
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 01 July 2021
Sec. Psychological Therapy and Psychosomatics
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.658416
This article is part of the Research Topic Recent Advances in Diagnosis and Treatment of Comorbid Conditions in Eating Disorders View all 8 articles
Parts of this article's content have been modified or rectified in:
Erratum: Is the Severity of the Clinical Expression of Anorexia Nervosa Influenced by an Anxiety, Depressive, or Obsessive-Compulsive Comorbidity Over a Lifetime?
 Elise Riquin1,2,3†
Elise Riquin1,2,3† Agathe Raynal4†
Agathe Raynal4† Lama Mattar5
Lama Mattar5 Christophe Lalanne6
Christophe Lalanne6 France Hirot7,8
France Hirot7,8 Caroline Huas7,9
Caroline Huas7,9 Jeanne Duclos10,11
Jeanne Duclos10,11 EVHAN group
EVHAN group Sylvie Berthoz8,12‡
Sylvie Berthoz8,12‡ Nathalie Godart7,8,9,13*‡
Nathalie Godart7,8,9,13*‡Purpose: The relationship between anxiety or depressive comorbidities, their chronology of onset, and the severity of anorexia nervosa (AN) is not well-studied. We hypothesize that the existence of a comorbidity, particularly before the onset of AN, is associated with greater severity of AN.
Methods: One hundred seventy-seven subjects were assessed. The prevalence of major depressive disorder (MDD), obsessive-compulsive disorder (OCD), generalized anxiety disorder (GAD), and social phobia (SP) as well as their chronology of onset were studied. The assessment criteria of AN severity were the overall clinical condition, body mass index (BMI) on admission, lowest BMI, intensity of the eating symptoms, age at the onset of AN, illness duration, number of hospitalizations, and quality of life.
Results: Patients with AN had the greatest clinical severity when they had a comorbid disorder over their lifetime, such as MDD, GAD, or SP. These comorbidities along with OCD were associated with a higher level of eating symptoms and a more altered quality of life. A profile of maximum severity was associated with a higher prevalence of MDD and GAD. Concerning the chronology of onset, the age at the start of AN was later in cases of MDD or GAD prior to AN.
Conclusion: There seems to be an association between severity of AN and both MDD and GAD. The chronology of onset of the comorbidity did not seem to be associated with the severity.
Anorexia nervosa (AN) is a condition that involves restriction of calorie intake, leading to a significantly low weight, intense fear of gaining weight, and dysmorphophobia (DSM-5) (1). Its lifetime prevalence is estimated between 0.9 and 2.2% (2). It is a particularly severe condition (3). In fact, half of people treated for AN in adolescence recover from this disorder, but they can have other psychiatric disorders as 21% have chronic eating disorders (EDs) and 5% die (4). Of the deaths, around half are due to somatic complications and half to suicide. Over an entire lifetime, AN is associated with psychiatric comorbidities, such as major depressive disorder (MDD) in up to 64% of cases with at least one anxiety disorder in up to 72% of cases and with obsessive-compulsive disorder (OCD) in up to 62% of cases (5). The etiopathogenesis of AN remains poorly understood; the trajectories of vulnerability and clinical expression are heterogeneous (6, 7). Better characterization of the phenotypic subgroups of patients could clarify the etiopathogenesis and also differentiate the therapeutic strategies (8).
AN can be described by different degrees of clinical severity based on the overall clinical condition (divided by intensity of the malnutrition and severity of the ED) and its consequences (impact on social adaptation, illness duration, response to care) (9). These degrees of severity are also found within the forms requiring hospitalization. The intensity of the clinical severity in AN was shown to be prognostic of the outcome (10). Thus, the factors for a poor prognosis are a long illness duration, a premorbid developmental abnormality (including premorbid OCD), obsessive-compulsive personality disorder, the intensity of malnutrition over the course of progression, the subsequent occurrence of bulimia, and the use of purgatives (11).
The relationship between anxiety-depressive (AD) comorbidity and prognosis has been studied. Some authors show that AD comorbidity could have a negative impact on the overall outcome and the body mass index (BMI) of patients, and others have find the opposite (12). The relationship between AD comorbidity and the severity of clinical expression of AN in terms of overall clinical condition or eating symptoms has been studied very little. The only data in the literature show that psychiatric comorbidities (including AD disorders, OCD, and personality disorders) are associated more with re-hospitalizations (13). Furthermore, concomitant depression increases the suicidal risk in AN (14): premorbid OCD contributes to poor prognosis; social phobia and agoraphobia affect the subjects' quality of life (QOL) and increase ED symptoms 6–12 years after inpatient treatment (15). However, to our knowledge, no studies to date consider the relationship between the severity of current AN and the lifetime existence of psychiatric comorbidities.
None of the previously cited studies concerning the relationship between AD comorbidity and clinical severity of AN in the acute phase of the disorder took into account the relative chronology of AN and AD onset. Chronology is a potential contributory factor for explaining the different profiles of progression in AN. In fact, we were able to show that although the comorbidity of OCD over a lifetime in AN is not associated with a particular outcome, the existence of premorbid OCD before AN is a poor prognostic factor for the future. This did not hold true for MDD, whether it was preexisting or on a lifetime scale (15).
As a result, we conducted this study to test the hypothesis that the existence of an AD or OCD comorbidity during a lifetime, particularly when it started before the AN, is associated with a more severe overall clinical expression of AN in the acute phase of the disorder, including in each area of clinical expression (nutritional state, age at the start of AN, illness duration, number of hospitalizations, eating symptoms, and QOL).
This study was part of a larger longitudinal multicentered study named EVHAN (Evaluation of Hospitalization for AN, Eudract number: 2007-A01110-53, registered in Clinical trials) between April 2009 and May 2011. The study protocol was approved by the Ile-de-France III Ethics Committee and the CNIL (Commission nationale de l'informatique et des libertés).
Inclusion criteria for the current study were as follows: being hospitalized for AN, admission BMI <15 and/or sudden and rapid weight loss, agreement to participate in the study, and being affiliated to the French Social Security health coverage system. Exclusion criteria were refusal to participate, insufficient command of the French language, existence of a potentially confounding pathology (e.g., diabetes, Crohn's disease, or other metabolic disorders), and being under the age of 13.
In accordance with the Helsinki declaration, written informed consent was obtained from each patient before inclusion and from the parents of those who were under 18 years old. Patients (if adults) or, in the case of children, both patients and their parents gave their written consent.
This study is part of a larger longitudinal multicentered study named EVHAN (Evaluation of Hospitalization for AN, Eudract number: 2007-A01110-53, registered in Clinical trials). The study protocol was approved by the Ile-de-France III Ethics Committee and the CNIL (Commission nationale de l'informatique et des libertés). In accordance with the Helsinki declaration, written informed consent was obtained from each patient before inclusion and also from the parents for those who were under 18 years old.
Sample patients were recruited from the inpatient treatment facilities of 11 centers in France (CHU-Bordeaux, Cochin—Maison des Adolescents, Institut Mutualiste Montsouris, MGEN—La Verrière, CHU-Nantes, CHU-Rouen, Robert Debre Hospital, Sainte-Anne Hospital, Saint-Etienne Hospital, Villejuif—Paul Brousse). Three hundred thirty-three consecutive patients with AN were involved in the EVHAN study between April 2009 and May 2011 (Figure 1). Inclusion criteria for the current study were as follows: being hospitalized for AN, admission BMI <15 and/or sudden and rapid weight loss, agreement to participate in the study, and being affiliated to the French Social Security health coverage system. Exclusion criteria were refusal to participate, insufficient command of the French language, existence of a potentially confounding pathology (e.g., diabetes, Crohn's disease, or other metabolic disorders), and being under the age of 13. In all, therefore, 222 patients (with full syndrome or subthreshold AN) were included in this study.
A current diagnosis of AN was based on the DSM-IV-TR criteria (16). These criteria were assessed using the Eating Disorder Examination (EDE-Q v. 5.2) and the Composite International Diagnostic Interview Short Form (CIDI 3.0) (17–19) (Kessler). The CIDI Short Form is an easy-to-use tool derived from the CIDI (17, 20) and has been widely used ever since for epidemiological studies in Canada (21–23) and Europe (24–27) and compared with the CIDI (18, 23). It consists of a structured interview developed under the aegis of the World Health Organization (WHO) for epidemiological and clinical research purposes.
The BMI criterion was <10th percentile up to 17 years of age and BMI <17.5 for 17 years of age and above (28). After the exclusion of patients for whom response to the short CIDI were missing, the sample size was 177 patients. Participants were assessed during the first 2 weeks of hospitalization.
The data were collected during the patient's hospitalization for AN. Each patient was selected by consecutive enrollment. Sociodemographic data and medical antecedents were collected, including age, age at AN onset, illness duration, number of hospitalizations for an ED, and duration of hospitalization. Nutritional status was assessed using the current BMI and the lowest BMI at the onset of the disorders.
Lifetime comorbidities (both current and/or past) were evaluated by questionnaires as follows:
The diagnoses of MDD, OCD, generalized anxiety disorder (GAD), and social phobia were made using the Composite International Diagnostic Interview Short Form (CIDI-SF) (18). Likewise, the chronology of onset was evaluated from the ages at AN onset, and each comorbidity was recorded with the CIDI-SF. The chronology of each onset was classified as follows: No comorbidity, comorbidity before AN onset, and comorbidity concomitant to or after AN onset.
The overall clinical condition was evaluated by the Global Outcome Assessment Scale (GOAS) by Morgan–Russell, a proxy questionnaire that assesses functioning over the past 6 months on five subscales exploring food, menstrual periods, mental state, psychosexual functioning, and socioeconomic status (28). It provides a score from 0 to 12. Lower scores indicate poorer clinical condition.
The severity of the ED symptoms was evaluated by the Eating Attitudes Test (EAT) (29, 30), which is a six-point format, self-report scale measuring a broad range of ED symptoms. It includes 26 items with three subscales: dieting, bulimia, and food preoccupation and oral control. It can range from 0 to 78 (maximum of symptoms) (29). Higher scores reflect a greater severity of ED symptoms.
QOL was assessed using the Eating Disorders Quality of Life (EDQOL), which is a 25-item self-reporting questionnaire, using four subscales (psychological, physical/cognitive, financial, professional/academic) (31). Possible ranges for each subscale and the total score are zero to four. Lower scores indicate a better QOL.
The data were processed on SPSS Statistics 19 package. A description of the sample was made using means, standard deviations, minimum, and maximum in the case of numerical variables and frequencies in cases of categorical variables. Chi-squared or Fisher exact tests were used for the categorical variables (i.e., comparison of prevalence between groups). The study of the relationship between AD comorbidity (categorical variable) and the continuous variables (overall clinical condition [GOAS], BMI, lowest BMI since the start of the disorder, intensity of the eating symptoms [EAT], age at AN onset, illness duration, number of hospitalizations and QOL [EDQOL]) was performed through comparison tests of means using Student's t-test. The study of relationships between the chronology of onset of the comorbidities in three classes (prior to AN, concomitant to or after AN, and absence) and the continuous variables was performed using ANOVA and then Student's t-test between groups when appropriate. The study of the relationships between comorbidities and their chronology of onset was carried out using Pearson's chi-squared test. Cluster analysis is a useful tool in the identification of subtypes. However, the selection of variables should be guided by a clearly stated theoretical framework in order to guide the interpretation of the results. According to the unsupervised approach, we primarily used an agglomerative (hierarchical) clustering procedure to partition the data set into subgroups whose characteristics might well-describe the typology observed through clinical inspection of the subjects. Hierarchical clustering using Ward's criterion and squared Euclidean distances were used to isolate homogeneous clusters of patients in order to delineate severity profiles based on the following variables: the overall psychosocial state of the subjects, assessed by five subscales of the GOAS (food, menstrual periods, mental state, psychosexual functioning, and socioeconomic status); symptoms of the eating disorder, assessed through three subscales of EAT (restriction, bulimia, and control); nutritional status, assessed through BMI on admission and minimum BMI; history of development of the disorder, assessed through age at AN onset, the illness duration, the number of hospitalizations; and their QOL, assessed through four subscales of the EDQOL (psychological, physical/cognitive, financial, professional/academic).
The study of the relationships between these various clusters and the presence or absence of an AD comorbidity and their chronology of onset was performed using Pearson's chi-squared tests.
The severity profiles were analyzed based on dendrograms derived from hierarchical clustering with a varying number of cluster solutions (two to six). Several solutions were then examined with the population of our sample combined into two to six clusters. Only the solutions with two and three clusters could be interpreted in terms of distribution of sample numbers and profile of means and were, therefore, selected and presented here.
A p < 0.05 was considered significant.
The sample included in this study comprises 177 patients (Figure 1) (for description, see Table 1); 84 (47.5%) of them had AN restrictive type (AN-R), and 93 (52.5%) had AN binge eating/purging type (AN-BP). The sample was 96% female (170 women and seven men). The general condition of the patients was very severe as reflected in the total GOAS score, the mean of which was 4.8 (SD: 1.4). The subscales for the eating symptoms (A) and menstrual periods (B) had lower means with, respectively, 1.41 (SD: 1.7) and 2.5 (SD: 4.25), reflecting eating symptoms among the most severe and nearly constant amenorrhea. The mean for the mental state was 5.8 (SD: 1.7). The dimensions of psychosexual functioning (D) and economic status (E) had means, respectively, of 7.0 (SD: 2) and 7.0 (SD: 2.2). The mean score of the EAT questionnaire was 35.1 (SD: 16.8). Last, the EDQOL score was low with a mean score of 2.3 (SD: 0.6).
More than half of the sample had experienced MDD over their lifetime, close to one third had had GAD or social phobia, and more than one quarter OCD. According to PTSD symptoms, 13 patients (7.3%) had this diagnosis. We had no information on age of onset or end. Concerning the concomitant comorbidities of the ED, more than one third of patients had experienced GAD, about one quarter MDD, and social phobia and one fifth OCD. The results are detailed in Table 2.
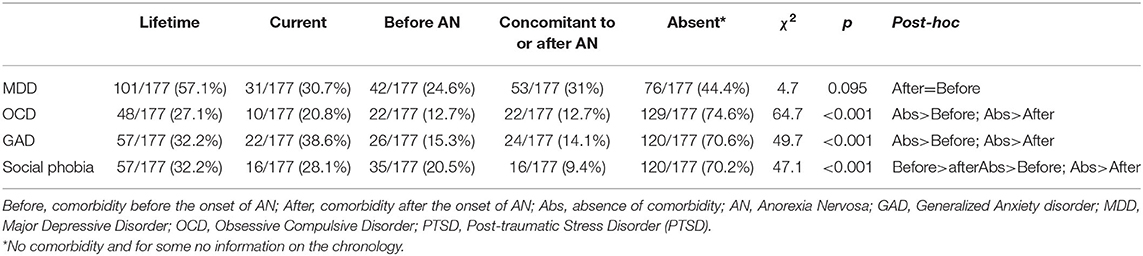
Table 2. Psychiatric disorder comorbidities over a lifetime: prevalence and chronology of onset (n = 177).
OCD and GAD, when they existed, occurred equally before and after AN (p < 0.0001). Social phobia, however, occurred more often before AN (p < 0.0001). There was no significant difference in the chronology of onset of MDD.
The results are described in the Table 3. When patients had experienced a comorbidity over their lifetime in terms of MDD, GAD, or social phobia, the clinical severity of the AN as measured by the GOAS score was significantly greater than in the case of no comorbidity at all (respectively, p < 0.0001, p = 0.004, p = 0.03). The difference was not significant for OCD.
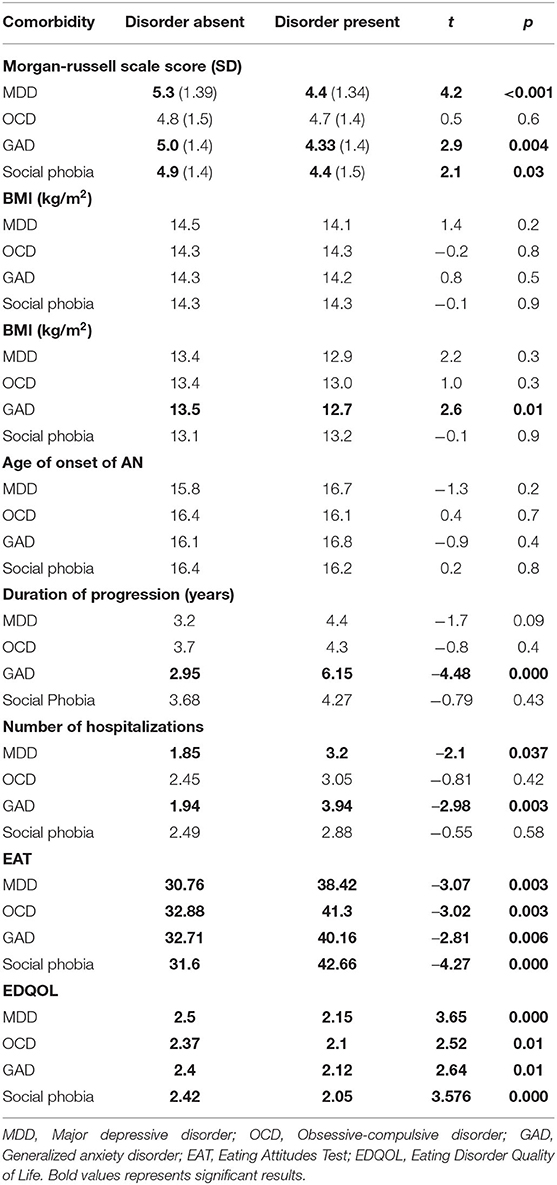
Table 3. Comparison of average scores on clinical condition assessment scales according to the presence or absence of a comorbidity (per Student's t-test).
All the studied comorbidities were significantly associated with a higher mean level of eating symptoms (EAT score) and a more altered QOL related to the ED (EDQOL score). GAD was associated with a significantly longer illness duration (p < 0.0001), more frequent hospitalizations before the current assessment (p = 0.003), and a poorer nutritional state over the course of the lifetime (minimum BMI) (p = 0.01). MDD was associated with more frequent hospitalizations (p = 0.037). BMI on admission and the age at AN onset did not differ significantly based on the existence of a comorbidity.
The results are described in the Supplementary Table A. We found very few differences between the groups of patients with comorbid disorders prior to AN and those with later comorbid disorders. Most of the observed differences were between the groups without comorbidities and the groups with comorbidities before or after, which confirms the results shown in the preceding paragraph for both of these groups.
The chronology of onset of disorders only shows a significant difference for the age of AN onset, which was later in cases of MDD (17.9 years vs. 15.2 years, p = 0.009) and GAD (18.0 years vs. 14.3 years, p = 0.009) before AN.
Considering the sample size of PTSD and the absence of chronology data, no subsequent analyses were carried out.
Table 4 shows the results of applying agglomerative clustering based on squared Euclidean distance with Ward criteria. As can be seen from the dendrogram, a solution consisting of three clusters with N = 88, 31, and 24 subjects seems to be an appropriate way to represent our results. The clusters are described in Figure 2.
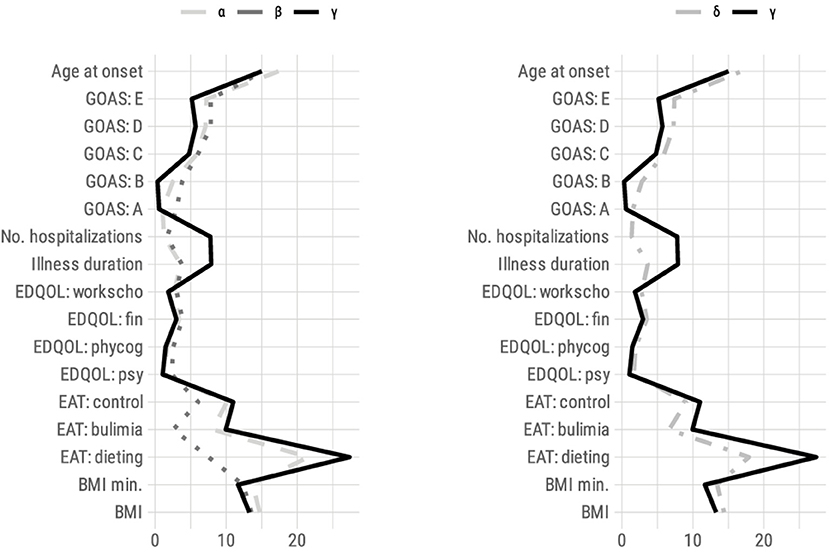
Figure 2. Profile of responses (mean) for all variables used in hierarchical clustering for each cluster solution (left: three clusters; right: two clusters). BMI, Body Mass Index; EAT, Eating Attitudes Test; EDQOL, Eating Disorder Quality of Life; psy, psychological; phy cog, physical/cognitive; end: financial; work scho, professional/academic; No: number; GOAS, Global Outcome Assessment Scale by Morgan-Russell.
The results are described in the Table 4. The γ cluster had the greatest severity profile of the three. It grouped patients that had average subscores on the GOAS supporting a more severe clinical expression of AN. As such, the eating dimension (A) was significantly affected; menstrual periods were the most altered (B); and the impact was most serious on the patient's mental state (C), psychosexual functioning (D), and socioeconomic status (E). The other assessment criteria also favored a more severe clinical expression of AN because this cluster was associated with the poorest nutritional state (BMI on admission and minimum BMI), the youngest age of AN onset, and the longest illness duration of the disorder, and the number of hospitalizations. The eating symptoms measured by the EAT scale were greater in the three subscales. The QOL was lower in all dimensions (EDQOL).
The α and β clusters had a more moderate severity profile than γ on all the variables. The α had variables that were mostly closer to β than γ with a slightly greater severity than β. However, α and β differed along two lines.
The α cluster showed a better nutritional status (higher BMI and minimum BMI) than the β and γ clusters. The age at onset was 2 years older on average; the illness duration and the number of hospitalizations were slightly lower. Conversely, the ED symptoms were very severe, close to those of the γ cluster (EAT score and GOAS subscore A).
The β cluster had a more severe nutritional impact (as measured by BMI and minimum BMI) than the α cluster. This can be explained by the fact that the α cluster assembled a significant majority of binge-purging type patients (AN-BP = 52, i.e., 59.1%), who normally have a better nutritional status and greater eating symptoms; conversely, the β cluster accounted for more restrictive type patients (AN-R = 23, i.e., 74.2%; χ2 = 10.2, p = 0.001). In addition, the β-cluster patients had a mean maximum BMI that was the lowest of the three groups (max BMI = 18.5 kg/m2) and those of the α cluster had the highest (max BMI = 20.9 kg/m2). In the β cluster, the illness duration and the number of hospitalizations were slightly higher. The age of onset was close to that of the γ cluster. Therefore, the severity seemed gradual: moderate for the α cluster (with the exception of the food symptoms) and β cluster (with the exception of the nutritional status), increased for all the criteria of the γ cluster. There was no significant difference in weight loss between the three clusters (maximum BMI over the lifetime minus admission BMI).
The prevalence of MDD and GAD comorbidities differed significantly according to the clusters. For these two disorders, the γ cluster was associated with more frequent comorbidities compared with the β cluster (75% MDD over the lifetime for patients of the γ cluster vs. 35.7% for the β cluster; OR = 5.4 [1.5–19.3]; 65% GAD over the lifetime for patients of the γ cluster vs. 17.9% for the β cluster). In addition, the prevalence of GAD was greater compared with the α cluster (α = 24.7%, OR = 5.7 [2.0–16.4]; β = 17.9% and γ = 65.0%, OR = 8.5 [2.3–32.4]). The prevalence distribution of OCD and social phobia was not significantly different between the clusters (Supplementary Table B).
When the sample was classified into two clusters, the α and β clusters were assembled to form the δ cluster. The γ cluster had more severe characteristics in all areas than the δ cluster (see Table 4).
MDD and GAD in a lifetime were associated significantly more frequently with a profile of major severity (respectively, p = 0.03 and p < 0.001) (γ cluster). This same tendency was also noted for social phobia (p = 0.063) (Table 5).
Comparison of severity profiles based on the chronology of onset of the comorbidities or their absence (Supplementary Table C).
The γ cluster had GAD associated with concomitant or later appearance relative to AN that was significantly different from the δ cluster.
The aim of this study was to investigate if the existence of an AD disorder comorbidity over a lifetime, especially when it started before AN, is associated with a more severe clinical expression of AN in the acute phase of the disorder.
Our main results confirm the first part of our initial hypothesis, namely, that patients with AN in the acute phase had greater overall severity (GOAS) when they experienced a comorbid disorder of MDD, GAD, or social phobia in their lifetime. When we tested the relationship between these three severity profiles and the comorbidities, we noticed that they differed for the prevalence of GAD and MDD. More specifically, the profile of maximum severity (γ) was associated with a significantly higher prevalence of MDD and GAD than one of the two profiles of lesser severity (β cluster). This later had the distinction of a more severe nutritional profile, with a major proportion of AN-R compared with the other (α). The solution in 2 clusters maintained this same difference (between γ and the δ cluster, which grouped together the two other profiles α and β).
On the other hand, the chronology of onset of the comorbidity or its absence was usually not associated with greater severity of AN regardless of the comorbid disorder. Most of the observed differences contrasted the absence of comorbidity against comorbidity before and/or after AN. The only differences observed were with MDD or GAD in which the age of onset of AN was later in the case of a comorbidity prior to AN compared with a later onset.
There is some evidence that emotional dysregulation, including anxiety and depression, are associated with poorer decision making in AN (32, 33). According to Bechara, decision making inherently relies on emotional processes that provide important implicit and explicit knowledge by which the individual is able to make fast and adaptive decisions (34). Van Elburg explains that “these emotional processes guide decision-making on several levels, including via bioregulatory processes, such as somatic marker signals, and occur both consciously and outside of awareness” (35). The literature highlights that an “affect-driven belief system profoundly influences the transformation of action into choices” and proposes that affect in particular plays a role in decision making that involves uncertainty, which is similar to the type of decision making mostly studied in AN (32, 35, 36). The severity of ED symptoms that we observed in conjunction with AD comorbidities could be explained by the link between anxiety and depression with poorer food decision making in AN.
The population of our study had frequent comorbidities (over a lifetime and concomitant), which is in accordance with the data in the literature. In our sample, the prevalence of MDD over the entire life was 57% (37) and that of GAD was 32.2% (5). This is considerable for a sample with a mean age of 20 years as the mean age of onset of GAD in the general population is 25 years (38). The prevalence of OCD varies in the literature between 10 and 62% (5, 39, 40); our sample falls within this interval (27%). The literature found a prevalence equivalent to that in our sample for social phobia, 32.2% (5, 37).
In addition, the patients had major severity criteria in all aspects: overall condition (GOAS), eating symptoms (EAT), mean BMI, and a very altered QOL. This homogeneity is associated with the sample under consideration; it is a sample of hospitalized patients except that the admission criteria are generally based on a highly deteriorated clinical state (41).
Our univariate analyses showed that, when patients experienced a comorbidity of MDD, GAD, or social phobia in their lifetime, the clinical severity of AN, in terms of general clinical condition, eating symptoms, and QOL was significantly greater than in the absence of these comorbid disorders. As the prevalence of OCD was lowest in the sample, the sample sizes were limited, and our analyses lacked power for this disorder; this might explain why no significant difference was found on the GOAS for OCD although the results went in this direction. As cited above, we studied AN in general without differentiating the restrictive (AN-R) subgroups or those with binge eating, vomiting, or use of purgatives (AN-BP) although several studies associate a higher prevalence of OCD with the restrictive subtype (38–40).
With regard to the severity of the disorder in terms of continuum of care, comorbid GAD was associated with both an average illness duration that was two times longer than for patients without a disorder (2.9 vs. 6.2 years) and more frequent hospitalizations. The duration of progression in the group with GAD is considerable. The average illness duration in a clinical population is 4 years (10). These hospitalizations and the duration of disorders considerably affect the subject's life, contribute to their social isolation, and create a real vicious circle, which perpetuates the disorder and increases complications (42). GAD was also associated with a poorer nutritional state over the lifetime (minimum BMI), and a minimum BMI in the course of life that is very low (<13) is a factor of excess mortality (43). The BMI on admission did not vary significantly based on the existence of a comorbidity as certain authors have already noted (44, 45).
When the relationship between the severity profiles established from AN assessment components is studied, the cluster of extreme severity (γ) was associated with a prevalence of MDD that was 5.4 times higher in comparison with β, and seven and 8.5 times higher for GAD over a lifetime in comparison with the less severe α and β profiles, respectively. The distribution of frequencies of OCD and social phobia did not differ significantly among the clusters.
Our hypothesis, which was that AN would be more severe when the comorbid disorders occurred before rather than after or were non-existent, was only verified for MDD or GAD. In univariate analyses, the age of onset of AN was higher when MDD and GAD occurred before AN. The other severity indices did not differ based on the chronology or absence of a disorder. A later age of onset was described as a poor outcome factor in terms of mortality (4, 11, 46). When the ages of onset of MDD and GAD were studied, they were found to be significantly lower in patients whose disorder started before the AN (MDD-before = 13.8 years and GAD-before = 11.3 years) compared with those whose disorder started concomitantly or after the AN (MDD-after = 16.5 years and GAD-after = 16.3 years). The age of onset of disorders before the ED were very early compared with the typical age of onset of these disorders (around 25 years for both of them) (38); this is in accordance with the literature, which shows that anxiety disorders in childhood are known risk factors for the development of EDs (47).
One of the study limitations is the estimation of the onset and the illness duration (AN and comorbid disorders), which was undertaken retrospectively. The chronology of onset is calculated from these data and is, therefore, imprecise, especially because the diagnoses of proven comorbidity, remission, and recovery include a time dimension (for example, MDD is considered cured after a complete remission phase of 4–6 months).
We performed many statistical comparisons owing to the diversity of the chosen assessment parameters. AN is a disorder with a complex clinical expression; assessing its severity can be performed using certain methods. The DSM-5 proposes graduating the severity only according to the BMI for adults or the percentile of BMI for children and adolescents. Based on this criterion, our sample is in the extreme category because the mean BMI was below 15 (14.3; SD: 1.5). As remarked by Machado et al. (48), this single criterion is not a good reflection of the overall severity of AN because it does not take into account the pathophysiology of the specific expression of symptoms of the ED, its developmental profile or its impact on daily life. We, therefore, explored all these dimensions in this study. Despite the large number of statistical tests performed, however, we have not made any correction on the comparisons. As recommended by Bender and Lange (49), we did not suggest adjusting the alpha level (multiple comparisons) because this study was an exploratory step; its design was not intended to test causal hypotheses. Recruitment took place in second- or third-line centers after failure of management. This explains the long duration of development as well as the number of hospitalizations observed. Our population was, therefore, not comparable to all anorexic patients, which limits the generalization of our results to less severe patients. Despite this selection, however, we were able to demonstrate severity profiles in the subjects related to the comorbidity.
All of these factors support our understanding that greater severity of the disorder on admission is associated with an increased prevalence of MDD and GAD. This severity is a risk factor for chronicity, somatic, and psychiatric morbidity and mortality (4). Huas et al. (50) have already found some of our criteria of severity to be factors associated with excess mortality, including an older age, long progression of the disorder, and more severe eating symptoms. As a result, it is important to look for a history of MDD or GAD at the hospital admission in the most severe patients. Early treatment of these disorders during hospitalization, particularly with psychotropics, could perhaps facilitate an improvement in their prognosis (5, 51). This early treatment is currently not put into practice because the present guidelines (41) recommend refeeding before introducing treatments. Indeed, an aspect of AD symptoms relates to malnutrition and its treatment through refeeding (12). This phenomenon has led some authors to suggest that anxiety disorders during AN are only “artifacts” related to malnutrition. Furthermore, these recommendations are also based on the fact that antidepressant treatments have not been proven to be effective on AN or on the occurrence of depression (52). However, no therapeutic trial has yet evaluated the impact of early treatment and targeting MDD and GAD in cases of severe AN. This could be of interest to the extent that these comorbid psychiatric disorders are linked to clinical severity, and they impact the duration of hospitalization and probably the subsequent prognosis in terms of morbidity and mortality given the associated clinical severity (53).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ile-de-France III Ethics Committee and the CNIL (Commission nationale de l'informatique et des libertés). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. In accordance with the Helsinki declaration, written informed consent was obtained from each patient before inclusion, and from the parents of those who were under 18 years old. Both classes of patient (either adults or children and their parents) gave written consent.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
AR and NG: design of the study. ER, NG, LM, SB, CH, FH, CL, and JD: revision of the manuscript. ER, AR, NG, LM, SB, CH, FH, and CL: article writing and revising. AR and NG: data analysis. All authors approved the submitted version.
EVHAN study was supported by grants from the French Ministry of Health (PHRCN 2007, 2011 AOM11197; ANR Jeune chercheur, Bourse de thèse Eiffel) and from CNAM-TS, Fondation de France, Fondation MGEN, EHESP, AP-HP, CIFRE, and Contrats d'interface INSERM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to acknowledge all the participants for their assistance in conducting the study.
Nathalie Godart1,2, Sylvie Berthoz1,2, Christophe Lalanne2, Jeanne Duclos1,2,3, Lama Mattar1,2, Hélène Roux1,2, Marie Raphaële Thiébaud1,2, Sarah Vibert1,2, Tamara Hubert2, Annaig Courty1,4, Damien Ringuenet4, Jean-pierre Benoit1,5, Corinne Blanchet1,5, Marie Rose Moro1,5, Laura Bignami6, Clémentine Nordon6, Frédéric Rouillon6,7, Solange Cook8, Catherine Doyen6,8, Marie-Christine Mouren Siméoni6, Priscille Gerardin9, Sylvie Lebecq9, Marc-Antoine Podlipski9, Claire Gayet9, Malaika Lasfar9, Marc Delorme10, Xavier Pommereau10, Stéphanie Bioulac10,11, Manuel Bouvard10,12, Jennifer Carrere10, Karine Doncieux13, Sophie Faucher13, Catherine Fayollet13, Amélie Prexl13, Stéphane Billard14,15, François Lang14,15, Virginie Mourier-Soleillant14, Régine Greiner14, Aurélia Gay14,15, Guy Carrot14,15, Sylvain Lambert16, Morgane Rousselet16,17, Ludovic Placé16,17, Jean-luc Venisse16,17, Marie Bronnec16,17, Bruno Falissard18, Christophe Genolini19, Christine Hassler18, Jean-Marc Tréluyer20, Olivier Chacornac20, Maryline Delattre20, Nellie Moulopo20, Christelle Turuban20 and Christelle Auger20. Lead author of the EVHAN Group: Nathalie Godart (bmF0aGFsaWUuZ29kYXJ0QGZzZWYubmV0)
1CESP, INSERM, University Paris-Sud, UVSQ, University Paris-Saclay, Paris, France
2Institut Mutualiste Montsouris, Paris, France
3University of Reims, EA 6291, Reims, France
4Hospital Paul Brousse, AP-HP, Villejuif, France
5Maison de Solenn, Hospital Cochin, AP-HP, Paris, France
6CMME, Saint Anne Hospital, Paris, France
7INSERM Center 894, Paris, France
8Hospital Robert Debré, AP-HP, Paris, France
9University Hospital of Rouen, France
10University Hospital of Bordeaux, Bordeaux, France
11USR CNRS 3413 SANPSY, Bordeaux, France
12University Victor Ségalen Bordeaux 2, Bordeaux, France
13Institut Marcel Rivière, La Verrière, Le Mesnil Saint-Denis, France
14University Hospital of Nord, Saint-Etienne, France
15University of Saint-Etienne, EA 4556 laboratory Epsylon, France
16University Hospital of Nantes, Nantes, France
17University of Nantes, EA 4275, France
18CESP, INSERM, University Paris-Sud, UVSQ, University Paris-Saclay, Villejuif, France
19UMR 1027, Toulouse, France
20URC-CIC Cochin Necker, AP-HP, Paris, France.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.658416/full#supplementary-material
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA (2013).
2. Roux H, Chapelon E, Godart N. Épidémiologie de l'anorexie mentale : revue de la littérature. (2013). Available online at: https://www.em-consulte.com/en/article/799997 (accessed April 29, 2019).
3. Zipfel S, Giel KE, Bulik CM, Hay P, Schmidt U. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry. (2015) 2:1099–111. doi: 10.1016/S2215-0366(15)00356-9
4. Steinhausen HC, Boyadjieva S, Griogoroiu-Serbanescu M, Neumärker KJ. The outcome of adolescent eating disorders. Eur Child Adolesc Psychiatry. (2003) 12:i91–8. doi: 10.1007/s00787-003-1112-x
5. Godart N, Flament MF, Perdereau F, Jeammet P. Comorbidity between eating disorders and anxiety disorders: a review. Int J Eat Disord. (2002) 32:253–70. doi: 10.1002/eat.10096
6. Godart N, Duclos J, Perdereau F. Étiopathogénie des troubles des conduites alimentaires. In: Masson E, editor. Manuel de Psychiatrie, 3ème édition. Paris: Elsevier Masson (2017).
7. Gorwood P, Blanchet-Collet C, Chartrel N, Duclos J, Dechelotte P, Hanachi M, et al. New insights in anorexia nervosa. Front Neurosci. (2016) 10:256. doi: 10.3389/fnins.2016.00256
8. Godart N, Berthoz S, Rein Z, Perdereau F, Lang F, Venisse JL, et al. Does the frequency of anxiety and depressive disorders differ between diagnostic subtypes of anorexia nervosa and bulimia? Int J Eat Disord. (2006) 39:772–8. doi: 10.1002/eat.20274
9. Maguire S, Surgenor LJ, Le Grange D, Lacey H, Crosby RD, Engel SG, et al. Examining a staging model for anorexia nervosa: empirical exploration of a four stage model of severity. J Eating Disord. (2017) 5:41. doi: 10.1186/s40337-017-0155-1
10. Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. (2002) 159:1284–93. doi: 10.1176/appi.ajp.159.8.1284
11. Steinhausen HC. Outcome of eating disorders. Child Adolesc Psychiatr Clin N Am. (2009) 18:225–42. doi: 10.1016/j.chc.2008.07.013
12. Mattar L, Huas C, Duclos J, Apfel A, Godart N. Relationship between malnutrition and depression or anxiety in anorexia nervosa: a critical review of the literature. J Affect Disord. (2011) 132:311–8. doi: 10.1016/j.jad.2010.09.014
13. Mekori E, Halevy L, Ziv SI, Moreno A, Enoch-Levy A, Weizman A, et al. Predictors of short-term outcome variables in hospitalised female adolescents with eating disorders. Int J Psychiatry Clin Pract. (2017) 21:41–9. doi: 10.1080/13651501.2016.1229794
14. Fedorowicz VJ, Falissard B, Foulon C, Dardennes R, Divac SM, Guelfi JD, et al. Factors associated with suicidal behaviors in a large French sample of inpatients with eating disorders. Int J Eat Disord. (2007) 40:589–95. doi: 10.1002/eat.20415
15. Carrot B, Radon L, Hubert T, Vibert S, Duclos J, Curt F, et al. Are lifetime affective disorders predictive of long-term outcome in severe adolescent anorexia nervosa? Eur Child Adolesc Psychiatry. (2017) 26:969–78. doi: 10.1007/s00787-017-0963-5
16. Collectif. DSM-IV-TR Manuel Diagnostique et Statistique des Troubles Mentaux. Unithèque. Consulté le 3 juillet. (2020). Available online at: https://www.unitheque.com/dsm-manuel-diagnostique-statistique-des-troubles-mentaux/elsevier-masson/Livre/3499 (accessed July 3, 2020).
18. Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen HU. The World Health Organization composite international diagnostic interview short-form (CIDI-SF). Int J Methods Psychiatr Res. (1998) 7:171–85. doi: 10.1002/mpr.47
19. Peterson CB, Crosby RD, Wonderlich SA, Joiner T, Crow SJ, Mitchell JE, et al. Psychometric properties of the eating disorder examination-questionnaire: factor structure and internal consistency. Int J Eat Disord. (2007) 40:386–9. doi: 10.1002/eat.20373
20. Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. (1988) 45:1069–77. doi: 10.1001/archpsyc.1988.01800360017003
22. Patten SB, Brandon-Christie J, Devji J, Sedmak B. Performance of the composite international diagnostic interview short form for major depression in a community sample. Chronic Dis Can. (2000) 21:68–72.
23. Patten SB, Wang JL, Beck CA, Maxwell CJ. Measurement issues related to the evaluation and monitoring of major depression prevalence in Canada. Chronic Dis Can. (2005) 26:100–6.
24. Isometsä E, Aro S, Aro H. Depression in Finland: a computer assisted telephone interview study. Acta Psychiatr Scand. (1997) 96:122–8. doi: 10.1111/j.1600-0447.1997.tb09916.x
25. Kovess V, Fournier L, Lesage A, Amiel-Lebigre F, Caria A. Two validation studies of the CIDIS: a simplified version of the CIDI. Psychiatric Netw. (2001) 4:10–24.
26. Kovess V, The State of Mental Health in The European Union Collaborators. The State of Mental Health in the European Union. European Communities (2004). Available online at: http://ec.europa.eu/health/ph_projects/2001/monitoring/fp_monitoring_2001_frep_06_en.pdf (accessed June 16, 2021).
27. Lehtinen V, Sohlman B, Kovess-Masféty V. Level of positive mental health in the European Union: results from the Eurobarometer 2002 survey. Clin Pract Epidemol Ment Health. (2005) 1:9. doi: 10.1186/1745-0179-1-9
28. Morgan H, Hayward A. Clinical Assessment of anorexia nervosa. The morgan-russell outcome assessment schedule. Br J Psychiatry. (1988) 152:367–71. doi: 10.1192/bjp.152.3.367
29. Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med. (1982) 12:871–8. doi: 10.1017/s0033291700049163
30. Leichner P, Steiger H, Puentes-Neuman G, Perreault M, Gottheil N. [Validation of an eating attitude scale in a French-speaking Quebec population]. Can J Psychiatry. (1994) 39:49–54. doi: 10.1177/070674379403900110
31. Engel SG, Wittrock DA, Crosby RD, Wonderlich SA, Mitchell JE, Kolotkin RL. Development and psychometric validation of an eating disorder-specific health-related quality of life instrument. Int J Eat Disord. (2006) 39:62–71. doi: 10.1002/eat.20200
32. Tchanturia K, Liao PC, Uher R, Lawrence N, Treasure J, Campbell IC. An investigation of decision making in anorexia nervosa using the iowa gambling task and skin conductance measurements. J Int Neuropsychol Soc. (2007) 13:635–41. doi: 10.1017/S1355617707070798
33. Fornasari L, Gregoraci G, Isola M, Laura Negri GA, Rambaldelli G, Cremaschi S, et al. Psychopathological and personality traits underlie decision making in recent onset medication naïve anorexia nervosa: a pilot study. Psychiatry Res. (2014) 216:89–96. doi: 10.1016/j.psychres.2013.12.052
34. Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games Econ Behav. (2005) 52:336–72. doi: 10.1016/j.geb.2004.06.010
35. van Elburg A, Danner UN, Sternheim LC, Lammers M, Elzakkers I. Mental capacity, decision-making and emotion dysregulation in severe enduring anorexia nervosa. Front Psychiatry. (2021) 12:545317. doi: 10.3389/fpsyt.2021.545317
36. Paulus GF, de Vaan LE, Verdam FJ, Bouvy ND, Ambergen TA, van Heurn LW. Bariatric surgery in morbidly obese adolescents: a systematic review and meta-analysis. Obes Surg. (2015) 25:860–78. doi: 10.1007/s11695-015-1581-2
37. Godart N, Radon L, Curt F, Duclos J, Perdereau F, Lang F, et al. Mood disorders in eating disorder patients: prevalence and chronology of ONSET. J Affect Disord. (2015) 185:115–22. doi: 10.1016/j.jad.2015.06.039
38. Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustün TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. (2007) 20:359–64. doi: 10.1097/YCO.0b013e32816ebc8c
39. Salbach-Andrae H, Lenz K, Simmendinger N, Klinkowski N, Lehmkuhl U, Pfeiffer E. Psychiatric comorbidities among female adolescents with anorexia nervosa. Child Psychiatry Hum Dev. (2008) 39:261–72. doi: 10.1007/s10578-007-0086-1
40. Halmi KA, Eckert E, Marchi P, Sampugnaro V, Apple R, Cohen J. Comorbidity of psychiatric diagnoses in anorexia nervosa. Arch Gen Psychiatry. (1991) 48:712–8. doi: 10.1001/archpsyc.1991.01810320036006
41. Haute Autorité de Santé. Anorexie Mentale : Prise en Charge. Haute Autorité de Santé. (2010). Available online at: https://www.has-sante.fr/jcms/c_985715/fr/anorexie-mentale-prise-en-charge (accessed June 08, 2021).
42. Treasure J, Schmidt U. The cognitive-interpersonal maintenance model of anorexia nervosa revisited: a summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. J Eat Disord. (2013) 1:13. doi: 10.1186/2050-2974-1-13
43. Hebebrand J, Himmelmann GW, Herzog W, Herpertz-Dahlmann BM, Steinhausen HC, Amstein M, et al. Prediction of low body weight at long-term follow-up in acute anorexia nervosa by low body weight at referral. Am J Psychiatry. (1997) 154:566–9. doi: 10.1176/ajp.154.4.566
44. Bühren K, Schwarte R, Fluck F, Timmesfeld N, Krei M, Egberts K, et al. Comorbid psychiatric disorders in female adolescents with first-onset anorexia nervosa. Eur Eat Disord Rev. (2014) 22:39–44. doi: 10.1002/erv.2254
45. Brand-Gothelf A, Leor S, Apter A, Fennig S. The impact of comorbid depressive and anxiety disorders on severity of anorexia nervosa in adolescent girls. J Nerv Ment Dis. (2014) 202:759–62. doi: 10.1097/NMD.0000000000000194
46. Lilenfeld LR, Wonderlich S, Riso LP, Crosby R, Mitchell J. Eating disorders and personality: a methodological and empirical review. Clin Psychol Rev. (2006) 26:299–320. doi: 10.1016/j.cpr.2005.10.003
47. Touchette E, Henegar A, Godart NT, Pryor L, Falissard B, Tremblay RE, et al. Subclinical eating disorders and their comorbidity with mood and anxiety disorders in adolescent girls. Psychiatry Res. (2011) 185:185–92. doi: 10.1016/j.psychres.2010.04.005
48. Machado PP, Grilo CM, Crosby RD. Evaluation of the DSM-5 severity indicator for anorexia nervosa. Eur Eat Disord Rev. (2017) 25:221–3. doi: 10.1002/erv.2508
49. Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol. (2001) 54:343–9. doi: 10.1016/s0895-4356(00)00314-0
50. Huas C, Caille A, Godart N, Foulon C, Pham-Scottez A, Divac S, et al. Factors predictive of ten-year mortality in severe anorexia nervosa patients. Acta Psychiatr Scand. (2011) 123:62–70. doi: 10.1111/j.1600-0447.2010.01627.x
51. Woodside BD, Staab R. Management of psychiatric comorbidity in anorexia nervosa and bulimia nervosa. CNS Drugs. (2006) 20:655–63. doi: 10.2165/00023210-200620080-00004
52. Mischoulon D, Eddy KT, Keshaviah A, Dinescu D, Ross SL, Kass AE, et al. Depression and eating disorders: treatment and course. J Affect Disord. (2011) 130:470–7. doi: 10.1016/j.jad.2010.10.043
Keywords: anorexia nervosa, anxiety, depression, nutritional status, body mass index
Citation: Riquin E, Raynal A, Mattar L, Lalanne C, Hirot F, Huas C, Duclos J, EVHAN group, Berthoz S and Godart N (2021) Is the Severity of the Clinical Expression of Anorexia Nervosa Influenced by an Anxiety, Depressive, or Obsessive-Compulsive Comorbidity Over a Lifetime? Front. Psychiatry 12:658416. doi: 10.3389/fpsyt.2021.658416
Received: 25 January 2021; Accepted: 29 April 2021;
Published: 01 July 2021.
Edited by:
Hana Flynn Zickgraf, University of South Alabama, United StatesReviewed by:
Beatrice Benatti, Luigi Sacco Hospital, ItalyCopyright © 2021 Riquin, Raynal, Mattar, Lalanne, Hirot, Huas, Duclos, EVHAN group, Berthoz and Godart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathalie Godart, bmF0aGFsaWUuZ29kYXJ0QGZzZWYubmV0
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.