
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Psychiatry, 23 April 2021
Sec. Mood Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.584416
This article is part of the Research TopicNovel Approaches to Improve Detection, Differentiation and Treatment in Mood Disorders View all 12 articles
Depression is one of the most common mental disorders, which causes global burden. Antidepressants and psychotherapies are the mainstay of treatment for depression, which have limited efficacy. Thus, alternative approaches for preventing and treating depression are urgently required. Recent clinical trials and preclinical researches have clarified that peripheral facial injection of botulinum neurotoxin type A (BoNT/A) is a rapid, effective and relative safe therapy for improving some symptoms of depression. Despite its safety and efficacy, the underlying therapeutic mechanisms of BoNT/A for depression remains largely unclear. In the present review, we updated and summarized the clinical and preclinical evidence supporting BoNT/A therapy for the treatment of depression. We further discussed the potential mechanisms underlying therapeutic effects of BoNT/A on depression. Notably, we recently identified that the anti-depressant effects of BoNT/A associated with up-regulation of 5-HT levels and brain-derived neurotrophic factor (BDNF) expression in the hippocampus in a preclinical mouse model. In summary, these studies suggest that BoNT/A therapy is a potential effective and safe intervention for the management of depression. However, fundamental questions remain regarding the future prospects of BoNT/A therapy, including safety, efficacy, dose-response relationships, identification of potential predictors of response, and the precise mechanisms underlying BoNT/A therapy.
Major depressive disorder (MDD) is a complex mental disease, which is characterized by symptoms of emotional, motivational, cognitional, and physiological domains (1). MDD is a highly prevalent disease of mental disorders which ranging from 6 to 18% across different countries, and it has substantially increased since 1990, possibly driven by global population growth and aging (2). According to World Health Organization (WHO), it was estimated that more than 350 million people suffer from MDD all over the world (3). In addition, many studies indicate women have been shown to be at greater risk for MDD than men (4). MDD is one of the top ten causes of disability all over the world. And MDD has been predicted to be a leading cause of global disease burden by 2030, in view of the overall disability and sufferings caused by it (5). For instance, the economic costs due to depression worldwide is estimated to be 1.15 trillion US dollars per year (3), and it is estimated that in the United States alone, this figure exceeds US$210 billion, resulting in 45% direct costs, 5% costs related to suicides and 50% costs related to work (3).
The symptoms of depression include low mood, decreased interest in the daily activities, decreased motivation, appetite and sleep disturbance, psychomotor agitation or retardation, cognitive impairment, and suicidal thought (6, 7). In addition, the patients with MDD have poorer physical health, including increased prevalence of cardiovascular disease, diabetes, and premature mortality compared with the general population (8). MDD which was untreated or partially treated has prodigious influence for the patients, their family, health-care system, and society (9, 10).
Clinically, the current treatments for depression are pharmacological and psychological interventions. Early clinical observations indicated that decreased monoamine function in the brain contributed to the pathogenesis of depression. Thus, antidepressants were developed in order to up-regulate monoamines levels in the brain either by inhibiting neuronal reuptake of them or by inhibiting their degradation. Although antidepressants are typically more efficacious than placebo in many clinical trials, some evidence suggested that ~50% of patients with depression were not responsive to antidepressant treatments (11). In addition, antidepressant medication may cause significant side effects, such as weight gain, increased risk of diabetes, and sexual dysfunction. Notably, cognitive behavioral therapy is also shown to have only moderate therapeutic effect on depression (12). Given the enormous disease burden of MDD and limited efficacy of current antidepressants or cognitive behavioral therapy, there is an imminent need to develop an alternative effective therapy for depression. To this end, there is growing evidence supporting botulinum neurotoxin type A (BoNT/A) therapy as useful method to treat major depression (13–16). In the present review, we have provided clinical and preclinical evidence supporting BoNT/A therapy for treatment of depression and discussed the potential therapeutic mechanisms and future perspectives.
Botulinum neurotoxins (BoNTs) are produced by Clostridium botulinum, of which there are 7 identified and different serotypes (A-G) (17, 18). BoNTs has a molecular weight of 150 kDa, which consist a light chain (LC; 50 kDa) and a heavy chain (HC; 100 kDa) (19–21). The main action of BoNTs occurs in the neuromuscular junction. In botulism poisoning, flaccid paralysis occurs by inhibiting the release of neurotransmitters from the peripheral cholinergic nerve terminals of the skeletal and autonomic nervous system (20). BoNTs are a typical example of bacterial exotoxins which target intracellular substrates. BoNTs have developed a structural organization aiming at delivering the metalloprotease domain into the host cell cytosol and by exploiting several physiologic functions of nerve terminals can achieve it (21). When local injection of BoNTs, they have limited diffusion, and their action can be reversible with time. Based on this above-mentioned feature, BoNTs (especial BoNT/A) have become the safe and most efficacious treatment for various kinds human syndromes that are characterized by hyperactivity of nerve terminals (21).
The classical mechanisms underlying the actions of BoNT/A is the inhibition of acetylcholine (ACh) release from the presynaptic nerve terminals, then reducing the activity of muscle fibers (22, 23). There are five major steps of BoNT/A transport in the nerve terminal, including binding and specificity, internalization into nerve terminals, membrane translocation, inter-chain disulfide reduction and SNARE protein cleavage (20), especially endocytosis, intracellular trafficking and transcytosis are important. It has been reported that HC/A can bind to and activate fibroblast growth factor receptor 3 (FGFR3), which is a tyrosine kinase receptor, in neuroblastoma cells (24). Recently, it was reported that by using non-toxic full-length BoNT/A(0) mutant, a catalytically inactive, BoNT/A(0) enters cortical neurons via a pathway dependent on FGFR3 receptor (25). Further study showed that BoNT/A(0) enters neurons through both dynamin-dependent and dynamin-independent endocytosis (25).
BoNT/A may also act similarly to the action of tetanus neurotoxins on the nervous system, which is the well-known example of retro-axonal transport inside motor axons (26). It is noteworthy that peripheral injection of BoNT/A can have a transynaptic action on the central neuronal circuits, including the spinal cord and higher brain regions (27, 28). So far, convincing evidence supporting BoNT/A1 retrograde transport to the CNS was supplied by tracking down the cleavage of the SNARE within the CNS neurons after peripheral injection, using a specified antibody for the novel epitope generated from the cleavage of SNAP-25 by BoNT/A1 (17, 29, 30). By injecting BoNT/A1 into the rat whisker pad, it caused the appearance of truncated SNAP-25 in the somatodendritic area of primary efferent facial motoneurons (31). It was also found that after the catalytically active BoNT/A was injected into the nasolabial muscle tissue of rats and mice, it was transported to the facial nucleus (FN) in the brain (27). Retrograde transportation of BoNT/A1 can also occur through primary sensory afferents, as it was observed that arrival of truncated SNAP-25 is not only in the trigeminal nucleus (29) but also in the spinal cord dorsal horn after subcutaneous or intramuscular injection (30). The transportation of BoNT/A1 includes undergoing retrograde from periphery to the ganglia and anterograde from ganglia to the afferent innervations in the brain stem and/or the spinal cord (28). Therefore, these results suggested that the intracellular transportation of BoNT/A1 may be an active retro-axonal transport, rather than through their passive diffusion or diffusion of split productions (31).
Currently, BoNTs therapy is widely administrated in clinical neurology, including dystonia, spasticity, autonomic disorders, and chronic pain (22, 23, 26) (Figure 1A). BoNTs can not only block the skeletal neuromuscular transmission, but also the autonomic innervation (24). Therefore, BoNTs therapy may also be beneficial for hyper-hidrotic disorders, urologic or gastrointestinal disorders (21). BoNT/A therapy had been applied for the management of many neurological disorders, such as dystonia and spasticity (25). Recently, a meta-analysis study including a total of 42 clinical trials reassessed the efficacy of BoNTs for movement disorders treatment, such as blepharospasm, hemifacial spasm, and laryngeal dystonia (27, 28). BoNT/A therapy was though as a choice of the pharmacological treatment in focal spasticity to improve limb position, functional ability and to release pain (29, 30). The application of BoNTs within the broad category of autonomic indications includes the hypersecretory disorders, such as hyperhidrosis and sialorrhea (31). Preclinical and clinical evidence have showed that BoNTs therapy had analgesic effects on neuropathic pain, trigeminal neuralgia, and chronic migraine (32). Notably, BoNT/A has been given official approval for preventive therapy of chronic migraine by Food and Drug Administration (FDA) in pain medicine (33). BoNTs can alleviate trigeminal neuralgia and can last for 6 months or more (34). Recently, BoNTs is also applied for the management of motor and non-motor symptoms in Parkinson's disease (PD) patients (35). Intriguingly, recent meta-analysis and literatures support that clinical application of BoNT/A may have antidepressant properties (14, 36, 37) (Figure 1B). In the current review, we summarized the clinical and preclinical studies on BoNT/A-induced antidepressant effects, discussed the therapeutic mechanisms, and proposed the future directions of BoNT/A therapy for depression.
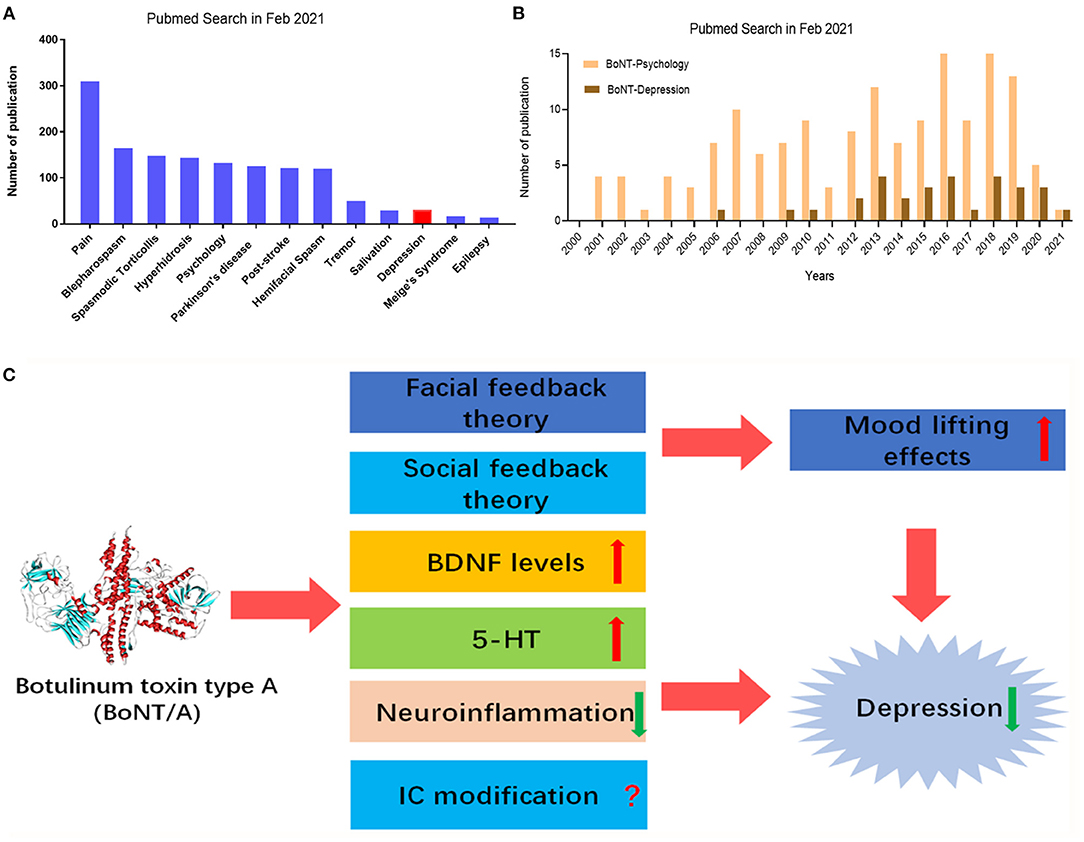
Figure 1. PubMed search for BoNT/A and neurological diseases, including depression. (A) Number of publications applying BoNT/A in Parkinson's disease, pain, hyperhidrosis, post-stroke, tremor, salivation, epilepsy, spasmodic torticollis, hemifacial spasm, blepharospasm, Meige's Syndrome, depression, and psychology. (B) Number of publications for applying BoNT/A in depression and psychology in the last 20 years. (C) Therapeutic mechanisms of BoNT/A in depression. Peripheral injection of BoNT/A produces antidepressant effects possible though multiple mechanisms, such as facial feedback and/or social feedback to lift mood, IC modifications, up-regulation of BDNF levels, increasing 5-HT levels, and dampening neuroinflammation. IC, insular cortex; BDNF, brain-derived neurotrophic factor; 5-HT, 5-hydroxytryptamine.
Although pharmacological and psychological treatment for depression are available, a considerable proportion of depressed patients are resistant to current standard treatment (38, 39). In esthetic medicine, the injection of BoNT/A in the glabellar region is a commonly used intervention (40). The contraction of the corrugator muscles is able to produce glabellar frown lines, which is also required for the facial expression of negative emotion, such as anger, fear, and/or sadness (41). Thus, it was concluded that BoNT/A therapy can actually make facial expression of emotion less negative, suggesting BoNT/A therapy may have mood-lifting effects in some depressed patients.
In 2006, Finzi et al. first reported open-label application of BoNT/A in 10 depressed patients led to significant improvement of self-rated depression score by using Beck Depression Inventory II (BDI-II) (15). Two months after the injection of BoNT/A, nine patients were not depressed and one patient reversed negative mood. Although there is no control experiment and the number of cases is too small, this study inspired people to further investigate the therapeutic effects of BoNT/A on depression. Subsequently, there are several randomized controlled trials (RCT) of BoNT/A therapy for the management of depression. In addition, injection of BoNT/A into frown muscles and glabellar region in humans was performed in most studies. Other injection sites were also increasingly used, such as bilateral lateral canthus (42) in humans. The injection sites of five reports were glabellar regions and the concentration of the injection were 29 U applying in females and 39 to 40 U for males. After that, Montgomery Asberg Depression Rating Scale (MADRS), BDI-II, and Hamilton Depression Scale (HAMD) were employed for the clinical evaluation of depression. They found that patients had a remission rate of more than 50% following BoNTs treatment, while females had a higher rate of remission than males (13). However, this study lasted only 6 weeks. In 2018, Finzi's another study showed that BoNTs injection was also effective for bipolar depression in men (43).
A randomized and placebo-controlled trial was conducted to evaluate the possible beneficial effect of injecting BoNT/A into the glabellar area as an adjuvant therapy for antidepression (44). This study lasted 16 weeks, making up for the shortcomings of abovementioned Finzi's research. Thirty participants randomly joined into the BoNT/A-treated (n = 15) or saline-treated (n = 15) group. The response rates of BoNT/A and placebo groups were 60.0 and 13.3%, respectively. This trial suggested that a single injection of BoNT/A in the glabellar region may rapidly reach an intense and sustained remission for some depressed patients, who did not response to former medication. This study is relatively perfect, except for a small number of samples. Lewis et al. assessed the effect of BoNT/A on mood by comparing the patients who received BoNT/A therapy in glabellar region and who had other cosmetic treatment (45). Total 25 female participants participated in this study. BoNT/A treatment group was lower than the control group in the scores of Irritability-Depression-Anxiety Scale (IDAS). For some participants, their first BoNT/A treatment was 2 weeks prior to the measurement, however one participant first BoNT/A treatment was 6 years ago. In order to avoid large skewness, the geometric mean is used to measure the central trend. The geometric mean was 195 days, which is the longest remission duration.
Brin et al. evaluated the antidepression effect of BoNT/A using two doses (30U and 50U) in females (36). At week 6, BoNT/A (50 U) treatment group did not separate from placebo group for any parameters. BoNT/A (30 U), administered in a standardized injection pattern in a single session, had a consistent efficacy signal across multiple depression symptom scales for 12 weeks or more. BoNT/A (30 U)/placebo MADRS differences of (observed ANCOVA) was ≥4.0 points (up to week 15) and ≥2.0 points (weeks 18–24), which is agree with the 2-point change threshold considered clinically relevant in MDD.
Magid et al. evaluated the effect of BoNT/A lasting 24 weeks (46). The response rates were 55% (6/11) in the BoNT/A-first group, 24% (4/17) in the BoNT/A-second group, and 0% (0/19) in the placebo group, respectively. The results suggested that the first injection of BoNT/A significantly decreased the BDI scores compared to that before treatment. An additional dose (no more than 20 U) were secondly injected to those patients who still had a severity score or greater for glabellar frown lines (47). Together, clinical studies (summarized in Table 1) have demonstrated that BoNT/A may be effective for the management of depression.
To date, preclinical animal studies of BoNTs treatment for depression are still limited, but there are some clues. A single facial injection of BoNT/A induced a rapid and continuous improvement about depression-like behaviors in naive and space-restriction-stressed (SRS) mice. The antidepressant-like effects of BoNT/A was reflected by a decreased duration of forced swimming test and tail suspension test immobility (14). Techniques currently used to assess antidepressants effects by using rodent models include olfactory bulbectomy, chronic mild stress, chronic forced swim test, novelty-suppressed feeding, novelty-induced hypophagia, social defeat stress, and learned helplessness (49). Given that different models have different sensitivity to behaviors tests, using different animal models to validate the anti-depressant effects of BoNT/A may be necessary in the future.
To date, the clinical trials and animal studies of BoNTs treatment of depression are still relatively lacking, and the number of cases needs to be increased to evaluate its safety and efficacy, especially the long-term efficacy. However, it was noticed that the publication about the application of BoNTs in depression is increasing (Figure 1B). Subsequently, we will discuss the therapeutic mechanisms underlying the effects of BoNTs on depression.
To date, the potentially mechanisms underlying the therapeutic effects of BoNT/A in the treatment of depression is still elusive. There are some review article or meta-analysis that discussed its efficacy and possible mechanisms (37, 50). Based on current clinical and preclinical evidence, several hypotheses were proposed to explain the therapeutic mechanisms of BoNT/A on depression to date. The facial feedback hypothesis claims that the injection of BoNT/A between the eyebrows interfere with emotional feedback. Because botulinum toxin can paralyze muscles, it is impossible to modify facial expression according to mood states, such as happy, sadness and anger, which often appear in depressed patients. Social feedback hypothesis indicated that happy facial expressions will get positive social feedback and improve mood. Finally, facial injection of BoNT/A causes structure or function changes in the brain to alleviate depression, for example, upregulation of brain-derived neurotrophic factor (BDNF) expression in the brain (51). We will further discuss these abovementioned hypotheses as follow (Figure 1C).
The possible relationship between facial expression and depression can be traced back to the Darwin period (52). Strack et al. tested the hypothesis that people's facial activity affects their emotional responses (53). These results suggest that inhibition and promotion mechanisms may contribute to the observed emotional response (53). Other researchers have presented supportive results, indicating that signaling between the emotional center of the brain and the facial muscles is bidirectional, which further supports the facial feedback hypothesis (54).
It was confirmed that after the injection of BoNTs between the eyebrow and the orbicularis muscle, the depressed emotional state was reduced compared with depressed patients who had a saline injection. The improvement of depressed emotional state produced by BoNTs therapy was more obvious, when dealing with mild emotional stimuli (55). Wollmer et al. analyzed existing studies in an attempt to find better predictors to reflect the effects of BoNTs on patients with MDD. After data analysis, they found that high tension was the main predictor of BoNTs response, which sensitivity, specificity and overall accuracy of 100, 56, and 87%, respectively (56). The better effect of BoNTs for mood may be by the intervention of a proprioceptive feedback loop from the facial musculature to the emotional brain (57). Tension may be associated with more dynamic activities, resulting in more facial expression changes (58). In addition, BoNTs therapy reduced anxiety caused by frowning muscles, supporting the facial feedback hypothesis (59).
Through its action of reversible paralysis of mimic muscles, peripheral injection BoNT/A is considered to be able to prevent the emotional facial expression, including anger, sadness, and fear from being perceived by seeing of the face. Thus, BoNT/A therapy may improve the social interaction with people around, improve social contact, and then have benign social feedback. Namely, people would enter a virtuous circle of positive mood and social feedback, resulting in the persistent improvement of self-esteem. Furthermore, it was demonstrated that brain limbic system and mirror neuron system is involved in the recognition of emotional facial expression (60, 61).
The down-regulation of the expression of several neurotrophic factors has been involved in the pathogenesis of MDD. The most prominent and widespread representative is a polypeptide BDNF, which promotes cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) phosphorylation through the activation of extracellular signal-regulated kinase (ERK). In the brain, especially in the hippocampus, BDNF plays a functional role in neuronal differentiation and survival, neurogenesis, synaptic plasticity, connectivity, maintenance of morphology, learning and memory (62). Intriguingly, BDNF levels in several brain regions are remarkably reduced in depression-like animals and depressed patients. Several commonly-used antidepressants, such as serotonin-selective reuptake inhibitors (SSRIs) (63) can up-regulate the level of BDNF expression in the hippocampus and the prefrontal cortex in order to exert antidepressant effects (64) (Figure 1). Our recent work also showed that facial injection of BoNT/A was also able to up-regulate the protein expression level of BDNF in the hippocampus not only at the mRNA but also at protein in mice. Furthermore, BoNT/A injection also activated the downstream ERK-CREB signaling pathways of BDNF in the hippocampus in stressed-mice (14). Thus, the up-regulation of BDNF levels may be a new mechanism underlying the therapeutic effects of BoNT/A in the management of depression.
The monoamine theory claims that there is an association between emotional disorders and reduced availability of the absence of 5-hydroxytryptamine (5-HT) or norepinephrine (NE) in the brain (65). Antidepressants can increase their brain content by inhibiting the reuptake of two important neurotransmitters (5-HT and NE) into nerve terminals (65). Some of the antidepressants based on monoamine theory include: tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOI), and SSRIs. Unilateral administration with BoNT/A into rat whisker significantly increased the NE levels in the striatum and 5-HT levels in the hypothalamus (14, 66). Interestingly, facial injection of BoNT/A significantly increased the 5-HT level in the hippocampus, hypothalamus, and prefrontal cortex in chronic stressed mice (14, 66). Thus, the results showed that BoNTs can up-regulate the levels of monoamines (e.g., 5-HT and NE) in the brain. Further clinical and basic studies need to identify the precise alteration of the neurotransmitters in the brain during BoNT/A therapy for depression.
Series of evidence support the importance of several brain areas associated with MDD, by using magnetic resonance imaging (MRI) (67–69) or meta-analysis the MRI data (70, 71). The most extensive research has been done on the insular cortex (IC), prefrontal cortex (PFC), anterior cingulate cortex (ACC), amygdala and hippocampus (62), which play a key role in sensation and emotion. IC is a brain region responsible for the coding of emotional and social aspects (51). Previous studies demonstrated abnormal insular connectivity may mediate mood disorders in the symptom of depression (72). A new hypothesis for the therapeutic effects of BoNTs on depression, refers to “insula cortex (IC) modification,” was recently proposed from transgenders' study (51). In patients suffering from mental illness, depression or bipolar disorder, it is possible to find both mental and physical dysphoria. The morphological changes of IC may be related to the difference between physical image and mental perception of one's body (51). Although the authors did not provide any evidence supporting BoNT/A therapy causes morphological alterations of IC, they proposed that the structural and functional modification of IC caused by BoNT/A therapy warrants further investigation.
An important part of the physiological stress-sensing system is the immune system, which interacts with main integrative systems of body, including the hypothalamic–pituitary–adrenal (HPA) axis, the autonomic nervous system and the central nervous system (CNS) (73). Immune disorders affecting CNS function would cause neuroinflammatory diseases, such as multiple sclerosis and autoimmune encephalitis. Low-level chronic systemic inflammation may play an important role in mediating the interface among psychological stress, depressive symptomatology, and association with depressive symptoms (74). However, there is no direct evidence to support the therapeutic mechanisms underlying the effects of BoNTs for depression involved in neuroinflammation. But there are some clues indicated the potential modulation effects of BoNTs on the development of neuroinflammation. The mechanisms underlying therapeutic effects of BoNTs on neuropathic pain are involved in restraining the release of inflammatory mediators and peripheral neurotransmitters from sensory nerves (75). Increased expression of inflammatory factors has been shown to be inhibited by monoclonal anti-Toll-like receptor 2 (TLR2) and inhibitors specific to intracellular proteins such as c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 mitogen-activated protein kinase (MAPK) (76). Furthermore, BoNT/A treatment did not decrease LPS-induced release of pro-inflammatory factors in the astroglia, suggesting BoNT/A may have little effect on astrocytes (77). In contrast, BoNT/A treatment decreased the upregulation of expression of microglia-derived pro-inflammatory factors (77, 78), suggesting that BoNT/A may modulate the development of neuroinflammation by the inhibition of microglia activation in the CNS. The direct evidence supporting that therapeutic effects of BoNT/A on depression may owe to inhibiting neuroinflammation is still lacking.
Clinical and preclinical experiments have showed that BoNTs may be beneficial for the management of depression. However, the therapeutic application of BoNTs for depression has not USA or EU or Evidence-based therapeutic approved indication. The therapeutic mechanisms of BoNTs in the treatment for depression are needed to be further clarified. In view of the heterogeneity of the clinical findings and various influencing factors (16), more clinical trials should be performed to evaluate the dose equivalence, especially considering the different BoNT/A1 formulations. Notably, the potency of the preparations of BoNTs can be expressed as Units (U), where 1 U corresponds to 1 LD50 in the mouse bioassay (79). It is noteworthy that the clinical effect of 1 unit is not interchangeable between different preparations, because the bioassay methods used by different brands are different, (80).
Functional magnetic resonance imaging (fMRI) was used to assess neural responses to BoNTs treatment by radionuclide imaging in human (81). However, it remains a major challenge in objectively reflecting emotional state especially in animal models. Interestingly, a recent study showed that mice can display stereotyped facial expressions responding to emotionally salient events, and upon targeted manipulations in emotion-relevant neuronal circuits (82). By using brain imaging methods and monitoring facial expression of emotion in rodents, the therapeutic mechanisms of BoNT/A on depression may be better understood.
Recently, recombinant techniques (including site-directed mutations) have been used to create engineering botulinum neurotoxins as potential novel drugs for improving their therapeutic efficacy (83, 84). For instance, Yin et al. generated a “gain-of-function” mutation by replacing two non-aromatic residues at an extended loop in the C-terminal receptor-binding domain of BoNT/B (85). This engineering BoNT/B showed enhanced binding to neuronal membrane, enhanced efficacy in paralyzing muscles, and lowered systemic diffusion (85). In addition, BoNTs were designed as novel analgesic by utilizing the ability of BoNT to cleave SNARE complexes (83). Another engineering BoNT designed by mixing BoNT/E LC to the N-terminus of BoNT/A produced locally-applied and long-acting analgesic effects in a rat neuropathic pain model (86). Scheps et al. designed a mutant with three mutations (T420E; F423M; Y426F) in the C-terminus of BoNT/A1 light chain, which exert a faster onset and a shorter duration than wild-type BoNT/A1 (87). There are several lines of evidence supporting that BoNT/A1 therapy is generally safe and may be used as a new, alternative option for the treatment of depression (37). However, safety issue must be considered for all recombinant engineering BoNTs. Together, these novel engineering botulinum neurotoxins warrant further investigation for depression treatment.
Although both clinical and preclinical studies have demonstrated that BoNT/A therapy may be an effective alternative intervention for depression, future investigations are needed to improve our understanding of the therapeutic mechanisms of BoNT/A for depression. Thus, studies on BoNT/A therapy may provide novel targets for the development of effective antidepressant drugs.
YL performed literature search and prepared the draft. TL and WL wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (81870874 and 81671270) and the Natural Science Foundation of Jiangsu Province, China (BK20170004), Jiangsu Key Laboratory of Neuropsychiatric Diseases (BM2013003), the Second Affiliated Hospital of Soochow University Preponderant Clinic Discipline Group Project Funding (XKQ2015002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Baldwin DS. A personal account of depressive illness and its antidepressant treatment. International Clin Psychopharmacol. (2019) 34:286–90. doi: 10.1097/YIC.0000000000000273
2. Global regional and national incidence prevalence and years lived with disability for 354 diseases and injuries for 195 countries and territories 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/s0140-6736(18)32279-7
3. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. doi: 10.1016/s0140-6736(17)32802-7
4. Stegenga BT, King M, Grobbee DE, Torres-González F, Švab I, Maaroos HI, et al. Differential impact of risk factors for women and men on the risk of major depressive disorder. Ann Epidemiol. (2012) 22:388–96. doi: 10.1016/j.annepidem.2012.04.011
5. Ii Timberlake M, Dwivedi Y. Linking unfolded protein response to inflammation and depression: potential pathologic and therapeutic implications. Mol Psychiatry. (2019) 24:987–94. doi: 10.1038/s41380-018-0241-z
6. Swinkels JA. [Revised Dutch GP guideline 'Depression': diagnosis not as simple as it appears]. Ned Tijdschr Geneeskd. (2012) 156:A5113.
7. Stewart JW, McGrath PJ, Quitkin FM, Klein DF. DSM-IV depression with atypical features: is it valid? Neuropsychopharmacology. (2009) 34:2625–32. doi: 10.1038/npp.2009.99
8. Rivera M, Porras-Segovia A, Rovira P, Molina E, Gutiérrez B, Cervilla J. Associations of major depressive disorder with chronic physical conditions, obesity and medication use: results from the PISMA-ep study. Eur Psychiatry. (2019) 60:20–7. doi: 10.1016/j.eurpsy.2019.04.008
9. Arnold JF, Zwiers MP, Fitzgerald DA, van Eijndhoven P, Becker ES, Rinck M, et al. Fronto-limbic microstructure and structural connectivity in remission from major depression. Psychiatry Res. (2012) 204:40–8. doi: 10.1016/j.pscychresns.2012.07.010
10. Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression—the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. (2010) 4:33. doi: 10.3389/fnsys.2010.00033
11. Furukawa TA, Maruo K, Noma H, Tanaka S, Imai H, Shinohara K, et al. Initial severity of major depression and efficacy of new generation antidepressants: individual participant data meta-analysis. Acta Psychiatr Scand. (2018) 137:450–8. doi: 10.1111/acps.12886
12. Ijaz S, Davies P, Williams CJ, Kessler D, Lewis G, Wiles N. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev. (2018) 5:Cd010558. doi: 10.1002/14651858.CD010558.pub2
13. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. (2014) 52:1–6. doi: 10.1016/j.jpsychires.2013.11.006
14. Li Y, Liu J, Liu X, Su CJ, Zhang QL, Wang ZH, et al. Antidepressant-Like Action of Single Facial Injection of Botulinum Neurotoxin A is Associated with Augmented 5-HT Levels and BDNF/ERK/CREB Pathways in Mouse Brain. Neurosci Bull. (2019) 35:661–72. doi: 10.1007/s12264-019-00367-8
15. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatolog Surg. (2006) 32:645–9; discussion 9–50. doi: 10.1111/j.1524-4725.2006.32136.x
16. Arnone D, Galadari H, Rodgers CJ, Östlundh L, Aziz KA, Stip E, et al. Efficacy of onabotulinumtoxinA in the treatment of unipolar major depression: systematic review, meta-analysis and meta-regression analyses of double-blind randomised controlled trials. J Psychopharmacol. (2021). doi: 10.1177/0269881121991827. [Epub ahead of print].
17. Cherington M. Botulism: update and review. Semin Neurol. (2004) 24:155–63. doi: 10.1055/s-2004-830901
18. Matak I, Lacković Z. Botulinum toxin A, brain and pain. Prog Neurobiol. (2014) 119–20:39–59. doi: 10.1016/j.pneurobio.2014.06.001
19. Rossetto O, Seveso M, Caccin P, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: turning bad guys into good by research. Toxicon. (2001) 39:27–41. doi: 10.1016/s0041-0101(00)00163-x
20. Park J, Chung ME. Botulinum toxin for central neuropathic pain. Toxins. (2018) 10:224. doi: 10.3390/toxins10060224
21. Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. (2017) 69:200–35. doi: 10.1124/pr.116.012658
22. Orsini M, Leite MA, Chung TM, Bocca W, de Souza JA, de Souza OG, et al. Botulinum neurotoxin type a in neurology: update. Neurol Int. (2015) 7:5886. doi: 10.4081/ni.2015.5886
23. Jankovic J. botulinum toxin: state of the art. Mov Disord. (2017) 32:1131–8. doi: 10.1002/mds.27072
24. Hosp C, Naumann MK, Hamm H. Botulinum toxin treatment of autonomic disorders: focal hyperhidrosis and sialorrhea. Semin Neurol. (2016) 36:20–8. doi: 10.1055/s-0035-1571214
25. Garcia-Ruiz PJ, Sanz-Cartagena P, Martinez-Castrillo JC, Ares-Pensado B, Aviles-Olmos I, Blazquez-Estrada M, et al. Myths and evidence on the use of botulinum toxin: neuropharmacology and dystonia. Rev Neurol. (2018) 66:163–72. doi: 10.33588/rn.6605.2017110
26. Jabbari B. Botulinum toxin treatment in neurology. Semin Neurol. (2016) 36:3–4. doi: 10.1055/s-0036-1571849
27. Hallett M, Albanese A, Dressler D, Segal KR, Simpson DM, Truong D, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon. (2013) 67:94–114. doi: 10.1016/j.toxicon.2012.12.004
28. Del Sorbo F, Albanese A. Botulinum neurotoxins for the treatment of focal dystonias: review of rating tools used in clinical trials. Toxicon. (2015) 107(Pt A):89–97. doi: 10.1016/j.toxicon.2015.09.010
29. Esquenazi A, Albanese A, Chancellor MB, Elovic E, Segal KR, Simpson DM, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon. (2013) 67:115–28. doi: 10.1016/j.toxicon.2012.11.025
30. Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. (2016) 86:1818–26. doi: 10.1212/wnl.0000000000002560
31. Evidente VG, Adler CH. An update on the neurologic applications of botulinum toxins. Curr Neurol Neurosci Rep. (2010) 10:338–44. doi: 10.1007/s11910-010-0129-z
32. Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes -an evidence based review. Toxicon. (2018) 147:120–8. doi: 10.1016/j.toxicon.2018.01.017
33. Begasse de Dhaem O, Gharedaghi MH, Rizzoli P. Modifications to the PREEMPT protocol for onabotulinumtoxina injections for chronic migraine in clinical practice. Headache. (2020) 60:1365–75. doi: 10.1111/head.13823
34. Kontzialis M, Kocak M. Imaging evaluation of trigeminal neuralgia. J Istanbul Univ Fac Dent. (2017) 51(3 Suppl 1):S62–8. doi: 10.17096/jiufd.27242
35. Wagle Shukla A, Malaty IA. Botulinum toxin therapy for parkinson's disease. Semin Neurol. (2017) 37:193–204. doi: 10.1055/s-0037-1602246
36. Brin MF, Durgam S, Lum A, James L, Liu J, Thase ME, et al. OnabotulinumtoxinA for the treatment of major depressive disorder: a phase 2 randomized, double-blind, placebo-controlled trial in adult females. Int Clin Psychopharmacol. (2020) 35:19–28. doi: 10.1097/yic.0000000000000290
37. Qian H, Shao F, Lenahan C, Shao A, Li Y. Efficacy and safety of botulinum toxin vs. placebo in depression: a systematic review and meta-analysis of randomized controlled trials. Front Psychiatry. (2020) 11:603087. doi: 10.3389/fpsyt.2020.603087
38. Ceylan D, Erer S, Zarifoglu M, Turkes N, Ozkaya G. Evaluation of anxiety and depression scales and quality of LIFE in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. (2019) 40:725–31. doi: 10.1007/s10072-019-3719-9
39. Weber A, Heger S, Sinkgraven R, Heckmann M, Elsner P, Rzany B. Psychosocial aspects of patients with focal hyperhidrosis. Marked reduction of social phobia, anxiety and depression and increased quality of life after treatment with botulinum toxin A. Br J Dermatol. (2005) 152:342–5. doi: 10.1111/j.1365-2133.2004.06334.x
40. Banegas RA, Farache F, Rancati A, Chain M, Gallagher CJ, Chapman MA, et al. The South American Glabellar Experience Study (SAGE): a multicenter retrospective analysis of real-world treatment patterns following the introduction of incobotulinumtoxinA in Argentina. Aesthet Surg J. (2013) 33:1039–45. doi: 10.1177/1090820x13503475
41. Feng Z, Sun Q, He L, Wu Y, Xie H, Zhao G, et al. Optimal dosage of botulinum toxin type A for treatment of glabellar frown lines: efficacy and safety in a clinical trial. Dermatol Surg. (2015) 41(Suppl 1):S56–63. doi: 10.1097/dss.0000000000000265
42. Lyu Alan FY, Lulu T, Xueyan G, Jing L, Yixian H, Xuping Z, et al. Clinical study on the efficacy and safety of botulinum toxin A in the treatment of Parkinson′s disease with depression. Chin J Neurol. (2019) 52:745–51.
43. Chugh S, Chhabria A, Jung S, Kruger THC, Wollmer MA. Botulinum toxin as a treatment for depression in a real-world setting. J Psychiatr Pract. (2018) 24:15–20. doi: 10.1097/pra.0000000000000277
44. Wollmer MA, de Boer C, Kalak N, Beck J, Götz T, Schmidt T, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. (2012) 46:574–81. doi: 10.1016/j.jpsychires.2012.01.027
45. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. (2009) 8:24–6. doi: 10.1111/j.1473-2165.2009.00419.x
46. Magid M, Reichenberg JS, Poth PE, Robertson HT, LaViolette AK, Kruger TH, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. (2014) 75:837–44. doi: 10.4088/JCP.13m08845
47. Hexsel D, Brum C, Siega C, Schilling-Souza J, Dal'Forno T, Heckmann M, et al. Evaluation of self-esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxinA for glabellar lines. Dermatol Surg. (2013) 39:1088–96. doi: 10.1111/dsu.12175
48. Finzi E, Kels L, Axelowitz J, Shaver B, Eberlein C, Krueger TH, et al. Botulinum toxin therapy of bipolar depression: a case series. J Psychiatr Res. (2018) 104:55–7. doi: 10.1016/j.jpsychires.2018.06.015
49. Ramaker MJ, Dulawa SC. Identifying fast-onset antidepressants using rodent models. Mol Psychiatry. (2017) 22:656–65. doi: 10.1038/mp.2017.36
50. Schulze J, Neumann I, Magid M, Finzi E, Sinke C, Wollmer MA, et al. Botulinum toxin for the management of depression: An updated review of the evidence and meta-analysis. J Psychiatr Res. (2021) 135:332–40. doi: 10.1016/j.jpsychires.2021.01.016
51. França K, Lotti T. Botulinum toxin for the treatment of depression. Dermatol Ther. (2017) 30:e12422. doi: 10.1111/dth.12422
53. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. (1988) 54:768–77. doi: 10.1037//0022-3514.54.5.768
54. Brennan C. Botulinum toxin type-A (BoNT-A) injections of the corrugator muscles for aesthetics and depression? Plast Surg Nurs. (2016) 36:167–9. doi: 10.1097/PSN.0000000000000159
55. Baumeister JC, Papa G, Foroni F. Deeper than skin deep—The effect of botulinum toxin-A on emotion processing. Toxicon. (2016) 118:86–90. doi: 10.1016/j.toxicon.2016.04.044
56. Wollmer MA, Kalak N, Jung S, de Boer C, Magid M, Reichenberg JS, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. (2014) 5:36. doi: 10.3389/fpsyt.2014.00036
57. Wollmer MA, Magid M, Kruger THC, Finzi E. The use of botulinum toxin for treatment of depression. In: Whitcup SM, Hallett M, editors. Botulinum Toxin Therapy. Handbook of Experimental Pharmacology, Vol. 263. Cham: Springer (2019). p. 265–78. doi: 10.1007/164_2019_272
58. Milev R. Response of depression to botulinum toxin treatment: agitation as a predictor. Front Psychiatry. (2015) 6:55. doi: 10.3389/fpsyt.2015.00055
59. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. (2016) 80:93–6. doi: 10.1016/j.jpsychires.2016.06.009
60. Leuchter AF, Cook IA, Hunter AM, Cai C, Horvath S. Resting-state quantitative electroencephalography reveals increased neurophysiologic connectivity in depression. PLoS ONE. (2012) 7:e32508. doi: 10.1371/journal.pone.0032508
61. Rajmohan V, Mohandas E. Mirror neuron system. Indian J Psychiatry. (2007) 49:66–9. doi: 10.4103/0019-5545.31522
62. Robinson MJES, Iyengar S, Bymaster F, Clark M, Katon W. Depression and pain. Front Biosci. (2009) 14:5031–51. doi: 10.2741/3585
63. Kishi T, Yoshimura R, Ikuta T, Iwata N. Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Front Psychiatry. (2017) 8:308. doi: 10.3389/fpsyt.2017.00308
64. Li K, Shen S, Ji YT, Li XY, Zhang LS, Wang XD. Melatonin augments the effects of fluoxetine on depression-like behavior and hippocampal BDNF-TrkB signaling. Neurosci Bull. (2018) 34:303–11. doi: 10.1007/s12264-017-0189-z
66. Ibragić S, Matak I, Dračić A, Smajlović A, Muminović M, Proft F, et al. Effects of botulinum toxin type A facial injection on monoamines and their metabolites in sensory, limbic and motor brain regions in rats. Neurosci Lett. (2016) 617:213–7. doi: 10.1016/j.neulet.2016.02.020
67. Arnone D. Functional MRI findings, pharmacological treatment in major depression and clinical response. Prog Neuro Psychopharmacol Biol Psychiatry. (2019) 91:28–37. doi: 10.1016/j.pnpbp.2018.08.004
68. Arnone D, McIntosh AM, Ebmeier KP, Munafò MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. (2012) 22:1–16. doi: 10.1016/j.euroneuro.2011.05.003
69. Wise T, Marwood L, Perkins AM, Herane-Vives A, Williams SCR, Young AH, et al. A morphometric signature of depressive symptoms in unmedicated patients with mood disorders. Acta Psychiatr Scand. (2018) 138:73–82. doi: 10.1111/acps.12887
70. Arnone D, Job D, Selvaraj S, Abe O, Amico F, Cheng Y, et al. Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp. (2016) 37:1393–404. doi: 10.1002/hbm.23108
71. Pezzoli S, Emsell L, Yip SW, Dima D, Giannakopoulos P, Zarei M, et al. Meta-analysis of regional white matter volume in bipolar disorder with replication in an independent sample using coordinates, T-maps, and individual MRI data. Neurosci Biobehav Rev. (2018) 84:162–70. doi: 10.1016/j.neubiorev.2017.11.005
72. Ambrosi E, Arciniegas DB, Madan A, Curtis KN, Patriquin MA, Jorge RE, et al. Insula and amygdala resting-state functional connectivity differentiate bipolar from unipolar depression. Acta Psychiatr Scand. (2017) 136:129–39. doi: 10.1111/acps.12724
73. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. (2016) 2:16065. doi: 10.1038/nrdp.2016.65
74. Inserra A, Mastronardi CA, Rogers G, Licinio J, Wong ML. Neuroimmunomodulation in major depressive disorder: focus on caspase 1, inducible nitric oxide synthase, and interferon-gamma. Mol Neurobiol. (2019) 56:4288–305. doi: 10.1007/s12035-018-1359-3
75. Park J, Park HJ. Botulinum Toxin for the treatment of neuropathic pain. Toxins. (2017) 9:260. doi: 10.3390/toxins9090260
76. Kim YJ, Kim JH, Lee KJ, Choi MM, Kim YH, Rhie GE, et al. Botulinum neurotoxin type A induces TLR2-mediated inflammatory responses in macrophages. PLoS ONE. (2015) 10:e0120840. doi: 10.1371/journal.pone.0120840
77. Rojewska E, Piotrowska A, Popiolek-Barczyk K, Mika J. Botulinum toxin type A-A modulator of spinal neuron-glia interactions under neuropathic pain conditions. Toxins. (2018) 10:145. doi: 10.3390/toxins10040145
78. Piotrowska A, Popiolek-Barczyk K, Pavone F, Mika J. Comparison of the expression changes after botulinum toxin type A and minocycline administration in lipopolysaccharide-stimulated rat microglial and astroglial cultures. Front Cell Infect Microbiol. (2017) 7:141. doi: 10.3389/fcimb.2017.00141
79. Sesardic T. Bioassays for evaluation of medical products derived from bacterial toxins. Curr Opin Microbiol. (2012) 15:310–6. doi: 10.1016/j.mib.2012.05.008
80. Rosales RL, Bigalke H, Dressler D. Pharmacology of botulinum toxin: differences between type A preparations. Eur J Neurol. (2006) 13(Suppl 1):2–10. doi: 10.1111/j.1468-1331.2006.01438.x
81. Zhang K, Zhu Y, Zhu Y, Wu S, Liu H, Zhang W, et al. Molecular, functional, and structural imaging of major depressive disorder. Neurosci Bull. (2016) 32:273–85. doi: 10.1007/s12264-016-0030-0
82. Dolensek N, Gehrlach DA, Klein AS, Gogolla N. Facial expressions of emotion states and their neuronal correlates in mice. Science. (2020) 368:89–94. doi: 10.1126/science.aaz9468
83. Steward L, Brin MF, Brideau-Andersen A. Novel native and engineered botulinum neurotoxins. Handb Exp Pharmacol. (2020) 263:63–89. doi: 10.1007/164_2020_351
84. Tang M, Meng J, Wang J. New engineered-botulinum toxins inhibit the release of pain-related mediators. Int J Mol Sci. (2019) 21:262. doi: 10.3390/ijms21010262
85. Yin L, Masuyer G, Zhang S, Zhang J, Miyashita SI, Burgin D, et al. Characterization of a membrane binding loop leads to engineering botulinum neurotoxin B with improved therapeutic efficacy. PLoS Biol. (2020) 18:e3000618. doi: 10.1371/journal.pbio.3000618
86. Wang J, Casals-Diaz L, Zurawski T, Meng J, Moriarty O, Nealon J, et al. A novel therapeutic with two SNAP-25 inactivating proteases shows long-lasting anti-hyperalgesic activity in a rat model of neuropathic pain. Neuropharmacology. (2017) 118:223–32. doi: 10.1016/j.neuropharm.2017.03.026
Keywords: depression, botulinum neurotoxin, hippocampus, brain-derived neurotrophic factor, 5-HT
Citation: Li Y, Liu T and Luo W (2021) Botulinum Neurotoxin Therapy for Depression: Therapeutic Mechanisms and Future Perspective. Front. Psychiatry 12:584416. doi: 10.3389/fpsyt.2021.584416
Received: 23 October 2020; Accepted: 29 March 2021;
Published: 23 April 2021.
Edited by:
Danilo Arnone, United Arab Emirates University, United Arab EmiratesReviewed by:
Michelle Magid, The University of Texas at Austin, United StatesCopyright © 2021 Li, Liu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tong Liu, dG9uZ2xpdUBudHUuZWR1LmNu; Weifeng Luo, bHdmd3h4QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.