- 1Department of Dynamic and Clinical Psychology, and Health Studies, Sapienza University of Rome, Rome, Italy
- 2Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy
- 3Unit of Neurology, Neurobiology, Neurophysiology and Psychiatry, Department of Medicine, Campus Bio-Medico University, Rome, Italy
- 4IRCCS – Fondazione Santa Lucia, Rome, Italy
A considerable body of literature reports that individuals with psychotic disorders often suffer from sexual dysfunctions (SDs), with these representing a major unmet need. Long-term antipsychotic drug treatment may be the main cause for SDs in psychotic patients, through a plethora of different mechanisms, including prolactin dyscrasia, histamine-mediated sedation, and serotonin-induced sexual demotivation. However, a few pieces of evidence treat sexuality in patients at risk or the onset of psychosis. For this purpose, we systematically reviewed literature of the last 10 years in order to investigate sexuality in ultra-high risk (UHR) for psychosis and first-episode psychosis (FEP). We included in our review 34 articles fitting our research criteria on SDs in UHR and FEP. Evidence of SDs in the transition from UHR to FEP emerges through the selected studies. In FEP, sexuality is affected by the severity of the psychotic symptoms and, in some cases, by the iatrogenic effects of psychopharmacological treatment. Further experimental and clinical studies should systematically investigate the role of sexual functioning in the transition from UHR to FEP and, consequently, clarify whether or not SDs could be considered a possible marker for the onset of psychosis in at-risk populations. Moreover, psychiatrists and clinical psychologists should take into consideration the role of sexual life in young people with prodromal mental symptoms or at the onset of psychosis. Focusing on a thorough sexual evaluation might be a major challenge that could break down barriers of mental health promotion among young people with schizophrenia-spectrum disorders and therefore achieve better clinical outcomes.
Introduction
Psychotic spectrum disorders (PSDs), e.g., schizophrenia. schizoaffective disorder, affective disorders with psychotic manifestations, are devastating conditions generally accompanied by a consistent number of medical comorbidities (1).
It is well-established in literature that individuals with psychiatric disorders, psychosis in particular, can often suffer from sexual dysfunctions (SDs) (2). Indeed, a major cause of impairment may be the patients' long-term drug treatment, which affects sexuality through a plethora of different mechanisms, including prolactin dyscrasia, histamine-mediated sedation, and serotonin-induced sexual demotivation (3, 4). Sexual function (SF) can be impaired in many domains, ranging from desire, arousal, erection, and ejaculation in males, to orgasm (5, 6). Therefore, it is easy to understand how the prevalence of sexual dysfunctions in psychotic patients is much higher than in the general population (7).
First-episode psychosis (FEP) is defined as the first time a person experiences psychosis or psychotic symptoms. Patients with FEP go through different periods of transition, with phases of well-being alternating with prodromal states characterized by (i) non-psychotic behavioral disorders (such as depression or obsessive-compulsive symptoms); (ii) attenuated psychotic symptoms that do not require treatment; (iii) psychotic symptoms that require initial antipsychotic drug therapy; (iv) a formal diagnosis of the schizophreniform disorder and, subsequently, of schizophrenia (8).
The trajectory to developing first-episode psychosis was systematized in the last years by the concept of ultra-high risk (UHR), a sub-clinical period characterized by one of the following conditions and criteria: the attenuated psychotic symptom (APS) criterion, the brief limited intermittent psychotic symptom (BLIPS) criterion, and the genetic risk and functional decline (GRFD) criterion (9). Clinicians and researchers consider this period and the presence of UHR criteria as a possible phase to prevent a possible imminent frank psychosis (10).
Within the context of these clinical scenarios, it is useful to understand the role of sexuality. Sexuality itself has a large number of meanings, namely symbolizations, perceptions, impulses. Sexuality can easily fit inside a delusional, erotomaniac, or persecutory frame (11). On the other hand, a recent and relevant overview article pointed out the importance of considering sexuality in the psychiatric field and severe mental illnesses (12).
It is well-established in literature how people with psychotic spectrum disorders have serious impairments in the theory of mind (ToM) domain (13). ToM refers to the mental ability, defined as mentalization, to understand other people's behavior, considering it the result of different mental states. Since interpersonal relationships and intimacy play a key role in early psychosexual development, it is easy to imagine how mentalization among these patients might be crucial during these years. A recent review article has indeed underscored how dating might be extremely challenging for people with psychotic disorders, as romantic and intimate relationships are often precluded for such patients (14). From a psychodynamic point of view, a disrupted ToM might result in a compromised capacity to integrate sexual states in one's own developing identity, therefore producing high levels of distress (15). To this end, it is not surprising that early evidence has indeed shown how a poor premorbid sociosexual functioning is associated with a greater severity of negative symptoms as well as current social withdrawal (16).
UHR and FEP are critical moments in the clinical history of psychosis. During these times, sexuality is indeed completely reworked in its symbolic-relational connotations, characterized by remarkable impairments, and delusional contents. In addition, it also remains a major unmet need in the life of psychotic patients. Some interesting studies have indeed found that clinicians often fail to evaluate sexual problems, with this having dramatic repercussions on the partner, on the couple, on the therapy itself, and eventually on the overall quality of life (QOL) (17). In a recent article, the Authors surveyed a group of 750 psychiatrists, investigating their clinical care algorithms; the findings suggested how little importance is generally given to sexual health (18). In particular, only up to 3% of clinicians reported conduct a thorough sexual assessment on people with severe mental illnesses.
Since sexual dysfunction deeply weighs on QOL (19), it is easy to understand how neglecting sexual health might represent a relevant barrier in mental health promotion and a dangerous risk factor among young people with a clinical high risk of developing full-blown schizophrenia. In keeping more closely with this issue, two recent review articles discuss how important it is to better assess intimacy and sexuality among young people with FEP and psychotic disorders in general (14, 20). However, to date, a comprehensive review of sexual dysfunction among patients with a clinical high risk of developing psychosis is still missing.
Considering that sexual health is a perfect crossover between mental and physical health and yet a major unmet need among people with UHR and FEP, the aim of this present article is to enhance awareness on this important topic. For this purpose, we reviewed the most relevant original experimental articles found in literature on the relationship between sexuality and psychosis in UHR and FEP individuals.
Methods
Search Strategies
For this review, a thorough analysis was conducted of literature focused on Sexual functioning, First Episode Psychosis, and Ultra-High Risk for psychosis.
A computerized search was performed to identify a full relevant experimental article in PubMed, Web of Science, and Scopus on sexual functioning in First-Episode Psychosis and Ultra-High Risk for psychosis, published from January 2010 up to December 2020.
The following search terms were used: “First Episode Psychosis” AND “Sexuality” OR “First Episode Psychosis” AND “Sexual Dysfunction” OR “First Episode Psychosis” AND “Sexual Behavior” OR “Ultra-High Risk for Psychosis” AND “Sexuality” OR “Ultra-High Risk for psychosis” AND “Sexual Dysfunction” OR “Ultra-High Risk for psychosis” AND “Sexual Behavior.”
Inclusion criteria were: English published studies with a high/medium level of evidence according to the Canadian Task Force on the Periodic Health Examination (21), i.e., randomized controlled trials (RCTs), case-control, and cross-sectional studies; the articles must include patients diagnosed with either FEP or UHR for psychosis according to the DSM-V (1) or ICD-10/ICD-11 (22).
Exclusion criteria were review articles, books/book chapters, editorials, theses; studies concerning characteristics of personality not connected to sexuality; chemical, biological, and other field studies different from sexology and sexual medicine. Figure 1 represents the flowchart showing further details on the literature search and the selection of articles included in this literature review.
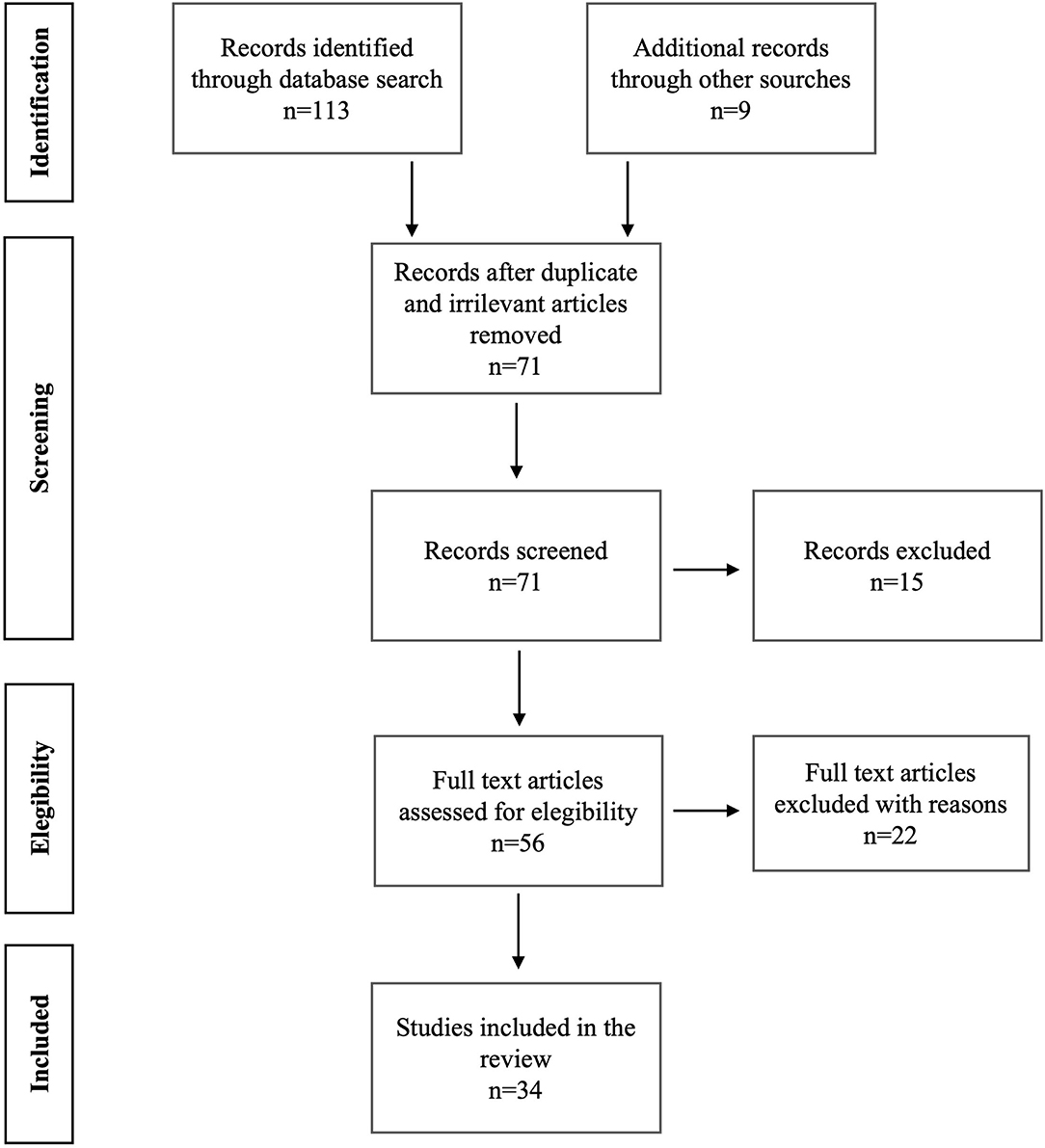
Figure 1. Prisma figure shows the search strategies performed in Pubmed, Scopus, and Web of Science. It also reports the literature results we found.
Results
A total of 113 articles were retrieved on PubMed, Scopus, and Web of Science. Subsequently, 47 articles were selected based on their abstracts. Finally, 34 articles, of which 1 was a RCT, 7 were retrospective studies and 26 were cross-sectional studies, fully satisfied the criteria for the review (Figure 1, Table 1). We broke down these literature results between UHR and FEP into the following paragraphs.

Table 1. Studies inserted in the review analysis about the sexuality in ultra-high risk for psychosis and first-episode psychosis.
Ultra-High Risk for Psychosis and Sexuality
Traditionally, sexual dysfunction in psychotic illnesses has always been linked to adverse side effects of psychopharmacology. However, sexuality can be severely impaired by numerous factors regardless of pharmacotherapy, like premorbid personality, i.e., schizoid or schizotypal, psychopathology itself, as a result of the negative effect of psychosis on personal and sexual relationships, and medical comorbidities that frequently occur in these patients (58). Indeed, a proof of concept of this was provided by a previous study where sexual function in UHR people was compared with healthy control subjects and with first-episode psychosis patients (28). The Authors demonstrated that UHR people that subsequently became full-blown psychotic patients primarily revealed greater sexual impairment compared to UHR individuals who did not develop psychosis. This evidence was not related to the gender or to the side effects of any antipsychotic treatment, as the UHR people were not undergoing any psychopharmacological therapy (28). In light of this study, it is possible to consider sexual dysfunctions as manifestations of UHR criteria-related psychotic symptoms. Moreover, it is also interesting to notice how the sexual content of thinking may be a direct consequence of early-life sexual abuse among young people with clinical high risk for psychosis.
UHR and Sexual Trauma
Although it is well-established how early trauma represents a vulnerability factor toward psychosis (59), previous research has also pointed out how the sexual nature of delusion demonstrates a close relationship with a past episode of sexual trauma or sexual abuse. For instance, being watched in the shower/toilet or undressing were the most frequent sexual delusions to be reported in UHR people (32). Generally, the relationship between sexual trauma and the UHR condition is mostly characterized by perceptual disturbances with sexual contents (35), symptomatologic elements that reveal once again to be predictive for psychosis.
In this regard, most of the experimental articles we reviewed affirmed that sexual abuse, physical abuse, and sexual trauma in childhood represent the main predictors for developing a frank psychosis in adulthood. More than 20% of people with UHR for psychosis had an experience of sexual abuse during childhood (24, 25, 31). Consistent with the literature, this dramatic evidence is mostly related both to the female gender and to higher levels of childhood trauma (33, 35).
Young individuals with at risk mental states who suffered from sexual abuse are also reported to have a higher severity and frequency of psychotic experiences (30, 53).
Moreover, recent evidence reported that UHR patients with a past clinical history of sexual abuse feature both cognitive and neuroanatomical impairments. In particular, patients with a history of physical trauma performed worse in attention, interference inhibition, working memory, and cognitive flexibility. Significant cortical thickness reductions in prefrontal and temporal brain regions were also observed in individuals at UHR for psychosis who reported moderate to high levels of childhood sexual abuse (29, 34). In addition, a study found that the striatal dopamine function, whose system is well-known to be impaired in PSDs, is increased in people with experiences of sexual abuse (27).
Therefore, it is possible to hypothesize that sexual trauma in childhood might represent a primum movens in people with UHR, with a devastating impact on the neuroanatomical and neurophysiological development.
First Episode Psychosis and Sexuality
The overall prevalence of sexual impairments in an FEP study population may vary from 13 to 64%, mostly depending on how patients were both selected and assessed (47, 49, 52). The European First Episode Schizophrenia Trial (EUFEST) reported dysfunctions in many domains of sexuality, i.e., orgasm (15%), erectile dysfunction (17%), and decreased libido (30.8%) in patients that were randomly assigned to various antipsychotics. Findings also showed that the main side effects caused by the increase in prolactin resulted in amenorrhea, galactorrhea, and gynecomastia (50).
As these patients are often young, it is important to investigate sexual health during the psychiatric evaluation (39). For instance, people with psychiatric disorders are more prone to engage in risky sexual behaviors, with psychotic patients ranking among the top positions (60). Notably, young people with FEP are more likely to have unprotected sexual intercourse compared to their peers. Previous research highlighted how younger age, absence of peer support, unemployment, and clinical status were associated with an increased probability of condom misuse in these patients (41). Sexual knowledge among such patients is poor, not only from a physical and technical standpoint (i.e., how to perform sexual intercourse or other sexual practices) but also in terms of prevention of sexually transmissible infections (STIs) (61).
Hence, it is easy to understand how people with FEP are at greater risk of contracting STIs, therefore needing significant prevention strategies and risk-reduction measures (42).
FEP and Sexual Trauma
It is not surprising to see that, similarly to UHR patients, consistent evidence of sexual abuse during childhood among people with FEP is also present in literature. A recent systematic review has indeed pointed out how its prevalence spans from 6 to 40%, with women suffering more sexual abuse compared to men (62). Moreover, the impact of childhood trauma tends to be greater on later-stage symptoms. For instance, FEP patients who suffered sexual abuse showed more positive and depressive symptoms as well as more severe delusions and hallucinations compared to their counterparts (37). Consistent with this, sexually abused patients with FEP show poor premorbid functioning, longer DUP, fewer years of education, past history of suicide attempts, and other psychiatric disorders; moreover, they are more likely to be diagnosed with a lifetime substance use disorder (43).
This evidence is backed up by an interesting model, known as the traumagenic neurodevelopmental model of psychosis (TN), which links childhood sexual maltreatments in people with FEP to functional and structural brain alterations during young adulthood (63). TN posits how hypothalamic-pituitary-adrenal axis (HPA) alterations, structural cerebral changes, such as frontal lobe and hippocampus abnormalities, dopamine system impairments, and psychiatric comorbidities are consistently more frequent in FEP patients who suffered from sexual trauma. For instance, sexual abuse exerts a divergent effect on HPA axis activity in patients with FEP and controls, with FEP showing a reduced cortisol awakening response and a less reactive HPA axis (45). Sexually maltreated people with FEP also reported higher levels of dissociative symptoms compared to controls (38). To this end, childhood sexual abuse, physical abuse, and parental separation are reported to be significantly associated with higher positive and negative symptoms (30, 36), more intense psychotic experiences (53), more frequent diagnosis of affective psychosis and higher rates of cannabis and heroin abuse (56) among young patients with FEP. Interestingly, recent evidence reported how sexual trauma may also impact at a molecular and genetic level, with sexually abused FEP young individuals showing a lower methylation of the FKBP5 gene (51) and lower brain-derived neurotrophic factor (BDNF) plasmatic levels (54).
lower brain-derived neurotrophic factor (BDNF) levelsŤ]
These findings underline and confirm what is extensively reported in literature; i.e., that early trauma deeply impacts on the development and severity of psychosis (64) (Figure 2).
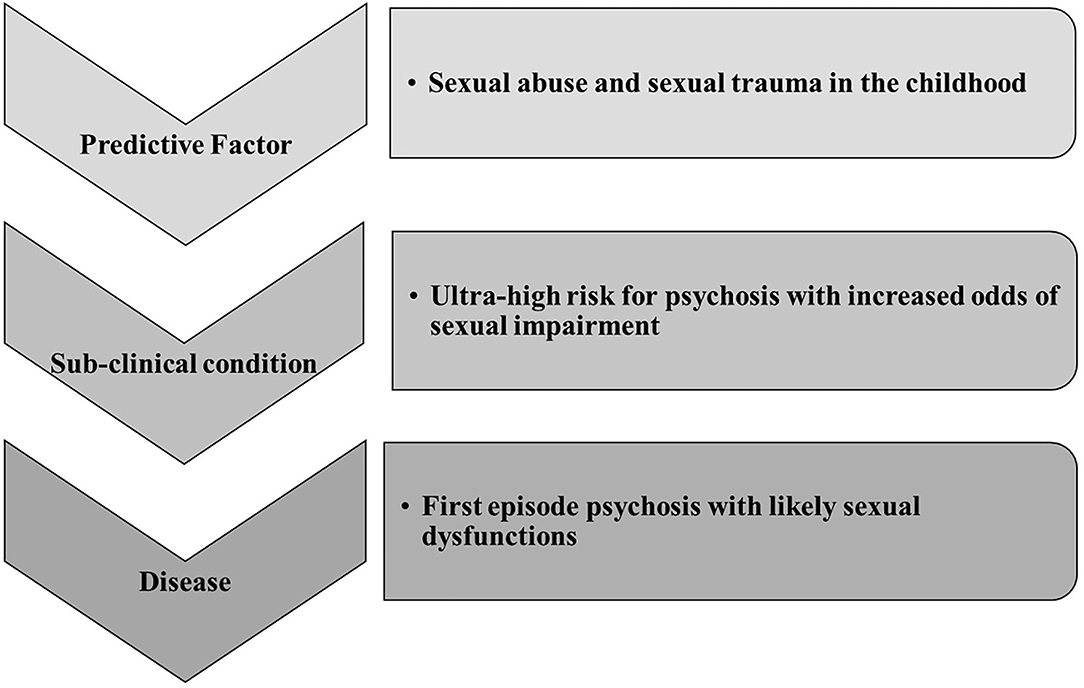
Figure 2. Our literature review is summarized in this figure showing the sexological trajectory of psychosis. It is based on literature finding mainly focused on sexual abuses experience. Sexual trauma can be considered a central etiological factor for UHR, FEP, and then psychosis. However, major clinical and research attention should be posed to sexual impairment during the UHR phase.
FEP and Gender
If in recent years psychopathology has focused more on what is generically defined as gender medicine, in the case of sexological evaluation in patients with FEP a genderized approach takes on clinical and theoretical relevance (65). For example, literature suggests that men show more negative and obsessive-compulsive symptoms, are more prone to drug abuse and develop symptoms earlier than women (66). To date, however, only a few studies offered a dichotomous perspective on gender differences of sexual dysfunctions in FEP (44, 46, 55, 65–67). Apart from the prevalence, which is slightly the same in both sexes, a conflicting result is found in two studies. In the first study, the authors described how vaginal lubrification and orgasm are significatively correlated with either general or positive PANSS scores, without finding any similar homolog association among male subjects (44). On the other hand, the second study reported that sexual functioning was negatively correlated with disorganized and positive symptoms in males and with higher scores in negative symptoms among females (46). Finally, a study on SF and metabolic structure among men found a correlation between higher Sexual Function Questionnaire scores (i.e., worse sexual outcome) and BMI, leptin levels, and waist-hip ratio (55). This highlights how obesity could play a mediating role between psychopathology and sexual dysfunction, with hormonal alterations in testosterone levels in young male patients with psychosis. A recent study evaluated 40 male subjects and found that men with FEP have a higher probability of developing erectile dysfunction (ED), with a DUP increasing the risk of both ED and hyperprolactinemia (48). Women, on the other hand, have more resources and social support during the pre-crisis phase, with levels of social functioning playing a protective role, although they show more depressive, anxious, and affective symptoms (67). Although these findings are scarce and poorly consistent with each other, they highlight the importance of considering a gender-based perspective in sexual function among patients with FEP in order to obtain a better therapeutic outcome.
Psychopharmacotherapy and Pathophysiology in FEP
Unlike UHR, people with FEP have generally had their first encounter with a specialist. As they are most commonly under psychopharmacological therapy, sexual dysfunctions are certainly enhanced by adverse side effects of antipsychotics (28). Rates of sexual dysfunction recurrence are indeed higher in FEP people under psychopharmacotherapies. To this end, a recent study conducted on a very large sample, within the EUFEST protocol, investigated these aspects during the first year of drug treatment, with particular attention to hormonal alterations and sexual dysfunctions (50). The authors indicated that other than age and general PANSS score, prolactin levels in the blood also predicted erectile dysfunctions in males.
Consistent with this, another study on 243 patients diagnosed with FEP and treated with antipsychotics found a prevalence in sexual impairment of 46% (2). The authors also reported that risperidone weighed on sexuality in a dose-dependent manner, causing more severe sexual dysfunctions compared to other active principles. Moreover, at the univariate logistic regression, risperidone showed a 7-fold increased risk of developing SDs.
However, a growing body of evidence stresses the importance of the disease pathophysiology as a major cause of sexual impairment in drug-naïve patients with FEP. Multiple studies point out how impaired sexuality may be either predicted by or correlated to heavier psychotic symptomatology and longer DUP, without showing any association with hormones blood levels, like prolactin, or testosterone (46, 47, 50, 52), although hyperprolactinemia may be a clinical feature of drug-naïve people with FEP (68). Moreover, these results are consistent with and endorsed by evidence from FEP individuals treated either with olanzapine or risperidone (57). Although patients in the drug groups reported a higher prevalence of sexual dysfunctions (without any difference between olanzapine and risperidone) compared to controls, no relationship between medication, hormones, frequency of sexual activity, and overall sexual satisfaction was found.
Discussion and Conclusions
When a physical, psychic, social, or relational problem arises, sexuality is almost always involved in its dysfunctional declination. Over time, a large body of literature has focused on the role of sexual dysfunction as a prodromal symptom of a possible psychotic onset (28). This omnipresent feature of sexuality during either a chronic or transient state of illness takes on considerable importance in young people. This is indeed testified by the families of FEP patients, whose major concerns for their offspring, along with substance abuse and self-esteem, is also sexuality and intimacy (69).
Similarly to major depression, in UHR individuals and people with FEP sexual dysfunctions may be considered as a prodrome or a consequence of either psychopathology or psychopharmacology.
Most of the early evidence indeed underscores a powerful intrinsic influence of psychosis itself on sexual functioning. Possible explanations for this might be found in the neurobiological mechanisms underpinning both sexual dysfunctions and schizophrenia. In a recent review article of five studies about the impact of psychosis on sexual functioning, Varga-Cáceres et al. hypothesized the role of a striatal dopaminergic dysregulation as a major cause of dysfunction in sexual life among unmedicated psychotic patients (20). Although the striatal dopaminergic pathway is key in reward-predicting cues, and therefore in sexual motivation (70), other possible explanations must be taken into account to better define the intricate relationship between these two conditions. In addition to dopamine and striatum, psychosis, and sexual disorders share in fact impairments both at other neurotransmitters and at different brain networks level. From a neurotransmitter perspective, we would like to focus on glutamate, oxytocin, and endocannabinoids. Firstly, it is well-established in literature that schizophrenia is characterized by high levels of glutamate in the prefrontal cortex (71). Interestingly, a recent article highlighted how glutamate afferents from the prefrontal cortex modulate the activation of the nucleus accumbens, a key hub for pleasure and reward, in female rodents during sexual intercourse (72). Keeping this in mind, we infer that impaired levels of glutamate in psychosis might be responsible for altered sexual functioning. Secondly, extensive data suggests that impairments in social life occurring in psychosis may also be explained by a dysfunctional oxytocinergic system, a key modulator of emotional, sexual, and social processes (73, 74). Disorders in ejaculation and consequently in orgasm may indeed be linked to a compromised dopamine and oxytocin pathways between the hypothalamus and the limbic structures (75). Thirdly, the endocannabinoid system (ECS) is considered a major modulator of the hedonic effects of natural rewards, such as sexual activity, with endogenous cannabinoids showing a significant relationship with sexual arousal (76, 77). As ECS has shown to be impaired in individuals with psychotic disorders, including those at the early stages of the illness without antipsychotic treatment (78), we might also hypothesize that altered levels of endocannabinoids might be another important etiology of sexual dysfunctions in psychosis.
From a brain network-based perspective, an altered mesocortical pathway may also be responsible for an impaired sexual drive in psychosis. The dorsolateral prefrontal cortex, an area involved in goal-directed behaviors and motivational significance to sexual stimuli (79), is mainly modulated by dopamine from the ventral tegmentum. As its functional alterations are largely reported in psychosis, it would explain why psychosis itself, featuring flattened affect, anhedonia, reduced social drive, and loss of motivation, may account for impaired sexual desire (80).
Finally, there have been several attempts to define psychosis as a disconnection syndrome. In the light of this, we might postulate that dysfunctions in the three major resting-state networks, i.e. default mode, salience, and central executive networks, which account for most of the behavioral, cognitive, and social deficits of psychosis, might also be an explanation for sexual deficits among people with psychotic disorders (81). In other words, as this model seems to explain the neural bases of intimacy and empathy, it might also be interpreted as a neurophysiological biotype for the comorbidity between psychosis and sexual dysfunctions.
On the other hand, extensive literature has grown on the fact that antipsychotics may impact sexual functioning, mostly because they affect the tuberoinfundibular pathway, therefore resulting in high levels of prolactinemia (82). For this reason, it is of vital importance to actively implement new therapeutical strategies to lower the incidence of these adverse effects. For instance, in addition to the well-known aripiprazole, whose switching (83) or augmentation (84) are now established among therapeutic options, new drugs are proving to decrease their impact on sexual functioning. In particular, both brexpiprazole (85) and the recently introduced SEP-363856 (86) have shown significantly low levels of prolactinemia in patients with schizophrenia.
After our screening of literature, several issues could be considered as lacking in the assessment and prevention of a possible mental disease. For example, a well-known phenomenon of compulsive masturbation related to psychiatric distress was not studied in light of the UHR or FEP condition (87), although it should be considered as one of the markers for the potential development of severe mental illness.
At the same time, hypersexuality and compulsive use of pornography could be indicators of psychosocial distress in more fragile personalities. Therefore, clinicians and researchers should take into consideration a possible relationship between these dysfunctional aspects of sexual behavior and a predisposition toward psychopathology, above all in young adults and adolescents (88, 89). Another understudied aspect, but likely central for psychologically-vulnerable males, is penile dysmorphia, a specific sexual symptom comparable to body dysmorphic disorder. If this symptom is often treated in the urological and andrological fields, with potential surgical approaches, a psychiatric assessment would be highly recommended when penile size becomes an obsession (90).
Hence, the behavioral spectrum related to the predictive role of sexual behavior toward psychopathology or dysfunctional condition is large and variegated. Literature mainly investigated sexual functioning and sexual history related to a traumatic experience in association with FEP and UHR, although other behavioral phenomena could be correlated to the exacerbation of psychopathology in youths. In this regard, clinical protocols should take into consideration more adequate assessment praxis along with the standardized psychometric procedure. To date, many psychometric tools are available to assess sexual behavior in all its facets, from sexual functioning to dysregulated and compulsive sexual behavior. These questionnaires should be integrated into psychodiagnostics in order to detect and possibly prevent a potential predictive association between sexual problems and possible mental disease.
Nevertheless, sexuality has to be considered as one of the major unmet needs and, likewise, sexual impairments as an important barrier to mental health promotion for young people with schizophrenia-spectrum diseases (91). Young people with UHR or FEP might be indeed facing what could be defined as a “syndemics.” Although this term is most specifically used within contexts of infectious diseases, syndemics refers to a biosocial model in which two or more medical conditions co-occur and interact leading to worse negative health outcomes (92). This model may also be applied to sexuality among young people with a clinical high risk of psychosis. Disease-related sexual dysfunctions, higher rates of sexual risky behavior, STIs, and sexual self-stigma, together with the well-known array of psychotic symptoms may, in fact, synergistically enhance feelings of worthlessness and increase social isolation (93). It is therefore easy to understand how this may dramatically affect an already delicate doctor-patient relationship, especially in a population of young individuals. Having such an unmet need might take away useful pathways of communication between the patient and the psychiatrist/clinical psychologist, therefore leading to worse clinical outcomes and higher rates of therapy drop-outs.
Thus, focusing on a thorough sexual evaluation might be a major challenge that could break down barriers of mental health promotion among young people with schizophrenia-spectrum diseases.
In conclusion, the clinician's defensive denial of a patient's sexuality can significantly affect the treatment, therefore hindering the achievement of a general well-being to which every therapeutic practice should aspire. With this in mind, psychiatry and clinical psychology should considerably include sexual assessment, as well as sexological expertise, in their research and clinical praxis.
Although this review benefits of a broad focus, our study suffers from a number of major limitations. Firstly, the vast majority of the studies were cross-sectional, with only one RCT. This means that no follow-up data was present; thus caution must be taken when generalizing these findings. Secondly, the high heterogeneity of studies did not allow to use a metanalytic approach, which would have been desirable in order to draw more consistent conclusions on this topic. Thirdly, none of the mentioned studies used a structural equation modeling approach, that would allow to gain information on the possible modulating or moderating effects of certain variables (i.e., gender, type of psychosis, etc.) on other variables (such as desire, arousal, orgasm, or overall sexual behavior). For this reason, with this article we aim to encourage future studies that will shed a brighter light on psychosis and sexual functioning.
Author Contributions
GC: manuscript drafting and conceptualization. TBJ: investigation and manuscript drafting. MR and RR: literature review. CN, AS, and EAJ: data curation and literature review. GDL: supervision and project administration. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mariano A, Di Lorenzo G, Jannini TB, Santini R, Bertinelli E, Siracusano A, et al. Medical comorbidities in 181 Patients with bipolar disorder vs. schizophrenia and related psychotic disorders: findings from a single-center, retrospective study from an acute inpatients psychiatric unit. Front Psychiatry. (2021) 12:702789. doi: 10.3389/fpsyt.2021.702789
2. Montejo AL, Majadas S, Rico-Villademoros F, Llorca G, De La Gandara J, Franco M, et al. Frequency of sexual dysfunction in patients with a psychotic disorder receiving antipsychotics. J Sex Med. (2010) 7:3404–13. doi: 10.1111/j.1743-6109.2010.01709.x
3. Nunes LV, Moreira HC, Razzouk D, Nunes SO, Mari de J. Strategies for the treatment of antipsychotic-induced sexual dysfunction and/or hyperprolactinemia among patients of the schizophrenia spectrum: a review. J Sex Marital Ther. (2012) 38:281–301. doi: 10.1080/0092623X.2011.606883
4. Park YW, Kim Y, Lee JH. Antipsychotic-induced sexual dysfunction and its management. World J Mens Health. (2012) 30:153–9. doi: 10.5534/wjmh.2012.30.3.153
5. Smith SM, O'Keane V, Murray R. Sexual dysfunction in patients taking conventional antipsychotic medication. Br J Psychiatry. (2002) 181:49–55. doi: 10.1192/bjp.181.1.49
6. Baggaley M. Sexual dysfunction in schizophrenia: focus on recent evidence. Hum Psychopharmacol. (2008) 23:201–9. doi: 10.1002/hup.924
7. Bourdeau G, Masse M, Lecomte T. Social functioning in early psychosis: are all the domains predicted by the same variables? Early Interv Psychiatry. (2012) 6:317–21. doi: 10.1111/j.1751-7893.2011.00337.x
8. Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry. (2017) 16:251–65. doi: 10.1002/wps.20446
9. Schultze-Lutter F, Michel C, Schmidt SJ, Schimmelmann BG, Maric NP, Salokangas RKR, et al. EPA guidance on the early detection of clinical high risk states of psychoses. European Psychiatry. (2015) 30:405–16. doi: 10.1016/j.eurpsy.2015.01.010
10. Phillips LJ, Yung AR, McGorry PD. Identification of young people at risk of psychosis: validation of Personal Assessment and Crisis Evaluation Clinic intake criteria. Aust N Z J Psychiatry. (2000) 34(Suppl.):S164–9. doi: 10.1177/000486740003401S25
12. Montejo AL, Montejo L, Baldwin DS. The impact of severe mental disorders and psychotropic medications on sexual health and its implications for clinical management. World Psychiatry. (2018) 17:3–11. doi: 10.1002/wps.20509
13. Jani M, Kasparek T. Emotion recognition and theory of mind in schizophrenia: a meta-analysis of neuroimaging studies. World J Biol Psychiatry. (2018) 19:S86–96. doi: 10.1080/15622975.2017.1324176
14. Cloutier B, Francoeur A, Samson C, Ghostine A, Lecomte T. Romantic relationships, sexuality, and psychotic disorders: a systematic review of recent findings. Psychiatr Rehabil J. (2021) 44:22–42. doi: 10.1037/prj0000409
15. Debbane M, Salaminios G, Luyten P, Badoud D, Armando M, Solida Tozzi A, et al. Attachment, neurobiology, and mentalizing along the psychosis continuum. Front Hum Neurosci. (2016) 10:406. doi: 10.3389/fnhum.2016.00406
16. Keefe RS, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Horvath TB, et al. Premorbid sociosexual functioning and long-term outcome in schizophrenia. Am J Psychiatry. (1989) 146:206–211. doi: 10.1176/ajp.146.2.206
17. Ostman M, Bjorkman AC. Schizophrenia and relationships. Clin Schizophr Relat Psychoses. (2013) 1:1–15. doi: 10.3371/CSRP.OSBJ.012513
18. Monteleone P, Amore M, Cabassi A, Clerici M, Fagiolini A, Girardi P, et al. Attitudes of Italian psychiatrists toward the evaluation of physical comorbidities and sexual dysfunction in patients with schizophrenia. implications for clinical practice. Front Psychiatry. (2019) 10:842. doi: 10.3389/fpsyt.2019.00842
19. Hou CL, Zang Y, Rosen RC, Cai MY, Li Y, Jia FJ, et al. Sexual dysfunction and its impact on quality of life in Chinese patients with schizophrenia treated in primary care. Compr Psychiatry. (2016) 65:116–121. doi: 10.1016/j.comppsych.2015.11.002
20. Vargas-Cáceres S, Cera N, Nobre P, Ramos-Quiroga JA. The impact of psychosis on sexual functioning: a systematic review. J Sex Med. (2021) 18:457–66. doi: 10.1016/j.jsxm.2020.12.007
21. Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. (2011) 128:305–10. doi: 10.1097/PRS.0b013e318219c171
22. World Health Organization. ICD-10 : International Statistical Classification of Diseases and Related Health Problems : Tenth Revision. 2nd ed. Geneva: World Health Organization (2004).
23. Adan Sanchez AY, McMillan E, Bhaduri A, Pehlivan N, Monson K, Badcock P, et al. High-risk sexual behaviour in young people with mental health disorders. Early Interv Psychiatry. (2019) 13:867–73. doi: 10.1111/eip.12688
24. Appiah-Kusi E, Fisher HL, Petros N, Wilson R, Mondelli V, Garety PA, et al. Do cognitive schema mediate the association between childhood trauma and being at ultra-high risk for psychosis? J Psychiatr Res. (2017) 88:89–96. doi: 10.1016/j.jpsychires.2017.01.003
25. Bechdolf A, Thompson A, Nelson B, Cotton S, Simmons MB, Amminger GP, et al. Experience of trauma and conversion to psychosis in an ultra-high-risk (prodromal) group. Acta Psychiatr Scand. (2010) 121:377–84. doi: 10.1111/j.1600-0447.2010.01542.x
26. Dragt S, Nieman DH, Veltman D, Becker HE, van de Fliert R, de Haan L, et al. Environmental factors and social adjustment as predictors of a first psychosis in subjects at ultra high risk. Schizophr Res. (2011) 125:69–76. doi: 10.1016/j.schres.2010.09.007
27. Egerton A, Valmaggia LR, Howes OD, Day F, Chaddock CA, Allen P, et al. Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophr Res. (2016) 176:171–6. doi: 10.1016/j.schres.2016.06.005
28. Marques TR, Smith S, Bonaccorso S, Gaughran F, Kolliakou A, Dazzan P, et al. Sexual dysfunction in people with prodromal or first-episode psychosis. Br J Psychiatry. (2012) 201:131–6. doi: 10.1192/bjp.bp.111.101220
29. Rapado-Castro M, Whittle S, Pantelis C, Thompson A, Nelson B, Ganella EP, et al. Does cortical brain morphology act as a mediator between childhood trauma and transition to psychosis in young individuals at ultra-high risk? Schizophr Res. (2020) 224:116–25. doi: 10.1016/j.schres.2020.09.017
30. Sahin S, Yuksel C, Guler J, Karadayi G, Akturan E, Gode E, et al. The history of childhood trauma among individuals with ultra high risk for psychosis is as common as among patients with first-episode schizophrenia. Early Interv Psychiatry. (2013) 7:414–20. doi: 10.1111/eip.12022
31. Schmidt SJ, Schultze-Lutter F, Bendall S, Groth N, Michel C, Inderbitzin N, et al. Mediators linking childhood adversities and trauma to suicidality in individuals at risk for psychosis. Front Psychiatry. (2017) 8:242. doi: 10.3389/fpsyt.2017.00242
32. Thompson A, Nelson B, McNab C, Simmons M, Leicester S, McGorry PD, et al. Psychotic symptoms with sexual content in the “ultra high risk” for psychosis population: frequency and association with sexual trauma. Psychiatry Res. (2010) 177:84–91. doi: 10.1016/j.psychres.2010.02.011
33. Thompson AD, Nelson B, Yuen HP, Lin A, Amminger GP, McGorry PD, et al. Sexual trauma increases the risk of developing psychosis in an ultra high-risk “prodromal” population. Schizophr Bull. (2014) 40:697–706. doi: 10.1093/schbul/sbt032
34. Üçok A, Kaya H, Ugurpala C, Çikrikçil i U, Ergül C, Yokuşoglu Ç, et al. History of childhood physical trauma is related to cognitive decline in individuals with ultra-high risk for psychosis. Schizophr Res. (2015) 169:199–203. doi: 10.1016/j.schres.2015.08.038
35. Velthorst E, Nelson B, O'Connor K, Mossaheb N, de Haan L, Bruxner A, et al. History of trauma and the association with baseline symptoms in an Ultra-High Risk for psychosis cohort. Psychiatry Res. (2013) 210:75–81. doi: 10.1016/j.psychres.2013.06.007
36. Ajnakina O, Trotta A, Oakley-Hannibal E, Di Forti M, Stilo SA, Kolliakou A, et al. Impact of childhood adversities on specific symptom dimensions in first-episode psychosis. Psychol Med. (2016) 46:317–26. doi: 10.1017/S0033291715001816
37. Bendall S, Hulbert CA, Alvarez-Jimenez M, Allott K, McGorry PD, Jackson HJ. Testing a model of the relationship between childhood sexual abuse and psychosis in a first-episode psychosis group: the role of hallucinations and delusions, posttraumatic intrusions, selective attention. J Nerv Ment Dis. (2013) 201:941–7. doi: 10.1097/NMD.0000000000000033
38. Braehler C, Valiquette L, Holowka D, Malla AK, Joober R, Ciampi A, et al. Childhood trauma and dissociation in first-episode psychosis, chronic schizophrenia and community controls. Psychiatry Res. (2013) 210:36–42. doi: 10.1016/j.psychres.2013.05.033
39. Brown A, Lubman DI, Paxton S. Sexual risk behaviour in young people with first episode psychosis. Early Interv Psychiatry. (2010) 4:234–42. doi: 10.1111/j.1751-7893.2010.00172.x
40. Visser RO, Smith AMA. Predictors of heterosexual condom use: characteristics of the situation are more important than characteristics of the individual. Psychol Health Med. (1999) 4:265–79. doi: 10.1080/135485099106207
41. Brown AP, Lubman DI, Paxton SJ. Psychosocial risk factors for inconsistent condom use in young people with first episode psychosis. Community Ment Health J. (2011) 47:679–87. doi: 10.1007/s10597-011-9370-4
42. Brown A, Lubman DI, Paxton SJ. Reducing sexually-transmitted infection risk in young people with first-episode psychosis. Int J Ment Health Nurs. (2011) 20:12–20. doi: 10.1111/j.1447-0349.2010.00700.x
43. Conus P, Cotton S, Schimmelmann BG, McGorry PD, Lambert M. Pretreatment and outcome correlates of sexual and physical trauma in an epidemiological cohort of first-episode psychosis patients. Schizophr Bull. (2010) 36:1105–14. doi: 10.1093/schbul/sbp009
44. Ciocca G, Usall J, Dolz M, Limoncin E, Gravina GL, Carosa E, et al. Sexual dysfunctions in people with first-episode psychosis assessed according to a gender perspective. Riv Psichiatr. (2015) 50:239–44. doi: 10.1708/2040.22166
45. Ciufolini S, Gayer-Anderson C, Fisher HL, Marques TR, Taylor H, Di Forti M, et al. Cortisol awakening response is decreased in patients with first-episode psychosis and increased in healthy controls with a history of severe childhood abuse. Schizophr Res. (2019) 205:38–44. doi: 10.1016/j.schres.2018.05.002
46. Del Cacho N, Vila-Badia R, Butjosa A, Cuadras D, Rubio-Abadal E, Rodriguez-Montes MJ, et al. Sexual dysfunction in drug- naive first episode nonaffective psychosis patients. relationship with prolactin and psychotic symptoms. gender differences. Psychiatry Res. (2020) 289:112985. doi: 10.1016/j.psychres.2020.112985
47. El Sayed El Taweel M, Zyada F, Sabry W. Sexual dysfunctions in drug-naive male patients with first-episode schizophrenia: a case–control study. Middle East Current Psychiatry. (2017) 24:168–73. doi: 10.1097/01.XME.0000520063.00808.3d
48. Gaber HD, El-Beeh KAM, Abd Al-Naser FAW, Hosny A. Erectile dysfunction in patients with first-episode psychosis. Andrologia. (2020) 52:e13793. doi: 10.1111/and.13793
49. Hui CL, Lee EH, Chang WC, Chan SK, Li YK, Lee JT, et al. Sexual dysfunction in Chinese patients with first-episode psychosis: prevalence, clinical correlates and functioning. Schizophr Res. (2013) 148:181–2. doi: 10.1016/j.schres.2013.06.004
50. Malik P, Kemmler G, Hummer M, Riecher-Roessler A, Kahn RS, Fleischhacker WW. Sexual dysfunction in first-episode schizophrenia patients: results from European First Episode Schizophrenia Trial. J Clin Psychopharmacol. (2011) 31:274–80. doi: 10.1097/JCP.0b013e3182199bcc
51. Misiak B, Karpinski P, Szmida E, Grazlewski T, Jablonski M, Cyranka K, et al. Adverse childhood experiences and methylation of the FKBP5 gene in patients with psychotic disorders. J Clin Med. (2020) 9:3792. doi: 10.3390/jcm9123792
52. Ravichandran D, Gopalakrishnan R, Kuruvilla A, Jacob KS. Sexual dysfunction in drug-naive or drug-free male patients with psychosis: prevalence and risk factors. Indian J Psychol Med. (2019) 41:434–9. doi: 10.4103/IJPSYM.IJPSYM_1_19
53. Reininghaus U, Kempton MJ, Valmaggia L, Craig TK, Garety P, Onyejiaka A, et al. Stress sensitivity, aberrant salience, and threat anticipation in early psychosis: an experience sampling study. Schizophr Bull. (2016) 42:712–22. doi: 10.1093/schbul/sbv190
54. Theleritis C, Fisher HL, Shafer I, Winters L, Stahl D, Morgan C, et al. Brain derived Neurotropic Factor (BDNF) is associated with childhood abuse but not cognitive domains in first episode psychosis. Schizophr Res. (2014) 159:56–61. doi: 10.1016/j.schres.2014.07.013
55. Theleritis C, Bonaccorso S, Habib N, Stahl D, Gaughran F, Vitoratou S, et al. Sexual dysfunction and central obesity in patients with first episode psychosis. Eur Psychiatry. (2017) 42:1–7. doi: 10.1016/j.eurpsy.2016.11.008
56. Tomassi S, Tosato S, Mondelli V, Faravelli C, Lasalvia A, Fioravanti G, et al. Influence of childhood trauma on diagnosis and substance use in first-episode psychosis. Br J Psychiatry. (2017) 211:151–6. doi: 10.1192/bjp.bp.116.194019
57. van Bruggen M, van Amelsvoort T, Wouters L, Dingemans P, de Haan L, Linszen D. Sexual dysfunction and hormonal changes in first episode psychosis patients on olanzapine or risperidone. Psychoneuroendocrinology. (2009) 34:989–95. doi: 10.1016/j.psyneuen.2009.01.013
58. de Boer MK, Castelein S, Wiersma D, Schoevers RA, Knegtering H. The facts about sexual (Dys)function in schizophrenia: an overview of clinically relevant findings. Schizophr Bull. (2015) 41:674–86. doi: 10.1093/schbul/sbv001
59. Stanton KJ, Denietolis B, Goodwin BJ, Dvir Y. Childhood trauma and psychosis: an updated review. Child Adolesc Psychiatr Clin N Am. (2020) 29:115–29. doi: 10.1016/j.chc.2019.08.004
60. Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev. (2005) 25:433–57. doi: 10.1016/j.cpr.2005.02.001
61. Shield H, Fairbrother G, Obmann H. Sexual health knowledge and risk behaviour in young people with first episode psychosis. Int J Ment Health Nurs. (2005) 14:149–54. doi: 10.1111/j.1440-0979.2005.00372.x
62. Vila-Badia R, Butjosa A, Del Cacho N, Serra-Arumi C, Esteban-Sanjusto M, Ochoa S, et al. Types, prevalence and gender differences of childhood trauma in first-episode psychosis. what is the evidence that childhood trauma is related to symptoms and functional outcomes in first episode psychosis? A systematic review. Schizophr Res. (2021) 228:159–79. doi: 10.1016/j.schres.2020.11.047
63. Longden E, Read J. Social adversity in the etiology of psychosis: a review of the evidence. Am J Psychother. (2016) 70:5–33. doi: 10.1176/appi.psychotherapy.2016.70.1.5
64. McKay MT, Cannon M, Chambers D, Conroy RM, Coughlan H, Dodd P, et al. Childhood trauma and adult mental disorder: a systematic review and meta-analysis of longitudinal cohort studies. Acta Psychiatr Scand. (2021) 143:189–205. doi: 10.1111/acps.13268
65. Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treatment. (2012) 2012:916198. doi: 10.1155/2012/916198
66. Koster A, Lajer M, Lindhardt A, Rosenbaum B. Gender differences in first episode psychosis. Soc Psychiatry Psychiatr Epidemiol. (2008) 43:940–46. doi: 10.1007/s00127-008-0384-3
67. Cotton SM, Lambert M, Schimmelmann BG, Foley DL, Morley KI, McGorry PD, et al. Gender differences in premorbid, entry, treatment, and outcome characteristics in a treated epidemiological sample of 661 patients with first episode psychosis. Schizophr Res. (2009) 114:17–24. doi: 10.1016/j.schres.2009.07.002
68. Riecher-Rossler A, Rybakowski JK, Pflueger MO, Beyrau R, Kahn RS, Malik P, et al. Hyperprolactinemia in antipsychotic-naive patients with first-episode psychosis. Psychol Med. (2013) 43:2571–82. doi: 10.1017/S0033291713000226
69. Iyer SN, Loohuis H, Pawliuk N, Joober R, Malla AK. Concerns reported by family members of individuals with first-episode psychosis. Early Interv Psychiatry. (2011) 5:163–7. doi: 10.1111/j.1751-7893.2011.00265.x
70. Georgiadis JR, Kringelbach ML. The human sexual response cycle: brain imaging evidence linking sex to other pleasures. Prog Neurobiol. (2012) 98:49–81. doi: 10.1016/j.pneurobio.2012.05.004
71. Kaminski J, Mascarell-Maricic L, Fukuda Y, Katthagen T, Heinz A, Schlagenhauf F. Glutamate in the dorsolateral prefrontal cortex in patients with schizophrenia: a meta-analysis of (1)H-Magnetic resonance spectroscopy studies. Biol Psychiatry. (2021) 89:270–7. doi: 10.1016/j.biopsych.2020.09.001
72. Moore KM, Oelberg WL, Glass MR, Johnson MD, Been LE, Meisel RL. Glutamate afferents from the medial prefrontal cortex mediate nucleus accumbens activation by female sexual behavior. Front Behav Neurosci. (2019) 13:227. doi: 10.3389/fnbeh.2019.00227
73. Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. (2010) 68:678–80. doi: 10.1016/j.biopsych.2010.04.039
74. Di Lorenzo G, Longo L, Jannini TB, Niolu C, Rossi R, Siracusano A. Oxytocin in the prevention and the treatment of post-traumatic stress disorder: a systematic review of randomized controlled trials. J. Psychopathol. (2020) 26:107–18.
75. Trovao JN, Serefoglu EC. Neurobiology of male sexual dysfunctions in psychiatric disorders: the cases of depression, anxiety, mania and schizophrenia. Int J Impot Res. (2018) 30:279–86. doi: 10.1038/s41443-018-0077-8
76. Klein C, Hill MN, Chang SC, Hillard CJ, Gorzalka BB. Circulating endocannabinoid concentrations and sexual arousal in women. J Sex Med. (2012) 9:1588–601. doi: 10.1111/j.1743-6109.2012.02708.x
77. Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. (2015) 16:579–94. doi: 10.36148/2284-0249-370
78. Minichino A, Senior M, Brondino N, Zhang SH, Godwlewska BR, Burnet PWJ, et al. Measuring disturbance of the endocannabinoid system in psychosis: a systematic review and meta-analysis. JAMA Psychiatry. (2019) 76:914–23. doi: 10.1001/jamapsychiatry.2019.0970
79. Cheng JC, Secondary J, Burke WH, Fedoroff JP, Dwyer RG. Neuroimaging and sexual behavior: identification of regional and functional differences. Curr Psychiatry Rep. (2015) 17:55. doi: 10.1007/s11920-015-0593-x
80. Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. (2010) 36:919–934. doi: 10.1093/schbul/sbq068
81. Nekovarova T, Fajnerova I, Horacek J, Spaniel F. Bridging disparate symptoms of schizophrenia: a triple network dysfunction theory. Front Behav Neurosci. (2014) 8:171. doi: 10.3389/fnbeh.2014.00171
82. Serretti A, Chiesa A. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol. (2011) 26:130–40. doi: 10.1097/YIC.0b013e328341e434
83. Potkin SG, Loze JY, Forray C, Baker RA, Sapin C, Peters-Strickland T, et al. Reduced sexual dysfunction with aripiprazole once-monthly versus paliperidone palmitate: results from QUALIFY. Int Clin Psychopharmacol. (2017) 32:147–54. doi: 10.1097/YIC.0000000000000168
84. Fujioi J, Iwamoto K, Banno M, Kikuchi T, Aleksic B, Ozaki N. Effect of adjunctive aripiprazole on sexual dysfunction in schizophrenia: a preliminary open-label study. Pharmacopsychiatry. (2017) 50:74–8. doi: 10.1055/s-0042-116323
85. Ivkovic J, Lindsten A, George V, Eriksson H, Hobart M. Effect of brexpiprazole on prolactin: an analysis of short- and long-term studies in schizophrenia. J Clin Psychopharmacol. (2019) 39:13–9. doi: 10.1097/JCP.0000000000000979
86. Koblan KS, Kent J, Hopkins SC, Krystal JH, Cheng H, Goldman R, et al. A Non-D2-Receptor-binding drug for the treatment of schizophrenia. N Engl J Med. (2020) 382:1497–506. doi: 10.1056/NEJMoa1911772
87. Castellini G, Fanni E, Corona G, Maseroli E, Ricca V, Maggi M. Psychological, relational, and biological correlates of ego-dystonic masturbation in a clinical setting. Sex Med. (2016) 4:e156–65. doi: 10.1016/j.esxm.2016.03.024
88. Fontanesi L, Marchetti D, Limoncin E, Rossi R, Nimbi F, Mollaioli D, et al. Hypersexuality and Trauma: a mediation and moderation model from psychopathology to problematic sexual behavior. J Affect Disord. (2020) 281:631–7. doi: 10.1016/j.jad.2020.11.100
89. Bothe B, Tóth-Király I, Griffiths MD, Potenza MN, Orosz G, Demetrovics Z. Are sexual functioning problems associated with frequent pornography use and/or problematic pornography use? Results from a large community survey including males and females. Addict Behav. (2021) 112:106603. doi: 10.1016/j.addbeh.2020.106603
90. Chidiebere EO, Abdalla AD, Shady E, Sami AA. Penile dysmorphic disorder: a secret obsession in men. Urol Sci. (2020) 31:85–6.
91. Galderisi S, Riva MA, Girardi P, Amore M, Carpiniello B, Aguglia E, et al. Schizophrenia and “unmet needs”: from diagnosis to care in Italy. Eur Psychiatry. (2020) 63:e26. doi: 10.1192/j.eurpsy.2019.8
92. Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. (2017) 389:941–950. doi: 10.1016/S0140-6736(17)30003-X
Keywords: ultra-high risk for psychosis, first-episode psychosis, sexuality, mental health promotion, sexual trauma
Citation: Ciocca G, Jannini TB, Ribolsi M, Rossi R, Niolu C, Siracusano A, Jannini EA and Di Lorenzo G (2021) Sexuality in Ultra-High Risk for Psychosis and First-Episode Psychosis. A Systematic Review of Literature. Front. Psychiatry 12:750033. doi: 10.3389/fpsyt.2021.750033
Received: 30 July 2021; Accepted: 30 September 2021;
Published: 27 October 2021.
Edited by:
Tim Bradshaw, The University of Manchester, United KingdomReviewed by:
Arghya Pal, All India Institute of Medical Sciences, Raebareli, IndiaLucia Sideli, Libera Università Maria SS. Assunta, Italy
Copyright © 2021 Ciocca, Jannini, Ribolsi, Rossi, Niolu, Siracusano, Jannini and Di Lorenzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tommaso B. Jannini, dC5qYW5uaW5pQG1lZC51bmlyb21hMi5pdA==
 Giacomo Ciocca
Giacomo Ciocca Tommaso B. Jannini
Tommaso B. Jannini Michele Ribolsi
Michele Ribolsi Rodolfo Rossi
Rodolfo Rossi Cinzia Niolu
Cinzia Niolu Alberto Siracusano
Alberto Siracusano Emmanuele A. Jannini
Emmanuele A. Jannini Giorgio Di Lorenzo
Giorgio Di Lorenzo