- 1Department of Psychiatry, Chaohu Hospital, Anhui Medical University, Hefei, China
- 2Anhui Psychiatric Center, Anhui Medical University, Hefei, China
- 3Department of Psychiatry, Wuxi Mental Health Center, Nanjing Medical University, Wuxi, China
Objectives: Dementia of the Alzheimer's type (DAT) is the most common chronic neurodegenerative disease. At present, the pathogenesis of DAT is not completely clear, and there are no drugs that can cure the disease. Once an individual is diagnosed with DAT, the survival time is only 3 to 9 years. Therefore, there is an urgent need to determine the etiology of DAT and the associated influencing factors to find a breakthrough in the treatment of DAT.
Methods: We studied the relationship between polymorphisms in several genes (including BIN1 and APOE) and DAT susceptibility and the effects of sex differences on DAT. Our study included 137 patients with DAT and 509 healthy controls (HCs).
Results: The APOE rs429358 polymorphism CC and CT genotypes were associated with an increased risk of DAT in women. We found a significant association between APOE ε4 and DAT. The frequency of the ε4 allele in the DAT group (15.5%) was higher than that in the HC group (8.7%). The BIN1 rs7561528 polymorphism was associated with a decreased risk of DAT in men.
Conclusions: APOE gene rs429358 and BIN1 gene 7561528 genes may affect the susceptibility to DAT in a Chinese Han population.
Introduction
Dementia of the Alzheimer's type (DAT) is the most common chronic neurodegenerative disease, affecting 44 million people worldwide, and is the fifth leading cause of death (1). The prevalence of dementia in China reached 9.19 million in 2010, with an average of 6.25 individuals per thousand people suffering from DAT (2). Although the rate of DAT progression varies, once diagnosed, the survival time of a patient with DAT is only 3–9 years (3). DAT is characterized by amyloid deposition in the hippocampus, early brain oxidative stress, and the development of nerve fiber tangles caused by the hyperphosphorylation of tau protein (4). The result of these pathological changes is a series of clinical features, such as learning and memory impairment, progressive aphasia, and decreased executive function (5). Genetic variants have been reported to seriously affect DAT susceptibility, with many genes, including BIN1, APOE, PICALM, and probably many more, being involved (6). Therefore, there is an urgent need to determine the etiology of DAT and the associated influencing factors to find a breakthrough in DAT treatment. Further understanding of DAT genetic susceptibility may be helpful for individual risk assessment and early intervention.
Apolipoprotein E (APOE) is a protein composed of 299 amino acids that is produced by hepatocytes, adipocytes, and other cells. APOE mainly transports lipids in the plasma and nervous system. In addition, APOE is involved in regulating the integrity and plasticity of synaptic proteins (7). APOE plays an important role in many diseases, including neurodegenerative diseases such as depression, dementia with Lewy bodies (DLB), Parkinson's disease (PD), and multiple sclerosis (MS). APOE has been reported to be associated with susceptibility to depression, and the APOE ε2 allele may be an important protective factor for depression (8). APOE participates in and promotes the development of DLB by directly participating in pathological changes in Lewy bodies and the maintenance of lipid homeostasis in the brain (9). In addition, the levels of APOE protein and its receptor in the plasma of patients with PD were found to be significantly increased. APOE ε4 is a risk factor for early-onset and cognitive impairment in PD (10). Finally, in patients with MS, the APOE allele is associated with poor speech learning task performance, persistent attention loss, and cortical impairment (11, 12).
At present, it is generally believed that the APOE gene is involved in the occurrence and development of DAT (13, 14). As a glycoprotein, APOE is a risk factor for DAT by participating in the inflammatory response of the nervous system and maintaining lipid homeostasis in the brain (15). The level of APOE in cerebrospinal fluid has been shown to be positively correlated with the level of phosphorylated tau protein. Therefore, the level of APOE may be a potential biological indicator of DAT (16). Animal experiments and epidemiological studies have confirmed that apolipoprotein E gene polymorphisms are associated with DAT (17, 18). APOE encodes three alleles: APOE ε2, APOE ε3 and APOE ε4. APOE ε2 is generally considered to be a protective factor for DAT, while APOE ε4 is the most high-risk genetic predictive factor known (19, 20). Therefore, we evaluated the impact of rs429258 and rs7412 on the risk of DAT in the Chinese population.
Bridging integrator 1 (BIN1), also known as amphiphysin 2, is located on chromosome 2q14.3. As a member of the BAR family, BIN1 is involved in the regulation of the immune response, presynaptic neurotransmitter release, and the maintenance of calcium homeostasis (21). As one of the most stable genetic risk factors for DAT, BIN1 is overexpressed in patient brains (22). The level of neuronal BIN1 in the brain tissue of patients with DAT has been shown to be significantly lower than that of healthy people. In contrast, the level of ubiquitous BIN1 was shown to be significantly increased, as was the level of BIN1 in peripheral blood (23). This finding shows that different subtypes of BIN1 play a unique role in the occurrence and development of DAT. Rs7561528 is located 25 kB upstream of the BIN1 coding region. It has been reported that it affects a load of tau and the expression of BIN1 mRNA in the brain of patients with DAT (24). This effect is due to the endocytosis of BIN1 protein and its specific binding to tau protein, thereby limiting the extracellular pathological transmission of tau protein (25).
Another site of high BIN1 expression is skeletal muscle. A study found that BIN1 is the pathogenic gene of central nuclear myopathy (CNM). After the mutation of the BIN1 gene, the degradation of actin was shown to increase, and troponin was shown to be destroyed (26). In addition, BIN1 has been shown to aggravate the occurrence of progressive myasthenia through selective ectopic splicing and early specific invagination of T tubules in patients with ankylosis and malnutrition (27). The effect of high expression of BIN1 in the brain is not fully understood.
Therefore, we conducted a case-control study to detect and analyze the correlation between DAT and several polymorphisms in genes, including BIN1 and APOE, in the Chinese Han population.
Materials and Methods
Participants
The case-control study population included 137 patients with DAT (mean age ± SD = 72.77 ± 9.18; 56.20% female). Two trained psychiatrists made the DAT diagnoses. A senior associate professor of psychiatry clinically validated these diagnoses. Healthy controls (HCs) included a total of 509 cognitively healthy elderly individuals from the same geographic area as the patients (mean age ± SD = 65.78 ± 6.27; 51.08% female). Individuals free from any neurological, systemic, or psychiatric diseases and with no family history of neurological diseases were included in the HC group. The DAT group and HC group did not differ in terms of sex distribution, were composed entirely of Han Chinese individuals and included no blood relatives. The Mini-Mental State Examination (MMSE) and Clinical Dementia Rating (CDR) scale were performed for all participants. DAT patients were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), criteria (28). According to the MMSE scale score, the selected participants in the HC group had the following characteristics: illiteracy >17 points, primary school >20 points, junior high school and above >22. The CDR scale score of HCs was 0. People under the age of 50 or with other neurological diseases, cancer, or a family history of autosomal-dominant dementia were not included in the study. People with a history of special drug usages and alcohol or drug dependence within the past 6 months were also excluded from this study. Participants were treated according to the ethical principles of the World Medical Association's Declaration of Helsinki. All participants signed the informed consent form, and the Institutional Ethical Committee approved the study. General demographic data, such as age, sex, education, occupation, and marital status, were collected.
SNP Selection
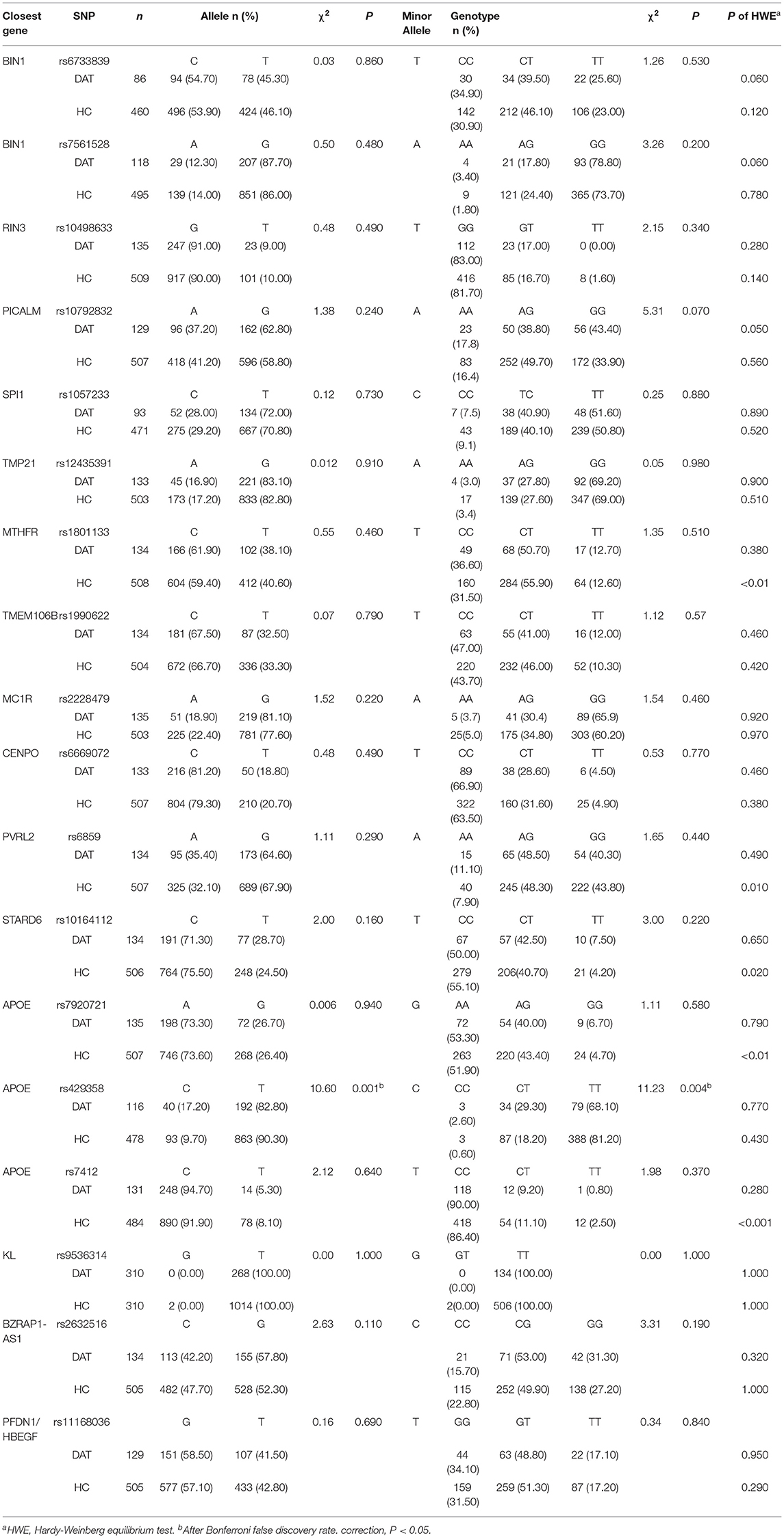
First, we selected SNPs in the public HapMap database. The criteria for selecting SNPs were a minor allele frequency of (MAF) ≥0.05 and r2 ≥0.8 in the Han Chinese population of Beijing (HCB). A total of eighteen SNPs were selected for evaluation in this study: two SNPs of BIN1, one of RIN3, one of PICALM, one SNP of SPI1, one of TMP21, one of MTHFR, one of TMEM106B, one of MC1R, one of CENPO, one of PVRL2, one of KL, one of BZRAP1-AS, one of PFDN1/HBEGF, and three of APOE (Table 2).
Genotyping
All participants were asked to fast for at least 8 h before morning blood collection. Approximately 5 ml of peripheral blood was collected from patients and controls, put into a test tube treated with EDTA and stored at 4°C. DNA was extracted from whole blood using a Tiangen DNA isolation kit (Tiangen Biotechnology, Beijing, China). TaqMan SNP genotyping analysis (Applied Biosystems, Foster City, CA, USA) and an ABI PRISM 7900 sequence detection system (Applied Biosystems) equipped with SDS2.1 software were used to analyze the selected SNPs of the genes. For quality control, researchers blinded to the status of the participant conducted the genotype analysis. Subsequently, 10% of the collected samples were genotyped again, and the results displayed 99.2% concordance. Two independent researchers scored the genotypic data. The deviation from the Hardy-Weinberg equilibrium expectation was evaluated for the entire study population.
Statistical Analysis
A t-test was performed with SPSS 24.0 statistical software (SPSS Inc., Chicago, IL, USA) to evaluate the difference in age and education level between DAT patients and HCs. The Pearson chi-square test was used to determine differences in sex and allele and genotype frequencies between the two groups. Hardy-Weinberg equilibrium, allele frequency, and genotype frequency measurements were performed using SHEsis software (http://analysis.bio-x.cn/myAnalysis.php). Bonferroni's false discovery rate correction was applied. After adjusting for sex, education level, and age as possible influencing factors, SNPStats (https://www.snpstats.net/start.htm) was used to evaluate the association between SNPs and the risk of DAT under five inheritance models, including codominant, dominant, recessive, overdominant, and log-additive models. P <0.05 (two-tailed) was defined as a statistically significant difference.
Results
General Demographic Characteristics
In this study, 646 participants, including 137 patients with DAT and 509 unrelated HC individuals, were enrolled. In the DAT group, which included 78 females and 59 males, the mean age was 72.88 ± 9.11 years, ranging from 50 to 90 years. In the HC group, which included 260 females and 249 males, the mean age was 65.78 ± 6.72 years, ranging from 50 to 90 years. The two groups were not significantly different in terms of the sex distribution (P = 0.223, respectively). The mean education level of DAT patients was 8.46 ± 3.09 years, and the mean education level of the control participants was 10.29 ± 2.73 years. Thus, there was no difference in educational level between the two groups (Table 1).
Allele and Genotype Frequency of the SNPs
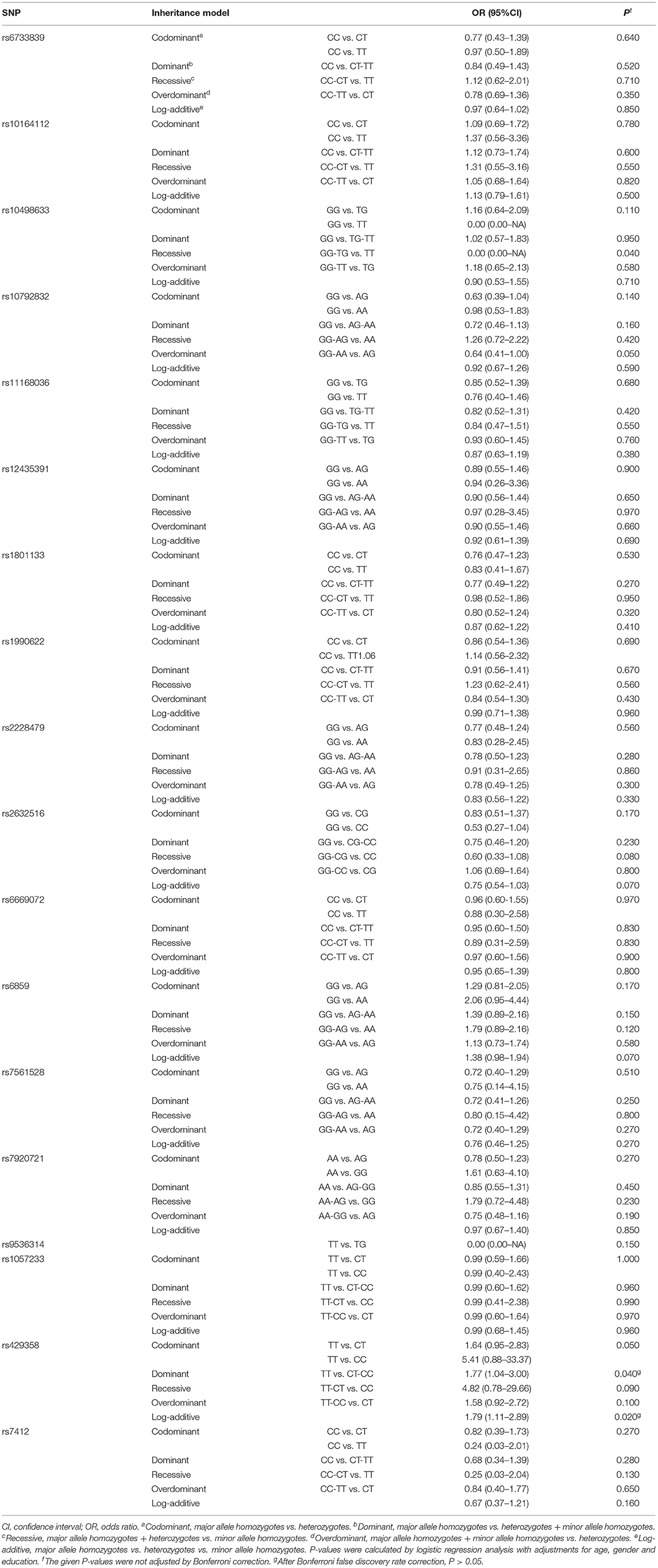
We detected and analyzed 18 DAT-related SNPs, including the BIN1 rs7561528 SNP. The allele frequencies and genotype frequencies of each gene polymorphism are listed in Table 2. The distribution of the APOE rs429358 and BIN1 rs7561528 polymorphisms in the population was in accordance with Hardy-Weinberg equilibrium. There were significant differences in the genotype frequencies (P = 0.001) and allele frequencies (P = 0.004) of rs429538 between the DAT group and the HC group. In the dominant model, after adjusting for age, sex, and education level, it was found that there was a significant difference in the APOE gene rs429538 polymorphism between the DAT group and the HC group (OR = 1.77, 95% CI = 1.04-3.00, P = 0.040). However, after Bonferroni's false discovery rate correction, the difference disappeared (adjusted P > 0.05, Table 3).
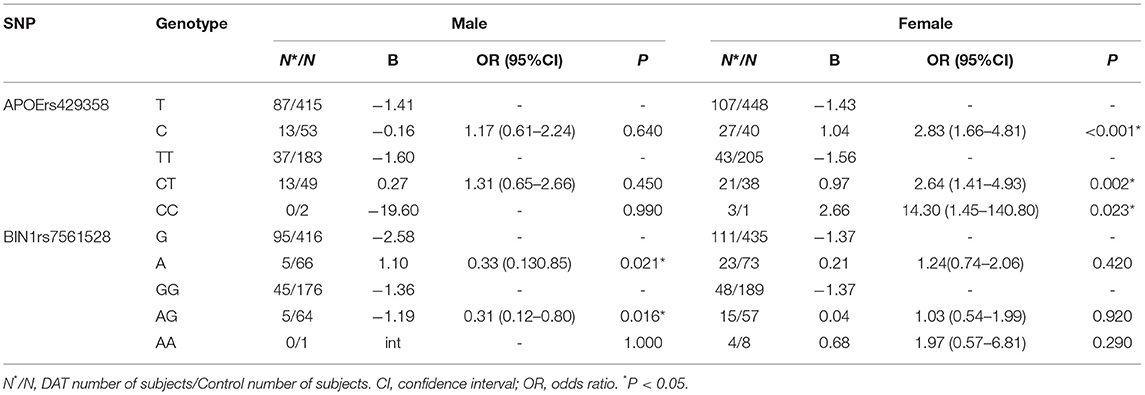
Further stratified analysis was conducted with female and male subgroups, and the results are shown in Table 4. The results showed that the rs429538 C allele was a risk factor for DAT in females (P <0.001, OR = 2.83). The rs429538 CT and CC genotypes increased the risk of DAT by 2.64 and 14.3 times, respectively. The rs429538 polymorphism had no effect on DAT risk in men. The risk of DAT in men carrying the BIN1 rs7561528 A allele was significantly different from that in men carrying the BIN1 rs7561528 G allele (P = 0.021, OR = 0.33). Among men, the AG genotype was a protective factor for DAT (OR = 0.31). There was no significant difference in the risk of DAT among women with the BIN1 rs7561528 polymorphism.
Association Between APOE Genotypes and DAT
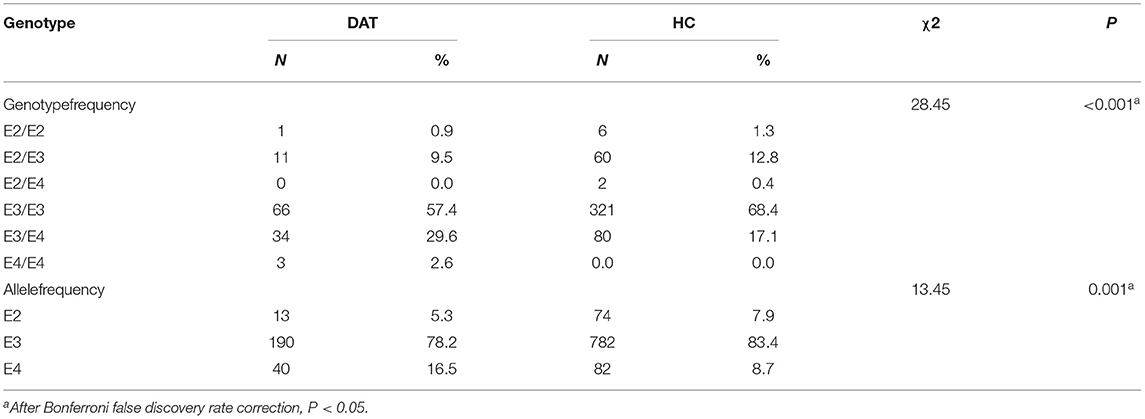
The frequencies of apolipoprotein E genotypes and alleles were also analyzed (P <0.001; P = 0.001). The frequency of the ε4 allele in the DAT group (16.5%) was higher than that in the HC group (8.7%). The ε4 allele increased the risk of DAT by 2.78-fold (see Tables 5, 6).
Discussion
Our study found that, in the dominant model, the APOE gene rs429358 polymorphism increased susceptibility to DAT. However, this difference disappeared after Bonferroni's false discovery rate correction. The rs429358 CC and CT genotypes were associated with an increased risk of DAT in women. The BIN1 rs7561528 polymorphism was associated with a decreased risk of DAT in men. These results suggest that there may be sex differences in the effect of APOE and BIN1 gene variations on DAT susceptibility.
Our results show that the ε4 allele of the APOE gene was overexpressed in DAT patients compared to HCs. Thus, considering ε2 gene carriers as a reference, the presence of the ε4 allele increased the risk of DAT. According to a genome-wide association (GWA) study, the APOE rs429358 polymorphism (APOE ε4 allele) is significantly associated with a reduced risk of longevity (29). In addition, the APOE rs429358 polymorphism is overexpressed in the brains of patients with PD and significantly affects cognitive dysfunction progression (30). Based on the analysis of gene polymorphisms of Hakkas in southern China, it was found that the risk of cerebral infarction in ε4 carriers was significantly higher than that in HCs (31). This finding suggests that ε4 may be a risk factor for longevity, PD, and cerebral infarction. Our research shows a significant increase in the risk of developing DAT among ε4 carriers, especially among women, consistent with the results of previous studies. Women who carry APOE ε4 have a much higher risk of developing DAT than men who carry APOE ε4. If a woman carries two ε4 alleles, her risk of developing DAT is 15 times higher than that of men who have the same genotype (32). Although the role of rs429358 in susceptibility to DAT in women is not fully understood, androgen may be a potential factor contributing to the increase in the prevalence of DAT. Recent studies have found that the decrease in androgen in DAT patients affects the APOE gene, leading to cognitive dysfunction and worsened development of DAT in these patients (33). A physiological dose of testosterone was suggested to be protective against on DAT. Androgen can alleviate the mitochondrial dysfunction caused by amyloid-beta (Aβ) and improve excessive oxidative stress in the brain tissue of patients with DAT (34). In addition, androgen is involved in the regulation of hippocampal formation and hippocampal-dependent cognitive behavior (35). Therefore, the high risk associated with the rs429358 polymorphism CC and CT genotypes may be related to the androgen deficiency in women.
In contrast, 65% to 80% of patients with DAT carry at least one copy of the APOE ε4 allele. Individuals with two APOE ε4 alleles had a 14 to 15-fold increased risk of DAT compared with those with no ε4 alleles (36). Mexicans who carry ε4 have a 13.3 times higher risk of developing dementia than those who are non-carriers (37). The inconsistency of risk results among different studies may be due to different races, environmental differences, and limited sample sizes.
Previous studies have suggested that APOE dysfunction causes disorders in maintaining cholesterol homeostasis in the brain by affecting cholesterol transport in the brain (37). This high-cholesterol state further promotes the formation of Aβ. It is well known that the accumulation of Aβ leads to the deposition of starch plaques in the terminal region of the cerebral cortex, which eventually leads to neurological degeneration and dementia by damaging nerve synapses (38). In addition, the different Aβ protein scavenging and proliferative functions of different subtypes of APOE may result in different risks of DAT among individuals carrying different APOE subtypes (39).
Rs7561528, the most significant SNP of the BIN1 gene, is considered a risk factor for late-onset Alzheimer's disease in the Caucasian population. The results of a large-scale meta-analysis suggest that rs7561528 A-allele carriers with the AG heterozygote genotype are not susceptible to DAT in any genetic inheritance pattern in mixed populations and East Asian populations. The AG heterozygote genotype of DAT is a protective factor for DAT in Caucasians and Asians (40). Unfortunately, this result has not been successfully replicated in the Han population in northern China (41). However, our study confirmed that male individuals with the A allele and AG genotype are not susceptible to DAT in the Chinese Han population.
Based on the analysis of other polymorphisms of the BIN1 gene in the Chinese Han population, rs67327804 and rs744373 were found to be risk factors for DAT (42, 43). These results suggest that mutations in the BIN1 gene are related to DAT in the Chinese Han population. A slight variation in BIN1 may lead to obvious pathological changes in individuals. For example, a BIN1 gene mutation can cause changes in internal olfactory structures and lead to hippocampal atrophy (44). However, variations in the BIN1 are not associated with the Aβ plaques observed in DAT. That is, the effect of BIN1 gene polymorphisms on DAT susceptibility may not be mediated by Aβ (45, 46).
There are several limitations to our study. First, our study had a cross-sectional design; therefore, the sequence of exposures, the timing of outcomes, and the causal relationship between exposures and outcomes were not considered. Second, although our sample size was significantly larger than those of previous reports, the limitations of our sample size, especially in the DAT group, resulted in our conclusions being less accurate or less persuasive. Therefore, these findings must be interpreted with caution. In the future, we will expand our sample size to further refine our conclusions. Third, our study did not take into account the linkage disequilibrium (LD) of SNPs. LD may affect the function of neighboring genes. Therefore, it is necessary to further understand the effect of LDs on samples. Fourth, since DAT is a multifactorial disease, other variables, such as marital, mental, and nutritional status, should be added to clinical data collection. Finally, although the Han people account for more than 90% of China's population, there are 56 ethnic groups in China; therefore, we should expand our research to different ethnic groups in the future.
Conclusion
In conclusion, a variety of genes and their polymorphic genotypes are involved in the pathogenesis of DAT. Our study suggests that the APOE gene and BIN1 play an important role in the pathogenesis of DAT in the Chinese Han population. Sex differences also affect susceptibility to DAT. These results are of great significance for understanding the function and influence of different polymorphic genes in DAT. We believe that these results will help provide new insights into the diagnosis and treatment of DAT.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary files, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Research and Ethics Committee of Wuxi Mental Health Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KZ: conception and design. HL and KZ: administrative support. YZ: provision of study materials or patients. XC, HY, ZW, and GW: collection and assembly of data. YZ and XL: data analysis and interpretation. All authors manuscript writing and final approval of manuscript.
Funding
We thank all of patients who volunteered to participate in the study. This study was supported by the National Natural Science Foundation of China (81801341), the Anhui Provincial Key R&D Programme (202004j07020030), the China International Medical Exchange Foundation (Z-2018-35-2002), and the National Clinical Key Specialty Project Foundation (CN). The funding body did not participate in the design, conduct, or writing of the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to all the patients and their families, and to the general practices that referred patients to our service and collaborated with the study.
References
1. Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. (2018) 25:59–70. doi: 10.1111/ene.13439
2. Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, et al. Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet. (2013) 381:2016–23. doi: 10.1016/s0140-6736(13)60221-4
3. Querfurth HW, Laferla FM. Alzheimer's disease. N Engl J Med. (2010) 362:329–44. doi: 10.1056/nejmra0909142
4. Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. (2018) 14:450–64. doi: 10.1016/j.redox.2017.10.014
5. Qiu Y, Jacobs DM, Messer K, Salmon DP, Feldman HH. Cognitive heterogeneity in probable Alzheimer disease: clinical and neuropathologic features. Neurology. (2019) 93:e778–90. doi: 10.1212/WNL.0000000000007967
6. Rasmussen IJ, Tybjaerg-Hansen A, Rasmussen KL, Nordestgaard BG, Frikke-Schmidt R. Blood-brain barrier transcytosis genes, risk of dementia and stroke: a prospective cohort study of 74,754 individuals. Eur J Epidemiol. (2019) 34:579–90. doi: 10.1007/s10654-019-00498-2
7. Zhao N, Liu CC, Qiao W, Bu G. Apolipoprotein e, receptors, and modulation of Alzheimer's disease. Biol Psychiatry. (2018) 83:347–57. doi: 10.1016/j.biopsych.2017.03.003
8. Feng F, Lu SS, Hu CY, Gong FF, Qian ZZ, Yang HY, et al. Association between apolipoprotein e gene polymorphism and depression. J Clin Neurosci. (2015) 22:1232–8. doi: 10.1016/j.jocn.2015.02.012
9. Zhao N, Attrebi ON, Ren Y, Qiao W, Sonustun B, Martens YA, et al. Apoe4 exacerbates α-synuclein pathology and related toxicity independent of amyloid. Sci Transl Med. (2020) 12:eaay1809. doi: 10.1126/scitranslmed.aay1809
10. Emamzadeh FN. Role of apolipoproteins and alpha-synuclein in Parkinson's disease. J Mol Neurosci. (2017) 62:344–55. doi: 10.1007/s12031-017-0942-9
11. Portaccio E, Goretti B, Zipoli V, Nacmias B, Stromillo ML, Bartolozzi ML, et al. APOE-epsilon4 is not associated with cognitive impairment in relapsing-remitting multiple sclerosis. Mult Scler. (2009) 15:1489–94. doi: 10.1177/1352458509348512
12. Mccomb M, Parambi R, Browne RW, Bodziak ML, Jakimovski D, Bergsland N, et al. Apolipoproteins ai and e are associated with neuroaxonal injury to gray matter in multiple sclerosis. Mult Scler Relat Disord. (2020) 45:102389. doi: 10.1016/j.msard.2020.102389
13. Serrano-Pozo A, Das S, Hyman BT. APOE. and Alzheimer's disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. (2021) 20:68–80. doi: 10.1016/s1474-4422(20)30412-9
14. Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. (2020) 581:71–6. doi: 10.1038/s41586-020-2247-3
15. Rebeck GW. The role of APOE on lipid homeostasis and inflammation in normal brains. J Lipid Res. (2017) 58:1493–9. doi: 10.1194/jlr.r075408
16. Toledo JB, Da X, Weiner MW, Wolk DA, Xie SX, Arnold SE, et al. CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol. (2014) 127:621–32. doi: 10.1007/s00401-013-1236-0
17. Pandey RS, Graham L, Uyar A, Preuss C, Howell GR, Carter GW. Genetic perturbations of disease risk genes in mice capture transcriptomic signatures of late-onset Alzheimer's disease. Mol Neurodegener. (2019) 14:50. doi: 10.1186/s13024-019-0351-3
18. Barrett MJ, Koeppel AF, Flanigan JL, Turner SD, Worrall BB. Investigation of genetic variants associated with Alzheimer disease in Parkinson disease cognition. J Parkinsons Dis. (2016) 6:119–24. doi: 10.3233/jpd-150706
19. Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. (2019) 179:312–39. doi: 10.1016/j.cell.2019.09.001
20. Yin Y, Wang Z. APOE and neurodegenerative diseases in aging. Adv Exp Med Biol. (2018) 1086:77–92. doi: 10.1007/978-981-13-1117-8_5
21. De Rossi P, Nomura T, Andrew RJ, Masse NY, Sampathkumar V, Musial TF, et al. Neuronal BIN1 regulates presynaptic neurotransmitter release and memory consolidation. Cell Rep. (2020) 30:3520–35. doi: 10.1016/j.celrep.2020.02.026
22. Sartori M, Mendes T, Desai S, Lasorsa A, Herledan A, Malmanche N, et al. BIN1 recovers tauopathy-induced long-term memory deficits in mice and interacts with Tau through Thr(348) phosphorylation. Acta Neuropathol. (2019) 138:631–52. doi: 10.1007/s00401-019-02017-9
23. Gao P, Ye L, Cheng H, Li H. The mechanistic role of bridging integrator 1 (BIN1) in Alzheimer's disease. Cell Mol Neurobiol. (2020) 41:1431–40. doi: 10.1007/s10571-020-00926-y
24. Karch CM, Goate AM. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. (2015) 77:43–51. doi: 10.1016/j.biopsych.2014.05.006
25. Calafate S, Flavin W, Verstreken P, Moechars D. Loss of BIN1 promotes the propagation of Tau pathology. Cell Rep. (2016) 17:931–40. doi: 10.1016/j.celrep.2016.09.063
26. Ceyhan-Birsoy O, Agrawal PB, Hidalgo C, Schmitz-Abe K, Dechene ET, Swanson LC, et al. Recessive truncating titin gene, TTN, mutations presenting as centronuclear myopathy. Neurology. (2013) 81:1205–14. doi: 10.1212/wnl.0b013e3182a6ca62
27. Fugier C, Klein AF, Hammer C, Vassilopoulos S, Ivarsson Y, Toussaint A, et al. Misregulated alternative splicing of bin1 is associated with T tubule alterations and muscle weakness in myotonic dystrophy. Nat med. (2011) 17:720–5. doi: 10.1038/nm.2374
28. Battle DE. Diagnostic and statistical manual of mental disorders (DSM). Codas. (2013) 25:191–2. doi: 10.1590/s2317-17822013000200017
29. Deelen J, Evans DS, Arking DE, Tesi N, Nygaard M, Liu X, et al. A meta-analysis of genome-wide association studies identifies multiple longevity genes. Nat Commun. (2019) 10:3669.
30. Tan MMX, Lawton MA, Jabbari E, Reynolds RH, Iwaki H, Blauwendraat C, et al. Genome-wide association studies of cognitive and motor progression in Parkinson's disease. Mov Disord. (2021) 36:424–33. doi: 10.1002/mds.28342
31. Wu H, Huang Q, Yu Z, Wu H, Zhong Z. The SNPs Rs429358 and Rs7412 of APOE gene are association with cerebral infarction but not SNPs Rs2306283 and Rs4149056 of SLCO1B1 gene in southern Chinese Hakka population. Lipids Health Dis. (2020) 19:202. doi: 10.1186/s12944-020-01379-4
32. Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. (2016) 160:134–47. doi: 10.1016/j.jsbmb.2016.03.012
33. Li Y, Li S, Xu S, Yu H, Tang L, Liu X, et al. Association of androgens and gonadotropins with amnestic mild cognitive impairment and probable Alzheimer's disease in Chinese elderly men. J Alzheimers Dis. (2020) 78:277–90. doi: 10.3233/JAD-200233
34. Grimm A, Biliouris EE, Lang UE, Gotz J, Mensah-Nyagan AG, Eckert A. Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-beta or hyperphosphorylated tau protein. Cell Mol Life Sci. (2016) 73:201–15. doi: 10.1007/s00018-015-1988-x
35. Atwi S, Mcmahon D, Scharfman H, Maclusky NJ. Androgen modulation of hippocampal structure and function. Neuroscientist. (2016) 22:46–60. doi: 10.1177/1073858414558065
36. Mahley RW. Apolipoprotein e: remarkable protein sheds light on cardiovascular and neurological diseases. Clin Chem. (2017) 63:14–20. doi: 10.1373/clinchem.2016.255695
37. Alavez-Rubio JS, Martinez-Rodriguez N, Escobedo-De-La-Pena J, Garrido-Acosta O, Juarez-Cedillo T. Relationship between genetic variants of ACAT1 and APOE with the susceptibility to dementia (SADEM study). Mol Neurobiol. (2021) 58:905–12. doi: 10.1007/s12035-020-02162-3
38. Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. (2013) 9:106–18. doi: 10.1038/nrneurol.2012.263
39. Uddin MS, Kabir MT, Al Mamun A, Abdel-Daim MM, Barreto GE, Ashraf GM, et al. APOE and Alzheimer's disease: evidence mounts that targeting APOE4 may combat Alzheimer's pathogenesis. Mol Neurobiol. (2019) 56:2450–65. doi: 10.1007/s12035-018-1237-z
40. Zhou F, Haina D. The bridging integrator 1 gene rs7561528 polymorphism contributes to Alzheimer's disease susceptibility in East Asian and Caucasian populations. Clin Chim Acta. (2017) 469:13–21. doi: 10.1016/j.cca.2017.03.013
41. Tan L, Yu JT, Zhang W, Wu ZC, Zhang Q, Liu QY, et al. Association of GWAS-linked loci with late-onset Alzheimer's disease in a northern han Chinese population. Alzheimers Dement. (2013) 9:546–53. doi: 10.1016/j.jalz.2012.08.007
42. Tan MS, Yu JT, Jiang T, Zhu XC, Guan HS, Tan L. Genetic variation in BIN1 gene and Alzheimer's disease risk in han Chinese individuals. Neurobiol Aging. (2014) 35:1781. doi: 10.1016/j.neurobiolaging.2014.01.151
43. Wang HF, Wan Y, Hao XK, Cao L, Zhu XC, Jiang T, et al. Bridging integrator 1 (BIN1) genotypes mediate Alzheimer's disease risk by altering neuronal degeneration. J Alzheimers Dis. (2016) 52:179–90. doi: 10.3233/jad-150972
44. Zhang X, Yu JT, Li J, Wang C, Tan L, Liu B, et al. Bridging integrator 1 (BIN1) genotype effects on working memory, hippocampal volume, and functional connectivity in young healthy individuals. Neuropsychopharmacology. (2015) 40:1794–803. doi: 10.1038/npp.2015.30
45. Holler CJ, Davis PR, Beckett TL, Platt TL, Webb RL, Head E, et al. Bridging integrator 1 (BIN1) protein expression increases in the Alzheimer's disease brain and correlates with neurofibrillary tangle pathology. J Alzheimers Dis. (2014) 42:1221–7. doi: 10.3233/jad-132450
Keywords: dementia with Alzheimer's type, APOE, BIN1 bridging integrator 1/amphiphysin-2 gene, single nucleotide polymorphism, genetic associations
Citation: Li X, Zhang Y, Chen X, Yuan H, Wang Z, Wang G, Zhang K and Liu H (2021) Association of Gene Polymorphisms in APOE and BIN1 With Dementia of Alzheimer's Type Susceptibility in Chinese Han Population. Front. Psychiatry 12:753909. doi: 10.3389/fpsyt.2021.753909
Received: 05 August 2021; Accepted: 21 September 2021;
Published: 18 October 2021.
Edited by:
Deana Davalos, Colorado State University, United StatesReviewed by:
Liu Sha, First Hospital of Shanxi Medical University, ChinaKuanjun He, Inner Mongolia University for Nationalities, China
Copyright © 2021 Li, Zhang, Chen, Yuan, Wang, Wang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanzhong Liu, aHVhbnpob25nbGl1QGFobXUuZWR1LmNu; Kai Zhang, emhhbmdrYWlAYWhtdS5lZHUuY24=
†These authors share first authorship
 Xiaoyue Li1†
Xiaoyue Li1† Yelei Zhang
Yelei Zhang Kai Zhang
Kai Zhang Huanzhong Liu
Huanzhong Liu