
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 16 July 2021
Sec. Molecular Psychiatry
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.667699
 Mimi Qiu1
Mimi Qiu1 Chenchen Zhang2
Chenchen Zhang2 Yu Dai1
Yu Dai1 Lingrui Zhang3
Lingrui Zhang3 Yang Wang4
Yang Wang4 Wei Peng4
Wei Peng4 Yalin Chen1
Yalin Chen1 Chao Wen5
Chao Wen5 Hui Li6*
Hui Li6* Tianmin Zhu1*
Tianmin Zhu1*Objective: Uncontrolled internet use may lead to the emergence of pathological internet use (PIU). PIU has become a global public health concern that can cause a range of psychotic symptoms, including anxiety, depression, and impulse control disorder. To date, we know very little about the principal biological factors related to PIU. Monoamine oxidase type A (MAOA) and serotonin (5-HT) 5-HT2A receptor (5-HT2AR) play critical roles in the development of behavioural and drug addictions. Thus, the aim of this study was to measure the relative expression of mRNA of MAOA and 5-HT2AR in peripheral blood mononuclear cells (PBMCs) of patients with PIU and to determine the correlations between these biological indicators and the comorbid symptoms of patients with PIU.
Methods: In this study, the mRNA of MAOA and 5-HT2AR was detected using real-time PCR in PBMCs of the patients with PIU (n = 24) and healthy controls (HCs, n = 25). The relationship between the mRNA levels of MAOA and 5-HT2AR and clinical symptoms in patients with PIU was further investigated.
Results: MAOA mRNA in PBMCs was significantly upregulated in patients with PIU compared with that in HCs. mRNA levels of 5-HT2AR were not found to differ significantly between HCs and patients with PIU. Correlation analyses further revealed a significant positive correlation between the relative expression of MAOA mRNA in PBMCs of patients with PIU and the Young's Internet Addiction Test and Self-Rating Depression Scale scores.
Conclusion: The present study revealed upregulated expression of MAOA mRNA in patients with PIU and an association between the expression of MAOA mRNA and clinical symptoms of PIU, suggesting that the neurobiological changes may be similar between PIU and substance addiction. Additionally, this study demonstrated a potential association between comorbid symptoms and mRNA levels of MAOA.
Pathological internet use (PIU) is defined as a behavioural addiction, which involves increasing use of the internet for a long time to obtain satisfaction, surfing the internet longer than intended, irritability during deprivation from the internet, and excessive internet use leading to functional deficits (1). Because of high rates of prevalence and psychiatric comorbidity, PIU has become a serious public health concern. The features of PIU include loss of interest or enjoyment in otherwise pleasurable activities, accompanied by other comorbid psychiatric symptoms such as impulse control disorder, anxiety, depression, insomnia, and attention-deficit/hyperactivity disorder (ADHD) (2–5). Studies have shown that 23.3, 26.3, and 21.7% of patients with PIU suffer from anxiety disorder, depression, and ADHD, respectively (6). Many researchers have confirmed the aetiological mechanism of PIU, and considerable evidence is available regarding changes in the central and peripheral nervous systems in patients with PIU (7, 8). However, the exact physio-pathological mechanism of PIU remains unclear.
Diminished activity of the serotonin (5-hydroxytryptamine, 5-HT) system has been implicated in the emergence of addictive diseases (9, 10). One of the main receptors for serotonin is the 5-HT2A receptor (5-HT2AR), which regulates different pathophysiological aspects, including mood, sleep, learning, and memory, of diseases of the nervous system (11–13). Intriguingly, considerable evidence has shed light on the importance of 5-HT2AR in the vulnerability and establishment of drug-related behaviours, suggesting a role of 5-HT2AR in transition to and maintenance of addiction (14, 15). In a previous positron emission tomography (PET) study, patients with PIU were found to have reduced serotonin 5-HT2AR in the left and right temporal cortex that was related to the low level of dopamine D2 receptors in the striatum (16). Moreover, studies have identified that abnormal levels of neurotransmitters such as serotonin (5-HT), norepinephrine (NE), and dopamine (DA) in individuals are closely associated with comorbid psychiatric symptoms of PIU (17–19).
Monoamine oxidase A (MAOA) is a mitochondrial enzyme that catalyses the degradation process of various amine neurotransmitters, including 5-HT, NE, and DA (20). Abnormalities of the MAOA gene have been found to be closely related to various neurological and mental disorders, such as behavioural addiction (21–23). Empirical studies have shown that an MAOA gene promoter allele with low activity is associated with pathological gambling (24). Additionally, MAOA-knockout mice have been reported to exhibit impaired nicotine preference but normal response to new stimuli (25). Therefore, MAOA is an important candidate gene for investigating differences in addiction sensitivity among individuals.
According to various studies, 5-HT and MAOA systems play pathophysiological and pathological roles in addictive diseases. We hypothesised that mRNA levels of MAOA and 5-HT2AR might be altered in PBMCs of patients with PIU having high rates of self-reported psychiatric symptoms. Therefore, the present study was designed to investigate the mRNA levels of MAOA and 5-HT2AR in PBMCs of patients with PIU and healthy controls (HCs). Furthermore, we investigated the association between MAOA and 5-HT2AR mRNA levels and comorbid symptoms of patients with PIU.
The clinical trial was conducted in China from June 2016 to January 2018. In this study, all subjects were screened according to the Beard's Diagnostic Questionnaire for Internet Addiction (26). A total of 49 college students (24 patients with PIU and 25 HCs) were selected for the clinical trial. All the selected students were native Chinese speakers. Inclusion criteria for the PIU group were as follows: (1) meeting the Beard's criteria for internet addiction; (2) aged between 18 and 30 years; (3) not having undergone any form of therapeutic intervention; and (4) not having any other organic or mental illnesses. Overall, 25 HCs were recruited that matched the PIU group in terms of age, gender, and internet age based on the following inclusion criteria: (1) not meeting the Beard's criteria; (2) aged between 18 and 30 years; and (3) not having other organic or mental illnesses. Participants with a history of substance addiction and pregnant or lactating women were excluded from this study.
We collected the baseline data, including those for gender, age, internet age, and weekly online time, of the participants through a self-designed questionnaire. Internet age is the actual age of the subject minus the age at which surfing the internet was started. The weekly internet surfing time is an estimate of the weekly internet surfing time of the participant in the past year. In addition, the following scales were used to examine the subjects' comorbid symptoms such as internet addiction, depression, anxiety, sleep quality, and impulsivity:
Young's Internet Addiction Test (IAT) (27) was used to assess the severity of internet addiction. The scale consists of 20 items, which are rated on a 5-point scale (1 = very rarely; 5 = very frequently), ranging from 20 to 100. A high score indicates more severe internet addiction. IAT has been validated in Chinese adolescents, with the Cronbach's alpha of 0.93(28).
Self-Rating Depression Scale (SDS) (29) was used to measure the depression symptoms of patients with PIU. It consists of 20 items, which are rated on a 4-point scale from 1 (never) to 4 (always). The total score was calculated as the sum of the 20 items multiplied by 1.25 and then converted into an integer in the rounded form. The higher the score, the higher is the degree of depression. The Chinese version of the SDS was verified previously (Cronbach's α = 0.75) (30).
Self-Rating Anxiety Scale (SAS) (31) consists of 20 items, which are rated on a 4-point scale (1 = never, 4 =always). A standard score was obtained by multiplying the raw score (the sum of the 20 items) by 1.25. The Chinese version of the SAS was widely used in Chinese samples, with good psychometric characteristics (32).
Pittsburgh Sleep Quality Index (PSQI) (33) was utilised to evaluate the subjective sleep quality over a one-month period. This index consists of 19 items and is used to assess seven components of sleep, namely subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disorders, sleep medication use, and daytime dysfunction. Each item is rated from 0 (no difficulty) to 3 (severe difficulty). The total score is obtained by adding the scores of seven components, ranging from 0 to 21. The higher the total score, the worse is the sleep. In addition, the reliability of the Chinese version of PSQI was verified, with Cronbach's alpha α = 0.81 (34).
Barratt Impulse Scale (BIS-11) (35) was used to assess the impulsivity of participants. The scale contains 30 items, which are rated on a 4-point scale (1 = never; 4 = always), evaluate three dimensions, namely attentional impulsivity, motor impulsivity, and non-planning impulsivity. The higher the score, the higher is the impulsivity. In a previous study, the reliability of the Chinese version of BIS-11 was verified (Cronbach's alpha α = 0.83) (36).
A total of 5 mL of whole peripheral blood samples were collected into tubes containing ethylene diamine tetra-acetic acid as an anticoagulant. Mononuclear cells were isolated using a gradient centrifuge (Thermo, Waltham, MA, USA). Total RNA was extracted from PBMCs by using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was dissolved in 20 μl RNase-free water provided in the kit. The RNA integrity was detected using the Agilent 2200 Bioanalyzer (Agilent, California, USA). Then, 1 μg of total RNA was reverse-transcribed into 20 μl first-strand cDNA by using the Fermentas cDNA synthesis kit (RevertAid™, Fermentas, USA) according to the manufacturer's instruction.
Nucleotide primers for real-time PCR amplification of beta-actin, MAOA, and 5-HT2AR were designed using primer blast software on the National Center for Biotechnology Information website. Primers used for real-time PCR are as follows:
β-actin: forward, 5′-GAAGATCAAGATCATTGCTCCT-3′ and reverse, 5′-TTGCTGATCCACA-3′ (amplicon size, 111-bp).
5-HT2AR: forward, 5′-GTAGGTATATCCATGCCAAT-3′ and reverse, 5′-AGGTGATCACCATGATGGTT-3′ (amplicon size, 177-bp).
MAOA: forward, 5′-CTGCCATCATGGGCTT-3′ and reverse, 5′-TTGCTGATCCACA-3′ (amplicon size, 154-bp).
For real-time PCR, the reaction volume was 25 μl/tube, and the following reagents were used: 2x TaqMan Real-time PCR Mix (12.5 μl); upstream primer (10 μm; 0.6 μl); downstream primer (10 μm; 0.6 μl); ddH2O (7.7 μl); and cDNA template (3 μl). The reaction was performed on an FTC-3000QPCR system (Funglyn Biotech, Toronto, Canada). Reaction conditions were as follows: pre-denaturing at 95°C for 10 min, denaturation at 95°C for 10 s, annealing at 53°C for 30 s, and 45 cycles of extension for 30 s at 60°C. The beta-actin gene was used as the housekeeping gene for normalising the target gene expression. Relative mRNA levels were calculated using the 2−ΔΔCt method (37).
All data were analysed using IBM SPSS Statistics 23.0 software (Chicago, USA) and GraphPad Prism 8 Software (California, USA). Shapiro–Wilk test was used to define the normal distribution of variables. Independent-sample t-test was performed for continuous variables with normal distribution, and Mann–Whitney U-test was performed for continuous variables with non-normal distribution (non-parametric data). Chi-square test was used for categorical variables. The relationships between 5-HT2AR and MAOA mRNAs and clinical scores were calculated using Spearman's correlation coefficient. Demographic and clinical characteristics that met normal distribution are presented as mean ± standard deviation (SD), whereas, those lacking normal distribution are expressed as median (inter-quartile range; IQR) or number (%) per group. A P-value of <0.05 was considered statically significant.
Table 1 shows the comparison of demographics and clinical scores between patients with PIU and HCs. No significant difference in age, gender, or internet age was observed between the two groups (P > 0.05). However, the weekly online time was higher in PIU group than in HC group (P < 0.001). In addition, consistent with the inclusion results, patients with PIU had higher IAT, SDS, SAS, PSQI, and BIS-11 scores (P < 0.001).
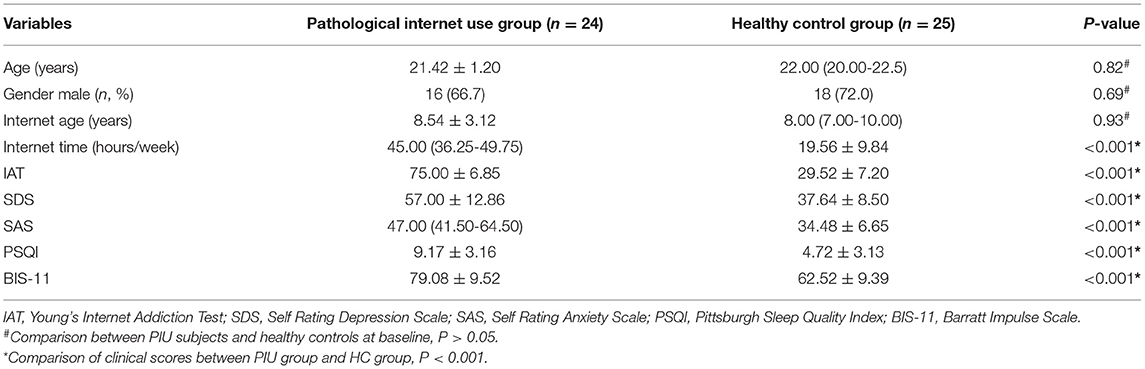
Table 1. Demographics and clinical characteristics of pathological internet use and healthy control groups.
Figure 1A shows that the mRNA level of MAOA was significantly higher in PBMCs of patients with PIU than in those of HCs (P < 0.05). No difference in beta-actin mRNA levels was observed between the two groups.
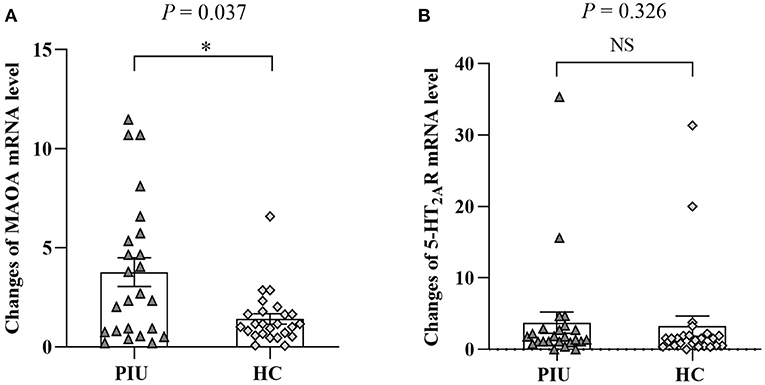
Figure 1. mRNA levels of MAOA (A) and 5-HT2AR (B) in PBMCs of PIU and HC groups. Bars represent means, error bars represent SEM, triangles, and diamonds represent individual data points (n = 24 in PIU and 25 in HC groups). *P < 0.05. NS, indicates not statistically.
Figure 1B shows the comparison of the mRNA level of 5-HT2AR in PBMCs between patients with PIU and HCs. There was no significant difference in 5-HT2AR expression between patients with PIU and HCs (P > 0.05).
Correlation analyses were conducted (Spearman's r) between the relative expression of MAOA mRNA in PBMCs and clinical symptoms data in PIU group. The relative expression of MAOA mRNA was found to be positively correlated to the IAT (r = 0.419, P = 0.042) and SDS scores (r = 0.506, P = 0.012) in the PIU group (Figures 2A,B). However, no prominent association was observed between the change in the mRNA level of MAOA and SAS, PSQI, or BIS-11 scores, although, the SAS, PSQI, and BIS-11 scores in patients with PIU were significantly higher than those in HCs.
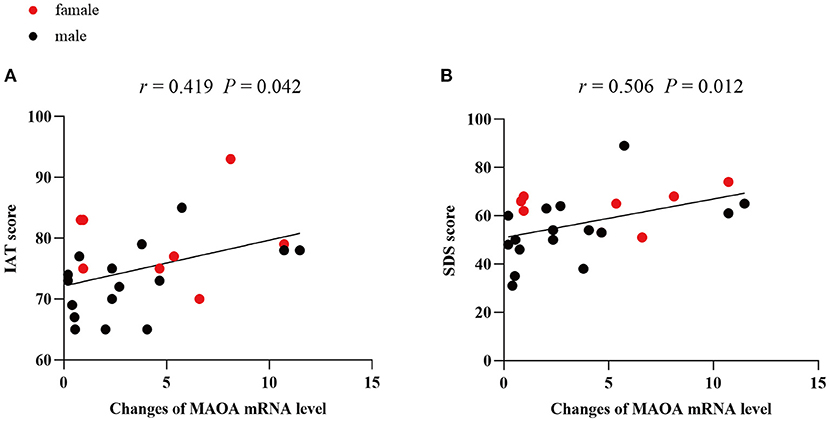
Figure 2. Correlations between mRNA levels of MAOA and clinical scores in PIU. (A) Correlation between the mRNA level of MAOA and IAT score of patients with PIU, (B) Correlation between the mRNA level of MAOA and SDS score of patients with PIU.
PIU is a relatively new and complex psychological phenomenon that is related to multiple comorbid symptoms. It has currently become a major public health problem in many countries (38). Various studies have indicated that pathological online behaviour can cause circadian rhythm disorder in patients with PIU, which may include prolonged sleep latency, shortened sleep time, and reduced sleep tendency, leading to sleep disorders, increased fatigue, and daytime sleepiness (39–41). Previous studies have reported that symptoms of depression and anxiety appear to have the most significant correlation with PIU (42). In a systematic review, Carli et al. (43) reported that one of the important underlying causes of depression associated with PIU may be related to sleep disorders caused by excessive internet use. Meanwhile, researchers have also found that patients with PIU are more impulsive and aggressive than those without PIU (44). The results of this study are consistent with those of the aforementioned studies. In addition, PIU has been found to be associated with various substance use disorders, including harmful alcohol use (45, 46). Nevertheless, the neurobiological evidence to confirm these statements is limited.
Serotonin 5-HT2AR is widely distributed in the central nervous system. Previous studies have reported that the function and expression level of 5-HT2AR were altered in addiction (47–49). However, determining changes in the expression of 5-HT2AR in the brain of living patients with behavioural and drug addiction is currently challenging. Based on the peripheral marker hypothesis, some researchers have suggested that changes in the neurotransmitter receptor expression level in the brain may be similar to those in the peripheral blood lymphocytes (50). Therefore, the exploration of the gene expression in peripheral blood is promising. For example, mRNA levels of 5-HT2AR in PBMCs from patients with major depression have been reported to be significantly upregulated compared with those in healthy subjects, which is consistent with the reported increase in 5-HT2AR binding sites in postmortem brain tissue (51–53). In Alzheimer's disease, a significant decrease in the mRNA level of 5-HT2AR in PBMCs was reported (54). We speculated that changes in mRNA level of 5-HT2AR in the brain may be reflected in PBMCs of patients with PIU. However, our results indicated no significant difference in the 5-HT2AR mRNA levels between PBMCs of patients with PIU and those of HCs. This could indicate that changes in 5-HT2AR expression are not involved in PIU. In this regard, studies on the expression level of 5-HT2AR during nicotine withdrawal in mice have reported no changes in the density and transcriptional level of 5-HT2AR in the midbrain (55). However, morphine tolerance and dependence studies in rats have demonstrated that 5-HT2AR is upregulated in the midbrain, pons, medulla oblongata, and amygdala (56). Consequently, the similarity of gene expression patterns between the brain and peripheral blood cells of behaviour addicts should be confirmed in future studies.
MAOA gene is located on the short arm of the X chromosome (Xp11.4–p11.23), and the variable number tandem repeat (VNTR) of the 30-bp repeat unit is present in the promoter region; the number of repetitions is 2–5. Simultaneously, different numbers of tandem repeats form different nucleotide sequences, which affect the transcriptional activity of MAOA (57). MAOA plays an important role in the catabolism of 5-HT, NE, and DA. Changes in these monoamines in the brain are essential for the regulation of motor, cognitive, and emotional functions.
Several psychiatric disorders such as anxiety, depression, alcohol dependence, drug abuse, and aggressive and impulsive behaviours have been found to be related to abnormalities in monoamine oxidase levels (58, 59). Brunner et al. (60) studied MAOA deficiency caused by spontaneous mutation in the MAOA gene in a Dutch family and found that male subjects with MAOA mutation and no activity have a significantly increased level of 5-HT in urine, mild mental retardation, impaired impulse control, and violent aggressive behaviours in response to sudden and stressful stimuli. A study in mice demonstrated that the MAOA gene knockout could elevate the concentrations of 5-HT and NE in the frontal cortex, hippocampus, and cerebellum, causing a considerable increase in fear and aggressive behaviour (61). Notably, the role of 5-HT2B receptor (5-HT2BR) gene and MAOA gene in regulating impulsive behaviour may be comparable. Doly et al. (62). found that 5-HT2BR gene may be involved in impulsivity by regulating the 5-HT and DA levels. It has been confirmed that the 5-HT2BR is located in the dopaminergic neurons that dominate the nucleus accumbens, one of the main components of the brain reward circuit, which is involved in the reinforcement and pleasure generated by substance abuse and addictive behaviour (63, 64). In previous studies on addiction, cannabis-related aggressive behaviour, and cocaine-crack use have been reported to be associated with the 5-HT2BR gene (65, 66). HTR2B Q20* carriers showed impulsive and aggressive behaviours, especially under the influence of alcohol (67). Therefore, the 5-HT2BR gene may be an important candidate gene for future research on behavioural addiction and substance abuse.
In addition, a study demonstrated that selective monoamine oxidase inhibitors decrease morphine-reinforced behaviour (68). Another study reported that oxycodone-injected mice exhibit higher levels of MAOA mRNA than mice injected with saline (69). Increases in MAOA mRNA level may suggest a high MAOA activity. Elevated MAOA activity in the brain is found in animals exposed to chronic stress and in patients with depression (70, 71). PET research further revealed higher density of MAOA in the brain of individuals with major depression than that in HCs (72). The recurrence of depressive symptoms is related to an increased MAOA density in the brain (73).
To the best of our knowledge, this is the first report on the mRNA level of MAOA in PBMCs of patients with PIU. Consistent with previous studies on major depression, our results demonstrate a remarkably increased MAOA mRNA level in the PIU group. Moreover, we observed that the IAT and SDS scores are positively correlated with the upregulated MAOA mRNA level. Thus, we speculate that the increased MAOA mRNA level might partly represent the underlying neurobiological mechanisms of PIU.
Our study has several limitations. First, it was a single-centre study, and cases were not collected from multiple cities. Second, although, PIU can be divided into sub-types, such as internet gaming disorder, online novel addiction, online gambling addiction, and online shopping addiction, we did not classify each sub-type of PIU; presence of multiple sub-types of internet addiction in individuals may have an impact on the experimental results. Third, the participants were restricted to college students, which limits the promotion of this research. Studies with a larger sample size and inclusion of multiple centres and different age groups along with a careful analysis of the relationship between the PIU and main comorbid symptoms and susceptibility genes will help in overcoming these limitations. In addition, in order to fully understand the relationship between the neurobiological changes of PIU and comorbid symptoms, further, exploration is needed to extend the PIU study to other genes.
Our study suggests that the increased levels of MAOA mRNA in PBMCs of patients with PIU may be associated with comorbid symptoms, and the increased MAOA mRNA level might be related to the changes in monoamine neurotransmitters. Although, our understanding of mRNA expression of PIU-related genes is inadequate, the present findings provide promising evidence to support that the increased MAOA mRNA level may be a cause of behavioural addiction.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Sichuan Traditional Chinese Medicine Regional Ethics Review Committee. The patients/participants provided their written informed consent to participate in this study.
TZ, HL, and YD conceptualized the study, designed the plan, and managed the project. TZ and HL supervised the study. MQ, CZ, LZ, CW, and YD conducted experiments. MQ, YW, WP, YC, and CW are statistically analyzed. MQ wrote the first draft of the manuscript. All authors read and approved the manuscript.
This research was supported by the Natural Science Foundation of China (81072852 and 81574047), the Key Research and Development Projects of Sichuan Science and Technology Department (2019YFS0175), the Xinglin Scholars Scientific Research Promotion Program of Chengdu University of Traditional Chinese Medicine (XSGG2019007), and the Training Funds of Academic and Technical Leader in Sichuan Province.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Kawabe K, Horiuchi F, Ochi M, Oka Y, Ueno S-I. Internet addiction: prevalence and relation with mental states in adolescents. Psychiatry Clin Neurosci. (2016) 70:405–12. doi: 10.1111/pcn.12402
2. Leménager T, Hoffmann S, Dieter J, Reinhard I, Mann K, Kiefer F. The links between healthy, problematic, and addicted Internet use regarding comorbidities and self-concept-related characteristics. J Behav Addict. (2018) 7:31–43. doi: 10.1556/2006.7.2018.13
3. Bernardi S, Pallanti S. Internet addiction: a descriptive clinical study focusing on comorbidities and dissociative symptoms. Compr Psychiatry. (2009) 50:510–6. doi: 10.1016/j.comppsych.2008.11.011
4. González-Bueso V, Santamaría JJ, Fernández D, Merino L, Montero E, Ribas J. Association between internet gaming disorder or pathological video-game use and comorbid psychopathology: a comprehensive review. Int J Environ Res Public Health. (2018) 15:668. doi: 10.3390/ijerph15040668
5. Fuchs M, Riedl D, Bock A, Rumpold G, Sevecke K. Pathological internet use-an important comorbidity in child and adolescent psychiatry: prevalence and correlation patterns in a naturalistic sample of adolescent inpatients. Biomed Res Int. (2018) 2018:1629147. doi: 10.1155/2018/1629147
6. Cheung LM, Wong WS. The effects of insomnia and internet addiction on depression in Hong Kong Chinese adolescents: an exploratory cross-sectional analysis. J Sleep Res. (2011) 20:311–7. doi: 10.1111/j.1365-2869.2010.00883.x
7. Weinstein A, Livny A, Weizman A. New developments in brain research of internet and gaming disorder. Neurosci Biobehav Rev. (2017) 75:314–30. doi: 10.1016/j.neubiorev.2017.01.040
8. Sadat-Shirazi M-S, Vousooghi N, Alizadeh B, Makki SM, Zarei SZ, Nazari S, et al. Expression of NMDA receptor subunits in human blood lymphocytes: a peripheral biomarker in online computer game addiction. J Behav Addict. (2018) 7:260–8. doi: 10.1556/2006.7.2018.35
9. Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. (2002) 27:576–86. doi: 10.1016/S0893-133X(02)00342-1
10. Adriaens AM, Polis IE, Vermeire ST, Waelbers T, Duchateau L, Sys SU, et al. The influence of morphine on cerebral 5-HT2A availability in dogs: a SPECT study. J Nucl Med. (2012) 53:1969–73. doi: 10.2967/jnumed.112.103796
11. Fajardo O, Galeno J, Urbina M, Carreira I, Lima L. Serotonin, serotonin 5-HT(1A) receptors and dopamine in blood peripheral lymphocytes of major depression patients. Int Immunopharmacol. (2003) 3:1345–52. doi: 10.1016/S1567-5769(03)00116-4
12. Zhang G, Stackman RW. The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol. (2015) 6:225. doi: 10.3389/fphar.2015.00225
13. Hu H. Reward and aversion. Annu Rev Neurosci. (2016) 39:297–324. doi: 10.1146/annurev-neuro-070815-014106
14. Müller CP, Homberg JR. The role of serotonin in drug use and addiction. Behav Brain Res. (2015) 277:146–92. doi: 10.1016/j.bbr.2014.04.007
15. Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. (2011) 61:421–32. doi: 10.1016/j.neuropharm.2011.03.022
16. Tian M, Chen Q, Zhang Y, Du F, Hou H, Chao F, et al. PET imaging reveals brain functional changes in internet gaming disorder. Eur J Nucl Med Mol Imaging. (2014) 41:1388–97. doi: 10.1007/s00259-014-2708-8
17. Zhang H-X, Jiang W-Q, Lin Z-G, Du Y-S, Vance A. Comparison of psychological symptoms and serum levels of neurotransmitters in Shanghai adolescents with and without internet addiction disorder: a case-control study. PLoS ONE. (2013) 8:e63089. doi: 10.1371/journal.pone.0063089
18. Liu M, Luo J. Relationship between peripheral blood dopamine level and internet addiction disorder in adolescents: a pilot study. Int J Clin Exp Med. (2015) 8:9943–8.
19. Hahn C, Kim D-J. Is there a shared neurobiology between aggression and Internet addiction disorder? J Behav Addict. (2014) 3:12–20. doi: 10.1556/JBA.3.2014.1.2
20. Naoi M, Riederer P, Maruyama W. Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: genetic and environmental factors involved in type A MAO expression. J Neural Transm. (2016) 123:91–106. doi: 10.1007/s00702-014-1362-4
21. Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. (1999) 22:197–217. doi: 10.1146/annurev.neuro.22.1.197
22. Bortolato M, Shih JC. Behavioral outcomes of monoamine oxidase deficiency: preclinical and clinical evidence. Int Rev Neurobiol. (2011) 100:13–42. doi: 10.1016/B978-0-12-386467-3.00002-9
23. Naoi M, Maruyama W, Shamoto-Nagai M. Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: from neurotransmitter imbalance to impaired neurogenesis. J Neural Transm. (2018) 125:53–66. doi: 10.1007/s00702-017-1709-8
24. Ibañez A, Perez de Castro I, Fernandez-Piqueras J, Blanco C, Saiz-Ruiz J. Pathological gambling and DNA polymorphic markers at MAO-A and MAO-B genes. Mol Psychiatry. (2000) 5:105–9. doi: 10.1038/sj.mp.4000654
25. Agatsuma S, Lee M, Zhu H, Chen K, Shih JC, Seif I, et al. Monoamine oxidase A knockout mice exhibit impaired nicotine preference but normal responses to novel stimuli. Hum Mol Genet. (2006) 15:2721–31. doi: 10.1093/hmg/ddl206
26. Beard KW, Wolf EM. Modification in the proposed diagnostic criteria for Internet addiction. Cyberpsychol Behav. (2001) 4:377–83. doi: 10.1089/109493101300210286
27. Young KS. Internet addiction: the emergence of a new clinical disorder. CyberPsychol Behav. (1996) 1:237–44 doi: 10.1089/cpb.1998.1.237
28. Lai C-M, Mak K-K, Watanabe H, Ang RP, Pang JS, Ho RCM. Psychometric properties of the internet addiction test in Chinese adolescents. J Pediatr Psychol. (2013) 38:794–807. doi: 10.1093/jpepsy/jst022
29. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
30. Cheung SK. Reliability and factor structure of the Chinese version of the depression self-rating scale. Educ Psychol Meas. (1996) 56:142–54. doi: 10.1177/0013164496056001011
31. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12:371–9. doi: 10.1016/S0033-3182(71)71479-0
32. Tao M, Gao JF. Reliability and validity of Zung's Self-Rating Anxiety Scale (SAS). J Chinese Neuropsychiatr Dis. (1994) 5:301–3. Available online at: https://www.cnki.com.cn/Article/CJFDTOTAL-ZSJJ405.022.htm
33. Smyth C. The Pittsburgh Sleep Quality Index (PSQI). J Gerontol Nurs. (1999) 25:10–1. doi: 10.3928/0098-9134-19991201-10
34. Tsai P-S, Wang S-Y, Wang M-Y, Su C-T, Yang T-T, Huang C-J, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res. (2005) 14:1943–52. doi: 10.1007/s11136-005-4346-x
35. Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. (1995) 51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1
36. Yang HQ, Yao SQ, Zhu XZ, Auerbach RP, AbelaJohn RZ. The Chinese version of the barratt impulsiveness scale 11th Version (BIS-11) in college students: Its reliability and validity. Chin Ment Health J. (2007) 223–5. Available online at: https://www.cnki.com.cn/Article/CJFDTOTAL-ZXWS200704004.htm
37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
38. Jorgenson AG, Hsiao RC-J, Yen C-F. Internet addiction and other behavioral addictions. Child Adolesc Psychiatr Clin N Am. (2016) 25:509–20. doi: 10.1016/j.chc.2016.03.004
39. Wang P-Y, Chen K-L, Yang S-Y, Lin P-H. Relationship of sleep quality, smartphone dependence, and health-related behaviors in female junior college students. PLoS ONE. (2019) 14:e0214769. doi: 10.1371/journal.pone.0214769
40. Chen Y-L, Gau SS-F. Sleep problems and internet addiction among children and adolescents: a longitudinal study. J Sleep Res. (2016) 25:458–65. doi: 10.1111/jsr.12388
41. Sami H, Danielle L, Lihi D, Elena S. The effect of sleep disturbances and internet addiction on suicidal ideation among adolescents in the presence of depressive symptoms. Psychiatry Res. (2018) 267:327–32. doi: 10.1016/j.psychres.2018.03.067
42. King DL, Delfabbro PH, Zwaans T, Kaptsis D. Clinical features and axis I comorbidity of Australian adolescent pathological Internet and video game users. Aust N Z J Psychiatry. (2013) 47:1058–67. doi: 10.1177/0004867413491159
43. Carli V, Durkee T, Wasserman D, Hadlaczky G, Despalins R, Kramarz E, et al. The association between pathological internet use and comorbid psychopathology: a systematic review. Psychopathology. (2013) 46:1–13. doi: 10.1159/000337971
44. Walther B, Morgenstern M, Hanewinkel R. Co-occurrence of addictive behaviours: personality factors related to substance use, gambling and computer gaming. Eur Addict Res. (2012) 18:167–74. doi: 10.1159/000335662
45. Lam LT. Risk factors of Internet addiction and the health effect of internet addiction on adolescents: a systematic review of longitudinal and prospective studies. Curr Psychiatry Rep. (2014) 16:508. doi: 10.1007/s11920-014-0508-2
46. Gentile DA, Choo H, Liau A, Sim T, Li D, Fung D, et al. Pathological video game use among youths: a two-year longitudinal study. Pediatrics. (2011) 127:e319–29. doi: 10.1542/peds.2010-1353
47. Zaniewska M, Alenina N, Wydra K, Fröhler S, Kuśmider M, McCreary AC, et al. Discovering the mechanisms underlying serotonin (5-HT)2A and 5-HT2C receptor regulation following nicotine withdrawal in rats. J Neurochem. (2015) 134:704–16. doi: 10.1111/jnc.13192
48. Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin J-P. 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci. (2004) 20:3073–84. doi: 10.1111/j.1460-9568.2004.03805.x
49. Li J-X, Shah AP, Patel SK, Rice KC, France CP. Modification of the behavioral effects of morphine in rats by serotonin 5-HT1A and 5-HT2A receptor agonists: antinociception, drug discrimination, and locomotor activity. Psychopharmacology. (2013) 225:791–801. doi: 10.1007/s00213-012-2870-2
50. Hepgul N, Cattaneo A, Zunszain PA, Pariante CM. Depression pathogenesis and treatment: what can we learn from blood mRNA expression? BMC Med. (2013) 11:28. doi: 10.1186/1741-7015-11-28
51. Amidfar M, Kim Y-K, Colic L, Arbabi M, Mobaraki G, Hassanzadeh G, et al. Increased levels of 5HT2A receptor mRNA expression in peripheral blood mononuclear cells of patients with major depression: correlations with severity and duration of illness. Nord J Psychiatry. (2017) 71:282–8. doi: 10.1080/08039488.2016.1276624
52. Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. (1990) 47:1038–47. doi: 10.1001/archpsyc.1990.01810230054009
53. Hrdina PD, Demeter E, Vu TB, Sótónyi P, Palkovits M. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. (1993) 614:37–44. doi: 10.1016/0006-8993(93)91015-K
54. Neshan M, Campbell A, Malakouti SK, Zareii M, Ahangari G. Gene expression of serotonergic markers in peripheral blood mononuclear cells of patients with late-onset Alzheimer's disease. Heliyon. (2020) 6:e04716. doi: 10.1016/j.heliyon.2020.e04716
55. Hayslett RL, Tizabi Y. Effects of donepezil, nicotine and haloperidol on the central serotonergic system in mice: implications for Tourette's syndrome. Pharmacol Biochem Behav. (2005) 81:879–86. doi: 10.1016/j.pbb.2005.06.010
56. Gulati A, Bhargava HN. Brain and spinal cord 5-HT2 receptors of morphine-tolerant-dependent and -abstinent rats. Eur J Pharmacol. (1989) 167:185–92. doi: 10.1016/0014-2999(89)90578-5
57. Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. (1998) 103:273–9. doi: 10.1007/s004390050816
58. Melas PA, Wei Y, Wong CCY, Sjöholm LK, Åberg E, Mill J, et al. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. Int J Neuropsychopharmacol. (2013) 16:1513–28. doi: 10.1017/S1461145713000102
59. Devor EJ, Cloninger CR, Hoffman PL, Tabakoff B. Association of monoamine oxidase (MAO) activity with alcoholism and alcoholic subtypes. Am J Med Genet. (1993) 48:209–13. doi: 10.1002/ajmg.1320480407
60. Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. (1993) 262:578–80. doi: 10.1126/science.8211186
61. Kim JJ, Shih JC, Chen K, Chen L, Bao S, Maren S, et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci USA. (1997) 94:5929–33. doi: 10.1073/pnas.94.11.5929
62. Doly S, Valjent E, Setola V, Callebert J, Hervé D, Launay J-M, et al. Serotonin 5-HT2B receptors are required for 3,4-methylenedioxymethamphetamine-induced hyperlocomotion and 5-HT release in vivo and in vitro. J Neurosci. (2008) 28:2933–40. doi: 10.1523/JNEUROSCI.5723-07.2008
63. Potenza MN, Fiellin DA, Heninger GR, Rounsaville BJ, Mazure CM. Gambling: an addictive behavior with health and primary care implications. J Gen Intern Med. (2002) 17:721–32. doi: 10.1046/j.1525-1497.2002.10812.x
64. Doly S, Quentin E, Eddine R, Tolu S, Fernandez SP, Bertran-Gonzalez J, et al. Serotonin 2B receptors in mesoaccumbens dopamine pathway regulate cocaine responses. J Neurosci. (2017) 37:10372–88. doi: 10.1523/JNEUROSCI.1354-17.2017
65. Lacoste J, Lamy S, Ramoz N, Ballon N, Jehel L, Maroteaux L, et al. A positive association between a polymorphism in the gene and cocaine-crack in a French Afro-Caribbean population. World J Biol Psychiatry. (2020) 21:784–9. doi: 10.1080/15622975.2018.1563721
66. Montalvo-Ortiz JL, Zhou H, D'Andrea I, Maroteaux L, Lori A, Smith A, et al. Translational studies support a role for serotonin 2B receptor (HTR2B) gene in aggression-related cannabis response. Mol Psychiatry. (2018) 23:2277–86. doi: 10.1038/s41380-018-0077-6
67. Tikkanen R, Tiihonen J, Rautiainen MR, Paunio T, Bevilacqua L, Panarsky R, et al. Impulsive alcohol-related risk-behavior and emotional dysregulation among individuals with a serotonin 2B receptor stop codon. Transl Psychiatry. (2015) 5:e681. doi: 10.1038/tp.2015.170
68. He S, Grasing K. L-methamphetamine and selective MAO inhibitors decrease morphine-reinforced and non-reinforced behavior in rats; insights towards selegiline's mechanism of action. Pharmacol Biochem Behav. (2006) 85:675–88. doi: 10.1016/j.pbb.2006.10.022
69. Mayer-Blackwell B, Schlussman SD, Butelman ER, Ho A, Ott J, Kreek MJ, et al. Self administration of oxycodone by adolescent and adult mice affects striatal neurotransmitter receptor gene expression. Neuroscience. (2014) 258:280–91. doi: 10.1016/j.neuroscience.2013.10.062
70. Chiuccariello L, Houle S, Miler L, Cooke RG, Rusjan PM, Rajkowska G, et al. Elevated monoamine oxidase a binding during major depressive episodes is associated with greater severity and reversed neurovegetative symptoms. Neuropsychopharmacology. (2014) 39:973–80. doi: 10.1038/npp.2013.297
71. Grunewald M, Johnson S, Lu D, Wang Z, Lomberk G, Albert PR, et al. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J Biol Chem. (2012) 287:24195–206. doi: 10.1074/jbc.M112.373936
72. Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, et al. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. (2006) 63:1209–16. doi: 10.1001/archpsyc.63.11.1209
73. Meyer JH, Wilson AA, Sagrati S, Miler L, Rusjan P, Bloomfield PM, et al. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry. (2009) 66:1304–12. doi: 10.1001/archgenpsychiatry.2009.156
Keywords: pathological internet use, monoamine oxidase type A, 5-HT2A receptor, mRNA level, comorbid symptoms
Citation: Qiu M, Zhang C, Dai Y, Zhang L, Wang Y, Peng W, Chen Y, Wen C, Li H and Zhu T (2021) mRNA Levels of MAOA and 5-HT2A Receptor in Patients With Pathological Internet Use: Correlations With Comorbid Symptoms. Front. Psychiatry 12:667699. doi: 10.3389/fpsyt.2021.667699
Received: 15 February 2021; Accepted: 22 June 2021;
Published: 16 July 2021.
Edited by:
Mira Jakovcevski, Max Planck Institute of Psychiatry (MPI), GermanyReviewed by:
Maarten Van Den Buuse, La Trobe University, AustraliaCopyright © 2021 Qiu, Zhang, Dai, Zhang, Wang, Peng, Chen, Wen, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianmin Zhu, dG16aHVAaG90bWFpbC5jb20=; Hui Li, dHRsaWh1aUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.