
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 17 May 2021
Sec. Child and Adolescent Psychiatry
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.658171
This article is part of the Research Topic Insights in Social Cognition: 2021 View all 10 articles
Background: Social anxiety disorder (SAD) has its typical onset in childhood and adolescence. Maladaptive processing of social information may contribute to the etiology and maintenance of SAD. During face perception, individuals execute a succession of visual fixations known as a scanpath which facilitates information processing. Atypically long scanpaths have been reported in adults with SAD, but no data exists from pediatric samples. SAD has also been linked to atypical arousal during face perception. Both metrics were examined in one of the largest eye-tracking studies of pediatric SAD to date.
Methods: Participants were children and adolescents with SAD (n = 61) and healthy controls (n = 39) with a mean age of 14 years (range 10–17) who completed an emotion recognition task. The visual scanpath and pupil dilation (an indirect index of arousal) were examined using eye tracking.
Results: Scanpaths of youth with SAD were shorter, less distributed, and consisted of a smaller number of fixations than those of healthy controls. These findings were supported by both frequentist and Bayesian statistics. Higher pupil dilation was also observed in the SAD group, but despite a statistically significant group difference, this result was not supported by the Bayesian analysis.
Conclusions: The results were contrary to findings from adult studies, but similar to what has been reported in neurodevelopmental conditions associated with social interaction impairments. Restricted scanpaths may disrupt holistic representation of faces known to favor adaptive social understanding.
Social anxiety disorder (SAD) is a highly disabling mental health disorder characterized by intense fear of social evaluation, often leading to extreme distress and avoidance of social interaction (1). SAD has a typical onset in late childhood or adolescence, and if left untreated often takes a chronic course (2). Current theoretical models suggest that etiological factors for SAD include genetic predispositions, temperament, cognitive biases, negative life events, peer-relations and parent behavior, that interact to produce SAD (3). The influential model by Clark and Wells (1995) (4) proposes that when an individual with social anxiety enters a social situation, a set of assumptions and beliefs are activated concerning how they think they have to act and what other people expect of them. A prediction of failure is made which causes the social situation to be perceived as dangerous and to protect the individual from harm, a number of behavioral, cognitive, attentional, affective and somatic processes are activated. These processes, however, are also involved in the long-term maintenance of social anxiety, which prevents habituation to social situations even as the individual engages in frequent interactions with others (5–7). Several models also implicate aberrant information processing in the maintenance or development of SAD (8).
So far, most studies of information processing in SAD have been conducted in adults (9–11). Adults with SAD tend to interpret social signals from others as indications of rejection or hostility (11), a bias that might be driven by disrupted allocation of attention, to socially threatening stimuli (9, 10). Individuals with SAD may also differ from healthy controls regarding the type of stimuli they perceive as threatening. According to the fear of positive evaluation hypothesis, individuals with SAD perceive social cues signaling both negative evaluation (such as an angry face) and positive evaluation (such as a smiling face) as threatening (12, 13).
Since an extensive literature has documented that the development of social attention and emotional brain mechanisms is protracted and often nonlinear, it is not clear whether these results generalize to the younger age range where SAD typically has its onset (14). For example, the period from late childhood to early adulthood is characterized by developmental changes in the relative balance between brain functions involved in automatic emotional reactivity such as the amygdala, and those involved in top-down control of attention and emotion regulation such as the anterior cingulate (ACC) and prefrontal cortex (14–16).
Late childhood and adolescence is a period of high plasticity and intense maturation of the social brain (17, 18). Therefore, aberrant information processing during this period is likely to have cascading developmental consequences (19). Conversely, late childhood and adolescence may be a period when interventions tailored to address disrupted information processing mechanisms are particularly likely to carry long-term benefits (14). To understand these mechanisms is therefore an important research priority.
There is some evidence that youth with SAD misinterpret signs of both negative and positive emotions in faces (20), and show atypical functioning of brain regions involved in face processing (21, 22). However, the patterns of visual attention linked to these observed SAD-linked anomalies are poorly understood.
Visual attention unfolds through a succession of fixations and saccades known as the scanpath. Fixated locations are highly prioritized for further cortical processing, and the scanpath is therefore a fundamental aspect of visual information processing. Cognitively demanding tasks typically lead to longer and more widely distributed scanpaths, reflecting a higher degree of mental effort and cognitive control of attention (23, 24). Stimuli signaling potential threats may also be viewed with longer scanpaths than neutral stimuli, possibly driven by increased allocation of attention (25). Some types of facial information can be identified with scanpaths consisting of as little as two fixations, including the identity of familiar individuals or emotional expressions which can be identified by a single feature (e.g., a furrowed eyebrow (26). More typically, novel faces are scanned with multiple fixations (27, 28). A spatially distributed scanpath is believed to facilitate encoding of the face into a holistic percept (26, 29), and improves face recognition (30). Holistic processing is a hallmark of face processing in healthy individuals (31), while individuals with impaired face processing abilities, including those with neurodevelopmental disorders, often use a piecemeal or detail focused strategy (32–34). Restricted scanpaths could be a marker of this detail-focused processing style (35).
So far, a small number of eye-tracking studies have been conducted in children and adolescents with SAD (36–42). These studies examined the relative distribution of attention between threat-related faces and other stimuli during free viewing tasks. Studies using free-viewing tasks are informative about how individuals with SAD spontaneously distribute their attention in the absence of an explicit task and are therefore likely reflecting multiple cognitive processes. However, results from free-viewing tasks are inconclusive, with reports of both avoidance (38, 39), prolonged monitoring of threat (41, 42), quicker orienting to angry faces (43) and equally quick orienting to and from emotional faces in youth with SAD and controls (36). Previous studies are limited by small sample sizes, typically ranging between 20 and 35 individuals [for reviews, see (9, 10)]. Studies in adults have suggested that individuals with SAD look less at the eyes of images of faces when accumulated looking time over several seconds is considered (9). Studies in children and adolescents have so far not reported reduced overall looking time at the eyes in SAD (38, 40). Looking time at the eyes or other regions of a face accumulated over several seconds is likely reflecting several attentional processes, and more temporally sensitive metrics may be needed to detect atypical social attention in child and adolescent SAD (41).
Scanpath measures could provide important information about information processing strategies in SAD but have so far not been examined in pediatric samples. Previous studies in adults with SAD reported a pattern of atypical scanpaths during face perception termed hyperscanning [e.g., (44)]. Hyperscanning can be defined as atypically long and widely distributed scanpaths (42–44). Typically, scanpath length is positively correlated with the number of executed fixations in both healthy individuals and individuals with SAD (25, 44–46). However, one study in adults with SAD reported the opposite pattern – i.e., that individuals who made longer scanpaths also made a smaller number of fixations. One reason for this unusual pattern may be that short fixations with a duration of <200 ms which may be more frequent during hyperscanning were discarded from these analyses (47, 48).
Hyperscanning was initially reported during free viewing of static images of faces (47, 48), and later also for dynamic stimuli during a public speaking task (44). Wermes and colleagues (45) extended these findings and found hyperscanning in adults with SAD but only after an anxiety induction procedure. In contrast, the type of visual stimuli (search for threat or neutral stimulus) did not modulate the results. Finally, Boll and colleagues (49) did not observe a group difference in scanpath length between adults with SAD and healthy controls during an emotion classification task.
Theoretical models of SAD propose that enhanced perception of threat during social-evaluative situations affect not only allocation of attention, but also physiological arousal (3). Heightened arousal is a common aspect of anxiety, and social fear could therefore potentially lead to hyperarousal. Consistent with this, brain imaging studies have shown amygdala hyperreactivity during face processing in SAD (21, 22). So far, little is known about potential links between atypical scanpaths and arousal in SAD.
Pupil dilation is an index of arousal directly controlled by joint activity in the sympathetic and parasympathetic branches of the autonomic nervous system. At least two components of the pupil response can be distinguished during stimulus processing. The first is a rapid constriction and subsequent dilation caused by changes in in luminance called the pupillary light reflex (PLR). The second and slower component (the pupil dilation response, PDR) is characterized by a relative increase in pupil size during periods of attention, mental effort, and arousal. This later response is modulated by cholinergic and noradrenergic activity (50, 51). Traditionally, only the PDR has been linked to cognitive processing, but recent studies indicate that also the PLR is affected by such factors (52).
In light of previous studies, it could therefore be expected that SAD would be associated with enhanced pupil dilation to emotional faces. However, this has not been found in the two studies published so far (38, 40). Keil and colleagues examined pupil dilation in 10–13 year old children and controls during face processing (38). Groups did not differ in the amplitude of their pupil dilation response measured during the whole trial interval (10 s), but the SAD group had a larger PLR than controls, which may reflect blunted cognitive modulation of the PLR. A recent study from our group examined the time course of pupil dilation in a group of adolescents with SAD as well as the amplitude of the response (40). Although adolescents with SAD did not differ from healthy controls in pupil dilation amplitude, an atypical time course was found, characterized by a decrease in pupil dilation over the course of stimulus presentation. We sought to extend these results in a larger sample and in the current study we examined pupil dilation amplitude which is the most commonly studied measure in the literature on pupil dilation (53).
The analysis plan was pre-registered in the Open Science Framework after data collection but prior to analysis (link: https://osf.io/dytnf).
The following hypotheses were tested:
Hypotheses 1: Youth with SAD will show longer scanpaths than healthy controls during face processing (longer total scanpath and more dispersion between fixations).
Hypothesis 2: Youth with SAD will show a blunted pupil dilation response during later stages of face processing (e.g., after initial adaptation to light).
We did not hypothesize group differences in accumulated looking time to the eyes or mouth but included exploratory analyses of these metrics.
Participants aged 10–17 years with SAD were recruited from an ongoing clinical trial evaluating the efficacy of internet-delivered cognitive behavioral therapy (ICBT) for pediatric SAD. Initially, 107 individuals with SAD were invited and 64 of these accepted to participate in the study and completed the experiment. Three participants with SAD were excluded from all analyses because of invalid data (see Recording and processing of eye tracking data and Statistical Analysis), resulting in a sample size of 61. A principal diagnosis of SAD according to DSM-5 (1) criteria was confirmed by an experienced clinical psychologist interviewing the child and parents jointly with the Anxiety Disorders Interview Schedule [ADIS; (54)]. The ADIS interview is normally conducted with the child and parents separately, so to ensure that the child's account was given sufficient attention during the interview, parents were instructed to let the child respond first to all questions. Exclusion criteria were initiation or dose modification of psychotropic drug within the past 6 weeks, current psychosis, eating disorder, severe depression, suicidal behavior, or other current severe mental disorder including autism spectrum disorder, or substance or alcohol abuse. Comorbid diagnoses were specific phobia (n = 5), generalized anxiety disorder (n = 8), depression (n = 4), attention deficit/hyperactivity disorder (n = 3), separation anxiety (n = 1) and panic disorder (n = 1). Two individuals with SAD medicated with selective serotonin reuptake inhibitors (SSRIs), three with stimulants (lisdexamphetamine), and one with melatonin. All results remained when participants on medication were excluded.
We planned for a control group of n = 40. Participants were randomly selected from the Swedish tax registry and contacted by mail. Initially, addresses of 326 10–17 year old children living in the Stockholm area were randomly selected from the Swedish tax registry. Families were sent a letter describing the purpose of the study and were later contacted over telephone and asked to participate. Of the initial sample, 153 families did not respond to the telephone calls, and 107 declined to participate. The remaining 66 participants were asked screening questions before inclusion. Of these, 18 were excluded because of current or previous mental health diagnoses (SAD: n = 4, ADHD: n = 10, bipolar disorder: n = 1, chromosome abnormalities, n = 1, obsessive compulsive disorder, n = 1, autism, n = 1). Seven participants were initially included but did not complete the testing procedure because no suitable time was found. The remaining 41 individuals were included and completed the testing procedure.
Control participants were assessed by a clinical psychologist using the MINI-KID (55). No participant in the control group had a mental health disorder according to the clinical assessment. Two individuals were excluded from analysis because of invalid data, resulting in a sample size of 39. As expected, both youth- and parent reports indicated higher levels of social anxiety in the SAD group than in healthy controls (see Table 1). All participants in the control group scored within one standard deviation of the mean scores on the Liebowitz Social Anxiety Scale – child version (LSAS-C) previously reported in normative samples of youths (56), and could therefore be considered healthy and non-anxious. The LSAS is a self-report measure of fear and avoidance in 24 different social and performance situations, available for youth as well as parents. Groups were matched on age and gender (Table 1). The sample is partly overlapping with the second cohort in a previous study by (41), where data from another experiment are reported.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the Stockholm regional research ethics committee (decision number 2017/1142-31/4).
Images from a standardized database of actors displaying emotional expressions were used as stimuli (57). In total, 24 images were shown to each participant, evenly distributed between three emotional expressions (angry, happy, fearful)1. The same actors appeared once with each expression, meaning that the stimulus set contained eight unique actors (50% male, 50% female). Stimulus images were cropped to show only the inner regions of the face. Each trial began with a fixation cross on a uniform gray screen for 2 s, followed by an emotional face presented for 4 s. Immediately after stimulus offset, participants were asked to identify with a mouse click whether the depicted person felt angry, happy, or fearful. Participants were not asked to make a speeded response. We chose a presentation time of 4 s to give the participants enough time to identify the emotional expressions, and also execute enough eye movements to calculate scanpath metrics. Example of stimulus images are shown in Figure 1.
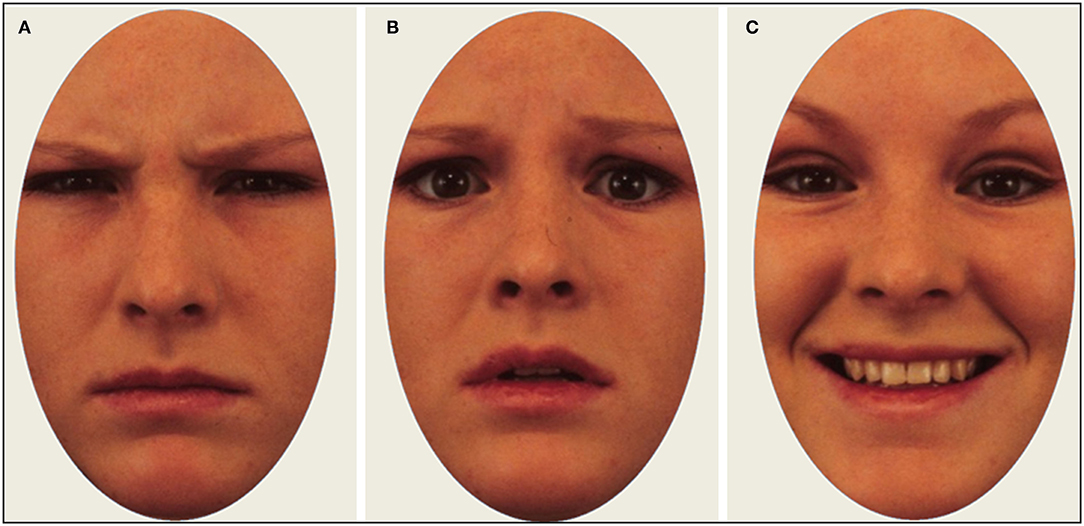
Figure 1. Example of stimulus images with actors displaying an angry (A), fearful (B), and happy (C) expression.
Data were recorded using a Tobii X-120 corneal reflection eye tracker (Tobii Inc, Danderyd, Sweden), which samples gaze at 120 Hz and pupil size at 40 Hz. Raw eye tracking data were processed using custom scripts written in MATLAB version 2019a (Mathworks, Inc). Fixations were identified using an I-VT filter (58) with parameters set according to recommendations by the manufacturer. Following linear interpolation of gaps in the data shorter than 100 ms, a moving average filter with a window size of 25 ms was applied to the x- and y-coordinates. Saccades were identified as periods with between-samples velocity exceeding 30°/s, and fixations were defined as periods between saccades. Subsequent fixations within 1° were merged. Fixations shorter than 50 ms were discarded.
Two pre-registered scanpath metrics were calculated: (1) The summed Euclidean distance between subsequent fixations (scanpath length); and (2) The root-mean-square (RMS) value of all fixations (scanpath dispersion). The RMS was calculated for each trial by (1) taking the average of the squared deviations from the mean of the x- and y-values of all fixations and (2) taking the square root of these values, and (3) averaging values for the x- and y-coordinates. Higher RMS values therefore reflect a higher degree of spatial dispersion of fixations, whereas scanpath length reflects the total distance that gaze travels during the entire stimulus presentations (see Figure 2 for illustrations). The RMS of individual fixations should not be confused with the RMS of the unfiltered samples constituting a fixation, which is sometimes used as a quality metric in eye-tracking studies.
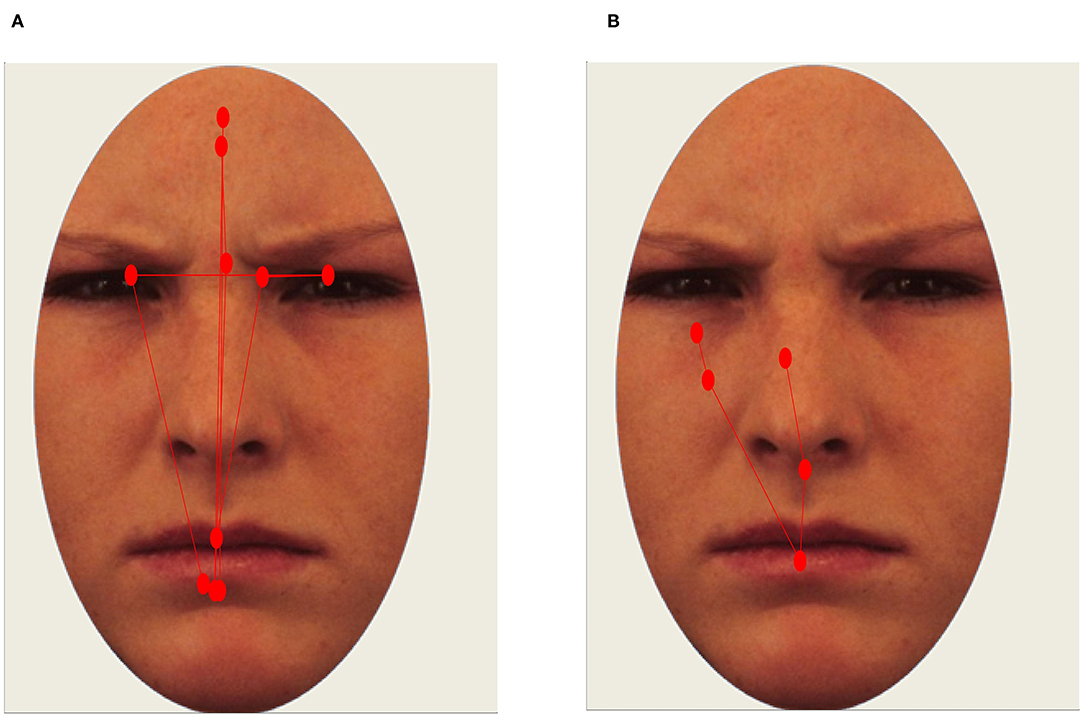
Figure 2. Example of a long scanpath [(A); scanpath length and RMS values above 75th percentile. Healthy participant] and restricted scanpath [(B); scanpath length and RMS values below 25th percentile. Participant with SAD]. Red circles represent fixations.
Scanpath length and dispersion are expressed in degrees of visual field. To account for the fact that more valid data from a trial would also result in longer scanpaths, we divided the scanpath measures by the total valid fixation time (defined as all successfully recorded samples identified as part of a fixation) in seconds. Previous studies of scanpaths in SAD have also analyzed the average number of fixations by trial [fixation count [e.g., 42–44]], a measure typically closely correlated with scanpath length. We included fixation count as an additional metric in the analyses to facilitate comparison with previous studies. Trials with <1,500 ms valid fixation time were discarded.
Pupil data were filtered according to procedures described in an earlier publication from our group (40). Gaps in the data shorter than 150 ms were replaced through linear interpolation and subsequently filtered by a moving median filter corresponding to 80 ms. A pupil dilation response was calculated for each trial and defined as the mean proportional change in pupil size during stimulus presentation relative to baseline pupil size. As in previous publications (40, 59), the initial increase in pupil size after stimulus onset that can largely be attributed to changes in screen luminance was discarded. Based on visual inspection of the data, pupil dilation response was defined as the mean pupil size during the 1,500–4,000 ms interval (see Supplementary Figure 1).
Baseline pupil size was measured during a 750 ms interval directly preceding the stimulus. An estimate of baseline pupil size was calculated for each participant by taking the mean pupil size during this interval from all valid trials. Values outside the +/− 3 SD range from the mean of each participant were considered outliers and were discarded.
For each individual, a mean of all valid trials within each condition was computed. All data from an individual and condition were rejected if <4 trials were valid. Six participants (3 with SAD) were excluded from all analyses because they were lacking data from all conditions. Cohens' d is reported as a standardized effect size of group differences. Age and sex were added as covariates in all analyses. A power analysis indicated that the study had 80% power to detect small to medium effect sizes of d = 0.2 or higher.
Hypotheses were tested using generalized linear mixed effects models (GLMM) with random intercept for participant (i.e., treating multiple observations from the same individual as repeated measures). Statistical tests for an effect were performed by comparing a model including the effect to the most complex model without the effect in question (the null model). For example: a main effect of group can be tested by comparing the full model Y ~ GROUP + (1|ID) to a null model including only the intercept: Y ~1+ (1|ID), where Y is the response variable. Both frequentist statistics (i.e., p-values) and Bayesian analyses were conducted. Bayesian statistics have been proposed as an alternative to null hypothesis significance testing (NHST). In a Bayesian analysis, the relative evidence for a hypothesis and the null hypothesis given the observed data can be quantified in terms of a Bayes factor. Consequently, researchers may potentially not only conclude that the null hypothesis could not be rejected, but also that it may be supported. Bayesian statistics may be more robust to false positive results than NHST (60). Therefore, we interpreted our results based on the Bayesian statistics when results from the two statistical approaches were conflicting.
In a frequentist framework, the full- and null models were compared using χ2-tests, yielding a p-value for the significance of the effect (61). The full- and null-models can also be compared using a Bayes factor (BF), expressing the relative likelihood of the two models. Following Wagenmakers (60), a BF is calculated from the Bayesian information criterion (BIC) values of the two models using the following equation:
Where BF10 is the Bayes factor favoring H1 over H0, with higher numbers indicating more evidence supporting H1. By reversing the terms, a BF01 can be calculated, with higher numbers indicating more support for H0. By convention, a BF > 3 indicates positive evidence for the hypothesis, a BF > 20 indicates strong support, and a BF > 150 very strong support (60). Post-hoc analyses were conducted to examine whether the observed group differences in scanpath metrics would also be linked to symptom levels of SAD. These analyses were conducted with linear regression models with the mean of all valid trials across conditions as dependent variable. For each participant, the highest value of the child and parent version of the LSAS was used as a measure of SAD symptoms. Sex and age were added as covariates. Bayes factors were computed by comparing a model including SAD symptoms to the next most complex model (the null model). Additional analyses were also conducted to compare accumulated looking time at the eyes and mouth.
No group differences were found in the number of completed trials (see Table 1). As can be seen in Table 1, although correct identification of emotion was close to ceiling in all conditions, the SAD group was more accurate than the HC group in identifying expressions of anger, whereas no group differences were found for happy or fearful faces.
A main effect of emotion was found on scanpath length, so that scanpaths were shorter during processing of happy compared to fearful and angry expressions. No effects of emotion were found on scanpath dispersion. Expressions of anger and fear elicited higher pupil dilation than expressions of happiness. These results are shown in Table 2. Groups did not differ in overall looking time at the eyes (χ2 = 0.12, p = 0.730, BF10 = 0.11, d = 0.07) or mouth (χ2 = 0.32, p = 0.575, BF10 = 0.12, d = 0.09). There were also no interactions between group and emotion in looking time at either region (see Supplementary Table 1).
Results are shown in Table 3 and Figure 3 and summarized here. Scanpaths were shorter in the SAD group than in healthy controls (χ2 = 9.38, p = 0.002, BF10 = 10.94, d = 0.54). This effect was not qualified by any interaction effect between group and emotion (χ2 = 3.60, p = 0.167, BF10 = 0.06).
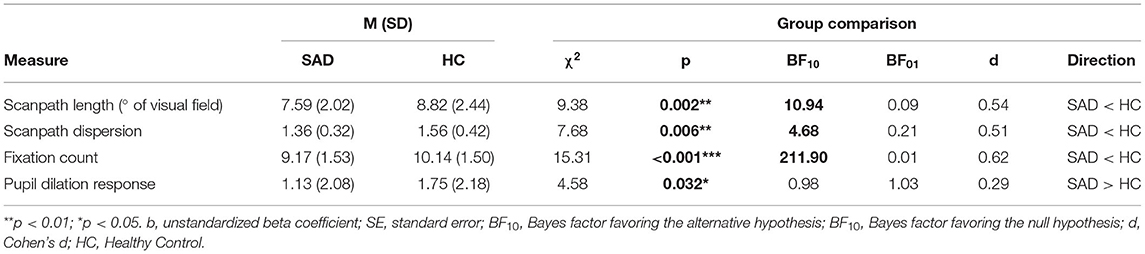
Table 3. Group differences between youth with SAD and healthy controls in scanpath length, scanpath dispersion and pupil dilation response.
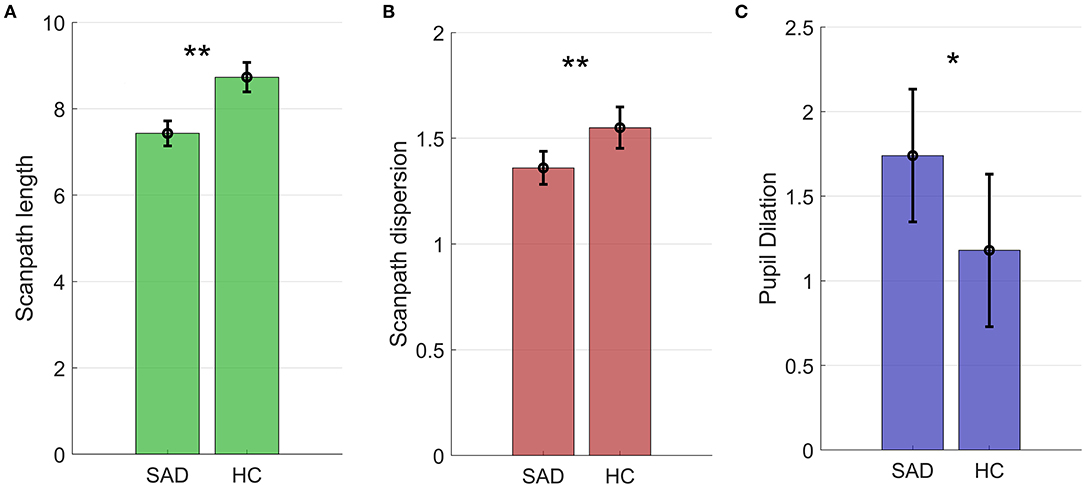
Figure 3. Scanpath length in degrees of the visual field (A), scanpath dispersion (B), and pupil dilation (C) in the SAD and HC groups. Figures show estimated marginal means and 95% confidence intervals *p < 0.01; **p < 0.05.
Scanpaths were also less dispersed in the SAD group compared to healthy controls (χ2 = 7.68, p = 0.006, BF10 = 4.68, d = 0.51; see Table 3 and Figure 2). Again, no interaction between group and emotion was found (χ2 = 0.43, p = 0.806; BF10 = 0.01). To sum up, restricted scanpaths were observed in the SAD group, a conclusion supported by both Bayesian and frequentist statistics.
As can be seen in Table 3, participants in the SAD group made a smaller number of fixations than healthy controls (χ2 = 15.31, p < 0.001, BF10 = 211.90, d = 0.62). No interaction between group and emotion was found (χ2 = 0.27, p = 0.88, BF10 = 0.01).
We conducted post-hoc analyses to examine whether symptom levels of SAD were linked to scanpath metrics. Higher levels of SAD symptoms were linked to shorter scanpath length (β = 0.24, p = 0.015, BF10 = 2.29) and smaller number of fixations (β = 0.32, p = 0.001, BF10 = 24.70). No relation was found between SAD symptoms and scanpath dispersion (β = 0.04, p = 0.32, BF10 = 0.17).
The SAD group had higher pupil dilation than controls. The difference was statistically significant (χ2 = 4.58, p = 0.032, d = 0.29), but the Bayes factor indicated that the data were marginally more likely under the null hypothesis (BF10 = 0.98, equivalent to BF01 = 1.03), and that the data were therefore inconclusive (see Figure 3 and Table 3). No interaction between group and emotion was found (χ2 = 0.05, p = 0.792, BF10 = 0.01).
Exploratory post-hoc analyses were conducted to examine links between pupil dilation and scanpath measures. No relations were found between pupil dilation and scanpath length (β = −0.11, p = 0.22, BF10 = 0.22, BF01 = 4.59), between pupil dilation and scanpath dispersion (β = −0.15, p = 0.10, BF10 = 0.40, BF01 2.50), or pupil dilation and number of fixations (β = 0.04, p = 0.69, BF10 = 0.40, BF01 = 9.29). The Bayes factors favored the null hypothesis.
Social anxiety disorder in children and adolescents is associated with cognitive biases that may maintain or exacerbate symptoms. One of the factors underlying these biases may be a pattern of disrupted allocation of attention during information processing. The current study examined visual scanpaths and pupil dilation during emotion recognition in children and adolescents with social anxiety disorder. Emotional faces are a social evaluative cue and are as such a disorder relevant stimulus in SAD. Compared to healthy controls, youth with SAD had shorter and less dispersed scanpaths, a finding supported by both frequentist and Bayesian statistics. Further analyses showed that higher levels of SAD symptoms were linked to shorter scanpaths and a smaller number of fixations, again suggesting restricted visual scanning in children with SAD.
These results were contrary to our registered hypothesis, and also contrary to what has been reported in adult studies (44, 47, 48) where it has been proposed that socially anxious individuals scan faces with prolonged scanpaths (10, 44). Our results suggest that this attention pattern is not present in pediatric populations. Instead, a pattern of restricted scanpaths was found. As noted in the introduction, under normal attentional circumstances, wider scanpaths are observed during periods of controlled attention and mental effort. The observed pattern of restricted scanpaths in the SAD group could therefore reflect difficulties with cognitive control and allocation of attention.
Restricted scanpaths may lead to a face processing strategy based on attention to single features rather than global configurations (i.e., holistic face processing). Importantly, the observed pattern of restricted scanpaths in SAD was not modulated by the emotional expression of the facial images. In fact, longer scanpaths to negative emotions (anger and fear) compared to positive (happiness) were observed in both groups, replicating previous findings in healthy populations (25, 62). It is possible that smiling faces with direct gaze may be interpreted as threatening by individuals with SAD, since they signal possible social evaluation (12, 13, 63).
Holistic as compared to detail-focused processing is a hallmark of normal face perception. However, a piecemeal strategy can sometimes facilitate detection of negative emotional expressions such as anger or sadness (64), which can be detected based on single features (65). In the current study, patients with SAD were more accurate in identifying expressions of anger than controls but did not differ in accuracy for happiness or fear. Although this finding should be interpreted with caution due to potential ceiling effects, it suggests that youth with SAD may show superior detection of angry facial affect.
The relation between restricted scanpaths and social anxiety may be bidirectional. Previous studies in nonclinical samples have demonstrated that negative mood is associated with disrupted holistic face processing (64, 66). Similarly, social anxiety may therefore disrupt holistic face processing. It is also possible that disrupted holistic processing exacerbates or maintains social anxiety to the extent that it reduces the ability to interpret ambiguous or complex facial information.
The observed pattern of restricted scanpaths may also reflect patients with SAD needing less information than healthy individuals to identify facial expressions as emotional. Longer scanpaths are associated with task difficulty and complexity (24, 62). The fact that longer scanpaths were observed in the control group could therefore reflect task difficulty. In support of this interpretation, Melfsen and Florin (20) reported that children with SAD had lower perceptual thresholds for identifying facial expressions as emotional. Interestingly, restricted scanpaths to stimuli with social content have previously been reported in other conditions with known social interaction impairments, including autism (35), schizophrenia (32) and schizotypy (29), suggesting that it may be a transdiagnostic mechanism. Atypical scanpaths were found in children with SAD despite the fact that they did not differ in overall looking time at the eyes or mouth.
Our finding of restricted scanpaths in the SAD group are opposite to what has been described in the adult studies (44, 48), pointing to a possible developmental difference between children and adults with SAD. It is possible that restricted scanning may have negative developmental consequences during this period, by restricting opportunities for learning. An interesting question for longitudinal studies is therefore whether restricted scanpaths predict worse social functioning or higher levels of SAD symptoms at later time points.
On the other hand, the transitional phase of adolescence may involve greater plasticity at the behavioral and neural level (14), thus interventions designed to alter these processing anomalies may yield greater and longer-lasting benefits. Interventions targeting SAD symptoms through attention training have been attempted, but evidence for their efficacy is so far limited (14). If these interventions are to be successful, a better understanding of the patterns of attention associated with SAD is needed. Our results suggest that restricted scanpaths may be a feasible target for interventions.
Our second aim was to examine pupil dilation during face processing. The SAD group had higher pupil dilation responses than healthy controls while viewing faces, regardless of their emotional expression. According to a conventional frequentist statistical analysis (i.e., inferential statistics based on p-values), this effect was statistically significant. However, the Bayesian analysis indicated that the hypothesis was marginally less likely than the null, rendering the result inconclusive. Bayesian statistics may be less vulnerable to false positives than frequentist statistics (60), and we believe that this result is therefore best interpreted as inconclusive. This means that we were not able to replicate previously reported findings of blunted pupillary reactivity during face perception in pediatric SAD, although it should be noted that these studies examined partly different pupil dilation metrics (38, 40). There was no relation between pupil dilation and any of the examined scanpath metrics, indicating no direct link between reduced scanpaths and hyperarousal.
Some limitations should be noted. Although the present study is one of the largest eye tracking studies of pediatric SAD to date, we were not able to compare individuals with SAD to groups with other mental health disorders. Future studies would also benefit from direct comparisons between child and adult populations with SAD. The generalizability of the findings may also be limited to treatment-seeking individuals with SAD, rather than to a broader population of youths with SAD. It should also be noted that the study did not include non-facial control stimuli. Therefore, it is not clear whether the findings are specific for faces or reflect a more general form of atypical attention. Future studies could benefit from the inclusion of additional experimental conditions, including nonsocial stimuli and more ambiguous and complex facial expressions. Finally, due to the limited sample rate of the equipment (40 Hz), we were not able to examine metrics which are sensitive to timing such as the time course of the pupil dilation response or the PLR amplitude, as was done in previous studies (38, 40). An interesting question for future studies is to examine whether scanpath lengths in children with SAD is related to other types of attention and perceptual judgement, including memory for faces. In a previous adult study (45), hyperscanning was found only after an anxiety induction procedure. An interesting question for future studies is whether scanpaths in youth with SAD are also affected by induction of state anxiety. Studies manipulating gaze behaviors (for example by instructing participants to scan either narrowly or broadly) could also examine whether scanpath length causally affects arousal.
Strengths of the study includes the use of a clinically well-characterized sample of treatment-seeking patients with SAD and a matched control group randomly selected from the general population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Etikprövningsmyndigheten (Swedish Ethical Review Authority). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin and by participants aged 15 - 17. Participants aged 10 - 14 gave verbal ascent.
JK and JH designed the study. JK analyzed the data and drafted the manuscript. JL, EL, and ES contributed to the interpretation of the data. All authors contributed to the article and approved the submitted version.
This research was supported by Riksbankens jubileumsfond (grant numbers P18-0068), Claes Groschinskys minnesfond, Region Stockholm and the regional agreement on medical training and clinical research between Region Stockholm and the Karolinska Institutet (PPG 20150032, The Swedish Research Council for Health, Working Life and Welfare (Forte 2014-4052), the Strategic Research Area Neuroscience (StratNEURO) and the Center for Psychiatry Research, Stockholm, Sweden. ES was supported by Region Stockholm (clinical research appointment 20170605). JL is receiving research funding from the UK Economic and Social Research Council (ES/T00004X/1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
A preprint version of this manuscript has been published on PsyArXiv (https://psyarxiv.com/m4d76/).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.658171/full#supplementary-material
1. ^The following images from the KDEF database were used as stimuli: ANSF07, ANSF09, ANSF33, ANSM07, ANSM08, ANSM09, ANSM32, HASF07, HASF09, HASF33, HASM07, HASM08, HASM08, HASM32, FESF07, FESF09, FESF33, FESM07, FESM08, FESM09, FESM32.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA: American Psychiatric Pub (2013).
2. Ruscio AM, Brown TA, Tat Chiu W, Sareen J, Stein MB, Kessler RC. Social fears and social phobia in the United States: results from the national comorbidity survey replication. Psychol Med. (2008) 38:15–28. doi: 10.1017/S0033291707001699
3. Wong QJJ, Rapee RM. The aetiology and maintenance of social anxiety disorder: a synthesis of complementary theoretical models and formulation of a new integrated model. J Affect Disord. (2016) 203:84–100. doi: 10.1016/j.jad.2016.05.069
4. Clark DM, Wells A. A cognitive model of social phobia. In: Heimbberg RG, Liebowitz M, Hope D, Scheier F, editors. Social Phobia: Diagnosis, Assessment, Treatment. New York, NT: Guilford Publications (1995). p. 69–93.
5. Avery SN, Blackford JU. Slow to warm up: the role of habituation in social fear. Soc Cogn Affect Neurosci. (2016) 11:1832–40. doi: 10.1093/scan/nsw095
6. Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Soc Cogn Affect Neurosci. (2013) 143–50. doi: 10.1093/scan/nsr078
7. Bas-Hoogendam JM, Steenbergen H, Blackford JU, Tissier RLM, Wee NJA, Westenberg PM. Impaired neural habituation to neutral faces in families genetically enriched for social anxiety disorder. Depress Anxiety. (2019) 36:1143–53. doi: 10.1002/da.22962
8. Spence SH, Rapee RM. The etiology of social anxiety disorder: an evidence-based model. Behav Res Ther. (2016) 86:50–67. doi: 10.1016/j.brat.2016.06.007
9. Chen J, Van Den Bos E, Westenberg PM. A systematic review of visual avoidance of faces in socially anxious individuals: influence of severity, type of social situation, and development. J Anxiety Disord. (2020) 70:102193. doi: 10.1016/j.janxdis.2020.102193
10. Chen NTM, Clarke PJF. Gaze-based assessments of vigilance and avoidance in social anxiety: a review. Curr Psychiatry Rep. (2017) 19:1–9. doi: 10.1007/s11920-017-0808-4
11. Hirsch CR, Clark DM. Information-processing bias in social phobia. Clin Psychol Rev. (2004) 24:799–825. doi: 10.1016/j.cpr.2004.07.005
12. Weeks JW, editors. The Wiley Blackwell Handbook of Social Anxiety Disorder. Malden, MA: Wiley Blackwell (2014).
13. Reichenberger J, Wiggert N, Wilhelm FH, Liedlgruber M, Voderholzer U, Hillert A, et al. Fear of negative and positive evaluation and reactivity to social-evaluative videos in social anxiety disorder. Behav Res Ther. (2019) 116:140–8. doi: 10.1016/j.brat.2019.03.009
14. Haller SP, Cohen Kadosh K, Scerif G, Lau JYF. Social anxiety disorder in adolescence: how developmental cognitive neuroscience findings may shape understanding and interventions for psychopathology. Dev Cogn Neurosci. (2015) 13:11–20. doi: 10.1016/j.dcn.2015.02.002
15. Caouette JD, Guyer AE. Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Dev Cogn Neurosci. (2014) 8:65–76. doi: 10.1016/j.dcn.2013.10.003
16. Thorell LB, Bohlin G, Rydell A-M. Two types of inhibitory control: predictive relations to social functioning. Int J Behav Dev. (2004) 28:193–203. doi: 10.1080/01650250344000389
17. Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, et al. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum Brain Mapp. (2016) 37:1684–95. doi: 10.1002/hbm.23129
18. Morales S, Fu X, Pérez-Edgar KE. A developmental neuroscience perspective on affect-biased attention. Dev Cogn Neurosci. (2016) 21:26–41. doi: 10.1016/j.dcn.2016.08.001
19. In-Albon T, Kossowsky J, Schneider S. Vigilance and avoidance of threat in the eye movements of children with separation anxiety disorder. J Abnorm Child Psychol. (2010) 38:225–35. doi: 10.1007/s10802-009-9359-4
20. Melfsen S, Florin I. Do socially anxious children show deficits in classifying facial expressions of emotions? J Nonverbal Behav. (2002) 26:109–26. doi: 10.1023/A:1015665521371
21. Frick A, Howner K, Fischer H, Kristiansson M, Furmark T. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Transl Psychiatry. (2013) 3:e312-e312. doi: 10.1038/tp.2013.85
22. Gentili C, Cristea IA, Angstadt M, Klumpp H, Tozzi L, Phan L, et al. Beyond emotions: a meta-analysis of neural response within face processing system in social anxiety. Exp Biol Med. (2016) 241:225–37. doi: 10.1177/1535370215603514
23. Dewhurst R, Foulsham T, Jarodzka H, Johansson R, Holmqvist K, Nyström M. How task demands influence scanpath similarity in a sequential number-search task. Vision Res. (2018) 149:9–23. doi: 10.1016/j.visres.2018.05.006
24. Barton JJS, Radcliffe N, Cherkasova M V, Edelman J, Itriligator JM. Information processing during face recognition: the effects of familiarity, inversion, and morphing on scanning fixations. Perception. (2006) 35:1089–105. doi: 10.1068/p5547
25. Green M, Williams L, Davidson D. In the face of danger: specific viewing strategies for faciala expressions of threat? Cogn Emot. (2003) 17:779–86. doi: 10.1080/02699930302282
26. Arizpe JM, Noles DL, Tsao JW, Chan AWY. Eye movement dynamics differ between encoding and recognition of faces. Vision. (2019) 3:1–24. doi: 10.3390/vision3010009
27. Bombari D, Mast FW, Lobmaier JS. Featural, configural, and holistic face-processing strategies evoke different scan patterns. Perception. (2009) 38:1508–21. doi: 10.1068/p6117
28. Schwarzer G, Huber S, Dümmler T. Gaze behavior in analytical and holistic face processing. Mem Cognit. (2005) 33:344–54. doi: 10.3758/BF03195322
29. Hills PJ, Eaton E, Pake JM. Correlations between psychometric schizotypy, scan path length, fixations on the eyes and face recognition. Q J Exp Psychol. (2016) 69:611–25. doi: 10.1080/17470218.2015.1034143
30. Chan CYH, Chan AB, Lee TMC, Hsiao JH. Eye-movement patterns in face recognition are associated with cognitive decline in older adults. Psychon Bull Rev. (2018) 25:2200–7. doi: 10.3758/s13423-017-1419-0
31. Farah M, Wilson K, Drain M, Tanaka J. What is" special" about face perception? Psychol Rev. (1998) 105:482. doi: 10.1037/0033-295X.105.3.482
32. Loughland CM, Williams LM, Gordon E. Visual scanpaths to positive and negative facial emotions in an outpatient schizophrenia sample. Schizophr Res. (2002) 55:159–70. doi: 10.1016/S0920-9964(01)00186-4
33. Nakahachi T, Yamashita K, Iwase M, Ishigami W, Tanaka C, Toyonaga K, et al. Disturbed holistic processing in autism spectrum disorders verified by two cognitive tasks requiring perception of complex visual stimuli. Psychiatry Res. (2008) 159:330–8. doi: 10.1016/j.psychres.2005.08.028
34. Falck-Ytter T. Face inversion effects in autism: a combined looking time and pupillometric study. Autism Res. (2008) 1:297–306. doi: 10.1002/aur.45
35. Heaton T, Freeth M. Reduced visual exploration when viewing photographic scenes in individuals with autism spectrum disorder. J Abnorm Psychol. (2016) 125:399–411. doi: 10.1037/abn0000145
36. Högström J, Nordh M, Larson Lindal M, Taylor E, Serlachius E, Kleberg JL. Visual attention to emotional faces in adolescents with social anxiety disorder receiving cognitive behavioral therapy. PLoS ONE. (2019) 14:e0225603. doi: 10.1371/journal.pone.0225603
37. Schmidtendorf S, Wiedau S, Asbrand J, Tuschen-caffier B, Heinrichs N. Attentional bias in children with social anxiety disorder. Cognit Ther Res. (2018) 42:273–88. doi: 10.1007/s10608-017-9880-7
38. Keil V, Hepach R, Vierrath S, Caffier D, Tuschen-Caffier B, Klein C, et al. Children with social anxiety disorder show blunted pupillary reactivity and altered eye contact processing in response to emotional faces: insights from pupillometry and eye movements. J Anxiety Disord. (2018) 58:61–9. doi: 10.1016/j.janxdis.2018.07.001
39. Kleberg JL, Högström J, Nord M, Bölte S, Serlachius E, Falck-Ytter T. Autistic traits and symptoms of social anxiety are differentially related to attention to others' eyes in social anxiety disorder. J Autism Dev Disord. (2017) 47:3814–21. doi: 10.1007/s10803-016-2978-z
40. Kleberg JL, Hanqvist C, Serlachius E, Högström J. Pupil dilation to emotional expressions in adolescent social anxiety disorder is related to treatment outcome. J Anxiety Disord. (2019) 65:26–33. doi: 10.1016/j.janxdis.2019.04.006
41. Kleberg JL, Högström J, Sundström K, Frick A, Serlachius E. Delayed gaze shifts away from others' eyes in children and adolescents with social anxiety disorder. J Affect Disord. (2021) 278:280–7. doi: 10.1016/j.jad.2020.09.022
42. Capriola-Hall NN, Ollendick TH, White SW. Gaze as an indicator of selective attention in adolescents with social anxiety disorder. Cognit Ther Res. (2020) 44:145–55. doi: 10.1007/s10608-019-10038-7
43. Seefeldt WL, Krämer M, Tuschen-Caffier B, Heinrichs N. Hypervigilance and avoidance in visual attention in children with social phobia. J Behav Ther Exp Psychiatry. (2014) 45:105–12. doi: 10.1016/j.jbtep.2013.09.004
44. Chen NTM, Thomas LM, Joseph P, Clarke F, Hickie IB, Guastella AJ. Hyperscanning and avoidance in social anxiety disorder: the visual scanpath during public speaking. Psychiatry Res. (2015) 225:667–72. doi: 10.1016/j.psychres.2014.11.025
45. Wermes R, Lincoln TM, Helbig-Lang S. Anxious and alert? Hypervigilance in social anxiety disorder. Psychiatry Res. (2018) 269:740–5. doi: 10.1016/j.psychres.2018.08.086
46. Hunnius S, de Wit TCJ, Vrins S, von Hofsten C. Facing threat: infants' and adults' visual scanning of faces with neutral, happy, sad, angry, and fearful emotional expressions. Cogn Emot. (2011) 25:193–205. doi: 10.1080/15298861003771189
47. Horley K, Williams LM, Gonsalvez C, Gordon E. Social phobics do not see eye to eye: a visual scanpath study of emotional expression processing. J Anxiety Disord. (2003) 17:33–44. doi: 10.1016/S0887-6185(02)00180-9
48. Horley K, Williams LM, Gonsalvez C, Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Res. (2004) 127:43–53. doi: 10.1016/j.psychres.2004.02.016
49. Boll S, Bartholomaeus M, Peter U, Lupke U, Gamer M. Attentional mechanisms of social perception are biased in social phobia. J Anxiety Disord. (2016) 40:83–93. doi: 10.1016/j.janxdis.2016.04.004
50. Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norephinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. (2005) 28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709
51. Joshi S. Pupillometry: arousal state or state of mind? Curr Biol. (2021) 31:R32–4. doi: 10.1016/j.cub.2020.11.001
52. Mathôt S. Pupillometry: psychology, physiology, and function. J Cogn. (2018) 1:1–23. doi: 10.5334/joc.18
53. Laeng B, Sirois S, Gredebäck G. Pupillometry. Perspect Psychol Sci. (2012) 7:18–27. doi: 10.1177/1745691611427305
54. Silverman W. Anxiety Disorders Interview Schedule for DSM-IV: Parent Interview Schedule, Vol 1. Oxford, UK: Oxford University Press (1996).
55. Sheehan D V, Lecrubier Y, Sheehan HK, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. - PsycNET. J Clin Psychiatry. (1998) 59:22–23. doi: 10.1037/t18597-000
56. De Oviedo U, Olivares E, Sánchez-García J, López-Pina R;, Antonio J. The liebowitz social anxiety scale for children and adolescents. Psicothema. (2009) 21:486–91.
57. Lundqvist D, Flyckt A, Öhman A. The karolinska directed emotional faces (KDEF). (1998). doi: 10.1037/t27732-000
58. Salvucci DD, Goldberg JH. Identifying fixations and saccades in eye-tracking protocols. In: Proceedings of the Eye Tracking Research and Applications Symposium. New York, NT: ACM Press (2000).
59. Kleberg JL, Frick MA, Brocki KC. Increased pupil dilation to happy faces in children with hyperactive/impulsive symptoms of ADHD. Dev Psychopathol. (2020) 1-11. doi: 10.1017/S0954579420000036
60. Wagenmakers E-J. A practical solution to the pervasive problems of p values. Psychon Bull Rev. (2007) 14:779–804. doi: 10.3758/BF03194105
61. Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. (2008) 59:390–412. doi: 10.1016/j.jml.2007.12.005
62. Gomez P, Von Gunten A, Danuser B. Eye gaze behavior during affective picture viewing: effects of motivational significance, gender, age, and repeated exposure. Biol Psychol. (2019) 146:107713. doi: 10.1016/j.biopsycho.2019.06.001
63. Bas-Hoogendam JM, van Steenbergen H, van der Wee NJA, Westenberg PM. Amygdala hyperreactivity to faces conditioned with a social-evaluative meaning– a multiplex, multigenerational fMRI study on social anxiety endophenotypes. NeuroImage Clin. (2020) 26:102247. doi: 10.1016/j.nicl.2020.102247
64. Curby KM, Johnson KJ, Tyson A. Face to face with emotion: holistic face processing is modulated by emotional state. Cogn Emot. (2012) 26:93–102. doi: 10.1080/02699931.2011.555752
65. Beaudry O, Roy-Charland A, Perron M, Cormier I, Tapp R. Featural processing in recognition of emotional facial expressions. Cogn Emot. (2014) 28:416–32. doi: 10.1080/02699931.2013.833500
Keywords: social anxiety disorder, eye tracking, visual scanpaths, social attention, child and adolescent, attention bias, emotion
Citation: Kleberg JL, Löwenberg EB, Lau JYF, Serlachius E and Högström J (2021) Restricted Visual Scanpaths During Emotion Recognition in Childhood Social Anxiety Disorder. Front. Psychiatry 12:658171. doi: 10.3389/fpsyt.2021.658171
Received: 25 January 2021; Accepted: 07 April 2021;
Published: 17 May 2021.
Edited by:
Veit Roessner, University Hospital Carl Gustav Carus, GermanyReviewed by:
Julian Schmitz, Leipzig University, GermanyCopyright © 2021 Kleberg, Löwenberg, Lau, Serlachius and Högström. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johan Lundin Kleberg, am9oYW4ubHVuZGluLmtsZWJlcmdAa2kuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.