
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Psychiatry , 22 December 2021
Sec. Public Mental Health
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.742169
This article is part of the Research Topic Real-World Implementation of the Biopsychosocial Approach to Healthcare: Pragmatic Approaches, Success Stories and Lessons Learned View all 23 articles
 Karly A. Murphy1,2*
Karly A. Murphy1,2* Arlene Dalcin1,2
Arlene Dalcin1,2 Emma E. McGinty3,4
Emma E. McGinty3,4 Stacy Goldsholl1
Stacy Goldsholl1 Ann Heller1
Ann Heller1 Gail L. Daumit1,2,3,4
Gail L. Daumit1,2,3,4People with serious mental illness (SMI) have a 2–3-fold higher mortality than the general population, much of which is driven by largely preventable cardiovascular disease. One contributory factor is the disconnect between the behavioral and physical health care systems. New care models have sought to integrate physical health care into primary mental health care settings. However, few examples of successful care coordination interventions to improve health outcomes with the SMI population exist. In this paper, we examine challenges faced in coordinating care for people with SMI and explore pragmatic, multi-disciplinary strategies for overcoming these challenges used in a cardiovascular risk reduction intervention shown to be effective in a clinical trial.
People with serious mental illness (SMI) experience excess mortality at rates 2–3 times higher than the general population, equivalent to a loss of life of 10–20 years (1, 2). Cardiovascular disease (CVD) is the leading cause of death for this group and is largely modifiable by addressing risk factors, including obesity, diabetes, hypertension, dyslipidemia, tobacco use, poor diet, and physical inactivity- which are highly prevalent in populations with SMI (1, 3, 4). Use of antipsychotic medications also contributes to metabolic changes with weight gain, hyperglycemia, and dyslipidemia (5).
People with SMI often face significant challenges accessing quality healthcare, including high rates of poverty, housing instability, unemployment, and interactions with the criminal justice system (6–8). Moreover, people with SMI are less likely to receive guideline-concordant care compared with the general population (9–11). For example, they are less likely to receive annual screenings for diabetes-related complications or recommended medications after a myocardial infarction (12–15). Cognitive dysfunction, communication challenges, and low health literacy may further impede care delivery to people with SMI (6, 16, 17). Finally, specialty mental healthcare has historically been delivered separately from physical healthcare services and created challenges in coordinating services for this vulnerable population (10, 18, 19).
Care delivery models have sought to better integrate delivery of behavioral and physical healthcare, but these models have faced challenges. Models such as Collaborative Care (20–22) or the Patient Centered Medical Home (23–25), where behavioral health is integrated into primary care settings, may have primary care providers (PCPs) who lack experience in treating individuals with SMI (24). In the Behavioral Health Home (BHH), where the coordination of care for physical healthcare is centered in the specialty mental healthcare setting (26, 27), behavioral health providers may not feel equipped to address CVD risk factors (28).
Given the lower rates of guideline-concordant care and persistent mortality gap for people with SMI, it is critical to understand successful examples of care coordination around physical health conditions. Yet descriptions are scarce regarding populations with SMI. In this paper, we use a clinical vignette of an individual with SMI, who was enrolled in a successful cardiovascular risk reduction intervention trial, to highlight challenges and opportunities in delivering guideline-concordant care for people with SMI and multiple CVD risk factors. We describe the care management and care coordination processes employed within the clinical intervention and future implementation lessons.
Care management and care coordination are complementary approaches to deliver healthcare across multiple providers and settings (Table 1). Care management is a team-based practice approach to align and manage health services according to a population's needs (29, 30). Strategies target providers (e.g., health risk assessment training, electronic decision support) or patients (e.g., health coaching, brochures) and involve a multi-disciplinary team of providers. Meanwhile, care coordination organizes patient care activities across participants involved with a patient's care (e.g., providers, patient, supporters of patient) (31). Activities include establishing accountability, communicating and sharing knowledge, facilitating transitions of care, assessing the patient's needs and goals, developing a care plan, monitoring and follow-up care, supporting patients' self-management goals, linking to community resources, and aligning resources with patient and population needs. This model has been shown to improve chronic disease care quality for the general population (29, 32, 33) and for those with SMI (34–38).
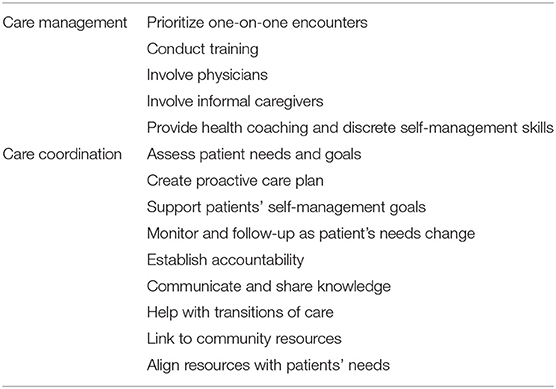
Table 1. Care management and care coordination activities from the Agency for Healthcare Research and Quality frameworks (30, 31).
The NHLBI-funded IDEAL trial was a successful 18-month randomized clinical trial that tested a comprehensive cardiovascular risk reduction program incorporating care management, with an emphasis on health behavior coaching, and care coordination at four community mental health outpatient programs for people with SMI who had at least one CVD risk factor (39). It demonstrated an overall reduction in 10 year CVD risk reduction by 13% in the intervention group compared to control (40).
The intervention's theoretical framework draws upon a bio-psychosocial approach and leverages behavioral change strategy and person-centered care in addressing physical health (39). Specifically, the 269 adult participants randomized to the intervention received a care plan tailored to their specific CVD risk factors (hypertension, diabetes, dyslipidemia, tobacco use, obesity), which was delivered jointly by a health coach and nurse. The health coach, based at the community mental health organization, conducted one-on-one sessions weekly for the first 6 months and then at least every 2 weeks thereafter. Sessions focused on individual health behaviors and collaboratively agreed upon goals. The nurse met with participants around CVD risk factor education, medication counseling, and accompanied participants to physical health visits with physicians as needed. In addition, the nurse coordinated care with physical and behavioral health providers (defined broadly as any healthcare worker including pharmacists, social workers). For participants interested in smoking cessation using pharmacotherapy, the nurse coordinated with participants' psychiatrists for varenicline, bupropion, and/or nicotine replacement therapy prescriptions (39). Both the health coach and nurse used a motivational interviewing approach and solution focused therapy techniques to facilitate health behavior change with participants (39).
The institutional review boards at Johns Hopkins University and Sheppard Pratt Health System approved the clinical trial. The patient provided permission to be featured in this clinical vignette, and identifying initial was changed to protect privacy.
Ms. E., a participant in her mid-40s, had a medical and psychiatric history notable for hypertension, type 2 diabetes mellitus, tobacco smoking, obesity, and schizophrenia. Significant medications were metformin 750 mg twice daily, propranolol 10 mg as needed, clozapine 300 mg daily, haloperidol 5 mg twice daily, fluoxetine 60 mg daily, valproic acid 1,500 mg daily, clonazepam 0.5 mg three times day, and benztropine 0.5 mg daily. On study enrollment, laboratory values were remarkable for an elevated A1c of 7.8% (reference range <5.7%), total cholesterol of 257 mg/dl (reference range: 0–200 mg/dl), and low-density lipoprotein (LDL) of 151 mg/dl (reference range: 0–100 mg/dl) with an estimated 10-year risk of 9.2% of having a heart attack or stroke based on atherosclerotic cardiovascular disease risk factors. Ms. E. lived in a residential program, where staff helped to schedule transportation.
On study entry, Ms. E. met with the health coach and nurse and identified diabetes self-management and tobacco smoking cessation as her primary health goals. Together, the health coach, nurse, and Ms. E. formulated a care plan to reach these goals. She met with the health coach weekly where she expressed anxiety around quitting smoking and her worry about developing cancer. By the first month, she set a quit date and focused on smoking cessation through behavioral change and pharmacotherapy, consistent with an evidence-based approach for persons with SMI (41). The nurse then coordinated earlier receipt of varenicline for smoking cessation as Ms. E.'s regular appointment with the prescribing psychiatrist was several months away. Ms. E. took the prescribed varenicline, continued to receive behavioral counseling for smoking cessation, and was able to quit smoking for 4 weeks.
When Ms. E. began smoking again, with inconsistent use of varenicline, she and the health coach identified triggers that led to relapse (e.g., feeling angry), potential solutions, and set a new quit date. They focused on discrete strategies, such as avoiding situations where she may be offered cigarettes or taking deep breaths. Ms. E. restarted varenicline and met with the coach weekly. However, after a series of falls, a director of the mental health center asked to discontinue varenicline out of concern that varenicline contributed. The nurse met with the director to discuss that varenicline was unlikely the source of the falls and emphasize that Ms. E. was at high risk for tobacco relapse. They agreed that Ms. E. could continue the medication. When Ms. E. underwent surgery for an ankle fracture, the psychiatrist held varenicline in anticipation of other medication changes in the perioperative period. After surgery, Ms. E. elected not to continue on varenicline; she successfully refrained from smoking and met bi-weekly with the health coach. After she was hospitalized for a mental health crisis, Ms. E. and her coach reviewed triggers for smoking and strategies to address triggers. Ms. E. was successful in remaining tobacco free for over a year at study end.
Attaining glycemic control was challenging for Ms. E. She met with the health coach weekly around diabetes self-management skills and received educational materials with high readability and simple messaging, such as “Avoid sugar drinks,” or “What counts as a fruit.” Ms. E. and her coach reviewed her blood glucose logs and foods that she had eaten. She found it difficult to regulate portion size and to remember to choose healthy snacks (e.g., sugar-free candies as a replacement for cigarettes). The coach helped her to make a list of foods that elevated blood sugars. Ms. E. also met with the nurse and reviewed diabetes self-management, medications, and prepared questions in advance of visits with her PCP. At the visit, the nurse advocated for initiation of statin therapy, consistent with guideline-concordant lipid management in persons with diabetes (42). Ms. E. was started on pravastatin.
Throughout the following year, Ms. E. worked closely with the health coach to review food choices, increase physical activity levels, and consistently take medication. She continued to struggle with changing dietary habits- eating when stressed, eating large portions, and eating when not hungry. Ms. E. set personal goals of cutting out sugary beverages and decreasing foods high in carbohydrates. After she fractured her ankle, she worried about gaining weight. She and her coach reviewed diabetes self-management topics and aligned smoking cessation strategies with smart snacking choices. Ms. E. then set defined goals, such as choosing water instead of juice. The nurse also coordinated with the residential counselor to reschedule canceled medical appointments, arrange transportation, and to obtain regular blood monitoring of glucose levels and cholesterol. At her 18-month follow up, Ms. E. had improved glycemic and cholesterol control with an A1c of 6.5% and an LDL of 65 mg/dl on an increased dose of metformin (1,000 mg twice daily) and pravastatin 40 mg daily. Over the study, 65 encounters (78 encounters anticipated per study protocol) were documented for Ms. E. between the coach and the nurse, of which 10 involved coordination with providers outside of the community mental health program.
This vignette of an individual with SMI who successfully stopped smoking tobacco and achieved glycemic control with the assistance of a health coach and nurse was drawn from an 18-month clinical trial in a community setting. It highlights the real-world challenges and the intensity of resources needed to reduce CVD risk factors for persons with SMI.
The care plan reflected the participant' health goals and CVD risk factors, and whether guideline-concordant care was due. It also included goals that the patient may not have prioritized (e.g., weight loss). Identifying the primary location of meals (e.g., residential facility, family) helped the health coach and nurse tailor nutrition-based education sessions (e.g., diabetes-focused) and took into account the patient's socioeconomic concerns.
Involving individuals with SMI in the creation and implementation of the care plan is fundamental to patient-centered care. This includes defining what is important to them and actions that they are willing or not to take. Motivational interviewing has been a successful approach for engaging people with SMI around health behavior change goals (43). Given that persons with SMI experience stigma within healthcare settings (16), it is important that their voice is heard from the onset and throughout care delivery.
Ms. E. experienced physical health setbacks and a mental health crisis during the intervention. Intervention staff checked in frequently and adapted coaching sessions to her evolving needs and concerns. They recognized that the ankle fracture impacted her physical activity levels and influenced her cravings for snacks and cigarettes. During transitions, the health coach addressed arising anxieties and helped Ms. E. to set well-defined goals.
Our experience found continuous engagement between trial staff and participants increased participants' confidence with self-management skills. As people with SMI may experience cognitive dysfunction, disability and low health literacy, materials were tailored to improve readability and to account for these considerations (6, 44). Some participants benefited from repetition of topics, updates or selecting new targets of behavior changes. Health coaches and trial staff advocated on participants' behalf to address organizational-level challenges. For example, one participant noted that he walked past staff members who were smoking when he entered the mental health clinic. Trial staff then spoke with clinic leadership to move the designated smoking area away from the front entrance, which was a culture shift for employees. Trial staff also worked with residential facility managers to change purchasing habits (e.g., increase diet soda availability).
In addition, goal setting, and skill building reinforced a behavioral change strategy well-suited to people with SMI (43, 45). We found that aligning multiple health goals was effective. For Ms. E, she and the health coach discussed using sugar free gum and candies to address oral cravings for smoking cessation while being mindful of underlying diabetes. Similarly, health coaches encouraged setting a discrete food goal (e.g., additional serving of vegetables) that would parallel a physical activity goal (e.g., number of steps) for weight loss.
This intervention had a high frequency and intensity of encounters between the participant, health coach, and nurse. Topics were reinforced over time, emphasized specific skills, and broke materials into small units. Coaches and nurse met participants where they were at, based on their psychiatric condition, cognitive skills, and behavioral change goals.
In addition, each health coach was embedded within a community mental health center and met with participants one-on-one. This approach likely improved rapport and facilitated communication between the coach and behavioral health team. Literature suggests that in-person encounters for care management are more effective than telephone encounters (46). Given the growth of telemedicine during the COVID-19 pandemic (47), programs should consider hybrid models for care management and care coordination services.
Care management and care coordination are inherently team-based practices, and successful teams need to have defined roles and responsibilities (48). During this intervention, accountability occurred when providers identified discrete expectations and goals, checked in regularly with one another, and closed feedback loops (31). The health coach and intervention nurse developed and implemented the care plan with the participant. The health coach led discussion on self-management skills and health behavior counseling, with regularly scheduled and ad hoc check-ins with participants. The nurse acted as a liaison with behavioral health providers, PCPs, medical sub-specialists, family, and residential program staff. The nurse sent an introductory letter about the intervention to PCPs and sometimes attended office visits. This approach helped to facilitate knowledge of how the nurse and health coach could enhance a participants' existing care plan. For example, the nurse measured participants' blood pressure and relayed that information to the PCP, thereby facilitating ambulatory blood pressure monitoring. Additional activities included advocating for aggressive management of CVD risk factors (e.g., statin prescription for cholesterol management) and educating caregivers about how to support individuals with SMI with health goals. Both the health coach and intervention nurse provided in-person and email updates to behavioral health staff of the progress of participants, if requested. If concerns related to social determinants of health arose (e.g., food insecurity), the health coach and/or nurse reached out to staff at the mental health center, which had existing ties with social services. This process required knowledge and understanding of who the participant was, their support network and living situation. Future work will need to incorporate clear expectations for roles and responsibilities and opportunities for formal and informal check-ins with team members.
When caring for people with SMI, it is essential that communication occur between the patient, behavioral health providers, and physical health providers and that communication loops are closed to reduce potential miscommunications. Transitions of care, are particularly high-risk settings (49). When Ms. E. had a somatic and later a mental health-related hospital admission, the health coach and nurse tailored counseling to match her needs to help to stay consistent with her health goals (e.g., smoking cessation).
The nurse and health coach regularly communicated in-person, by phone, and email. Frequent communication occurred with behavioral and physical health providers, with more open communication correlated to improved control of CVD risk factors for participants. However, challenges around communication arose. No shared electronic health records were available between behavioral and physical health providers, a problem observed in care coordination programs across other healthcare systems (50). Intervention staff relied on phone messages or faxes for communication with external providers, sometimes leading to delays in having messages returned from providers outside of the mental health center.
This vignette illustrates how care management and care coordination processes were successfully employed to reduce CVD risk factors for person with SMI within the formalized structure of an intervention. By outlining the frequent, high-intensity care coordination and care management processes required to care for patients with SMI, we filled a gap in the literature. It is imperative that health systems implement protocolized care coordination and care management processes to address CVD risk factor care for populations with SMI.
Future program implementation may wish to start in settings that care for high number of individuals with SMI. Enhanced primary care models and BHHs are natural settings because they focus on populations with SMI, and in the case for BHHs, have an existing funding through the Medicaid waiver program (27, 51). Models that integrate somatic and behavioral health across acute and outpatient settings may also be of interest given their focus on individuals with SMI to maintain overall health after hospital admission (52).
Implementation plans will need to include accountability and address communication gaps between behavioral and physical health providers. For behavioral health providers, addressing physical health concerns may feel out of scope for their usual practice; therefore, it is essential that implementation plans include connections with (a) PCPs who can lead on physical health management, and (b) care managers and health coaches who work across behavioral and physical healthcare sectors.
Finally, financing remains a challenge. Alternative payment models, such as Accountable Care Organizations or the Medicaid waiver program, could provide funding streams to support care coordination as described here (53, 54). Future policies will need to provide support for health systems to implement these processes. This vignette is a first step in highlighting the discrete, intensive care coordination and care management processes needed to care for populations with SMI and considerations for program implementation and sustainability.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Johns Hopkins University School of Medicine. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
KM and GD contributed to the conception and design. KM wrote the first draft of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We gratefully acknowledge funding from NIMH grant P50 MH115842 and NHBLI grant UG3154280.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. (2006) 3:A42. Available online at: http://www.cdc.gov/pcd/issues/2006/apr/05_0180.htm
2. Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. (2015) 72:1172–81. doi: 10.1001/jamapsychiatry.2015.1737
3. Daumit GL, Anthony CB, Ford DE, Fahey M, Skinner EA, Lehman AF, et al. Pattern of mortality in a sample of Maryland residents with severe mental illness. Psychiatry Res. (2010) 176:242–5. doi: 10.1016/j.psychres.2009.01.006
4. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. (2007) 298:1794–6. doi: 10.1001/jama.298.15.1794
5. De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. (2011). 8:114–26. doi: 10.1038/nrendo.2011.156
6. Mueser KT, McGurk SR. Schizophrenia. Lancet. (2004). 363:2063–72. doi: 10.1016/S0140-6736(04)16458-1
7. Draine J, Salzer MS, Culhane DP, Hadley TR. Role of social disadvantage in crime, joblessness, and homelessness among persons with serious mental illness. Psychiatr Serv. (2002) 53:565–73. doi: 10.1176/appi.ps.53.5.565
8. Shim R, Koplan C, Langheim FJP, Manseau MW, Powers RA, Compton MT. The social determinants of mental health: an overview and call to action. Psychiatr Ann. (2014) 44:22–6. doi: 10.3928/00485713-20140108-04
9. McGinty EE, Baller J, Azrin ST, Juliano-Bult D, Daumit GL. Quality of medical care ssfor persons with serious mental illness: a comprehensive review. Schizophr Res. (2015) 165:227–35. doi: 10.1016/j.schres.2015.04.010
10. Horvitz-Lennon M, Kilbourne AM, Pincus HA. From silos to bridges: meeting the general health care needs of adults with severe mental illnesses. Health Aff . (2006). 25:659–69. doi: 10.1377/hlthaff.25.3.659
11. Murphy KA, Stone EM, Presskreischer R, McGinty EE, Daumit GL, Pollack CE. Cancer screening among adults with and without serious mental illness: a mixed methods study. Med Care. (2021) 59:327–33. doi: 10.1097/MLR.0000000000001499
12. Banta JE, Morrato EH, Lee SW, Haviland MG. Retrospective analysis of diabetes care in California Medicaid patients with mental illness. J Gen Intern Med. (2009) 24:802–8. doi: 10.1007/s11606-009-0994-9
13. McGinty EE, Blasco-Colmenares E, Zhang Y, Dosreis SC, Ford DE, Steinwachs DM, et al. Post-myocardial-infarction quality of care among disabled Medicaid beneficiaries with and without serious mental illness. Gen Hosp Psychiatry. (2012) 34:493–99. doi: 10.1016/j.genhosppsych.2012.05.004
14. Frayne SM, Halanych JH, Miller DR, Wang F, Lin H, Pogach L, et al. Disparities in diabetes care: impact of mental illness. Arch Intern Med. (2005). 165:2631–8. doi: 10.1001/archinte.165.22.2631
15. Druss BG, Bradford WD, Rosenheck RA, Radford MJ, Krumholz HM. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. (2001). 58:565–72. doi: 10.1001/archpsyc.58.6.565
16. Kaufman EA, McDonell MG, Cristofalo MA, Ries RK. Exploring barriers to primary care for patients with severe mental illness: frontline patient and provider accounts. Issues Ment Health Nurs. (2012) 33:172–80. doi: 10.3109/01612840.2011.638415
17. Lester H, Tritter JQ, Sorohan H. Patients' and health professionals' views on primary care for people with serious mental illness: focus group study. BMJ. (2005) 330:1122. doi: 10.1136/bmj.38440.418426.8F
18. Davis K, Reedy W, Little N. Integrated illness management and recovery for older adults with serious mental illness. Am J Geriatr Psychiatry. (2013). 21:S20–1. doi: 10.1016/j.jagp.2012.12.051
19. Druss BG, Goldman HH. Integrating health and mental health services: a past and future history. Am J Psychiatry. (2018) 175:1199–204. doi: 10.1176/appi.ajp.2018.18020169
20. Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. (2010) 363:2611–20. doi: 10.1056/NEJMoa1003955
21. Kronenberg C, Doran T, Goddard M, Kendrick T, Gilbody S, Dare CR, et al. Identifying primary care quality indicators for people with serious mental illness: a systematic review. Br J Gen Pract. (2017). 67:e519–30. doi: 10.3399/bjgp17X691721
22. Woltmann E, Grogan-Kaylor A, Perron B, Georges H, Kilbourne AM, Bauer MS. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. (2012). 169:790–804. doi: 10.1176/appi.ajp.2012.11111616
23. Grove LR, Olesiuk WJ, Ellis AR, Lichstein JC, DuBard CA, Farley JF, et al. Evaluating the potential for primary care to serve as a mental health home for people with schizophrenia. Gen Hosp Psychiatry. (2017). 47:14–9. doi: 10.1016/j.genhosppsych.2017.03.002
24. Minkoff K, Parks J. Primary health-behavioral health integration for the population of individuals with serious mental illness. In: O'Donohue W, Maragakis A, O'Donohue W, Maragakis A, editors. Integrated Primary Behavioral Care: Role in Medical Homes Chronic Disease Management. Cham: Springer International Publishing (2015). p. 171–99. doi: 10.1007/978-3-319-19036-5_10
25. Sklar M, Aarons GA, O'Connell M, Davidson L, Groessl EJ. Mental health recovery in the patient-centered medical home. Am J Public Health. (2015) 105:1926–34. doi: 10.2105/AJPH.2015.302683
26. Druss BG, von Esenwein SA, Glick GE, Deubler E, Lally C, Ward MC, et al. Randomized trial of an integrated behavioral health home: the Health Outcomes Management and Evaluation (HOME) study. Am J Psychiatry. (2017) 174:246–55. doi: 10.1176/appi.ajp.2016.16050507
27. Murphy KA, Daumit GL, Stone E, McGinty EE. Physical health outcomes and implementation of behavioural health homes: a comprehensive review. Int Rev Psychiatry. (2018) 30:224–41. doi: 10.1080/09540261.2018.1555153
28. Smith TE, Sederer LI. A new kind of homelessness for individuals with serious mental illness? The need for a “mental health home.” Psychiatr Serv. (2009). 60:528–33. doi: 10.1176/ps.2009.60.4.528
29. Mitchell P, Wynia M, Golden R, McNellis B, Okun S, Webb CE, et al. Core Principles & Values of Effective Team-Based Health Care. In: Perspectives N, editor. Washington, DC: National Academy of Medicine (2012). doi: 10.31478/201210c
30. Schottenfeld L, Peterson D, Peikes D, Ricciardi R, Burak H, McNellis R, et al. Creating Patient-Centered Team-Based Primary Care. Rockville, MD: Agency for Healthcare Research and Quality (2016).
31. McDonald KM SE, Albin L, Pineda N, Lonhart J, Sundaram V, Smith-Spangler C, et al. Care Coordination Atlas Version 4 (Prepared by Stanford University under subcontract to American Institutes for Research on Contract No. HHSA290–2010-00005I). Rockville, MD: Agency for Healthcare Research and Quality (2014).
32. Bodenheimer T. Lessons from the trenches–a high-functioning primary care clinic. N Engl J Med. (2011) 365:5–8. doi: 10.1056/NEJMp1104942
33. Shi L, Lee DC, Chung M, Liang H, Lock D, Sripipatana A. Patient-centered medical home recognition and clinical performance in U.S. Community Health Centers. Health Serv Res. (2017) 52:984–1004. doi: 10.1111/1475-6773.12523
34. Swietek KE, Domino ME, Beadles C, Ellis AR, Farley JF, Grove LR, et al. Do medical homes improve quality of care for persons with multiple chronic conditions? Health Serv Res. (2018) 53:4667–81. doi: 10.1111/1475-6773.13024
35. Jackson GL, Powers BJ, Chatterjee R, Bettger JP, Kemper AR, Hasselblad V, et al. The patient centered medical home. A Systematic Review. Ann Intern Med. (2013). 158:169–78. doi: 10.7326/0003-4819-158-3-201302050-00579
36. Domino ME, Wells R, Morrissey JP. Serving persons with severe mental illness in primary care–based medical homes. Psychiatr Serv. (2015) 66:477–83. doi: 10.1176/appi.ps.201300546
37. Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries:15 randomized trials. JAMA. (2009) 301:603–18. doi: 10.1001/jama.2009.126
38. Olesiuk WJ, Farley JF, Domino ME, Ellis AR, Morrissey JP. Do medical homes offer improved diabetes care for medicaid enrollees with co-occurring schizophrenia? J Health Care Poor Underserved. (2017) 28:1030–41. doi: 10.1353/hpu.2017.0094
39. Dalcin AT, Jerome GJ, Appel LJ, Dickerson FB, Wang NY, Miller ER, et al. Need for cardiovascular risk reduction in persons with serious mental illness: design of a comprehensive intervention. Front Psychiatry. (2018) 9:786. doi: 10.3389/fpsyt.2018.00786
40. Daumit GL, Dalcin AT, Dickerson FB, Miller ER, Evins AE, Cather C, et al. Effect of a comprehensive cardiovascular risk reduction intervention in persons with serious mental illness: a randomized clinical trial. JAMA Netw Open. (2020) 3:e207247. doi: 10.1001/jamanetworkopen.2020.7247
41. Cather C, Pachas GN, Cieslak KM, Evins AE. Achieving smoking cessation in individuals with schizophrenia: special considerations. CNS Drugs. (2017) 31:471–81. doi: 10.1007/s40263-017-0438-8
42. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care. (2018) 41(Suppl 1):S73–85. doi: 10.2337/dc18-S008
43. Swanson AJ, Pantalon MV, Cohen KR. Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. J Nerv Ment Dis. (1999). 187:630–5. doi: 10.1097/00005053-199910000-00007
44. Velligan DI, Bow-Thomas CC, Huntzinger C, Ritch J, Ledbetter N, Prihoda TJ, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry. (2000). 157:1317–23. doi: 10.1176/appi.ajp.157.8.1317
45. McCracken S, Corrigan P. Motivational interviewing for medication adherence in individuals with schziophrenia. In: Arkowitz H, Westra HA, Miller WR, Rollnick S, editors. Motivational Interviewing in the Treatment of Psychological Problems. New York, NY: GuildfordPress (2007), 249–76.
46. Kim SE, Michalopoulos C, Kwong RM, Warren A, Manno MS. Telephone care management's effectiveness in coordinating care for Medicaid beneficiaries in managed care: a randomized controlled study. Health Serv Res. (2013) 48:1730–49. doi: 10.1111/1475-6773.12060
47. Chen JA, Chung WJ, Young SK, Tuttle MC, Collins MB, Darghouth SL, et al. COVID-19 and telepsychiatry: early outpatient experiences and implications for the future. Gen Hosp Psychiatry. (2020) 66:89–95. doi: 10.1016/j.genhosppsych.2020.07.002
48. Shoemaker SJ, Parchman ML, Fuda KK, Schaefer J, Levin J, Hunt M, et al. A review of instruments to measure interprofessional team-based primary care. J Interprof Care. (2016) 30:423–32. doi: 10.3109/13561820.2016.1154023
49. Arbaje AI, Kansagara DL, Salanitro AH, Englander HL, Kripalani S, Jencks SF, et al. Regardless of age: Incorporating principles from geriatric medicine to improve care transitions for patients with complex needs. J Gen Intern Med. (2014) 29:932–9. doi: 10.1007/s11606-013-2729-1
50. Samal L, Dykes PC, Greenberg JO, Hasan O, Venkatesh AK, Volk LA, et al. Care coordination gaps due to lack of interoperability in the United States: a qualitative study and literature review. BMC Health Serv Res. (2016) 16:143. doi: 10.1186/s12913-016-1373-y
51. Grove LR, Gertner AK, Swietek KE, Lin CC, Ray N, Malone TL, et al. Effect of enhanced primary care for people with serious mental illness on service use and screening. J Gen Intern Med. (2021) 36:970–7. doi: 10.1007/s11606-020-06429-2
52. Wittink MN, Cross W, Goodman J, Jackson H, Lee HB, Olivares T, et al. Taking the long view in an inpatient medical unit: a person-centered, integrated team approach for patients with severe mental illnesses. Psychiatr Serv. (2020) 71:885–92. doi: 10.1176/appi.ps.201900385
53. Miller BF, Ross KM, Davis MM, Melek SP, Kathol R, Gordon P. Payment reform in the patient-centered medical home: enabling and sustaining integrated behavioral health care. Am Psychol. (2017) 72:55–68. doi: 10.1037/a0040448
Keywords: serious mental illness, care coordination, care management, cardiovascular risk, behavioral coaching
Citation: Murphy KA, Dalcin A, McGinty EE, Goldsholl S, Heller A and Daumit GL (2021) Applying Care Coordination Principles to Reduce Cardiovascular Disease Risk Factors in People With Serious Mental Illness: A Case Study Approach. Front. Psychiatry 12:742169. doi: 10.3389/fpsyt.2021.742169
Received: 15 July 2021; Accepted: 30 November 2021;
Published: 22 December 2021.
Edited by:
Marsha Wittink, University of Rochester, United StatesReviewed by:
Frederike Jörg, University Medical Center Groningen, NetherlandsCopyright © 2021 Murphy, Dalcin, McGinty, Goldsholl, Heller and Daumit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karly A. Murphy, a2J1cmtlMzRAamhtaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.