- 1Department of Psychiatry, Chaohu Hospital, Anhui Medical University, Hefei, China
- 2School of Mental Health and Psychological Sciences, Anhui Medical University, Hefei, China
- 3Maanshan Fourth People's Hospital, Maanshan, China
Objectives: Leptin is a crucial regulator of energy balance and is associated with obesity. In recent years, it has also been recognized as involved in the psychopathological mechanism. Our study aimed to elucidate the relationships between serum leptin levels, body mass index (BMI), and psychopathology symptoms in patients with schizophrenia.
Methods: A cross-sectional assessment of 324 inpatients with schizophrenia was conducted. Schizophrenia symptoms were measured using the Positive and Negative Syndrome Scale (PANSS) and the Brief Psychiatric Rating Scale (BPRS). Serum leptin levels were assessed by the Enzyme-Linked Immunosorbent Assay (ELISA).
Results: Significant differences in sex, BMI, and negative symptom subscale (PANSS-N) scores were found between the groups with high and low leptin levels in the study. Leptin levels were positively correlated with BMI (B = 2.322, t = 9.557, P < 0.001) and negatively correlated with PANSS-N scores (B = −0.303, t = −2.784, P = 0.006).
Conclusions: Our results suggest that the increase in leptin levels is responsible for antipsychotic-induced weight gain and improved psychopathological symptoms.
Introduction
Schizophrenia is a chronic syndrome with a variety of clinical symptoms and biological characteristics (1), and its symptoms are usually divided into three categories (general psychopathological symptoms and positive and negative symptoms) (2). The life expectancy of people with schizophrenia is about 11–20 years shorter than that of the general population, and the average life expectancy is about 80–85% of that of the general population. Studies show that most people with schizophrenia die from complications such as cardiovascular disease. This phenomenon is mainly due to the higher risk of weight gain and other adverse metabolic effects caused by antipsychotics in patients with schizophrenia (3–5). Although the pathophysiological mechanisms of metabolic disorders, including weight gain induced by antipsychotics, remain unclear, associations between metabolic changes and psychopathological symptoms have been reported (6).
With the increasing use of second-generation antipsychotics (SGA), metabolic side effects are becoming more common, such as weight gain, changes in blood lipids, and glucose intolerance (7). Among second-generation antipsychotics, weight gain is the most common metabolic side effect in patients with schizophrenia who take these medications. The obesity rate is about 26–55% of mental disorders, 4.3 times higher than that of the general population (8). A meta-analysis comparing the efficacy of antipsychotics found that clozapine and olanzapine, while more effective than other drugs, were also more likely to cause weight gain than other drugs (9). Furthermore, the weight gain induced by olanzapine was dose-dependent in the short term after treatment (10). There is some evidence that antipsychotics with a faster track of weight gain during early treatment (such as olanzapine and clozapine) are more likely to gain weight than drugs with a slower track of weight gain. This phenomenon is significantly associated with clinical efficacy (11, 12). Ziprasidone and aripiprazole were also associated with weight gain in patients with schizophrenia who were first treated with antipsychotics (13, 14). However, the weight gain effect induced by aripiprazole was significantly lower than that of olanzapine (15). Pimavanserin is adjuvant clozapine, can significantly increase the weight of patients (16, 17). In addition, first-generation antipsychotics (FGA), such as chlorpromazine and thiouracil, have also been found to cause significant weight gain (18). Therefore, there is reason to suspect that the early clinical efficacy of the drug is associated with an increased cardiovascular burden.
Weight gain caused by medication, if not treated and managed, may lead to more serious consequences, that is, metabolic syndrome (19). Although clozapine and olanzapine have unique advantages in treating refractory schizophrenia, they are most likely to cause metabolic abnormalities (20). Clozapine and olanzapine also directly increase the risk of hyperlipidemia and hypertension and are unrelated to their effects on obesity and glucose tolerance (21, 22). Clozapine, in particular, increases the above risks (23). These risks will increase rapidly in a short time after treatment, endangering the lives of patients (24).
If there is a correlation between antipsychotic-induced weight gain and treatment effectiveness, the nature of the relationship is worth exploring. There are three hypotheses for this link:
1. The weight gain of patients directly improves the symptoms during treatment.
2. Clinical efficacy will lead to weight gain.
3. Antipsychotics induce weight gain and symptom improvement in patients in (a) interdependent/interdependent or (b) independent/mutually exclusive manner.
This means:
1. Antipsychotic-induced weight gain may be necessary for treatment effectiveness.
2. Weight gain caused by antipsychotics is a side effect that can be safely targeted without affecting the final therapeutic effect (e.g., lifestyle or drug intervention).
Leptin is an anorexic hormone produced by adipocytes and whose levels increase with weight gain (25). The energy-related role of leptin has been widely studied. As a satiety factor, leptin plays a vital role in maintaining energy balance by interacting with neural pathways in the brain, especially those involving the hypothalamus (26). Moreover, there was evidence that leptin promotes cognitive and behavioral functions in both rodents and humans (27). Recently, attention has been focused on the effect of leptin on psychopathological symptoms in patients with schizophrenia.
Antipsychotics can increase body weight, with a concurrent increase in leptin levels. Several studies have reported a positive relationship between weight gain and clinical status improvement after treatment. In addition, elevated serum leptin levels have been positively correlated with overall psychopathological improvement and are considered a predictor of clinical improvement (28). Although there are suggestions that leptin may be a biomarker for the prognosis of schizophrenia, it remains unclear whether this hormone is associated with particular psychopathological parameters. Few studies have explored leptin changes in patients with psychopathologies, especially in Chinese patients with schizophrenia. Therefore, our research aims to explore (1) serum leptin levels in patients with schizophrenia and (2) whether there was a relationship between serum leptin levels, BMI, and psychopathological parameters.
Materials and Methods
Participants
We collected data on inpatients with schizophrenia from three hospitals in Anhui Province (the Chaohu Hospital of Anhui Medical University, Hefei Fourth People's Hospital, and Ma'an shan Fourth People's Hospital). The obesity rate in schizophrenia is estimated at 20% and in the general population at 10% (29), with a prevalence difference of about 10% between the two groups. Considering the following relevant parameters, α = 0.05, 1–β = 0.82, R = 0.5, 323 patients should be included through PASS11 calculation.
The inclusion criteria were as follows: (1) patients aged 18–75 years; (2) patients diagnosed with schizophrenia using the International Classification of Diseases, 10th Revision (ICD-10); (3) those with a disease duration of more than 5 years and was hospitalized for more than 1 month and (4) those with the ability to provide written informed consent and participate in psychopathology assessments. The exclusion criteria were as follows: (1) patients diagnosed with other mental disorders using the ICD-10; (2) those with severe physical illnesses, including severe neuroendocrine or metabolic disease; (3) those who abuse alcohol or other substances; and (4) pregnant or lactating women.
The study initially involved 443 patients, and 324 were eventually included, with an effective rate of 73.14%. Of the 119 excluded patients, 85 refused to participate in further interviews, nine refused to provide blood samples, nine were discharged from the hospital, and 16 had incomplete blood test data. In general, the study included three groups of people: (1) medically stable patients who chose to be hospitalized for family reasons; (2) patients who relapse due to irregular drug use; and (3) long-term hospitalized patients with unstable conditions. All aspects of the study protocol were reviewed and approved by the ethics. All of the participants provided written informed consent to participate in this study.
Demographic Data
The general information was collected by query and recorded on the questionnaires by professional psychiatrists trained for the task according to a uniform set of criteria. Height (m) and weight (kg) were measured. Researchers reviewed the histories of schizophrenia inpatients. We divided the subjects into three groups according to their current medication status, including the typical antipsychotics group (such as sulpiride, haloperidol), the atypical antipsychotics group (such as olanzapine, clozapine, risperidone, quetiapine, ziprasidone, aripiprazole, amisulpride), and the combination group (use both types of antipsychotics). Using the defined daily dose (DDD) recommended by the WHO as an indicator, each drug's equivalent dose of chlorpromazine was calculated for further analysis (30).
Clinical Assessment
To verify the diagnosis of schizophrenia, two independent psychiatrists conducted the Structured Clinical Interview for the ICD-10 with each patient. The psychopathological symptoms were evaluated using the Positive and Negative Syndrome Scale (PANSS) and the Brief Psychiatric Rating Scale (BPRS). When interrater inconsistency was observed, the raters underwent consistency training and received clarifications regarding the rating scale. The intraclass correlation coefficient (ICC) of the scores given by the four scale evaluators was 0.89. The PANSS consists of three subscales: the positive symptoms subscale (PANSS-P, items P1–P7), the negative symptoms subscale (PANSS-N, items N1–N7), and the general psychopathology symptoms subscale (PANSS-G, items G1–G16) (31).
Measurement of Serum Leptin
All three hospitals stopped serving dinner after 6:30 p.m., but as a precaution, subjects were still asked to fast for more than 8 h before blood collection. Then, venous blood was collected between 6:00 and 8:00 a.m., and each subject's samples were stored in two 5 ml vacutainer tubes containing potassium EDTA. The plasma was labeled with anonymous codes after centrifugation (15 min at 1,000×g) and stored at −80°C. After all subjects were enrolled, the leptin levels in serum specimens were measured using an ELISA kit purchased from Cusabio Biotechnology Company (Code: CSB-E04649h). In brief, serum leptin was measured according to the instructions. The detection range was 0.156–10 ng/ml. An intra-lot coefficient of variation <8% and an inter-lot coefficient <10% were calculated.
Statistical Analysis
All statistical analyses were calculated by SPSS 24.0 (SPSS Inc., Chicago, IL, USA), including independent sample t-test (normally distributed continuous variables) and Mann-Whitney U-test (non-normally distributed continuous variables). The chi-squared test compared the categorical variables, including sex, smoking, drinking, obesity, and current antipsychotic drug treatment. Relationships between BMI, serum leptin levels, and psychopathology symptoms were examined by Spearman's correlation analysis. Then, taking the serum leptin levels (ng/ml) as the dependent variable, multiple stepwise regression was performed to examine the relationship between serum leptin and psychopathology symptoms among participants with schizophrenia. Subsequently, linear regressions were performed to examine the relationship between leptin, BMI, negative symptoms. Moreover, multiple tests were adjusted using Bonferroni correction, and P < 0.05 (two-tailed) indicated statistically significant differences.
Results
General Information
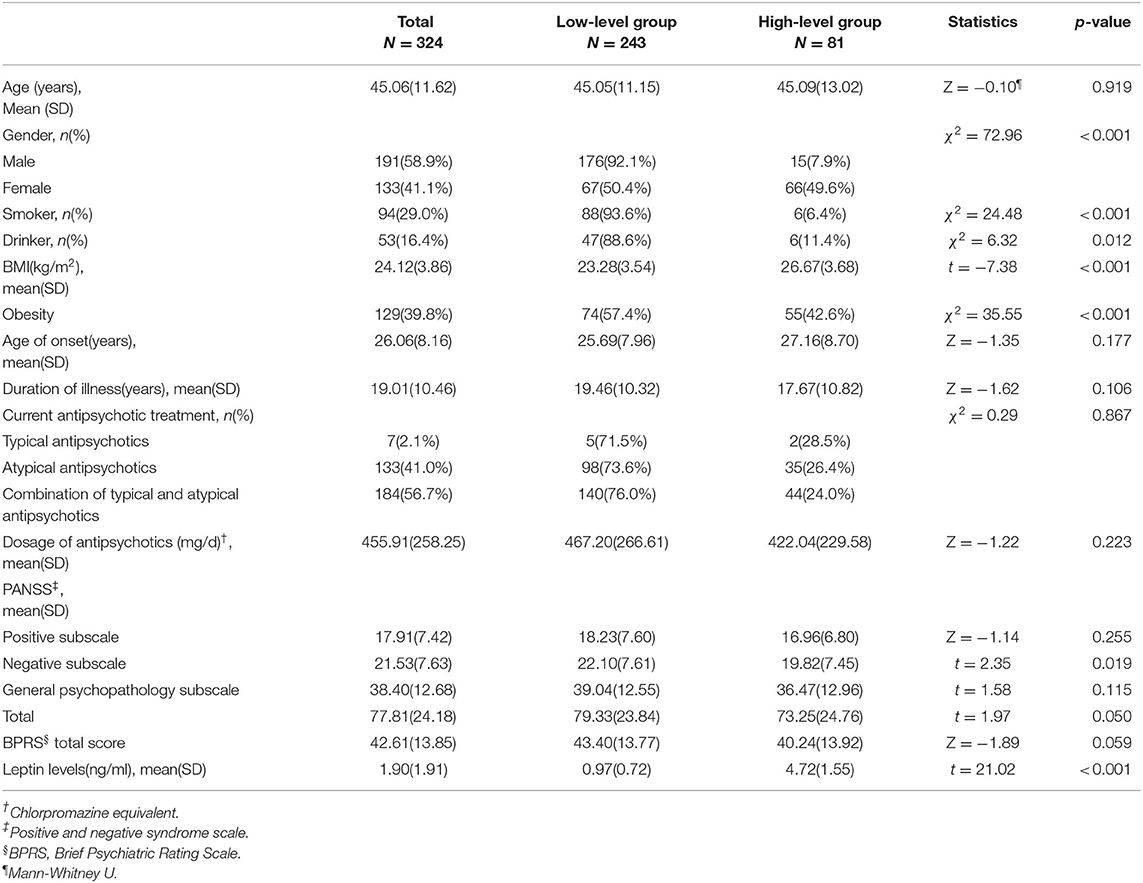
The data of 324 inpatients with schizophrenia were analyzed, comprising 191 males and 133 females. Across all participants, the leptin data had a positively skewed distribution (skewness = 1.5, kurtosis = 2.1). Patients were grouped according to quartiles of the distribution of serum leptin levels. Those in the upper quartile were considered the high-level group (81 patients; 2.86 ng/mL), and the remainder were considered the low-level group (243 patients; 2.86 ng/mL). Table 1 shows the baseline characteristics of the two groups. The average age of study subjects was 45.06 ± 11.62 years, and the onset age was 26.06 ± 8.16 years. The disease duration was 19.01 ± 10.46 years. Moreover, there were significant differences in sex, BMI, and PANSS-N scores between the low-level and high-level groups (P < 0.05). The high leptin level group had a significantly higher BMI than the low leptin level group (26.67 ± 3.68 vs. 23.28 ± 3.54, P < 0.001). In contrast, Negative subscale scores showed lower levels in the high leptin level group (19.82 ± 7.45) than in the low leptin level group (22.10 ± 7.61). There were no differences in age of onset, disease duration, type and dose of antipsychotics between the two groups (P > 0.05, Table 1).
The Correlations Between Leptin and Psychopathology Symptoms
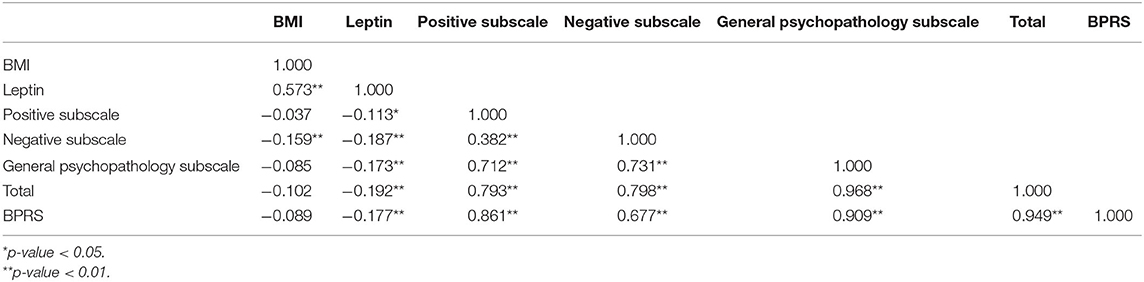
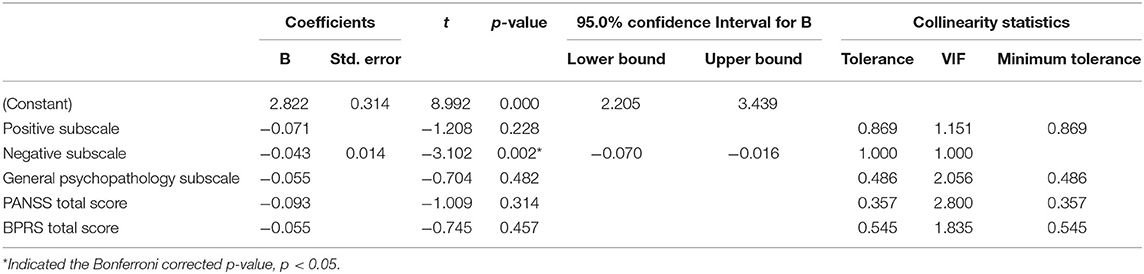
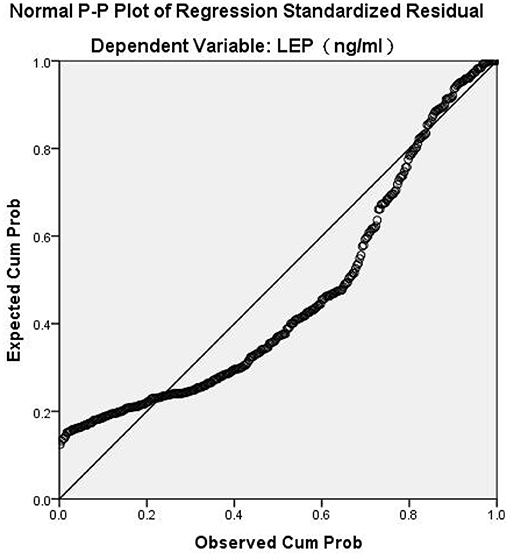
Spearman's correlation analysis revealed that leptin was positively correlated with BMI (r = 0.573, P < 0.001) and negatively correlated with all psychopathology symptoms (P < 0.05). BMI was negatively associated with negative symptoms (r = −0.159, P = 0.004). BMI had no significant correlation with PANSS-P scores, PANSS-G scores, total PANSS scores, or the BPRS scores (P > 0.05). In general, our results indicated that leptin, BMI, and PANSS-N scores were all correlated with each other (Table 2). As shown in Table 3, Multiple stepwise regression analysis showed a negative relationship between serum leptin levels and PANSS-N (B = −0.043, t = −3.102, P = 0.002). Multiple stepwise regression analyses of leptin and psychopathic symptoms revealed that the residuals conformed to a normal distribution. It means the model was stable (Figure 1).
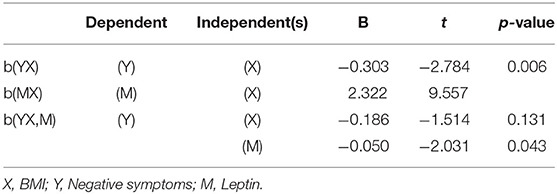
Regression Analysis Between Leptin, BMI, and Negative Symptoms
Table 4 showed that BMI had a positive effect on serum leptin (B = 2.322, t = 9.557, P < 0.001) and a negative effect on PANSS-N scores (B = −0.303, t = −2.784, P = 0.006). After adding leptin to the model, the effect of BMI on PANSS-N scores disappeared (B = −0.186, t = −1.514, P = 0.131), indicating that leptin may be a predictor for the negative symptoms.
Discussion
Weight gain caused by antipsychotics is related to psychopathological improvement. When the patient's weight increased by 7%, the PANSS score decreased by 12% (32). The BPRS score of patients with more than 7% weight gain decreased by 45.6%, while the BPRS score of the rest of the patients decreased by only 31.9% (33). Previous studies have found that the higher the BMI of Chinese patients with chronic schizophrenia, the higher the plasma orexin-A level and the less negative symptoms (34). Many studies have shown a significant relationship between antipsychotic-induced weight gain and good treatment outcomes (20, 35). For example, for clozapine and olanzapine, there is a significant correlation between weight gain and clinical response to these antipsychotics, but the reason is not clear (36, 37). Most studies have shown a correlation between antipsychotic-induced weight gain and treatment benefits based on the above definition. Lower baseline weight was associated with weight gain induced by antipsychotics (38). In studies of controlled baseline weight or BMI, 90% of studies showed a link between symptom improvement and weight gain (33).
Weight gain and improvement caused by antipsychotics may result from the pharmacodynamic properties of antipsychotics, but the relationship between them is not necessarily interdependent. It has also been reported that weight loss in patients with schizophrenia through life intervention and drug intervention does not affect the efficacy of antipsychotic drugs (39). In summary, there is a correlation between antipsychotic-induced weight gain and the therapeutic benefit (40). However, the direct cause-effect relationship between antipsychotic-induced weight gain and the therapeutic benefit is unclear, or weight gain is not an absolute requirement for clinical efficacy.
In this study, serum leptin was negatively correlated with positive, negative, general pathological symptoms and PANSS total BPRS score in patients with schizophrenia. Multiple stepwise regression analyses showed that serum leptin level was significantly correlated with negative symptoms. In summary, this study showed that serum leptin levels in patients with schizophrenia were negatively correlated with the three subscales of PANSS (PANSS-P, PANSS-N, PANSS-G), the total scale score, and the BPRS score. This result is consistent with the reported changes in serum leptin levels and the severity of negative symptoms (41, 42). A large body of evidence suggests that dopamine system dysfunction is associated with negative symptoms of schizophrenia, leading to a lack of will and pleasure (43–45). More studies have shown that the role of leptin in protecting cell survival, promoting apoptosis, and dopamine regulation may be the main mechanism for improving the psychopathological symptoms of schizophrenia (46). A previous study found that plasma leptin levels were negatively correlated with PANSS depression factor scores (r = −0.255, Bonferroni corrected P = 0.028) (47). These findings support the hypothesis that leptin is a predictor of the reduction of negative symptoms in schizophrenia.
If leptin can relieve psychopathological symptoms, then weight gain in patients with schizophrenia is of concern. The leptin level in cerebrospinal fluid has been positively correlated with plasma leptin level and BMI (48). Another study found that leptin levels in schizophrenic patients treated with antipsychotics were higher than those in healthy controls, which was associated with weight gain caused by antipsychotics (49, 50). In addition, studies on patients without medication have shown that low leptin levels are associated with schizophrenia, and antipsychotics will increase leptin levels. Therefore, it is beneficial to study the relationship between leptin, BMI, and psychopathological symptoms. Interestingly, there were no statistically significant differences in age of onset, disease duration, type or the dose of antipsychotics between the high-leptin and low-leptin groups. It suggests that the antipsychotic was not the focus of the study.
The serum leptin level in women (3.24 ± 2.07 ng/ml) was higher than that in men (0.97 ± 1.05 ng/ml). There are three possible reasons for this phenomenon: Firstly, the location of fat deposition and the proportion of fat in the body (51). Subcutaneous fat is dominant in females, while visceral fat is more abundant in males. Subcutaneous fat produces more leptin than visceral fat; therefore, males' serum leptin levels are lower than females' (52). Another possibility is the effect of sex hormones: oestradiol promotes the secretion and release of leptin in cultured adipose tissue, but this process does not occur in men (53). Testosterone has been shown to have antiregulatory effects, suggesting that males' leptin concentrations may be lower than those in females, even when body fat ratios are similar (54, 55). Finally, it is possible that leptin production is higher in the female brain than in the male brain, leading to higher circulating leptin levels in female blood (56, 57).
Several limitations of this study need to be emphasized. Firstly, due to the cross-sectional design, this study cannot obtain causality. These findings must be carefully interpreted and explained in future longitudinal studies. Secondly, we focused on the effect of leptin on weight gain and included other relevant indicators, such as lipoprotein and triglyceride. In addition, leptin gene and leptin receptor gene polymorphisms may affect antipsychotic drug-induced weight gain. However, our study did not evaluate the effect of leptin receptor gene polymorphism on prognosis. Finally, we believe that different kinds of antipsychotics may have different effects on body weight. Therefore, further research is needed to evaluate and control these effects.
Conclusion
It is the first study to suggest that leptin may play a role in the relationship between antipsychotics-induced weight gain and favorable treatment response in schizophrenia. These findings help us further understand the relationship between leptin, weight gain, and antipsychotic response in patients with schizophrenia. Our findings may help monitor the recovery of patients with schizophrenia and develop new treatment options.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Chaohu Hospital affiliated with Anhui Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YeZ, YY, and XN collected and analyzed the data. KZ and HL gave administrative support. YeZ and XL wrote the paper. YuZ, XY, YY, TZ, and LX provided insightful discussion for the manuscript. All authors contributed to data interpretation and approved the final version for publication.
Funding
This research was supported by grants from the National Natural Science Foundation of China (Grant No. 81771449) and the Anhui Province Key Scientific and Technological Projects (1804h08020263).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hennekens CH, Newcomer JW. Series introduction and cardiovascular disease in patients with schizophrenia. J Clin Psychiatry. (2007) 68:e12. doi: 10.4088/JCP.0507e12
2. Berrocal-Izquierdo N, Bernardo M. Schizophrenia and cerebrovascular disease. A description of a series and bibliographic reivew. Actas Esp Psiquiatr. (2014) 42:74–82.
3. Laursen TM, Wahlbeck K, Hällgren J, Westman J, Ösby U, Alinaghizadeh H, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS ONE. (2013) 8:e67133. doi: 10.1371/journal.pone.0067133
4. Kredentser MS, Martens PJ, Chochinov HM, Prior HJ. Cause and rate of death in people with schizophrenia across the lifespan: a population-based study in Manitoba, Canada. J Clin Psychiatry. (2014) 75:154–61. doi: 10.4088/JCP.13m08711
5. Hermes E, Nasrallah H, Davis V, Meyer J, McEvoy J, Goff D, et al. The association between weight change and symptom reduction in the CATIE schizophrenia trial. Schizophr Res. (2011) 128:166–70. doi: 10.1016/j.schres.2011.01.022
6. Zheng W, Jiang WL, Zhang X, Cai DB, Sun JW, Yin F, et al. Use of the RBANS to evaluate cognition in patients with schizophrenia and metabolic syndrome: a meta-analysis of case-control studies. Psychiatr Q. (2021). doi: 10.1007/s11126-021-09889-9. [Epub ahead of print].
7. Muench J, Hamer AM. Adverse effects of antipsychotic medications. Am Fam Physician. (2010) 81:617–22.
8. Wang J, Zhang Y, Yang Y, Liu Z, Xia L, Li W, et al. The prevalence and independent influencing factors of obesity and underweight in patients with schizophrenia: a multicentre cross-sectional study. Eat Weight Disord. (2020) 26:1365–74. doi: 10.1007/s40519-020-00920-9
9. Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet (London, England). (2013) 382:951–62. doi: 10.1016/S0140-6736(13)60733-3
10. Fernø J, Ersland KM, Duus IH, González-García I, Fossan KO, Berge RK, et al. Olanzapine depot exposure in male rats: dose-dependent lipogenic effects without concomitant weight gain. Eur Neuropsychopharmacol. (2015) 25:923–32. doi: 10.1016/j.euroneuro.2015.03.002
11. Garcia-Rizo C. Antipsychotic-induced weight gain and clinical improvement: a psychiatric paradox. Front Psychiatry. (2020) 11:560006. doi: 10.3389/fpsyt.2020.560006
12. Mezquida G, Savulich G, Garcia-Rizo C, Garcia-Portilla MP, Toll A, Garcia-Alvarez L, et al. Inverse association between negative symptoms and body mass index in chronic schizophrenia. Schizophr Res. (2018) 192:69–74. doi: 10.1016/j.schres.2017.04.002
13. Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS ONE. (2014) 9:e94112. doi: 10.1371/journal.pone.0094112
14. de Silva VA, Suraweera C, Ratnatunga SS, Dayabandara M, Wanniarachchi N, Hanwella R. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. (2016) 16:341. doi: 10.1186/s12888-016-1049-5
15. Orsolini L, Tomasetti C, Valchera A, Vecchiotti R, Matarazzo I, Vellante F, et al. An update of safety of clinically used atypical antipsychotics. Expert Opin Drug Saf. (2016) 15:1329–47. doi: 10.1080/14740338.2016.1201475
16. Orsolini L, De Berardis D, Volpe U. Up-to-date expert opinion on the safety of recently developed antipsychotics. Expert Opin Drug Saf. (2020) 19:981–98. doi: 10.1080/14740338.2020.1795126
17. Fava M, Dirks B, Freeman MP, Papakostas GI, Shelton RC, Thase ME, et al. A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. (2019) 80:12928. doi: 10.4088/JCP.19m12928
18. Sapra M, Lawson D, Iranmanesh A. Fat distribution in schizophrenia patients: a pilot study comparing first- and second-generation antipsychotics. J Clin Psychopharmacol. (2018) 38:68–71. doi: 10.1097/JCP.0000000000000810
19. Masuda T, Misawa F, Takase M, Kane JM, Correll CU. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry. (2019) 76:1052–62. doi: 10.1001/jamapsychiatry.2019.1702
20. Raben AT, Marshe VS, Chintoh A, Gorbovskaya I, Müller DJ, Hahn MK. The Complex relationship between antipsychotic-induced weight gain and therapeutic benefits: a systematic review and implications for treatment. Front Neurosci. (2017) 11:741. doi: 10.3389/fnins.2017.00741
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. (2016) 173:166–73. doi: 10.1176/appi.ajp.2015.15030332
22. Grajales D, Ferreira V, Valverde ÁM. Second-generation antipsychotics and dysregulation of glucose metabolism: beyond weight gain. Cells. (2019) 8:1336. doi: 10.3390/cells8111336
23. Gupta S, Rajaprabhakaran R. Paradoxical hypertension associated with clozapine. Am J Psychiatry. (1994) 151:148. doi: 10.1176/ajp.151.1.148a
24. De Berardis D, Rapini G, Olivieri L, Di Nicola D, Tomasetti C, Valchera A, et al. Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine. Ther Adv Drug Safety. (2018) 9:237–56. doi: 10.1177/2042098618756261
25. Farr OM, Gavrieli A, Mantzoros CS. Leptin applications in 2015: what have we learned about leptin and obesity? Curr Opin Endocrinol Diabetes Obes. (2015) 22:353–9. doi: 10.1097/MED.0000000000000184
26. Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: an epigenetic landscape. Life Sci. (2015) 140:57–63. doi: 10.1016/j.lfs.2015.05.003
27. Van Doorn C, Macht VA, Grillo CA, Reagan LP. Leptin resistance and hippocampal behavioral deficits. Physiol Behav. (2017) 176:207–13. doi: 10.1016/j.physbeh.2017.03.002
28. Smith RC, Leucht S, Davis JM. Maximizing response to first-line antipsychotics in schizophrenia: a review focused on finding from meta-analysis. Psychopharmacology. (2019) 236:545–59. doi: 10.1007/s00213-018-5133-z
30. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. (2003) 64:663–7. doi: 10.4088/JCP.v64n0607
31. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
32. Holden JM, Holden UP. Weight changes with schizophrenic psychosis and psychotropic drug therapy. Psychosomatics. (1970) 11:551–61. doi: 10.1016/S0033-3182(70)71576-4
33. Kemp DE, Correll CU, Tohen M, Delbello MP, Ganocy SJ, Findling RL, et al. Associations among obesity, acute weight gain, and response to treatment with olanzapine in adolescent schizophrenia. J Child Adolesc Psychopharmacol. (2013) 23:522–30. doi: 10.1089/cap.2012.0099
34. Liu Z, Zhang Y, Zhao T, Wang J, Xia L, Zhong Y, et al. A higher body mass index in Chinese in patients with chronic schizophrenia is associated with elevated plasma orexin-A levels and fewer negative symptoms. Nord J Psychiatry. (2020) 74:525–32. doi: 10.1080/08039488.2020.1755995
35. Tsuneyama N, Suzuki Y, Sawamura K, Sugai T, Fukui N, Watanabe J, et al. Effect of serum leptin on weight gain induced by olanzapine in female patients with schizophrenia. PLoS ONE. (2016) 11:e0149518. doi: 10.1371/journal.pone.0149518
36. Lally J, Gallagher A, Bainbridge E, Avalos G, Ahmed M, McDonald C. Increases in triglyceride levels are associated with clinical response to clozapine treatment. J Psychopharmacol. (2013) 27:401–3. doi: 10.1177/0269881112472568
37. Sharma E, Rao NP, Venkatasubramanian G. Association between antipsychotic-induced metabolic side-effects and clinical improvement: a review on the Evidence for “metabolic threshold”. Asian J Psychiatr. (2014) 8:12–21. doi: 10.1016/j.ajp.2013.11.017
38. Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. (2020) 7:64–77. doi: 10.1016/S2215-0366(19)30416-X
39. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. (2010) 35:1520–30. doi: 10.1038/npp.2010.21
40. Mackin P, Watkinson HM, Young AH. Prevalence of obesity, glucose homeostasis disorders and metabolic syndrome in psychiatric patients taking typical or atypical antipsychotic drugs: a cross-sectional study. Diabetologia. (2005) 48:215–21. doi: 10.1007/s00125-004-1641-y
41. Atmaca M, Kuloglu M, Tezcan E, Gecici O, Ustundag B. Weight gain, serum leptin and triglyceride levels in patients with schizophrenia on antipsychotic treatment with quetiapine, olanzapine and haloperidol. Schizophr Res. (2003) 60:99–100. doi: 10.1016/S0920-9964(02)00305-5
42. Venkatasubramanian G, Chittiprol S, Neelakantachar N, Shetty TK, Gangadhar BN. A longitudinal study on the impact of antipsychotic treatment on serum leptin in schizophrenia. Clin Neuropharmacol. (2010) 33:288–92. doi: 10.1097/WNF.0b013e3181fa2a6f
43. Maia TV, Frank MJ. An integrative perspective on the role of dopamine in schizophrenia. Biol Psychiatry. (2017) 81:52–66. doi: 10.1016/j.biopsych.2016.05.021
44. Ellenbroek BA, Prinssen EP. Can 5-HT3 antagonists contribute toward the treatment of schizophrenia? Behav Pharmacol. (2015) 26:33–44. doi: 10.1097/FBP.0000000000000102
45. Xu P, Klaasen NG, Opmeer EM, Pijnenborg GHM, van Tol MJ, Liemburg EJ, et al. Intrinsic mesocorticolimbic connectivity is negatively associated with social amotivation in people with schizophrenia. Schizophr Res. (2019) 208:353–9. doi: 10.1016/j.schres.2019.01.023
46. Davis C, Mudd J, Hawkins M. Neuroprotective effects of leptin in the context of obesity and metabolic disorders. Neurobiol Dis. (2014) 72:61–71. doi: 10.1016/j.nbd.2014.04.012
47. Xu J, Jiao Y, Xing M, Lin Y, Su Y, Ding W, et al. Increased plasma leptin as a novel predictor for psychopathological depressive symptoms in chronic schizophrenia. Gen Psychiatr. (2018) 31:e100018. doi: 10.1136/gpsych-2018-100018
48. Page-Wilson G, Meece K, White A, Rosenbaum M, Leibel RL, Smiley R, et al. Proopiomelanocortin, agouti-related protein, and leptin in human cerebrospinal fluid: correlations with body weight and adiposity. Am J Physiol Endocrinol Metab. (2015) 309:E458–65. doi: 10.1152/ajpendo.00206.2015
49. Endomba FT, Tankeu AT, Nkeck JR, Tochie JN. Leptin and psychiatric illnesses: does leptin play a role in antipsychotic-induced weight gain? Lipids Health Dis. (2020) 19:22. doi: 10.1186/s12944-020-01203-z
50. Panariello F, Polsinelli G, Borlido C, Monda M, De Luca V. The role of leptin in antipsychotic-induced weight gain: genetic and non-genetic factors. J Obes. (2012) 2012:572848. doi: 10.1155/2012/572848
51. Christen T, Trompet S, Noordam R, van Klinken JB, van Dijk KW, Lamb HJ, et al. Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides. (2018) 107:25–31. doi: 10.1016/j.peptides.2018.07.008
52. Korac A, Srdic-Galic B, Kalezic A, Stancic A, Otasevic V, Korac B, et al. Adipokine signatures of subcutaneous and visceral abdominal fat in normal-weight and obese women with different metabolic profiles. Arch Med Sci. (2021) 17:323–36. doi: 10.5114/aoms/92118
53. Chun KA, Kocarnik JM, Hardikar SS, Robinson JR, Berndt SI, Chan AT, et al. Leptin gene variants and colorectal cancer risk: sex-specific associations. PLoS ONE. (2018) 13:e0206519. doi: 10.1371/journal.pone.0206519
54. Liu CC, Huang SP, Hsieh TJ, Lee CH, Cheng KH, Huang TY, et al. Fatty liver index is associated with the risk of testosterone deficiency in aging men without metabolic syndrome. Andrology. (2021) 9:863–72. doi: 10.1111/andr.12979
55. Lima TFN, Nackeeran S, Rakitina E, Lima GFN, Arora H, Kargi AY, et al. Association of leptin with total and free testosterone: results from the national health and nutrition examination surveys. Androgens. (2020) 1:94–100. doi: 10.1089/andro.2020.0007
56. Couillard C, Mauriege P, Prud'homme D, Nadeau A, Tremblay A, Bouchard C, et al. Plasma leptin concentrations: gender differences and associations with metabolic risk factors for cardiovascular disease. Diabetologia. (1997) 40:1178–84. doi: 10.1007/s001250050804
Keywords: leptin, body mass index, psychopathological symptoms, schizophrenia, antipsychotics
Citation: Zhang Y, Li X, Yao X, Yang Y, Ning X, Zhao T, Xia L, Zhang Y, Zhang K and Liu H (2021) Do Leptin Play a Role in Metabolism–Related Psychopathological Symptoms? Front. Psychiatry 12:710498. doi: 10.3389/fpsyt.2021.710498
Received: 16 May 2021; Accepted: 17 August 2021;
Published: 10 September 2021.
Edited by:
Jolanta Kucharska-Mazur, Pomeranian Medical University, PolandReviewed by:
Domenico De Berardis, Azienda Usl Teramo, ItalyTakefumi Suzuki, University of Yamanashi, Japan
Copyright © 2021 Zhang, Li, Yao, Yang, Ning, Zhao, Xia, Zhang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Zhang, emhhbmdrYWlAYWhtdS5lZHUuY24=; Huanzhong Liu, aHVhbnpob25nbGl1QGFobXUuZWR1LmNu
†These authors share first authorship
 Yelei Zhang1,2†
Yelei Zhang1,2† Lei Xia
Lei Xia Kai Zhang
Kai Zhang Huanzhong Liu
Huanzhong Liu