- 1Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, United States
- 2The School of Psychological Sciences, University of Melbourne, Melbourne, VIC, Australia
- 3La Trobe University, Melbourne, VIC, Australia
- 4Saint Joseph University, Faculty of Medicine, Beirut, Lebanon
- 5Autism Speaks, New York, NY, United States
- 6Department of Psychology, John Carroll University, University Heights, OH, United States
- 7Genomic Medicine Institute, Cleveland, OH, United States
- 8Neurological Institute, Cleveland Clinic, Cleveland, OH, United States
- 9Departments of Human Genetics, Pediatrics and Psychiatry, University of California, Los Angeles, Los Angeles, CA, United States
- 10Translational Neuroscience Center, Department of Neurology, Boston Children's Hospital, Harvard Medical School, Boston, MA, United States
- 11Department of Genetics and Genome Sciences, Case Western University School of Medicine, Cleveland, OH, United States
- 12Department of Neurology, Rosamund Stone Zander Translational Neuroscience Center, Boston Children's Hospital, Harvard Medical School, Boston, MA, United States
Germline heterozygous PTEN mutations have been associated with high prevalence of autism spectrum disorder (ASD) and elevated rates and severity of broadly defined behavioral problems. However, limited progress has been made toward understanding whether PTEN mutation is associated with specific psychiatric co-morbidity profiles when compared to idiopathic ASD. The current study aimed to utilize a cross-measure approach to compare concurrent psychiatric characteristics across children and adolescents with PTEN mutation with (PTEN-ASD; n = 38) and without ASD (PTEN-No ASD; n = 23), and ASD with macrocephaly but no PTEN mutation (macro-ASD; n = 25) using the Child Behavior Checklist (CBCL) and the Aberrant Behavior Checklist (ABC). There were significant group effects for the CBCL Internalizing and Externalizing broad symptom score, the majority of specific CBCL syndrome scores, and all ABC subscale scores. Post-hoc comparisons revealed greater behavioral symptoms in the ASD groups (PTEN-ASD and macro-ASD) compared to the PTEN-no ASD group on nearly all subtest scores examined. There were no statistically significant differences between the PTEN-ASD and macro-ASD groups; however, there was a trend for the macro-ASD group showing higher levels of aggressive behaviors. Our findings provide evidence of specific behavior profiles across PTEN-No ASD, PTEN-ASD, and macro-ASD groups and highlight the importance of early identification of behavioral vulnerabilities in individuals with PTEN mutations in order to provide access to appropriate evidence-based interventions.
Introduction
Germline mutations in the gene encoding phosphatase and tensin homolog tumor suppressor (PTEN) result in a range of physical, behavioral and cognitive features including macrocephaly, executive functioning deficits, elevated rates of intellectual disability and high prevalence of autism spectrum disorder (ASD) (1–4). Indeed, pathogenic PTEN mutations are identified in ~2% of all ASD cases and up to 20% of cases with ASD and macrocephaly (2, 5–7) with 17% weighted average reported across more than 10 studies (8). Furthermore, regardless of age, at least 23% of individuals with PTEN mutations meet DSM-5 diagnostic criteria for ASD (3, 9, 10), and research has started to explore whether individuals with PTEN mutations differ in terms of presentation and severity of core ASD symptoms from individuals with idiopathic ASD (2, 11). In addition, recent studies have suggested a high rate of behavioral difficulties in individuals with PTEN mutations across the lifespan (12). However, it remains unclear whether the profile of behavioral difficulties and neuropsychiatric symptoms varies between those with and without a comorbid ASD diagnosis and how it compares to that of individuals with idiopathic ASD.
Prior research has reported a variety of neuropsychiatric phenotypes in individuals with PTEN mutations (3, 13, 14). For instance, clinical presentations in a study of nine patients with PHTS ranged from asymptomatic macrocephaly to clinical diagnosis of anxiety, bipolar disorder, obsessive-compulsive disorder (OCD), psychosis and adult-onset movement disorder (13). Another retrospective chart review of 47 patients aged between 1 and 26 years of age showed that apart from ASD diagnosis, which was found in 50% of individuals, 34% of the cohort had at least one behavioral/psychological diagnosis with ADHD (24%) and anxiety (15%) being the most common (3).
In a recent study by our group, behavioral problems were examined across three patient groups: individuals with PTEN mutations with and without ASD diagnosis (PTEN-ASD and PTEN-no ASD) and individuals with idiopathic ASD with macrocephaly (macro-ASD) (2). No differences were found between the two ASD groups on the severity of broad internalizing and externalizing symptom spectra; however, the PTEN-no ASD group had significantly lower scores than both ASD groups. However, no comparison of the severity of more specific symptom domains within internalizing and externalizing spectra was conducted and the potential impact of cognitive level on detected group differences was not explored.
In summary, although evidence of a relationship between germline heterozygous PTEN mutations and ASD is well-established and research has shown elevated rates and severity of broadly defined behavioral problems, a significant knowledge gap exists in terms of the impact on the expression and fine-grained profile of psychiatric and behavioral symptoms and disruptive behaviors. Therefore, in the current study, we utilized a cross-measurement approach to increase our understanding of whether PTEN mutation with or without ASD is associated with specific comorbidity profiles when compared to idiopathic ASD, and whether individual variations in IQ affect these profiles.
Methods
Participants
Participants were recruited at four sites: Cleveland Clinic, Stanford University, University of California at Los Angeles, and Boston Children's Hospital. Individuals 3–21 years old with a clinical diagnosis of ASD with history of macrocephaly and individuals with or without a verified PTEN mutation without deleterious copy number variation beyond the PTEN region were invited to participate. ASD diagnoses were made based on the Autism Diagnostic Interview–Revised [ADI-R; (15)], Autism Diagnostic Observation Schedule, Second Edition [ADOS-2; (16)] and DSM-5 criteria. Individuals with ASD and macrocephaly, defined by a head circumference at or above the 98th percentile were included in the macro-ASD group. PTEN-No ASD participants were required to have scores below the broad spectrum cut-off on the ADOS-2, T scores below 60 on the Social Responsiveness Scale [SRS-2; (17)], and deemed not to have ASD by clinical evaluation. Participants with ASD and intellectual disability (IQ <70 with adaptive function deficits) were included in the study, but those with intellectual disability without ASD were excluded to reduce sample heterogeneity. Individuals with a clinically significant medical disease that would prohibit participation in the study procedures were excluded from the study. See Table 1 for the descriptive statistics of the sample.
Eighty-six participants met all inclusion/exclusion criteria and were included in this study. Participants were divided into the following three subgroups based on clinical diagnosis and the results of PTEN genotyping: ASD + PTEN mutation (PTEN-ASD; n = 38; Mage = 8.93 years, SDage = 4.75), ASD with macrocephaly, no PTEN mutation (macro-ASD; n = 25; Mage = 11.99 years; SDage = 5.15), and PTEN mutation without ASD (PTEN-no ASD; n = 23; Mage = 8.94 years; SDage = 4.85).
Measures
Participants' cognitive level was assessed using the Stanford Binet, Fifth Edition or the Mullen Scales of Early Learning (18, 19). Participants with a history of an ASD diagnosis received confirmatory diagnostic assessment with the Autism Diagnostic Interview- Revised (ADI-R) (15) and/or the Autism Diagnostic Observation Schedule- Second Edition (ADOS-2) (16). The ADI-R and ADOS-2 were administered by trained research staff and supervised by a research-reliable clinician.
The Child Behavior Checklist [CBCL; (20)] is a norm-referenced, parent-report questionnaire measure designed to assess behavioral, emotional, and social problems in children aged 1.5–5 years and 6–18 years (20). Each item is rated on a three-point Likert scale ranging from 0 (Not True) to 2 (Very True or Often True). In addition to total and Internalizing and Externalizing scores, both versions of the CBCL provide the following overlapping syndrome scores: Anxious/Depressed, Withdrawn, Somatic Problems, which constitute Internalizing Problems scores, and Attention Problems and Aggressive Problems, which constitute Externalizing Problems scores. In the present report, the T scores and combined specific overlapping subscales across the two different versions of the CBCL were used.
The Aberrant Behavior Checklist [ABC; (21)] is a 58-item rating scale used to assess a range of maladaptive behaviors including Irritability, Hyperactivity, Lethargy/Withdrawal, Stereotypy and Inappropriate Speech. Each item is rated on a four-point Likert scale ranging from 0 (not at all problem) to 3 (the problem is severe in degree). In this study, we did not utilize the Stereotypy scale given that the focus was on the profile of behavioral problems/co-occurring symptoms rather than on core ASD symptoms.
Results
Preliminary Analyses
There were no age, sex, race nor ethnicity differences between the three groups (Table 1). However, the PTEN-ASD and macro-ASD groups had significantly lower FSIQ (F = 14.11, p <0.001, ηp2 = 0.29) scores than the PTEN-no ASD group.
Main Analyses
CBCL: Table 2 shows the percentage of individuals across the three clinical groups scoring in the normative, at-risk and clinical range across the CBCL syndrome scales. Twenty-eight and 24 percent of macro-ASD participants met the cut-off criteria for clinically significant Internalizing and Externalizing problems, respectively. In the PTEN-ASD group, 47.8% of participants scored in the clinical and 15.8% in at-risk range for Internalizing problems and 34.2% in the clinical range for Externalizing symptoms. In the PTEN-no ASD group, 30.4 and 21.7% of participants scored in the clinical range for Internalizing and Externalizing symptoms, respectively.
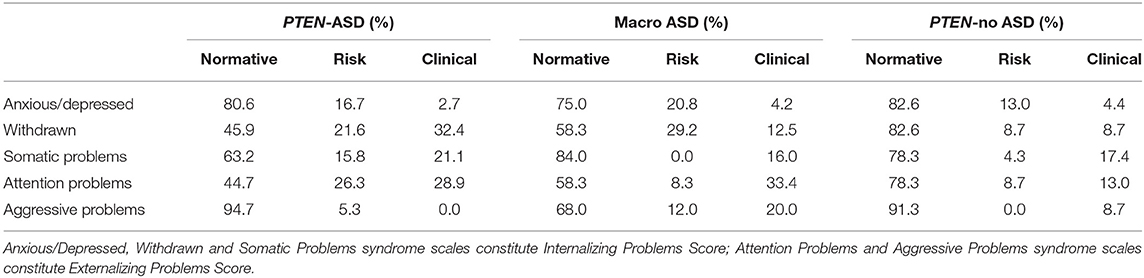
Table 2. Frequency of psychopathologies across PTEN-ASD, Macro ASD and PTEN-no ASD groups based on the CBCL cut-off scores.
There was a significant group effect for both Internalizing (F = 4.57, p = 0.013, ηp2 = 0.10) and Externalizing (F = 8.80, p <0.001, ηp2 = 0.18) problems scores with post-hoc comparisons showing that the PTEN-no ASD group had significantly lower Internalizing problems when compared to the PTEN-ASD group (p = 0.011, d = 0.06) and significantly lower Externalizing problems than both the PTEN-ASD (p = 0.003, d = 0.07) and macro-ASD groups (p <0.001, d = 0.08). When examining group effects on the syndrome scales, there were significant effects for Withdrawn (F = 6.46, p = 0.003, ηp2 = 0.14), Attention Problems (F = 4.09, p = 0.020, ηp2 = 0.09), and Aggressive Problems (F = 4.27, p = 0.017, ηp2 = 0.09), but not for Anxious/Depressive or Somatic Complaints. Post-hoc analysis showed that while both macro-ASD and PTEN-ASD groups had higher Withdrawn scores than PTEN-no ASD, differences reached significance only for the macro-ASD vs. PTEN-no ASD contrasts for Attention and Aggressive Problems syndrome scales. See Table 3 for descriptives.
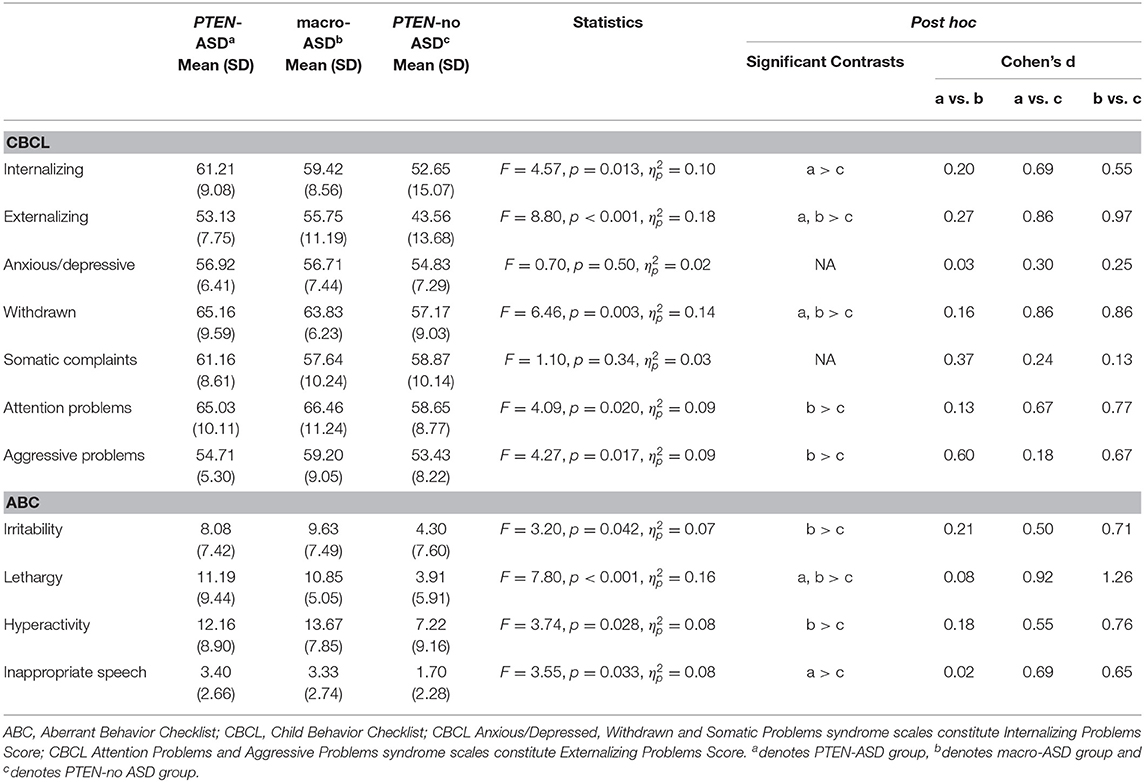
Table 3. Comparison of CBCL and ABC score severity across PTEN-ASD, Macro ASD and PTEN-no ASD groups.
ABC: There were significant effects for all ABC subscale scores (Irritability [F = 3.20, p = 0.042, ηp2 = 0.07], Lethargy [F = 7.80, p <0.001, ηp2 = 0.16], Hyperactivity [F = 3.74, p = 0.028, ηp2 = 0.08] and Inappropriate Speech [F = 3.55, p = 0.033, ηp2 = 0.08]) with post-hoc analysis showing that the PTEN-no ASD group had significantly lower scores than (i) both ASD groups on Lethargy, (ii) macro-ASD on Irritability and Hyperactivity, and (iii) PTEN-ASD on Inappropriate Speech.
Adjustment for FSIQ: Given that the PTEN-ASD and macro-ASD groups had significantly lower FSIQ (F = 14.11, p <0.001, ηp2 = 0.29) scores than the PTEN-no ASD group, a series of univariate models were conducted to explore the contribution of FSIQ to the noted differences in CBCL and ABC scores. After adjusting for FSIQ score, there were still significant, albeit decreased in terms of effect size group differences for CBCL Internalizing (F = 3.28, p = 0.043, ηp2 = 0.08; Covariate; FSIQ: p = 0.68, ηp2 = 0.002) and Externalizing (F = 5.05, p = 0.009, ηp2 = 0.13; Covariate; FSIQ: p = 0.076, ηp2 = 0.05) problems, CBCL Aggressive Problems (F = 4.94, p = 0.01, ηp2 = 0.12; Covariate; FSIQ: p = 0.20, ηp2 = 0.02), and ABC Lethargy (F = 5.19, p = 0.008, ηp2 = 0.13; Covariate; FSIQ: p = 0.11, ηp2 = 0.03). Group differences were no longer significant for CBCL Withdrawn (F = 1.96, p = 0.15, ηp2 = 0.05; Covariate; FSIQ: p = 0.20, ηp2 = 0.02), CBCL Attention Problems (F = 1.15, p = 0.32, ηp2 = 0.03; Covariate; FSIQ: p = 0.37, ηp2 = 0.01), ABC Irritability (F = 1.94, p = 0.15, ηp2 = 0.05; Covariate; FSIQ: p = 0.007, ηp2 = 0.10), ABC Hyperactivity (F = 1.69, p = 0.19, ηp2 = 0.05; Covariate; FSIQ: p = 0.01, ηp2 = 0.09), or ABC Inappropriate Speech (F = 1.55, p = 0.22, ηp2 = 0.04; Covariate; FSIQ: p = 0.17, ηp2 = 0.03).
Discussion
The current study aimed to describe specific problem behavior profiles in individuals with germline pathogenic mutations in the PTEN gene. Individuals with PTEN-ASD were compared with individuals with PTEN-no ASD and those with macro-ASD on broad Internalizing and Externalizing spectra, as well more specific symptom clusters. In addition, we aimed to investigate the contribution of cognitive functioning on the problem behavior profiles across these three clinical groups.
We first explored differences between individuals with ASD but with different genetic status. No significant differences were found between PTEN-ASD and macro-ASD groups in Internalizing or Externalizing Problems, nor on any of the syndrome subscales of the CBCL nor the ABC subscales. These findings are consistent with previous investigations (2) and extend prior findings to a greater level of specificity. While behavior comparisons of PTEN-ASD and macro-ASD subgroups are quite limited within the literature, prior analyses have suggested that the PTEN-ASD subgroup displays less emotion dysregulation than other ASD groups (22). Furthermore, clinical observations have described these individuals as being generally happy and more flexible with changes in environment and routine than those with idiopathic ASD (2, 11), in line with our finding that there is approximately a half-standard deviation difference between the groups. Our findings that for internalizing Problems, PTEN-ASD group (but not MACRO-ASD group) had significantly higher scores than PTEN-no ASD group, and that for Attention Problems and Aggressive Problems, Macro-ASD group (but not PTEN-ASD group) had significantly higher scores than PTEN-no ASD group, provide some support to the noted subjective clinical observations. However, this study may not have been sufficiently powered to detect some of the key differences, and future longitudinal time points may be needed to identify smaller profile differences.
The influence of ASD diagnosis on problem behavior expression was also examined. Individuals in the PTEN-no ASD group had significantly lower scores than both ASD groups for CBCL Internalizing, Externalizing, Withdrawn, Attention, and Aggressive Problems scores and for ABC Irritability, Lethargy, Hyperactivity and Inappropriate Speech scores. Differences on CBCL domains such as Anxious/Depressed and Somatic Problems scales were not significant. Although the majority of scores were in the normative range across all CBCL scales, it is important to note that apart for the CBCL Anxious/Depressed scale (where only 4.4% were in the clinical range), at least 8.7% of individuals with PTEN-no ASD had clinically significant behavior problems, particularly in terms of Somatic (17.4%) and Attention Problem (13%) syndrome scales and both Internalizing (30.4%) and Externalizing (21.7%) problems, a significantly higher rate of noted problems in the general population (23). These observations indicate that some, perhaps all, individuals with PTEN mutations, regardless of their ASD status, will also require clinical assessment and, if positive, treatment. Finally, differences between groups remained significant, albeit with reduced effect sizes, after adjusting for FSIQ scores.
This investigation has several limitations. The sample size of the different groups is small, which is related to the low prevalence rates of PTEN mutations. Larger studies are warranted to confirm the current observations. Additionally, findings reported here are based on parent-report measures, and future investigations should use a multi-method assessment approach and include clinician-administered standardized assessments. Further, despite clinical utility and robust psychometric properties of the ABC and the CBCL in ASD, it is important to highlight the fact that the presence of ASD is associated with atypical expression of psychiatric and behavioral symptoms, in particular anxiety and depression (24–27). Therefore, it will be important for future studies to include instruments such as the Anxiety Disorders Interview Schedule—Autism Addendum (28), the Parent-Rated Anxiety Scale for Youth With Autism Spectrum Disorder (29) or the Anxiety Scale for Children with Autism Spectrum Disorder (30) to ensure that these potentially important and clinically impactful symptoms are not missed or under-sampled. It will also be important to investigate continuities and discontinuities in the presentation and structure of neurodevelopmental and neuropsychiatric symptoms in neurogenetic syndromes when compared to other clinical groups.
Despite noted limitations, reported findings have important clinical implications. More specifically, elevated levels of behavioral problems in individuals with PTEN mutations highlight the importance of early identification of behavioral vulnerabilities to facilitate access to appropriate evidence-based interventions. Importantly, our findings also highlight areas for future investigations, including the mechanisms underlying behavioral problems. In particular, it will be crucial to utilize a multi-level approach to fully characterize the associations between PTEN pathway proteins with individual differences in cognitive, behavioral, and clinical profiles (31). In addition, prospective research should attempt to further understand the impact of both cognitive functioning and ASD traits and explore the role of factors such as emotion regulation, cognitive control and intolerance of uncertainty that have been shown as key risk factors across a range of psychopathologies, in both general clinical (32–34), ASD (35–41) and most recently PTEN (42) cohorts. This critical step will be essential in order to inform the development and personalized delivery of effective treatments.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study received IRB approvals from relevant sites that participated: Cleveland Clinic, Stanford University, University of California at Los Angeles, and Boston Children's Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
CE, MSa, TF, and AH designed the study. CE, MSa, AH, RB, PK, SS, and JM-A collected the data. MU, GR, and MSt had full access to the data and conducted the analyses. MSt and MU drafted the initial manuscript. All authors critically reviewed and provided the feedback on the initial version of manuscript and approved the final version of the manuscript.
Consortium Affiliations
• Simon K. Warfield, PhD, Department of Radiology, Boston Children's Hospital, Boston, MA, United States.
• Benoit Scherrer, PhD, Department of Radiology, Boston Children's Hospital, Boston, MA, United States.
• Kira Dies, ScM, CGC, Department of Neurology, Boston Children's Hospital, Boston, MA, United States.
• Rajna Filip-Dhima, MS, Department of Neurology, Boston Children's Hospital, Boston, MA, United States.
• Amanda Gulsrud, PhD, UCLA Semel Institute for Neuroscience & Human Behavior, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States.
• Ellen Hanson, PhD, Department of Developmental Medicine, Boston Children's Hospital, Boston, MA, United States.
Funding
This study was funded, in part, by the National Institute of Health Developmental Synaptopathies Consortium (U54NS092090; PI/Network Director: Sahin; Project 2 Leaders/PIs: Eng & Busch) and the Ambrose Monell Foundation (to CE). The Developmental Synaptopathies Consortium (U54NS092090) was part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS). The content was solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). CE was the Sondra J. and Stephen R. Hardis Endowed Chair of Cancer Genomic Medicine at the Cleveland Clinic and an ACS Clinical Research Professor. MSa was the Rosamund Stone Zander Chair at Boston Children's Hospital. MU was currently supported by the Australian Research Council Discovery Early Career Researcher Award (DE180100632).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We were sincerely indebted to the generosity of the families and patients in PTEN clinics across the United States who contributed their time and effort to this study. We would also like to thank the PTEN Hamartoma Syndrome Foundation and the PTEN Research Foundation for their continued support in PTEN research.
References
1. Busch RM, Chapin JS, Mester J, Ferguson L, Haut JS, Frazier TW, et al. Cognitive characteristics of PTEN hamartoma tumor syndromes. Genet Med. (2013) 15:548. doi: 10.1038/gim.2013.1
2. Busch RM, Srivastava S, Hogue O, Frazier TW, Klaas P, Hardan A, et al. Neurobehavioral phenotype of autism spectrum disorder associated with germline heterozygous mutations in PTEN. Transl Psychiatry. (2019) 9:1–9. doi: 10.1038/s41398-019-0588-1
3. Hansen-Kiss E, Beinkampen S, Adler B, Frazier T, Prior T, Erdman S, et al. A retrospective chart review of the features of PTEN hamartoma tumour syndrome in children. J Med Genet. (2017) 54:471–8. doi: 10.1136/jmedgenet-2016-104484
4. Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. (2012) 18:400–7. doi: 10.1158/1078-0432.CCR-11-2283
5. Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. (2005) 42:318–21. doi: 10.1136/jmg.2004.024646
6. Buxbaum JD, Cai G, Chaste P, Nygren G, Goldsmith J, Reichert J, et al. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am J Med Genet B: Neuropsychiatr Genet. (2007) 144:484–91. doi: 10.1002/ajmg.b.30493
7. Libero LE, Nordahl CW, Li DD, Amaral DG. Macrocephaly and megalencephaly in autism spectrum disorder. In: Autism Imaging and Devices. Boca Raton: CRC Press (2017). p. 187–204.
8. Tilot AK, Frazier TW, Eng C. Balancing proliferation and connectivity in PTEN-associated autism spectrum disorder. Neurotherapeutics. (2015) 12:609–19. doi: 10.1007/s13311-015-0356-8
9. Ciaccio C, Saletti V, D'Arrigo S, Esposito S, Alfei E, Moroni I, et al. Clinical spectrum of PTEN mutation in pediatric patients. A bicenter experience. Eur J Med Genet. (2019) 62:103596. doi: 10.1016/j.ejmg.2018.12.001
10. Tan MH, Mester J, Peterson C, Yang Y, Chen JL, Rybicki LA, et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am J Human Genet. (2011) 88:42–56. doi: 10.1016/j.ajhg.2010.11.013
11. Frazier TW, Embacher R, Tilot AK, Koenig K, Mester J, Eng C. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol Psychiatry. (2015) 20:1132. doi: 10.1038/mp.2014.125
12. Yehia L, Keel E, Eng C. The clinical spectrum of PTEN mutations. Annu Rev Med. (2020) 71:103–16. doi: 10.1146/annurev-med-052218-125823
13. Balci TB, Davila J, Lewis D, Boafo A, Sell E, Richer J, et al. Broad spectrum of neuropsychiatric phenotypes associated with white matter disease in PTEN hamartoma tumor syndrome. Am J Med Genet B: Neuropsychiatr Genet. (2018) 177:101–9. doi: 10.1002/ajmg.b.32610
14. Macken WL, Tischkowitz M, Lachlan KL. PTEN Hamartoma tumor syndrome in childhood: a review of the clinical literature. Am J Med Genet C Semin Med Genet. (2019) 181:591–610. doi: 10.1002/ajmg.c.31743
15. Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services (2003).
16. Lord C, DiLavore PC, Gotham K. Autism Diagnostic Observation Schedule. Torrance, CA: Western Psychological Services (2012).
17. Constantino JN, Gruber CP. Social Responsiveness Scale: SRS-2. Torrance, CA: Western Psychological Services (2012).
18. Roid GH, Pomplun M. The Stanford-Binet Intelligence Scales. New York, NY: The Guilford Press (2012).
20. Achenbach TM, Rescorla LA. Manual for ASEBA School Age Forms and Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth and Families (2001).
21. Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. (1985) 89:485–91. doi: 10.1037/t10453-000
22. Frazier TW. Autism Spectrum Disorder Associated with Germline Heterozygous PTEN Mutations. Cold Spring Harbor Perspect Med. (2019) 9:a037002. doi: 10.1101/cshperspect.a037002
23. Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. (2000) 21:265–71. doi: 10.1542/pir.21-8-265
24. Halim AT, Richdale AL, Uljarevi, ć M. Exploring the nature of anxiety in young adults on the autism spectrum: a qualitative study. Res Autism Spectrum Disord. (2018) 55:25–37. doi: 10.1016/j.rasd.2018.07.006
25. Kerns CM, Rump K, Worley J, Kratz H, McVey A, Herrington J, et al. The differential diagnosis of anxiety disorders in cognitively-able youth with autism. Cogn Behav Pract. (2016) 23:530–47. doi: 10.1016/j.cbpra.2015.11.004
26. Uljarević M, Nuske H, Vivanti G. Anxiety in Autism Spectrum Disorder. In: Mazzone L. Vitiello B, editors. Psychiatry Comorbidity in Autism Spectrum Disorders. Switzerland: Springer (2016). doi: 10.1007/978-3-319-29695-1_2
27. Uljarević M, Hedley D, Foley K-R, Magiati I, Cai RY, Dissanayake C, et al. Anxiety and depression from adolescence to old age in Autism Spectrum Disorder. J Autism Dev Disord. (2019) 50:3155–65. doi: 10.1007/s10803-019-04084-z
28. Kerns CM, Renno P, Kendall PC, Wood JJ, Storch EA. Anxiety disorders interview schedule–autism addendum: Reliability and validity in children with autism spectrum disorder. J Clin Child Adolesc Psychol. (2017) 46:88–100. doi: 10.1080/15374416.2016.1233501
29. Scahill L, Lecavalier L, Schultz RT, Evans AN, Maddox B, Pritchett J, et al. Development of the parent-rated anxiety scale for youth with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. (2019) 58:887–96. doi: 10.1016/j.jaac.2018.10.016
30. Rodgers J, Wigham S, McConachie H, Freeston M, Honey E, Parr JR. Development of the anxiety scale for children with autism spectrum disorder (ASC-ASD). Autism Res. (2016) 9:1205–15. doi: 10.1002/aur.1603
31. Frazier TW, Jaini R, Busch RM, Wolf M, Sadler T, Klaas P, et al. Cross-level analysis of molecular and neurobehavioral function in a prospective series of patients with germline heterozygous PTEN mutations with and without autism. Mol Autism. (2021) 12:5. doi: 10.1186/s13229-020-00406-6
32. Bottesi G, Noventa S, Freeston MH, Ghisi M. Seeking certainty about Intolerance of Uncertainty: addressing old and new issues through the Intolerance of Uncertainty Scale-Revised. PLoS ONE. (2019) 14:e0211929. doi: 10.1371/journal.pone.0211929
33. McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. (2017) 174:676–85. doi: 10.1176/appi.ajp.2017.16040400
34. Sheppes G, Suri G, Gross JJ. Emotion regulation and psychopathology. Annu Rev Clin Psychol. (2015) 11:379–405. doi: 10.1146/annurev-clinpsy-032814-112739
35. Cai RY, Richdale AL, Dissanayake C, Uljarevi, ć M. Brief report: Inter-relationship between emotion regulation, intolerance of uncertainty, anxiety, and depression in youth with autism spectrum disorder. J Autism Dev Disord. (2018) 48:316–25. doi: 10.1007/s10803-017-3318-7
36. Cai RY, Richdale AL, Uljarevi, ć M, Dissanayake C, Samson AC. Emotion regulation in autism spectrum disorder: where we are and where we need to go. Autism Res. (2018) 11:962–78. doi: 10.1002/aur.1968
37. Samson AC, Phillips JM, Parker KJ, Shah S, Gross JJ, Hardan AY. Emotion dysregulation and the core features of autism spectrum disorder. J Autism Dev Disord. (2014) 44:1766–72. doi: 10.1007/s10803-013-2022-5
38. South M, Rodgers J. Sensory, emotional and cognitive contributions to anxiety in autism spectrum disorders. Front Hum Neurosci. (2017) 11:20. doi: 10.3389/fnhum.2017.00020
39. Cai R-Y, Richdale AL, Dissanayake C, Trollor J, Uljarević M. Emotion regulation in autism: Reappraisal and suppression interactions. Autism. (2019) 23:737–49. doi: 10.1177/1362361318774558
40. Cai RY, Richdale AL, Dissanayake C, Uljarević M. Resting heart rate variability, emotion regulation, psychological wellbeing and autism symptomatology in adults with and without autism. Int J Psychophysiol. (2019) 137:54–62. doi: 10.1016/j.ijpsycho.2018.12.010
41. Cai RY, Richdale AL, Dissanayake C, Uljarević M. How does emotion regulation strategy use and psychological wellbeing predict mood in adults with and without autism spectrum disorder? A naturalistic assessment. J Autism Dev Disord. (2019) 50:1786–99. doi: 10.1007/s10803-019-03934-0
Keywords: PTEN, macrocephaly, autism, behavioral problems, internalizing and externalizing
Citation: Steele M, Uljarević M, Rached G, Frazier TW, Phillips JM, Libove RA, Busch RM, Klaas P, Martinez-Agosto JA, Srivastava S, Eng C, Sahin M and Hardan AY (2021) Psychiatric Characteristics Across Individuals With PTEN Mutations. Front. Psychiatry 12:672070. doi: 10.3389/fpsyt.2021.672070
Received: 25 February 2021; Accepted: 27 April 2021;
Published: 17 August 2021.
Edited by:
Milica Pejovic-Milovancevic, Institute of Mental Health (Belgrade), SerbiaReviewed by:
Bryan Weston Luikart, Dartmouth College, United StatesMarija Raleva, Saints Cyril and Methodius University of Skopje, North Macedonia
Copyright © 2021 Steele, Uljarević, Rached, Frazier, Phillips, Libove, Busch, Klaas, Martinez-Agosto, Srivastava, Eng, Sahin and Hardan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Y. Hardan, aGFyZGFuYXlAc3RhbmZvcmQuZWR1
 Morgan Steele1
Morgan Steele1 Mirko Uljarević
Mirko Uljarević Gaëlle Rached
Gaëlle Rached Thomas W. Frazier
Thomas W. Frazier Robyn M. Busch
Robyn M. Busch Patricia Klaas
Patricia Klaas Mustafa Sahin
Mustafa Sahin