- 1Department of Clinical Psychology, National Institute of Mental Health and Neuro Sciences, Bangalore, India
- 2Department of Psychology, Texas State University, San Marcos, TX, United States
Neuropsychological functions in obsessive-compulsive disorder (OCD) have been extensively investigated. Despite some common findings across studies indicating deficient test performance across cognitive domains with small to medium effect sizes, results remain inconsistent and heterogeneous. However, multiple past attempts to identify moderators that may account for such variability have been unrewarding. Typical moderators including symptom severity, age at onset, medication status, and comorbid conditions failed to provide sufficient explanatory power. It has then been posited that these inconsistencies may be attributed to the inherent heterogeneous nature of the disorder (i.e., symptom dimensions), or to the natural fluctuation in symptom severity. However, recent meta-analyses suggest that these factors may not account for the persistent unexplained variability. Other potential factors—some of which are unique to neuropsychological testing—received scarce research attention, including definition of cognitive impairments, specificity and selection of test and outcome measures, and their limited ecological validity. Other moderators, particularly motivational aspects, and metacognitive factors (e.g., self-efficacy) were not previously addressed despite their potential association to OCD, and their documented impact on cognitive function. The aim of the present mini-review is to provide an updated succinct overview of the current status of the neuropsychological literature in OCD and expanding upon oft-neglected potential moderators and their putative impact on neuropsychological findings in OCD. Our goal is to highlight important avenues for further research and provide a road map for investigators in order to advance our understanding of cognitive functions in OCD that has been stagnant in the past decade.
Introduction
Neuropsychological Findings in OCD
Decades of research into cognitive function in Obsessive-Compulsive Disorder (OCD), including a number of systematic reviews and meta analyses (1–6), reveal deficient test performance across multiple cognitive domains. Although all meta-analyses consistently report underperformance with small to medium effect sizes in OCD compared to non-clinical controls (see Table 1), a hallmark finding in this literature is significant heterogeneity and inconsistency across studies.
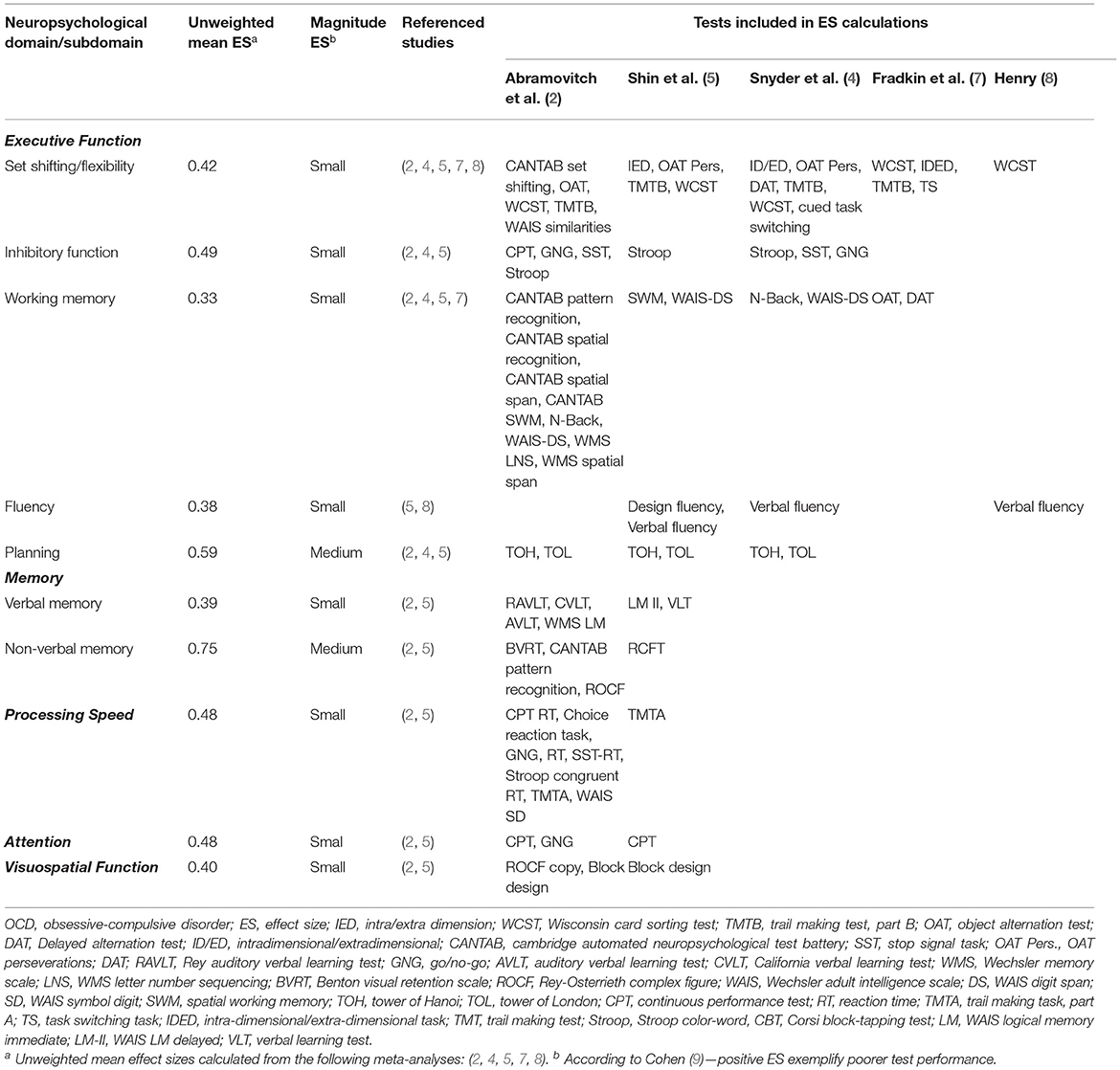
Table 1. Unweighted mean effect sizes for neuropsychological test performance across domains in adult OCD.
Indeed, a recent meta-analysis of familial cognitive endophenotypes in OCD also found significant heterogeneity across major executive functions (6). Such inconsistencies suggest that some moderators or latent factors may explain this heterogeneity. However, moderator analyses examining multiple potential variables, including demographic (e.g., sex, age, education) and clinical variables (e.g., age of onset, OCD symptom-severity, medication, comorbidities) found no meaningful moderation effects (2, 4, 5). Moreover, although moderator analyses in meta-analytic reviews usually utilize a meta-regression procedure, some meta-analyses endeavored to examine such potential moderators as the primary outcome. However, these studies, including examinations of correlations between cognitive function and symptom severity (10), and with OCD dimensions (11, 12), found no meaningful effects accounting for such heterogeneity. Moreover, this inconsistency is further obfuscated by research and meta-analysis in pediatric OCD yielding a substantially divergent picture compared with adult OCD (13). Of note, similar extent of heterogeneity has been reported in a meta-analysis examining cognitive functions across studies utilizing the same tasks and outcome measures (as opposed to calculating domain effect sizes from different tests) (5).
Notably, the magnitude of these effects (small to medium) in OCD do not amount to what is typically considered a cognitive impairment (2). It is also important to note that the pattern of cognitive dysfunction is not specific to OCD, and a recent umbrella review did not identify any viable disorder-specific biological or cognitive markers for OCD (14). Moreover, similar effect sizes and somewhat similar heterogeneity trends were recently identified across DSM disorders (15–18). This lead to the conclusion that the C Factor (i.e., cognitive dysfunction) is transdiagnostic, and that there is no reliable disorder-specific neuropsychological profile (19). Considering that OCD is associated with functional impairments (20–22), this state of affairs raises the question of whether OCD is linked to meaningful cognitive deficits at all, and if not, whether neuropsychological tests may be poor predictors of everyday functional impairment in OCD. In this review, we outline under-researched factors and several potential latent constructs that ought to be investigated in order to promote our understanding of neuropsychological findings in OCD. These include factors associated with psychometric and interpretive aspects of neuropsychological testing, and state/trait structures associated with OCD or with psychopathology in general.
Methodological and Psychometric Issues
Test Selection
One major factor contributing to neuropsychological heterogeneity in OCD is the utilization of different tests under the same general neuropsychological domain. This problem is seen across populations, where different tests assessing a general cognitive domain often yield different results (3). Indeed, researchers have been sounding the alarm about this issue for two decades (1, 23). This problem is evidenced for example in the context of inhibitory function—the most widely researched cognitive domain in OCD. Given the hypothesis that people with OCD struggle to inhibit their urge to perform compulsions, cognitive and behavioral inhibitory dysfunction has been subject to much interest from researchers, and at one point was proposed as an endophenotypic marker for OCD (24). However this was later largely recanted by the authors (25). Notwithstanding, the general domain of inhibitory function is commonly assessed using a number of tests, primarily the Stop Signal Task (SST), the Stroop test, and Go/No-Go/Continuous Performance Tests. However, these tests yield different effect sizes in OCD (2), which may not be surprising because they measure different subdomains of inhibitory function and are associated with different neuroanatomical and neurochemical processes (26–28). Whereas, the Stroop test assesses interference control, the Go/No-Go paradigm assesses response inhibition (inhibition of prepotent motor ‘program’), and the SST assesses response cancellation (29). Since most studies use these tests interchangeably to measure “response inhibition,” the heterogeneity of effect sizes under this construct may be to some extent, a result of problematic conceptualization of such studies, and not a characteristic of OCD. The same problem arises in the context of other neuropsychological domains, including, but not limited to, other executive functions. Unfortunately, despite the decades-old calls to increase precision in test selection and construct definitions (in neuropsychological research in general, as well as specifically in OCD), this problem is still evident in OCD research. This may be a contributing factor to the longstanding issue of unexplained heterogeneity. It is therefore important that neuropsychological studies in psychiatry/psychopathology research involve neuropsychologists, with a careful consideration of underlying constructs, task impurity, psychometrics, and ecological validity (30).
Selection of Outcome Measures
The ‘task impurity problem’ in neuropsychology is a longstanding issue inherent to cognitive testing, where a several interrelated but distinct cognitive demands are reflected in a single test score (31). This problem, characteristic of most cognitive tests, but more so in tests assessing higher order executive function, poses an interpretive hurdle (32). Several solutions to mitigate this problem have been offered such as utilizing and cross-referencing from more than one test to assess an executive function (33). Carefully attending to the construct validity of specific outcomes within a test should be standard practice in neuropsychological research. For instance, the Wisconsin Card Sorting Test (WCST) is frequently used to assess cognitive flexibility, set-shifting, and concept formation, but performance on the WCST also requires working memory, attention, as well as planning, strategizing, inhibitory control, feedback processing, rule extraction, and self-monitoring (7, 34, 35). Indeed, a recent meta-analysis (7) examined the notion that OCD is associated with deficits in flexibility/set shifting (36)—constructs known for their heterogeneity in OCD—by parceling out different cognitive processes from the same tasks. Differentiating performance on shifting vs. “control” (i.e., non-shifting) outcome measures from the same tests, the authors found no evidence for such deficits in OCD (7). Thus, together with the need to carefully select neuropsychological tests to assess specific domains of cognitive function, an even more careful approach should be taken when selecting outcome measures for analyses within the selected tests.
Ecological Validity
The goal of assessment of neuropsychological functions is to predict task performance in real-life settings (37). Therefore, there is great importance in evaluating Ecological Validity—the “functional and predictive relationship between the patient's performance on a set of neuropsychological tests and the patient's behavior in a variety of real-world settings” (38)—in neuropsychological research. Traditionally, neuropsychological tests are associated with moderate degree of ecological validity (31), but evidence points to significantly limited ecological validity in psychopathology (19). Indeed, emotional problems in everyday life have been termed “the conditional neurological lesion” (39), and neuropsychology researchers have long recognized that individuals may display intact performance on a task in a quiet room but may show significant difficulties in everyday settings due to the marked impact of psychopathological symptoms on cognitive functions (31). Conversely, assessment settings may provoke anxiety and potentially negatively impact performance, compared to everyday settings where individuals may not feel they are being evaluated. Unfortunately these important situational factors are rarely addressed in the context of neuropsychological studies in psychopathology in general, and in OCD in particular (19). Limited research suggests that this problem is evident in OCD. For instance, despite consistently reported non-verbal memory deficits in OCD (i.e., poor performance on the Rey Complex Figure test), everyday memory functioning in OCD was found to be unimpaired relative to non-psychiatric controls (40). Similarly, in the context of tests of inhibitory function, although suboptimal test performance has been reported in OCD, behavioral impulsivity (the corresponding real-life behavioral construct) in OCD is found to be consistently lower or equivalent compared to non-clinical controls (41). In fact, a study that directly examined performance on different neuropsychological tasks of inhibitory control in OCD found no associations with real-life behavioral impulsivity (42). Another study assessed performance on executive function tasks as well as on a questionnaire of real-life behaviors reflecting executive function [i.e., the Behavior Rating Inventory of Executive Function (BRIEF)] before and after a 14-week CBT treatment in a sample of youth with OCD (43). This study found no change post-treatment on neuropsychological tasks, but a meaningful improvement on real-life functions as assessed by the BRIEF.
In the context of neuropsychological testing, assessment of ecological validity assumes two general approaches, veridicality, which is the degree that a neuropsychological test corresponds empirically to outcome measures of everyday function, and verisimilitude, which is the degree to which test demands mimic the demands of everyday environments (31). The vast majority of neuropsychological research uses veridicality testing, which is generally known to have modest association with real-life functions (44). For example, the most common tests utilized in OCD research to assess planning are the Tower of London and the Tower of Hanoi tests, in which the primary demand is to copy a structure of beads or discs while adhering to task rules. This test, that involves planning, may be far removed from the real-life demand of planning a vacation for example. Unfortunately, there is a dearth of research into cognitive function in OCD that utilizes tests assuming the verisimilitude approach. These tests may assess complex everyday tasks, such as the Multiple Errands Test [MET; (45)], a test that mimics real-life scenarios related to chores and shopping. Furthermore, with the advancement and availability of virtual reality (VR) technology, verisimilitude tests may become more prevalent in research settings, and in fact may provide a unique integration between veridicality and verisimilitude approaches (46). However, studies that assess cognitive function using VR technology are practically non-existent in OCD. Notably, many of these tests possess very good psychometric properties (47), and researchers are encouraged to consider utilizing such tests to aid in elucidating the nature of cognitive deficiencies in OCD, as well as their relationship to everyday function and psychopathological mechanisms.
State/Trait Personal Variables
Correspondence With Clinical and Functional Indices
Despite previous research suggesting that neuropsychological performance may be related to symptom severity, severity has not emerged as a significant moderator of performance on meta-analyses (4, 5, 10). Furthermore, the relationship of test performance to treatment (pharmacological or psychological) is extremely inconsistent (48). While several studies have examined neuropsychological performance as a predictor of treatment outcome (49–52), or in the context of sensitivity to treatment (53–55), results from such studies are extremely sparse and inconsistent, and overall there are no replicable results suggesting that cognitive functions are reliable predictors of response to treatment. This is not surprising, given the lack of associations between neuropsychological test performance and severity measures in the first place.
However, the above inconsistencies and lacunae present a conundrum, as they preclude a meaningful understanding of neuropsychological performance in OCD with regard to psychopathological mechanisms or real-world functioning. Particularly striking is the near-total absence of studies examining correspondence of neuropsychological performance with functional, vocational, and academic indices in OCD, a disorder linked to notable academic and occupational dysfunction (20–22). Moreover, there is little correspondence between neuropsychological assessment of executive function and ratings of real-life functioning (43, 56). This problem however, is not unique to OCD and has been reported across disorders (57, 58) and may partly relate to level of awareness of such difficulties, and the discrepancy between the constructs measured in cognitive tests, and how these are expressed in everyday life. Unfortunately, self-report scales developed uniquely for cognitive difficulties in OCD [e.g., Cognitive Assessment Instrument of Obsessions and Compulsions (CAIOC-13); (59)], have not been examined in relation to neuropsychological test scores.
We recommend that future studies examine the correspondence between neuropsychological performance and functional correlates. This is an essential and highly needed research that would enable the field to learn about the driving factors underlying everyday functional impairments in OCD, and equally important, help to determine what extent of underperformance on cognitive tests may be regarded as indicating real-life functional impairment.
Affective, Motivational, and Metacognitive Factors
Affective states (e.g., anxiety or depression), motivation, effort, and internal distractions (e.g., intrusive obsessive thoughts) have long been noted as confounds in neuropsychological testing (60), but may carry particular value in explaining discrepant findings. For instance, in some studies, individuals with OCD report greater anxiety about their performance, distracting OCD thoughts, and negative momentary influences during neuropsychological testing (61). In addition, testing of motivation and effort is recommended as an essential part of standard neuropsychological assessments (62), since discrepant performance may indicate sub-optimal effort, attributable to multiple causes including anhedonia, somatization, or secondary gain (19, 63).
Metacognition is the capacity to assess, reflect, control, and evaluate one's cognitions (64). Metacognition is known to be altered across disorders (65). Some aspects of meta-cognition that may impact cognitive function include self-efficacy (66), self-stigma (67), attitudes toward neuropsychological testing (61), and hyper monitoring of one's performance (68). For instance, several explanations of deficient performance in OCD have implicated heightened monitoring of errors or perceived errors, and sensitivity to novelty, including findings regarding post-error slowing on the SST (69, 70), difficulties on simpler/initial test items relative to subsequent/more complex items on the same test (71, 72), and an “always on guard” style of responding even when task demands are relaxed (73). These findings have contributed to the understanding that OCD may be characterized, not by impulsivity, but by over-cautious and inflexible performance monitoring (74–77). Importantly, as depicted by the Executive Overload Model of OCD, such hypercontrol and sensitivity to novel stimuli is related to a surge in obsessive thoughts and may cause an “executive overload” and adversely affect test performance (78).
Other metacognitive processes impacting attention/working memory are evidenced from studies on non-clinical samples—negative expectations relating to task difficulty/own ability (79), stereotype threat (80), rumination and emotional arousal (81), and threat to self-esteem (82). Threat to self-esteem, and lower self-esteem, is posited to affect multiple cognitive functions, including attention through increased state anxiety and (metacognitive) diversion of attentional resources to task-irrelevant stimuli (83). In OCD, stigma/self-stigma from negative stereotypes about cognitive dysfunction in this disorder appear to adversely impact neuropsychological test performance (84). Metacognitive processes, particularly as they relate to self-monitoring and subsequent reframing may also have a facilitatory effect on cognition. Such processes may be utilized to mitigate negative influences on test performance, and assist in selection and use of task-relevant strategies. Hence metacognitive techniques have been included in cognitive remediation interventions (85, 86); however applications to OCD are few, and bear further investigation (87, 88).
Psychological processes impacting test performance are often overlooked and it is recommended that such processes be closely investigated in future studies. Studies so far have employed several approaches to address this issue, such as breaking down test performance to component processes [e.g., (7, 32)], employing experimental modification to classic tasks [e.g., (73)], use of self-reports to assess metacognitive processes during [e.g., (89)], or after [e.g., (61)] neuropsychological task performance. Such research may be crucial to clarifying inconsistencies in findings and improving goodness-of-fit to psychopathological models and real-world correlates of functioning in OCD.
Discussion
A vast body of literature indicates that OCD is associated with underperformance on neuropsychological tests across multiple domains. However, attempts to integrate cognitive dysfunction with contemporary OCD models or psychopathological mechanisms have been unfruitful, and unexplained heterogeneity remains a major problem. Indeed, moderator analyses across multiple meta-analyses failed to identify any variable or combinations of variables that may account for this heterogeneity. Further attempts to resolve this issue included meta-analyses directly examining moderators, such as symptom severity (10) and OCD dimensions (11, 12), which did not yield meaningful results. In addition, these findings seem to be non-specific to OCD, and such cognitive dysfunction is seen across DSM disorders with very similar effect sizes. Indeed, recently Abramovitch et al. (19) conducted a systematic review of meta-analyses examining cognitive functions across disorders and concluded that psychopathology (defined categorically or dimensionally) is characterized by cognitive dysfunction. This transdiagnostic finding—termed the C Factor (for cognitive dysfunction)—raises the question about common factors across disorders that, like the p factor (90), may have better explanatory power.
However, analyses of moderators that may explain such heterogeneity depend on moderators that researchers choose to assess. These are largely circumscribed to demographic and classic clinical factors. It is important to consider that observable cognitive functioning may be the final product of intricate dynamics involving genetic, neurophysiological underpinnings, neuropsychological functions, psychological factors such as metacognitive biases, and state-related changes in affect and symptoms. Despite mounting evidence, assessment of psychological aspects including motivational and metacognitive factors related to performance is not part of standard neuropsychological research—even though best practice in neuropsychology requires that a conclusion regarding the results of any neuropsychological assessment be made only if effort has been assessed as part of the test battery (62). In particular, the marked inconsistencies in OCD research make assessing these aspects imperative. We recommend that future research consider state/trait personal variables that may impact test performance in OCD, which may also increase interpretive power, and goodness-of-fit with psychopathological models.
Notwithstanding, given that it is becoming increasingly clear that the ecological validity of classic neuropsychological tests in the context of psychopathology (and particularly in OCD) is poor, we recommend that researchers take a careful approach toward selection of tests and outcome measures, as well as with regards to interpretation of their results. Neuropsychological research in OCD would benefit from a careful consideration of tasks and outcome variables, and incorporation of assessment of everyday function is crucial. We also encourage researchers in the field to utilize the verisimilitude approach, incorporating tests that mimic the demands of real-life situations, instead of focusing solely on tests that may be correlated with real-life functions. In addition, self-report systems tapping into real-life functions related to cognitive domains (e.g., the BRIEF) would be of added value. Formation of an international neuropsychological consortium of researchers may be a potential venue to discuss these and other issues, and work toward clearer delineation of suitable tests.
In sum, following decades of exhaustive foundational research on neuropsychology in OCD, subsequent efforts may need to be broader (e.g., consider the role of other factors impacting cognitive dysfunction), deeper (e.g., explore tests and constructs in relation to neuropsychological methods, clinical, and functional correlates), and finer (e.g., undertake more nuanced investigations of test performance), in order to advance the field.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. (2004) 65:185–236. doi: 10.1016/j.biopsycho.2003.07.007
2. Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev. (2013) 33:1163–71. doi: 10.1016/j.cpr.2013.09.004
3. Abramovitch A, Cooperman A. The cognitive neuropsychology of obsessive-compulsive disorder: a critical review. J Obsess Compul Relat Disord. (2015) 5:24–36. doi: 10.1016/j.jocrd.2015.01.002
4. Snyder HR, Kaiser RH, Warren SL, Heller W, Hall F, Hospital M. Obsessive-compulsive disorder is associated with broad impairments in executive function: a meta-analysis. Clin Psychol Sci. (2016) 3:301–30. doi: 10.1177/2167702614534210
5. Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. (2014) 44:1121. doi: 10.1017/S0033291713001803
6. Bora E. Meta-analysis of neurocognitive deficits in unaffected relatives of obsessive-compulsive disorder (OCD): comparison with healthy controls and patients with OCD. Psychol Med. (2020) 50:1257–66. doi: 10.1017/S0033291720001634
7. Fradkin I, Strauss AY, Pereg M, Huppert JD. Rigidly applied rules? Revisiting inflexibility in obsessive compulsive disorder using multilevel meta-analysis. Clin Psychol Sci. (2018) 64:481–505. doi: 10.1177/2167702618756069
8. Henry J. A meta-analytic review of Wisconsin Card Sorting Test and verbal fluency performance in obsessive-compulsive disorder. Cogn Neuropsychiatry. (2006) 11:156–76. doi: 10.1080/13546800444000227
9. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum. (1988).
10. Abramovitch A, McCormack B, Brunner D, Johnson M, Wofford N. The impact of symptom severity on cognitive function in obsessive- compulsive disorder : a meta-analysis. Clin Psychol Rev. (2019) 67:36–44. doi: 10.1016/j.cpr.2018.09.003
11. Leopold R, Backenstrass M. Neuropsychological differences between obsessive-compulsive washers and checkers: a systematic review and meta-analysis. J Anxiety Disord. (2015) 30:48–58. doi: 10.1016/j.janxdis.2014.12.016
12. Bragdon LB, Gibb BE, Coles ME. Does neuropsychological performance in OCD relate to different symptoms? A meta-analysis comparing the symmetry and obsessing dimensions. Depress Anxiety. (2018). 761–74. doi: 10.1002/da.22785
13. Abramovitch A, Abramowitz JS, Mittelman A, Stark A, Ramsey K, Geller DA. Research Review: neuropsychological test performance in pediatric obsessive-compulsive disorder - a meta-analysis. J Child Psychol Psychiatry Allied Discip. (2015) 56:837–47. doi: 10.1111/jcpp.12414
14. Fullana MA, Abramovitch A, Via E, López-Sola C, Goldberg X, Reina N, et al. Diagnostic biomarkers for obsessive-compulsive disorder: a reasonable quest or ignis fatuus? Neurosci Biobehav Rev. (2020) 118:504–13. doi: 10.1016/j.neubiorev.2020.08.008
15. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. (2013) 139:81–132. doi: 10.1037/a0028727
16. Stefanopoulou E, Manoharan A, Landau S, Geddes JR, Goodwin G, Frangou S. Cognitive functioning in patients with affective disorders and schizophrenia: a meta-analysis. Int Rev Psychiatry. (2009) 21:336–56. doi: 10.1080/09540260902962149
17. Boonstra AM, Oosterlaan J, Sergeant JA, Buitelaar JK. Executive functioning in adult ADHD: a meta-analytic review. Psychol Med. (2005) 35:1097–108. doi: 10.1017/S003329170500499X
18. Bora E, Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophr Bull. (2015) 41:1095–104. doi: 10.1093/schbul/sbu198
19. Abramovitch A, Short T, Schweiger A. The C factor: cognitive dysfunction as a transdiagnostic dimension in psychopathology. Clin Psychol Rev. (2021) 86:102007. doi: 10.1016/j.cpr.2021.102007
20. Markarian Y, Larson MJ, Aldea MA, Baldwin SA, Good D, Berkeljon A, et al. Multiple pathways to functional impairment in obsessive-compulsive disorder. Clin Psychol Rev. (2010) 30:78–88. doi: 10.1016/j.cpr.2009.09.005
21. Huppert J, Simpson H, Nissenson K, Leibowitz M, Foa E. Quality of life and functional impairment in obsessive–compulsive disorder: a comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. (2009) 26:39–45. doi: 10.1002/da.20506
22. Pérez-Vigil A, De La Cruz LF, Brander G, Isomura K, Jangmo A, Feldman I, et al. Association of obsessive-compulsive disorder with objective indicators of educational attainment: a nationwide register-based sibling control study. JAMA Psychiatry. (2018) 75:47–55. doi: 10.1001/jamapsychiatry.2017.3523
23. Abramovitch A, Mittelman A, Tankersley AP, Abramowitz JS, Schweiger A. Neuropsychological investigations in obsessive-compulsive disorder: a systematic review of methodological challenges. Psychiatry Res. (2015) 228:112–20. doi: 10.1016/j.psychres.2015.04.025
24. Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. (2005) 29:399–419. doi: 10.1016/j.neubiorev.2004.11.006
25. Chamberlain SR, Leppink EW, Redden SA, Grant JE. Are obsessive-compulsive symptoms impulsive, compulsive or both? Compr Psychiatry. (2016) 68:111–8. doi: 10.1016/j.comppsych.2016.04.010
26. Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl). (2008) 199:439–56. doi: 10.1007/s00213-008-1127-6
27. Morooka T, Ogino T, Takeuchi A, Hanafusa K, Oka M, Ohtsuka Y. Relationships between the color-word matching Stroop task and the Go/NoGo task: toward multifaceted assessment of attention and inhibition abilities of children. Acta Med Okayama. (2012) 66:377–86. doi: 10.18926/AMO/49385
28. Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. (2001) 13:250–61. doi: 10.1006/nimg.2000.0685
29. van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA. Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front Hum Neurosci. (2014) 8:1–22. doi: 10.3389/fnhum.2014.00419
30. Abramovitch A, Schweiger A. Misuse of cognitive neuropsychology in psychiatry research: the intoxicating appeal of neo-reductionism. Behav Ther. (2015) 38:187–91.
31. Chaytor N, Schmitter-Edgecombe M. The ecological validity of neuropsychological tests: a reviewof the literature on everyday cognition skills. Neuropsychol Rev. (2003) 13:181–97. doi: 10.1023/B:NERV.0000009483.91468.fb
32. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks : a latent variable analysis. Cogn Psychol. (2000) 41:49–100. doi: 10.1006/cogp.1999.0734
33. Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. 5th ed. New York: Oxford University Press. (2012).
34. Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. (2002) 53:401–33. doi: 10.1146/annurev.psych.53.100901.135220
35. Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol. (2015). 6:328. doi: 10.3389/fpsyg.2015.00328
36. Gruner P, Pittenger C. Cognitive inflexibility in obsessive-compulsive disorder. Neuroscience. (2017) 345:243–55. doi: 10.1016/j.neuroscience.2016.07.030
37. Spooner DM, Pachana NA. Ecological validity in neuropsychological assessment: a case for greater consideration in research with neurologically intact populations. Arch Clin Neuropsychol. (2006) 21:327–37. doi: 10.1016/j.acn.2006.04.004
38. Sbordone RJ. Ecological validity: some critical issues for the neuropsychologist. In: Sbordone RJ, Long CJ, editor. Ecological Validity of Neuropsychological Testing. Boca Raton, FL: St Lucie Press (1996). p. 16.
39. Sbordone RJ, Guilmette TJ. Ecological validity: prediction of everyday and vocational functioning from neuropsychological test data. In: Sweet JJ, editor. Forensic Neuropsychology: Fundamentals and Practice. Lisse: Swets and Zeitlinger (1999). p. 227–54.
40. Jelinek L, Moritz S, Heeren D, Naber D. Everyday memory functioning in obsessive – compulsive disorder. J Int Neuropsychol Soc. (2006) 12:746–9. doi: 10.1017/S1355617706060899
41. Abramovitch A, McKay D. Behavioral impulsivity in obsessive-compulsive disorder. J Behav Addict. (2016) 5:395–7. doi: 10.1556/2006.5.2016.029
42. Sohn SY, Kang JI, Namkoong K, Kim SJ. Multidimensional measures of impulsivity in obsessive-compulsive disorder: cannot wait and stop. PLoS ONE. (2014) 9:e111739. doi: 10.1371/journal.pone.0111739
43. Hybel KA, Mortensen EL, Lambek R, Højgaard DRMA, Thomsen PH, Anna K, et al. Executive function predicts cognitive-behavioral therapy response in childhood obsessive-compulsive disorder. Behav Res Ther. (2017) 99:11–8. doi: 10.1016/j.brat.2017.08.009
44. Kingstone A, Smilek D, Ristic J, Friesen CK, Eastwood JD. Attention, researchers! It Is time to take a look at the real world. Curr Dir Psychol Sci. (2003) 12:176–80. doi: 10.1111/1467-8721.01255
45. Alderman N, Burgess PW, Knight C, Henman C. Ecological validity of a simplified version of the multiple errands shopping test. J IntNeuropsychol Soc. (2003) 9:31–44. doi: 10.1017/S1355617703910046
46. Parsons TD. Virtual reality for enhanced ecological validity and experimental control in the clinical, affective and social neurosciences. Front Hum Neurosci. (2015) 9:1–19. doi: 10.3389/fnhum.2015.00660
47. Poncet F, Swaine B, Dutil E, Chevignard M, Pradat-Diehl P. How do assessments of activities of daily living address executive functions: a scoping review. Neuropsychol Rehabil. (2017) 27:618–66. doi: 10.1080/09602011.2016.1268171
48. Vandborg SK, Hartmann TB, Bennedsen BE, Pedersen AD, Eskildsen A, Videbech PBH, et al. Do cognitive functions in obsessivecompulsive disorder change after treatment? A systematic review and a double case report. Nord J Psychiatry. (2012) 66:60–7. doi: 10.3109/08039488.2011.626869
49. Flessner CA, Allgair A, Garcia A, Freeman J, Sapyta J, Franklin ME, et al. The impact of neuropsychological functioning on treatment outcome in pediatric obsessive–compulsive disorder. Depress Anxiety. (2010) 27:365–71. doi: 10.1002/da.20626
50. Cavedini P, Riboldi G, D'annucci A, Belotti P, Cisima M, Bellodi L. Decision-making heterogeneity in obsessive-compulsive disorder: ventromedial prefrontal cortex function predicts different treatment outcomes. Neuropsychologia. (2002) 40:205–11. doi: 10.1016/S0028-3932(01)00077-X
51. McNamara JPH, Reid AM, Balkhi AM, Bussing R, Storch EA, Murphy TK, et al. Self-regulation and other executive functions relationship to pediatric OCD severity and treatment outcome. J Psychopathol Behav Assess. (2014) 36:432–42. doi: 10.1007/s10862-014-9408-3
52. Bolton D, Raven P, Madronal-Luque R, Marks IM. Neurological and neuropsychological signs in obsessive compulsive disorder: interaction with behavioural treatment. Behav Res Ther. (2000) 38:695–708. doi: 10.1016/S0005-7967(99)00139-4
53. Vandborg SK, Hartmann TB, Bennedsen BE, Pedersen AD, Thomsen PH. Are there reliable changes in memory and executive functions after cognitive behavioural therapy in patients with obsessive-compulsive disorder? Cogn Neuropsychiatry. (2015) 20:128–43. doi: 10.1080/13546805.2014.981649
54. Roh K, Shin M, Kim M, Ha T, Shin Y, Lee K, et al. Persistent cognitive dysfunction in patients with obsessive-compulsive disorder : a naturalistic study. Psychiatry Clin Neurosci. (2005) 59:539–45. doi: 10.1111/j.1440-1819.2005.01411.x
55. Kuelz AK, Riemann D, Halsband U, Vielhaber K, Unterrainer J, Kordon A, et al. Neuropsychological impairment in obsessive-compulsive disorder - improvement over the course of cognitive behavioral treatment. J Clin Exp Neuropsychol. (2006) 28:1273–87. doi: 10.1080/13803390500507246
56. Negreiros J, Best J, Yamin D, Belschner L, Lin S, Stewart S. Test-based versus parent ratings of executive function in pediatric Obsessive-Compulsive Disorder. J Obs Relat Disord. (2020) 24:100495. doi: 10.1016/j.jocrd.2019.100495
57. Burdick KE, Endick CJ, Goldberg JF. Assessing cognitive deficits in bipolar disorder: are self-reports valid? Psychiatry Res. (2005) 136:43–50. doi: 10.1016/j.psychres.2004.12.009
58. Svendsen AM, Kessing L V, Munkholm K, Vinberg M, Miskowiak KW. Is there an association between subjective and objective measures of cognitive function in patients with affective disorders? Nord J Psychiatry. (2012) 66:248–53. doi: 10.3109/08039488.2011.626870
59. Dittrich WH, Johansen T, Fineberg NA. Cognitive Assessment Instrument of Obsessions and Compulsions (CAIOC-13) - a new 13-item scale for evaluating functional impairment associated with OCD. Psychiatry Res. (2011). 187:283–90. doi: 10.1016/j.psychres.2010.10.031
60. Chaytor N, Schmitter-Edgecombe M, Burr R. Improving the ecological validity of executive functioning assessment. Arch Clin Neuropsychol. (2006) 21:217–27. doi: 10.1016/j.acn.2005.12.002
61. Moritz S, Hauschildt M, Saathoff K, Jelinek L. Does impairment in neuropsychological tests equal neuropsychological impairment in obsessive-compulsive disorder (OCD)? Momentary influences, testing attitude, and motivation are related to neuropsychological performance in OCD. J Obsess Compul Relat Disord. (2017) 14:99–105. doi: 10.1016/j.jocrd.2017.06.005
62. Heilbronner RL, Sweet JJ, Attix DK, Krull KR, Henry GK, Hart RP. Official position of the American Academy of clinical neuropsychology on serial neuropsychological assessments: the utility and challenges of repeat test administrations in clinical and forensic contexts. Clin Neuropsychol. (2010) 24:1267–78. doi: 10.1080/13854046.2010.526785
63. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 2nd ed. New York: Oxford University Press. (2006).
64. Rouault M, McWilliams A, Allen MG, Fleming SM. Human metacognition across domains: insights from individual differences and neuroimaging. Pers Neurosci. (2018) 1:1–13. doi: 10.1017/pen.2018.16
65. Sun X, Zhu C, So SHW. Dysfunctional metacognition across psychopathologies: a meta-analytic review. Eur Psychiatry. (2017) 45:139–53. doi: 10.1016/j.eurpsy.2017.05.029
66. Bandura A, Caprara GV, Barbaranelli C, Gerbino M, Pastorelli C. Role of affective self-regulatory efficacy in diverse spheres of psychosocial functioning. Child Dev. (2003) 74:769–82. doi: 10.1111/1467-8624.00567
67. Rüsch N, Corrigan PW, Wassel A, Michaels P, Larson JE, Olschewski M, et al. Self-stigma, group identification, perceived legitimacy of discrimination and mental health service use. Br J Psychiatry. (2009) 195:551–2. doi: 10.1192/bjp.bp.109.067157
68. Flavell JH. Metacognition and cognitive monitoring: a new area of cognitive-developmental inquiry. Am Psychol. (1979) 34:906–11. doi: 10.1037/0003-066X.34.10.906
69. Silveira VP, Frydman I, Fontenelle LF, Mattos P, de Oliveira-Souza R, Moll J, et al. Exploring response inhibition and error monitoring in obsessive-compulsive disorder. J Psychiatr Res. (2020) 126:26–33. doi: 10.1016/j.jpsychires.2020.04.002
70. Berlin GS, Lee HJ. Response inhibition and error-monitoring processes in individuals with obsessive-compulsive disorder. J Obsessive Compuls Relat Disord. (2018) 16:21–7. doi: 10.1016/j.jocrd.2017.11.001
71. Foa EB, Mathews A, Abramowitz JS, Amir N, Przeworski A, Riggs DS, et al. Do patients with obsessive – compulsive disorder have deficits in decision-making? Cognit Ther Res. (2003) 27:431–45. doi: 10.1023/A:1025424530644
72. Kashyap H, Kumar KJ, Kandavel T, Reddy YCJ. Neuropsychological functioning in obsessive- compulsive disorder : are executive functions the key deficit? Compr Psychiatry. (2013) 54:533–40. doi: 10.1016/j.comppsych.2012.12.003
73. Kalanthroff E, Anholt GE, Henik A. Always on guard: test of high vs. low control conditions in obsessive-compulsive disorder patients. Psychiatry Res. (2014) 219:322–8. doi: 10.1016/j.psychres.2014.05.050
74. Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Zangen A, Dar R. From self-induced to perceived errors - a generalized over-monitoring activity in obsessive-compulsive disorder. Eur Neuropsychopharmacol. (2019) 29:1083–91. doi: 10.1016/j.euroneuro.2019.07.240
75. Riesel A, Endrass T, Auerbach LA, Kathmann N. Overactive performance monitoring as an endophenotype for obsessive-compulsive disorder: evidence from a treatment study. Am J Psychiatry. (2015) 172:665–73. doi: 10.1176/appi.ajp.2014.14070886
76. Ischebeck M, Endrass T, Simon D, Kathmann N. Auditory novelty processing is enhanced in obsessive–compulsive disorder. Depress Anxiety. (2011) 28:915–23. doi: 10.1002/da.20886
77. Riesel A, Kathmann N, Klawohn J. Flexibility of error-monitoring in obsessive-compulsive disorder under speed and accuracy instructions. J Abnorm Psychol. (2019) 128:671–8. doi: 10.1037/abn0000463
78. Abramovitch A, Dar R, Hermesh H, Schweiger A. Comparative neuropsychology of adult obsessive-compulsive disorder and attention deficit/hyperactivity disorder: implications for a novel executive overload model of OCD. J Neuropsychol. (2012) 6:161–91. doi: 10.1111/j.1748-6653.2011.02021.x
79. Ashcraft MH, Kirk EP. The relationships among working memory, math anxiety, and performance. J Exp Psychol Gen. (2001) 130:224–37. doi: 10.1037/0096-3445.130.2.224
80. Beilock SL, Rydell RJ, McConnell AR. Stereotype threat and working memory: mechanisms, alleviation, and spillover. J Exp Psychol Gen. (2007) 136:256–76. doi: 10.1037/0096-3445.136.2.256
81. Schmader T, Forbes CE. Shen Zhang, Berry Mendes W. A metacognitive perspective on the cognitive deficits experienced in intellectually threatening environments. Pers Soc Psychol Bull. (2009) 35:584–96. doi: 10.1177/0146167208330450
82. Keinan G, Friedland N, Kahneman D, Roth D. The effect of stress on the suppression of erroneous competing responses. Anxiety Stress Coping. (1999) 12:455–76. doi: 10.1080/10615809908249321
83. Braunstein-Bercovitz H, Dimentman-Ashkenazi I, Lubow RE. Stress affects the selection of relevant from irrelevant stimuli. Emotion. (2001) 1:182–92. doi: 10.1037/1528-3542.1.2.182
84. Moritz S, Spirandelli K, Happach I, Lion D, Berna F. Dysfunction by disclosure? Stereotype threat as a source of secondary neurocognitive malperformance in obsessive-compulsive disorder. J Int Neuropsychol Soc. (2018) 24:584–92. doi: 10.1017/S1355617718000097
85. Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. (2011) 92:519–30. doi: 10.1016/j.apmr.2010.11.015
86. Tate R, Kennedy M, Ponsford J, Douglas J, Velikonja D, Bayley M, et al. INCOG recommendations for management of cognition following traumatic brain injury, Part III: executive function and self-awareness. J Head Trauma Rehabil. (2014) 29:338–52. doi: 10.1097/HTR.0000000000000068
87. Kashyap H, Reddy P, Mandadi S, Narayanaswamy JC, Sudhir PM, Reddy YCJ. Cognitive training for neurocognitive and functional impairments in obsessive compulsive disorder: a case report. J Obsessive Compuls Relat Disord. (2019) 23:100480. doi: 10.1016/j.jocrd.2019.100480
88. Park HS, Shin Y-W, Ha TH, Shin MS, Kim YY, Lee YH, et al. Effect of cognitive training focusing on organizational strategies in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. (2006) 60:718–26. doi: 10.1111/j.1440-1819.2006.01587.x
89. Quiles C, Verdoux H, Prouteau A. Assessing metacognition during a cognitive task: impact of on-line metacognitive questions on neuropsychological performances in a non-clinical sample. J Int Neuropsychol Soc. (2014) 20:547–54. doi: 10.1017/S1355617714000290
Keywords: cognitive function, neuropsychology, obsessive-compulsive disorder, ecological validity, neurocognitive
Citation: Kashyap H and Abramovitch A (2021) Neuropsychological Research in Obsessive-Compulsive Disorder: Current Status and Future Directions. Front. Psychiatry 12:721601. doi: 10.3389/fpsyt.2021.721601
Received: 07 June 2021; Accepted: 30 September 2021;
Published: 01 November 2021.
Edited by:
Roseli Gedanke Shavitt, University of São Paulo, BrazilReviewed by:
Nicole C. R. McLaughlin, Butler Hospital, United StatesMarcelo Camargo Batistuzzo, Pontifical Catholic University of São Paulo, Brazil
Copyright © 2021 Kashyap and Abramovitch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amitai Abramovitch, YWJyYW1vdml0Y2hAdHhzdGF0ZS5lZHU=
†These authors have contributed equally to this work
 Himani Kashyap
Himani Kashyap Amitai Abramovitch
Amitai Abramovitch