
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Psychiatry , 27 October 2021
Sec. Psychopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.748958
This article is part of the Research Topic Addressing Comorbidity between Mental Disorders and Neurological Conditions in the Elderly View all 24 articles
Purpose: Delirium is common in geriatric with Parkinson's disease (PD). Treatments for delirium have generally been neuroleptics; however, antipsychotics have potential effect to block striatal dopamine D2 receptors and worsen symptom of parkinsonism. We explored whether naloxone can alleviate delirium in PD and other forms of parkinsonism.
Patients and Methods: Patients with parkinsonism who met the delirium criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) received naloxone infusions once or twice daily. Treatment effects were evaluated by the delirium rating scale–revised 98 (DRS-R98), including non-cognitive and cognitive subscales; the Richmond agitation–sedation scale (RASS); and the mini mental status examination (MMSE).
Results: Two patients with primary parkinsonism, one with vascular PD were observed. The daily dose of naloxone was 2.08 ± 0.64 mg (range: 1–4 mg). Medication time last from 1 h to 7 days without side effects observed. Following with naloxone infusions, DRS-R98 scores decreased within 12 h and MMSE scores increased. The psychotic symptoms, disorientation, and attention deficits were alleviated significantly, while RASS scores decreased with naloxone treatment.
Conclusion: Naloxone alleviated psychotic symptoms, improved cognitive dysfunction, and irritability in patients with delirium in the context of PD. The preliminary findings point out that the opioid system may be involved in the pathophysiology of delirium, which may be one of potential treat targets for delirium of PD.
Nigrostriatal dopamine deficiency is well-recognized as the primary biochemical abnormality in Parkinson's disease (PD). Delirium is common in geriatric with PD, particularly in those receiving anticholinergics and dopamine agonists (1), and increases the risk of dementia, motor impairment, and mortality (2). The pathophysiology of delirium in PD is poorly understood. Neurotransmitter imbalances, such as loss of acetylcholine and/or an excess of dopamine, are thought to be common pathways (3–5). To date, first-line treatments are neuroleptics for delirium, such as haloperidol. Quetiapine is recommended with the fewest side effects in PD (6). However, antipsychotics except pimavanserin have the potential effect to block striatal dopamine D2 receptors and thus worsen motor features in parkinsonian, and all of them are considered relating to increase mortality in elderly dementia patients (7). The U.S. Food and Drug Administration (FDA) has not approved any agents for delirium treatment.
Researchers found that naloxone enhanced the release of acetylcholine (8, 9) and reduced the content of dopamine (10) in specific cerebral areas in rats. Other studies reported that naloxone could improve spatial working memory impairments by induced scopolamine in animals (11). It also was reported to alleviate or eliminate auditory and visual hallucinations in individuals with schizophrenia (12, 13). Based on known findings, we speculated that naloxone could improve delirium in PD. Delirium is characterized with cognitive defects, including memory impairments, and psychotic symptoms such as hallucinations. We present a case series of three patients with parkinsonism followed up 2 years, they were treated by naloxone infusion for delirium.
The patient's demographics, concomitant diagnosis, clinical profile, and naloxone use are summarized in Table 1. Efficacy of naloxone was evaluated daily during delirium episodes using the delirium rating scale–revised 98 severity scale (DRS-R98) and the Richmond agitation–sedation scale (RASS). Cognitive status was assessed by the mini mental status examination (MMSE) before and after naloxone treatment. All assessments were blinded. Informed consent was obtained from the guardians of all patients, and the ethical issues were approved by the Ethics Committee of the hospital.
Delirium duration (7.75 ± 5.32, range: 1–12 h) (the patient 1 including two treatment intervals) was much shorter than the natural course of delirium (9.03 ± 10.11, range: 0.08–20 days) after naloxone intervention in three patients. Thirteen domain scores of the DRS-R98 severity scale indicated that perceptual disturbances, the hallucinations (2.75 ± 0.50) and sleep-wake cycle disturbances (2.22 ± 1.41) were more prominent problems in view of non-cognitive subscale; long-term memory impairment and visuospatial impairment (both 2.25 ± 0.95) were more severity in view of cognitive subscale before naloxone intervention. Compared to pre-treatment, the occurrence of perceptual disturbances and hallucinations (2.75) significantly descended followed by sleep-wake cycle disturbances (2.22), affective labilities, long-term memory impairment and visuospatial impairment (2.00, respectively), delusions (1.88), thought process abnormalities, motor agitation, orientation problems, and attention deficits (1.75, respectively). The scores are shown in Table 2. The daily dosage of naloxone was 2.08 ± 0.64 mg (range: 1–4 mg). The mean duration of naloxone treats was 4.01 ± 3.58 days (range: 1 h−7 days). We observed that naloxone was safe and well-tolerated in all three patients.
This patient was a 66-year-old man, who was diagnosed with PD 8 years previously. For the past 2 years, he had been taking 100 mg/400 mg of benserazide/levodopa and 2 mg of benzhexol four times a day. However, the disease continued to worsen, so entacapone (100 mg) was added twice daily. Twenty days ago, he began to experience hallucinations that thieves were hiding on the second floor of his house, he once hurriedly jumped into a pool in front of his house to chase them. His confusion and behavioral dysfunction were most serious at night, with severe insomnia. According to DSM-5, he was diagnosed with persistent hyperactive delirium; the delirium was due to PD and anti-PD medications.
Benzhexol was discontinued, and benserazide/levodopa was reduced to three times with concomitant of entacapone twice a day. On the night of admission, he was irritable and refused to take medications. The next day morning, the patient reported two thieves were running after him during the night, then naloxone was medicated (Figure 1A). The treatment caused the patient somnolence whole day, while psychotic symptoms were not detected on day 1 and next day. aloxone was withheld on day 3 and the patient regained full orientation and showed apparent improvement in delayed recall. However, on the afternoon of the fourth day, he suddenly tore up a box and threw it, and poured water on the bed. He was given a second round of naloxone treatment, and became calm and fell asleep quickly that night. On the morning of the fifth day, he regained clarity of awareness, but still displayed static tremor and bradykinesia. He explained that the previous night he had feared that he was surrounded by about 20 villains who wanted to rob him. Thereafter, naloxone treatment was maintained for 3 days without the recurrence of delirium. There were not antipsychotics or benzodiazepines used in all processes.
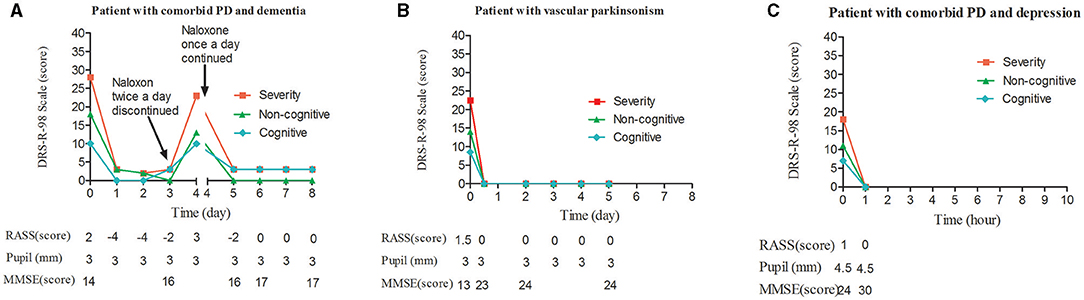
Figure 1. Change in DRS-R98 scores over the study period, and corresponding measurements. (A) DRS-R98 severity and RASS scores decreased during each of the two treatment intervals. In the first, under twice-daily naloxone, the RASS was −4, indicating the agitated patient was over sedated on days 1 and 2. The patient was delirium-free and mildly sedated on day 3 without naloxone treatment. On day 4, DRS-R98 severity and RASS scores were 23 and 3, respectively, indicating that delirium had returned. In the second interval, under nightly naloxone only, the patient was delirium-free and mildly sedated on day 5. MMSE scores were 14–16 and stabilized at 17 with remaining cognitive deficits. Corresponding pupil diameter was normal. (B) DRS-R98 severity and RASS scores decreased over time. A change in RASS score from 1.5 to 0 indicated that the mildly agitated patient became calm after sleeping 12 h at night. The MMSE score increased from 13 to 23 and stabilized at 24 with remaining cognitive deficits. Corresponding pupil diameter was normal. (C) DRS-R98 severity and RASS scores decreased over time. RASS score of 1–0 indicated that the patient recovered from a restless to a normal state. The MMSE score increased from 24 to 30 indicating no cognitive deficits; however, pupil diameter remained 4.5 mm (normally 3 mm). DRS-R98, delirium rating scale-revised 98 (severity scale only; 13 items; maximum score, 39; delirium, >15; resolution, <12); MMSE, mini mental status examination (30 point cognitive test; maximum score 30); RASS, Richmond agitation sedation scale (10 levels; 4, combative; 3, very agitated; 2, agitated; 1, restless; 0, alert and calm; −1, drowsy; −2, light sedation; −3, sedation; −4, deep sedation; −5, cannot be aroused).
During 3 months of follow up, the patient remained functionally and mental stable with combination of 50 mg/200 mg of benserazide/levodopa three times a day and 200 mg of entacapone twice a day.
The patient was a 76-year-old woman diagnosed with secondary vascular PD after a cerebral infarction 6 years previously. She had been taking 50 mg/200 mg of benserazide/levodopa and 100 mg of amantadine three times a day, in combination with 20 mg piribedil twice a day. Her essential hypertension was controlled by nifedipine with normotension. One week before admission, the woman began to manifest disturbances of awareness, composed of vivid, horrible, or absurd auditory and visual hallucinations that were richer at night. Her symptoms were more serious in the 3 days before admission, with insomnia almost whole night. Her diagnosis was persistent hyperactive anti-PD medication-induced delirium. Amantadine and piribedil were stopped, while benserazide/levodopa was continued. In addition, naloxone was administrated at night (Figure 1B). Her delirium vanished completely just after a whole night's good sleep, which notably improved her psychotic symptoms, orientation, and delayed recall. Naloxone was maintained for 5 days and there was no recurrence of delirium. No concurrent interventions were applied.
The patient remained mental stable after 3 months with the same dose of benserazide/levodopa.
A 56-year-old woman began suffering from fatigue and muscle soreness 6 years previously, leading to irritation, anxiety, and occasional suicidal ideation. The diagnosis was presumed to be PD and depression, but her symptoms sometimes worsened with adjusting dose of benserazide/levodopa and occasional fluoxetine. She was restless, and then admitted to our hospital. For the first 3 weeks of hospitalization, the patient received 20 mg of paroxetine with benserazide/levodopa (150 mg/600 mg), carbidopa/levodopa (100 mg/400 mg), and 100 mg of entacapone daily. However, the patient's symptoms remained refractory. Her physician regarded the depressive symptoms as the side effect of levodopa, so anti-PD medications were tapered to stopped. Paroxetine was continued and increased to 40 mg/day. Consequently, the patient developed a lumbering gait with stiffness in the limbs. Then this status was thought to be paroxetine-induced parkinsonism, so the paroxetine dose was halved and supplemented with 150 mg of venlafaxine in prolonged-release capsules, 1.5 mg of lorazepam, and 0.8 mg of alprazolam daily. The patient's parkinsonism worsened. Benzhexol (2 mg) was then used twice daily for 2 days in combination with 0.3 mg scopolamine in order to reduce muscle tone.
Unfortunately, although the patient's muscle tension relieved, she developed a clouding of consciousness characterized by impaired time perception, disorganized speech, and uncontrolled limb movements. She sometimes babbled “porridge on the mosquito net,” or ordered her family to open the door or buy vegetables. She was diagnosed with acute hyperactive delirium; delirium due to PD and anticholinergic drugs. Naloxone infusion was carried out (Figure 1C). Her delirium was resolved after 1 h of naloxone treatment, which also visibly improved deficits of immediate and delayed recall, although her pupil diameter remained 4.5 mm. During the course of naloxone infusion, she didn't receive any other medications. Over the next 2 days, the patient was able to walk around the ward. After that, her Parkinson's symptoms reappeared with muscle rigidity, bradykinesia, and hyperreflexia, and a diagnosis of PD with depression was confirmed.
Follow-up treatment used a combination of benserazide/levodopa (150 mg/600 mg) and paroxetine (40 mg) with no anticholinergic drugs, and her delirium did not recur.
We observed that naloxone improved dramatically delirium in three hospitalized patients with primary PD and secondary vascular PD. The severity of delirium was alleviated rapidly, determined with simultaneous decreases in DRS-R98 scores in both the non-cognitive and cognitive domains. Psychotic symptoms, disorientation, and attention deficits were the first and most symptoms to be improved obviously, and patients became noticeably calmer.
In patient 1 and 2, concomitant use of dopamine preparation and/or dopamine agonists and anticholinergic drugs contributed to the development of delirium (1). Both PD and cognitive decline may be important risk factors for delirium (6, 14). We emphasized that the management of anti-PD-medication-induced delirium is keeping a balance between reducing the causative agent and maintaining motor function. However, we believe that naloxone was associated with the rapid delirium resolution as we observed. First, there was a rapid onset of sedation and the elimination of psychosis in two patients. Second, the dosage of 2 mg twice a day caused over-sedation in patient 1 on initial 2 days, so naloxone dosage had to be halved on the second intervention. These results indicate that naloxone has a dose-dependent effect on delirium, because naloxone itself has not pharmacological sedative effect. Third, premature discontinuation of naloxone caused delirium relapsing in patient 1. It suggested it is necessary to maintain a full course of treatment with naloxone. In this study, various dose of benserazide/levodopa was managed individually for patients involved, since the parkinson's symptom was required to be managed at stable level.
Inouye and colleagues observed that poly-pharmacy is a risk factor for delirium (15). The combination of scopolamine and benzhexol with paroxetine, venlafaxine, and benzodiazepines may have played a role for third patient's delirium. Paroxetine has potent anticholinergic effect, which can increase the risk of delirium. Anticholinergic drugs are believed to play a fundamental role in delirium (16, 17). However, we consider that resolution methods for delirium unlikely were consequence of attenuation to the effect of anticholinergics. We think naloxone playing a crucial role in delirium resolution, because the patient's symptoms of delirium disappeared after 1 h treatment, but the effects of anticholinergic agents remained, it is verified from her pupils remaining enlarged and locomotion continually improved.
The acetylcholinergic neurotransmitter system is key for the function of alertness, attention, memory, and learning in the central nervous system. Hypocholinergia results in the hallmark “clouding of consciousness” and inattention seen in delirium. Elevation of dopamine can result in frank hallucinations, which is one of causes of agitation in the delirious patient (18, 19). Similar to shown in animals, in patients with delirium, we presumed that naloxone could synergistically complement neurotransmitter imbalances, promoting the release of acetylcholine (8, 9) to improve orientation, attention, and memory impairment, and limiting the increase in dopamine (10) to reduce psychotic symptoms.
Interestingly, naloxone had a rapid-onset effect on sedation or sleep ameliorated in three patients. These findings is consistent with Berger and colleagues' case report, in which Mr. A became “clearly less agitated” after naloxone injection (12), and is also consistent with our previous finding that naloxone could control agitated behavior in delirium after a stroke (20). Accumulating evidences indicate that all three opioid receptors (mu, delta, and kappa) mediate the mechanisms associated with emotional responses and mood control (21). Particularly, naloxone has been proved to active the mu receptor, which in turn interact with dopaminergic and acetylcholine system facilitating neurotransmitter releasing to alleviate cognitive and psychotic symptom (9, 10, 22). This phenomenon suggests a causal relationship with the gamma-aminobutyric acid (GABA)ergic system (23, 24). Agitation in delirium is very complex that needs to be addressed. Some previous studies have reported that antipsychotics such as haloperidol contribute mainly to sedation in the treatment of delirium (25, 26).
Notably, naloxone intervention was free of side effects in our patients, so we could avoid anticholinergic effects and extrapyramidal symptoms that occur frequently on antipsychotics treatment.
There are several limitations in this observational study. The three cases involved in the pilot study limited the statistical power for validity of conclusion. Poly-pharmacy in some cases involved and uncertain neurobiological mechanism make it difficult to explain the efficacy of naloxone on delirium occurred with patients with PD. Few rating scales involved limited the efficacy for evaluating the multiply domains in cognitive functioning for patients in the study. This case series highlighted the clinical significance that naloxone contributed potentially to manage the symptoms in delirium of patients with PD.
The research gives insight into why naloxone may be a promising agent for the treats of delirium in the context of PD. However, we think further pharmacological studies on how effects of naloxone on brain neurotransmitter in patients with delirium are necessary to prove this observation.
Naloxone was safe and well-tolerated in our studies. It alleviated psychotic symptoms, improved cognitive dysfunction, and calmed irritability in patients with PD delirium. The results imply that the opioid system is perhaps related to complex interactions between dopamine, acetylcholine, and GABA, which implicate in underlying pathophysiology of delirium. Further observations need provide pilot data to support a double-blind, placebo-controlled clinical trial to demonstrate the efficacy of naloxone in the treatment of delirium in PD and parkinsonism.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Shunde Wuzhongpei hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HJ and JZ: concept and design, data acquisition, analysis and interpretation, statistical analysis, and drafting the manuscript. QH: data acquisition, analysis, and statistical analysis. JP: critical revision of the manuscript. TJ: data analysis. BD: data interpretation and supervision. XD: concept and design and critical revision of the manuscript. All authors read and approved the final manuscript.
This study was supported by Foshan Scientific and Technological projects (200808112), the grants from Shanghai Municipal Health Commission (2019ZB0201), and Social Welfare Scientific Research Project (Key Program) of Zhongshan City (2018B1006). This research received no specific grant from any funding agency in the public, commercial, ornot-for-profit sectors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Handling Editor ZL declared a shared affiliation, though no other collaboration, with one author HJ at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Bronwen Gardner, Ph.D., from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
1. Papapetropoulos S, Mash DC. Psychotic symptoms in Parkinson's disease. From description to etiology. Neurology. (2005) 252:753–64. doi: 10.1007/s00415-005-0918-5
2. Serrano-Dueñas M, Bleda MJ. Delirium in Parkinson's disease patients. A five-year follow-up study. Parkinsonism Relat Disord. (2005) 11:387–92. doi: 10.1016/j.parkreldis.2005.05.002
3. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. (2000) 5:132–48. doi: 10.153/SCNP00500132
4. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. (2013) 21:1190–222. doi: 10.1016/j.jagp.2013.09.005
5. Zhong XM, Shi HS, Shen ZW, Hou L, Luo X, Chen X, et al. 1H-proton magnetic resonance spectroscopy differentiates dementia with Lewy bodies from Alzheimer's disease. J Alzheimers Dis. (2014) 40:953–66. doi: 10.3233/JAD-131517
6. Vardy ER, Teodorczuk A, Yarnall AJ. Review of delirium in patients with Parkinson's disease. Neurology. (2015) 262:2401–10. doi: 10.1007/s00415-015-7760-1
7. Combs BL, Cox AG. Update on the treatment of Parkinson's disease psychosis: role of pimavanserin. Neuropsychiatr Dis Treat. (2017) 13:737–44. doi: 10.2147/NDT.S108948
8. Ragozzino ME, Gold PE. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience. (1995) 68:981–8. doi: 10.1016/0306-4522(95)00204-V
9. Mizuno T, Kimura F. Medial septal injection of naloxone elevates acetylcholine release in the hippocampus and induces behavioral seizures in rats. Brain Res. (1996) 713:1–7. doi: 10.1016/0006-8993(95)01287-7
10. Hirose N, Murakawa K, Takada K, Oi Y, Suzuki T, Nagase H, et al. Interactions among mu- and delta-opioid receptors, especially putative delta1- and delta2-opioid receptors, promote dopamine release in the nucleus accumbens. Neuroscience. (2005) 135:213–25. doi: 10.1016/j.neuroscience.2005.03.065
11. Duan X, Ma GY, Zhang YM. Intervention effect of naloxone on scopolamine-induced impairment of spatial working memory in rats. Chin J Clin Rehabil. (2005) 9:248–51. doi: 10.1007/s10512-005-0264-9
12. Berger PA, Watson SJ, Akil H, Barchas JD. The effects of naloxone in chronic schizophrenia. Am J Psychiatry. (1981) 138:913–8. doi: 10.1176/ajp.138.7.913
13. Pickar D, Vartanian F, Bunney WE Jr, Maier HP, Gastpar MT, Prakash R, et al. Short-term naloxone administration in schizophrenic and manic patients. A world health organization collaborative study. Arch Gen Psychiatry. (1982) 39:313–9. doi: 10.1001/archpsyc.1982.04290030047009
14. Davis DHJ, Skelly DT, Murray C, Hennessy E, Bowen J, Norton S, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry. (2015) 23:403–15. doi: 10.1016/j.jagp.2014.08.005
15. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons: predictive model and interrelationship with baseline vulnerability. JAMA. (1996) 275:852–7. doi: 10.1001/jama.275.11.852
16. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. (2004) 80:388–93. doi: 10.1136/pgmj.2003.017236
17. Catic AG. Identification and management of in-hospital drug-induced delirium in older patients. Drugs Aging. (2011) 28:737–48. doi: 10.2165/11592240-000000000-00000
18. Zaal IJ, Slooter AJ. Delirium in critically ill patients: epidemiology, pathophysiology, diagnosis and management. Drugs. (2012) 72:1457–71. doi: 10.2165/11635520-000000000-00000
19. Caplan JP. Haloperidol and delirium: management or treatment? J Crit Care Med. (2009) 37:354–5. doi: 10.1097/CCM.0b013e318193023a
20. Duan X, Li CY, Qiu B, Ma GY. Naloxone treatment for poststroke agitated delirium in hospitalized older adults: a pilot study. J Am Geriatr Soc. (2016) 64:663–5. doi: 10.1111/jgs.14006
21. Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. (2013) 36:195–206. doi: 10.1016/j.tins.2012.11.002
22. Gunne LM, Lindstrom L, Terenius L. Naloxone-induced reversal of schizophrenic hallucinations. J Neural Transm. (1977) 40:13–9. doi: 10.1007/BF01250276
23. Ashabi G, Oryan S, Ahmadi R, Valizadegan F. The effects of hippocampal opioidergic and septal GABAergic system interactions on anxiety-like behavior in rats. Life Sci. (2011) 89:821–618. doi: 10.1016/j.lfs.2011.09.009
24. Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, et al. Presynaptic inhibition of GABA release in the BNST by kappa opioid receptor signaling. Biol Psychiatry. (2012) 71:725–32. doi: 10.1016/j.biopsych.2011.11.015
25. Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. (2013) 1:515–23. doi: 10.1016/S2213-2600(13)70166-8
Keywords: naloxone, delirium, Parkinson's disease, parkinsonism, anticholinergic drugs, anti-PD medications
Citation: Jin H, Zhang J, Hu Q, Ping J, Jiang T, Du B and Duan X (2021) Naloxone Alleviate the Severity of Delirium in Hospitalized Patients With Parkinsonism: Three Case Reports. Front. Psychiatry 12:748958. doi: 10.3389/fpsyt.2021.748958
Received: 04 August 2021; Accepted: 29 September 2021;
Published: 27 October 2021.
Edited by:
Zezhi Li, Shanghai JiaoTong University, ChinaReviewed by:
Shuwei Xie, University of Nebraska Medical Center, United StatesCopyright © 2021 Jin, Zhang, Hu, Ping, Jiang, Du and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Duan, ZHVhbnhpbnNkQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.