- 1Division of Developmental Medicine, Department of Pediatrics, Boston Children's Hospital, Boston, MA, United States
- 2Department of Psychology, University of Virginia, Charlottesville, VA, United States
- 3Department of Psychology, Georgia State University, Atlanta, GA, United States
- 4Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
Variability in functional brain network connectivity has been linked to individual differences in cognitive, affective, and behavioral traits in adults. However, little is known about the developmental origins of such brain-behavior correlations. The current study examined functional brain network connectivity and its link to behavioral temperament in typically developing newborn and 1-month-old infants (M [age] = 25 days; N = 75) using functional near-infrared spectroscopy (fNIRS). Specifically, we measured long-range connectivity between cortical regions approximating fronto-parietal, default mode, and homologous-interhemispheric networks. Our results show that connectivity in these functional brain networks varies across infants and maps onto individual differences in behavioral temperament. Specifically, connectivity in the fronto-parietal network was positively associated with regulation and orienting behaviors, whereas connectivity in the default mode network showed the opposite effect on these behaviors. Our analysis also revealed a significant positive association between the homologous-interhemispheric network and infants' negative affect. The current results suggest that variability in long-range intra-hemispheric and cross-hemispheric functional connectivity between frontal, parietal, and temporal cortex is associated with individual differences in affect and behavior. These findings shed new light on the brain origins of individual differences in early-emerging behavioral traits and thus represent a viable novel approach for investigating developmental trajectories in typical and atypical neurodevelopment.
Introduction
Spontaneous brain activity is characterized by intrinsic dynamics of synchronized low-frequency fluctuations within structurally and functionally connected brain networks (1, 2). Much research has been focused on mapping the human connectome and delineating its anatomical and functional properties (3). Individual variability in functional connectivity profiles can accurately identify specific individuals likened to a fingerprint (4) and is linked to individual differences in cognitive, affective, and behavioral traits in adults (2, 5, 6). More generally, the study of functional brain network connectivity has been argued to be one of the most promising and effective ways in bridging between brain and behavior (7).
From a developmental perspective, functional connectivity within brain networks can be detected from very early in human brain development. A host of studies employing resting-state functional magnetic resonance imaging (rs-fMRI) have mapped and identified functional networks in newborn infants (8–12). In fact, a body of work relying on progress in fetal rs-fMRI suggests that the basic organization and architecture of the functional connectome emerges during the late second trimester of pregnancy (10, 11, 13, 14). In addition, early prenatal and postnatal experiences, such as premature birth, have begun to shape the development of these networks already within the first week of life (15, 16). The existing research with fetuses and infants points to a developmental progression whereby functional connectivity in primary short-range sensory-motor and homologous-interhemispheric networks are already in place at birth, whereas functional connectivity in higher-order cortical networks across longer ranges involving frontal, temporal, and parietal cortex shows more protracted development during infancy (9, 14). For example, there is evidence to suggest that higher-order networks, such as the default mode network (DMN), exist in a rudimentary form even in the fetus and newborn (14, 17); however, functional network integration and synchronization continues to develop during infancy and beyond (18). Other higher-order networks, such as the fronto-parietal network, show an even more protracted development as it is still considered immature by the end of the first year of postnatal life [see (17), for a review]. Moreover, it is these networks with prolonged development, such as the fronto-parietal network, that go on to have the greatest inter-person variability and are considered to be unique identifiers of individuals (4). Taken together, much progress has been made in mapping the functional connectome in early human brain development; however, to date, little is known about whether and how functional brain network connectivity in these networks is linked to early affective, cognitive, and behavioral traits. This is a particularly important question considering that many mental health disorders are: (a) accompanied by alterations in functional connectivity and (b) are argued to have deep developmental origins (2, 19–21).
Infant and child temperament is considered to reflect robust, biologically-based individual differences in affective and behavioral traits (22) linked to adult personality traits and long-term developmental outcomes (23–26). More specifically, profiles of temperament such as high negative emotionality and low levels of regulatory behaviors are considered early indicators of mental health disorders (26–31). To date, a host of studies in the adult literature have shown that high negative emotionality (and specifically high levels of neuroticism), low levels of regulatory functioning, and associated mental health outcomes are characterized by: (1) hypoconnectivity within the fronto-parietal network (FPN; composed of regions in the anterior cingulate cortex, dorsolateral prefrontal cortex, and parietal cortex) implicated in the cognitive control of attention and emotion (2) hyperconnectivity within the default mode network [DMN; composed of regions in the medial prefrontal cortex (mPFC), the precuneus, the posterior and anterior cingulate cortex, the inferior parietal cortex, and the lateral temporal cortex] involved in internally-oriented thought, mind-wandering, social cognition and (3) hypoconnectivity within the homologous-interhemispheric network (HIN; examining cross-hemispheric connections between frontal, temporal, and parietal lobes) involved in emotion regulation (2, 32–36). Considering these alterations in functional connectivity associated with adult personality traits and the possibility that they might have their origins in early human brain development, it is important to examine variability in these brain networks and how this links to individual differences in affective and behavioral traits.
Therefore, the current study followed two major goals. First, we aimed to identify and map individual variability in the three functional brain networks (FPN, DMN, HIN) in young infants using functional near-infrared spectroscopy (fNIRS). FNIRS is a non-invasive, portable, and safe, optical neuroimaging technique for assessing functional connectivity in cortical brain networks during infancy [see the following papers for other examples of functional connectivity analysis using fNIRS with infants (37–39)]. To capture functional connectivity patterns in young infants using fNIRS, we pre-defined the following three long-range brain networks including available channels in specific frontal, temporal, and parietal regions: (1) the FPN was created by measuring functional connectivity between the dorsolateral prefrontal cortex and inferior parietal cortex; (2) the DMN was created by measuring functional connectivity between the lateral temporal cortex and medial prefrontal cortex (note that our probe layout did not allow us to measure activity from superior parietal cortical regions including the precuneus, which is typically included in the DMN); and (3) the HIN was created by measuring functional connectivity between homologous cross-hemispheric connections in frontal, temporal, parietal cortex. In addition, based on previous work measuring functional connectivity using fNIRS in adults (40), we created a so-called control network, computing functional connectivity between left frontal cortex and right temporal cortex and right frontal cortex and left temporal cortex. This served as a non-functional control network, because these regions are not known to have any functional associations and show much lower levels of functional connectivity than established functional brain networks (40). We hypothesized that functional connectivity within the three functional brain networks (FPN, DMN, and HIN) will be significantly greater than in the control network, attesting to the existence of these long-range cortical networks in young infants. In this context, it is important to mention that, to our knowledge, there is no prior work demonstrating long-range functional connectivity in FPN and DMN in newborns and 1-month-old infants (14, 17), whereas functional connectivity in HIN has been shown to exist in the fetal brain (13).
Critically, we also examined whether and how variability in functional brain network connectivity maps onto individual differences in infant temperament, which can be readily and reliably assed through parental report (22). Brain networks assessed during the first few weeks of life have been linked to behavioral temperament 6 months later (41, 42). In addition, previous work with older infants has shown that the networks of interest in the present study appear to be supporting negative emotionality and regulatory functioning already in the first year of life. For example, a study of 5 to 6-month-old infants found that the DMN (indexed by the medial Prefrontal Cortex response) and FPN (indexed by the dorsolateral Prefrontal Cortex response) are already involved in regulatory functions, such as switching between self- oriented and other-oriented thought (43). Moreover, 6- to 12-month old infants who displayed greater DMN functional connectivity showed greater negative emotionality (44). Finally, a structural fMRI study found that the length of the corpus callosum (thought to underly the homologous-interhemispheric network) was negatively associated with emotional control problems at 4 years of age (45). However, to our knowledge, no prior work has examined the concurrent relation between behavioral temperament and functional brain connectivity in infants younger than 5 months of age. Specifically, we focused our investigation on three critical dimensions of infant temperament (regulation/orienting, negative emotionality, positive emotionality/surgency), which have been previously identified in a factor analysis (46). Based on prior work with adults and with older infants linking functional connectivity in FPN to cognitive control of attention and behavior (2, 33, 34, 47, 48), we hypothesized that infants' regulation/orienting behaviors will be associated with functional connectivity in the FPN with greater connectivity in this network being linked to enhanced regulation and orienting. In contrast, we predicted the opposite pattern of association for the DMN, whereby lower connectivity is hypothesized to be linked to enhanced regulation based on prior work on DMN function with older infants and adults (2, 33, 34, 44, 48). Moreover, we expected that infants' reduced functional connectivity in the HIN will be linked to higher levels of negative emotionality, based on previous findings linking reduced cross-hemispheric connectivity to negative emotionality and related mental health outcomes in infants and adults (33–36, 45). Critically, in our analysis, we expected to see these predicted associations only for the specific functional networks and not for the (non-functional) control network. Finally, considering that there is little work informing how surgency/positive emotionality is linked to network connectivity, we did not have a specific hypothesis regarding this trait, but still included it in our analysis because surgency/positive emotionality has been identified as an important factor in previous work (46). Together, the current study presents a systematic examination of functional connectivity in long-range brain networks and its links to behavioral temperament in a sample of young, healthy, infants.
Materials and Methods
Seventy-five newborn and 1-month-old infants (M [age] = 25 days; Median [age] = 24 days; ranging from 9 days to 56 days; 32 females; 43 males) were included in the final sample used for the present analyses (see Table 1 for a description of the socio-demographic characteristics for the present sample). Participants were recruited from a local hospital. The diverse sample of infants were representative of the surrounding Mid-Atlantic college town area such that the majority of infants were Caucasian (n = 50 Caucasian; n = 14 Black; n = 3 South Asian; n = 3 Pacific Islander; n = 2 Asian; n = 3 Other), from highly-educated parents (n = 31 obtained a Graduate Degree; n = 19 Bachelor's Degree; n = 12 some College/Associates Degree; n = 11 High School Diploma/GED; n = 2 some High School), and low to medium-income families (n = 21 $15–45,000; n = 18 $75–110,000; n = 11 $45–75,000; n = 11 $110–175,000; n = 8 $175,000+; n = 5 less than $15,000; n = 1 did not respond). All participants were born at term, with normal birth weight (>2,500 g), and did not have any hearing or visual impairments. Thirty-three additional infants were tested but were excluded from the present analyses for the following reasons: n = 25 were excluded because they failed to reach our pre-determined inclusion criterion of having at least 100 s of continuous data with non-disruptive behaviors (see below); n = 4 were excluded because of inaccurate placement of the cap; n = 4 were excluded because more than 33% of the measured fNIRS channels had poor light intensity readings, more specifically, a signal-to-noise ratio of less than 1.5 (37, 50). Note that the current attrition rate (30%) is lower than in previous infant fNIRS studies (51). Moreover, temperament profiles (negative emotionality, regulation/orienting, and surgency/positive emotionality) were compared using independent samples t-tests between infants that were included and excluded from the present analyses and no significant differences were found between the two groups (all p-values > 0.29). All parents gave informed consent for their infants to participate in accordance with the Declaration of Helsinki and families received a payment for their participation. All procedures were approved by and carried out in accordance with The University of Virginia Institutional Review Board for Health Sciences (Protocol number 20381).
Infant Temperament
Infant temperament was assed using parental reports of the 91-item Infant Behavior Questionnaire Revised Short Form [IBQ-R; (46)]. Parents filled out the questionnaire online using Qualtrics survey platform prior to their appointment. This measure has been widely used and shown to be reliable and valid at the newborn time point [see the following papers for examples of prior work using this measure with newborns (52–54)]. The questionnaire asks parents to report their infant's behavior during the previous 2 weeks and rate the occurrence/frequency of the behavior on a 1 (Never) to 7 (Always) scale. Based on prior work using factor analysis (46), three general temperament dimensions were computed summarizing information from various sub-scales: (1) negative emotionality (contributing sub-scales: fear, distress to limitations, falling reactivity, sadness), (2) regulation/orienting (contributing sub-scales: low intensity pleasure, cuddliness, duration of orienting, soothability), and (3) surgency/positive emotionality (contributing sub-scales: activity level, smiling and laughing, high intensity pleasure, perceptual sensitivity, approach, vocal reactivity) (46). If parents reported the behavior was not applicable at the current time then this item was given a value of 0. Chronbach's alpha coefficients were calculated to determine reliability of the temperament measures and all values were in acceptable ranges for each of the three dimensions: surgency/positive emotionality α = 0.78, regulation/orienting α = 0.78, and negative emotionality α = 0.91. Finally, correlation analyses between Edinburgh Postnatal Depression Scale scores [assessed at the same time as behavioral temperament; (49)] and behavioral temperament scores were conducted in order to statistically account for any variance in maternal-reported behavioral temperament that may be related to maternal mental health. Here, we did not find any significant associations (all p-values > 0.24). Therefore, maternal depression was not used as a covariate in later analyses.
Procedure
The resting state fNIRS task took place in a quiet, dimly-lit testing area. Infants were seated on their parents' lap and placed ~60 cm from the screen (23-inch monitor). The infants were fitted with a fNIRS fabric cap (EasyCap, Germany) which was secured in place using infant overalls and outside netting. The experimental paradigm was presented using the Presentation software package (Neurobehavioral Systems, USA). A non-social stimulus was created by selecting non-social clips from a popular infant video (Baby Einstein - Kids2 Inc.) that featured videos of toys, stuffed animals, and still images of everyday objects, which was accompanied by classical music (55). Similar screen-saver-like videos have been used in prior work examining functional connectivity using fNIRS [see (38)]. This video was played for a total of 7 min while fNIRS data were being recorded. The clips were segmented into 30 s intervals and the order of presentation was randomized for each infant. Parents were asked to remain quiet throughout the fNIRS recording session. Sessions were video-recorded using a camera mounted above the screen. This allowed for later offline coding of infants' alertness and cap placement.
Data Acquisition
Infants' fNIRS data were recorded using a NIRx Nirscout system and NirStar acquisition software. The fNIRS method quantifies concentration changes of oxygenated hemoglobin (oxyHb) and deoxygenated hemoglobin (deoxyHb) in the cerebral cortex through shining specific frequencies of light that are selectively absorbed by these chromophores [for more information regarding this technique see (56)]. The fNIRS system used contains 16 source-detector pairs (~2.0 cm apart) resulting in a total of 49 channels positioned over frontal and temporal-parietal regions [see (57–60) for infant work using the identical channel positioning/layout]. The system emits two wavelengths of light in the Near-Infrared spectrum, 760 and 850 nm, and captures both deoxyHb and oxyHb. The diodes have a power of 25 mW/wavelength and data were recorded at a preset default sampling rate of 3.91 Hz.
Behavioral Coding
Infants' behavior during the fNIRS recording session was coded by a trained research assistant using video recordings of the experimental session. Specifically, coders identified timepoints where the parents were talking and where the infants were crying, excessively moving, or looking at the parents. These periods were then removed from the analysis. To assess the reliability of the attentional coding done by the primary coder, an additional trained coder also coded infant behavior from selected subsample of infants (25.3%; n = 9). This analysis showed that inter-rater reliability for amount of data included was excellent (Cronbach's α = 0.94). In line with previous studies, infants were only included in the present analysis if they had at least 100 s of disruption-free (see aforementioned behaviors) data (37). Moreover, as it takes a minimum of 8 s for the Hemodynamic response function to return to baseline after a stimulus-evoked event, the onset of useable data was delayed for 8 s (37, 38). However, unlike Bulgarelli et al. (37, 38), the time series of fNIRS obtained in the current study was continuous. On average, infants contributed 317.59 s of data (SD = 115.46 s; range = 100–420 s). Furthermore, the amount of data included in the current analysis is comparable to other functional connectivity work using fNIRS with older infants (38). In addition, we coded infants' state of alertness on a 1 (Deep Sleep) to 6 (Crying) scale. On average infants were rated as being in an Active Light Sleep to Drowsy State (M = 2.68, SD = 1.27). Finally, we assessed how infants' behavior throughout the session, specifically the amount of useable data related to functional network connectivity in each of the networks (see Supplementary Material).
Data Analysis
The fNIRS data were analyzed using the functional connectivity program, FC-NIRS (50). First, channels were assessed for light intensity quality and channels were removed if the signal-to-noise ratio was less than 1.5 (50). In order to be included in the present analyses, infants needed to have at least 70% of their channels passing this threshold (37). Next, data were band-pass filtered [using a 0.08 Hz low-pass filter, to remove fast fluctuations related to heart rate, and a high-pass filter of 0.01 Hz, to remove changes that were too slow and related to drift; (37, 61)]. This range of 0.01 to 0.08 Hz was chosen on the basis of prior work (37, 40). This range was also selected because it falls well below the reported range for cardiac fluctuations (greater than 1 Hz), providing us with greater confidence that the measured changes reflect hemodynamic events tied to cortical activity rather than (systemic) cardiovascular system activity [e.g., heart rate (62, 63)]. Finally, concentration changes were calculated using the modified Beer-Lambert law (partial path length factor: 6.0) (64).
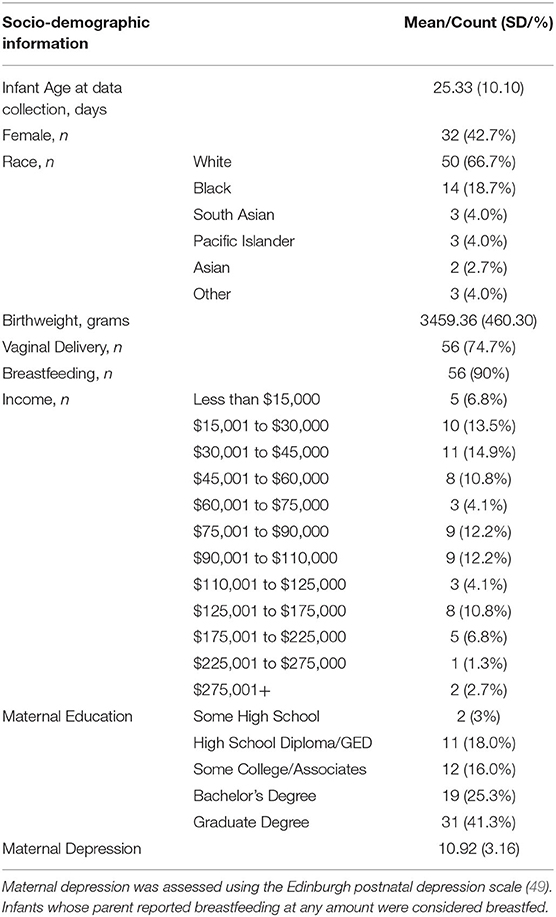
For each infant, we obtained a 49 by 49 correlation matrix corresponding to all of the relations between all of the channels measured. Considering that negative values are difficult to interpret in terms of their neurobiological basis, and based on prior work, we first checked data to see if there were any negative values and found out there were none (65, 66). In order to standardize the values, Fisher Z-transformations were performed on all correlation matrices. Networks of interest were created by selecting channels that corresponded to specific regions of interest. Brain networks were composed based on the anatomical information available in Kabdebon et al. (67), a meta-analysis of resting state fMRI (2), a large resting state fMRI functional connectivity analysis of newborn infants (68), and prior work infant and adult work using rs-fNIRS (35, 38, 40, 69). Based on this information four networks were created: (1) The FPN was created by averaging all correlations between three channels in the dorsolateral prefrontal cortex (corresponding with the F3, F4, F5, F6 electrodes) and two channels in the parietal area (corresponding with CP3 and CP4 electrodes); (2) The DMN was created by averaging all correlations between three channels in the medial prefrontal cortex (corresponding with the Fpz electrode) and four channels in the lateral temporal cortex (corresponding with FT7, T7, FT8, T8 electrodes); (3) The HIN was created by averaging all correlations between the 21 channels in the left hemisphere (including frontal, temporal, and parietal cortical regions) with their corresponding (homologous) channels in the right hemisphere; and, (4) a (non-functional) control network was created by averaging all correlations between three channels in the left frontal area (corresponding with the F7 electrode) with three channels in the right temporal area (corresponding with the T8 electrode) and three channels in the right frontal area (corresponding with F8 electrode) with three channels in the left temporal area (corresponding with the T7 electrode; see Figure 1 for schematic of network configurations). Cortical projections onto a standard MNI newborn (0–2 months old) atlas (70) were created using NIRSite (Nirx) by using 10-20 system references from the cap layout.
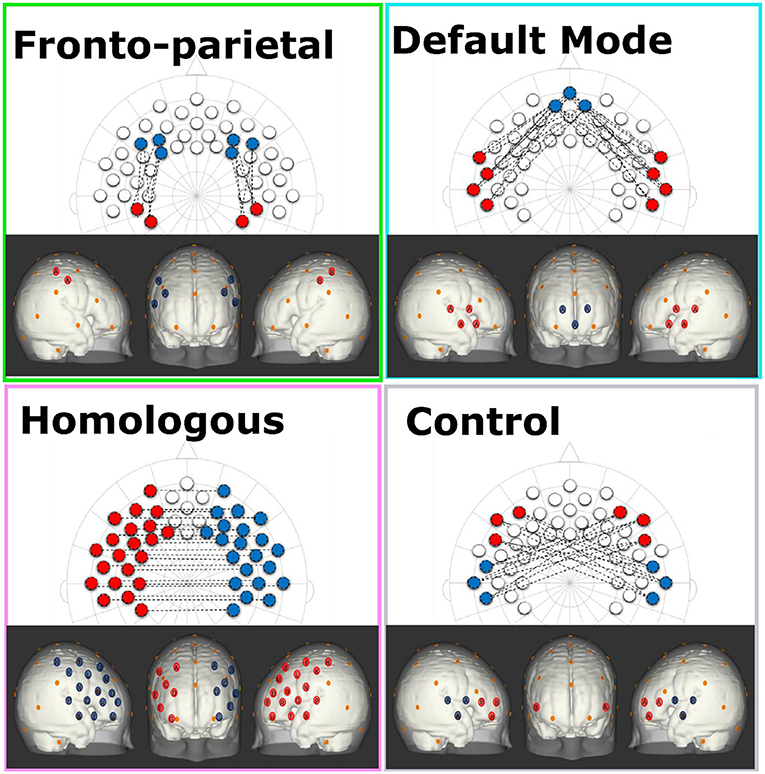
Figure 1. This figure shows the configurations for each of the network patterns in both a 2-dimensional 10-20 system layout and estimated projections onto cortical space of a 0–2 month-old Atlas (70). Each network consists of the average of all of the connections between red and blue channels of the same letter. In addition, the orange dots represent relevant 10-20 landmarks.
All analyses were conducted for both oxyHb and deoxyHb (for deoxyHb results please see Supplementary Material). Moreover, statistical outliers—values that were more than 3 SD above the mean—were removed for the subsequent analyses (FPN n = 2, negative emotionality n = 1).
Results
A series of Spearman's rho correlations were used to identify significant associations between variables of interest and potential socio-demographic factors (for a schematic representation for all associations see Supplementary Figure 1). Any demographic variables found to be significantly associated with a study variable of interest were then included in the subsequent models assessing differences in said study variable as a covariate. Negative Emotionality was significantly associated with both infant age (Spearman's rho correlation rs = 0.47, p < 0.001) and family income (Spearman's rho correlation rs = 0.29, p = 0.011). Regulation/orienting was significantly associated with Education (Spearman's rho correlation rs = −0.30, p = 0.009). However, there were no significant associations found between any of the functional connectivity measures and any of the covariates.
Similarly, we tested for associations between alertness levels, amount of usable data collected during the fNIRS testing session, and the study variables of interest. Here, we found that the level of alertness was negatively related to functional connectivity levels in the three networks of interest (HIN rs = −0.27, p = 0.021; FPN rs = −0.38, p < 0.001; DMN rs = −0.30, p = 0.008). This analysis also revealed that the amount of data included was positively associated with connectivity for the DMN (rs = 0.27, p = 0.020) and negatively associated with behavioral temperament (negative emotionality rs = −0.27, p = 0.022; regulation/orienting rs = −0.24, p = 0.041). Additional analyses with alertness and amount of data as covariates can be found in the Supplementary Material.
Functional Connectivity Across Networks
As a first step, a series of one-sample t-tests were conducted to assess whether Fisher-transformed correlation between individual channels within the pre-defined networks of interest differed from zero. As shown in Figure 2, this analysis identified significant functional connectivity between individual channels within the pre-defined networks of interest (see Supplementary Table 1). Next, we conducted a series of one-sample t-tests to assess connectivity at the network level (combining across all channels of interest). Here, all networks (FPN, DMN, HIN, control) were found to be greater than zero [FPN, t(72) = 9.07, p < 0.001, q-value < 0.001; DMN, t(74) = 6.88, p < 0.001, q-value < 0.001; HIN, t(74) = 9.43, p < 0.001, q-value < 0.001; Control, t(74) = 3.86, p < 0.001, q-value < 0.001; see Figure 3). To analyze differences in overall connectivity levels across networks an omnibus repeated measures ANOVA with network type (FPN, DMN, HIN, control) as a within-subjects factor was conducted. This analysis revealed a significant within-subjects effect across network types, F(3, 216) = 18.78, p < 0.001, η2 = 0.207. Post-hoc analyses with Bonferroni adjustments for multiple comparisons were conducted to assess which networks significantly differed from one another. Importantly, all functional networks of interest had significantly higher connectivity than the (non-functional) control network [M = 0.05; SD = 0.12; range: −0.20–0.44; HIN vs. Control t(74) = 5.06, p < 0.001; FPN vs. Control, t(72) = 6.11, p < 0.001; DMN vs. Control, t(74) = 4.15, p < 0.001]. In addition, we found that there was significantly greater connectivity in the FPN (M = 0.21; SD = 0.20; range: −0.16–0.72) compared to both the HIN (M = 0.13; SD = 0.12; range: −0.12–0.48), t(72) = 3.63, p = 0.003, and the DMN (M = 0.13; SD = 0.16; range: −0.28–0.73), t(72) = 3.63, p = 0.010. However, there was no significant difference found between the level of connectivity for the HIN from the DMN, p = 1.00 (see Figure 3).
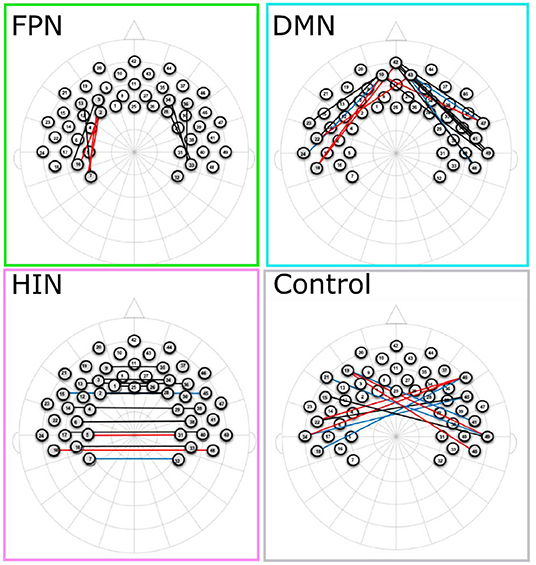
Figure 2. This figure shows the channels that are significantly different than zero for each of the networks. Channels in red, blue, and black represent significant changes for oxyHb, deoxyHb, and both oxy and deoxyHb respectively.
Figure 3. This figure shows the average levels of functional connectivity (oxyHb) and range of variability for each network. The boxplot horizontal lines from bottom to top reflect values for the lower quartile, median, and upper quartile respectively. *p < 0.05.
Functional Connectivity and Behavioral Temperament
In order to assess how functional connectivity patterns differentially predicted temperament characteristics, three separate regressions with all four network types (FPN, DMN, HIN, control) predicting each of the three domains of temperament (negative emotionality, regulation/orienting, surgency/positive emotionality) were conducted.
Regulation/Orienting
A multiple linear regression using the entry method was conducted with the socio-demographic covariate (education) and four network types (FPN, DMN, HIN, control) as the predictors and regulation/orienting as the outcome variable. The regression model was statistically significant, F(5, 72) = 4.84, p = 0.001, R2 = 0.27. More specifically, connectivity in the DMN was negatively associated with regulation/orienting (B = −1.02, SE = 0.42, p = 0.018, q-value = 0.054); whereas, connectivity in the FPN was positively associated with regulation/orienting (B = 0.71, SE = 0.35, p = 0.049, q-value = 0.074; see Figure 4). Neither the HIN nor the Control network were found to be related to regulation/orienting, all p-values > 0.24.
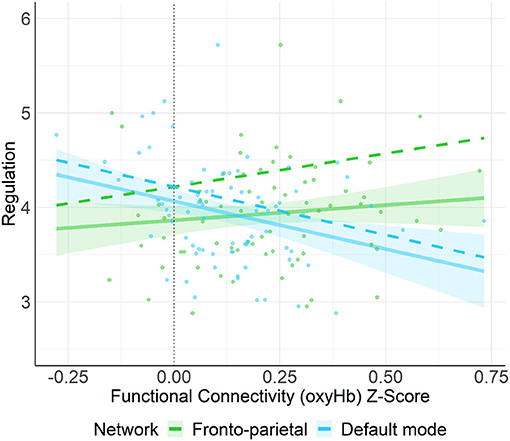
Figure 4. This figure shows the unadjusted (solid line) and adjusted relation (covariates not shown: HIN, Control, maternal education) between functional connectivity (oxyHb) Z-score and regulation/orienting. Here, we found that connectivity in the FPN was positively associated with regulation/orienting (p = 0.049) whereas, connectivity in the DMN was negatively associated with regulation/orienting (p = 0.018). Shaded regions represent 90% confidence intervals for the raw (unadjusted) data.
Negative Emotionality
A multiple linear regression using the entry method was conducted with the socio-demographic covariates (age, income) and four network types (FPN, DMN, HIN, control) as the predictors and negative emotionality as the outcome variable. The regression model was statistically significant, F(6, 64) = 5.50, p < 0.001, R2 = 0.34. More specifically, we found a significant positive relation between HIN connectivity and negative emotionality, (B = 1.60, SE = 0.75, p = 0.038, q-value = 0.114; See Figure 5). However, none of the other networks (functional nor control) were found to be related to Negative Emotionality, all p-values > 0.57.
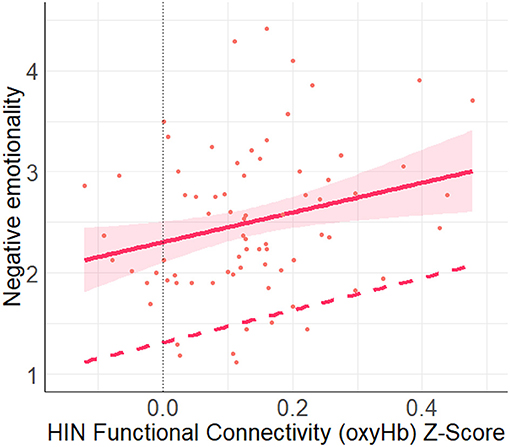
Figure 5. This figure shows the unadjusted (solid line) and adjusted relation (covariates: FPN, DMN, Control, income, age) between HIN functional connectivity (oxyHb) Z-score and negative emotionality. Here, we found a significant positive relation between the HIN and negative emotionality (p = 0.038). Shaded regions represent 90% confidence interval for the raw (unadjusted) data.
Surgency/Positive Emotionality
A linear regression was conducted with the four network types (FPN, DMN, HIN, control) predicting surgency/positive emotionality using the entry method. Here, the regression model was not statistically significant, p = 0.39. Moreover, none of the network types were significantly associated with surgency/positive emotionality (all p's > 0.14).
Discussion
The current study examined functional connectivity in brain networks using fNIRS and behavioral temperament using parental report in young infants. Our results show that functional connectivity in long-range cortical brain networks (FPN, DMN, and HIN) can be identified in very young infants and that functional connectivity in these networks varied considerably among infants. This supports the suitability of fNIRS in assessing functional connectivity and its variability in newborn infants. Importantly, our results also show that such variability in functional brain network connectivity systematically maps onto individual differences in infant behavioral temperament. Overall, the current findings provide novel insights into the brain origins of individual differences in affect and behavior, pointing to the early perinatal foundation of human temperament.
In line with our hypothesis, functional connectivity within the three brain networks (FPN, DMN, and HIN) was significantly greater than in the control network and significantly greater than a zero-value, indicating the existence of these long-range cortical brain networks in young infants. This provides further evidence that functional brain networks exist from early in ontogeny and are detectable in young infants (12, 17, 71). To our knowledge, this is the first study to demonstrate long-range functional connectivity in FPN and DMN in young infants, suggesting a remarkably early emergence of long-range connectivity in higher-order brain networks linked to cognitive control and self-referential processes, respectively. The current findings are noteworthy also in regard to the fact that both networks involve regions in prefrontal cortex, providing new evidence from newborns and 1-month-old infants supporting the view that prefrontal cortex plays a critical role in human brain function from very early in development (14, 72–75).
In addition to the general difference in connectivity between the functional and the (non-functional) control network, we also found that activity in the FPN was significantly greater than in the DMN and HIN (whereas there was no difference in connectivity levels found between the DMN and HIN). One possible interpretation of this finding is that functional connectivity in the FPN might have been enhanced when compared to the other functional networks because, like other resting-state studies with infants, the participants were presented with a video accompanied by music during the fNIRS measurement (37). In other words, the FPN might have been more engaged because infants were attending to external audio-visual stimuli [note that all infants were exposed to the same video (audio-visual) stimulus]. Here, it is important to mention that prior work with adults using fMRI shows that functional connectivity in higher-order cortical resting-state networks can be reliably acquired during the presentation of videos and corresponds to functional connectivity acquired in the absence of any stimulus (4, 76). Nonetheless, based on recent work showing that preterm infants display enhanced functional connectivity in higher-order cognitive networks in response to music (55), we speculate that enhanced functional connectivity in FPN might at least be partly explained by having newborn infants listen to music in the current study. Clearly, future research with infants which systematically compares stimulation protocols is needed to examine whether and how functional connectivity is influenced by the measurement context and the stimulation protocol used. Overall, our functional connectivity analysis supports the notion that intrinsic functional connectivity in cortical brain networks and its variability can be effectively mapped in newborn infants using fNIRS.
Having established functional connectivity in these brain networks as variable and distinct from a (non-functional) control network then allowed for the examination of specific associations between brain network connectivity and infant behavioral temperament. Our results confirmed our hypothesis and showed that infants' regulation/orienting behaviors were associated with functional connectivity in the FPN with greater connectivity in this network being associated with enhanced regulation and orienting. This result is in line with prior work linking functional connectivity in FPN to cognitive control of attention and behavior in adults (2, 48) and more recent work with infants (43). The current results further showed the opposite pattern of association for functional connectivity in DMN, with greater connectivity associated with reduced regulation and orienting, which is in agreement with our hypothesis based on the DMN previously being linked to self-referential, stimulus-independent thought and mind-wandering in adults (2, 48) and infants (43). To obtain such opposing effects of functional connectivity in FPN and DMN is reminiscent of seminal findings supporting the existence of anti-correlated brain networks in adults (77) and may suggest that similar organizational principles are at play in newborn infants. However, it should be emphasized that functional connectivity in the FPN and DMN in the current study was not anticorrelated as such, but rather had opposing effects on infants' behavioral and attentional regulation.
Our results concerning behavioral and attentional regulation and their functional connectivity correlates in infants are principally in line with prior research showing hyperconnectivity in the DMN and hypoconnectivity in the FPN in adults with negative emotionality and related mental health outcomes (33, 34, 48). Moreover, our data show that infants' functional connectivity in the HIN was associated with negative emotionality. Contrary to prior work with adults indicating that hypoconnectivity is associated with negative emotionality and depression (33, 35, 36) and work with infants indicating that corpus callosum length (thought to underly the HIN) is negatively associated with later emotion regulation abilities (45), the current infant data show that greater connectivity between homologous brain regions in both hemispheres was associated with greater negative affect. It is unclear why the direction of the association (positive vs. negative) would differ as a function of age (newborn infants in the current study and preschool aged and adults in previous work), but it is worth noting that the experience and display of negative affect only gradually emerges during infancy and may thus not be fully present in newborn infants (53).
Taken together, the current findings demonstrate specific associations between functional brain network connectivity and behavioral temperament in newborn infants. This suggests a remarkably early emergence of functional networks with behavioral relevance and highlights the importance of evaluating individual differences reflected in intrinsic brain connectivity. Although there are many advantages in the current approach of using fNIRS to examine functional brain connectivity, including its cost-effective and non-confining application, there are some limitations that need to be mentioned. First, because fNIRS is limited in monitoring activity from (superficial) cortical structures (78), our approach did not allow us to measure activity from deeper cortical and subcortical regions and include those in our network analyses. Second, from a developmental perspective, it should be noted that our analysis is limited to only one age group and comprised of very young infants. It is thus critically important to further assess the development of variability in these brain networks and their associations with behavioral temperament over developmental time to determine its long-term effects and the robustness of these associations (69). Third, it is important to note that these associations were assessed in a population of healthy infants, meaning there were no known birth or health complications at the time of the visit. Therefore, given the breadth of work examining how preterm birth and other medical complications (e.g., hypoxia) impact brain development, it will be important to test whether or not these associations generalize to other populations (16, 79, 80).
In conclusion, the current study provides novel insights into the use of fNIRS in identifying neural endophenotypes—variability in functional brain network connectivity—linked to behavioral temperament traits in early human development. The present findings support the notion that functionally distinct neural networks are implicated in regulatory and emotional behaviors already in newborn infants, adding a critical developmental component to efforts directed at mapping how the individual functional connectome links to affective, cognitive, and behavioral traits. The current findings shed light on the brain origins of individual differences in early-emerging behavioral traits and provide the basis for future research examining the genetic and environmental factors contributing to and the long-term developmental consequences of this brain-behavior correlation. More generally, the current study provides early ontogenetic evidence for the idea that studying functional brain network connectivity is an effective way in helping bridge the gap between brain and behavior.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Virginia Institutional Review Board for Health Sciences (Protocol number 20381). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
CK and TG contributed to conception and design of the study, and wrote the first draft of the manuscript. CK and KF collected the data. CK performed the statistical analysis. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was supported by Danone North America, Gut Microbiome, Yogurt and Probiotics Fellowship Grant, Jefferson Scholars Foundation and UVA Data Science Fellowship (to CK) and National Science Foundation #2017229 and UVA Brain Institute Seed fund (to TG).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to all families who participated in this study as well as Sarah Thomas, Christina Marlow, Kate Haynes, Carolynn McElroy, Julia Larsen, Heath Yancey, Sujal Sigdel, and Shefalika Prasad for assistance with infant data collection at the University of Virginia. This manuscript is available as a pre-print on BioRxiv (link: https://www.biorxiv.org/content/10.1101/2020.07.15.204271v1).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.685754/full#supplementary-material
References
1. Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. (2006) 103:13848–53. doi: 10.1073/pnas.0601417103
2. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. (2015) 72:603–11. doi: 10.1001/jamapsychiatry.2015.0071
3. Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the human connectome project. Neuroimage. (2013) 80:144–68. doi: 10.1016/j.neuroimage.2013.05.039
4. Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. (2015) 18:1664–71. doi: 10.1038/nn.4135
5. Jauniaux J, Khatibi A, Rainville P, Jackson PL. A meta-analysis of neuroimaging studies on pain empathy: investigating the role of visual information and observers' perspective. Soc Cogn Affect Neurosci. (2019) 14:789–813. doi: 10.1093/scan/nsz055
6. Picó-Pérez M, Alemany-Navarro M, Dunsmoor JE, Radua J, Albajes-Eizagirre A, Vervliet B, et al. Common and distinct neural correlates of fear extinction and cognitive reappraisal: a meta-analysis of fMRI studies. Neurosci Biobehav Rev. (2019) 104:102–15. doi: 10.1016/j.neubiorev.2019.06.029
7. Friston KJ. Functional and effective connectivity: a review. Brain Connect. (2011) 1:13–36. doi: 10.1089/brain.2011.0008
8. Fransson P, Skiöld B, Engström M, Hallberg B, Mosskin M, Aden U, et al. Spontaneous brain activity in the newborn brain during natural sleep–an fMRI study in infants born at full term. Pediatr Res. (2009) 66:301–5. doi: 10.1203/PDR.0b013e3181b1bd84
9. Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W. Development of human brain cortical network architecture during infancy. Brain Struct Funct. (2015) 220:1173–86. doi: 10.1007/s00429-014-0710-3
10. Schöpf V, Kasprian G, Brugger PC, Prayer D. Watching the fetal brain at 'rest'. Int J Dev Neurosci. (2012) 30:11–7. doi: 10.1016/j.ijdevneu.2011.10.006
11. Thomason ME, Hect JL, Rauh VA, Trentacosta C, Wheelock MD, Eggebrecht AT, et al. Prenatal lead exposure impacts cross-hemispheric and long-range connectivity in the human fetal brain. Neuroimage. (2019) 191:186–92. doi: 10.1016/j.neuroimage.2019.02.017
12. Zhang H, Shen D, Lin W. Resting-state functional MRI studies on infant brains: a decade of gap-filling efforts. Neuroimage. (2019) 185:664–84. doi: 10.1016/j.neuroimage.2018.07.004
13. Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, Anderson AL, et al. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. (2013) 5:173ra124. doi: 10.1126/scitranslmed.3004978
14. van den Heuvel MI, Thomason ME. Functional connectivity of the human brain in utero. Trends Cogn Sci. (2016) 20:931–9. doi: 10.1016/j.tics.2016.10.001
15. Rogers CE, Lean RE, Wheelock MD, Smyser CD. Aberrant structural and functional connectivity and neurodevelopmental impairment in preterm children. J Neurodev Disord. (2018) 10:38. doi: 10.1186/s11689-018-9253-x
16. Smyser CD, Wheelock MD, Limbrick DD Jr, Neil JJ. Neonatal brain injury and aberrant connectivity. Neuroimage. (2019) 185:609–23. doi: 10.1016/j.neuroimage.2018.07.057
17. Gao W, Lin W, Grewen K, Gilmore JH. Functional connectivity of the infant human brain: plastic and modifiable. Neuroscientist. (2017) 23:169–84. doi: 10.1177/1073858416635986
18. Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. (2018) 19:123. doi: 10.1038/nrn.2018.1
19. Hu ML, Zong XF, Mann JJ, Zheng JJ, Liao YH, Li ZC, et al. A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull. (2017) 33:73–84. doi: 10.1007/s12264-016-0090-1
20. Steward T, Menchon JM, Jiménez-Murcia S, Soriano-Mas C, Fernandez-Aranda F. Neural network alterations across eating disorders: a narrative review of fMRI studies. Curr Neuropharmacol. (2018) 16:1150–63. doi: 10.2174/1570159x15666171017111532
22. Rothbart MK. Temperament, development, and personality. Curr Dir Psychol Sci. (2007) 16:207–12. doi: 10.1111/j.1467-8721.2007.00505.x
23. Asendorpf JB, Denissen JJ, van Aken MA. Inhibited and aggressive preschool children at 23 years of age: personality and social transitions into adulthood. Dev Psychol. (2008) 44:997–1011. doi: 10.1037/0012-1649.44.4.997
24. Caspi A, Harrington H, Milne B, Amell JW, Theodore RF, Moffitt TE. Children's behavioral styles at age 3 are linked to their adult personality traits at age 26. J Pers. (2003) 71:495–513. doi: 10.1111/1467-6494.7104001
25. Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. (1996) 53:1033–9. doi: 10.1001/archpsyc.1996.01830110071009
26. Tang A, Crawford H, Morales S, Degnan KA, Pine DS, Fox NA. Infant behavioral inhibition predicts personality and social outcomes three decades later. Proc Natl Acad Sci USA. (2020) 117:9800–7. doi: 10.1073/pnas.1917376117
27. Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, et al. A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. J Am Acad Child Adolesc Psychiatry. (2017) 56:1097–105. doi: 10.1016/j.jaac.2017.10.007
28. Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. (2009) 48:928–35. doi: 10.1097/CHI.0b013e3181ae09df
29. Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Dev Psychopathol. (2007) 19:729–46. doi: 10.1017/S0954579407000363
30. Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. (2018) 75:336–46. doi: 10.1001/jamapsychiatry.2017.4602
31. Pérez-Edgar KE, Guyer AE. Behavioral inhibition: temperament or prodrome? Curr Behav Neurosci Rep. (2014) 1:182–90. doi: 10.1007/s40473-014-0019-9
32. Banich MT, Karol DL. The sum of the parts does not equal the whole: evidence from bihemispheric processing. J Exp Psychol Hum Percept Perform. (1992) 18:763. doi: 10.1037/0096-1523.18.3.763
33. Bjørnebekk A, Fjell AM, Walhovd KB, Grydeland H, Torgersen S, Westlye LT. Neuronal correlates of the five factor model (FFM) of human personality: multimodal imaging in a large healthy sample. Neuroimage. (2013) 65:194–208. doi: 10.1016/j.neuroimage.2012.10.009
34. Mincic AM. Neuroanatomical correlates of negative emotionality-related traits: a systematic review and meta-analysis. Neuropsychologia. (2015) 77:97–118. doi: 10.1016/j.neuropsychologia.2015.08.007
35. Patashov D, Goldstein D, Balberg M. Homologous connectivity maps can discriminate diseased from healthy brains. Paper presented at the Optics and the Brain. Tucson (2019).
36. Wang L, Li K, Zhang Q-E, Zeng Y-W, Jin Z, Dai W-J, et al. Interhemispheric functional connectivity and its relationships with clinical characteristics in major depressive disorder: a resting state fMRI study. PLoS ONE. (2013) 8:e60191. doi: 10.1371/journal.pone.0060191
37. Bulgarelli C, Blasi A, de Klerk CC, Richards JE, Hamilton A, Southgate V. Fronto-temporoparietal connectivity and self-awareness in 18-month-olds: a resting state fNIRS study. Dev Cogn Neurosci. (2019) 38:100676. doi: 10.1016/j.dcn.2019.100676
38. Bulgarelli C, de Klerk C, Richards JE, Southgate V, Hamilton A, Blasi A. The developmental trajectory of fronto-temporoparietal connectivity as a proxy of the default mode network: a longitudinal fNIRS investigation. Hum Brain Mapp. (2020) 41:2717–40. doi: 10.1002/hbm.24974
39. Homae F, Watanabe H, Otobe T, Nakano T, Go T, Konishi Y, et al. Development of global cortical networks in early infancy. J Neurosci. (2010) 30:4877–82. doi: 10.1523/jneurosci.5618-09.2010
40. Sasai S, Homae F, Watanabe H, Taga G. Frequency-specific functional connectivity in the brain during resting state revealed by NIRS. Neuroimage. (2011) 56:252–7. doi: 10.1016/j.neuroimage.2010.12.075
41. Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, et al. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev Cogn Neurosci. (2016) 18:12–25. doi: 10.1016/j.dcn.2015.09.006
42. Thomas E, Buss C, Rasmussen JM, Entringer S, Ramirez JS, Marr M, et al. Newborn amygdala connectivity and early emerging fear. Dev Cogn Neurosci. (2019) 37:100604. doi: 10.1016/j.dcn.2018.12.002
43. Xu M, Hoshino E, Yatabe K, Matsuda S, Sato H, Maki A, et al. Prefrontal function engaging in external-focused attention in 5- to 6-month-old infants: a suggestion for default mode network. Front Hum Neurosci. (2017) 10:676. doi: 10.3389/fnhum.2016.00676
44. Graham AM, Pfeifer JH, Fisher PA, Carpenter S, Fair DA. Early life stress is associated with default system integrity and emotionality during infancy. J Child Psychol Psychiatry. (2015) 56:1212–22. doi: 10.1111/jcpp.12409
45. Kok R, Lucassen N, Bakermans-Kranenburg MJ, van IMH, Ghassabian A, Roza SJ, et al. Parenting, corpus callosum, and executive function in preschool children. Child Neuropsychol. (2014) 20:583–606. doi: 10.1080/09297049.2013.832741
46. Gartstein MA, Rothbart MK. Studying infant temperament via the revised infant behavior questionnaire. Infant Behav Dev. (2003) 26:64–86. doi: 10.1016/S0163-6383(02)00169-8
47. Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. (2015) 17:690–703. doi: 10.1016/j.chom.2015.04.004
48. Kaiser RH, Andrews-Hanna JR, Spielberg JM, Warren SL, Sutton BP, Miller GA, et al. Distracted and down: neural mechanisms of affective interference in subclinical depression. Soc Cogn Affect Neurosci. (2015) 10:654–63. doi: 10.1093/scan/nsu100
49. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. (1987) 150:782–6.
50. Xu J, Liu X, Zhang J, Li Z, Wang X, Fang F, et al. FC-NIRS: a functional connectivity analysis tool for near-infrared spectroscopy data. BioMed Res Int. (2015) 2015:248724. doi: 10.1155/2015/248724
51. Cristia A, Dupoux E, Hakuno Y, Lloyd-Fox S, Schuetze M, Kivits J, et al. An online database of infant functional near infrared spectroscopy studies: a community-augmented systematic review. PLoS ONE. (2013) 8:e58906. doi: 10.1371/journal.pone.0058906
52. Rigato S, Stets M, Bonneville-Roussy A, Holmboe K. Impact of maternal depressive symptoms on the development of infant temperament: cascading effects during the first year of life. Soc Dev. (2020) 29:1115–33. doi: 10.1111/sode.12448
53. Stifter CA, Fox NA. Infant reactivity: physiological correlates of newborn and 5-month temperament. Dev Psychol. (1990) 26:582. doi: 10.1037/0012-1649.26.4.582
54. Worobey J, Blajda VM. Temperament ratings at 2 weeks, 2 months, and 1 year: differential stablity of activity and emotionality. Dev Psychol. (1989) 25:257. doi: 10.1037/0012-1649.25.2.257
55. Lordier L, Meskaldji D-E, Grouiller F, Pittet MP, Vollenweider A, Vasung L, et al. Music in premature infants enhances high-level cognitive brain networks. Proc Natl Acad Sci USA. (2019) 116:12103–8. doi: 10.1073/pnas.1817536116
56. Lloyd-Fox S, Széplaki-Köllod B, Yin J, Csibra G. Are you talking to me? Neural activations in 6-month-old infants in response to being addressed during natural interactions. Cortex. (2015) 70:35–48. doi: 10.1016/j.cortex.2015.02.005
57. Altvater-Mackensen N, Grossmann T. The role of left inferior frontal cortex during audiovisual speech perception in infants. Neuroimage. (2016) 133:14–20. doi: 10.1016/j.neuroimage.2016.02.061
58. Grossmann T, Missana M, Krol KM. The neurodevelopmental precursors of altruistic behavior in infancy. PLoS Biol. (2018) 16:e2005281. doi: 10.1371/journal.pbio.2005281
59. Kelsey CM, Krol KM, Kret ME, Grossmann T. Infants' brain responses to pupillary changes in others are affected by race. Sci Rep. (2019) 9:4317. doi: 10.1038/s41598-019-40661-z
60. Krol KM, Puglia MH, Morris JP, Connelly JJ, Grossmann T. Epigenetic modification of the oxytocin receptor gene is associated with emotion processing in the infant brain. Dev Cogn Neurosci. (2019) 37:100648. doi: 10.1016/j.dcn.2019.100648
61. Lu C, Zhang Y-J, Biswal B, Zang Y-F, Peng D, Zhu C-Z. Use of fNIRS to assess resting state functional connectivity. J Neurosci Methods. (2009) 186:242–9. doi: 10.1016/j.jneumeth.2009.11.010
62. Elwell C, Springett R, Hillman E, Delpy DT. Oscillations in cerebral haemodynamics. In: Eke A, and Delpy DT, editors. Oxygen Transport to Tissue XXI. New York, NY: Springer (1999). p. 57–65.
63. Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhäupl K, et al. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage. (2000) 12:623–39. doi: 10.1006/nimg.2000.0657
64. Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. (1997) 20:435–42. doi: 10.1016/s0166-2236(97)01132-6
65. Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. (2009) 101:3270–83. doi: 10.1152/jn.90777.2008
66. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. (2009) 44:893–905. doi: 10.1016/j.neuroimage.2008.09.036
67. Kabdebon C, Leroy F, Simmonet H, Perrot M, Dubois J, Dehaene-Lambertz G. Anatomical correlations of the international 10–20 sensor placement system in infants. Neuroimage. (2014) 99:342–56. doi: 10.1016/j.neuroimage.2014.05.046
68. Eyre M, Fitzgibbon SP, Ciarrusta J, Cordero-Grande L, Price AN, Poppe T, et al. The Developing Human Connectome Project: typical and disrupted perinatal functional connectivity. bioRxiv. (2020). doi: 10.1101/2020.01.20.912881
69. Imai M, Watanabe H, Yasui K, Kimura Y, Shitara Y, Tsuchida S, et al. Functional connectivity of the cortex of term and preterm infants and infants with Down's syndrome. Neuroimage. (2014) 85:272–8. doi: 10.1016/j.neuroimage.2013.04.080
70. Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. (2011) 54:313–27. doi: 10.1016/j.neuroimage.2010.07.033
71. Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, et al. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci USA. (2009) 106:6790–5. doi: 10.1073/pnas.0811221106
72. Grossmann T. Mapping prefrontal cortex functions in human infancy. Infancy. (2013) 18:303–24. doi: 10.1111/infa.12016
73. Grossmann T. The role of medial prefrontal cortex in early social cognition. Front Hum Neurosci. (2013) 7:340. doi: 10.3389/fnhum.2013.00340
74. Grossmann T. The early development of social brain functions in infancy. Psychol Bull. (2015) 141:1266–97. doi: 10.1037/bul0000002
75. Powell LJ, Kosakowski HL, Saxe R. Social origins of cortical face areas. Trends Cogn Sci. (2018) 22:752–63. doi: 10.1016/j.tics.2018.06.009
76. Vanderwal T, Kelly C, Eilbott J, Mayes LC, Castellanos FX. Inscapes: a movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage. (2015) 122:222–32. doi: 10.1016/j.neuroimage.2015.07.069
77. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. (2005) 102:9673–8. doi: 10.1073/pnas.0504136102
78. Lloyd-Fox S, Blasi A, Elwell C. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev. (2010) 34:269–84. doi: 10.1016/j.neubiorev.2009.07.008
79. Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA. (2010) 107:20015–20020. doi: 10.1073/pnas.1007921107
Keywords: functional near infrared spectroscopy, functional connectivity, default mode network, fronto parietal network, infancy, temperament
Citation: Kelsey CM, Farris K and Grossmann T (2021) Variability in Infants' Functional Brain Network Connectivity Is Associated With Differences in Affect and Behavior. Front. Psychiatry 12:685754. doi: 10.3389/fpsyt.2021.685754
Received: 25 March 2021; Accepted: 14 May 2021;
Published: 09 June 2021.
Edited by:
Peter B. Marschik, University Medical Center Göttingen, GermanyReviewed by:
Trinh Nguyen, University of Vienna, AustriaHelmet Karim, University of Pittsburgh, United States
Copyright © 2021 Kelsey, Farris and Grossmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tobias Grossmann, dGczbnlAdmlyZ2luaWEuZWR1
 Caroline M. Kelsey
Caroline M. Kelsey Katrina Farris
Katrina Farris Tobias Grossmann
Tobias Grossmann