- 1The Clinical Hospital of Chengdu Brain Science Institute, MOE Key Lab for Neuroinformation, University of Electronic Science and Technology of China, Chengdu, China
- 2School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu, China
- 3Chengdu Forth People' s Hospital, Chengdu Mental Health Center, Chengdu, China
- 4Neuropsychiatry Program, Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston, Houston, TX, United States
- 5Chengdu Medical College School of Nursing, Chengdu, China
Objective: Metabolic and other medical conditions are frequently comorbid with schizophrenia. As they might be the side-effects of antipsychotic treatment, studying first-episode drug-naïve schizophrenia (FDSZ) provides a unique opportunity to investigate a direct pathogenic link between metabolic changes and schizophrenia. Here, we presented the methods and baseline unique metabolic profile of FDSZ patients without medical comorbidities unveiling subthreshold indices of metabolic disturbances.
Method: Drug-naïve individuals diagnosed with schizophrenia but without any previous medical conditions were invited to participate in the study. Participants were submitted to structured psychiatric and cognitive assessments, laboratory and neuroimaging tests. Subjects will be followed after antipsychotic treatment at 6, 24 and 48 weeks.
Results: During an 8-month-period, out of 103 patients presenting with first episode psychosis, 67 subjects (43.3% men, 56.7% women) were enrolled in the study. They had a mean ± SD age of 32.1 ± 8.7 years, with a mean BMI of 21.1 kg/m2 and 11.3 ± 3.6 years of schooling. Less than 1/3 reported a family history of mental illness. Upon laboratory assessment, 10.4%, 7.5%, and 11.9% of patients were identified with hyperhomocysteinemia, hypertriglyceridemia and hyperprolactinemia, respectively, with percentages of women relatively higher than men except for hypertriglyceridemia.
Conclusions: First episode schizophrenia patients, especially women, present subclinical metabolic abnormalities, independent of antipsychotic treatment.
Introduction
Schizophrenia is a severe disabling psychiatric disorder marked by psychotic and negative symptoms alongside cognitive deficits. While its pathophysiology has not been fully elucidated, it is recognized that metabolic abnormalities (MAs) are frequent comorbid in these patients, contributing to their higher burden of medical diseases and complications. The average life expectancy of men and women with schizophrenia is, respectively, 15 and 12 years shorter than for those without schizophrenia (1), in which comorbidities of metabolic and cardiovascular diseases are widely recognized as major contributors (2). Actually, patients affected by schizophrenia are at increased risk for metabolic and cardiovascular diseases, including hypertension, type II diabetes mellitus, coronary artery disease (3, 4), in which various MAs have been described as playing an important role. Moreover, MAs have been associated with poorer functional outcomes (5), worse quality of life (6, 7), and non-compliance with antipsychotic therapy (8) in this group of individuals. Interestingly, previous studies showed that first-episode schizophrenia populations without or minimal antipsychotic exposure present with higher prevalence of MAs, including lipid profile disturbances, reduced uric acid levels, elevated homocysteine and prolactin levels (9–13). Until now, the underlying mechanism of increased subthreshold metabolic dysregulation in schizophrenia is still unclear.
Accumulating evidence indicates that complex interactions between sex and metabolism might affect the incidence of MAs in the schizophrenia population (14), and yet previously reported data on sex-specific association with MAs in schizophrenia are still controversial. Some reports demonstrate a higher prevalence of MAs among female patients (15–17), while other reports even show the opposite results (18), and others show no sex difference (19, 20). Many factors may lead to these contradictory results, such as antipsychotic treatment and previous medical comorbidities. It has long been recognized that taking antipsychotics increases the risk of MAs (21–24). Previous reviews and meta-analyses indicated that MAs increase after the initial exposure to antipsychotics in children and adolescents (25, 26) and in first-episode psychosis patients (27, 28). Accordingly, MAs have become a matter of concern in the early stages of antipsychotic treatment. For example, one recent real-world study by Tao Li et al. (29) revealed that insulin resistance and lipid metabolism disorders occurred as early as 2 weeks after the initiation of antipsychotic treatment in first-episode schizophrenia patients. However, considerable debate exists about whether schizophrenia itself can contribute directly to metabolic dysfunction. Distinguishing pre-existing risk from antipsychotic induced effects is important for the understanding of the basis of MAs in the context of schizophrenia. First episode and drug naïve (FEDN) patients without medical comorbidities provide a unique opportunity to minimize confounding factors and examine MAs in schizophrenia patients without antipsychotics. Moreover, sex-specific MAs in FEDN schizophrenia without medical comorbidities have not been fully investigated.
Therefore, we designed a research project in a naturalistic setting, the First-Episode Drug-Naïve Schizophrenia without Metabolic and Cardiovascular Diseases Study, to investigate metabolic problems that might increase cardiovascular risk in the early stage of schizophrenia. The aim of the current report is to present the methods and the baseline characteristics of subjects enrolled in the study, in order to understand whether schizophrenia itself is associated with MAs.
Study Design
Subjects
For identification of cases, the Chengdu Fourth People's Hospital (Chengdu Mental Health Center, CMHC) recruited patients between May 1, 2018, and Dec 31, 2018.
At the time of enrollment of the study, participants were between the age of 18 and 55 years, experiencing a first psychotic episode of schizophrenia. Participants had never taken antipsychotics before. All patients met the diagnostic criteria of schizophrenia, as defined in the International Classification of Diseases, Eleventh Edition (ICD-11) (30).
Exclusion criteria were: (1) concurrent diagnosis of psychiatric disorders defined in ICD-11 other than schizophrenia; (2) concurrent treatment with anti-diabetic or lipid-lowering agents or special diets to lower glucose or lipid levels, or use of immunosuppressive agents; (3) diagnosis of diabetes, dyslipidemia, or any endocrine disease; (4) ongoing infections or allergies, history of alcohol or other substance use, autoimmune disorders, pregnancy or breastfeeding, known medical conditions that might affect metabolism, and history of diabetes or lipid disorders.
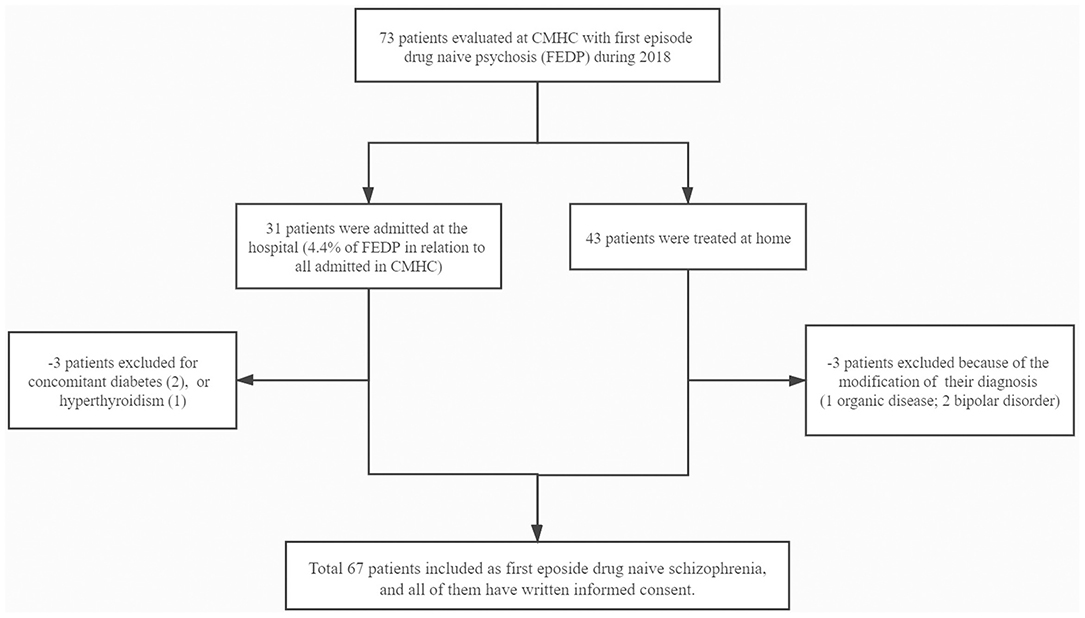
The study was approved by the ethics committees of the West China Hospital of Sichuan University (ER136, 2017) and CMHC (ER15, 2017), as well as all subjects participated after providing a written, informed consent (See Figure 1).
Methods
The study was conducted in four phases. On phase 1, two trained interviewers visited these participants and their families at CMHC, explained in detail the nature of the survey and invited them to participate. In the case of acceptance, all participant signed a written informed consent and was submitted to a detailed and structured evaluation that included: socio-demographic information, previous and current medical diagnoses, additional psychiatric medical information (medications in use with specific dose regimens, frequency of attendance to medical consultation), and general physical examination with weight and height, blood pressure and heart rate record. Positive and Negative Syndrome Scale (PANSS) (31) to assess schizophrenia symptoms; Hamilton Anxiety Scale (HAMA) (32) to assess anxiety; Hamilton Depression Scale-24 items (HAMD-24) (33) to assess depression. In addition, each subject was asked about suicide ideation, suicide plan, and suicide attempt, with items taken directly from the National Comorbidity Survey (34). These items were: “Have you ever seriously thought about committing suicide?” “Have you ever made a plan for committing suicide?” “Have you ever attempted suicide?” The clinical evaluation was conducted by team composed of four board certified psychiatrists, all of them with at least three years of experience in psychiatric assessment. Training sessions in order to standardize the clinical procedures were previously carried out by the group.
On phase 2, participants were submitted to a structured cognitive examination including: Brief Assessment of Cognition in Schizophrenia (BACS) (35) to evaluate the overall cognitive functioning; Wisconsin Card Sorting Test (WCST) (36) to evaluate executive functioning; Raven's Standard Progress Matrices (SPM) (37) to test the ability of observation and reasoning; Wechsler Adult Intelligence Scale-Revised (WAIS-R) (38) to measure the general intelligence abilities; and the Stroop Color and Word test (SCWT) (39) to test attention and executive functions. Neuropsychological and functional assessments were conducted by a team of three neuropsychologists, two psychologists and one occupational therapist, all with at least 3 years of clinical experience.
On phase 3, all participants were invited to provide a blood sample for laboratory tests, including uric acid (UA), homocysteine (HC), total cholesterol (TC), triglyceride (TG) and prolactin (PRL). Previous studies have reported that first-episode schizophrenia populations present with decreased UA levels, increased HC, TC, TG and PRL levels (9–13). Considering that a substantial body of evidence has accumulated that indicates hypouricemia (40, 41), hyperhomocysteinemia (42), hypercholesterolemia (43), hypertriglyceridemia (44) and hyperprolactinemia (45) are associated with cardiovascular risk, it was necessary to investigate hypouricemia, hyperhomocysteinemia, hypercholesterolemia, hypertriglyceridemia and hyperprolactinemia in these patients. As described in previous studies (46–50), hypouricemia was defined when UA concentration was <2.0 mg/dL; hyperhomocysteinemia, hypercholesterolemia, hypertriglyceridemia and hyperprolactinemia were defined when HC, TC, TG and PRL concentration exceeded 15.0 μmol/L, 200.0 mg/dL, 1.70 mmol/L, and 20.0 ng/mL, respectively. Blood samples were drawn after overnight fasting of at least 12 h between 06:00 to 07:00 a.m. Blood was sampled using anticoagulant-free tubes and kept for 1 h at 4 °C (for platelet activation) before serum was isolated (centrifugation at 3,000 rpm for 20 min at 4 °C). They also underwent neuroimaging test through a 3.0 Tesla SIEMENS Trio Tim scanner equipped with a 32-chanel head coil located at West China Hospital, Sichuan University, to measure volume T1& T2, DTI and rest-state functional MRI.
On phase 4, all patients are expected to be followed at 6, 24 and 48 weeks after enrollment. They will repeat clinical, neuropsychological and functional evaluations in each time point. In addition, all patients will be evaluated regarding clinical treatment outcomes, including: Clinical Global Impressions Scale (CGIS) (51), Rating Scale for Extrapyramidal Side Effects (RSESE) (52), Udvalg for Kliniske Undersogelser (UKU) (53), Barnes Akathisia Rating Scale (BARS) (54) and Abnormal Involuntary Movement Scale (AIMS) (55).
Statistical Analysis
Descriptive tables and measures were used to establish a demographic, psychopathological and cognition-related profile of the patients enrolled in this study.
Group differences of the female and male were compared using one-way ANOVA for continuous variables and chi square for categorical variables. Since the scores of PANSS, HAMD-24 and HAMA were normally distributed in the male and female (Kolmogorov-Smirnov one-sample test; both p > 0.05), the principal outcome analysis consisted of one-way analysis of variance (ANOVA). Where there was a significance in ANOVA, the effect of age, education, smoking, and body mass index (BMI) were tested by adding these variables to the analysis model as covariates.
The PASW Statistics 18.0 software (SPSS Inc., Chicago, IL, USA) was used to do all statistical analysis. Data were presented as rate (%) or mean ± SD. All p-values were 2-tailed at the significant level of <0.05.
Results
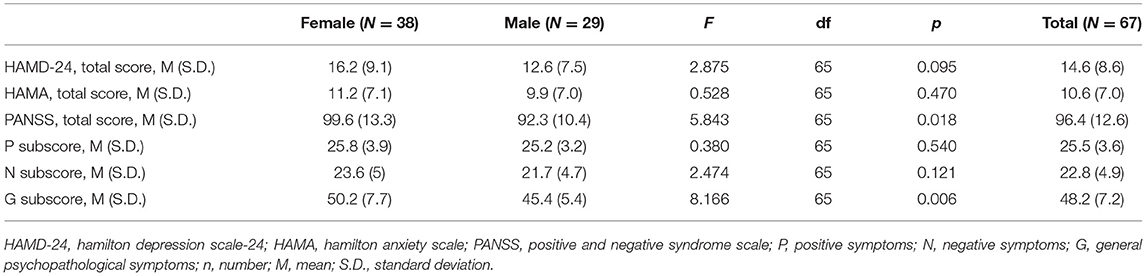
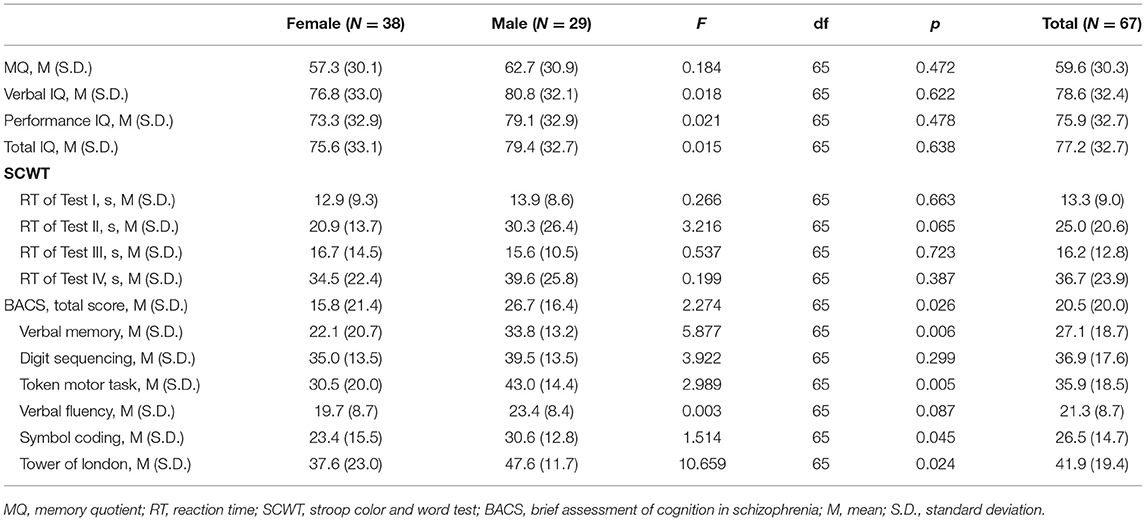
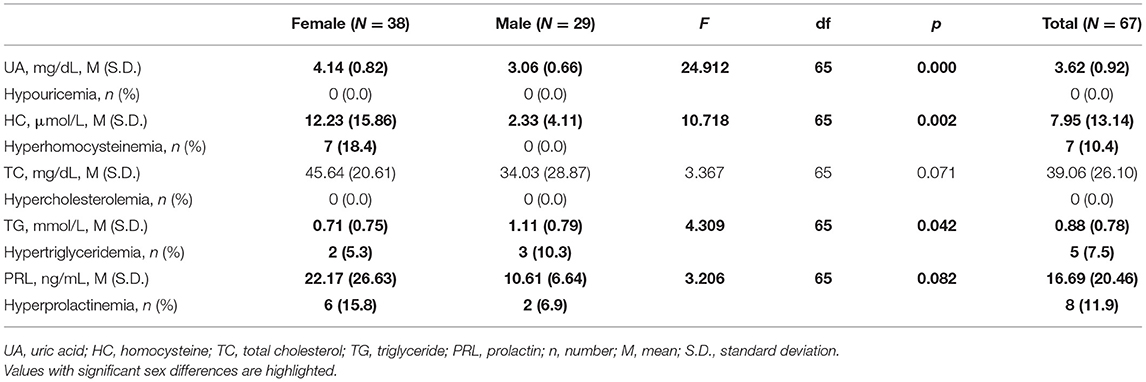
We evaluated 73 subjects presenting with first episode of psychosis at the CMHC from May 2018 to December 2018, of whom 31 needed to be hospitalized for further observation (Figure 1). Thirty six subjects were excluded from the study due to the following reasons: (1) two patients had diabetes and one had hyperthyroidism; (2) three had their psychiatric diagnosis changed (one organic disease, two bipolar disorder). Finally, we fully evaluated 67 individuals from Chengdu, Sichuan Province, China, being 38 (56.7%) women and 29 (43.3%) men, aged 21.1 ± 3.4 years, with educational level of 11.3 ± 3.6 years. Tables 1–3 depicted data on baseline general characteristics.
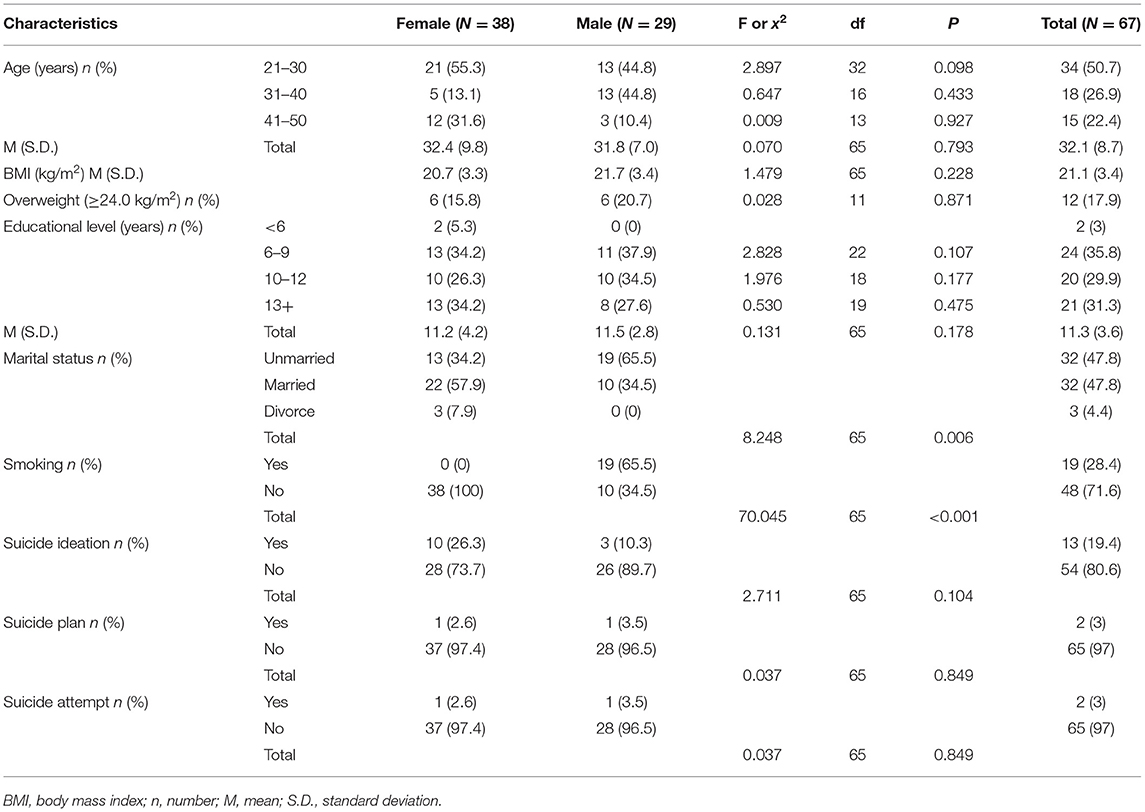
Table 1. Distribution of participants by demographics characteristics (Chengdu, China, 2018) by sex.
As shown in Table 1, there was no significant difference in age (32.4 ± 9.8 years vs. 31.8 ± 7.0 years), education (11.2 ± 4.2 years vs. 11.5 ± 2.8 years), BMI (20.7 ± 3.3 kg/m2 vs. 21.7 ± 3.4 kg/m2), suicidal ideation rate (26.3 vs. 10.3%), suicidal plan rate (2.6 vs. 3.5%) and suicidal attempt rate (2.6 vs. 3.5%) between males and females, but smoking rate (0.0 vs. 65.5%) and married status were significant different (all p < 0.01). In addition, we found similar years of education, BMI and suicide risk distributions in both sexes, while the distribution of age was different. Only 7.9% of women were divorced, while only men admitted to be smoking. Up to 19.4% subjects reported severe suicidal ideation, with 26.3% for female and 10.3% for male.
In Table 2, we observe that total PANSS score (F = 5.843, df = 65, p < 0.05) and general psychopathology subscale (F = 8.166, df = 65, p < 0.01) were significantly higher in women than in men. This difference was still significant when adjusting for age, education, BMI and smoking (p < 0.05). No significant differences were observed in HAMD-24, HAMA, positive and negative subscales of PANSS between groups (all p > 0.05). As shown in Table 3, there was no significant difference in Memory quotient (MQ), verbal IQ, performance IQ, total IQ, reaction time (RT) of SCWT, digit sequencing and verbal fluency in BACS (all p > 0.05) when comparing men vs. women. However, BACS Composite score (F = 2.274, df = 65, p < 0.05), verbal memory (F = 5.877, df = 65, p < 0.01), token motor task (F = 2.989, df = 65, p < 0.01), symbol coding (F = 1.514, df = 65, p < 0.05), and tower of London (F = 10.659, df = 65, p < 0.01) in BACS were significantly higher in men than in women.
Table 4 shows levels of UA, HC, TC, TG and PRL in patients by sex. 10.4% (Female = 18.4% vs. Male = 0.0%) of patients had hyperhomocysteinemia; 7.5% (Female = 5.3% vs. Male = 10.3%) had hypertriglyceridemia and 11.9% (Female = 15.8% vs. Male = 6.9%) had hyperprolactinemia. There are significant differences in UA (p < 0.001), HC (p < 0.01) and TG (p < 0.05) between groups. These differences were still significant when adjusting for age, education, BMI and smoking (p < 0.05). Additionally, no significant differences were shown in levels of TC (p > 0.05) and PRL (p > 0.1).
Discussion
Understanding the mechanisms underlying MAs before and during antipsychotic therapy of schizophrenia is very important due to practical implications in the management of these patients (56–58). In this report, our results indicate that FEDN patients already have metabolic changes before their antipsychotic treatment. Importantly, such phenomenon is more prevalent in women.
Accumulating evidence indicates that subthreshold metabolic dysregulation might be present in the premorbid phase of the illness (59) and in antipsychotic-naïve patients with first-episode psychosis (60), which has been confirmed in our report. We did not find hypercholesterolemia but hypertriglyceridemia in FEDN patients. In line with this finding, a recent meta-analysis by Frydecka et al. (2017) (9) revealed that patients with first-episode non-affective psychosis had significantly lower levels of TC as well as significantly higher levels of TG compared to controls. Lipid metabolic dysregulation observed in drug-naïve patients suggest that schizophrenia-spectrum disorders might share overlapping genetic background with cardio-metabolic phenotypes. Actually, the pathophysiology of schizophrenia seems to involve alterations in biosynthesis of TC, fatty acids (FAs), phospholipids (PLs) and sphingolipids (61–64). In addition, several neuropathological studies demonstrated alterations in the levels of cholesteryl esters (TC esters), TG, polyunsaturated FAs and PLs in prefrontal and frontal cortex of patients with schizophrenia (53).
Our results also showed that FEDN patients had higher prevalence of hyperhomocysteinemia and hyperprolactinemia. Previous studies have also shown significantly increased HC and PRL levels in antipsychotic-naïve schizophrenia and related disorders (11, 20). For example, Kirkpatrick et al. (13) reported significant differences in PRL levels in patients with schizophrenia compared with controls. Interestingly, HC can induce reactive oxygen species production, implicated in schizophrenia pathophysiology (65), by PAR-4 (protease-activated receptor-4) activation. Moreover, given that PRL is under negative control by dopamine (66), high level of PRL means dopamine dysfunction (67, 68). In this context, it is tempting to speculate that oxidative stress imbalance is one potential common pathway shared by MAs and schizophrenia. In addition, UA is a well-known antioxidant, so lower serum UA concentrations might be regarded as a peroxidation state (66). Although our sample did not show hypouricemia, UA level in the male was significant lower than that in the female, which is similar to the meta-analysis by Qiu He et al. (66).
To the best of our knowledge, this is the first report of sex differences in the frequency of hyperhomocysteinemia, hypertriglyceridemia and hyperprolactinemia in FEDN schizophrenia patients without medical comorbidities. We showed that nearly 10% of patients had metabolic disorders, independent of medical comorbidities and antipsychotic exposure. Some recent meta-analysis and case-control studies on antipsychotic-naïve patients with first-episode psychosis have found comparable results (14, 18, 69, 70). However, it is unclear whether these studies excluded subjects with medical comorbidities, especially type two diabetes, making complicated to rule out the confounding effects of medical comorbidities. For example, Yongjie Zhou et al. (14) reported that over 30% patients present with hypertriglyceridemia or hypercholesterolemia. They reported a much higher prevalence than we did, which might be due to the fact that they included FEDN patients with comorbid type II diabetes mellitus. In other words, what the previous studies with untreated patients with schizophrenia did not address is the extent to which the disease itself presents an increased risk for metabolic dysfunction independent of medical comorbidities. The results of our study showed that FEDN patients without medical comorbidities, especially women, were exceptionally vulnerable to metabolic abnormalities. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study (71) found a higher prevalence for metabolic syndrome (MetS) in women (48.5%) than in men (42.6%). However, this study also recruited patients with antipsychotics that may affect the prevalence of MetS. A previous meta-analysis of the Chinese population showed that MetS was more common in women than in men (27.0 vs. 19.2 %) (72), which are consistent with our findings.
Our study has several limitations worthy of attention. First, although we shall recognize the heterogeneity that characterizes different regions of China (73), the Chengdu FEDN schizophrenia patients are able to typically represent the Han Chinese population. Second, our methods of measuring metabolite biomarkers were hypothesis driven and focused on assaying single metabolites, however, such high homogeneous and well-defined subject groups might be benefit from exploratory high-throughput metabolomic methods capable of quantifying hundreds of metabolites at one time (74). Third, current evidence suggests that the emergence of schizophrenia is associated with epigenetic characteristics (75). Genetic variation, such as microRNAs (17) and long noncoding RNAs (lncRNAs) (76), should be considered as another important parameter to be analyzed. Forth, environmental aspects during early stages of life, such as childhood trauma (77), and poor adult lifestyles (78) should be considered in future studies, which could contribute to the consistence of MAs and schizophrenia. Fifth, we did not include healthy controls for comparison, but definitions of hypouricemia, hyperhomocysteinemia, hypercholesterolemia, hypertriglyceridemia and hyperprolactinemia are based on reference values established for the Chinese population. Accordingly, the current results reflect the prevalence and sex differences of pathological MAs in subjects with schizophrenia. Last but not least, the sample size of patients we included was relatively small and the current study was limited to its cross-sectional design. However, in our next steps, we will integrate multiple dimensions (socio-demographics vs. psychopathological/cognitive vs. metabolic vs. neuroimaging), providing a comprehensive view on the MAs influencing psychiatric/cognitive performance in the early phases of schizophrenia. Due to the longitudinal nature of the study, we will eventually explore long term effects of metabolic abnormalities on the pathophysiology of schizophrenia.
Conclusions
In conclusion, in this manuscript, we reported the baseline general characteristics of FEDN schizophrenia patients with a unique metabolic profile. A total of 67 patients were enrolled in this study, and 10.4%, 7.5%, and 11.9% of them were identified with hyperhomocysteinemia, hypertriglyceridemia and hyperprolactinemia, respectively. These results indicate that subclinical metabolic abnormalities may be present in patients with first episode schizophrenia.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the West China Hospital of Sichuan University and Chengdu Mental Health Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JD and LJ completed the inclusion and exclusion of patients. LJ, QZ, XB, XZ, and YL evaluated the psychopathological symptoms. WC and LZ completed the blood sample test and EEG examination, respectively. QZ, HH, JD, and AT analyzed the data and wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This Study is partially supported by the National Natural Science Foundation (81761128023) and the Science & Technology Department of Sichuan Province, China (2017JY0031, JD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the partnership from Huaxi MR Research Center (which subsidized the magnetic resonance exams). We are deeply grateful to Prof. Qiyong Gong for the local coordination of the operational arrangements. We also greatly thank all patients and their families for the generous collaboration and engagement on the research project.
References
1. Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. (2013) 170:324–33. doi: 10.1176/appi.ajp.2012.12050599
2. Casey DE. Metabolic issues and cardiovascular disease in patients with psychiatric disorders. Am J Med Suppl. (2005) 118:15–22. doi: 10.1016/j.amjmed.2005.01.046
3. Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. (2013) 39:306–18. doi: 10.1093/schbul/sbr148
4. Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. (2015) 14:339–47. doi: 10.1002/wps.20252
5. Vancampfort D, Sweers K, Probst M, Maurissen K, Knapen J, Minguet P, et al. Association of the metabolic syndrome with physical activity performance in patients with schizophrenia. Diabetes Metab. (2011) 37:318–23. doi: 10.1016/j.diabet.2010.12.007
6. De Hert M, Peuskens B, van Winkel R, Kalnicka D, Hanssens L, Van Eyck D, et al. Body weight and self-esteem in patients with schizophrenia evaluated with B-WISE®. Schizophr Res. (2006) 88:222–6. doi: 10.1016/j.schres.2006.07.025
7. Vancampfort D, Probst M, Scheewe T, Maurissen K, Sweers K, Knapen J, et al. Lack of physical activity during leisure time contributes to an impaired health related quality of life in patients with schizophrenia. Schizophr Res. (2011) 129:122–7. doi: 10.1016/j.schres.2011.03.018
8. Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res. (2004) 66:51–7. doi: 10.1016/S0920-9964(02)00498-X
9. Misiak B, Stańczykiewicz B, Łaczmański Ł, Frydecka D. Lipid profile disturbances in antipsychotic-naive patients with first-episode non-affective psychosis: a systematic review and meta-analysis. Schizophr Res. (2017) 190:18–27. doi: 10.1016/j.schres.2017.03.031
10. Chen S, Broqueres-You D, Yang G, Wang Z, Li Y, Wang N, et al. Relationship between insulin resistance, dyslipidaemia and positive symptom in Chinese antipsychotic-naive first-episode patients with schizophrenia. Psychiatry Res. (2013) 210:825–9. doi: 10.1016/j.psychres.2013.08.056
11. Liu Y, Tao H, Yang X, Huang K, Zhang X, Li C. Decreased serum oxytocin and increased homocysteine in first-episode schizophrenia patients. Front Psychiatry. (2019) 10:217. doi: 10.3389/fpsyt.2019.00217
12. He Q, You Y, Yu L, Yao L, Lu H, Zhou X, et al. Uric acid levels in subjects with schizophrenia: a systematic review and meta-analysis. Psychiatry Res. (2020) 292:113305. doi: 10.1016/j.psychres.2020.113305
13. González-Blanco L, Greenhalgh AMD, Garcia-Rizo C, Fernandez-Egea E, Miller BJ, Kirkpatrick B. Prolactin concentrations in antipsychotic-naive patients with schizophrenia and related disorders: a meta-analysis. Schizophr Res. (2016) 174:156–60. doi: 10.1016/j.schres.2016.03.018
14. Zhou Y, Song X, Guo Y, Lang X, Li Z, Zhang XY. Sex differences in metabolic disorder patterns of first-episode drug-naive patients with schizophrenia. Psychoneuroendocrinology. (2021) 124:105061. doi: 10.1016/j.psyneuen.2020.105061
15. Bener A, Al-Hamaq AO, Dafeeah EE. A two fold risk of metabolic syndrome in a sample of patients with schizophrenia: do consanguinity and family history increase risk? Diabetes Metab Syndr. (2014) 8:24–9. doi: 10.1016/j.dsx.2013.10.003
16. Huang MC, Lu ML, Tsai CJ, Chen PY, Chiu CC, Jian DL, et al. Prevalence of metabolic syndrome among patients with schizophrenia or schizoaffective disorder in Taiwan. Acta Psychiatr Scand. (2009) 120:274–80. doi: 10.1111/j.1600-0447.2009.01401.x
17. Huang X, Bao C, Lv Q, Zhao J, Wang Y, Lang X, et al. Sex difference in cognitive impairment in drug-free schizophrenia: association with miR-195 levels. Psychoneuroendocrinology. (2020) 119:104748. doi: 10.1016/j.psyneuen.2020.104748
18. Chen S, Broqueres-You D, Yang G, Wang Z, Li Y, Yang F, et al. Male sex may be associated with higher metabolic risk in first-episode schizophrenia patients: a preliminary study. Asian J Psychiatry. (2016) 21:25–30. doi: 10.1016/j.ajp.2015.12.001
19. Fan X, Liu EY, Freudenreich O, Park JH, Liu D, Wang J, et al. Higher white blood cell counts are associated with an increased risk for metabolic syndrome and more severe psychopathology in non-diabetic patients with schizophrenia. Schizophr Res. (2010) 118:211–7. doi: 10.1016/j.schres.2010.02.1028
20. Hägg S, Lindblom Y, Mjörndal T, Adolfsson R. High prevalence of the metabolic syndrome among a Swedish cohort of patients with schizophrenia. Int Clin Psychopharmacol. (2006) 21:93–8. doi: 10.1097/01.yic.0000188215.84784.17
21. Howell S, Yarovova E, Khwanda A, Rosen SD. Cardiovascular effects of psychotic illnesses and antipsychotic therapy. Heart. (2019) 105:1852–9. doi: 10.1136/heartjnl-2017-312107
22. Ijaz S, Bolea B, Davies S, Savović J, Richards A, Sullivan S, et al. Antipsychotic polypharmacy and metabolic syndrome in schizophrenia: a review of systematic reviews. BMC Psychiatry. (2018) 18:1–13. doi: 10.1186/s12888-018-1848-y
23. Jeon SW, Kim Y-K. Unresolved issues for utilization of atypical antipsychotics in schizophrenia: antipsychotic polypharmacy and metabolic syndrome. Int J Mol Sci. (2017) 18:2174. doi: 10.3390/ijms18102174
24. Khalil RB. Atypical antipsychotic drugs, schizophrenia, and metabolic syndrome in non–Euro-American societies. Clin Neuropharmacol. (2012) 35:141–7. doi: 10.1097/WNF.0b013e31824d5288
25. Nielsen RE, Laursen MF, Vernal DL, Bisgaard C, Jakobsen H, Steinhausen H-C, et al. Risk of diabetes in children and adolescents exposed to antipsychotics: a nationwide 12-year case-control study. J Am Acad Child Adol Psychiatry. (2014) 53:971–9. e6. doi: 10.1016/j.jaac.2014.04.023
26. Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. (2009) 302:1765–73. doi: 10.1001/jama.2009.1549
27. Balõtšev R, Haring L, Koido K, Leping V, Kriisa K, Zilmer M, et al. Antipsychotic treatment is associated with inflammatory and metabolic biomarkers alterations among first-episode psychosis patients: A 7-month follow-up study. Early Int Psychiatry. (2019) 13:101–9. doi: 10.1111/eip.12457
28. Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M. Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull. (2013) 39:295–305. doi: 10.1093/schbul/sbs082
29. Tao Y-J, Hu L, He Y, Cao B-R, Chen J, Ye Y-H, et al. A real-world study on clinical predictors of relapse after hospitalized detoxification in a Chinese cohort with alcohol dependence. PeerJ. (2019) 7:e7547. doi: 10.7717/peerj.7547
31. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261. doi: 10.1093/schbul/13.2.261
32. Hamilton M. The assessment of anxiety states by rating. Bri J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
33. Hamilton M. A rating scale for depression. J Neurol Neuro Psychiatry. (1960) 23:56. doi: 10.1136/jnnp.23.1.56
34. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the national comorbidity survey. Arch Gen Psychiatry. (1999) 56:617. doi: 10.1001/archpsyc.56.7.617
35. Keefe RS. Brief Assessment of Cognition in Schizophrenia (BACS). Durham, NC: Duke University Medical Center, NeuroCog Trials (1999).
36. Heaton RK, Staff P. Wisconsin card sorting test: computer version 2. Odessa Psychol Assess Res. (1993) 4:1–4.
37. Burke HR. Raven's progressive matrices: a review and critical evaluation. J Gen Psychol. (1958) 93:199–228. doi: 10.1080/00221325.1958.10532420
38. Silverstein A. Two-and four-subtest short forms of the wechsler adult intelligence scale-revised. J Cons Clin Psychol. (1982) 50:415. doi: 10.1037/0022-006X.50.3.415
39. Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. (1935) 18:643–62. doi: 10.1037/h0054651
40. Lee SY, Park W, Suh YJ, Lim MJ, Kwon S-R, Lee J-H, et al. Association of serum uric acid with cardiovascular disease risk scores in Koreans. Int J Environ Res Public Health. (2019) 16:4632. doi: 10.3390/ijerph16234632
41. Hu L, Hu G, Xu BP, Zhu L, Zhou W, Wang T, et al. U-shaped association of serum uric acid with all-cause and cause-specific mortality in US adults: a cohort study. J Clin Endocrinol Metab. (2020) 105:e597–e609. doi: 10.1210/clinem/dgz068
42. Kim J, Kim H, Roh H, Kwon Y. Causes of hyperhomocysteinemia and its pathological significance. Arch Pharm Res. (2018) 41:372–83. doi: 10.1007/s12272-018-1016-4
43. Trinder M, Francis GA, Brunham LR. Association of monogenic vs polygenic hypercholesterolemia with risk of atherosclerotic cardiovascular disease. JAMA Cardiol. (2020) 5:390–9. doi: 10.1001/jamacardio.2019.5954
44. Sniderman AD, Couture P, Martin SS, DeGraaf J, Lawler PR, Cromwell WC, et al. Hypertriglyceridemia and cardiovascular risk: a cautionary note about metabolic confounding. J Lipid Res. (2018) 59:1266–75. doi: 10.1194/jlr.R082271
45. Krogh J, Selmer C, Torp-Pedersen C, Gislason GH, Kistorp C. Hyperprolactinemia and the association with all-cause mortality and cardiovascular mortality. Horm Metab Res. (2017) 49:411–7. doi: 10.1055/s-0043-107243
46. Maesaka JK, Cusano AJ, Thies HL, Siegal FP, Dreisbach AW. Hypouricemia in acquired immunodeficiency syndrome. Am J Kidney Dis. (1990) 15:252–7. doi: 10.1016/S0272-6386(12)80770-0
47. Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. New Eng J Med. (1991) 324:1149–55. doi: 10.1056/NEJM199104253241701
48. Melkersson KI, Hulting AL, Brismar KE. Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychoses. J Clin Psychiatry. (2000) 61:742–9. doi: 10.4088/jcp.v61n1006
50. Verhelst J, Abs R. Hyperprolactinemia. Treat Endocrinol. (2003) 2:23–32. doi: 10.2165/00024677-200302010-00003
51. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. (2007) 4:28. doi: 10.1161/01.CIR.85.1.212
52. Simpson G, Angus J. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. (1970) 45(S212):11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x
53. Chen K-P. Reliability and validity of the short version of udvalg for kliniske undersogelser in antipsychotic treatment. Psychiatr Quart. (2017) 88:787–96. doi: 10.1007/s11126-017-9494-y
54. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. (1989) 154:672–6. doi: 10.1192/bjp.154.5.672
55. Fann W, Stafford J, Malone R, Frost J Jr, Richman B. Clinical research techniques in tardive dyskinesia. Am J Psychiatry. (1977) 134:759–62. doi: 10.1176/ajp.134.7.759
56. Graham KA, Cho H, Brownley KA, Harp JB. Early treatment-related changes in diabetes and cardiovascular disease risk markers in first episode psychosis subjects. Schizophr Res. (2008) 101:287–94. doi: 10.1016/j.schres.2007.12.476
57. Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA. Increased mortality in schizophrenia due to cardiovascular disease–a non-systematic review of epidemiology, possible causes, and interventions. Front Psychiatry. (2014) 5:137. doi: 10.3389/fpsyt.2014.00137
58. Leppik L, Parksepp M, Janno S, Koido K, Haring L, Vasar E, et al. Profiling of lipidomics before and after antipsychotic treatment in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci. (2020) 270:59–70. doi: 10.1007/s00406-018-0971-6
59. Larson MK, Walker EF, Compton MT. Early signs, diagnosis and therapeutics of the prodromal phase of schizophrenia and related psychotic disorders. Exp Rev Neurother. (2010) 10:1347–59. doi: 10.1586/ern.10.93
60. Petruzzelli MG, Margari M, Peschechera A, de Giambattista C, De Giacomo A, Matera E, et al. Hyperprolactinemia and insulin resistance in drug naive patients with early onset first episode psychosis. BMC Psychiatry. (2018) 18:1–7. doi: 10.1186/s12888-018-1827-3
61. Polymeropoulos MH, Licamele L, Volpi S, Mack K, Mitkus SN, Carstea ED, et al. Common effect of antipsychotics on the biosynthesis and regulation of fatty acids and cholesterol supports a key role of lipid homeostasis in schizophrenia. Schizophr Res. (2009) 108:134–42. doi: 10.1016/j.schres.2008.11.025
62. Zhou X, Long T, Haas GL, Cai H, Yao JK. Reduced levels and disrupted biosynthesis pathways of plasma free fatty acids in first-episode antipsychotic-naïve schizophrenia patients. Front Neurosci. (2020) 14:784. doi: 10.3389/fnins.2020.00784
63. Sethom M, Fares S, Bouaziz N, Melki W, Jemaa R, Feki M, et al. Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostag Leukotr Essent Fatty Acids. (2010) 83:131–6. doi: 10.1016/j.plefa.2010.07.001
64. Tkachev D, Mimmack ML, Huffaker SJ, Ryan M, Bahn S. Further evidence for altered myelin biosynthesis and glutamatergic dysfunction in schizophrenia. Int J Neuropsychopharmacol. (2007) 10:557–63. doi: 10.1017/S1461145706007334
65. Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circulat Physiol. (2005) 289:H2649–56. doi: 10.1152/ajpheart.00548.2005
67. MohanKumar SM, Kasturi BS, Shin AC, Balasubramanian P, Gilbreath ET, Subramanian M, et al. Chronic estradiol exposure induces oxidative stress in the hypothalamus to decrease hypothalamic dopamine and cause hyperprolactinemia. Am J Physiol Regul Integr Comp Physiol. (2011) 300:R693–R9. doi: 10.1152/ajpregu.00481.2010
68. Levine S, Muneyyirci-Delale O. Stress-induced hyperprolactinemia: pathophysiology and clinical approach. Obstetr Gynecol Int. (2018) 1–6. doi: 10.1155/2018/9253083
69. Cordes J, Bechdolf A, Engelke C, Kahl KG, Balijepalli C, Lösch C, et al. Prevalence of metabolic syndrome in female and male patients at risk of psychosis. Schizophr Res. (2017) 181:38–42. doi: 10.1016/j.schres.2016.09.012
70. Kraal AZ, Ward KM, Ellingrod VL. Sex differences in antipsychotic related metabolic functioning in schizophrenia spectrum disorders. Psychopharmacol Bull. (2017) 47:8. doi: 10.1007/978-94-007-0831-0_5
71. McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the clinical antipsychotic trials of intervention effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. (2005) 80:19–32. doi: 10.1016/j.schres.2005.07.014
72. Li R, Li W, Lun Z, Zhang H, Sun Z, Kanu JS, et al. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. (2016) 16:1–10. doi: 10.1186/s12889-016-2870-y
73. Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2019) 6:211–24. doi: 10.1016/S2215-0366(18)30511-X
74. Yang X, Sun L, Zhao A, Hu X, Qing Y, Jiang J, et al. Serum fatty acid patterns in patients with schizophrenia: a targeted metabonomics study. Trans Psychiatry. (2017) 7:e1176. doi: 10.1038/tp.2017.152
75. Roth TL, Lubin FD, Sodhi M, Kleinman JE. Epigenetic mechanisms in schizophrenia. Biochim Biophys Acta. (2009) 1790:869–77. doi: 10.1016/j.bbagen.2009.06.009
76. Ren Y, Cui Y, Li X, Wang B, Na L, Shi J, et al. A co-expression network analysis reveals lncRNA abnormalities in peripheral blood in early-onset schizophrenia. Prog Neuro Psychopharmacol Biol Psychiatry. (2015) 63:1–5. doi: 10.1016/j.pnpbp.2015.05.002
77. Morgan C, Fisher H. Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma—a critical review. Schizophr Bull. (2007) 33:3–10. doi: 10.1093/schbul/sbl053
Keywords: first-episode drug-naï ve schizophrenia, metabolic abnormalities, Homocysteine, triglyceride, prolactin
Citation: Zhang Q, He H, Bai X, Jiang L, Chen W, Zeng X, Li Y, Teixeira AL and Dai J (2021) Unveiling the Metabolic Profile of First-Episode Drug-Naïve Schizophrenia Patients: Baseline Characteristics of a Longitudinal Study Among Han Chinese. Front. Psychiatry 12:702720. doi: 10.3389/fpsyt.2021.702720
Received: 29 April 2021; Accepted: 15 June 2021;
Published: 09 July 2021.
Edited by:
Bin Zhang, Southern Medical University, ChinaReviewed by:
Zezhi Li, Shanghai JiaoTong University, ChinaYanhui Liao, Sir Run Run Shaw Hospital, China
Copyright © 2021 Zhang, He, Bai, Jiang, Chen, Zeng, Li, Teixeira and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio L. Teixeira, QW50b25pby5MLlRlaXhlaXJhQHV0aC50bWMuZWR1; Jing Dai, amluZy5kYWlAdWVzdGMuZWR1LmNu
†These authors share first authorship
 Qi Zhang
Qi Zhang Hui He
Hui He Xia Bai
Xia Bai Liping Jiang
Liping Jiang Wei Chen
Wei Chen Xiaoying Zeng
Xiaoying Zeng Yanjia Li
Yanjia Li Antonio L. Teixeira
Antonio L. Teixeira Jing Dai
Jing Dai