
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 10 September 2021
Sec. Mood Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.714014
Physical activity may prevent anxiety, but the importance of exercise intensity, sex-specific mechanisms, and duration of the effects remains largely unknown. We used an observational study design to follow 395,369 individuals for up to 21 years to investigate if participation in an ultralong-distance cross-country ski race (Vasaloppet, up to 90 km) was associated with a lower risk of developing anxiety. Skiers in the race and matched non-skiers from the general population were studied after participation in the race using the Swedish population and patient registries. Skiers (n = 197,685, median age 36 years, 38% women) had a significantly lower risk of developing anxiety during the follow-up compared to non-skiers (adjusted hazard ratio, HR 0.42). However, among women, higher physical performance (measured as the finishing time to complete the race, a proxy for higher exercise dose) was associated with an increased risk of anxiety compared to slower skiing women (HR 2.00). For men, the finishing time of the race did not significantly impact the risk of anxiety. Our results support the recommendations of engaging in physical activity to decrease the risk of anxiety in both men and women. The impact of physical performance level on the risk of anxiety requires further investigations among women.
Anxiety disorders are common mental health problems, estimated to currently affect up to 10% of the population globally (1) and twice as common among women compared to men (2, 3). The onset is typically early in life, during childhood, adolescence, or early adulthood. Additionally, co-morbidity with depression or other mental illnesses is common (2). Several reports reveal poorer physical health and shorter life expectancy among patients with anxiety disorders (4, 5). Unfortunately, up to half of the patients do not receive enough symptom relief when treated with first-line treatments, such as selective serotonin reuptake inhibitors (SSRI) or cognitive-behavioral therapy (CBT) (6). Due to the high prevalence, early-onset, and frequency of treatment-resistance among individuals with anxiety disorders, their contribution to years lived with disability and economic burden for society is substantial (7).
Physical activity has been pointed out as a promising strategy to mitigate the burden of anxiety, both when it comes to preventing the disease (8–10) as well as to alleviate the symptoms (11–13). Moreover, increasing the physical activity levels may also improve the physical health among these individuals, thereby reducing their comorbidity with other disorders and increasing their life expectancy.
Little is known when it comes to the impact of exercise dose, intensity or physical fitness level on the risk of developing anxiety disorders (14–17). Further, it remains unclear whether physical activity and fitness impact the risk of developing anxiety disorders equally in men and women. Importantly, several studies indicate that physical activity may affect anxiety levels differently among men and women (3, 10, 18–22).
Contrariwise, there are also studies indicating that physical activity may not reduce anxiety symptoms (23, 24), or at least not as much as psychopharmaceuticals do (23, 25). Further, it has been argued that the association between high physical activity and a lower risk of getting diagnosed with anxiety disorders may be driven by reverse causation (26–29). For instance, anxious symptoms before diagnosis may prevent vulnerable individuals from engaging in physical activity. Indeed, patients diagnosed with anxiety are less active than healthy controls (29–31) and stress has been indicated as a major obstacle for exercise participation according to a meta-analysis including psychiatric patients (32). As most studies in the field are cross-sectional, it is difficult to draw any conclusions regarding the causality in the association between physical activity and anxiety (30, 33–35). A recent meta-analysis including 14 prospective studies revealed that low physical activity predicted future anxiety (9). However, the longest follow-up time was <10 years and a majority of the included studies had <5 years of follow-up, which implicate a significant risk of bias due to reverse causation. Further, in a Swedish national cohort, Hallgren et al. followed over 27 000 middle-aged participants for 13 years (12). In their cross-sectional analysis, higher physical activity was associated with lower odds of anxiety symptoms, but no significant prospective association between activity and subsequent anxiety diagnosis was found. Long follow-up periods are needed and exclusion of individuals diagnosed with mental disorders within the first years after study inclusion to reduce the potential bias due to reverse causation.
We aimed to investigate the association between a physically active lifestyle and future development of anxiety disorders in men and women separately using a population-based cohort with a long-term perspective. Additionally, we investigate the impact of fitness level as a proxy for exercise dose on anxiety. We compared participants in the world's largest long-distance cross-country ski race (Vasaloppet) with matched non-skiers from the general population, to include a total of 395,369 individuals with up to 21 years of follow-up. To the best of our knowledge, the association between a physically active lifestyle and the development of anxiety disorders has not been investigated in such a large study population, including both men and women, with a long follow-up time before.
This observational study design has been described previously (36) and has been approved by the Ethical Review Board in Uppsala, Sweden, (D.nr 2010/305). The study population includes all Swedes who participated in the world's largest long-distance (30–90 km), cross-country ski race (Vasaloppet) between 1989 and 2010 (n = 197,685), together with frequency-matched, individuals from the general population (n = 197,684) (Supplementary Figure 1). Statistics Sweden was used for frequency matching by drawing non-skier controls from the population registry according to region of residency, age group (5-year intervals), sex, and year of participation in ski race as described previously (37). In general, Vasaloppet skiers have higher leisure-time physical activity, smoke less, have a healthier diet, and lower mortality compared to the general Swedish population (38, 39). Individuals with severe disease were excluded as previously described (e.g., cancer, chronic neurologic disease, dementia, heart-, and lung disease) (40) to reduce bias due to the inability to participate in the race because of poor physical health. In addition, we excluded skiers and non-skiers with dementia [all-cause, Alzheimer's disease (AD), vascular dementia (VaD), Parkinson disease dementia, Lewy body dementia, senile dementia], Parkinson disease, meningitis/encephalitis, epilepsy, psychiatric disorders (depressive episode, schizophrenia, bipolar disorder, anxiety disorders, and mental disorders due to the use of alcohol) (see Supplementary Table 1).
In addition to ski race participation, skiers were monitored for finishing time in the race in three categories with finishing time of 100–150, 150–200, and above 200% of the winning finishing time for each sex, respectively. The finishing time analysis was used as a measurement of physical fitness and a proxy for the more extreme doses of exercise. Information on date of birth, sex, and education level was derived from Swedish registries (Swedish National Patient Registry for diagnoses and Statistics Sweden for socio-economic data) (37). The total study cohort (n = 395,369) was followed in the Swedish National Patient Registry (described below) throughout 2010.
The Swedish National Patient Registry was used to retrieve psychiatric and somatic diagnoses. It provides information on all primary and secondary diagnoses in patients attending hospital-based care in Sweden since 1987. The register additionally includes hospital-based out-patient visits since 2001 and covers 99% of all hospital-based diagnoses. Primary care diagnoses are not included in the registry. Anxiety disorders were defined according to the International Classification of Diseases (ICD), tenth revision (ICD10), or ninth revision (ICD9). Diagnoses included are (F40, F41, F42, 300A, 300B, 300C, 300D, 300D, 3000, 3001, 3002, 3003).
R statistical software package was used for analyses. P < 0.05 were considered statistically significant. Demographic data are presented as median and interquartile range (IQR) or numbers (n) and percent (%). Mann-Whitney U tests were used to estimate numeric group differences and categorical group differences were estimated with Pearson's χ2 test. Cox regression models were used to compare the risk of anxiety for skiers vs. non-skiers. The risks of anxiety disorders are presented as hazard ratios (HR) with 95% confidence intervals (CI). Numbers at risk were derived from survival tables specifying the number of individuals entering each 5-year interval, as presented in the graph. The time variable was calculated as years between participation in the ski race (and the same year for the matched non-skier) and event or censoring. The event was an anxiety disorder. Censoring appeared when subjects died or at the time of register outtake. Date of death for deceased study individuals was available through the Causes of Death Register (CDR), held at the National Board of Health and Welfare. Schoenfeld residuals were modeled graphically to assess the proportionality assumption. Men and women were also analyzed separately since sex was suggested to be a possible effect modifier. The impact of finishing time (fitness level) was assessed by trichotomizing the finishing time to 100–150%, 150–00%, and above 200% of the winning finishing time for men and women separately. Adjustments were done for sex, age, and education in the adjusted cox model. In primary sensitivity analyses, all individuals who developed anxiety disorders within 5 years of inclusion were excluded. In additional sensitivity analysis, all individuals who developed any psychiatric disorders (depression, anxiety, schizophrenia, or bipolar disorder, see Supplementary Table 1) within 5 years of inclusion were excluded.
Table 1 shows the demographic data comparing the skiers and non-skiers. A total of 395,369 individuals were followed over 3975,881 person-years. After a median follow-up of 10 (IQR 5–15) years, a total of 1,649 individuals were newly diagnosed with anxiety disorders. Participation in the long-distance ski race was associated with a lower risk of developing anxiety disorders in the follow-up compared to non-skiers (unadjusted HR 0.38, 95% CI 0.34–0.42, Table 2, Figure 1A). Compared to non-skiers, skiers had a higher education than non-skiers (Table 1), but adjustments for age, sex, and education did not alter the results (adjusted cox model, Table 2). The effect remained even when individuals that developed anxiety within 5 years of the ski race (baseline) were excluded (unadjusted HR 0.41, 95% CI 0.36–0.47, Table 2, Figure 1B). Additional sensitivity analysis excluding all individuals who developed any psychiatric disorders within 5 years of inclusion did not alter the results (see Supplementary Table 2). Taken together, skiers in this race had a 62% lower relative risk of getting diagnosed with anxiety disorders compared to matched non-skiers.
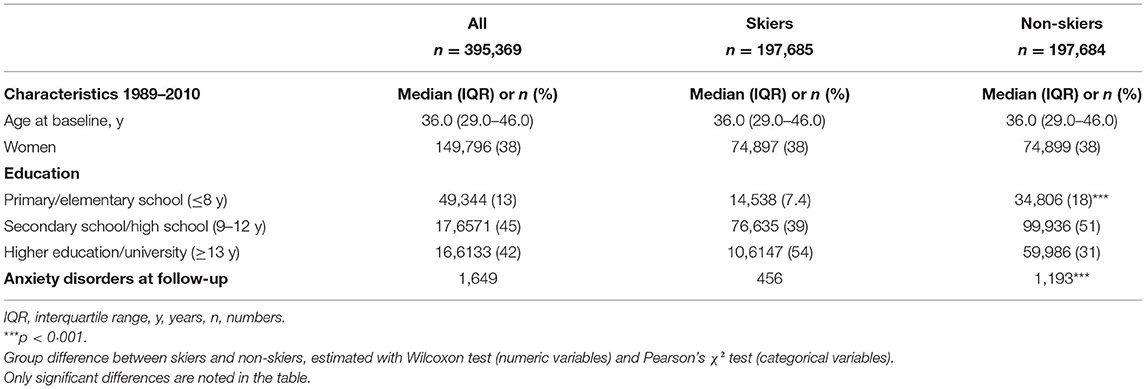
Table 1. Characteristics of the study population, presented for the whole cohort, and by skiers and non-skiers separately.
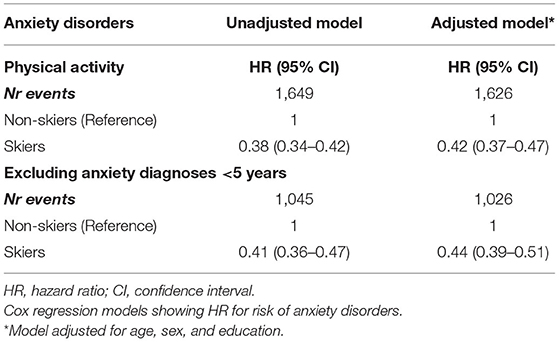
Table 2. Association between physical activity and incident anxiety disorders, based on participation in a long-distance ski race (skiers) compared to non-skiers.
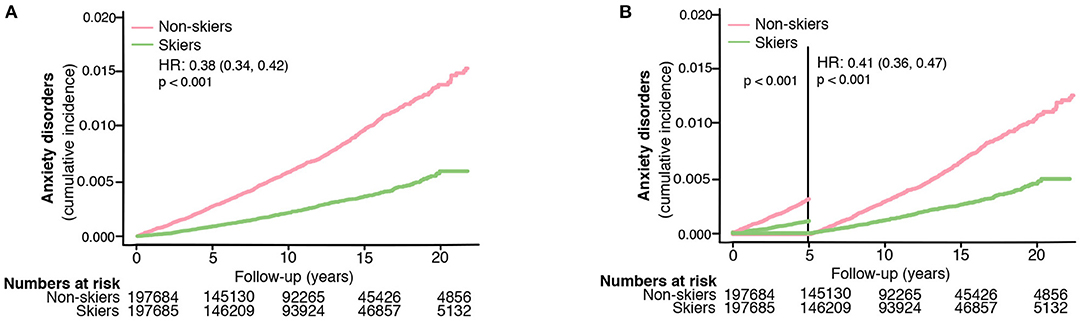
Figure 1. The risk of developing anxiety disorders in skiers compared to non-skiers (A) and the risk of developing anxiety disorders more than 5 years after completing the ski race (B). HR represents hazard ratios from an unadjusted cox regression.
The association between ski race participation and lower incidence of anxiety was seen in both men and women (unadjusted HR 0.37, 95% CI, 0.32–0.43 for men and unadjusted HR 0.39, 95% CI, 0.33–0.46 for women, Figures 2A,B).
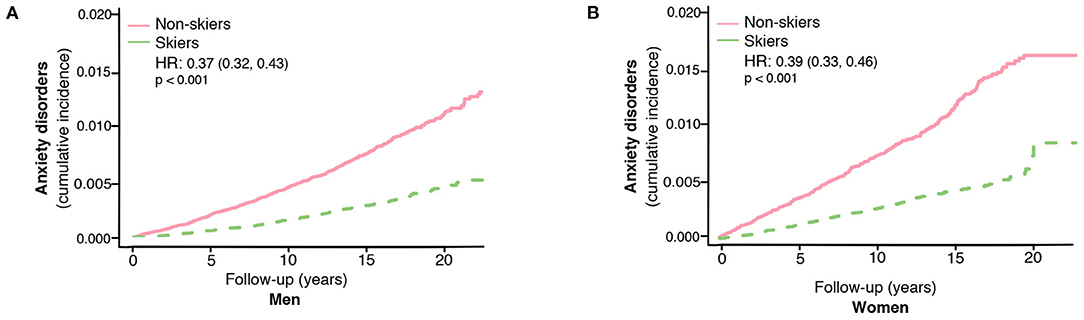
Figure 2. The risk of developing anxiety disorders in skiers compared to non-skiers in men (A) and women separately (B). HR represents hazard ratios from an unadjusted cox regression.
We could not detect any impact of the ski race finishing time (a proxy for the effect of extreme exercise) on the risk of anxiety disorders among skiing men (unadjusted HR 0.73, 95% CI, 0.47–1.12, Table 3, Figure 3A). Opposingly, women completing the race with the shortest finishing time had a higher risk of developing anxiety compared to slower skiers (unadjusted HR 2.00, 95% CI, 1.23–3.26, Table 3, Figure 3B). Adjustments for age and education did not alter the results (adjusted cox model, Table 3). However, this association among the women became non-significant when excluding cases diagnosed with anxiety within the first 5 years (unadjusted HR 1.67, 95% CI, 0.86–3.23 for women, Table 3, Figures 3C,D).
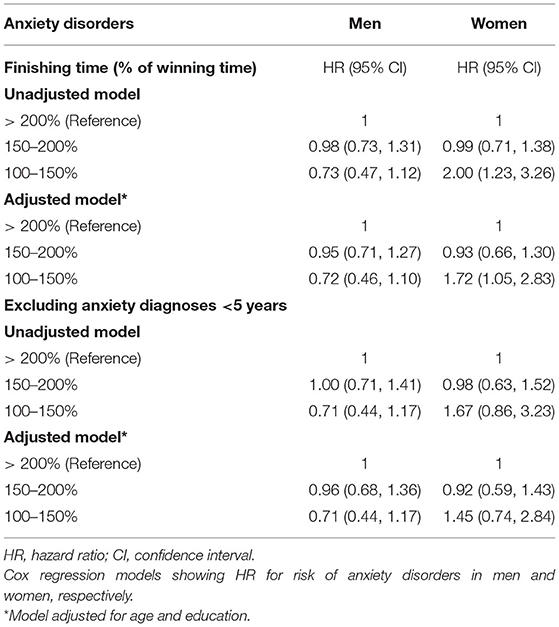
Table 3. Association between ski race finishing time and incident anxiety disorders in men and women.
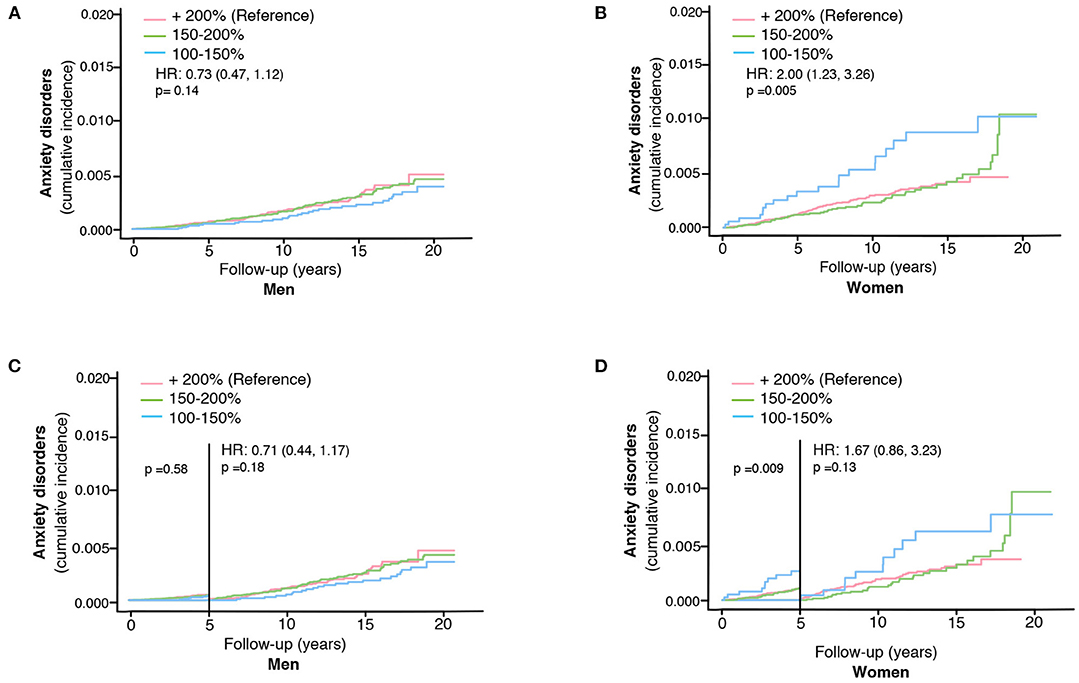
Figure 3. The impact of ski race finishing time on the risk of developing anxiety disorders in skiers in men (A) and women separately (B). The impact of ski race finishing time on the risk of developing anxiety disorders more than 5 years after completing the ski race in men (C) and women (D). HR represents hazard ratios from an unadjusted cox regression for the fastest (100–150% of winning finishing time) group, using +200% as the reference group.
We found that having a physically active lifestyle (being a skier) is associated with around 60% lower risk of developing anxiety disorders compared to matched individuals from the general population in an observational study following almost 400,000 individuals for up to 21 years. Our results were the same when excluding all individuals diagnosed with anxiety disorders within the first 5 years after study inclusion. Moreover, analysis of ski race finishing time (a proxy for the level of fitness) revealed a sex-specific association between the dose of exercise and incident anxiety.
Importantly, our study offers new knowledge about how a physically active lifestyle may affect the development of anxiety disorders in both men and women, adding to the findings made by Nyberg et al. They found low cardiovascular fitness to be associated with a higher risk of getting diagnosed with anxiety disorders in their study with up to 42-year follow-up of over 1 million Swedish men (8). As their study does not include women and as physical activity has been suggested to affect the risk of anxiety differently in men and women, our study adds important knowledge. We found participation in the ski race to be associated with a long-term lower risk of developing anxiety disorders in both men and women. This association remained when cases diagnosed within the first 5 years following inclusion were excluded.
We are not able to investigate the mechanisms behind the potential protective effects of exercise on the development of anxiety in our study. Nevertheless, several studies have tried to elucidate this. The ability of physical activity to pre-occupy the mind and offer distraction from other, potentially anxious, thoughts may explain its beneficial effects (41). As such, the natural environment during cross-country skiing may be specifically beneficial (42). Interestingly, physical activity has been shown to shift the recruitment of neurons in the rodent striatum during aversive events from those expressing dopamine D2 receptors, involved in stress vulnerability, toward others expressing D1 receptors involved in reward and stress resilience (43). Further, many patients with anxiety disorders have abnormal cortisol response after stress (44), and individuals with higher cardiovascular fitness or randomized to be physically active before being subjected to stress have a lower cortisol response (45). Moreover, exercise may reduce inflammation (46) and oxidative stress (47), systems suggested to be linked to anxiety (47–51), albeit investigated to a lesser extent compared to their involvement in depressive disorders (52–55). Exercise is also a well-known inducer of brain-derived neurotrophic growth factor (BDNF), which appears to be decreased in patients with anxiety disorders (56) and increasing levels have been linked to reduced anxiety in rodents following exercise (57, 58). However, the BDNF response to exercise seems to vary based on BDNF gene polymorphisms (59) and sex (58, 60, 61), where women tend to have less increase in BDNF following exercise (60, 61).
Interestingly, we found differences between men and women when analyzing the impact of finishing time of the ski race (a proxy for extreme exercise or higher fitness level) on the risk of anxiety disorders. Among male skiers, finishing time did not significantly affect the risk of developing anxiety disorders. However, among women, fast skiing was associated with a 2-fold higher risk of developing anxiety disorders compared to being a slower skier. Importantly, the cumulative incidence of anxiety disorders among fast skiing women was still lower than that of the matched non-skiing females from the general population. Thus, on a group level, physically high-performing women (fast skiers) may still benefit from a physically active lifestyle even though the optimal dose of exercise may be lower. To the best of our knowledge, this association between physical performance and the risk for anxiety disorders in women specifically has not been reported before. Previous studies have indicated that the effect of physical activity on anxiety may differ between men and women (3, 10, 18–22), but results are rather inconclusive. Some studies suggest that physical activity may have more pronounced effects against anxiety among women (21, 22, 62), whereas others report the opposite (10, 19). Interestingly, the impact of physical performance (being a fast skier) on the risk of anxiety disorders differs between male and female skiers in our study. Nyberg et al. reported that lower physical fitness was associated with a higher risk of developing anxiety disorders in their study, but that study only included males.
Even though our study does not investigate why faster skiing is associated with an increased risk of developing anxiety compared to slower skiers among women, possible reasons behind this has been discussed previously. For example, it may be caused by differences in the physiological response to exercise, where women have reported greater stress and exhaustion following exercise (19). Asztalos et al. suggested that the optimal intensity of physical activity to improve mental health may be high for men and milder for women (63). However, another study reveals a more beneficial effect of exercise on state anxiety in women if exercise was performed at a higher intensity (64). A possible explanation to the higher risk of anxiety among the fast skiing women in our study could be that confounding psychological factors linked to anxiety may be more frequent among these high-performing female skiers. For instance, appearance anxiety is more common among female exercisers (20, 65). Further, the individual's self-perception of physical fitness may correlate better with anxiety than the actual fitness level (66). These factors were not possible to investigate in our study, but female runners with pronounced physique anxiety are at higher risk for developing exercise dependence (67). Hence, psychological factors may drive a high exercise level in some of the high performing female skiers and this may be the reason behind their higher risk of anxiety. Thus, the relation between symptoms of anxiety and exercise behavior may not be linear. Even though many studies indicate that anxiety may prevent people from engaging in physical activity (30–32), it is also possible that symptoms linked to anxiety drive certain persons to exert more extreme exercise behaviors and this may be more pronounced among women (67). Consequently, the increased physical performance among these women may rather be a symptom of already present anxiety than causing anxiety disorders per se. Importantly, this association between faster skiing and higher risk for anxiety disorders among women becomes non-significant if individuals diagnosed during the first 5 years after inclusion are excluded. This indicates that this association may, at least to some extent, be driven by reverse causation. Studies investigating the driving factors behind these differences between men and women when it comes to extreme exercise behaviors are needed. In our recently published study on the development of depression in this study population, we saw a similar pattern regarding the difference in the impact of fast skiing on the risk for future depression among men and women (36). Future studies considering the impact of exercise intensity on the risk of developing anxiety disorders in men and women separately are warranted, especially with designs allowing for conclusions about directionality and causality of the association between physical activity and anxiety as our study design does not allow for these conclusions. An ongoing trial with exercise interventions of different intensities as a treatment for patients already diagnosed with anxiety will hopefully increase our knowledge regarding this within the near future (68).
Limitations of the study include that the physical activity level is not the only factor distinguishing our skiing population from their matched non-skiers in the general population. This population of skiers smokes less and has a better diet compared to the control population of non-skiers (38, 39). We were not able to control for this as we lack data on this for the majority of the participants. However, the results were not altered when we adjusted for age, sex, and education. Moreover, we do not have any detailed information about the physical activity in our cohort. We use baseline participation in the long-distance ski race (30–90 km) as a proxy of a physically active lifestyle. The race is physically demanding and requires preparatory exercise long term before the race. Nevertheless, it is possible that the reference group of non-skiers to some extent include physically active and this may attenuate the true association. Still, the participants in this ski race have reported a higher average time spent with physical activity than the matched non-skiing population (38, 39). Furthermore, as outcome measurement, we use anxiety diagnoses registered in the national wide patient registry. Although this registry is one of the largest in the world, and that diagnoses set in the primary care are likely to be imported into this registry given our long follow-up time, our data will only contain diagnoses and not the presence of anxiety symptoms. This means that our study does not consider the impact of symptoms related to undiagnosed anxiety disorders, which still may impact life quality and lifestyle physical activity. However, to reduce the influence of reverse causation on our results, we excluded individuals already diagnosed with severe disorders that may prevent their participation in the ski race. In our sensitivity analysis we additionally excluded those diagnosed with anxiety or other psychiatric disorders during the first 5 years after inclusion. Nonetheless, it is not possible to eliminate other factors that may lead to reverse causation, such as the influence of individual personality traits to exercise engagement and anxiety disorder vulnerability (11, 21, 26, 69). Therefore, we identify a need for future studies to gain deeper knowledge about the impact of these confounding psychological factors, taking both environmental, genetic, and epigenetic background into account.
In conclusion, our study setup offered a unique possibility to study the effect of a physically active lifestyle on the development of anxiety disorders by following 395,369 individuals during a period of up to 21 years and analyzing diagnoses set in the Swedish patient registry. We found that having a physically active lifestyle (being a skier) is associated with a substantially lower risk of developing anxiety disorders among both men and women.
To the best of our knowledge, this is the largest population-based study to date, confirming a long-term association of a physically active lifestyle on the later development of anxiety disorders in both men and women seen in previous studies with shorter follow up times. Our results suggest that the preventive effects of physical activity on anxiety disorders may be greater than previously reported. Randomized intervention trials, as well as long-term objective measurements of physical activity in prospective studies, are required to assess the validity and causality of this association.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical Review Board in Uppsala, Sweden. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MS drafted the article, interpreted the results, and prepared the figures and tables. UH and SJ was responsible for setting up the Vasaloppet Registry. TD drafted the idea of our study. All authors participated in the discussion about how to analyse and interpret the results as well as critically revising the manuscript.
We were funded by the Strategic Research Area MultiPark (Multidisciplinary Research in neurodegenerative diseases) at Lund University, the Swedish Alzheimer foundation, the Swedish Brain Foundation, Crafoord Foundation, Swedish Dementia Association, G&J Kock Foundation, Olle Engkvist Foundation, the Swedish Medical Research Council, the Swedish Parkinson Foundation, the A.E. Berger Foundation, the Thurings Foundation, and the Swedish mental health foundation. LB was supported by the National Institutes of Mental Health and the MJ Fox Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to Vasaloppet Registry for providing us with the research material.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.714014/full#supplementary-material
1. Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med. (2013) 43:897–910. doi: 10.1017/S003329171200147X
3. Brunes A, Gudmundsdottir SL, Augestad LB. Gender-specific associations between leisure-time physical activity and symptoms of anxiety: the HUNT study. Soc Psychiatry Psychiatr Epidemiol. (2015) 50:419–27. doi: 10.1007/s00127-014-0915-z
4. Kandola A, Vancampfort D, Herring M, Rebar A, Hallgren M, Firth J, et al. Moving to beat anxiety: epidemiology and therapeutic issues with physical activity for anxiety. Curr Psychiatry Rep. (2018) 20:63. doi: 10.1007/s11920-018-0923-x
5. Henriksson M, Nyberg J, Schioler L, Hensing G, Kuhn GH, Soderberg M, et al. Cause-specific mortality in Swedish males diagnosed with non-psychotic mental disorders in late adolescence: a prospective population-based study. J Epidemiol Community Health. (2018) 72:582–8. doi: 10.1136/jech-2018-210461
6. Patterson B, Van Ameringen M. Augmentation strategies for treatment-resistant anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. (2016) 33:728–36. doi: 10.1002/da.22525
7. Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. (2016) 3:171–8. doi: 10.1016/S2215-0366(15)00505-2
8. Nyberg J, Henriksson M, Aberg MAI, Rosengren A, Soderberg M, Aberg ND, et al. Cardiovascular fitness in late adolescent males and later risk of serious non-affective mental disorders: a prospective, population-based study. Psychol Med. (2018) 48:416–25. doi: 10.1017/S0033291717001763
9. Schuch FB, Stubbs B, Meyer J, Heissel A, Zech P, Vancampfort D, et al. Physical activity protects from incident anxiety: a meta-analysis of prospective cohort studies. Depress Anxiety. (2019) 36:846–58. doi: 10.1002/da.22915
10. Strohle A, Hofler M, Pfister H, Muller AG, Hoyer J, Wittchen HU, et al. Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychol Med. (2007) 37:1657–66. doi: 10.1017/S003329170700089X
11. Kroencke L, Harari GM, Katana M, Gosling SD. Personality trait predictors and mental well-being correlates of exercise frequency across the academic semester. Soc Sci Med. (2019) 236:112400. doi: 10.1016/j.socscimed.2019.112400
12. Hallgren M, Nguyen TT, Herring MP, McDowell CP, Gordon BR, Stubbs B, et al. Associations of physical activity with anxiety symptoms and disorders: findings from the Swedish National March Cohort. Gen Hosp Psychiatry. (2019) 58:45–50. doi: 10.1016/j.genhosppsych.2019.03.001
13. Ensari I, Greenlee TA, Motl RW, Petruzzello SJ. Meta-analysis of acute exercise effects on state anxiety: an update of randomized controlled trials over the past 25 years. Depress Anxiety. (2015) 32:624–34. doi: 10.1002/da.22370
14. Dunn AL, Trivedi MH, O'Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. (2001) 33(6 Suppl):S587–97; discussion 609–10. doi: 10.1097/00005768-200106001-00027
15. Baumeister SE, Leitzmann MF, Bahls M, Dorr M, Schmid D, Schomerus G, et al. Associations of leisure-time and occupational physical activity and cardiorespiratory fitness with incident and recurrent major depressive disorder, depressive symptoms, and incident anxiety in a general population. J Clin Psychiatry. (2017) 78:e41–e7. doi: 10.4088/JCP.15m10474
16. Loprinzi PD, Addoh O, Wong Sarver N, Espinoza I, Mann JR. Cross-sectional association of exercise, strengthening activities, and cardiorespiratory fitness on generalized anxiety, panic and depressive symptoms. Postgrad Med. (2017) 129:676–85. doi: 10.1080/00325481.2017.1336054
17. Carmack CL, Boudreaux E, Amaral-Melendez M, Brantley PJ, de Moor C. Aerobic fitness and leisure physical activity as moderators of the stress-illness relation. Ann Behav Med. (1999) 21:251–7. doi: 10.1007/BF02884842
18. Bhui K, Fletcher A. Common mood and anxiety states: gender differences in the protective effect of physical activity. Soc Psychiatry Psychiatr Epidemiol. (2000) 35:28–35. doi: 10.1007/s001270050005
19. Medina JL, DeBoer LB, Davis ML, Rosenfield D, Powers MB, Otto MW, et al. Gender moderates the effect of exercise on anxiety sensitivity. Ment Health Phys Act. (2014) 7:147–51. doi: 10.1016/j.mhpa.2014.08.002
20. Portman RM, Bradbury J, Lewis K. Social physique anxiety and physical activity behaviour of male and female exercisers. Eur J Sport Sci. (2018) 18:257–65. doi: 10.1080/17461391.2017.1417485
21. Brunes A, Augestad LB, Gudmundsdottir SL. Personality, physical activity, and symptoms of anxiety and depression: the HUNT study. Soc Psychiatry Psychiatr Epidemiol. (2013) 48:745–56. doi: 10.1007/s00127-012-0594-6
22. Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. (2015) 9:366–78. doi: 10.1080/17437199.2015.1022901
23. Bartley CA, Hay M, Bloch MH. Meta-analysis: aerobic exercise for the treatment of anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 45:34–9. doi: 10.1016/j.pnpbp.2013.04.016
24. Mailey EL, Wojcicki TR, Motl RW, Hu L, Strauser DR, Collins KD, et al. Internet-delivered physical activity intervention for college students with mental health disorders: a randomized pilot trial. Psychol Health Med. (2010) 15:646–59. doi: 10.1080/13548506.2010.498894
25. Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. (2011) 41:15–28. doi: 10.2190/PM.41.1.c
26. Hearon BA, Harrison TJ. Not the exercise type? Personality traits and anxiety sensitivity as predictors of objectively measured physical activity and sedentary time. J Health Psychol. (2020) 2020:1359105320906242. doi: 10.1177/1359105320906242
27. Broman-Fulks JJ, Abraham CM, Thomas K, Canu WH, Nieman DC. Anxiety sensitivity mediates the relationship between exercise frequency and anxiety and depression symptomology. Stress Health. (2018) 34:500–8. doi: 10.1002/smi.2810
28. Moshier SJ, Szuhany KL, Hearon BA, Smits JA, Otto MW. Anxiety sensitivity uniquely predicts exercise behaviors in young adults seeking to increase physical activity. Behav Modif. (2016) 40:178–98. doi: 10.1177/0145445515603704
29. Hiles SA, Lamers F, Milaneschi Y, Penninx B. Sit, step, sweat: longitudinal associations between physical activity patterns, anxiety and depression. Psychol Med. (2017) 47:1466–77. doi: 10.1017/S0033291716003548
30. Stubbs B, Koyanagi A, Hallgren M, Firth J, Richards J, Schuch F, et al. Physical activity and anxiety: a perspective from the World Health Survey. J Affect Disord. (2017) 208:545–52. doi: 10.1016/j.jad.2016.10.028
31. Difrancesco S, Lamers F, Riese H, Merikangas KR, Beekman ATF, van Hemert AM, et al. Sleep, circadian rhythm, and physical activity patterns in depressive and anxiety disorders: A 2-week ambulatory assessment study. Depress Anxiety. (2019) 36:975–86. doi: 10.1002/da.22949
32. Firth J, Rosenbaum S, Stubbs B, Gorczynski P, Yung AR, Vancampfort D. Motivating factors and barriers towards exercise in severe mental illness: a systematic review and meta-analysis. Psychol Med. (2016) 46:2869–81. doi: 10.1017/S0033291716001732
33. Khanzada FJ, Soomro N, Khan SZ. Association of physical exercise on anxiety and depression amongst adults. J Coll Physicians Surg Pak. (2015) 25:546–8.
34. Mucke M, Ludyga S, Colledge F, Gerber M. Influence of regular physical activity and fitness on stress reactivity as measured with the trier social stress test protocol: a systematic review. Sports Med. (2018) 48:2607–22. doi: 10.1007/s40279-018-0979-0
35. Teychenne M, Costigan SA, Parker K. The association between sedentary behaviour and risk of anxiety: a systematic review. BMC Public Health. (2015) 15:513. doi: 10.1186/s12889-015-1843-x
36. Svensson M, Brundin L, Erhardt S, Madaj Z, Hallmarker U, James S, et al. Long distance ski racing is associated with lower long-term incidence of depression in a population based, large-scale study. Psychiatry Res. (2019) 281:112546. doi: 10.1016/j.psychres.2019.112546
37. Hallmarker U, Lindback J, Michaelsson K, Arnlov J, Asberg S, Wester P, et al. Survival and incidence of cardiovascular diseases in participants in a long-distance ski race (Vasaloppet, Sweden) compared with the background population. Eur Heart J Qual Care Clin Outcomes. (2018) 4:91–7. doi: 10.1093/ehjqcco/qcy005
38. Farahmand BY, Ahlbom A, Ekblom O, Ekblom B, Hallmarker U, Aronson D, et al. Mortality amongst participants in Vasaloppet: a classical long-distance ski race in Sweden. J Intern Med. (2003) 253:276–83. doi: 10.1046/j.1365-2796.2003.01122.x
39. Carlsson S, Olsson L, Farahmand BY, Hallmarker U, Ahlbom A. [Skiers in the long-distance ski race invest in their health]. Lakartidningen. (2007) 104:670–1.
40. Hallmarker U, Michaelsson K, Arnlov J, Hellberg D, Lagerqvist B, Lindback J, et al. Risk of recurrent ischaemic events after myocardial infarction in long-distance ski race participants. Eur J Prev Cardiol. (2016) 23:282–90. doi: 10.1177/2047487315578664
41. Mikkelsen K, Stojanovska L, Polenakovic M, Bosevski M, Apostolopoulos V. Exercise and mental health. Maturitas. (2017) 106:48–56. doi: 10.1016/j.maturitas.2017.09.003
42. Berto R. The role of nature in coping with psycho-physiological stress: a literature review on restorativeness. Behav Sci. (2014) 4:394–409. doi: 10.3390/bs4040394
43. Greenwood BN. The role of dopamine in overcoming aversion with exercise. Brain Res. (2019) 1713:102–8. doi: 10.1016/j.brainres.2018.08.030
44. Zorn JV, Schur RR, Boks MP, Kahn RS, Joels M, Vinkers CH. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology. (2017) 77:25–36. doi: 10.1016/j.psyneuen.2016.11.036
45. Wood CJ, Clow A, Hucklebridge F, Law R, Smyth N. Physical fitness and prior physical activity are both associated with less cortisol secretion during psychosocial stress. Anxiety Stress Coping. (2018) 31:135–45. doi: 10.1080/10615806.2017.1390083
46. Svensson M, Lexell J, Deierborg T. Effects of physical exercise on neuroinflammation, neuroplasticity, neurodegeneration, and behavior: what we can learn from animal models in clinical settings. Neurorehabil Neural Repair. (2015) 29:577–89. doi: 10.1177/1545968314562108
47. Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. (2010) 208:545–52. doi: 10.1016/j.bbr.2009.12.039
48. Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. (1998) 10:313–8. doi: 10.1006/cyto.1997.0290
49. Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, et al. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. (2001) 6:475–80. doi: 10.1038/sj.mp.4000872
50. Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. (2010) 68:261–75. doi: 10.1016/j.neures.2010.08.007
51. Moylan S, Eyre HA, Maes M, Baune BT, Jacka FN, Berk M. Exercising the worry away: how inflammation, oxidative and nitrogen stress mediates the beneficial effect of physical activity on anxiety disorder symptoms and behaviours. Neurosci Biobehav Rev. (2013) 37:573–84. doi: 10.1016/j.neubiorev.2013.02.003
52. Hallberg L, Janelidze S, Engstrom G, Wisen AG, Westrin A, Brundin L. Exercise-induced release of cytokines in patients with major depressive disorder. J Affect Disord. (2010) 126:262–7. doi: 10.1016/j.jad.2010.02.133
53. Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. (2011) 16:751–62. doi: 10.1038/mp.2010.52
54. Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. (2014) 45:77–86. doi: 10.1016/j.psyneuen.2014.03.019
55. Wang AK, Miller BJ. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. (2018) 44:75–83. doi: 10.1093/schbul/sbx035
56. Suliman S, Hemmings SM, Seedat S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front Integr Neurosci. (2013) 7:55. doi: 10.3389/fnint.2013.00055
57. Lapmanee S, Charoenphandhu J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N. Agomelatine, venlafaxine, and running exercise effectively prevent anxiety- and depression-like behaviors and memory impairment in restraint stressed rats. PLoS ONE. (2017) 12:e0187671. doi: 10.1371/journal.pone.0187671
58. Onakomaiya MM, Porter DM, Oberlander JG, Henderson LP. Sex and exercise interact to alter the expression of anabolic androgenic steroid-induced anxiety-like behaviors in the mouse. Horm Behav. (2014) 66:283–97. doi: 10.1016/j.yhbeh.2014.04.008
59. Smits JA, Powers MB, Rosenfield D, Zvolensky MJ, Jacquart J, Davis ML, et al. BDNF Val66Met polymorphism as a moderator of exercise enhancement of smoking cessation treatment in anxiety vulnerable adults. Ment Health Phys Act. (2016) 10:73–7. doi: 10.1016/j.mhpa.2016.01.001
60. Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. (2015) 60:56–64. doi: 10.1016/j.jpsychires.2014.10.003
61. Dinoff A, Herrmann N, Swardfager W, Lanctot KL. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci. (2017) 46:1635–46. doi: 10.1111/ejn.13603
62. McDowell CP, Campbell MJ, Herring MP. Sex-related differences in mood responses to acute aerobic exercise. Med Sci Sports Exerc. (2016) 48:1798–802. doi: 10.1249/MSS.0000000000000969
63. Asztalos M, De Bourdeaudhuij I, Cardon G. The relationship between physical activity and mental health varies across activity intensity levels and dimensions of mental health among women and men. Public Health Nutr. (2010) 13:1207–14. doi: 10.1017/S1368980009992825
64. Cox RH, Thomas TR, Hinton PS, Donahue OM. Effects of acute 60 and 80% VO2max bouts of aerobic exercise on state anxiety of women of different age groups across time. Res Q Exerc Sport. (2004) 75:165–75. doi: 10.1080/02701367.2004.10609148
65. Corazza O, Simonato P, Demetrovics Z, Mooney R, van de Ven K, Roman-Urrestarazu A, et al. The emergence of exercise addiction, body dysmorphic disorder, and other image-related psychopathological correlates in fitness settings: a cross sectional study. PLoS ONE. (2019) 14:e0213060. doi: 10.1371/journal.pone.0213060
66. Abadie BR. Relating trait anxiety to perceived physical fitness. Percept Mot Skills. (1988) 67:539–43. doi: 10.2466/pms.1988.67.2.539
67. Cook B, Karr TM, Zunker C, Mitchell JE, Thompson R, Sherman R, et al. The influence of exercise identity and social physique anxiety on exercise dependence. J Behav Addict. (2015) 4:195–9. doi: 10.1556/2006.4.2015.020
68. Nyberg J, Henriksson M, Aberg ND, Wall A, Eggertsen R, Westerlund M, et al. Effects of exercise on symptoms of anxiety, cognitive ability and sick leave in patients with anxiety disorders in primary care: study protocol for PHYSBI, a randomized controlled trial. BMC Psychiatry. (2019) 19:172. doi: 10.1186/s12888-019-2169-5
Keywords: exercise, psychiatric disorders, mental health, women, men, long-term effect
Citation: Svensson M, Brundin L, Erhardt S, Hållmarker U, James S and Deierborg T (2021) Physical Activity Is Associated With Lower Long-Term Incidence of Anxiety in a Population-Based, Large-Scale Study. Front. Psychiatry 12:714014. doi: 10.3389/fpsyt.2021.714014
Received: 24 May 2021; Accepted: 03 August 2021;
Published: 10 September 2021.
Edited by:
Suraj Bahadur Thapa, University of Oslo, NorwayReviewed by:
Felice Iasevoli, University of Naples Federico II, ItalyCopyright © 2021 Svensson, Brundin, Erhardt, Hållmarker, James and Deierborg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina Svensson, bWFydGluYS5zdmVuc3NvbkBtZWQubHUuc2U=; Tomas Deierborg, dG9tYXMuZGVpZXJib3JnQG1lZC5sdS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.