- 1Endogenous Disorders Department, Mental Health Research Institute, Tomsk National Research Medical Center of the Russian Academy of Sciences, Tomsk, Russia
- 2University Hospital, Siberian State Medical University, Tomsk, Russia
- 3Fundamental Psychology and Behavioral Medicine Department, Siberian State Medical University, Tomsk, Russia
- 4Molecular Genetics and Biochemistry Laboratory, Mental Health Research Institute, Tomsk National Research Medical Center of the Russian Academy of Sciences, Tomsk, Russia
- 5PharmacoTherapy, -Epidemiology and -Economics, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, Netherlands
- 6Addictive Disorders Department, Mental Health Research Institute, Tomsk National Research Medical Center of the Russian Academy of Sciences, Tomsk, Russia
- 7Psychiatry, Addiction Psychiatry and Psychotherapy Department, Siberian State Medical University, Tomsk, Russia
Objective: The purpose of this study was to compare the prevalence of MetS and the associated sociodemographic, clinical, and pharmacotherapeutic characteristics of patients with schizophrenia in three psychiatric hospitals in the West Siberian region.
Methods: Patients with a clinical diagnosis of schizophrenia (ICD-10: F20) and an age between 18 and 60 years were included in the study after giving informed consent. Metabolic syndrome was diagnosed according to the International Diabetes Federation criteria. This research was carried out at three Western Siberian psychiatric hospitals in Kemerovo, Tomsk, and Omsk. The study population included respectively 94, 131, and 91 inpatients with schizophrenia. We carried out schizophrenia symptoms assessment by PANSS, antipsychotic therapy evaluation, anthropometry, and biochemical analysis. Statistical Analysis included the Shapiro–Wilk test, non-parametric Kruskal–Wallis H-test for independent samples, Mann–Whitney U-test for independent samples, the chi-square test, stepwise multiple regression analyses. The level of significance was p < 0.05.
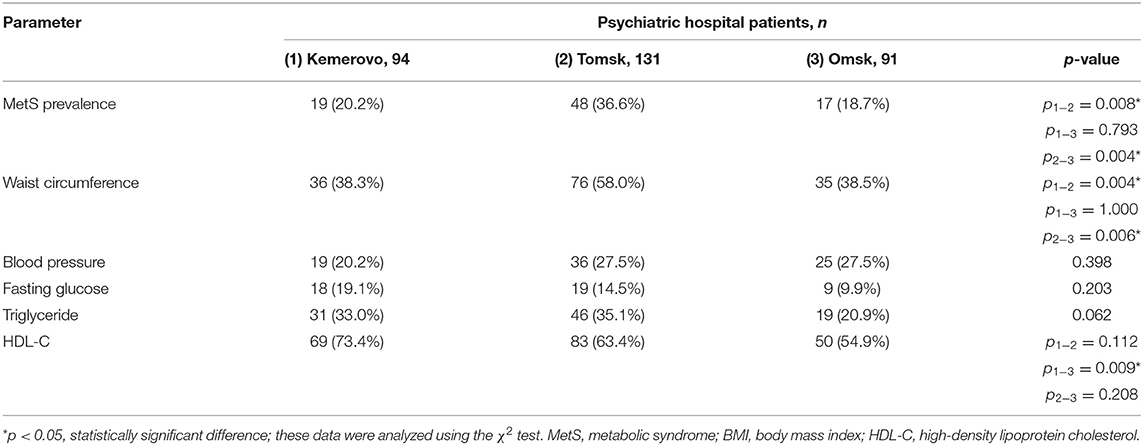
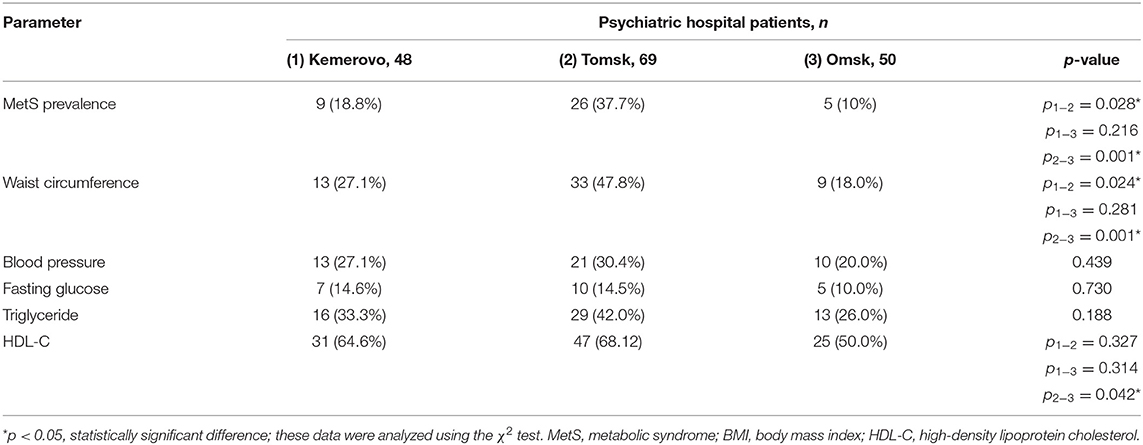
Results: The metabolic syndrome prevalence was higher among patients in Tomsk (36.6%), compared with Kemerovo (20.2%, p = 0.008) or Omsk (18.7%, p = 0.004), mainly due to the high prevalence of abdominal obesity, while men from Tomsk were more susceptible to this condition than men from other regions (p < 0.05). Patients from Omsk had the highest severity schizophrenia symptoms according to PANSS, and patients from Tomsk had the lowest severity of positive symptoms according to PANSS. Patients from Tomsk had the minimum duration of antipsychotic therapy compared with the patient from Kemerovo (p = 0.017) and from Omsk (p = 0.000019), but most patients from Tomsk received second-generation atypical antipsychotics, while patients from Omsk received mainly conventional antipsychotics (p = 0.0001). Multiple regression analysis showed that metabolic syndrome associated with schizophrenia duration and body mass index, although the association was not so strong (adjusted R2 = 0.2435, p < 0.0001).
Discussion: The study illustrates that in different psychiatric hospitals within the same region, the prevalence of metabolic syndrome in patients with schizophrenia can vary significantly, which dictates the need to look for opportunities to minimize the risk of its occurrence, taking into account the experience of each hospital.
Introduction
Schizophrenia is a chronic and severe mental disorder. There is generally accepted that its prevalence in the population is relatively stable at 1% (1).
Schizophrenia associated with a high global disease burden (2) and a decreased life expectancy of 11–20 years compared to the general population (3). One of the most common causes of death in patients with schizophrenia is cardiovascular diseases (4), closely associated with the presence of metabolic syndrome (MetS), which prevalence in patients with schizophrenia is significantly higher than in the general population (5). MetS in patients with schizophrenia is a complex phenomenon, which is partly due to the disorder itself, and partly due to adverse events of antipsychotic treatment (6, 7).
There is some evidence that the prevalence of the MetS is heterogeneous depending on the region of the world and even region of the country, and the prevalence of its cluster components has some associations with cross-culturally and cross-ethnicity (8–10). Also, the contribution of certain socioeconomic factors to the prevalence of the MetS is discussed: its components were much less frequent among individuals with a higher educational level, higher income, and occupational status, and those having a life partner (11). The prevalence of MetS among patients with schizophrenia was 23.9% in a meta-analysis conducted by Iranian researchers. The prevalence was higher among women than among men (34 vs. 10.8%). There is noteworthy that no relationship between the prevalence of MetS and age or duration of illness (12). A study from Turkey showed a higher prevalence of MetS among patients with schizophrenia and first-episode psychosis compared with the randomly selected healthy volunteers, regardless of the MetS criteria used. The MetS patients had a higher mean of age, a longer duration of disease and treatment compared to patients without MetS (13). Similar results were found in a study in Palestine, there were also discovered that systolic blood pressure, high triglycerides, high fasting plasma glucose, and low high-density lipoprotein cholesterol (HDL-C) were significant predictors of MetS (14). Spanish researchers, in addition to assessing the prevalence of MetS, also found that patients with schizophrenia who met the remission criteria were younger and had a lower body mass index (BMI) (15). In a Singapore study, the prevalence of MetS in patients with schizophrenia was 46.0% (16). Thus, the frequency of MetS and its components in patients with schizophrenia is not constant and depends on many factors that should be studied comprehensively.
The described differences in the epidemiology of both schizophrenia and MetS indicate the importance of comparative population and subpopulation studies to identify potential risk groups. While wide variations have been reported little is known about the factors underlying and the importance of understanding them cannot be overemphasized. A large meta-analysis by Indian researchers showed a similar to European patients' prevalence of MetS (29.83%) in persons with schizophrenia (17). Another study reported a lower prevalence of MetS in drug-naive patients with schizophrenia than in the general population (18).
Researchers from Japan found a greater prevalence of MetS in the outpatient group compared with the inpatient group with schizophrenia. This difference can be explained by the peculiarities of the Japanese mental health care system, which provides for constant lifestyle management in hospitals (19, 20). Similar data were obtained in Indonesia: MetS was even less common in inpatients with schizophrenia than in the general population, regardless of antipsychotic treatment (21). The authors explain these differences by the financial burden of patients and the inability to buy extra food during hospitalization.
Separate studies conducted in Korea and Taiwan compare a cohort of patients with schizophrenia with the general population, revealing a high prevalence of MetS in the first group, which is consistent with data from European studies (22, 23). However, to date, few comparisons have been made between individual subpopulations of patients with schizophrenia. Among them, we can highlight the study of differences in the prevalence and characteristics of MetS in outpatients with schizophrenia in different countries (19), or living in communities (24). In several studies, MetS and its components were compared in schizophrenia patients with mood disorders (25, 26). There are practically no comparative studies of MetS in schizophrenia inpatients in different psychiatric hospitals, geographically located within the same region, which allow one to assess the role of antipsychotic pharmacotherapeutic schemes and combinations characteristic of individual hospitals in the development of MetS.
The question of the effect of antipsychotic treatment on MetS in patients with schizophrenia is remaining open. A major recent review noted differences between antipsychotics in terms of metabolic side-effects, with olanzapine and clozapine exhibiting the worst profiles and aripiprazole, brexpiprazole, cariprazine, lurasidone, and ziprasidone the most benign profiles. The risk factors of antipsychotic-induced metabolic change were increased baseline weight, male sex, and non-white ethnicity (27). At the same time, much less data exist on the effect of antipsychotic polypharmacy on the prevalence of MetS. There was also shown a possible protective effect of drug combinations including aripiprazole for diabetes and hyperlipidemia, compared to other combinations and/or monotherapy (28). Other authors report that the prevalence of MetS does not differ in schizophrenia whether patients are receiving polypharmacy and monotherapy (29). In a study comparing polypharmacy and clozapine monotherapy, clozapine had a more significant effect on the development of MetS (28). There is also evidence that antipsychotic polypharmacy can increase the risk of the pre-metabolic syndrome, even after adjusting for patients' lifestyle characteristics (30, 31). We have not come across studies comparing the prevalence of MetS and its components in inpatients with schizophrenia in hospitals within the same region, for example, a country or area. This formulation of the question can reveal subjective differences, for example, the approaches to treatment adopted in a particular hospital.
Thus, despite the existence of differences between subpopulations of patients with schizophrenia, the amount of data on this topic remains insufficient, which determines the relevance of this study. The purpose of this study was to compare the prevalence of MetS and the associated sociodemographic, clinical, and pharmacotherapeutic characteristics of patients with schizophrenia in three psychiatric hospitals in the West Siberian region. We hypothesized that there may be differences in MetS prevalence in patients with schizophrenia taking antipsychotic therapy across multiple psychiatric hospitals within the same region, associated with differing sociodemographic, clinical, and pharmacotherapeutic characteristics. Western Siberia is an industrial and scientific region with a continental climate, long cold winters, short summers, located in the geographic center of Russia, known for its hydrocarbon deposits and an extensive network of gas and oil production. The administrative centers are large cities, but the region has a relatively low population density, due to which psychiatric hospitals are concentrated in large cities. Mental health care in this region, as well as throughout Russia, is funded by the state and is free of charge for patients. Hospitals, as a rule, do not have significant differences among themselves in the organization of mental health care.
Materials and Methods
Study Population
This research was carried out from September to December 2019 at three Western Siberian psychiatric hospitals in Kemerovo, Tomsk, and Omsk. The study population included respectively 94, 131, and 91 inpatients with schizophrenia who written informed consent and met the following inclusion criteria:
• presence of schizophrenia diagnosed according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10: F20);
• antipsychotics therapy for at least 6 months before entering the study;
• age range 18–60 years.
Exclusion criteria:
• a history of eating disorders;
• a history of alcohol or drug abuse;
• diabetes;
• treatment for organic comorbidities.
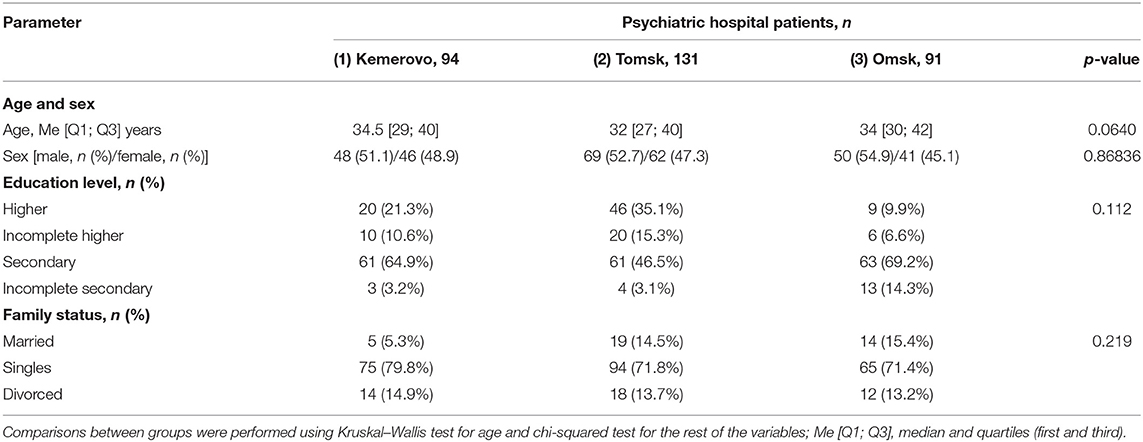
The groups of patients recruited in each of the hospitals did not differ in sex, age, educational level, and marital status (Table 1). All patients received therapy with conventional or/and second-generation atypical antipsychotics.
Ethics Approval Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Mental Health Research Institute of the Tomsk National Research Medical Center of the Russian Academy of Sciences (protocol number 187, date of approval 2018-04-24). All participants provided written informed consent to participate in this study.
Schizophrenia Criteria and Symptom Severity Assessment
We used the World Health Organization World Mental Health Composite International Diagnostic Interview (WHO WMH-CIDI) for schizophrenia diagnostics. Symptom severity assessment was carried out with the Positive and Negative Syndrome Scale (PANSS) (32). Information about work capacity or disability as a result of mental disorder, duration of the disorder, and duration of antipsychotic use were taken from available medical records. The doses of antipsychotics that patients received were calculated to uniformity for chlorpromazine equivalent (CPZeq) (33).
Definition of MetS
We used the criteria of the IDF for MetS diagnostics (34). These criteria require that MetS is diagnosed in a patient with central obesity (WC more than 94 cm in men and more than 80 cm in women) and the presence of any two of the following four signs:
• the concentration of triglycerides in serum is higher than 1.7 mmol/L (150 mg/dl) or lipid-lowering therapy is carried out;
• the concentration of high-density lipoprotein in serum is below 1.03 mmol/L (40 mg/dl) in men and 1.29 mmol/L (50 mg/dl) in women;
• the arterial blood pressure level is systolic above 130 mmHg or diastolic above 85 mmHg (or with treatment of previously diagnosed hypertension);
• serum glucose concentration is >5.6 mmol/L (100 mg/dl; or previously diagnosed type 2 diabetes).
Blood Sampling and Biochemical Parameters
After 12 h of overnight fasting, blood samples were taken into BD Vacutainer® with a clot activator (BD, Franklin Lakes, NJ, USA) for biochemical tests of glucose, HDL-C, and triglycerides using commercial kits (Cormay, Łomianki, Poland).
Statistical Analysis
We used the Shapiro–Wilk test to assess the data correspondence to the normal distribution at the first stage. The significance of intergroup differences was evaluated using the non-parametric Kruskal–Wallis H-test for independent samples. The pairwise analysis was performed using Mann–Whitney U-test for independent samples. The chi-square (χ2) test was used to analyze the categorical variables. Bonferroni correction was applied for multiple comparisons to erroneous inferences prevent. Descriptive statistics are presented as the median with 25 and 75% quartiles, Me (Q1; Q3). We conducted stepwise multiple regression analyses to examine the relevance of variables for the presence of the MetS. We entered into the model variables that in univariate analyses were different between patients in three groups (psychiatric hospitals in Kemerovo, Tomsk, and Omsk). The level of significance was p < 0.05.
Results
Employment Status of the Study Population
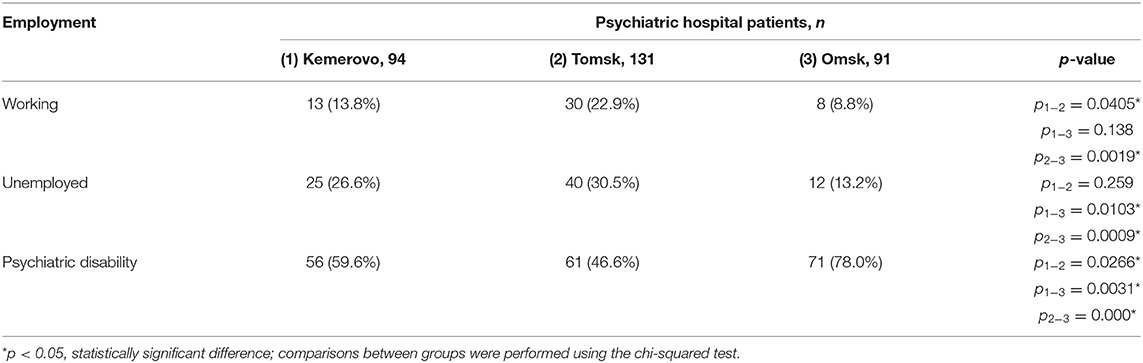
More patients from Tomsk had a job compared to a patient from Kemerovo and Omsk (p = 0.0405 and 0.0019, respectively). There were fewer unemployed patients from Omsk compared to patients from Kemerovo and Tomsk (p = 0.0103 and 0.0099, respectively). However, the majority of patients from Omsk had psychiatric disability compared to patients from Kemerovo and Tomsk (p = 0.031 and 0.000, respectively). A psychiatric disability was also more common in Kemerovo than in Tomsk patients (p = 0.0266; Table 2).
Schizophrenia Clinical Characteristics Comparison
Patients from Omsk had a longer disease duration than patients from Tomsk (p = 0.032) and Kemerovo (p = 0.075). Patients from Tomsk had a minimum score of PANSS positive symptoms compared with the patient from Kemerovo (p = 0.00001) and from Omsk (p = 0.00000). The maximum total PANSS score was in patients from Omsk (p = 0.035 and 0.001 compared to patients from Kemerovo and Tomsk, respectively), mainly due to negative symptoms (p = 0.002 compared to patients from Kemerovo; Table 3). The minimum PANSS positive symptoms score was recorded among males from Tomsk, as compared to males from Kemerovo (p = 0.0029) and from Omsk (p = 0.00007). The PANSS total score was higher in males from Omsk than in males from Tomsk (p = 0.0346; Table 4). The minimum positive PANSS symptoms score was recorded in females from Tomsk compared with females from Kemerovo (p = 0.0046) and from Omsk (p = 0.00057). Females from Omsk had a higher PANSS negative symptoms score than females from Kemerovo (p = 0.0176), as well as a higher total PANSS score than females from Tomsk (p = 0.047; Table 5).
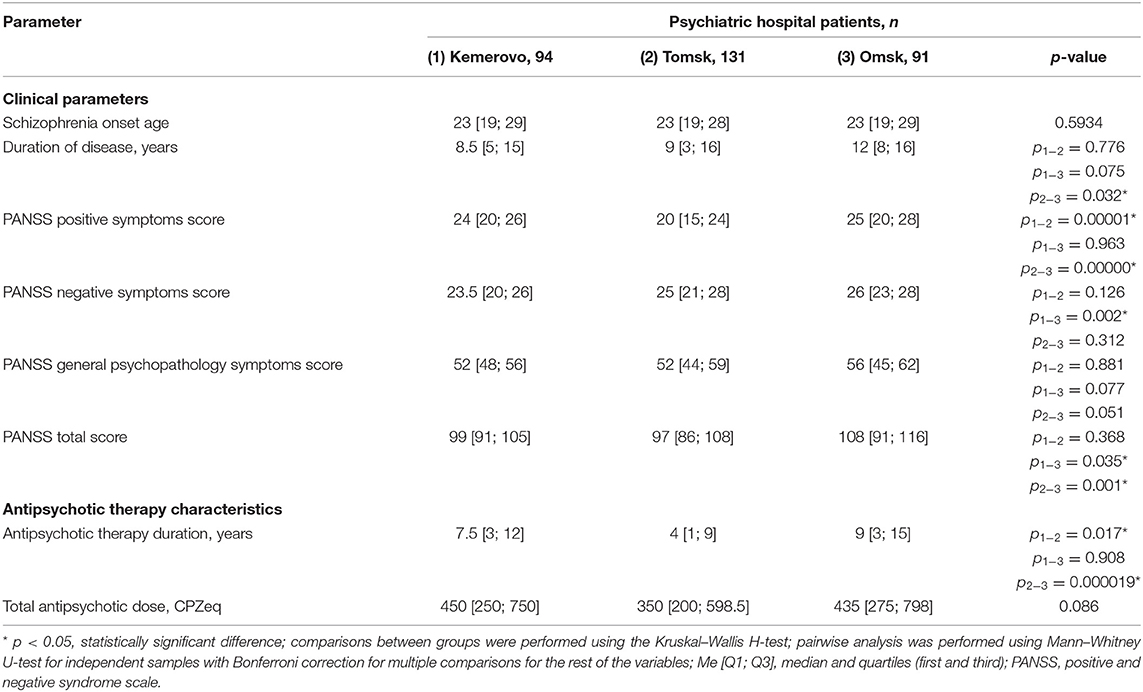
Table 3. Comparative characteristics and evaluation of clinical parameters and antipsychotic therapy in the study population (Me [Q1; Q3]).
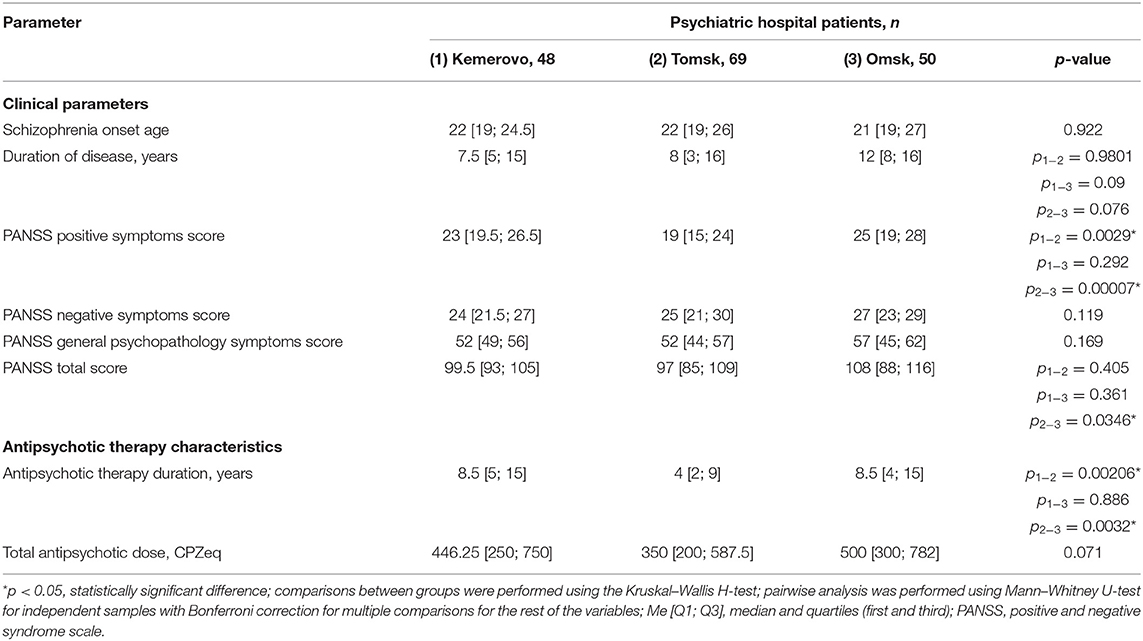
Table 4. Comparative characteristics and evaluation of clinical parameters and antipsychotic therapy in the male part of the study population (Me [Q1; Q3]).
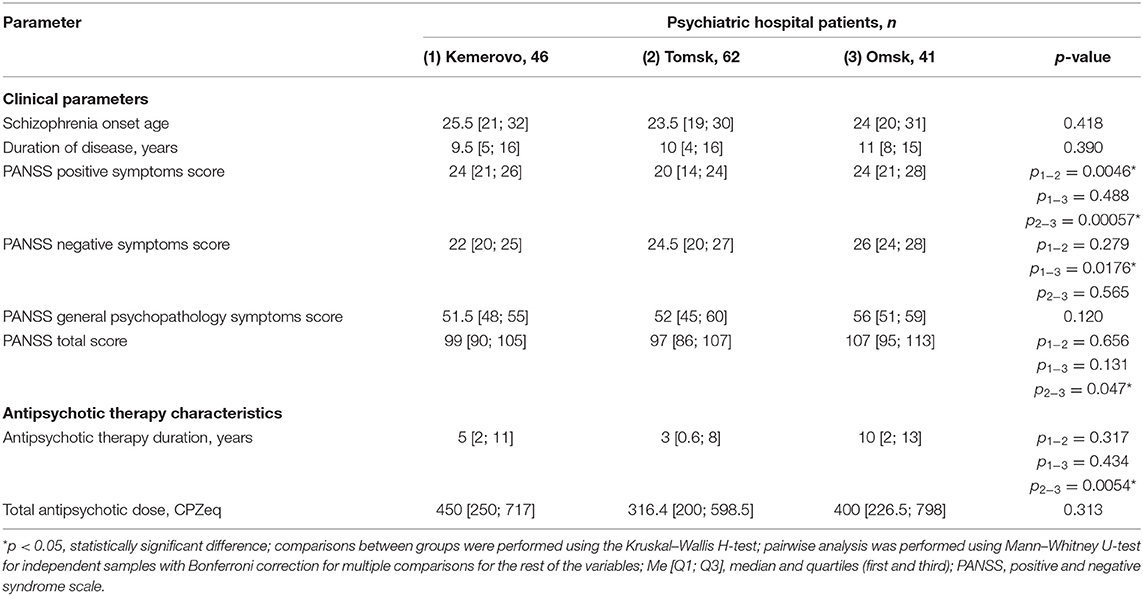
Table 5. Comparative characteristics and evaluation of clinical parameters and antipsychotic therapy in the female part of the study population (Me [Q1; Q3]).
Comparison of Antipsychotic Therapy
Patients from Tomsk had the minimum duration of antipsychotic therapy (Table 3) compared with the patient from Kemerovo (p = 0.017) and from Omsk (p = 0.000019). The minimum duration of antipsychotic therapy was recorded in males from Tomsk as compared to males from Kemerovo (p = 0.00206) and from Omsk (p = 0.0032; Table 4). Females from Omsk had a longer duration of antipsychotic therapy than females from Tomsk (p = 0.0054; Table 5). Most patients from Tomsk received atypical antipsychotics, while patients from Omsk received mainly conventional antipsychotics (p = 0.0001; Table 6). The majority of patients from Tomsk took antipsychotic monotherapy as compared with patients from Kemerovo and Omsk (p = 0.0186 and 0.001, respectively). Patients from Omsk most often received a combination of two antipsychotics compared to patients from Kemerovo and Tomsk (p = 0.046 and 0.0019, respectively). The patients from Kemerovo were more likely to take three antipsychotics at the same time as compared with the patients from Tomsk (p = 0.0224; Table 6).
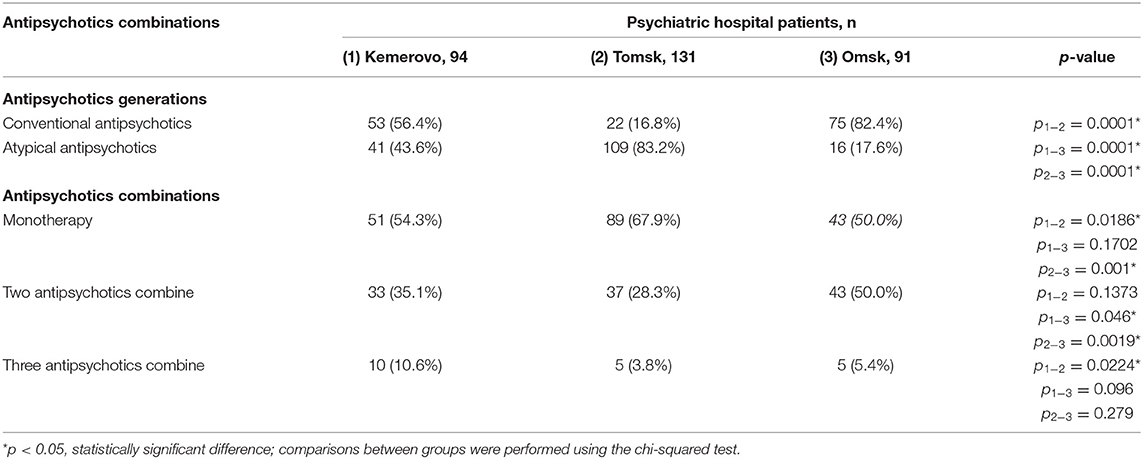
Table 6. Comparative characteristics and evaluation of antipsychotics generations and antipsychotics combinations, n (%).
Anthropometric Parameters
Patients from Tomsk, both in the general group (Table 7) and when divided by sex (Tables 8, 9), were characterized by a larger waist circumference and BMI than patients from Kemerovo or Omsk (p < 0.05 in each case).
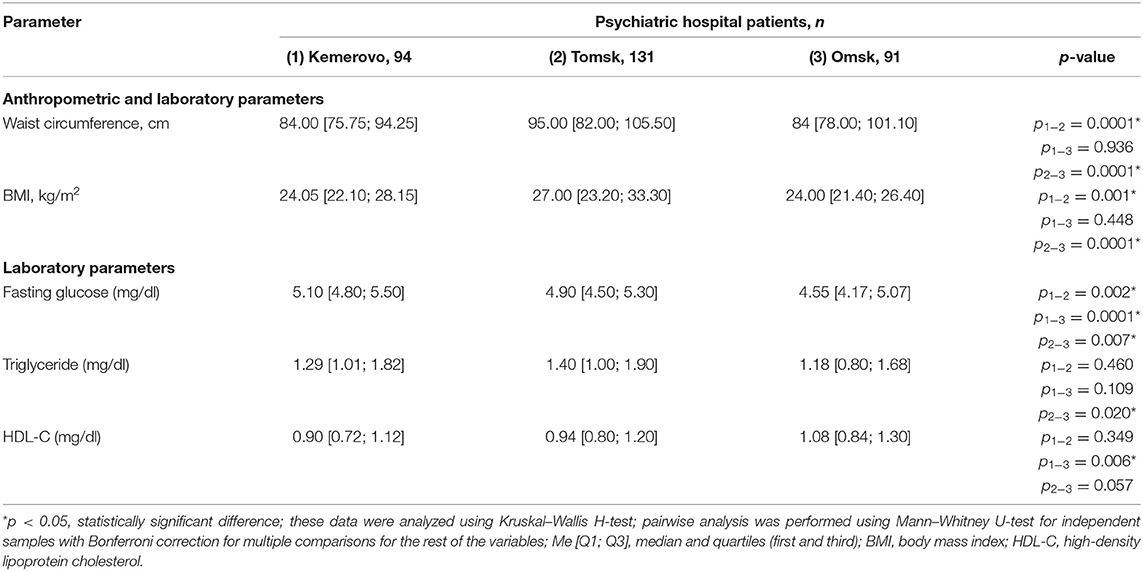
Table 7. Comparative characteristics and evaluation of anthropometric and laboratory parameters in the study population (Me [Q1; Q3]).
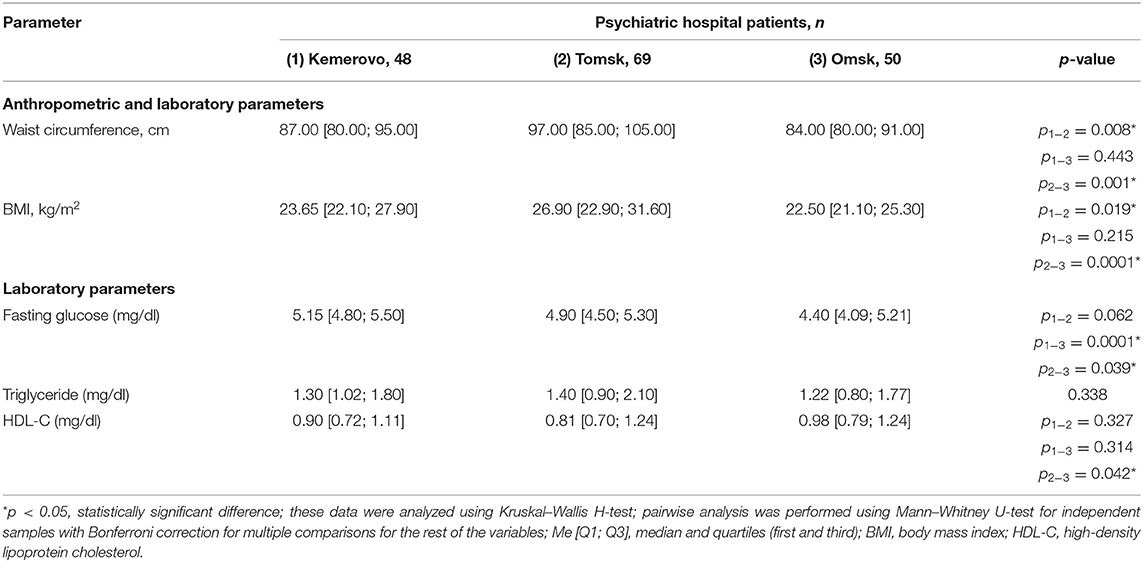
Table 8. Comparative characteristics and evaluation of anthropometric and laboratory parameters in the male part of the study population (Me [Q1; Q3]).
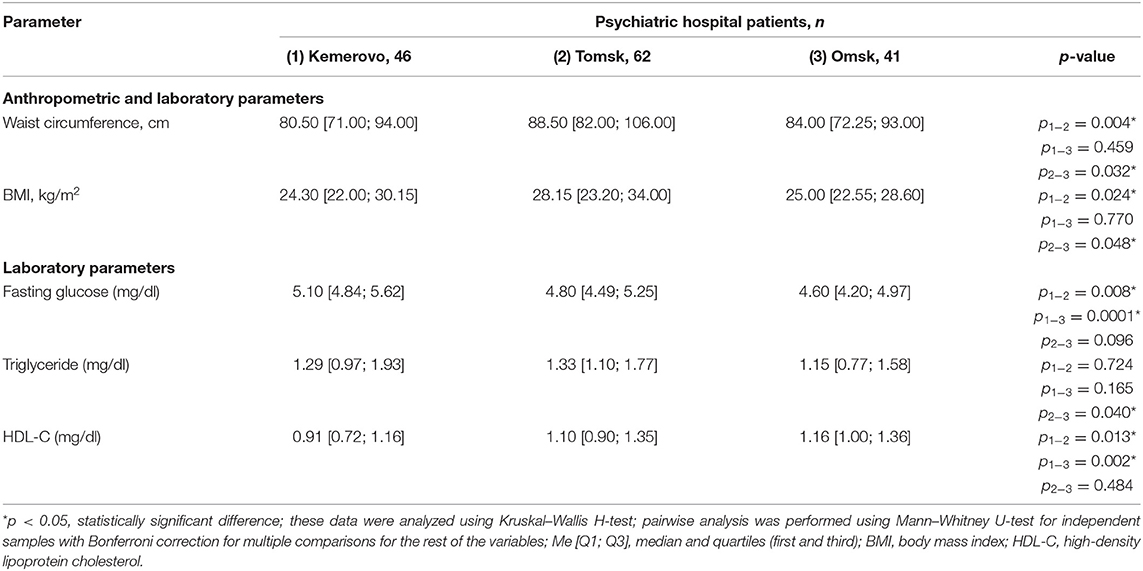
Table 9. Comparative characteristics and evaluation of anthropometric and laboratory parameters in the female part of the study population (Me [Q1; Q3]).
Laboratory Parameters
The maximum level of fasting glucose in the blood serum was registered in patients from Kemerovo (Table 7), the minimum—from Omsk (p < 0.05 in each case). Moreover, the maximum and minimum fasting glucose values were achieved mainly due to the level of this indicator in females (Table 9) and males (Table 8), respectively (p < 0.05 in each case). The triglyceride level was higher in patients from Tomsk (Table 7), mainly females (Table 9; p = 0.040) than from Omsk (p = 0.020). Females from Kemerovo had a lower HDL-C (Table 9) level than females from other regions (p < 0.05 in each case).
Prevalence of MetS and Its Criteria
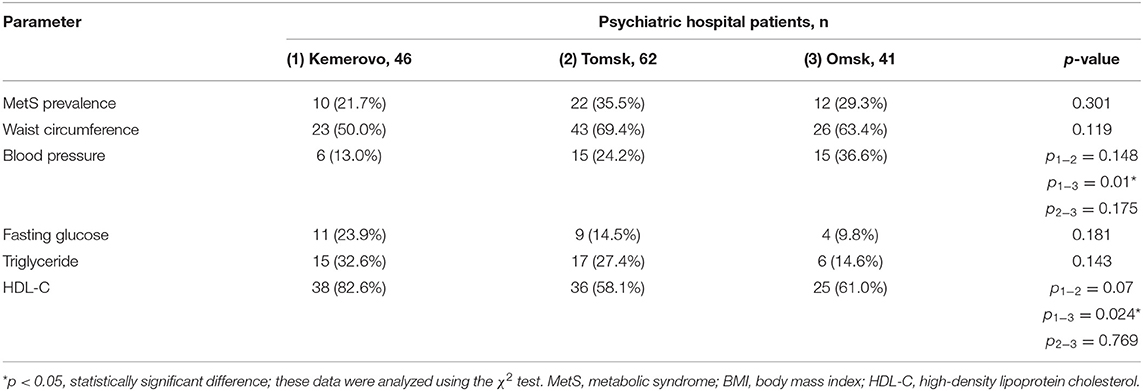
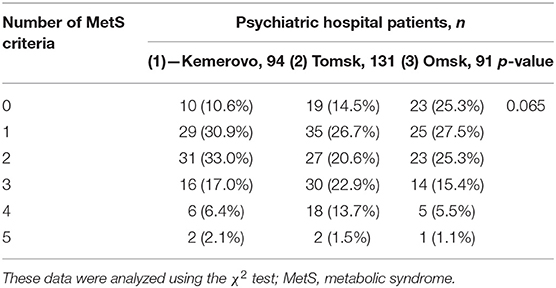
The prevalence of MetS was higher among patients in Tomsk (Table 10), compared with Kemerovo [p = 0.008; Odds Ratio (OR): 2.283, 95% Confidence Interval (CI): 1.233–4.228] or Omsk (p = 0.004; OR: 2.517, 95% CI: 1.333–4.754), mainly due to the high prevalence of abdominal obesity (Table 10), while men from Tomsk (Table 11) were more susceptible to this condition than men from other regions (p < 0.05 in each case). The incidence of elevated HDL-C levels was higher in individuals from Kemerovo than in individuals from Omsk (Table 10), mainly due to women (p < 0.05 in each case; Table 12). Blood pressure was higher in the female part of the study population in female patients from Omsk compared to female patients from Kemerovo (p = 0.01; Table 12). The number of MetS criteria was comparable in all the regions studied (Table 13); there were also no gender differences.
Determinants of MetS in Multiple Regression Analysis
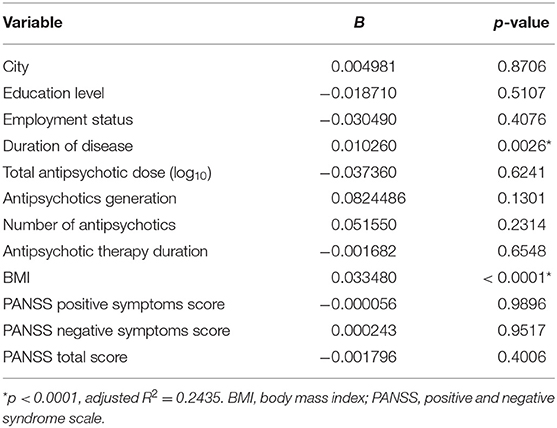
The rest step in the statistical analysis was multiple regression to determine the potential impact of factors such as duration of disease, education level, employment status, city, PANSS score, BMI, and antipsychotic therapy. Chlorpromazine equivalents data were converted using a decimal logarithm to reduce asymmetry. MetS was associated with a duration of disease (p = 0.0026) and BMI (p < 0.0001) [adjusted R2 = 0.2435, p < 0.0001] as a result of the multiple regression model (Table 14).
Discussion
Employment Status
Patients from Tomsk were employed more often than the rest of the patient groups and had more frequent MetS compared to the Kemerovo subpopulation. This is consistent with Lin et al. (35) and Moreira et al. (36), that there are some differences in the higher prevalence of MetS in people with a lower socioeconomic level compared to a high one in the general population, which is apparently related to differences in diet and lifestyle. Also, Moreira et al. (36) pointed out that employment status should be considered in combination with other factors, in particular, the level of education and physical activity in the MetS development context. Patients from different hospitals included in our study had similar lifestyles and did not differ in educational level, which underlines the importance of the results of this part of the study.
Schizophrenia Clinical Characteristics Comparison
It is known that the duration of schizophrenia increases the incidence of MetS in patients (5). This pattern was not reflected in the results of our study when comparing the duration of illness between patients from different hospitals and the presence of MetS in them. On the other hand, it is necessary to clarify that we found differences in the symptoms of schizophrenia between patients of different hospitals, which could affect the results obtained. Such differences were found in both males and females. The peculiarities described are more attributable to differences in study designs than to the contra data identified. For example, a higher incidence of obesity in chronic schizophrenia was noted in women (37), and according to Lin et al. (35) a higher prevalence of MetS is associated with older age, female sex, low psychotic symptoms, and clozapine use. In contrast, our study participants did not take clozapine, and the samples from different hospitals did not initially differ by sex.
Comparison of Antipsychotic Therapy
Individuals diagnosed with schizophrenia often lead unhealthy lifestyles, such as being inactive, exercising little, eating poorly, and smoking a lot, which contributes to the MetS development. These are due in part to the negative symptoms of schizophrenia, lack of motivation, poor understanding of their health, and the sedative effects of antipsychotic treatment (38). Patients from Tomsk mostly received therapy with atypical antipsychotics, while patients from Omsk received conventional antipsychotics. The revealed differences in the prevalence of MetS among patients from Tomsk and Omsk may also be associated with antipsychotic therapy since it is well-known that the use of second-generation antipsychotics is more often accompanied by the development of metabolic disorders (27). We have previously shown that 6-week therapy with atypical antipsychotics led to a disruption of most parameters of fat metabolism, glucose, and lipid metabolism (39). Also, patients from Tomsk most often took antipsychotic monotherapy, patients from Omsk—a combination of two antipsychotics, and patients from Kemerovo were more likely to take three antipsychotics. Although the use of antipsychotic polypharmacy is controversial due to the possible increase in side effects, in routine clinical practice it often continues to be used in the treatment of patients with schizophrenia (28, 40). In our study, patients received a combination of conventional and atypical second-generation antipsychotics, excluding clozapine. A number of studies have shown conflicting data on the relationship between antipsychotic polypharmacy and MetS, with some of them demonstrating a possible increase in the risk of developing MetS, while others—its reduction (40, 41). Safe combinations of antipsychotic therapy in terms of the development of MetS are discussed when antipsychotics usually used in clinical practice are combined with a third-generation drug (aripiprazole). Presumably, this effect of aripiprazole may be associated with its partial agonist activity against the 5-HT1A serotonin receptor, which possibly balances the effects exerted through the 5-HT2C receptor, as well as with the effect on the orexin and histamine systems (42, 43). Also, it is known that antipsychotic polypharmacy does not increase the risk of MetS in schizophrenia patients when the total antipsychotic load in chlorpromazine equivalent is comparable to the equivalent dose in monotherapy (40). Our study also showed that polypharmacy does not play a significant role in the formation of MetS.
Anthropometric Parameters
The highest prevalence of MetS, mainly due to an increase in the waist and BMI, was observed in patients from Tomsk, both in the general group and among males and females separately. This fits into the differences in approaches to antipsychotic therapy between hospitals discussed above and the general pattern of the formation of MetS, revealed in many studies, in particular, a large systematic review found that in patients with severe mental illness, a higher BMI was associated with lower levels of physical activity as a consequence of the lack of resources or professional support (44). Our previous study also showed the role of the bone component of body composition in the formation of MetS (45).
Laboratory Parameters
BMI, HDL, triglycerides, waist circumference are all the strong risk factors for the development of MetS in drug naïve schizophrenia patients (46), and an unfavorable lipid profile is combined with increased BMI and waist circumference values (47). It is also known that women with chronic schizophrenia have more severe lipid metabolism dysfunction than men (37). We found differences in the level of fasting glucose in the blood serum between patients from Kemerovo and Omsk both in the general group and among males and females separately. The triglyceride level was higher in patients from Tomsk, mainly females than from Omsk. Females from Kemerovo had a lower HDL-C level than females from other regions. The data obtained ultimately influenced the prevalence of MetS and its components.
Prevalence of MetS and Its Criteria
The prevalence of MetS was higher among patients in Tomsk, compared with other groups. Also, we found the sex differences in MetS criteria between groups. The minimum incidence of MetS was observed in patients from Omsk. At the same time, patients from Omsk had the highest severity of the disease according to PANSS, and patients from Tomsk had the lowest severity of positive symptoms according to PANSS. Other researchers previously showed that a deterioration in the lipid profile may be associated with an improvement in the clinical manifestations of schizophrenia (48). According to Lally et al. (49), an increase in triglyceride levels strongly predicted clinical improvement as well as an improvement in positive and negative PANSS scores. It is also known that people with schizophrenia had poor diets, including eating few fruits and vegetables, and a large number of instant foods, convenience foods, and sugar (50, 51). On this side, the lower prevalence of MetS in patients from Omsk can be explained by a rather meager diet due to economic conditions, since most patients from this group had a psychiatric disability. In addition, patients from Omsk were less likely to receive atypical antipsychotics. The number of MetS criteria was comparable in patients of all psychiatric hospitals.
Determinants of MetS in Multiple Regression Analysis
Statistical analysis showed that MetS was associated with schizophrenia duration and BMI. As a result of constructing a multiple regression model, in which the value of the presence/absence of MetS acted as a dependent variable, there was a weak regression equation, that is, the studied predictors are weakly related to this variable. According to the results of other multivariate logistic regression analysis, the risk of developing MetS in people with mental disorders, is associated with different factors: in men, they include low educational level, high BMI, long duration of mental illness, concomitant chronic physical diseases and diagnosed bipolar disorder, while in women, aggravating factors include high BMI, marriage, and older age (52). The results obtained in our study indicate that the duration of schizophrenia, BMI, and the characteristics of antipsychotic therapy are not key factors in the development of MetS in patients with schizophrenia. It is assumed that there is a possible general genetic, endocrinological, and inflammatory predisposition to MetS and schizophrenia. In obedience to a systematic review (53), several genes are strongly associated with MetS in schizophrenia patients such as the fat mass and obesity associated gene (FTO), leptin and leptin receptor genes (LEP, LEPR), methylenetetrahydrofolate reductase (MTHFR) gene, and the serotonin receptor 2C gene (HTR2C). The regulation of homeostasis systems, including the hypothalamus-pituitary-adrenal system and the inflammatory response, is often disrupted in patients with schizophrenia. These pathophysiological imbalances are also associated with the development of MetS (54). Nevertheless, it would be unjustified to ignore clinical, anthropometric, and pharmacological factors when assessing the risk of MetS in patients with schizophrenia.
We did not find studies, even published in Russian, that even approximately allowed us to compare the prevalence of MetS in the general population of Kemerovo, Tomsk, and Omsk. Such data would be helpful to comprehend the results of our study more fully. At the same time, the following clinical implications proceed from it. First, if our study is replicated in other regions by comparing several hospitals and finding similar differences, it would raise questions about the influence of pharmacological traditions on the long-term effects of treatment. Second, there will be also a question of comparing the styles of use of antipsychotics by individual psychiatrists. Third, we once again draw attention in general to the problem of the safety of schizophrenia treatment.
Conclusion
Taken together, we performed a comparison of the metabolic syndrome prevalence in patients with schizophrenia from three hospitals in one region. Based on the results above schizophrenia patients of different psychiatric hospitals within the same region had some differences in MetS and its components prevalence, schizophrenia clinical characteristics antipsychotic treatment peculiarities. Future studies are needed to comprehending the reasons for these differences in the context of treatment and rehabilitation approaches improving.
Study Limitations
Our study had limitations such as crossover design, which did not allow us to establish the temporal relationship between schizophrenia and MetS, as well as the lack of reliable information about which antipsychotics patients received during the entire period of illness. In addition, we did not assess patients' lifestyle (e.g., physical activity, sedentary, diet, and smoking) in this study.
Study Strengths
We consider the strengths of our study to be the assessment with standardized instruments, the collection of a large number of laboratory and anthropometric parameters, and the rigorous way of MetS diagnostic, are making the study reproducible.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Mental Health Research Institute of the Tomsk National Research Medical Center of the Russian Academy of Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
EK, AK, and SI: conceptualization. EK and SI: methodology and data curation. AK, IM, and IP: software. EK, IM, and AK: validation and writing—original draft preparation. IM, AG, and IP: formal analysis. IM, AG, AB, and VG: investigation. EK and AK: resources. EK, IM, AL, and VG: writing—review and editing. AS and SI: supervision. NB: project administration. AB: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation (project no. 19-75-10012).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the Kemerovo Regional Clinical Psychiatric Hospital (chief physician—Dr. Veronika A. Sorokina), the Tomsk Clinical Psychiatric Hospital (chief physician—Dr. Sergey M. Andreev), and the N.N. Solodnikova Clinical Psychiatric Hospital of Omsk (chief physician—Dr. Andrey I. Cheperin) for their help in recruiting patients for this investigation.
References
1. McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. (2008) 30:67–76. doi: 10.1093/epirev/mxn001
2. Moreno-Küstner B, Martín C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS One. (2018) 13:e0195687. doi: 10.1371/journal.pone.0195687
3. Laursen TM, Wahlbeck K, Hällgren J, Westman J, Ösby U, Alinaghizadeh H, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. (2013) 8:e67133. doi: 10.1371/journal.pone.0067133
4. Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. (2015) 2:452–64. doi: 10.1016/S2215-0366(15)00115-7
5. Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. (2013) 39:306–18. doi: 10.1093/schbul/sbr148
6. Rojo LE, Gaspar PA, Silva H, Risco L, Arena P, Cubillos-Robles K, et al. Metabolic syndrome and obesity among users of second generation antipsychotics: a global challenge for modern psychopharmacology. Pharmacol Res. (2015) 101:74–85. doi: 10.1016/j.phrs.2015.07.022
7. Cordes J, Bechdolf A, Engelke C, Kahl KG, Balijepalli C, Lösch C, et al. Prevalence of metabolic syndrome in female and male patients at risk of psychosis. Schizophr Res. (2017) 181:38–42. doi: 10.1016/j.schres.2016.09.012
8. Sigit FS, Tahapary DL, Trompet S, Sartono E, Willems van Dijk K, Rosendaal FR, et al. The prevalence of metabolic syndrome and its association with body fat distribution in middle-aged individuals from Indonesia and the Netherlands: a cross-sectional analysis of two population-based studies. Diabetol Metab Syndr. (2021) 12:2. doi: 10.1186/s13098-019-0503-1
9. Herningtyas EH, Ng TS. Prevalence and distribution of metabolic syndrome and its components among provinces and ethnic groups in Indonesia. BMC Public Health. (2019) 19:377. doi: 10.1186/s12889-019-6711-7
10. Scuteri A, Laurent S, Cucca F, Cockcroft J, Cunha PG, Mañas LR, et al. Metabolic syndrome across Europe: different clusters of risk factors. Eur J Prev Cardiol. (2015) 22:486–91. doi: 10.1177/2047487314525529
11. Montano D. Association between socioeconomic determinants and the metabolic syndrome in the German Health Interview and Examination Survey for Adults (DEGS1) - A mediation analysis. Rev Diabet Stud. (2017) 14:279–94. doi: 10.1900/RDS.2017.14.279
12. Shojaeimotlagh V, Hashiehbaf A, Karami M, Monjazebi F, Gheshlagh RG. Prevalence of metabolic syndrome in Iranian patients with schizophrenia: a systematic review and meta-analysis. Diabetes Metab Syndr. (2019) 13:143–7. doi: 10.1016/j.dsx.2018.08.014
13. Sahpolat M, Ari M. Higher prevalence of metabolic syndrome and related factors in patients with first-episode psychosis and schizophrenia: a cross-sectional study in Turkey. Nord J Psychiatry. (2021) 75:73–8. doi: 10.1080/08039488.2020.1815080
14. Sweileh WM, Zyoud SH, Dalal SA, Ibwini S, Sawalha AF, Ali I. Prevalence of metabolic syndrome among patients with schizophrenia in Palestine. BMC Psychiatry. (2012) 12:235. doi: 10.1186/1471-244X-12-235
15. Gutiérrez-Rojas L, Azanza JR, Bernardo M, Rojo L, Mesa F, Martínez-Ortega JM. Prevalencia del síndrome metabólico en pacientes españoles con esquizofrenia y sobrepeso. El estudio CRESSOB [Prevalence of metabolic syndrome in Spanish patients with schizophrenia and overweight. The CRESSOB Study]. Actas Esp Psiquiatr. (2014) 42:9–17. (in Spanish)
16. Lee J, Nurjono M, Wong A, Salim A. Prevalence of metabolic syndrome among patients with schizophrenia in Singapore. Ann Acad Med Singap. (2012) 41:457–62.
17. Ganesh S, Ashok AH, Kumar CN, Thirthalli J. Prevalence and determinants of metabolic syndrome in patients with schizophrenia: a systematic review and meta-analysis of Indian studies. Asian J Psychiatr. (2016) 22:86–92. doi: 10.1016/j.ajp.2016.05.006
18. Rawat VS, Ganesh S, Bijjal S, Shanivaram Reddy K, Agarwal V, Devi R, et al. Prevalence and predictors of metabolic syndrome in patients with schizophrenia and healthy controls: a study in rural South Indian population. Schizophr Res. (2018) 192:102–7. doi: 10.1016/j.schres.2017.04.039
19. Sugai T, Suzuki Y, Yamazaki M, Shimoda K, Mori T, Ozeki Y, et al. Difference in prevalence of metabolic syndrome between Japanese outpatients and inpatients with schizophrenia: a nationwide survey. Schizophr Res. (2016) 171:68–73. doi: 10.1016/j.schres.2016.01.016
20. Sugai T, Suzuki Y, Yamazaki M, Shimoda K, Mori T, Ozeki Y, et al. High prevalence of obesity, hypertension, hyperlipidemia, and diabetes mellitus in Japanese outpatients with schizophrenia: a Nationwide Survey. PLoS One. (2016) 11:e0166429. doi: 10.1371/journal.pone.0166429
21. Marthoenis M, Aichberger MC, Puteh I, Syahrial S, Schouler-Ocak M. Metabolic syndrome among psychiatric inpatients with schizophrenia in Indonesia. Asian J Psychiatr. (2015) 15:10–4. doi: 10.1016/j.ajp.2015.04.004
22. Yang CY, Lo SC, Peng YC. Prevalence and predictors of metabolic syndrome in people with schizophrenia in inpatient rehabilitation wards. Biol Res Nurs. (2016) 18:558–66. doi: 10.1177/1099800416653184
23. Sun MJ, Jang MH. Risk factors of metabolic syndrome in community-dwelling people with schizophrenia. Int J Environ Res Public Health. (2020) 17:6700. doi: 10.3390/ijerph17186700
24. Sugawara N, Yasui-Furukori N, Sato Y, Kishida I, Yamashita H, Saito M, et al. Comparison of prevalence of metabolic syndrome in hospital and community-based Japanese patients with schizophrenia. Ann Gen Psychiatry. (2011) 10:21. doi: 10.1186/1744-859X-10-21
25. Lasić D, Uglešić B, Vujnović Z, Krnić S. PAI-1 as a component of the metabolic syndrome in depression and schizophrenia - Croatian experience. Psychiatr Danub. (2015) 27:71–2.
26. Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. (2015) 14:339–47. doi: 10.1002/wps.20252
27. Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. (2020) 7:64–77. doi: 10.1016/S2215-0366(19)30416-X
28. Ijaz S, Bolea B, Davies S, Savović J, Richards A, Sullivan S, et al. Antipsychotic polypharmacy and metabolic syndrome in schizophrenia: a review of systematic reviews. BMC Psychiatry. (2018) 18:275. doi: 10.1186/s12888-018-1848-y
29. Aly El-Gabry DM, Abdel Aziz K, Okasha T, Azzam H, Okasha A. Antipsychotic polypharmacy and its relation to metabolic syndrome in patients with schizophrenia: an Egyptian Study. J Clin Psychopharmacol. (2018) 38:27–33. doi: 10.1097/JCP.0000000000000815
30. Softic R, Sutovic A, Avdibegovic E, Osmanović E, Bećirović E, Hajdukov MM. Metabolic syndrome in schizophrenia - who is more to blame: FGA polypharmacy or clozapine monotherapy? Psychiatr Danub. (2015) 27:378–84.
31. Misawa F, Shimizu K, Fujii Y, Miyata R, Koshiishi F, Kobayashi M, et al. Is antipsychotic polypharmacy associated with metabolic syndrome even after adjustment for lifestyle effects?: a cross-sectional study. BMC Psychiatry. (2011) 11:118. doi: 10.1186/1471-244X-11-118
32. Kay SR, Opler LA, Fiszbein A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
33. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. (2010) 67:255–62. doi: 10.1016/j.biopsych.2009.08.040
34. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
35. Lin YC, Lai CL, Chan HY. The association between rehabilitation programs and metabolic syndrome in chronic inpatients with schizophrenia. Psychiatry Res. (2018) 260:227–32. doi: 10.1016/j.psychres.2017.11.081
36. Moreira GC, Cipullo JP, Ciorlia LA, Cesarino CB, Vilela-Martin JF. Prevalence of metabolic syndrome: association with risk factors and cardiovascular complications in an urban population. PLoS One. (2014) 9:e105056. doi: 10.1371/journal.pone.0105056
37. Li Q, Chen D, Liu T, Walss-Bass C, de Quevedo JL, Soares JC, et al. Sex differences in body mass index and obesity in Chinese patients with chronic schizophrenia. J Clin Psychopharmacol. (2016) 36:643–48. doi: 10.1097/JCP.0000000000000594
38. Ho C, Zhang M, Mak A, Ho R. Metabolic syndrome in psychiatry: advances in understanding and management. Adv Psychiatr Treat. (2014) 20:101–12. doi: 10.1192/apt.bp.113.011619
39. Kornetova EG, Kornetov AN, Mednova IA, Dubrovskaya VV, Boiko AS, Bokhan NA, et al. Changes in body fat and related biochemical parameters associated with atypical antipsychotic drug treatment in schizophrenia patients with or without metabolic syndrome. Front Psychiatry. (2019) 10:803. doi: 10.3389/fpsyt.2019.00803
40. Correll CU, Rummel-Kluge C, Corves C, Kane JM, Leucht S. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. (2009) 35:443–57. doi: 10.1093/schbul/sbn018
41. Fleischhacker WW, Heikkinen ME, Olié JP, Landsberg W, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. (2010) 13:1115–25. doi: 10.1017/S1461145710000490
42. Riordan HJ, Antonini P, Murphy MF. Atypical antipsychotics and metabolic syndrome in patients with schizophrenia: risk factors, monitoring, and healthcare implications. Am Health Drug Benefits. (2011) 4:292–302.
43. Coccurello R, Moles A. Potential mechanisms of atypical antipsychotic-induced metabolic derangement: clues for understanding obesity and novel drug design. Pharmacol Ther. (2010) 127:210–51. doi: 10.1016/j.pharmthera.2010.04.008
44. Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry. (2017) 16:308–15. doi: 10.1002/wps.20458
45. Kornetova EG, Dubrovskaya VV, Kornetov AN, Lobacheva OA, Ivanova SA, Semke AV. Morphophenotypic predictor of the development of visceral obesity in patients with schizophrenia receiving antipsychotic therapy. Bull Sib Med. (2018) 17:54–64. doi: 10.20538/1682-0363-2018-4-54-64 (In Russian).
46. Anjum S, Bathla M, Panchal S, Singh GP, Singh M. Metabolic syndrome in drug naïve schizophrenic patients. Diabetes Metab Syndr. (2018) 12:135–40. doi: 10.1016/j.dsx.2017.11.006
47. Bora K, Pathak MS, Borah P, Das D. Variation in lipid profile across different patterns of obesity - observations from Guwahati, Assam. J Clin Diagn Res. (2015) 9:OC17–21. doi: 10.7860/JCDR/2015/15334.6787
48. Chen SF, Hu TM, Lan TH, Chiu HJ, Sheen LY, Loh EW. Severity of psychosis syndrome and change of metabolic abnormality in chronic schizophrenia patients: severe negative syndrome may be related to a distinct lipid pathophysiology. Eur Psychiatry. (2014) 29:167–71. doi: 10.1016/j.eurpsy.2013.04.003
49. Lally J, Gallagher A, Bainbridge E, Avalos G, Ahmed M, McDonald C. Increases in triglyceride levels are associated with clinical response to clozapine treatment. J Psychopharmacol. (2013) 27:401–3. doi: 10.1177/0269881112472568
50. Heald A, Pendlebury J, Anderson S, Narayan V, Guy M, Gibson M, et al. Lifestyle factors and the metabolic syndrome in Schizophrenia: a cross-sectional study. Ann Gen Psychiatry. (2017) 16:12. doi: 10.1186/s12991-017-0134-6
51. Teasdale SB, Ward PB, Samaras K, Firth J, Stubbs B, Tripodi E, et al. Dietary intake of people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. (2019) 214:251–59. doi: 10.1192/bjp.2019.20
52. Tzeng WC, Chiang YS, Feng HP, Chien WC, Tai YM, Chen MJ. Gender differences in metabolic syndrome risk factors among patients with serious mental illness. Int J Ment Health Nurs. (2020) 29:254–65. doi: 10.1111/inm.12670
53. Malan-Müller S, Kilian S, van den Heuvel LL, Bardien S, Asmal L, Warnich L, et al. A systematic review of genetic variants associated with metabolic syndrome in patients with schizophrenia. Schizophr Res. (2016) 170:1–17. doi: 10.1016/j.schres.2015.11.011
Keywords: schizophrenia, psychosis, antipsychotics, metabolic syndrome, pathology
Citation: Kornetova EG, Kornetov AN, Mednova IA, Goncharova AA, Gerasimova VI, Pozhidaev IV, Boiko AS, Semke AV, Loonen AJM, Bokhan NA and Ivanova SA (2021) Comparative Characteristics of the Metabolic Syndrome Prevalence in Patients With Schizophrenia in Three Western Siberia Psychiatric Hospitals. Front. Psychiatry 12:661174. doi: 10.3389/fpsyt.2021.661174
Received: 03 February 2021; Accepted: 09 June 2021;
Published: 02 July 2021.
Edited by:
Felice Iasevoli, University of Naples Federico II, ItalyReviewed by:
Sarah Tosato, University of Verona, ItalyMassimo Tusconi, University of Cagliari, Italy
Stefano Barlati, University of Brescia, Italy
Copyright © 2021 Kornetova, Kornetov, Mednova, Goncharova, Gerasimova, Pozhidaev, Boiko, Semke, Loonen, Bokhan and Ivanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena G. Kornetova, a29ybmV0b3ZhQHNpYm1haWwuY29t
 Elena G. Kornetova
Elena G. Kornetova Alexander N. Kornetov
Alexander N. Kornetov Irina A. Mednova
Irina A. Mednova Anastasia A. Goncharova1
Anastasia A. Goncharova1 Valeria I. Gerasimova
Valeria I. Gerasimova Ivan V. Pozhidaev
Ivan V. Pozhidaev Anastasiia S. Boiko
Anastasiia S. Boiko Anton J. M. Loonen
Anton J. M. Loonen Nikolay A. Bokhan
Nikolay A. Bokhan Svetlana A. Ivanova
Svetlana A. Ivanova