- 1Department of Psychiatry, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, United States
- 2Research Foundation for Mental Hygiene, New York State Psychiatric Institute, New York, NY, United States
Cannabis is increasingly used by individuals with mental health diagnoses and often purported to treat anxiety and various other psychiatric symptoms. Yet support for using cannabis as a psychiatric treatment is currently limited by a lack of evidence from rigorous placebo-controlled studies. While regulatory hurdles and other barriers make clinical trials of cannabis challenging to conduct, addiction researchers have decades of experience studying cannabis use in human laboratory models. These include methods to control cannabis administration, to delineate clinical and mechanistic aspects of cannabis use, and to evaluate potential treatment applications for cannabis and its constituents. In this paper, we review these human laboratory procedures and describe how each can be applied to study cannabis use in patients with psychiatric disorders. Because anxiety disorders are among the most common psychiatric illnesses affecting American adults, and anxiety relief is also the most commonly-reported reason for medicinal cannabis use, we focus particularly on applying human laboratory models to study cannabis effects in individuals with anxiety and related disorders. Finally, we discuss how these methods can be integrated to study cannabis effects in other psychiatric conditions and guide future research in this area.
Introduction
Societal attitudes and public policy regarding cannabis use have shifted dramatically over the past two decades. Americans increasingly view cannabis as harmless (1), and as of December 2020, 36 states and the District of Columbia (DC) have legalized medicinal cannabis, while 15 states and DC permit recreational cannabis use. As acceptance of cannabis grows, more Americans are using, with 4.8 million more adults reporting near-daily cannabis use in 2018 compared to 2008 (2). Meanwhile, cannabis is purported to treat a variety of ailments. Despite limited evidence to support many of these claims, cannabis products are increasingly marketed as treatments for various medical conditions, including psychiatric disorders (3, 4). Thus, there is an urgent need to examine cannabis' purported mental health benefits and rigorously test its effects in patients with psychiatric disorders. In this paper, we describe how investigators can apply human laboratory methods to address these issues.
Why Study the Effects of Cannabis in Psychiatric Populations?
Americans increasingly use cannabis medicinally (i.e., with the intent to treat one or more symptoms) (5). Psychiatric symptoms including anxiety, depression, and stress are among the most common reasons for which patients report seeking medicinal cannabis (6, 7). Of 9,003 American adults who responded to a randomized, nationally-representative survey, 7% endorsed medicinal cannabis use, among whom 47 and 39%, respectively used cannabis to treat anxiety and depression (5). Among 2,774 American cannabis users in another survey, 13.6 and 12.7% reported using cannabis as a substitute for anxiolytics and antidepressant medication, respectively (8). Cannabis use to treat psychiatric symptoms may have further increased during the COVID-19 pandemic: Among 1,202 American medicinal cannabis users surveyed before and after the pandemic's onset, self-reported cannabis consumption increased by 91% more for those with mental health conditions vs. those without (9).
Although interest in, access to, and use of cannabis is increasing, surprisingly few studies have directly examined its effects in those with medical or psychiatric illness. As a result, there is limited evidence supporting using cannabis as a treatment. A 2017 report by the National Academies of Sciences, Engineering, and Medicine found sufficient evidence to support treating only two conditions with medicinal cannabinoids (i.e., cannabis constituents or analogs): Chronic pain (for which both oral cannabinoids and cannabis showed efficacy) and multiple sclerosis-related spasticity (for which only oral cannabinoids were effective) (10). The psychiatric literature is even sparser: A 2019 meta-analysis including 88 studies found insufficient evidence to support medicinal cannabis treatment of any psychiatric condition (7).
Despite this dearth of research, individuals with psychiatric illness increasingly use cannabis for both recreational and medicinal purposes (11). Yet lacking evidence of cannabis' effects on psychiatric symptoms, patients and clinicians have little information to guide decisions about how to use it. Whereas, FDA approval for medications requires release of detailed information about different doses, routes of administration, indications, and potential adverse effects, this information for a highly variable plant is largely unknown. Advertising from the now-billion-dollar cannabis industry has filled the information void and often includes claims about cannabis' purported psychiatric benefits (3, 12). Meanwhile, cannabis is a federally-illegal substance legalized by individual states, with scant consensus regarding how it should be used. For instance, a physician may recommend medicinal cannabis to a patient with PTSD in New York, but not in Iowa, while physicians in Colorado have discretion to recommend cannabis for any condition they determine it might help (including psychiatric illnesses) (13, 14). This creates a landscape that is bewildering to clinicians, who generally report feeling ill-equipped to make evidence-based recommendations about cannabis (15), and to patients, who may develop unrealistic expectations about what cannabis can and cannot do.
Regulatory and Scientific Challenges to Studying Cannabis Use in Human Volunteers
Clinical trials of cannabis are challenging to conduct in the US, partially due to cannabis' Schedule I labeling by the Drug Enforcement Agency (DEA). Researchers studying cannabis and other Schedule 1 substances must obtain special federal and state licensure (which can take months to years) following extensive monitoring and DEA-approved storage procedures (e.g., storing cannabis in a gun safe) (14). Thus, cannabis studies are typically limited to highly-specialized research environments. Moreover, current researchers may only use cannabis produced by the National Institute of Drug Abuse (NIDA) (14); in contrast, a range of cannabis products are sold in dispensaries and other commercial outlets or can be obtained illicitly (and may differ substantially from NIDA cannabis) (16).
In addition to regulatory factors, several scientific issues complicate studies of cannabis in human volunteers. Because cannabis is most often smoked or vaporized, different patterns of inhalation can lead to substantial variability in serum cannabinoid concentrations compared to, for example, intravenous administration (17, 18). Standardized methods to administer cannabis can minimize this variability. Smoking and vaporizing involve different preparation and delivery procedures than oral or intravenous administration (e.g., inserting plant material into a cigarette), requiring researchers to use different blinding techniques with these methods.
Cannabis' effects are susceptible to expectancy, such that individuals who anticipate receiving active cannabis but instead receive placebo nonetheless report experiencing cannabis-like effects (19). Psychiatric symptoms are also responsive to placebos, even those administered without deception (20). Thus, placebo control is essential for cannabis trials in psychiatric populations. Because many participants may be familiar with cannabis effects (for example, 16% of all Americans were estimated to have used cannabis in the past year in 2018) (2), placebo selection is also important to consider.
Dissecting the mechanistic properties and clinical effects of cannabis can also be difficult. Cannabis is pharmacologically diverse, containing over 140 unique chemical constituents (“phytocannabinoids”). Many phytocannabinoids are likely psychoactive, and the neurobiological mechanisms of even the two best-studied, Δ-9 tetrahydrocannabinol (THC) and cannabidiol (CBD), are incompletely understood (21). The properties of different cannabis varietals vary with their phytocannabinoid composition, the form, dose, and frequency in which they are administered, and the users' history of cannabinoid exposure (22). Disentangling the contributions of these factors can be difficult outside of controlled settings.
While few of cannabis' potential clinical benefits have been rigorously tested, its abuse potential has been well-documented (23). This poses an additional challenge to its study in individuals with psychiatric illnesses [who may be at increased risk for developing cannabis use disorder (CUD), among other adverse effects] (24). Investigators need to consider designs that can distinguish between cannabis' effects on psychiatric symptoms (e.g., anxiolysis/anxiogenesis) and unrelated drug effects (e.g., intoxication), while also minimizing the risk that participants develop CUD or experience other cannabis-related harms.
Given the barriers involved in clinical research, cannabis' effects on psychiatric outcomes have mostly been examined through observational studies and surveys (7, 25, 26). These studies tend to rely on participants' retrospective self-reports of cannabis effects, which are subject to recall biases; in recruiting medicinal cannabis users (who by definition believe cannabis to be potentially helpful), they also involve selection bias. As noted above, both cannabis effects (19) and psychiatric symptoms (20) are influenced by expectancy. Given its pharmacologic diversity (22), accounting for the different effects of cannabis' various constituents (e.g., THC vs. CBD) is daunting even in controlled studies. In observational research, it is nearly impossible: Labeling of commercially-available cannabis products is frequently inaccurate (27, 28), state-run cannabis testing facilities have demonstrated systematic differences in the cannabinoid concentrations they report, and even experienced cannabis users have difficulty determining the THC/CBD content of the products they use from their subjective responses (29, 30). Further, cannabis that is smoked or vaporized vs. taken orally in tinctures or capsules will produce markedly different plasma cannabinoid concentrations (31).
Though observational research and surveys can be useful tools, their limitations make them insufficient to fully elucidate cannabis' clinical risks and benefits or its potential role in psychiatric treatment. Randomized, placebo-controlled trials remain the gold-standard tests of efficacy, yet only a few have examined cannabis' potential medicinal properties (of which only a subset involved patients with psychiatric disorders). Although small trials have tested psychiatric applications of synthetic cannabinoids (32) [e.g., nabilone, a synthetic THC analog that is approved by the US Food and Drug Administration (FDA) for treating cancer chemotherapy and HIV-related nausea and vomiting] and cannabinoid isolates (33) (e.g., various CBD preparations), recreational and medicinal users overwhelmingly ingest cannabinoids through inhaling smoked or vaporized cannabis flower (6, 16). While understanding cannabis' effects when used as it is most commonly in daily settings is critically important, a 2016 systematic review identified only one cannabis trial for any psychiatric indication (34). This open-label trial of smoked cannabis for PTSD lacked a placebo control or systematic method of cannabis administration (35). Since then, we have conducted two small placebo-controlled studies of smoked cannabis at our site: One tested its effects in individuals at high risk for psychotic disorders (36), and another tested its effects in patients with obsessive-compulsive disorder (OCD) (37).
Rationale for Using Human Laboratory Methods to Study Cannabis Use in Psychiatric Populations
Given the current political, social, medical, and legal climate, the public health need for controlled studies of cannabis effects in psychiatrically ill populations has never been more urgent. Whereas, psychiatric cannabis trials are nascent, addiction researchers have explored cannabis effects in human laboratory studies for decades (38). Human laboratory methods were developed to study problematic use of psychoactive drugs like cannabis and to identify new ways of treating individuals with substance use disorders. These procedures enable investigators to study and control methods of administration and to blind participants/investigators for rigorous testing of clinical effects. Researchers have also devised strategies to delineate factors contributing to the development and maintenance of CUD and other substance use disorders. Finally, the human laboratory has proved to be an efficient venue in which to screen for potential therapeutic effects of psychoactive substances like cannabis and cannabinoids before testing them in large-scale clinical trials. Herein, we review some of these human laboratory methods and describe how they could be applied to examine the effects of cannabis and cannabinoids in patients with psychiatric illnesses.
Using Human Laboratory Methods to Study the Effects of Cannabis and Cannabinoids in Psychiatric Populations
Overview: Substance use researchers have developed human laboratory methods to directly examine the effects of cannabis and its constituents. These include methods to control cannabis administration (e.g., dosing and blinding procedures), to delineate clinical and mechanistic aspects of cannabis use (e.g., intoxication and other acute effects, positive and negative reinforcement, dose-dependency, and tolerance), and to evaluate potential treatments (e.g., screening potential uses of cannabis in psychiatric treatment, testing treatments for comorbid psychiatric illness and CUD, and identifying cannabis-drug interactions). Below, we review these human laboratory procedures and describe their potential applications to explore cannabis effects in patients with psychiatric illnesses. Because anxiety disorders are among the most common psychiatric illnesses affecting American adults (39), and anxiety relief is also the most commonly-reported reason for medicinal cannabis use (5), we focus particularly on how human laboratory procedures could be applied to study cannabis effects in individuals with anxiety and related disorders. These procedures and associated applications are summarized in Table 1.
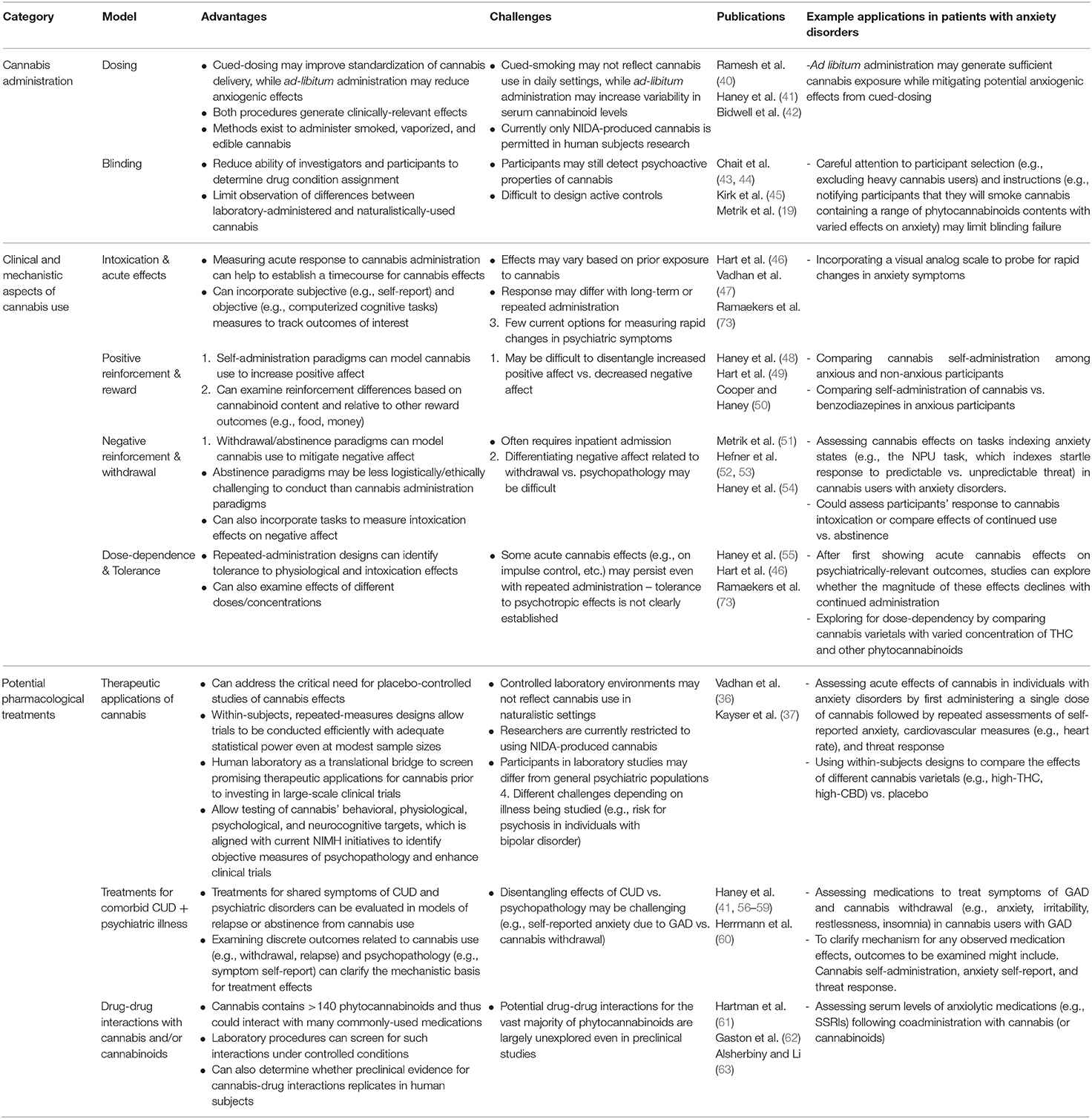
Table 1. Human laboratory procedures to model cannabis use and potential applications in patients with anxiety disorders.
Methods to Control Cannabis Administration
Procedures to Control Dosing
Cued-smoking procedures have been developed to help standardize cannabis administration (64). Investigators provide participants a specific amount of cannabis containing known concentrations of constituents (e.g., THC, CBD), and then guide them through the process of smoking, controlling the duration of inhalation and the amount of time that smoke is held within the lungs (see Figure 1 for details). Similar methods exist for controlled administration of vaporized (31, 65) and edible (31) cannabis formulations. Following cannabis administration, participants' subjective, physiological, and/or neurocognitive responses can be measured at precise timepoints (38).
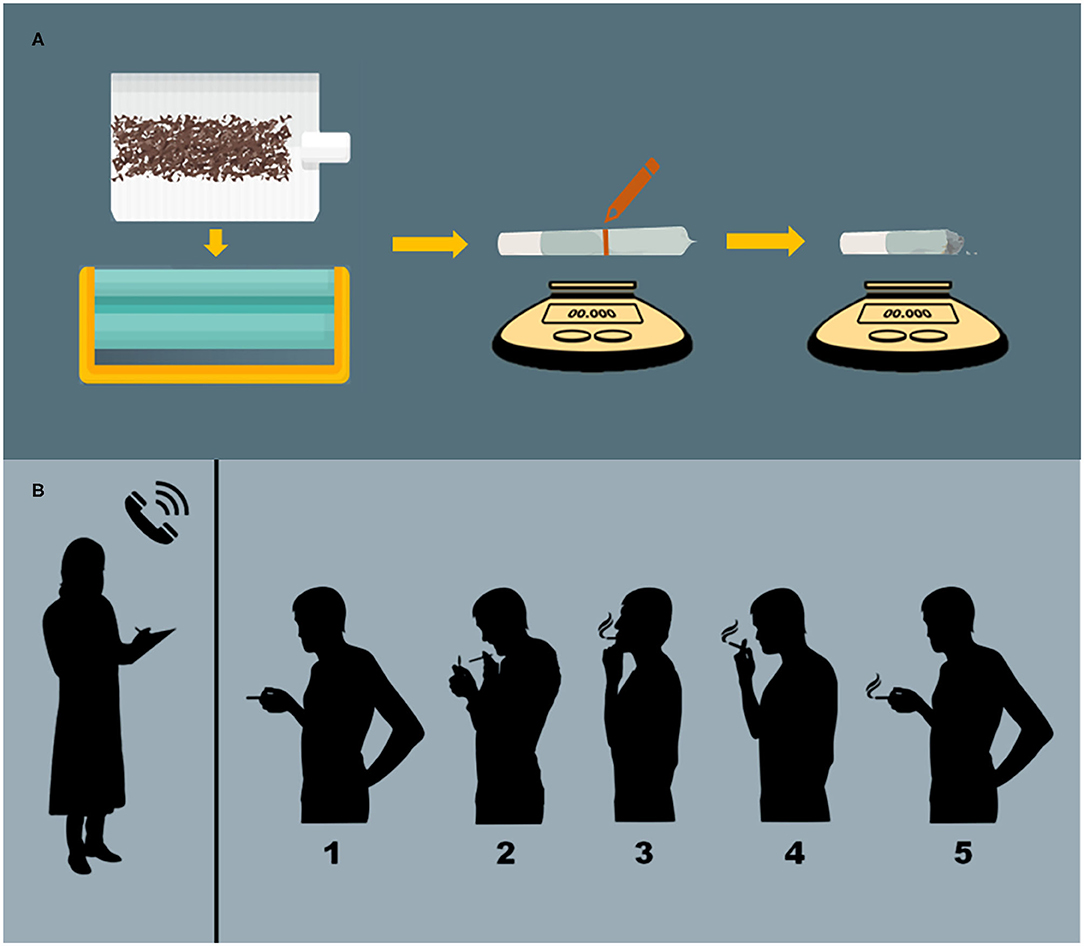
Figure 1. Administration procedures. (A) Preparation of cannabis cigarettes. Cannabis cigarettes are machine-rolled using cigarette paper and then inserted into a plastic cigarette holder. A line is drawn at the half-way point and the participant is instructed to smoke 50% of the cigarette. Cannabis consumption is verified via pre- and post-administration weighing of cigarettes. (B) Cued-smoking procedure. From a separate room with a two-way mirror, an investigator (who has no other contact with participants) guides participants through cued-smoking procedures. (1) The participant is presented with the cannabis cigarette, and then instructed to (2) “Prepare” (light the cigarette and prepare to smoke), (3) “Inhale” (5 s), (4) “Hold smoke in lungs” (10 s), and (5) “Exhale.” This cycle is repeated, allowing a 40 s interval between puffs, until 50% of the cigarette is pyrolized.
Though potentially improving standardization, cued-smoking procedures may not reflect how cannabis is used in daily settings. Moreover, asking participants to smoke a specific percentage of a cannabis cigarette (rather than allowing them to titrate to their desired level) may induce discomfort or anxiety in some individuals if, for example, they are required to smoke more cannabis than they are comfortable smoking (51). Some studies have accounted for such effects by instructing participants to smoke ad libitum over the course of a predefined time period (e.g., 10 min) (42, 66). Ad libitum cannabis administration may increase variability in serum cannabinoid concentrations, but recent studies suggest it nonetheless yields clinically-relevant effects (42, 66–68). Thus, in a study of patients with anxiety disorders, ad libitum procedures may generate sufficient cannabis exposure while also mitigating potential anxiogenic effects due to administration procedures (rather than cannabis itself) that might occur with cued-smoking.
Despite attempts to standardize administration procedures, cannabis smokers adjust their inhalation patterns as a function of cannabinoid content (i.e., decrease inhalation as THC content increases, and vice versa) (40, 69). As a result, both cued-smoking and ad libitum administration yield relatively consistent serum cannabinoid concentrations, even when accounting for differences in potency (i.e., THC content) (69). Nonetheless, participants experience clinically-relevant effects when guided through these smoking procedures. Indeed, even heavy users who are tolerant to cannabis will become intoxicated from controlled administration of low-potency cannabis in the human laboratory (41).
Procedures to Improve Blinding
Placebo-controlled trials assume that participants and investigators are blinded to drug conditions (i.e., that inactive and active agents are indistinguishable). Blinding is critical in cannabis research because cannabis users experience significant expectancy effects when exposed to cannabis-related cues (e.g., cigarette appearance and smell, the act of smoking) (43, 45, 70), and also report subjective cannabis-like effects when they anticipate receiving active cannabis but instead receive placebo (19). Moreover, participants' observation of differences between laboratory-administered cannabis and the cannabis they use outside of the lab may influence expectancy (71). As described above, psychiatric symptoms are also particularly sensitive to expectancy effects; thus, adequate blinding is essential to studying cannabis effects in psychiatric illness. Fortunately, human laboratory researchers have developed extensive procedures to improve blinding to cannabis dosing conditions (44).
In the cannabis administration procedures outlined above, blinding is maintained through the following methods (detailed in Figure 1): (36, 37, 41). First, cigarettes are machine-rolled using cigarette paper. They are then inserted into a plastic cigarette holder and a line is drawn at the half-way point, after which the cigarette is presented to the participant. The participant is then guided through the smoking procedure until 50% of the cigarette is smoked (verified by pyrolization to the half-way mark on the cigarette). Smoking only half of a cigarette prevents participants and investigators from seeing the color of its contents (which might vary across conditions or differ from the cannabis participants use in daily settings) and masks the moisture content of the cigarette (which affects burn time and may be higher in placebo vs. active cannabis). Smoking through a plastic cigarette holder also prevents participants from squeezing and possibly occluding the end of the cigarette with their lips, and ensures more consistent puff-to-puff delivery of smoke components, which vary (often increase) with successive puffs (44). Once participants have smoked to the 50% mark, consumption can also be verified via pre- and post-administration weighing of cigarettes (41).
Another approach to the blinding problem is to instruct participants that they will smoke cannabis containing a wide range of THC and other cannabinoids, some of which are intoxicating and others which are not, and ask them to guess their treatment assignment after study completion (72). Across a variety of human laboratory studies (19, 69), individuals receiving placebo cannabis often guess that they instead received a low-potency (but still active) varietal, suggesting the presence of expectancy effects. Investigators can also assess participants' self-report of psychological and physiological effects from active vs. placebo cannabis (19, 40). Other proposed approaches have included recruiting cannabis-naïve participants, which may improve blinding but also potentially increase risk for addiction and other adverse effects (e.g., panic attacks), or using active controls, which may be challenging in that it is unclear which substance suitably mimics the effects of cannabis (euphoria, dry mouth, tachycardia, etc.) without affecting other relevant outcomes (71). Finally, using within-subjects designs, investigators can compare different cannabis varietals with varied concentrations of THC and other cannabinoids (36, 37) while also reducing participants' ability to determine their assigned condition by increasing the range of phytocannabinoids concentrations they could possibly receive.
The blinding approaches above could easily be applied to study how cannabis affects individuals with anxiety disorders. That said, the instructions participants receive should be designed carefully to limit potential expectancy effects on self-reported anxiety: For example, investigators may inform patients that they will smoke cannabis with different concentrations of THC/CBD (rather than active cannabis vs. placebo), which may have a range of effects on anxiety (rather than being anxiolytic or anxiogenic). Excluding heavy cannabis users (e.g., weekly or greater) may reduce the chances that experienced participants guess their assigned condition (in addition to mitigating tolerance effects); to limit risk for adverse cannabis effects, researchers could recruit participants with at least some prior experience using cannabis without negative effects (e.g., >1 lifetime use without experiencing a panic attack).
Methods to Dissect Clinical and Mechanistic Aspects of Cannabis Use
Intoxication and Other Acute Effects
Acute cannabis effects can be examined in laboratory studies by obtaining self-reports, physiological assessments, and/or neurocognitive tests at specific intervals following cannabis administration; these methods also permit exploration of cannabis' acute effects on psychiatric outcomes. Cannabis studies typically ask participants to self-report ratings of intoxication, including how “high” they feel, cannabis “liking,” and “good/bad effect.” Because THC produces dose-dependent increases in heart rate, researchers often integrate serial physiological assessments to establish a timeline for acute cannabis effects. Laboratory studies have also included repeated self-report assessments to probe acute changes in psychiatric symptoms: (36, 37). For example, patients with OCD in our cannabis trial were asked to complete standardized scales of obsessions, compulsions, and anxiety following cannabis administration (37). Other studies have used computerized cognitive tasks [administered once (46) or serially (47)] or obtained neuroimaging assessments (73) to examine acute cannabis effects on neurocognitive outcomes.
Selecting appropriate self-report instruments may be challenging for psychiatry researchers, since many validated scales measure symptoms over long-term (i.e., weeks to months) rather than rapid timeframes (i.e., minutes to hours) (74). While better ways to assess acute changes in psychiatric symptoms are needed, pending their development, studies of rapid-acting treatments (e.g., ketamine) often use a simple visual analog scale (VAS) to identify symptomatic changes (75, 76). In the above laboratory study in patients with OCD, we used a VAS to explore patients' self-report of change in obsessions and compulsions (on a scale from 1 to 10); (37) similar measures could easily be developed to explore cannabis-related symptomatic changes in patients with anxiety or other psychiatric disorders.
Positive and Negative Reinforcement
Behavioral pharmacology studies in non-treatment seeking cannabis smokers demonstrate that cannabis is positively reinforcing: Given the option to self-administer different cannabis varietals in a laboratory setting, participants will administer THC-containing cannabis more often than cannabis containing minimal THC (50). Depending on THC content, participants in these paradigms will also choose to receive THC-containing cannabis over non-drug alternatives like money (49) or a preferred food (48). The incentive-sensitization model describes how positive reinforcement may contribute to increased cannabis use among those with psychiatric illness: Individuals who associate cannabis with pleasure develop greater motivational salience toward cannabis-related cues, which elicits more approach behaviors and attentional bias toward cannabis cues that ultimately increase the likelihood of further cannabis use (77). Several psychiatric conditions including attention-deficit-hyperactivity disorder (ADHD) involve deficits in motivation and attention, reflecting dysfunction in reward-related (particularly dopaminergic) neural circuits (78, 79). Individuals with such deficits may be more susceptible to positive reinforcement from cannabis, which is consistent with epidemiological data supporting higher rates of cannabis use for those with untreated ADHD than in the general population (80).
To date, most laboratory investigations of cannabis' capacity for positive reinforcement have been in cannabis users or adults with CUD. However, self-administration paradigms could also be used to delineate cannabis-related positive reinforcement effects in participants with psychiatric disorders. One example would be for researchers to compare self-administration of cannabis among adults with anxiety disorders and controls matched for their patterns of cannabis use. Another would be to offer anxious participants the choice to receive either cannabis or anxiolytic medications known to be positively-reinforcing (e.g., benzodiazepines) (81).
There is also substantial evidence that cannabis is negatively reinforcing, meaning that individuals use it to escape or reduce the effects of aversive states (e.g., negative affect, withdrawal) (82). Laboratory models of cannabis-associated negative reinforcement typically focus on withdrawal states, admitting participants to an inpatient unit where their access to cannabis is controlled and/or stopped completely (54, 83) and then assessing symptoms of cannabis withdrawal (e.g., disrupted sleep, negative mood) and self-administration. These procedures also have identified differences in cognitive (e.g., reward valuation) (52) and physiological processes (threat response) (53) between cannabis users and controls. Specifically, compared to non-users, heavy cannabis users who abstained from cannabis for 3 days showed greater uncertainty aversion on a reward valuation task (52), while both abstinent and non-abstinent cannabis users had increased startle responses to unpredictable threat (a physiological marker of anxiety states) (53).
According to the affect-motivational model, negative reinforcement drives cannabis use by some individuals with affective psychopathology (e.g., depression/anxiety disorders), who may use cannabis situationally to attenuate affective symptoms (82). Supporting this idea, both depressive and anxiety disorders are linked to higher-than-average rates of cannabis use (82), and alleviating depression/anxiety symptoms is among the most commonly-cited reasons for which individuals seek medicinal cannabis treatment (5, 84). Moreover, preliminary neuroimaging data in both cannabis users (85) and non-cannabis using healthy volunteers (86, 87) suggest that THC acutely reduces functional activity in brain regions involved in emotional processing, particularly when evaluating negative face emotions.
Laboratory probes for negative reinforcement could test whether cannabis use alleviates symptoms or other aversive states in individuals with specific psychiatric diagnoses. Investigators might do this by assessing for differences in disease-relevant outcomes (e.g., symptom self-report, physiological measures, neurocognitive task performance) under conditions of continued use vs. abstinence, or following active vs. placebo cannabis administration. In the case of anxiety disorders, the neutral/predictable/unpredictable shock (NPU) task offers an example of an outcome that is sensitive to both disease- and cannabis-related effects. The NPU task, which indexes startle response to unpredictable vs. predictable threat, can discriminate between anxiety and fear states (88), has been used to screen for the effects of anxiolytic medications (89), and has identified effects related to cannabis withdrawal along with differences between cannabis users and controls (53). The task could easily integrate into laboratory models of intoxication or withdrawal, providing a powerful tool to evaluate for cannabis-related effects on anxiety.
Dose-Dependency and Tolerance
Dose-dependent cannabis effects have also been identified using human laboratory procedures (40, 90). These studies consistently find that cardiovascular outcomes and (to a lesser extent) self-rated subjective responses are sensitive to variation in THC content (40). Dose-response relationships for subjective responses have been more difficult to establish, possibly due to stronger influence of expectancy effects on self-report outcomes. Performance on error-monitoring tasks (e.g., the Flanker task) and other neurocognitive measures has also been shown to vary with THC dose (90).
Tolerance to the effects of THC-containing cannabis develops rapidly over the course of a few days. Cannabis users who were admitted to an inpatient unit where they received smoked cannabis initially reported acute increases in euphoria and intoxication (e.g., “high,” “good drug effect”), but the magnitude of these effects declined over several days of repeated administration. Moreover, tolerance developed dose-dependently (i.e., was greater when high-THC cannabis was administered compared to low-THC) (55). Tolerance to cannabis' physiological effects (e.g., tachycardia) developed dose-dependently over a similar timeframe in other studies (46). In contrast, cannabis' effects on neurocognitive functions like impulse control may persist even with sustained administration (91).
Similar designs could help to determine whether dose-dependency or tolerance moderate cannabis effects on psychiatrically-relevant outcomes. One strategy would be to recruit individuals with anxiety disorders to receive several cannabis varietals with varied THC content to determine whether THC dose moderates self-reported anxiety, blood pressure/heart rate, and or cognitive/physiological measures (e.g., the NPU task). If dose-dependent THC effects are identified, investigators could then assess whether tolerance develops following repeated administration. Establishing whether dose-dependency or tolerance occur will be critical in determining cannabis' potential role in treating anxiety or other psychiatric symptoms.
Methods to Evaluate Pharmacological Treatments
Laboratory procedures already used to screen for CUD treatments can also be applied to study cannabis' role in psychiatric treatment, specifically by screening for potential uses of cannabis to treat symptoms of psychiatric disorders, evaluating medications to treat comorbid psychiatric and CUD symptoms, and assessing for cannabis-drug interactions. Examples of each application are provided below.
Potential Uses of Cannabis to Treat Psychiatric Illness
The human laboratory can serve as a translational bridge to move promising preclinical findings into clinical studies of cannabis. In this regard, a critical use for laboratory paradigms is to test the safety, tolerability, and clinical effects of cannabis in psychiatrically ill individuals. Findings from observational cannabis studies are often difficult to apply in real-world clinical scenarios because (as described above) these designs rarely capture the types of cannabis participants use, or how they ingest it. In contrast, human laboratory procedures permit delivery of precise amounts of cannabis in various forms (e.g., smoked, vaporized, or edible) and containing known phytocannabinoid concentrations. As a result, investigators are able to more accurately determine how the dose, formulation, and contents of cannabis relate to its clinical effects.
Human laboratory paradigms can also be used to validate cannabinoids' hypothesized targets. For example, investigators might test how cannabis acutely modulates brain function using task-based fMRI, or alters cognitive or physiological outcomes during paradigms like the NPU task. Evidence that cannabis meaningfully changes these outcomes could inform mechanistic understanding of its effects in psychiatric illness and may suggest potential treatment applications for further testing.
Finally, the human laboratory is an ideal venue for conducting preliminary tests of cannabis' efficacy as a psychiatric treatment. By incorporating placebo control and rigorous blinding procedures, laboratory paradigms are better able to count for expectancy effects than observational studies or surveys. Compared to clinical trials, human laboratory studies are also faster, cost less, and enable tighter control over potential confounds. Many use within-subjects designs that can achieve adequate statistical power with a smaller number of participants, facilitating testing of clinical effects, mechanistic hypotheses, and potential response moderators [e.g., age (92), gender (67, 93), genetics (94, 95), psychiatric history (96), and prior cannabis exposure (97)]. Laboratory models can thus function as a key intermediary step between preclinical research and clinical trials, rapidly generating data about the odds that cannabis treatment will succeed, which would then guide decisions about the utility of large-scale, resource-intensive clinical trials (98).
Treatments for Comorbid Psychiatric Disorders and CUD
Psychiatric comorbidity is common among adults with CUD, and conversely, psychiatrically-ill individuals are at greater-than-average risk for CUD (24). Though few laboratory studies have explored treatments for these combined conditions, CUD-relevant outcomes have been modeled extensively in the laboratory. These include relapse, operationally defined in the human laboratory as self-administration of cannabis following a period of abstinence. Though it would be unethical to offer cannabis to individuals seeking treatment for cannabis use, relapse can be modeled in non-treatment seeking cannabis users. Participants are typically admitted to an inpatient unit where they remain abstinent for several days. Then, they are given the choice to purchase individual puffs of a cannabis cigarette. Money not spent on cannabis self-administration is given to participants at study end. The initial puff, which reflects “relapse” to cannabis use, carries the greatest cost, while the cost of subsequent puffs decreases (56). Around 50% of participants in studies following these procedures will choose to “relapse”; (38) thus, investigators can explore how treatments influence the decision to resume cannabis use (56–59). Using similar methods, future studies might explore whether medications (e.g., SSRIs) moderate risk for cannabis relapse among individuals with anxiety disorders and CUD.
Cannabis self-administration models have also been used to test medications targeting symptoms of cannabis withdrawal. In one such paradigm, daily cannabis users, abstinent from cannabis for several days, were treated with nabilone at either 6 mg or 8 mg/day vs. placebo. Nabilone improved withdrawal-associated irritability and insomnia while significantly reducing the choice to pay money to self-administer cannabis following abstinence (i.e., a laboratory model of relapse) (56). A follow-up study found that adding zolpidem to nabilone more robustly targeted insomnia, with this combination yielding improved negative mood, anorexia, and insomnia while decreasing cannabis relapse rates compared to placebo or zolpidem alone (60). Both cannabis withdrawal and generalized anxiety disorder (GAD) involve symptoms of anxiety, irritability, restlessness, and insomnia, which may lead those with GAD to experience withdrawal symptoms more frequently or intensely, increasing their risk for continued cannabis use and relapse (99). Thus, using similar laboratory methods, investigators could examine medications or psychotherapies hypothesized to effectively treat these shared symptoms.
Finally, laboratory researchers have evaluated potential treatments to help individuals with CUD achieve abstinence. A straightforward approach used in many studies is to provide non-treatment-seeking cannabis users with either a medication or placebo, and then assess for between-group differences in cannabis self-administration (i.e., whether cannabis use is maintained, reduced, or stopped) (41, 56). Researchers have also used this procedure to explore the abstinence-promoting effects of contingency management paradigms (which offer participants monetary incentives to abstain from cannabis) (100) and cognitive behavioral therapy (CBT) (101). One preliminary study found that a modified form of CBT targeting both anxiety and CUD symptoms reduced self-reported anxiety and cannabis use among individuals with CUD and anxiety disorders; (102) future studies might examine whether this intervention moderates laboratory models of abstinence in this population.
Drug-Drug Interactions Between Cannabis and Psychotropic Medications
Two substances administered simultaneously may interact by pharmacodynamic (i.e., affecting the same receptor or target) and/or pharmacokinetic (i.e., affecting absorption, distribution, metabolism, or excretion) mechanisms. The most commonly reported drug-drug interactions involve pharmacokinetic changes to the activity of cytochrome P450 (CYP450) enzymes, leading to altered drug metabolism. With over 140 phytocannabinoid constitutents (103), cannabis can potentially interact with a range of medications. Animal studies suggest that THC and CBD be substrates for and inducers/inhibitors of CYP450 enzymes (63). With a diverse array of targets including 5HT1A receptors, CBD also has a variety of potential pharmacodynamic interactions with psychotropic drugs (104).
While not all drug-drug interactions identified in animal models are clinically relevant, human trials of both THC and CBD have shown that they interact with common medications. In patients with epilepsy, co-administration of CBD modified serum levels of various antileptics including topiramate, clobazam, and zonisamide (62). Conversely, in adult cannabis users, alcohol increased serum THC levels when co-administered with cannabis (61). Preliminary studies also suggest that cannabis and its constituents can interact with warfarin, oxymorphone, disulfiram, pentobarbital, and cocaine, among other agents (63). Interactions between cannabis/cannabinoids and most psychotropic medications (including anxiolytics) have not been rigorously tested. The human laboratory may be an ideal venue to assess for these potential interactions under controlled conditions.
Integrating Human Laboratory Procedures to Study Cannabis Effects in Psychiatric Illness: Example From a Study in Adults With OCD
Our human laboratory study of smoked cannabis in adults with OCD offers one example of how these paradigms could be applied to screen for therapeutic cannabis effects and inform future clinical and translational research (37). Considering preclinical evidence that cannabinoids affect key cognitive processes and neural circuits implicated in OCD (105), along with anecdotal reports from our clinic patients who suggested that cannabis relieved their symptoms, we conducted a randomized, placebo-controlled, within-subjects study. Twelve adult participants with OCD received three cannabis varietals over the course of three laboratory sessions: High-THC (7.0% THC/0.18% CBD), high-CBD (0.4% THC/10.4% CBD), and placebo (0% THC/CBD). Cannabis was administered using cued-smoking procedures, and serial measurements of OCD symptoms, state anxiety, intoxication, and cardiovascular measures were obtained over 3 h. We found that OCD symptoms and state anxiety decreased immediately following cannabis administration in all three conditions. However, there were no differences in OCD symptoms as a function of cannabis condition. Further, placebo cannabis yielded greater reductions in state anxiety than either active varietal. High-THC cannabis significantly increased heart rate and self-reported intoxication compared to both high-CBD and placebo, demonstrating that the cannabis exposure was sufficient to produce physiological and subjective effects.
This human laboratory study integrated several of the procedures reviewed above, including cued-smoking and blinding methods, self-report scales measuring psychiatric symptoms and intoxication, and physiological assessments. Findings have important clinical, public health, and research implications. Our data suggest that smoked cannabis may have little short-term benefit to individuals with OCD, which would argue against clinical use of cannabis as an acute OCD treatment, inclusion of OCD among the indications for physician-recommended cannabis, or conduct of large-scale clinical trials of smoked cannabis for the acute treatment OCD. Alternatively, finding acute benefits from active cannabis over placebo would have supported further study of its potential clinical utility in OCD: This might have included laboratory examinations of the potential risks and benefits of longer-term cannabis use in OCD (i.e., repeat administration over days to weeks), larger-scale trials assessing its acute efficacy for treating OCD symptoms, or mechanistic studies exploring the basis for the preliminary clinical effects that were observed. Because our preliminary study did not support these larger trials, we were able to quickly move on to pursue alternative research directions.
We then asked a different empirical question: Can THC facilitation of extinction improve the efficacy of existing therapeutic approaches? In a small pilot trial, we tested the effects of 4 weeks of daily treatment with nabilone (an FDA-approved synthetic THC analog) in patients with OCD, and found that nabilone had little effect on OCD symptoms as monotherapy, but appeared to enhance the effects of exposure-based psychotherapy when both were combined (106). This finding was consistent with animal (107, 108) and human neuroimaging data (109–112) suggesting that THC facilitates extinction learning, which is thought to occur during exposure treatment for OCD (113). Thus, THC may have therapeutic benefit to individuals with OCD when paired with exposure treatment.
Based on these findings, in an upcoming fMRI study, we will test the hypothesis that nabilone facilitates extinction learning by impacting relevant brain circuitry. In a separate study, we will also assess whether anxious individuals respond similarly to those with OCD following acute cannabis challenge (i.e., experience smaller anxiety reductions with active cannabis vs. placebo). Using a similar human laboratory design, we will examine the acute effects of smoked cannabis on self-reported anxiety, physiological response to threat, and intoxication in adults with anxiety disorders and high trait anxiety. These novel research directions demonstrate how human laboratory paradigms can guide clinical and translational research involving the effects of cannabis and cannabinoids in psychiatric illness, whether results are positive or negative.
Discussion
Human laboratory models have been used to understand why individuals use cannabis, to define factors that may contribute to CUD, and to test potential treatments for problematic cannabis use. Applying these procedures can also help elucidate the relationship between cannabis use and psychiatric disorders. Laboratory methods permit controlled administration of cannabis under blinded conditions and assessment of interactions between psychiatric symptoms and discrete cannabis-related outcomes (e.g., intoxication, positive and negative reinforcement, dose-dependency, and tolerance). Finally, the human laboratory can be a powerful translational venue in which to screen potential applications of cannabis or its constituents to treat psychiatric symptoms, evaluate treatments for comorbid psychiatric illness and CUD, and identify cannabis-drug interactions.
A key strength of laboratory models is that they can resolve the acute effects of cannabis on discrete behavioral (e.g., self-administration, choice of non-cannabis rewards), psychological (i.e., self-reported or clinically-assessed symptoms), physiological (e.g., cardiovascular and pharmacokinetic measures), and neurocognitive outcomes (e.g., performance on computerized cognitive tasks, neuroimaging assessment). Laboratory researchers can explore endpoints that are directly related to cannabis use (e.g., models of cannabis relapse) and those that are not (e.g., performance on a social-stress paradigm) (114), and can incorporate both subjective (i.e., self-report) and objective (e.g., physiological) assessments. This ability to test cannabis effects across various levels of analysis is consistent with the US National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) (115) and other initiatives aimed at developing more objective measurements of psychopathology (116). Moreover, by incorporating fMRI and other neurobiological measures (73), laboratory models might reveal targets to index cannabis effects that could then be explored in future treatment studies. Thus, the goals and designs of human laboratory research are also well-matched to experimental medicine approaches to psychiatric treatment development (117).
Of course, human laboratory research is not without limitations. First, while tight control over various confounding factors is a key strength of laboratory paradigms, this may also limit their generalizability, as real-world settings are rarely so well-regulated. Whether laboratory studies accurately capture cannabis effects on psychopathology or predict medication efficacy also depends on the chosen design and outcome measures. For example, a study of cannabis effects in specific phobia that does not incorporate symptom provocations may fail to detect an anxiolytic signal even when one exists (since patients with specific phobia typically have minimal anxiety in the absence of phobic triggers). In contrast, a finding that cannabis acutely reduces scores on the Depression, Anxiety, and Stress Scale; DASS) in patients with GAD may lead investigators to conclude that cannabis has anxiolytic effects, when in fact participants misinterpreted reduced stress and tension as reflecting anxiety relief (as prior studies in cannabis users suggest they may do) (118).
Second, participant selection is critical to consider given that the risks of cannabis are different for individuals with different psychiatric disorders: Adults with GAD may be at relatively low risk from participating in a study modeling acute effects from smoking one cannabis cigarette, but the same paradigm would involve different risks and ethical concerns in children with GAD (e.g., increased risk for psychosis) or adults with panic disorder (e.g., panic attacks). Even among participants with the same disorder, individual factors like age (92), gender (67, 93), or genetics (94, 95) may influence the response to cannabis and need to be considered when designing studies. Beyond participant selection, volunteers for laboratory studies may differ from general psychiatric populations in important ways: For example, they tend to have fewer medical comorbidities in order to pass inclusion criteria allowing cannabis to be safely administered (98). Moreover, individuals motivated to participate in a cannabis study presumably have neutral or positive expectations about its effects, which could positively bias study results.
Finally, in the US, only cannabis produced by NIDA can be used in human subjects research (14). Yet the available NIDA cannabis varietals differ substantially in their phytocannabinoid contents compared to cannabis available in the community through both legal (119) and illicit means (120). In particular, THC concentrations on average are lower with NIDA cannabis, which has raised concerns about the generalizability of research involving NIDA preparations. However, there are dozens of studies showing that daily, heavy cannabis users (who are presumably tolerant to THC) become intoxicated and show reliable increases in heart rate after smoking NIDA cannabis (41). Thus, despite differences in cannabinoid content between NIDA and community-obtained cannabis, human laboratory models may nonetheless provide clinically-relevant information about cannabis effects in human subjects.
Conclusion
In summary, human laboratory procedures have a rich history in the field of substance use research. Laboratory methods can also be applied to examine how psychopathology relates to cannabis use, clarify the risks and benefits of cannabis use to individuals with psychiatric disorders, and screen for potential applications of cannabis in psychiatric treatment. Exactly which designs and endpoints best capture specific psychopathologies remains to be determined and should be explored. In addition, while placebo-controlled studies in the human laboratory may provide the necessary groundwork to justify future cannabis trials, further research is needed to verify that promising findings from laboratory models of cannabis treatment are indeed replicable in psychiatric clinical trials. Nonetheless, these laboratory models are powerful tools that can address the increasingly critical need to understand the relationship between cannabis use and psychiatric illness. By improving understanding of cannabis' risks, benefits, and potential treatment applications for patients with psychiatric disorders, laboratory models can enhance the way we conceptualize, diagnose, and treat individuals who suffer from both anxiety and other mental health disorders as well as problematic cannabis use.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
RK, MH, and HS contributed to the conceptual framework, literature review, methodological design, and manuscript writing and preparation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a National Institute of Mental Health T32 Training Grant in Mood, Anxiety and Related Disorders (grant no. T32MH15144) an NIMH Loan Repayment (grant no. L30MH120715, both to RK) and by generous donations from the Youth Anxiety Center (New York Presbyterian Hospital, Columbia University Vagelos College of Physicians and Surgeons, Weill Cornell Medical College, New York, NY, to HS and RK).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. (2018) 43:195–212. doi: 10.1038/npp.2017.198
2. Lipari RN, Park-Lee E. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. (2019). Available online at: https://www.samhsa.gov/data/ (accessed August 26, 2019)
3. Richter KP, Levy S. Big marijuana — Lessons from big tobacco. N Engl J Med. (2014) 371:399–401. doi: 10.1056/nejmp1406074
4. Subritzky T, Lenton S, Pettigrew S. Legal cannabis industry adopting strategies of the tobacco industry. Drug Alcohol Rev. (2016) 35:511–3. doi: 10.1111/dar.12459
5. Azcarate PM, Zhang AJ, Keyhani S, Steigerwald S, Ishida JH, Cohen BE. Medical reasons for marijuana use, forms of use, and patient perception of physician attitudes among the US population. J Gen Intern Med. (2020) 35:1979–86. doi: 10.1007/s11606-020-05800-7
6. Sexton M, Cuttler C, Finnell JS, Mischley LK. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. (2016) 1:131–8. doi: 10.1089/can.2016.0007
7. Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. The Lancet Psychiatry. (2019) 6:995–1010. doi: 10.1016/S2215-0366(19)30401-8
8. Corroon J, Mischley L, Sexton M. Cannabis as a substitute for prescription drugs – a cross-sectional study. J Pain Res. (2017) 10:989–98. doi: 10.2147/JPR.S134330
9. Vidot DC, Islam JY, Marlene Camacho-Rivera, Harrell MB, Rao DR, Chavez JV, et al. The COVID-19 cannabis health study: Results from an epidemiologic assessment of adults who use cannabis for medicinal reasons in the United States. J Addict Dis. (2020) 39:26-36. doi: 10.1080/10550887.2020.1811455
10. National Academies of Sciences Engineering. The Health Effects of Cannabis and Cannabinoids. Washington, DC: National Academies Press (2017). doi: 10.17226/24625
11. Turna J, Simpson W, Patterson B, Lucas P, Van Ameringen M. Cannabis use behaviors and prevalence of anxiety and depressive symptoms in a cohort of Canadian medicinal cannabis users. J Psychiatr Res. (2019) 111:134–9. doi: 10.1016/j.jpsychires.2019.01.024
12. Shover CL, Vest NA, Chen D, Stueber A, Falasinnu TO, Hah JM, et al. Association of state policies allowing medical cannabis for opioid use disorder with dispensary marketing for this indication. JAMA Netw Open. (2020) 3:e2010001. doi: 10.1001/jamanetworkopen.2020.10001
13. State Medical Marijuana Laws. Available online at: https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (accessed July 12, 2020)
14. Haney M. Perspectives on cannabis research—Barriers and recommendations. JAMA Psychiatry. (2020) 77:994–5. doi: 10.1001/jamapsychiatry.2020.1032
15. Alexander SP. Barriers to the wider adoption of medicinal Cannabis. Br J Pain. (2020) 14:122–32. doi: 10.1177/2049463720922884
16. Spindle TR, Bonn-Miller MO, Vandrey R. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr Opin Psychol. (2019) 30:98–102. doi: 10.1016/j.copsyc.2019.04.002
17. Ohlsson A, Lindgren J-E, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. (1980) 28:409–16. doi: 10.1038/clpt.1980.181
18. Lindgren JE, Ohlsson A, Agurell S, Hollister L, Gillespie H. Clinical effects and plasma levels of Δ9-Tetrahydrocannabinol (Δ9-THC) in heavy and light users of cannabis. Psychopharmacology. (1981) 74:208–12. doi: 10.1007/BF00427095
19. Metrik J, Rohsenow DJ, Monti PM, McGeary J, Cook TAR, de Wit H, et al. Effectiveness of a marijuana expectancy manipulation: Piloting the balanced-placebo design for marijuana. Exp Clin Psychopharmacol. (2009) 17:217–25. doi: 10.1037/a0016502
20. Guevarra DA, Moser JS, Wager TD, Kross E. Placebos without deception reduce self-report and neural measures of emotional distress. Nat Commun. (2020) 11:1–8. doi: 10.1038/s41467-020-17654-y
21. Lutz B. Neurobiology of cannabinoid receptor signaling. Dialogues Clin Neurosci. (2020) 22:207–22. doi: 10.31887/DCNS.2020.22.3/blutz
22. Jikomes N, Zoorob M. The cannabinoid content of legal cannabis in washington state varies systematically across testing facilities and popular consumer products. Sci Rep. (2018) 8:1–15. doi: 10.1038/s41598-018-22755-2
23. Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: findings from the national epidemiologic survey on alcohol and related conditions-III. Am J Psychiatry. (2016) 173:588–99. doi: 10.1176/appi.ajp.2015.15070907
24. Gorelick DA. Psychiatric comorbidity of cannabis use disorder. In: Montoya I, Weiss S, editors. Cannabis Use Disorders Cham: Springer International Publishing (2020). p. 113–25. doi: 10.1007/978-3-319-90365-1_13
25. Hindley G, Beck K, Borgan F, Ginestet CE, McCutcheon R, Kleinloog D, et al. Psychiatric symptoms caused by cannabis constituents: a systematic review and meta-analysis. Lancet Psychiatry. (2020) 7:344–53. doi: 10.1016/S2215-0366(20)30074-2
26. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. (2019) 233:181–92. doi: 10.1016/j.socscimed.2019.06.005
27. Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. J Am Med Assoc. (2015) 313:2491–3. doi: 10.1001/jama.2015.6613
28. Cash MC, Cunnane K, Fan C, Romero-Sandoval EA. Mapping cannabis potency in medical and recreational programs in the United States. PLoS ONE. (2020) 15:e0230167. doi: 10.1371/journal.pone.0230167
29. Freeman TP, Morgan CJA, Hindocha C, Schafer G, Das RK, Curran HV. Just say “know”: how do cannabinoid concentrations influence users' estimates of cannabis potency and the amount they roll in joints? Addiction. (2014) 109:1686–94. doi: 10.1111/add.12634
30. Kruger DJ, Kruger JS, Collins RL. Frequent cannabis users demonstrate low knowledge of cannabinoid content and dosages. Drugs Educ Prev Policy. (2020). doi: 10.1080/09687637.2020.1752150. [Epub ahead of print].
31. Newmeyer MN, Swortwood MJ, Abulseoud OA, Huestis MA. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked vaporized, and oral cannabis administration. Drug Alcohol Depend. (2017) 175:67–76. doi: 10.1016/j.drugalcdep.2017.02.003
32. Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond B Biol Sci. (2012) 367:3353–63. doi: 10.1098/rstb.2011.0381
33. Sarris J, Sinclair J, Karamacoska D, Davidson M, Firth J. Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review. BMC Psychiatry. (2020) 20:24. doi: 10.1186/s12888-019-2409-8
34. Wilkinson ST, Radhakrishnan R, D'Souza DC. Systematic review of evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. (2016) 77:8:1050–64. doi: 10.4088/JCP.15r10036
35. Reznik I. P.4.a.011 Post-traumatic stress disorder and medical cannabis use: a naturalistic observational study. Eur Neuropsychopharmacol. (2012) 22, (Supplement 2):S363–S364. doi: 10.1016/s0924-977x(12)70563-1
36. Vadhan NP, Corcoran CM, Bedi G, Keilp JG, Haney M. Acute effects of smoked marijuana in marijuana smokers at clinical high-risk for psychosis: a preliminary study. Psychiatry Res. (2017) 257:372–4. doi: 10.1016/j.psychres.2017.07.070
37. Kayser RR, Haney M, Raskin M, Arout C, Simpson HB. Acute effects of cannabinoids on symptoms of obsessive-compulsive disorder: a human laboratory study. Depress Anxiety. (2020) 37:801–11. doi: 10.1002/da.23032
38. Arout CA, Herrmann E, Haney M. Human laboratory models of cannabis use disorder. In: Montoya I, Weiss S, editors. Cannabis Use Disorders. Cham: Springer (2019). doi: 10.1007/978-3-319-90365-1_9
39. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. (2005) 62:593–602. doi: 10.1001/archpsyc.62.6.593
40. Ramesh D, Haney M, Cooper ZD. Marijuana's dose-dependent effects in daily marijuana smokers. Exp Clin Psychopharmacol. (2013) 21:287–93. doi: 10.1037/a0033661
41. Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. (2016) 41:1974–82. doi: 10.1038/npp.2015.367
42. Bidwell LC, Bidwell LC, Ellingson JM, Ellingson JM, Karoly HC, Yorkwilliams SL, et al. Association of naturalistic administration of cannabis flower and concentrates with intoxication and impairment. JAMA Psychiatry. (2020) 77:787–96. doi: 10.1001/jamapsychiatry.2020.0927
43. Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology. (1988) 94:206–12. doi: 10.1007/bf00176846
44. Chait LD, Pierri J. Some physical characteristics of NIDA marijuana cigarettes. Addict Behav. (1989) 14:61–7. doi: 10.1016/0306-4603(89)90017-8
45. Kirk JM, Doty P, De Wit H. Effects of expectancies on subjective responses to oral delta9-tetrahydrocannabinol. Pharmacol Biochem Behav. (1998) 59:287–93. doi: 10.1016/s0091-3057(97)00414-0
46. Hart CL, Van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. (2001) 25:757–65. doi: 10.1016/S0893-133X(01)00273-1
47. Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. J Clin Exp Neuropsychol. (2007) 29:357–64. doi: 10.1080/13803390600693615
48. Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. (1997) 8:101–12.
49. Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Δ9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. (2005) 181:237–43. doi: 10.1007/s00213-005-2234-2
50. Cooper ZD, Haney M. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol. (2008) 13:188–95. doi: 10.1111/j.1369-1600.2007.00095.x
51. Metrik J, Aston ER, Kahler CW, Rohsenow DJ, McGeary JE, Knopik VS. Marijuana's acute effects on cognitive bias for affective and marijuana cues. Exp Clin Psychopharmacol. (2015) 23:339–50. doi: 10.1037/pha0000030
52. Hefner KR, Starr MJ, Curtin JJ. Altered subjective reward valuation among drug-deprived heavy marijuana users: a version to uncertainty. J Abnorm Psychol. (2016) 125:138–50. doi: 10.1037/abn0000106
53. Hefner KR, Starr MJ, Curtin JJ. Heavy marijuana use but not deprivation is associated with increased stressor reactivity. J Abnorm Psychol. (2018) 127:348–58. doi: 10.1037/abn0000344
54. Haney M, Cooper ZD, Bedi G, Herrmann E, Comer SD, Reed SC, et al. Guanfacine decreases symptoms of cannabis withdrawal in daily cannabis smokers. Addict Biol. (2019) 24:707–16. doi: 10.1111/adb.12621
55. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. (1999) 141:395–404.
56. Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology. (2013) 38:1557–65. doi: 10.1038/npp.2013.54
57. Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. (2008) 197:157–68. doi: 10.1007/s00213-007-1020-8
58. Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD, et al. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology. (2010) 211:233–44. doi: 10.1007/s00213-010-1888-6
59. Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, et al. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol Psychiatry. (2013) 73:242–8. doi: 10.1016/j.biopsych.2012.07.028
60. Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology. (2016) 233:2469–78. doi: 10.1007/s00213-016-4298-6
61. Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney G, et al. Controlled cannabis vaporizer administration: blood and plasma cannabinoids with and without alcohol. Clin Chem. (2015) 61:850–69. doi: 10.1373/clinchem.2015.238287
62. Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. (2017) 58:1586–92. doi: 10.1111/epi.13852
63. Alsherbiny M, Li C. Medicinal cannabis—Potential drug interactions. Medicines. (2018) 6:3. doi: 10.3390/medicines6010003
64. Foltin RW, Brady JV, Fischman MW, Emurian CS, Dominitz J. Effects of smoked marijuana on social interaction in small groups. Drug Alcohol Depend. (1987) 20:87–93. doi: 10.1016/0376-8716(87)90079-2
65. Russo EB. Current therapeutic cannabis controversies and clinical trial design issues. Front Pharmacol. (2016) 7:309. doi: 10.3389/fphar.2016.00309
66. Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, et al. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Netw Open. (2018) 1:e184841. doi: 10.1001/jamanetworkopen.2018.4841
67. Matheson J, Sproule B, Di Ciano P, Fares A, Le Foll B, Mann RE, et al. Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology. (2020) 237:305–16. doi: 10.1007/s00213-019-05369-y
68. Matheson J, Mann RE, Sproule B, Huestis MA, Wickens CM, Stoduto G, et al. Acute and residual mood and cognitive performance of young adults following smoked cannabis. Pharmacol Biochem Behav. (2020) 194:172937. doi: 10.1016/j.pbb.2020.172937
69. Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. (2009) 103:107–13. doi: 10.1016/j.drugalcdep.2009.01.023
70. Fillmore MT, Mulvihill LE, Vogel-Sprott M. The expected drug and its expected effect interact to determine placebo responses to alcohol and caffeine. Psychopharmacology. (1994) 115:383–8. doi: 10.1007/BF02245081
71. Casarett D. The achilles heel of medical cannabis research—inadequate blinding of placebo-controlled trials. JAMA Intern Med. (2018) 178:9–10. doi: 10.1001/jamainternmed.2017.5308
72. Ellis RJ, Toperoff W, Vaida F, Van Den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. (2009) 34:672–80. doi: 10.1038/npp.2008.120
73. Ramaekers JG, van Wel JH, Spronk D, Franke B, Kenis G, Toennes SW, et al. Cannabis and cocaine decrease cognitive impulse control and functional corticostriatal connectivity in drug users with low activity DBH genotypes. Brain Imaging Behav. (2016) 10:1254–63. doi: 10.1007/s11682-015-9488-z
74. Ballard ED, Luckenbaugh DA, Richards EM, Walls TL, Brutsché NE, Ameli R, et al. Assessing measures of suicidal ideation in clinical trials with a rapid-acting antidepressant. J Psychiatr Res. (2015) 68:68–73. doi: 10.1016/j.jpsychires.2015.06.003
75. Wilkinson ST, Katz RB, Toprak M, Webler R, Ostroff RB, Sanacora G. Acute and longer-term outcomes using ketamine as a clinical treatment at the yale psychiatric hospital. J Clin Psychiatry. (2018) 79:17m11731. doi: 10.4088/JCP.17m11731
76. Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. (2013) 38:2475–83. doi: 10.1038/npp.2013.150
77. Metrik J, Aston ER, Kahler CW, Rohsenow DJ, McGeary JE, Knopik VS, et al. Cue-elicited increases in incentive salience for marijuana: Craving, demand, and attentional bias. Drug Alcohol Depend. (2016) 167:82–8. doi: 10.1016/j.drugalcdep.2016.07.027
78. Blum K, Chen ALC, Braverman ER, Comings DE, Chen TJH, Arcuri V, et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr Dis Treat. (2008) 4:893–917. doi: 10.2147/ndt.s2627
79. Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. (2011) 16:1147–54. doi: 10.1038/mp.2010.97
80. Capusan AJ, Bendtsen P, Marteinsdottir I, Larsson H. Comorbidity of adult ADHD and its subtypes with substance use disorder in a large population-based epidemiological study. J Atten Disord. (2019) 23:1416–26. doi: 10.1177/1087054715626511
81. Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory animals - Implications for problems of long-term use and abuse. Psychopharmacology. (1997) 134:1–37. doi: 10.1007/s002130050422
82. Wycoff AM, Metrik J, Trull TJ. Affect and cannabis use in daily life: a review and recommendations for future research. Drug Alcohol Depend. (2018) 191:223–33. doi: 10.1016/j.drugalcdep.2018.07.001
83. Bedi G, Cooper ZD, Haney M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict Biol. (2013) 18:872–81. doi: 10.1111/j.1369-1600.2011.00427.x
84. Glodosky NC, Cuttler C. Motives matter: cannabis use motives moderate the associations between stress and negative affect. Addict Behav. (2020) 102:106188. doi: 10.1016/j.addbeh.2019.106188
85. Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJA, et al. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol. (2015) 25: 325–34. doi: 10.1016/j.euroneuro.2014.11.014
86. Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, et al. Modulation of effective connectivity during emotional processing by δ9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. (2010) 13:421–32. doi: 10.1017/S1461145709990617
87. Bossong MG, van Hell HH, Jager G, Kahn RS, Ramsey NF, Jansma JM. The endocannabinoid system and emotional processing: a pharmacological fMRI study with {increment}9-tetrahydrocannabinol. Eur Neuropsychopharmacol. (2013) 23:1687–97. doi: 10.1016/j.euroneuro.2013.06.009
88. Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nat Protoc. (2012) 7:527–32. doi: 10.1038/nprot.2012.001
89. Grillon C, Ernst M. A way forward for anxiolytic drug development: Testing candidate anxiolytics with anxiety-potentiated startle in healthy humans. Neurosci Biobehav Rev. (2020) 119:348–54. doi: 10.1016/j.neubiorev.2020.09.024
90. Kowal MA, Van Steenbergen H, Colzato LS, Hazekamp A, Van Der Wee NJA, Manai M, Durieux J, Hommel B. Dose-dependent effects of cannabis on the neural correlates of error monitoring in frequent cannabis users. Eur Neuropsychopharmacol. (2015) 25:1943–53. doi: 10.1016/j.euroneuro.2015.08.001
91. Ramaekers J, van Wel J, Spronk D, Toennes SW, Kuypers KPC, Theunissen EL, et al. Cannabis and tolerance: acute drug impairment as a function of cannabis use history. Sci Rep. (2016) 6:26843. doi: 10.1038/srep26843
92. Sexton M, Cuttler C, Mischley LK. A survey of cannabis acute effects and withdrawal symptoms: differential responses across user types and age. J Altern Complement Med. (2019) 25:326–35. doi: 10.1089/acm.2018.0319
93. Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. (2014) 136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013
94. Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. (2010) 35:967–75. doi: 10.1038/npp.2009.200
95. Palmer RHC, McGeary JE, Knopik VS, Bidwell LC, Metrik JM. CNR1 and FAAH variation and affective states induced by marijuana smoking. Am J Drug Alcohol Abuse. (2019) 45:514–26. doi: 10.1080/00952990.2019.1614596
96. Chao T, Radoncic V, Hien D, Bedi G, Haney M. Stress responding in cannabis smokers as a function of trauma exposure, sex, and relapse in the human laboratory. Drug Alcohol Depend. (2018) 185:23–32. doi: 10.1016/j.drugalcdep.2017.11.021
97. Colizzi M, McGuire P, Giampietro V, Williams S, Brammer M, Bhattacharyya S. Previous cannabis exposure modulates the acute effects of delta-9-tetrahydrocannabinol on attentional salience and fear processing. Exp Clin Psychopharmacol. (2018) 26:582–98. doi: 10.1037/pha0000221
98. Brezing CA, Levin FR. The Current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. (2018) 43:173–94. doi: 10.1038/npp.2017.212
99. Livne O, Shmulewitz D, Lev-Ran S, Hasin DS. DSM-5 cannabis withdrawal syndrome: demographic and clinical correlates in U.S. adults. Drug Alcohol Depend. (2019) 195:170–7. doi: 10.1016/j.drugalcdep.2018.09.005
100. Schuster RM, Hanly A, Gilman J, Budney A, Vandrey R, Evins AE. A contingency management method for 30-days abstinence in non-treatment seeking young adult cannabis users. Drug Alcohol Depend. (2016) 167:199–206. doi: 10.1016/j.drugalcdep.2016.08.622
101. Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. (2006) 74:307–16. doi: 10.1037/0022-006X.74.2.307
102. Buckner JD, Ecker AH, Beighley JS, Zvolensky MJ, Schmidt NB, Shah SM, et al. Integrated cognitive behavioral therapy for comorbid cannabis use and anxiety disorders. Clin Case Stud. (2016) 15:68–83. doi: 10.1177/1534650115590857
103. Russo EB, Marcu J. Cannabis pharmacology: the usual suspects and a few promising leads. Adv Pharmacol. (2017) 80:67–134. doi: 10.1016/BS.APHA.2017.03.004
104. Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. (2012) 367:3364–78. doi: 10.1098/rstb.2011.0389
105. Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, et al. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. (2013) 64:396–402. doi: 10.1016/j.neuropharm.2012.06.063
106. Rabinak CA, Angstadt M, Lyons M, Mori S, Milad MR, Liberzon I, et al. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol Learn Mem. (2014) 113:125–34. doi: 10.1016/j.nlm.2013.09.009
107. Rabinak CA, Peters C, Marusak HA, Ghosh S, Phan KL. Effects of acute Δ9-tetrahydrocannabinol on next-day extinction recall is mediated by post-extinction resting-state brain dynamics. Neuropharmacology. (2018) 143:289–98. doi: 10.1016/j.neuropharm.2018.10.002
108. Hammoud MZ, Peters C, Hatfield JRB, Gorka SM, Phan L, Milad MR, et al. Influence of Δ9-tetrahydrocannabinol on long-term neural correlates of threat extinction memory retention in humans. Neuropsychopharmacology. (2019) 44:1769–77. doi: 10.1038/s41386-019-0416-6
109. Dougherty DD, Brennan BP, Stewart SE, Wilhelm S, Widge AS, Rauch SL. Neuroscientifically informed formulation and treatment planning for patients with obsessive-compulsive disorder. JAMA Psychiatry. (2018) 75:1081. doi: 10.1001/jamapsychiatry.2018.0930
110. Childs E, Lutz JA, de Wit H. Dose-related effects of delta-9-THC on emotional responses to acute psychosocial stress. Drug Alcohol Depend. (2017) 177:136–44. doi: 10.1016/j.drugalcdep.2017.03.030
111. Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. (2010) 167:748–51. doi: 10.1176/appi.ajp.2010.09091379
112. Insel TR, Wang PS. Rethinking mental illness. J Am Med Assoc. (2010) 303:1970–1. doi: 10.1001/jama.2010.555
113. Insel TR, Gogtay N. National institute of mental health clinical trials new opportunities, new expectations. JAMA Psychiatry. (2014) 71:745–6. doi: 10.1001/jamapsychiatry.2014.426
114. Kayser RR, Snorrason I, Haney M, Lee FS, Simpson HB. The endocannabinoid system: a new treatment target for obsessive compulsive disorder? Cannabis Cannabinoid Res. (2019) 4:77–87. doi: 10.1089/can.2018.0049
115. Kayser RR, Raskin M, Snorrason I, Hezel DM, Haney M, Simpson HB. Cannabinoid augmentation of exposure-based psychotherapy for obsessive-compulsive disorder. J Clin Psychopharmacol. (2020) 40:207–10. doi: 10.1097/JCP.0000000000001179
116. Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. (2002) 418:530–4. doi: 10.1038/nature00839
117. Morena M, Berardi A, Colucci P, Palmery M, Trezza V, Hill MN, et al. Enhancing endocannabinoid neurotransmission augments the efficacy of extinction training and ameliorates traumatic stress-induced behavioral alterations in rats. Neuropsychopharmacology. (2018) 43:1284–96. doi: 10.1038/npp.2017.305
118. Temple EC, Driver M, Brown RF. Cannabis use and anxiety: is stress the missing piece of the puzzle? Front Psychiatry. (2014) 5:168. doi: 10.3389/fpsyt.2014.00168
119. Vergara D, Bidwell LC, Gaudino R, Torres A, Du G, Ruthenburg TC, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Sci Rep. (2017) 7:46528. doi: 10.1038/srep46528
Keywords: cannabis (marijuana), cannabinoids, psychiatric disorders, anxiety disorders, human laboratory research, clinical and translational research
Citation: Kayser RR, Haney M and Simpson HB (2021) Human Laboratory Models of Cannabis Use: Applications for Clinical and Translational Psychiatry Research. Front. Psychiatry 12:626150. doi: 10.3389/fpsyt.2021.626150
Received: 05 November 2020; Accepted: 03 February 2021;
Published: 25 February 2021.
Edited by:
Danilo De Gregorio, McGill University, CanadaReviewed by:
Anthony Ecker, Baylor College of Medicine, United StatesWayne Denis Hall, The University of Queensland, Australia
Copyright © 2021 Kayser, Haney and Simpson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reilly R. Kayser, cmVpbGx5LmtheXNlckBueXNwaS5jb2x1bWJpYS5lZHU=
 Reilly R. Kayser
Reilly R. Kayser Margaret Haney1,2
Margaret Haney1,2 Helen Blair Simpson
Helen Blair Simpson