
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 30 September 2021
Sec. Psychological Therapies
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.727496
This article is part of the Research Topic Can Psychedelic Therapies open a New Frontier in Mental Healthcare (Or Will the Bubble Burst?) View all 22 articles
 James B. Close1*
James B. Close1* Julia Bornemann1
Julia Bornemann1 Maria Piggin2
Maria Piggin2 Sandra Jayacodi3
Sandra Jayacodi3 Lisa Xiaolu Luan1
Lisa Xiaolu Luan1 Robin Carhart-Harris1
Robin Carhart-Harris1 Meg Jo Spriggs1
Meg Jo Spriggs1Within the context of scientific research, patient and public involvement (PPI) is defined as research performed “with” or “by” patients and members of the public, rather than “to,” “about”, or “for” them. When carried out systematically and thoughtfully, PPI has the potential to strengthen the quality and impact of research by fostering accountability, transparency, and relevance. There exist numerous guidelines, frameworks and tools for supporting PPI, however, these do not account for the unique challenges faced in psychedelic research. This paper describes the co-design of guidance intended to help build, evaluate and improve PPI in psychedelic research. A steering group was formed to design and run a co-design workshop alongside public collaborators. Insights from this workshop were analyzed and refined into a comprehensive and readily usable guide for planning PPI specific to the field of psychedelic research. Core values emerging from the process focused on the essential importance of trust, learning, purpose and inclusivity. It is hoped that this guidance will be a starting point for incorporating PPI in future psychedelic research, so that it can grow and adapt as this burgeoning field of research progresses.
Research into “classic” tryptamine psychedelics [e.g., psilocybin, lysergic acid diethylamide (LSD), and dimethyltryptamine (DMT)] is proliferating at a rapid rate. Database searches reveal more than a doubling in the number of peer-reviewed publications relating to psychedelics in the last decade (1). Among this surge is a growing number of clinical and non-clinical trials exploring the action of psychedelics in human subjects (2, 3). Around the globe, research centers choosing to focus on psychedelics shape the manner and direction in which we choose to approach this frontier. Historically, academic research has been carried out with little to no involvement of those outside the core research team, with patients and members of the public seen as a sources of data on which to build and test hypotheses (4). This is changing, rising ethical standards are placing more weight on addressing the inherent power imbalance between researchers as “knowledge creators,” and those who stand to benefit from that knowledge (5).
Within the context of scientific enquiry, patient, and public involvement (PPI) is defined as research performed “with” or “by” patients and members of the public, rather than “to,” “about”, or “for” them (6). Involving patients and public in research is becoming more commonplace, and has risen from a “desirable” to “essential” criteria for many research funding bodies, such as UK Research & Innovation (UKRI), an assembly of six organizations with a combined budget of over £8 billion (7). Furthermore, updated legislation now makes it a duty for healthcare providers to involve patients in the commissioning and decision making process in several governing organizations including the UK's National Institute for Health Research (NIHR) (8), the United Nations Children's Fund (UNICEF) (9), and several Canadian regional health boards (10). Such a collective reprioritization signifies a new gold standard for conducting research, particularly in clinical populations where those with lived experience offer a pragmatic, real world perspective on the development of interventions. Neglecting to recognize the reality of how data will be used downstream can lead to impractical “blue sky” research which ultimately fails to get adopted (11).
Besides a moral and ethical imperative for involving patients and the public in research, a growing evidence base indicates how PPI can strengthen research by improving priority setting (12), recruitment and retention (13, 14), diversity among participants (15, 16), and impact and dissemination of findings (17, 18). Other purported benefits of PPI can be difficult to capture, leaving them open to criticism, for example, a public contributor could gain more agency, or a researcher may improve their ability to explain concepts in plain language (19, 20).
There is limited evidence of PPI taking place in psychedelic studies to date. None of the 16 contemporary clinical trials on classic psychedelics describe PPI activities in their methods or give acknowledgment to public contributors (21–36). While this may not entirely rule out public involvement in these studies, it highlights how reporting is non-essential for the majority of publishers. Notably, a number of these psychedelic studies have come under criticism for a lack of diversity among participants (37, 38), a concern not exclusive to psychedelic research which continues in spite of efforts such as the NIH Revitalization Act (39) and the NIHR INCLUDE project (40, 41). PPI may offer one way to balance inequalities; a study using the psychoactive drug 3,4-Methylenedioxymethamphetamine (MDMA) described PPI methods as a means of improving the relevance of recruitment materials to underrepresented groups (42, 43). Additionally, psychedelic research organizations such as The Chacruna Institute embed PPI principles into their mission statement with the purpose of recognizing the contributions of the traditional healers who have long used naturally occurring psychedelic compounds (44). These are only some examples of the possible benefits of PPI to psychedelic research that remain to be explored.
In spite the growing internal and external incentives for PPI in research, delivery remains a challenge. As a supplementary activity for many, established researchers lack awareness around benefits and methods of PPI (5, 45). Doctoral students often cite restraints on resources or time leading to “tokenistic” or abbreviated attempts at PPI that fail to be recorded in the literature (17). Barriers to PPI in research may result in issues such as stagnant participation rates for people of color, or the overall decline in community engagement over the course of the COVID-19 pandemic (46).
Various tools have been developed to help support PPI in research; a 2019 systematic review identified and classified 64 frameworks (47), these were rarely adopted outside the research groups that developed them, showing how a one-size-fits all approach to PPI guidance may not work. While a number of existing frameworks may be used to help inform PPI in psychedelic research (8, 48–50), none of these account for the unique position which this field of research finds itself, namely: a highly complex socio-political landscape; rapidly changing drug policy; a wealth of knowledge stemming from a long tradition of psychedelic use pre-dating modern research; an underrepresentation of women, Black, Asian, and ethnically diverse populations; the social stigma associated with recreational use; and the 20-year hiatus in psychedelic research. All of these factors are not only important considerations for researchers conducting studies, but also for those taking part, particularly considering the potential for long-term impact on a participant's life (51). In response, the aim of this project was to co-develop guidance for planning PPI in psychedelic research which: (1) is sensitive to the unique challenges faced in psychedelic research, (2) draws on the experience of researchers, those with experience participating in psychedelic trials, and those with experience in PPI, (3) is harmonious with UK standards for PPI, and (4) is rated highly on quality measures for PPI guidance.
The Oxford English Dictionary (OED) defines the word guidance as “help or advice about how to do something.” In an attempt to make the guidance generalizable between both clinical and non-clinical psychedelic trials, we do not aim to provide study specific suggestions. Instead, we hope that guidance will be adaptable and broadly applicable to all types of research conducted in the psychedelic space.
A steering group was formed to plan, oversee and review all activities over the course of the production process. Group members comprised of four psychedelic researchers including one clinical academic, a public advisor and a PPI lead. Prior to starting, co-design objectives and methods were circulated, appraised and agreed upon in meetings between group members. Due to existing high quality PPI guidance, it was agreed that a completely original approach would not be necessary or appropriate. For this reason, and to ensure our approach accorded with national standards, we grounded our co-design process in the UK Standards for Public Involvement (8). This is a framework intended for use in public involvement research and is based on six core areas: inclusive opportunities, working together, support and learning, governance, communications, and impact.
For the purpose of this project, co-design was defined as the deliberate involvement of those outside the central research team in the development and evaluation of research improvement initiatives (52). A number of co-design methods have been described, most originating from outside academic settings (53–55). We chose to adapt and supplement an existing method developed by Greenhalgh et al. (47) as this was specifically created for designing custom PPI frameworks, and has been used successfully elsewhere (56, 57). In this method public collaborators and researchers worked together in an interactive participatory approach to systematically capture and build on suggestions.
We identified four distinct collaborator groups as key to bringing a wide breadth of knowledge and experience to discussions. These were: (1) former participants from clinical psychedelic trials; (2) former participants from non-clinical psychedelic trials; (3) PPI experienced public advisors; and (4) researchers from within the field of psychedelics. Collaborators were recruited using a convenience sampling strategy (58), the opportunity was e-mailed to participants of previous psychedelics studies at Imperial College Centre for Psychedelic Research and online via NIHR's People in Research platform (https://www.peopleinresearch.org/). Additional collaborators were found through the Imperial Patient Experience Research Centre (PERC), a core facility of the Imperial Biomedical Research Centre. All collaborators were offered compensation for their time at rates recommended by the NIHR's Payment Guidance for Researchers and Professionals (59). When appropriate, positive action (60) was taken to ensure a diverse age, gender and ethnicity demographic.
A facilitated workshop was used to identify and explore salient issues around public involvement in psychedelic research. One month before the workshop collaborators were sent details about the workshop structure and were signposted to information about PPI with the opportunity to ask any questions. Due to COVID-19, there were government imposed restrictions on meeting in groups at the time of the study, and so the workshop was hosted online using a remote platform (Zoom Video Communications, Inc.) and lasted ~2 h. An introduction outlined the aims and structure of the workshop including the important distinction between our focus on PPI for psychedelic trials rather than a focus on participant experience on a psychedelic trial (see Figure 1). Key collaborator groups were split into four breakout rooms each facilitated by one researcher from the steering group. Discussions in each room were themed according to a UK National Standard for Public Involvement and were clarified before opening up the discussion. All collaborators consented to recording of breakout discussions which were also annotated by scribes present at the time. Records and personal data from discussions were stored according to GDPR regulations (61). Notes were retrospectively checked for accuracy against recordings by the lead author.
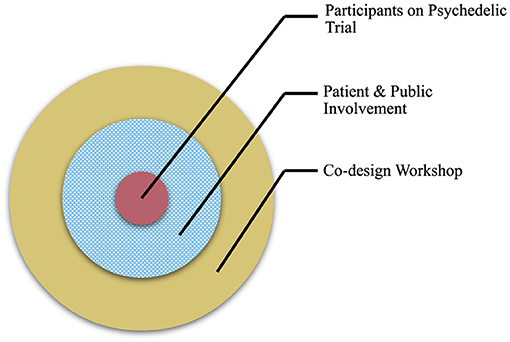
Figure 1. A diagram to illustrate the aim of the co-design workshop. Workshop activities (outer ring) focused on issues related to public contributors and PPI in psychedelic research (middle ring), rather than participants enrolled on a psychedelic study (inner circle).
Insights from the workshop were pooled and analyzed using conceptual mapping, a systematic process for delineating and identifying latent themes and patterns in large quantities of data (62). Once completed, results were formatted into a structure based on the NIHR Values and Principles Framework (49). Finally, all collaborators were invited to review the draft guidance and make further suggestions which were integrated and finalized by the steering group.
The Imperial Research Governance and Integrity Team (RGIT) was consulted about all proposed activities prior to starting. As this study is classified as PPI, those in attendance are considered as collaborators not sources of data, and therefore no ethical approval is required.
The Guidance for Reporting Involvement of Patients and the Public (GRIPP) 2 checklist was used to help improve the design and reporting of PPI in this project (63). All instances of when and how public collaborators (non-psychedelic researchers) influenced the guidance were logged and discussed among steering group members. Quality checks on the final guidance document were performed using an modified framework (45) originally developed for the analysis of PPI strategies (Table 1). This draws on the 4Pi National Involvement Standards (64) to outline five key domains (principles, purpose, presence, process, and impact) and provides supporting questions against which PPI strategies can be evaluated. We modified questions to support our objectives which were not to develop strategy as such, but rather guide the planning of such strategies. Domains are rated as “unmet,” “partially met,” or “fully met” depending on how many criteria are met.
Once organized by the steering group, the co-design workshop was attended by a total of 26 people, a breakdown of the groups in attendance is shown in Table 2. Workshop and breakout discussions ran without interruption from technical issues, and were captured on both video recording and in writing.
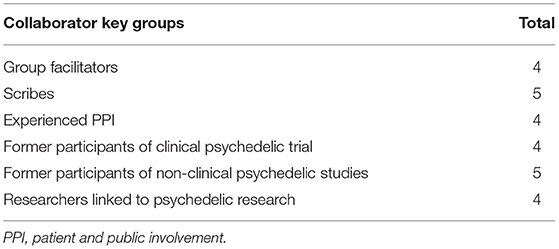
Table 2. Breakdown of groups attending a co-design workshop aimed developing guidance for PPI in psychedelic research.
Concept mapping of the workshop is shown in Figure 2. Discussions exposed the main themes relevant to PPI in psychedelic research, these could be classified by four distinct but interrelating values and principles described below and illustrated in Figure 3. Suggestions for how these principles could be incorporated into the psychedelic research were arranged into the guidance document by steering group members.

Figure 2. A scaled version of the conceptual map of content from the co-design workshop. Themes are grouped into four distinct but interrelated areas defined by core values of trust, purpose, inclusivity, and learning.
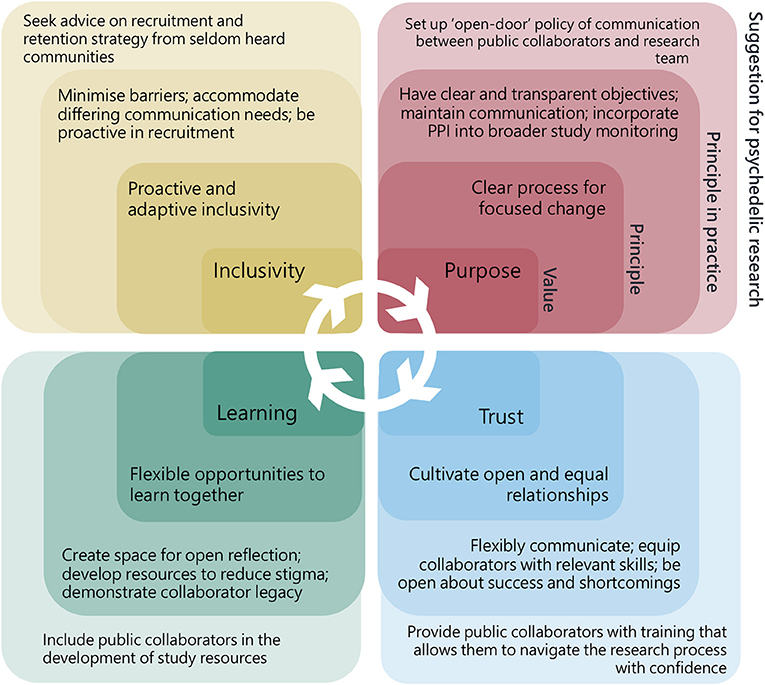
Figure 3. Values, principles, principles in practice, and suggestions for patient and public involvement in psychedelic research. Every value is relevant to all four areas of PPI and also bring more specific principles on which to build specific actions.
Public collaborators highlighted the need for those working on research in psychedelics to “reach out” and be proactive in seeking patient and public contributors. Stigma and misunderstanding about psychedelic compounds, and in some cases, mental health, could act as barriers to involvement in more hesitant groups for whom the only point of reference for psychedelics might come from their community, laws or popular culture. Relying on a single PPI activity advert could therefore lead to a homogenous group of self-motivated individuals, lacking a diversity in opinions. Solutions to such problems included ways to “enable” involvement, for example, by being flexible and offering a choice of different means to access PPI opportunities (e.g., in person, by post, or online).
Two of the groups raised the importance of demonstrating open awareness and deliberate attempts to address inclusion in all areas of research, from participant recruitment, to engagement with the media, and other members of the core research team. If underrepresented groups are not coming forward, it was suggested that researchers could utilize PPI strategies to engage with these populations, for example, by going directly to community groups and grass-roots organizations to ask how to better connect with relevant groups on the topic of psychedelics.
Those who had taken part in a psychedelic study all stressed the significance of their experience and desire to get involved in the research, but that they lacked opportunity to contribute further once they had ended their participation in a study. Several suggestions were raised around how former participants could contribute, such as through feedback forms or post-trial forums. It was observed how non-researchers could bring useful skills into the research team, for example some collaborators at the workshop drew on professional knowledge of project management and public relations when giving advice. Building spaces in which skills might be harnessed, recognized, and results fed-back would help contributors feel more valued.
Equally, the group of psychedelic researchers confirmed how much there was to learn from both former-participants and experienced public collaborators, especially in a field which is growing and both participants and researchers are significantly affected by outcomes. Both public contributors and researchers recognized the need to develop their own knowledge and skills before being able to fully contribute. Educational resources could be made available to cover a more detailed background of psychedelics or public involvement theory and could also be co-produced with specific populations in mind.
Those psychedelic researchers outside the steering group had a limited understanding around the motives for undertaking PPI, recognizing that clear objectives would be an important part of any PPI strategy. A similar point was raised separately among the group experienced in PPI, who also pointed out the need to revisit and update objectives when implementing a strategy.
Suggestions for how PPI could influence psychedelic research changed with context, for example, impact might look very different in a psychedelic survey study to a clinical trial. There was an agreement amongst all discussion groups that some PPI outputs could be useful regardless of study specific context. A proposal was made that PPI activities adapt to include study specific populations if indicated. This might allow for more “change management” principles to feedforward into future studies and ensure PPI at the earliest stage of research design and funding applications.
Those from outside of research expressed a need for transparency around research activities, naming an individual accountable for PPI changes within the psychedelic research team was seen as a useful way to ensure any changes were actioned, and would give a clear point of contact for public contributors. In some cases, it might not be possible to be completely transparent, such as in trials where research design requires blinding conditions.
Non-researcher discussion groups all expressed a need to be treated as equals by researchers. Feeling left out of the research process might be easily, if unintentionally, done through inaccessible language or deficient planning. This could be addressed by engaging with public collaborators while in the planning phase and when creating study documentation.
Additionally, there was a call for researchers to be open about both the positive and negative factors affecting psychedelic research such as specific political issues, resource limitations, and conflicts of interest. Honesty and humility were also seen as important personal qualities for engaging with public collaborators. Suggestions for how researchers could be more open include: circulating meeting minutes, incorporating PPI into all stages of the research cycle and including in funding decision-making. Ensuring trust is a value which guides all communications, through thoughtfully moderating the language.
External collaborators contributed to the development of this guidance at every point from conception to completion. Most material used to develop content originating from the workshop but some was used by the steering group, these additions were reviewed and approved by collaborators.
When evaluated against the quality framework (Table 1), the guidance fully met the criteria of principles, purpose and presence domain. The “process” domain was partially met for not explicitly describing accountability lines. As the guidance is not explicit about mechanisms to assess impact and evidence for success criteria, the impact domain was also partially met.
Guidance for creating a patient and public involvement strategy in psychedelic research were developed by researchers and patient and public collaborators. Using co-design methods, we identified fundamentals to PPI in psychedelic research and built these into a guidance document to help inform psychedelic research teams and their collaborators. Four key values: purpose, inclusivity, learning, and trust emerged as central to PPI in psychedelic research.
To our knowledge there is no published guidance which seeks to address PPI in the field of psychedelic research. At a fundamental level, our document holds a number of parallels with existing PPI guidance (49, 64) including the UK National Standards for Public Involvement upon which our workshop was based (8). This is likely a reflection of the common premise of PPI, which is grounded in ideals of equity and democracy. Significantly, the majority of content is original and distinct from generalized as it places more emphasis on the fundamental importance of building trusting relationships with public collaborators in research specific to psychedelics. Trust has long been placed at the center of guidance related to psychedelic research (65–69) in which participants are encouraged to “trust, open, and let go” (70). In this context, the responsibility lies with the researcher to be trustworthy, rather than a participant to be trusting (71). We propose that psychedelic research built on open and trusting PPI will result in superior outcomes and experiences for participants. The vocabulary used when developing participant information, outcome measures and interventions for psychedelic research might feel alienating or less socially acceptable to some audiences (72). Seeking the advice of others can help build empathy and help researchers to understand the fears and beliefs of potential participants of psychedelic research. This is particularly important, as researchers often knowingly or unknowingly act in a guiding capacity for participants, and can consequently influence expectancy bias (73).
Our guidelines promote a proactive approach to inclusivity, by prompting researchers to make an active effort to ensure opportunities for engagement are given to a diverse audience. Similarly, “positive action,” a principle relating to employment practices, aims to improve workforce diversity by guiding how candidates are fairly recruited and promoted (60). For many, there is an assumption that with higher ethical standards, contemporary psychedelic research will not follow the same path of discrimination, cultural appropriation (43) and other abuses of power seen in early psychedelic studies (74, 75). However, over 80% of participants in clinical trials are White (38), suggesting that more work has to be done to re-balance this representation. In addition, when conducted in naturalistic settings, psychedelic research risks amounting to cultural appropriation if indigenous communities are not consulted (43). Our guidance recognizes that making psychedelic research accessible requires more than an “open door” recruitment policy. Although unintentional, a passive approach can lead to a perpetuation of the problem of inequality in a field less accessible to minority populations (37) whom are already more inclined to mistrust medical and research institutions (76). A good example of positive actions are described by Williams et al. (42) who use culturally informed research design to help participants from minority backgrounds relate and engage with research into MDMA assisted therapy. Cultural competency is an essential attribute for psychedelic researchers and clinicians (77), we hope the guidance presented here will prompt researchers to take similar purposeful actions, to address gender, socioeconomic, educational and age disparities in psychedelic research (78).
Participating in a psychedelic trial demands great courage, and is often reported as one the most meaningful experiences of a lifetime (51). Creating a space in which contributions can be acknowledged after the completion of participation in the psychedelic study emerged as a priority in the guidance. Our guidance recognizes research as an iterative process (79); for researchers only seeking answers to specific research questions a great opportunity for mutual learning can be lost in failing to engage participants beyond the end of a psychedelic trial. Collaboration with former-participants could feedforward to strengthen future research, especially when knowledge is managed and shared across trials with this specific purpose. Such transparent PPI processes have the potential to counter threats to the legitimacy and potential of the psychedelic renaissance (1).
Psychedelic researchers face a great task in conducting high quality research. Besides the numerous hurdles of procuring heavily regulated drugs, researchers are expected to balance participant welfare with a need to complete trials in a timely and cost-effective manner. As our guidance shows, carrying out good PPI in psychedelics is not straightforward, and as it stands PPI is rarely included as part of undergraduate training for researchers. Subsequently, researchers must seek funding, experience and skills for PPI under their own volition. Greater education and resources for PPI would not only be beneficial to the field of psychedelic research, but medical research more broadly.
There are a number of limitations to our guidelines and the methods used to develop them. At the time of publication, the guidelines were yet to be tested over the course of a whole research cycle, and may need to be adapting accordingly (45). Furthermore, this rapidly evolving field of research makes it difficult to capture the impact of PPI activities. Future studies in this area could seek to evaluate the effects of PPI strategies and the guidance upon which they are based.
One of the appeals of psychedelics shared by researchers and participants alike is the promise of discovery. The quality of present-day psychedelic research means all stakeholders are on the same journey into unknown territory. For many, there is an assumption that with higher ethical standards, contemporary psychedelic research will not follow the same path of discrimination and other abuses of power seen in early psychedelic studies. We hope that the guidelines presented here will assist in greater incorporation of the voices of the wider community in psychedelic research, and that it will continue to develop alongside this exciting field of research. PPI when carried out systematically and thoughtfully, offers a means of creating more valuable psychedelic research through directly addressing the complex power dynamic between research team and the public at large.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JC conceptualized the study, acted as lead author and main point of contact for collaborators. JB and MS contributed to the initial design of the study. JB, JC, LL, MP, MS, and SJ oversaw the design, planning and running of the workshop, and contributed to processing the proceedings. JC wrote the first draft. JB, JC, LL, RC-H, MP, MS, and SJ reviewed the manuscript prior to submission. All authors contributed to the article and approved the submitted versions.
Funding for this project was provided by the Imperial College London, Centre for Psychedelic Research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to make a special acknowledgment to all of the members of the public who contributed to this work. We would also like to thank the students who assisted in facilitating the workshop: Hannah Douglass, Tommaso Barba, Rebecca Harding, Caterina Radu, and Rhodes Willoughby.
1. Petranker R, Anderson T, Farb N. Psychedelic research and the need for transparency: Polishing Alice's Looking Glass. Front Psychol. (2020) 11:1681. doi: 10.3389/fpsyg.2020.01681
2. Andersen KA, Carhart-Harris R, Nutt DJ, Erritzoe D. Therapeutic effects of classic serotonergic psychedelics: a systematic review of modern-era clinical studies. Acta Psychiatr Scand. (2021) 143:101–18. doi: 10.1111/acps.13249
3. Wheeler SW, Dyer NL. A systematic review of psychedelic-assisted psychotherapy for mental health: an evaluation of the current wave of research and suggestions for the future. Psychol Conscious Theor Res Pract. (2020) 7:279. doi: 10.1037/cns0000237
4. Thornton H. Patient and public involvement in clinical trials. Br Med J. (2008) 336: 903–4. doi: 10.1136/bmj.39547.586100.80
5. Tembo D, Hickey G, Montenegro C, Chandler D, Nelson E, Porter K, et al. Effective engagement and involvement with community stakeholders in the co-production of global health research. BMJ. (2021) 372:n178. doi: 10.1136/bmj.n178
6. INVOLVE. Briefing Notes for Researchers: Involving the Public in NHS, Public Health Social Care Research Eastleigh: NIHR. NIHR (2012). Available online at: https://www.nihr.ac.uk/documents/briefing-notes-for-researchers-public-involvement-in-nhs-health-and-social-care-research/27371 (accessed May 15, 2021).
10. Frankish CJ, Kwan B, Ratner PA, Higgins JW, Larsen C. Challenges of citizen participation in regional health authorities. Soc Sci Med. (2002) 54:1471–80. doi: 10.1016/S0277-9536(01)00135-6
11. Ioannidis JP. Why most clinical research is not useful. PLoS Med. (2016) 13:e1002049. doi: 10.1371/journal.pmed.1002049
12. Kelly S, Lafortune L, Hart N, Cowan K, Fenton M, Brayne C. Dementia priority setting partnership with the James Lind Alliance: using patient and public involvement and the evidence base to inform the research agenda. Age Ageing. (2015) 44:985–93. doi: 10.1093/ageing/afv143
13. Crocker JC, Ricci-Cabello I, Parker A, Hirst JA, Chant A, Petit-Zeman S, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ. (2018) 363:k4738. doi: 10.1136/bmj.k4738
14. Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. (2014) 14:89. doi: 10.1186/1472-6963-14-89
15. Conneely M, Boland P, O'Neill A, Byrne D, Cronin S, Quinn D, et al. A protocol for the establishment and evaluation of an older adult stakeholder panel for health services research. HRB Open Res. (2020) 3:1. doi: 10.12688/hrbopenres.12979.1
16. Oliver S, Liabo K, Stewart R, Rees R. Public involvement in research: making sense of the diversity. J Health Serv Res Policy. (2015) 20:45–51. doi: 10.1177/1355819614551848
17. Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. (2014) 17:637–50. doi: 10.1111/j.1369-7625.2012.00795.x
18. Esmail L, Moore E, Rein A. Evaluating patient and stakeholder engagement in research: moving from theory to practice. J Comp Effect Res. (2015) 4:133–45. doi: 10.2217/cer.14.79
19. Russell J, Fudge N, Greenhalgh T. The impact of public involvement in health research: what are we measuring? Why are we measuring it? Should we stop measuring it? Res Involv Engag. (2020) 6:1–8. doi: 10.1186/s40900-020-00239-w
20. Bagley HJ, Short H, Harman NL, Hickey HR, Gamble CL, Woolfall K, et al. A patient and public involvement (PPI) toolkit for meaningful and flexible involvement in clinical trials–a work in progress. Res Involv Engag. (2016) 2:1–14. doi: 10.1186/s40900-016-0029-8
21. Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. (2006) 67:1735–40. doi: 10.4088/JCP.v67n1110
22. Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archiv Gen Psychiatry. (2011) 68:71–8. doi: 10.1001/archgenpsychiatry.2010.116
23. Garcia-Romeu A, R Griffiths R, W Johnson M. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev. (2014) 7:157–64. doi: 10.2174/1874473708666150107121331
24. Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. (2016) 3:619–27. doi: 10.1016/S2215-0366(16)30065-7
25. Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. (2016) 30:1165–80. doi: 10.1177/0269881116675512
26. Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. (2016) 30:1181–97. doi: 10.1177/0269881116675513
27. Palhano-Fontes F, Barreto D, Onias H, Andrade KC, Novaes MM, Pessoa JA, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. (2019) 49:655–63. doi: 10.1017/S0033291718001356
28. Gasser P, Kirchner K, Passie T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: a qualitative study of acute and sustained subjective effects. J Psychopharmacol. (2015) 29:57–68. doi: 10.1177/0269881114555249
29. Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. (2021) 384:1402–11. doi: 10.1056/NEJMoa2032994
30. Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PCR, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. (2015) 29:289–99. doi: 10.1177/0269881114565144
31. Osório FdL, Sanches RF, Macedo LR, Dos Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Brazil J Psychiatry. (2015) 37:13–20. doi: 10.1590/1516-4446-2014-1496
32. Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. (2014) 28:983–92. doi: 10.1177/0269881114548296
33. Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Mental Dis. (2014) 202:513. doi: 10.1097/NMD.0000000000000113
34. Carhart-Harris RL, Bolstridge M, Day C, Rucker J, Watts R, Erritzoe D, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology. (2018) 235:399–408. doi: 10.1007/s00213-017-4771-x
35. Agin-Liebes GI, Malone T, Yalch MM, Mennenga SE, Ponté KL, Guss J, et al. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J Psychopharmacol. (2020) 34:155–66. doi: 10.1177/0269881119897615
36. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcoh Abuse. (2017) 43:55–60. doi: 10.3109/00952990.2016.1170135
37. Williams MT, Labate BC. Diversity, equity, and access in psychedelic medicine. J Psyched Stud. (2020) 4:1–3. doi: 10.1556/2054.2019.032
38. Michaels TI, Purdon J, Collins A, Williams MT. Inclusion of people of color in psychedelic-assisted psychotherapy: a review of the literature. BMC Psychiatry. (2018) 18:245. doi: 10.1186/s12888-018-1824-6
39. Health NIo. Revitalization Act of (1993)- Clinical Research Equity Regarding Women and Minorities (1993).
40. Witham MD, Anderson E, Carroll C, Dark PM, Down K, Hall AS, et al. Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials. (2020) 21:1–9. doi: 10.1186/s13063-020-04613-7
41. National Institute for Health CEN. NICE Guideline on Chronic Pain [Draft for Consultation]. National Institute for Health and CE (2020). p. 28.
42. Williams MT, Reed S, Aggarwal R. Culturally informed research design issues in a study for MDMA-assisted psychotherapy for posttraumatic stress disorder. J Psyched Stud. (2020) 4:40–50. doi: 10.1556/2054.2019.016
43. George JR, Michaels TI, Sevelius J, Williams MT. The psychedelic renaissance and the limitations of a White-dominant medical framework: a call for indigenous and ethnic minority inclusion. J Psyched Stud. (2020) 4:4–15. doi: 10.1556/2054.2019.015
44. Herzberg G, Butler J. Blinded by the White: Addressing Power and Privilege in Psychedelic Medicine. Chacruna (2019).
45. Matthews R, Kaur M, French C, Baker A, Reed J. How helpful are Patient and Public Involvement strategic documents-Results of a framework analysis using 4Pi National Involvement Standards. Res Involv Engag. (2019) 5:31. doi: 10.1186/s40900-019-0164-0
46. Ramsbottom A, O'Brien E, Ciotti L, Takacs J. Enablers and barriers to community engagement in public health emergency preparedness: a literature review. J Commun Health. (2018) 43:412–20. doi: 10.1007/s10900-017-0415-7
47. Greenhalgh T, Hinton L, Finlay T, Macfarlane A, Fahy N, Clyde B, et al. Frameworks for supporting patient and public involvement in research: systematic review and co-design pilot. Health Expect. (2019) 22:785–801. doi: 10.1111/hex.12888
48. Hewlett S, Wit Md, Richards P, Quest E, Hughes R, Heiberg T, et al. Patients and professionals as research partners: challenges, practicalities, and benefits. Arthr Care Res. (2006) 55:676–80. doi: 10.1002/art.22091
49. INVOLVE. Public Involvement in Research: Values and Principles Framework. (2016). Available online at: https://www.invo.org.uk/wp-content/uploads/2017/08/Values-Principles-framework-Jan2016.pdf (accessed May 25, 2021).
50. Johnson H, Ogden M, Brighton LJ, Etkind SN, Oluyase AO, Chukwusa E, et al. Patient and public involvement in palliative care research: what works, and why? A qualitative evaluation. Palliat Med. (2020) 35:151–60. doi: 10.1177/0269216320956819
51. Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. (2006) 187:268–83. doi: 10.1007/s00213-006-0457-5
52. Bevir M, Needham C, Waring J. Inside co-production: ruling, resistance, and practice. Soc Policy Administr. (2019) 53:197–202. doi: 10.1111/spol.12483
53. O'Brien J, Fossey E, Palmer VJ. A scoping review of the use of co-design methods with culturally and linguistically diverse communities to improve or adapt mental health services. Health Soc Care Commun. (2021) 29:1–17. doi: 10.1111/hsc.13105
54. Niedderer K, Tournier I, Coleston-Shields DM, Craven M, Gosling J, Garde J, et al. Designing With and for People With Dementia: Developing a Mindful Interdisciplinary Co-Design Methodology (2020).
55. Eyles H, Jull A, Dobson R, Firestone R, Whittaker R, Te Morenga L, et al. Co-design of mHealth delivered interventions: a systematic review to assess key methods and processes. Curr Nutr Rep. (2016) 5:160–7. doi: 10.1007/s13668-016-0165-7
56. Morris RL, Ruddock A, Gallacher K, Rolfe C, Giles S, Campbell S. Developing a patient safety guide for primary care: a co-design approach involving patients, carers and clinicians. Health Expect. (2021) 24:42–52. doi: 10.1111/hex.13143
57. McCarron TL, Noseworthy T, Moffat K, Wilkinson G, Zelinsky S, White D, et al. A co-designed framework to support and sustain patient and family engagement in health-care decision making. Health Expect. (2020) 23:825–36. doi: 10.1111/hex.13054
58. Patton M. Qualitative Research & Evaluation Methods: Integrating Theory and Practice. VitalSource Bookshelf. (2015).
59. Payment Guidance for Researchers Professionals. Available online at: https://www.nihr.ac.uk/documents/payment-guidance-for-researchers-and-professionals/27392 (accessed May 8, 2021).
60. Government Equalities Office. Employers: Quick Start Guide to Positive Action in Recruitment and Promotion Online. (2011). Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/85014/positive-action-recruitment.pdf (accessed May 15, 2021).
61. Information Commissioner's Office. Guide to the UK General Data Protection Regulation (UK GDPR). (2018). Available online at: https://ico.org.uk/for-organisations/guide-to-data-protection/guide-to-the-general-data-protection-regulation-gdpr/ (accessed May 23, 2021).
62. Daley B. Using concept maps in qualitative research. In: Concept Maps: theory, Methodology, Technology: Proceedings of the First International Conference on Concept Mapping. Servicio de Publicaciones. (2004). p. 191–198.
63. Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Res Involv Engag. (2017) 3:13. doi: 10.1186/s40900-017-0062-2
64. Faulkner A, Yiannoullou S, Kalathil J, Crepaz-Keay D, Singer F, James N, et al. Involvement for Influence. 4PI National Involvement Standards. London: National survivor user network (NSUN) (2015).
65. Metzner R. Allies for Awakening: Guidelines for Productive and Safe Experiences with Entheogens. Berkeley, CA: Green Earth Foundation & Regent Press (2015).
66. Phelps J. Developing guidelines and competencies for the training of psychedelic therapists. J Hum Psychol. (2017) 57:450–87. doi: 10.1177/0022167817711304
68. Penn AD, Phelps J, Rosa WE, Watson J. Psychedelic-assisted psychotherapy practices and human caring science: toward a care-informed model of treatment. J Hum Psychol. (2021). doi: 10.1177/00221678211011013
69. Watts R, Luoma J. The use of the psychological flexibility model to support psychedelic assisted therapy. J Cont Behav Sci. (2019). doi: 10.1016/j.jcbs.2019.12.004
70. Richards WA. Psychedelic psychotherapy: insights from 25 years of research. J Hum Psychol. (2017) 57:323–37. doi: 10.1177/0022167816670996
71. Wilkins CH. Effective engagement requires trust and being trustworthy. Med Care. (2018) 56(10 Suppl. 1):S6. doi: 10.1097/MLR.0000000000000953
72. Corrigan K, Haran M, McCandliss C, McManus R, Cleary S, Trant R, et al. Psychedelic perceptions: mental health service user attitudes to psilocybin therapy. Irish J Med Sci. (2021) 15:1–13. doi: 10.1007/s11845-021-02668-2
73. Muthukumaraswamy S, Forsyth A, Lumley T. Blinding and expectancy confounds in psychedelic randomised controlled trials. Expert Rev Clin Pharmacol. (2021) 14:1133–52. doi: 10.1080/17512433.2021.1933434
74. Sessa B. The History of Psychedelics in Medicine. Handbuch Psychoaktive Substanzen Springer Reference Psychologie. Berlin: Springer (2016). doi: 10.1007/978-3-642-55214-4_96-1
75. Smart RG, Storm T, Baker EF, Solursh L. A controlled study of lysergide in the treatment of alcoholism. I. The effects on drinking behavior. Q J Stud Alcoh. (1966) 27:469–82. doi: 10.15288/qjsa.1966.27.469
76. Woodall A, Morgan C, Sloan C, Howard L. Barriers to participation in mental health research: are there specific gender, ethnicity and age related barriers? BMC Psychiatry. (2010) 10:103. doi: 10.1186/1471-244X-10-103
77. Sonstein SA, Seltzer J, Li R, Silva H, Jones CT, Daemen E. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clin Res. (2014) 28:17–23. Available online at: https://mrctcenter.org/wp-content/uploads/2016/02/2014-06-Moving-from-Compliance-to-Competency-CenterWatch.pdf (accessed May 14, 2021).
78. Willoughby R, Close J, Bournemann J, Spriggs M. Diversity of participants of contemporary psychedelic research: a profile of survey respondents and trial participants (thesis). Centre for Psychedelic Research, Imperial College London, London, United Kingdom (2021).
79. A Rough Guide to Public Involvement. (2020). Available online at: https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/perc/PERCs-Rough-Guide-to-Public-Involvement—Jan-2020.pdf (accessed May 28, 2021).
Keywords: psychedelics, research, patient, public, involvement, PPI, guideline, strategy
Citation: Close JB, Bornemann J, Piggin M, Jayacodi S, Luan LX, Carhart-Harris R and Spriggs MJ (2021) Co-design of Guidance for Patient and Public Involvement in Psychedelic Research. Front. Psychiatry 12:727496. doi: 10.3389/fpsyt.2021.727496
Received: 18 June 2021; Accepted: 06 September 2021;
Published: 30 September 2021.
Edited by:
Graham Campbell, Hammersmith Medicines Research, United KingdomReviewed by:
Soni Kewalramani, Amity University, IndiaCopyright © 2021 Close, Bornemann, Piggin, Jayacodi, Luan, Carhart-Harris and Spriggs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James B. Close, amNsb3NlQGljLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.