- 1Department of Pediatrics, Dokkyo Medical University, Mibu, Japan
- 2Department of Urology, Fujita Health University, Toyoake, Japan
- 3Clinical Research Center, Chiba University Hospital, Chiba, Japan
The balance between antioxidant capacity and oxidative stress-induced free radicals may be crucial in the pathophysiological development factor of autism spectrum disorder (ASD). We measured the following urinary and plasma biomarker levels of oxidative stress and antioxidants. As urinary biomarkers, (1) hexanoyl-lysine (HEL), which is a new biomarker of oxidative stress, (2) the total antioxidant capacity (TAC), and (3) 8-hydroxy-2′-deoxyguanosine (8-OHdG), as a product of oxidative modifications to DNA; and the plasma levels of (4) the antioxidant protein superoxide dismutase (SOD), which is the crucial defense again oxygen reactive species, and (5) transferrin and (6) ceruloplasmin, which are biomarkers of iron and copper neurotransmission and oxidant-antioxidant systems. We examined the relationship between these urinary and plasma biomarkers and behavioral symptoms in 19 individuals with ASD (mean age, 10.8 ± 5.2 years) and 10 age-matched healthy controls (mean age, 14.2 ± 7.0 years). Behavioral symptoms were estimated using the Aberrant Behavior Checklist (ABC). Urinary TAC levels were significantly lower, whereas urinary HEL levels were significantly increased in the ASD group as compared with the control group. The five ABC subscale and total scores were significantly raised in the autism group than in the control group. The results of a linear regression analysis revealed that plasma SOD levels may be a more accurate predictor of differences in ABC scores between individuals with ASD and control individuals. The present study firstly revealed the important findings that the cooperation between the urinary antioxidant TAC and plasma SOD levels may contribute to the ABC subscale scores of stereotypy. Urinary TAC activity and antioxidant protein SOD may be associated with incomplete mineral body store and antioxidant-related transcription factor and browning reactions. Consequently, a critical imbalance between TAC urinary levels and plasma SOD levels may be an important contributor to autistic behavioral symptoms.
Introduction
A lot of evidence indicated that interactions between genetic and environmental factors at the time of early childhood are critically important in the development of autism spectrum disorders (ASD) (1). A lack of balance between the excessive production of reactive oxygen species and antioxidant capacity may contribute to the pathophysiology of ASD in predisposed individuals (2, 3). Recent ASD studies reported a reduced total antioxidant capacity (TAC) (3), higher levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), a biomarker of oxidative modifications to DNA (4), and elevated urinary hexanoyl-lysine (HEL) levels (5, 6). Urinary TAC levels are a measure of oxidative stress (7), while plasma superoxide dismutase (SOD) levels are a biomarker of the antioxidant status (8). Although a series of oxidative stress-related biomarkers, including urinary HEL, TAC, and 8-OHdG levels, may provide important and useful information on brain damage induced by oxidative stress (6), they have not yet been examined in detail. Recent studies on ASD reported significantly lower urinary TAC levels, indicating heightened vulnerability to oxidative damage (3) and significantly increased urinary 8-OHdG concentrations (7). Urinary HEL levels were found to be heightened in children with ASD, on the other hand, higher HEL levels associated with the hyperactivity component of the Childhood Autism Rating Scale (5). We already reported significantly lower levels of urinary TAC (9) and significantly higher levels of urinary HEL (10) with no significant differences in 8-OHdG levels in the two groups (8, 9, 11). Nevertheless, the relationships between core ASD behavioral symptoms and urinary HEL, TAC, and 8-OHdG levels have not yet been elucidated.
Plasma (9) and serum (10, 12) levels of copper-binding antioxidant ceruloplasmin (Cp) and plasma (9) or serum (12) levels of iron-binding protein transferrin (Tf) have been identified as pathophysiological factors. Furthermore, erythrocyte (10, 13, 14) and plasma (15, 16) levels of SOD crucially contribute to the pathophysiology of ASD. The relationships of plasma antioxidant proteins, such as Tf, Cp, and SOD, to urinary oxidative stress biomarkers and TAC remain unclear.
We previously reported that increased plasma levels of docosahexaenoic acid (DHA)/arachidonic acid (ARA) and eicosapentaenoic acid (EPA)/ARA may be associated with reduced plasma levels of neuroprotective properties of Cp, diminishing the defensive activity against brain damage, and may thus impart to the pathophysiology of autistic behaviors in individuals with ASD (9, 15). Importantly, this study firstly examined the role of a set of urinary and plasma oxidative stress-related biomarkers, and added antioxidant activity possessed essential polyunsaturated fatty acids. Furthermore, this study explored the association of oxidative stress-related biomarkers between these urinary and plasma biomarkers in ASD.
The enzymes cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) have been shown to convert ARA into eicosanoids (16). As the antioxidant effect is related to eicosanoid synthesis regulation (17), decreased plasma ARA levels may reduce the antioxidant capacity.
Regarding the antioxidant capacity of essential fatty acids, DHA plays an important role in brain development and function and has also been shown to enhance the antioxidant and cognitive activities of the brain (18). A previous study demonstrated that DHA altered antioxidant enzyme activity in a valproic acid-induced rat model of ASD (19). A pretreatment with EPA prevented lipid peroxidation by inhibiting lipid oxidation (20) and also enhanced the antioxidant capacity (21). Furthermore, α-linolenic acid (ALA) exhibits antioxidant activity (22). Linolenic acid (LA) is an antioxidant that is present in the human diet (22), and γ-linolenic acid (GLA) has antioxidant potential of bioprocessed substrates (23). Dihomo-γ-linolenic acid (DGLA) has also been shown to exhibit antioxidant activity (24).
Based on these findings, we examined important avenues in research on the role of urinary and plasma antioxidants in the behavioral symptoms of individuals with ASD. The aims of the present study were 3-fold: we examined the associations between the urinary concentrations of HEL, TAC, and 8-OHdG, and plasma levels of Cp, Tf, SOD, and PUFAs, such as ARA, DHA, EPA, LA, ALA, GLA, and DGLA; their associations among the core symptoms of behavioral symptoms in individuals with ASD; and the relationship between urinary TAC levels and plasma SOD levels because SOD 1 is a powerful antioxidant enzyme that binds copper and zinc ions under neurodegenerative conditions (25).
This is a first study of the role of a set of urinary and plasma oxidative stress-related biomarkers and the association of oxidative stress-related biomarkers between these urinary and plasma biomarkers in ASD.
Previous clinical studies reported that urinary TAC levels (26) and the serum antioxidant capacity (26) were affected by a tryptophan-enriched diet and Mediterranean diet, respectively; therefore, we assessed daily food and nutrient intakes by using a semiconstructive questionnaire for the Japanese participants (DHQ) [the junior high school version (DHQ15)].
Methods
Participants
Participants comprised 29 young and physically healthy individuals. These participants were recruited to Fujimoto Medical Clinic in Kobe city, Japan and Dokkyo Medical University in Japan between May 2018 and October 2019 via a local advertisement. They and their mothers applied to this study to obtain information on their children's urinary antioxidant capacity.
Diagnosis of ASD was conducted using the Diagnostic and Statistical Manual of Mental Disorders-V (DSM-V) criteria (27) and certified under the Autism Diagnostic Interview-Revised (ADI-R). These diagnostic procedures were internationally agreed diagnostic classification systems according to ICD 10 Research Diagnostic Guidelines. Diagnostic interviews were conducted with all individuals who were assessed as possible ASD among the 29 participants. Diagnostic procedures were conducted by the psychiatrist (K.Y.) and pediatrician (G.I.) who were professional medical doctors in the field of developmental disorders. Among the 29 participants, 19 were diagnosed with ASD (12 males and seven females, mean age: 10.8 ± 5.2 years old, age range: 6–15 and 22 years old in one subject), and the remaining 10 were normal healthy controls (six males and four females, mean age: 14.2 ± 7.0 years old, age range: 5–17 and 21 years old in one control subjects). The 10 normal controls were estimated to be physically and mentally healthy during initial physical and mental screening tests before the study. The 19 individuals with ASD exhibited the core symptoms of the DSM-V diagnostic criteria for ASD without any abnormal neurological symptoms (e.g., seizures or neurological diseases). The 19 individuals with ASD and 10 healthy control subjects were matched for food intake, age, sex, and full intelligent quotient (IQ) scores (Table 1). Any other abnormalities were detected in physical (resting blood pressure and pulse rate) or clinical laboratory examinations (hematology and plasma chemistry, including plasma fatty acids) in the two groups at initial health screening tests before the study. The IQs of these individuals were assessed by using the Wechsler Intelligence Scale (WISC-IV) for children and adolescents aged 6–16 years old or the respective scale for adults (WAIS-R) (Table 1). Comorbid neuropsychiatric diseases were explored using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Additional criteria of inclusion were as follows: (a) the absence of any other medical or comorbid psychiatric diseases; (b) a baseline verbal or full IQ >70 (28) or the respective scale for adults (WAIS-R) because subjects with high-functioning ASD were estimated to have a total IQ of at least 70 (29); and (c) no treatment with antidepressants, anxiolytic medications, or neuroleptics within 3 months prior to the initiation of the present study.
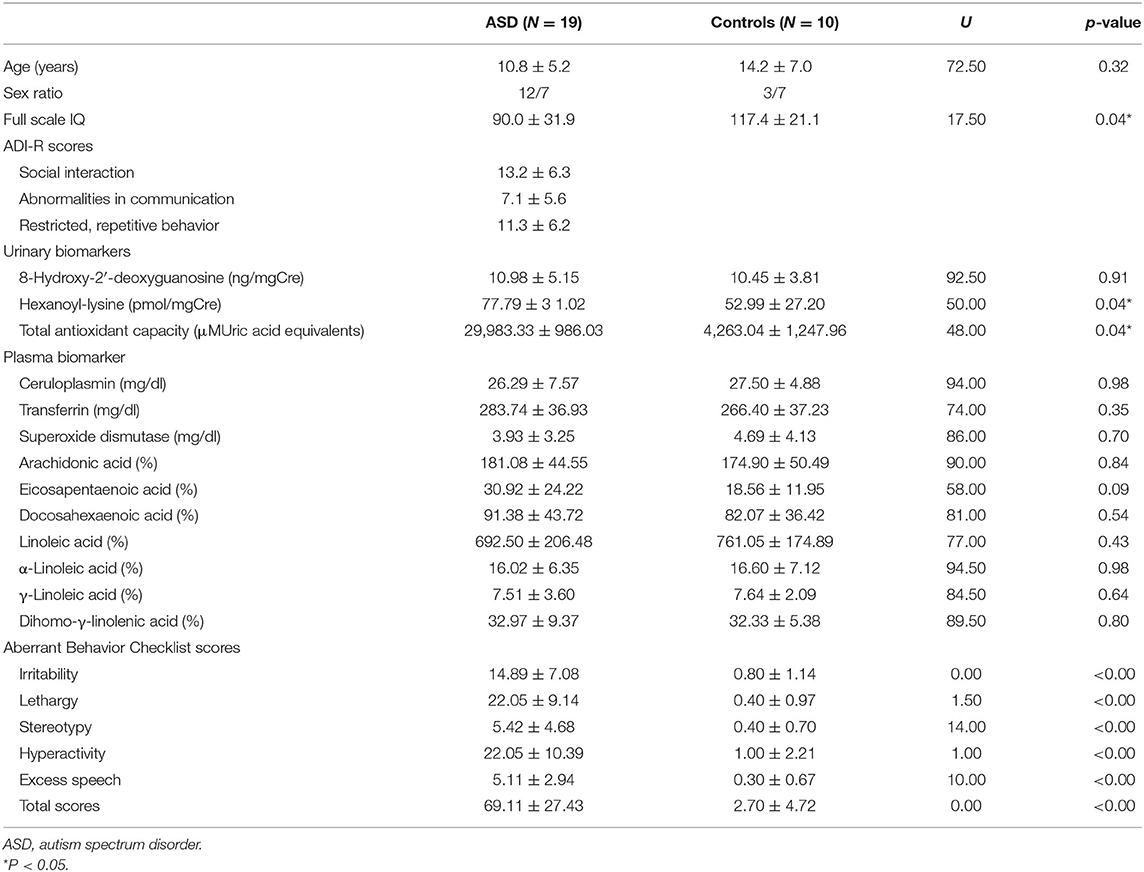
Table 1. Clinical characteristics and urinary and plasma variables, and ABC scores in ASD control groups.
The present study was conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. It was performed with the approval of the Ethics Committee of Dokkyo Medical University and the Fujimoto Medical Clinic in Kobe city, Japan. These ethics committees were registered with the Pharmaceuticals and Medical Devices Agency of Japan to register IRB information (http://www.info.pmda.go.jp/). The majority of subjects in this study were under the legal age of 20 years; therefore, we obtained parental permission and assessed data on these individuals (e.g., recognizing whether each participant's urinary TAC and plasma SOD levels were within the standard values according to SRL Inc., Tokyo, Japan). The standard values of TAC, HEL, and 8-OHdG levels were within the standard ranges established by the Department of Pediatrics, Tokyo Metropolitan Fuchu Medical Center for the Disabled, Tokyo, Japan. Written informed consent was given by the participants and/or their parents. This study was registered at the Clinical Trials Registry, Japan Medical Association, June 14, 2010; renewal, December 31, 2015; ID: JMA-IIA00162.
Management of Food Intake and Assessment of Nutrient Intake
TAC levels in urine may be influenced by food intake (30, 31). Furthermore, plasma fatty acid levels may be confounded by a prior dietary intake (28). To manage dietary intake, all 29 subjects received the “Japanese Food Guide” (32). Moreover, to assess daily food and nutrient intakes, a semiconstructive questionnaire for the Japanese participants (DHQ) was performed using the DHQ15 (DHQ Support Center in Tokyo, Japan (http://www.ebnjapan.org/). DHQ15 consists of 72 questions on the intake frequency of 150 various food and beverage items and cooking methods. The DHQ15 was completed 1 month before the study on randomly selected subsamples of 10 individuals with ASD and 10 normal controls. The estimated intake of nutrients was calculated using a dedicated program for the DHQ system (DHQ Support Center, Tokyo, Japan) (33). The validity of DHQ15 in young adolescents has been already verified (34).
Measures for Small Sample Size
As the small sample size in the present study, the present findings might not be generalizable. Therefore, efforts are needed for comparing with larger sample size and better controlled studies (35). Furthermore, the mean intraindividual variability in plasma and urine variables is expressed as coefficients of variation which was assessed to the reliability of data (36). Therefore, the coefficients of variation were used to analyze the reliability of plasma and urine analyses.
Urinary HEL, TAC, and 8-OHdG Levels
Their blood and urine sample using clean voided methods were collected in the order of medical consultation. Voided urine was gathered and immediately held at −80°C until analyzed. After the procedures of dissolution, urine samples were centrifuged to remove all insoluble materials. Specialists at the Department of Pediatrics, Tokyo Metropolitan Fuchu Medical Center for the Disabled (Tokyo, Japan) measured urinary HEL, 8-OHdG, and TAC levels. The standard values for TAC, HEL, and 8-OHdG levels were within normal ranges (TAC, 3,700–4,000; HEL, 50–125; 8-OHdG, 9.0–9.7 μM uric acid equivalents in eight normal healthy subjects aged 6–37 years) (37).
Urinary HEL Levels
Urinary HEL levels were measured in duplicate using a competitive ELISA kit (Japan International Cooperation Agency-JICA, Shizuoka, Japan) (38).
Urinary 8-OHdG Levels
Void urine were centrifuged, and the supernatants obtained after dilution were used in duplicate for assessments with a competitive enzyme-linked immunosorbent assay kit (8-OHdG check ELISA kit, JalCA, Japan Institute for the Control of Aging, Shizuoka, Japan). Results were then corrected to the urinary concentration of creatinine, and urinary 8-OHdG/creatinine levels were used in subsequent analyses (39).
Urinary TAC
As described in the instruction manual by the manufacturer (Oxford Biomedical Research), urinary TAC levels were assessed using ELISA (40). This assay measures TAC levels in samples based on the combined activities of the constituents (Oxford Biomedical Research).
Measurement of Plasma SOD, Cp, and Tf Levels
Whole blood samples were collected by venipuncture into EDTA tubes after a 5-h fast and immediately placed on ice. Although a 9–12-h fast before triglyceride measurements was previously reported to be appropriate (41), another study indicated that fasting for 8 h was sufficient. Besides triglycerides, lipid parameters without fasting are useful when examining for dyslipidemia in children (42) and plasma lipid profiles may be evaluated in non-fasted blood samples (43). Therefore, the 5-h fast employed in the present study was acceptable. After 20 min of supine rest in a quiet room to minimize the effects of circadian variations, blood was collected from participants at 11:00 am−12:30 pm. Samples were kept at −80°C for the later analysis of plasma SOD, Cp, and Tf levels at a clinical analytical laboratory (SRL Inc., Tokyo, Japan).
Plasma SOD Levels
SOD concentration in plasma were assayed from the rate of decrease in nitrite produced by hydroxylamine and superoxide anions based on the nitrite method using a Versa max instrument (Molecular Devices Co., Tokyo, Japan). Human plasma was assayed using a SOD Assay Kit (Takara Bio, Tokyo, Japan) based on the cytochrome c method, and plasma SOD levels were expressed as units per milliliter. The sensitivity of the assay was 0.3 U/ml. Intraassay and interassay coefficients were 2.11 and 2.10 U/ml, respectively.
Plasma Cp Concentration
A Bering BN II Nephelometer (Siemens Healthcare Diagnostics K.K., USA) was used to estimate plasma Cp levels. Assay sensitivity was 3.0 mg/dl. Intra- and interassay coefficients were 10.2 and 10.1 mg/dl, respectively.
Plasma Tf Concentration
A standard turbidimetric assay and automated biochemical analyzer (JCA-BM8000 series, JEOL Ltd., Tokyo, Japan) were utilized to estimate plasma Tf levels. Intra- and interassay coefficients were 108.1 and 107.4 mg/dl, respectively.
Estimation of Autistic Behaviors
The ABC was used to evaluate the behavioral symptoms of the 19 individuals with ASD and 10 normal controls. This scale is a broad-band–estimating instrument that captures a various behavior problems including self-injurious and aggressive behaviors. The ABC was used as a dependent variable in this study to capture a broad range of behavior problems (40). A subscale of ABC is useful for the longitudinal changes of the subject's behavioral problems (44). The ABC can discriminate between disruptive behavior disorders and the behavioral symptoms of ASD (45). The associations between ABC subscales and other behavioral scales, and demographic variables provided useful measures of behavioral problems in ASD (46). The following subscales were employed: (1) irritability (15 items); (2) social withdrawal (16 items); (3) stereotypic behavior (seven items); (4) hyperactivity (16 items); and (5) excessive speech (four items).
Statistical Analysis
Specialist in statistics was all blinded to the study groups. The assumption of normality was checked. As the data were not normally distributed, the non-parametric Mann–Whitney U-test was employed. Multiple regression analyses were performed to investigate potential confounding factors, such as urine and plasma biomarkers, and assessment scales of behavioral symptoms (45). A multiple linear regression was employed to confirm the relationships between the five ABC subscale and total scores, urinary TAC, HL, and 8-OHdG levels, and plasma Cp, Tf, and SOD levels. Statistical analyses were conducted using SPSS version 18.0 (IBM Tokyo, 2009).
Results
Study Population
The 19 individuals with ASD showed DSM-V core behavioral and social symptoms: the stereotyped repetitive behaviors (n = 11); restricted interests that are abnormal in intensity or focus (n = 6); hyperreactivity to sensory input or unusual interests in sensory aspects of the environment (n = 2).
The mean ABC total score for our patients was 69.1 ± 27.4 (Table 1). A recent study reported an ABC total score of 23.6 ± 3.7 in 530 children with ASD aged 3–12 years in the autism health network (47). Therefore, our patients exhibited severe autistic behavioral symptoms. No significant differences were observed in age between the two groups (p = 0.31).
CV in Plasma and Urine Variables
The CVs in plasma and urine variables were 41.6% in the ASD group and 37.2% in the control group.
Urinary Concentrations of Oxidative Stress-Related Biomarkers
Urinary HEL levels were significantly higher (p = 0.040), whereas urinary TAC levels were significantly lower (p = 0.031) in the 19 individuals with ASD than in the 10 normal controls. No significant differences were observed in urinary 8-OHdG levels or plasma SOD levels between the groups (Table 1).
Predictor Variables
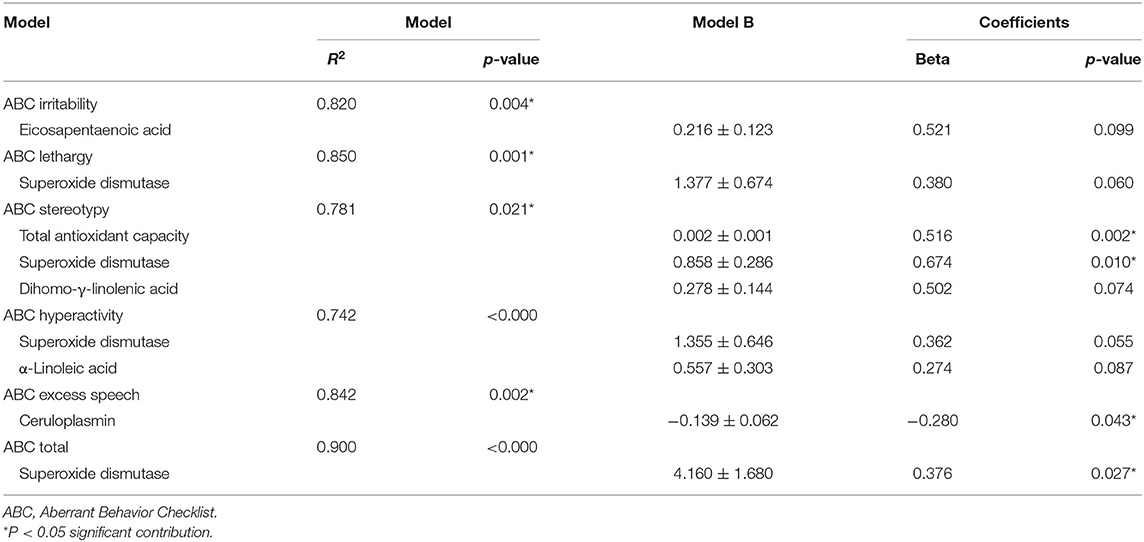
As shown in Table 2, plasma SOD levels (B = 4.160 ± 1.680, β = 0.376; p = 0.027) significantly contributed to ABC total scores. Urinary TAC levels (B = 0.002 ± 0.001, β = 0.561; p = 0.020) and plasma SOD levels (B = 0.856 ± 0.286, β = 3.001; p = 0.010) contributed to the stereotypy subscale score.
Collectively, these results suggest that plasma SOD levels and urinary TAC levels are accurate predictors of differences in the biomarkers in the ABC total scores and stereotypy subscale scores, respectively, from the control group (Table 2).
Assessment of Nutrient Intake
There were no significant differences in weight, height, energy intake, or the intakes of protein (p = 1.00), cholesterol (p = 0.49), omega-6 (p = 0.44), iron p = 0.55), copper (p = 0.55), omega-3 (p = 0.50), or omega-6 PUFAs (p = 0.44) between the two groups (Table 3).
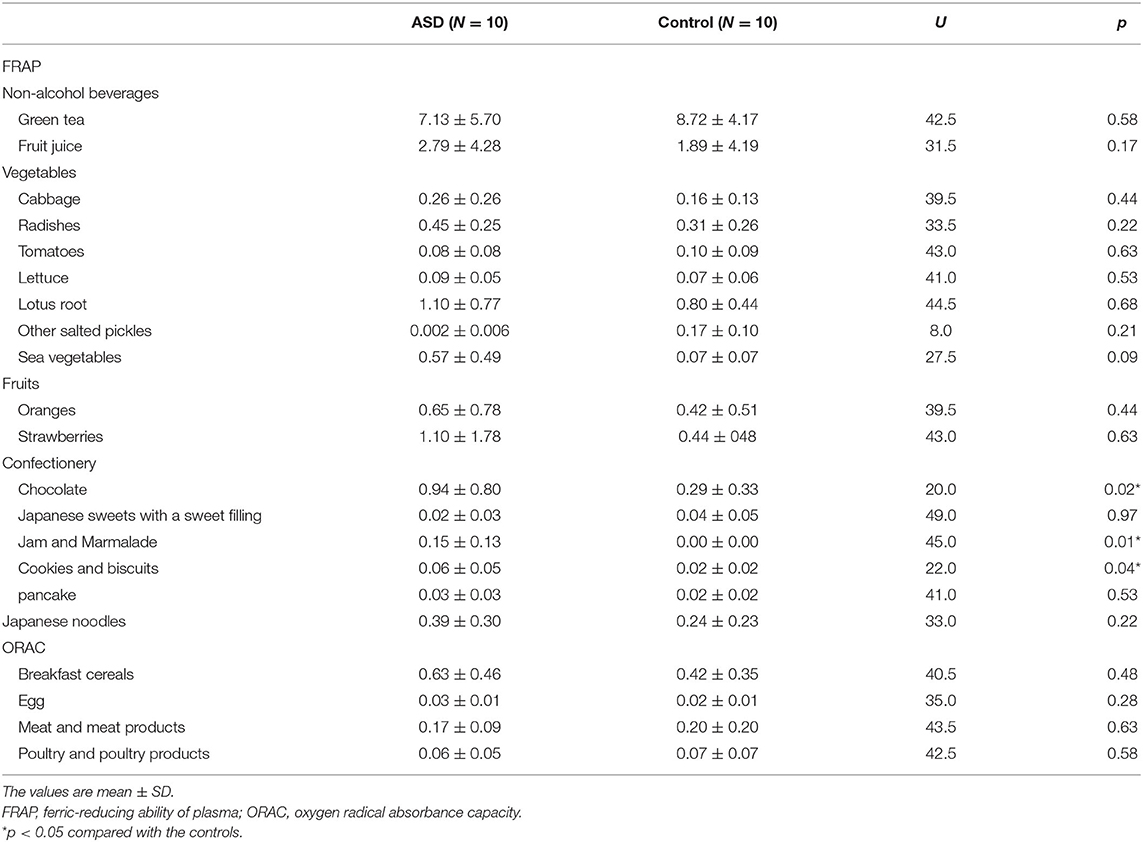
Table 3. Dietary TAP in the random subsamples that include 10 individuals with 10 individuals with ASD and 10 normal controls.
Main Findings
Urinary antioxidant biomarker HEL were increased, while, urinary antioxidant biomarker TAP levels were reduced in the ASD group. Consequently, a critical imbalance between urinary TAC levels and plasma SOD levels may be an important contributor to autistic behavioral symptoms.
Discussion
We calculated the coefficient of variation (CV) (mean/SD) in plasma and urine variables to analyze the reliability (36) of plasma and urine analyses. The CVs in the ASD and control groups were 41.65 and 37.2%, respectively. The JAMA clinical trial report indicated that the CV in plasma variable concentration was 46.1 and 32% in two groups with sunscreen spray and lotion 4.3 and 1.8 ng/ml, respectively (48). Furthermore, a previous study of microRNA expression levels indicated the CV of 20–40% (49). Although the variations in plasma and urine variables were large, the CVs in this study appeared to be reasonable.
In this study, age difference between ASD subjects and controls was 4.5 years (Table 1). These ages in the two groups were not significantly different (p = 0.328). A lot of previous studies on randomized clinical trials included subjects with age range of 5–17 years (50) or 18–35 years (51). In a recent clinical report, 1,260 adolescent girls with ages 10–17 years were included in food survey study (52). Thus, age ranges in this study appeared to be reasonable.
This study revealed that TAC concentrations in urine were significantly reduced, while HEL concentrations in urine were significantly increased in the 19 individuals with ASD than in the 10 healthy controls. Furthermore, there were no significant differences in 8-OHdG concentrations in urine between the two groups. The results of a linear regression analysis revealed that plasma SOD and Tf concentrations and urinary TAC concentrations were accurate predictors of differences in the biomarkers and the ABC total scores and subscale scores of stereotypy and excess speech between the ASD and control groups. These results indicate that an important relationship exists between urinary TAC levels and plasma SOD levels, and also that plasma SOD levels contribute to ABC scores (Table 2). Furthermore, plasma SOD levels may be a more accurate predictor of differences in ABC scores between the two groups.
Regarding the role of SOD in ASD, reduced serum SOD levels have been contributed to the pathophysiology and progression of ASD in 96 children, suggesting that SOD is a risk factor for the development of ASD (53). SOD levels were previously shown to be higher in autistic children than in typically developed children, and this increase was associated with the upregularized expression of nitrotyrosine, a marker of oxidative damage, in the immune cells of ASD subjects (54). SOD levels in serum were significantly reduced in 30 children with ASD as compared with those in the control individuals with no severe ASD symptoms, suggesting the utility of reduced serum SOD levels as an early diagnostic biomarker (55). Previous animal studies reported that an injection of SOD induced ASD-like repetitive behavior (56), and that SOD 1 knockout mice exhibited impaired motivational behavior (57). SOD levels were reduced in a rat model of maternal diabetes (58), and SOD 1 genetic mice exhibited bizarre behavior (59). Therefore, SOD may regulate social behavior in animals.
The present results indicated a relationship between plasma SOD levels and autistic behavior, and, thus, provide insights into the contribution of SOD to autistic behavior, such as stereotypy. It is important to note that urinary TAC levels and plasma SOD concentrations were secure indicator to distinguish the ASD group from the control group. These results indicate additional and useful information on the crucial imbalance between heightened oxidative stress and deficit urinary antioxidant systems in the pathophysiological factors in ASD subjects.
Regarding the relationship between urinary TAC levels and plasma SOD levels, accumulating evidence indicates that the imbalance between oxidative stress-induced reactive oxygen species and urinary TAC levels correlates with ASD. A previous report demonstrated that SOD levels were higher in autistic children than in typically developed children, and this increase was associated with the up-regularized expression of nitrotyrosine, a marker of oxidative damage, in the immune cells of ASD subjects (60). Of reference, plasma SOD may affect urinary TAC activity (10). Other clinical reports indicated significant decreases in plasma SOD and urinary TAC concentrations in 47 patients with sarcoma (51). Collectively, these findings revealed concomitant changes in plasma and urine SOD and TAC levels, which is consistent with the present results.
In the present study, the linear regression analysis showed that urinary TAC levels significantly contributed to the ABC subscale scores of stereotypy. Urinary antioxidant levels were previously shown to be significantly lower in autistic children and positively correlated with symptom severity (61). We previously reported reduced urinary TAC levels (9, 11) and increased urinary oxidative stress biomarker HEL (15) in 20 autistic children. Furthermore, significant reductions were observed in urinary TAC levels with increases in the severity of ASD (61). In comparisons with 24 age-matched healthy controls, reduced urinary TAC levels with no consequent heightened urinary catalase activity and total thiol molecules, which are the measures of the antioxidant activities (3), were reported in 29 subjects with ASD with age of 6–12 years (3). Moreover, plasma and erythrocyte TAC levels were significantly lower in 34 adolescent individuals with Asperger syndrome (mean age: 12.89 ± 2.58 years) than in 34 age-matched controls (62). These findings suggest that urinary TAC levels in association with a low detoxifying capacity, as indicated by reduced plasma SOD levels, in ASD individuals contribute to the characteristic behavioral symptoms of ASD. Importantly, this study demonstrated for the first time a relationship between urinary TAC levels and plasma SOD levels that contribute to the characteristic symptoms of ASD, such as stereotypy.
Assessment of daily nutrient intake using DHQ15 revealed no significant differences in the intake of fat, protein, vitamin B2, vitamin B6, vitamin B12, vitamin C, omega-6, or omega-3 PUFAs between randomly selected subsamples of 10 individuals with ASD and those of 10 controls. Regarding the association between dietary intake and urinary TAC levels, virgin olive oil (63) and tryptophan-enriched cereal (26) were increase urinary TAC levels, whereas refined potato starch reduced urinary TAC concentrations (64). Thus, the effects of food intake on urinary TAC levels may vary, and differences between the groups examined did not appear to influence urinary TAC levels. The intake of TAC from chocolate, biscuits, cookies, jam, and marmalade was significantly increased in the ASD group than in the control group. Products of cocoa, such as chocolate, are good sources of dietary antioxidants (65). Cookies containing chocolate chips increased antioxidant capacity (63), Furthermore, biscuits containing 5% cocoa held antioxidant properties (66). Jam and marmalade productions increased the antioxidant capacity (67), and bilberry jams contained antioxidant compounds (68, 69). Although chocolate, cookies, biscuits, jam, and marmalade contained potent antioxidant abilities, urinary TAC levels were significantly lower in the ASD group than in the control group.
Previous studies on the antioxidant capacity in subjects with ASD implied vulnerability to oxidative stress because of an unbalance in intracellular and extracellular antioxidant activities or a chronically reduced detoxifying capacity (62, 70).
A recent study on antioxidant networks indicated that antioxidant enzymes, such as SOD, glutathione peroxidase, and oxidized glutathione, function as an antioxidant network within specific intracellular or extracellular components of the antioxidant system (71). Such vulnerable antioxidant systems may be due to genetic and dietary factor (72) or the integrative concept of balance between oxygen- and capacity-limited thermal tolerances (73). Dysregulation of antioxidant enzymes such as SOD, glutathione peroxidase, and glutathione reductase were included in neutrophils and monocytes as in antioxidant network in peripheral innate immune cells (54).
SOD and catalase were also antioxidant defense system (74), and SOD/catalase activities may contribute to ASD pathophysiology (75). Interestingly, an inverse relationship between SOD activity and catalase due to changes in the toxic effects of polyvinyl chloride has been reported (76). Additionally, reactive oxygen species competes between oxidative stress (2, 77) and contribute to the development of ASD (75). These antioxidant enzymes such as SOD, glutathione peroxidase, glutathione reductase, catalase, and reactive oxygen species (ROS) have been found to contribute to the pathophysiology of ASD (2).
Glutathione depletion and the activities of antioxidant enzymes such as SOD and ROS levels were closely associated with the cytoprotective effects of gamma-aminobutyric acid (GABA) against oxidative stress via the increased expression of interleukin 8 related to the transcription factor [nuclear factor erythroid 2-related factor 2 (Nrf2)] (78), which has an antioxidant activity (79), and upregulating expression of phase II antioxidant genes (80). Moreover, formula-induced Maillard reaction was related to SOD, catalase, glutathione peroxidase, and messenger RNA levels of miRNA-21 and miRNA-155 (81). Importantly, accumulating evidence indicated that browning or Maillard reaction, which was induced by oxidative reactions of food and cookie, produce strong activity of antioxidant (82). This reaction may contribute to increased SOD activity (83, 84). Several previous reports on the antioxidant activities in individuals with ASD indicated vulnerability to oxidative stress because of an imbalance in intracellular and extracellular antioxidant capacities (85) or an endogenous reduced detoxifying capacity (62, 70). Such antioxidant defense systems may be involved in the endogenous antioxidant system. A present finding on antioxidant networks indicated that antioxidant enzymes, such as SOD, glutathione peroxidase, and oxidized glutathione may operate as an antioxidant network in the specific intracellular or the extracellular antioxidant system (71).
In the present study, the linear regression analysis indicated that plasma Cp levels contributed to the ABC subscale scores of Excess speech. Plasma Cp levels were previously shown to be reduced in 28 autistic children with a mean age of 13.2 years (12). Reduced plasma Cp levels may induce or be a consequence of neurodegeneration, such as Alzheimer's disease, while decreased plasma SOD activity may exacerbate defective Cp activity, indicating an intrinsic relationship between plasma Cp and SOD levels (86). Elevated serum Cp levels have been associated with impulsiveness in patients with Parkinson's disease (87). Cerebrospinal fluid Cp can be induced in neurodegenerative disorders (88). Collectively, these findings implicate Cp in neurodegeneration in ASD.
The present study had some limitations: (a) plasma and urinary concentrations of glutathione peroxidation (GPX) are frequently used as antioxidant in clinical studies (89, 90). However, urinary GPX concentrations were sensitive to selenium (79, 91). Thus, urinary GPX may not reflect serum total antioxidant capacity. Additionally, the present study revealed an informative set of antioxidant biomarkers including HEL and 8-OHdG concentrations in urine, and Cp, Tf, and SOD concentrations in plasma, for the first time discovered important findings on impaired antioxidant systems; (b) the male/female ratio in the present study was 13/7 (1.8/1.0), which is consistent with several clinical studies reported previously (92). Furthermore, the ratio of normal controls to cases in the present study was (2:1), which was similar to that in a randomized clinical trial on a new drug (experimental group/control group ratio of 2:1) (93). (c) Although there is no significant difference in ages between ASD and control groups, the age range of both groups are different. Of reference, a lot of previous clinical studies indicated large age ranges in patients and healthy controls. Several clinical studies, for example, showed that 34.68 ± 15.43 years old in 65 patients with diabetes mellitus and 47.33 ± 19.58 years old in 27 healthy controls (94), and that 70.5 ± 2.1 years old in 50 heart failure patients in New York, and 47.9 ± 9.7 years old in 28 healthy university population (95); (d) given the small sample sizes, this study employed two types of measures: first, an endogenous antioxidant system is needed for comparing larger sample size and better-controlled studies described in the Journal of American Medical Association (JAMA) (96). Of reference, the present findings were supported by previous studies (10, 12–15, 97, 98). The present findings on increased urinary HEL levels and decreased urinary TAC levels (8, 11) were supported by previous studies. According to the previous studies, the CV in this study is reasonable (49, 50). To measure small sample size in this study, the mean intraindividual variability in plasma and urine variables is expressed as CV (36).
Conclusion
The present findings are the first to report the reduced urinary TAC levels and increased urinary HEL levels in individuals with ASD without significant changes in urinary 8-OHdG levels. Importantly, the cooperation between the urinary antioxidant TAC and plasma SOD levels may contribute to stereotypy. Therefore, the role of redox regulation in both plasma SOD and urinary TAC may play an important role in autistic stereotypy. Additionally, the results of linear regression analysis revealed that SOD concentrations in plasma are a more adequate parameter for discriminating the ASD group from the control group. Therefore, the generation of ROS and TAC balance was defective in individuals with ASD. Urinary TAC demonstrated that elements and incomplete mineral body store or nitric oxide may be related to urinary activities of urinary TAC. The activities of antioxidant protein SOD were related to transcription factor and browning or Maillard reactions. These present results suggest that the endogenous antioxidant defense system is defective in youth in subjects with ASD.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Dokkyo Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. The animal study was reviewed and approved by the Ethical Committee of Dokkyo Medical University. Written informed consent was obtained from the owners for the participation of their animals in this study. Written informed consent was obtained from the individual(s) and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
KY and GI conceptualized and designed this study and contributed to the writing of the manuscript. KY, GI, and HS contributed to the collection of the plasma and urinary samples. YK and YS conducted statistical analysis. KY analyzed the data. KY, GI, HS, and RS contributed to the interpretation of the data and results. All of the authors approved the final manuscript and submission to the Frontiers in Psychiatry.
Funding
This research was founded by a Grant-in-Aid for scientific research (C) (2016–2018; No. 26461777) by the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all of the parents and patients who kindly volunteered to participate in providing clinical information. We also thank comedical staffs in the Dokkyo Medical University and the Fujimoto Clinic for assisting this study.
References
1. Stoltenberg C, Schjølberg S, Bresnahan M, Hornig M, Hirtz D, Dahl C, et al. The Autism Birth Cohort: a paradigm for gene-environment-timing research. Mol Psychiatry. (2010) 15:676–80. doi: 10.1038/mp.2009.143
2. Manivasagam T, Arunadevi S, Essa MM, SaravanaBabu C, Borah A, Thenmozhi AJ, et al. Role of oxidative stress and antioxidants in autism. Adv Neurobiol. (2020) 24:193–206. doi: 10.1007/978-3-030-30402-7_7
3. Ranjbar A, Rashedi V, Rezaei M. Comparison of urinary oxidative biomarkers in Iranian children with autism. Res Dev Disabil. (2014) 35:2751–5. doi: 10.1016/j.ridd.2014.07.010
4. Graille M, Wild P, Sauvain JJ, Hemmendinger M, Guseva Canu I, Hopf NB. Urinary 8-OHdG as a biomarker for oxidative stress: a systematic literature review and meta-analysis. Int J Mol Sci. (2020) 21:3743. doi: 10.3390/ijms21113743
5. Ghezzo A, Visconti P, Abruzzo PM, Bolotta A, Ferreri C, Mazzanti G, et al. Oxidative stress and erythrocyte membrane alterations in children with autism: correlation with clinical features. PLoS ONE. (2013) 8:e66418. doi: 10.1371/journal.pone.0066418
6. Tokuda F, Sando Y. Matsui H. N epsilon-(hexanoyl) lysine, a new oxidative stress marker, is increased in metabolic syndrome, but not in obstructive sleep apnea. Am J Med Sci. (2009) 338:127–33. doi: 10.1097/MAJ.0b013e3181a478e5
7. Ming X, Stein TP, Brimacombe M, Johnson WG, Lambert GH, Wagner GC. Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukot Essent Fatty Acids. (2005) 73:379–84. doi: 10.1016/j.plefa.2005.06.002
8. Yui K, Tanuma N, Yamada H, Kawasaki Y. Reduced endogenous urinary total antioxidant power and its relation of plasma antioxidant activity of superoxide dismutase in individuals with autism spectrum disorder. Int J Dev Neurosci. (2017) 60:70–7. doi: 10.1016/j.ijdevneu.2016.08.003
9. Yui K, Imataka G, Kawasaki Y, Yamada H. Increased ω-3 polyunsaturated fatty acid/arachidonic acid ratios and upregulation of signaling mediator in individuals with autism spectrum disorders. Life Sci. (2016) 145:205–12. doi: 10.1016/j.lfs.2015.12.039
10. Tórsdóttir G, Hreidarsson S, Kristinsson J, Snaedal J, Jóhannesson Y. Ceruloplasmin, superoxide dismutase and copper in autistic patients. Basic Clin Pharmacol Toxicol. (2005) 96:146–8. doi: 10.1111/j.1742-7843.2005.pto960210.x
11. Yui K, Tanuma N, Yamada H, Kawasaki Y. Decreased total antioxidant capacity has a larger effect size than increased oxidant levels in urine in individuals with autism spectrum disorder. Environ Sci Pollut Res Int. (2017) 24:9635–44. doi: 10.1007/s11356-017-8595-3
12. Chauhan A, Chauhan V, Brown WT, Cohen I. Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin–the antioxidant proteins. Life Sci. (2004) 75:2539–49. doi: 10.1016/j.lfs.2004.04.038
13. Yorbik O, Sayal A, Akay CD, Akbiyik DI, Sohmen T. Investigation of antioxidant enzymes in children with autistic disorder. Prostaglands Leukot Essent Fatty Acids. (2002) 67:341–3. doi: 10.1054/plef.2002.0439
14. Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L. Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem. (2009) 42:1032–40. doi: 10.1016/j.clinbiochem.2009.03.011
15. Yui K, Imataka G, Kawasaki Y, Yamada H. Down-regulation of a signaling mediator in association with lowered plasma arachidonic acid levels in individuals with autism spectrum disorders. Neurosci Lett. (2016) 610:223–8. doi: 10.1016/j.neulet.2015.11.006
16. Wang MH, Hsiao G, Al-Shabrawey M, Wang MH. Eicosanoids and oxidative stress in diabetic retinopathy. Antioxidants. (2020) 9:520. doi: 10.3390/antiox9060520
17. Storniolo CE, Moreno JJ. Resveratrol analogs with antioxidant activity inhibit intestinal epithelial cancer Caco-2 cell growth by modulating arachidonic acid cascade. J Agric Food Chem. (2019) 67:819–28. doi: 10.1021/acs.jafc.8b05982
18. Hashimoto M, Hossain S, Al Mamun A, Matsuzaki K, Arai H. Docosahexaenoic acid: one molecule diverse functions. Crit Rev Biotechnol. (2017) 37:579–97. doi: 10.1080/07388551.2016.1207153
19. Gao J, Wu H, Cao Y, Liang S, Sun C, Wang P, Wang J, Sun H, Wu L. Maternal DHA supplementation protects rat offspring against impairment of learning and memory following prenatal exposure to valproic acid. J Nutr Biochem. (2016)35:87–95. doi: 10.1016/j.jnutbio.2016.07.003
20. Sherratt SCR, Mason RP. Eicosapentaenoic acid and docosahexaenoic acid have distinct membrane locations and lipid interactions as determined by X-ray diffraction. Chem Phys Lipids. (2018) 212:73–9. doi: 10.1016/j.chemphyslip.2018.01.002
21. Peng J, Xiong J, Cui C, Huang N, Zhang H, Wu X, et al. Maternal eicosapentaenoic acid feeding decreases placental lipid deposition and improves the homeostasis of oxidative stress through a sirtuin-1 (SIRT1) independent manner. Mol Nutr Food Res. (2019) 63:e1900343. doi: 10.1002/mnfr.201900343
22. Saffaryazdi A, Ganjeali A, Farhoosh R, Cheniany M. Variation in phenolic compounds, alpha-linolenic acid and linoleic acid contents and antioxidant activity of purslane (Portulaca oleracea L) during phenological growth stages. Physiol Mol Biol Plants. (2020) 26:1519–29. doi: 10.1007/s12298-020-00836-9
23. Dulf FV, Vodnar DC, Toşa MI, Dulf EH. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. (2020) 310:125927. doi: 10.1016/j.foodchem.2019.125927
24. Timoszuk M, Bielawska K, Skrzydlewska E. Evening Primrose (Oenothera biennis) Biological activity dependent on chemical composition. Antioxidants (Basel). (2018) 7:108. doi: 10.3390/antiox7080108
25. Kaur SJ, McKeown SR, Rashid S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene. (2016) 577:109–18. doi: 10.1016/j.gene.2015.11.049
26. Bravo R, Matito S, Cubero J, Paredes SD, Franco L, Rivero M, et al. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age. (2013) 35:1277–85. doi: 10.1007/s11357-012-9419-5
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth edition. Arlington: American Psychiatric Publishing (2013).
28. Zhong Y, Wang K, Jiang L, Wang J, Zhang X, Xu J, et al. Dietary fatty acid intake, plasma fatty acid levels, and the risk of age-related macular degeneration (AMD): Jan 19, a dose-response meta-analysis of prospective cohort studies. Eur J Nutr. (2021) 2445:4. doi: 10.1007/s00394-020-02445-4
29. Koyama T, Kamio Y, Inada N, Kurita H. Sex differences in WISC-III profiles of children with high-functioning pervasive developmental disorders. J Autism Dev Disord. (2009) 39:135–41. doi: 10.1007/s10803-008-0610-6
30. González-Flores D, Gamero E, Garrido M, Ramírez R, Moreno D, Delgado J, et al. Urinary 6-sulfatoxymelatonin and total antioxidant capacity increase after the intake of a grape juice cv. Tempranillo stabilized with HHP. Food Funct. (2012) 3:34–9. doi: 10.1039/C1FO10146C
31. Garrido M, Paredes SD, Cubero J, Lozano M, Toribio-Delgado AF, Muñoz JLR, et al. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J Gerontol A Biol Sci Med Sci. (2010) 65:909–14. doi: 10.1093/gerona/glq099
32. Ministry of Health Labour and Welfare Ministry of Agriculture Forestry and Fishers. Japanese Food Guide. Tokyo: Ministry of Health, Labour and Welfare, and Ministry of Agriculture, Forestry and Fishers (2012).
33. Kobayashi Y, Hattori M, Wada S, Iwase H, Kadono M, Tatsumi H, et al. Assessment of daily food and nutrient intake in Japanese type 2 diabetes mellitus patients using dietary reference intakes. Nutrients. (2013) 5:2276–88. doi: 10.3390/nu5072276
34. Okuda M, Sasaki S, Bando N, Hashimoto M, Kunitsugu I, Sugiyama S, et al. Carotenoid, tocopherol, and fatty acid biomarkers and dietary intake estimated by using a brief self-administered diet history questionnaire for older Japanese children and adolescents. J Nutr Sci Vitaminol. (2009) 55:231–41. doi: 10.3177/jnsv.55.231
35. Morgan J. Review use of proper statistical techniques for research studies with small samples. Charity Am J Physiol Lung Cell Mol Physiol. (2017) 313:L873–7. doi: 10.1152/ajplung.00238.2017
36. Lago-Fuentes C, Aiello P, Testa M, Muñoz I, Calvo MM. Validity and reliability of a new device to measure type of actions in indoor sports. Int J Sports Med. (2021) 42:253–8. doi: 10.1055/a-1244-9985
37. Miyata R, Tanuma N, Sakuma H, Hayashi H. Circadian rhythms of oxidative stress markers and melatonin metabolite in patients with Xeroderma pigmentosum group A. Oxid Med Cell Longev. (2016) 2016:5741517. doi: 10.1155/2016/5741517
38. MatsuI Y, Satoh K, Miyazaki T, Shirabe S, Atarashi R, Mutsukura K, et al. High sensitivity of an ELISA kit for detection of the gamma-isoform of 14-3-3 proteins: usefulness in laboratory diagnosis of human prion disease. BMC Neurol. (2011) 11:120. doi: 10.1186/1471-2377-11-120
39. Boonla C, Wunsuwan R, Tungsanga K, Tosukhowong P. Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol Res. (2007) 35:185–91. doi: 10.1007/s00240-007-0098-0
40. Rojahn J, Schroeder SR, Mayo-Ortega L, Oyama-Ganiko R, LeBlanc J, Marquis J, et al. Validity and reliability of the behavior problems inventory, the Aberrant Behavior Checklist, and the Repetitive Behavior Scale-Revised among infants and toddlers at risk for intellectual or developmental disabilities: a multi-method assessment approach. Res Dev Disabil. (2013) 34:1804–14. doi: 10.1016/j.ridd.2013.02.024
41. Simundic AM, Cornes M, Grankvist K, Lippi G, Nybo M. Standardization of collection requirements for fasting samples: for the Working Group on Preanalytical Phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin Chim Acta. (2014) 432:33–7. doi: 10.1016/j.cca.2013.11.008
42. Kubo T, Takahashi K, Furujo M, Hyodo Y, Tsuchiya H, Hattori M, et al. Usefulness of non-fasting lipid parameters in children. J Pediatr Endocrinol Metab. (2017) 30:77–83. doi: 10.1515/jpem-2016-0271
43. Watts GF, Sypniewska G, Wiklund O, Borén J. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cutpoints-A joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem. (2016) 62:930–46. doi: 10.1373/clinchem.2016.258897
44. Doyle CA, McDougle CJ. Dialogues Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Clin Neurosci. (2012) 14:263–79. doi: 10.31887/DCNS.2012.14.3/cdoyle
45. Karabekiroglu K, Aman MG. Validity of the aberrant behavior checklist in a clinical sample of toddlers. Child Psychiatry Hum Dev. (2009) 40:99–110. doi: 10.1007/s10578-008-0108-7
46. Kaat AJ, Lecavalier L, Aman MG. Validity of the aberrant behavior checklist in children with autism spectrum disorder. J Autism Dev Disord. (2014) 44:1103–16. doi: 10.1007/s10803-013-1970-0
47. Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod. (2019) 34:1567–75. doi: 10.1093/humrep/dez079
48. Matta MK, Zusterzeel R, Pilli NR, Patel V, Volpe DA, Florian J, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. (2019) 321:2082–91. doi: 10.1001/jama.2019.5586
49. Otsu H, Watanabe M, Inoue N, Masutani R, Iwatani Y. Intraindividual variation of microRNA expression levels in plasma and peripheral blood mononuclear cells and the associations of these levels with the pathogenesis of autoimmune thyroid diseases. Clin Chem Lab Med. (2017) 55:626–35. doi: 10.1515/cclm-2016-0449
50. Wang B, Eastwood PR, Becker A, Isensee C, Wong JWY, Huang RC, et al. Concurrent developmental course of sleep problems and emotional/behavioral problems in childhood and adolescence as reflected by the dysregulation profile. Sleep. (2019) 42:zsy243. doi: 10.1093/sleep/zsy243
51. Olson CM, Groth SW, Graham ML, Reschke JE, Strawderman MS, Fernandez ID. The effectiveness of an online intervention in preventing excessive gestational weight gain: the e-moms roc randomized controlled trial. BMC Pregnancy Childbirth. (2018) 18:148. doi: 10.1186/s12884-018-1767-4
52. Yunus FM, Jalal C, Afsana K, Podder R, Vandenberg A. DellaValle DM. Iron-fortified lentils to improve iron (Fe) status among adolescent girls in Bangladesh-study protocol for a double-blind community-based randomized controlled trial. Trials. (2019) 20:251. doi: 10.1186/s13063-019-3309-4
53. Wang L, Jia J, Zhang J, Li K. Serum levels of SOD and risk of autism spectrum disorder: a case-control study. Int J Dev Neurosci. (2016) 51:12–6. doi: 10.1016/j.ijdevneu.2016.04.004
54. Nadeem A, Ahmad SF, Attia SM, Al-Ayadhi LY, Al-Harbi NO, Bakheet SA. Dysregulated enzymatic antioxidant network in peripheral neutrophils and monocytes in children with autism. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 88:352–9. doi: 10.1016/j.pnpbp.2018.08.020
55. Afrazeh M, Saedisar S, Khakzad MR, Hojati M. Measurement of serum superoxide dismutase and its relevance to disease intensity autistic children. Maedica. (2015) 10:315–8.
56. Horspool AM, Chang HC. Superoxide dismutase SOD-1 modulates C. elegans pathogen avoidance behavior. Sci Rep. (2017) 2017:45128. doi: 10.1038/srep45128
57. Yoshihara D, Fujiwara N, Kitanaka N, Kitanaka J, Sakiyama H, Eguchi H, et al. The absence of the SOD1 gene causes abnormal monoaminergic neurotransmission and motivational impairment-like behavior in mice. Free Radic Res. (2016) 50:1245–56. doi: 10.1080/10715762.2016.1234048
58. Wang X, Lu J, Xie W, Lu X, Liang Y, Li M, et al. Maternal diabetes induces autism-like behavior by hyperglycemia-mediated persistent oxidative stress and suppression of superoxide dismutase 2. Proc Natl Acad Sci USA. (2019) 116:23743–52. doi: 10.1073/pnas.1912625116
59. Kreilaus F, Guerra S, Masanetz R, Menne V, Yerbury J, Karl T. Novel behavioural characteristics of the superoxide dismutase 1 G93A (SOD1) mouse model of amyotrophic lateral sclerosis include sex-dependent phenotypes. Genes Brain Behav. (2020) 19:12604. doi: 10.1111/gbb.12604
60. Ilhan M, Turgut S, Turan S, Cekic SD, Ergen HA, Dursun CK, et al. The assessment of total antioxidant capacity and superoxide dismutase levels, and the possible role of manganese superoxide dismutase polymorphism in acromegaly. Endocr J. (2018) 65:91–9. doi: 10.1507/endocrj.EJ17-0300
61. Priya L, Damodaran M, Arumugam G. Urinary oxidative stress markers in children with autism. Redox Rep. (2011) 16:216–22. doi: 10.1179/1351000211Y.0000000012
62. Parellada M, Moreno C, Mac-Dowell K, Leza JC, Giraldez M, Concepción Bailón C, et al. Plasma antioxidant capacity is reduced in Asperger syndrome. J Psychiatr Res. (2012) 46:394–401. doi: 10.1016/j.jpsychires.2011.10.004
63. Garrido M, González-Flores D, Marchena AM, Prior EJ, García-Parria J, Barriga C, et al. A lycopene-enriched virgin olive oil enhances antioxidant status in humans. J Sci Food Agric. (2013) 3:1820–6. doi: 10.1002/jsfa.5972
64. Vinson JA, Demkosky CA, Navarre DA, Smyda MA. High-antioxidant potatoes: acute in vivo antioxidant source and hypotensive agent in humans after supplementation to hypertensive subjects. J Agric Food Chem. (2012) 60:6749–54. doi: 10.1021/jf2045262
65. Todorovic V, Redovnikovic IR, Todorovic Z, Jankovic G, Dodevska M, Sobaji S. Polyphenols, methylxanthines, and antioxidant capacity of chocolates produced in Serbia. J Food Compost Anal. (2015) 41:137–43. doi: 10.1016/j.jfca.2015.01.018
66. Ajibola GF, Oyerinde VO, Adeniyan OS. Physiochemical and antioxidant properties of whole-wheat biscuits incorporated with Moringa oleifera leaves and cocoa powder. J Sci Res Rep. (2015) 7:195–206. doi: 10.9734/JSRR/2015/18070
67. Kamiloglu S, Pasli AA, Ozcelik B, Van Camp J, Capanoglu E. Influence of different processing and storage conditions on in vitro bioaccessibility of polyphenols in black carrot jams and marmalades. Food Chem. (2015) 186:74–82. doi: 10.1016/j.foodchem.2014.12.046
68. Poiana MA, Alexa E, Mateescu C. Tracking antioxidant properties and color changes in low-sugar bilberry jam as effect of processing, storage and pectin concentration. Chem Cent J. (2012) 6:4. doi: 10.1186/1752-153X-6-4
69. Poiana MA, Munteanu MF, Bordean DM, Gligor R, Alexa E. Assessing the effects of different pectins addition on color quality and antioxidant properties of blackberry jam. Chem Cent J. (2013) 7:121. doi: 10.1186/1752-153X-7-121
70. Alabdali A, Al-Ayadhi L, El-Ansary A. A key role for an impaired detoxification mechanism in the etiology and severity of autism spectrum disorders. Behav Brain Funct. (2014) 10:14. doi: 10.1186/1744-9081-10-14
71. Martínez-Noguera FJ, Alcaraz PE, Ortolano-Ríos R, Dufour SP, Cristian Marín-Pagán C. Differences between professional and amateur cyclists in endogenous antioxidant system profile. Antioxidants. (2021) 10:282. doi: 10.3390/antiox10020282
72. Slyskova J, Lorenzo Y, Karlsen A, Carlsen MH, Novosadova V, Blomhoff R, et al. Both genetic and dietary factors underlie individual differences in DNA damage levels and DNA repair capacity. DNA Repair. (2014) 16:66–73. doi: 10.1016/j.dnarep.2014.01.016
73. Pörtner HO, Bock C, Mark FC. Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J Exp Biol. (2017) 20:2685–96. doi: 10.1242/jeb.134585
74. Zoroglu SS, Armutcu F, Ozen S, Gurel A, Sivasli E, Yetkin O, et al. Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. Eur Arch Psychiatry Clin Neurosci. (2004) 254:143–7. doi: 10.1007/s00406-004-0456-7
75. Yenkoyan K, Harutyunyan H, Harutyunyan A. A certain role of SOD/CAT imbalance in pathogenesis of autism spectrum disorders. Free Radic Biol Med. (2018) 123:85–95. doi: 10.1016/j.freeradbiomed.2018.05.070
76. Xia X, Sun M, Zhou M, Chang Z, Li L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae Sci Total Environ. (2020) 716:136479. doi: 10.1016/j.scitotenv.2019.136479
77. Pangrazzi L, Balasco L, Bozzi Y, Pangrazzi L, Balasco L, Bozzi Y. Oxidative stress and Immune system dysfunction in autism spectrum disorders. Int J Mol Sci. (2020) 21:3293. doi: 10.3390/ijms21093293
78. Choe H, Lee H, Lee J, Kim Y. Protective effect of gamma-aminobutyric acid against oxidative stress by inducing phase II enzymes in C2C12 myoblast cells. J Food Biochem. (2021) 45:e13639. doi: 10.1111/jfbc.13639
79. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. (2013) 53:401–26. doi: 10.1146/annurev-pharmtox-011112-140320
80. Liu H, Jiang Y, Guan H, Li F, Sun-Waterhouse D, Chen Y, et al. Enhancing the antioxidative effects of foods containing rutin and alpha-amino acids via the Maillard reaction: a model study focusing on rutin-lysine system. Food Biochem. (2020) 44:e13086. doi: 10.1111/jfbc.13086
81. Firmin S, Elmhiri G, Crepin D, Souidi M, Taouis M, Abdennebi-Najar L. Formula derived Maillard reaction products in post-weaning intrauterine growth-restricted piglets induce developmental programming of hepatic oxidative stress independently of microRNA-21 and microRNA-155. J Dev Orig Health Dis. (2018) 9:566–72. doi: 10.1017/S2040174417001015
82. Sun Y, Hayakawa S, Ogawa M, Fukada K, Izumori K. Influence of a rare sugar, d-Psicose, on the physicochemical and functional properties of an aerated food system containing egg albumen. J Agric Food Chem. (2008) 56:4789–96. doi: 10.1021/jf800050d
83. Nguyen NH, Tran GB, Nguyen CT. Anti-oxidative effects of superoxide dismutase 3 on inflammatory diseases. J Mol Med. (2020) 98:59–69. doi: 10.1007/s00109-019-01845-2
84. Jarosiewicz M, Krokosz A, Marczak A, Bukowska B. Changes in the activities of antioxidant enzymes and reduced glutathione level in human erythrocytes exposed to selected brominated flame retardants. Chemosphere. (2019) 227:93–9. doi: 10.1016/j.chemosphere.2019.04.008
85. Bjørklund G, Meguid NA, El-Bana MA, Tinkov AA, Saad K, Dadar M, et al. Oxidative stress in autism spectrum disorder. Mol Neurobiol. (2020) 57:2314–32. doi: 10.1007/s12035-019-01742-2
86. Snaedal J, Kristinsson J, Gunnarsdóttir S, Baldvinsson OM, Jóhannesson T. Copper, ceruloplasmin and superoxide dismutase in patients with Alzheimer's diseas. A case-control study. Dement Geriatr Cogn Disord. (1998) 9:239–42. doi: 10.1159/000017067
87. Bakeberg MC, Riley M, Byrnes M, Jefferson A, Ghosh S, Horne MK, et al. Elevated serum ceruloplasmin levels are associated with higher impulsivity in people with Parkinson's disease. Parkinsons Dis. (2020) 2020:8296203. doi: 10.1155/2020/8296203
88. Zanardi A, Alessio M. Ceruloplasmin deamidation in neurodegeneration: from loss to gain of function. Int J Mol Sci. (2021) 22:663. doi: 10.3390/ijms22020663
89. Ammar A, Trabelsi K, Boukhris O, Glenn JM, Bott N, Masmoudi L, et al. Effects of aerobic-, anaerobic- and combined-based exercises on plasma oxidative stress biomarkers in healthy untrained young adults. Int J Environ Res Public Health. (2020) 17:2601. doi: 10.3390/ijerph17072601
90. Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim BiophysActa. (2013) 1830:3289–303. doi: 10.1016/j.bbagen.2012.11.020
91. Vinceti M, Mandrioli J, Borella P, Michalke B, Tsatsakis A, Finkelstein Y. Selenium neurotoxicity in humans: bridging laboratory and epidemiologic studies. Toxicol Lett. (2014) 230:295–303. doi: 10.1016/j.toxlet.2013.11.016
92. Deng X, Wang K, Wu L, Yang C, Yang T, Zhao L, et al. Intraspinal hemangioblastomas: analysis of 92 cases in a single institution: clinical article. J Neurosurg Spine. (2014) 21:260–9. doi: 10.3171/2014.1.SPINE13866
93. Li J, Zhang C, Wu Z, Wang G, Zhao H, Li J. The Mechanism and clinical outcome of patients with Corona virus disease 2019 whose nucleic acid test has changed from negative to positive, and the therapeutic efficacy of favipiravir: a structured summary of a study protocol for a randomised controlled trial. Trials. (2019) 21:488. doi: 10.1186/s13063-020-04430-y
94. Vasquez-Muñoz M, Arce-Alvarez A, von Igel M, Veliz C, Ruiz-Esquide G, Ramirez-Campillo R, et al. Oscillatory pattern of glycemic control in patients with diabetes mellitus. Sci Rep. (2021) 11:5789. doi: 10.1038/s41598-021-84822-5
95. Accardi AJ, Matsubara BS, Gaw RL, Daleiden-Burns A, Heywood JT. Clinical utility of fluid volume assessment in heart failure patients using bioimpedance spectroscopy. Front Cardiovasc Med. (2021) 8:636718. doi: 10.3389/fcvm.2021.636718
96. Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. J Am Med Assoc. (2005) 13:218–28. doi: 10.1001/jama.294.2.218
97. Meguid N, Dardir AA, Abdel-Raouf ER, Hashish A. Evaluation of oxidative stress in autism: defective antioxidant enzymes and increased lipid peroxidation. Biol Trace Elem Res. (2011) 143:58–65. doi: 10.1007/s12011-010-8840-9
Keywords: autism spectrum disorder, urinary total antioxidant capacity, HEL, 8-OHdG, plasma ceruloplasmin, transferrin and superoxide dismutase, aberrant behavior checklist
Citation: Imataka G, Yui K, Shiko Y, Kawasaki Y, Sasaki H, Shiroki R and Yoshihara S (2021) Urinary and Plasma Antioxidants in Behavioral Symptoms of Individuals With Autism Spectrum Disorder. Front. Psychiatry 12:684445. doi: 10.3389/fpsyt.2021.684445
Received: 24 March 2021; Accepted: 21 June 2021;
Published: 03 September 2021.
Edited by:
Francesca Bonomini, University of Brescia, ItalyReviewed by:
Grace Eugenia Sameve, UNICEF Indonesia, IndonesiaKonstantin Boris Yenkoyan, Yerevan State Medical University, Armenia
Elif Ari, Bahçeşehir University, Turkey
Copyright © 2021 Imataka, Yui, Shiko, Kawasaki, Sasaki, Shiroki and Yoshihara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kunio Yui, eXVpMTZAYmVsbC5vY24ubmUuanA=
 George Imataka
George Imataka Kunio Yui
Kunio Yui Yuki Shiko
Yuki Shiko Yohei Kawasaki3
Yohei Kawasaki3 Ryoichi Shiroki
Ryoichi Shiroki Shigemi Yoshihara
Shigemi Yoshihara