- 1Department of Clinical Laboratory, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan
- 2Department of Sleep-Wake Disorders, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan
- 3Department of Psychiatry, Faculty of Medicine, Shimane University, Izumo, Japan
- 4Department of Radiology, Faculty of Medicine, Shimane University, Izumo, Japan
Background: The ventral tegmental area (VTA; a dopaminergic nucleus) plays an important role in the sleep-wake regulation system including orexin system. In addition to neuronal activity, there is increasing evidence for an important role of glial cells (i.e., astrocytes and microglia) in these systems. The present study examined the utility of magnetic resonance spectroscopy (MRS) for detecting neural and/or glial changes in the VTA to distinguish responders from non-responders before treatment with the orexin receptor antagonist suvorexant.
Methods: A total of 50 patients were screened and 9 patients were excluded. The remaining 41 patients with insomnia who have or not a psychiatric disease who were expected to receive suvorexant treatment were included in this study. We compared MRS signals in the VTA between responders to suvorexant and non-responders before suvorexant use. Based on previous reports, suvorexant responders were defined as patients who improved ≥3 points on the Pittsburgh Sleep Quality Index after 4 weeks of suvorexant use. MRS data included choline (reflects non-specific cell membrane breakdown, including of glial cells) and N-acetylaspartate (a decrease reflects neuronal degeneration).
Results: Among 41 examined patients, 20 patients responded to suvorexant and 21 patients did not. By MRS, the choline/creatine and phosphorylcreatine ratio in the VTA was significantly high in non-responders compared with responders (p = 0.039) before suvorexant treatment. There was no difference in the N-acetylaspartate/creatine and phosphorylcreatine ratio (p = 0.297) between the two groups.
Conclusions: Changes in glial viability in the VTA might be used to distinguish responders to suvorexant from non-responders before starting treatment. These findings may help with more appropriate selection of patients for suvorexant treatment in clinical practice. Further, we provide novel possible evidence for a relationship between glial changes in the VTA and the orexin system, which may aid in the development of new hypnotics focusing on the VTA and/or glial cells.
Introduction
An orexin receptor antagonist was recently developed for clinical use to promote sleep (1). Orexin neurons promote wakefulness via excitation of brainstem nuclei including the ventral tegmental area (VTA) (2). Recent studies have also emphasized the importance of dopaminergic activity in the VTA on sleep-wake regulation (3). For example, electroencephalogram detected firing pattern changed across sleep-wake cycle, or optogenetic or chemogenic stimulation of the VTA was found to initiate and maintain wakefulness (4, 5).
There is accumulating evidence for an important role of glial cells in controlling the orexin system and sleep-wake regulation, in addition to the roles of neurons in sleep disturbances in various neurodegenerative disorders (6). For example, morphological changes of astrocytes can result in decreased concentrations of extracellular adenosine, an important sleep promoting molecule (7). Pharmacological inhibition of microglial activity was also reported to reduce the compensatory increase in slow wave activity following sleep deprivation (8). Further, the orexin system can regulate inflammation and associated insomnia via control of microglial reactivity (9). Finally, astrocytes were found to convey orexin activity to remote brain areas utilizing a local network (10).
In the present study, we examined the hypothesis that differences in neuronal and/or glial cell function in the VTA are important for the responses of patients to the orexin receptor antagonist suvorexant and that these differences can be detected by magnetic resonance spectroscopy (MRS). MRS is a technique that measures metabolite changes related to neuronal or glial cell viability (11). To our knowledge, no studies using MRS have examined the brainstem in patients with insomnia. We conducted a 4-week cohort study of patients with insomnia to examine whether MRS findings can distinguish responders from non-responders before starting suvorexant treatment.
Methods
Ethics Statement
The present study was approved by the ethics committee of Shimane University Hospital (Shimane, Japan) (Approval No: 20170814-1) in August 2017. Detailed information on the aims and protocols of the study were explained to the participant. The participants provided their written informed consent in this study.
Participants
This 4-week cohort study was conducted in the Psychiatry Department of Shimane University Hospital from October 2017 to February 2019. Consecutive patients with insomnia symptoms were enrolled using the following inclusion criteria: (a) met the diagnostic criteria of insomnia regardless of comorbid psychiatric disease; (b) were expected to be treated with suvorexant for at least 4 weeks and did not use medicines that are prohibited to combine with suvorexant; (c) were intended to receive magnetic resonance imaging (MRI) examination to detect and/or exclude organic diseases; (d) agreed with the extended MRS examination in addition to routine MRI; and (e) who or whose caregivers could answer a sleep questionnaire. Patients were included in this study regardless of the type of primary disease. We excluded patients based on the following exclusion criteria: (a) in a coma; (b) were strongly sedated with anesthetics; (c) were allergic to suvorexant; (d) had a history of drug abuse or drug dependency; (e) were pregnant or were planning pregnancy; (f) were breastfeeding (suvorexant has not been proven to be safe in infants); (g) could not swallow the suvorexant pill; or (h) participated in another trial within 3 months before entering this study. We recruited patients in acute psychiatric ward. Therefore, almost all patients were in acute phase of psychiatric disease. Patients were diagnosed using Diagnostic criteria of Mental Disease 5th edition (DSM-5) by specialized psychiatrists.
Measurements
All patients were questioned with the Pittsburgh Sleep Quality Index (PSQI) (12) before and after 4 weeks of suvorexant use. The PSQI measures sleep quality and quantity based on recall of sleep behaviors over the prior 4-week period. The 19 PSQI questions measure seven categories of sleep, including quality, latency, duration, efficacy, disturbances, hypnotic use, and daytime dysfunction. Domain scores are coded from 0 to 3 and a global PSQI score is obtained by summing across the domains. The global PSQI score ranges from 0 to 21 and a score >5 is considered to reflect a patient with low sleep quality. The sensitivity and specificity of Japanese version of the PSQI estimates 85.7 and 86.6% of primary insomnia, and Cronbach's alpha is 0.77 (13).
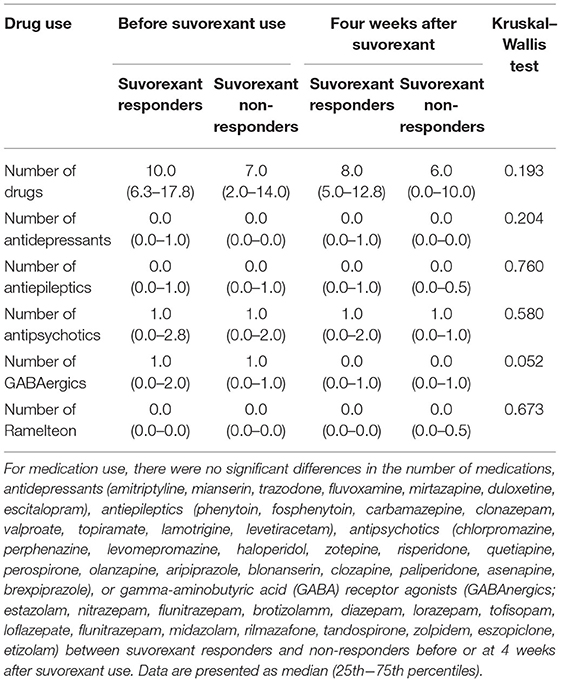
We set the observational period as 4 weeks because a previous trial using suvorexant showed sleep improvement within this timeframe (14) and as the PSQI measures symptoms of insomnia over 4 weeks. We defined a responder to suvorexant as a patient who showed a ≥3 PSQI global score decrease, as previously reported (15, 16). The background (including gender, age, body mass index, Eastern Cooperative Oncology Group (ECOG) performance status (17), Charlson comorbid index (18), and current smoking and alcohol consumption), psychiatric disorders (DSM-5), and medication use (antidepressants, antiepileptics, antipsychotics, and hypnotics including gamma-aminobutyric acid receptor agonists and ramelteon) of the patients were also evaluated to examine for confounding factors.
MRS
MRS can be used to determine the concentration of metabolites in the brain (11) including choline [which reflects non-specific cellular membrane breakdown (19)], creatine and phosphorylcreatine [the level of creatine and phosphorylcreatine is constant and is used as a reference value (20)], N-acetylaspartate [NAA; an NAA signal decrease reflects neuronal and/or myelin degeneration (21)], and myo-inositol [a marker of microglial activation (22)]. Participants were scanned using a PRESS (point-resolved spectral sequencing) sequence with a repetition time (TR)/echo time (TE) of 1,500 ms/35 ms, respectively.
The spectral acquisition was applied to the VTA. A region of interest was set for the VTA using a voxel size of 10 × 10 × 10 mm, while 133 frames were collected for all participants (Figure 1) (23). Other orexin projecting areas including the locus coeruleus (13 mm3) (24), dorsal raphe nuclei (70 mm3) (25), laterodorsal/pedunculopontine tegmental nuclei (100 mm3) (26), and the tuberomammillary nucleus (in the posterior hypothalamus; 130 mm3) (27) were too small to allow for signal acquisition compared with the VTA (360 mm3) (28). MRS data were analyzed using commercial software (Siemens Syngo VE11C; Neuro 3D, Siemens, Erlangen, Germany) (29). To ascertain the adequate signal-noise ratio, the attending MRI specialized clinical technician checked full width at half maximum (FWHM) every MRS examination and confirmed FWHM <20 Hz.
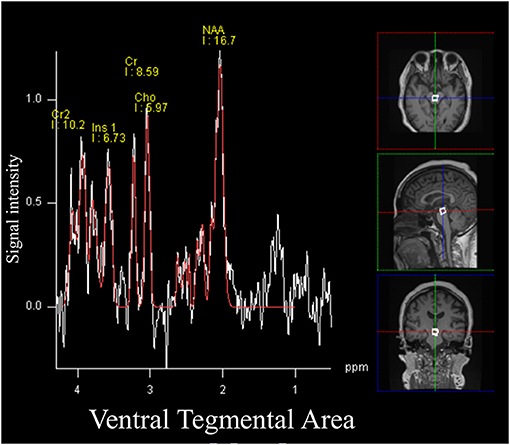
Figure 1. Magnetic resonance spectroscopy (MRS) signals and the volume of interest for the ventral tegmental area. All participants underwent a single voxel point-resolved spectral sequencing scan. The voxel size was set as 1.0 × 1.0 × 1.0 cm.
MRI Acquisition
MRI was obtained on a 3.0T MAGNETOM PRISMA system (Siemens) using a 20-channel phased array coil. For conventional MRI scans, the parameters for T1-weighted imaging were TR/TE = 410 ms/13 ms, respectively, and field of view (FOV) = 22 cm × 22 cm, the parameters for T2-weighted imaging were TR/TE = 3400 ms/100 ms, respectively, and FOV = 22 × 22 cm, and the parameters for T2-fluid-attenuated inversion recovery imaging were TR/TE = 12,000 ms/130 ms, respectively, and FOV = 22 × 22 cm. Additional MRI sequences included three-dimensional T1 magnetization-prepared rapid gradient echo (TR = 2,300 ms, TE = 2.4 ms, inversion time = 991 ms, bandwidth = 200 Hz, number of averages = 1, matrix size = 154 × 256, coronal section thickness = 1.2 mm, flip angle = 8°, acquisition time = 3:23 min).
Explanatory Examination
To assess the influence of diseases comorbid with insomnia, such as schizophrenia, depression, and neurocognitive disorders, on the choline/creatine and phosphorylcreatine ratio in the VTA, we compared the choline/creatine and phosphorylcreatine ratio between responders and non-responders stratified by each diagnostic criteria of comorbid psychiatric disease. To evaluate the association between insomnia severity and the choline/creatine and phosphorylcreatine ratio in the VTA, we constructed a scatter plot of the baseline PSQI global score and the choline/creatine and phosphorylcreatine ratio in the VTA.
Statistical Analysis
A Mann-Whitney U test was used to compare differences in MRS signals, including the choline/creatine and phosphorylcreatine ratio and the NAA/creatine and phosphorylcreatine ratio, between suvorexant responders and non-responders. Other signals including myo-inositol are too weak to detect differences in small cohorts of patients. In this preliminary study, differences in myo-inositol signals between suvorexant responders and non-responders were examined using the Mann–Whitney U test for explanatory purposes. The correlations between MRS signals and difference in PSQI global score before and after 4-week of suvorexant treatment, and PSQI global score before the treatment were calculated using Spearman's test for explanatory purposes. The background data of the patients are presented as mean ± standard deviation. The Chi-square test and the Kruskal-Wallis test were used to compare the background data. The Kruskal–Wallis test was performed to compare drug use between suvorexant responders and non-responders before and after the 4 weeks of suvorexant treatment. These data are presented as median (interquartile range). A power analysis with 90% power indicated that a sample size of N = 20 was required, assuming an effect size of d = 1.0, based on previous studies using MRS in the brainstem (30, 31). All statistical analyses were performed using statistical software (SPSS v23; IBM, Tokyo, Japan). Significance was set at p = 0.05.
Results
A total of 50 patients were recruited into this study (Figure 2). One patient withdrew consent to receive an MRI scan and two patients did not meet the inclusion criteria (one did not fulfill insomnia diagnostic criteria and one started clarithromycin [a cytochrome P450, family 3, subfamily A, competitive inhibitor], which is prohibited for use with suvorexant). Thus, 47 patients were included in this study. However, six more patients were excluded; MRS data could not be used for two patients (one patient could not stop moving during MRS examination and one patient exhibited iron deposition near the globus pallidus that disturbed the MRS signals), two patients did not attend the follow-up examination, and two patients did not complete the 4-week observation period (one patient developed liver failure and one stopped taking suvorexant because of somnolence during the daytime and could not complete the follow-up questionnaire). Thus, a total of 41 patients were included in the final analysis. Of these 41 patients, 20 patients responded to suvorexant and 21 patients did not.
Suvorexant responders showed trends toward being younger (54.7 ± 13.9 years vs. 61.1 ± 14.3 years, respectively) and having a higher body mass index (24.1 ± 7.6 vs. 21.7 ± 4.3, respectively), a lower Charlson comorbid index (0.4 ± 0.7 vs. 1.0 ± 1.2, respectively), and a higher PSQI score before suvorexant use (15.0 ± 2.7 vs. 11.1 ± 4.8, respectively) compared with non-responders (Table 1). The PSQI global score was increased (worsened) among non-responders during the observational period. As shown in Supplementary Table 1, component 1(Subjective sleep quality), component 2 (Sleep latency), component 3 (Sleep duration), and component 4 (Habitual sleep efficiency) were increased. The diseases of the patients according to the DSM-5 are shown in Table 2. Patients with depression were more prevalent in suvorexant responders compared with non-responders. There were seven patients with schizophrenia and schizoaffective disorders in the responders group and six patients in the non-responders group. There were three patients with dementia in the responders group and four patients in the non-responders group. Medication use in responders and non-responders before and after the 4-week suvorexant treatment is shown in Table 3 (median number of drug use for each patient) and Supplementary Table 2 (the number of patients who use each drug). There were no differences in the number of antidepressants, antiepileptics, antipsychotics, or gamma-aminobutyric acid receptor agonists used between the responders and non-responders.
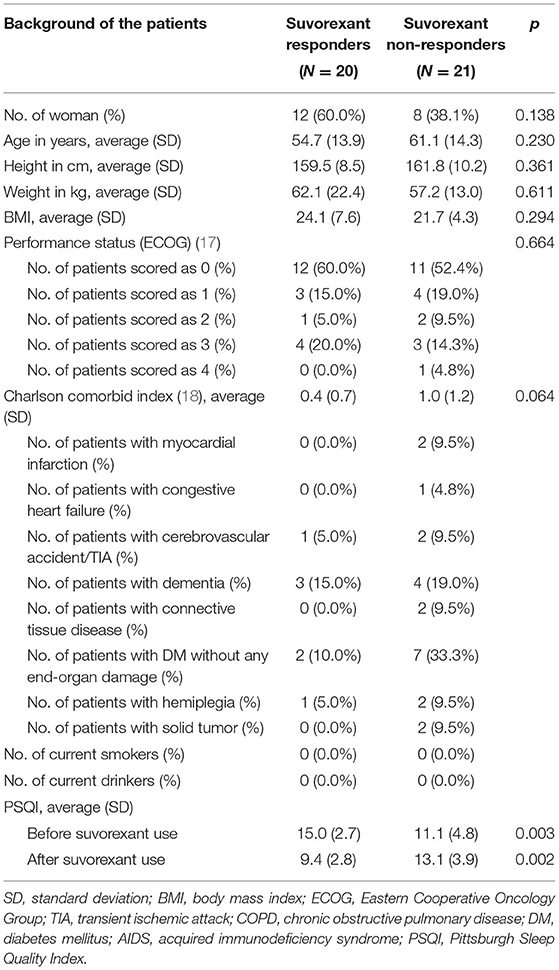
Table 1. Comparison of the background of the patients for responders to suvorexant and non-responders.
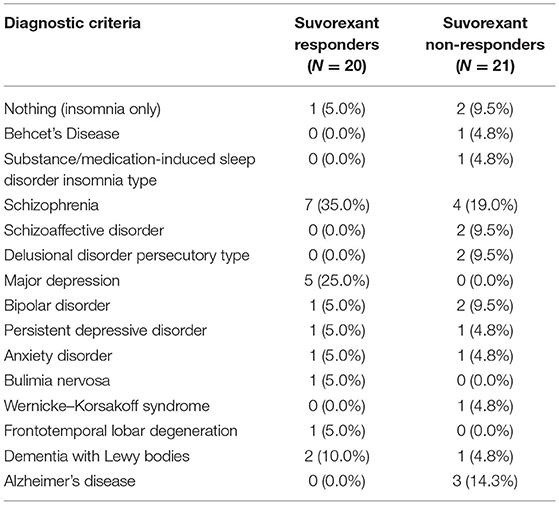
Table 2. Psychiatric disease comorbid with insomnia diagnosed by the Diagnostic and Statistical Manual of Mental Disorders-5 criteria.
The primary outcome of our study was that the choline/creatine and phosphorylcreatine ratio in the VTA was significantly lower in suvorexant responders (0.85; median, 0.76–0.95 for the 25th−75th percentiles) compared with non-responders (1.00; median, 0.85–1.29 for 25th−75th percentile; p = 0.039) (Figure 3). There were no differences in the NAA/creatine and phosphorylcreatine or the myo-inositol/creatine and phosphorylcreatine ratio between responders and non-responders (NAA/creatine and phosphorylcreatine: 1.86 [1.48–2.23] vs. 1.83 [1.69–2.47], respectively, p = 0.297; myo-inositol/creatine and phosphorylcreatine: 0.633 [0.56–3.90] vs. 0.681 [0.51–0.90], respectively, p = 0.979).
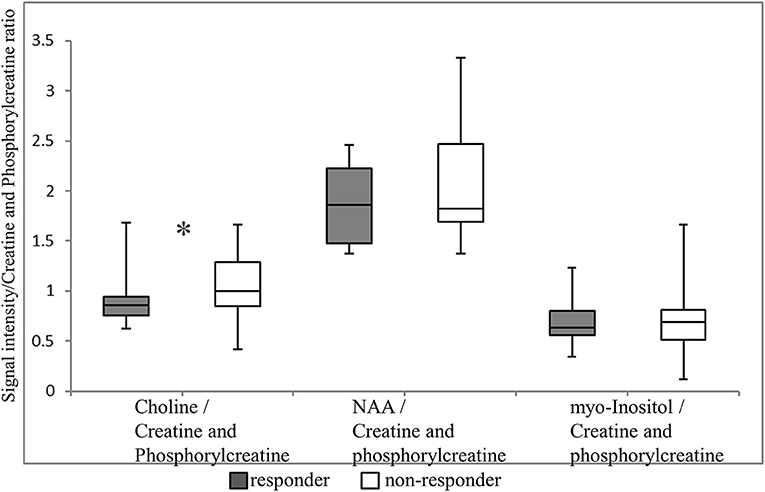
Figure 3. Magnetic resonance spectroscopy (MRS) signals in the ventral tegmental area. Each MRS signal is shown as a box plot. The upper and lower whiskers represent the maximum and minimum ratios, respectively. The upper, middle, and lower bars represent the 75th percentiles, median, and 25th percentiles, respectively. *p = 0.039. NAA, N-acetylaspartate.
The choline/creatine and phosphorylcreatine ratios in the VTA between responders and non-responders stratified by each diagnostic criteria of comorbid psychiatric disease are shown in Table 4. The majority of the subcategories of comorbid psychiatric disease in the suvorexant responders group showed a lower choline/creatine and phosphorylcreatine ratio. Furthermore, most of the subcategories in the non-responders group showed a higher choline/creatine and phosphorylcreatine ratio.
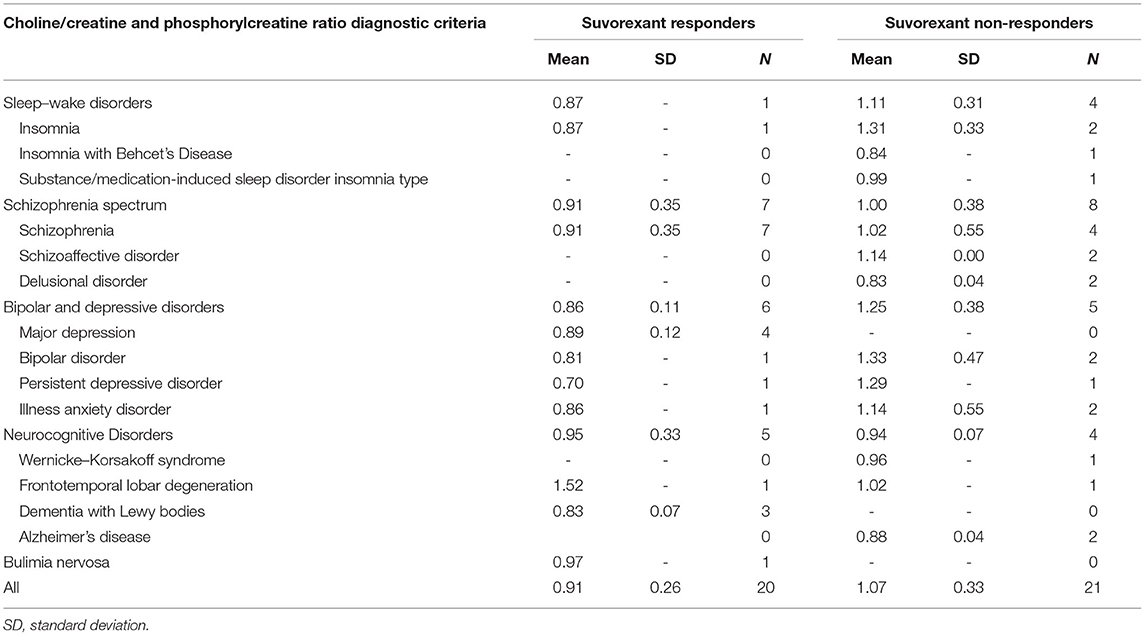
Table 4. The choline/creatine and phosphorylcreatine ratio in the ventral tegmental area between responders and non-responders stratified by each diagnostic criteria of comorbid psychiatric disease.
A scatter plot of the baseline PSQI global score and the choline/creatine and phosphorylcreatine ratio in the VTA is shown in Figure 4. The average baseline PSQI global score of non-responders was low compared with suvorexant responders. However, the baseline PSQI global scores of non-responders were widely distributed, with some patients having high baseline PSQI scores.
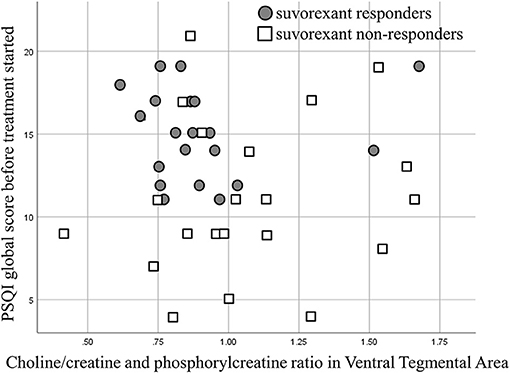
Figure 4. A scatter plot showing the baseline Pittsburgh Sleep Quality Index (PSQI) global score and the choline/creatine and phosphorylcreatine ratio.
Another scatter plot of the choline/creatine and phosphorylcreatine ratio in the VTA and the differences in PSQI global score before and after 4-week of suvorexant treatment is shown in Figure 5. The correlation was not significant (correlation coefficient was 0.292, p = 0.064). The correlation of the choline/creatine and phosphorylcreatine ratio in the VTA and the PSQI global score before the treatment was not significant (correlation coefficient was −0.0112, p = 0.487).
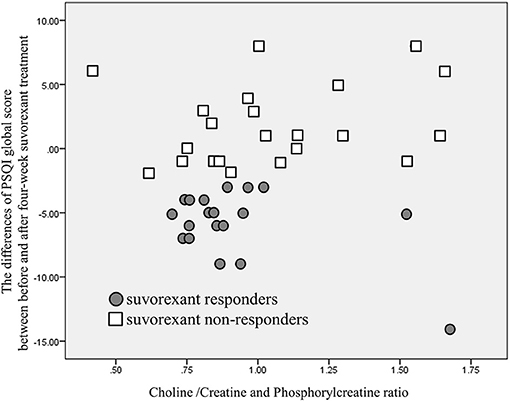
Figure 5. A scatter plot of choline/creatine and phosphorylcreatine ratio and the differences in PSQI global score before and after 4-week of suvorexant treatment. Although there is a tendency that lower choline/creatine and phosphorylcreatine ratio may relate to a greater decrease of PSQI global score, there is no significant correlation of choline /creatine and phosphorylcreatine ratio and the differences of PSQI global score (p = 0.064).
Discussion
A key finding of the present study was that signal changes in the VTA detected by MRS might be used to distinguish responders to suvorexant from non-responders in patients with insomnia before starting treatment. Specifically, patients with relatively high choline/creatine and phosphorylcreatine signal ratio in the VTA may show a poor response to suvorexant.
Although we observed relatively high cholinergic signal in suvorexant non-responders, there were no differences in NAA between the two groups. Relatively high cholinergic signal was reported to reflect non-specific cell membrane breakdown (19), while a decreased NAA signal reflects neuronal and/or myelinic degeneration (21). As such, our data suggest that preservation of non-neural cells such as glia may be associated with the response to suvorexant. Although previous studies have emphasized the importance of glia in sleep-wake regulation (7–10), to our knowledge there are no reports examining the relationship between glial changes and the effect of suvorexant. It is also possible that MRS differences in the choline/creatine and phosphorylcreatine ratio may reflect differences in cellular states. Future studies examining the relationship between specific changes in glia and suvorexant function may provide a further understanding of the mechanisms underlying suvorexant resistance.
In the present study, signal changes in the VTA detected by MRS may predict the response to suvorexant. The VTA plays a key role in sleep-wake regulation (4, 5), while several studies have examined the role of glia in the VTA (32, 33) and the role of the VTA in sleep (34). Furthermore, recent animal studies showed suvorexant can restore stress-induced aberrant dopamine system activity in VTA (35). However, to our knowledge, there are no reports of a direct relationship between glial changes in the VTA and sleep function. Our findings may suggest a potential connection between glial function in the VTA and the effect of suvorexant. Thus, the development of drugs to prevent glial changes in the VTA may be useful for patients who are resistant to suvorexant.
The findings from the present study suggest that it may be possible to distinguish suvorexant responders from non-responders before suvorexant treatment is started. Previous studies have examined the predictive factors for insomnia treatment, although not for the prediction of treatment prognosis using suvorexant. Further, there are inconsistent findings in these studies, including the severity of insomnia (36, 37), sleep duration (38, 39), and comorbid psychiatric disease (40, 41). Further studies examining the accuracy of prediction of suvorexant treatment may help clinicians in the selection of optimal therapy based on prognosis prediction.
The present study has several potential limitations. First, we collected patients in an acute psychiatry ward and almost all patients had comorbid psychiatric diseases. The majority of subcategories of comorbid psychiatric disease in suvorexant responders showed a relatively low choline/creatine and phosphorylcreatine ratio, whereas most of the subcategories of comorbid psychiatric disease in non-responders showed a relatively high choline/creatine and phosphorylcreatine ratio (Table 4), as same as the primary outcome of the present study. However, because of the limited number of patients in each diagnostic group, a contribution of comorbid psychiatric diseases to our findings remains possible. Furthermore, this background diversity of our patients may limit the relevance of our findings to chronic insomnia patients without any psychiatric comorbidity. In addition, psychiatric disease and stress also affect glial activity (42, 43). The present study could not detect the causal relationship between suvorexant response and psychiatric stress. Furthermore, in the present study, we could not detect the significant association between choline/creatinine and phosphorylcreatine ratio and the differences in PSQI global score before and after 4-week of suvorexant treatment, although there is a tendency that lower ratio of choline/creatinine and phosphorylcreatine ratio may be correlated with a greater decrease of PSQI global score, as shown in Figure 5. A larger sample of participants may confirm the correlation of choline/creatinine and phosphorylcreatine ratio signal and status of symptoms. Second, this study was not randomized. Therefore, the backgrounds of the patients were different between suvorexant responders and non-responders. For example, the baseline PSQI was higher in the suvorexant responder group. Although some of the non-responders had a high baseline PSQI global score (Figure 4), a randomized control study is needed to eliminate the effect of differences in baseline characteristics. Moreover, as shown in Table 3, the number of drug use was not significantly different between responders and non-responders and before and after treatment. However, there is a possibility that other drugs may modify the results. Furthermore, the assessors of the PSQI and MRS data were not blinded to the treatment groups. Thus, large blinded studies are required to eliminate these potential biases. Third, although it is important that which psychiatric disease is severely affected by degenerative cells in VTA, the limited number of participants in the present study prevents conclusive discussion. The present study cannot address the differences by diagnosis, nor can it address the association between the orexin system and the prognosis of suvorexant treatment by diagnosis. The present study warrants the need for future study with a larger number of subjects. Fourth, we did not use the linear combination model (44), which may have reduced the accuracy of our findings. During the study period, the linear combination model was not freely available. Therefore, we planned to use Siemens Syngo VE11C; Neuro 3D. From 16th February 2021, the linear combination model has become open access. Future studies should use the linear combination model. Fifth, in the present study, objective sleep measurements, such as polysomnography or actigraphy were not used. Further studies using objective sleep measurement are needed. Sixth, the size of the region of interest may have been too small to eliminate noise. As such, we also examined signals other than choline and NAA for explanatory purposes. Sixth, the size of the region of interest was 2.7 times larger than the VTA. The VTA is surrounded by nuclei such as the substantia nigra, which is another major source of dopaminergic neurons (45). Thus, further studies are required to separate the role of VTA from other dopaminergic nuclei. Finally, suvorexant is currently available only in United States, Canada, Australia, Russia, and Japan, and is not approved in other industrialized countries. And a lower dosage was approved, while the clinical trials examined the high dosage of 40 mg (46, 47). Modest effect and next day somnolence may prevent the usage of suvorexant worldwide.
Conclusion
We found that changes in cholinergic signals in the VTA may be useful for distinguishing responders to suvorexant from non-responders before starting treatment, which may help clinicians with decisions on optimal drug selection. Furthermore, the present study may demonstrate a role for the VTA and glia in the orexin system. Thus, future studies and drug development considering the relation between glia in VTA and insomnia may be useful.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: Clinical datasets of participants presented in this article are not readily available due to ethical reasons. Please contact bWluYWdha2lAbWVkLnNoaW1hbmUtdS5hYy5qcA== for questions. Requests to access these datasets should be directed to Masatoshi Inagaki, bWluYWdha2lAbWVkLnNoaW1hbmUtdS5hYy5qcA==.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Shimane University Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MIz: conceptualization, methodology, validation, data curation, formal analysis, investigation, and writing—original draft preparation. SM, KO, MH, and SH: writing—review and editing. MN and HK: supervision. HA: data curation, formal analysis, investigation, and writing—review and editing. MIn: writing—review and editing and supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
MIn received grants from the Research for Promotion of Cancer Control Programs during this study. He has also received lecture fees from Technomics, Fuji Keizai, Novartis, Yoshitomiyakuhin, Pfizer, MSD, Meiji Seika Pharma, Eisai, Otsuka, Sumitomo Dainippon Pharma, Mochida, Janssen, Takeda, and Eli Lilly. His institute has received research funds from Otsuka, Eisai, Daiichi-Sankyo, Pfizer, Astellas, MSD, Takeda, Fujifilm, Shionogi, and Mochida. HK has received lecture fees from Eisai, FUJIFILM Toyama Chemical Co., Ltd, Guerbet Japan, and Bayer. His institute has received research funds from Fuji Pharma Co., Ltd., Eisai, Guerbet Japan, Daiichi-Sankyo, Nihon Medi-Physics Co., Ltd., and FUJIFILM Toyama Chemical Co., Ltd. MIz has received lecture fee from Eisai. This study has NOT supported any institute including MSD, Merck unit in Japan, which sells suvorexant.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Sanae Yamashita, Sakura Hino, Masako Yano, and Tomoko Araki for supporting our work and Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.714376/full#supplementary-material
Data Sheet 1. STROBE checklist.
References
1. Gotter AL, Stevens J, Garson SL, Cui D, Coleman PJ, Harrell CM, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. (2011) 25:52–61. doi: 10.3109/01677063.2011.566953
2. Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci. (2011) 32:451–62. doi: 10.1016/j.tips.2011.03.007
3. Gompf HS, Anaclet C. The neuroanatomy and neurochemistry of sleep-wake control. Curr Opin Physiol. (2020) 15:143–51. doi: 10.1016/j.cophys.2019.12.012
4. Oishi Y, Lazarus M. The control of sleep and wakefulness by mesolimbic dopamine systems. Neurosci Res. (2017) 118:66–73. doi: 10.1016/j.neures.2017.04.008
5. Eban-Rothschild A, Appelbaum L, De Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. (2018) 43:937–52. doi: 10.1038/npp.2017.294
6. Irfan M, Cherukuri CM, Khawaja IS, Schenck CH. Sleep disturbances in neurodegenerative disorders. Psychiatr Ann. (2018) 48:296–302. doi: 10.3928/00485713-20180515-01
7. Haydon PG. Astrocytes and the modulation of sleep. Curr Opin Neurobiol. (2017) 44:28–33. doi: 10.1016/j.conb.2017.02.008
8. Frank MG. The role of glia in sleep regulation and function. Handb Exp Pharmacol. (2019) 253:83–96. doi: 10.1007/164_2017_87
9. Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, et al. Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neurosci Lett. (2015) 606:140–4. doi: 10.1016/j.neulet.2015.08.033
10. Burt J, Alberto CO, Parsons MP, Hirasawa M. Local network regulation of orexin neurons in the lateral hypothalamus. Am J Physiol Integr Comp Physiol. (2011) 301:R572–80. doi: 10.1152/ajpregu.00674.2010
11. Duarte JMN, Lei H, Mlynárik V, Gruetter R. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. Neuroimage. (2012) 61:342–62. doi: 10.1016/j.neuroimage.2011.12.038
12. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
13. Doi Y, Minowa M, Uchiyama M, Okawa M, Kim K, Shibui K, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. (2000) 97:165–72. doi: 10.1016/S0165-1781(00)00232-8
14. Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, et al. Suvorexant in patients with insomnia: pooled analyses of three-month data from phase-3 randomized controlled clinical trials. J Clin Sleep Med. (2016) 12:1215–25. doi: 10.5664/jcsm.6116
15. Germain A, Moul DE, Franzen PL, Miewald JM, Reynolds CF, Monk TH, et al. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. J Clin Sleep Med. (2006) 2:403–6. doi: 10.5664/jcsm.26654
16. Joffe H, Partridge A, Giobbie-Hurder A, Li X, Habin K, Goss P, et al. Augmentation of venlafaxine and selective serotonin reuptake inhibitors with zolpidem improves sleep and quality of life in breast cancer patients with hot flashes: a randomized, double-blind, placebo-controlled trial. Menopause. (2010) 17:908–16. doi: 10.1097/gme.0b013e3181dbee1b
17. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern cooperative oncology Group. Am J Clin Oncol. (1982) 5:649–55. doi: 10.1097/00000421-198212000-00014
18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
19. Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm. (2000) 107:1027–63. doi: 10.1007/s007020070051
20. Pouwels PJW, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. (1998) 39:53–60. doi: 10.1002/mrm.1910390110
21. Block W, Träber F, Flacke S, Jessen F, Pohl C, Schild H. In-vivo proton MR-spectroscopy of the human brain: assessment of N-acetylaspartate. (NAA) reduction as a marker for neurodegeneration. Amino Acids. (2002) 23:317–23. doi: 10.1007/s00726-001-0144-0
22. Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. (2002) 82:736–54. doi: 10.1046/j.1471-4159.2002.01041.x
23. Baroncini M, Jissendi P, Catteau-Jonard S, Dewailly D, Pruvo JP, Francke JP, et al. Sex steroid hormones-related structural plasticity in the human hypothalamus. Neuroimage. (2010) 50:428–33. doi: 10.1016/j.neuroimage.2009.11.074
24. Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. (2016) 537:357–62. doi: 10.1038/nature19325
25. Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, et al. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. (1999) 46:473–83. doi: 10.1016/S0006-3223(99)00043-8
26. Cho KH, Cho YJ, Lee BI, Heo K. Atrophy of the pedunculopontine nucleus region in patients with sleep-predominant seizures: a voxel-based morphometry study. Epilepsia. (2016) 57:e151–4. doi: 10.1111/epi.13431
27. Bocchetta M, Gordon E, Manning E, Barnes J, Cash DM, Espak M, et al. Detailed volumetric analysis of the hypothalamus in behavioral variant frontotemporal dementia. J Neurol. (2015) 262:2635–42. doi: 10.1007/s00415-015-7885-2
28. Nakamura Y, Okada N, Kunimatsu A, Kasai K, Koike S. Anatomical templates of the midbrain ventral tegmental area and substantia nigra for Asian populations. Front Psychiatry. (2018) 9:383. doi: 10.3389/fpsyt.2018.00383
29. Vora M, Kumar S, Sharma S, Sharma S, Makhaik S, Sood R. Advanced magnetic resonance neuroimaging in bulbar and limb onset early amyotrophic lateral sclerosis. J Neurosci Rural Pract. (2015) 7:102. doi: 10.4103/0976-3147.165423
30. Zhang J, Lei D, Ma J, Wang M, Shen G, Wang H, et al. Brain metabolite alterations in children with primary nocturnal enuresis using proton magnetic resonance spectroscopy. Neurochem Res. (2014) 39:1355–62. doi: 10.1007/s11064-014-1320-4
31. Zhang L, Xu Y, Zhuang J, Peng H, Wu H, Zhao ZZ, et al. Metabolic abnormality of pontine tegmentum in patients with REM sleep behavior disorder analyzed using magnetic resonance spectroscopy. Clin Neurol Neurosurg. (2016) 148:137–41. doi: 10.1016/j.clineuro.2016.07.002
32. Xin W, Edwards N, Bonci A. VTA dopamine neuron plasticity – the unusual suspects. Eur J Neurosci. (2016) 44:2975–83. doi: 10.1111/ejn.13425
33. Gomez JA, Perkins JM, Beaudoin GM, Cook NB, Quraishi SA, Szoeke EA, et al. Ventral tegmental area astrocytes orchestrate avoidance and approach behavior. Nat Commun. (2019) 10:1455. doi: 10.1038/s41467-019-09131-y
34. Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. (2007) 32:1232–41. doi: 10.1038/sj.npp.1301251
35. Elam HB, Perez SM, Donegan JJ, Lodge DJ. Orexin receptor antagonists reverse aberrant dopamine neuron activity and related behaviors in a rodent model of stress-induced psychosis. Transl Psychiatry. (2021) 11:114. doi: 10.1038/s41398-021-01235-8
36. Van Houdenhove L, Buyse B, Gabriëls L, Van Den Bergh O. Treating primary insomnia: clinical effectiveness and predictors of outcomes on sleep, daytime function and health-related quality of life. J Clin Psychol Med Settings. (2011) 18:312–21. doi: 10.1007/s10880-011-9250-7
37. Lacks P, Powlishta K. Improvement following behavioral treatment for insomnia: clinical significance, long-term maintenance, and predictors of outcome. Behav Ther. (1989) 20:117–34. doi: 10.1016/S0005-7894(89)80122-4
38. Bathgate CJ, Edinger JD, Krystal AD. Insomnia patients with objective short sleep duration have a blunted response to cognitive behavioral therapy for Insomnia. Sleep. (2017) 40: zsw012. doi: 10.1093/sleep/zsw012
39. Lovato N, Lack L, Kennaway DJ. Comparing and contrasting therapeutic effects of cognitive-behavior therapy for older adults suffering from insomnia with short and long objective sleep duration. Sleep Med. (2016) 22:4–12. doi: 10.1016/j.sleep.2016.04.001
40. Espie CA, Inglis SJ, Harvey L. Predicting clinically significant response to cognitive behavior therapy for chronic insomnia in general medical practice: analyses of outcome data at 12 months posttreatment. J Consult Clin Psychol. (2001) 69:58–66. doi: 10.1037/0022-006X.69.1.58
41. Lancee J, Van Den Bout J, Van Straten A, Spoormaker VI. Baseline depression levels do not affect efficacy of cognitive-behavioral self-help treatment for insomnia. Depress Anxiety. (2013) 30:149–56. doi: 10.1002/da.22004
42. Nikulina EM, Johnston CE, Wang J, Hammer RP. Neurotrophins in the ventral tegmental area: Role in social stress, mood disorders and drug abuse. Neuroscience. (2014) 282:122–38. doi: 10.1016/j.neuroscience.2014.05.028
43. Chocyk A, Dudys D, Przyborowska A, Majcher I, Maćkowiak M, Weogonekdzony K. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neuroscience. (2011) 173:1–18. doi: 10.1016/j.neuroscience.2010.11.037
44. Zhu H, Barker PB. MR Spectroscopy and Spectroscopic Imaging of the Brain. In: Baltes C, Princz-Kranz F, Rudin M, Mueggler T. Detecting Amyloid-β Plaques in Alzheimer's Disease. In: Modo M, Bulte JWM. Magnetic Resonance Neuroimaging. New York, NY: Springer (2010). p. 511–533; 203–226. doi: 10.1007/978-1-61737-992-5_26
45. Zhang Y, Larcher KM-H, Misic B, Dagher A. Anatomical and functional organization of the human substantia nigra and its connections. Elife. (2017) 6:1–23. doi: 10.7554/eLife.26653
46. Herring WJ, Connor KM, Ivgy-May N, Snyder E, Liu K, Snavely DB, et al. Suvorexant in patients with Insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. (2016) 79:136–48. doi: 10.1016/j.biopsych.2014.10.003
47. Michelson D, Snyder E, Paradis E, Chengan-Liu M, Snavely DB, Hutzelmann J, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. (2014) 13:461–71. doi: 10.1016/S1474-4422(14)70053-5
Keywords: magnetic resonance spectroscopy, insomnia, suvorexant, orexin, glia, ventral tegmental area, psychiatric patients
Citation: Izuhara M, Miura S, Otsuki K, Nagahama M, Hayashida M, Hashioka S, Asou H, Kitagaki H and Inagaki M (2021) Magnetic Resonance Spectroscopy in the Ventral Tegmental Area Distinguishes Responders to Suvorexant Prior to Treatment: A 4-Week Prospective Cohort Study. Front. Psychiatry 12:714376. doi: 10.3389/fpsyt.2021.714376
Received: 25 May 2021; Accepted: 31 July 2021;
Published: 23 August 2021.
Edited by:
Victor B. Fenik, Albany Medical College, United StatesReviewed by:
Axel Steiger, Ludwig Maximilian University of Munich, GermanyArturo Garay, Centro de Educación Médica e Investigaciones Clínicas Norberto Quirno (CEMIC), Argentina
Copyright © 2021 Izuhara, Miura, Otsuki, Nagahama, Hayashida, Hashioka, Asou, Kitagaki and Inagaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masatoshi Inagaki, bWluYWdha2lAbWVkLnNoaW1hbmUtdS5hYy5qcA==
 Muneto Izuhara
Muneto Izuhara Shoko Miura3
Shoko Miura3 Koji Otsuki
Koji Otsuki Michiharu Nagahama
Michiharu Nagahama Sadayuki Hashioka
Sadayuki Hashioka Hiroya Asou
Hiroya Asou Hajime Kitagaki
Hajime Kitagaki