
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Psychiatry, 31 January 2022
Sec. Social Neuroscience
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.752274
This article is part of the Research TopicRising Stars in Social Cognition in PsychiatryView all 7 articles
 Peter Mundy1,2*†
Peter Mundy1,2*† Jenifer Bullen3†
Jenifer Bullen3†Differences in social attention development begin to be apparent in the 6th to 12th month of development in children with Autism Spectrum Disorder (ASD) and theoretically reflect important elements of its neurodevelopmental endophenotype. This paper examines alternative conceptual views of these early social attention symptoms and hypotheses about the mechanisms involved in their development. One model emphasizes mechanism involved in the spontaneous allocation of attention to faces, or social orienting. Alternatively, another model emphasizes mechanisms involved in the coordination of attention with other people, or joint attention, and the socially bi-directional nature of its development. This model raises the possibility that atypical responses of children to the attention or the gaze of a social partner directed toward themselves may be as important in the development of social attention symptoms as differences in the development of social orienting. Another model holds that symptoms of social attention may be important to early development, but may not impact older individuals with ASD. The alterative model is that the social attention symptoms in infancy (social orienting and joint attention), and social cognitive symptoms in childhood and adulthood share common neurodevelopmental substrates. Therefore, differences in early social attention and later social cognition constitute a developmentally continuous axis of symptom presentation in ASD. However, symptoms in older individuals may be best measured with in vivo measures of efficiency of social attention and social cognition in social interactions rather than the accuracy of response on analog tests used in measures with younger children. Finally, a third model suggests that the social attention symptoms may not truly be a symptom of ASD. Rather, they may be best conceptualized as stemming from differences domain general attention and motivation mechanisms. The alternative argued for here that infant social attention symptoms meet all the criteria of a unique dimension of the phenotype of ASD and the bi-directional phenomena involved in social attention cannot be fully explained in terms of domain general aspects of attention development.
Autism Spectrum Disorder (ASD) has a genomic, neurodevelopmental etiology with an early onset that occurs in as many as 1 in 54 people (1–3). Symptoms include significant differences in the development of social and communication behaviors, as well as restricted or repetitive patterns of behavior and interests and differences in sensory responses (4). However, the behavioral symptoms used for the diagnosis of ASD change over age, which significantly complicates the diagnosis and nosology of ASD (5). Moreover, about 30% of individuals with ASD are comorbid for intellectual disability (IQ < 75) and/or minimal verbal development (2), but 70% display low average to very advanced verbal and intellectual abilities (6). Accordingly, ASD is a behaviorally defined syndrome, but the diagnosis, treatment, and study of ASD is complicated by the considerable heterogeneity in its behavioral expression (7). The heterogeneity of ASD creates challenges for the study of its central biological and psychological mechanisms. Nevertheless, the detailed study of symptoms can provide a critical source of information about the psychological and bio-behavioral mechanisms of a neurodevelopmental syndrome (8). In this review, we examine the value of research on early social attention symptoms for providing information that is essential to understanding the nature, diagnosis, and treatment of ASD.
Conceptually, early social attention involves at least two types of phenomena (9, 10). One involves the tendency of infants prioritizing orienting to other people and biologically relevant stimuli. A second type involves the impact of another person on the attention of the child. Distinct methodological paradigms guide research on these two types of early social attention. The study of prioritizing attention to other people employs the social orienting paradigm (Figure 1). This paradigm assesses bias for allocating attention to faces, eyes, and the sounds people make, as well as dot display representations of biological motion [e.g., (14–17)]. Studies of the social attention responses to the presence of others involves the joint attention paradigm (Figure 2). The latter assesses responses to gaze shifts and direction of attention of another person. It also assesses behaviors involving monitoring and leading the gaze and attention of other people to initiate social attention coordination [e.g., (22–25)]. In this paradigm, gaze following behaviors are referred to as responding to joint attention (RJA) and gaze leading as initiating joint attention [IJA, (26)].
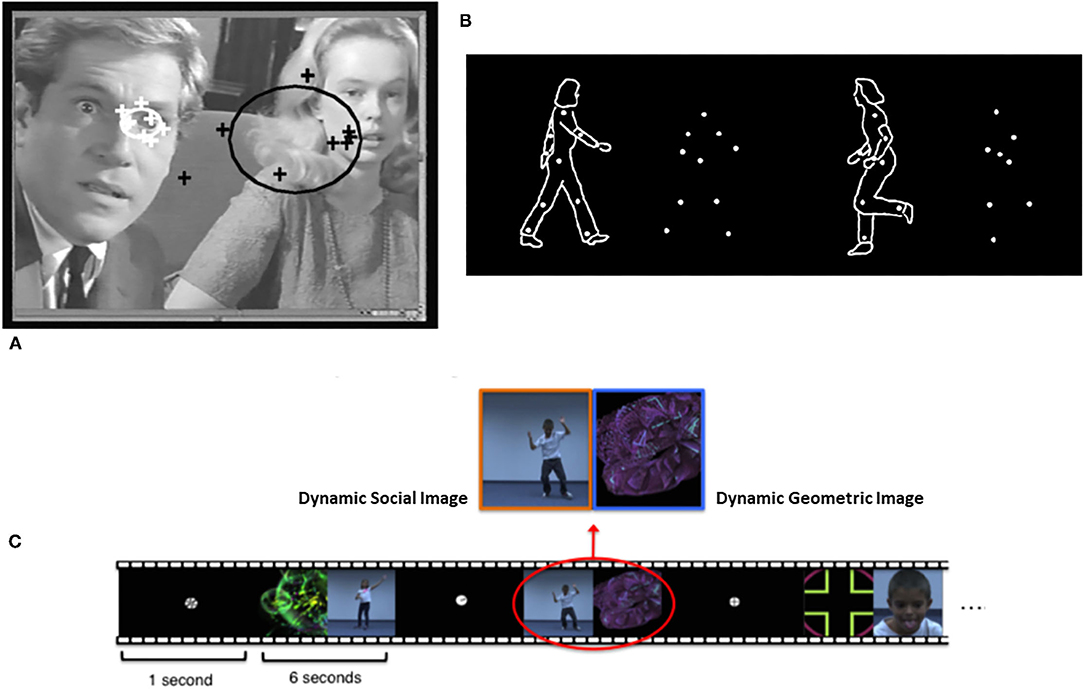
Figure 1. Illustrations of (A) eye-tracking, (B) biological motion, and (C) paired-preference measures of social-orienting from Klin et al. (11), Pierce et al. (12), and Puce and Perrett (13), respectively. Figures reprinted with permissions from, a) Nature, Springer Nature, b) Philosophical Transactions. Biological Sciences, Royal Society, and c) Biological Psychiatry, Science Direct, Elsevier.
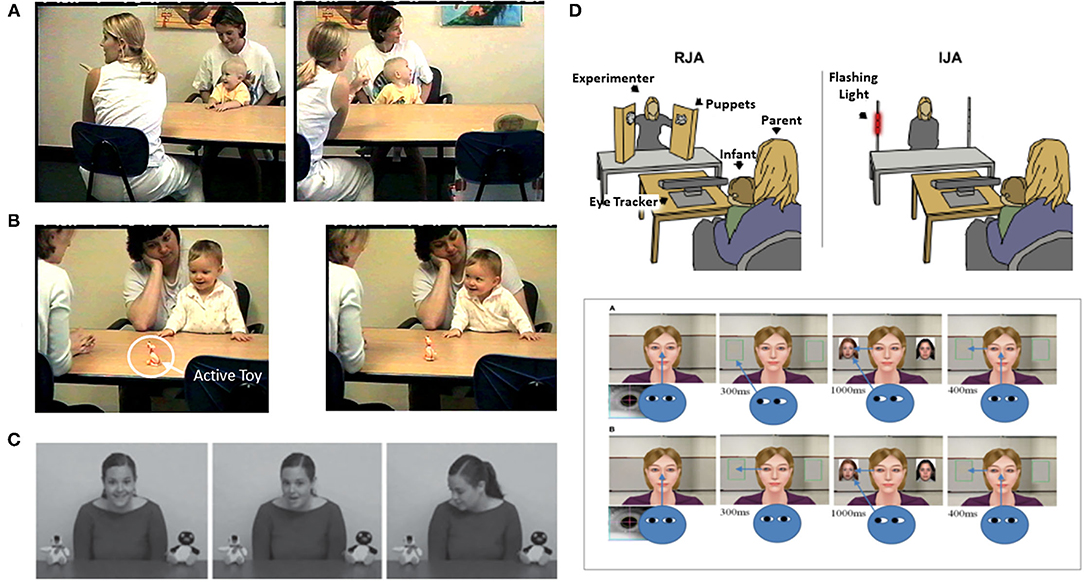
Figure 2. Illustrations of measures of IJA or Initiating Joint Attention/gaze leading [(B,D,E) upper panels] and RJA or Responding to Joint Attention/Gaze following [(A,C,E) lower panel] from Gredebäck et al. (18), Kim and Mundy (19), Mundy (20), and Nyström et al. (21). Figures reprinted with permissions from, a,b) Child Development, John Wiley, c) Developmental Science, John Wiley, d) Biological Psychiatry, Science Direct, Elsevier, and e) Frontiers in Human Neuroscience, Frontiers.
These two types of social attention are distinguished by several characteristics. Social orienting is most often measured in analog social paradigms employing eye tracking to people's faces in videos or pictures. Initiating joint attention on the other hand requires a responsive social partner so it is always measured in real time social interactions, either with a person or a contingently responsive avatar in a virtual reality paradigm (Figure 2). Responding to joint attention is measured either in vivo social interactions or analog paradigms with videos or pictures of gaze shifts (Figure 2). A more fundamental distinguishing feature of joint attention is that it involves monitoring one's own attention, the attention of a social partner, and the common object of attention. Thus, joint attention involves spatial and triadic attention that is fundamental to referential cognition (27). Alternatively, social orienting involves dyadic social attention, but not necessarily triadic or referential information processing.
Research has long indicated that preschool and early elementary school-aged children with ASD display significant differences in both types of social attention compared to peers with typical development, as well as those with other neurodevelopmental conditions [e.g., (15, 24, 28–30)]. Since both types of social attention develop in the first year of life, these early studies suggested that social attention was disrupted in the first postnatal year of development of ASD. This was confirmed subsequently with the advent of the infant-siblings research paradigm (31). Infant siblings of children diagnosed with ASD exhibit a recurrence rate estimated to be 18–19% (32). Therefore, large collaborative longitudinal studies of infant siblings can provide information on the first months of development of infants who go on to receive the diagnosis of ASD. This type of research now indicates that the onset of social attention symptoms is likely no later than the 6th to 12th month period of infant development [e.g., (12, 23, 33–35)] and social attention symptoms currently constitute a significant part of the evidence-based diagnostic and screening instruments used with preschool and early elementary school-aged children (36–38).
Their early diagnostic value notwithstanding, the early emergence of social attention symptoms also likely reflects primary if not congenital neurodevelopmental mechanisms of the complex endophenotype of ASD (9, 16, 39–42). Moreover, early social attention symptoms are thought to be associated with significant differences in early social-information processing that contribute to the developmental perturbations in learning, social communication, and cognition that are characteristic of older autistic children (16, 39, 43–45). This “developmental cascade hypothesis” of social attention symptoms has motivated researchers to develop methods to target interventions for joint attention, social orienting, and eye contact in young children (46, 47). Thus, the acquisition of a precise understanding of the psychological and bio-behaviors processes involved in the emergence of early social attention is a goal of autism science with both implications for basic and clinical science.
There are, of course, alternative models of development that impact not only research approaches and methods, but also the recognition of the merit of social attention as an essential construct in autism research. First, it has not been clear whether social orienting and joint attention paradigms assess components of a unified social attention construct or developmentally distinct phenomenon (15, 28, 44). Second, it is not clear whether social attention symptoms are a characteristic of ASD that are limited to infancy and not necessarily prominent in the childhood and adult presentations of ASD (5). Third, there is a debate about whether or not social attention is a unique and domain specific dimension of development, or one better conceptualized in terms of “more basic” domain general cognitive and attention processes (9, 10). These alternative viewpoints raise important questions for research. However, their lack of resolution may also inhibit exploration of the potential power of a more unified approach to research on social attention in ASD.
A primary goal of this review is to examine research that addresses these issues in order to advance theory and the study of the psychological, bio-behavioral development of the social attention symptoms of ASD. In this regard this review will adopt an interpersonal perspective on social-cognitive neuroscience that suggests that the nature of social attention and social interaction are such that the neurocognitive mechanisms involved in their development may build on domain general mechanisms. However, the bidirectional and interpersonal nature of their domain of application (i.e., social communication interaction) leads to the development of domain specific neurocognitive processes in early development that guide social behaviors and social learning across the lifespan [e.g., (20, 48–51)]. This perspective recognizes the value of research employing tasks that measure the frequency, location, and accuracy of attention allocation to analog social targets, However, it maintains that the science of social attention development must also be informed by in vivo social interaction measurement that includes measures of how rapidly and efficiently social attention can be engaged in the dynamic and complex process of interacting in social pairs and groups. It also holds that being the object of attention of others is as fundamental to social attention development as is allocating attention to other people (52, 53).
The review has been organized to address several specific hypotheses about the social attention symptoms in ASD, including, but not limited to the following.
• Developmental differences in social attention constitute a unique diagnostic dimension of ASD and that experimental and clinical social orienting and joint attention measures converge on a common construct of social attention.
• Social orienting and joint attention behaviors and symptoms emerge concurrently rather than sequentially in typical and atypical development.
• Social orienting and joint attention reflect types of atypical gaze processing as much or more than atypical face processing.
• Social motivation factors impact social attention development in ASD and these involve processes associated with responses to the perception of being the object of attention of other people, as well as processes involved in the allocation of attention to people.
• Imaging and genetic studies indicate that social orienting and joint attention symptoms may reflect common neurodevelopmental processes, some of which are associated with social-cognition. Thus, infant social attention and childhood social cognition may constitute a developmentally continuous axis of social symptoms in ASD from infancy through childhood.
• In childhood, symptoms may involve differences in the spontaneous and efficient use of social attention and social cognition, rather than differences in the capacity for, or accuracy of social attention or social cognition.
• Social attention is valid and distinct dimension of human development that is related to but not fully explained by domain general attention and cognitive processes.
A secondary goal of this review is to provide an examination of new findings and hypotheses in the literature on social attention to provide a compendium of information that contributes to the foundation for next generation of research on this significant topic in autism research.
A wealth of evidence has accrued to indicate that that differences in social attention are valid markers of the development of ASD in preschool children [e.g., (15, 24, 28–30, 54, 55)]. Indeed, estimates of the signal detection of characteristics of social attention measures for the identification of ASD in preschool samples are substantial, ranging from 0.92 to 0.82 for sensitivity and 0.92 to 0.81 for specificity (24, 56).
Many of these studies informed the development of evidence-based diagnostic instruments for ASD, such as the Autism Diagnostic Observation Schedule [ADOS-2, (36, 57)], which is a diagnostic instrument used worldwide (58–60). Structured clinician observations on the Social Affect (SA) scale of the ADOS-2 assess the social symptoms of ASD, and Modules 1 and 2 of the ADOS-2 provide measure of the symptoms for preschool children. Five of the ten ADOS-2 SA scale items in these modules involve observations of social attention behaviors (see Table 1), and these appear to constitute a distinct preschool “joint attention” symptom factor within the SA scale (36). Although not specified in the Gotham et al. study, “unusual eye contact” may be considered to be a social orienting item, while the other four SA items involve joint attention (see Table 1).
Recent studies have also confirmed that data from experimental social orienting and joint attention measures display convergent validity, or significant correlations with structured clinical observations of social attention on SA scale of the ADOS-2 in studies of infants (21, 61, 62). The latter two studies also provided evidence of divergent validity such that experimental social attention measures were not related to the non-social Restricted and Repetitive Behavior (RRB) score of the ADOS-2. Four additional studies provide evidence of significant correlations between ADOS-2 SA scores and experimental social attention measures in older children, some of whom were verbally fluent (63–65). Hence, a modest but consistent set of data indicates that experimental and clinical measures converge on a common social attention symptom construct in studies of infant siblings, as well as older children with ASD.
Social attention items also make substantial contributions to other clinical instruments. The Modified Checklist for ASD in Toddlers [M-CHAT-R/F, (37)] is a prominent screening instrument that includes joint attention items, and the Screening Tool for ASD in Two Year-Olds [STAT, (38)] includes a joint attention subscale. The Early Social Cognitive Battery [ESB, (62)], the ASD Observation Scale for Infants [AOSI, (66)], the Joint Attention Observation Scale (67), and the Social Attention and Communication Surveillance Tool (68) all include items to assess social attention. Moreover, a Social-Orienting Continuum and Response Scale score (SOC-RS) may be derived from secondary coding of videos of ADOS-2 administrations (69).
One symptom dimension alone cannot be used as a definitive diagnostic indicator of all the social symptoms of all individuals with ASD, or across all phases of development (5, 70). Nevertheless, social attention currently constitutes a major reliable and valid symptom dimension in early screening and diagnostic assessment of ASD. Moreover, individual differences among younger children with ASD on the social attention measures of diagnostic instruments may have prognostics validity as well. Six studies indicate that joint attention factor score, or scores for individual joint attention items, from the ADOS-2 Modules 1 and 2 correlated concurrently and predictively with individual differences in cognitive, language, and social adaptive outcomes in ASD children and adolescents (71–76). These observations also attest to the construct validity of the joint attention factor with the SA scale of the ADOS 2 used with non-verbal and minimally verbal children.
Paradoxically, neither the Diagnostic and Statistical Manual-5 (4) nor the International Classification of Disease-11 (77) nosologies explicitly refer to social orienting, joint attention, or social attention in their formal descriptions of the social symptoms of ASD. Similarly, social attention (social orienting and joint attention) is not classified as a “construct,” or a “subconstruct” that is a distinctive biological or psychological dimension for research on mental disorders in the United States National Institute of Mental Health Research Diagnostic Criteria (RDoC) matrix. This may be due in no small part to the debate about whether social attention constitutes a unique domain of cognition or is best conceived as an application of basic domain general attention processes and development.
To be sure the RDoC model leans more to the former than the later. It maintains that social attention is part of the construct of Social Communication, which is distinguishable from other cognitive systems, such as perception, cognitive control, memory, or attention because of its domain specific role in the development and guidance of social interaction. The RDoC system holds that “The underlying neural substrates of social communication evolved to support both automatic/reflexive and volitional control, including the motivation and ability to engage in social communication. Receptive aspects may be implicit or explicit, examples include affect recognition, facial recognition and characterization. Productive aspects include eye contact, expressive reciprocation, and gaze following.” (https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/definitions-of-the-rdoc-domains-and-constructs, Nov. 21, 2021).
However, the RDoC primarily classifies joint attention and social orienting as behavioral measures of the subconstructs of Facial Communication and Perception and Understanding of Others. This rather fragmented approach to social attention may not be surprising since it is a relatively new construct in cognitive science (78–80). The RDoC matrix of constructs is largely based on a foundation of information provided by workshops held through 2012. Nevertheless, much of the more recent research reviewed in this paper indicates that social attention meets the criteria of a construct as defined in the RDoC system and that it constitutes a valid diagnostic dimension of ASD. That is to say social attention: (a) can be studied along a span of functioning from normal to abnormal across the lifespan, (b) can be reliably measured, and (c) reflects processes that can be studied across genetic, neurocircuit, behavioral, and/or self-report units of analysis.
Moreover, recent research on social attention provides unique insights regarding the bio-behavioral mechanisms involved in the development of the social symptoms of this syndrome. We have already reviewed research that attests to the reliability and validity of measurement of social attention and social attention symptoms. It is also the case that research over the last 20 years has also made the argument for the fundamental role that social attention, and especially eye gaze perception and its interpretation plays in the development of human cognitive systems (52, 81–83). Finally, research suggests that social attention symptoms, and more specifically disrupted eye gaze perception, is a valid and distinct RDoC construct for the study of social bio-behavioral mechanisms across mental health conditions, including ASD (84). This research will be reviewed in the next sections of the paper, and we will return to the alternative hypothesis that social attention is best conceptualized as part of the development of domain general attention processes in more detail in a last section of the review.
Historically, the influential social orienting model of social attention development [e.g., (15, 28, 44)] asserted that social orienting impairments have a neonatal onset in the first weeks of life in the course of development of ASD. These impairments purportedly reflect developmental perturbations of basal ganglia and ventral cortical neural systems involved in a social-motivation mechanism that bias neonatal attention to people and especially face processing (44). In contrast, the presumptive onset of joint attention impairment in ASD in this model was considerably later in the last third of the first year of life. Accordingly, the impoverishment of social orienting and face processing was thought to be a congenital developmental disturbance that diminished early social information processing, leading to a cascade of subsequent impairments in joint attention and other aspects of social communication development (15, 16, 28, 44). Social orienting, therefore, reflected primary aspects of the endophenotype of ASD (16), but joint attention impairments were less primary in this regard (16, 44, 85). Recent research, though, has challenged aspects of this model.
Jones and Klin (34) observed that 2- to 6-month-old male infant siblings who went on to receive the diagnosis of ASD did not display evidence of less looking to faces and eyes at the end of the neonatal period of development (see Figure 3). One of the issues that may have impacted the observations of Jones and Klin (34) is the bias for attention to faces may be weak or highly variable in the first months of life in typical development, but become more evident or consistent among 6- to 12-month-olds (86–88). Hence, the typical pattern of social attention development in neonates may not be sufficiently robust or reliable to readily detect contrasting atypical patterns of development. However, social attention differences clearly begin to become reliably detected by 6- to 8-months in infant siblings (12, 33, 61, 89–92).
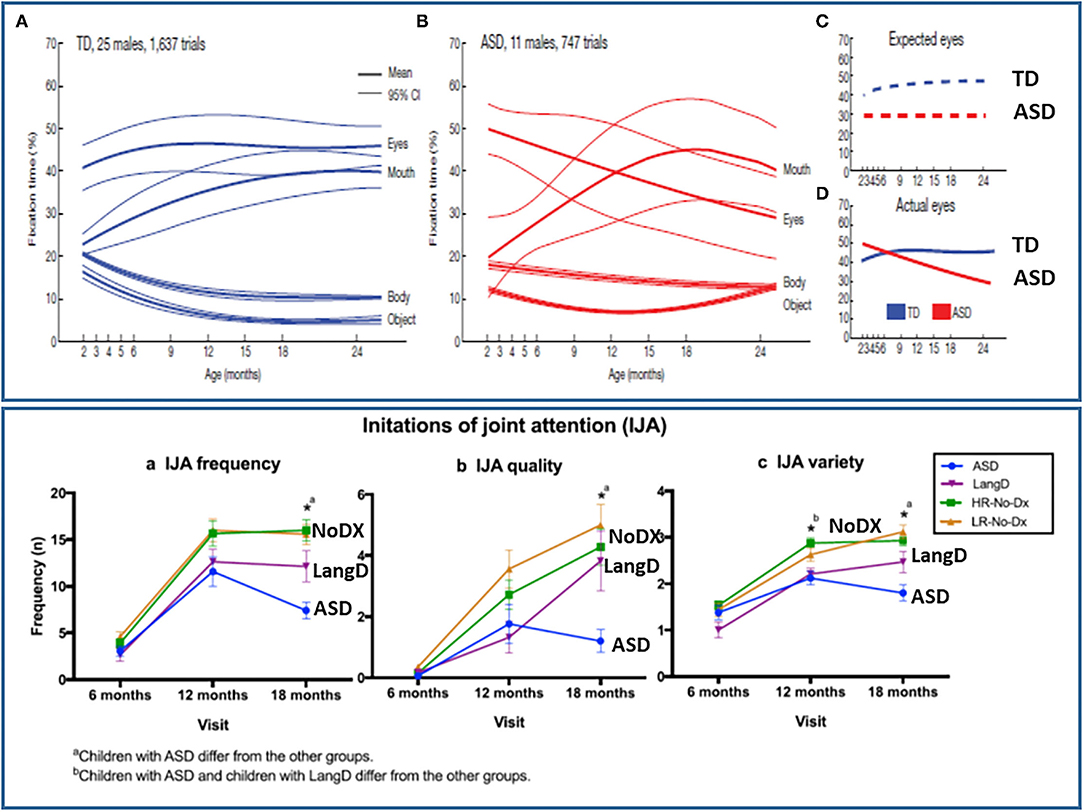
Figure 3. Illustration of the onset of social attention symptoms in the 6th to 12th month of development for social orienting (top panel) and joint attention (bottom panel) from Jones and Klin (34) and Franchini et al. (23), respectively. Figures reprinted with permissions from, (Top panel) Nature, Springer Nature, and (Bottom panel) Journal of Abnormal Child Psychology, Springer Nature.
Jones and Klin, however, also observed that a negative growth trajectory of social attention between 2- to 6-months significantly differentiated the ASD sample from controls. This provided some evidence of the very early but post-neonatal onset of a disturbance of the typical pattern of social attention development in ASD in this period of development. However, the specific nature of the atypical social attention development was not clear. It could reflect the gradual onset of an attenuation of processes associated with spontaneously social orienting to faces across early development. However, studies of the temporal dynamics of social attention indicate that individuals with both ASD and typically developing children orient to faces comparably and also display a decay, or habituation of attention to faces over time (93, 94). However, diagnostic group differences were apparent because the social attention of typical peers recovers periodically such that alternating attention to and from a social partner is the typical pattern. This pattern of recovery and alternation of attention to faces is significant less robust in ASD (93, 94). Processes related to alternating between social and non-social attention are also thought to be a critical component of early joint attention development, especially IJA [(41, 95), see Figure 2]. Thus, it may be that a lack of development of the processes that typically leads the tendency to frequently alternate between social and non-social attention, which is involved in both social orienting and joint attention development, led to the gradual decline in the total among of social attention observed in the 2- to 6-month old observed by Jones and Klin (34). A related observation is that 6-month-old infant siblings who do or do not develop ASD display similar durations in attention to caregivers faces, but the siblings who went on to develop ASD shifted their gaze to and from their parents' faces significantly less than non-ASD sibs (96).
While it is not yet clear what processes are involved in the decline of social attention in the first 6 months of life, Johnson (97) and Jones and Klin (34) noted that the pattern of the data contradicted the supposition that a neonatal development of ASD is characterized by a robust attenuation of social orienting and social information processing that could have a cascading negative effect on subsequent development. Accordingly, the revised hypothesis is that the disturbance of social orienting in ASD likely begins to emerge between 3 and 4 months of age as a result of problems in the shift between subcortical and cortical mechanisms of attention regulation (16, 50, 97). This same developmental period, and mechanism, has also been proposed as the starting point of the disturbance of joint attention in ASD (20, 41, 98).
Evidence also indicates that joint attention develops at the same time as social orienting, rather than later in development. Gaze following or RJA emerges between 2- and 8-months in typical development (18, 25, 94, 99–101), which is the same time for the measurable onset of social orienting. For example, studies of gaze following (18) and preferential orienting to biological motion (102) both indicate that these social attention behaviors develop between 2- and 4-months in infancy. Rudimentary joint attention (e.g., gaze following) has also been observed in 2- to 5-day old infants (103). In comparative research gaze following has also been observed in neonatal birds, reptiles and mammals (104, 105). It a basic, fundamental and well-conserved social attention function across species (82). Symptoms associated with attenuated gaze following and RJA appear in infant siblings can be measured by 9 months of age (106) and, more attention to gaze shifts at 8 months is related to lower ASD symptom development (89).
Initiating joint attention behaviors, such as alternating gaze (see Figure 2), may be more specific to human development (95) and can be reliably measured by 6- and 10-months of age (21, 23, 25, 107). Moreover, atypical IJA becomes measurable in infant siblings between 6- and 12-months of age (23) in the same timeframe that social orienting problems in ASD were most clearly observed by Jones and Klin [(34), see Figure 3]. Gangi et al. (108) and Nyström et al. (21) also have observed differences in IJA in infant sibling samples in the 6- to 12-month phase of development.
Thus, the development of social orienting and joint attention symptoms in ASD may be concurrent rather than sequential in development. None of these observations, however, disprove the social orienting model's hypothesis of a precursor relation between social orienting and joint attention. Definitive testing of this hypothesis will require studies that include both joint attention and social orienting measures in careful longitudinal or experimental-intervention studies of the relations between these types of social attention. Very few studies, though, have included both joint attention and social orienting measures in the same study of typical or atypical social attention development. The few studies that have combined the study of joint attention and social orienting have observed significant correlations between these measures in research on ASD is consistent with the hypothesis that they reflect a common factor in development (15, 56, 109–112).
This is not surprising since social orienting and joint attention paradigms often assess similar behaviors. For example, IJA measures of alternating gaze (Figure 2) involve attracting an infant's attention to an active toy or event, and then measuring their spontaneous social orienting to the face and eyes of a social partner. This is similar to a paired-preference social orienting paradigm that measures preference to orient to a social stimulus in the context of a competing non-social stimulus [(12), see Figure 1]. Indeed, it would be rare for a child to engage in a joint attention behavior without attending or social orienting to their social partner. Given research that joint attention and social orienting develop in the same months of early development, overlap in the behaviors measured and are correlated in samples of children with ASD, a parsimonious hypothesis is that they reflect a common or unified social attention construct in ASD research. Indeed, as noted previously, they likely reflect a common neurodevelopmental starting point at 3–4 months of age (16, 41, 97).
If the social orienting and joint attention symptoms reflect a common social attention construct then combining their measurement paradigms in research on ASD may be useful for several reasons. One of these is that the psychometrics of combination of measures, may be superior to the psychometrics of either measure on its own in terms error of measurement and reliability. There is relatively little data available on the test-retest reliability of individual social attention measures. Nevertheless, the interclass correlation (ICC) test-retest reliabilities of individual infant social and non-social attention measures may be expected to range from fair to good (ICCs = 0.40 to 0.75), but with lower ICCs likely at younger and younger ages (113–116). Robust clinical or biometric applications of measures require test-retest reliability exceeding 0.80 (117). This level of reliability may be difficult to obtain with research that employs only one or the other social attention paradigm. However, a multivariate latent construct measurement model may be expected to improve reliability and power in social attention research on ASD (118, 119). Attempts to utilize a multivariate, latent construct approach to social attention measurement have begun to appear in the literature (119), but much more research is needed to understand the utility of this approach.
Combining measures may also provide additive information or incremental validity in social attention research on ASD. For example, Dawson et al. (56) observed that the combination of an auditory social orienting and visual joint attention measures differentiated ASD preschoolers from controls better than either type of measure alone. Dawson et al., also observed that both joint attention and social orienting correlated with language development in these children. However, joint attention mediated the relations between social orienting to language in the ASD sample (56). These observations suggest that, even though joint attention and social orienting behaviors may reflect a common construct they likely reflect distinct but complimentary information about the mechanisms involved in the social attention symptoms of ASD. This may be expected since there are differences as well as similarities in the task demands of social orienting and joint attention. Recall that social orienting is a dyadic form of social attention, while joint attention is a triadic form of social attention. Triadic social attention involves information processing of the spatial relations between two or more people and a third object or event in order share a common point of reference vis-à-vis an object or event in the environment (27). This “referential” component of joint attention is especially important to early language learning in ASD and typical development (120, 121), but is not measured with social orienting social attention paradigms. This may account for the observations of Dawson et al. (56). Thus, joint attention may have incremental validity relative to social orienting measures in understanding some of the developmental cascade of impacts of social attention symptoms for young children with ASD. On the other hand, social orienting measurement methods may have advantages relative to joint attention assessments in such things as observing critical information about the development of temporal patterns of social attention in early development (94).
The Dawson et al. (56) study illustrates the benefits of a more integrated approach that views social orienting and joint attention measures as complimentary assessments and sources of information about social attention phenomena in ASD research. The next two sections of the paper consider how a more integrated approach may advance hypotheses about the neurodevelopmental and metabolic mechanisms involved in the early development of social attention symptoms in ASD.
The social motivation hypothesis of social attention in ASD (40, 44) suggests that a bottom-up system of striatal, amygdala, and orbital neural networks upregulates the perceptual salience of social stimuli and motivates the allocation of attention to faces and eyes (42, 83, 122). Presumably, pituitary neuropeptides (e.g., oxytocin), as well as dopaminergic signaling play a role in this system as well, but the exact nature of the social reward processes involved require further characterization (44, 123, 124).
Direct gaze, or eye contact, is particularly powerful in capturing the attention of infants. Hence, the mechanism of this so called “eye contact effect” (83, 125, 126) may be central to the social motivation hypothesis (44). The eye contact effect refers to evidence that the perception of eye contact or gaze directed to one's self impacts arousal, cognition, attention engagement, stimulus salience, and motivation in infants, children, and adults (52, 83, 126–128).
Hypothetically, the eye contact effect is a function of a “fast track” neural network composed of the superior colliculus, pulvinar, and amygdala [(83), Figure 4]. It serves to prioritize information about eye-gaze directed both to and away from an individual, in combination with cortical processing, within 150–170 ms of perception (129). Senju and Johnson (83) and others (130–132) suggest that the neurocognitive mechanisms of eye gaze processing of the fast track modulator are distinct from those involved in processing the identity or expressions of faces. This is an important observation because, as noted previously, theory suggests that social attention symptoms stem from abnormal face processing in ASD (42, 44, 85) and this notion was inculcated into the US RDoC matrix such that gaze direction processing and joint attention are classified as measures of facial communication. The direction of causal relations between face processing and social attention however, is not clear.
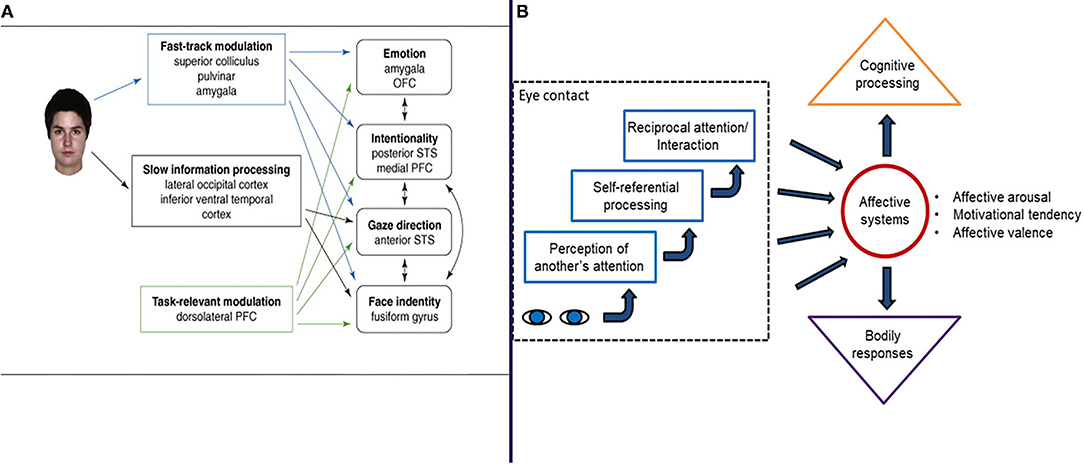
Figure 4. Two illustration of the mechanisms and processes involved in the eye contact effect from Senju and Johnson (83) (A) and Hietanen (52) (B), respectively. Figures reprinted with permissions from, (Left panel) Trends in Cognitive Science, Elsevier, and (Right panel) Frontiers in Psychology, Frontiers.
Autism Spectrum Disorder social attention symptoms may not necessarily be explained in terms of face processing, per se. Rather, it is equally plausible that eye contact effects and gaze processing may play a distinct role in the social attention symptoms (133) and in face processing phenomenon observed in ASD (134, 135). This assertion stems from numerous observations. First, research suggests that gaze processing mechanisms appear to contribute to differences in the development of face detection and face processing (25, 136–138). Indeed, the early cortical face processing indicated by N-170 ERP data may be mediated by eye region processing (136). Second, face expression and eye gaze are processed independently (135). Third, gaze direction modulates fusiform activity in face processing (130) and diminished gaze fixation may account for fusiform hypoactivation to faces observed in studies of ASD (134). Fourth, imaging studies reveal little evidence of fusiform activation in typical processing during joint attention tasks [see (20)]. Finally, as Tso et al. (84) have noted, disrupted gaze perception may be a distinct dimension of atypical social behavior across clinical conditions in human psychopathology, and this does appear to the case for individuals with ASD, as well as infant siblings (89, 139, 140). These observations do not lead to a definitive conclusion regarding the relative role of face processing and gaze processing on ASD development. However, they do suggest that more research is needed before accepting the hypothesis that the social attention symptoms of ASD stem from or are well-described in terms of atypical face processing.
Research and theory on the eye contact also has important implications for the characterization of motivation in social attention development (52, 126). Chevallier et al. (44) interpreted the eye contact effect and fast track modulator as central to mechanisms involved in a decreased tendency to spontaneously orient to faces and eyes. However, it is also the case that the eye contact effect and fast track modulator involves a motivation mechanism associated with the perception of another person's attention being directed toward one's self (52) Hence, being the perceived object of attention of others hypothetically has an impact on arousal, which impacts motivation related to, stimulus salience and a sense of relatedness to others (52, 140). This possible mechanism has received relatively little attention in the research on social attention and social motivation and ASD, but may be significant nonetheless.
The eye contact effect begins to become apparent between 2- and 6-months of age (100, 141, 142). Reddy (53) proposed that the early awareness and reactions to being the object of the attention of caregivers after 2 months of life is a likely catalyst for joint attention development. In this regard, Rayson et al. (25) recently reported a set of seminal observations. Recall that IJA involves gaze leading or the awareness, that another person is attending to oneself and following one's line of regard (26, 143). Rayson et al., observed that 6- to 9-month-olds displayed increased attention engagement to objects and reduced parietal EEG alpha power indicative of cortical arousal in response to the experience of gaze leading on IJA trials. Rayson et al., also observed that the reduction of alpha power was greater in the older infants raising the possibility that a developmental change in cortical arousal to gaze leading may occur between 6- and 9-months. Related, independent observations also indicate the cortical response to eye contact may be detected by 5 months (100) and 10- to 12-month-olds are more aware or responsive to gaze shifts directed to themselves than are 8- to 9-month-olds (141, 144). These data suggest that responses to and awareness of being the object of attention of others may increase in the 6- to 12-month phase of development. This coincides with the timing of social orienting and joint attention symptom development in ASD (23, 34) and is process to consider in future research on the genesis of social attention symptoms in ASD. In this regard, neural responsiveness to gaze directed toward and away from 6- to 10-month-old infant siblings has also been observed to predict the diagnosis of ASD at 36 months (139).
Returning to the Rayson et al. (25) study, this research group also reported that 9-month-olds in their study displayed a preference for the avatar face stimuli observed on gaze leading IJA trials, but not on control trials. Moreover, individual differences in alpha power among the infants significantly correlated with this 9-month face preference effect. This is consistent with other reports that gaze leading effects the salience of face stimuli on IJA trials in adults (26, 145, 146). However, the 6-month-olds in the Rayson et al., study did not display evidence of the increased salience of face stimuli observed on IJA trials, raising the possibility that the gaze leading, and the eye contact effects on stimulus salience including preference for faces may increase in the 6- to 9-month period of typical development during which social attention symptom emerge in ASD.
These observations provide a foundation for a revised social motivation hypothesis of social attention symptoms in ASD. Neurocognitive responses to the perception of eye contact, and/or the awareness of being the object of other person's attention may play a role in the typical increase in the salience of faces in infancy. Hence, problems in this developmental process may impact both social orienting and joint attention symptom development in ASD in the 6- to 12-month period of development. Several recent studies provide evidence that older individuals with ASD may be less responsive to being the object of attention of others (143, 147–150). However, research on this phenomenon infant siblings, perhaps using methods similar to those describe by Rayson et al. (25), will be needed to examine this hypothesis. Notably, though, other social symptoms that emerge in the period of development may be related to problems in the awareness of or response to being the object of attention of other people, such as the attenuated response to name in young children with ASD (151).
Of course, several other motivation mechanisms may impact social attention development. Negative states of arousal in response to eye contact could characterize ASD and interfere with early social attention development (140). However, early in development in 2-year-olds with ASD do not display evidence of aversion to looking to eyes compared to typical and developmentally delayed peers (152). Moreover, motivation related to social distancing in older individuals with ASD appear to be characterized by diminished social approach rather than social aversion (153) and the eye contact effect is not associated with evidence of avoidant behavioral inhibition in children with ASD (154).
Heightened interest and attention to non-social objects, rather than a diminished salience for faces, may also be central to motivation factors that impact the social attention symptoms of young children with ASD (12, 155–157). This pattern of perceptual bias could result in diminished social attention and eye contact effects, but for reasons very different from those described in “social” motivation models. A fourth possibility is that different admixtures of these processes contribute to individual differences in social attention across individuals with ASD. Furthermore, an important caveat here is that the motivation mechanisms involved in social attention symptoms may reflect ASD differences in domain general sensitivity to reward, rather that processes specific to social motivation per se (158).
Thus, the continued examination of alternative models of motivation in social attention remains vital to understanding the nature of the social attention symptoms of ASD. One of the many approaches to future studies of the social motivation hypothesis may take is to consider studies of early intervention effects on social attention in ASD. Understanding the active ingredient in such interventions may reasonably be expected provide clues about the motivation processes involved in the development of the social attention symptoms of ASD.
Several studies suggest that targeted early intervention can improve social attention in preschool children with ASD, including the spontaneous initiation of joint attention [e.g., (46, 159, 160)]. It would be useful to understand if a change in process related to the social motivation models of social attention play a role in the effect of intervention on these symptoms. In this regard, one of the active ingredients in interventions that target IJA appears to be the systematic imitation (mimicking) of the actions of children with ASD by an interventionist (161–163). The mechanisms by which mimicry affects the initiation of joint attention, however, are not yet clear. Mimicry is a form of joint engagement that involves the interventionists' coordination of their actions with the actions of a child during play or daily routines. Hence, it is a form of joint action. Joint action and joint attention share related perceptual, mental and social affiliative mechanisms (164–166). Because they share common mechanisms it may be that the repeated experience of mimicry (joint action) directly scaffolds the social behavioral and even neurodevelopment of joint attention in some young children with ASD.
Another plausible hypothesis, though, is that mimicry provides additional, or more obvious information to a child that indicates that they are the focus of another person's attention. This could elicit or kindle latent social motivation processes analogous to those associated with the eye contact effect. Indeed, studies of typical development indicate that mimicry is linked to increased activation of reward centers of the brain, such as the ventral striatum [Kuhn et al. (167)]. Other research suggests that IJA also is associated with similar reward related striatal activation (168–170). On the other hand, neither mimicry (171) nor response to gaze shifts (172) is linked to the activation of brain centers for reward in individuals with ASD to the same extent as it is in comparisons groups.
This literature suggests that a better understanding of mimicry effects and their possible relation to reward processes may be useful in understanding how mimicry impacts social attention development in ASD. Moreover, the idea that mimicry is an active ingredient in early intervention for social attention leads to several testable hypotheses. Behavioral or cortical measures of mimicry may predict responsiveness to early joint attention in ASD, or serve as important outcome measures. In addition, response to mimicry and intervention related increases in IJA in may be expected to be associated with the types of cortical and behavioral response to gaze leading described by Rayson et al. (25), and perhaps increased salience of faces in ASD. Research on the relations between mimicry and neural network reward mechanisms and their change in response to intervention, though methodologically challenging, may prove singularly informative regarding processes associated with intervention effects and in addressing theory on the role of social motivation the social attention symptoms of ASD.
It may also be important to understand whether mimicry is processed through children's overt or covert attention to the interventionist. Mimicry intervention or imitating the child's action in joint attention intervention often occurs when a child is focused on object play rather than on the interventionist (159). In such cases it is likely that some level covert attention to the social partner is involved in the awareness and impact of mimicry on the child. Covert attention regulates mature social attention (173), yet with few exceptions (174) we know little about its role in the early typical or atypical development of social attention. Nevertheless, a substantive literature on covert orienting of attention in infancy exists that can provide a foundation for future research on this topic (175, 176). It may be useful to take advantage of this literature in future research and intervention on the early social attention symptoms of ASD.
The relations between early and later social attention development in ASD are currently unclear and require more study. For example, maturational or experiential effects may lead to evidence of negative arousal or aversion to eye contact in some older individuals with ASD (134, 177), which may be related to increases in social anxiety across age (178). So, it is reasonable to assume that the mechanisms of social attention change during childhood and especially adolescence (179), and to anticipate that there is considerable heterogeneity in the social motivation tendencies of older children and adolescents with ASD such that no one process or level of social motivation applies to all individuals with ASD (180, 181). For example, girls with ASD may exhibit more attention to faces than boys with ASD (182). Moreover, in the study of social attention in older children, one rarely considered issue is that social attention likely involves effort (18, 127, 183) and that social attention may be more effortful in some or many people with ASD (157, 184). If so, any increased effort required for social attention may play a role in difference in motivation for social attention among people with ASD.
Across typical development social attention and especially joint attention needs to be rapidly, consistently and frequently engaged in order to stay with the dynamic shifts of points of common reference and to maintain adaptive social attention coordination in group social interactions with peers, adults or in the classroom (41, 48, 185). The developmental increase in efficiency of execution of joint attention is illustrated by studies that indicate that the latency to respond to gaze shifts or joint attention bids decreases from about 3.25 s at 2-months, to about 1.5 s at 8-months (18), to about 0.80 s at 18-months (186), and finally to 0.67 s in adolescence (187). Response latency provides an index of efficiency of execution of a mental-behavioral process and mental effort (188). Therefore, the relative latency of engagement or processing of social attention may indicate the ease with which it is deployed in social interactions across development or individuals (186). A set of related studies illustrates one facet of how increased effort of social attention may impact older individuals with ASD. Differences in joint attention affect the ability of children with ASD to use pronouns [e.g., (29, 189)]. In older individuals with ASD, Mizuno et al. (190) have reported that adults with ASD require significantly longer to process deictic shifting in a linguistic visual perspective taking in response to pronouns. It was not that the older individuals were less accurate in processing pronouns, but their longer latency in processing pronouns was indicative of less efficient processing of the mental, referential social-attention coordination, or perspective taking, that is elicited by pronouns.
The possibility that social attention may be less efficient or more effortful is important to recognize in research with older individuals. Some studies report little evidence of differences in the frequency or accuracy of looks to social stimuli (191) inviting the conclusion that social attention is typical in older individuals. However, other studies suggest that social attention remains less efficient or more effortful in older individuals with ASD (156, 192–194); Liu et al., (in submission). Indeed, one recent study suggests that adolescents with ASD may display less efficient covert processing of eye contact (195). If social attention is less efficient or more effortful it may decrease its use, or the success of use in guiding social interactions for some people with ASD. In any event, the example of the potential role of effort or the efficiency of use of social attention illustrates that the concepts and methods used to study social attention will likely need to go beyond infant and preschool paradigms to arrive at a veridical picture of social attention, as well as the social attention symptoms of older individuals with ASD (196).
Social motivation theory provides only one view of the mechanisms involved in the social attention symptoms of ASD. Another perspective is provided by theory and research that suggests that early social attention and later social-cognitive neurodevelopment are related (82, 138, 197–200). This view is echoed in the US RDoC which classifies gaze following as a behavioral measure of the construct of Perception and Understanding of Others. This refers to social cognition, and especially the ability to mentally represent or “mentalize” the intentions, perspective and emotional status of another person (201). Like social attention symptoms, problems with social cognition are a common characteristic of ASD in children and adults (201–204). However, the impact of social cognitive differences on the social behaviors or social competence autistic people is less clear, especially in older individuals (205).
When social cognition is measured in terms of accuracy on social cognitive test items, research results, even from the same research group, can vary from no evidence of relations between social cognition and social interactions in ASD [e.g., (206)] to observing that social cognition makes some direct and indirect contributions to functional and social skills in adults with ASD [e.g., (207)]. However, measurement issues may play a role in observations of variability regarding social cognitive effects just as they do in research on social attention. That is because social cognitive symptoms in older individuals with ASD do not necessarily involve an inability to demonstrate accurate responses on social cognition tests. Rather, they may be best observed with measures that assess differences in the tendency to spontaneously or rapidly engage in social cognition during social interactions (150, 208, 209). This is consistent with the notion that social cognitive mentalizing relies on the ability to rapidly compare one's own perspective and another person's perspective in working memory to determine the congruence or incongruence of perspectives and interpret the behavior and intentions of other people (95, 210, 211).
Hypothetically, mentalizing begins with social information processing associated with social orienting and the bi-directional practice of processing one's own attention and another person's attention in triadic “self-other-object” joint attention contexts in infancy (20, 95, 197, 212, 213). Repeated experience with sharing visual perspectives to objects or events during joint attention with social partners hypothetically allows infants to construct the internal mental representations and executive processes required for social cognitive mentalizing (41, 107). Accordingly, differences in social attention and social cognition may be thought to form a continuous developmental axis of the social symptoms of ASD from infancy through adulthood (201, 214, 215).
The continuity in social attention and social cognitive symptom presentation likely stems in part from common neurodevelopmental substrates. Because joint attention and social cognition play an integral role in perspective taking that involves the integrated triadic processing of self-referenced and other-referenced information, they are thought to involve the processing across a widely distributed frontal, temporal, parietal system [(20), cf. (216)]. In addition, joint attention and social orienting are thought to involve motivation processes supported by mid-brain reward networks (20) and the interaction between bottom-up salience network regulation of attention and top-down executive control of social attention (79) that begins between 3 and 4 months of age (16, 41, 217, 218).
Consistent with these assumptions, studies indicate that both social orienting and joint attention are associated with patterns of cortical activation and neural network connectivity that are similar to those observed for social cognitive mentalizing (22, 89, 100, 170, 183, 199, 200, 215, 219–224). Recent reviews have summarized this literature and suggest that the social attention symptoms in ASD involve at least four distinct but interacting functional neural networks, or circuits in RDoC terminology [for details see (20, 138)]. A brief description of the current understanding of functions of these circuits is as follows.
First, a medial prefrontal cortical network (mMPC) observed in joint attention is thought to play a role in triadic self-other-object/event processing (211) that is involved in sharing, comparing or adopting another person's perspective (210, 225, 226). More specifically the ventral mPFC joins with the posterior cingulate cortex, hippocampus, and nucleus accumbens in salience processing of self-referenced information, while the dorsal mPFC joins with the temporal-parietal cortex and middle temporal gyrus in social perspective taking and related social episodic memory retrieval (227).
The second and third networks involves structures of the orbitofrontal cortex and insula (OCI) and systems of the anterior and posterior cingulate cortex (ACC-PCC). The OCI and ACC networks are involved in the intentional sharing of information and the awareness of sharing information with other people (123, 228, 229). The PCC node is also involved in self-referenced information processing. However, in conjunction with dMPC, the PCC is associated with regulating the balance and integration of processing self-references aspects of attention and with external processing of another person's attention (230, 231).
A fourth network involves integrated functions of the superior temporal-parietal cortex, amygdala, insula and striatum (STAIS). Elements of STAIS activation are common to both joint attention and gaze direction processing (132, 232, 233) as well as social orienting (183, 199, 220). This network processes the eye movements and eye contact, biological motion, reward processing, and the assignment of valence to external stimuli, goal-related motivation, episodic and working memory, and decision-making functions involved in the allocation of social attention (234–237). The amygdala, insula, and the striatum are also involved in the fast track regulation of the eye contact effect (83), as well as a sense of social relatedness and intersubjectivity with a social partner that occurs during joint attention (128). The activation of the striatum in association with IJA in four studies (168–170, 187) also aligns well with the hypothesized involvement of motivation in spontaneous social orienting and joint attention. Notably the STAIS network and the mPfC network display less organized activity to eye contact, averted gaze, and self-relevant rewards in people with ASD (238–240).
These observations comport with the hypothesis that interactions of top-down social cognitive, and bottom-up salience processing networks play a prominent role in the neurodevelopmental mechanisms of human social attention (79) and the social attention symptoms of ASD [e.g., (16)]. Moreover, some of the functions of these networks may involve the comparative and integrated processing of self-referenced and other-referenced salience and perspective taking information [e.g., (211)]. This is consistent with the idea that it is vital to understand the first- and second-person bi-directional nature of social attention in the neuroscience of social cognition (49, 51, 211), and in the social attention symptoms of ASD (41, 213).
To date, the explicit study of bi-directional effects has not played a major role in research on social attention in ASD. However, research on the effects of being the object of attention of others has begun to shift social attention research toward a bi-directional paradigm. Several other observations may propel this shift. For example, eye contact plays a role in the acquisition of understanding of the bidirectional association between our own actions and the actions of others (241). Second, phenomena associated with being the object of other people's attention are similar to those described as “audience effects” in comparative and social psychology (242). Audience effects refer to the change in behavior caused by being observed by another person or persons. Two recent studies suggest such audience effects may be attenuated in in ASD (147, 150). This raises the possibility that integrating the wealth of research and paradigms on the typical development of audience effects and its potential developmental change in adolescence (243) may be important to future studies of social attention and social development in ASD (244). Finally, the development of hyper-scanning imaging paradigms may enable the examination of synchronization of cortical activity across social partners to offer a new method for exploring the bidirectional effects of social attention, eye contact (245), and joint attention (126, 246, 247). This new paradigm may contribute novel insights about the nature of the social attention symptoms of ASD.
The research on the social-cognitive cortical networks involved in social attention addresses the criteria that a valid symptom dimension of ASD should be assessable in terms of fundamental neural circuit substrates.
In addition to research on neurocircuits, though, theory suggests that neurotransmitters such as dopamine and the pituitary neuro-peptides oxytocin and vasopressin may also play a role in the social attention symptoms in ASD (44, 123, 124). A small literature on the relations between the genes that regulate these neurotransmitters and social attention has begun to address this hypothesis.
Polymorphisms of the dopamine receptor gene DRD2 (Taq1A) and the dopamine transporter gene (DAT1) have been implicated in attention bias to positive facial expressions and the cognitive processing of faces in ASD (248, 249). Yamaguchi et al. (250) observed that administration of D1 and D2 agonists were associated with decreased social orienting in Japanese macaques, but the D1 agonist was also associated with increased non-social orienting (250). This is noteworthy because, as previously noted, a preference for non-social orienting may be involved in ASD social attention symptoms [e.g., (12)].
DRD4 and DRD2 have also been related to lower IJA in the first year among infant siblings of children with ASD (35). Polymorphisms of the former are also associated with ADHD (251). Methylphenidate upregulation of dopamine transporter availability in the striatum used in ADHD treatments has also been observed to increase both IJA and RJA in children with ASD (252). In another study, variability of the DRD4 gene also predicted performance on a measure of social cognitive development that included a joint attention items in preschool children (253). Similarly, decreased phasic dopamine release in the putamen correlated with poorer theory of mind skills in a small sample of ASD adults (254).
The pituitary peptides oxytocin and vasopressin may also play a central role in social attention symptom development in ASD (44, 124). In a comparative study, individual differences in RJA that were observed in male chimpanzees were associated with the DupB+_ DupB−/− polymorphism of the AVPR1A arginine vasopressin receptor gene (255). Additionally, the intranasal administration of oxytocin increased eye contact in bonobos, but decreased it in chimpanzees (256). These findings suggest that comparative primate studies provide important animal models for developmental research on the mechanism processes involved in social attention symptoms in ASD (250).
In a study of infants, Wade et al. (257) observed that GG haplotypes of oxytocin gene OXTR were associated with a measure of RJA and cooperation in 18-month-olds. Tops et al. (258) has also reported that the more efficient GG genotype of the oxytocin receptor gene was associated with increased social orienting and processing of auditory stimuli. In addition, Domes et al. (259) observed that oxytocin mediates individual differences in covert attention to social cues (259). This is notable given the previous discussion of the role of covert attention in social communication and social attention development [e.g., (173)]. Moreover, the capacity to switch attention between interoceptive signals and exteroceptive cues, as well as developmental shifts in bias to attend to social and non-social stimuli, may also be modulated by oxytocin (260, 261). This is significant since switching attention between self-referenced or interoceptive, and other referenced or exteroceptive social attention cues may be central to joint attention and social cognition (20, 211).
Other recent behavioral genetic studies are also informative. Constantino et al. (262) observed a concordance rate of 0.91 for social orienting to eyes among 18- to 24- month-old monozygotic twins. Wang et al. (263) reported a concordance rate of 0.50 for monozygotic adolescent twins on a biological motion task. Wang et al., also observed a high level of concordance for gaze following (0.58) and concordance across gaze following and the biological motion measures (0.91) in monozygotic adolescent twins (264). Finally, the observation of associations between differences in DNA methylation and changes in attention to face stimuli with direct gaze in a sample of infant siblings provided the first evidence of possible epigenetic effects on social attention and eye contact effects in development (265).
It is difficult to draw conclusions from this small literature except that it is feasible to study genetic factors associated social attention and social attention symptoms in ASD directly with children or with comparative and behavioral genetic paradigms. In this regard, Skuse and Gallagher (266) suggest that it may be especially important to study the interactive roles of dopamine and oxytocin in the atypical development of social attention in ASD and developmental disorders more generally. To be sure, the development of brief eye-tracking measures of different kinds of both social orienting and joint attention measures (21, 34) have increased the feasibility of both behavioral and metabolic genetic studies of social attention development and social attention symptoms in ASD. So does the availability of individual difference data from joint attention factor within Modules 1 and 2 of ADOS (36).
It remains vital to continue to study what, if anything, distinguishes the development of social attention from non-social attention (9, 10, 52, 267). Domain general and domain specific attention measures may be useful as early diagnostic indicators (191, 268–270). Moreover, domain general processes such as ocular motor control (271), sex differences (272), or predictive processing (213) may moderate the development of social attention symptoms development in ASD. Atypical sensory processing may also influence social attention development (273–275). However, joint attention may also predict the second-year sensory regulation in infants at risk for ASD (276).
It is also the case that young children with ASD also have difficulty with attention shifting and attention disengagement (277, 278), which could contribute to atypical social attention, or at least joint attention problems in ASD (278). Domain general measures of executive functions and inhibition are also correlated joint attention, perspective taking, and social cognition in typical development and in ASD (186, 279–281). Some research also suggests that different patterns of executive functions are involved in the development of IJA and RJA (111, 186, 279, 281).
Bedford et al. (282), however, have provided an essentially informative study regarding the role of inhibition and attention disengagement in the social attention symptoms of ASD. They observed that domain general visual attention disengagement and domain specific social attention provided unique and additive predictive information about the development of ASD in infant siblings. Thus, rather than one dimension being primary or explanatory, social, and non-social attention measures provided additive and complimentary information about the development of ASD (282, 283). There are several reasons why this might be the case.
Neurocognitive development involves adaptations to different types of environmental demands, which lead to the differentiation of neural structures and functional circuits (284). To the extent that the task demands of social and non-social attention differ, they may stem from common cortical mechanisms, but distinct neurocognitive mechanisms or circuits may become involved in each over the course of development. Thus, social and non-social attention symptoms in ASD likely share important common neurocognitive mechanisms (9), but measurable bio-behavioral distinctions increase as activity dependent neurocognitive neural network functional adaptations occur in response to the different social and non-social tasks demands (285). Therefore, it is possible for social and non-social tasks to have different task demands and therefore engage different sets of cognitive processes.
In terms of the different demands of social and non-social tasks, it is especially difficult to conceive of processes associated with the bi-directional nature of social attention that are analogous to those evoke in attention to non-social stimuli (286). It may go without saying that this is especially the case for phenomenon associated with being the object of attention of other people or self-referenced social attention (49). It is also difficult to conceive of a non-social attention analogy to the dynamic neural coupling hypothesis of social cognition (287) and emergence of evidence of dynamic neural coupling between social partners during eye contact (126, 128) joint attention (229, 234, 246, 247, 288), and social cognition (289).
The “social brain” hypothesis makes a similar but phylogenetic argument whereby social attention and the bi-directional exchange of information with other people has been fundamental to human evolution, which was supported by the development of specific functional social attention and social cognitive brain networks [e.g., see (48, 80, 290–292) for review]. Evidence that supports this hypothesis includes but is not limited to observations of the specific neurons in primates and humans that are uniquely responsive to social information (293, 294), the specific behavioral characteristics and cortical mechanisms of reflexive spatial orienting to social stimuli vs. non-social stimuli (78, 172), and the observation that human social attention is associated with substantial heritability estimates (262, 264, 295). Thus, there is a reasonable body of theory and evidence consistent with the contention that social attention constitutes a valid and distinct construct within the broader field of cognitive science and more specifically regarding attention research on typical development and ASD.
This review was intended to consider research related to seven hypotheses about the nature of social attention symptoms in ASD. These are reconsidered here in light of the studies examined in this review.
The primary hypothesis was that early developmental differences in social attention constitute a unique diagnostic dimension of ASD. Most of the research reviewed here was relevant to this hypothesis in one way or another. The validity of this hypothesis would appear to be clear since social attention measures constitute a major component of evidence based diagnostic assessments such at the ADOS (36). However, the lack of recognition of social attention symptoms in the US DSM (4) and ICD-11 (77) nosologies and diagnostic descriptions of ASD illustrates the need to explicitly evaluate this hypothesis. The studies reviewed here indicate that differences in social attention are among the earliest social symptoms of ASD emerging in infant siblings between 6- and 12-months of age (23, 34). These symptoms distinguish young children with ASD from other children with developmental conditions as well as those with typical development (24, 56) and can be reliably measured by both experimental and clinical measures which converge on the same construct [e.g., (21, 62)]. There is evidence for the prognostic as well as diagnostic validity of social symptom measures [e.g., (76, 89)] and evidence suggests that social attention is an important target for early intervention (46, 47). In addition, research suggests that infant social attention symptoms constitute part of a developmentally continuous axis of symptom presentation that includes social cognitive symptom development in childhood (20). Finally, the developmental processes involved in social attention can be studied using cognitive, motivation, parent report, neurodevelopmental, and genetic paradigms across infants, children, and adolescents [e.g., (35, 40, 145, 187, 219, 296, 297)]. The neural circuits and genetic factors involved in social attention development have also begun to be described in imaging and metabolic studies [e.g., (20, 35, 138)]. It is difficult to imagine what other evidence would be needed to support the assertion that differences in social attention constitute a valid and significant symptom dimension of ASD.
Differences in theory about the development of social attention may have delayed the acknowledgment of the validity of this symptom dimension in ASD. One hypothesis has been that social orienting emerged before joint attention in early development and reflected a more primary aspect of the endophenotype of ASD [e.g., (153)]. A related idea was that atypical problem in face processing was pivotal to the emergence of social attention symptoms [e.g., (44)]. Subsequently, there was a tendency for research to focus on only one type of social attention paradigm, and to emphasize which type of social attention was more primary in ASD development. However, cross study comparisons indicate that social orienting and joint attention behaviors emerge concurrently rather than sequentially in typical development [e.g., (18, 102)], as well as in ASD [e.g., (21, 33)]. Further, processing of eye gaze may be as fundamental or more fundamental to social attention development than face processing per se [e.g., (134, 136)] and the development of social orienting and joint attention may share common neurodevelopmental and genetic substrates [e.g., (199, 233)]. A unified measurements model of social attention that combines measures of social orienting and joint attention may be more powerful way forward in research on social attention in ASD (118, 119). However, a unified measurement model that allows for the direct comparison of data from joint attention and social orienting measures on the same samples of children in future research is still needed to truly examine the relations between these two types of social attention in the development of ASD.
Such a unified measurement model of social attention will also be important in future research because it is difficult to assess the socially interactive nature of social attention development with only one paradigm. The nature and development of social attention development involves bi-directional processes of both attending to a social partner and being affected by the social attention of another person directed to one's self [e.g., (25, 52)]. This fundamental assertion has implications for the social motivation theory of social attention. Whereas, this theory has focused on hypothetical processes involved in the motivation to spontaneously attend to other people (44), recent research and theory suggest that processes associated with typical or atypical responses to being the object of attention also need to be considered in research on the motivation for social attention [e.g., (147, 148)]. The bi-directional conceptualization of social attention also argues for the value of in-vivo measures of social attention in social interaction to assess develop more veridical models of the processes involved in its nature and development (205). This does not mean, however, that non-interactive social attention measures are not valid. Rather, interpretations of social attention research may be best served by synthesizing information across both in-vivo and analog measures. By the same token though, we must recognize the limits of any one paradigm significantly constrain the conclusions we can draw about social attention from data any one paradigm. It also means that innovations in research paradigms, such as the advent of hyper-scanning imaging methods that allow for the examination of bi-directional effects across interactive social partners may be necessary to advance the understanding of functions of cortical systems involved in social attention (128, 246).
Finally, the bi-directional conceptualization of social attention encapsulates perhaps the best argument for why social attention constitutes a unique dimension of cognitive and attention development (49, 286). That is to say, bi-directional process (e.g., signal sending and signal receiving) are not part of the task demands that sculpt domain general attention development. The difference in the task demand of social and non-social attention over development from the age of 3–4 months to adulthood may be expected to be significant enough to sculpt significantly different neuro-circuits for domains specific social vs. domain general attention (41, 98).
A final hypothesis examined in this review was that social attention symptoms of ASD may change over age. Measures of accuracy or frequency of different type of social attention behaviors may be valid in the study of preschool development. However, for adaptive behavior guidance in the increasingly complex and rapid dynamics of group social interactions in childhood, social attention, and social cognition need to be able to be engaged spontaneously and efficiently [e.g., (209)]. Therefore, paradigms that measure the latency, effort and spontaneity of social attention (and social cognition) may be needed to comprehensively assess the presence and impact of these related symptom dimensions in older individuals with ASD (192, 298). Studies of accuracy alone may lead to erroneous conclusions about the role social attention and social cognitive symptoms play in the later development of individuals with ASD.
In conclusion, we should recognize that no hypotheses are unequivocally proved or disproved by the evidence reviewed in this paper. Nevertheless, a goal has been to improve and expand the conceptualization of social attention and the social attention symptoms of ASD. To the degree that goal has been achieved we hope this paper contributes to advances in the questions that need to be asked and answered in future research on social attention in typical and atypical development.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
PM developed the concept for this review and wrote the initial draft. PM and JB collaborated on two revisions of conceptual content and organization of the manuscript. Both authors approved the final version of the paper for submission. Both authors contributed to the article and approved the submitted version.
The work of PM and JB was supported by the Lisa Capps Endowment for Neurodevelopmental Disorders and Education, Department of Psychiatry and Behavioral Science, University of California at Davis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors note that the paper by Salley and Colombo (10) served as a catalyst for the development of this article.
1. Girault JB, Piven J. The neurodevelopment of autism from infancy through toddlerhood. Neuroimaging Clin. (2020) 30:97–114. doi: 10.1016/j.nic.2019.09.009
2. Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ. (2020) 69:1–47. doi: 10.15585/mmwr.ss6903a1
3. Ozonoff S, Heung K, Byrd R, Hansen R, Hertz-Picciotto I. The onset of ASD: patterns of symptom emergence in the first years of life. ASD Res. (2008) 1:320–8. doi: 10.1002/aur.53
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Pub (2013). doi: 10.1176/appi.books.9780890425596
5. Lord C, Jones RM. Annual research review: re-thinking the classification of ASD spectrum disorders. J Child Psychol Psychiatry. (2012) 53:490–509. doi: 10.1111/j.1469-7610.2012.02547.x
6. Kaboski J, McDonnell CG, Valentino K. Resilience and autism spectrum disorder: applying developmental psychopathology to optimal outcome. Rev J Autism Dev Disord. (2017) 4:175–89. doi: 10.1007/s40489-017-0106-4
7. Lord C, Charman T, Haydal A, Carbone P, Anagnostou E, Boyd B, et al. Lancet commission on the future of care and clinical research in autism. Lancet Psychiatry. (2021). 1–64. doi: 10.1016/S0140-6736(21)01541-5
8. Wilshire CE, Ward T, Clack S. Symptom descriptions in psychopathology: how well are they working for us? Clin Psychol Sci. (2021) 9:323–9. doi: 10.1177/2167702620969215
9. Braithwaite EK, Gui A, Jones EJH. Social attention: what is it, how can we measure it, and what can it tell us about autism and ADHD? Progr Brain Res. (2020) 254:271–303. doi: 10.1016/bs.pbr.2020.05.007
10. Salley B, Colombo J. Conceptualizing social attention in developmental research. Soc Dev. (2016) 25:687–703. doi: 10.1111/sode.12174
11. Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philos Trans R Soc Lond Ser B Biol Sci. (2003) 358:345–60. doi: 10.1098/rstb.2002.1202
12. Pierce K, Marinero S, Hazin R, McKenna B, Barnes CC, Malige A. Eye-tracking reveals abnormal visual preference for geometric images as an early biomarker of an ASD subtype associated with increased symptom severity. Biol Psychiatry. (2016) 79:657. doi: 10.1016/j.biopsych.2015.03.032
13. Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philos Trans R Soc Lond B Biol Sci. (2003) 358:435–45. doi: 10.1098/rstb.2002.1221
14. Annaz D, Campbell R, Coleman M, Milne E, Swettenham J. Young children with ASD spectrum disorder do not preferentially attend to biological motion. J ASD Dev Disord. (2012) 42:401–8. doi: 10.1007/s10803-011-1256-3
15. Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with ASD fail to orient to naturally occurring social stimuli. J ASD Dev Disord. (1998) 28:479–85. doi: 10.1023/A:1026043926488
16. Klin A, Shultz S, Jones W. Social visual engagement in infants and toddlers with ASD: early developmental transitions and a model of pathogenesis. Neurosci Biobehav Rev. (2015) 50:189–203. doi: 10.1016/j.neubiorev.2014.10.006
17. Shi J, Weng X, He S, Jiang Y. Biological motion cues trigger reflexive attentional orienting. Cognition. (2010) 117:348–54. doi: 10.1016/j.cognition.2010.09.001
18. Gredebäck G, Fikke L, Melinder A. The development of joint visual attention: a longitudinal study of gaze following during interactions with mothers and strangers. Dev Sci. (2010) 13:839–48. doi: 10.1111/j.1467-7687.2009.00945.x
19. Kim K, Mundy P. Joint attention, social-cognition, and recognition memory in adults. Front Hum Neurosci. (2012) 6:172. doi: 10.3389/fnhum.2012.00172
20. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and ASD spectrum disorder. Eur J Neurosci. (2018) 47:497–514. doi: 10.1111/ejn.13720
21. Nyström P, Thorup E, Bölte S, Falck-Ytter T. Joint attention in infancy and the emergence of ASD. Biol Psychiatry. (2019) 86:631–8. doi: 10.1016/j.biopsych.2019.05.006
22. Eggebrecht AT, Elison JT, Feczko E, Todorov A, Wolff JJ, Kandala S, et al. Joint attention and brain functional connectivity in infants and toddlers. Cereb Cortex. (2017) 27:1709–20. doi: 10.1093/cercor/bhw403
23. Franchini M, Hamodat T, Armstrong VL, Sacrey LA, Brian J, Bryson SE, et al. Infants at risk for ASD spectrum disorder: frequency, quality, and variety of joint attention behaviors. J Abnorm Child Psychol. (2019) 47:907–20. doi: 10.1007/s10802-018-0471-1
24. Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of ASD: the contribution of non-verbal communication measures. J Child Psychol Psychiatry. (1986) 27:657–69. doi: 10.1111/j.1469-7610.1986.tb00190.x
25. Rayson H, Bonaiuto JJ, Ferrari PF, Chakrabarti B, Murray L. Building blocks of joint attention: early sensitivity to having one's own gaze followed. Dev Cogn Neurosci. (2019) 37:100631. doi: 10.1016/j.dcn.2019.100631
26. Bayliss AP, Murphy E, Naughtin CK, Kritikos A, Schilbach L, Becker SI. “Gaze leading”: initiating simulated joint attention influences eye movements and choice behavior. J Exp Psychol. Gen. (2013) 142:76. doi: 10.1037/a0029286
27. Butterworth G, Jarrett N. What minds have in common is space: spatial mechanisms serving joint visual attention in infancy. Brit J Dev Psychol. (1991) 9:55–72. doi: 10.1111/j.2044-835X.1991.tb00862.x
28. Klin A. Young autistic children's listening preferences in regard to speech: a possible characterization of the symptom of social withdrawal. J ASD Dev Disord. (1991) 21:29–42. doi: 10.1007/BF02206995
29. Loveland KA, Landry SH. Joint attention and language in ASD and developmental language delay. J ASD Dev Disord. (1986) 16:335–49. doi: 10.1007/BF01531663
30. Wetherby AM, Prutting CA. Profiles of communicative and cognitive-social abilities in autistic children. J Speech Lang Hear Res. (1984) 27:364–77. doi: 10.1044/jshr.2703.364
31. Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with ASD at 4 and 14 months: social engagement, communication, and cognition. J Child Psychol Psychiatry. (2006) 47:511–23. doi: 10.1111/j.1469-7610.2005.01528.x
32. Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for ASD spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. (2011) 128:488–95. doi: 10.1542/peds.2010-2825
33. Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with ASD spectrum disorders. Biol Psychiatry. (2013) 74:195–203. doi: 10.1016/j.biopsych.2012.11.022
34. Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with ASD. Nature. (2013) 504:427–31. doi: 10.1038/nature12715
35. Gangi DN, Messinger DS, Martin ER, Cuccaro ML. Dopaminergic variants in siblings at high risk for ASD: associations with initiating joint attention. ASD Res. (2016) 9:1142–50. doi: 10.1002/aur.1623
36. Gotham K, Risi S, Pickles A, Lord C. The ASD Diagnostic observation schedule: revised algorithms for improved diagnostic validity. J ASD Dev Disord. (2007) 37:613. doi: 10.1007/s10803-006-0280-1
37. Robins DL, Casagrande K, Barton M, Chen CMA, Dumont-Mathieu T, Fein D. Validation of the modified checklist for ASD in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics. (2014) 133:37–45. doi: 10.1542/peds.2013-1813
38. Stone WL, Coonrod EE, Turner LM, Pozdol SL. Psychometric properties of the STAT for early ASD screening. J ASD Dev Disord. (2004) 34:691–701. doi: 10.1007/s10803-004-5289-8
39. Dawson G, Bernier R, Ring RH. Social attention: a possible early indicator of efficacy in ASD clinical trials. J Neurodev Disord. (2012) 4:11. doi: 10.1186/1866-1955-4-11
40. Jones EJ, Venema K, Earl RK, Lowy R, Webb SJ. Infant social attention: an endophenotype of ASD-related traits? J ASD Dev Disord. (2017) 58:270–81. doi: 10.1111/jcpp.12650
41. Mundy P, Sullivan L, Mastergeorge AM. A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Res. (2009) 2:2–21. doi: 10.1002/aur.61
42. Schultz RT. Developmental deficits in social perception in ASD: the role of the amygdala and fusiform face area. Int J Dev Neurosci. (2005) 23:125–41. doi: 10.1016/j.ijdevneu.2004.12.012
43. Brandes-Aitken A, Braren S, Gandhi J, Perry RE, Rowe-Harriott S, Blair C. Joint attention partially mediates the longitudinal relation between attuned caregiving and executive functions for low-income children. Dev Psychol. (2020) 56:1829. doi: 10.1037/dev0001089
44. Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of ASD. Trends Cogn Sci. (2012) 16:231–9. doi: 10.1016/j.tics.2012.02.007
45. Mundy P, Crowson M. Joint attention and early social communication: implications for research on intervention with autism. J Autism Dev Disord. (1997) 27:653–76. doi: 10.1023/A:1025802832021
46. Kasari C, Paparella T, Freeman S, Jahromi LB. Language outcome in ASD: randomized comparison of joint attention and play interventions. J Consult Clin Psychol. (2008) 76:125. doi: 10.1037/0022-006X.76.1.125
47. Rollins PR, De Froy A, Campbell M, Hoffman RT. Mutual gaze: an active ingredient for social development in toddlers with ASD: a randomized control trial. J ASD Dev Disord. (2021) 51:1921–38. doi: 10.1007/s10803-020-04672-4
48. Pan Y, Novembre G, Olsson A. The interpersonal science of social learning. Perspect Psychol Sci. (2021) 2021:17456916211008429. doi: 10.1177/17456916211008429
49. Redcay E, Schilbach L. Using second-person neuroscience to elucidate the mechanisms of social interaction. Nat Rev Neurosci. (2019) 20:495–505. doi: 10.1038/s41583-019-0179-4
50. Shultz S, Klin A, Jones W. Neonatal transitions in social behavior and their implications for autism. Trends Cogn Sci. (2018) 22:452–69. doi: 10.1016/j.tics.2018.02.012
51. Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, et al. Toward a second-person neuroscience 1. Behav Brain Sci. (2013) 36:393–414. doi: 10.1017/S0140525X12000660
52. Hietanen JK. Affective eye contact: an integrative review. Front Psychol. (2018) 9:1587. doi: 10.3389/fpsyg.2018.01587
53. Reddy V. On being the object of attention: implications for self–other consciousness. Trends Cogn Sci. (2003) 7:397–402. doi: 10.1016/S1364-6613(03)00191-8
54. Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with ASD. Psychol Sci. (2003) 14:151–7. doi: 10.1111/1467-9280.01434
55. Mundy P, Sigman M, Kasari C. Joint attention, developmental level, and symptom presentation in ASD. Dev Psychopathol. (1994) 6:389–401. doi: 10.1017/S0954579400006003
56. Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in ASD: social orienting, joint attention, and attention to distress. Dev Psychol. (2004) 40:271. doi: 10.1037/0012-1649.40.2.271
57. Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The ASD diagnostic observation schedule—generic: a standard measure of social and communication deficits associated with the spectrum of ASD. J ASD Dev Disord. (2000) 30:205–23. doi: 10.1023/A:1005592401947
58. Kanne SM, Randolph JK, Farmer JE. Diagnostic and assessment findings: a bridge to academic planning for children with ASD spectrum disorders. Neuropsychol Rev. (2008) 18:367–84. doi: 10.1007/s11065-008-9072-z
59. Rudra A, Banerjee S, Singhal N, Barua M, Mukerji S, Chakrabarti B. Translation and usability of ASD screening and diagnostic tools for ASD spectrum conditions in India. ASD Res. (2014) 7:598–607. doi: 10.1002/aur.1404
60. Smith L, Malcolm-Smith S, de Vries PJ. Translation and cultural appropriateness of the ASD diagnostic observation schedule-2 in Afrikaans. ASD. (2017) 21:552–63. doi: 10.1177/1362361316648469
61. Jones EJ, Venema K, Earl R, Lowy R, Barnes K, Estes A, et al. Reduced engagement with social stimuli in 6-month-old infants with later ASD spectrum disorder: a longitudinal prospective study of infants at high familial risk. J Neurodev Disord. (2016) 8:7. doi: 10.1186/s11689-016-9139-8
62. Taylor LJ, Charman T, Howlin P, Slonims V, Green J. Brief report: associations between preverbal social communication skills, language and symptom severity in children with ASD: an investigation using the early sociocognitive battery. J ASD Dev Disord. (2020) 1–9. doi: 10.1007/s10803-020-04364-z
63. Franchini M, Wood de Wilde H, Glaser B, Gentaz E, Eliez S, Schaer M. Brief report: a preference for biological motion predicts a reduction in symptom severity 1 year later in preschoolers with ASD spectrum disorders. Front Psychiatry. (2016) 7, 143. doi: 10.3389/fpsyt.2016.00143
64. Romero V, Fitzpatrick P, Roulier S, Duncan A, Richardson MJ, Schmidt RC. Evidence of embodied social competence during conversation in high functioning children with autism spectrum disorder. PLoS ONE. (2018) 13:e0193906. doi: 10.1371/journal.pone.0193906
65. Vivanti G, Fanning PA, Hocking DR, Sievers S, Dissanayake C. Social attention, joint attention and sustained attention in autism spectrum disorder and Williams syndrome: convergences and divergences. J Autism Dev Disord. (2017) 47:1866–77. doi: 10.1007/s10803-017-3106-4
66. Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The ASD observation scale for infants: scale development and reliability data. J ASD Dev Disord. (2008) 38:731–8. doi: 10.1007/s10803-007-0440-y
67. Nygren G, Sandberg E, Gillstedt F, Ekeroth G, Arvidsson T, Gillberg C. A new screening programme for ASD in a general population of Swedish toddlers. Res Dev Disabil. (2012) 33:1200–10. doi: 10.1016/j.ridd.2012.02.018
68. Barbaro J, Wang C, Wang J, Liu G, Liang Y, Wang J, et al. A pilot investigation of the Social Attention and Communication Surveillance (SACS) tool for the early identification of autism in Tianjin, China (SACS-C). Front Neurol. (2020) 11:1389. doi: 10.3389/fneur.2020.597790
69. Mosconi MW, Reznick JS, Mesibov G, Piven J. The Social Orienting Continuum and Response Scale (SOC-RS): a dimensional measure for preschool-aged children. J ASD Dev Disord. (2009) 39:242–50. doi: 10.1007/s10803-008-0620-4
70. Happé F, Ronald A, Plomin R. Time to give up on a single explanation for ASD. Nat Neurosci. (2006) 9:1218–20. doi: 10.1038/nn1770
71. Bal VH, Fok M, Lord C, Smith IM, Mirenda P, Szatmari P, et al. Predictors of longer-term development of expressive language in two independent longitudinal cohorts of language-delayed preschoolers with autism spectrum disorder. J Child Psychol Psychiatry. (2020) 61:826–35. doi: 10.1111/jcpp.13117
72. Harrison AJ, Lu ZL, McLean RL, Sheinkopf SJ. Cognitive and adaptive correlates of an ADOS-derived joint attention composite. Res Autism Spectr Disord. (2016) 29:66–78. doi: 10.1016/j.rasd.2016.07.001
73. Maljaars J, Noens I, Scholte E, van Berckelaer-Onnes I. Language in low-functioning children with autistic disorder: differences between receptive and expressive skills and concurrent predictors of language. J Autism Dev Disord. (2012) 42:2181–91. doi: 10.1007/s10803-012-1476-1
74. Sano M, Yoshimura Y, Hirosawa T, Hasegawa C, An KM, Tanaka S, et al. Joint attention and intelligence in children with autism spectrum disorder without severe intellectual disability. Autism Res. (2021) 14:2603–12. doi: 10.1002/aur.2600
75. Thurm A, Lord C, Lee LC, Newschaffer C. Predictors of language acquisition in preschool children with autism spectrum disorders. J Autism Dev Disord. (2007) 37:1721–34. doi: 10.1007/s10803-006-0300-1
76. Zachor DA, Ben-Itzchak E. From toddlerhood to adolescence, trajectories and predictors of outcome: long-term follow-up study in autism spectrum disorder. Autism Res. (2020) 13:1130–43. doi: 10.1002/aur.2313
77. World Health Organization. International Classification of Diseases, 11th Revision. Geneva: World Health Organization (2019). Available online at: https://icd.who.int/en (accessed November 21, 2021).
78. Birmingham E, Kingstone A. Human social attention. Prog Brain Res. (2009). 176:309–320. doi: 10.1016/S0079-6123(09)17618-5
79. Klein JT, Shepherd SV, Platt ML. Social attention and the brain. Curr Biol. (2009) 19:R958–62. doi: 10.1016/j.cub.2009.08.010
80. Nummenmaa L, Calder AJ. Neural mechanisms of social attention. Trends Cogn Sci. (2009) 13:135–43. doi: 10.1016/j.tics.2008.12.006
81. Capozzi F, Ristic J. How attention gates social interactions. Ann N Y Acad Sci. (2018) 1426:179–98. doi: 10.1111/nyas.13854
82. Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychol Bull. (2007) 133:694. doi: 10.1037/0033-2909.133.4.694
83. Senju A, Johnson MH. The eye contact effect: mechanisms and development. Trends Cogn Sci. (2009) 13:127–34. doi: 10.1016/j.tics.2008.11.009
84. Tso IF, Lasagna CA, Fitzgerald KD, Colombi C, Sripada C, Peltier SJ, et al. Disrupted eye gaze perception as a biobehavioral marker of social dysfunction: an RDoC investigation. J Psychiatry Brain Sci. (2020) 5:e200021. doi: 10.20900/jpbs.20200021
85. Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in ASD: insights from behavioral and electrophysiological studies. Dev Neuropsychol. (2005) 27:403–24. doi: 10.1207/s15326942dn2703_6
86. Frank MC, Vul E, Johnson SP. Development of infants' attention to faces during the first year. Cognition. (2009) 110:160–70. doi: 10.1016/j.cognition.2008.11.010
87. Leppänen JM. Using eye tracking to understand infants' attentional bias for faces. Child Dev Perspect. (2016) 10:161–5. doi: 10.1111/cdep.12180
88. Thiele M, Hepach R, Michel C, Haun D. Infants' preference for social interactions increases from 7 to 13 months of age. Child Dev. (2021) 92:2577–94. doi: 10.1111/cdev.13636
89. Bussu G, Llera A, Jones EJ, Tye C, Charman T, Johnson MH, et al. Uncovering neurodevelopmental paths to autism spectrum disorder through an integrated analysis of developmental measures and neural sensitivity to faces. J Psychiatry Neurosci. (2021) 46:E34. doi: 10.1503/jpn.190148
90. Elsabbagh M, Fernandes J, Webb SJ, Dawson G, Charman T, Johnson MH, et al. Disengagement of visual attention in infancy is associated with emerging ASD in toddlerhood. Biol Psychiatry. (2013) 74:189–94. doi: 10.1016/j.biopsych.2012.11.030
91. Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for ASD. Dev Sci. (2009) 12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x
92. Chawarska K, Volkmar F, Klin A. Limited attentional bias for faces in toddlers with ASD spectrum disorders. Arch Gen Psychiatry. (2010) 67:178–85. doi: 10.1001/archgenpsychiatry.2009.194
93. Hedger N, Chakrabarti B. Autistic differences in the temporal dynamics of social attention. Autism. (2021) 25:1615–26. doi: 10.1177/1362361321998573
94. Del Bianco T, Mason L, Charman T, Tillman J, Loth E, Hayward H, et al. Temporal profiles of social attention are different across development in autistic and neurotypical people. Biol Psychiatry. (2021) 6:813–24. doi: 10.1016/j.bpsc.2020.09.004
95. Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: the origins of cultural cognition. Behav Brain Sci. (2005) 28:675–91. doi: 10.1017/S0140525X05000129
96. Ibanez LV, Messinger DS, Newell L, Lambert B, Sheskin M. Visual disengagement in the infant siblings of children with an autism spectrum disorder (ASD). Autism. (2008) 12:473–85. doi: 10.1177/1362361308094504
97. Johnson M. ASD: demise of the innate social orienting hypothesis. Curr Biol. (2014) 24:R30–1. doi: 10.1016/j.cub.2013.11.021
98. Mundy P, Newell L. Attention, joint attention, and social cognition. Curr Dir Psychol Sci. (2007) 16:269–74. doi: 10.1111/j.1467-8721.2007.00518.x
99. D'Entremont B, Hains SM, Muir DW. A demonstration of gaze following in 3-to 6-month-olds. Infant Behav Dev. (1997) 20:569–72. doi: 10.1016/S0163-6383(97)90048-5
100. Grossmann T, Lloyd-Fox S, Johnson MH. Brain responses reveal young infants' sensitivity to when a social partner follows their gaze. Dev Cogn Neurosci. (2013) 6:155–61. doi: 10.1016/j.dcn.2013.09.004
101. Tremblay H, Rovira K. Joint visual attention and social triangular engagement at 3 and 6 months. Infant Behav Dev. (2007) 30:366–79. doi: 10.1016/j.infbeh.2006.10.004
102. Sifre R, Olson L, Gillespie S, Klin A, Jones W, Shultz S. A longitudinal investigation of preferential attention to biological motion in 2-to 24-month-old infants. Sci Rep. (2018) 8:1–10. doi: 10.1038/s41598-018-20808-0
103. Farroni T, Massaccesi S, Pividori D, Johnson MH. Gaze following in newborns. Infancy. (2004) 5:39–60. doi: 10.1207/s15327078in0501_2
104. Jaime M, Lopez JP, Lickliter R. Bobwhite quail (Colinus virginianus) hatchlings track the direction of human gaze. Anim Cogn. (2009) 12:559–65. doi: 10.1007/s10071-009-0214-3
105. Wilkinson A, Mandl I, Bugnyar T, Huber L. Gaze following in the red-footed tortoise (Geochelone carbonaria). Anim Cogn. (2010) 13:765–9. doi: 10.1007/s10071-010-0320-2
106. Stallworthy I, Lasch C, Berry D, Wolff JJ, Pruett JR Jr, Marrus N, et al. Variability in responding to joint attention cues in the first year is associated with autism outcome. J Am Acad Child Adolesc Psychiatry. (2021) 2021:S0890-8567(21)00294-X. doi: 10.1016/j.jaac.2021.03.023
107. Brandone AC, Stout W, Moty K. Triadic interactions support infants' emerging understanding of intentional actions. Dev Sci. (2020) 23:e12880. doi: 10.1111/desc.12880
108. Gangi DN, Ibañez LV, Messinger DS. Joint attention initiation with and without positive affect: risk group differences and associations with ASD symptoms. J ASD Dev Disord. (2014) 44:1414–24. doi: 10.1007/s10803-013-2002-9
109. Adamson LB, Deckner DF, Bakeman R. Early interests and joint engagement in typical development, ASD, and Down syndrome. J ASD Dev Disord. (2010) 40:665–76. doi: 10.1007/s10803-009-0914-1
110. Franchini M, Glaser B, de Wilde HW, Gentaz E, Eliez S, Schaer M. Social orienting and joint attention in preschoolers with ASD spectrum disorders. PLoS ONE. (2017) 12:e0178859. doi: 10.1371/journal.pone.0178859
111. Schietecatte I, Roeyers H, Warreyn P. Exploring the nature of joint attention impairments in young children with ASD spectrum disorder: associated social and cognitive skills. J ASD Dev Disord. (2012) 42:1–12. doi: 10.1007/s10803-011-1209-x
112. Tsang T, Johnson S, Jeste S, Dapretto M. Social complexity and the early social environment affect visual social attention to faces. ASD Res. (2019) 12:445–57. doi: 10.1002/aur.2060
113. Colombo J, Mitchell DW, Horowitz FD. Infant visual attention in the paired-comparison paradigm: test-retest and attention-performance relations. Child Dev. (1988) 1198–210. doi: 10.2307/1130483
114. Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, Parlade MV. Individual differences and the development of joint attention in infancy. Child Dev. (2007) 78:938–54. doi: 10.1111/j.1467-8624.2007.01042.x
115. Munsters NM, van Ravenswaaij H, van den Boomen C, Kemner C. Test-retest reliability of infant event related potentials evoked by faces. Neuropsychologia. (2019) 126:20–6. doi: 10.1016/j.neuropsychologia.2017.03.030
116. Singer LT. Randomized clinical trials in infancy: methodologic issues. Semin Neonatol. (2001) 6:393–401. doi: 10.1053/siny.2001.0060
117. Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic. (1981) 86:127–37.
118. Chawarska K, Ye S, Shic F, Chen L. Multilevel differences in spontaneous social attention in toddlers with ASD spectrum disorder. Child Dev. (2016) 87:543–57. doi: 10.1111/cdev.12473
119. Frazier TW, Uljarevic M, Ghazal I, Klingemier EW, Langfus J, Youngstrom EA, et al. Social attention as a cross-cultural transdiagnostic neurodevelopmental risk marker. ASD Res. (2021) 14:1873–85. doi: 10.1002/aur.2532
120. Bottema-Beutel K. Associations between joint attention and language in autism spectrum disorder and typical development: a systematic review and meta-regression analysis. Autism Res. (2016) 9:1021–35. doi: 10.1002/aur.1624
121. Çetinçelik M, Rowland CF, Snijders TM. Do the eyes have it? A systematic review on the role of eye gaze in infant language development. Front Psychol. (2021) 11:3627. doi: 10.3389/fpsyg.2020.589096
122. Moore T, Zirnsak M. Neural mechanisms of selective visual attention. Annu Rev Psychol. (2017) 68:47–72. doi: 10.1146/annurev-psych-122414-033400
123. Mundy P. Annotation: the neural basis of social impairments in ASD: the role of the dorsal medial-frontal cortex and anterior cingulate system. J Child Psychol Psychiatry. (2003) 44:793–809. doi: 10.1111/1469-7610.00165
124. Stavropoulos KK, Carver LJ. Research review: social motivation and oxytocin in ASD–implications for joint attention development and intervention. J Child Psychol Psychiatry. (2013) 54:603–18. doi: 10.1111/jcpp.12061
125. Becchio C, Bertone C, Castiello U. How the gaze of others influences object processing. Trends Cogn Sci. (2008) 12:254–8. doi: 10.1016/j.tics.2008.04.005
126. Noah JA, Zhang X, Dravida S, Ono Y, Naples A, McPartland JC, et al. (2020). Real-time eye-to-eye contact is associated with cross-brain neural coupling in angular gyrus. Front Hum Neurosci. 14:19. doi: 10.3389/fnhum.2020.00019
127. Jarick M, Laidlaw KE, Nasiopoulos E, Kingstone A. Eye contact affects attention more than arousal as revealed by prospective time estimation. Atten Percept Psychophys. (2016) 78:1302–7. doi: 10.3758/s13414-016-1085-8
128. Koike T, Sumiya M, Nakagawa E, Okazaki S, Sadato N. What makes eye contact special? Neural substrates of on-line mutual eye-gaze: a hyperscanning fMRI study. eNeuro. (2019) 6:ENEURO.0284-18.2019. doi: 10.1523/ENEURO.0284-18.2019
129. Conty L, Gimmig D, Belletier C, George N, Huguet P. The cost of being watched: Stroop interference increases under concomitant eye contact. Cognition. (2010) 115:133–9. doi: 10.1016/j.cognition.2009.12.005
130. George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. (2001) 13:1102–12. doi: 10.1006/nimg.2001.0769
131. Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. (2000) 3:80–4. doi: 10.1038/71152
132. Hooker CI, Paller KA, Gitelman DR, Parrish TB, Mesulam MM, Reber PJ. Brain networks for analyzing eye gaze. Cogn Brain Res. (2003) 17:406–18. doi: 10.1016/S0926-6410(03)00143-5
133. Wallace S, Coleman M, Pascalis O, Bailey A. A study of impaired judgment of eye-gaze direction and related face-processing deficits in autism spectrum disorders. Perception. (2006) 35:1651–64. doi: 10.1068/p5442
134. Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in ASD. Nat Neurosci. (2005) 8:519–26. doi: 10.1038/nn1421
135. Graham R, LaBar KS. Neurocognitive mechanisms of gaze-expression interactions in face processing and social attention. Neuropsychologia. (2012) 50:553–66. doi: 10.1016/j.neuropsychologia.2012.01.019
136. Itier RJ, Alain C, Sedore K, McIntosh AR. Early face processing specificity: it's in the eyes! J Cogn Neurosci. (2007) 19:1815–26. doi: 10.1162/jocn.2007.19.11.1815
137. Simpson EA, Maylott SE, Mitsven SG, Zeng G, Jakobsen KV. Face detection in 2-to 6-month-old infants is influenced by gaze direction and species. Dev Sci. (2020) 23:e12902. doi: 10.1111/desc.12902
138. Stephenson L, Edwards SG, Bayliss A. From gaze perception to social cognition: a neurocognitive model of joint and shared attention. Perspect Psychol Sci. (2021) 16:553–76. doi: 10.1177/1745691620953773
139. Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, et al. Infant neural sensitivity to dynamic eye gaze is associated with later emerging ASD. Curr Biol. (2012) 22:338–42. doi: 10.1016/j.cub.2011.12.056
140. Senju A, Johnson MH. Atypical eye contact in autism: models, mechanisms and development. Neurosci Biobehav Rev. (2009) 33:1204–14. doi: 10.1016/j.neubiorev.2009.06.001
141. Beier JS, Spelke ES. Infants' developing understanding of social gaze. Child Dev. (2012) 83:486–96. doi: 10.1111/j.1467-8624.2011.01702.x
142. Farroni T, Massaccesi S, Pividori D, Johnson MH. Direct gaze modulates face recognition in young infants. Cognition. (2007) 102:396–404. doi: 10.1016/j.cognition.2006.01.007
143. Edwards SG, Stephenson LJ, Dalmaso M, Bayliss AP. Social orienting in gaze leading: a mechanism for shared attention. Proc R Soc B Biol Sci. (2015) 282:11–41. doi: 10.1098/rspb.2015.1141
144. Boyer TW, Harding SM, Bertenthal BI. The temporal dynamics of infants' joint attention: effects of others' gaze cues and manual actions. Cognition. (2020) 197:104151. doi: 10.1016/j.cognition.2019.104151
145. Grynszpan O, Martin JC, Fossati P. Gaze leading is associated with liking. Acta Psychol. (2017) 173:66–72. doi: 10.1016/j.actpsy.2016.12.006
146. Willemse C, Marchesi S, Wykowska A. Robot faces that follow gaze facilitate attentional engagement and increase their likeability. Front Psychol. (2018) 9:70. doi: 10.3389/fpsyg.2018.00070
147. Chevallier C, Parish-Morris J, Tonge N, Le L, Miller J, Schultz RT. Susceptibility to the audience effect explains performance gap between children with and without autism in a theory of mind task. J Exp Psychol Gen. (2014) 143:972. doi: 10.1037/a0035483
148. Grynszpan O, Bouteiller J, Grynszpan S, Le Barillier F, Martin JC, Nadel J. Altered sense of gaze leading in ASD. Res ASD Spect Disord. (2019) 67:101441. doi: 10.1016/j.rasd.2019.101441
149. Mundy P, Kim K, McIntyre N, Lerro L, Jarrold W. Brief report: joint attention and information processing in children with higher functioning ASD spectrum disorders. J ASD Dev Disord. (2016) 46:2555–60. doi: 10.1007/s10803-016-2785-6
150. Zwickel J, White SJ, Coniston D, Senju A, Frith U. Exploring the building blocks of social cognition: spontaneous agency perception and visual perspective taking in autism. Soc Cogn Affect Neurosci. (2011) 6:564–71. doi: 10.1093/scan/nsq088
151. Hatch B, Iosif AM, Chuang A, de la Paz L, Ozonoff S, Miller M. Longitudinal differences in response to name among infants developing ASD and risk for ADHD. J ASD Dev Disord. (2020). 51:827–36. doi: 10.1007/s10803-020-04369-8
152. Moriuchi JM, Klin A, Jones W. Mechanisms of diminished attention to eyes in ASD. Am J Psychiatry. (2017) 174:26–35. doi: 10.1176/appi.ajp.2016.15091222
153. Kim K, Rosenthal MZ, Gwaltney M, Jarrold W, Hatt N, McIntyre N, et al. A virtual joy-stick study of emotional responses and social motivation in children with autism spectrum disorder. J Autism Dev Disord. (2015) 45:3891–9. doi: 10.1007/s10803-014-2036-7
154. Kylliäinen A, Wallace S, Coutanche MN, Leppänen JM, Cusack J, Bailey AJ, et al. Affective–motivational brain responses to direct gaze in children with ASD spectrum disorder. J Child Psychol Psychiatry. (2012) 53:790–7. doi: 10.1111/j.1469-7610.2011.02522.x
155. Kohls G, Yerys B, Schultz RT. Striatal development in autism: repetitive behaviors and the reward circuitry. Biol Psychiatry. (2014) 76:358. doi: 10.1016/j.biopsych.2014.07.010
156. Tillmann J, Tuomainen J, Swettenham J. The effect of visual perceptual load on auditory awareness of social vs. non-social stimuli in individuals with autism. J Autism Dev Disord. (2021) 51:1028–38. doi: 10.1007/s10803-020-04587-0
157. Tschida JE, Yerys BE. A systematic review of the positive valence system in autism spectrum disorder. Neuropsychol Rev. (2021) 31:58–88. doi: 10.1007/s11065-020-09459-z
158. Clements CC, Zoltowski AR, Yankowitz LD, Yerys BE, Schultz RT, Herrington JD. Evaluation of the social motivation hypothesis of autism: a systematic review and meta-analysis. JAMA Psychiatry. (2018) 75:797–808. doi: 10.1001/jamapsychiatry.2018.1100
159. Kasari C, Gulsrud A, Shire S, Strawbridge C. The JASPER Model for Children with Autism: Promoting Joint Attention, Symbolic Play, Engagement, and Regulation. New York: Guilford Publications, NYC. (2021).
160. Schertz HH, Odom SL, Baggett KM, Sideris JH. Effects of joint attention mediated learning for toddlers with autism spectrum disorders: an initial randomized controlled study. Early Child Res Q. (2013) 28:249–58. doi: 10.1016/j.ecresq.2012.06.006
161. Ezell S, Field T, Nadel J, Newton R, Murrey G, Siddalingappa V, et al. Imitation effects on joint attention behaviors of children with ASD. Psychology. (2012) 3:681. doi: 10.4236/psych.2012.39103
162. Gulsrud AC, Hellemann G, Shire S, Kasari C. Isolating active ingredients in a parent-mediated social communication intervention for toddlers with ASD spectrum disorder. J Child Psychol Psychiatry. (2016) 57:606–13. doi: 10.1111/jcpp.12481
163. Ingersoll B. Brief report: effect of a focused imitation intervention on social functioning in children with ASD. J ASD Dev Disord. (2012) 42:1768–73. doi: 10.1007/s10803-011-1423-6
164. Milward SJ, Carpenter M. Joint action and joint attention: drawing parallels between the literatures. Soc Personal Psychol Compass. (2018) 12:e12377. doi: 10.1111/spc3.12377
165. Sebanz N, Bekkering H, Knoblich G. Joint action: bodies and minds moving together. Trends Cogn Sci. (2006) 10:70–6. doi: 10.1016/j.tics.2005.12.009
166. Wolf W, Launay J, Dunbar RI. Joint attention, shared goals, and social bonding. Brit J Psychol. (2016) 107:322–37. doi: 10.1111/bjop.12144
167. Kuhn S, Muller BCN, van Baaren RB, Wietzker A, Dijksterhuis A, Brass M. Why do I like you when you behave like me? Neural mechanisms mediating positive consequences of observing someone being imitated. Soc Neurosci. (2010) 5:384–92. doi: 10.1080/17470911003633750
168. Gordon I, Eilbott JA, Feldman R, Pelphrey KA, Vander Wyk BC. Social, reward, and attention brain networks are involved when online bids for joint attention are met with congruent versus incongruent responses. Soc Neurosci. (2013) 8:544–54. doi: 10.1080/17470919.2013.832374
169. Pfeiffer UJ, Schilbach L, Timmermans B, Kuzmanovic B, Georgescu AL, Bente G, et al. Why we interact: on the functional role of the striatum in the subjective experience of social interaction. Neuroimage. (2014) 101:124–37. doi: 10.1016/j.neuroimage.2014.06.061
170. Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, et al. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J Cogn Neurosci. (2010) 22:2702–15. doi: 10.1162/jocn.2009.21401
171. Hsu CT, Neufeld J, Chakrabarti B. Reduced reward-related neural response to mimicry in individuals with ASD. Eur J Neurosci. (2018) 47:610–8. doi: 10.1111/ejn.13620
172. Greene DJ, Mooshagian E, Kaplan JT, Zaidel E, Iacoboni M. The neural correlates of social attention: automatic orienting to social and nonsocial cues. Psychol Res PRPF. (2009) 73:499–511. doi: 10.1007/s00426-009-0233-3
173. Laidlaw K, Foulsahm T, Kuhn G, Kingstone A. Potential social interactions are important to social attention. Proc Nat Acad Sci USA. (2011) 108:5548–53. doi: 10.1073/pnas.1017022108
174. Lunghi M, Di Giorgio E, Benavides-Varela S, Simion F. Covert orienting of attention in 3-month-old infants: the case of biological motion. Infant Behav Dev. (2020) 58:101422. doi: 10.1016/j.infbeh.2020.101422
175. Richards JE. Localizing the development of covert attention in infants with scalp event-related potentials. Dev Psychol. (2000) 36:91. doi: 10.1037/0012-1649.36.1.91
176. Xie W, Richards JE. The relation between infant covert orienting, sustained attention and brain activity. Brain Topogr. (2017) 30:198–219. doi: 10.1007/s10548-016-0505-3
177. Trevisan D, Roberts N, Lin C, Birmingham E. How do adults and teens with self-declared Autism Spectrum Disorder experience eye contact? A qualitative analysis of first and accounts. PLoS ONE. (2017) 11:e0188446. doi: 10.1371/journal.pone.0188446
178. Kleberg JL, Högström J, Nord M, Bölte S, Serlachius E, Falck-Ytter T. Autistic traits and symptoms of social anxiety are differentially related to attention to others' eyes in social anxiety disorder. J Autism Dev Disord. (2017) 47:3814–21. doi: 10.1007/s10803-016-2978-z
179. Kilford EJ, Garrett E, Blakemore SJ. The development of social cognition in adolescence: an integrated perspective. Neurosci Biobehav Rev. (2016) 70:106–20. doi: 10.1016/j.neubiorev.2016.08.016
180. Mundy P. Individual differences, social attention and the social-motivation hypothesis of ASD. Behav Brain Sci. (2019) 42:1504–49. doi: 10.1017/S0140525X18002509
181. Scheeren AM, Koot HM, Begeer S. Stability and change in social interaction style of children with ASD spectrum disorder: a 4-year follow-up study. ASD Res. (2020) 13:74–81. doi: 10.1002/aur.2201
182. Harrop C, Jones DR, Sasson NJ, Zheng S, Nowell SW, Parish-Morris J. Social and object attention is influenced by biological sex and toy gender-congruence in children with and without ASD. ASD Res. (2020) 13:763–76. doi: 10.1002/aur.2245
183. Thompson J, Parasuraman R. Attention, biological motion, and action recognition. Neuroimage. (2012) 59:4–13. doi: 10.1016/j.neuroimage.2011.05.044
184. Mosner MG, Kinard JL, McWeeny S, Shah JS, Markiewitz ND, Damiano-Goodwin CR, et al. (2017). Vicarious effort-based decision-making in autism spectrum disorders. J Autism Dev Disord. 47, 2992–3006. doi: 10.1007/s10803-017-3220-3
185. McParland A, Gallagher S, Keenan M. Investigating gaze behaviour of children diagnosed with autism spectrum disorders in a classroom setting. J Autism Dev Disord. (2021) 51:4663–78. doi: 10.1007/s10803-021-04906-z
186. Vaughn Van Hecke AV, Mundy P, Block JJ, Delgado CE, Parlade MV, Pomares YB, et al. Infant responding to joint attention, executive processes, and self-regulation in preschool children. Infant Behav Dev. (2012) 35:303–11. doi: 10.1016/j.infbeh.2011.12.001
187. Oberwelland E, Schilbach L, Barisic I, Krall SC, Vogeley K, Fink GR, et al. Look into my eyes: investigating joint attention using interactive eye-tracking and fMRI in a developmental sample. Neuroimage. (2016) 130:248–60. doi: 10.1016/j.neuroimage.2016.02.026
188. Shenhav A, Musslick S, Lieder F, Kool W, Griffiths TL, Cohen JD, et al. Toward a rational and mechanistic account of mental effort. Annu Rev Neurosci. (2017) 40:99–124. doi: 10.1146/annurev-neuro-072116-031526
189. Kelty-Stephen E, Fein DA, Naigles LR. Children with ASD use joint attention and linguistic skill in pronoun development. Lang Acquis. (2020) 27:410–33. doi: 10.1080/10489223.2020.1769626
190. Mizuno A, Liu Y, Williams DL, Keller TA, Minshew NJ, Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. (2011) 134:2422–35. doi: 10.1093/brain/awr151
191. Fischer J, Koldewyn K, Jiang Y, Kanwisher N. Children with ASD spectrum disorder demonstrate normal attentional preference for faces and normal attentional disengagement. J Vis. (2013) 13:942. doi: 10.1167/13.9.942
192. Birmingham E, Johnston KHS, Iarocci G. Spontaneous gaze selection and following during naturalistic social interactions in school-aged children and adolescents with ASD spectrum disorder. Canad J Exp Psychol. (2017) 71:243. doi: 10.1037/cep0000131
193. Freeth M, Morgan E, Bugembe P, Brown A. How accurate are autistic adults and those high in autistic traits at making face-to-face line-of-sight judgements? Autism. (2020) 24:1482–93. doi: 10.1177/1362361320909176
194. Zhao S, Uono S, Yoshimura S, Kubota Y, Toichi M. Atypical gaze cueing pattern in a complex environment in individuals with ASD. J Autism Dev Disord. (2017) 47:1978–86. doi: 10.1007/s10803-017-3116-2
195. Akechi H, Stein T, Senju A, Kikuchi Y, Tojo Y, Osanai H, et al. Absence of preferential unconscious processing of eye contact in adolescents with autism spectrum disorder. Autism Res. (2014) 7:590–7. doi: 10.1002/aur.1397
196. Freeth M, Bugembe P. Social partner gaze direction and conversational phase; factors affecting social attention during face-to-face conversations in autistic adults? Autism. (2019) 23:503–13. doi: 10.1177/1362361318756786
197. Burnside K, Wright K, Poulin-Dubois D. Social orienting predicts implicit false belief understanding in preschoolers. J Exp Child Psychol. (2018) 175:67–79. doi: 10.1016/j.jecp.2018.05.015
198. Carpenter M, Tomasello M. Joint attention, cultural learning, and language acquisition: implications for children with ASD. In: Wetherby AM, Prizant BM, editors. Communication and Language Intervention Series, Vol. 9. ASD Spectrum Disorders: A Transactional Developmental Perspective. Baltimore, MD: Paul H Brookes Publishing (2000) p. 31–54.
199. Ramot M, Walsh C, Reimann GE, Martin A. Distinct neural mechanisms of social orienting and mentalizing revealed by independent measures of neural and eye movement typicality. Commun Biol. (2020) 3:1–11. doi: 10.1038/s42003-020-0771-1
200. Itier RJ, Batty M. Neural bases of eye and gaze processing: the core of social cognition. Neurosci Biobehav Rev. (2009) 33:843–63. doi: 10.1016/j.neubiorev.2009.02.004
201. Baron-Cohen S. Mindblindness: An Essay on ASD and Theory of Mind. Boston, MA: MIT Press (1997).
202. Isaksson J, Van't Westeinde A, Cauvet É, Kuja-Halkola R, Lundin K, Neufeld J, et al. Social cognition in ASD and other neurodevelopmental disorders: a co-twin control study. J ASD Dev Disord. (2019) 49:2838–48. doi: 10.1007/s10803-019-04001-4
203. Pellicano E. The development of core cognitive skills in autism: a 3-year prospective study. Child Dev. (2010) 81:1400–16. doi: 10.1111/j.1467-8624.2010.01481.x
204. Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in ASD. Ment Retard Dev Disabil Res Rev. (2004) 10:259–71. doi: 10.1002/mrdd.20040
205. Risko EF, Laidlaw KE, Freeth M, Foulsham T, Kingstone A. Social attention with real versus reel stimuli: toward an empirical approach to concerns about ecological validity. Front Hum Neurosci. (2012) 6:143. doi: 10.3389/fnhum.2012.00143
206. Morrison KE, DeBrabander KM, Jones DR, Ackerman RA, Sasson NJ. Social cognition, social skill, and social motivation minimally predict social interaction outcomes for autistic and non-autistic adults. Front Psychol. (2020) 11:3282. doi: 10.3389/fpsyg.2020.591100
207. Sasson NJ, Morrison KE, Kelsven S, Pinkham AE. Social cognition as a predictor of functional and social skills in autistic adults without intellectual disability. Autism Res. (2020) 13:259–70. doi: 10.1002/aur.2195
208. Livingston LA, Carr B, Shah P. Recent advances and new directions in measuring theory of mind in autistic adults. J Autism Dev Disord. (2019) 49:1738–44. doi: 10.1007/s10803-018-3823-3
209. Senju A. Atypical development of spontaneous social cognition in autism spectrum disorders. Brain Dev. (2013) 35:96–101. doi: 10.1016/j.braindev.2012.08.002
210. Meyer ML, Taylor SE, Lieberman MD. Social working memory and its distinctive link to social cognitive ability: an fMRI study. Soc Cogn Affect Neurosci. (2015) 10:1338–47. doi: 10.1093/scan/nsv065
211. Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. (2006) 16:235–9. doi: 10.1016/j.conb.2006.03.001
212. Striano T, Reid VM. Social cognition in the first year. Trends Cogn Sci. (2006) 10:471–6. doi: 10.1016/j.tics.2006.08.006
213. Hoehl S, Berthenthal B. An interactionist perspective on the development of coordinated social attention. Adv Child Dev Behav. (2021) 61:1–41. doi: 10.31234/osf.io/s5tgz
214. Mundy P, Sigman M. The theoretical implications of joint-attention deficits in ASD. Dev Psychopathol. (1989) 1:173–83. doi: 10.1017/S0954579400000365
215. Yamaguchi M, Kuhlmeier VA, Wynn K, VanMarle K. Continuity in social cognition from infancy to childhood. Dev Sci. (2009) 12:746–52. doi: 10.1111/j.1467-7687.2008.00813.x
216. Wolf D, Mittelberg I, Rekittke LM, Bhavsar S, Zvyagintsev M, Haeck A, et al. Interpretation of social interactions: functional imaging of cognitive-semiotic categories during naturalistic viewing. Front Hum Neurosci. (2018) 12:296. doi: 10.3389/fnhum.2018.00296
217. Canfield RL, Kirkham NZ. Infant cortical development and the prospective control of saccadic eye movements. Infancy. (2001) 2:197–211. doi: 10.1207/S15327078IN0202_5
218. Johnson MH. Cortical maturation and the development of visual attention in early infancy. J Cogn Neurosci. (1990) 2:81–95. doi: 10.1162/jocn.1990.2.2.81
219. Caruana N, Brock J, Woolgar A. A frontotemporoparietal network common to initiating and responding to joint attention bids. Neuroimage. (2015) 108:34–46. doi: 10.1016/j.neuroimage.2014.12.041
220. Dayan E, Sella I, Mukovskiy A, Douek Y, Giese MA, Malach R, et al. The default mode network differentiates biological from non-biological motion. Cereb Cortex. (2016) 26:234–45. doi: 10.1093/cercor/bhu199
221. Elison JT, Wolff JJ, Heimer DC, Paterson SJ, Gu H, Hazlett HC, et al. Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Dev Sci. (2013) 16:186–97. doi: 10.1111/desc.12015
222. Kühn-Popp N, Kristen S, Paulus M, Meinhardt J, Sodian B. Left hemisphere EEG coherence in infancy predicts infant declarative pointing and preschool epistemic language. Soc Neurosci. (2016) 11:49–59. doi: 10.1080/17470919.2015.1024887
223. Redcay E, Kleiner M, Saxe R. Look at this: the neural correlates of initiating and responding to bids for joint attention. Front Hum Neurosci. (2012) 6:169. doi: 10.3389/fnhum.2012.00169
224. Shepherd SV. Following gaze: gaze-following behavior as a window into social cognition. Front Integr Neurosci. (2010) 4:5. doi: 10.3389/fnint.2010.00005
225. D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. (2007) 19:935–44. doi: 10.1162/jocn.2007.19.6.935
226. Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self-and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. (2012) 24:1742–52. doi: 10.1162/jocn_a_00233
227. Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, et al. Segregation of the human medial prefrontal cortex in social cognition. Front Hum Neurosci. (2013) 7:232. doi: 10.3389/fnhum.2013.00232
228. Baek EC, Scholz C, O'Donnell MB, Falk EB. The value of sharing information: a neural account of information transmission. Psychol Sci. (2017) 28:851–61. doi: 10.1177/0956797617695073
229. Koike T, Tanabe HC, Adachi-Abe S, Okazaki S, Nakagawa E, Sasaki AT, et al. Role of the right anterior insular cortex in joint attention-related identification with a partner. Soc Cogn Affect Neurosci. (2019) 14:1131–45. doi: 10.1093/scan/nsz087
230. Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. (2014) 137:12–32. doi: 10.1093/brain/awt162
231. Herold D, Spengler S, Sajonz B, Usnich T, Bermpohl F. Common and distinct networks for self-referential and social stimulus processing in the human brain. Brain Struct Funct. (2016) 221:3475–85. doi: 10.1007/s00429-015-1113-9
232. Delbruck E, Yang M, Yassine A, Grossman ED. Functional connectivity in ASD: atypical pathways in brain networks supporting action observation and joint attention. Brain Res. (2019) 1706:157–65. doi: 10.1016/j.brainres.2018.10.029
233. Materna S, Dicke PW, Thier P. Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: a functional magnetic resonance imaging study. J Cogn Neurosci. (2008) 20:108–19. doi: 10.1162/jocn.2008.20008
234. Dravida S, Noah JA, Zhang X, Hirsch J. Joint Attention during live person-to-person contact activates rtpj, including a sub-component associated with spontaneous eye-to-eye contact. Front Hum Neurosci. (2020) 14:201. doi: 10.3389/fnhum.2020.00201
235. Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. (2000) 80:953–78. doi: 10.1152/physrev.2000.80.3.953
236. Kirby LA, Moraczewski D, Warnell K, Velnoskey K, Redcay E. Social network size relates to developmental neural sensitivity to biological motion. Dev Cogn Neurosci. (2018) 30:169–77. doi: 10.1016/j.dcn.2018.02.012
237. Odriozola P, Uddin L, Lynch C, Kochalka J, Chen T, Menon V. Insula response and connectivity during social and non-social attention in children with ASD. Soc Cogn Affect Neurosci. (2016) 11:433–44. doi: 10.1093/scan/nsv126
238. Georgescu AL, Kuzmanovic B, Schilbach L, Tepest R, Kulbida R, Bente G, et al. Neural correlates of “social gaze” processing in high-functioning autism under systematic variation of gaze duration. NeuroImage Clin. (2013) 3:340–51. doi: 10.1016/j.nicl.2013.08.014
239. Pitskel NB, Bolling DZ, Hudac CM, Lantz SD, Minshew NJ, Vander Wyk BC, et al. Brain mechanisms for processing direct and averted gaze in individuals with autism. J Autism Dev Disord. (2011) 41:1686–93. doi: 10.1007/s10803-011-1197-x
240. Sumiya M, Okamoto Y, Koike T, Tanigawa T, Okazawa H, Kosaka H, et al. Attenuated activation of the anterior rostral medial prefrontal cortex on self-relevant social reward processing in individuals with ASD spectrum disorder. NeuroImage Clin. (2020) 102249. doi: 10.1016/j.nicl.2020.102249
241. Sato A, Itakura S. Intersubjective action-effect binding: eye contact modulates acquisition of bidirectional association between our and others' actions. Cognition. (2013) 127:383–90. doi: 10.1016/j.cognition.2013.02.010
243. Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol Sci. (2013) 24:1554–62. doi: 10.1177/0956797613475633
244. Hamilton AFDC, Lind F. Audience effects: what can they tell us about social neuroscience, theory of mind and autism? Cult Brain. (2016) 4:159–77. doi: 10.1007/s40167-016-0044-5
245. Hirsch J, Zhang X, Noah JA, Ono Y. (2017). Frontal temporal and parietal systems synchronize within and across brains during live eye-to-eye contact. NeuroImage 157, 314–330. doi: 10.1016/j.neuroimage.2017.06.018
246. Goelman G, Dan R, Stößel G, Tost H, Meyer-Lindenberg A, Bilek E. Bidirectional signal exchanges and their mechanisms during joint attention interaction–a hyperscanning fMRI study. Neuroimage. (2019) 198:242–54. doi: 10.1016/j.neuroimage.2019.05.028
247. Lachat F, Hugueville L, Lemarechal J, Conty L, George N. Oscillatory brain correlates of live joint attention: a dual-EEG study. Front Hum Neurosci. (2012) 6:156. doi: 10.3389/fnhum.2012.00156
248. Gong P, Shen G, Li S, Zhang G, Fang H, Lei L, et al. Genetic variations in COMT and DRD2 modulate attentional bias for affective facial expressions. PLoS ONE. (2013) 8:e81446. doi: 10.1371/journal.pone.0081446
249. Sjaarda CP, Sabbagh M, Wood S, Ward-King J, McNaughton AJ, Hudson ML, et al. Homozygosity for the 10-repeat dopamine transporter (DAT1) allele is associated with reduced EEG response in males with ASD. Res ASD Spectr Disord. (2019) 60:25–35. doi: 10.1016/j.rasd.2018.12.003
250. Yamaguchi Y, Atsumi T, Poirot R, Lee YA, Kato A, Goto Y. Dopamine-dependent visual attention preference to social stimuli in nonhuman primates. Psychopharmacology. (2017) 234:1113–20. doi: 10.1007/s00213-017-4544-6
251. Wu J, Xiao H, Sun H, Zou L, Zhu LQ. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. (2012) 45:605–20. doi: 10.1007/s12035-012-8278-5
252. Jahromi LB, Kasari CL, McCracken JT, Lee LS, Aman MG, McDougle CJ, et al. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J ASD Dev Disord. (2009) 39:395–404. doi: 10.1007/s10803-008-0636-9
253. Lackner C, Sabbagh MA, Hallinan E, Liu X, Holden JJ. Dopamine receptor D4 gene variation predicts preschoolers' developing theory of mind. Dev Sci. (2012) 15:272–80. doi: 10.1111/j.1467-7687.2011.01124.x
254. Zürcher NR, Walsh EC, Phillips RD, Cernasov PM, Tseng CEJ, Dharanikota A, et al. A simultaneous [11 C] raclopride positron emission tomography and functional magnetic resonance imaging investigation of striatal dopamine binding in autism. Transl Psychiatry. (2020) 11:1–11. doi: 10.1038/s41398-020-01170-0
255. Hopkins WD, Keebaugh AC, Reamer LA, Schaeffer J, Schapiro SJ, Young LJ. Genetic influences on receptive joint attention in chimpanzees (Pan troglodytes). Sci Rep. (2014) 4:1–7. doi: 10.1038/srep03774
256. Brooks J, Kano F, Sato Y, Yeow H, Morimura N, Nagasawa M, et al. Divergent effects of oxytocin on eye contact in bonobos and chimpanzees. Psychoneuroendocrinology. (2021) 125:105119. doi: 10.1016/j.psyneuen.2020.105119
257. Wade M, Hoffmann TJ, Wigg K, Jenkins JM. Association between the oxytocin receptor (OXTR) gene and children's social cognition at 18 months. Genes Brain Behav. (2014) 13:603–10. doi: 10.1111/gbb.12148
258. Tops M, Van IJzendoorn MH, Riem MM, Boksem MA, Bakermans-Kranenburg MJ. Oxytocin receptor gene associated with the efficiency of social auditory processing. Front Psychiatry. (2011) 2:60. doi: 10.3389/fpsyt.2011.00060
259. Domes G, Sibold M, Schulze L, Lischke A, Herpertz SC, Heinrichs M. Intranasal oxytocin increases covert attention to positive social cues. Psychol Med. (2013) 43:1747–53. doi: 10.1017/S0033291712002565
260. Nishizato M, Fujisawa TX, Kosaka H, Tomoda A. Developmental changes in social attention and oxytocin levels in infants and children. Sci Rep. (2017) 7:1–10. doi: 10.1038/s41598-017-02368-x
261. Yao S, Becker B, Zhao W, Zhao Z, Kou J, Ma X, et al. Oxytocin modulates attention switching between interoceptive signals and external social cues. Neuropsychopharmacology. (2018) 43:294. doi: 10.1038/npp.2017.189
262. Constantino JN, Kennon-McGill S, Weichselbaum C, Marrus N, Haider A, Glowinski AL, et al. Infant viewing of social scenes is under genetic control and is atypical in ASD. Nature. (2017) 547:340–4. doi: 10.1038/nature22999
263. Wang Y, Wang L, Xu Q, Liu D, Chen L, Troje NF, et al. Heritable aspects of biological motion perception and its covariation with autistic traits. Proc Nat Acad Sci U.S.A. (2018) 115:1937–42. doi: 10.1073/pnas.1714655115
264. Wang L, Wang Y, Xu Q, Liu D, Ji H, Yu Y, et al. Heritability of reflexive social attention triggered by eye gaze and walking direction: common and unique genetic underpinnings. Psychol Med. (2020) 50:475–83. doi: 10.1017/S003329171900031X
265. Gui A, Jones EJ, Wong CC, Meaburn E, Xia B, Pasco G, et al. Leveraging epigenetics to examine differences in developmental trajectories of social attention: a proof-of-principle study of DNA methylation in infants with older siblings with ASD. Infant Behav Dev. (2020) 60:101409. doi: 10.1016/j.infbeh.2019.101409
266. Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. (2009) 13:27–35. doi: 10.1016/j.tics.2008.09.007
267. Bottini S. Social reward processing in individuals with ASD spectrum disorder: a systematic review of the social motivation hypothesis. Res ASD Spectr Disord. (2018) 45:9–26. doi: 10.1016/j.rasd.2017.10.001
268. Bradshaw J, Shic F, Holden AN, Horowitz EJ, Barrett AC, German TC, et al. The use of eye tracking as a biomarker of treatment outcome in a pilot randomized clinical trial for young children with autism. Autism Res. (2019) 12:779–93. doi: 10.1002/aur.2093
269. Gliga T, Bedford R, Charman T, Johnson MH, Baron-Cohen S, Bolton P, et al. Enhanced visual search in infancy predicts emerging ASD symptoms. Curr Biol. (2015) 25:1727–30. doi: 10.1016/j.cub.2015.05.011
270. Mo S, Liang L, Bardikoff N, Sabbagh MA. Shifting visual attention to social and non-social stimuli in ASD spectrum disorders. Res ASD Spectr Disord. (2019) 65:56–64. doi: 10.1016/j.rasd.2019.05.006
271. Brenner LA, Turner KC, Müller RA. Eye movement and visual search: are there elementary abnormalities in autism?. J Autism Dev Disord. (2007) 37:1289–309. doi: 10.1007/s10803-006-0277-9
272. Harrop C, Jones D, Zheng S, Nowell S, Schultz R, ParishMorris J. (2019). Visual attention to faces in children with autism spectrum disorder: are there sex differences? Mol Autism. 10:1–10. doi: 10.1186/s13229-019-0276-2
273. Baranek GT, Woynaroski TG, Nowell S, Turner-Brown L, DuBay M, Crais ER, et al. Cascading effects of attention disengagement and sensory seeking on social symptoms in a community sample of infants at-risk for a future diagnosis of ASD spectrum disorder. Dev Cogn Neurosci. (2018) 29:30–40. doi: 10.1016/j.dcn.2017.08.006
274. Dakopolos AJ, Jahromi LB. Differences in sensory responses among children with ASD spectrum disorder and typical development: links to joint attention and social competence. Infant Child Dev. (2019) 28:e2117. doi: 10.1002/icd.2117
275. Jones EJ, Dawson G, Webb SJ. Sensory hypersensitivity predicts enhanced attention capture by faces in the early development of ASD. Dev Cogn Neurosci. (2018) 29:11–20. doi: 10.1016/j.dcn.2017.04.001
276. Nowell SW, Watson LR, Crais ER, Baranek GT, Faldowski RA, Turner-Brown L. Joint attention and sensory-regulatory features at 13 and 22 months as predictors of preschool language and social-communication outcomes. J Speech Lang Hear Res. (2020) 63:3100–16. doi: 10.1044/2020_JSLHR-20-00036
277. Elsabbagh M, Gliga T, Pickles A, Hudry K, Charman T, Johnson MH, et al. The development of face orienting mechanisms in infants at-risk for ASD. Behav Brain Res. (2013) 251:147–54. doi: 10.1016/j.bbr.2012.07.030
278. Landry R, Bryson SE. Impaired disengagement of attention in young children with ASD. J Child Psychol Psychiatry. (2004) 45:1115–22. doi: 10.1111/j.1469-7610.2004.00304.x
279. Billeci L, Narzisi A, Campatelli G, Crifaci G, Calderoni S, Gagliano A, et al. Disentangling the initiation from the response in joint attention: an eye-tracking study in toddlers with ASD spectrum disorders. Transl Psychiatry. (2016) 6:e808. doi: 10.1038/tp.2016.75
280. Jahromi LB, Chen Y, Dakopolos AJ, Chorneau A. Delay of gratification in preschoolers with and without ASD spectrum disorder: individual differences and links to executive function, emotion regulation, and joint attention. ASD. (2019) 23:1720–31. doi: 10.1177/1362361319828678
281. Nichols KE, Fox N, Mundy P. Joint attention, self-recognition, and neurocognitive function in toddlers. Infancy. (2005) 7:35–51. doi: 10.1207/s15327078in0701_4
282. Bedford R, Pickles A, Gliga T, Elsabbagh M, Charman T, Johnson MH. Additive effects of social and non-social attention during infancy relate to later ASD spectrum disorder. Dev Sci. (2014) 17:612–20. doi: 10.1111/desc.12139
283. Ramsey R, Ward R. Putting the nonsocial into social neuroscience: a role for domain-general priority maps during social interactions. Perspect Psychol Sci. (2020) 15:1076–94. doi: 10.1177/1745691620904972
284. Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. (2010) 20:327–48. doi: 10.1007/s11065-010-9148-4
285. Ganguly K, Poo MM. Activity-dependent neural plasticity from bench to bedside. Neuron. (2013) 80:729–41. doi: 10.1016/j.neuron.2013.10.028
286. Gobel MS, Kim HS, Richardson DC. The dual function of social gaze. Cognition. (2015) 136:359–64. doi: 10.1016/j.cognition.2014.11.040
287. Hasson U, Frith CD. (2016). Mirroring and beyond: coupled dynamics as a generalized framework for modeling social interactions. Philos Trans R Soc Lond B Biol Sci. 371:20150366. doi: 10.1098/rstb.2015.0366
288. Yoshioka A, Tanabe HC, Sumiya M, Nakagawa E, Okazaki S, Koike T, et al. Neural substrates of shared visual experiences: a hyperscanning fMRI study. Soc Cogn Affect Neurosci. (2021) 1:12. doi: 10.1093/scan/nsab082
289. Misaki M, Kerr KL, Ratliff EL, Cosgrove KT, Simmons WK, Morris AS, et al. Beyond synchrony: the capacity of fMRI hyperscanning for the study of human social interaction. Soc Cogn Affect Neurosci. (2021) 16:84–92. doi: 10.1093/scan/nsaa143
290. Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. (2003) 4:165–78. doi: 10.1038/nrn1056
291. Bayliss AP, Bartlett J, Naughtin CK, Kritikos A. A direct link between gaze perception and social attention. J Exp Psychol Hum Percept Perform. (2011) 37:634. doi: 10.1037/a0020559
292. Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. (2012) 16:559–72. doi: 10.1016/j.tics.2012.09.006
293. Demolliens M, Isbaine F, Takerkart S, Huguet P, Boussaoud D. Social and asocial prefrontal cortex neurons: a new look at social facilitation and the social brain. Soc Cogn Affect Neurosci. (2017) 12:1241–8. doi: 10.1093/scan/nsx053
294. Harris LT, McClure SM, Van Den Bos W, Cohen JD, Fiske ST. Regions of the MPFC differentially tuned to social and nonsocial affective evaluation. Cogn Affect Behav Neurosci. (2007) 7:309–16. doi: 10.3758/CABN.7.4.309
295. Wang Q, Hoi SP, Wang Y, Lam CM, Fang F, Yi L. Gaze response to others' gaze following in children with and without ASD. J Abnorm Psychol. (2020) 129:320–9. doi: 10.1037/abn0000498
296. Jarick M, Kingstone A. The duality of gaze: eyes extract and signal social information during sustained cooperative and competitive dyadic gaze. Front Psychol. (2015) 6:1423. doi: 10.3389/fpsyg.2015.01423
297. Mundy P, Novotny S, Swain-Lerro L, McIntyre N, Zajic M, Oswald T. Joint-attention and the social phenotype of school-aged children with ASD. Grant Submiss. (2017) 47:1423–35. doi: 10.1007/s10803-017-3061-0
Keywords: joint attention, social orienting, social motivation, face processing, diagnosis, intervention, social-cognitive neuroscience, genetics
Citation: Mundy P and Bullen J (2022) The Bidirectional Social-Cognitive Mechanisms of the Social-Attention Symptoms of Autism. Front. Psychiatry 12:752274. doi: 10.3389/fpsyt.2021.752274
Received: 02 August 2021; Accepted: 20 December 2021;
Published: 31 January 2022.
Edited by:
Ahmad Abu-Akel, University of Lausanne, SwitzerlandReviewed by:
Katherine Rice Warnell, Texas State University, United StatesCopyright © 2022 Mundy and Bullen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Mundy, cGNtdW5keUB1Y2RhdmlzLmVkdQ==
†ORCID: Peter Mundy orcid.org/0000-0002-2484-5206
Jenifer Bullen orcid.org/0000-0002-2199-082X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.