- 1School of Psychological Science, University of Western Australia, Perth, WA, Australia
- 2Centre for Sleep Science, School of Human Sciences, University of Western Australia, Perth, WA, Australia
- 3Department of Pulmonary Physiology and Sleep Medicine, West Australian Sleep Disorders Research Institute, Sir Charles Gairdner Hospital, Nedlands, WA, Australia
- 4College of Medicine and Public Health, Flinders Health and Medical Research Institute, Flinders University, Bedford Park, SA, Australia
- 5School of Population and Global Health, University of Western Australia, Perth, WA, Australia
Study Objectives: To determine cognitive profiles in individuals with short sleep duration insomnia (SSDI) and normal sleep duration insomnia (NSDI; also, paradoxical insomnia), compared to healthy sleepers.
Method: Polysomnographic (PSG) and neuropsychological data were analysed from 902 community-based Raine Study participants aged 22 ± 0.6 years of whom 124 met criteria for insomnia (53 with NSDI and 71 with or SSDI) and 246 were classified as healthy with normal sleep (i.e., without insomnia or other sleep disorders). Measurements of self- report (attention and memory) and laboratory-assessed (attention, episodic memory, working memory, learning, and psychomotor function) cognition and mood, and PSG-based sleep stages (% total sleep time; %TST) were compared between these 3 groups.
Results: In comparison to the healthy sleeper group, both insomnia groups had poorer self-reported attention, memory, mood, and sleep, and poorer laboratory-assessed attention (inconsistency). The NSDI group had less consistent working memory reaction time than healthy-sleepers or those with SSDI. The SSDI group had more inconsistency in executive function (shifting), and showed greater %TST in stage N1 and N3, and less REM sleep than either healthy-sleepers or those with NSDI.
Conclusions: Individuals with NSDI demonstrated greater working memory inconsistency, despite no laboratory assessed sleep problems, implicating early signs of pathophysiology other than disturbed sleep. Those with SSDI demonstrated different sleep architecture, poorer attention (inconsistency), and greater executive function (inconsistency) compared to healthy-sleepers and those with NSDI, implicating sleep disturbance in the disease process of this phenotype.
Introduction
Insomnia is a highly prevalent sleep disorder (1), characterised by self-reported dissatisfaction with sleep quality or quantity, frequently expressed as difficulty initiating or maintaining sleep, or experiencing non-restorative sleep over many days, and accompanied by significant distress or daytime impairment (2). Risk factors for developing insomnia include female gender, older age, and chronic illness or pain (3). Comorbidities include other sleep disorders and psychiatric disorders, the latter being present in ~40% (1), obesity and metabolic problems (4). Consequences include an increased risk of accidents, poorer work productivity, higher pain levels, and more emotional and mental health problems (5). Cognitive problems are often found using laboratory-based computerised neuropsychological tests in individuals with insomnia (6, 7).
In a recent systematic review and meta-analysis of insomnia and cognitive performance, Wardle-Pinkston et al. (6) reported small to medium differences in the cognitive domains of complex attention, working memory, episodic memory, and executive function between individuals with and without insomnia. An earlier meta-analysis by Fortier-Brochu et al. (8) also reported small to medium effects for episodic memory, working memory, and executive functions (problem solving), whilst finding no differences in attention. Further, an investigation by Ballesio et al. (9), focussing on executive functions, found small effects for reaction times, but not accuracy, in the subdomains of inhibition, flexibility, and working memory.
The findings of these three papers contrast with those from a meta-analysis by Fulda and Shulz (10) who reported no differences in working memory, episodic memory, or attention, but did find contrasting ability between those with and without insomnia in a different aspect of executive functioning (generativity). Taken together, these reviews indicate that insomnia is associated with small to moderate, but variable, effects on cognition within the domains of working memory, episodic memory, and executive function.
Such inconsistencies may result from three possibilities: (1) Treating insomnia as a homogenous disorder, when it is not; (2) Assessing cognition using measures that are not sensitive to subtle changes in cognition, and/or; (3) Using older samples where age and comorbidity may confound the results. The review by Wardle-Pinkston et al. proposed that results may be variable due to differences in lab-assessed (i.e., objective) sleep factors between individuals with insomnia and psychometric testing sensitivity (6). These concepts are expanded upon below.
Two different phenotypes of insomnia are consistently identified: insomnia with short sleep duration (SSDI) and insomnia with normal sleep duration (NSDI; also called paradoxical insomnia or sleep state misperception) (11). These two phenotypes have different daytime symptoms (12), nocturnal symptoms (13), self-reported cognitive problems (14, 15), and underlying biology (16), all of which may also be associated with dissimilar objective neurocognitive challenges.
Individuals with SSDI have a short sleep period, and can accurately self-report wakefulness in the presence of lab-assessed wakefulness (13). They experience daytime fatigue, and self-reported problems with attention and memory (13). Further, SSDI is associated with self-reported and lab-assessed cognitive difficulties, mood disruption, physiological hyperarousal, and a higher risk of hypertension, diabetes, and all-cause mortality (17). SSDI also appears to be a biological marker of genetic predisposition to chronic insomnia (17).
In contrast, people with NSDI report short sleep time whilst lab-assessments indicate normal sleep time, and report being awake when PSG indicates sleep (16), termed sleep state-misperception. NSDI is also associated with self-reported and objective cognitive difficulties, mood disruption, and cortical hyperarousal (17). The Default Mode Network (DMN), is associated with self-referential information processing and has been shown to remain active in patients with insomnia, when it would deactivate in a healthy sleeper (16, 18, 19). Problems with the DMN are thought to result in deficits in self-referential and goal-directed behaviours (i.e., executive functions) (20), and have been implicated in mood disorders (12) and the development of dementia (15). This process may underlie the paradoxical experience of feeling awake while biologically asleep in those with NSDI (21).
Further, some of the inconsistent findings across reviews may be due to using measures of cognition that are not sensitive to small and/or early changes. All reviews [bar Fulda and Shulz, (10)] report small to moderate effects across papers, and propose that cognitive test sensitivity may account for differences across studies. To-date, the studies examining cognition in OSA have utilised traditional measures of accuracy and mean reaction time. None has explored intra-individual variability, which may be a more sensitive measure of cognitive performance. A measure of intra-individual variability, inconsistency, refers to intra-individual, short-term fluctuations in performance across trials, within a task, and can be measured using the intra-individual standard deviation (ISD) of reaction time on a trial-by-trial basis. Intra-individual variability has been demonstrated to be more sensitive than accuracy and mean reaction time to subtle cognitive changes, that may be exhibited in mild or early onset of disease (22, 23).
Finally, being middle-aged or older is itself associated with subtle decline in cognition and is frequently associated with greater comorbidity and disease burden (24–27). Declines in cognition are demonstrated in psychometric assessments using measures of reaction time, accuracy (frequently show an accuracy/speed trade-off), and in studies incorporating inconsistency measures (27). Given that the majority of studies of cognition in insomnia have recruited participants of middle to older age [e.g., in Wardle-Pinkston et al. (6) the mean age of participants across studies was 44.9 years], ageing effects in cognition may have confounded findings.
The present research aimed to contrast the cognitive function of individuals with SSDI and NSDI using self-report and computerised assessments providing measures of accuracy, speed, and inconsistency of performance, examining the cognitive constructs of attention, learning, working memory, executive function, and psychomotor funtion, in a sample of young adults.
We asked:
1. Do individuals with SSDI or NSDI differ from age-matched healthy sleeper controls from the same sample and, if yes, on what aspects of cognition?
2. How are the cognitive profiles of individuals with SSDI and NSDI different?
3. How are mood, daytime function outcomes, and sleep profiles of individuals with SSDI and NSDI different?
With regard to sleep, we hypothesised that: The SSDI group would have poorer self report and lab-assessed sleep than the NSDI and healthy sleepers, and; The NSDI group would have poor self-report but not lab assessed sleep than the healthy sleepers. Second, with regard to cognition, we hypothesised that: The NSDI and SSDI groups would report similar problems for self-report cognition, mood, and daytime function, and these would be greater than those reported by healthy sleepers, and; The SSDI group would show more extensive lab-assessed cognitive difficulties than the NSDI group.
Materials and Methods
Participants
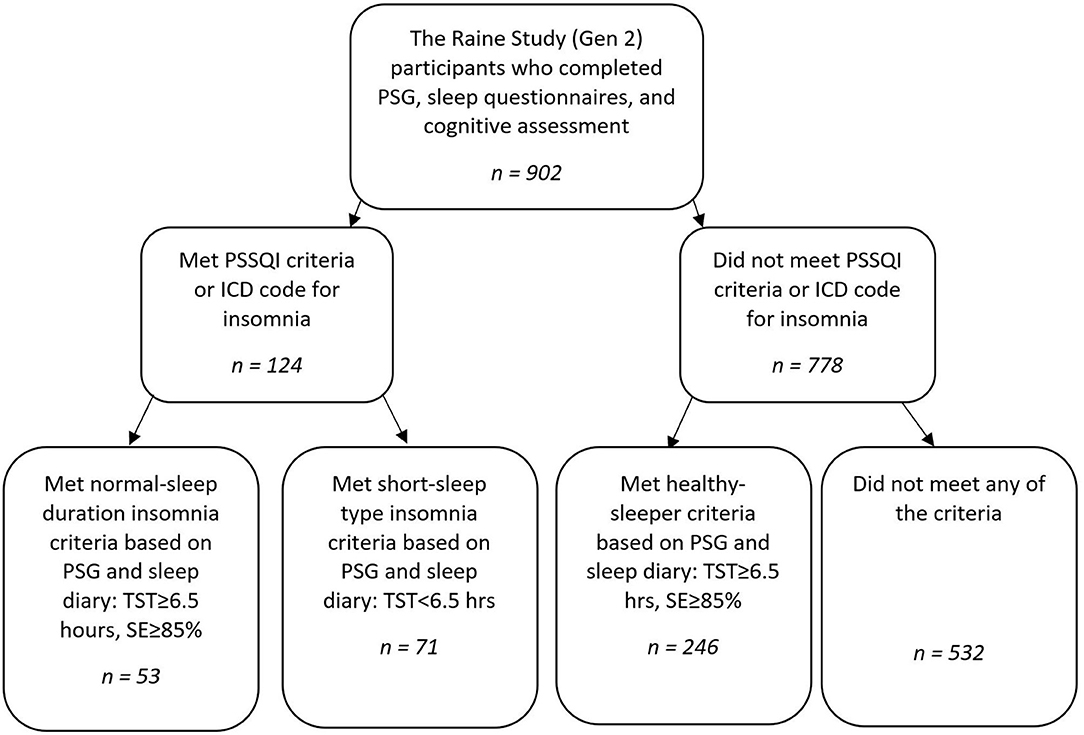
The present analyses used data from the Raine Study, a multigenerational longitudinal epidemiological study established in 1989 (for more study details visit; rainestudy.org.au). Data from Generation 2 (Gen2) participants1 who completed the sleep study, actigraphy, and cognitive testing at the Gen2-22-year follow-up were used (n = 902).
Individuals with either a diagnosis of insomnia (DSM-5) or who met the insomnia criterion on the insomnia symptom questionnaire (ISQ; n = 156) were separated into NSDI (n = 63) or SSDI (n = 93) based on the Research Diagnostic Criteria (RDC) for PSG (28). For NSDI the RDC requires that PSG shows scored total sleep time (TST) ≥6.5 h, sleep efficiency (SE; TST/Time in bed × 100) ≥85%. For SSDI, the RDC requires short-sleep duration, PSG-based TST <6.5 h.
While participants were categorised by sleep study, they were retained if their self-report sleep diary data validated short or normal sleep duration: normal-sleep duration (n = 53) or short-sleep duration (n = 71). This was to ascertain if this single night of sleep under PSG was typical of an individual's sleep.
A healthy sleeper sample was constructed from those participants from the Raine Study who were without insomnia or other sleep disorders, had SE ≥85%, and TST ≥6.5 h on PSG (n = 324). Participants were retained if they reported normal sleep duration (TST>= 6.5 h) on their sleep diary (n = 246).
Participants who did not meet the insomnia selection criteria, healthy sleeper criteria, had a history of neurological, neurodevelopmental, significant psychiatric history, shift-work, or other sleep disorder/s (e.g., sleep apnoea with apnoea hypopnoea index≥15 and/or restless legs syndrome) were excluded (n = 532) from analyses.
A flow chart of study group selection is shown in Figure 1.
Materials
Sleep Study/PSG
Participants were administered full overnight Level 1 polysomnography (PSG) [Compumedics E-Series (Compumedics, Melbourne Australia)] and scored using Compumedics PSG 3 software at the Centre for Sleep Science, University of Western Australia. Equipment placement, sleep staging and event scoring was completed by experienced sleep technologists according to American Academy of Sleep Medicine criteria (29).
Cogstate Computerised Battery
This computerised battery (30) provided tasks of attention (Card Identification), executive functioning (Set Shifting), learning (Continuous Paired Associates), psychomotor function (Detection Test), and working memory (One-back Task). The tasks show good correlations to traditional neuropsychological assessments (30) even when measured in populations that exhibit subtle cognitive changes (31). The Cogstate records both accuracy (whether the trial was answered correctly) and speed (time to make a correct response) on a trial-by-trial basis, allowing calculation of accuracy, mean response time, and variability in response time (inconsistency) using intra-individual standard deviations, for each task assessed.
To date, a few small sample studies examining cognition in obstructive sleep apnoea have included cognitive inconsistency amongst their measures. All have found greater IIV (i.e., more inconsistency or less cognitive stability) in those with obstructive sleep apnoea compared to healthy controls (32). IIV has not been reported in insomnia.
Prospective and Retrospective Memory Questionnaire
A 16-item questionnaire (33) that provides a self-reported assessment of prospective and retrospective memory errors. The scale demonstrates good construct validity and internal reliability (α = 0.80–89) (33). Scores were generated for prospective and retrospective subscales.
Attention-Related Cognitive Errors Scale
This 12-item questionnaire (34) provides a measure of everyday mistakes made when not paying sufficient attention to a task. The values are summed to give an overall score. ARCES scores are highly correlated with other scales assessing errors of sustained attention (35).
Pittsburgh Sleep Symptom Questionnaire-Insomnia/Insomnia Symptom Questionnaire
A 13-item self-report tool (36) designed and used to identify insomnia symptoms and provide a case definition of insomnia. The items are consistent with the RDC (28) for insomnia and compared with interview methods of diagnosing insomnia the ISQ has good internal reliability (α = 0.89), moderate sensitivity (50–67%) and good specificity (91%) (36).
Functional Outcomes of Sleep Questionnaire-10 (FOSQ-10)
This questionnaire (37) has 10-items designed to assess the impact of daytime sleepiness on daytime activity. The FOSQ-10 has good internal consistency (α = 0.87) and demonstrates changes over time with successful treatment of sleep disorders (38). Scores are reported for general productivity, vigilance, social outcomes, activity level, and sexual desire.
Pittsburgh Sleep Quality Index
An 18-item questionnaire (39) that is designed to assess sleep habits, disturbances, and daytime impairments. This scale shows good internal consistency (α = 0.73) and shows a strong correlation with other scales assessing daytime function (40).
Epworth Sleepiness Scale
This self-administered 8-item scale (41) assesses sleepiness in several every-day situations. The questionnaire has good internal consistency (α = 0.73–0.90). Excessive sleepiness is a score of 10 or more.
Depression Anxiety and Stress Questionnaire-21 Item
This well-established 21-item questionnaire (42) assesses symptoms of depression, anxiety, and stress. It has demonstrated good internal reliability (α = 0.82–0.97) (42) and is an appropriate mood measure for sleep disordered populations, as it does not contain sleep-related items (43). Scores were generated for depression, anxiety, and stress subscales.
Procedure
Neuropsychological testing and questionnaires were administered to all participants the night of or the morning after their sleep study at the Centre for Sleep Science. There was no adaptation night for this sleep study, hence we examined if this night was representative of an individual's habitual sleep against a weeklong sleep diary.
Analysis
Descriptive statistics for the SSDI, NSDI, and healthy-sleeper groups are presented in Table 1.
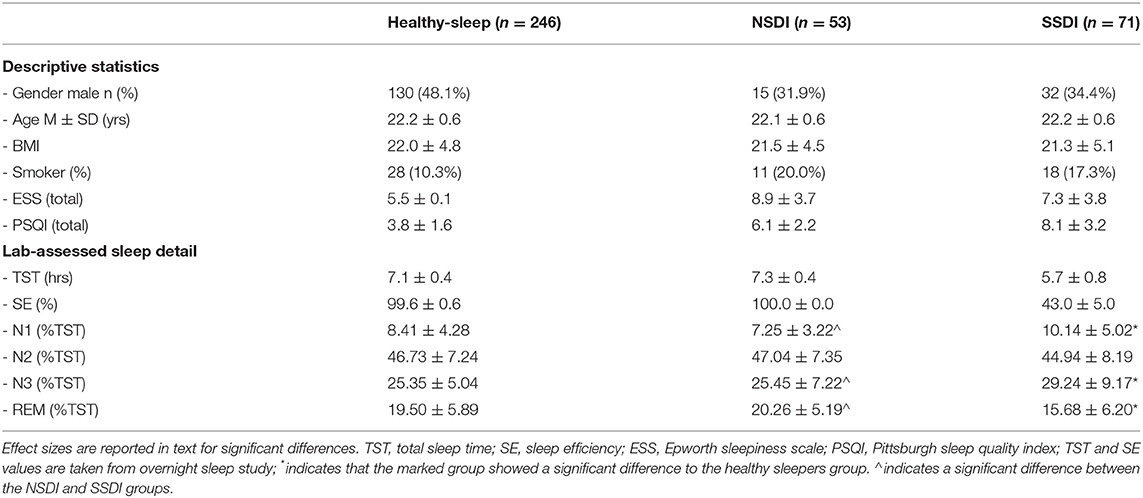
Table 1. Descriptive statistics and lab-assessed sleep detail (PSG staged sleep; Stage as % of total sleep time) for the healthy sleepers, those with NSDI, and SSDI.
Residuals of the dependent variables were checked for and approximated normality as assessed through visual inspection of normality plots, p-p plots, and metrics of skew and kurtosis. Due to the differences in sample size, homogeneity of variance was assessed and was not violated. Independence of observations was met as all participants were counted in only one group (healthy, long-sleep duration, or short-sleep).
MANOVAs were conducted to compare the effect of group (normal-sleep duration insomnia, short-sleep type insomnia, and healthy sleepers) on lab-assessed sleep differences (PSG—-percentage N1, N2, N3, and REM), lab-based (Cogstate) and self-report cognition (retrospective and prospective memory subscales of the PRMQ and ARCES total score), self-reported mood (DASS-21- stress, anxiety and depression subscale scores), and self-reported daytime function (FOSQ—-general productivity, vigilance, social outcomes, activity level and sexual desire subscale scores). Lab-based (Cogstate) domains of cognition examined were attention, executive functioning, working memory, learning, and psychomotor function. For all domains, tests of accuracy, speed, and inconsistency of performance were assessed for group differences. Where tests of sphericity were violated an adjusted F value is reported. An alpha level of 0.05 was used, except where omnibus group interaction or main effect differences were found, then post-hoc Bonferroni-corrected tests were conducted (0.05 × number of tests).
Results
Overall, these data show that the cohort was relatively young (22-yrs), that lab-assessed sleep efficiency was poor in those with SSDI, and that both phenotypes reported high levels of sleepiness, and low levels of sleep quality (as per cut-offs on the PSQI).
Lab-Assessed Sleep Differences
Means for sleep study data are presented in Table 1.
Interactions were significant for group by sleep stage, Roy's Largest Root, F(3, 427) = 2267.69, p < 0.001. Indicating that time spent in sleep stages varied by group.
Post-hoc comparisons indicated that participants with SSDI spent a larger percentage of TST in N1 compared to the healthy sleepers [p < 0.001; Cohen's d = 0.39 (95% CI, LL = 0.12, UL = 0.65)] and to those with NSDI [p < 0.001; Cohen's d = 0.67 (95% CI, LL = 0.31, UL = 1.02)]. Those with SSDI also spent a higher percentage of TST in N3 than those with healthy sleep [p < 0.001; Cohen's d = 0.63 (95% CI, LL = 0.36, UL = 0.89)] or with NSDI [p < 0.001; Cohen's d = 0.45 (95% CI, LL = 0.09, UL = 0.81)]. Finally, those with SSDI had lower %REM than healthy sleep [p < 0.001; Cohen's d = 0.72 (95% CI, LL = 0.46, UL = 0.99)] and NSDI [p < 0.001; Cohen's d = 0.88 (95% CI, LL = 0.52, UL = 1.23)].
In summary, participants with SSDI demonstrated a greater percentage of their total sleep time in NREM sleep and less time in REM sleep than the healthy sleepers or those with NSDI.
Lab-Assessed Cognition
Interactions were not significant for accuracy or reaction time, however, a significant group by cognitive test interaction was found for inconsistency, Roy's Largest Root, F(8, 1212) = 3.15, p = 0.002, revealing a different pattern of cognitive performance for each group.
For attention (inconsistency) the healthy sleeper group showed more consistent response times than the NSDI [p < 0.001; Cohen's d = 0.91 (95% CI, LL = 0.61, UL = 1.20)] and the SSDI (p = 0.006; Cohen's d = 0.44 (95% CI, LL = 0.18, UL = 0.71) groups. For working memory (inconsistency), the healthy sleepers had more consistent response times than the NSDI [p = 0.005; Cohen's d = 0.31 (95% CI, LL = 0.01, UL = 0.61)] but not the SSDI group. For executive function, shifting (inconsistency) healthy sleepers were more consistent in their response times than the SSDI group [p < 0.001; Cohen's d = 0.55 (95% CI, LL = 0.29, UL = 0.82)], but not the NSDI. Means for all lab-assessed cognitive tests are presented in Table 2.
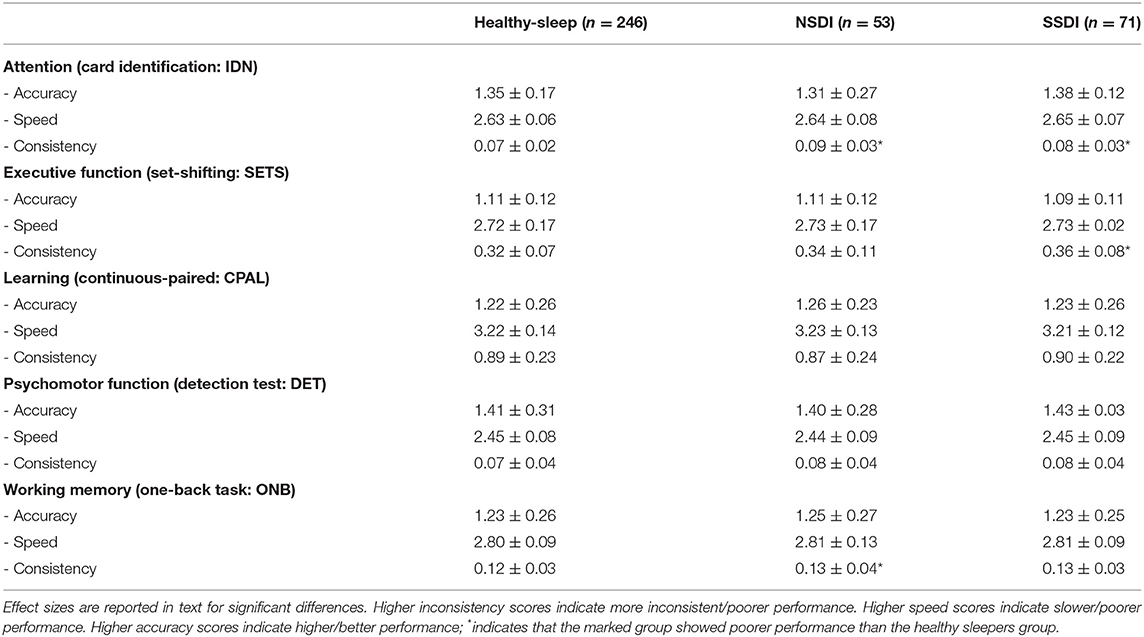
Table 2. Means and standard deviations for lab-assessed cognitive assessments (CogState) for the healthy sleepers, those with NSDI, and SSDI.
There were no group differences on psychomotor function or learning, nor any interactions with group.
Taken together, these results indicate that the healthy participant group experienced better lab-assessed cognition overall and that both insomnia groups demonstrated greater inconsistency; the NSDI group demonstrated poorer working memory than the healthy sleepers; and, the SSDI group demonstrated poorer executive function, shifting, than the healthy sleepers.
Self-Report Cognition
Interactions were significant for group by self-report cognitive test, Roy's largest root, F(3, 423) = 11.29, p < 0.001.
Post-hoc comparisons indicated that all mean scores for all self-reported cognition assessments for the healthy sleeper group were significantly better than the NSDI (p < 0.001) and SSDI groups (p < 0.001), however the insomnia groups did not differ. Means are presented in Table 3.
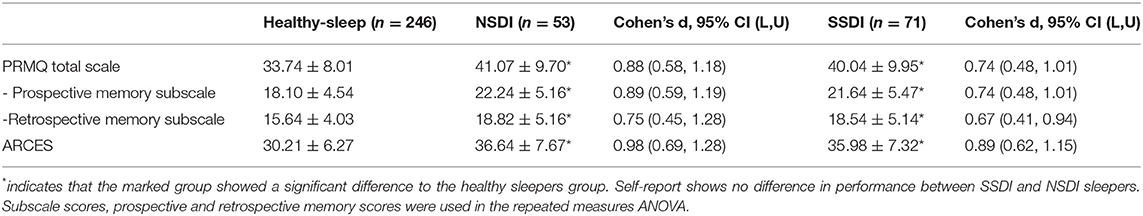
Table 3. Means and standard deviations for the self-reported cognition assessments (PRMQ and ARCES) for the healthy sleepers, those with NSDI, and SSDI.
These results indicate that people from both the SSDI and NSDI groups reported poorer attention, retrospective, and prospective memory than individuals with healthy sleep, and that the insomnia groups did not differ from one another.
Self-Reported Mood and Daytime Function
Interactions were significant for group by mood [Roy's Largest Root: F(3, 402) = 5.868, p < 0.001] and daytime function by group [Roy's Largest Root: F(5, 391) = 26.596, p < 0.001].
Post-hoc comparisons indicated that mean scores for all assessments of self-reported mood (p < 0.001) and functional sleep outcomes (p < 0.001, with the exception of the FOSQ vigilance subscale, p = 0.003) were significantly higher in both insomnia groups than in healthy sleepers, though the insomnia groups did not differ. Means are presented in Table 4.
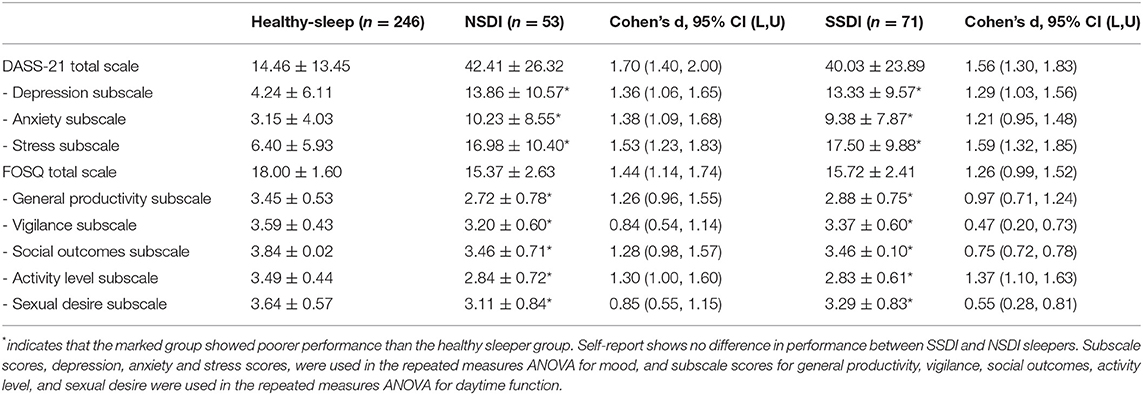
Table 4. Means and standard deviations for self-reported mood and daytime function assessments (DASS-21 and FOSQ questionnaires) for the healthy sleepers, those with NSDI, and SSDI.
These results suggest that both the NSDI and SSDI groups report poorer mood across higher depression, stress, and anxiety, and report that their poor sleep impacts on their ability to function day-to-day, with regards to productivity, vigilance, social outcomes, activity levels, and sexual desire, than healthy sleepers.
Discussion
This paper aimed to characterise the two main insomnia phenotypes (SSDI and NSDI) with detailed lab and self-report cognitive and mood assessments, in a large sample of Gen2 participants from the Raine Study. The results demonstrate that those with SSDI and NSDI self-report problems with attention and memory, daytime function due to poor sleep. Further, those with SSDI and NSDI show greater inconsistency in performance on objective attention tasks. Those with SSDI show less consistent executive functioning and those with NSDI show less consistent working memory, than those with healthy sleep. Further, those with SSDI demonstrated sleep architecture that was different from NSDI and healthy sleepers, while those with NSDI showed relatively healthy lab-assessed sleep. These differences are summarised in Figure 2.
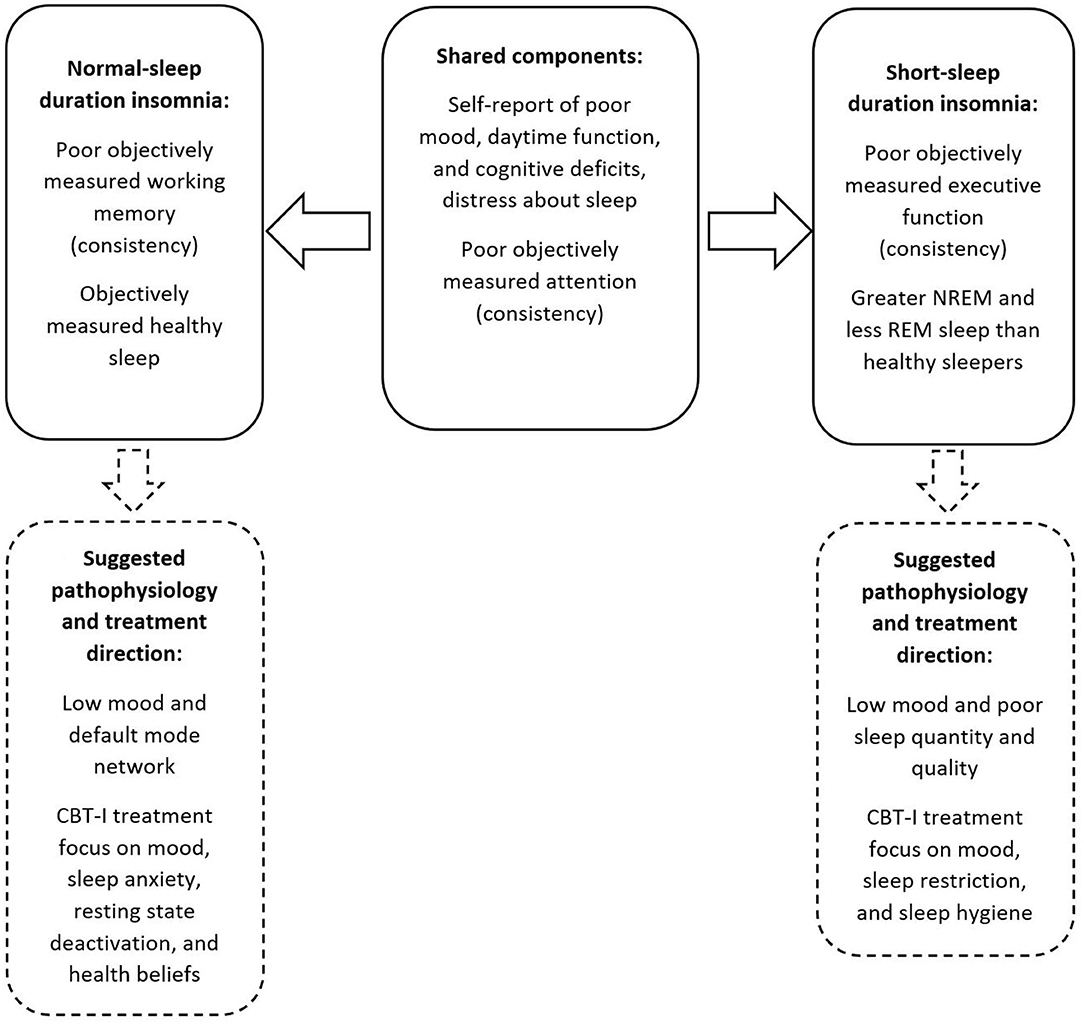
Figure 2. Summary of the shared and separable profiles of SSDI and NSDI, and suggested pathophysiology and treatment focus.
Self-Reported Mood, Function, and Sleep
In line with the literature (1, 6, 13), those with both SSDI and NSDI self-report problems with cognition (attention and memory), daytime function, mood, and sleep quality. As self-reported sleep quality, daytime function, and mood are core components of an insomnia diagnosis (2), this result is not surprising. Further, these findings suggest that SSDI and NSDI do not differ in terms of self-report measures of cognition, however they do differ on objective assessments.
Lab-Assessed Cognition and Sleep
Both phenotypes of insomnia exhibit inconsistency in attention. Whilst Wardle-Pinkston et al. (6) also reported attention problems in insomnia in their meta-analysis, Fulda and Schulz (10) and Fortier Brochu et al. (8) did not, but all three studies reported accuracy. This sample, using a younger sample than previous studies, found no evidence of problems with attention accuracy, but did find greater inconsistency in the speed of responding to attention trials in insomnia. Likewise, whilst past studies, also with older participants, have reported deficits in working memory and executive function accuracy (6, 8, 10), we found more subtle effects in inconsistency of responding in these domains. These differences were varied across the insomnia phenotypes: those with NSDI were less consistent in working memory despite relatively healthy sleep, while those with SSDI were less consistent in executive functioning, and had different sleep architecture (more N1 and N3, and less REM, as a percentage of total sleep time) than healthy sleepers and those with NSDI.
A high amount of N3 sleep, seen here in those with SSDI, has been noted to indicate rebound sleep. This is considered ‘recovery sleep’ as shown after sleep deprivation or chronic sleep restriction (44). This supports the diagnosis of SSDI, provided by PSG, diary, and self-report symptoms in this study, and supports the idea that the cognitive problems shown in SSDI are the result of chronic sleep loss. Conversely, those with NSDI showed subtle objective and self-reported cognitive deficits, despite no evidence of lab-based sleep loss.
That separable cognitive profiles and sleep profiles were demonstrated for the different phenotypes suggests future directions for providing a differential diagnosis. Currently, as in this paper, overnight sleep study or actigraphy are used to assess the mismatch between self-report and lab-assessed sleep and distinguish short- from normal normal-sleep duration insomnia (28). However, sleep studies, actigraphy, and full neuropsychological assessment can be expensive and time consuming, and are activities requiring a high degree of specialised training. The present paper suggests that cognitive tasks such as those used here, may provide further information to profile those with insomnia. When computerised, these tasks are relatively quick (7-min in healthy participants), and easy to administer. However, these results require replication by other groups and the ability of these differences to discriminate groups requires validation.
Working memory and executive functions are separable but related components of cognition. Working memory is a limited capacity cognitive system that can hold information ready for processing for a limited time (45). The executive function factor assessed here, shifting, is related to working memory, as it assists with shifting attentional control quickly (46). Other aspects of executive function [updating, generativity, fluid problem solving, and inhibition (46, 47)] were not assessed in the present paper, and as such we have an incomplete picture of executive function in these subtypes of insomnia. A complete examination of executive functions in insomnia phenotypes will provide greater understanding of how executive functions are impacted and deepen our understanding of different cognitive profiles in insomnia.
There were no psychomotor or learning problems for either phenotype uncovered in these analyses. These findings are in line with past studies of psychomotor function (6, 8, 10) and in contrast to past explorations of learning in insomnia (6). Learning, in the current sample, may not have been problematic as Generation-2 from the Raine Study were young (Age, M = 22 years, at the time of assessment). Young age is protective for cognition. Wardle-Pinkston et al. (6) investigated age as a moderator in their analyses showing that older age was associated with larger effect sizes in the differences between healthy sleepers and individuals with insomnia. To explore the impact of age, future research could investigate phenotypic differences in the progression of cognitive deficits in longitudinal datasets, or compare cognitive function in older and younger samples. The Raine Study will make an excellent space to explore this concept as data continue to be collected on the same individuals, their parents (Generation 1), their grandparents, (Generation 0) and now the children of Generation 2 (i.e., Generation 3).
Where there were cognitive differences, for both phenotypes of insomnia these were in maintaining consistent response times throughout a testing trial, whilst accuracy and speed were not impacted (6, 8, 10). This finding, taken together with the small effects evidenced in meta-analyses and inconsistent findings across the field, identifies a need for sensitive measures of cognition that capture moment-to-moment performance stability, such as intra-individual variability (IIV) (48). Previous studies have not examined inconsistency, reporting only measures of accuracy and speed, meaning it is possible these consistency differences were present in earlier samples. The literature on IIV indicates it is a sensitive measure of early cognitive change and is predictive of later cognitive dysfunction and decline (49). Future follow-up assessments of the Raine Study participants will be able to track those showing early cognitive instability to see if clearer cognitive problems, in accuracy, speed, and/or a wider set of cognitive domains, develop.
Further, the present sample were relatively healthy, young individuals involved in research from before birth to their mid-twenties, possibly leading to selection bias. As comorbidity, including overweight and psychological diagnoses, can independently impact sleep, sleep disorders, and cognition (50), future studies may wish to investigate the interaction of comorbidity and/or other demographic features with cognition in those with insomnia.
Pathophysiology
Hyperarousal is a core pathophysiological feature of insomnia, in general, and is explained in two different models: a psychological model and a physiological model. The psychological model posits that worry and rumination about life stress, and about sleep itself, disrupt sleep, whereas, the physiological model posits that hyperarousal is due to a higher level of neuroendocrine and metabolic functioning, which disrupts sleep.
While it is possible that cognition and sleep, in both insomnia phenotypes, are impacted by psychological processes, as certainly belief impacts biological health in other areas, including stress (51) and treatment uptake (52, 53), such a model, with one road to pathology, does not explain the different cognitive and sleep profiles of these disorders, as evidenced here. There is some discussion in the literature of differing types of hyperarousal across the two phenotypes. Short sleep is associated with physiological hyperarousal while NSDI is associated with cortical hyperarousal (17). While physiological hyperarousal is a heightened stress state due to negative thoughts, cortical hyperarousal is increased activation of the reticular formation causing an increase in wakefulness. The result from the present paper provide further evidence of two different disorders that may have different underlying causes of hyperarousal.
Short-Sleep Duration Insomnia
Disrupted sleep appears to be a core feature of short-sleep duration insomnia, with less total time asleep, a higher % of NREM, and lower percent of REM sleep, when compared to those with healthy sleep and NSDI. This pattern of sleep architecture is similar to that seen in attention deficit hyperactivity disorder (ADHD) (54), another disorder of hyperarousal. This suggests potential for some shared pathophysiology, for example, problems in the dorsolateral pons, an area of the brain implicated in modulating arousal and sleep states (55, 56). This biological basis for poor sleep may then impact mood and thinking, and then move into a more cyclic relationship between these features.
Further, short sleep duration is associated with chronic insomnia (17). As chronicity in many other disorders is associated with poorer health and cognitive outcomes (24), it is surprising that the results of the present paper do not indicate more severe cognitive problems for those with SSDI. However, the sample was young and relatively healthy, and as cognitive problems were only witnessed in inconsistency (an indicator of early cognitive change), it is possible these individuals have not had sufficient exposure to insomnia, and/or that young age may provide a “buffer” for cognitive problems. For example, N3 declines with age, whereas in the present sample, there was greater quantity of N3 sleep, perhaps reflecting a homeostatic way of compensating for sleep loss that may not be present in an older sample. Examination of the impact of chronicity among these two phenotypes is an important future direction.
Normal-Sleep Duration Insomnia
By contrast, poor lab-assessed sleep is not a core feature of NSDI, despite less consistent response times to cognitive tasks in comparison to healthy sleepers. This suggests that something else is affecting cognition and mood than the sleep disruption seen in SSDI.
The DMN is a network of interacting brain regions that is active when a person is at rest (18) and is thought to be responsible for “off-line” cognitive functions. This network has been implicated in the paradoxical experience of feeling awake while lab-assessed assessments detect sleep, and has been purported to be responsible for cognitive problems in other neurological disorders, including schizophrenia (57), where there are also working memory deficits and problems with self-referential thoughts (58). As such, NSDI appears not to be a sleep disorder per se, rather a disorder of neural networks.
Impact on Diagnosis and Treatment
Different pathophysiology, as suggested by these results, directly impacts the most appropriate therapy and suggests a need for early differential diagnosis.
Cognitive behavioural therapy for insomnia (CBTi) is considered a first-line treatment for insomnia with results superior to benzodiazepines (59). Among other aspects, CBTi includes core components to address unhelpful health beliefs, low mood, and unconsolidated sleep. These factors are important modifiable precipitating and maintaining features of insomnia. While CBTi demonstrates good results, not all patients receive benefits from this treatment (59). It is plausible that those who do not benefit are those for whom the underlying cause has not been addressed. For example, if short-sleep duration is due to biologically-based hyperarousal then CBTi may have limited ability to address such predisposing factors.
Further work to understand these potentially different disorders and their pathophysiology is crucial to inform therapy and provide more individualised treatments.
Data Availability Statement
The datasets presented in this article are not readily available as the data belong to the Raine Study. Requests to access this data can be made to this administering institution. Requests to access the datasets should be directed to https://rainestudy.org.au/.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Ethics Committee, University of Western Australia. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
PE was supported by a NHMRC Senior Research Fellowship (1136548).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the Raine Study participants and their families for their ongoing participation in the study and the Raine Study team for study co-ordination and data collection. We acknowledge The University of Western Australia, Centre for Sleep Science for utilisation of the facility and the support of their sleep study technicians. We also thank the NHMRC for their long-term contribution to funding the study over the last 30 years. The core management of the Raine Study was funded by the University of Western Australia, Curtin University, Telethon Kids Institute, Women and Infants Research Foundation, Edith Cowan University, Murdoch University, The University of Notre Dame Australia and the Raine Medical Research Foundation. The Raine Study Gen2-22-year follow-up was funded by NHMRC project grants 1027449, 1044840, and 1021858. Funding was also generously provided by Safe Work Australia.
Footnotes
1. ^The Raine Study index participants, who were born into the study between 1990 and 1991.
References
1. Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. (2007) 3(Suppl. 5):S7–10. doi: 10.5664/jcsm.26929
2. APA. Diagnostic and Statistical Manual - 5. Arlington, VA: American Psychiatric Association Publishing (2013). doi: 10.1176/appi.books.9780890425596
3. LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. (2009) 32:1027–37. doi: 10.1093/sleep/32.8.1027
4. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. (2016) 29:409–12. doi: 10.1097/YCO.0000000000000284
5. Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: Results of the (1991). National Sleep Foundation Survey. II. Sleep. (1999) 22(Suppl. 2):S354–8.
6. Wardle-Pinkston MS, Slavish DC, Taylor DJ. Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Med. Rev. (2019) 48:101205. doi: 10.1016/j.smrv.2019.07.008
7. Fortier-Brochu E, Morin CM. Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep. (2014) 34:1787–98. doi: 10.5665/sleep.4172
8. Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: A meta-analysis. Sleep Med Rev. (2012) 16:83–94. doi: 10.1016/j.smrv.2011.03.008
9. Ballesio A, Aquino JV, Ferlazzo F, Lombardo C. Executive functions in insomnia disorder: a systematic review and exploratory meta-analysis. Front Psychol. (2019) 10:101. doi: 10.3389/fpsyg.2019.00101
10. Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. (2001) 5:243–445. doi: 10.1053/smrv.2001.0157
11. Vgontzas AN, Fernandex-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. (2013) 17:241–54. doi: 10.1016/j.smrv.2012.09.005
12. Mohan A, Roberto AJ, Mohan A, Lorenzo A, Jones K, Carney MJ, et al. The significance of the Default Mode Network (DMN) in neurological and neuropsychiatric disorders: a review. Yale J Biol Med. (2016) 89:49–57.
13. Goldman-Mellor S, Caspi A, Gregory AM, Harrington H, Poulton R, Moffitt TE. Is insomnia associated with deficits in neuropsychological functioning? Evidence from a population-based study. Sleep. (2015) 38:623–31. doi: 10.5665/sleep.4584
14. Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Me Rev. (2018) 38:39–49. doi: 10.1016/j.smrv.2017.03.005
15. Contreras JA, Goni J, Risacher SL, Amico E, Yoder K, Dzemidzic M, et al. Cognitive complaints in older adults at risk for Alzheimer's disease are associated with altered resting-state networks. Alzheimers Dement. (2017) 6:40–9. doi: 10.1016/j.dadm.2016.12.004
16. Marques DR, Gomes AA, Caetano G, Castelo-Branco M. Insomnia disorder and brain's default-mode network. Curr Neurol Neurosci Rep. (2018) 18:45. doi: 10.1007/s11910-018-0861-3
17. Vgontzas AN, Fernandez-Mendoza J. Insomnia with short sleep duration: nosological, diagnostic, and treatment implications. Sleep Med Clin. (2013) 1:309–22. doi: 10.1016/j.jsmc.2013.04.009
18. Grecius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. PNAS. (2003) 100:253–8. doi: 10.1073/pnas.0135058100
19. Nie X, Shao Y, Liu S, Li HJ, Wan AL, Nie S, et al. Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatr Dis Treatment. (2015) 11:3085–93. doi: 10.2147/NDT.S95224
20. Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cognit Sci. (2013) 16:584–92. doi: 10.1016/j.tics.2012.10.008
21. Edinger JD, Krystal AD. Subtyping primary insomnia: is sleep state misperception a distinct clinical entity? Sleep Med Rev. (2003) 7:203–14. doi: 10.1053/smrv.2002.0253
22. Bliese PD, Wesensten NJ, Balkin TJ. Age and individual variability in performance during sleep restriction. J Sleep Res. (2006) 15:376–85. doi: 10.1111/j.1365-2869.2006.00557.x
23. Koscik RL, Berman SE, Clark LR, Mueller KD, Okonkwo OC, Gleason CE, et al. Intraindividual cognitive variability in middle age predicts cognitive impairment 8-10 years later: results from the wisconsin registry for Alzheimer's prevention. J Int Neuropsychol Soc. (2016) 22:1016–25. doi: 10.1017/S135561771600093X
24. Kim J, Park E, An M. the cognitive impact of chronic diseases on functional capacity in community-dwelling adults. J Nursing Res. (2019) 27:1–8. doi: 10.1097/jnr.0000000000000272
25. Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. (2012) 2012:344. doi: 10.1136/bmj.d7622
26. Davis JW, Chung R, Juarez DT. Prevalence of comorbid conditions with aging among patients with diabetes and cardiovascular disease. Hawaii Med J. (2011) 70:209–13.
27. Wiliams BR, Hultsch DF, Strauss EH, Hunter MA, Tannock R. Inconsistency in reaction time across the life span. Neuropsychology. (2005) 19:88–96. doi: 10.1037/0894-4105.19.1.88
28. Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. (2004) 27:1567–96. doi: 10.1093/sleep/27.8.1567
29. AASM. AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine (2012).
30. Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. (2009) 24:165–78. doi: 10.1093/arclin/acp010
31. Patel SK, Meier AM, Fernandez N, Lo TTY, Moore C, Degado N. Convergent and criterion validity of the CogState computerized brief battery cognitive assessment in women with and without breast cancer. Clin Neuropsychol. (2017) 31:1375–86. doi: 10.1080/13854046.2016.1275819
32. Simões EN, Padilla CS, Bezerra MS, Schmidt SL. Analysis of attention subdomains in obstructive sleep apnea patients. Front Psychiatry. (2018) 9:435. doi: 10.3389/fpsyt.2018.00435
33. Crawford JR, Henry JD, Ward AL, Blake J. The prospective and retrospective memory questionnaire (PRMQ): Latent structure, normative data and discrepancy analysis for proxy-ratings. Br J Clin Psychol. (2005) 44:1–23. doi: 10.1348/014466505X28748
34. Carriere JS, Cheyne JA, Smilek D. Everyday attention lapses and memory failures: the affective consequences of mindlessness. Consciousness Cognit. (2008) 17:835–47. doi: 10.1016/j.concog.2007.04.008
35. Cheyne JA, Carriere JS, Smilek D. Absent-mindedness: Lapses of conscious awareness and everyday cognitive failures. Consciousness Cognit. (2006) 15:578–92. doi: 10.1016/j.concog.2005.11.009
36. Okun ML, Kravitz HM, Sowers MF, Moul DE, Buysse DJ, Hall M. Psychometric evaluation of the insomnia symptom questionnaire: a self-report measure to identify chronic insomnia. J Clin Sleep Med. (2009) 5:41–51. doi: 10.5664/jcsm.27391
37. Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. (1997) 20:835–43.
38. Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the functional outcomes of sleep questionnaire. Sleep. (2009) 32:915–9. doi: 10.1093/sleep/32.7.915
39. Buysse DJ, Reynolds CF, Monk TH. Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
40. Raniti MB, Waloszek JM, Schwartz O, Allen NB, Trinder J. Factor structure and psychometric properties of the Pittsburgh Sleep Quality Index in community-based adolescents. Sleep. (2018) 41:1–12. doi: 10.1093/sleep/zsy066
41. Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
42. Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd ed. Sydney: Psychology Foundation (1995). doi: 10.1037/t39835-000
43. Nanthakumar S, Bucks R, Skinner T. Are we overestimating the prevalence of depression in chronic illness using questionnaires? Meta-analytic evidence in obstructive sleep apnoea. Health Psychol. (2014) 35:423–32. doi: 10.1037/hea0000280
44. Shrivastava D, Jung S, Saadat M, Sirohi R, Crewson K. How to interpret the results of a sleep study. J Community Hosp Intern Med Perspect. (2014) 4:24983. doi: 10.3402/jchimp.v4.24983
45. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press (2004).
46. Miyake A, Friedman NP. The nature and organisation of individual differences in executive functions: four general conclusions. Curr Direct Psychol Sci. (2012) 21:8–14. doi: 10.1177/0963721411429458
47. Fisk JE, Sharp CA. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J Clin Experi Neuropsychol. (2004) 26:874–90. doi: 10.1080/13803390490510680
48. Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. (2004) 27:423–33.
49. Wahl HW, Schmitt M, Danner D, Coppin A. Is the emergence of functional ability decline in early old age related to change in speed of cognitive processing and also to change in personality? J Ageing Health. (2010) 22:691–712. doi: 10.1177/0898264310372410
50. Robinson OJ, Vytal K, Cornwell BR, Grillon C. The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front Hum Neurosci. (2013) 7:203. doi: 10.3389/fnhum.2013.00203
51. Moore SA, Zoellner LA, Mollenholt N. Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behav Res Therapy. (2008) 46:993–1000. doi: 10.1016/j.brat.2008.05.001
52. Skinner TC, McNeil L, Olaithe M, Eastwood P, Hillman D, Phang J, et al. Predicting uptake of Continuous Positive Airway Pressure (CPAP) therapy in Obstructive Sleep Apnoea (OSA): a belief-based theoretical approach. Sleep Breath. (2013) 17:1229–40. doi: 10.1007/s11325-013-0828-1
53. Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnea: implications for future interventions. Indian J Med Res. (2010) 131:245–58.
54. Gruber R, Xi T, Frenette S, Robert M, Vannasinh P, Carrier J. Sleep disturbances in prepubertal children with attention deficit hyperactivity disorder: a home polysomnography study. Sleep. (2009) 32:343–50. doi: 10.1093/sleep/32.3.343
55. Lanfranchi PA, Fradette L, Gagnon JF, Colombo R, Montplaisir J. Cardiac autonomic regulation during sleep in idiopathic REM sleep behavior disorder. Sleep. (2007) 30:1019–25. doi: 10.1093/sleep/30.8.1019
56. Hauw JJ, Hausser-Hauw C, De Girolami U, Hasboun D, Seilhean D. Neuropathology of sleep disorders: a review. J Neuropathol Experi Neurol. (2011) 70:243–52. doi: 10.1097/NEN.0b013e318211488e
57. Lee H, Lee DK, Park K, Kim CE, Ryu S. Default mode network connectivity is associated with long-term clinical outcome in patients with schizophrenia. NeuroImage: Clin. (2019) 22:1–6. doi: 10.1016/j.nicl.2019.101805
58. Van Snellenberg JX, Girgis RR, Horga G, van de Giessen E, Slifstein M, Ojeil N, et al. Mechanisms of working memory impairment in schizophrenia. Biol Psychiatry. (2016) 80:617–26. doi: 10.1016/j.biopsych.2016.02.017
Keywords: insomnia, neuropsychology, phenotypes, paradoxical, short-sleep, cognition
Citation: Olaithe M, Ree M, McArdle N, Donaldson S, Pushpanathan M, Eastwood PR and Bucks RS (2021) Cognitive Dysfunction in Insomnia Phenotypes: Further Evidence for Different Disorders. Front. Psychiatry 12:688672. doi: 10.3389/fpsyt.2021.688672
Received: 31 March 2021; Accepted: 22 June 2021;
Published: 19 July 2021.
Edited by:
Luigi De Gennaro, Sapienza University of Rome, ItalyReviewed by:
Xiangdong Tang, Sichuan University, ChinaEdward F. Pace-Schott, Harvard Medical School, United States
Mercedes Atienza, Universidad Pablo de Olavide, Spain
Copyright © 2021 Olaithe, Ree, McArdle, Donaldson, Pushpanathan, Eastwood and Bucks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle Olaithe, bWljaGVsbGUub2xhaXRoZUB1d2EuZWR1LmF1
†These authors have contributed equally to this work and share senior authorship
 Michelle Olaithe
Michelle Olaithe Melissa Ree1,2
Melissa Ree1,2 Sara Donaldson
Sara Donaldson Maria Pushpanathan
Maria Pushpanathan Romola S. Bucks
Romola S. Bucks