
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 08 April 2021
Sec. Schizophrenia
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.608231
This article is part of the Research Topic Understanding Early Detection Markers in Schizophrenia View all 9 articles
 Xiao-Jiao Bi1†
Xiao-Jiao Bi1† Lei Hu1†
Lei Hu1† Dong-Dong Qiao1
Dong-Dong Qiao1 Chao Han1
Chao Han1 Meng-Meng Sun1
Meng-Meng Sun1 Kai-Yan Cui1
Kai-Yan Cui1 Li-Na Wang1
Li-Na Wang1 Li-Min Yang1
Li-Min Yang1 Lan-Fen Liu1*
Lan-Fen Liu1* Zhe-Yu Chen2,3*
Zhe-Yu Chen2,3*Background: Neural precursor cell-expressed developmentally downregulated 4 (NEDD4) polymorphisms and childhood trauma (CT) are associated with schizophrenia. However, whether NEDD4 interacts with CT on symptoms of schizophrenia remains unknown. This study aimed to investigate the gene–environment interaction effect.
Methods: We recruited 289 schizophrenia patients and 487 controls and genotyped rs2303579, rs3088077, rs7162435, rs11550869, and rs62043855 in their NEDD4 gene.
Results: We found significant differences in the rs2303579 and rs3088077 between the two groups. Patients with the rs2303579 CC genotype had higher scores compared with other genotype (P = 0.026) in the test of positive schizophrenia syndrome scores, whereas patients with the rs3088077 TT (P = 0.037) and rs7162435 CC genotypes (P = 0.009) had higher scores compared with the other genotypes in the test of excitement factor. Patients with a family history of psychosis (FH+) reported higher negative scores (P = 0.012) than those without. Patients exposed to physical abuse (PA) reported a lower language learning and memory score (P = 0.017) and working memory score (P = 0.047) than those not. Patients exposed to sexual abuse (SA) reported a lower reasoning and problem-solving skills score (P = 0.025); those exposed to emotional neglect (EN) reported a lower social cognition score (P = 0.044); and those exposed to physical neglect reported a lower social cognition score (P = 0.036) but higher visual learning and memory score (P = 0.032). Rs3088077 could interact with EN to increase risk for schizophrenia. Optimal model rs62043855 × EA, rs3088077 × rs7162435 × rs11550869 × SA × EN and rs2303579 × rs7162435 × rs11550869 × rs62043855 × EA × PA could explain positive symptom, excitement symptom and working memory, respectively, in FH+ group.
Conclusion: The study highlighted that the combined interaction of NEDD4 and CT may be associated with symptoms of schizophrenia especially for those with FH+.
Schizophrenia has a lifetime risk of ~1% and is manifested by a disruption in cognition and emotion, along with negative and positive symptoms. Genetic factors account for more than 80% of the variance in susceptibility, and risk likely results from multiple loci with small effects (1). According to the neurodevelopment hypothesis, variations in the genes implicated in neuronal function and synaptic plasticity mechanisms may be associated with schizophrenia (2, 3). Studies have confirmed that the interaction of genes and environmental factors can affect the symptoms of psychological diseases, whereas childhood abuse is an important environmental factor that can affect the occurrence and development of schizophrenia (4). Systematic reviews of the literature and meta-analyses have demonstrated a clear relation between childhood trauma (CT) and the severity of psychotic symptom, increased prevalence of substance use, and a more refractory and prolonged illness course (5). Meanwhile the impact of CT on symptoms of schizophrenia may be enhanced due to genetic effects, particularly in the negative sub-domai (6), with a family history of psychosis (FH+) being considered the strongest genetic risk factor for schizophrenia. The course and severity of schizophrenia and long-term occupational outcomes are reportedly affected by FH+ (7). For FH+ and exposure to pyelonephritis increases by five times the risk of schizophrenia (8).
The neural precursor cell expressed developmentally down-regulated 4 (NEDD4) is a ubiquitin ligase that is critical to all stages of neuronal development, such as neuronal cell fate determination, neurite outgrowth, axon guidance, and neuronal cell survival in the developing mammalian brain (9–12). The NEDD4 protein involved in the pathological process of neurodegeneration diseases, such as Parkinson's, has been found both in animal models (13) and postmortem studies (14). Srinivasan et al. found that rs1912403 in NEDD4 is a risk factor for Parkinson's disease in white people (15), Nonetheless, whether NEDD4 plays a beneficial or detrimental role remains controversial.
Studies associating NEDD4 and mental illness are relatively lacking. Lien et al. using genomic linkage, suggested that 15q21.3, where NEDD4 is located, is related to the social isolation and introverted score of non-schizophrenic relatives (16). Jahanshad et al. found that rs17819300 and rs1781928 of NEDD4 are related to abnormal brain connections (17). Furthermore, Warnica et al. in a study on the micro-RNA of copy number variation in patients with schizophrenia, reported that the NEDD4 gene may be one of the target genes of schizophrenia (18). Our previous study revealed that the rs2303579 and rs62043855 loci in NEDD4 are associated with cognitive dysfunction in schizophrenia patients in the Chinese Han population (19).
As gene–environment interactions (G × Es) are increasingly assumed to play a crucial role in schizophrenia (20) studies have investigated the role of multiple gene variants and their combined effects on susceptibility and phenotype of schizophrenia. To our knowledge, however, whether the interaction between NEDD4 and childhood abuse affects the clinical symptoms of schizophrenia patients remains unknown. Thus, the current study aimed to investigate the potential involvement of G × Es of NEDD4 and CT on the clinical symptoms of schizophrenia.
The study sample included 289 patients with schizophrenia recruited from Shandong Mental Health Center and 487 healthy controls (HCs) recruited from the community via advertisements. All Chinese Han patients aged 18–65 years fulfilling the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) criteria for schizophrenia were recruited. They had a certain level of education to understand and comply with the relevant requirements of the study, not taken any antipsychotics for at least 1 month before being recruited, and not undergone convulsive electroconvulsive therapy within 6 months. The diagnosis, along with a review of psychiatric case records, was independently checked and verified by two senior psychiatrists. Patients with serious physical diseases or those who fulfilled ICD-10 criteria for organic mental disorders, mental and behavioral disorders from psychoactive substances, schizoaffective disorder, or affective disorder were excluded. Women who were pregnant, lactating, or planning to become pregnant, and those who participated in other clinical research within 4 weeks before enrolment were also excluded.
We used PANSS to obtain the clinical characteristics of schizophrenia. After the patients' admission to hospital, psychiatrists who passed the consensus assessment performed the rating scale within 3 days. To conduct further factor analyses, we used the five components of PANSS, which are positive, negative, excitement, anxiety/depression, and cognitive defect (21).
We employed a short-form version of CTQ-SF to retrospectively elicit information on a range of maltreatment experiences from schizophrenia participants. The CTQ-SF is a 28-item tool using a five-point Likert scale ranging from 1 (never true) to 5 (very often true). The reliability and validity of the Chinese version of CTQ-SF have been analyzed by Xing-Fu et al. (22) and Zhang (23) and their results suggested that it is a good psychometric instrument for the evaluation of Chinese childhood abuse. Five types of CT are measured in the CTQ-SF: emotional abuse (EA), physical abuse (PA), sexual abuse (SA), emotional neglect (EN), and physical neglect (PN). In the current study, the CTQ-SF cut-off scores were as follows: PA ≥ 8, SA ≥ 6, EA ≥ 9, PN ≥ 8, and EN ≥ 10 (24). We used low-to-moderate exposure cut-off scores to capture cases with even the lowest severity of CT. We handed out the questionnaire the day after the patient was admitted and asked that it be returned within 3 days.
We used the Chinese version of MCCB to evaluate the patients' cognitive function. This tool consists of 10 sub-tests covering seven dimensions, namely, assessment of processing speed, attention and alertness, working memory, language learning and memory, visual learning and memory, reasoning and problem-solving skills, and social cognition (25). According to the patient's condition, we collected MCCB data within 7 days of enrolment to the study.
All of the DNA samples we used originated from venous blood (5 mL) in EDTA-containing tubes. The improved potassium iodide method was used to extract genomic DNA after processing the blood samples. Five single nucleotide polymorphisms (SNPs) of NEDD4, namely, rs2303579, rs3088077, rs7162435, rs11550869, and rs62043855, were genotyped using TaqMan probe system reported in our previous study (26).
Data were analyzed using IBM SPSS Statistics for Windows, Version 20.0 and generalized multifactor dimensionality reduction (GMDR Software Beta, version 0.7). We used chi-squared tests to analyze the Hardy–Weinberg equilibrium (HWE) and differences between the various qualitative data of the study subjects, including sex, FH, course of disease, genotype, and CTQ score. Meanwhile, differences in the PANSS and MCCB scores of the patients in the case group were compared using one-way analysis of variance. To control for the impact of sex, age, and course of disease on clinical symptoms, we used clinical symptoms as dependent variables and the five SNP genotypes as independent variable in conducting analysis of covariance (ANCOVA). These continuous variables were presented as mean ± standard deviation.
The interaction between the NEDD4 gene and childhood abuse was first analyzed by logistic regression. Heterozygous genotype was set as reference category, equal to 0, to correspond to the other two dummy variables in the five SNPs. The dummy genotypes variables were used as the dependent variables, whereas environmental factors were used as the independent variables in the logistic regression analysis. Second, the G × Es on the clinical symptoms of schizophrenia patients grouped by FH and course of disease were analyzed by GMDR, a free resource accessible at http://www.healthsystem.virginia.edu/internet/Addiction-Genomics/ (27). A Age and sex were included as covariates in all the interactions analyses. Given that the selected traits used for score calculation were quantitative ones, we selected the linear regression method to calculate the statistical scores. All interactions were tested using 10-fold cross-validations in an exhaustive search that considered all possible combinations. The model with the highest testing balance accuracy and cross-validation consistency was selected as the optimal model. P < 0.05 was considered as statistically significant.
Among these 289 patients with schizophrenia, there were 185 females with a mean age of 35.21 ± 11.464 years and 104 males with a mean age of 29.21 ± 9.031 years. 487 HCs (female = 282) aged 32.53 ± 10.386 were enrolled in the study. There were no statistically significant differences found between patients and HCs for sex and age (P > 0.05). Of those patients with schizophrenia, there were 200 patients with FH+ and another 89 cases without family history of psychosis (FH–). Besides, when grouped by course of schizophrenia, we found 70 patients were first episode and 219 were recurrent. All of those patients completed MCCB and PANSS and all the blood samples were successfully genotyped. We did not find any significant differences between genotype distributions and sex, FH, course of disease, respectively (Table 1).
A total of 289 CTQ-SF questionnaires were distributed out of which 136 completed questionnaires were returned and used for the study. Among them, 36 were males, 100 were females, 86 were FH positive, 50 were negative, 42 were first-episode patients, and 94 were relapsed patients. Those who exposed to five types of CT based on our cut-off scores did not differ significantly from course of disease, FH (Supplementary Table 1). However, significant differences were found between childhood emotional neglect and sex with female patients reporting more emotional neglect (χ2 = 13.926, P = 0.000368) (Table 2).
All SNPs in testing HWE were not statistically significant (data not show). The genotype frequencies of rs2303579 (χ2 = 11.769, P = 0.003) and rs3088077 (χ2 = 18.382, P = 0.000102) loci were significantly different between the patients group and HCs, while the genotype frequencies of rs7162435 (χ2 = 5.289, P = 0.071), rs11550869 (χ2 =1.041, P = 0.594), and rs62043855 (χ2= 1.473, P = 0.479) loci were not significantly different (Table 3).
One-way analysis of variance was used to compare the scores of PANSS, MCCB among the patients with three different genotypes of five SNPs locus in NEDD4 gene, with or without five types of CTQ, FH+ or FH-. We found that patients with rs2303579 CC genotype had significantly higher scores (33.03 ± 4.841) than other genotypes (F = 3.688, P = 0.026) in the test of positive scores. Rs3088077 TT genotype (15.31 ± 3.825, F = 3.33, P = 0.037) and rs7162435 CC genotype (15.58 ± 6.043, F = 4.783, P = 0.009) both had significantly higher scores than other genotypes in the test of excitement scores, while no significant difference was found in rs11550869 and rs62043855 (Table 4). Additionally, FH+ patients got higher negative scores (32.91 ± 7.769, F = 6.366, P = 0.012) than those FH- patients (Table 4). While we did not find any significant differences between FH+ and FH- groups in MCCB scores (Supplementary Table 2). The ANCOVA results were consistent with the results of the one-way analysis of variance. After controlling for the effects of sex, age, and disease course, the genotype of rs2303579, rs3088077, and rs7162435 were related to scores of positive and excitement symptoms, respectively (Supplementary Table 3).
When it came to CTQ, no significant difference between patients with or without five types of CTQ was found in any test of PANSS (Supplementary Table 4). But we found that those patients who exposed PA got a significantly lower language learning and memory score (42.27 ± 10.322, F = 5.866, P = 0.017) and significantly lower working memory score (87.24 ± 19.484, F = 4.016, P = 0.047) than who not (Table 5). In addition, those patients who exposed SA got a significantly lower reasoning and problem solving skills score (41.51 ± 9.601, F = 5.129, P = 0.025), those exposed EN got a significantly lower social cognition score (47.17 ± 11.759, F = 4.150, P = 0.044), and those exposed PN got a significantly lower social cognition score (47.88 ± 11.200, F = 4.487, P = 0.036) but a significantly higher visual learning and memory score (44.33 ± 11.758, F = 4.671, P = 0.032) compared with the other groups (Table 5).
The G × Es between 5 SNPs on NEDD4 gene and 5 types of childhood abuse were tested using both logistic regression and GMDR. Our logistic regression results showed that rs3088077 could interact with environment factor EN (OR = 4.626, P = 0.044) (Table 6) in increasing the risk rs3088077 for schizophrenia. No interaction was found between other SNPs and 5 types of childhood abuse.
Using the GMDR, controlling for sex and age as covariates, we analyzed the interactions between NEDD4 and childhood abuse on symptoms of schizophrenia in five components of PANSS and seven dimensions of MCCB. We tested all the combinations within the two aspects in FH+ and FH– subgroups. As the results shown in Table 7, we found the optimal model rs62043855 × EA which had a cross-validation consistency of 10/10 (P‡ = 0.0107, P§ = 0.002) could explain positive symptoms, the optimal model rs3088077 × rs7162435 × rs11550869 × SA × EN which had a cross-validation consistency of 10/10 (P‡ = 0.001, P§ = 0.000) could explain excitement symptoms, the optimal model rs2303579 × rs7162435 × rs11550869 × rs62043855 × EA × PA which had a cross-validation consistency of 8/10 (P‡ = 0.0107, P§ = 0.000) could explain working memory in FH+ group.
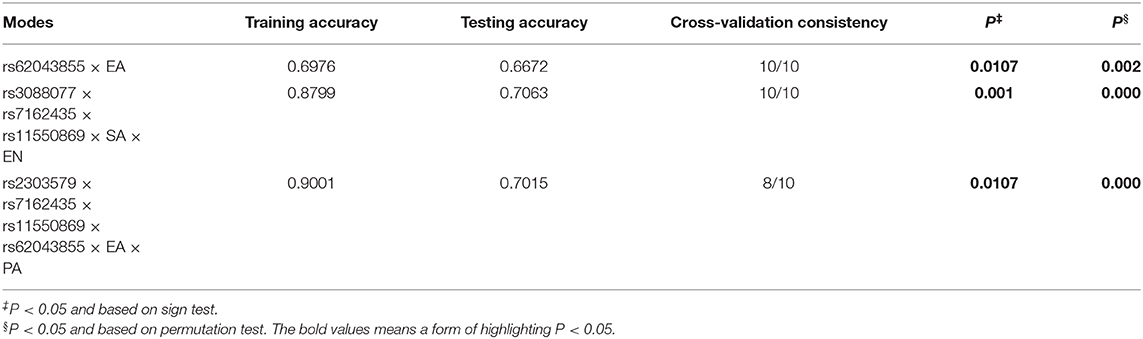
Table 7. GMDR analyses on NEDD4-CTQ interactions on clinical symptoms in schizophrenia patients grouped by FH.
There is indirect evidence supporting the inference that the NEDD4 gene might interact with CT for schizophrenia. However, to the best of our knowledge, the present study is the first to link the NEDD4 gene, CT, and schizophrenia. We used two different clinical symptom assessment methods to evaluate the clinical symptoms and two different statistical methods to explore the effect of G × Es. Our results indicated that the interaction between NEDD4 and CT may play an essential role in the development of the clinical symptoms of schizophrenia.
Consistent with the results of previous studies, our findings indicate a relation between NEDD4 and susceptibility to schizophrenia. Among the five SNPs of NEDD4, the polymorphism rs3088077 of 3'UTR and exonic polymorphism rs2303579 showed significant associations with schizophrenia. This connection is possible from the functional perspective of NEDD4. The NEDD4 protein is a critical ubiquitin ligase to many biological functions of the nervous system (28). It regulates the turnover and trafficking of ion channels and G protein-coupled receptors present in neurons, such as the GluA1 AMPA receptor, GluN2D-containing NMDA receptor (29, 30), voltage-gated sodium channels, calcium channels, and the newly discovered metabotropic glutamate receptor 7 (31). A recent study also found that NEDD4 could regulate hydrogen peroxide-induced cell proliferation and death through the inhibition of Hippo signaling in human bone marrow-derived stem cells (32). The effects of NEDD4 on the neurotransmitters modulate long-term potentiation and long-term memory (33), leading to the impairment of prefrontal cortex-mediated cognitive functions (34, 35). Our research have showed links between symptoms of schizophrenia such as excitement and cognitive dysfunction and several SNPs in NEDD4 (19, 26).
The abnormal expression of the NEDD4 gene may cause abnormalities in the nervous system, which may cause clinical symptoms in patients with schizophrenia. One animal study revealed that levels of NEDD4 and mRNA are reduced in the brain of mice fed with antipsychotic drugs, suggesting that NEDD4 may be a target for new drug interventions (36). Given the current understanding of the biological function NEDD4, NEDD4 may be inferred to be related with the development of not only schizophrenia but also other mental diseases. Indeed, a recent article explored the possible relation between NEDD4 and depression. Maternal immune activation suppressing NEDD4-related signal pathways has been shown to impede offspring's dendrite development and cause depressive-like behaviors (37). However, clinical studies on the correlation between NEDD4 and depression have not been conducted.
Our findings not only confirmed the possible connection between NEDD4 and schizophrenia but also explored the optimal model of NEDD4 × childhood abuse interaction on schizophrenic clinical symptoms. Childhood abuse has always been an environmental factor of great concern (38). The present study underlined that PA, SA, EN, and PN, determined through the CTQ-SF, were related to cognitive symptoms, consistent with previous findings showing childhood adversity as a risk factor for schizophrenia (39) and EN and PN being associated with cognitive profiles (40).
Meanwhile, previous studies have found a positive dose-response relation between SA and auditory hallucinations (41) but our results failed to show a link between CTQ and positive symptoms as well as the other factors assessed by PANSS. From our perspective, it is difficult for a single adverse experience to act on a single symptom in schizophrenia, a complicated disease. The correlation between environmental factors and symptoms calls for an evaluation of the risk factors, provision of preventative interventions, and creation of a better environment among patients with schizophrenia (42). Given that biological factors cannot compensate for all the adverse environmental factors, when the biological aspect cannot be handled, the disease will appear. We emphasized G × Es for this reason.
Interestingly, our results showed that women reported significantly more EN than men. Individuals who do not develop health-harming behaviors are more likely to have experienced safe, nurturing childhoods (43), whereas girls who have experienced EN have increased relative risk of health-related risky behaviors, such as depression, suicidal ideation, planned suicide, brawls, drunkenness, smoking, and abnormal eating behaviors (44). All of these results re-emphasize the importance of the environment. Emotional satisfaction may reduce the incidence of health-related risky behavior and mental illness, especially for women.
Regarding G × Es, a growing body of evidence suggests that early environmental risk factors and genetic predispositions for psychiatric disorders have an important influence on schizophrenia (20). Early environmental risk factors include maternal environmental risk factors (45) and CT (6). In our study, we found that NEDD4 could enhance the effect of EN, more apparently so in FH+ patients. When FH was used as a grouping condition, the combination of rs62043855 × EA, rs3088077 × rs7162435 × rs11550869 × SA × EN and rs2303579 × rs7162435 × rs11550869 × rs62043855 × EA × PA explained positive symptoms, excitement symptoms and working memory, respectively, in FH+ group. Consistent with previous findings, we found that many genes interacted with CT to affect symptoms of schizophrenia. For example, EA interacts with the forkhead box P2 gene, involved in the development of speech and language, with respect to auditory verbal hallucinations in schizophrenia (46). We also found that the interaction between NEDD4 and CT had a certain impact on the clinical symptoms of schizophrenia. Whether the strengthening effect of NEDD4 on the negative environment has a role in other mental and psychological diseases, such as depression, needs to be explored further.
FH+, which acts as another important environmental risk factor with regard to schizophrenia, can be targeted in interventions. FH+ may affect patients' clinical symptoms in many ways, such as in the aspects of family growth environment and parent–child attachment. Our results showed that patients with FH+ had a higher score for negative symptoms. Regarding this finding, we have two speculations. Firstly, the genetic factors of patients with FH+ can promote social withdrawal and emotional indifference in patients with schizophrenia. Secondly, patients with FH+ may have more CT in their growing environment. As patients had not learned good ways of expressing emotions, they would tend to shrink back when sick. Therefore, our results further emphasized the importance of early intervention. FH is an obvious and reliable sign. For such families, it is necessary to provide good family and social support for the young members.
Psycho-educational programs (47), family interventions (48), and other psychosocial interventions should become essential components of the effective treatment of schizophrenia. Specifically, interventions can proceed from the following aspects. Firstly, from the patient's perspective, increased disease health education can improve treatment compliance and reduce stigma. By improving individualized treatment measures for patients through precision medicine and other methods combined with supportive psychotherapy, health professionals can improve patients' confidence in the treatment, social function, and outcomes. Secondly, from the aspect of the family, for FH+ patients, health professionals can expand disease health education and family therapy to improve the family members' understanding of the patient and the disease. The development of NEDD4-related locus testing items is necessary, particularly for the next generation who show positive susceptibility genotypes. More attention should be paid to the childhood abuse experience. By improving family support, providing good family atmosphere, improving parent–child interaction, and reducing bad family relations and attachment relationship affects, health professionals can help reduce the impact of FH and G × Es on the next generation. Lastly, in terms of the government and society, although China has made considerable progress in the management of severe mental illnesses, its investment in the treatment and rehabilitation of schizophrenia should be increased, along with the rehabilitation exercise measures and designated assistance for patients in the community. Alternative ways to reduce the financial and psychological burden of patients' families should likewise be explored.
Our study had several limitations. Firstly, we only chose five SNPs of NEDD4 related to neurodevelopment based on current research, and only 136 patients with gender imbalance completed the CTQ-SF, which resulted in the reduction of our sample size. Secondly, the number of subjects required to obtain proper power for identifying G × Es in GMDR remains unknown (49). Moreover, our G × Es model was conducted in a case-only sample, which could estimate the interaction but not the main effect. Finally, the interaction model by GMDR was a statistical model; the biological effect of the combined G × Es remains unclear. These issues thereby limit the strength of our conclusions and data interpretation.
Our study highlighted the importance of the combined interactions of NEDD4 and CT in the clinical symptoms of patients with schizophrenia. We provided evidence for the need to pay more attention on patients with FH+: FH+ patients with NEDD4 gene susceptibility who have experienced more CT may have more severe clinical symptoms. These G × Es models may better elucidate schizophrenia, which will help clinicians assess comprehensively the symptoms of patients and personalize diagnoses and therapeutic options. Meanwhile, preventing CT for children with FH of psychosis may reduce the risk of the disorder and the severity of clinical symptoms. However, given the limitations of our study, the relation between NEDD4 and psychiatric disorders still needs to be explored further, and the impact of its interaction with CT on clinical symptoms still requires further confirmation by more clinical studies.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by The Ethical Committee of Shandong Mental Health Center. The patients/participants provided their written informed consent to participate in this study.
L-FL supervised the whole research project. Z-YC was responsible for project design and quality control. X-JB did the literature review, the genotyping, and the statistical analyses. LH contributed to quality control and training of this subject. CH contributed to the genotyping. M-MS is responsible for the database establishment and management. D-DQ, K-YC, L-NW, and L-MY contributed to the sample collection and clinical assessment. All authors contributed to the interpretation of results and have worked on the preparation of the final version of the manuscript.
This work was supported by Shandong Natural Science Foundation of China [grant numbers No. ZR2017MH094].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the patients and support of their families in the study and all members of the school staff for supporting our research.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.608231/full#supplementary-material
1. Singh S, Kumar A, Agarwal S, Phadke SR, Jaiswal Y. Genetic insight of schizophrenia: past and future perspectives. Gene. (2014) 535:97–100. doi: 10.1016/j.gene.2013.09.110
2. Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. (2009) 35:528–48. doi: 10.1093/schbul/sbn187
3. Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. (2014) 506:179–84. doi: 10.1038/nature12929
4. Morgan C, Fisher H. Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma–a critical review. Schizophr Bull. (2007) 33:3–10. doi: 10.1093/schbul/sbl053
5. Stanton KJ, Denietolis B, Goodwin BJ, Dvir Y. Childhood trauma and psychosis: an updated review. Child Adolesc Psychiatr Clin N Am. (2020) 29:115–29. doi: 10.1016/j.chc.2019.08.004
6. Pinckaers FME, Rotee ILM, Nwosu CV, Krolinski P, Smeets APW, Guloksuz S, et al. Evidence for interaction between genetic liability and childhood trauma in the development of psychotic symptoms. Soc Psychiatry Psychiatr Epidemiol. (2019) 54:1045–54. doi: 10.1007/s00127-019-01711-z
7. Kakela J, Panula J, Oinas E, Hirvonen N, Jaaskelainen E, Miettunen J. Family history of psychosis and social, occupational and global outcome in schizophrenia: a meta-analysis. Acta Psychiatr Scand. (2014) 130:269–78. doi: 10.1111/acps.12317
8. Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. (2009). 166:1025–30. doi: 10.1176/appi.ajp.2009.08010031
9. Kawabe H, Neeb A, Dimova K, Young SM Jr, Takeda M, Katsurabayashi S, et al. Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron. (2010) 65:358–72. doi: 10.1016/j.neuron.2010.01.007
10. Kawabe H, Brose N. The ubiquitin E3 ligase Nedd4-1 controls neurite development. Cell Cycle. (2010) 9:2477–8. doi: 10.4161/cc.9.13.12236
11. Drinjakovic J, Jung H, Campbell DS, Strochlic L, Dwivedy A, Holt CE. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron. (2010) 65:341–57. doi: 10.1016/j.neuron.2010.01.017
12. Hsia HE, Kumar R, Luca R, Takeda M, Courchet J, Nakashima J, et al. Ubiquitin E3 ligase Nedd4-1 acts as a downstream target of PI3K/PTEN-mTORC1 signaling to promote neurite growth. Proc Natl Acad Sci USA. (2014) 111:13205–10. doi: 10.1073/pnas.1400737111
13. Davies SE, Hallett PJ, Moens T, Smith G, Mangano E, Kim HT, et al. Enhanced ubiquitin-dependent degradation by Nedd4 protects against alpha-synuclein accumulation and toxicity in animal models of Parkinson's disease. Neurobiol Dis. (2014) 64:79–87. doi: 10.1016/j.nbd.2013.12.011
14. Kwak YD, Wang B, Li JJ, Wang R, Deng Q, Diao S, et al. Upregulation of the E3 ligase NEDD4-1 by oxidative stress degrades IGF-1 receptor protein in neurodegeneration. J Neurosci. (2012) 32:10971–81. doi: 10.1523/JNEUROSCI.1836-12.2012
15. Srinivasan BS, Doostzadeh J, Absalan F, Mohandessi S, Jalili R, Bigdeli S, et al. Whole genome survey of coding SNPs reveals a reproducible pathway determinant of Parkinson disease. Hum Mutat. (2009) 30:228–38. doi: 10.1002/humu.20840
16. Lien YJ, Tsuang HC, Chiang A, Liu CM, Hsieh MH, Hwang TJ, et al. The multidimensionality of schizotypy in nonpsychotic relatives of patients with schizophrenia and its applications in ordered subsets linkage analysis of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. (2010) 153B:1–9. doi: 10.1002/ajmg.b.30948
17. Jahanshad N, Rajagopalan P, Hua X, Hibar DP, Nir TM, Toga AW, et al. Genome-wide scan of healthy human connectome discovers SPON1 gene variant influencing dementia severity. Proc Natl Acad Sci USA. (2013) 110:4768–73. doi: 10.1073/pnas.1216206110
18. Warnica W, Merico D, Costain G, Alfred SE, Wei J, Marshall CR, et al. Copy number variable microRNAs in schizophrenia and their neurodevelopmental gene targets. Biol Psychiatry. (2015) 77:158–66. doi: 10.1016/j.biopsych.2014.05.011
19. Han C, Cui K, Bi X, Wang L, Sun M, Yang L, et al. Association between polymorphism of the NEDD4 gene and cognitive dysfunction of schizophrenia patients in Chinese Han population. BMC Psychiatry. (2019) 19:405. doi: 10.1186/s12888-019-2386-y
20. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. (2019) 21:100. doi: 10.1007/s11920-019-1091-3
21. Si TM, Yang JZ, Su L, Wang XL, Kong QM, Zhou M, et al. The reliability, validity of PANSS and its implication. Chin Mental Health J. (2004) 18:45–7. doi: 10.3321/j.issn:1000-6729.2004.01.016
22. Xing-Fu Z, Ya-Lin Z, Long-Fei L, Yun-Fei Z, He-Zhan L, Shi-Chang Y. Evaluation on reliability and validity of Chinese version of childhood trauma questionnaire. Chinese J Clin Rehabil. (2005) 9:209–11. doi: 10.3321/j.issn:1673-8225.2005.20.052
23. Zhang M. Reliability and validity of the Chinese version of CTQ-SF. Chin J Public Health. (2011) 27:669–70.
24. Li XB, Li QY, Liu JT, Zhang L, Tang YL, Wang CY. Childhood trauma associates with clinical features of schizophrenia in a sample of Chinese inpatients. Psychiatry Res. (2015) 228:702–7. doi: 10.1016/j.psychres.2015.06.001
25. Zou YZ, Cui JF, Wang J, Chen N, Tan SP, Zhang D, Xu Z, et al. Clinical reliability and validity of the Chinese version of Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery. Chin J Psychiatry. (2009) 42:29–33. doi: 10.3760/cma.j.issn.1006-7884.2009.01.009
26. Bi X, Cui K, Han C, Sun M, Wang L, Yang L, et al. Association of NEDD4 gene polymorphisms with schizophrenia and its clinical characteristics in Chinese Han population. Chin J Med Genet. (2015) 32:385–90. doi: 10.3760/cma.j.issn.1003-9406.2015.03.019
27. Lou XY, Chen GB, Yan L, Ma JZ, Zhu J, Elston RC, et al. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet. (2007) 80:1125–37. doi: 10.1086/518312
28. Boase NA, Kumar S. NEDD4: The founding member of a family of ubiquitin-protein ligases. Gene. (2015) 557:113–22. doi: 10.1016/j.gene.2014.12.020
29. Lin A, Hou Q, Jarzylo L, Amato S, Gilbert J, Shang F, et al. Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J Neurochem. (2011) 119:27–39. doi: 10.1111/j.1471-4159.2011.07221.x
30. Gautam V, Trinidad JC, Rimerman RA, Costa BM, Burlingame AL, Monaghan DT. Nedd4 is a specific E3 ubiquitin ligase for the NMDA receptor subunit GluN2D. Neuropharmacology. (2013) 74:96–107. doi: 10.1016/j.neuropharm.2013.04.035
31. Lee S, Park S, Lee H, Han S, Song JM, Han D, et al. Nedd4 E3 ligase and beta-arrestins regulate ubiquitination, trafficking, and stability of the mGlu7 receptor. Elife. (2019) 8:e44502. doi: 10.7554/eLife.44502
32. Jeon SA, Kim DW, Cho JY. Neural precursor cell-expressed, developmentally down-regulated 4 (NEDD4) regulates hydrogen peroxide-induced cell proliferation and death through inhibition of Hippo signaling. FASEB J. (2019) 33:14772–83. doi: 10.1096/fj.201901404R
33. Camera D, Coleman HA, Parkington HC, Jenkins TA, Pow DV, Boase N, et al. Learning, memory and long-term potentiation are altered in Nedd4 heterozygous mice. Behav Brain Res. (2016) 303:176–81. doi: 10.1016/j.bbr.2016.01.054
34. Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. (2012) 73:962–77. doi: 10.1016/j.neuron.2011.12.033
35. Wei J, Xiong Z, Lee JB, Cheng J, Duffney LJ, Matas E, et al. Histone modification of Nedd4 Ubiquitin ligase controls the loss of AMPA receptors and cognitive impairment induced by repeated stress. J Neurosci. (2016) 36:2119–30. doi: 10.1523/JNEUROSCI.3056-15.2016
36. Duncan CE, Chetcuti AF, Schofield PR. Coregulation of genes in the mouse brain following treatment with clozapine, haloperidol, or olanzapine implicates altered potassium channel subunit expression in the mechanism of antipsychotic drug action. Psychiatr Genet. (2008) 18:226–39. doi: 10.1097/YPG.0b013e3283053019
37. Hu Y, Hong XY, Yang XF, Ma RH, Wang X, Zhang JF, et al. Inflammation-dependent ISG15 upregulation mediates MIA-induced dendrite damages and depression by disrupting NEDD4/Rap2A signaling. Biochim Biophys Acta Mol Basis Dis. (2019) 1865:1477–89. doi: 10.1016/j.bbadis.2019.02.020
38. Koola MM, Ahmed AO, Sebastian J, Duncan EJ. Childhood physical and sexual abuse predicts suicide risk in a large cohort of veterans. Prim Care Companion CNS Disord. (2018) 20:18m02317. doi: 10.4088/PCC.18m02317
39. Wells R, Jacomb I, Swaminathan V, Sundram S, Weinberg D, Bruggemann J, et al. The impact of childhood adversity on cognitive development in schizophrenia. Schizophr Bull. (2020) 46:140–53. doi: 10.1093/schbul/sbz033
40. Garcia M, Montalvo I, Creus M, Cabezas A, Sole M, Jose AM, et al. Sex differences in the effect of childhood trauma on the clinical expression of early psychosis. Compr Psychiat. (2016) 68:86–96. doi: 10.1016/j.comppsych.2016.04.004
41. Chae S, Sim M, Lim M, Na J, Kim D. Multivariate analysis of relationship between childhood trauma and psychotic symptoms in patients with schizophrenia. Psychiatry Investig. (2015) 12:397–401. doi: 10.4306/pi.2015.12.3.397
42. Turner S, Harvey C, Hayes L, Castle D, Galletly C, Sweeney S, et al. Childhood adversity and clinical and psychosocial outcomes in psychosis. Epidemiol Psychiatr Sci. (2019) 29:e78. doi: 10.1017/S2045796019000684
43. Bellis MA, Hughes K, Leckenby N, Jones L, Baban A, Kachaeva M, et al. Adverse childhood experiences and associations with health-harming behaviours in young adults: surveys in eight eastern European countries. Bull World Health Organ. (2014) 92:641–55. doi: 10.2471/BLT.13.129247
44. Bu-Yi Y. Childhood emotional neglect and associations with health-harming behaviors in female adolescents: survey in Beijing. CJCHC. (2016) 24:628–31. doi: 10.11852/zgetbjzz2016-24-06-22
45. Ensink J, de Moor M, Zafarmand MH, de Laat S, Uitterlinden A, Vrijkotte TGM, et al. Maternal environmental risk factors and the development of internalizing and externalizing problems in childhood: the complex role of genetic factors. Am J Med Genet B Neuropsychiatr Genet. (2020) 183:17–25. doi: 10.1002/ajmg.b.32755
46. McCarthy-Jones S, Green MJ, Scott RJ, Tooney PA, Cairns MJ, Wu JQ, et al. Preliminary evidence of an interaction between the FOXP2 gene and childhood emotional abuse predicting likelihood of auditory verbal hallucinations in schizophrenia. J Psychiatr Res. (2014) 50:66–72. doi: 10.1016/j.jpsychires.2013.11.012
47. Caqueo-Urizar A, Gutierrez-Maldonado J. Satisfaction with mental health services in a Latin American community of carers of patients with schizophrenia. Community Ment Health J. (2009) 45:285–9. doi: 10.1007/s10597-009-9220-9
48. Norman R, Lecomte T, Addington D, Anderson E. Canadian treatment guidelines on psychosocial treatment of schizophrenia in adults. Can J Psychiatry. (2017) 62:617–23. doi: 10.1177/0706743717719894
Keywords: childhood trauma, gene-environment interaction, NEDD4, phenotype, schizophrenia
Citation: Bi X-J, Hu L, Qiao D-D, Han C, Sun M-M, Cui K-Y, Wang L-N, Yang L-M, Liu L-F and Chen Z-Y (2021) Evidence for an Interaction Between NEDD4 and Childhood Trauma on Clinical Characters of Schizophrenia With Family History of Psychosis. Front. Psychiatry 12:608231. doi: 10.3389/fpsyt.2021.608231
Received: 19 September 2020; Accepted: 05 March 2021;
Published: 08 April 2021.
Edited by:
Kyriaki Sidiropoulou, University of Crete, GreeceReviewed by:
Giulia Agostoni, Vita-Salute San Raffaele University, ItalyCopyright © 2021 Bi, Hu, Qiao, Han, Sun, Cui, Wang, Yang, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan-Fen Liu, bGl1bGY1MjFAMTYzLmNvbQ==; Zhe-Yu Chen, emhleXVjaGVuQHNkdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.