
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry, 22 October 2021
Sec. Child and Adolescent Psychiatry
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.738368
This article is part of the Research TopicWomen in Psychiatry 2021: Child and Adolescent PsychiatryView all 8 articles
Objectives: Neurocognitive functions might indicate specific pathways in developing attention deficit hyperactivity disorder (ADHD). We focus on reward-related dysfunctions and analyze whether reward-related inhibitory control (RRIC), approach motivation, and autonomic reactivity to reward-related stimuli are linked to developing ADHD, while accounting for comorbid symptoms of oppositional defiant disorder (ODD), and callous-unemotional (CU) traits.
Methods: A sample of 198 preschool children (115 boys; age: m = 58, s = 6 months) was re-assessed at age 8 years (m = 101.4, s = 3.6 months). ADHD diagnosis was made by clinical interviews. We measured ODD symptoms and CU traits using a multi-informant approach, RRIC (Snack-Delay task, Gift-Bag task) and approach tendency using neuropsychological tasks, and autonomic reactivity via indices of electrodermal activity (EDA).
Results: Low RRIC and low autonomic reactivity were uniquely associated with ADHD, while longitudinal and cross-sectional links between approach motivation and ADHD were completely explained by comorbid ODD and CU symptoms.
Conclusion: High approach motivation indicated developing ADHD with ODD and CU problems, while low RRIC and low reward-related autonomic reactivity were linked to developing pure ADHD. The results are in line with models on neurocognitive subtypes in externalizing disorders.
Attention deficit hyperactivity disorder (ADHD) and oppositional defiant and conduct disorder (ODD/CD) frequently occur together—about 50% of ADHD cases also develop ODD/CD (1). Longitudinal research has revealed a common developmental progression from preschool symptoms of ADHD to comorbid symptoms of ODD/CD in childhood and adolescence (2, 3). In recent years, research on externalizing disorders has pointed to a further distinguishable, early-developing psychopathological dimension, i.e., so-called callous-unemotional (CU) traits. CU traits, which comprise reduced guilt and remorse, callousness, low empathy, and deficient prosocial emotions, overlap with the dimensions of ADHD and ODD/CD symptoms (2, 4).
ADHD, ODD/CD and CU traits have been found to be associated with diverse neurocognitive dysfunctions (1). As these dysfunctions might represent markers of etiological subtypes or predictors of specific developmental pathways, the question of whether a dysfunction is uniquely related to ADHD or pertains to a specific combination with comorbid symptoms is an important issue of research (5). However, longitudinal research on this issue is scarce, especially between preschool and school age.
Dysfunctional processing of reward has been found to be prevalent in ADHD and in externalizing disorders. The “trait impulsivity” model, for example, postulates that early emerging impulsivity is a crucial vulnerability factor (externalizing liability) and indicative of a developmental pathway from ADHD to ODD and other externalizing disorders such as CD and substance use disorder (6). The impulsivity concept combines the two components of high subcortically mediated (bottom-up) approach motivation and low top-down inhibitory (cognitive) control (IC) mediated by the forebrain (7–9). In a further model, Blair et al. (2) proposed that deficient decision making, which comprises a high risk of impulsivity, represents the lowest common denominator for conduct problems. Similar to the trait-impulsivity model, the dysfunction is thought to involve subcortical bottom-up processes and prefrontal top-down control, and to be present in children showing multiple facets of externalizing problems, including children with ADHD, ODD/CD, and CU traits.
There is broad empirical evidence of low reward-related IC (RRIC) and other executive function deficits in children with ADHD as well as those with ODD/CD (10, 11). Low RRIC, dysfunctional reward-related decision making, and “delay aversion” have been assumed to characterize an ADHD subtype (12) with comorbid ODD/CD symptoms (13–15). CU traits, however, have sometimes been assumed to be associated with a rather good inhibitory control capacity and fewer cognitive deficits (4, 16, 17), making it possible that children with ADHD symptoms and comorbid CU traits show fewer RRIC deficits. Research on this issue is sparse. In particular, there are very few studies on the association between CU traits and IC in the context of ADHD development.
There is relatively broad evidence that children with ADHD show cortical arousal deficits. Cognitive deficits of the disorder have been assumed to be caused by difficulties in regulating arousal according to situational demands (1, 18, 19). Cortical and peripheral sympathetic arousal are linked via the locus coeruleus and the brain norepinephrine system. Measures of sympathetic electrodermal activity (EDA) have thus been taken as indicators of the arousal regulation dysfunction in ADHD (18). Bellato et al. (18) systematically reviewed the results of 55 studies on autonomic nervous system function in ADHD, and found that children and adolescents with ADHD showed hypoarousal (indicated, e.g., by low EDA). The authors concluded, however, that reactivity to rewarding, emotional stimuli, as well as the role of comorbidity, have not yet been sufficiently studied.
It has been shown that low resting-state sympathetic arousal in children with ADHD can be caused by comorbid ODD/CD problems, low anxiety, and psychopathic personality traits (20, 21). These characteristics overlap with CU traits (4, 22). Studies analyzing sympathetic arousal (using the cardiac pre-ejection period) during reward-related tasks found associations with comorbid ODD: Tenenbaum et al. (23) compared healthy children and children with ADHD, ADHD+ODD, and ADHD+CD regarding their sympathetic activity during a risky decision-making task and found the lowest activity in children with ADHD+ODD. In a sample of children with ADHD, Beauchaine et al. (24) found that sympathetic activity during a rewarded simple-matching task was associated with parent-reported conduct behavior problems. However, Conzelmann et al. (25) compared the electrodermal reactivity (EDR) to neutral, positive, and aversive stimuli between unmedicated boys with ADHD and healthy controls, and found lower EDR in the boys with ADHD in all three conditions, irrespective of comorbidity. As these studies did not assess CU traits, it is possible that comorbid CU traits and/or ODD symptoms explain low arousal in response to reward-related tasks.
Based on the research reviewed above, we examined the following hypotheses: (a) Low RRIC is associated with developing ADHD. This association overlaps with (i.e., can be explained by) comorbid ODD symptoms. We do not hypothesize an overlap with CU traits, as this top-down control component of impulsivity might not be impaired in children with CU traits. (b) High reward-related approach behavior is associated with developing ADHD. This association can be explained by ODD symptoms and CU traits, as this bottom-up component of impulsivity can be expected to be common to all three psychopathological domains. (c) Low autonomic reactivity to reward-related stimuli is linked to ADHD. This association overlaps with ODD symptoms and CU traits.
A sample of 198 preschool children (115 boys, 58%) was recruited from childcare facilities. The children were 4–5 years old (T1; m = 58, s = 6 months) at the first assessment wave and 8 years old (T2; m = 101.4, s = 3.65 months) at the second wave. Inclusion criteria were: IQ>80, lack of motor and sensory disabilities, lack of chronic physical and mental diseases, no indication of a trauma experienced by the child, and no continuous pharmacological treatment. To determine eligibility, a telephone interview and a screening questionnaire on the ADHD symptoms of the child [FBB-ADHS-V by (26), see below] were used. Children with high ADHD symptoms were oversampled. Of the 198 children, 179 participated in the 8-years assessment (retention rate of 89%). There were no differences between children who participated in the 8-years assessment and those who dropped out with respect to gender (Chi2 = 0.22; t = 0.00) and age of the child, ADHD symptoms, symptoms of anxiety/depression, and oppositional symptoms of the child (t-scores between −1.78 and 0.96).
At T1, all children were medication naïve. At 8 years (T2), three children were medicated with methylphenidate, and were therefore excluded from the analyses of the 8-years data. Table 1 contains descriptive data of the sample. Parents gave their written informed consent to participate in the study, and received an expense allowance of 50 Euros at T1 and 70 Euros at the T2 assessment. The study was approved by the Ethics Committee of the Medical Faculty, University of Marburg.
Reward-related inhibitory control. RRIC was measured using the Snack-Delay task by Kochanska (27). In this task, the child is instructed to wait for the ringing of a bell before he/she can retrieve a sweet that is covered by a transparent cup. After a practice trial, six trials followed, with delay intervals between 10 and 40 s. Waiting vs. approach behaviors are scored (27). The task is widely used for the assessment of RRIC in ADHD and has shown good psychometric properties (28). In the present study, tasks were carried out and scored by trained investigators. Interrater reliability was checked in 20% of cases and proved to be very good (ICC = 0.99).
Approach motivation. The Stranger-with-Toys (SWT) task (29) was used to capture behavioral approach motivation, i.e., the tendency to immediately approach a rewarding stimulus while disregarding possible risks associated with the unfamiliarity of the adult and the situation. In the past, similar tasks have been used to measure “exuberance” (30). In the SWT task, the child sits at a table with one rather boring toy. A stranger enters the room, bringing along a transparent bag of interesting toys, which she successively unpacks and plays with while not attending to the child. After 3 min, she invites the child to play with her together with the toys and continues to talk kindly to the child for a further 2 min. The latency (seconds) until the child's first spontaneous utterance directed to the stranger is scored. The measure has proven to be highly stable (0.74 across 2 years), and to show significant associations with parent ratings of the child's approach vs. withdrawal behavior, observed approach behavior in peer interactions (29), and ADHD symptoms of preschool children (31). Interrater reliability (checked in 20% of cases) was very good (ICC = 0.90).
ADHD and ODD symptoms of the child. The ADHD scale of the Parental Account of Childhood Symptoms (PACS) interview in the modified preschool version (Pre-PACS) (32) was conducted with the mother. The preschool version of the PACS interview has demonstrated good psychometric properties, and has proven to be suitable for the assessment of ADHD symptoms as a dimensional variable (33). Parents and teachers completed the preschool version of the ADHD rating scale (FBB-ADHS-V) by (26). This questionnaire is suitable for capturing ADHD symptoms according to the DSM-5 and ICD-10, and has shown high reliability and validity. In the present study, dimensional ADHD symptom scores were summed up (after z-transformation). Cronbach's Alpha of this summary score was 0.63.
Parents completed the ODD rating scale of the questionnaire (FBB-SSV), which has also shown good psychometric properties (26).
Anxiety and depressive symptoms of the child. The Anxious/Depressed scale of the German version of the Child Behavior Checklist (CBCL4-18) by Döpfner et al. (34) was employed for control purposes. The scale shows significant associations with anxiety and emotional disorders, indicating good validity (34).
Reward-related inhibitory control. At T2, we conducted the Gift-Bag task by Kochanska (27). In this task, the experimenter places a red paper bag containing a gift for the child on the table in front of the child. The experimenter then leaves the room for 5 min. The child is instructed not to look while awaiting the experimenter's return with the mother. Approach behavior was scored in accordance with Kochanska (27). Interrater reliability (20% of cases) proved to be very good (Kappa = 1.0).
Approach motivation. The interview on attractive toys (Int-AT) task adapted from Asendorpf (29) was conducted. As in the preschool task, approach behavior is provoked by a series of attractive toys and has to override a mild obstacle introduced by the unfamiliarity of the experimenter and the situation. The child is told that he/she will receive a gift for participating, but that prior to this, an interview on the attractiveness of a series of toys has to be conducted by a colleague. After 3 min of waiting (with a small book), an unfamiliar adult enters the room and places six different toys in front of the child and asks six questions, with a break of 10 s between the child's answer and the next question. The latency in seconds until the child's first spontaneous utterance toward the experimenter is scored.
Autonomic reactivity. Arousal level and reactivity of the sympathetic nervous system can be validly measured by indices of the EDA (35). We analyzed the electrodermal reactivity to the six questions of the Int-AT task. Baseline EDA (3 min) was recorded before the Int-AT task. The procedure was videotaped. Video and EDA recordings were synchronized. The measurement of EDA followed the guidelines by Boucsein et al. (35) using a BioPac MP150 system. EDA was measured as skin conductance level (in microsiemens) with two silver-silver chloride (Ag/AgCl) disposable electrodes attached to the middle phalanges of the middle and ring finger of the non-dominant hand. The mean skin conductance level (SCL) during baseline was calculated. To assess the child's sympathetic reactivity, the mean amplitude of the SCRs elicited by the six questions of the Int-AT task was determined.
ADHD diagnoses. At T2, the ADHD diagnostic module of the Child and Adolescent Psychiatric Interview (CAPA) by Angold et al. (36) was conducted with the mothers. The CAPA is a well-validated, widely established clinical interview. Diagnoses were made according to the DSM-5. Of the 179 children, n = 31 (15.7%) received an ADHD research diagnosis. Parents and teachers completed the ADHD questionnaire (FBB-ADHS of DISYPS-III) by Döpfner and Görtz-Dorten (37). In the present study, the parent (r = 0.63, p < 0.001) and the teacher (r = 0.54, p < 0.001) ADHD questionnaire scores were significantly associated with the ADHD diagnosis.
ODD symptoms. For the assessment of ODD symptoms, mothers and fathers completed the oppositional symptoms scale (of the FBB-SSV questionnaire; DISYPS-III) by Döpfner and Görtz-Dorten (37). Teachers and mothers, moreover, completed the conduct problems scale of the Strengths and Difficulties Questionnaire (SDQ) (38). We created a dimensional ODD symptom score by summing up the z-transformed scores of the mother (SDQ and FBB-SSV), father (FBB-SSV), and teacher (SDQ) (r's between 0.37 and 0.77, Cronbach's Alpha: 0.83).
CU traits. CU traits were assessed using the “prosocial behavior” scale of the SDQ and the “callous-unemotional” scale of the Antisocial Process Screening Device (APSD) (39). Mothers and teachers completed these questionnaires. The items of the two scales have proven to validly capture CU traits in 4–9-year-old children (40–42). Additionally, mothers and fathers completed the CU scale (of the FBB-SSV; DISYPS-III) by Döpfner and Görtz-Dorten (37). In the present study, the mother, father and teacher CU scores correlated significantly (r's between 0.23 and 0.44). We built a composite score by summing up the z-transformed scores (Cronbach's Alpha: 0.67).
Further control variables. Symptoms of anxiety disorders and depression were assessed by use of the screen interview of the DISYPS-III by Döpfner and Görtz-Dorten (37). The verbal IQ of the child was estimated by two subtests (Similarities and Vocabulary) of the Wechsler Intelligence Scale for Children [WISC-IV; (43)].
Correlation coefficients among the study variables were calculated for descriptive purposes. In those cases where gender of child was significantly associated with a neuropsychological/physiological predictor variable, we adjusted for gender in all respective analyses.
To test the hypotheses on the associations of (a) T1 and T2 RRIC, (b) T1 and T2 approach tendency and (c) T2 electrodermal reactivity with T2 ADHD diagnosis, we conducted logistic regression analyses. In analyses (a) and (b), we adjusted for T1 ADHD symptoms (model 2) to assess whether the predictor variables predict T2 ADHD over and above T1 ADHD symptoms. In all analyses (a, b, c), ODD and CU symptoms were covaried in model 3. For control purposes we additionally adjusted for anxiety/depressive symptoms and the approximated verbal IQ of the child in model 4.
In a next step, for the significant predictor-ADHD links, we tested whether ODD and/or CU symptoms significantly explain this link (i.e., the common variance between the predictor variable with the T2 ADHD diagnosis). For this purpose, we partitioned the total predictor-ADHD link (common variance) into the direct link (common variance between predictor and ADHD not explainable by the comorbid dimension) and the indirect link (i.e., common variance explainable by the comorbid dimension) using ordinary least squares (OLS) regression, and tested these links using the bootstrapping method recommended by Preacher and Hayes (44). The path-analytic procedure is suitable for analyzing the role of third variables (e.g., mediators, confounders, suppressor variables) in relationships between two variables (45). Calculations were conducted using the SPSS macro “Indirect” (44) and IBM SPSS Statistics software (IBM Corp.).
Consistent with our hypothesis, the T1 Snack-Delay task and the T2 Gift-Bag task were significantly associated with the T2 ADHD diagnosis (Table 3A, model 1). Associations remained statistically significant after adjusting for the T1 ADHD symptoms score (Table 3A, model 2). We further hypothesized that the link between RRIC and ADHD is shared with ODD symptoms. However, the T1 Snack-Delay task was only marginally significantly associated with the T2 ODD score, and the T2 Gift-Bag task was not correlated with the T2 ODD score (Table 2). Moreover, covariation of ODD and CU scores (Table 3A, model 3) did not change the significant associations between the RRIC tasks and T2 ADHD diagnosis. Hence, the results indicate a unique association between low RRIC and ADHD.
The T1 SWT task and the T2 Int-AT task were significantly associated with the T2 ADHD diagnosis (Table 3B, model 1) indicating high approach motivation in children with ADHD. After adjusting for T1 ADHD symptoms, the prediction by the T1 SWT task was no longer significant (Table 3B, model 2). This finding indicates significant common variance between ADHD symptoms and high approach motivation already at T1. We further hypothesized that the link between approach motivation and ADHD can be explained by comorbid ODD and CU symptoms. Adjustment for the ODD and CU scores led to a reduction in the associations of T1 and T2 approach motivation task with T2 ADHD (Table 3B, model 3). Next, we analyzed whether T2 ODD and CU scores explain the link between T1 SWT task and T2 ADHD (Table 4). The indirect links via the ODD and via the CU score were significant. After accounting for the indirect link via the comorbidity scores, the SWT task-ADHD association was no longer significant. Hence, T1 approach motivation predicted ADHD with comorbid ODD and CU problems. Regarding the link of the T2 Int-AT task with T2 ADHD, indirect effects by CU and ODD scores were not statistically significant (Table 4).
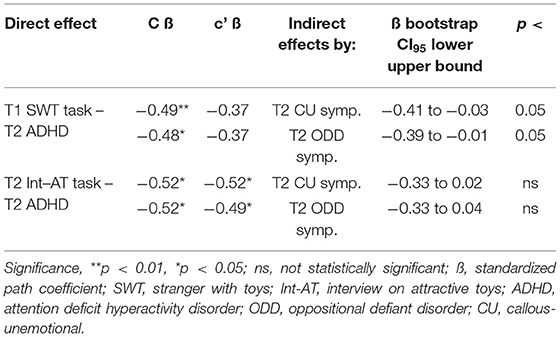
Table 4. Path-analytic estimation of the direct and indirect links of approach motivation with ADHD, ODD, and CU symptoms.
As expected, children with ADHD showed lower mean SCRs to the questions on the attractiveness of the toys than did the other children (Table 2; Table 3C, model 1). Mean SCRs were not significantly correlated with ODD symptoms and CU traits (Table 2). Adjusting for ODD and CU scores did not change the significant association with the ADHD diagnosis (Table 3C, model 3). Hence, low sympathetic reactivity to the stimuli was uniquely associated with ADHD.
For the purpose of comparison, we additionally assessed baseline SCL. As shown in Table 2, the score was significantly associated with preschool ADHD symptoms. Associations with preschool and school-age ODD symptom scores just failed to reach statistical significance (p's < 0.10). Children with high symptoms showed a lower baseline/resting arousal level.
In the present study, it was analyzed whether reward-related dysfunctions and sympathetic arousal are linked to ADHD development, and whether or not these links can be explained by ODD symptoms and CU traits. We found low RRIC, measured at preschool and school age, to be uniquely related to ADHD. Preschool RRIC significantly predicted ADHD development. The association between preschool approach motivation with school-age ADHD was significantly and completely explainable by comorbid ODD symptoms as well as by CU traits. Children with ADHD showed low autonomic responses to reward-related stimuli. This link was unique for ADHD, i.e., could not be explained by symptoms of ODD or CU traits. We discuss these findings in greater detail in the following.
We expected that RRIC deficits in ADHD can be explained by comorbid ODD symptoms. Contrary to this expectation, however, we found that the school-age ADHD diagnosis was significantly and uniquely associated with preschool- and school-age low RRIC. The associations could not be explained by comorbid ODD symptoms or CU traits. This result appears to correspond with a recent model on conduct problem development by Waller et al. (22). In this model, three pathways are distinguished, of which an ADHD pathway is characterized by low cognitive control. Hence, in this early stage of ADHD development, low cognitive control in the reward-related context might rather uniquely pertain to ADHD.
Corresponding to our expectations, high approach motivation at preschool and school age was associated with ADHD. Moreover, as expected, ODD symptoms and CU traits significantly and completely explained the link between preschool approach motivation and ADHD. Hence, high approach motivation at preschool age might indicate risk for the development of comorbid ADHD/ODD symptoms/CU traits. Several models have proposed that impulsivity forms the basis of externalizing disorders (7, 15). Blair et al. (2) assumed that deficient decision making in the context of reward and punishment (implying risk of impulsivity) is common to ADHD, externalizing disorders and CU traits. In the present study, we assessed the tendency to approach a gratification while overriding signals of threat (due to unfamiliarity). Thus, it seems probable that the SWT task captures the respective neurocognitive dysfunction at an early developmental stage. Based on these findings, it might be worthwhile to cross-validate and further refine the neuropsychological assessment of approach motivation as a risk predictor of the comorbid pathway.
In line with our hypothesis, children with an ADHD diagnosis showed comparably low mean SCRs to the reward-related stimuli at the 8-years assessment. The associations were unique for ADHD. ODD and CU symptoms were not associated with low SCRs. This finding corresponds to the results of Conzelmann et al. (25), who reported that children with ADHD showed low SCRs (regardless of the emotional valence of the stimuli), which were not explained by comorbidity. Hypoarousal, i.e., low, dysregulated cortical arousal, is an etiologically significant dysfunction in ADHD. Low arousal is thought to be implicated in cognitive and attentional deficits due to difficulties in the regulation of wakefulness and alertness states according to environmental demands (1, 18, 19). Our finding appears to be in line with this perspective.
Our study has several strengths, including the consideration of neuropsychological and psychophysiological characteristics as well as different psychopathological domains in a longitudinal design; the multi-informant approach (mother, father, and teacher reports) to the assessment of comorbid symptoms; the use of dimensional scores reflecting the expression of comorbid symptoms; and the analysis of a sample with increased ADHD symptoms, allowing for a sensitive and reliable description of ADHD development. A limitation might be seen in the lack of measurement of autonomic reactivity to neutral and negative stimuli. Such a measurement would have facilitated the comparison with previous research. Moreover, in future research, it would be interesting to assess further neuropsychological, biological and psychosocial characteristics and to analyze the role of ADHD symptom presentation (i.e., inattention or hyperactivity/impulsive symptoms) in order to increasingly refine the characterization of the developmental pathways. Due to possible influences on our findings the overlap between RRIC and the concept of delay aversion should be analyzed. As a further limitation of our study, it is not possible to draw any causal inferences from the findings. The reward-processing dysfunctions might constitute intermediate phenotypes involved in the development of the specific psychopathological problems, or may merely be correlates of these problems. In either case, however, an identification of neuropsychological/physiological predictors or indicators of emerging psychopathological pathways can be useful for risk identification and the application of tailored interventions.
Taken together, we assessed the development of ADHD while accounting for ODD symptoms and CU traits between preschool and school age. In line with current theorizing, we found that high approach motivation was linked to ADHD with comorbid ODD/CU symptoms. Low reward-related cognitive control (RRIC) and low autonomic reactivity to reward-related stimuli were specific for ADHD and might reflect the arousal regulation dysfunction of the disorder at an early stage of development. Given the scarcity of longitudinal data in this area, our results need to be cross-validated. The neurocognitive variables might constitute age-specific markers of clinically more homogenous pathways.
The datasets presented in this article are not readily available because they are part of a ongoing longitudinal study. Requests to access the datasets should be directed to dXJzdWxhLnBhdWxpLXBvdHRAbWVkLnVuaS1tYXJidXJnLmRl.
The studies involving human participants were reviewed and approved by Ethics Committee of the Faculty of Medicine of the University of Marburg, Marburg, Germany. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
AC, KB, FD, PS, and SS organized the database. UP-P performed the statistical analysis. SS wrote the first draft of the manuscript. SS and UP-P wrote sections of the manuscript. All authors contributed to conception, design of the study, contributed to manuscript revision, read, and approved the submitted version.
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, grant number Be2573/3-1,2) and the University Hospital Marburg (grant number: 17/2018 MR).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar J, Ramos-Quiroga JA. Attention deficit/hyperactivity disorder. Nat Rev Dis Prim. 1:1–23 (2015). doi: 10.1038/nrdp.2015.20
2. Blair RJ, Leibenluft E, Pine DS. Conduct disorder and callous-unemotional traits in youth. N Engl J Med. (2014) 371:2207–16. doi: 10.1056/NEJMra1315612
3. Sonuga-Barke EJ, Auerbach J, Campbell SB, Daley D, Thompson M. Varieties of preschool hyperactivity: multiple pathways from risk to disorder. Dev Sci. (2005) 8:141–50. doi: 10.1111/j.1467-7687.2005.00401.x
4. Frick PJ, Ray JV, Thornton LC, Kahn RE. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol Bull. (2014) 140:1–57. doi: 10.1037/a0033076
5. Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry. (2014) 76:350–3. doi: 10.1016/j.biopsych.2014.01.006
6. Beauchaine TP, McNulty T. Comorbidities and continuities as ontogenic processes: toward a developmental spectrum model of externalizing psychopathology. Dev Psychopathol. (2013) 25:1505–28. doi: 10.1017/S0954579413000746
7. Beauchaine TP, Zisner AR, Sauder CL. Trait impulsivity and the externalizing spectrum. Ann Rev Clin Psychol. (2017) 13:343–68. doi: 10.1146/annurev-clinpsy-021815-093253
8. Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, et al. Behavioral and neural correlates of delay of gratification 40 years later. Proc Natl Acad Sci USA. (2011) 108:14998–5003. doi: 10.1073/pnas.1108561108
9. Nigg JT. Annual research review: on the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J Child Psychol Psychiatry Allied Dis. (2017) 58:361–83. doi: 10.1111/jcpp.12675
10. Hobson CW, Scott S, Rubia K. Investigation of cool and hot executive function in ODD/CD independently of ADHD. J Child Psychol Psychiatry. (2011) 52:1035–43. doi: 10.1111/j.1469-7610.2011.02454.x
11. Pauli-Pott U, Becker K. Neuropsychological basic deficits in preschoolers at risk for ADHD: a meta-analysis. Clin Psychol Rev. (2011) 31:626–37. doi: 10.1016/j.cpr.2011.02.005
12. Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev. (2014) 38:125–34. doi: 10.1016/j.neubiorev.2013.07.012
13. Alegria AA, Radua J, Rubia K. Meta-Analysis of fMRI studies of disruptive behavior disorders. Am J Psychiatry. (2016) 173:1119–30. doi: 10.1176/appi.ajp.2016.15081089
14. Kamradt JM, Nigg JT, Friderici KH, Nikolas MA. Neuropsychological performance measures as intermediate phenotypes for attention-deficit/hyperactivity disorder: a multiple mediation analysis. Dev Psychopathol. (2017) 29:259–72. doi: 10.1017/S0954579416000195
15. Martel MM, Levinson CA, Lee CA, Smith TE. Impulsivity symptoms as core to the developmental externalizing spectrum. J Abn Child Psychol. (2017) 45:83–90. doi: 10.1007/s10802-016-0148-6
16. Fanti KA, Kimonis ER, Hadjicharalambous MZ, Steinberg L. Do neurocognitive deficits in decision making differentiate conduct disorder subtypes? Eur Child Adoles Psy. (2016) 25:989–96. doi: 10.1007/s00787-016-0822-9
17. Graziano PA, Landis T, Maharaj A, Ros-Demarize R, Hart KC, Garcia A. Differentiating preschool children with conduct problems and callous-unemotional behaviors through emotion regulation and executive functioning. J Clin Child Adolesc. (2019) 1–13. doi: 10.1080/15374416.2019.1666399. [Epub ahead of print].
18. Bellato A, Arora I, Hollis C, Groom MJ. Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neurosci Biobehav Rev. (2020) 108:182–206. doi: 10.1016/j.neubiorev.2019.11.001
19. Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry. (2005) 57:1248–55. doi: 10.1016/j.biopsych.2004.09.010
20. Fairchild G, Baker E, Eaton S. Hypothalamic-Pituitary-adrenal axis function in children and adults with severe antisocial behavior and the impact of early adversity. Curr Psychiatry Rep. (2018) 20:84. doi: 10.1007/s11920-018-0952-5
21. Herpertz SC, Wenning B, Mueller B, Qunaibi M, Sass H, Herpertz-Dahlmann B. Psychophysiological responses in ADHD boys with and without conduct disorder: implications for adult antisocial behavior. J Am Acad Child Adolesc Psychiatry. (2001) 40:1222–30. doi: 10.1097/00004583-200110000-00017
22. Waller R, Hyde LW, Grabell AS, Alves ML, Olson SL. Differential associations of early callous-unemotional, oppositional, and ADHD behaviors: multiple domains within early-starting conduct problems? J Child Psychol Psychiatry. (2015) 56:657–66. doi: 10.1111/jcpp.12326
23. Tenenbaum RB, Musser ED, Raiker JS, Coles EK, Gnagy EM, Pelham WE. Specificity of reward sensitivity and parasympathetic-based regulation among children with attention-deficit/hyperactivity and disruptive behavior disorders. J Abnor Child Psychol. (2018) 46:965–77. doi: 10.1007/s10802-017-0343-0
24. Beauchaine TP, Gatzke-Kopp L, Neuhaus E, Chipman J, Reid MJ, Webster-Stratton C. Sympathetic- and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. J Consult Clin Psychol. (2013) 81:481–93. doi: 10.1037/a0032302
25. Conzelmann A, Gerdes ABM, Mucha RF, Weyers P, Lesch KP, Bahne CG, et al. Autonomic hypoactivity in boys with attention-deficit/hyperactivity disorder and the influence of methylphenidate. World J Biol Psychia. (2014) 15:56–65. doi: 10.3109/15622975.2013.829584
26. Döpfner M, Görtz-Dorten A. DISYPS-III. Diagnostik-System Für Psychische Störungen nach ICD-10 und DSM-5 für Kinder und Jugendliche - IIHogrefe I. Bern: Hogrefe (2017).
27. Kochanska G. Family Study. Effortful Control Batteries. Iowa City, IA: University of Iowa (2009).
28. Carlson SM. Developmentally sensitive measures of executive function in preschool children. Dev Neuropsychol. (2005) 28:595–616. doi: 10.1207/s15326942dn2802_3
29. Asendorpf JB. Development of inhibition during childhood: evidence for situational specificity and a two-factor model. Dev Psychol. (1990) 26:721–30. doi: 10.1037/0012-1649.26.5.721
30. Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. (2001) 72:1–21. doi: 10.1111/1467-8624.00262
31. Pauli-Pott U, Dalir S, Mingebach T, Roller A, Becker K. Attention deficit/hyperactivity and comorbid symptoms in preschoolers: differences between subgroups in neuropsychological basic deficits. Child Neuropsychol. (2014) 20:230–44. doi: 10.1080/09297049.2013.778236
32. Daley D. Preschool-Parent Account of Child Symptoms (Pre-Pacs). Nottingham: University of Nottingham (2010).
33. Sonuga-Barke EJ, Dalen L, Remington B. Do executive deficits and delay aversion make independent contributions to preschool attention-deficit/hyperactivity disorder symptoms? J Am Acad Child Adol Psychiatry. (2003) 42:1335–42. doi: 10.1097/01.chi.0000087564.34977.21
34. Döpfner M, Schmeck K, Berner W, Lehmkuhl G, Poustka F. Reliabilität und validität der child-behavior-checklist. Z Kinder Jugendpsychiatr Psychother. (1994) 22:189–205.
35. Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, et al. Publication recommendations for electrodermal measurements. Psychophysiology. (2012) 49:1017–34. doi: 10.1111/j.1469-8986.2012.01384.x
36. Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The child and adolescent psychiatric-assessment (Capa). Psychol Med. (1995) 25:739–53. doi: 10.1017/S003329170003498X
37. Döpfner M, Görtz-Dorten A. DISYPS-III. Diagnostik-System Für Psychische Störungen nach ICD-10 und DSM-5 für Kinder und Jugendliche – IIHogrefe I. Bern (2017).
38. Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. (1997) 38:581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x
39. Frick PJ, Hare R. The Antisocial Process Screening Device (APSD): Technical Manual. Toronto, ON: Multi-Health Systems (2001).
40. Dadds MR, Fraser J, Frost A, Hawes DJ. Disentangling the underlying dimensions of psychopathy and conduct problems in childhood: a community study. J Consult Clin Psych. (2005) 73:400–10. doi: 10.1037/0022-006X.73.3.400
41. Koglin U, Petermann F. Callous-unemotional traits: behavioral problems und prosocial behavior in kindergarten children. Kindh Entwickl. (2012) 21:141–50. doi: 10.1026/0942-5403/a000080
42. Pasalich DS, Dadds MR, Hawes DJ, Brennan J. Do callous-unemotional traits moderate the relative importance of parental coercion versus warmth in child conduct problems? An observational study. J Child Psychol Psychiatry. (2011) 52:1308–15. doi: 10.1111/j.1469-7610.2011.02435.x
43. Petermann F, Petermann U. Wechsler Intelligence Scale for Children - Fourth Edition, deutsche Version. Frankfurt am Main: Pearson Assessment & Information GmbH (2011).
44. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. (2008) 40:879–91. doi: 10.3758/BRM.40.3.879
Keywords: ADHD, externalizing disorders, callous-unemotional traits, developmental pathways, cognitive control, neurocognitive markers
Citation: Schloß S, Derz F, Schurek P, Cosan AS, Becker K and Pauli-Pott U (2021) Reward-Related Dysfunctions in Children Developing Attention Deficit Hyperactivity Disorder—Roles of Oppositional and Callous-Unemotional Symptoms. Front. Psychiatry 12:738368. doi: 10.3389/fpsyt.2021.738368
Received: 08 July 2021; Accepted: 13 September 2021;
Published: 22 October 2021.
Edited by:
Li Yang, Peking University Sixth Hospital, ChinaReviewed by:
Br Yang, Shenzhen Children's Hospital, ChinaCopyright © 2021 Schloß, Derz, Schurek, Cosan, Becker and Pauli-Pott. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ursula Pauli-Pott, dXJzdWxhLnBhdWxpLXBvdHRAbWVkLnVuaS1tYXJidXJnLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.