
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 17 March 2021
Sec. Schizophrenia
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.642403
This article is part of the Research TopicCardiovascular and Physical Health in Severe Mental IllnessView all 14 articles
 Pruntha Kanagasundaram1,2†
Pruntha Kanagasundaram1,2† Jiwon Lee1,3†
Jiwon Lee1,3† Femin Prasad1,3†
Femin Prasad1,3† Kenya A. Costa-Dookhan1,3†
Kenya A. Costa-Dookhan1,3† Laurie Hamel1
Laurie Hamel1 Madeleine Gordon3,4
Madeleine Gordon3,4 Gary Remington1,3,5
Gary Remington1,3,5 Margaret K. Hahn1,3,5,6‡
Margaret K. Hahn1,3,5,6‡ Sri Mahavir Agarwal1,3,5,6*‡
Sri Mahavir Agarwal1,3,5,6*‡Introduction: Antipsychotic-induced dyslipidemia represents a common adverse effect faced by patients with schizophrenia that increases risk for developing further metabolic complications and cardiovascular disease. Despite its burden, antipsychotic-induced dyslipidemia is often left untreated, and the effectiveness of pharmacological interventions for mitigating dyslipidemia has not been well-addressed. This review aims to assess the effectiveness of pharmacological interventions in alleviating dyslipidemia in patients with schizophrenia.
Methods: Medline, PsychInfo, and EMBASE were searched for all relevant English articles from 1950 to November 2020. Randomized placebo-controlled trials were included. Differences in changes in triglycerides, HDL cholesterol, LDL cholesterol, and VLDL cholesterol levels between treatment and placebo groups were meta-analyzed as primary outcomes.
Results: Our review identified 48 randomized controlled trials that comprised a total of 3,128 patients and investigated 29 pharmacological interventions. Overall, pharmacological interventions were effective in lowering LDL cholesterol, triglycerides, and total cholesterol levels while increasing the levels of HDL cholesterol. Within the intervention subgroups, approved lipid-lowering agents did not reduce lipid parameters other than total cholesterol level, while antipsychotic switching and antipsychotic add-on interventions improved multiple lipid parameters, including triglycerides, LDL cholesterol, HDL cholesterol, and total cholesterol. Off label lipid lowering agents improved triglycerides and total cholesterol levels, with statistically significant changes seen with metformin.
Conclusion: Currently available lipid lowering agents may not work as well in patients with schizophrenia who are being treated with antipsychotics. Additionally, antipsychotic switching, antipsychotic add-ons, and certain off label interventions might be more effective in improving some but not all associated lipid parameters. Future studies should explore novel interventions for effectively managing antipsychotic-induced dyslipidemia.
Registration: PROSPERO 2020 CRD42020219982; https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020219982.
Dyslipidemia refers to abnormalities in lipid levels such as increases in total and low-density lipoprotein (LDL) cholesterols, low concentrations of high-density lipoprotein (HDL) cholesterols, and high triglyceride levels. This metabolic abnormality causes almost a third of ischemic heart disease and a fifth of global cerebrovascular disease (1). Patients with schizophrenia are at an increased risk of developing cardiovascular disease in part due to the illness itself (2–7), as well as a higher prevalence of well-known lifestyle factors that promote cardiovascular disease risk, namely sedentary lifestyle, poor diet, and smoking (8, 9). Antipsychotics are the cornerstone of treatment in schizophrenia and are widely prescribed across other psychiatric conditions (10). However, their use is associated with severe metabolic adverse effects, including weight gain, dyslipidemia, insulin resistance, and risk of type 2 diabetes mellitus (T2D) in a population burdened with premature cardiovascular mortality.
While the prevalence of dyslipidemia and consequent effects on morbidity and mortality are high worldwide among the general population, particular subgroups may be at a greater risk. In particular, patients with schizophrenia are at an increased risk of dyslipidemia and its associated influence on cardiovascular disease and metabolic dysfunction (11, 12). Despite its high prevalence and associated cardiovascular risk, dyslipidemia often goes untreated among patients with schizophrenia. Reported rates for non-treatment are almost 90% (13–15), and patients with schizophrenia are often medically underserved and disadvantaged in their physical health care (16–18). As shown by results from a study by the National Institute of Mental Health, namely the Recovery After an Initial Schizophrenia Episode–Early Treatment Program (RAISE-ETP), at baseline more than half of patients (161/394 or 56.5%) had dyslipidemia and only 0.5% were receiving treatment (16).
Previous discussions addressing antipsychotic-induced metabolic abnormalities in patients with schizophrenia have largely focused on weight gain or metabolic syndrome, and not dyslipidemia per se (19–21). Only a few studies have investigated approved lipid lowering agents for treating dyslipidemia in schizophrenia (22–30). More commonly, as reported in a 2014 review by Tse et al. a wide variety of pharmacological agents have been investigated to treat dyslipidemia in patients with schizophrenia, including treatment with omega-3 fatty acids, fluvoxamine, topiramate, metformin, sibutramine, telmisartan, ramelteon, and valsartan (31). Antipsychotic switching and adding aripiprazole have also been evaluated as strategies to improve lipid outcomes in patients with schizophrenia (32–38). Given the variety of approaches used to address dyslipidemia in this patient population, as well as the absence of clear clinical guidelines, it is important to summarize the available evidence and guide clinical decision making. Hence, we performed a systematic review and meta-analysis of randomized controlled trials to compare the effects of pharmacological interventions vs. placebo treatment in antipsychotic-induced dyslipidemia in patients with schizophrenia.
The protocol for the review is registered on PROSPERO (PROSPERO 2020 CRD42020219982; https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020219982). PRISMA guidelines were used for study design and reporting.
We searched for studies published between 1950 and November 2020 using Medline, PsychInfo and EMBASE databases, with the following search string: psychotic disorder OR schizophrenia OR schizoaffective OR schizophreniform OR psychosis OR first episode AND hyperlipidemia OR triglycerides OR cholesterol OR lipid OR LDL cholesterol OR VLDL cholesterol OR HDL cholesterol. The search was limited to studies conducted in human participants and published in English. References cited in previously published literature reviews and meta-analyses pertaining to interventions for metabolic disturbances in the schizophrenia population were reviewed for additional studies.
Articles were initially screened using title and abstract, based on the study's relevance to our meta-analysis. Thereafter, articles were further screened to ensure that studies met the following inclusion criteria: (a) randomized placebo-controlled trial; (b) diagnosis of schizophrenia spectrum disorders comprising the majority (>50%) of study populations; (c) patients with current metabolic abnormalities; (d) an active pharmacological intervention used to improve metabolic abnormalities or an antipsychotic switching/add-on method if the antipsychotic change is aimed to improve metabolic parameters; and (e) primary outcome listed as lipids or other metabolic measures if lipid outcomes were included in the list of metabolic measures.
Studies were excluded from analysis during the final screening stage if (a) not aimed at improving metabolic measures; (b) comparing different modes of antipsychotic administration (i.e., deltoid, sublingual, gluteal etc.); (c) comparing effectiveness between different antipsychotics; (d) evaluating non-pharmacological intervention (e.g., behavioral interventions, dietary modulations etc.); or, (e) evaluating strategies to prevent dyslipidemia (i.e., patients did not have metabolic abnormalities or dyslipidemia at baseline).
The primary outcomes included the following lipid parameters: total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, and very low-density lipoprotein cholesterol (VLDL) cholesterol. Additional secondary outcome data were also extracted including body weight, body mass index (BMI), waist circumference, waist to hip ratio, fasting blood glucose, fasting insulin, hemoglobin A1c (HbA1c), diastolic blood pressure, systolic blood pressure, the homeostatic model assessment of insulin resistance (HOMA-IR), and total positive and negative symptom scale (total PANSS). Outcomes were extracted for both the intervention and placebo groups, where the placebo groups were used as comparators. Outcomes were extracted by two authors (PK and KC-D) and were checked by authors, FP and JL. For studies that examined multiple doses of the same intervention, the data pertaining to the higher dose were extracted.
Review Manager 5.4 (Revman 5.4.0 (Mac Version) Cochrane Collaboration, Oxford) was used to analyze the data extracted from the final list of included articles. Continuous outcomes were reported using mean differences (MD) with 95% confidence intervals (CIs), following the inverse variance statistical method and random effects model to account for study heterogeneity. Missing standard deviations (SDs) were calculated using other available statistics that were reported. Endpoint data were primarily used unless not available, in which case mean change data were used. Endpoint and change data were combined during the analysis, as we used mean difference rather than standardized mean difference (39). For Emsley et al. which was a double-blind trial with an open-label extension (27), data were extracted at the endpoint of the double-blind phase. Study heterogeneity was calculated using the I2 statistic, with significant heterogeneity being classified as I2 ≥ 50%. Significant statistical differences were classified as p < 0.05. Changes in lipid profiles (i.e., HDL cholesterol, LDL cholesterol, VLDL cholesterol, triglycerides, and total cholesterol) were assessed for all interventions pooled and for the following 4 subgroups: lipid lowering agents; antipsychotic switching or antipsychotic add-on interventions; the off-label lipid lowering agent metformin; and other off-label lipid lowering agents.
All included studies were judged for risk of bias in random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias using the Cochrane Risk of Bias tool (40). Studies were judged to have either a low, high, or unclear risk of bias. Sensitivity analyses were conducted after removing studies found to be at high risk of bias to examine their impact on findings.
Of the 244 full-text articles screened, 48 articles (n = 3,128) met criteria for inclusion (Figure 1, Supplementary Table 1). Forty-three studies were double-blind, 2 were open-label, one had blinding but did not specify level, and the remaining 2 studies did not provide information on blinding. All studies included adult populations (18 years or older). The average age (±SD) of participants receiving interventions was 40.1 (±12.8) years, vs. 40.6 (±9.6) for those receiving placebo. A total of 74% of participants in both the intervention and placebo groups were male, with 89.2% diagnosed with schizophrenia. Trials were 4–24 weeks long, with a mean duration (±SD) of 13.1 (±5.7) weeks. Studies comprised a total of 29 interventions. Lipid lowering agents included omega-3 fatty acids [(26–28, 30), N = 4, n = 250] and pravastatin [(29), N = 1, n = 49]. Antipsychotic switching or add-on interventions included the following: switching to quetiapine [(41), N = 1, n = 133]; adding aripiprazole [(33, 34, 38), N = 3, n = 322]; and, switching to aripiprazole [(35–37), N = 3, n = 390]. Off label lipid modulating agents included the following: metformin [(42–47), N = 6, n = 565], reboxetine [(48), N = 1, n = 54], nizatidine [(49), N = 1, n = 54], atomoxetine [(50), N = 1, n = 29], combination of metformin and sibutramine [(51), N = 1, n = 28], rosiglitazone [(52, 53), N = 2, n = 47], ramelteon [(54), N = 1, n = 20], telmisartan [(55), N = 1, n = 43], vitamin D and probiotic combination [(56), N = 1, n = 60], sibutramine [(17, 57), N = 2, n = 55]; dehydroepiandrosterone [DHEA; (58), N = 1, n = 43], exenatide [(59), N = 1, n = 40], orlistat [(60), N = 1, n = 63], vitamin D [(61), N = 1, n = 47], liraglutide [(62), N = 1, n = 97], intranasal insulin [(63), N = 1, n = 39], minocycline [(64), N = 1, n = 55], fluvoxamine [(65, 66), N = 2, n = 153], naltrexone and bupropion combination [(67), N = 1, n = 21], melatonin [(68, 69), N = 2, n = 80], pioglitazone [(70), N = 1, n = 52], Liuyu decoction, traditional Chinese medicine [(71), N = 1, n = 154], a combination of celery, dill, and green tea [(72), N = 1, n = 60], naltrexone [(73, 74), N = 2, n = 47], Konjac powder [(75), N = 1, n = 59], and resveratrol [(76), N = 1, n = 19]. Baseline antipsychotic use by participants included olanzapine (N = 27), clozapine (N = 25), risperidone (N = 11), quetiapine (N = 8), aripiprazole (N = 5), ziprasidone (N = 2), paliperidone (N = 2), haloperidol (N = 1), fluphenazine (N = 1), flupenthixol (N = 1), clopenthixol (N = 1), and sulpiride (N = 1), chlorpromazine (N = 1), perphenazine (N = 1), zuclopenthixol (N = 1), chlorprothixene (N = 1), amisulpride (N = 1), sertindole (N = 1), and sulpiride (N = 1).
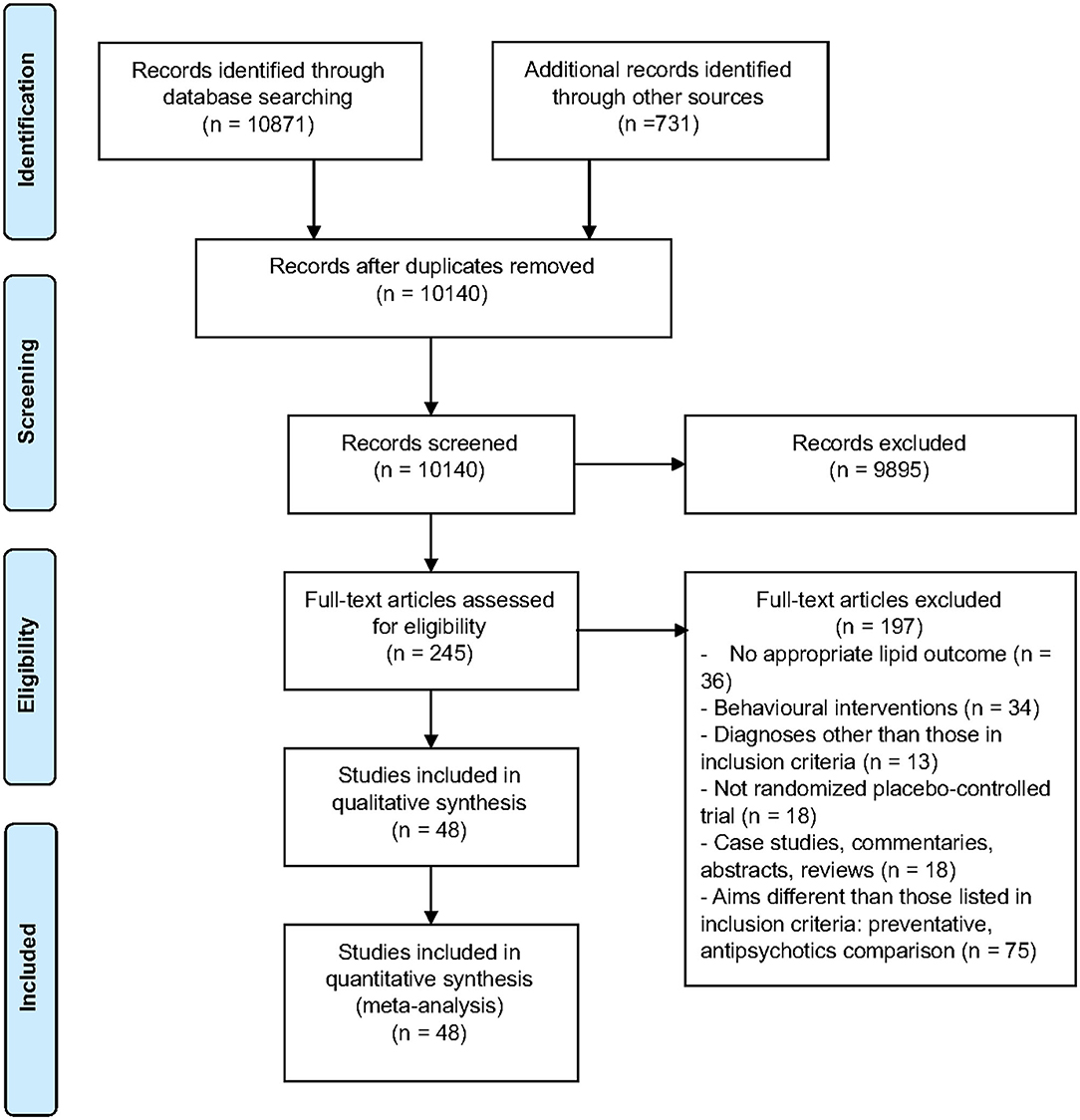
Figure 1. PRISMA flow chart describing the selection process of included articles for the systematic review and meta-analysis.
Compared to placebo, pharmacological interventions were associated with a pooled mean difference of −13.08 mg/dL (CI: −20.82, −5.33; p = 0.0009) for triglycerides (Figure 2), 0.43 mg/dL (CI: −0.85, 1.70; p = 0.51) for HDL (Figure 3), −4.19 mg/dL (CI: −7.71, −0.67; p = 0.02) for LDL cholesterol (Figure 4), −3.27 mg/dL (CI: −7.38, 0.84; p = 0.12) for VLDL cholesterol (Figure 5), and −7.96 mg/dL (CI: −11.14, −4.77; p < 0.00001) for total cholesterol (Figure 6). Heterogeneity was low to moderate for most outcomes: I2 = 71% for HDL, I2 = 60% for LDL cholesterol, I2 = 0% for VLDL cholesterol, I2 = 52% for triglycerides, I2 = 37% for total cholesterol.
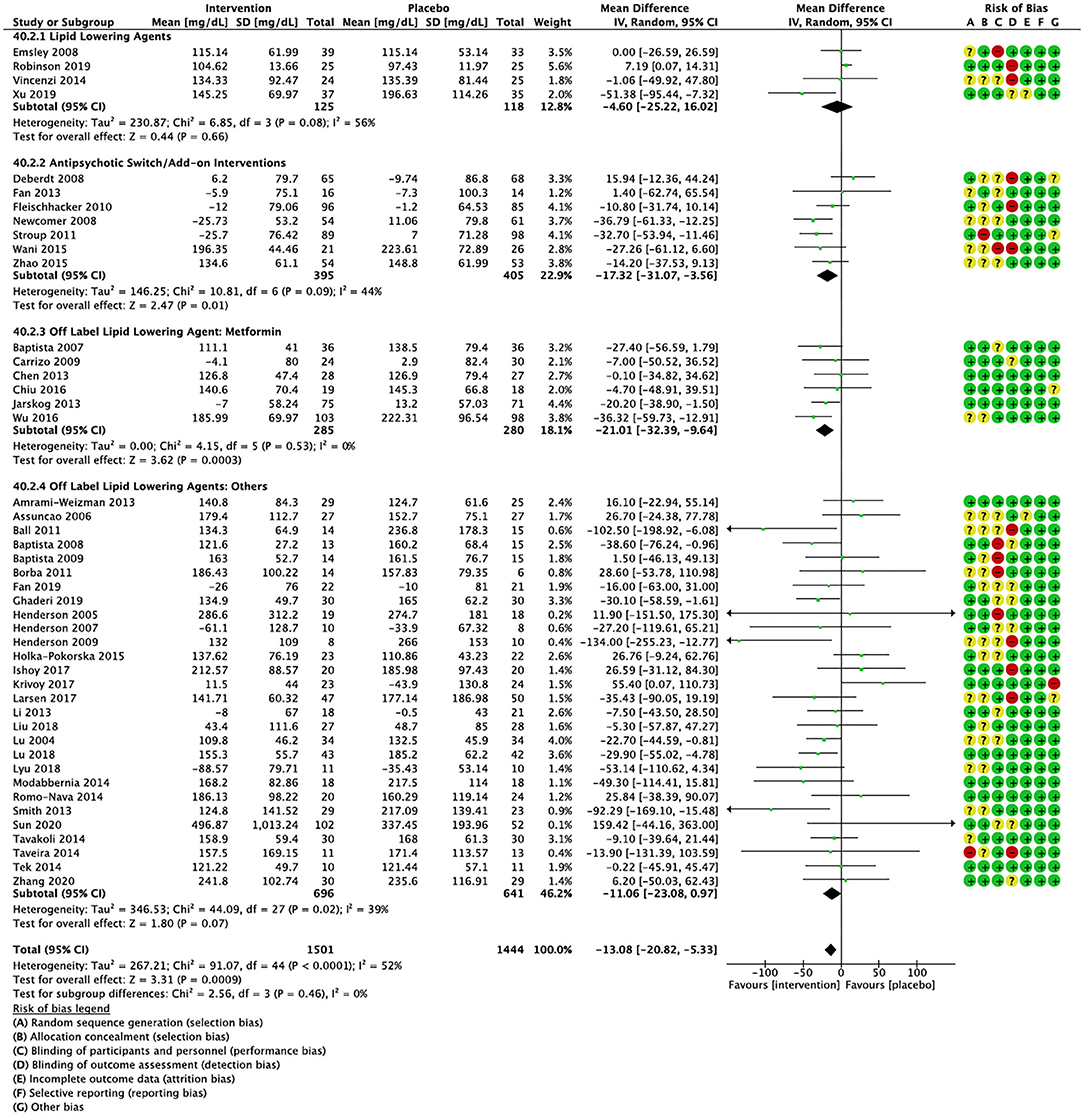
Figure 2. Forest plot showing the pooled mean difference in changes in triglycerides (mg/dL) for all interventions as compared to placebo along with subgroup analyses and Risk of Bias assessments.
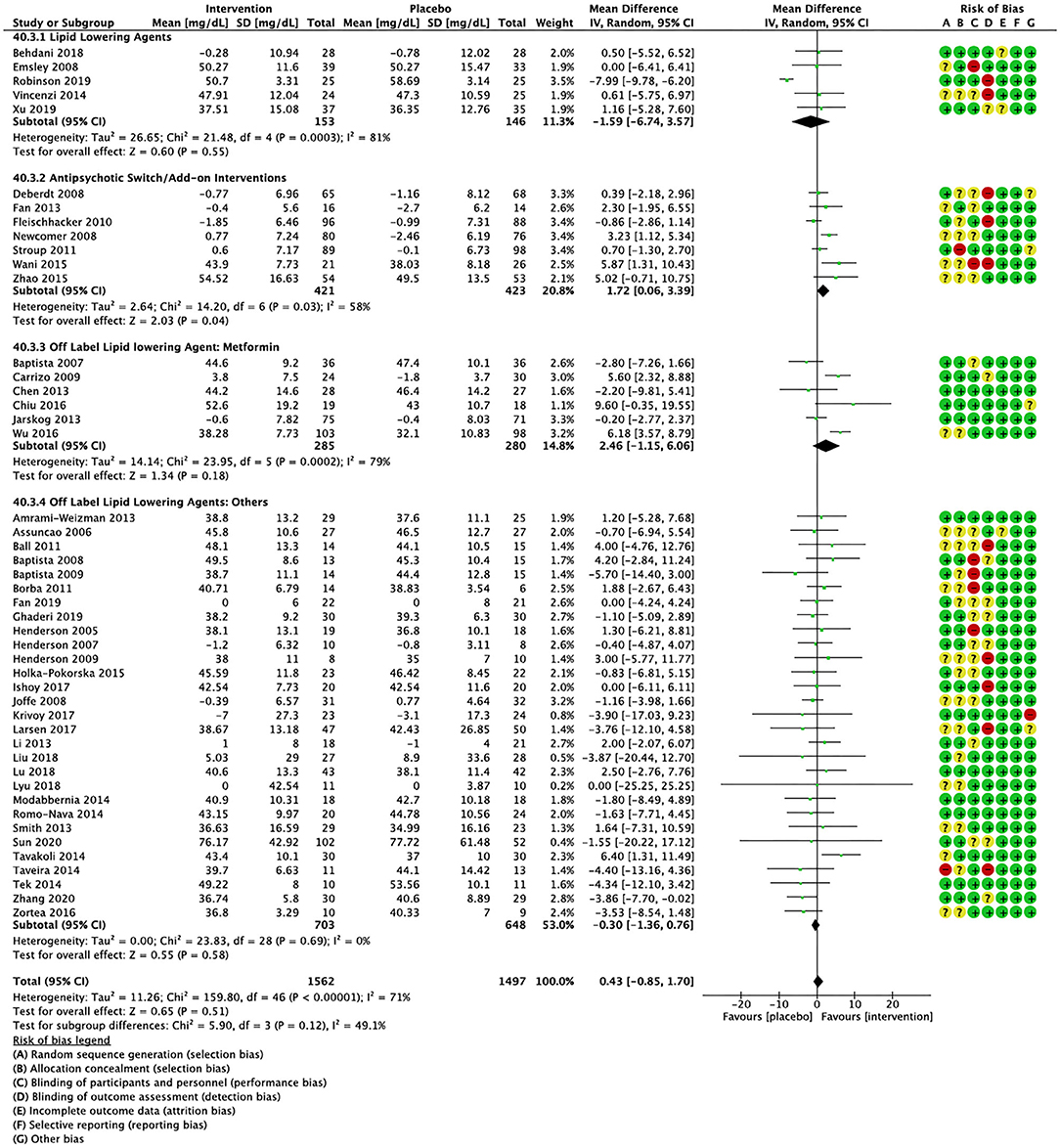
Figure 3. Forest plot showing the pooled mean difference in changes in high-density lipoprotein cholesterol (mg/dL) for all interventions as compared to placebo along with subgroup analyses and Risk of Bias assessments.
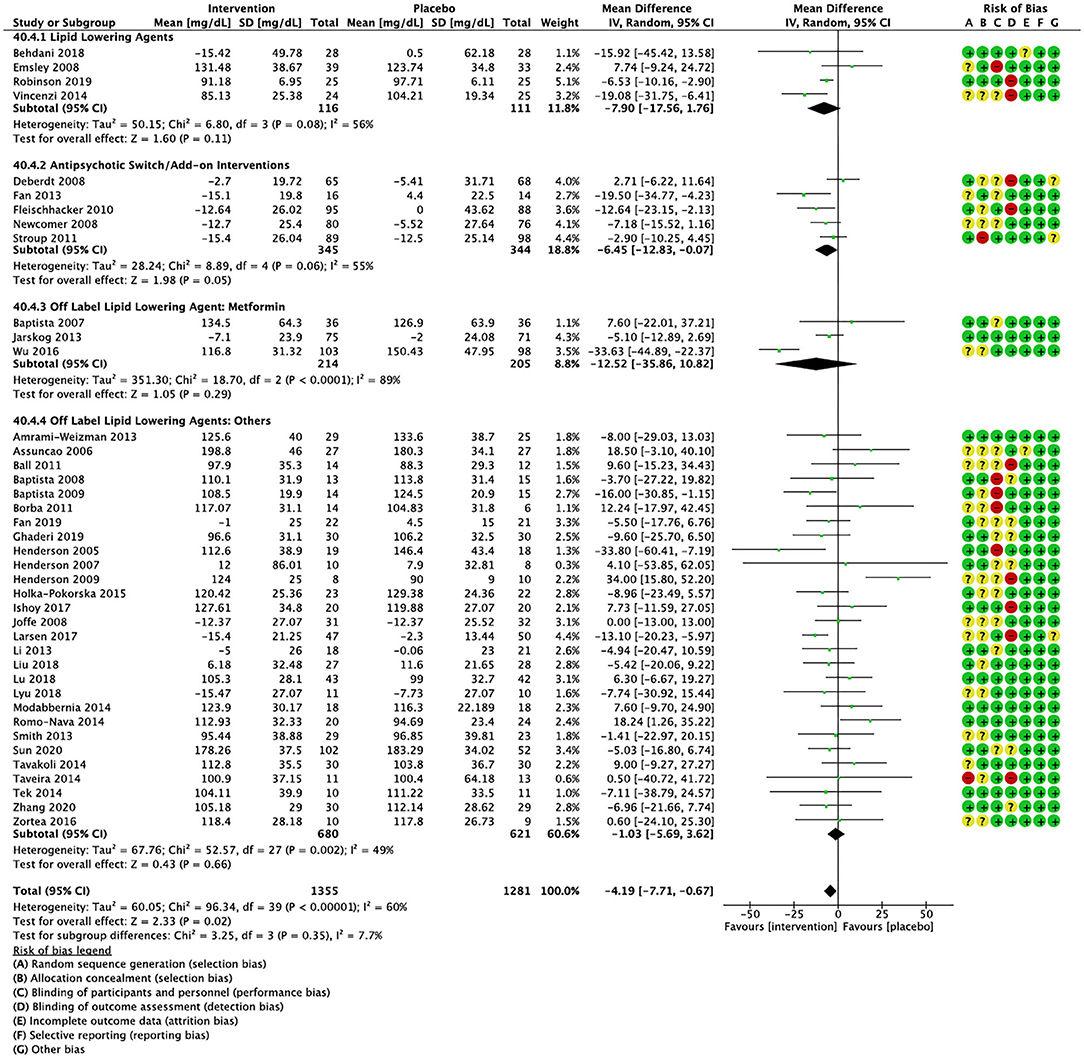
Figure 4. Forest plot showing the pooled mean difference in changes in low-density lipoprotein cholesterol (mg/dL) for all interventions as compared to placebo along with subgroup analyses and Risk of Bias assessments.
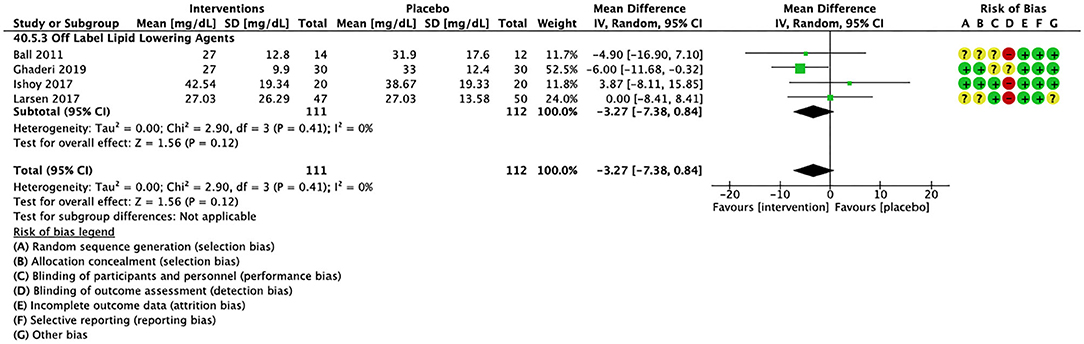
Figure 5. Forest plot showing the pooled mean difference in changes in very low-density lipoprotein cholesterol (mg/dL) for all interventions as compared to placebo and Risk of Bias assessments.
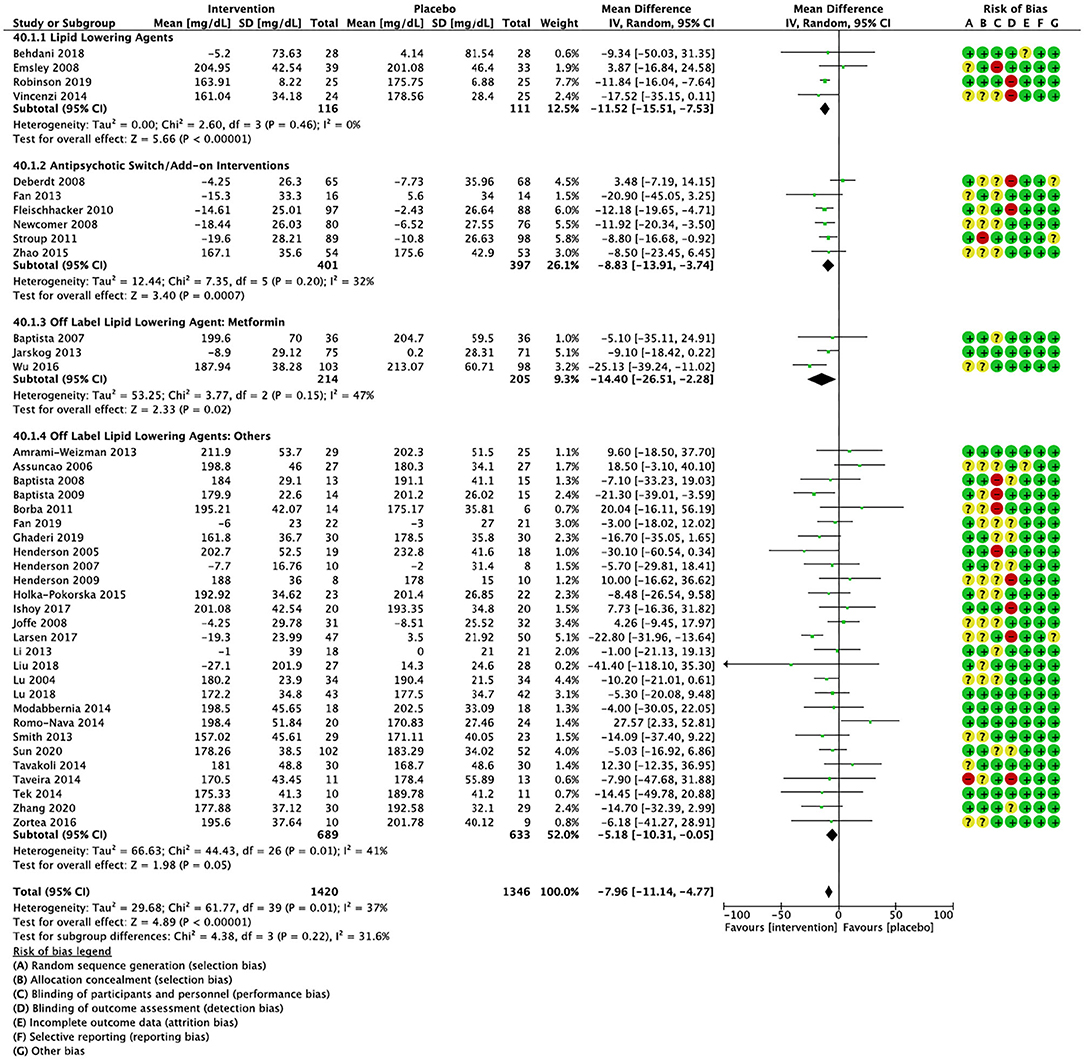
Figure 6. Forest plot showing the pooled mean difference in changes in total cholesterol (mg/dL) for all interventions as compared to placebo along with subgroup analyses and Risk of Bias assessments.
Lipid lowering agents were associated with significant reductions in total cholesterol compared to placebo (Figure 6; N = 4, n = 227; WMD = −11.52 mg/dL, CI: −15.51, −7.53; p < 0.00001; I2 = 0). There were no significant differences in triglycerides (Figure 2; N = 4, n = 243; I2 = 56), HDL cholesterol (Figure 3; N = 5, n = 299; I2 = 81), and LDL cholesterol (Figure 4; N = 4, n = 227; I2 = 56) levels. None of the lipid lowering agent studies examined VLDL cholesterol.
Antipsychotic switch/add-on strategies were associated with significant decreases in triglycerides (Figure 2; N = 7, n = 800; WMD = −17.32 mg/dL, CI: −31.07, −3.56; p = 0.01; I2 = 44), LDL cholesterol (Figure 4; N = 5, n = 689; WMD = −6.45 mg/dL, CI: −12.83, −0.07; p = 0.05; I2 = 55), and total cholesterol levels (Figure 6; N = 6, n = 798; WMD = −8.83 mg/dL; CI: −13.91, −3.74; p = 0.0007; I2 = 32) in comparison to placebo. A significant increase was noted for HDL cholesterol level (Figure 3; N = 7, n = 844; WMD = 1.72 mg/dL; CI: 0.06, 3.39; p = 0.04; I2 = 58). None of the antipsychotic switching/add-on studies examined VLDL cholesterol.
Metformin was associated with significant reductions in triglycerides (Figure 2; N = 6, n = 565; WMD = −21.01 mg/dL, CI: −32.39, −9.64; p = 0.0003; I2 = 0) and total cholesterol (Figure 6; N = 3, n = 419; WMD = −14.40 mg/dL; CI: −26.51, −2.28; p = 0.02; I2 = 47) compared to placebo. No significant changes were noted for HDL cholesterol (Figure 3; N = 6, n = 565; I2 = 79) and LDL cholesterol (Figure 4; N = 3, n = 419; I2 = 89). None of the metformin studies examined VLDL cholesterol.
For other off label lipid lowering agents, there was a statistically significant reduction in total cholesterol levels (Figure 6; N = 27, n = 1,322; WMD = −5.18 mg/dL, CI: −10.31, −0.05; p = 0.05; I2 = 41) along with a decreasing trend in levels of triglycerides that was nonsignificant (Figure 2; N = 28, n = 1,337; WMD = −11.06, CI: −23.08, 0.97; p = 0.07; I2 = 39). There were no significant differences for LDL cholesterol (Figure 4; N = 28, n = 1,301; I2 = 49), HDL cholesterol (Figure 3; N = 29, n = 1,351; I2 = 0), and VLDL cholesterol (Figure 5; N = 4; n = 223; I2 = 0).
Cumulatively, the pharmacological interventions reviewed in this paper were associated with significant reductions in body weight (Supplementary Figure 1; N = 38, n = 2,380; WMD = −1.13 kg, CI: −2.18, −0.08; p = 0.03), BMI (Supplementary Figure 2; N = 36, n = 2,174; WMD = −0.42 kg/m2, CI: −0.85, 0.01; p = 0.05), and waist circumference (Supplementary Figure 3; N = 29, n = 1,532; WMD = −1.34 cm, CI: −2.34, −0.34; p = 0.009) compared to placebo. As for glucose-related parameters, interventions led to significant decreases in blood insulin (Supplementary Figure 4; N = 24, n = 1,636; WMD = −1.64 mIU/mL, CI: −2.76, −0.52; p = 0.004) and HOMA-IR (Supplementary Figure 5; N = 16, n = 867; WMD = −0.52, CI: −0.89, −0.15; p = 0.005) compared to placebo. Blood glucose levels showed a decreasing trend, but the difference was not significant (Supplementary Figure 6; N = 46, n = 3,048; WMD = −1.17 mg/dL, CI: −2.44, −0.11; p = 0.07). Differences in HbA1c levels were also not significant (Supplementary Figure 7; N = 19, n = 1,097). Total PANSS scores showed a trend toward improvement in the intervention group, but the difference again was not statistically significant (Supplementary Figure 8; N = 13, n = 1,005, WMD = −2.15; CI: −4.45, 0.16; p = 0.07). Finally, there were no significant differences in systolic blood pressure (Supplementary Figure 9; N = 16, n = 892) and diastolic blood pressure (Supplementary Figure 10; N = 15, n = 845).
Risk of bias in random sequence generation was deemed to be low in 29 studies, high in 1, and unclear in 18 (Supplementary Figure 11). Outcomes did not change significantly after the study with high risk of bias was removed.
In this systematic review and meta-analysis, we examined different pharmacological interventions used to treat antipsychotic-induced dyslipidemia in schizophrenia spectrum disorders. The 29 pharmacological interventions analyzed were cumulatively effective in lowering total cholesterol, LDL cholesterol, and triglycerides, while increasing HDL cholesterol. However, improvements were not significant with VLDL cholesterol. Amongst the subgroups analyzed, we found that antipsychotic switching/add-on proved most effective in improving lipid parameters commonly dysregulated in schizophrenia, namely triglycerides and HDL cholesterol (77). Notably, the off-label lipid lowering agent metformin was more promising than approved lipid lowering agents in decreasing triglycerides and total cholesterol levels. However, other off label agents only showed a trend in improving lipid parameters.
Our findings suggest that off label strategies can be effectively employed to ameliorate antipsychotic-induced dyslipidemia. In particular, metformin shows considerable promise, improving lipid parameters and showing consistent association with a decrease in triglycerides and total cholesterol levels. Similar findings for triglycerides and total cholesterol levels were previously demonstrated in a review by Jiang et al. (78), and in the context of schizophrenia would benefit through evidence specific to long-term outcomes. Prior studies indicate that a 40 mg/dL reduction in LDL cholesterol and triglycerides translates into a 20% and 4–5% decrease in risk for developing cardiovascular disease, respectively, independent of baseline risk (79). Given this, our review suggests that the available strategies for targeting dyslipidemia are inadequate, reinforcing the need for novel, more effective interventions. Furthermore, while our findings provide strong evidence for antipsychotic switch/add-on interventions, study duration ranged from 6 to 24 weeks, which does not provide adequate time to assess the long-term effects of these treatments on dyslipidemia. While aripiprazole and quetiapine are both second generation antipsychotics with less severe metabolic side-effects compared to others like olanzapine and clozapine, they have their own metabolic burden that cannot be ignored and needs to be better understood over the longer term (80, 81). Similarly, while our findings suggest that lipid lowering agents are not effective in improving dyslipidemia, these results may have been limited by the short duration of the included studies. The small number of studies also did not permit examination of the possible effects of dose of lipid-lowering agents.
Current studies provide general support for the potential effectiveness of pharmacological interventions, but further research is warranted to refining recommendations pertaining to individual treatments. Currently in many studies, lipid management was not a primary focus; of the 48 reviewed studies, only 26 identified lipid profile as a primary outcome measure, in contrast to 22 where it was positioned as a secondary outcome. More studies focused on this area of research sets the stage for additional insights and the increased power necessary to detect not only beneficial outcomes, but also the elucidation of specific variables contributing to effective treatment. At present, the significant heterogeneity among studies within intervention categories limit generalizations that can be made with respect to mechanisms or interacting variables. Factors affecting outcomes may include specific antipsychotic treatment, diagnosis and stage of illness, co-morbid health conditions, concomitant medications, and duration of antipsychotic and/or lipid intervention treatment. Differential pharmacological interventions may, in fact, vary as a function of patient population. Moreover, to date, many interventions are confined to a single study, precluding pooled data or comparisons between interventions. Our review restricted the population to schizophrenia patients, even though antipsychotics are used to treat patients with other psychiatric illnesses such as affective disorders who also share the metabolic burden (82) and may benefit from the reviewed interventions. Finally, while behavioral and lifestyle interventions remain first-line treatments for dyslipidemia, our review restricted the search to pharmacological interventions.
Given the prevalence of dyslipidemia in schizophrenia, along with the associated increased risk of metabolic complications and cardiovascular disease, it is imperative that such studies be undertaken. At present, dyslipidemia is often left untreated (13–15); indeed, the physical health of this population is generally overlooked while the focus is directed to managing psychotic symptoms (83, 84). The integration of psychiatric and medical care falls short at present (85); however, this overview of dyslipidemia, its prevalence and current treatment underscores the need to ensure a more comprehensive model of care be implemented.
The original contributions generated for the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
PK, SMA, and MKH contributed to developing the original protocol. PK and KC-D contributed to the original screening, data extraction, risk of bias assessments, and writing the first draft of the manuscript (introduction, methods, and results). FP and JL wrote and registered the protocol with PROSPERO, re-ran the search, updated study selection and risk of bias, and contributed to final data extraction and synthesis prior to manuscript submission, as well as updating the first draft. LH assisted with editing and writing the final draft. SMA was involved in supervising all aspects of the review. GR and MKH contributed to editing the final draft. All authors contributed to the article and approved the submitted version.
SMA is supported by in part by an Academic Scholars Award from the Department of Psychiatry, University of Toronto and has grant support from the Canadian Institutes of Health Research, PSI foundation, Ontario, and the CAMH. MKH was awarded the Cardy Family Schizophrenia Research Chair. GR has received research support from the Canadian Institutes of Health Research (CIHR), University of Toronto, Research Hospital Fund–Canada Foundation for Innovation (RHF-CFI), and HLS Therapeutics Inc. KC-D has received support through scholarships from the Canadian Institutes of Health Research (Canada Graduate Scholarship Masters Award) and the Banting and Best Diabetes Center, University of Toronto. JL is supported by the Ontario Graduate Scholarship and an award from the Institute of Medical Science, University of Toronto.
MKH has received Alkermes consultation fees. GR has received advisory board support from HLS Therapeutics and consultant fees from Mitsubishi Tanabe Pharma Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.642403/full#supplementary-material
1. World Health Organization. Global Status Report on Noncommunicable Diseases 2014. Geneva: World Health Organization (2014).
2. Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders baseline results from the RAISE-ETP study. JAMA Psychiatry. (2014) 71:1350–63. doi: 10.1001/jamapsychiatry.2014.1314
3. Kaddurah-Daouk R, McEvoy J, Baillie R, Zhu H, Yao JK, Nimgaonkar VL, et al. Impaired plasmalogens in patients with schizophrenia. Psychiatry Res. (2012) 198:347–52. doi: 10.1016/j.psychres.2012.02.019
4. Schwarz E, Prabakaran S, Whitfield P, Major H, Leweke FM, Koethe D, et al. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J Proteome Res. (2008) 7:4266–77. doi: 10.1021/pr800188y
5. Thakore JH. Metabolic disturbance in first-episode schizophrenia. Br J Psychiatry Suppl. (2004) 47:S76–9. doi: 10.1192/bjp.184.47.s76
6. Verma SK, Subramaniam M, Liew A, Poon LY. Metabolic risk factors in drug-naive patients with first-episode psychosis. J Clin Psychiatry. (2009) 70:997–1000. doi: 10.4088/JCP.08m04508
7. Petruzzelli MG, Marzulli L, Giannico OV, Furente F, Margari M, Matera E, et al. Glucose metabolism, thyroid function, and prolactin level in adolescent patients with first episode of schizophrenia and affective disorders. Front Psychiatry. (2020) 11:775. doi: 10.3389/fpsyt.2020.00775
8. Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. (1999) 29:697–701. doi: 10.1017/S0033291798008186
9. Strassnig M, Brar JS, Ganguli R. Self-reported body weight perception and dieting practices in community-dwelling patients with schizophrenia. Schizophr Res. (2005) 75:425–32. doi: 10.1016/j.schres.2004.04.007
10. Carton L, Cottencin O, Lapeyre-Mestre M, Geoffroy PA, Favre J, Simon N, et al. Off-Label prescribing of antipsychotics in adults, children and elderly individuals: a systematic review of recent prescription trends. Curr Pharm Des. (2015) 21:3280–97. doi: 10.2174/1381612821666150619092903
11. Paton C, Esop R, Young C, Taylor D. Obesity, dyslipidaemias and smoking in an inpatient population treated with antipsychotic drugs. Acta Psychiatr Scand. (2004) 110:299–305. doi: 10.1111/j.1600-0447.2004.00372.x
12. Saari K, Jokelainen J, Veijola J, Koponen H, Jones PB, Savolainen M, et al. Serum lipids in schizophrenia and other functional psychoses: a general population northern Finland 1966 birth cohort survey. Acta Psychiatr Scand. (2004) 118:510–9. doi: 10.1111/j.1600-0447.2004.00358.x
13. Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. ResSchizophr Res. (2006) 86:15–22. doi: 10.1016/j.schres.2006.06.026
14. Correll CU, Druss BG, Lombardo I, O'Gorman C, Harnett JP, Sanders KN, et al. Findings of a US national cardiometabolic screening program among 10,084 psychiatric outpatients. Psychiatr Serv. (2010) 61:892–8. doi: 10.1176/ps.2010.61.9.892
15. Falissard B, Mauri M, Shaw K, Wetterling T, Doble A, Giudicelli A, et al. The METEOR study: frequency of metabolic disorders in patients with schizophrenia. Focus on first and second generation and level of risk of antipsychotic drugs. Int Clin Psychopharmacol. (2011) 26:291–302. doi: 10.1097/YIC.0b013e32834a5bf6
16. De Hert M, Cohen D, Bobes J, Cetkovich-Bakmas M, Leucht S, Ndetei DM, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. (2011) 10:138–51. doi: 10.1002/j.2051-5545.2011.tb00036.x
17. Henderson DC, Copeland PM, Daley TB, Borba CP, Cather C, Nguyen DD, et al. A double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. Am J Psychiatry. (2005) 162:954–62. doi: 10.1176/appi.ajp.162.5.954
18. Jeste DV, Gladsjo JA, Lindamer LA, Lacro JP. Medical comorbidity in schizophrenia. Schizophr Bull. (1996) 22:413–30. doi: 10.1093/schbul/22.3.413
19. Maayan L, Correll CU. Management of antipsychotic-related weight gain. Expert Rev Neurother. (2010) 10:1175–200 doi: 10.1586/ern.10.85
20. Mizuno Y, Suzuki T, Nakagawa A, Yoshida K, Mimura M, Fleischhacker WW, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. (2014) 40:1385–403. doi: 10.1016/S0920-9964(14)70289-0
21. Papanastasiou E. Interventions for the metabolic syndrome in schizophrenia: a review. Ther Adv Endocrinol Metab. (2012) 3:141–62. doi: 10.1177/2042018812458697
22. Ojala K, Repo-Tiihonen E, Tiihonen J, Niskanen L. Statins are effective in treating dyslipidemia among psychiatric patients using second-generation antipsychotic agents. J Psychopharmacol. (2008) 22:33–8. doi: 10.1177/0269881107077815
23. De Hert M, Kalnicka D, van Winkel R, Wampers M, Hanssens L, Van Eyck D, et al. Treatment with rosuvastatin for severe dyslipidemia in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. (2006) 67:1889–96. doi: 10.4088/JCP.v67n1208
24. Vincenzi B, Borba CP, Gray DA, Copeland PM, Wang X, Fan X, et al. An exploratory study examining lipid-lowering medications in reducing fasting serum lipids in schizophrenia patients treated with atypical antipsychotics. Ann Clin Psychiatry. (2013) 25:141–8.
25. Landry P, Dimitri E, Tessier S, Légaré N. Efficacy of lipid-lowering medications in patients treated with clozapine: a naturalistic study. J Clin Psychopharmacol. (2008) 28:348–9. doi: 10.1097/JCP.0b013e3181727592
26. Behdani F, Roudbaraki SN, Saberi-Karimian M, Tayefi M, Hebrani P, Akhavanrezayat A, et al. Assessment of the efficacy of omega-3 fatty acids on metabolic and inflammatory parameters in patients with schizophrenia taking clozapine and sodium valproate. Psychiatry Res. (2018) 261:243–47. doi: 10.1016/j.psychres.2017.12.028
27. Emsley R, Niehaus DJ, Oosthuizen PP, Koen L, Ascott-Evans B, Chiliza B, et al. Safety of the omega-3 fatty acid, eicosapentaenoic acid (EPA) in psychiatric patients: results from a randomized, placebo-controlled trial. Psychiatry Res. (2008) 161:284–91. doi: 10.1016/j.psychres.2007.06.029
28. Robinson DG, Gallego JA, John M, Hanna LA, Zhang JP, Birnbaum ML, et al. A potential role for adjunctive omega-3 polyunsaturated fatty acids for depression and anxiety symptoms in recent onset psychosis: results from a 16 week randomized placebo-controlled trial for participants concurrently treated with risperidone. Schizophr Res. (2019) 204:295–303. doi: 10.1016/j.schres.2018.09.006
29. Vincenzi B, Stock S, Borba CP, Cleary SM, Oppenheim CE, Petruzzi LJ, et al. A randomized placebo-controlled pilot study of pravastatin as an adjunctive therapy in schizophrenia patients: effect on inflammation, psychopathology, cognition and lipid metabolism. Schizophr Res. (2014) 159:395–403. doi: 10.1016/j.schres.2014.08.021
30. Xu F, Fan W, Wang W, Tang W, Yang F, Zhang Y, et al. Effects of omega-3 fatty acids on metabolic syndrome in patients with schizophrenia: a 12-week randomized placebo-controlled trial. Psychopharmacology. (2019) 236:1273–79. doi: 10.1007/s00213-018-5136-9
31. Tse L, Procyshyn RM, Fredrikson DH, Boyda HN, Honer WG, Barr AM. Pharmacological treatment of antipsychotic-induced dyslipidemia and hypertension. Int Clin Psychopharmacol. (2014) 29:125–37. doi: 10.1097/YIC.0000000000000014
32. Chen Y, Bobo WV, Watts K, Jayathilake K, Tang T, Meltzer HY. Comparative effectiveness of switching antipsychotic drug treatment to aripiprazole or ziprasidone for improving metabolic profile and atherogenic dyslipidemia: a 12-month, prospective, open-label study. J Psychopharmacol. (2012) 26:1201–10. doi: 10.1177/0269881111430748
33. Fan X, Borba CP, Copeland P, Hayden D, Freudenreich O, Goff DC, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. (2013) 127:217–26. doi: 10.1111/acps.12009
34. Fleischhacker WW, Heikkinen ME, Olié JP, Landsberg W, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. (2010) 13:1115–25. doi: 10.1017/S1461145710000490
35. Newcomer JW, Campos JA, Marcus RN, Breder C, Berman RM, Kerselaers W, et al. A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry. (2008) 69:1046–56. doi: 10.4088/JCP.v69n0702
36. Stroup TS, McEvoy JP, Ring KD, Hamer RH, LaVange LM, Swartz MS, et al. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. (2011) 168:947–56. doi: 10.1176/appi.ajp.2011.10111609
37. Wani RA, Dar MA, Chandel RK, Rather YH, Haq I, Hussain A, et al. Effects of switching from olanzapine to aripiprazole on the metabolic profiles of patients with schizophrenia and metabolic syndrome: a double-blind, randomized, open-label study. Neuropsychiatr Dis Treat. (2015) 11:685–93. doi: 10.2147/NDT.S80925
38. Zhao J, Song X, Ai X, Gu X, Huang G, Li X, et al. Adjunctive aripiprazole treatment for risperidone-induced hyperprolactinemia: an 8-week randomized, open-label, comparative clinical trial. PLoS ONE. (2015) 10:e0139717. doi: 10.1371/journal.pone.0139717
39. Higgins JP, Green S. Chapter 7: selecting studies and collecting data. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. (2011). Available online at: www.handbook.cochrane.org (accessed March 2011).
40. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
41. Deberdt W, Lipkovich I, Heinloth AN, Liu L, Kollack-Walker S, Edwards SE, et al. Double-blind, randomized trial comparing efficacy and safety of continuing olanzapine versus switching to quetiapine in overweight or obese patients with schizophrenia or schizoaffective disorder. Ther Clin Risk Manag. (2008) 4:713–20. doi: 10.2147/TCRM.S3153
42. Baptista T, Rangel N, Fernández V, Carrizo E, El Fakih Y, Uzcátegui E, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophr Res. (2007) 93:99–108. doi: 10.1016/j.schres.2007.03.029
43. Carrizo E, Fernández V, Connell L, Sandia I, Prieto D, Mogollón J, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. (2009) 113:19–26. doi: 10.1016/j.schres.2009.05.007
44. Chen CH, Huang MC, Kao CF, Lin SK, Kuo PH, Chiu CC, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. (2013) 74:e424–30. doi: 10.4088/JCP.12m08186
45. Chiu CC, Lu ML, Huang MC, Chen PY, Lin YK, Lin SK, et al. Effects of low dose metformin on metabolic traits in clozapine-treated schizophrenia patients: an exploratory twelve-week randomized, double-blind, placebo-controlled study. PLoS ONE. (2016) 11:e0168347. doi: 10.1371/journal.pone.0168347
46. Jarskog LF, Hamer RM, Catellier DJ, Stewart DD, Lavange L, Ray N, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. (2013) 170:1032–40. doi: 10.1176/appi.ajp.2013.12010127
47. Wu RR, Zhang FY, Gao KM, Ou JJ, Shao P, Jin H, et al. Metformin treatment of antipsychotic-induced dyslipidemia: an analysis of two randomized, placebo-controlled trials. Mol Psychiatry. (2016) 21:1537–44. doi: 10.1038/mp.2015.221
48. Amrami-Weizman A, Maayan R, Gil-Ad I, Pashinian A, Fuchs C, Kotler M, et al. The effect of reboxetine co-administration with olanzapine on metabolic and endocrine profile in schizophrenia patients. Psychopharmacology. (2013) 230:23–7. doi: 10.1007/s00213-013-3199-1
49. Assunção SSM, Ruschel SI, Rosa LCR, Campos JAO, Alves MJO, Bracco OL, et al. Weight gain management in patients with schizophrenia during treatment with olanzapine in association with nizatidine. Braz J Psychiatry. (2006) 28:270–76. doi: 10.1590/S1516-44462006000400005
50. Ball MP, Warren KR, Feldman S, McMahon RP, Kelly DL, Buchanan RW. Placebo-controlled trial of atomoxetine for weight reduction in people with schizophrenia treated with clozapine or olanzapine. Clin Schizophr Relat Psychoses. (2011) 5:17–25. doi: 10.3371/CSRP.5.1.3
51. Baptista T, Uzcátegui E, Rangel N, El Fakih Y, Galeazzi T, Beaulieu S, et al. Metformin plus sibutramine for olanzapine-associated weight gain and metabolic dysfunction in schizophrenia: a 12-week double-blind, placebo-controlled pilot study. Psychiatry Res. (2008) 159:250–3. doi: 10.1016/j.psychres.2008.01.011
52. Baptista T, Rangel N, El Fakih Y, Uzcátegui E, Galeazzi T, Beaulieu S, et al. Rosiglitazone in the assistance of metabolic control during olanzapine administration in schizophrenia: a pilot double-blind, placebo-controlled 12-week trial. Pharmacopsychiatry. (2009) 42:14–9. doi: 10.1055/s-0028-1085438
53. Henderson DC, Fan X, Sharma B, Copeland PM, Borba CP, Boxill R, et al. A double-blind, placebo-controlled trial of rosiglitazone for clozapine-induced glucose metabolism impairment in patients with schizophrenia. Acta Psychiatr Scand. (2009) 119:457–65. doi: 10.1111/j.1600-0447.2008.01325.x
54. Borba CP, Fan X, Copeland PM, Paiva A, Freudenreich O, Henderson DC. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. (2011) 31:653–8. doi: 10.1097/JCP.0b013e31822bb573
55. Fan X, Copeland P, Nawras S, Harrington A, Freudenreich O, Goff DC, et al. Adjunctive telmisartan treatment on body metabolism in clozapine or olanzapine treated patients with schizophrenia: a randomized, double blind, placebo controlled trial. Psychopharmacology. (2019) 236:1949–57. doi: 10.1007/s00213-019-5181-z
56. Ghaderi A, Banafshe HR, Mirhosseini N, Moradi M, Karimi MA, Mehrzad F, et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry. (2019) 19:77. doi: 10.1186/s12888-019-2059-x
57. Henderson DC, Fan X, Copeland PM, Borba CP, Daley TB, Nguyen DD, et al. A double-blind, placebo-controlled trial of sibutramine for clozapine-associated weight gain. Acta Psychiatr Scand. (2007) 115:101–5. doi: 10.1111/j.1600-0447.2006.00855.x
58. Holka-Pokorska JA, Radzio R, Jarema M, Wichniak A. The stabilizing effect of dehydroepiandrosterone on clinical parameters of metabolic syndrome in patients with schizophrenia treated with olanzapine—a randomized, double-blind trial. Psychiatr Pol. (2015) 49:363–76. doi: 10.12740/PP/30180
59. Ishøy PL, Knop FK, Broberg BV, Bak N, Andersen UB, Jørgensen NR, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes Obes Metab. (2017) 19:162–71. doi: 10.1111/dom.12795
60. Joffe G, Takala P, Tchoukhine E, Hakko H, Raidma M, Putkonen H, et al. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. (2008) 69:706–11. doi: 10.4088/JCP.v69n0503
61. Krivoy A, Onn R, Vilner Y, Hochman E, Weizman S, Paz A, et al. Vitamin D supplementation in chronic schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled clinical trial. EBioMedicine. (2017) 26:138–45. doi: 10.1016/j.ebiom.2017.11.027
62. Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI, Svensson CK, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. (2017) 74:719–28. doi: 10.1001/jamapsychiatry.2017.1220
63. Li J, Li X, Liu E, Copeland P, Freudenreich O, Goff DC, et al. No effect of adjunctive, repeated dose intranasal insulin treatment on body metabolism in patients with schizophrenia. Schizophr Res. (2013) 146:40–5. doi: 10.1016/j.schres.2013.01.034
64. Liu F, Xie L, Zhang B, Ruan Y, Zeng Y, Xu X, et al. No effect of adjunctive minocycline treatment on body metabolism in patients with schizophrenia. J Clin Psychopharmacol. (2018) 38:125–8. doi: 10.1097/JCP.0000000000000841
65. Lu ML, Chen TT, Kuo PH, Hsu CC, Chen CH. Effects of adjunctive fluvoxamine on metabolic parameters and psychopathology in clozapine-treated patients with schizophrenia: a 12-week, randomized, double-blind, placebo-controlled study. Schizophr Res. (2018) 193:126–33. doi: 10.1016/j.schres.2017.06.030
66. Lu ML, Lane HY, Lin SK, Chen KP, Chang WH. Adjunctive fluvoxamine inhibits clozapine-related weight gain and metabolic disturbances. J Clin Psychiatry. (2004) 65:766–71. doi: 10.4088/JCP.v65n0607
67. Lyu X, Du J, Zhan G, Wu Y, Su H, Zhu Y, et al. Naltrexone and bupropion combination treatment for smoking cessation and weight loss in patients with schizophrenia. Front Pharmacol. (2018) 9:181. doi: 10.3389/fphar.2018.00181
68. Modabbernia A, Heidari P, Soleimani R, Sobhani A, Roshan ZA, Taslimi S, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. (2014) 53:133–40. doi: 10.1016/j.jpsychires.2014.02.013
69. Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, Saracco Alvarez R, Becerra-Palars C, Moreno J, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. (2014) 16:410–21. doi: 10.1111/bdi.12196
70. Smith RC, Jin H, Li C, Bark N, Shekhar A, Dwivedi S, et al. Effects of pioglitazone on metabolic abnormalities, psychopathology, and cognitive function in schizophrenic patients treated with antipsychotic medication: a randomized double-blind study. Schizophr Res. (2013) 143:18–24. doi: 10.1016/j.schres.2012.10.023
71. Sun F, Ren Z, Jiang Y, Fang X, Wang N, Jin W. A placebo-controlled study on the treatment of metabolic syndrome of qi stagnation and dampness obstruction related to atypical antipsychotics with traditional chinese medicine (TCM). Evid Based Complement Alternat Med. (2020) 2020:5103046. doi: 10.1155/2020/5103046
72. Tavakoli E, Rezaei O, Fadai R. A comparison on composition of celery, dill and green tea as three medicinal plants with placebo to treat schizophrenia patients' metabolic syndrome. Afinidad. (2014) 80:1118–23.
73. Taveira TH, Wu WC, Tschibelu E, Borsook D, Simonson DC, Yamamoto R, et al. The effect of naltrexone on body fat mass in olanzapine-treated schizophrenic or schizoaffective patients: a randomized double-blind placebo-controlled pilot study. J Psychopharmacol. (2014) 28:395–400. doi: 10.1177/0269881113509904
74. Tek C, Ratliff J, Reutenauer E, Ganguli R, O'Malley SS. A randomized, double-blind, placebo-controlled pilot study of naltrexone to counteract antipsychotic-associated weight gain: proof of concept. J Clin Psychopharmacol. (2014) 34:608–12. doi: 10.1097/JCP.0000000000000192
75. Zhang L, Han Y, Zhao Z, Liu X, Xu Y, Cui G, et al. Beneficial effects of konjac powder on lipid profile in schizophrenia with dyslipidemia: a randomized controlled trial. Asia Pac J Clin Nutr. (2020) 29:505–12. doi: 10.6133/apjcn.202009_29(3).0009
76. Zortea K, Franco VC, Francesconi LP, Cereser KM, Lobato MI, Belmonte-de-Abreu PS. Resveratrol supplementation in schizophrenia patients: a randomized clinical trial evaluating serum glucose and cardiovascular risk factors. Nutrients. (2016) 8:73. doi: 10.3390/nu8020073
77. Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry. (2007) 68 (Suppl. 1):20–7.
78. Jiang WL, Cai DB, Yin F, Zhang L, Zhao XW, He J, et al. Adjunctive metformin for antipsychotic-induced dyslipidemia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Transl Psychiatry. (2020) 10:117. doi: 10.1038/s41398-020-0785-y
79. Marston NA, Giugliano RP, Im K, Silverman MG, O'Donoghue ML, Wiviott SD, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. (2019) 140:1308–17. doi: 10.1161/CIRCULATIONAHA.119.041998
80. Briles JJ, Rosenberg DR, Brooks BA, Roberts MW, Diwadkar VA. Review of the safety of second-generation antipsychotics: are they really “atypically” safe for youth and adults? Prim Care Companion CNS Disord. (2012) 14:PCC.11r01298. doi: 10.4088/PCC.11r01298
81. Komossa K, Rummel-Kluge C, Schmid F, Hunger H, Schwarz S, El-Sayeh HG, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. (2009) 4:CD006569. doi: 10.1002/14651858.CD006569.pub3
82. Mazereel V, Detraux JD, Vancampfort D, van Winkel R, De Hert M. Impact of psychotropic medication effects on obesity and the metabolic syndrome in people with serious mental illness. Front Endocrinol. (2020) 11:573479. doi: 10.3389/fendo.2020.573479
83. Millar H. Management of physical health in schizophrenia: a stepping stone to treatment success. Eur Neuropsychopharmacol. (2008) 18 (Suppl. 2):S121–8. doi: 10.1016/j.euroneuro.2008.02.002
84. Berry A, Drake RJ, Yung AR. Examining healthcare professionals' beliefs and actions regarding the physical health of people with schizophrenia. BMC Health Serv Res. (2020) 20:771. doi: 10.1186/s12913-020-05654-z
Keywords: schizophrenia, antipsychotics, dyslipidemia, systematic review, meta-analysis
Citation: Kanagasundaram P, Lee J, Prasad F, Costa-Dookhan KA, Hamel L, Gordon M, Remington G, Hahn MK and Agarwal SM (2021) Pharmacological Interventions to Treat Antipsychotic-Induced Dyslipidemia in Schizophrenia Patients: A Systematic Review and Meta Analysis. Front. Psychiatry 12:642403. doi: 10.3389/fpsyt.2021.642403
Received: 16 December 2020; Accepted: 16 February 2021;
Published: 17 March 2021.
Edited by:
Błażej Misiak, Wroclaw Medical University, PolandReviewed by:
Takefumi Suzuki, University of Yamanashi, JapanCopyright © 2021 Kanagasundaram, Lee, Prasad, Costa-Dookhan, Hamel, Gordon, Remington, Hahn and Agarwal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sri Mahavir Agarwal, TWFoYXZpci5BZ2Fyd2FsQGNhbWguY2E=
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.