
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 15 March 2021
Sec. Aging Psychiatry
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.617773
This article is part of the Research Topic Cerebrovascular Diseases and Neuropsychiatric Disorders in Aging View all 7 articles
Objective: The associations of vascular risk factors (VRFs), apolipoprotein E (APOE), and translocase of outer mitochondrial membrane 40 (TOMM40) with cognitive function have been investigated mostly in western societies. In the present study, we sought to examine the associations of VRFs [i.e., current smoking, current drinking, physical inactivity, obesity, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), diabetes, and hypertension] and variants located in APOE (ε2/3/4) and TOMM40 (rs2075650) with global cognitive function in Chinese older adults, with a focus on their potential interactions.
Methods: This is a cross-sectional study that included 422 permanent residents (mean age 69.2 years, 54.3% female) living in Beijing, who were free of dementia. Data were collected through interviews, clinical examinations, and laboratory tests. The two genetic polymorphisms were genotyped, and participants were dichotomized as carriers vs. non-carriers of APOE ε4 or TOMM40 G. Global cognitive function was assessed with the Mini-Mental State Examination (MMSE). Data were analyzed with multivariable linear regression models.
Results: Physical inactivity and diabetes were independently associated with a lower MMSE score (all p < 0.05). When four putative VRFs (i.e., current smoking, physical inactivity, high LDL-C, and diabetes) were aggregated, an increasing number of having these factors was associated with a decreasing MMSE score in a dose–response manner (p = 0.001). TOMM40 polymorphisms, independent of the APOE ε4 allele, interacted with aggregated VRFs to influence cognitive performance, such that having one or more of these VRFs was particularly detrimental to the cognition of TOMM40 carriers. Further analyses revealed interactions of the TOMM40 polymorphism with (i) physical inactivity and (ii) diabetes, such that having either physical inactivity or diabetes in combination with carrying a TOMM40 G allele, compared to having neither, was significantly associated with a markedly lower MMSE score (all p < 0.05).
Conclusion: This study provides some evidence supporting the association of vascular risk factors with poor cognitive performance among dementia-free Chinese older adults and further revealed their interactions with the TOMM40 polymorphism. The results underscore the vulnerability of global cognitive function to VRFs, which could be reinforced by carrying the TOMM40 rs2075650 G allele. These findings have potential implications for developing tailored intervention programs to maintain cognitive function.
The proportion of older people has been growing rapidly in China over the past half-century, alongside social and economic development. In 2010, there were 178 million people aged 60 years and older in China, accounting for ~13.3% of the total population. Therefore, age-related disorders such as Alzheimer's disease (AD) and dementia have posed a tremendous burden to individuals and the society at large in China (1, 2). Recently, great efforts have been made to identify modifiable risk factors for early-stage cognitive disorders, such as mild cognitive impairment, which may help identify high-risk individuals and further facilitate the development of interventions to delay the onset of dementia. The associations of individual vascular risk factors (VRFs) and their aggregation with poor cognitive function have been well-established in western countries (3, 4). In China, some studies have reported the associations of individual VRFs (e.g., hypertension and diabetes) with cognitive impairment (5, 6). However, very few studies have examined the association of aggregated VRFs with global cognitive impairment. Modifiable or manageable VRFs often coexist among the elderly, and the concurrent presence of multiple VRFs plays a critical role in age-related cognitive decline (7). Thus, exploring the association of aggregated VRFs and cognitive function among Chinese older adults is of high relevance for developing the appropriate strategies for the prevention of age-related cognitive impairment and dementia in China.
The ε4 allele of the apolipoprotein E (APOE) gene is a known genetic risk factor for AD and global cognitive decline in old age. Evidence from meta-analysis has suggested that the APOE ε4 allele was associated with poorer performance on tests of global cognitive function (8, 9). However, most of the previous studies have been carried out among western societies, and very few studies are performed in Chinese older adults. Besides, translocase of outer mitochondrial membrane 40 (TOMM40), roughly 15 kb upstream to the APOE gene, is responsible for encoding an essential protein for cell viability on the outer mitochondrial membrane. Several single-nucleotide polymorphisms (SNPs) of TOMM40, such as rs2075650, have been identified to be associated with the risk of developing AD in different populations, including Han Chinese (10, 11). Linkage disequilibrium (LD) between TOMM40 rs2075650 and APOE rs429358 has been reported in various populations (12–14); thus, the association of TOMM40 rs2075650 with AD is often considered as a proxy for that of the APOE ε4 allele. However, genetic association analyses among APOE ε4 non-carriers found that TOMM40 rs2075650 is still associated with AD (15, 16), suggesting that TOMM40 might play a role in AD independent of the APOE ε4 allele. A few studies have explored the contribution of TOMM40 rs2075650 to cognitive dysfunction, and the results are inconsistent across different populations. For example, a genome-wide association study has revealed that TOMM40 rs2075650 was associated with cognitive aging in Swedish cohorts, but not in UK cohorts (17). Furthermore, whether TOMM40 rs2075650 is associated with global cognitive function independently of the APOE ε4 allele is still unclear, especially in Chinese older adults.
Emerging evidence has indicated that VRFs and susceptibility genetic polymorphisms could interact synergistically to exacerbate the cognitive decline. For instance, some studies have reported that VRFs (e.g., diabetes) could interact with APOE ε4 to adversely affect global cognitive function (18–20) and specific cognitive domains, such as memory and executive function (21, 22). So far, no study has examined the interaction of VRFs with TOMM40 rs2075650. Of note, population-based studies that explore potential interactive effects of individual and aggregated VRFs with APOE ε4, or with TOMM40 polymorphisms on cognition are generally lacking in the Chinese population.
Therefore, in this community-based study, we aim to examine (i) the relationship between individual and aggregated VRFs and global cognitive performance; (ii) the relationship of APOE genotype and TOMM40 polymorphisms with cognition; and (iii) the potential interactions between VRFs and two susceptibility polymorphisms on global cognitive function in Chinese older adults. We hypothesized that VRFs, APOE ε4, and TOMM40 G carriers were independently associated with poor cognitive performance and that carrying genetic polymorphisms may strengthen the associations of VRFs with poor cognitive function.
This is a community-based study performed in Beijing, China. The details of this study design and data collection procedures have been fully described previously (23). Briefly, at baseline (2011), 473 participants (age ≥60 years) were randomly selected from permanent residents living in the XiCheng and ChaoYang Districts in Beijing, which represented the old downtown and the newly developed area, respectively. Of these, 40 participants were excluded due to lack of blood samples and an additional 11 participants were excluded owing to probable AD or other types of dementia defined according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria (DSM-IV), leaving 422 participants for the final analysis of cross-sectional associations.
This study was approved by the Ethics Committee at the Institute of Psychology, Chinese Academy of Sciences, Beijing, China. Written informed consent was given by each participant at each visit.
At baseline, research assistants with psychological background collected data on demographics or cardiovascular or lifestyle-related factors (e.g., smoking, alcohol consumption, and leisure activity). Physical activity was measured via the question “How often do you regularly participate in physical activity at least 20 min per day (never, 1–3 days/week, 4–6 days/week, or every day)?” Psychiatrists conducted the clinical assessment, including health history and use of medications, a routine physical examination, and neuropsychological tests (e.g., Neuropsychiatric Inventory and the Clinical Dementia Rating). Depressive symptoms were assessed using the 30-item Geriatric Depression Scale (GDS-30) (24). Weight and height were measured in light clothes with no shoes. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Arterial blood pressure was measured on the right arm in a sitting position.
After an overnight fast, peripheral blood samples were taken. Plasma glucose, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured using standard enzymatic methods on routine automated chemistry systems. Genomic DNA was isolated from whole blood samples.
Smoking and alcohol intake were dichotomized into current and non-current (never and former). Physical inactivity was defined as participating in any physical activity ≤ 3 days/week during leisure time. Obesity was defined as a BMI ≥28 kg/m2, a commonly recommended cutoff point for Chinese adults (25). High TC was defined as TC >6.2 mmol/l or use of hypolipidemic drugs, high TG as TG ≥2.3 mmol/l or receiving hypolipidemic drugs, low HDL-C as HDL-C <1.0 mmol/l in men or HDL-C <1.3 mmol/l in women or use of hypolipidemic drugs, and high LDL-C as LDL-C ≥4.1 mmol/l or using hypolipidemic drugs (26). Diabetes was defined as fasting plasma glucose ≥7.0 mmol/l or the current use of oral blood glucose-lowering medications or insulin injections (27). Hypertension was defined as having a self-reported history of hypertension, current use of antihypertensive medication, or blood pressure ≥140/90 mm Hg (28).
APOE and TOMM40 polymorphisms were genotyped using the Sequenom iPLEX Gold assay and the MassARRAY MALDI-TOF mass spectrometry platform (San Diego, CA, USA). The APOE genotype was dichotomized as carriers vs. non-carriers of the APOE ε4 allele, and TOMM40 rs2075650 as carriers vs. non-carriers of the G allele.
The presence of depressive symptoms was defined as the GDS-30 score >10. Heart disease was defined as having a myocardial infarction, atrial fibrillation, heart failure, or angina.
Global cognitive functioning was assessed with the Chinese version of the Mini-Mental State Examination (MMSE). Most items of the MMSE test were translated literally from the original version without modification, while some items were adopted to meet the Chinese cultural context (29). It is a validated and widely used test of assessing global cognitive function with orientation, memory, attention-calculation, language, and registration components. The total MMSE score ranges from 0 to 30, with higher scores denoting better cognitive performance.
The participants' characteristics by APOE ε4 and TOMM40 G carrier status were compared using independent t-tests for continuous variables and χ2 tests for categorical variables.
We dichotomized each of the VRFs, APOE ε4 and TOMM40 G carrier status, depressive symptoms, and heart disease as presence vs. absence. Multivariable linear regression models were used to analyze the associations of the MMSE score with various factors. Firstly, we estimated the β coefficients and 95% confidence interval (CI) of the MMSE score associated with individual and aggregated VRFs, APOE, and TOMM40 polymorphisms. The aggregated VRF measure was calculated by summing up the total number of concurrently presented putative VRFs that were associated with the MMSE score in multivariable linear regression analysis (30). In order not to miss any potential risk factors associated with the MMSE score, we used a conservative p-value of 0.35 for the inclusion of the putative VRFs, as previously reported (31, 32). The aggregated VRF was treated as either a continuous or categorical variable in the analyses.
We also assessed the interactions of individual and aggregated VRFs with genetic polymorphisms by simultaneously including the two independent variables and their cross-product terms in the same model. To examine the joint effects of VRFs with a genetic polymorphism, we divided subjects into 4 groups: those with neither VRFs nor the genetic polymorphism (reference), with only VRFs (e.g., diabetes), with only the genetic polymorphism (e.g., APOE ε4 allele), or with both VRFs and genetic polymorphism.
We reported results from two models: model 1 was adjusted for demographic variables (i.e., age, sex, and education) and model 2 was further adjusted for the presence of depressive symptoms and heart disease. Given the moderate LD between APOE rs429358 and TOMM40 rs2075650 in Han Chinese people (12), in models 1 and 2, we additionally adjusted for each other to verify their independent associations with the MMSE score. IBM SPSS Statistics 21.0 for Windows (IBM Corp., Armonk, NY) was used for all statistical analyses.
The characteristics of the study participants were summarized as shown in Table 1. Of the 422 participants, the mean age was 69.2 (SD, 6.2) years, 54.3% were women, and 95.5% were Han Chinese. Among these participants, 68 (16.1%) carried at least one APOE ε4 allele and 81 (19.2%) carried TOMM40 G. The frequency distributions of the two genes were similar to the previous report from the large-scale study in Beijing (33). The APOE ε4 carriers and non-carriers did not differ significantly in the mean age and years of education, or in the distribution of sex, current smoking, alcohol intake, physical inactivity, obesity, high cholesterol, high triglycerides, low HDL-C, high LDL-C, hypertension, diabetes, depression, and heart disease (for all comparisons, p > 0.05). Furthermore, there were no significant differences between TOMM40 G carriers and non-carriers on foregoing factors (for all comparisons, p > 0.05), except for high LDL-C (p = 0.05). The mean MMSE score was 27.1 (SD, 3.5), and there were no significant differences in MMSE score between APOE ε4 carriers and non-carriers (p = 0.66) and between TOMM40 G carriers and non-carriers (p = 0.11).
Associations of individual VRFs with MMSE score were summarized as shown in Table 2. Specifically, physical inactivity was significantly associated with a low MMSE score in model 1 (p = 0.01), and the results remained significant when depression and heart disease were further adjusted for in model 2 (p = 0.02). Similarly, diabetes was also significantly associated with a low MMSE score in model 1 (p = 0.001), and the significance did not change at all in model 2. No other VRFs were significantly associated with the MMSE score.
When a conservative p-value of 0.35 was used to include all the potential risk factors, four putative VRFs (i.e., current smoking, physical inactivity, high LDL-C, and diabetes) were counted to calculate the aggregated VRF score (Table 2). As a continuous variable (0–4), an increasing VRF score was significantly associated with a lower MMSE score (p = 0.001) (Table 3). As a categorical variable (categorized into 0, 1, and ≥2), having 2 or more of these VRFs, in comparison with having none, was significantly associated with a lower MMSE score, even when further adjusting for the presence of depressive symptoms and heart disease (p = 0.001) (Table 3, models 1 and 2).
Carrying the APOE ε4 allele was not significantly associated with MMSE score after adjusting for age, sex, education, and TOMM40 in model 1 (β: 0.41, 95% CI: −0.62 to 1.44, p = 0.44), and the results were similar when depressive symptoms and heart disease were additionally controlled for in model 2 (β: 0.47, 95% CI: −0.55 to 1.50, p = 0.36). Carrying the TOMM40 G allele was marginally associated with a low MMSE score when age, sex, education, and APOE were adjusted for (β: −0.84, 95% CI: −1.79 to 0.10, p = 0.08), and the results were similar in the fully adjusted model (β: −0.87, 95% CI: −1.81 to 0.08, p = 0.07).
First, we analyzed the interactions of aggregated VRFs with APOE genotype and TOMM40 polymorphisms. We did not detect a significant interaction between aggregated VRFs and APOE ε4 in model 1 and the fully adjusted model (all p for interaction > 0.05). However, a marginally significant interaction between aggregated VRFs and the TOMM40 polymorphism was detected (p = 0.06), which became statistically evident when further adjusting for depressive symptoms and heart disease (p = 0.05). Further analysis stratified by TOMM40 indicated that among TOMM40 G carriers, having 1 or more of these VRFs was potentially correlated with a lower MMSE score in model 1 (β: −1.38, 95% CI: −2.80 to 0.03, p = 0.05). However, among TOMM40 G non-carriers, an association of aggregated VRFs with a lower MMSE score was not significant (β: −0.28, 95% CI: −0.82 to 0.28, p = 0.31). The results were similar in the fully adjusted model. These associations detected in foregoing genetic analyses were independent of the APOE ε4 carrier status.
We also explored the interactions of individual VRFs with APOE ε4, or with TOMM40 G. Interactions between individual VRFs and APOE ε4 were not significant after TOMM40 adjustment in model 1 and fully adjusted model (all p > 0.05). However, the interaction between physical inactivity and TOMM40 G was statistically significant (p = 0.01). The joint-effect analysis showed that compared to people with neither physical inactivity nor TOMM40 G, individuals with only physical inactivity or only TOMM40 G did not have a significantly lower MMSE score, but people having both physical inactivity and TOMM40 G had a markedly lower MMSE score in model 1 (β: −2.52, 95% CI: −3.88 to −1.16, p = 0.001), and the results were similar in the fully adjusted model (Figure 1A). The results also revealed a significant interaction between diabetes with TOMM40 G (p = 0.03). Further analysis indicated that compared to people with neither diabetes nor TOMM40 G, individuals with diabetes alone had a significantly lower MMSE score in model 1 (β: −1.18, 95% CI: −1.189 to −0.47, p = 0.001) and people having both diabetes and TOMM40 G had a markedly lower MMSE score in model 1 (β: −2.10, 95% CI: −3.49 to −0.71, p = 0.003), and the results were similar in the fully adjusted model (Figure 1B); moreover, these associations were independent of the APOE polymorphism. No other individual VRFs were found to interact with TOMM40 G to affect cognitive performance.
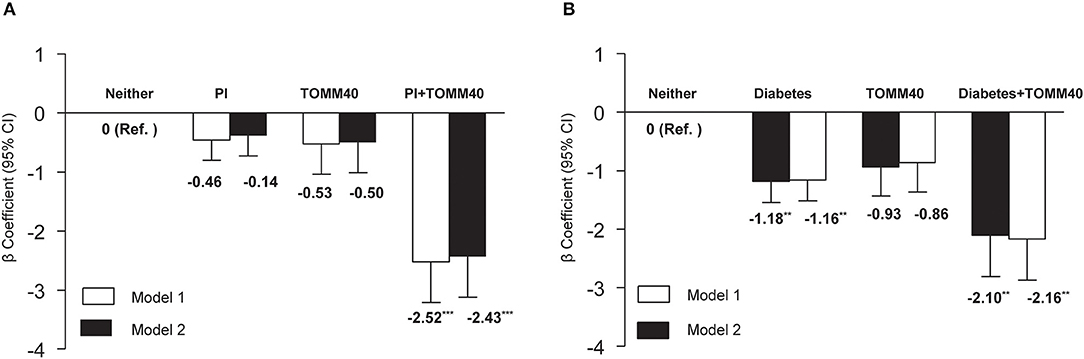
Figure 1. (A) Joint effects of physical inactivity (PI) and TOMM40 polymorphisms, and (B) diabetes and TOMM40 polymorphisms on MMSE score. Model 1 was controlled for age, sex, education, and APOE polymorphism, and model 2 was additionally controlled for the presence of depressive symptoms and heart disease. **p < 0.01; ***p < 0.001.
The primary aim of the current study was to investigate the associations of VRFs, APOE, and TOMM40 polymorphisms with cognitive performance in Chinese older adults who were free of dementia, with a focus on potential interactions between VRFs and the two genetic polymorphisms. We found that physical inactivity and diabetes were independently associated with poor global cognitive performance. When four putative VRFs (i.e., current smoking, physical inactivity, high LDL-C, and diabetes) were aggregated, an increasing number of concurrently having these factors was associated with a decreasing MMSE score in a dose–response manner. Furthermore, we detected an interaction between the aggregated VRFs and TOMM40 G, such that having 1 or more VRFs, in combination with TOMM40 G, was associated with markedly low cognitive function in Chinese older adults. We also revealed interactions of physical inactivity with TOMM40 G, and diabetes with TOMM40 G independent of the APOE polymorphism, such that TOMM40 G in combination with either physical inactivity or diabetes was associated with a lower MMSE score.
Physical activity has been shown to benefit cognition in older adults (34). For example, a population-based study with 104,909 participants from 20 countries showed that physical activity was associated with global cognitive performance (35). In line with previous literature, we found that low physical activity was associated with poor cognitive performance in Chinese older adults (36). In addition, diabetes was reported to be associated with deficits in global cognitive function as assessed by the MMSE in a previous study (37). We replicated this result by detecting an association of diabetes with a lower MMSE score in older adults. However, given the relatively small sample of our study and the potential false-positive associations due to multiple comparisons, caution is needed when interpreting these results.
Some individual VRFs (e.g., smoking and hypertension) have been linked with poor cognitive performance in other studies (3, 38, 39); however, these factors were not associated with MMSE score in the present study. It has been reported that the associations of some VRFs, such as hypertension, obesity, and high cholesterol with cognitive function, largely depend on age. That is, having these factors in midlife or young–old age, but not necessarily in very old, was associated with age-related cognitive impairment and dementia (40–42). Indeed, 51.8% of the participants in this study were aged over 70 years. This might partly contribute to the lack of positive associations between some VRFs and poor cognitive function in our study. Further large prospective cohort studies that consist of different age groups are necessary to further clarify the associations of VRFs with cognitive decline.
VRFs are often coexisting together in middle-aged or older populations. Accumulating evidence showed that people who are exposed to multiple VRFs simultaneously often perform much worse on different cognitive tests (7, 43) and have a much higher risk of developing dementia than those who have a single VRF (44). However, most of these studies are performed in western countries. Our results extend prior work by showing that aggregated VRFs are associated with poor global cognitive function among community-dwelling Chinese older adults. These results imply that early interventions targeting multiple modifiable risk factors among cognitively healthy older adults may partly ameliorate cognitive decline and may delay the onset of dementia syndrome.
Carrying the APOE ε4 allele in our study participants was not associated with the MMSE score. Although some studies from western countries (e.g., Northern Europe) have found APOE ε4-related decline in global cognitive function (45, 46), a population-based study carried out in Chinese older adults, actually, failed to observe a significant association of APOE ε4 with MMSE score (47). We speculate that the ethnic differences in genetic susceptibility may partially contribute to the discrepancy of research findings across studies. Indeed, compared to the proportion of people carrying APOE ε4 in the Nordic population (around 25–30%), the proportion in our study is relatively low (16.1%), and only 1.4% were homozygous for ε4. In addition, episodic memory and executive functioning were reported to be particularly affected by carrying the ε4 allele (48–50). Thus, global cognitive function as assessed with the MMSE may not be as sensitive to the influence of the APOE ε4 allele. Further large-scale population-based studies are needed to investigate the associations between other cognitive domains and the APOE ε4 allele in dementia-free Chinese older adults.
Our results extend prior work by showing that TOMM40 G carriers showed poor global cognitive function independent of the APOE ε4 allele in Chinese older adults. However, large-scale population-based studies are recommended to further examine the relationship between TOMM40 G and cognitive function. TOMM40 is mainly responsible for mitochondrial function. Mitochondrial dysfunction is implicated in neurodegenerative disorders, and TOMM40 may be influential in this regard by influencing mitochondrial neurotoxicity. Mitochondrial dysfunction is common in AD, and it may be the primary event that causes deposition of Aβ deposition, degeneration of synapses, and neuronal death in AD (51, 52). Future AD therapies aimed at boosting mitochondrial function may offer an alternative treatment strategy.
We detected interactions of VRFs (i.e., physical inactivity, diabetes, and aggregated VRFs) with TOMM40 G on global cognitive performance. The interactive effects suggest that carrying TOMM40 G, in combination with VRFs (i.e., physically inactive, diabetes), and particularly aggregated multiple VRFs, has substantially poorer performance in a global cognitive test. This is the first study to reveal synergistic effects of individual and aggregated VRFs with TOMM40 G on global cognitive function. This finding deserves further confirmation in different ethnic groups in China. The biological mechanisms behind the interactions are not fully understood. Previous research found that the TOMM40 rs2075650 G allele, independent of the APOE ε4 allele, was associated with depression and obesity, which are risk factors for dementia (53, 54). TOMM40 rs2075650 was also found to be specifically associated with bilateral hippocampus and right amygdala volume (55). Furthermore, evidence has indicated that VRFs can accelerate cognitive decline by aggravating mitochondrial damage (56). It is possible that TOMM40 in combination with different VRFs influences mitochondrial function and further leads to brain structure changes and eventually affects human behavior (52). The mechanisms for these VRFs-TOMM40 interactions deserve further exploration. Our results highlight the potential importance of interventions by targeting multiple VRFs to maintain cognitive function, especially among TOMM40 G carriers.
This is one of the first community-based studies of Chinese older adults to examine the associations of VRFs and genetic polymorphisms (APOE and TOMM40) with global cognitive performance as assessed with the MMSE test. We randomly recruited the study sample from the communities. However, the limitations of our study deserve mention. Firstly, we cannot determine the temporal and causal relationships from a cross-sectional study, and the finding of any association might be subject to bias due to selective survival. However, selective survival is likely to dilute the true associations given that exposures (VRFs and APOE ε4) and outcomes (poor cognitive performance) are known to be associated with mortality. Secondly, the study sample is relatively small, especially for genetic studies, which might have inadequate power to detect a weakly-to-moderately strong association between those factors and the MMSE score. Thirdly, MMSE is a validated and widely used test of global cognitive functioning with orientation, memory, attention calculation, language, and registration components; however, it lacks specific items that test executive functions and psychomotor speed. The cognitive domains that are often impaired by exposures to VRFs and related cerebrovascular disease therefore might not be sensitive enough to detect cognitive impairment. Thus, it is worth examining the relationship of VRFs and genetic polymorphisms with different cognitive domains in future studies. Finally, physical activity was assessed based on self-reported information, and we did not have detailed information regarding frequency, duration, intensity, types, or energy expenditure of physical activity. Thus, it could be difficult to define physical activity accurately. Future studies that use objective measurements of physical activity, such as accelerometers and measures of aerobic fitness, as well as self-reported questionnaires that provide proxies of energy expenditure may help more properly address this issue.
This community-based study provides evidence supporting associations between individual and aggregated VRFs with poor cognitive function. In addition, we found a marginally significant association of TOMM40 with cognitive performance independent of the APOE gene and further detected interactions between VRFs (e.g., physical inactivity, diabetes, and aggregated VRFs) and TOMM40 G but not for the APOE ε4 variant. Research efforts to identify therapeutic approaches for preclinical and clinical dementia have so far largely focused on the amyloid pathway, but almost all the clinical trials have yielded limited benefits. These results imply that early interventions by targeting multiple modifiable risk factors among cognitively healthy older adults may partly ameliorate cognitive decline, therefore delaying the onset of the dementia syndrome, especially for carriers of risk genes. Large-scale community-based longitudinal studies and interventions are needed to further clarify the causal relationships and mechanisms between VRFs and cognitive function and their interactive effects with genetic polymorphisms on cognition.
The datasets generated and analyzed during the current study are not available publicly as ethical clearance was not obtained to share data publicly. However, the data are available from the corresponding author on reasonable request.
This study was approved by the Ethics Committee at the Institute of Psychology, Chinese Academy of Sciences, Beijing, China. Written informed consent was given by each participant at each visit.
WG and JL developed the research questions and designed the study. WG analyzed the data and wrote the manuscript. JL was the principal investigator of this project. JL and CQ supervised the study. JL, CQ, and QS critically revised the paper. All authors approved the final version of the paper.
We would like to thank all the study participants for their valuable contribution to the project. This work was supported in part by grants from National Key Research and Development Program of China (2018YFC2000300, 2018YFC2001701, 2016YFC1305900, 2017YFB1401203, and 2020YFC2003000), the National Natural Science Foundation of China (32071079, 31861133011, 31671157, and 31711530157), and Beijing Municipal Science & Technology Commission (Z171100008217006 and Z171100000117006), the Swedish Research Council (grants nos: 2017-00740, 2017-05819, and 2020-01574), the Swedish Foundation for International Cooperation in Research and Higher Education (STINT, grant no.: CH2019-8320) for the China-Sweden Mobility Program, and the Taishan Scholar Program of Shandong Province, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all the study participants for their participation. The present study was previously published on Research Square as a preprint. We thank the platform that makes our research communication faster.
1. Xu J, Wang J, Wimo A, Fratiglioni L, Qiu C. The economic burden of dementia in China, 1990–2030: implications for health policy. Bull World Health Organ. (2017) 95:18–26. doi: 10.2471/BLT.15.167726
2. Jia J, Wei C, Chen S, Li F, Tang Y, Qin W, et al. The cost of Alzheimer's disease in China and re-estimation of costs worldwide. Alzheimers Dement. (2018) 14:483–91. doi: 10.1016/j.jalz.2017.12.006
3. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. (2015) 11:718–26. doi: 10.1016/j.jalz.2015.05.016
4. Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health. (2014) 14:510. doi: 10.1186/1471-2458-14-510
5. Yuan J-Q, Lv Y-B, Chen H-S, Gao X, Yin Z-X, Wang W-T, et al. Association between late-life blood pressure and the incidence of cognitive impairment: a community-based prospective cohort study. J Am Med Dir Assoc. (2019) 20:177–82.e2. doi: 10.1016/j.jamda.2018.05.029
6. Zhang L, Yang J, Liao Z, Zhao X, Hu X, Zhu W, et al. Association between diabetes and cognitive function among people over 45 years old in China: a cross-sectional study. Int J Environ Res Public Health. (2019) 16:1294. doi: 10.3390/ijerph16071294
7. Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol. (2015) 12:267–77. doi: 10.1038/nrcardio.2014.223
8. Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging. (2011) 32:63–74. doi: 10.1016/j.neurobiolaging.2009.02.003
9. Davies G, Armstrong N, Bis JC, Bressler J, Chouraki V, Giddaluru S, et al. Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53 949). Mol Psychiatry. (2015) 20:183–92. doi: 10.1038/mp.2014.188
10. Ma X-Y, Yu J-T, Wang W, Wang H-F, Liu Q-Y, Zhang W, et al. Association of TOMM40 polymorphisms with late-onset Alzheimer's disease in a Northern Han Chinese population. Neuromolecular Med. (2013) 15:279–87. doi: 10.1007/s12017-012-8217-7
11. Huang H, Zhao J, Xu B, Ma X, Dai Q, Li T, et al. The TOMM40 gene rs2075650 polymorphism contributes to Alzheimer's disease in Caucasian, and Asian populations. Neurosci Lett. (2016) 628:142–6. doi: 10.1016/j.neulet.2016.05.050
12. Xiao Q, Liu Z-J, Tao S, Sun Y-M, Jiang D, Li H-L, et al. Risk prediction for sporadic Alzheimer's disease using genetic risk score in the Han Chinese population. Oncotarget. (2015) 6:36955–64. doi: 10.18632/oncotarget.6271
13. Yashin AI, Fang F, Kovtun M, Wu D, Duan M, Arbeev K, et al. Hidden heterogeneity in Alzheimer's disease: insights from genetic association studies and other analyses. Exp Gerontol. (2018) 107:148–60. doi: 10.1016/j.exger.2017.10.020
14. Blue EE, Cheng A, Chen S, Yu C-E. Association of uncommon, noncoding variants in the APOE region with risk of Alzheimer disease in adults of European ancestry. JAMA Netw Open. (2020) 3:e2017666. doi: 10.1001/jamanetworkopen.2020.17666
15. Naj AC, Jun G, Reitz C, Kunkle BW, Perry W, Park YS, et al. Effects of multiple genetic loci on age at onset in late-onset Alzheimer disease: a genome-wide association study. JAMA Neurol. (2014) 71:1394–404. doi: 10.1001/jamaneurol.2014.1491
16. Soyal SM, Kwik M, Kalev O, Lenz S, Zara G, Strasser P, et al. A TOMM40/APOE allele encoding APOE-E3 predicts high likelihood of late-onset Alzheimer's disease in autopsy cases. Mol Genet Genomic Med. (2020) 8:e1317. doi: 10.1002/mgg3.1317
17. Davies G, Harris SE, Reynolds CA, Payton A, Knight HM, Liewald DC, et al. A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol Psychiatry. (2014) 19:76–87. doi: 10.1038/mp.2012.159
18. Kim IY, Grodstein F, Kraft P, Curhan GC, Hughes KC, Huang H, et al. Interaction between apolipoprotein E genotype and hypertension on cognitive function in older women in the Nurses' health study. PLoS ONE. (2019) 14:e0224975. doi: 10.1371/journal.pone.0224975
19. Yasuno F, Tanimukai S, Sasaki M, Ikejima C, Yamashita F, Kodama C, et al. Effect of plasma lipids, hypertension and APOE genotype on cognitive decline. Neurobiol Aging. (2012) 33:2633–40. doi: 10.1016/j.neurobiolaging.2011.12.028=
20. Zhen J, Lin T, Huang X, Zhang H, Dong S, Wu Y, et al. Association of ApoE genetic polymorphism and type 2 diabetes with cognition in non-demented aging Chinese adults: a community based cross-sectional study. Aging Dis. (2018) 9:346–57. doi: 10.14336/AD.2017.0715
21. de Frias CM, Schaie KW, Willis SL. Hypertension moderates the effect of APOE on 21-year cognitive trajectories. Psychol Aging. (2014) 29:431–9. doi: 10.1037/a0036828
22. Andrews S, Das D, Anstey KJ, Easteal S. Interactive effect of APOE genotype and blood pressure on cognitive decline: the PATH through life study. J Alzheimers Dis. (2015) 44:1087–98. doi: 10.3233/JAD-140630
23. Yu J, Li J, Huang X. The Beijing version of the montreal cognitive assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. (2012) 12:156. doi: 10.1186/1471-244X-12-156
24. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. (1983) 17:37–49. doi: 10.1016/0022-3956(82)90033-4
25. Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96. doi: 10.1046/j.1440-6047.11.s8.9.x
26. Third report of the National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. (2002) 106:3143–421.
27. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2013) 36:S67–74. doi: 10.2337/dc13-s067
28. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. (2003) 289:2560–72. doi: 10.1001/jama.289.19.2560
29. Katzman R, Zhang M, Wang Z, Liu WT, Yu E, Wong S-C, et al. A Chinese version of the mini-mental state examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. (1988) 41:971–8. doi: 10.1016/0895-4356(88)90034-0
30. Wang R, Fratiglioni L, Laukka EJ, Lövdén M, Kalpouzos G, Keller L, et al. Effects of vascular risk factors and APOE ε4 on white matter integrity and cognitive decline. Neurology. (2015) 84:1128–35. doi: 10.1212/WNL.0000000000001379
31. Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology. (2009) 73:674–80. doi: 10.1212/WNL.0b013e3181b59bf3
32. Li J, Wang Y, Zhang M, Xu Z, Gao C, Fang C, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. (2011) 76:1485–91. doi: 10.1212/WNL.0b013e318217e7a4
33. Li J, Fu J, Zheng Z, Zhu X, Han X, Li J. Effects of cognitive-and sleep-related single nucleotide polymorphisms on cognitive functions in the Han Chinese community-dwelling elderly. Preprint (2020).
34. Lü J, Fu W, Liu Y. Physical activity and cognitive function among older adults in China: a systematic review. J Sport Health Sci. (2016) 5:287–96. doi: 10.1016/j.jshs.2016.07.003
35. de Souto Barreto P, Delrieu J, Andrieu S, Vellas B, Rolland Y. Physical activity and cognitive function in middle-aged and older adults: an analysis of 104,909 people from 20 countries. Mayo Clin Proc. (2016) 91:1515–24. doi: 10.1016/j.mayocp.2016.06.032
36. Paulo TR, Tribess S, Sasaki JE, Meneguci J, Martins CA, Freitas IF, et al. A cross-sectional study of the relationship of physical activity with depression and cognitive deficit in older adults. J Aging Phys Act. (2016) 24:311–21. doi: 10.1123/japa.2014-0253
37. Dybjer E, Nilsson PM, Engström G, Helmer C, Nägga K. Pre-diabetes and diabetes are independently associated with adverse cognitive test results: a cross-sectional, population-based study. BMC Endocr Disord. (2018) 18:91. doi: 10.1186/s12902-018-0318-3
38. Momtaz YA, Hamid TA, Haron SA, Bagat MF, Mohammadi F. Prevalence of hypotension and its association with cognitive function among older adults. Aging Ment Health. (2018) 22:447–52. doi: 10.1080/13607863.2016.1268093
39. Huang CQ, Dong BR, Zhang YL, Wu HM, Liu QX. Association of cognitive impairment with smoking, alcohol consumption, tea consumption, and exercise among Chinese nonagenarians/centenarians. Cogn Behav Neurol. (2009) 22:190–6. doi: 10.1097/WNN.0b013e3181b2790b
40. Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. (2014) 14:643. doi: 10.1186/1471-2458-14-643
41. Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. (2015) 9:93–113. doi: 10.1016/j.orcp.2014.05.001
42. Gardner RC, Liang Y, Ngandu T, Laatikainen T, Soininen H, Tuomilehto J, et al. Cardiovascular health metrics from mid- to late-life and risk of dementia: a population-based cohort study in Finland. PLoS Med. (2020) 17:e1003474. doi: 10.1371/journal.pmed.1003474
43. Joosten H, van Eersel ME, Gansevoort RT, Bilo HJ, Slaets JP, Izaks GJ. Cardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjects. Stroke. (2013) 44:1543–9. doi: 10.1161/STROKEAHA.111.000496
44. Reitz C, Tang M-X, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol. (2010) 67:835–41. doi: 10.1001/archneurol.2010.136
45. Josefsson M, Larsson M, Nordin S, Adolfsson R, Olofsson J. APOE-ε4 effects on longitudinal decline in olfactory and non-olfactory cognitive abilities in middle-aged and old adults. Sci Rep. (2017) 7:1286. doi: 10.1038/s41598-017-01508-7
46. Qiu C, Winblad B, Fratiglioni L. Cerebrovascular disease, APOE ϵ 4 allele and cognitive decline in a cognitively normal population. Neurol Res. (2006) 28:650–6. doi: 10.1179/016164106X130443
47. Sun S, Fu J, Chen J, Pang W, Hu R, Li H, et al. ApoE type 4 allele affects cognitive function of aged population in Tianjin City, China. Am J Alzheimers Dis Other Demen. (2015) 30:503–7. doi: 10.1177/1533317514566114
48. Lim YY, Williamson R, Laws SM, Villemagne VL, Bourgeat P, Fowler C, et al. Effect of APOE genotype on amyloid deposition, brain volume, and memory in cognitively normal older individuals. J Alzheimers Dis. (2017) 58:1293–302. doi: 10.3233/JAD-170072
49. Marioni RE, Campbell A, Scotland G, Hayward C, Porteous DJ, Deary IJ. Differential effects of the APOE e4 allele on different domains of cognitive ability across the life-course. Eur J Hum Genet. (2016) 24:919–23. doi: 10.1038/ejhg.2015.210
50. Luck T, Then FS, Luppa M, Schroeter ML, Arélin K, Burkhardt R, et al. Association of the apolipoprotein E genotype with memory performance and executive functioning in cognitively intact elderly. Neuropsychology. (2015) 29:382–7. doi: 10.1037/neu0000147
51. Perez Ortiz JM, Swerdlow RH: Mitochondrial dysfunction in Alzheimer's disease. Role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol. (2019) 176:3489–507. doi: 10.1111/bph.14585
52. Ferencz B, Karlsson S, Kalpouzos G. Promising genetic biomarkers of preclinical Alzheimer's disease: the influence of APOE and TOMM40 on brain integrity. Int J Alzheimers Dis. (2012) 2012:421452. doi: 10.1155/2012/421452
53. McFarquhar M, Elliott R, McKie S, Thomas E, Downey D, Mekli K, et al. TOMM40 rs2075650 may represent a new candidate gene for vulnerability to major depressive disorder. Neuropsychopharmacology. (2014) 39:1743–53. doi: 10.1038/npp.2014.22
54. Kulminski AM, Loika Y, Culminskaya I, Huang J, Arbeev KG, Bagley O, et al. Independent associations of TOMM40 and APOE variants with body mass index. Aging Cell. (2019) 18:e12869. doi: 10.1111/acel.12869
55. Shen L, Kim S, Risacher SL, Nho K, Swaminathan S, West JD, et al. Whole genome association study of brain-wide imaging phenotypes for identifying quantitative trait loci in MCI and AD: A study of the ADNI cohort. Neuroimage. (2010) 53:1051–63. doi: 10.1016/j.neuroimage.2010.01.042
56. Pratchayasakul W, Sa-nguanmoo P, Sivasinprasasn S, Pintana H, Tawinvisan R, Sripetchwandee J, et al. Obesity accelerates cognitive decline by aggravating mitochondrial dysfunction, insulin resistance and synaptic dysfunction under estrogen-deprived conditions. Horm Behav. (2015) 72:68–77. doi: 10.1016/j.yhbeh.2015.04.023
Keywords: aging, vascular risk factors, APOE, TOMM40, cognitive function, population-based study
Citation: Gui W, Qiu C, Shao Q and Li J (2021) Associations of Vascular Risk Factors, APOE and TOMM40 Polymorphisms With Cognitive Function in Dementia-Free Chinese Older Adults: A Community-Based Study. Front. Psychiatry 12:617773. doi: 10.3389/fpsyt.2021.617773
Received: 15 October 2020; Accepted: 01 February 2021;
Published: 15 March 2021.
Edited by:
Tao Liu, Beihang University, ChinaReviewed by:
Karen Anne Mather, University of New South Wales, AustraliaCopyright © 2021 Gui, Qiu, Shao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Li, lijuan@psych.ac.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.