- 1Specialty of Psychiatry, University of Sydney School of Medicine, The University of Sydney, Sydney, NSW, Australia
- 2Brain Dynamics Centre, The Westmead Institute for Medical Research, The University of Sydney, Sydney, NSW, Australia
- 3Child and Youth Mental Health Service, Western Sydney Local Health District, North Parramatta, NSW, Australia
Introduction: Cognitive impairments are a common and significant issue for young people with a severe mental illness. Young people with schizophrenia, bipolar disorder and major depression all experience significant cognitive problems that impede their ability to return to work or study. These neurocognitive problems are frequently exacerbated by social cognitive deficits that interfere with their ability to integrate into the community and understand the social and emotional nuances about them. This study aimed to assess if the addition of a social cognitive remediation treatment to a neurocognitive remediation therapy improved functional outcome.
Methods: Five youth mental health services were trained in both the Neuropsychological Educational Approach to Remediation (NEAR) and the Social Cognition and Interaction Training (SCIT) treatments. Participants were randomised between receiving either NEAR + SCIT or NEAR + treatment as usual (TAU) over a 20-week period, with all participants receiving the NEAR treatment first. Symptoms, neurocognition, social cognition and functioning were examined at baseline, end of treatment and at 3 months follow-up and compared between the two arms of the study. The primary outcome was function.
Results: Thirty-nine participants were randomised to treatment (Schizophrenia spectrum = 28, Bipolar disorder = 7, Major Depression = 2). The trial was curtailed by Covid-related service restrictions. There was an overall significant improvement in function over time with a trend towards a greater improvement in the NEAR + SCIT arm. No changes in symptoms, neurocognitive or social cognitive measures were seen. While 74% completed treatment only 49% agreed to follow up at 3 months affecting our ability to interpret the findings. Attrition did not differ by arm.
Conclusions: In a pragmatic, service-based research project, treatment aimed at improving cognition enhanced functional outcome in young people with a range of severe mental illnesses. There was a trend towards improved function in young people who had a combined NEAR + SCIT approach.
Clinical Trial Registration: Identifier: ACTRN12622000192785.
Introduction
Remission of psychotic symptoms is a common primary treatment metric used to assess treatment response in severe mental illnesses (SMI) such as schizophrenia, schizophreniform disorder or bipolar disorder, however functional recovery, a more important but frequently secondary outcome, remains elusive for many. A significant contributor to this failure to achieve functional recovery is the detrimental effects of neurocognitive and social cognitive deficits in SMI. Initially, investigations of neurocognitive and social cognitive deficits centred upon schizophrenia (1–3), however there is now a more holistic approach that these deficits are common to all SMI (4), albeit with different degrees of severity.
Neurocognition and social cognition are important because of their contribution to the ability of the individual to operate in a complex society, however their effects appear to be different though overlapping (5). While neurocognitive deficits are basic to the impact of cognition on function, both due to a direct effect and via its influence on social cognition, social cognition may have a greater effect on community functioning overall both by its direct effect and via moderating neurocognitive deficits (6). This suggests that targeting neurocognitive deficits alone is not sufficient to improve overall outcome. Unfortunately, the cognitive deficits in SMI are not ameliorated by standard antipsychotic therapies (7). This along with the recognition of the important role played by cognitive deficits in the outcome of SMI (6) has generated new treatment approaches such as cognitive remediation therapy (CRT) and more recently, social cognitive remediation therapy (SCRT). These treatments have a small to moderate effect upon cognitive function and this is noted to generalise to functional improvement (8, 9). This positive effect on functional recovery is helped by combining cognitive treatments with other psychosocial approaches such as supported employment (10), however, the effectiveness of combining the two approaches to cognitive remediation—therapy for neurocognitive as well as social cognitive deficits—is less frequently investigated. This is surprising given that neurocognition, at least in some analyses, appears to be a foundation for the mediating effect of social cognition upon eventual community functioning (6, 11, 12).
Of the studies that have examined the usefulness of combining neurocognitive and social cognitive remediation strategies, most have been in chronically unwell, predominantly male participants (13–16). While CRT clearly improved neurocognition (8), this effect was enhanced by the combination of neurocognitive and social cognitive remediation therapies (15). But improvement in social cognition required the specific treatment of that domain (13, 16). Treatment of cognition translated into better community function (15), though whether the combination of both treatments is necessary for this effect is not clear (13, 16). This study aimed to test the effectiveness of combining CRT with SCRT against CRT alone in improving community functioning in a group of young people with severe mental illness. In addition, it followed participants up over 3 months to see if any improvements were maintained longer term. We predicted that the combined treatment of neurocognitive and social cognitive remediation would have a superior effect on functional outcome over neurocognitive remediation alone.
Method
This trial was a single blind randomised controlled trial conducted in five youth mental health services across Sydney, Australia. All participants were aged between 17 and 25 years of age; had a diagnosis of a severe mental illness (first episode psychosis, schizophrenia, schizophreniform disorder, bipolar disorder, or major depressive disorder); neurocognitive or social cognitive deficits; were able to provide consent (and parent/guardian if required); and had reasonable English skills. Participants were excluded if they had a developmental delay (IQ <75); current substance abuse or substance dependence other than caffeine or nicotine; a history of head injury (> 10 min unconsciousness); or had been treated with electroconvulsive therapy in the last 6 months.
Participants were randomised between two arms on a 1:1 ratio. Participants in the treatment arm were provided with a combination of Cognitive Remediation Therapy [using Neuropsychological Educational Approach to Remediation or NEAR (17)] and social cognitive remediation (using Social Cognition and Interaction Therapy or SCIT (18). This was compared to a control arm of CRT (NEAR) + the additional treatment available at the service where the treatment was provided.
NEAR (17) is a manualised CRT designed to address cognitive deficits by utilising commercially available educational software to create a rich learning environment that is intrinsically motivating and rewarding. The treatment was provided over 10 weeks, two times per week to participants in groups averaging four people. All participants received NEAR.
SCIT (18) is a manualised treatment designed to address social cognitive deficits. It consisted of 20 1-h sessions over 10 weeks. Training was run in small groups of three to six people using a manual-driven suite of activities. The training approach of SCIT is such that participants receive repeated exposure and practise of the skills that underlie complex mental-state reasoning abilities. The CRT-only group had a range of additional active comparator treatments including physical exercise, social skills groups, individual therapy, or no additional treatment. Both arms received the same duration of treatment-−20 weeks of twice-weekly treatment. All therapists were trained and supervised for the duration of the study, however adherence to the manualised treatments was not formally audited.
Participants were randomised in blocks of four participants from a central register that was operated by administrative staff independent to the services involved. The randomisation sequence was stratified by site and was generated using an online randomisation generator (Sealed Envelope https://www.sealedenvelope.com/simple-randomiser/v1/lists) by a statistician affiliated with the University of Sydney and independent to the research team. Allocation was blind to the research psychologist performing all cognitive and clinical assessments. Allocation was revealed to the treating clinical team by the administrative staff after consent had been obtained and baseline measures taken.
Participants were assessed at baseline, at the completion of treatment and 3 months following the completion of treatment, on a broad range of clinical, cognitive, and functional measures by a psychologist blind to allocation. Initial demographic and clinical details was collected using a semi-structured interview that detailed age, duration of illness, age of onset of illness, treatment history, medication dose, years of education, past employment history, relationship history. Clinical psychopathology was rated using the Positive and Negative Syndrome Scale-−6 items (PANSS-6) (19), the Calgary Depression Scale for Schizophrenia (CDSS) (20) and the Depression Anxiety and Stress Scale (DASS-21) (21).
Neuropsychological Function was assessed using a battery of neuropsychological measures to assess aspects of attention, concentration, vigilance, verbal learning, executive functioning, planning and premorbid intelligence including The two-subtest versions of the Wechsler Abbreviated Scale of Intelligence (WASI) was used to provide an estimate of IQ (22). The two subtests include Vocabulary and Matrix Reasoning. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) assesses five indexes of neurocognition such as immediate memory, delayed memory, attention, construction visuospatial and language (23). Information processing speed and attentional control were assessed with the Trail-Making Test Part A and B. Social cognition was assessed using the Hinting Task (24) as a measure of Theory of Mind (ToM), the Penn Emotion Recognition Test (ER40) (25) as a test of emotion recognition; and the Ambiguous Intentions Hostility Questionnaire (AIHQ-A) as a test of attributional style (26).
Community functioning was assessed using the Social and Occupational Functioning Assessment Scale (SOFAS), which is an interviewer rated scale based on the evaluation of the participants' social and occupational functioning (27) and via the Activity and Participation Questionnaire (APQ) (28). The Assessment of Quality of Life (AQoL-8D) was used to measure subjective satisfaction and well-being (29).
Statistical Analysis
Participants were assessed on the battery of neurocognitive and functional measures at baseline, post-treatment, and follow-up at 3 months after the treatment. Repeated measures analysis of variance (ANOVA) was used to assess any potential interactions across time and between treatment groups. A chi-squared analysis was conducted to compare the demographics between the treatment and control groups inclusive of the diagnosed SMI and medication. Statistical analyses were conducted using SPSS Statistical Packaging for the Social Sciences (30).
Results
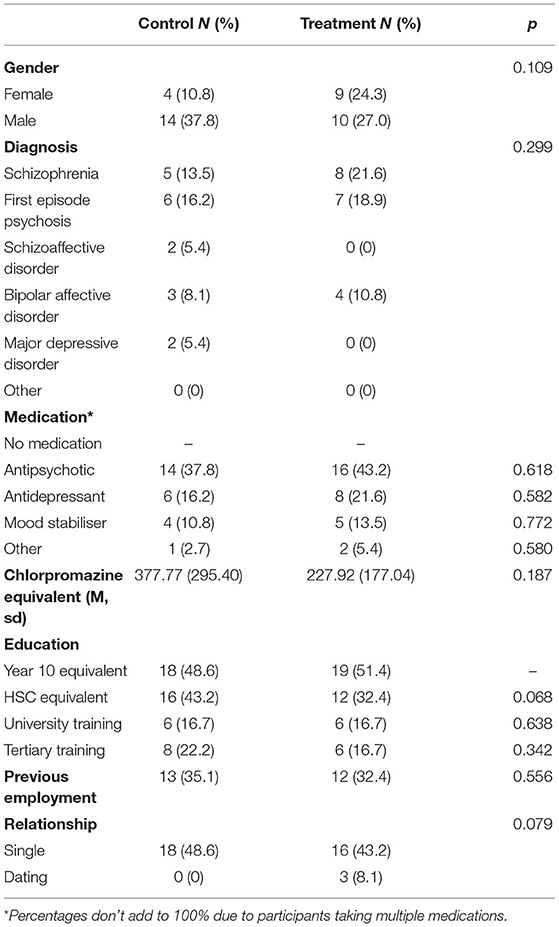
A total of 39 participants were randomised between the two arms of the study (see Figure 1). The study group had an average age of 21.7 yrs (sd 3.0 yrs) and a duration of illness of 3.1 yrs (sd 2.5 yrs). Sixty five percentage of the group were male. There were no significant differences between treatment and control groups based on gender, diagnosis of SMI, medication, education, previous employment, and relationship status (see Table 1). It should be noted that percentages for medication does not add up to 100% due to some participants taking multiple medications, which is clinically common. Drop out through the trial was high with only 49% of participants completing the 3-month follow-up. There was no difference in participant attrition between the arms.
The neurocognitive and functional outcomes at baseline, post-treatment and follow-up for the treatment and control groups are summarised in Table 2. Repeated measures ANOVA indicated that there was a significant main effect of time from baseline to post-treatment on the hinting task (F = 8.880, df = 2, p < 0.001). Pairwise comparisons adjusted for multiple comparisons revealed that this effect was only significant from baseline to follow-up (p = 0.005), and was not significant when measuring from baseline to post-treatment (p = 0.073), or post-treatment to follow-up (p = 0.160). There was no significant difference between groups and no interaction effect. Similarly, there was a significant main effect of time for the SOFAS measure from baseline to post-treatment (F = 5.500, df = 2, p = 0.009), as can be seen from Figure 2. Again, pairwise comparisons showed that this effect was only significant when comparing baseline to follow-up (p = 0.010), and was not significant when comparing baseline to post-treatment (p = 0.480) or post-treatment to follow-up (p = 0.183), and there was no significance between groups and no interaction effect. There were no significant differences between groups or over time for any other neurocognitive, clinical, or functional measures (all p > 0.05).
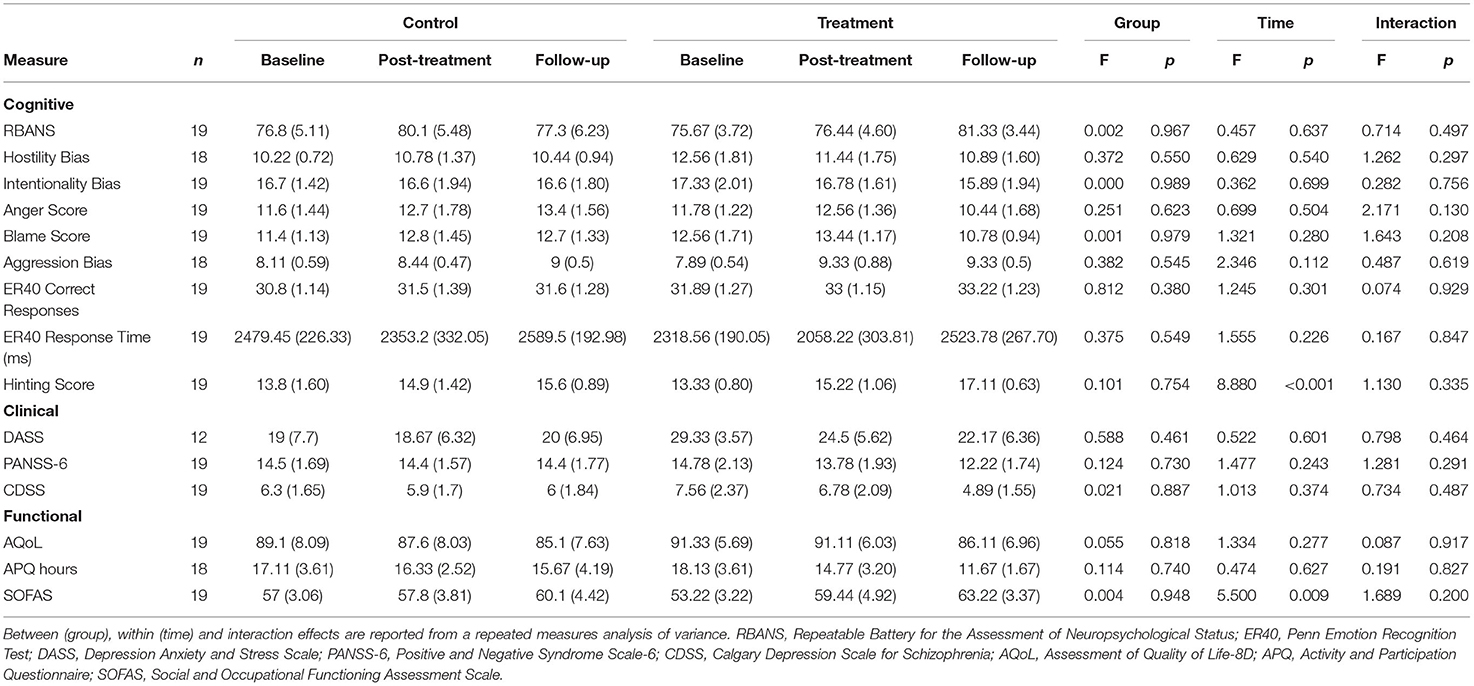
Table 2. Results: Neurocognitive and social functioning scales means and standard errors at baseline, post-treatment and follow-up for the control and treatment arms.
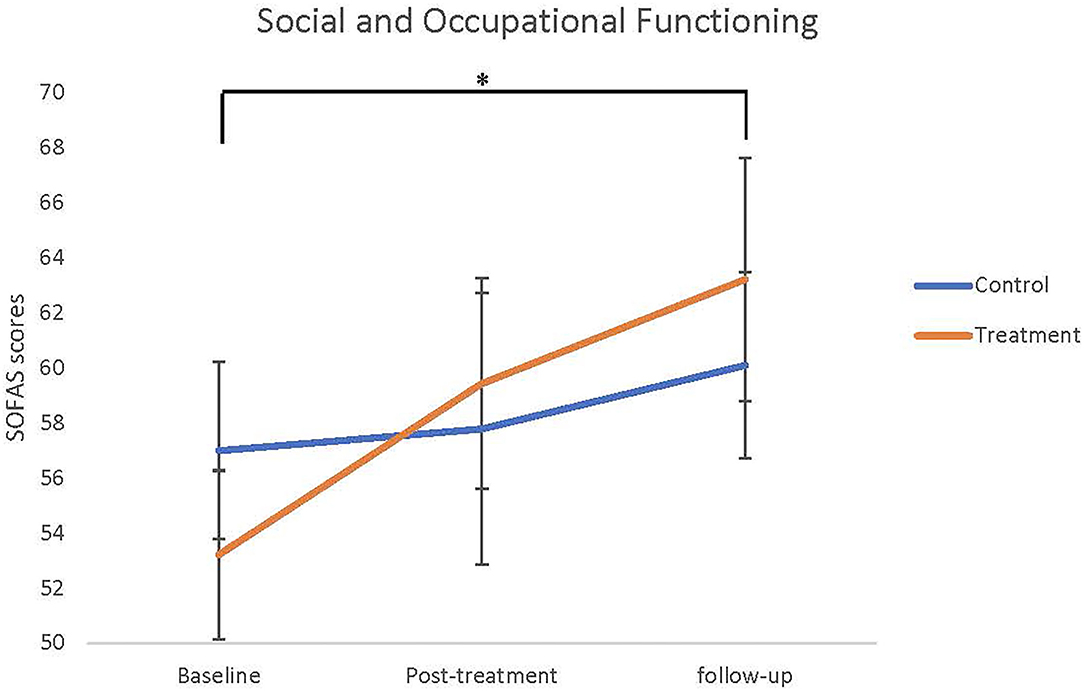
Figure 2. Social and Occupational Functioning Assessment Scale (SOFAS) scores means (SEMs) as baseline, post-treatment, and follow-up. Asterisk indicated significant main effect of time from baseline to follow-up, but there were no differences between groups and interaction.
Discussion
This study observed a positive effect on functioning in young people whether they received NEAR alone or NEAR and SCIT together. There was a weak trend suggesting that the combination of NEAR and SCIT improved functioning at 3 months follow-up. This finding is consistent with previous studies that found an improvement in functioning with exposure to both CRT and SCRT (16, 31, 32), with the suggestion that a broader based approach to remediation is more likely to lead to improvements in community functioning (9). The young people who persisted in therapy in the Advantage treatment group had a change of 10 SOFAS points as against 3 points for active control group over the nearly 9 months. This is a clinically significant improvement (33). Other studies have had mixed results with some finding a greater propensity to change in younger participants, such as ours compared to older, more chronically unwell people (34) or noting little (35) or no change in community functioning (36).
In contrast to our expectations, there were no significant differences in neurocognition or social cognition between groups or across time in either treatment arm. A possible explanation is that the RBANS was not sensitive to change in this group of young people that were less chronically unwell and relatively better educated. However, the group scores indicated a moderate level of neurocognitive impairment and ceiling effects are not a reason for the lack of change (37). The lack of change in social cognitive scores was also surprising. The lack of significant improvement in cognition accompanying an improvement in function was noted by Revell in her meta-analysis of cognitive remediation in early schizophrenia which found that higher quality blinded studies did not observe as much change as non-blinded studies (38). In our study the rater was blind to assignment. Another possibility is that young people may be less able to benefit from intensive training on computer assisted cognitive remediation compared to older and more chronically ill participants as they have more recently been exposed to teaching and training. Our sample was young and comparatively well-educated. We also note that the diagnostic heterogeneity of our sample with several subjects with affective disorders may have lessened the effect size of the cognitive improvement.
Participant attrition was high. Our participants were involved in two sequential treatment programs of 10 weeks duration followed by a further 3-month follow-up, totalling a commitment of nearly 9 months with the breaks required for testing. The biggest dropout rate was during the second phase of the study, when participants received either SCIT or TAU and after during follow-up. This was consistent across sites and suggests treatment fatigue among the young people coming for treatment. We note that high rates of participant attrition is not uncommon amongst long-term interventional studies. For example, Fisher and colleagues' (16) attrition loss of 45% of their participants over 33 weeks training was despite compensation for their attendance. In a randomised controlled trial of cognitive enhancement therapy in young people, Wojtalik et al. lost 52% of their sample over 18 months of treatment (35). This is in accordance with our own experience and that of the literature, that treatment programs limited to 12–16 weeks have a lower attrition rate. For example, Vidarsdottir et al. (36) combined NEAR, SCIT and additional compensatory cognitive training into a more intense 12 week program that in content was similar to our program and in a very similar group of young people. Their participant attrition rate was only 12%.
Although our study was not conclusive, it does support the importance of addressing social cognitive deficits as well as neurocognitive deficits in people with severe mental illness to improve community outcomes. Recovery in people with severe mental illness like schizophrenia has not improved despite the availability of new medications and psychotherapies (39). Recent large studies have underlined the contribution that social and neurocognition make to community function and the complex interaction at play with psychopathology—both negative symptoms and positive symptoms (40–42). The use of targeted cognitive remediation strategies early in the course of illness may help improve the long-term outcome of people with severe mental illness. Further work is required to explore the dynamics of how this is achieved.
Our study has several limitations. It was curtailed by the start of the global Covid-19 pandemic bringing recruitment to an end and is underpowered. The control arm consisted of a treatment-as-usual intervention that was specific to each site, as such there is variability across the sites for those in the control group. A standardised active control condition would have controlled for any potential confounding factors due to site-specific treatment-as-usual therapies. Although all therapists were trained using the standard manuals for the treatments, and continued supervision provided, therapy sessions were not recorded and monitored for treatment fidelity. Our study identified changes in function using the SOFAS which is a crude measure of function in the community. Further work would benefit from the use of a more reliable and valid measure such as the UCSD Performance-based Skills Assessment which has been noted to have a better correlation with cognitive performance (43). Nonetheless, the study had strengths in that it was a multi-site investigation, run in standard youth mental health teams, supporting the clinical utility of the interventions. All assessments were blinded to treatment allocation. Participants represented a group of subjects under-represented in the literature, which has concentrated on older, more chronically unwell subjects. In addition, participants were followed up enabling us to observe if changes were maintained after the end of treatment.
In conclusion, this study suggests that there are advantages for community functioning in combining neurocognitive with social cognitive remediation therapy. The provision of treatment would be assisted by a more concentrated and intense treatment program that delivered the therapy over a shorter period. The availability of computer assisted cognitive remediation is now being enhanced by the development of similar programs targeting social cognition. We look forward to combining these programs and investigating the role of additional psychosocial interventions that are known to synergise the effects of CRT (8) to improve the outcomes for young people with SMI.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Western Sydney Local Health District Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AH, CD, JW, and MK designed the proposal, recruited the participants, and contributed to the interpretation and writing of the paper. CM contributed to the analysis, interpretation, and writing of the paper. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by a Western Sydney Local Health District Research & Education Network Research Grant and by a Health Education and Training Institute Mental Health Research Award to JW. The funding sources had no input into any aspect of the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge Ms. Emily McAulay for her assistance with recruitment and treatment.
References
1. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. (1998) 12:426–45. doi: 10.1037/0894-4105.12.3.426
2. Mesholam-Gately R, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. (2009) 23:315–36. doi: 10.1037/a0014708
3. Bora E, Yucel M, Pantelis C. Theory of mindX impairment in schizophrenia: meta-analysis. Schizophr Res. (2009) 109:1–9. doi: 10.1016/j.schres.2008.12.020
4. Cotter J, Granger K, Backx R, Hobbs M, Looi CY, Barnett JH. Social cognitive dysfunction as a clinical marker: a systematic review of meta-analyses across 30 clinical conditions. Neurosci Biobehav Rev. (2018) 84:92–9. doi: 10.1016/j.neubiorev.2017.11.014
5. Green MF. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J Clin Psychiatry. (2016) 77(Suppl. 2):8–11. doi: 10.4088/JCP.14074su1c.02
6. Halverson TF, Orleans-Pobee M, Merritt C, Sheeran P, Fett A-K, Penn DL. Pathways to functional outcomes in schizophrenia spectrum disorders: meta-analysis of social cognitive and neurocognitive predictors. Neurosci Biobehav Rev. (2019) 105:212–9. doi: 10.1016/j.neubiorev.2019.07.020
7. Nielsen RE, Levander S, Kjaersdam Telléus G, Jensen SO, Østergaard Christensen T, Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia–a meta-analysis of randomized clinical trials. Acta Psychiatr Scand. (2015) 131:185–96. doi: 10.1111/acps.12374
8. Vita A, Barlati S, Ceraso A, Nibbio G, Ariu C, Deste G, et al. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. (2021) 78:848–58. doi: 10.1001/jamapsychiatry.2021.0620
9. Roelofs RL, Wingbermühle E, Egger JIM, Kessels RPC. Social cognitive interventions in neuropsychiatric patients: a meta-analysis. Brain Impair. (2017) 18:138–73. doi: 10.1017/BrImp.2016.31
10. van Duin D, de Winter L, Oud M, Kroon H, Veling W, van Weeghel J. The effect of rehabilitation combined with cognitive remediation on functioning in persons with severe mental illness: systematic review and meta-analysis. Psychol Med. (2019) 49:1414–25. doi: 10.1017/S003329171800418X
11. Fett A-KJ, Viechtbauer W, Dominguez M-d-G, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. (2011) 35:573–88. doi: 10.1016/j.neubiorev.2010.07.001
12. Gard DE, Fisher M, Garrett C, Genevsky A, Vinogradov S. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophr Res. (2009) 115:74–81. doi: 10.1016/j.schres.2009.08.015
13. Horan WP, Kern RS, Tripp C, Hellemann G, Wynn JK, Bell M, et al. Efficacy and specificity of Social Cognitive Skills Training for outpatients with psychotic disorders. J Psychiatric Res. (2011) 45:1113–22. doi: 10.1016/j.jpsychires.2011.01.015
14. Lindenmayer J-P, McGurk SR, Khan A, Kaushik S, Thanju A, Hoffman L, et al. Improving social cognition in schizophrenia: a pilot intervention combining computerized social cognition training with cognitive remediation. Schizophr Bull. (2013) 39:507–17. doi: 10.1093/schbul/sbs120
15. Lindenmayer J-P, Khan A, McGurk SR, Kulsa MKC, Ljuri I, Ozog V, et al. Does social cognition training augment response to computer-assisted cognitive remediation for schizophrenia? Schizophr Res. (2018) 201:180–6. doi: 10.1016/j.schres.2018.06.012
16. Fisher M, Nahum M, Howard E, Rowlands A, Brandrett B, Kermott A, et al. Supplementing intensive targeted computerized cognitive training with social cognitive exercises for people with schizophrenia: an interim report. Psychiatr Rehabil J. (2017) 40:21–32. doi: 10.1037/prj0000244
17. Medalia A, Revheim N, Herlands T. Cognitive Remediation for Psychological Disorders: Therapist Guide. New York, NY: Oxford University Press (2009). p. 1–176.
18. Combs DR, Adams SD, Penn DL, Roberts D, Tiegreen J, Stem P. Social Cognition and Interaction Training (SCIT) for inpatients with schizophrenia spectrum disorders: preliminary findings. Schizophr Res. (2007) 91:112–6. doi: 10.1016/j.schres.2006.12.010
19. Østergaard SD, Lemming OM, Mors O, Correll CU, Bech P. PANSS-6: a brief rating scale for the measurement of severity in schizophrenia. Acta Psychiatr Scand. (2016) 133:436–44. doi: 10.1111/acps.12526
20. Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry. (1993) 163 (Suppl. 22):39–44. doi: 10.1192/S0007125000292581
21. Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. Sydney, NSW: Psychology Foundation (1995).
23. Randolph C. RBANS Update : Repeatable Battery for the Assessment of Neuropsychological Status. Repeatable Battery for the Assessment of Neuropsychological Status. Bloomington, MN: NCS Pearson (2012).
24. Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res. (1995) 17:5–13. doi: 10.1016/0920-9964(95)00024-G
25. Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. (2003) 160:1768–74. doi: 10.1176/appi.ajp.160.10.1768
26. Combs DR, Penn DL, Wicher M, Waldheter E. The Ambiguous Intentions Hostility Questionnaire (AIHQ): a new measure for evaluating hostile social-cognitive biases in paranoia. Cogn Neuropsychiatry. (2007) 12:128–43. doi: 10.1080/13546800600787854
27. Rybarczyk B. Social and Occupational Functioning Assessment Scale (SOFAS). In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York (2011). p. 2313.
28. Stewart G, Sara G, Harris M, Waghorn G, Hall A, Sivarajasingam S, et al. A brief measure of vocational activity and community participation: development and reliability of the Activity and Participation Questionnaire. Australian N Zeal J Psychiatry. (2010) 44:258–66. doi: 10.3109/00048670903487175
29. Richardson J, Iezzi A, Khan MA, Maxwell A. Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. Patient. (2014) 7:85–96. doi: 10.1007/s40271-013-0036-x
31. Fernandez-Gonzalo S, Turon M, Jodar M, Pousa E, Hernandez Rambla C, García R, et al. A new computerized cognitive and social cognition training specifically designed for patients with schizophrenia/schizoaffective disorder in early stages of illness: a pilot study. Psychiatry Res. (2015) 228:501–9. doi: 10.1016/j.psychres.2015.06.007
32. Peña J, Ibarretxe-Bilbao N, Sánchez P, Iriarte MB, Elizagarate E, Garay MA, et al. Combining social cognitive treatment, cognitive remediation, and functional skills training in schizophrenia: a randomized controlled trial. NPJ Schizophr. (2016) 2:16037. doi: 10.1038/npjschz.2016.37
33. Samara MT, Engel RR, Millier A, Kandenwein J, Toumi M, Leucht S. Equipercentile linking of scales measuring functioning and symptoms: examining the GAF, SOFAS, CGI-S, and PANSS. Eur Neuropsychopharmacol. (2014) 24:1767–72. doi: 10.1016/j.euroneuro.2014.08.009
34. Deste G, Barlati S, Galluzzo A, Corsini P, Valsecchi P, Turrina C, et al. Effectiveness of cognitive remediation in early versus chronic schizophrenia: a preliminary report. Front Psychiatry. (2019) 10:236. doi: 10.3389/fpsyt.2019.00236
35. Wojtalik JA, Mesholam-Gately RI, Hogarty SS, Greenwald DP, Litschge MY, Sandoval LR, et al. Confirmatory efficacy of cognitive enhancement therapy for early schizophrenia: results from a multisite randomized trial. Psychiatr Serv. (2021) 0:appi.ps.202000552. doi: 10.1176/appi.ps.202000552
36. Vidarsdottir OG, Roberts DL, Twamley EW, Gudmundsdottir B, Sigurdsson E, Magnusdottir BB. Integrative cognitive remediation for early psychosis: results from a randomized controlled trial. Psychiatry Research. (2019) 273:690–8. doi: 10.1016/j.psychres.2019.02.007
37. Loughland CM, Lewin TJ, Carr VJ, Sheedy J, Harris AW. RBANS neuropsychological profiles within schizophrenia samples recruited from non-clinical settings. Schizophr Res. (2007) 89:232–42. doi: 10.1016/j.schres.2006.08.022
38. Revell ER, Neill JC, Harte M, Khan Z, Drake RJ. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. (2015) 168:213–22. doi: 10.1016/j.schres.2015.08.017
39. Jääskeläinen E, Juola P, Hirvonen N, McGrath JJ, Saha S, Isohanni M, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. (2013) 39:1296–306. doi: 10.1093/schbul/sbs130
40. Mucci A, Galderisi S, Gibertoni D, Rossi A, Rocca P, Bertolino A, et al. Factors associated with real-life functioning in persons with schizophrenia in a 4-year follow-up study of the Italian Network for research on psychoses. JAMA Psychiatry. (2021) 78:550–9. doi: 10.1001/jamapsychiatry.2020.4614
41. Hajdúk M, Penn DL, Harvey PD, Pinkham AE. Social cognition, neurocognition, symptomatology, functional competences and outcomes in people with schizophrenia – a network analysis perspective. J Psychiatr Res. (2021) 144:8–13. doi: 10.1016/j.jpsychires.2021.09.041
42. Galderisi S, Rucci P, Mucci A, Rossi A, Rocca P, Bertolino A, et al. The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry. (2020) 19:81–91. doi: 10.1002/wps.20700
Keywords: cognitive remediation, social cognition, schizophrenia, social cognition rehabilitation, bipolar disorder, youth mental health, outcome, community function
Citation: Harris AWF, Kightley M, Williams J, Ma C and Dodds C (2022) Does Adding Social Cognitive Remediation Therapy to Neurocognitive Remediation Therapy Improve Outcomes in Young People With a Severe Mental Illness?—The Advantage Trial. Front. Psychiatry 12:789628. doi: 10.3389/fpsyt.2021.789628
Received: 05 October 2021; Accepted: 10 December 2021;
Published: 14 March 2022.
Edited by:
Padmavati Ramachandran, Schizophrenia Research Foundation, IndiaReviewed by:
Armida Mucci, University of Campania Luigi Vanvitelli, ItalyYann Quidé, University of New South Wales, Australia
Copyright © 2022 Harris, Kightley, Williams, Ma and Dodds. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony W. F. Harris, YW50aG9ueS5oYXJyaXNAc3lkbmV5LmVkdS5hdQ==
 Anthony W. F. Harris
Anthony W. F. Harris Michelle Kightley3
Michelle Kightley3 Cassandra Ma
Cassandra Ma