- 1Department of Psychiatry, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 2Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy
- 3Department of Clinical and Experimental Medicine, Psychiatry Unit, University of Foggia, Foggia, Italy
- 4Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, Section of Neurosciences, University of Salerno, Salerno, Italy
- 5Department of Neurosciences, Mental Health and Sensory Organs, S. Andrea Hospital, University of Rome “La Sapienza”, Rome, Italy
Background: Negative symptoms represent a heterogeneous dimension with a strong impact on functioning of subjects with schizophrenia (SCZ). Five constructs are included in this dimension: anhedonia, asociality, avolition, blunted affect, and alogia. Factor analyses revealed that these symptoms cluster in two domains: experiential domain (avolition, asociality, and anhedonia) and the expressive deficit (alogia and blunted affect), that might be linked to different neurobiological alterations. Few studies investigated associations between N100, an electrophysiological index of early sensory processing, and negative symptoms, reporting controversial results. However, none of these studies investigated electrophysiological correlates of the two negative symptom domains.
Objectives: The aim of our study was to evaluate, within the multicenter study of the Italian Network for Research on Psychoses, the relationships between N100 and negative symptom domains in SCZ.
Methods: Auditory N100 was analyzed in 114 chronic stabilized SCZ and 63 healthy controls (HCs). Negative symptoms were assessed with the Brief Negative Symptom Scale (BNSS). Repeated measures ANOVA and correlation analyses were performed to evaluate differences between SCZ and HCs and association of N100 features with negative symptoms.
Results: Our findings demonstrated a significant N100 amplitude reduction in SCZ compared with HCs. In SCZ, N100 amplitude for standard stimuli was associated with negative symptoms, in particular with the expressive deficit domain. Within the expressive deficit, blunted affect and alogia had the same pattern of correlation with N100.
Conclusion: Our findings revealed an association between expressive deficit and N100, suggesting that these negative symptoms might be related to deficits in early auditory processing in SCZ.
Introduction
Negative symptoms represent an unmet therapeutic need in the care of subjects with schizophrenia (SCZ) (1, 2). Indeed, these symptoms do not respond satisfactorily to current available treatments and are regarded as one of the main determinants of the poor outcome of SCZ (1–6). According to the present conceptualization, negative symptoms are described as five individual symptoms: avolition (reduced interest and motivation for goal-directed activities), asociality (diminished social drive or interest and desire for affiliation), anhedonia (reduced ability to experience or anticipate pleasure), blunted affect (reduced intensity and range of emotional expression), and alogia (reduced spontaneous speech and loss of conversational fluency) (2, 7–10). Different factor analytic studies demonstrated the existence of two negative symptom domains, which are named as experiential domain, including anhedonia, avolition, and asociality, and the expressive deficit domain, including blunted affect and alogia (2, 8, 10–14). Clustering into two domains is also supported by studies that showed how these domains are associated with different behavioral and neurobiological alterations (8, 10, 15, 16). The experiential domain is associated with abnormalities in different aspects of the motivational processes, which might be related to the motivational value system (research domain criteria-RDoC-positive valence system) (17, 18) or to the salience system (8, 19). The former refers to motivational aspects such as reward prediction, value encoding, action outcome contingency learning, and the integration of goal-directed behavior and experienced value (8, 10, 16, 20–45). On the other side, the salience system refers to motivational aspects related to orientation toward salient stimuli (aversive or rewarding stimuli), cognitive activation, and general motivation (8, 46–48). Another hypothesis, which has not been entirely supported by previous studies (8), poses at the bases of the experiential domain deficits in the executive control of behavior (16, 49–53).
The pathophysiology of the expressive deficit domain has been less investigated, in comparison to the experiential domain (8). Symptoms that belong to the expressive deficit have been found to relate to deficits in neurocognitive and social cognition abilities and to neurological soft signs, suggesting that these symptoms are probably subtended by a diffuse neurodevelopmental disconnectivity (8, 54, 55). In particular, it is possible that the expressive deficit domain is related to limited availability of cognitive resources. According to this hypothesis, alogia might depend on deficits in semantic memory organization and verbal fluency. Furthermore, it has been suggested that in “high-load” situations (e.g., social situations) subjects might allocate less cognitive resources to speech production due to the high cognitive demands required from the surrounding environment (15). Another hypothesis has indicated emotion expression and emotion perception deficits as possible candidate mechanisms that subtend this domain and in particular blunted affect (15, 56, 57).
Electrophysiology (EEG), which is a non-invasive and inexpensive technique with a high temporal resolution, represents a valid method to identify abnormalities of cortical brain functions and to investigate the neurophysiological bases of different psychopathological aspects, such as negative symptoms (58–61). Specifically, the analysis of event-related potentials (ERPs) represents an objective tool to study mental processes, due to its high temporal resolution in capturing responses to internal and external events (62, 63). However, so far, findings regarding associations between ERPs and negative symptoms are scattered/scarce and often inconsistent. Three studies investigated abnormalities of reward anticipation and evaluation processes (assessed using the stimulus preceding negativity-SPN, P300, and N200) and their eventual association with negative symptoms (64–66). Wynn et al. (66) found that the SPN was associated with trait anhedonia and with the total negative symptoms score. P300 (64) and N200 (65) amplitude did not correlate with the two negative symptom domains, while the P300 amplitude was found to be associated with social anhedonia (64). However, other studies found that these ERP indices correlated also with other psychopathological aspects, for instance P300 was associated also to positive and disorganized dimensions. The inconsistence about previous findings might be due to different factors, such as the heterogeneity of negative symptoms, the improper conceptualization of these symptoms, the use of assessment instruments often not in line with the current conceptualization of negative symptoms and the small sample sizes of the studies.
Another ERP that has been extensively studied in SCZ is the N100, which is thought to measure early perceptual processing. N100 is one of the largest auditory and visually evoked ERP and can be visualized as a negative deflection peaking between 80 and 120 ms after the stimulus onset (67). The N100 has gained attention due to the fact that its alterations (a reduction in N100 amplitude and delayed latency of its peak) represent well-replicated findings in SCZ, since the early phases of the disorder (67–78). Furthermore, aberrations of N100 in schizophrenia include also deficits in N100 gating ratio probably due to decreased N100 amplitude to initial stimulus, whereas the N100 amplitudes to the repeated stimulus did not systematically vary between patients and controls (79). Previous findings demonstrated that abnormalities in sensory gating and decreased N100 amplitude might be associated with deficits in processing of auditory salience, auditory verbal hallucinations (80–82), antipsychotic intake (67), and attention deficits (83, 84). Subjects with primary and persistent negative symptoms demonstrated a reduction of N100 but not of other ERP components such as P300 (69, 85), suggesting a link between early information processing and primary negative symptoms.
Alterations in N100, reflecting deficits in gating and early sensory processing, are consistently found in SCZ, and contribute to poor outcome (86–88). Indeed, some “cascade” models have hypothesized that impairment in early visual and auditory processing might contribute to deterioration of higher-level processing, such as social cognition. These deficits might be related to negative symptoms and might contribute to impairment in functioning (86–88).
Therefore, it seems to be of great interest the investigation of eventual associations between N100 impairment and negative symptoms. However, also in this case findings are inconsistent. In particular, two studies investigated abnormalities in ERPs in subjects with deficit schizophrenia (subjects with primary and persistent negative symptoms) as compared to subjects with non-deficit schizophrenia and healthy controls (HCs) (69, 89). One study (69) reported an association between N100 and primary and persistent negative symptoms. The authors of this study found that subjects with deficit schizophrenia, as compared to subjects with non-deficit schizophrenia and HCs, had a reduction in N100 amplitude for target tones and also topographic abnormalities for standard tones in brain areas involved in the evaluation of motivational relevance of events, auditory discrimination, and memory retrieval (69).
In contrast, the study conducted by Li et al. failed to find any specific associations between primary and persistent negative symptoms and N100, since both subjects with deficit and those with non-deficit schizophrenia presented the same alterations in N100 (reduced amplitude and delayed latency) as compared to HCs (89). Other two studies did not find a significant correlation between N100 and negative symptom severity (90, 91). While in a small sample of men with recent-onset psychosis, a correlation was found between N100 and negative symptom severity (85). In particular, the authors reported that men with recent onset psychosis had lower right-anterior N100, as compared to HCs, and that this abnormality correlated with the severity of negative symptoms, measured with the positive and negative syndrome scale (PANSS) (92). Starting from the assumption that the main generator of the anterior N100 is the anterior cingulate cortex, these results suggested that negative symptoms might be due to abnormalities in anterior cingulate cortex in modulating signal to noise ratio (85).
However, the majority of the above-mentioned studies (85, 89–91) used first generation rating scales, such as the PANSS (92) and the scale for the assessment of negative symptoms (SANS) (93) to assess negative symptoms. These assessment instruments present some limitations, as they include aspects that actually are not conceptualized as negative symptoms, but are mostly related to cognitive functions and disorganization (2). Furthermore, the study of Li et al. (89) used a proxy from the PANSS for categorizing subjects with deficit and non-deficit schizophrenia. However, it has been demonstrated that the proxy for categorizing DS and NDS patients has some problems in terms of face validity and temporal stability (2). The association between N100 abnormalities with the two negative symptom domains in SCZ has never been investigated.
Therefore, in the light of above observations, our study aims to fill the gap investigating in SCZ the relationships between N100 and the two negative symptom domains, evaluated with state-of-the-art instruments, in a large sample of SCZ.
To achieve this aim, the study investigated: (1) the differences in N100 parameters between subjects with SCZ and HCs; (2) the associations between N100 parameters with negative symptom domains in SCZ.
Methods
Study Participants
The study has been conducted as part of the add-on EEG study of the Italian Network for Research on Psychoses (3). One hundred and forty-eight SCZ and 70 HCs were recruited for the study, at five research sites in Naples, Foggia, Rome “Tor Vergata”, Rome “Sapienza”, and Salerno. The SCZ sample included individuals seen at the outpatient units of the five mentioned Italian university psychiatric clinics. All patients had a diagnosis of schizophrenia according to DSM-IV, confirmed with the Structured Clinical Interview for DSM IV-Patient version (SCID-I-P), and an age between 18 and 65 years.
The HCs sample was recruited from the community at the same sites mentioned above. Inclusion criteria for HCs were the absence of a current or lifetime Axis I or II psychiatric diagnosis. Exclusion criteria for both groups were: (a) a history of head trauma with loss of consciousness; (b) a history of moderate to severe mental retardation or of neurological diseases; (c) a history of alcohol and/or substance abuse in the last six months; (d) current pregnancy or lactation; and (e) inability to provide an informed consent. Schizophrenia with treatment modifications and/or hospitalization due to symptom exacerbation in the last three months were excluded.
The Ethics Committee of the involved institutions approved the electrophysiological add-on study. The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All participants signed a written informed consent to participate after receiving a detailed explanation of the study procedures and goals.
Clinical and Neurocognitive Assessments
All subjects recruited were evaluated for sociodemographic variables such as age, education, and gender, through a clinical form filled using every available source of information.
For SCZ, a semi-structured interview, the Brief Negative Symptom Scale (BNSS) was used to assess negative symptoms (94, 95). The scale includes 13 items, organized into six subscales (blunted affect, alogia, avolition, anhedonia, asociality, and a control subscale named distress). All the items are rated on a 7-point (0–6) scale, thus ranging from absent (0) to moderate (3) to extremely severe (6) symptoms (except distress for which the severity rating is reversed: 0 normal distress and 6 absent).
With regard to the two domains, the experiential domain was computed by summing the scores on the subscales anhedonia, avolition, and asociality; the expressive deficit was computed by summing the scores on the subscales blunted affect and alogia (94).
The PANSS was used to rate the severity of positive symptoms and disorganization (92). All items are rated on a 7-point scale from 1 to 7, ranging from absent (1) to moderate (4) to extremely severe (7). We also assessed depressive symptoms using the Calgary Depression Scale for Schizophrenia (CDSS) (96) and extrapyramidal symptoms using the St. Hans rating scale (SHRS) (97).
EEG Recording Procedure
EEGs were recorded using two highly comparable EEG recording systems: EASYS2 (Brainscope, Prague) and Galileo MIZAR-sirius (EBNeuro, Florence). Before starting the study, a harmonization of the amplifier settings and recording procedure was carried out to ensure the same settings in all the centers. All EEGs were recorded using a cap electrode system with 29 unipolar leads (Fpz, Fz, Cz, Pz, Oz, F3, F4, C3, C4, FC5, FC6, P3, P4, O1, O2, Fp1, Fp2, F7, F8, T3, T4, T5, T6, AF3, AF4, PO7, PO8, right mastoid, and left mastoid), which were placed following the 10–20 system. All the leads were referenced to the linked earlobes (a resistor of 10 kΩ was interposed between the earlobe leads). A ground electrode was placed on the forehead.
For artifact monitoring, a horizontal electro-oculogram (hEOG) was recorded from the epicanthus of each eye, and a vertical EOG (vEOG) from the leads beneath and above the right eye. All impedances of the leads were kept below 5 kΩ. The EEG data were filtered with a band-pass of 0.15–70 Hz and recorded with a sampling rate of 512 Hz.
A calibration was performed for all channels, using a 50 μV sine wave, before each recording session. Subjects were seated in a reclining chair, in a sound attenuated room, minimizing eye movement or muscle tension. Subjects performed an auditory “odd-ball” task during which 320 standard stimuli (1,500 Hz, 80 dB) and 80 target stimuli, deviant for their frequency (1,000 Hz, 80 dB), were played. Patient were asked to press the button as fast as possible upon the appearance of every target stimulus. Participants who scored <60% on the behavioral target detection task were excluded from the analysis.
Participants were instructed not to drink coffee or tea and to abstain from smoking cigarettes in the 2 h before the beginning of the recording session and did not take psychotropic medications in the morning. Information on the quality of sleep during the night prior to the recording was collected and the EEG session was postponed if the subject reported a non-restoring sleep.
EEG Data Preprocessing
One expert from the coordinating center (Naples) using Brain Vision Analyzer software (Brain Products, Munich, Germany) performed all the pre-processing analyses on data collected by the different recording sites. Data were parsed into epochs of 1,000 ms duration, which were time-locked to the onset of the cue and spanned from a 100 ms pre-stimulus period up to 900 ms post-stimulus. The recorded EEG was digitally filtered offline using a band-pass filter of 0.01–30 Hz. N100 waves were extracted in each subject by the averaging method in order to improve the signal/noise ratio, ruling out baseline activity not related to the stimulus. The N100 components for standard and target tones were analyzed separately. Trials with drifts larger than ±100 μV in any scalp electrode were rejected. If following artifacts and noisy trials removal, <40 usable target trials (50% of target trials) remained, the subject was excluded from the analysis. Data were baseline-corrected using the 100 ms time window preceding stimuli. N100 peaks were automatically marked using the “peak finder” function of Brain Analyzer, as the most negative peak point ranging from 80 to 120 ms post-stimulus. We analyzed amplitude and latency of N100 from the Fz, Cz, and Pz electrodes. Target stimuli also elicited a later auditory ERP known as the P3b, which is related to the allocation of attentive resources toward task relevant tones. Although the current study aimed to characterize the very early processing stages of auditory perception rather than higher-order processing phases, a control analysis was carried out to verify whether the later component was associated with negative symptoms. Findings concerning the difference between patients and controls for this component are reported elsewhere (Giordano et al., unpublished).
Statistical Analysis
All statistical analyses were computed using SPSS Version 22.0 (IBM Corporation, 2014). Normality tests were performed on demographic, clinical, and electrophysiological variables to test distribution of data in order to set up parametric or non-parametric tests.
Mann-Whitney U-Tests and χ2-tests were used to compare SCZ and HCs on demographic characteristics. N100 amplitude and latency were entered separately into a two-factor repeated measures ANOVA design, incorporating electrode × stimulus type × group, with electrode and stimulus type as within subjects' variables and group as between subjects' factors. The Huynh-Feldt correction was applied. Significant main and interaction effects were further analyzed by post-hoc comparisons with Bonferroni adjusted alpha level using independent samples t-test and Mann-Whitney U-test.
Pearson or Spearman rank correlations, based on normality test results, were performed to test the relationships between N100 amplitude and latency for standard and target stimuli separately at the three midline electrodes (Fz, Cz, and Pz) with negative symptom severity (BNSS total score) in SCZ. For all the correlations considered, Bonferroni-Holm correction was applied in order to control for type-I error inflation, accordingly to the number of tests (three tests for each stimulus type, p < 0.016). Only when a significant correlation of BNSS total score with N100 measures was observed, correlations of the same measures with the two negative symptom domains (experiential domain and expressive deficit), and their component symptoms were further assessed (p-value threshold corrected accordingly to the number of symptom domains). Furthermore, if correlations with negative symptoms were statistically significant, we performed partial correlations to exclude the influence of positive and extrapyramidal symptoms, disorganization, and depression.
Results
Participants
One hundred and forty-eight SCZ and 70 HCs were originally enrolled as part of the add-on EEG study. However, 23 SCZ and 4 HCs did not complete the paradigm for the electrophysiological recording. Furthermore, 11 SCZ and 3 HCs were excluded either for the presence of artifacts in the ERP recordings or for poor behavioral performance on the active target recognition task. Thus, the final study sample consisted of 114 SCZ and 63 HCs.
Demographic and Clinical Characteristics
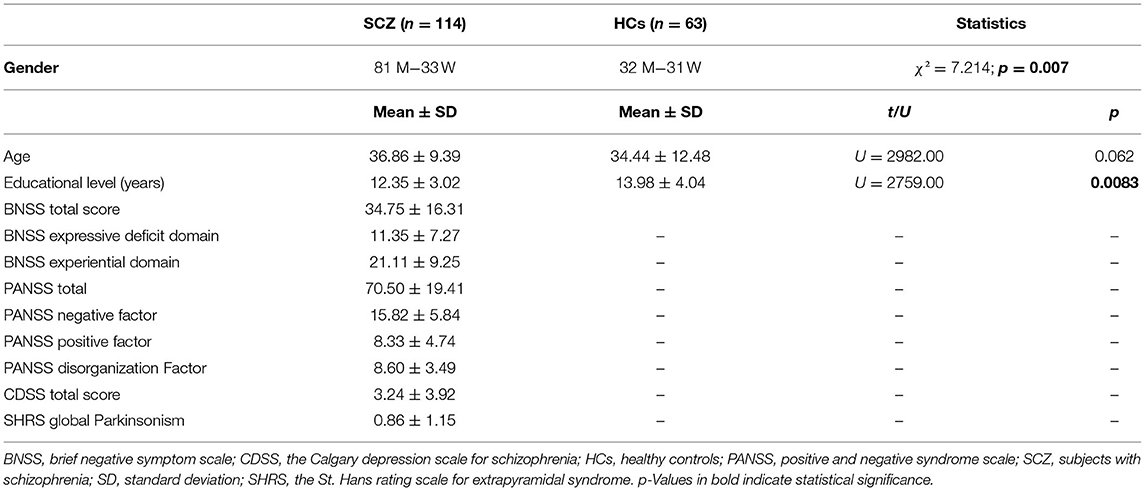
Data on relevant demographic and clinic characteristics are provided in Table 1. The gender ratio was significantly different between the two groups (χ2 = 7.214; p < 0.01) since in the SCZ group the number of male subjects was higher, as compared to HCs; the mean age was not significantly different between the two sample groups (U = 2982.00; p > 0.05). Furthermore, as expected, SCZ had significantly lower education as compared to HCs (U = 2759.00; p < 0.01). Schizophrenia were characterized by mild to moderate severity of the negative symptoms (BNSS total score of 34.75) and absent to mild severity of both positive and disorganization dimensions (PANSS mean dimension score <9 for both). They had a low mean level of depression (CDSS total score <4) and of Parkinsonism (SHRS Parkinsonism score <1).
Group Comparison on N100 Amplitude and Latency
Mean values of N100 amplitude (Table 2) and latency (Table 3) were calculated for SCZ and HCs.

Table 2. Comparisons of N100 mean amplitude for standard and target stimuli between subjects with schizophrenia and healthy controls.
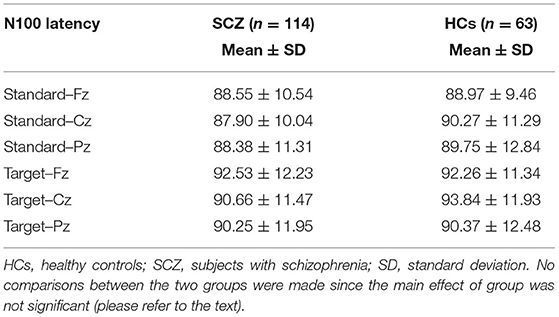
Table 3. N100 mean latency for standard and target stimuli in subjects with schizophrenia and healthy controls.
No significant electrode × stimulus × group interaction [F(1.724, 301.67) = 0.906; p > 0.05] on N100 amplitude was detected. A significant main effect of the electrode was recorded [F(1.772, 310.18) = 354.03; p < 0.001; highest peaks amplitude recorded on Fz and Cz electrodes], while no significant electrode × group interaction was detected (p > 0.05). A significant main effect of the stimulus type was observed [F(1, 175) = 27.658; p < 0.001; higher peak amplitude on target trials], but this was not influenced by group (p > 0.05). Finally, a main effect of group was found [F(1, 175) = 20.272; p < 0.001]. Given the above group main effect and influence of stimulus type and electrode, the difference between the two groups were further investigated at each electrode level, separately for standard and target stimuli. Post-hoc analysis showed that remarkable reductions (p < 0.001) in N100 amplitude could be observed both in standard and target stimuli (lower N100 absolute value in SCZ; Figure 1; Table 2).
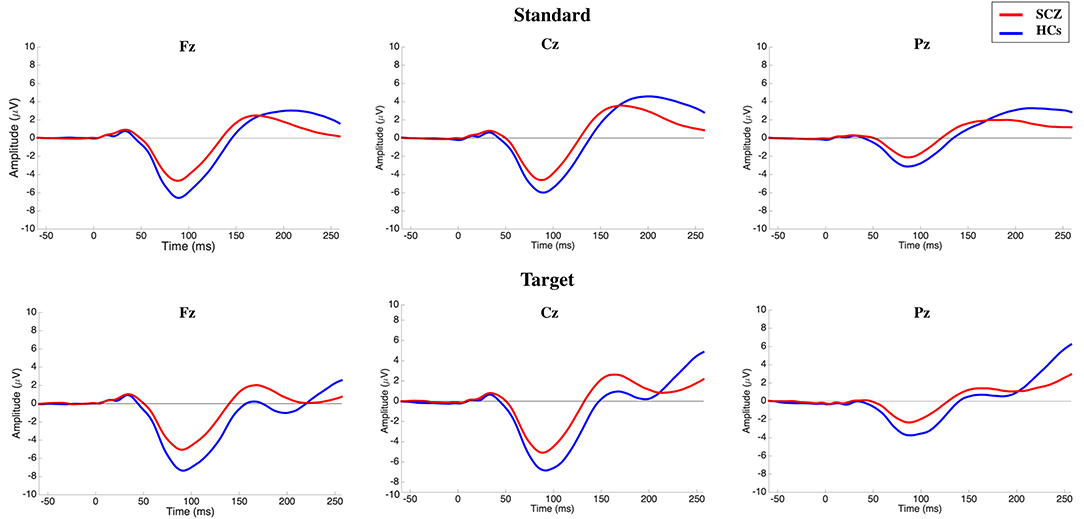
Figure 1. Grand-average N100 waves elicited by standard (top) and target (bottom) stimuli on the three midlines electrodes for subjects with schizophrenia (SCZ) and healthy controls (HCs). Following stimulus onset, the N100 component was measured in a time window within 80–120 ms. The potential was filtered between 0.01 and 30 Hz to optimize scoring of the N100 component.
No significant interaction group × electrodes × stimulus type was detected for N100 latency [F(1.757, 307.55) = 0.550; p > 0.05]. No significant electrode × group interaction or main effect of electrode was found (p > 0.05). A significant main effect was detected for stimulus type [F(1, 175) = 15.976; p < 0.001; longer latency for target stimuli], which was not affected by the group variable (p > 0.05).
Finally, no significant main effect of group was detected [F(1, 175) = 0. 542; p > 0.05]. Given the absence of a main effect of group, no post-hoc analysis for N100 latency was implemented (Table 3).
Correlation Analysis Between N100 Characteristics and Negative Symptoms
Correlations between N100 features and severity of negative symptoms, assessed through the BNSS total score, were initially performed. Correlations between BNSS total score and N100 amplitude and latency are reported in Tables 4, 5, respectively. We found that N100 amplitude recorded at Fz elicited by standard stimuli correlated with the BNSS total score (rs = 0.241; p = 0.011) (Figure 2; Table 4). No significant associations between N100 latency and BNSS total score were observed (Table 5).
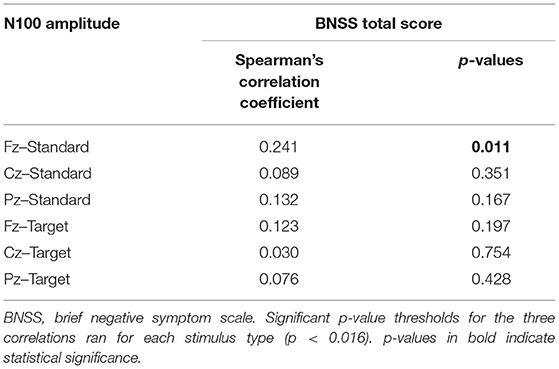
Table 4. Correlations between N100 amplitude for standard and target stimuli and BNSS total score in SCZ.
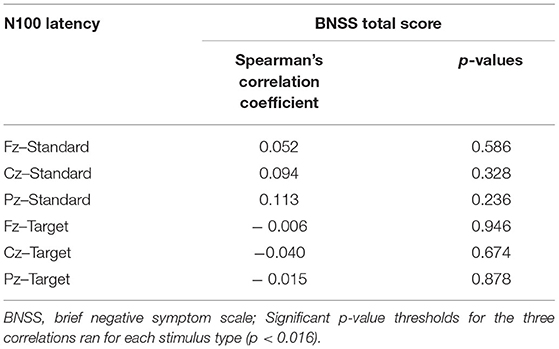
Table 5. Correlations between N100 latency for standard and target stimuli and BNSS total score in SCZ.
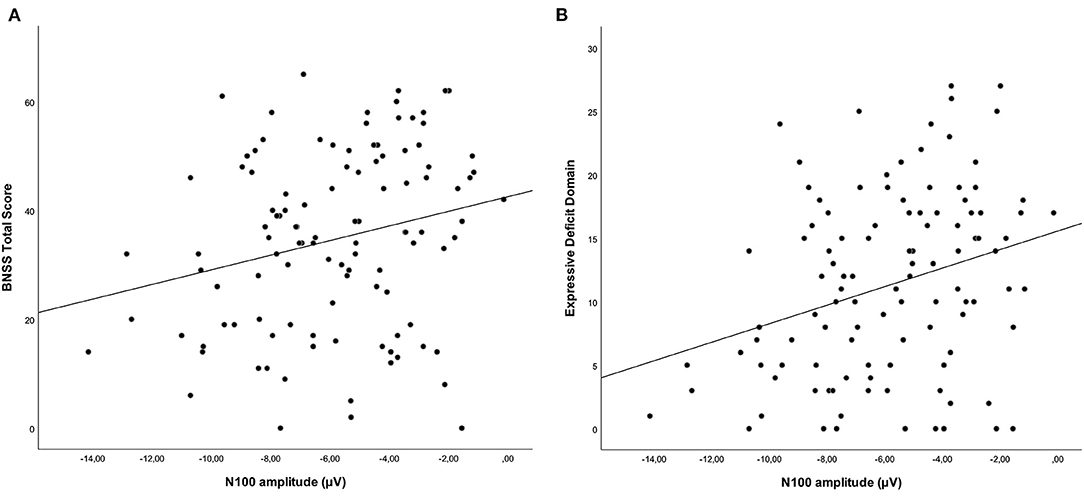
Figure 2. Correlations between standard-stimuli N100 amplitude (Fz electrode) with the BNSS total score (A) (rs = 0.241; p = 0.011) and the expressive deficit domain (B) (rs = 0.296; p = 0.002) in subjects with schizophrenia. Both correlations remained significant after controlling for the possible confounding effects of positive symptoms, extrapyramidal side effects, depression, and disorganization.
Furthermore, when we considered the two domains of negative symptoms, we found a different pattern of correlations between these domains and N100 amplitude. In particular, while a correlation was observed between N100 amplitude (standard stimuli–Fz electrode) and the expressive deficit domain (rs = 0.296; p = 0.002) (Figure 2; Table 6), no significant correlation was found for the experiential domain (rs = 0.188; p = 0.051) (Table 6). Since the p-value of this last correlation was close to the threshold value, we performed an exploratory analysis focusing on the correlations between N100 amplitude and all the symptoms constituting the experiential domain (avolition, anhedonia, and asociality). The correlations between the N100 amplitude and avolition (rs = 0.075; p = 0.445) and asociality (rs = 0.040; p = 0.686) were not statistically significant, while the correlation with anhedonia did not survive correction for multiple tests (rs = 0.205; p = 0.035).
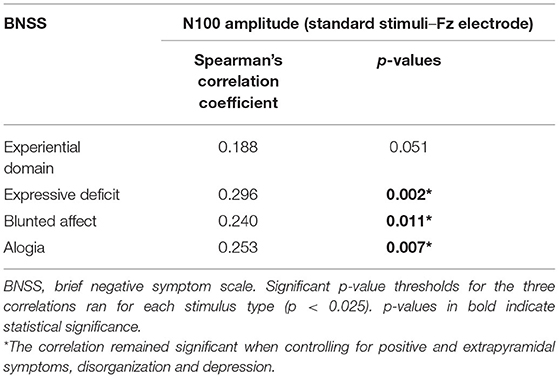
Table 6. Correlations between N100 amplitude (standard stimuli–Fz electrode) and negative symptom domains.
Finally, within the expressive deficit, both blunted affect (rs = 0.240; p = 0.011) and alogia (rs = 0.253 p = 0.007) had the same pattern of correlation with N100 (Table 6). All correlations remained significant after controlling for the possible confounding effects of positive symptoms, extrapyramidal side effects, depression, and disorganization.
Control Analysis of Correlations of P3b With Negative Symptoms
No association of P3b with negative symptoms was observed in the study. In particular, no significant correlations were found between P3b amplitude and the BNSS total (rs = −0.054; p = 0.575) or the experiential (rs = −0.053; p = 0.577) and the expressive deficit (rs = −0.060; p = 0.533) domains. Finally, no significant correlations were found between P3b latency and the BNSS total (rs = −0.046; p = 0.635) and the experiential (rs = −0.037; p = 0.701) and the expressive deficit (rs = −0.083; p = 0.387) domains.
Discussion
The current study aimed to investigate auditory-elicited N100 in SCZ and its association with negative symptom domains. The two main aims were: (1) to identify differences in N100 amplitude between SCZ and HCs; (2) to investigate the presence of associations between N100 and negative symptom domains (experiential and expressive deficit) in SCZ. The main results of our study included: (1) N100 amplitude was reduced in SCZ, compared to HCs, while no significant differences were detected in N100 latency between the two groups; (2) negative symptoms, assessed by BNSS scale, showed an association with N100 amplitude for standard stimuli; (3) expressive deficit, but not the experiential domain, was associated with N100 amplitude; and (4) both blunted affect and alogia were associated with N100 amplitude.
N100 amplitude was reduced in SCZ compared to HCs, for both standard and target stimuli. These results are in line with previous literature findings, which robustly documented diminished N100 amplitude in SCZ (67–71, 73, 76, 77). Abnormalities of N100 are already detectable in early stages of the disease and in high-risk individuals (74, 75) and, therefore, have been proposed as indicators of brain functional changes related to schizophrenia vulnerability (98). In line with this hypothesis, N100 amplitude deficit has also been recorded in unaffected first-degree relatives of subjects with SCZ (72). Using topographic analysis, such as low-resolution electromagnetic tomography analysis (LORETA), it is possible to detect the main brain areas involved in N100 generation: the primary auditory cortex, the dorsolateral prefrontal cortex, and the anterior cingulate (69, 99). Abnormalities in these areas, along with widespread connectivity alterations are consistently reported in neuroimaging studies conducted in SCZ (8, 100, 101).
The N100 is regarded as an index of early visual and auditory processing, which is also influenced by selective attention and unpredictability of the stimuli. Therefore, the reduction in N100 amplitude in SCZ is interpreted as a deficit in early sensory processing of the stimulus, an aspect well documented in schizophrenia both through behavioral and neurophysiology studies, since the earliest stages of the disease (67, 70, 75, 79, 85). Deficits in early visual and auditory processing, along with aberrations in the integration of simultaneous and multisensory stimulation, might lead to impairment also in higher-level functions (86, 102–105).
The second part of our study aimed to evaluate the relationship between N100 and negative symptoms. Previous studies have found an association between dysfunctions in N100 elicitation in SCZ and auditory hallucinations (80–82), antipsychotic intake (67), attention deficits (83, 84), and negative symptoms (69, 85).
As reported in the Introduction, the association between N100 abnormalities and negative symptoms remains unclear since results reported by different studies are inconsistent (69, 85, 89–91). However, the majority of the above-mentioned studies (85, 89–91) used first generation rating scales, such as the PANSS (92) and the SANS (93) to assess negative symptoms. These assessment instruments present some limitations, as they include aspects that actually are not conceptualized as negative symptoms, but are mostly related to cognitive functions and disorganization (2). In addition, previous studies did not investigate associations between N100 and the two negative symptom domains.
In a large sample of stabilized subjects with chronic schizophrenia, our study demonstrated a relationship between N100 abnormalities with negative symptoms. The strength of this finding stem from fact that negative symptoms were evaluated with the BNSS, a second-generation rating scale in line with the current conceptualization of negative symptoms, and that as documented by partial correlation analysis, this outcome was not mediated by positive symptoms, extrapyramidal side effects, disorganization, or depression, frequently causing secondary negative symptoms within negative symptoms, the expressive deficit domain was strongly correlated with N100 amplitude, as compared to the experiential domain. In particular, although the p-value of association between the experiential domain and the N100 was close to threshold, none of the symptoms belonging to this domain was significantly correlated with N100 amplitude, while the expressive deficit domain and its subcomponent symptoms were correlated with N100.
The presence of an association of N100 amplitude with only one of the two negative symptom domains is in agreement with previous results that suggest the existence of separate neurobiological mechanisms at the core of the experiential domain and expressive deficit (8, 10, 15, 16, 37, 58).
Indeed, neuroimaging studies have provided a rich evidence of the possible faulty neuronal circuits underlying the two domains of negative symptoms. The experiential domain seems to be related to abnormalities in brain networks regulating different aspects of motivation, and probably to impairment in executive functions. On the other side, the pathophysiological mechanisms at the basis of the expressive deficit domain remain less understood (8, 10, 15, 16). This domain of negative symptoms has been related to deficit in neurocognitive skills, social cognition abilities, and neurological soft signs, which comprise also subtle deficits in sensory integration, along with motor coordination, and sequencing of complex motor acts (8). These associations seem to pinpoint that this domain is related to a diffuse neurodevelopmental disconnectivity.
According to the hypothesis of the limited cognitive resource, expressive deficit symptoms, in particular alogia, might depend on deficits in different cognitive functions, such as semantic memory organization and verbal fluency. Starting from limited cognitive resources, in “high-load” situations (e.g., social situations) subjects are exposed to high cognitive demands and might allocate less cognitive resources to speech production (15). As we have reported above, reduced N100 amplitude is an index of deficits in sensory processing and sensory gating, a well-replicated finding in SCZ. It has been proposed that alterations in sensory gating of N100 cause sensory flooding and defective processing of information to the brain, contributing to the symptoms of SCZ (91). Given the relationship between N100 and sensory processing deficits, our study demonstrated a connection between deficits in sensory processing with negative symptom severity, in particular those belonging to the expressive deficit. A possible interpretation of this connection is based on some “cascade” models that have hypothesized that impairment in early sensory processing might contribute to deficits in higher-level processing which are related to negative symptoms, leading to poor functioning (86–88, 106).
Certain limitations of this study should be taken into account. For instance, age and pharmacological treatment might have had an impact on our results. In our study, we used a sample in which subjects were matched for age: therefore, we could exclude the effect of age on the differences between HCs and SCZ. With regard to medication, we excluded the confounding effect of medication on correlation between N100 and negative symptoms, using partial correlation analysis in which we controlled for extrapyramidal symptoms that might cause secondary negative symptoms. However, further studies including drug-naïve subjects at their first episode, as well as subjects at high risk for psychosis, using a proper characterization of negative symptoms, are needed in order to disentangle different neurobiological underpinnings of negative symptom domains.
Conclusions
In conclusion, in line with previous studies, our results suggested that chronic individuals with schizophrenia are affected by neurophysiological abnormalities in early stages of auditory processing, as indexed by reduced N100 amplitude. In addition, we reported a correlation between reductions of N100 amplitude and severity of the expressive deficit domain, while no correlation was found with the experiential domain. These results reinforce the hypothesis of separate neurophysiological correlates of the two negative symptom domains. Furthermore, previous models have hypothesized a concatenation of pathological features starting from impairment in early sensory processing up to deficits in higher-level processing that could lead to negative symptoms, and finally might contribute to poor functioning in real life. Further studies, including large sample sizes, a proper characterization of negative symptoms, and an analysis of pathways to functional outcome, are needed.
Improving knowledge in the pathophysiology of different aspects of negative symptoms and their relative contribution to poor functioning is one of the main goals of the research, since it could help in the design and implementation of effective treatments for negative symptoms, which unfortunately still represent an unmet need in the care of SCZ.
Italian Network for Research on Psychoses
Members of the Italian Network for Research on Psychoses participating in the add-on EEG study include: Eleonora Merlotti and Giuseppe Piegari, University of Campania “Luigi Vanvitelli”; Girolamo Francavilla and Flavia A. Padalino, University of Foggia; Cinzia Niolu and Michele Ribolsi, University of Rome Tor Vergata; Roberto Brugnoli and Paolo Girardi, University of Rome “La Sapienza”; Giulio Corrivetti and Francesca Marciello, University of Salerno.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Comitato Etico Università degli Studi della Campania Luigi Vanvitelli—A.O.U. Luigi Vanvitelli and A.O.R.N. Ospedali dei Colli. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The project idea was initiated by SG, involving a collaboration with GMG, FB, AP, GDL, and MM. SG and GMG planned the experimental procedures. GMG, FB, and AP performed the analyzes of the data and wrote the first draft of the manuscript. All authors were responsible for the interpretation of the analyzes, contributed to critically revising the content, and approved the final manuscript for submission to Frontiers in Psychiatry.
Funding
This study was funded by the Italian Ministry of Education (grant number: 2010XP2XR4), the Italian Society of Psychopathology (SOPSI), the Italian Society of Biological Psychiatry (SIPB), Roche, Switzerland; Lilly, United States; AstraZeneca, United Kingdom; Lundbeck Foundation, Denmark; and Bristol-Myers Squibb, United Kingdom. These entities had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the paper for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Galderisi S, Kaiser S, Bitter I, Nordentoft M, Mucci A, Sabé M, et al. EPA guidance on treatment of negative symptoms in schizophrenia. Eur. Psychiatry. (2021) 64:e21. doi: 10.1192/j.eurpsy.2021.13
2. Galderisi S, Mucci A, Dollfus S, Nordentoft M, Falkai P, Kaiser S, et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur Psychiatry. (2021) 64:e23. doi: 10.1192/j.eurpsy.2021.11
3. Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry. (2014) 13:275–87. doi: 10.1002/wps.20167
4. Galderisi S, Rucci P, Kirkpatrick B, Mucci A, Gibertoni D, Rocca P, et al. Interplay among psychopathologic variables, personal resources, context-related factors, and real-life functioning in individuals with schizophrenia: a network analysis. JAMA Psychiatry. (2018) 75:396–404. doi: 10.1001/jamapsychiatry.2017.4607
5. Galderisi S, Rucci P, Mucci A, Rossi A, Rocca P, Bertolino A, et al. The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry. (2020) 19:81–91. doi: 10.1002/wps.20700
6. Mucci A, Galderisi S, Gibertoni D, Rossi A, Rocca P, Bertolino A, et al. Factors associated with real-life functioning in persons with schizophrenia in a 4-year follow-up study of the Italian Network for Research on Psychoses. JAMA Psychiatry. (2021) 78:550–9. doi: 10.1001/jamapsychiatry.2020.4614
7. Galderisi S, Färden A, Kaiser S. Dissecting negative symptoms of schizophrenia: history, assessment, pathophysiological mechanisms and treatment. Schizophr Res. (2017) 186:1–2. doi: 10.1016/j.schres.2016.04.046
8. Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. (2018) 5:664–77. doi: 10.1016/s2215-0366(18)30050-6
9. Kirkpatrick B, Fenton WS, Carpenter WT Jr., Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. (2006) 32:214–9. doi: 10.1093/schbul/sbj053
10. Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. (2017) 16:14–24. doi: 10.1002/wps.20385
11. Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. (2020) 16:519–34. doi: 10.2147/ndt.s225643
12. Gaebel W, Falkai P, Hasan A. The revised German evidence- and consensus-based schizophrenia guideline. World Psychiatry. (2020) 19:117–9. doi: 10.1002/wps.20706
13. Kirkpatrick B, Fischer B. Subdomains within the negative symptoms of schizophrenia: commentary. Schizophr Bull. (2006) 32:246–9. doi: 10.1093/schbul/sbj054
14. Reed GM, First MB, Kogan CS, Hyman SE, Gureje O, Gaebel W, et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry. (2019) 18:3–19. doi: 10.1002/wps.20611
15. Begue I, Kaiser S, Kirschner M. Pathophysiology of negative symptom dimensions of schizophrenia - current developments and implications for treatment. Neurosci Biobehav Rev. (2020) 116:74–88. doi: 10.1016/j.neubiorev.2020.06.004
16. Kaiser S, Lyne J, Agartz I, Clarke M, Mørch-Johnsen L, Faerden A. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment. Schizophr Res. (2017) 186:39–45. doi: 10.1016/j.schres.2016.07.013
17. Cuthbert BN, Morris SE. Evolving concepts of the schizophrenia spectrum: a research domain criteria perspective. Front Psychiatry. (2021) 12:641319. doi: 10.3389/fpsyt.2021.641319
18. Sanislow CA. RDoC at 10: changing the discourse for psychopathology. World Psychiatry. (2020) 19:311–2. doi: 10.1002/wps.20800
19. Menon V. Brain networks and cognitive impairment in psychiatric disorders. World Psychiatry. (2020) 19:309–10. doi: 10.1002/wps.20799
20. Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. (2010) 36:919–34. doi: 10.1093/schbul/sbq068
21. Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. (2010) 36:143–50. doi: 10.1093/schbul/sbn061
22. Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. (2007) 93:253–60. doi: 10.1016/j.schres.2007.03.008
23. Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. (2007) 116:268–78. doi: 10.1037/0021-843x.116.2.268
24. Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. (2007) 12:213–21. doi: 10.1080/13546800601005900
25. Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. (2008) 34:819–34. doi: 10.1093/schbul/sbn071
26. Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. (2007) 62:756–64. doi: 10.1016/j.biopsych.2006.09.042
27. Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. (2010) 67:902–11. doi: 10.1016/j.biopsych.2009.10.020
28. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam's razor. Schizophr Bull. (2010) 36:359–69. doi: 10.1093/schbul/sbn094
29. Mann CL, Footer O, Chung YS, Driscoll LL, Barch DM. Spared and impaired aspects of motivated cognitive control in schizophrenia. J Abnorm Psychol. (2013) 122:745–55. doi: 10.1037/a0033069
30. Pizzagalli DA. The “anhedonia paradox” in schizophrenia: insights from affective neuroscience. Biol Psychiatry. (2010) 67:899–901. doi: 10.1016/j.biopsych.2010.02.022
31. Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophr Bull. (2012) 38:1111–7. doi: 10.1093/schbul/sbs114
32. Morris RW, Quail S, Griffiths KR, Green MJ, Balleine BW. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol Psychiatry. (2015) 77:187–95. doi: 10.1016/j.biopsych.2014.06.005
33. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. (2014) 40(Suppl 2):S107–16. doi: 10.1093/schbul/sbt197
34. Strauss J. Reconceptualizing schizophrenia. Schizophr Bull. (2014) 40(Suppl 2):S97–100. doi: 10.1093/schbul/sbt156
35. Mucci A, Dima D, Soricelli A, Volpe U, Bucci P, Frangou S, et al. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol Med. (2015) 45:1765–78. doi: 10.1017/s0033291714002943
36. Amodio A, Quarantelli M, Mucci A, Prinster A, Soricelli A, Vignapiano A, et al. Avolition-apathy and white matter connectivity in schizophrenia: reduced fractional anisotropy between amygdala and insular cortex. Clin EEG Neurosci. (2018) 49:55–65. doi: 10.1177/1550059417745934
37. Giordano GM, Stanziano M, Papa M, Mucci A, Prinster A, Soricelli A, et al. Functional connectivity of the ventral tegmental area and avolition in subjects with schizophrenia: a resting state functional MRI study. Eur Neuropsychopharmacol. (2018) 28:589–602. doi: 10.1016/j.euroneuro.2018.03.013
38. Grant PM, Best MW, Beck AT. The meaning of group differences in cognitive test performance. World Psychiatry. (2019) 18:163–4. doi: 10.1002/wps.20645
39. Harvey PD, Strassnig MT. Cognition and disability in schizophrenia: cognition-related skills deficits and decision-making challenges add to morbidity. World Psychiatry. (2019) 18:165–7. doi: 10.1002/wps.20647
40. Culbreth AJ, Moran EK, Kandala S, Westbrook A, Barch DM. Effort, avolition and motivational experience in schizophrenia: analysis of behavioral and neuroimaging data with relationships to daily motivational experience. Clin Psychol Sci. (2020) 8:555–68. doi: 10.1177/2167702620901558
41. Davidson M. Cognitive impairment as a diagnostic criterion and treatment target in schizophrenia. World Psychiatry. (2019) 18:171–2. doi: 10.1002/wps.20651
42. Falkai P, Schmitt A. The need to develop personalized interventions to improve cognition in schizophrenia. World Psychiatry. (2019) 18:170. doi: 10.1002/wps.20650
43. Moritz S, Silverstein SM, Dietrichkeit M, Gallinat J. Neurocognitive deficits in schizophrenia are likely to be less severe and less related to the disorder than previously thought. World Psychiatry. (2020) 19:254–5. doi: 10.1002/wps.20759
44. Reichenberg A, Velthorst E, Davidson M. Cognitive impairment and psychosis in schizophrenia: independent or linked conditions? World Psychiatry. (2019) 18:162–3. doi: 10.1002/wps.20644
45. Sahakian BJ, Savulich G. Innovative methods for improving cognition, motivation and wellbeing in schizophrenia. World Psychiatry. (2019) 18:168–70. doi: 10.1002/wps.20649
46. Bissonette GB, Roesch MR. Development and function of the midbrain dopamine system: what we know and what we need to. Genes Brain Behav. (2016) 15:62–73. doi: 10.1111/gbb.12257
47. Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. (2010) 68:815–34. doi: 10.1016/j.neuron.2010.11.022
48. Bowie CR. Cognitive remediation for severe mental illness: state of the field and future directions. World Psychiatry. (2019) 18:274–5. doi: 10.1002/wps.20660
49. Faerden A, Vaskinn A, Finset A, Agartz I, Ann Barrett E, Friis S, et al. Apathy is associated with executive functioning in first episode psychosis. BMC Psychiatry. (2009) 9:1. doi: 10.1186/1471-244x-9-1
50. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. (2006) 16:916–28. doi: 10.1093/cercor/bhj043
51. Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annu Rev Clin Psychol. (2013) 9:409–33. doi: 10.1146/annurev-clinpsy-050212-185538
52. Hartmann-Riemer MN, Hager OM, Kirschner M, Bischof M, Kluge A, Seifritz E, et al. The association of neurocognitive impairment with diminished expression and apathy in schizophrenia. Schizophr Res. (2015) 169:427–32. doi: 10.1016/j.schres.2015.10.032
53. Cohen AS, Schwartz E, Le TP, Fedechko T, Kirkpatrick B, Strauss GP. Using biobehavioral technologies to effectively advance research on negative symptoms. World Psychiatry. (2019) 18:103–4. doi: 10.1002/wps.20593
54. Melle I. Cognition in schizophrenia: a marker of underlying neurodevelopmental problems? World Psychiatry. (2019) 18:164–5. doi: 10.1002/wps.20646
55. Galderisi S, Caputo F, Giordano G, Mucci A. Aetiopathological mechanisms of negative symptoms in schizophrenia. Die Psychiatrie. (2016) 13:121–9. doi: 10.1055/s-0038-1669683
56. Barch DM. Nonsocial and social cognitive function in psychosis: interrelationships, specificity and innovative approaches. World Psychiatry. (2019) 18:117–8. doi: 10.1002/wps.20653
57. Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. (2019) 18:146–61. doi: 10.1002/wps.20624
58. Giordano GM, Koenig T, Mucci A, Vignapiano A, Amodio A, Di Lorenzo G, et al. Neurophysiological correlates of Avolition-apathy in schizophrenia: a resting-EEG microstates study. Neuroimage Clin. (2018) 20:627–36. doi: 10.1016/j.nicl.2018.08.031
59. Kotov R, Jonas KG, Carpenter WT, Dretsch MN, Eaton NR, Forbes MK, et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): I. Psychosis superspectrum. World Psychiatry. (2020) 19:151–72. doi: 10.1002/wps.20730
60. Hasey GM, Kiang M. A review of recent literature employing electroencephalographic techniques to study the pathophysiology, phenomenology, and treatment response of schizophrenia. Curr Psychiatry Rep. (2013) 15:388. doi: 10.1007/s11920-013-0388-x
61. Ince E, Üçok A. Relationship between persistent negative symptoms and findings of neurocognition and neuroimaging in schizophrenia. Clin EEG Neurosci. (2017) 49:27–35. doi: 10.1177/1550059417746213
62. Phillips JM, Maxwell CR, Ehrlichman RS, Siegel SJ. Event-related potentials (ERPs) in the study of schizophrenia: how preclinical ERP studies have contributed to our understanding of schizophrenia. In: Lajtha A, Javitt D, Kantrowitz J, editors. Handbook of Neurochemistry and Molecular Neurobiology: Schizophrenia. Springer (2009) p. 525–43.
63. Qiu Y-Q, Tang Y-X, Chan RCK, Sun X-Y, He J. P300 aberration in first-episode schizophrenia patients: a meta-analysis. PLoS ONE. (2014) 9:e97794-e. doi: 10.1371/journal.pone.0097794
64. Vignapiano A, Mucci A, Ford J, Montefusco V, Plescia GM, Bucci P, et al. Reward anticipation and trait anhedonia: an electrophysiological investigation in subjects with schizophrenia. Clin Neurophysiol. (2016) 127:2149–60. doi: 10.1016/j.clinph.2016.01.006
65. Vignapiano A, Mucci A, Merlotti E, Giordano GM, Amodio A, Palumbo D, et al. Impact of reward and loss anticipation on cognitive control: an event-related potential study in subjects with schizophrenia and healthy controls. Clin EEG Neurosci. (2018) 49:46–54. doi: 10.1177/1550059417745935
66. Wynn JK, Horan WP, Kring AM, Simons RF, Green MF. Impaired anticipatory event-related potentials in schizophrenia. Int J Psychophysiol. (2010) 77:141–9. doi: 10.1016/j.ijpsycho.2010.05.009
67. Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia – a critical review. Psychiatry Res. (2008) 161:259–74. doi: 10.1016/j.psychres.2008.03.017
68. Bruder G, Kayser J, Tenke C, Rabinowicz E, Friedman M, Amador X, et al. The time course of visuospatial processing deficits in schizophrenia: an event-related brain potential study. J Abnorm Psychol. (1998) 107:399–411. doi: 10.1037//0021-843x.107.3.399
69. Mucci A, Galderisi S, Kirkpatrick B, Bucci P, Volpe U, Merlotti E, et al. Double dissociation of N1 and P3 abnormalities in deficit and nondeficit schizophrenia. Schizophr Res. (2007) 92:252–61. doi: 10.1016/j.schres.2007.01.026
70. Sumich A, Kumari V, Dodd P, Ettinger U, Hughes C, Zachariah E, et al. N100 and P300 amplitude to Go and No-Go variants of the auditory oddball in siblings discordant for schizophrenia. Schizophr Res. (2008) 98:265–77. doi: 10.1016/j.schres.2007.09.018
71. Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. (2009) 165:27–37. doi: 10.1016/j.psychres.2008.04.013
72. Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, et al. Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry. (2008) 64:1051–9. doi: 10.1016/j.biopsych.2008.06.018
73. Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia. Arch Gen Psychiatry. (2011) 68:654–64. doi: 10.1001/archgenpsychiatry.2011.17
74. Del Re E, Spencer K, Oribe N, Mesholam-Gately R, Goldstein J, Shenton M, et al. Clinical high risk and first episode schizophrenia: auditory event-related potentials. Psychiatry Res. (2014) 231:126–33. doi: 10.1016/j.pscychresns.2014.11.012
75. Hsieh MH, Lin Y-T, Chien Y-L, Hwang T-J, Hwu H-G, Liu C-M, et al. Auditory event-related potentials in antipsychotic-free subjects with ultra-high-risk state and first-episode psychosis. Front Psychiatry. (2019) 10:223. doi: 10.3389/fpsyt.2019.00223
76. Perrottelli A, Giordano GM, Brando F, Giuliani L, Mucci A. EEG-based measures in at-risk mental state and early stages of schizophrenia: a systematic review. Front Psychiatry. (2021) 12:653642. doi: 10.3389/fpsyt.2021.653642
77. Boutros NN, Brockhaus-Dumke A, Gjini K, Vedeniapin A, Elfakhani M, Burroughs S, et al. Sensory-gating deficit of the N100 mid-latency auditory evoked potential in medicated schizophrenia patients. Schizophr Res. (2009) 113:339–46. doi: 10.1016/j.schres.2009.05.019
78. Oribe N, Hirano Y, Kanba S, del Re EC, Seidman LJ, Mesholam-Gately R, et al. Early and late stages of visual processing in individuals in prodromal state and first episode schizophrenia: an ERP study. Schizophr Res. (2013) 146:95–102. doi: 10.1016/j.schres.2013.01.015
79. Rosburg T. Auditory N100 gating in patients with schizophrenia: a systematic meta-analysis. Clin Neurophysiol. (2018) 129:2099–111. doi: 10.1016/j.clinph.2018.07.012
80. Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. (2007) 64:286–96. doi: 10.1001/archpsyc.64.3.286
81. Ford JM, Dierks T, Fisher DJ, Herrmann CS, Hubl D, Kindler J, et al. Neurophysiological studies of auditory verbal hallucinations. Schizophr Bull. (2012) 38:715–23. doi: 10.1093/schbul/sbs009
82. Thoma RJ, Meier A, Houck J, Clark VP, Lewine JD, Turner J, et al. Diminished auditory sensory gating during active auditory verbal hallucinations. Schizophr Res. (2017) 188:125–31. doi: 10.1016/j.schres.2017.01.023
83. Lijffijt M, Moeller FG, Boutros NN, Steinberg JL, Meier SL, Lane SD, et al. Diminished P50, N100 and P200 auditory sensory gating in bipolar I disorder. Psychiatry Res. (2009) 167:191–201. doi: 10.1016/j.psychres.2008.04.001
84. Smith AK, Edgar JC, Huang M, Lu BY, Thoma RJ, Hanlon FM, et al. Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. Am J Psychiatry. (2010) 167:1264–75. doi: 10.1176/appi.ajp.2010.09071059
85. Sumich A, Harris A, Flynn G, Whitford T, Tunstall N, Kumari V, et al. Event-related potential correlates of depression, insight and negative symptoms in males with recent-onset psychosis. Clin Neurophysiol. (2006) 117:1715–27. doi: 10.1016/j.clinph.2006.04.017
86. de Jong JJ, de Gelder B, Hodiamont PP. Sensory processing, neurocognition, and social cognition in schizophrenia: towards a cohesive cognitive model. Schizophr Res. (2013) 146:209–16. doi: 10.1016/j.schres.2013.02.034
87. Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. (2009) 5:249–75. doi: 10.1146/annurev.clinpsy.032408.153502
88. Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. (2006) 163:448–54. doi: 10.1176/appi.ajp.163.3.448
89. Li Z, Zheng B, Deng W, Liu X, Zheng Z, Li T. Multi-components of evoked-brain potentials in deficit and nondeficit schizophrenia. Asia Pac Psychiatry. (2013) 5:69–79. doi: 10.1111/appy.12030
90. Pinheiro AP, Del Re E, Mezin J, Nestor PG, Rauber A, McCarley RW, et al. Sensory-based and higher-order operations contribute to abnormal emotional prosody processing in schizophrenia: an electrophysiological investigation. Psychol Med. (2013) 43:603–18. doi: 10.1017/S003329171200133X
91. Shen CL, Chou TL, Lai WS, Hsieh MH, Liu CC, Liu CM, et al. P50, N100, and P200 auditory sensory gating deficits in schizophrenia patients. Front Psychiatry. (2020) 11:868. doi: 10.3389/fpsyt.2020.00868
92. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
93. Andreasen NC. Scale for the assessment of negative symptoms (SANS). Brit J Psychiatry. (1989) 155:53–8.
94. Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. (2010) 37:300–5. doi: 10.1093/schbul/sbq059
95. Mucci A, Galderisi S, Merlotti E, Rossi A, Rocca P, Bucci P, et al. The Brief Negative Symptom Scale (BNSS): independent validation in a large sample of Italian patients with schizophrenia. Eur Psychiatry. (2015) 30:641–7. doi: 10.1016/j.eurpsy.2015.01.014
96. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. (1990) 3:247–51. doi: 10.1016/0920-9964(90)90005-r
97. Gerlach J, Korsgaard S, Clemmesen P, Lauersen AM, Magelund G, Noring U, et al. The St. Hans Rating Scale for extrapyramidal syndromes: reliability and validity. Acta Psychiatr Scand. (1993) 87:244–52. doi: 10.1111/j.1600-0447.1993.tb03366.x
98. Ahveninen J, Jaaskelainen IP, Raij T, Bonmassar G, Devore S, Hamalainen M, et al. Task-modulated “what” and “where” pathways in human auditory cortex. Proc Natl Acad Sci USA. (2006) 103:14608–13. doi: 10.1073/pnas.0510480103
99. Gallinat J, Mulert C, Bajbouj M, Herrmann WM, Schunter J, Senkowski D, et al. Frontal and temporal dysfunction of auditory stimulus processing in schizophrenia. Neuroimage. (2002) 17:110–27. doi: 10.1006/nimg.2002.1213
100. Goghari VM, Sponheim SR, MacDonald AW III. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev. (2010) 34:468–86. doi: 10.1016/j.neubiorev.2009.09.004
101. Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. (2014) 24:645–92. doi: 10.1016/j.euroneuro.2014.03.008
102. de Jong JJ, Hodiamont PP, de Gelder B. Modality-specific attention and multisensory integration of emotions in schizophrenia: reduced regulatory effects. Schizophr Res. (2010) 122:136–43. doi: 10.1016/j.schres.2010.04.010
103. Van den Stock J, de Jong SJ, Hodiamont PP, de Gelder B. Perceiving emotions from bodily expressions and multisensory integration of emotion cues in schizophrenia. Soc Neurosci. (2011) 6:537–47. doi: 10.1080/17470919.2011.568790
104. Williams LE, Light GA, Braff DL, Ramachandran VS. Reduced multisensory integration in patients with schizophrenia on a target detection task. Neuropsychologia. (2010) 48:3128–36. doi: 10.1016/j.neuropsychologia.2010.06.028
105. Stein BE, Stanford TR, Rowland BA. Multisensory integration and the society for neuroscience: then and now. J Neurosci. (2020) 40:3–11. doi: 10.1523/JNEUROSCI.0737-19.2019
Keywords: schizophrenia, negative symptoms, EEG, ERP, N100
Citation: Giordano GM, Brando F, Perrottelli A, Di Lorenzo G, Siracusano A, Giuliani L, Pezzella P, Altamura M, Bellomo A, Cascino G, Del Casale A, Monteleone P, Pompili M, Galderisi S, Maj M and the Italian Network for Research on Psychoses (2021) Tracing Links Between Early Auditory Information Processing and Negative Symptoms in Schizophrenia: An ERP Study. Front. Psychiatry 12:790745. doi: 10.3389/fpsyt.2021.790745
Received: 07 October 2021; Accepted: 19 November 2021;
Published: 20 December 2021.
Edited by:
Ingrid Melle, University of Oslo, NorwayReviewed by:
Joshua T. Kantrowitz, Columbia University, United StatesStefano Barlati, University of Brescia, Italy
Copyright © 2021 Giordano, Brando, Perrottelli, Di Lorenzo, Siracusano, Giuliani, Pezzella, Altamura, Bellomo, Cascino, Del Casale, Monteleone, Pompili, Galderisi, Maj and the Italian Network for Research on Psychoses. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvana Galderisi, c2lsdmFuYS5nYWxkZXJpc2lAZ21haWwuY29t
 Giulia M. Giordano
Giulia M. Giordano Francesco Brando
Francesco Brando Andrea Perrottelli1
Andrea Perrottelli1 Giorgio Di Lorenzo
Giorgio Di Lorenzo Alberto Siracusano
Alberto Siracusano Luigi Giuliani
Luigi Giuliani Pasquale Pezzella
Pasquale Pezzella Mario Altamura
Mario Altamura Antonello Bellomo
Antonello Bellomo Giammarco Cascino
Giammarco Cascino Antonio Del Casale
Antonio Del Casale Palmiero Monteleone
Palmiero Monteleone Maurizio Pompili
Maurizio Pompili Silvana Galderisi
Silvana Galderisi