079) 1.794*** (0.831, 2.757) | 63.33* 98.11*** | 17.09* 901.29*** | <0.001 | 9 12 | 558 919 | −12.918*** (−18.726, −7.109) −19.410*** (−26.392, −12.428) | −0.732*** (−1.157, −0.307) −1.750** (−2.869, −0.631) | 73.25*** 96.76*** | 31.18*** 384.18*** | <0.001 |
| Length of intervention ≤ 6 weeks >6 weeks | 10 14 | 1,021 677 | 9.701*** (6.909, 12.494) 7.792*** (4.770, 10.814) | 1.878** (0.572, 3.183) 1.384*** (0.664, 2.104) | 96.24*** 94.48*** | 729.98*** 177.78*** | <0.001 | 8 13 | 746 731 | −15.975*** (−24.535, −7.414) −16.870*** (−22.591, −11.149) | −1.747* (−3.406, −0.088) −1.032*** (−1.500, −0.565) | 96.63*** 86.39*** | 145.86*** 138.64*** | <0.001 |
| Form of delivery Group–delivered Individual–delivered | 10 14 | 1,034 664 | 8.915*** (5.658, 12.172) 8.185*** (5.317, 11.053) | 2.319** (0.995, 3.644) 1.021*** (0.484, 1.557) | 98.53*** 80.33*** | 865.70*** 53.59*** | <0.001 | 10 11 | 909 568 | −18.678*** (−26.363, −10.992) −14.012*** (−19.398, −8.625) | −1.862** (−3.198, −0.526) −0.793*** (−1.173, −0.412) | 97.53*** 68.15*** | 382.00*** 32.32*** | <0.001 |
| Drug treatment No drug used Hypnotics Other | 16 4 4 | 1,338 155 205 | 8.801*** (6.334, 11.267) 8.920*** (5.581, 12.259) 7.139 (−0.789, 15.066) | 2.052*** (1.085, 3.019) 0.844*** (0.520, 1.167) 0.639 (−0.130, 1.409) | 97.60*** 0.00 88.28*** | 882.33*** 1.19 25.25*** | <0.001 | 14 5 2 | 1,140 233 104 | −16.076*** (−21.724, −10.427) −18.240*** (−27.033, −9.447) −17.089 (−38.040, 3.862) | −1.634*** (−2.610, −0.658) −0.588** (−1.010, −0.167) −0.879 (−2.405, 0.648) | 96.28*** 21.52 75.68* | 438.32*** 5.42 4.11* | <0.001 |
| Comorbid disease None Psychiatric Chronic | 10 9 5 | 714 712 272 | 7.901*** (4.943, 10.859) 8.639*** (4.754, 12.524) 9.149** (3.953, 14.345) | 0.631*** (0.478, 0.783) 2.682** (1.148, 4.215) 1.343*** (0.591, 2.095) | 66.28*** 98.86*** 90.59*** | 39.63*** 800.55*** 39.13*** | <0.001 | 8 7 6 | 642 430 405 | −12.726** (−21.113, −4.338) −21.382*** (−25.870, −16.895) −14.774** (−24.092, −5.455) | −0.482** (−0.781, −0.182) −2.637** (−4.317, −0.958) −0.807*** (−1.267, −0.347) | 77.14** 87.73*** 88.72*** | 25.06** 63.11*** 53.07*** | <0.001 |
| Treatment rationale No Yes | 22 2 | 1,551 147 | 9.194*** (7.141, 11.247) 1.690 (−5.784, 9.163) | 1.746*** (1.024, 2.468) 0.155 (−0.694, 1.004) | 96.19*** 86.99** | 892.26*** 7.69** | <0.001 | 19 2 | 1,337 140 | −16.140*** (−20.911, −11.369) −18.343 (−41.539, 4.853) | −1.377*** (−2.124, −0.630) −0.726 (−1.878, 0.427) | 94.10*** 81.70* | 442.00*** 5.47* | <0.001 |
| Sleep hygiene No Yes | 6 18 | 248 1,450 | 8.307*** (3.654, 12.960) 8.590*** (6.156, 11.024) | 0.703*** (0.450, 0.955) 1.910*** (1.022, 2.797) | 65.73** 97.47*** | 21.48** 863.02*** | <0.001 | 5 16 | 253 1,224 | −21.756*** (−26.820, −16.693) −15.184*** (−20.628, −9.739) | −0.957*** (−1.316, −0.597) −1.443** (−2.334, −0.552) | 0.00 95.59*** | 4.22 442.81 | <0.001 |
| Relapse prevention No Yes | 17 7 | 1,261 437 | 7.476*** (4.920, 10.031) 10.834*** (7.572, 14.097) | 1.386** (0.573, 2.198) 2.180** (0.891, 3.469) | 97.01***87.34*** | 837.06*** 55.92*** | <0.001 | 14 7 | 950 527 | −15.303*** (−21.471, −9.135) −19.871*** (−24.995, −14.746) | −1.337** (−2.290, −0.384) −1.254** (−2.117, −0.391) | 95.89*** 56.53* | 433.40*** 14.13* | <0.001 |
| Relaxation training No Yes | 11 13 | 790 908 | 6.787***(3.737, 9.837) 9.779***(7.087, 12.472) | 1.228***(0.563, 1.893) 1.946**(0.805, 3.088) | 95.83*** 93.96*** | 62.51*** 282.25*** | <0.001 | 9 12 | 515 962 | −14.383*** (−21.427, −7.340) −18.259*** (−24.314, −12.204) | −0.963*** (−1.419, −0.507) −1.564** (−2.721, −0.406) | 89.41*** 93.10*** | 77.27*** 127.32*** | <0.001 |
| Basic sleep information No Yes | 13 11 | 964 734 | 8.744*** (6.270, 11.218) 8.431*** (4.691, 12.171) | 2.244*** (1.154, 3.334) 0.649*** (0.384, 0.914) | 97.32*** 85.66*** | 813.36*** 79.70*** | <0.001 | 13 8 | 832 645 | −15.742*** (−20.939, −10.544) −18.835** (−29.656, −8.014) | −1.727** (−2.771, −0.683) −0.625** (−1.006, −0.244) | 95.25*** 79.58*** | 413.47*** 30.51*** | <0.001 |
Subgroup analysis for sleep efficiency and sleep onset latency.
MD, mean difference; SMD, standardized mean difference; CI, confidence interval.
p < 0.05,
p < 0.01,
p < 0.001.
In addition, sleep onset latency time was significantly improved in the CBT intervention group (mean difference = −16.43, 95% CI −21.04 to −11.81, p < 0.001, I2 = 93.75) in comparison with the control group, with large effect size (SMD = −1.31, 95% CI −2.00 to −0.63, p < 0.001). Subgroup analysis was also conducted for sleep onset latency, and the results are presented in Table 4. We found that the subgroup variable that provided greatest effect in improving sleep onset latency was comorbid disease. There was a significantly greater improvement in sleep onset latency when patients had comorbid psychiatric diseases (mean difference = −21.382, 95% CI −25.870 to −16.895, p < 0.001, I2 = 87.73) compared with patients who had comorbid chronic diseases (mean difference = −14.774, 95% CI −24.092 to −5.455, p < 0.01, I2 = 88.72) and patients who had no comorbid diseases (mean difference = −12.726, 95% CI −21.113 to −4.338, p < 0.01, I2 = 77.14). Other effective CBT characteristics and components included drop-out rate <20%, number of sessions <6, duration of one session ≥60 min, group delivered, relapse prevention, relaxation training, and basic sleep information.
Wake time after sleep onset was also significantly improved in the CBT intervention group (mean difference = −25.74, 95% CI −32.46 to −19.02, p < 0.001, I2 = 92.56) compared with the control group, with large effect size (SMD = −1.44, 95% CI −2.14 to −0.74, p < 0.001). The results of subgroup analysis for wakening after sleep onset are displayed in Table 5. The subgroup variables that provided greatest effect in improving wake time after sleep onset were duration of intervention session and relaxation training. The subgroup analysis indicated that wakening after sleep onset was more significantly improved in studies providing intervention sessions longer than 1 h (mean difference = −30.149, 95% CI −39.022 to −21.296, p < 0.001, I2 = 94.17) compared with studies providing sessions shorter than 1 h (mean difference = −17.375, 95% CI −20.768 to −13.987, p < 0.001, I2 = 0.00). A greater improvement in wakening after sleep onset was observed in studies that included relaxation training (mean difference = −30.034, 95% CI −40.118 to −19.950, p < 0.001, I2 = 94.89) compared with studies that did not include relaxation training (mean difference = −19.597, 95% CI −25.892 to −13.302, p < 0.001, I2 = 62.68). Other effective CBT characteristics and components included group delivered, comorbid psychiatric diseases, sleep hygiene, and relapse prevention.
Table 5
| Subgroups | Wake after sleep onset | Depression | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (n) | Participant (n) | MD (95% CI) | SMD (95% CI) | MD I2 (%) | MD Q–test | p Between | Study (n) | Participant (n) | MD (95% CI) | SMD (95% CI) | MD I2 (%) | MD Q-test | p Between | |
| Dropout rate <20% ≥20% | 15 5 | 1,168 219 | −25.810*** (−33.770, −17.850) −25.133*** (−38.650, −11.615) | −1.549** (−2.475, −0.623) −1.086*** (−1.610, −0.562) | 93.85*** 73.45** | 152.11*** 16.20** | <0.001 | 8 7 | 461 292 | −4.725** (−7.598, −1.853) −2.087 (−4.313, 0.138) | −1.726* (−3.163, −0.289) −0.686 (−1.398, 0.026) | 97.44*** 62.28 | 221.19*** 12.46 | <0.001 |
| Number of sessions <6 sessions ≥6 sessions | 7 13 | 569 818 | −25.781*** (−36.605, −14.956) −25.564*** (−34.024, −17.104) | −0.545*** (−0.806, −0.283) −1.909*** (−2.917, −0.902) | 42.04 95.61*** | 10.49 204.31*** | <0.001 | 5 10 | 287 466 | −2.571 (−6.156, 1.013) −4.055** (−6.467, −1.643) | −0.239 (−0.580, 0.102) −1.692** (−2.787, −0.598) | 56.75* 98.52*** | 10.00* 284.23*** | <0.001 |
| Duration of one session <1 h ≥1 h | 7 13 | 430 957 | −17.375*** (−20.762, −13.987) −30.149*** (−39.002, −21.296) | −0.880** (−1.414, −0.346) −1.735** (−2.764, −0.705) | 0.00 94.17*** | 6.59 142.31*** | <0.001 | 6 9 | 252 501 | −4.830* (−8.699, −0.961) −2.813** (−4.777, −0.850) | −1.217 (−2.545, 0.112) −1.136* (−2.095, −0.178) | 92.17*** 96.80*** | 86.79*** 160.42*** | <0.001 |
| Length of intervention ≤ 6 weeks >6 weeks | 7 13 | 716 671 | −28.502*** (−38.685, −18.318) −23.996*** (−32.979, −15.012) | −1.956* (−3.632, −0.280) −1.147*** (−1.747, −0.546) | 93.85*** 87.67*** | 40.32*** 115.79*** | <0.001 | 3 12 | 263 490 | −1.240*** (−1.704, −0.775) −4.031** (−6.391, −1.671) | −0.494* (−0.912, −0.077) −1.374** (−2.320, −0.429) | 0.00 96.87*** | 0.63 284.44*** | <0.001 |
| Form of delivery Group-delivered Individual-delivered | 10 10 | 849 538 | −30.329*** (−41.395, −19.263) −19.934*** (−25.494, −14.374) | −1.887** (−3.213, −0.560) −0.963*** (−1.386, −0.539) | 96.17*** 47.72 | 135.77*** 16.72 | <0.001 | 6 9 | 432 321 | −3.047** (−5.187, −0.908) −3.955* (−7.009, −0.901) | −1.468* (−2.841, −0.095) −0.707** (−1.231, −0.184) | 97.37*** 90.25*** | 149.21*** 102.55*** | <0.001 |
| Drug treatment No drug used Hypnotics Other | 13 5 2 | 1,047 236 104 | −26.880*** (−36.232, −17.529) −22.412*** (−30.592, −14.233) −28.393* (−53.079, −3.708) | −1.795** (−2.820, −0.771) −0.607** (−0.965, −0.249) −1.230 (−2.823, 0.363) | 96.23*** 0.00 81.54* | 206.58*** 1.24 5.42* | <0.001 | 10 1 4 | 508 40 205 | −4.259** (−6.761, −1.757) −5.600* (−10.754, −0.446) −1.605 (−5.165, 1.955) | −1.630** (−2.744, −0.516) −0.660* (−1.285, −0.036) −0.248 (−0.811, 0.315) | 98.55 N/A 63.86 | 282.91*** 0.00 9.32* | <0.001 |
| Comorbid disease None Psychiatric Chronic | 8 7 5 | 650 430 307 | −22.820*** (−32.381, −13.258) −31.968*** (−45.169, −18.766) −19.743*** (−29.190, −10.295) | −0.565*** (−0.819, −0.311) −2.736** (−4.381, −1.090) −0.949*** (−1.482, −0.415) | 52.33* 97.58*** 71.19* | 14.37* 109.84*** 12.26* | <0.001 | 1 8 6 | 40 276 437 | −2.300 (−8.335, 3.735) −2.922** (−5.084, −0.759) −4.623* (−8.368, −0.877) | −0.243 (−0.882, 0.396) −1.036* (−2.049, −0.023) −1.627* (−3.192, −0.061) | N/A 93.67*** 94.80*** | 0.00 125.19*** 144.30*** | <0.001 |
| Treatment rationale No Yes | 18 2 | 1,247 140 | −27.238*** (−34.324, −20.152) −12.513* (−24.985, −0.041) | −1.574*** (−2.334, −0.814) −0.300 (−0.655, 0.056) | 93.22*** 0.00 | 208.59*** 0.61 | <0.001 | 11 4 | 510 243 | −4.341*** (−6.656, −2.025) −1.594 (−4.900, 1.712) | −1.532** (−2.525, −0.539) −0.238 (−0.695, 0.220) | 98.22 63.66 | 286.07*** 8.11* | <0.001 |
| Sleep hygiene No Yes | 4 16 | 163 1,224 | −19.641*** (−28.584, −10.699) −27.058*** (−35.085, −19.031) | −0.640*** (−0.950, −0.330) −1.647*** (−2.505, −0.790) | 0.00 94.83*** | 1.15 210.35*** | <0.001 | 3 12 | 102 651 | −2.961** (−4.834, −1.089) −3.726** (−6.133, −1.319) | −0.593** (−0.982, −0.204) −1.337** (−2.295, −0.380) | 0.00 98.39 | 1.17 291.81 | <0.001 |
| Relapse prevention No Yes | 13 7 | 860 527 | −24.200*** (−31.025, −17.375) −27.126*** (−41.376, −12.876) | −1.469** (−2.387, −0.550) −1.391* (−2.540, −0.241) | 89.18*** 90.08*** | 123.21*** 89.22*** | <0.001 | 7 8 | 449 304 | −1.273*** (−1.495, −1.052) −5.120*** (−7.856, −2.384) | −0.654 (−1.329, 0.021) −1.765* (−3.204, −0.327) | 0.00 93.81*** | 8.12 91.71*** | <0.001 |
| Relaxation training No Yes | 10 10 | 553 834 | −19.597*** (−25.892, −13.302) −30.034*** (−40.118, −19.950) | −0.949*** (−1.404, −0.495) −1.912** (−3.221, −0.604) | 62.68* 94.89*** | 19.93* 79.11*** | <0.001 | 7 8 | 313 440 | −2.239* (−4.182, −0.296) −4.651** (−7.616, −1.686) | −0.877* (−1.616, −0.139) −1.569* (−3.059, −0.080) | 83.57** 96.81*** | 19.99** 221.23*** | <0.001 |
| Basic sleep information No Yes | 11 9 | 704 683 | −27.019*** (−36.458, −17.579) −22.827*** (−30.934, −14.721) | −2.192*** (−3.312, −1.072) −0.497*** (−0.686, −0.308) | 96.59*** 25.36 | 199.25*** 11.78 | <0.001 | 7 8 | 418 335 | −3.103*** (−4.804, −1.403) −4.021* (−7.561, −0.480) | −1.613** (−2.716, −0.509) −0.353* (−0.673, −0.032) | 96.49*** 86.04*** | 155.60*** 86.41*** | <0.001 |
Subgroup analysis for wake after sleep onset and depression.
MD, mean difference; SMD, standardized mean difference; CI, confidence interval.
p < 0.05,
p < 0.01,
p < 0.001.
The meta-analysis also showed that there was a significant improvement in number of awakenings (mean difference = −0.686, 95% CI −0.91 to −0.46, p < 0.001, I2 = 72.02) in the CBT intervention group compared with the control group, with large effect size (SMD = −1.18, 95% CI −2.10 to −0.26, p < 0.05).
The Q-test results were consistent with the I2 results in all analyses, indicating the same levels of significance of heterogenicity. The p-values between groups were all smaller than 0.001, suggesting that there was a significant difference between groups for each of the subgroup variables.
Overall Effect of Face-to-Face CBT on Psychiatric Diseases
A total of 15 studies measured the effect of CBT on depression (31, 34–36, 43, 44, 46, 47, 49–52, 57–59) and the total number of participants in these studies was 753. We found that there was a significant improvement of depressive symptoms measured by depression scales (mean difference = −3.67, 95% CI −5.66 to −1.68, p < 0.001, I2 = 97.58) in CBT intervention groups compared with the control group, and there was a large effect size (SMD = −1.14, 95% CI −1.85 to −0.42, p < 0.01).
Subgroup analysis was also conducted on depression. The results for subgroup analysis on depression were presented in Table 5. The subgroup analysis showed that relapse prevention had greatest effect on depression. Studies that included relapse prevention reported a significantly greater improvement on depressive conditions (mean difference = −5.120, 95% CI −7.856 to −2.384, p < 0.001, I2 = 93.81) than studies that did not include relapse prevention (mean difference = −1.273, 95% CI −1.495 to −1.052, p < 0.001, I2 = 0.00). Other characteristics of the studies including drop-out rate <20%, number of sessions ≥6 sessions, duration of 1 session <1 h, length of intervention >6 weeks, individual delivered, using sleep drugs, and patients with co-morbid chronical diseases had significant effect on the reduction of depression. CBT intervention components including sleep hygiene, relaxation training, and basic sleep information had significant effect on the improvement of depression. The Q-test results were consistent with the I2 results, indicating the same levels of heterogenicity. The p between groups were all smaller than 0.001, suggesting that there was a significant difference between groups for each of the subgroup variables.
Anxiety was measured in eight studies (31, 35, 43, 47, 51, 52, 57, 59), and the total number of participants in these studies was 493. Compared with control groups, CBT intervention groups only reported a slight improvement in anxiety symptoms measured by anxiety scales (mean difference = −0.66, 95% CI −1.53 to 0.22, p > 0.05, I2 = 88.09), with moderate effect size (SMD = −0.62, 95% CI −1.55 to 0.32, p > 0.05). The Q-test result was consistent with the I2 result, indicating a high level of heterogenicity.
Overall Effect of Face-to-Face CBT on Fatigue
Fatigue was reported as outcome measurement in six studies (34, 38, 48, 53, 55, 62), and the total number of participants in these studies was 426. The meta-analysis found that there was a significant reduction of daytime fatigue measured by different fatigue scales (mean difference = −4.74, 95% CI −9.34 to −0.14, p < 0.05, I2 = 99.94) in CBT intervention groups compared with the control group with a large effect size (SMD = −2.23, 95% CI −3.87 to −0.58, p < 0.01). The Q-test result was consistent with the I2 result, indicating a high level of heterogenicity.
Overall Effect of Face-to-Face CBT on Quality of Life
The scores of SF 12 and SF 36 quality of life survey were reported in three studies (34, 42, 62). The scores of physical health section and mental health section were collected from all of the three studies. Patients in the CBT intervention groups showed a significant improvement in physical health measured by SF 12 and SF 36 survey (1.90, 95% CI 1.02 to 2.78, p < 0.001, I2 = 0.00), with a medium effect size (SMD = 0.42, 95% CI 0.08 to 0.76, p < 0.05). Mental health measured by SF 12 and SF 36 survey was also improved in CBT intervention group (4.95, 95% CI 1.01 to 8.90, p < 0.05, I2 = 80.07) compared with control group, with a large but not statistically significant effect size (SMD = 1.09, 95% CI −0.59 to 2.77, p > 0.05). The Q-test results were consistent with the I2 results, indicating the same levels of heterogenicity.
Publication Bias
The results of the Egger's regression test for all of the research outcomes were summarized in Table 2. The funnel plots of the outcome variables were provided in Supplementary Figures 1, 2. A symmetric distribution of mean difference in all outcomes was observed upon the visual inspection of the funnel plots, indicating that there was no significant publication bias. The results of Egger's test of all variables had no significant results including Insomnia Severity Index (t = 1.05, 95% CI −0.89 to 2.69, p > 0.05), Pittsburgh Sleep Quality Index (t = 0.20, 95% CI −3.99 to 4.52, p > 0.05), total sleep time (t = −0.18, 95% CI −1.19 to 1.00, p > 0.05), sleep efficiency (t = 1.51, 95% CI −0.36 to 2.27, p > 0.05), sleep onset latency (t = −0.87, 95% CI −2.02 to 0.84, p > 0.05), wakening after sleep onset (t = −0.42, 95% CI −1.76 to 1.18, p > 0.05), number of awakenings (t = 0.98, 95% CI −1.22 to 2.71, p > 0.05), depression (t = 0.04, 95% CI −1.82 to 1.89, p > 0.05), anxiety (t = −0.86, 95% CI −1.89 to 0.90, p > 0.05), physical health (t = 1.00, 95% CI −9.78 to 11.45, p > 0.05), and mental health (t = −3.18, 95% CI −14.87 to 8.91, p > 0.05). These results suggested that there is no significant publication bias for these outcomes. The study by Pigeon et al. (53) was excluded in the analysis of fatigue because it provided a high publication bias for this outcome. The Egger's t-value for fatigue after excluding this study was −1.77 (95% CI −5.01 to 1.43, p > 0.05), which suggested that there is no significant publication bias for fatigue in the rest of the studies. Removing any single study in other outcomes did not change the overall results. Therefore, all of the included studies contributed to the overall publication bias results in all outcomes except fatigue.
Discussion
This study included 31 randomized controlled trial studies to assess the effect of face-to-face delivered CBT on different health outcomes in patients with insomnia. The results from this meta-analysis study showed that face-to-face delivered CBT had a significant positive effect in improving all of the sleep outcomes (Insomnia Severity Index, Pittsburgh Sleep Quality Index, total sleep time, sleep efficiency, sleep onset latency, wakening after sleep onset, and number of awakenings), one of the psychiatric disease outcomes (depression), fatigue, and quality-of-life related physical and mental health. Face-to-face delivered CBT did not show significant effects on the other psychiatric disease outcome (anxiety).
Efficacy of Face-to-Face CBT Intervention on Sleep Outcomes
The meta-analysis results were consistent with the findings from previous studies (12, 65), suggesting that face-to-face CBT had an overall significant effect in improving sleep quality and reducing insomnia symptoms. Previous studies (65, 66) mentioned that one of the major goals of CBT interventions on insomnia was to initially limit sleep opportunities at wrong time points in order to increase the pressure for sleep at right time points. Ultimately, the increased sleep drive would lead to an improvement of homeostatic regulation of sleep, and then right sleep opportunities at right time points. Variables such as sleep efficiency, sleep onset latency, and wake after sleep onset were related to homeostatic regulation of sleep, and these variables reported a relatively immediate improvement during the intervention, indicating an improvement of homeostatic regulation (65, 66). Other variables, including total sleep time and number of awakening, may show greater improvements over time as participants continue to practice the skills acquired during CBT intervention and gradually increase their opportunity for sleep (65, 66).
In randomized controlled trial studies, especially studies which were delivered in groups, frequent drop-out of participants may bring negative thoughts, such as untrust of the intervention and therapists, to other participants in the study. Participants would be less focused during the intervention sessions, and the overall effect of intervention would be reduced. Moreover, larger number of intervention sessions and longer duration of single sessions give the therapists a better chance to deliver the contents of the intervention in detail. Participants in these studies will get better understandings of the intervention contents and strategies and more likely to apply the strategies to their real life. We noticed that one of the included studies by Ellis (41) provided only one session of CBT intervention, but this study provided similar results on sleep outcomes compared with other studies which provided larger number of sessions. Considering this study was aimed to treat acute insomnia at early phase, further study was needed to confirm the effects of brief CBT on acute insomnia. In addition, longer intervention period could lead to the impatience of the participants and becoming unwilling to participate. Another important point is the form of delivery of the intervention. Patients in group-delivered CBT could discuss the contents of the interventions with peers, which promoted better understanding of the contents than patients who received CBT treatment alone. Also, the effect of face-to-face CBT was more significant when the patients had co-morbid psychiatric diseases or taking drugs such as hypnotics and antidepressants, which was consistent with the findings in previous study (67). A possible explanation for this result is that psychiatric diseases could be a main cause of insomnia. Although the studies included in this study all used CBT that was specially adapted for treating insomnia, some of the components such as cognitive therapy and relaxation training, still remained the functions of identifying and relieving anxious thoughts, which was effective in treating psychiatric disease symptoms. As a result, depressive or anxious symptoms were relieved due to CBT itself or other drug treatments. The relief of psychiatric disease symptoms led to a better mental health status, and therefore, insomnia was treated more effectively.
Sleep hygiene component was widely used as an individual treatment option in previous studies (68), which aimed to provide a better environment that is suitable for sleep. Although the effect of sleep hygiene alone on insomnia was very limited, a well-established bedroom environment is still advantageous when patients attempted to make behavioral changes (68). Consistent with the findings from previous study (69), relaxation training component was found to be effective. Relaxation training component taught the patients how to fall asleep more easily, establishing a positive mental condition toward insomnia, and patients with positive mental status are more determined to make behavioral changes and change their old sleep habits in order to improve sleep quality (69). As insomnia was a chronic condition, sustaining improvement is required after CBT intervention is finished. Therefore, relapse prevention is very important in CBT intervention program because the presence of relapse prevention component ensured the long-term effect of the intervention. In contrast, the rarely used components such as treatment rationale and basic sleep information did not seem to have great effects on sleep. These components only provided background information of disease and intervention to the patients, with limited effect on thoughts or behavioral changes. As the number of studies utilizing these components was limited, the effect of these components on sleep outcomes was uncertain.
Efficacy of Face-to-Face CBT Intervention for Psychiatric Diseases
It has been reported in prior study that face-to-face CBT has a significant effect in improving depressive symptoms (65), which is similar to our findings. A possible explanation for the result would be face-to-face CBT intervention established a regularized sleep-wake cycle, and previous study mentioned that patients with a regularized sleep-wake cycle was more likely to experience a reduction in daytime symptoms, including depression and pain (65).
In previous studies, traditional cognitive behavioral showed an overall significant effect on anxiety (70, 71), which was quite different from our findings. However, all of the studies included in this study used CBT for insomnia (CBT-I), a specially adapted form of CBT targeting insomnia only. Different to the traditional CBT interventions, the contents in the CBT-I intervention focused on sleep problems and topics on anxiety were not discussed. On the other hand, only five studies reported anxiety in this study. Due to the limited number of studies, the results of meta-analysis on anxiety may not be representative.
Efficacy of Face-to-Face CBT Intervention on Fatigue
It is recognized that the most direct consequence of poor sleep quality caused by insomnia is fatigue during the day (72). Face-to-face CBT has already shown a significant effect in improving sleep quality. It is plausible that patients receiving CBT intervention have developed a more efficient rest in bed, which led to an apparent reduction in daytime fatigue.
Efficacy of Face-to-Face CBT Intervention on Quality of Life
The proposed mechanism for the improvement of physical and mental health is that face-to-face CBT intervention established a regularized sleep-wake cycle, which enabled the participants to get enough rest across the night. The adequate rest of the body would be helpful in reducing daytime symptoms such as depression, fatigue, and pain, leading to an overall increase in physical health. In this context, negative thoughts were rectified, and patients would have a more positive attitude toward life after the treatment. The improvement of both physical health and mental health contributed to the overall improvement of quality of life.
Strength, Limitations, and Implications of Study
This study included a large number of high quality randomized controlled trial studies on the effectiveness of face-to-face delivered CBT in treating insomnia published in the last 22 years since the first RCT study (51) was published, and the results from subgroup analysis explained the contribution of specific subgroup variables to the overall effects. However, there are a number of limitations for the study. First, articles published in languages other than English were not included in this study. Second, although some of the research outcomes including Pittsburgh Sleep Quality Index, anxiety, fatigue, and scores of SF-12 and SF-36 health surveys were good indicators for sleep quality and health, they were reported in a very limited number of studies, making it hard to conclude if face-to-face CBT had a significant effect on those outcomes. Third, the effect of some CBT components was hard to measure because those components were presented in only one or two of the included studies, and we were not able to measure the heterogenicity and p-value between groups for those components. Therefore, further studies that include more trials with larger sample sizes, published in different languages, are needed to confirm the results of this study.
The using of face-to-face CBT when treating insomnia was highly recommended. When designing the CBT treatment plans, it is recommended that an appropriate design can include more than six sessions, duration of one session >1 h, length of intervention shorter than 6 months, and group delivered intervention. The retention of participants should be encouraged in the program, and the inclusion of the following components was recommended: sleep restriction, stimulus control, cognitive therapy sleep hygiene, relapse prevention, and relaxation training. Treatment rationale and basic sleep information was not recommended, but the inclusion of these components could still be considered if the circumstances permitted.
Conclusion
In conclusion, face-to-face delivered CBT is effective in improving the sleep-related outcomes, including Insomnia Severity Index, Pittsburgh Sleep Quality Index, total sleep time, sleep efficiency, sleep onset latency, and wake after sleep onset in patients with insomnia. It was also found that face-to-face delivered CBT can improve other health outcomes in insomnia patients, including depression, fatigue, physical health, and mental health. Face-to-face delivered CBT is more effective when delivered through a larger number of sessions with longer duration of each session, and when delivered in groups. Apart from the most traditional components such as sleep restriction, stimulus control, and cognitive therapy, other components including sleep hygiene, relapse prevention, and relaxation training also showed promising effects and can be considered to add into study designs and treatment plans. More studies with larger sample sizes are required in further studies to confirm the findings.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
DX: data collection, data analysis, and writing of original draft. JS: conceptualization, methodology, data collection, and reviewing and editing of writing. EC and SB: critically reviewing and editing of writing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.798453/full#supplementary-material
Supplementary Figure 1Funnel plots for sleep outcomes. (A) Insomnia Severity Index, (B) Pittsburgh Sleep Quality Index, (C) total sleep time, (D) sleep efficiency, (E) sleep onset latency, (F) wake after sleep onset.
Supplementary Figure 2Funnel plots for sleep and health outcomes. (A) Number of awakenings, (B) depression, (C) anxiety, (D) fatigue, (E) Physical health, (F) mental health.
References
1.
American Psychiatric AssociationDAssociationAP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association (2013). 10.1176/appi.books.9780890425596
2.
BhaskarSHemavathyDPrasadS. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care. (2016) 5:780–4. 10.4103/2249-4863.201153
3.
Lee-ChiongTL. Sleep: A Comprehensive Handbook. Hoboken, NJ: John Wiley & Sons (2005). 10.1002/0471751723
4.
Institute of Medicine Committee on Sleep Medicine and Research. The National Academies Collection: Reports funded by National Institutes of Health. In: ColtenHRAltevogtBM, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press. (2006).
5.
MedicGWilleMHemelsME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. (2017) 9:151–61. 10.2147/NSS.S134864
6.
MitchellMDGehrmanPPerlisMUmscheidCA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. (2012) 13:1–11. 10.1186/1471-2296-13-40
7.
Kay-StaceyMAttarianH. Advances in the management of chronic insomnia. BMJ. (2016) 354:i2123. 10.1136/bmj.i2123
8.
RothbaumBOMeadowsEAResickPFoyDW. Cognitive-Behavioral Therapy. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. New York, NY: The Guilford Press (2000). p. 320–5.
9.
BuysseDJ. Insomnia. Jama. (2013) 309:706–16. 10.1001/jama.2013.193
10.
BelangerLSavardJMorinCM. Clinical management of insomnia using cognitive therapy. Behav Sleep Med. (2006) 4:179–98. 10.1207/s15402010bsm0403_4
11.
RiemannDPerlisML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. (2009) 13:205–14. 10.1016/j.smrv.2008.06.001
12.
TrauerJMQianMYDoyleJSRajaratnamSMCunningtonD. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. (2015) 163:191–204. 10.7326/M14-2841
13.
van der ZweerdeTBisdounisLKyleSDLanceeJvan StratenA. Cognitive behavioral therapy for insomnia: a meta-analysis of long-term effects in controlled studies. Sleep Med Rev. (2019) 48:101208. 10.1016/j.smrv.2019.08.002
14.
LiberatiAAltmanDGTetzlaffJMulrowCGøtzschePCIoannidisJPet al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. 10.1016/j.jclinepi.2009.06.006
15.
LeonardoRPICO. Model for clinical questions. Evid Based Med Pract. (2018) 3:2. 10.4172/2471-9919.1000115
16.
VerhagenAPde VetHCde BieRAKesselsAGBoersMBouterLMet al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. (1998) 51:1235–41. 10.1016/S0895-4356(98)00131-0
17.
MorinCM. Insomnia: Psychological Assessment and Management. New York, NY: Guilford Press. (1993).
18.
BuysseDJReynoldsCFIIIMonkTHBermanSRKupferDJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. 10.1016/0165-1781(89)90047-4
19.
CashinAGMcAuleyJH. Clinimetrics: physiotherapy evidence database (PEDro) scale. J Physiother. (2020) 66:59. 10.1016/j.jphys.2019.08.005
20.
BeckATSteerRABrownGK. Beck Depression Inventory (BDI-II). New York, NY: Pearson. (1996). 10.1037/t00742-000
21.
EatonWWSmithCYbarraMMuntanerCTienA. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R). In MaruishME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Adults.Mahwah, NJ: Lawrence Erlbaum Associates Publishers. (2004). p. 363–77. 10.1037/t29280-000
22.
HamiltonM. The Hamilton Rating Scale for Depression. Assessment of Depression:New York, NY: Springer (1986). p. 143–52. 10.1007/978-3-642-70486-4_14
23.
YesavageJA. Geriatric depression scale. Psychopharmacol Bull. (1988) 24:709–11.
24.
ZungWW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. 10.1001/archpsyc.1965.01720310065008
25.
BeckATEpsteinNBrownGSteerRA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. (1988) 56:893–7. 10.1037/0022-006X.56.6.893
26.
ZigmondASSnaithRP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. 10.1111/j.1600-0447.1983.tb09716.x
27.
BielingPJAntonyMMSwinsonRP. The State–Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther. (1998) 36:777–88. 10.1016/S0005-7967(98)00023-0
28.
KruppLBLaRoccaNGMuir-NashJSteinbergAD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. (1989) 46:1121–3. 10.1001/archneur.1989.00520460115022
29.
SmetsEGarssenB. Bonke Bd, De Haes J. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. (1995) 39:315–25. 10.1016/0022-3999(94)00125-O
30.
WareJEKosinskiMAKellerSD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: Health Institute, New England Medical Center (1994).
31.
HedgesLVOlkinI. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press. (2014).
32.
PerlisMLJungquistCSmithMPosnerDA. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide.New York, NY: Springer (2005). p. 1–182.
33.
DerSimonianRKackerR. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. (2007) 28:105–14. 10.1016/j.cct.2006.04.004
34.
ArnedtJTConroyDAArmitageRBrowerKJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. (2011) 49:227–33. 10.1016/j.brat.2011.02.003
35.
AyabeNOkajimaINakajimaSInoueYWatanabeNYamaderaWet al. Effectiveness of cognitive behavioral therapy for pharmacotherapy-resistant chronic insomnia: a multi-center randomized controlled trial in Japan. Sleep Med. (2018) 50:105–12. 10.1016/j.sleep.2018.05.038
36.
BotheliusKKyhleKEspieCABromanJE. Manual-guided cognitive-behavioural therapy for insomnia delivered by ordinary primary care personnel in general medical practice: a randomized controlled effectiveness trial. J Sleep Res. (2013) 22:688–96. 10.1111/jsr.12067
37.
CarneyCEEdingerJDKuchibhatlaMLachowskiAMBogouslavskyOKrystalADet al. Cognitive behavioral insomnia therapy for those with insomnia and depression: a randomized controlled clinical trial. Sleep. (2017). 40:zsx019. 10.1093/sleep/zsx019
38.
ChenHYChengICPanYJChiuYLHsuSPPaiMFet al. Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney Int. (2011) 80:415–22. 10.1038/ki.2011.151
39.
CurrieSRClarkSHodginsDCEl-GuebalyN. Randomized controlled trial of brief cognitive–behavioural interventions for insomnia in recovering alcoholics. Addiction. (2004) 99:1121–32. 10.1111/j.1360-0443.2004.00835.x
40.
DrakeCLKalmbachDAArnedtJTChengPTonnuCVCuamatzi-CastelanAet al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. (2019). 42:zsy217. 10.1093/sleep/zsy217
41.
EllisJGCushingTGermainA. Treating acute insomnia: a randomized controlled trial of a “single-shot” of cognitive behavioral therapy for insomnia. Sleep. (2015) 38:971–8. 10.5665/sleep.4752
42.
EspieCAMacMahonKMKellyHLBroomfieldNMDouglasNJEnglemanHMet al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. (2007) 30:574–84. 10.1093/sleep/30.5.574
43.
GarlandSNRoscoeJAHecklerCEBarillaHGehrmanPFindleyJCet al. Effects of armodafinil and cognitive behavior therapy for insomnia on sleep continuity and daytime sleepiness in cancer survivors. Sleep Med. (2016) 20:18–24. 10.1016/j.sleep.2015.12.010
44.
HouYHuPLiangYMoZ. Effects of cognitive behavioral therapy on insomnia of maintenance hemodialysis patients. Cell Biochem Biophys. (2014) 69:531–7. 10.1007/s12013-014-9828-4
45.
JacobsGDPace-SchottEFStickgoldROttoMW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. (2004) 164:1888–96. 10.1001/archinte.164.17.1888
46.
Jansson-FröjmarkMLintonSJFlinkIKGranbergSDanermarkBNorell-ClarkeA. Cognitive-behavioral therapy for insomnia co-morbid with hearing impairment: a randomized controlled trial. J Clin Psychol Med Settings. (2012) 19:224–34. 10.1007/s10880-011-9275-y
47.
JungquistCRO'BrienCMatteson-RusbySSmithMTPigeonWRXiaYet al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. (2010) 11:302–9. 10.1016/j.sleep.2009.05.018
48.
LovatoNLackLWrightHKennawayDJ. Evaluation of a brief treatment program of cognitive behavior therapy for insomnia in older adults. Sleep. (2014) 37:117–26. 10.5665/sleep.3320
49.
ManberREdingerJDGressJLSan Pedro-SalcedoMGKuoTFKalistaT. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. (2008) 31:489–95. 10.1093/sleep/31.4.489
50.
McCraeCSWilliamsJRoditiDAndersonRMundtJMMillerMBet al. Cognitive behavioral treatments for insomnia and pain in adults with comorbid chronic insomnia and fibromyalgia: clinical outcomes from the SPIN randomized controlled trial. Sleep. (2019). 42:zsy234. 10.1093/sleep/zsy234
51.
MorinCMColecchiCStoneJSoodRBrinkD. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. Jama. (1999) 281:991–9. 10.1001/jama.281.11.991
52.
Norell-ClarkeAJansson-FröjmarkMTillforsMHolländareFEngströmI. Group cognitive behavioural therapy for insomnia: effects on sleep and depressive symptomatology in a sample with comorbidity. Behav Res Ther. (2015) 74:80–93. 10.1016/j.brat.2015.09.005
53.
PigeonWRMoynihanJMatteson-RusbySJungquistCRXiaYTuXet al. Comparative effectiveness of CBT interventions for co-morbid chronic pain and insomnia: a pilot study. Behav Res Ther. (2012) 50:685–9. 10.1016/j.brat.2012.07.005
54.
SadlerPMcLarenSKleinBHarveyJJenkinsM. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. (2018). 41:zsy104. 10.1093/sleep/zsy104
55.
SavardJSimardSIversHMorinCM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. (2005) 23:6083–96. 10.1200/JCO.2005.09.548
56.
SchillerHSöderströmMLekanderMRajaleidKKecklundGA. randomized controlled intervention of workplace-based group cognitive behavioral therapy for insomnia. Int Arch Occup Environ Health. (2018) 91:413–24. 10.1007/s00420-018-1291-x
57.
SivertsenBOmvikSPallesenSBjorvatnBHavikOEKvaleGet al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. Jama. (2006) 295:2851–8. 10.1001/jama.295.24.2851
58.
SmithMTFinanPHBuenaverLFRobinsonMHaqueUQuainAet al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. (2015) 67:1221–33. 10.1002/art.39048
59.
SoeffingJPLichsteinKLNauSDMcCraeCSWilsonNMAguillardRNet al. Psychological treatment of insomnia in hypnotic-dependant older adults. Sleep Med. (2008) 9:165–71. 10.1016/j.sleep.2007.02.009
60.
SongMLParkKMMotamediGKChoYW. Cognitive behavioral therapy for insomnia in restless legs syndrome patients. Sleep Med. (2020) 74:227–34. 10.1016/j.sleep.2020.07.011
61.
TalbotLSMaguenSMetzlerTJSchmitzMMcCaslinSERichardsAet al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. (2014) 37:327–41. 10.5665/sleep.3408
62.
TaylorDJPetersonALPruiksmaKEHaleWJYoung-McCaughanSWilkersonAet al. Impact of cognitive behavioral therapy for insomnia disorder on sleep and comorbid symptoms in military personnel: a randomized clinical trial. Sleep. (2018). 41:zsy069. 10.1093/sleep/zsy069
63.
TaylorDJPetersonALPruiksmaKEYoung-McCaughanSNicholsonKMintzJ. Internet and in-person cognitive behavioral therapy for insomnia in military personnel: a randomized clinical trial. Sleep. (2017). 40:zsx075. 10.1093/sleep/zsx075
64.
VitielloMVMcCurrySMShortreedSMBaldersonBHBakerLDKeefeFJet al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. (2013) 61:947–56. 10.1111/jgs.12275
65.
KoffelEAKoffelJBGehrmanPRA. meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. (2015) 19:6–16. 10.1016/j.smrv.2014.05.001
66.
SmithMTPerlisMLParkASmithMSPenningtonJGilesDEet al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. (2002) 159:5–11. 10.1176/appi.ajp.159.1.5
67.
WuJQApplemanERSalazarRDOngJC. Cognitive Behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. (2015) 175:1461–72. 10.1001/jamainternmed.2015.3006
68.
ChungKFLeeCTYeungWFChanMSChungEWLinWL. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. (2018) 35:365–75. 10.1093/fampra/cmx122
69.
GarciaMCKozasaEHTufikSMelloLEAHachulH. The effects of mindfulness and relaxation training for insomnia (MRTI) on postmenopausal women: a pilot study. Menopause. (2018) 25:992–1003. 10.1097/GME.0000000000001118
70.
CarpenterJKAndrewsLAWitcraftSMPowersMBSmitsJAJHofmannSG. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. (2018) 35:502–14. 10.1002/da.22728
71.
HallJKellettSBerriosRBainsMKScottS. Efficacy of cognitive behavioral therapy for generalized anxiety disorder in older adults: systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry. (2016) 24:1063–73. 10.1016/j.jagp.2016.06.006
72.
YangXHZhangBLGuYHZhanXLGuoLLJinHM. Association of sleep disorders, chronic pain, and fatigue with survival in patients with chronic kidney disease: a meta-analysis of clinical trials. Sleep Med. (2018) 51:59–65. 10.1016/j.sleep.2018.06.020
Summary
Keywords
cognitive behavioral therapy (CBT), face-to-face, insomnia, quality of sleep, health outcomes
Citation
Xu D, Cardell E, Broadley SA and Sun J (2021) Efficacy of Face-to-Face Delivered Cognitive Behavioral Therapy in Improving Health Status of Patients With Insomnia: A Meta-Analysis. Front. Psychiatry 12:798453. doi: 10.3389/fpsyt.2021.798453
Received
20 October 2021
Accepted
17 November 2021
Published
23 December 2021
Volume
12 - 2021
Edited by
Liye Zou, Shenzhen University, China
Reviewed by
Sitong Chen, Victoria University, Australia; Yu Qian, University of Macau, China
Updates
Copyright
© 2021 Xu, Cardell, Broadley and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Sun j.sun@griffith.edu.au
This article was submitted to Public Mental Health, a section of the journal Frontiers in Psychiatry
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.