- 1Department of General Surgery, Molecular Oncology, and Immunotherapy, Rostock University Medical Center, Rostock, Germany
- 2Department of Plastic Surgery, Southern University of Science and Technology Hospital, Shenzhen, China
- 3Institute for Experimental Surgery, Rostock University Medical Center, Rostock, Germany
- 4Department of General Surgery, Carson International Cancer Research Center, Shenzhen University General Hospital, Shenzhen University Clinical Medical Academy, Shenzhen, China
- 5Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, School of Biomedical Engineering, Shenzhen University Health Science Center, Shenzhen, China
- 6Guangdong Key Laboratory of Regional Immunity and Diseases, Shenzhen University Health Science Center, Shenzhen, China
Background: If the diagnosis of neuroendocrine neoplasm (NEN) increases the risk of patients to commit suicide has not been investigated so far. Identifying NEN patients at risk to commit suicide is important to increase their life quality and life expectancy.
Methods and findings: Cancer cases were extracted from the Surveillance, Epidemiology, and End Results program and were divided into the NEN and the non-NEN cohorts. Subsequently, the NEN patients were randomly split into a training data set and a validation data set. Analyzing the training data set, we developed a score for assessing the risk to commit suicide for patients with NEN. In addition, we validated the score using the validation data set and evaluated, if this score could also be applied to other cancer entities by using the test data set, a non-NEN cohort. The odds ratio (OR) of suicide between NEN and non-NEN patients was determined. Moreover, the performance of a score was evaluated by the receiver operating characteristic curve and the area under the curve (AUC). Compared to non-NEN, NEN significantly increased the risk of suicide to 1.8-fold (NEN vs. non-NEN; OR, 1.832; P < 0.001). In addition, we observed that age, gender, race, marital status, tumor stage, histologic grade, surgery, and chemotherapy were associated with suicide among NEN patients; and a synthesized score based on these factors could significantly distinguish suicide individuals from non-suicide individuals in the training data set (AUC, 0.829; P < 0.001) and in the validation data set (AUC, 0.735; P < 0.001). This score also had a good performance when it was assessed by the test data set (AUC, 0.690; P < 0.001). This demonstrates that the score might also be applicable to other cancer entities.
Conclusions: This population-based study suggests that NEN patients have a higher risk of suicide than non-NEN patients. In addition, this study provided a score, which can identify NEN patients at high-risk of committing suicide. Thus, this score in combination with current screening and prevention strategies for suicide may improve life quality and life expectancy of NEN patients.
Introduction
Neuroendocrine neoplasm (NEN) arises from neuroendocrine cells and is commonly observed in lung, stomach, intestines, and pancreas (1, 2). Recent evidence proved that the incidence of lung and gastroenteropancreatic NEN increased 6.4-fold from 1973 to 2012 in the USA (3). Interestingly, distinct from other tumors, NEN is indolent. In addition, the survival time of these patients is longer than that of patients suffering from adenocarcinoma or squamous cell carcinoma (3). Hence, improving the surveillance and quality of life after diagnosis of NEN has become an important clinical issue.
Suicide has developed into an enormous public health problem all around the globe, and it is one of the leading causes of death in the USA (4–6). Notably, several studies suggest that the suicide incidence of cancer survivors is significantly higher than that of patients suffering from chronic diseases or in the general population (7, 8). However, suicide can potentially be prevented, when individuals at risk for suicide are identified and proper psychological support is given. A mandatory prerequisite is the effective recognition of patients at elevated risk of suicide.
Previous studies suggest that NEN is positively associated with psychiatric disorder symptoms, such as depression, anxiety, or psychosis (9–11). In addition, it is well-known that psychiatric disease is a master contributor to suicides (12). Thus, we hypothesized that NEN may increase the risk of suicide when directly compared to non-NEN. However, to our knowledge, no study addressing this issue has been published. Even though several studies have emphasized the screening of anxiety and depression (13), and some studies have investigated the risk factors of suicide (14–16), none of them provided and evaluated a strategy, which can effectively identify NEN patients at an elevated risk of suicide.
Thus, the primary purpose of this study was to evaluate if NEN increases the risk of suicide. Secondly, we developed and evaluated a score, which may identify NEN patients at high risk for committing suicide. In this study, we observed that NEN significantly increase the risk of suicide, and we developed and validated a score to identify patients at high-risk for committing of suicide. The score in combination with current screening and psychological management of potential suicides might help to prevent or at least reduce suicidal death in patients suffering from neuroendocrine neoplasms.
Patients and Methods
Data Source and Study Population
Surveillance, Epidemiology, and End Results (SEER) program is an authoritative source of cancer patients' information in the USA. Dependent on the number of registries, the SEER program has four databases: SEER 9, SEER 13, SEER 18, and SEER 21. This study was performed with the data obtained from SEER 18, which includes ~28% of the USA population (17).
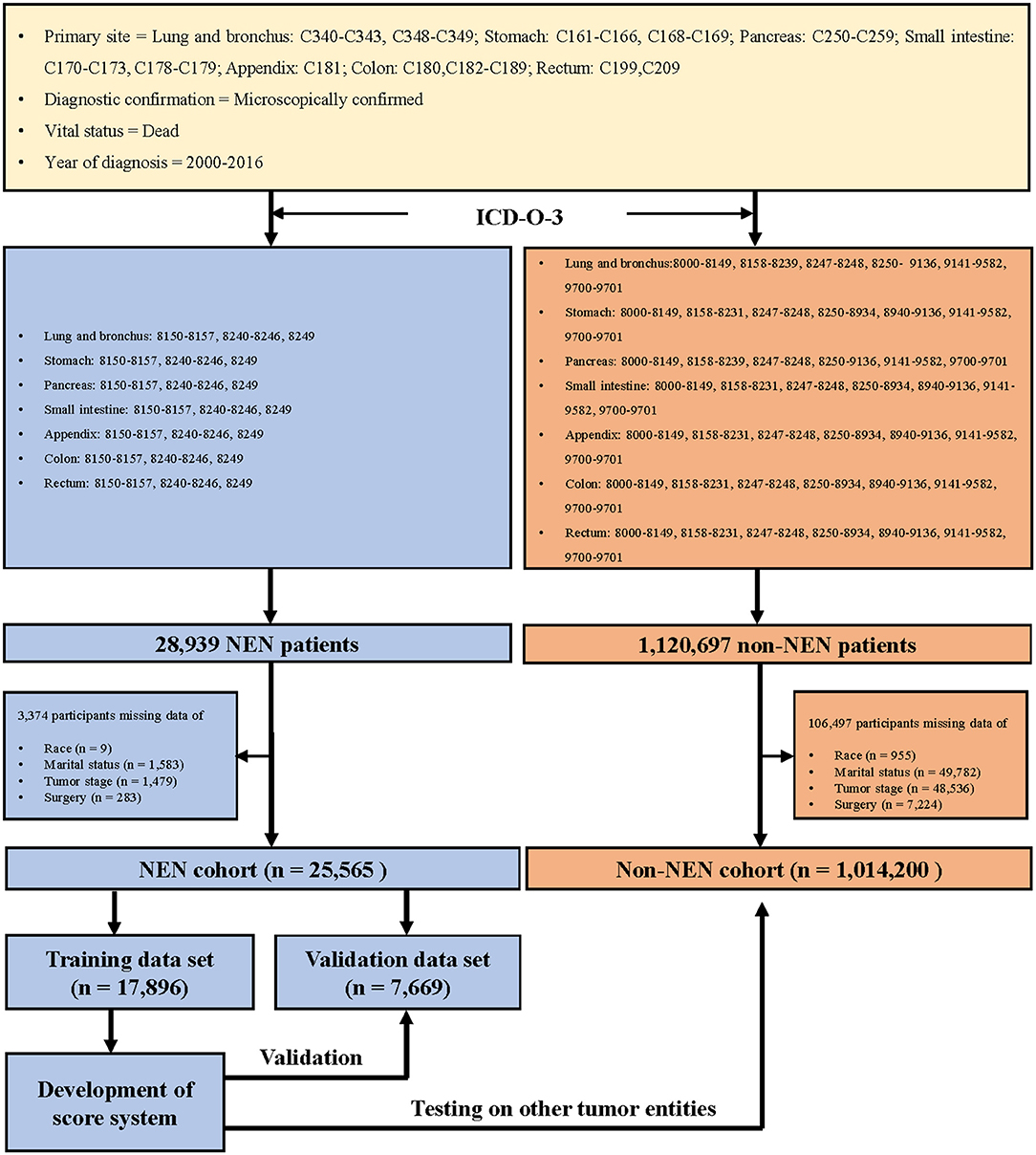
We collected the information of patients who were diagnosed with cancer during the years 2000 to 2016, with the support of SEER*Stat 8.3.6 version (18), and the topography codes (3) (lung and bronchus: C340–C343, C348–C349; stomach: C161–C166, C168–C169; pancreas: C250–C259; small intestine: C170–C173, C178–C179; appendix: C181; colon: C180 and C182–C189; rectum: C199 and C209). In addition, the patients were classified as NEN or non-NEN patients with the help of the International Classification of Disease for Oncology codes, 3rd edition (3) (Figure 1).
Study Variables
Notably, the SEER program does not record the suicidal ideation or previous suicide attempts. We defined suicidal behavior dependent on the code of 50,220, which suggested that the patients died of suicide and self-inflicted injury (16). In order to determine the proportionate mortality ratio (PMR) of suicide, we identified the dead cases from the SEER program.
In order to determine the risk factors of suicide, we defined the following variables as covariates: population characteristics (age at diagnosis, gender, race, and marital status); tumor characteristics (tumor stage and histologic grade), and treatment (surgery and chemotherapy). Notably, the histologic grade of tumors was not defined according to the recommendations of WHO and the European Neuroendocrine Tumor Society classification (19, 20). It was determined based on the morphological description in the pathology reports. For example, SEER grade I suggested that tumors were classified as well-differentiated; grade II suggested that tumors were classified as moderately differentiated; grade III suggested that tumors were classified as poorly differentiated, and grade IV suggested that tumors were classified as undifferentiated or anaplastic (17).
Statistical Analysis
The mortality was calculated using SEER*Stat software and presented as per 100,000 population (Supplementary Table 1). The PMR of suicide was calculated by the following formula: (the number of patients committing suicide/the number of patients died) × 100% (Figure 2). The difference of the ratio between the NEN cohort and the non-NEN cohort was evaluated by Chi-squared test. In order to evaluate if NEN was a risk factor for committing suicide, a univariate logistic regression was performed, and the variables which have P < 0.05 were included in the multiple logistic regression (enter method, Table 1).
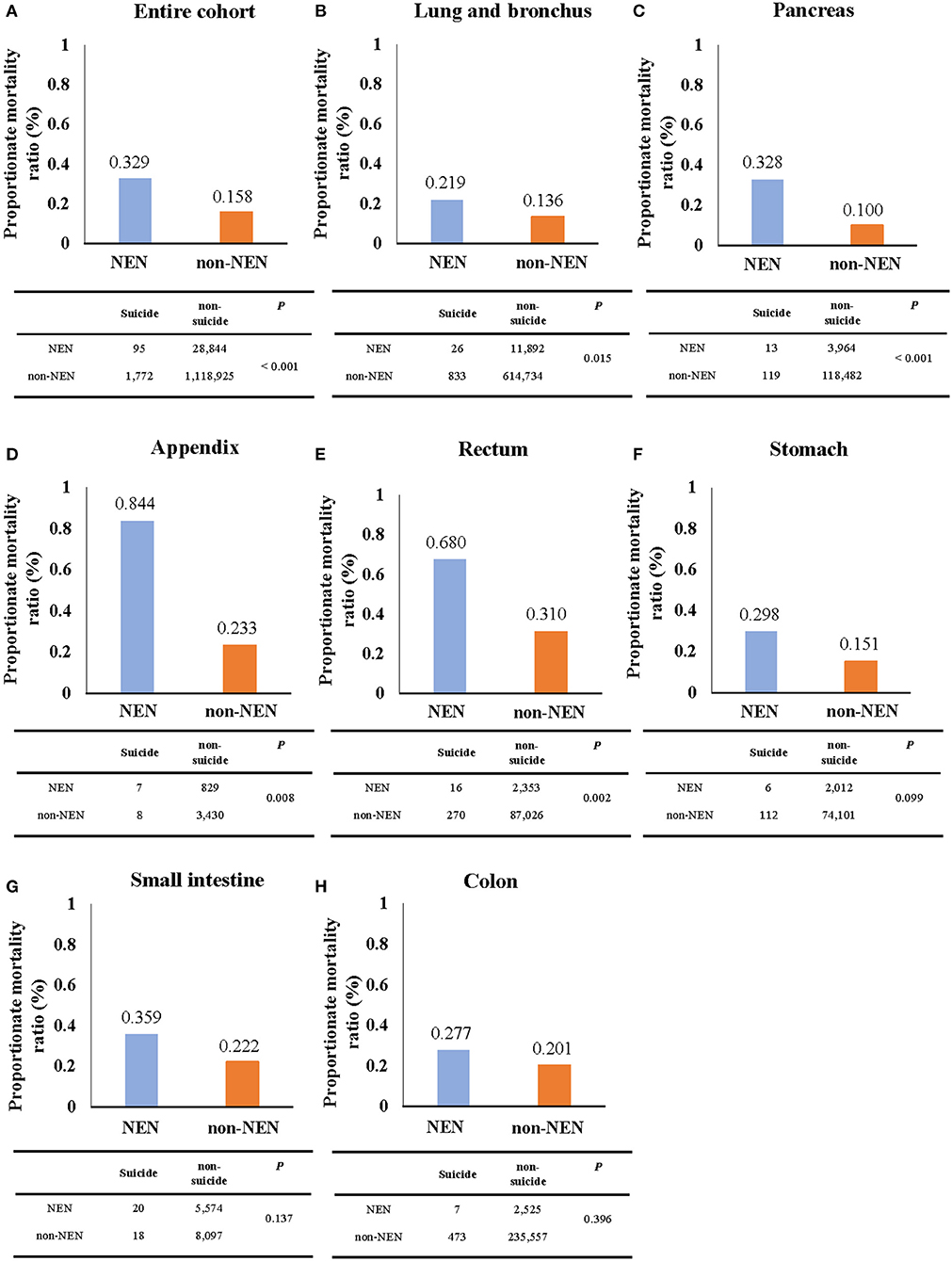
Figure 2. The proportionate mortality ratio (PMR) of suicide. The PMR of suicide of NEN patients was significantly higher than that of non-NEN patients in cohorts with all listed types of cancer (A) as well as only lung and bronchus (B), pancreatic (C), appendix (D), and rectum cancer (E). However, no significant difference for PMR of suicide between NEN and non-NEN patients was found, when analyzing stomach cancer (F), small intestine cancer (G), and colon cancer cohorts (H). The P-values were determined by Chi-squared test, and P < 0.05 indicates a significant difference.
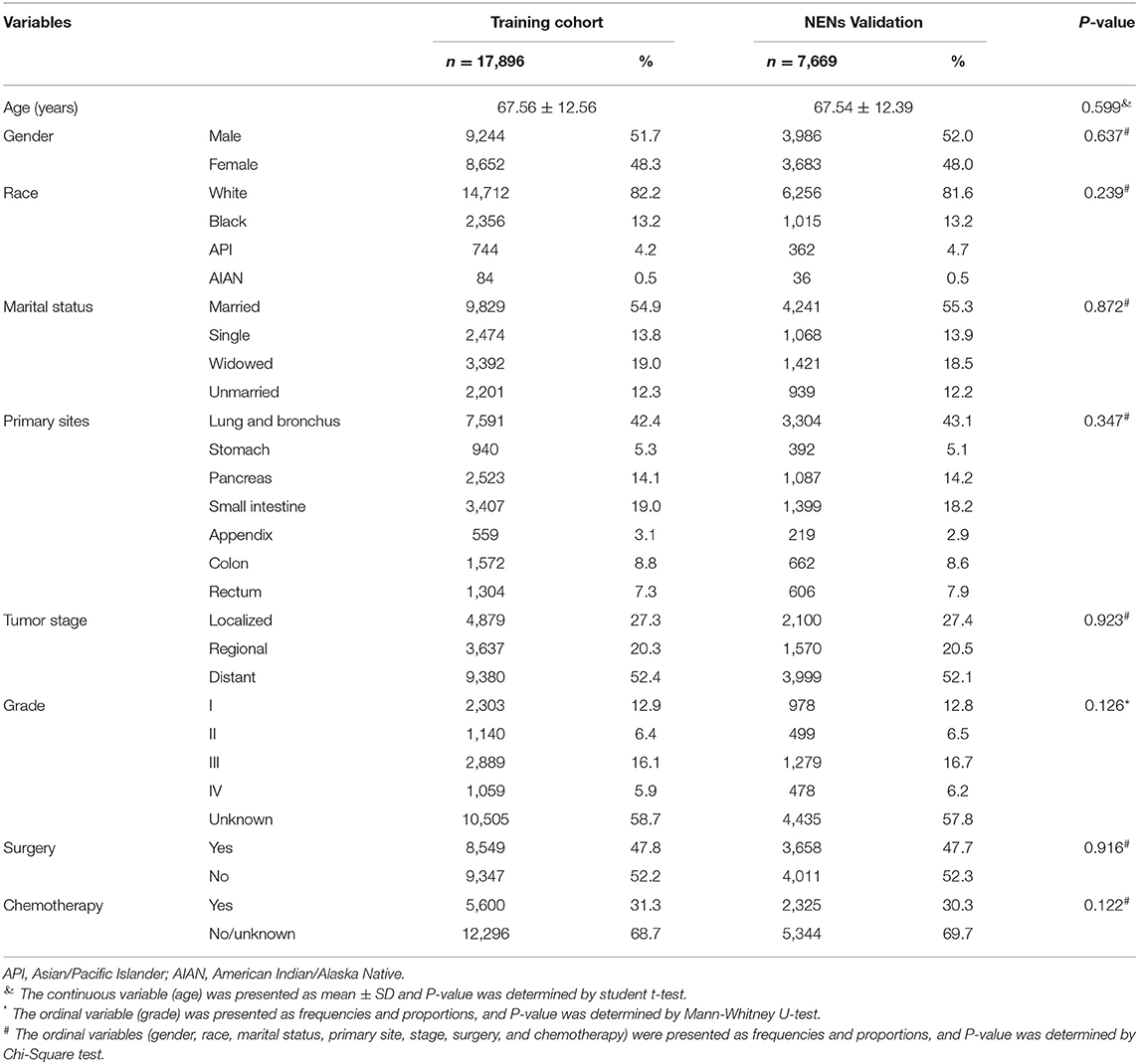
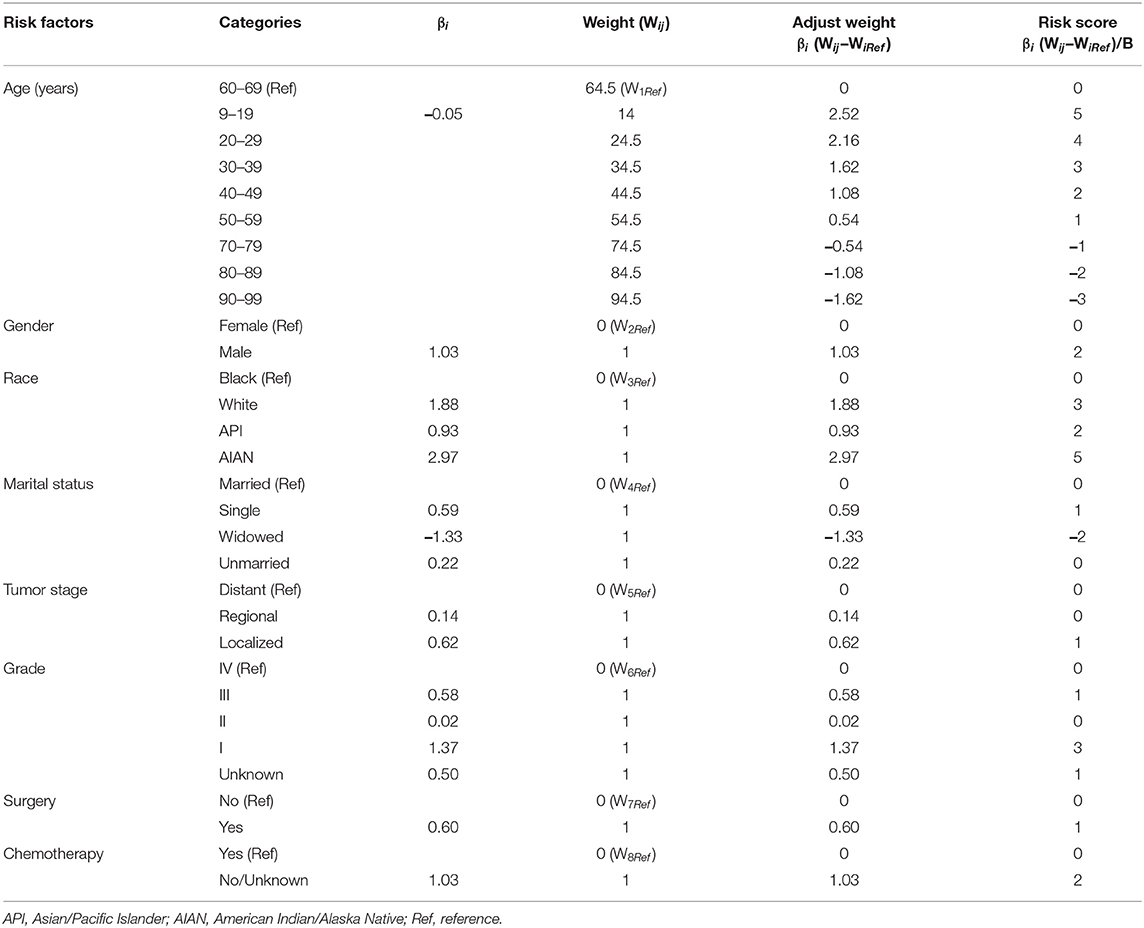
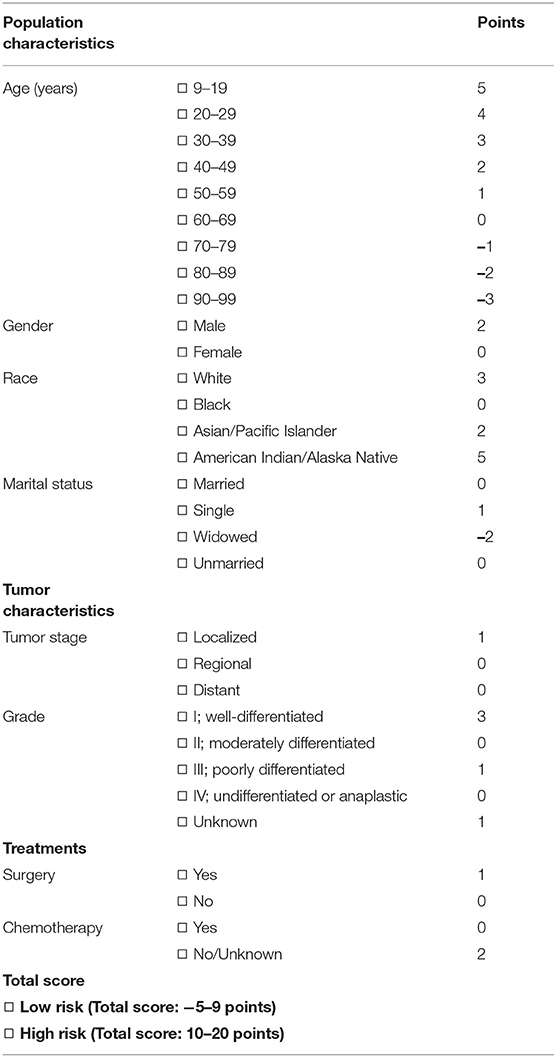
To develop the score, we followed the suggestion of the TRIPOD statement (21), 70 and 30% of the NEN patients were randomly split into a training data set and a validation data set, respectively (for basic characteristics of patients in both data sets see Table 2). To determine the risk factors for committing suicide, univariate logistic regression analyses were performed using the training data set; the odds ratios (ORs) and 95% confidence intervals (CIs) of variables were calculated (Figure 3). The β coefficient (βi) of each category was determined by multiple logistic regression analysis (Table 3). In order to determine the weight of each category, the age was converted into a categorical variable, and the weight was assigned as the value of average age (22, 23). In addition, for the other categorical variables such as gender, race, marital status, tumor stage, histologic grade, surgery, and chemotherapy, the weight of the reference category (WiRef) was defined as 0 and the weight of the other categories (Wij) were assigned as 1. Subsequently, the weight of each category was adjusted by the following formula: βi × (Wij–WiRef). In order to determine the constant score B (Table 3) of the system, we defined B to reflect the decrease of risk when age has increased 10 years (22): B = −0.05 × 10 = −0.5. Finally, the score of each category was computed by βi × (Wij–WiRef)/B (22–24).
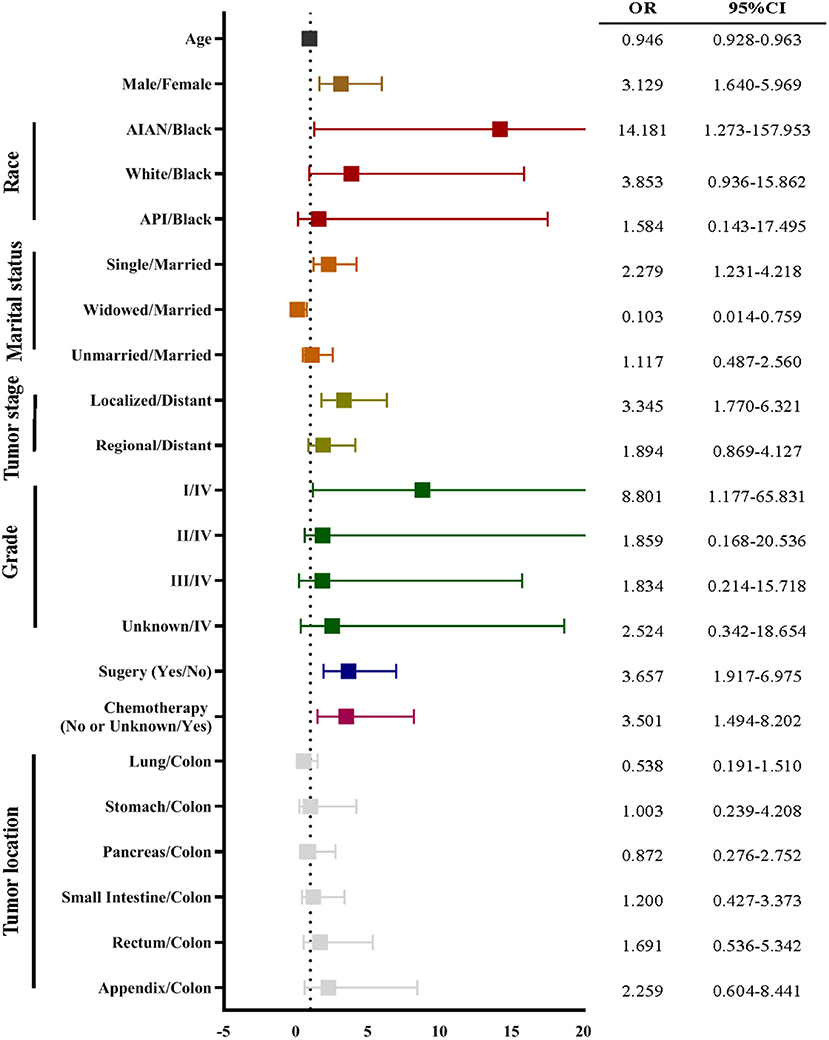
Figure 3. The univariate logistic regression of suicide using the training data set. The univariate logistic regression demonstrated that age, gender, race, marital status, cancer stage, cancer grade, surgery, and chemotherapy were associated with suicide of neuroendocrine neoplasm (NEN). The odds ratio (OR) was presented by a square, and the 95% confidence interval (CI) was presented by the horizontal lines with bars.
In order to evaluate and validate this score, the performance of this strategy was determined by receiver operating characteristic (ROC) curve analysis using the training data set, the validation data set, and non-NEN cohort, respectively. The area under the curve (AUC) was presented. When the AUC is higher than 0.5, it suggests that the score can discriminate between suicidal patients and non-suicidal patients. All statistical analyses were performed using SPSS 19.0 (IBM, Armonk, New York), and P < 0.05 was considered to indicate statistical significance.
Results
NEN Increases the Risk of Suicide
During the period of 2000–2016, 95 NEN patients (mortality, 0.00629/100,000; 95% CI, 0.00503/100,000-0.00776/100,000) and 1,773 non-NEN patients (mortality, 0.13089/100,000; 95% CI, 0.12483/100,000-0.13718/100,000) died of suicide (Figure 2A and Supplementary Table 1). Interestingly, we observed that the PMR of the NEN cohort was over 2-fold higher than that of the non-NEN cohort (Figure 2A). In addition, we evaluated the PMR in different tumor sites. Again, we observed that the PMR of NEN patients was higher than that of non-NEN patients in lung and bronchus cancer (Figure 2B), pancreatic cancer (Figure 2C), cancer of the appendix (Figure 2D), rectum cancer (Figure 2E), stomach cancer (Figure 2F), small intestine cancer (Figure 2G), and colon cancer (Figure 2H). In order to evaluate if NEN is a risk factor for suicide, the multiple logistic regression analysis was subsequently performed. We observed that NEN significantly increased the risk of suicide by 1.8-fold in comparison to non-NEN (Table 1).
Development of a Score for Predicting Suicides Among NEN Patients
In order to develop a score for predicting suicide, we excluded 3,374 participants, because the data of race, marital status, tumor stage, or surgical treatment were not recorded in the SEER program. Thus, 25,565 NEN patients were included in the subsequent analyses, and were randomly split into a training data set and a validation data set. The clinicopathological characteristics of each data set were summarized in Table 2. We did not observe significant differences of any clinicopathological characteristics between the two data sets. The ORs and 95% CIs of each variable were calculated using the training data set. We observed that being young, male, American Indian/Alaska Native, single, localized tumor, well-differentiated, surgery, and non-chemotherapy were significantly (P < 0.05) associated with suicide (Figure 3). In order to develop the score system, the βi and the weight of each category were determined as indicated in Table 3, and the score of each category was determined by βi × (Wij-WiRef)/B (Tables 3, 4).
Validation of the Score for Predicting Suicide Among NEN
The performance of the score in predicting suicide was evaluated by ROC curve using the training data set. There, we observed that the score could indeed significantly (P < 0.001) discriminate suicide patients from non-suicide patients (AUC, 0.829; 95% CI, 0.775–0.883; Figure 4A). To evaluate if the score is significantly superior to each of the single predictors such as age, gender, chemotherapy, surgery, tumor stage, race, material status, and tumor grade, we additionally performed the ROC curves of these predictors (Supplementary Figure 1). These analyses demonstrated that the AUC of the score was significantly higher than that of each single predictor (Figure 4B). In addition, in order to validate the performance of the score, the ROC curve analysis was performed using the validation data set of NEN cohort. We observed that the AUC of the validation data set was 0.735 (95% CI, 0.647–0.823; P < 0.001, Figure 4C). Moreover, we tested if this score was also able to predict the suicide of patients in the test data set (non-NEN cohort). The AUC of this test data set was 0.690 (95% CI, 0.678–0.702; P < 0.001, Figure 4D). This suggests that this score has acceptable performance and is a stable predictive system for suicide among patients with NEN as well as among patients with other tumor entities. In order to determine the optimal cut-off of this score system, the Youden's index was determined by using the training data set. We observed that the optimal cut-off of the score is 10. This optimal cut-off had a sensitivity of 63.50% and a specificity of and 87.5%.
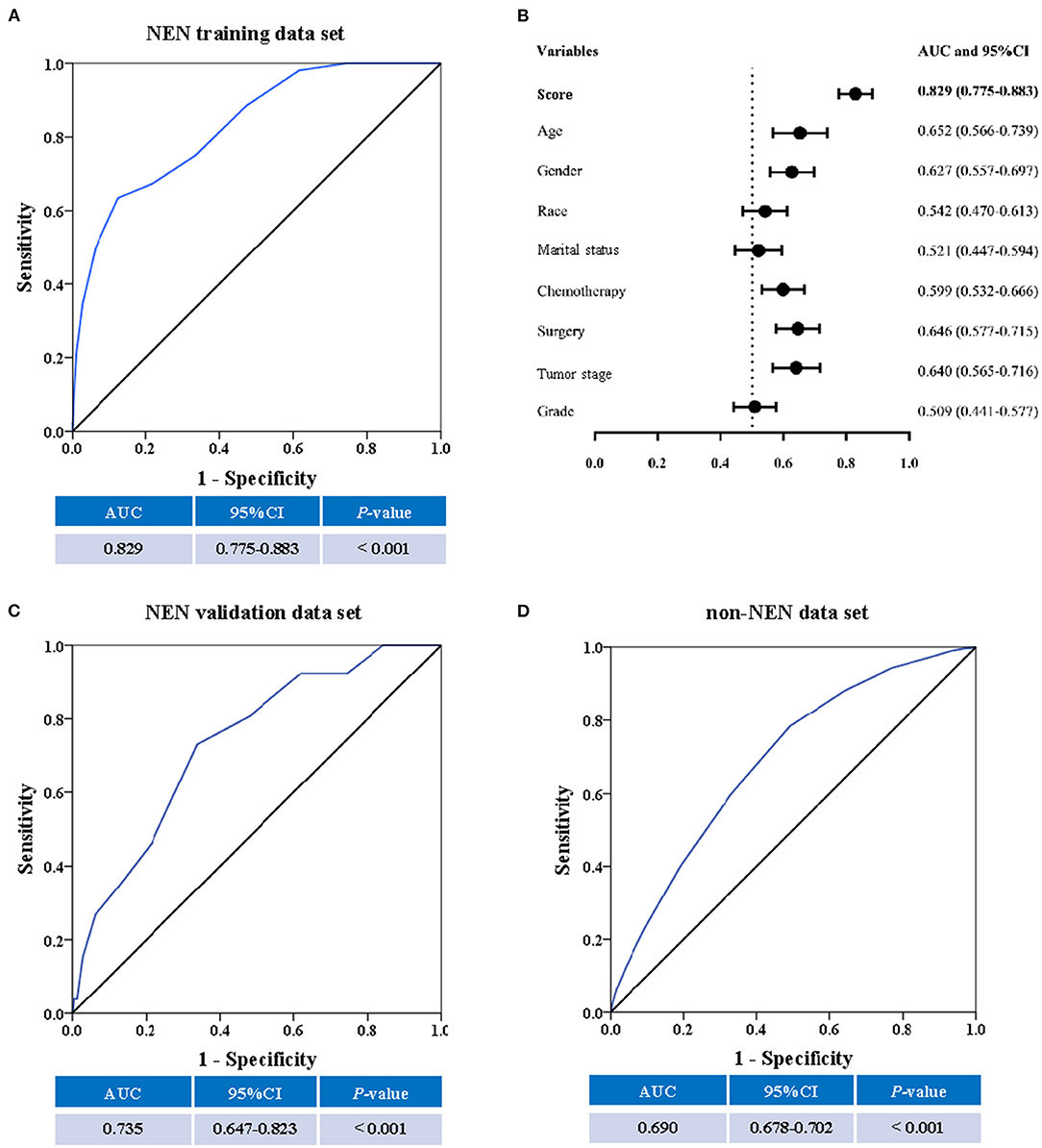
Figure 4. The predictive ability of the score. The receiver operating characteristic (ROC) curve, the area under the curve (AUC) and the confidence interval (CI) suggest a significant discriminatory power of the score when distinguishing between suicide patients from non-suicide patients (A). In addition, the discriminatory power of the score is significantly superior to single factors such as age, gender, race, marital status, chemotherapy, surgical procedure, cancer stage, and cancer grade (B). Moreover, the SEER score could distinguish suicide from non-suicide patients in the validation cohort (C), and the non-NEN cohort (D).
Discussion
Suicide is regarded to be a serious public health problem, especially among cancer patients (8, 14). Although, previous studies (7, 8) have determined the incidence of suicide in several cancers within the SEER program, they did not evaluate the incidence of suicide in NEN. In this study, we observed that the PMR of NEN patients is significantly higher than non-NEN patients, especially when the cancer was located in the appendix or pancreas. This suggests that suicide may be more frequent in NEN patients than in non-NEN patients.
We proved, for the first time, that NEN is an independent risk factor for suicide. This is supported by the observation that NEN increases the risk of suicide to 1.8-fold in direct comparison to non-NEN (Table 1). However, the underlying reasons why NEN patients more frequently commit suicide are still unclear. Park et al. (25) suggested that the Bcl-1 polymorphisms of neuron-specific glucocorticoid receptor (NR3C1) may contribute to the susceptibility to suicide in cancer patients. This hypothesis is supported by the concept that dysregulation of NR3C1 leads to depression and suicide by disturbing the function of the hypo-thalamic-pituitary-adrenal axis (26, 27).
Consistent with previous studies (16, 28, 29), we observed that some features of cancer patients such as being young, male, a member of the white race, unmarried, or surgery as well as having cancer in the localized or regional stage increased the risk of committing suicide. However, in contrast to Osazuwa-Peters's study (15), we observed that patients whose tumor was located in lung and bronchus, stomach, or pancreas had a significantly reduced risk of committing suicide, when compared to patients whose tumor was located in colon. In Osazuwa-Peters's study, the authors defined the colon and rectal tumor as an entire cohort. Notably, Tamas et al. (30) suggests that the biological and clinical characters of colon cancer and rectal cancer are different. Hence, we split colorectal cancer into two distinct cohorts, and we observed that tumor located in rectum significantly increased the risk of suicide, when compared to tumor located in colon.
Currently, most cancer patients die of non-cancer causes, such as accidents, heart diseases, and suicide (29). However, the suicide among cancer patients can be directly prevented and several strategies have been established (13, 31, 32). For example, treatments of psychiatric disorders, screening programs for high-risk persons, education for the general public, and restricting access to lethal means (33). Indeed, some studies proved that physician education and restricting access to lethal means, such as guns, domestic gas and barbiturates, could reduce suicide rates (33, 34).
Some screening questionnaires, such as nine-item patient health questionnaire (PHQ-9), hospital anxiety and depression scale (HADS), and Diagnostic and Statistical Manual of Mental Disorders (DSM), have been used for depression screening in cancer patients (13). In addition, the pan-Canadian guideline (35) and the American society of clinical oncology guideline (13) suggest that all patients with cancer should be screened for depressive symptoms at their initial visit and during the treatment until the end-of-life. Notably, most of these tools were originally used to detect depression and anxiety in the general population. They consequently did not include tumor-specific factors, such as tumor stage and grade as well as treatment variables. This may reduce the sensitivity and specificity of these screening tools. Indeed, Hartung et al. (36) demonstrated that the screening performance of both the PHQ-9 and the HADS was limited, when they were applied for depression screening in cancer patients. Thus, our score in combination with current screening tools might successfully identify cancer patients at high-risk of committing suicide.
Our study has two clinical implications: First, prevention of suicide in NEN patients is especially necessary. This conclusion bases on the observation that compared to non-NEN, NEN increases the risk of suicide by 1.8-fold. Thus, a routine screening among NEN patients, especially when the cancer is localized in the appendix or pancreas, may effectively reduce the suicide rate of these patients. In addition, raising awareness of physicians to this topic may be important for patients suffering from pancreatic neuroendocrine tumors (pNET). It is well-known that pancreatic ductal adenocarcinoma (PDAC) is a lethal disease, and the 5-year survival of patients is only around 10% (37). However, the 5-year survival of pNET patients is 50% (3), and there is a high risk that pNET patients actually think they suffer from a PDAC. This may increase the depression and anxiety of these pNET patients. Indeed, the present study suggests that the pancreatic NEN patients have a more than 3-fold higher risk of suicide in comparison with non-NEN patients (Figure 2C). These results imply that patients with longer survival might have an elevated risk to experience depression symptoms and succeed in their suicide attempts when compared to ones with shorter overall survival. Thus, physicians' education may help these patients eliminating depression as well as anxiety, and thus reducing the suicide rate. Second, this study proved that the score (Table 4), which includes general population characteristics (age at diagnosis, gender, race, marital status), tumor characteristics (tumor stage and histologic grade), and treatment parameters (surgery, chemotherapy) can significantly distinguish individuals having committed suicide from those that did not. This supports the hypothesis that incorporation of this score into current screening strategies may prevent suicide among patients suffering from NEN.
Limitations
Notably, there are some limitations of our studies. First, even though SEER program is an authoritative source of information on cancer incidence and mortality, this database did not identify patients who committed unsuccessful suicide attempts. We, therefore, failed to calculate the incidence of suicide, and only determined the PMR of suicide among NEN cohort and non-NEN cohort. Second, we identified the participants of this study by the cause of death, however, we were unable to evaluate whether there was misclassification bias in our study. Previous studies suggest that suicide may be misclassified as accidents or unintentional death (38, 39). Third, suicide is a complex behavior, and several factors, such as psychiatric disorders, occupation, childhood adversity, family history, and antidepressants can increase the risk of suicide (40, 41). However, the SEER program did not provide these information, and we could not evaluate these factors in this study. In addition, although, this SEER scale has a probability of over 80% to correctly distinguish suicide patients from non-suicide individuals; the false negative rate is 36.5% and the false positive rate is 12.5% when considering 10 as the optimal cut-off point.
Conclusion
In conclusion, this study, for the first time, demonstrated that patients with NEN have a higher risk of committing suicide than patients with non-NEN. In addition, we developed and validated a suicide score, which can distinguish suicide and non-suicide individuals among these patients. This score may improve the current screening strategies and may reduce the suicide rate especially among patients with NEN.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XZ and PG co-supervised the study. LL wrote the manuscript. LL and YS analyzed and interpreted the data. XZ, LL, DZ, ML, and CM performed the critical revision and language check of manuscript. All authors contributed to the article and approved the submitted version.
Funding
LL was supported by the China Scholarship Council (Grant No.: 201908080127). The study was supported by National Natural Science Foundation of China (Grant No.: 81973646); Characteristic Innovation Projects of Universities in Guangdong Province (Grant No.: 2018KTSCX193); Special Scientific Research Project of New Coronary Pneumonia Epidemic Prevention and Control of Guangdong Provincial Department of Education (Grant No.: 2020KZDZX1170); Shenzhen Key Medical Discipline Construction Fund; Sanming Project of Medicine in Shenzhen; China Postdoctoral Science Foundation (Grant No.: 2020M682887); Guangdong Basic and Applied Basic Research Fund (Guangdong Natural Science Fund; Grant No.: 2020A1515110083); Shenzhen Science and Technology Innovation Commission (Grant No.: RCBS20200714114958333). These founders support the open access publication fees.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.638152/full#supplementary-material
Supplementary Figure 1. The performance of predictive factors to distinguish between suicide patients from non-suicide patients. The receiver operating characteristic (ROC) curves, the area under the curve (AUC), and the confidence interval (CI) suggest that age (A), gender (B), chemotherapy (C), surgery (D), and stage (E) could significantly distinguish suicide patients from non-suicide patients, however, race (F), marital status (G), and grade (H) did not have this ability to discriminate.
Supplementary Table 1. Mortality in patients dead from suicide, non-suicide, and all causes.
Abbreviations
NEN, neuroendocrine neoplasm; SEER, Surveillance, Epidemiology, and End Results; PMR, proportionate mortality ratio; OR, odds ratios; CIs, confidence intervals; βi, β coefficient; WiRef, weight of the reference category; Wij, weight of the other categories; ROC, receiver operating characteristic; AUC, area under the curve; NR3C1, neuron-specific glucocorticoid receptor; PHQ-9, nine-item patient health questionnaire; HADS, hospital anxiety and depression scale; pNET, pancreatic neuroendocrine tumors; PDAC, pancreatic ductal adenocarcinoma.
References
1. Boyar Cetinkaya R, Aagnes B, Thiis-Evensen E, Tretli S, Bergestuen DS, Hansen S. Trends in incidence of neuroendocrine neoplasms in Norway: a report of 16,075 cases from 1993 through 2010. Neuroendocrinology. (2017) 104:1–10. doi: 10.1159/000442207
2. Zhang X, Ma L, Bao H, Zhang J, Wang Z, Gong P. Clinical, pathological, and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: a retrospective study. BMC Endocr Disord. (2014) 14:54. doi: 10.1186/1472-6823-14-54
3. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
5. Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiol Rev. (2008) 30:133–54. doi: 10.1093/epirev/mxn002
6. Jones DS, Podolsky SH, Greene JA. The burden of disease and the changing task of medicine. N Engl J Med. (2012) 366:2333–8. doi: 10.1056/NEJMp1113569
7. Anguiano L, Mayer DK, Piven ML, Rosenstein D. A literature review of suicide in cancer patients. Cancer Nurs. (2012) 35:E14–26. doi: 10.1097/NCC.0b013e31822fc76c
8. Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J Clin Oncol. (2008) 26:4731–8. doi: 10.1200/JCO.2007.13.8941
9. Larsson G, Sjoden PO, Oberg K, Eriksson B, von Essen L. Health-related quality of life, anxiety and depression in patients with midgut carcinoid tumours. Acta Oncol. (2001) 40:825–31. doi: 10.1080/02841860152703445
10. Lewis AR, Wang X, Magdalani L, D'Arienzo P, Bashir C, Mansoor W, et al. Health-related quality of life, anxiety, depression and impulsivity in patients with advanced gastroenteropancreatic neuroendocrine tumours. World J Gastroenterol. (2018) 24:671–9. doi: 10.3748/wjg.v24.i6.671
11. Russo S, Boon JC, Kema IP, Willemse PH, den Boer JA, Korf J, et al. Patients with carcinoid syndrome exhibit symptoms of aggressive impulse dysregulation. Psychosom Med. (2004) 66:422–5. doi: 10.1097/00006842-200405000-00022
12. Miret M, Ayuso-Mateos JL, Sanchez-Moreno J, Vieta E. Depressive disorders and suicide: epidemiology, risk factors, and burden. Neurosci Biobehav Rev. (2013) 37:2372–4. doi: 10.1016/j.neubiorev.2013.01.008
13. Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. (2014) 32:1605–19. doi: 10.1200/JCO.2013.52.4611
14. Zaorsky NG, Zhang Y, Tuanquin L, Bluethmann SM, Park HS, Chinchilli VM. Suicide among cancer patients. Nat Commun. (2019) 10:207. doi: 10.1038/s41467-018-08170-1
15. Osazuwa-Peters N, Simpson MC, Zhao L, Boakye EA, Olomukoro SI, Deshields T, et al. Suicide risk among cancer survivors: head and neck versus other cancers. Cancer. (2018) 124:4072–9. doi: 10.1002/cncr.31675
16. Turaga KK, Malafa MP, Jacobsen PB, Schell MJ, Sarr MG. Suicide in patients with pancreatic cancer. Cancer. (2011) 117:642–7. doi: 10.1002/cncr.25428
17. Liu P, Zhang X, Shang Y, Lu L, Cao F, Sun M, et al. Lymph node ratio, but not the total number of examined lymph nodes or lymph node metastasis, is a predictor of overall survival for pancreatic neuroendocrine neoplasms after surgical resection. Oncotarget. (2017) 8:89245–55. doi: 10.18632/oncotarget.19184
18. Surveillance Research Program. National Cancer Institute SEER*Stat Software (seer.cancer.gov/seerstat) version <8.3.6>. Released on August 8, 2019
19. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2019) 76:182–8. doi: 10.1111/his.13975
20. Cavalcanti MS, Gonen M, Klimstra DS. The ENETS/WHO grading system for neuroendocrine neoplasms of the gastroenteropancreatic system: a review of the current state, limitations and proposals for modifications. Int J Endocr Oncol. (2016) 3:203–19. doi: 10.2217/ije-2016-0006
21. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. (2015) 162:55–63. doi: 10.7326/M14-0697
22. Antonopoulos AS, Odutayo A, Oikonomou EK, Trivella M, Petrou M, Collins GS, et al. Development of a risk score for early saphenous vein graft failure: An individual patient data meta-analysis. J Thorac Cardiovasc Surg. (2019) 160:116–27. doi: 10.1016/j.jtcvs.2019.07.086
23. Halls MC, Berardi G, Cipriani F, Barkhatov L, Lainas P, Harris S, et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg. (2018) 105:1182–91. doi: 10.1002/bjs.10821
24. Hammond ER, Crum RM, Treisman GJ, Mehta SH, Marra CM, Clifford DB, et al. The cerebrospinal fluid HIV risk score for assessing central nervous system activity in persons with HIV. Am J Epidemiol. (2014) 180:297–307. doi: 10.1093/aje/kwu098
25. Park S, Hong JP, Lee JK, Park YM, Park Y, Jeon J, et al. Associations between the neuron-specific glucocorticoid receptor (NR3C1) Bcl-1 polymorphisms and suicide in cancer patients within the first year of diagnosis. Behav Brain Funct. (2016) 12:22. doi: 10.1186/s12993-016-0104-1
26. Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. (2000) 23:477–501. doi: 10.1016/S0893-133X(00)00159-7
27. McGirr A, Diaconu G, Berlim MT, Pruessner JC, Sable R, Cabot S, et al. Dysregulation of the sympathetic nervous system, hypothalamic-pituitary-adrenal axis and executive function in individuals at risk for suicide. J Psychiatry Neurosci. (2010) 35:399–408. doi: 10.1503/jpn.090121
28. Gunnes MW, Lie RT, Bjorge T, Ghaderi S, Syse A, Ruud E, et al. Suicide and violent deaths in survivors of cancer in childhood, adolescence, and young adulthood-a national cohort study. Int J Cancer. (2017) 140:575–80. doi: 10.1002/ijc.30474
29. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol. (2017) 28:400–7. doi: 10.1093/annonc/mdw604
30. Tamas K, Walenkamp AM, de Vries EG, van Vugt MA, Beets-Tan RG, van Etten B, et al. Rectal and colon cancer: not just a different anatomic site. Cancer Treat Rev. (2015) 41:671–9. doi: 10.1016/j.ctrv.2015.06.007
31. Zalsman G, Hawton K, Wasserman D, van Heeringen K, Arensman E, Sarchiapone M, et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry. (2016) 3:646–59. doi: 10.1016/S2215-0366(16)30030-X
32. Holland JC, Bultz BD. The NCCN guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. (2007) 5:3–7. doi: 10.6004/jnccn.2007.0003
33. Mann JJ, Apter A, Bertolote J, Beautrais A, Currier D, Haas A, et al. Suicide prevention strategies: a systematic review. JAMA. (2005) 294:2064–74. doi: 10.1001/jama.294.16.2064
34. Rutz W, von Knorring L, Walinder J. Frequency of suicide on Gotland after systematic postgraduate education of general practitioners. Acta Psychiatr Scand. (1989) 80:151–4. doi: 10.1111/j.1600-0447.1989.tb01318.x
35. Howell D, Keller-Olaman S, Oliver TK, Hack TF, Broadfield L, Biggs K, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. (2013) 20:e233–46. doi: 10.3747/co.20.1302
36. Hartung TJ, Friedrich M, Johansen C, Wittchen HU, Faller H, Koch U, et al. The Hospital Anxiety and Depression Scale (HADS) and the 9-item Patient Health Questionnaire (PHQ-9) as screening instruments for depression in patients with cancer. Cancer. (2017) 123:4236–43. doi: 10.1002/cncr.30846
37. Hsu CP, Hsu JT, Liao CH, Kang SC, Lin BC, Hsu YP, et al. Three-year and five-year outcomes of surgical resection for pancreatic ductal adenocarcinoma: long-term experiences in one medical center. Asian J Surg. (2018) 41:115–23. doi: 10.1016/j.asjsur.2016.11.009
38. Rockett IR, Caine ED. Self-injury is the eighth leading cause of death in the United States: it is time to pay attention. JAMA Psychiatry. (2015) 72:1069–70. doi: 10.1001/jamapsychiatry.2015.1418
39. Oquendo MA, Volkow ND. Suicide: a silent contributor to opioid-overdose deaths. N Engl J Med. (2018) 378:1567–9. doi: 10.1056/NEJMp1801417
40. Devries K, Watts C, Yoshihama M, Kiss L, Schraiber LB, Deyessa N, et al. Violence against women is strongly associated with suicide attempts: evidence from the WHO multi-country study on women's health and domestic violence against women. Soc Sci Med. (2011) 73:79–86. doi: 10.1016/j.socscimed.2011.05.006
Keywords: neuroendocrine neoplasms, suicide, score, proportionate mortality ratio, risk factors
Citation: Lu L, Shang Y, Zechner D, Mullins CS, Linnebacher M, Zhang X and Gong P (2021) Development and Validation of a Score for Screening Suicide of Patients With Neuroendocrine Neoplasms. Front. Psychiatry 12:638152. doi: 10.3389/fpsyt.2021.638152
Received: 05 December 2020; Accepted: 19 May 2021;
Published: 11 June 2021.
Edited by:
Nicola Susan Gray, Swansea University, United KingdomReviewed by:
Robert Snowden, Cardiff University, United KingdomKrystallenia i Alexandraki, National and Kapodistrian University of Athens, Greece
Copyright © 2021 Lu, Shang, Zechner, Mullins, Linnebacher, Zhang and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianbin Zhang, emhhbmd4aWFuYmluQGhvdG1haWwuY29t; eGlhbmJpbi56aGFuZ0BzenUuZWR1LmNu; Peng Gong, ZG9jdG9yZ29uZ3BlbmdAc3p1LmVkdS5jbg==
†These authors have contributed equally to this work
 Lili Lu
Lili Lu Yuru Shang
Yuru Shang Dietmar Zechner3
Dietmar Zechner3 Michael Linnebacher
Michael Linnebacher Xianbin Zhang
Xianbin Zhang