- 1Affiliated Wuhan Mental Health Center, Tongji Medical College of Huazhong, University of Science and Technology, Wuhan, China
- 2First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 3Key Laboratory of Psychiatry and Mental Health of Hunan Province, China National Clinical Research Center for Mental Health Disorders, Mental Health Institute of the Second Xiangya Hospital, National Technology Institute of Psychiatry, Central South University, Changsha, China
Background: Cytokine levels can be changed in methamphetamine (METH) use disorders (MUDs) and primary psychosis. The present study assessed serum levels of some kinds of interleukins (ILs) in METH-associated psychosis (MAP) and their relationships with psychotic symptoms and cognitive dysfunction.
Methods: Serum IL-2R, IL-6, IL-8, and IL-10 levels were examined by chemiluminescence assays in MAP patients (n = 119) and healthy controls (n = 108). The Positive and Negative Syndrome Scale (PANSS) and Montreal Cognitive Assessment (MOCA) were administered.
Results: Serum levels of IL-6 and IL-8 were significantly increased in MAP patients (all p < 0.05). There was a negative relationship between IL-2R levels and PANSS positive (P) subscale scores (r = −0.193, p = 0.035). IL-6, IL-8 and IL-10 levels were all negatively correlated with the naming, delayed recall and orientation subscores on the MOCA (r = −0.209, p = 0.022; r = −0.245, p = 0.007; r = −0.505, p < 0.001, respectively).
Conclusions: Our results indicate that immune disturbances are related to MAP and that IL-2R, IL-6, IL-8, and IL-10 are associated with the severity of psychotic symptoms and cognitive function impairment.
Introduction
Methamphetamine (METH), a drug in the class of amphetamine-type stimulants (ATS), has been a global health issue for the past several decades, with an estimated 29 million people using ATS in 2017 (1). From 2010 to 2018, the number of registered ATS users has increased from 0.36 million to 1.35 in China (2). METH use places a heavy burden on families, society and the health care system.
Methamphetamine-associated psychosis (MAP) resulting from methamphetamine use, such as schizophrenia, depression, and other neuropsychiatric disorders (3–5). Moreover, patients with MUD have cognitive function deficits relative to healthy control subjects, including impairments of attention, executive function, and working memory (6). Most studies on MUDs have focused on neurotoxic effects, and the role of neuroinflammation in these toxic effects has been identified as important. METH exposure induces the release of a number of proinflammatory markers, including interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α), that cause neuroplasticity in the brain (7).
MUD also results in dysregulation in the peripheral immune system, leading to an imbalanced expression of cytokines. The expression of pro- and anti-inflammatory cytokines has been implicated in METH-related neuronal injury, which may be associated with MUD (8). Some studies have found that peripheral cytokines penetrate the blood-brain barrier directly via active transport mechanisms or indirectly via vagal nerve stimulation (9) and can relay messages responsible for inducing changes in motor function and motivation.
Many studies have examined the relationships between serum cytokine levels and the degree of psychosis in patients with primary mental disorders, such as schizophrenia and mood disorders (10, 11). However, studies examining MAP and cytokine levels are rare. The aim of our study was to examine the association of the intensity of psychiatric symptoms and the exacerbation of cognitive functions with imbalance in the immune system, which is reflected by serum cytokine concentrations, in MAP patients.
Materials and Methods
Subjects
Patients were recruited from Wuhan Mental Health Center (WMHC), a Wuhan city-owned psychiatric hospital. All patients met the following inclusion criteria: age 20–64 years and Han Chinese; confirmed diagnosis of MAP based on the Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) by the consensus of two psychiatrists. A diagnosis of MAP was made if the following symptoms were present: (1) prominent hallucinations or delusions, a score ≥4 on at least one Positive and Negative Syndrome Scale (PANSS) (12) psychosis item (delusions, conceptual disorganization, hallucinatory behavior, grandiosity, or suspiciousness/persecution); (2) symptoms that met the criterion developed during or soon after substance intoxication or withdrawal or after exposure to METH, and the psychotic symptoms lasted for more than 2 weeks; (3) psychotic symptoms that were not part of a psychotic disorder (such as schizophrenia, schizophreniform disorder, or schizoaffective disorder, and if the psychotic symptom onset was prior to substance or medication use or persist longer than 1 month after substance intoxication or withdrawal, then another psychotic disorder is likely); (4) psychotic symptoms that do not only occur during delirium; and (5) use of METH at least once per week during the 3 months prior to enrollment and a positive urine screen for METH. The patients had not received any antipsychotic drugs, immunomodulators or antioxidants for at least 12 months before entry into the study.
Healthy participants were recruited in Wuhan by advertisements. None of them had taken any immunomodulators, antipsychotic drugs, or antioxidants during the 1 year before the study. None of the healthy subjects had a personal or family history of psychiatric disorder.
Subjects with primary psychosis, polydrug abuse or major medical conditions were excluded.
The study was approved by the WMHC Ethics Committee, and written informed consent was obtained from the participating patients and healthy individuals before enrollment, which is when the data were retrospectively collected.
Clinical Measurements
The severity of psychotic symptoms was estimated with the PANSS. Cognitive functions were assessed using the Montreal Cognitive Assessment (MOCA) (13). Two psychiatrists who had simultaneously attended a training session before the use of the PANSS and MOCA administered these measures.
Investigation of IL-2R, IL-6, IL-8, and IL-10 Levels in Serum
Blood samples were drawn from 7 to 9 a.m. after an overnight fast. The serum was separated by centrifugation and then stored at −70°C. Serum IL-2R, IL-6, IL-8, and IL-10 concentrations were analyzed with chemiluminescence assays. The reference ranges of IL-2R, IL-6, IL-8, and IL-10 were 223–710 U/mL, 0–5.9 pg/mL, 0–62 pg/mL, and 0–9.1 pg/mL, respectively.
Wuhan CMLabs is an independent clinical laboratory that holds a laboratory accreditation certificate from the China National Accreditation Service for Conformity Assessment (CNAS Number: MT0324), the College of American Pathologists certification (CAP Number: 8997670, AU-ID: 1734258) and biosafety laboratory certification, Hubei, China (BLS-2[2020]01-01-001).
Statistical Analysis
Differences between patients and healthy controls were evaluated using independent t-tests for continuous variables and chi-square tests for demographic and clinical variables. Given that the IL data were not normally distributed in the two groups (Shapiro-Wilk test; IL-2R: z = 0.127, p = 0.000; IL-6: z = 0.327, p = 0.000; IL-8: z = 0.268, p = 0.000; IL-10: z = 0.352, p = 0.000 for patients; IL-2R: z = 0.933, p = 0.000; IL-6: z = 0.569, p = 0.000; IL-8: z = 0.819, p = 0.000; IL-10: z = 0.877, p = 0.000 for controls), a nonparametric test (Mann-Whitney test) was used for comparisons between the two groups.
We performed all correlation analyses using Pearson's correlation coefficient. We performed a multivariate regression analysis for each serum cytokine concentration, correcting for confounding variables, including age, education level and duration and frequency of METH use.
Results
Demographic Data
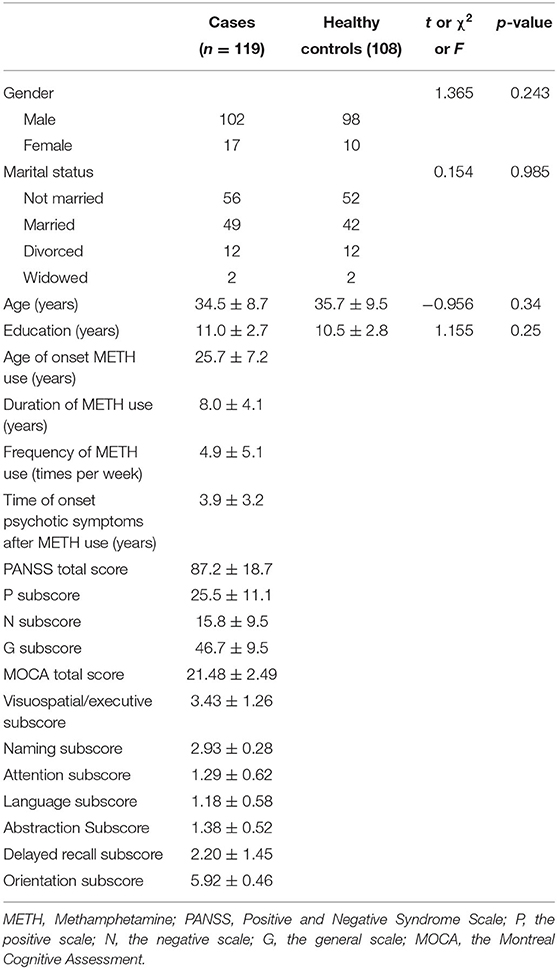
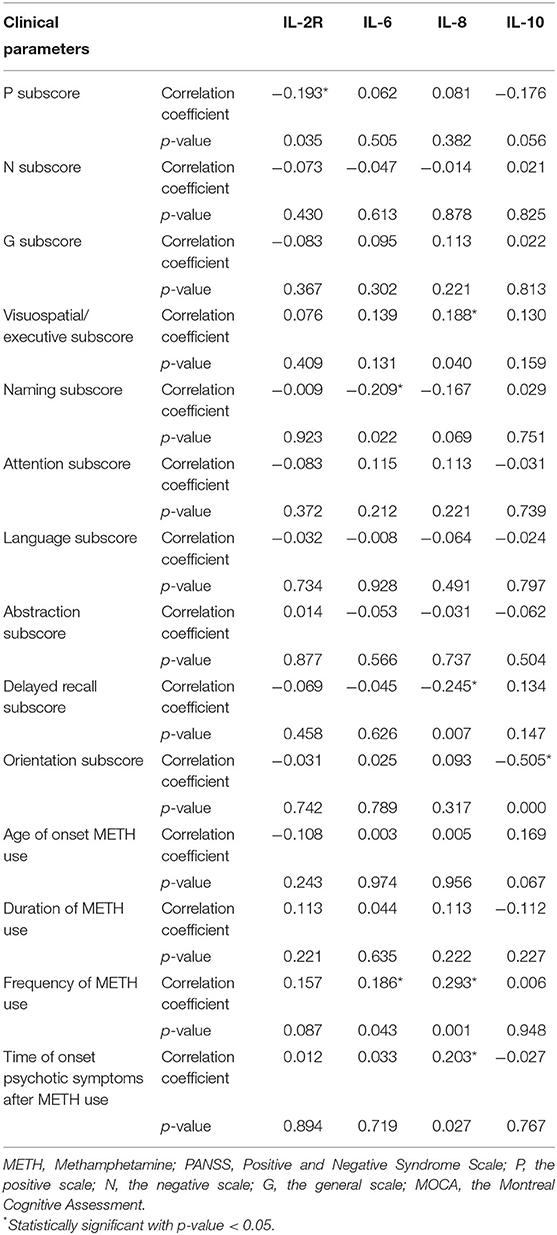
The study included 119 MAP inpatients with psychotic disorders, and the mean age of the patients was 34.5 ± 8.7 years. The mean age of the 108 controls was 35.3 ± 9.5 years. Age, gender, marital status and education did not differ significantly between the two groups; further details are shown in Table 1. There were insignificant correlations between gender, age and marital status, and serum IL-2R, IL-6, IL-8, and IL-10 levels are shown either for the whole group or for the two groups separately. However, the frequency of METH use and the time of onset psychotic symptoms after METH use were significantly correlated with IL-8 (r = 0.293, n = 119, p = 0.001, and r = 0.203, n = 119, p = 0.027).
Serum Measures
Table 2 shows the comparison of the mean serum cytokine levels in MAP patients with those in normal controls.
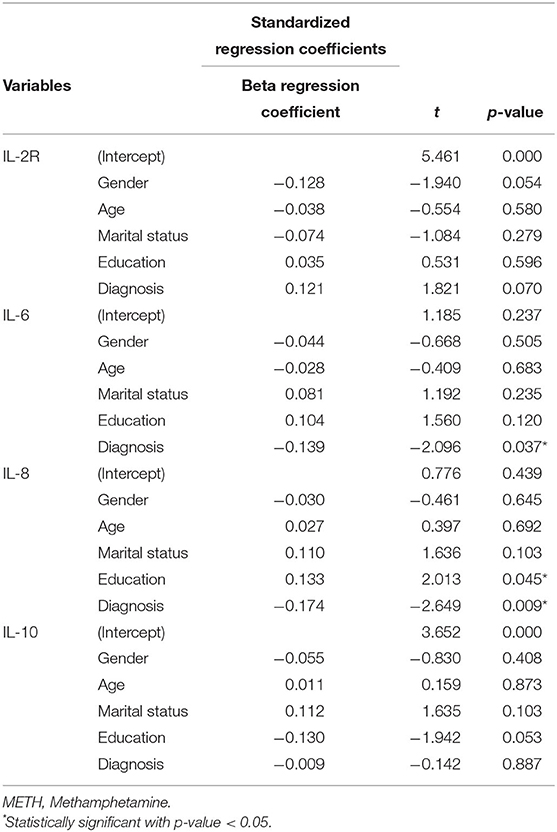
Table 2. Multivariate regression analyses of the association between serum cytokine concentration and MAP, as measured in patients compared to healthy controls.
Serum IL-2R Levels
The serum IL-2R levels were higher in the MAP patients {median [quartile range (QR)]: 338.0 (168.0)} than in the normal controls [median (QR): 380.5 (161.3); (z = −2.38; p = 0.017)]. In the multivariate regression analysis, however, this difference was not significant after adjusting for covariance in gender, age, marital status and education (p = 0.07) (Table 2).
Serum IL-6 Levels
Serum IL-6 levels were higher in the MAP patients [median (QR): 3.8 (3.6)] than in the controls [median (QR): 3.3 (4.0); (z = −2.69; p = 0.007)]. In the multivariate regression analysis, this difference was still consistent after adjusting for covariation in age, gender, marital status and education (p = 0.037) (Table 2).
Serum IL-8 Levels
Serum IL-8 levels were insignificantly different between the MAP group [median (QR): 163 (413)] and the control group [median (QR): 154.5 (164.8); (z = −0.852; p = 0.394)]. In the multivariate regression analysis, this difference was significant after gender, age, marital status and education were used as covariates (p = 0.009) (Table 2).
Serum IL-10 Levels
Serum IL-10 levels were not significantly different between the MAP group [median (QR): 3.45 (1.85)] and the control group [median (QR): 3.47 (1.74); (z = −0.211; p = 0.833)]. In the multivariate regression analysis, this difference was still consistent after adjusting for covariation in gender, age, marital status and education (p = 0.887) (Table 2).
The Relationship Among Cytokine Levels in Patients and Controls
Correlation analysis showed a significant and positive relationship between serum IL-6 and IL-8 levels in the MAP patients (r = 0.363, p < 0.001) and healthy controls (r = 0.476, p < 0.001) (Figure 1).
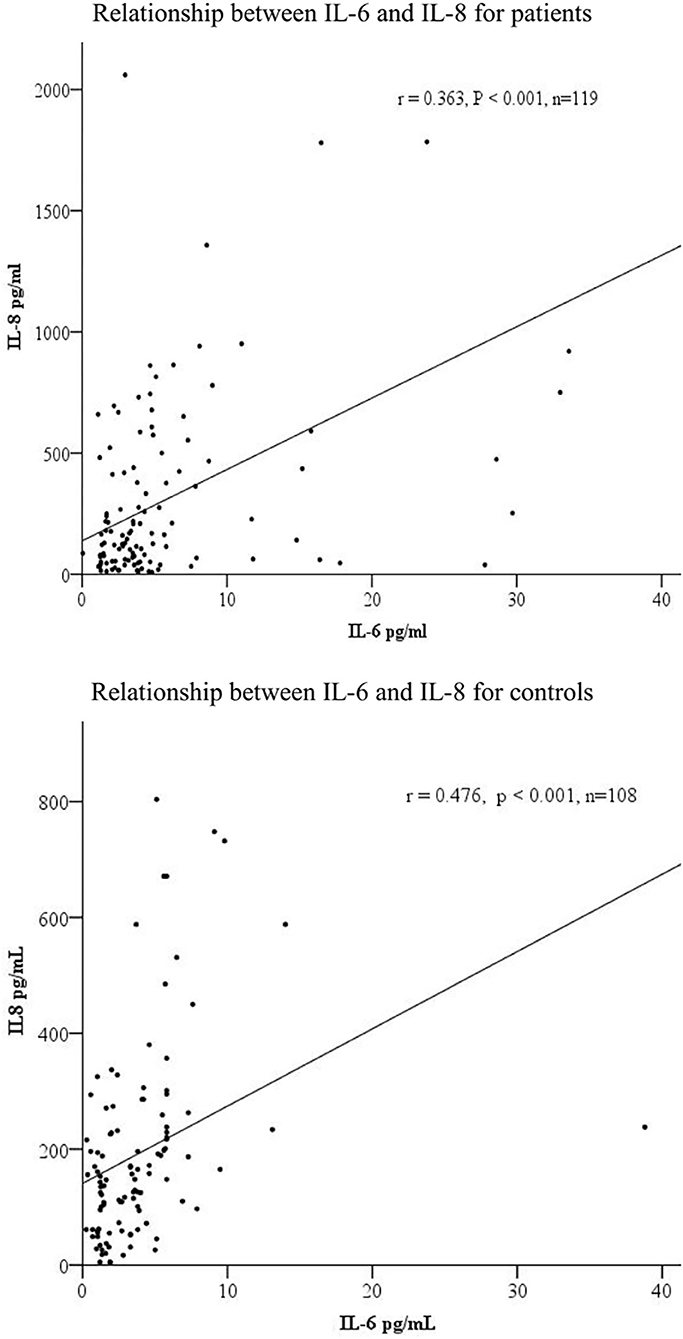
Figure 1. The correlations among serum IL-6 and IL-8 levels in patients (n = 119) and controls (n = 108).
The Relationship Between IL-2R, IL-6, IL-8, and IL-10 Levels and Clinical Symptoms
PANSS scores were found to have a statistically significant negative correlation with IL-2R levels (positive subscore: r = −0.193, p = 0.035). Correlations were found between IL-6, IL-8, and IL-10 levels and some clinical parameters, especially the severity of cognitive impairment. There was a significant negative relationship between serum IL-6 levels and MOCA scores (naming subscore: r = −0.209, p = 0.022). IL-8 levels were also found to have significant negative relationships with the delayed recall subscores on the MOCA (r = −0.245, p = 0.007), frequency of METH use (r = 0.293, p = 0.001), and time of psychotic symptom onset after METH use (r = 0.203, p = 0.027). There were significant and negative relationships between IL-10 and the orientation subscores on the MOCA (r = −0.505, p < 0.001). Further details are shown in Table 3.
Cytokine Levels and the Severity of Clinical Symptoms
To further confirm the correlations between IL levels and the severity of psychotic symptoms, patients were placed in one of two subgroups based on their mean scores on the PANSS positive (P) subscale (25.5 ± 11.1), the negative (N) subscale (15.8 ± 9.5) and the general psychopathology (G) subscale (46.8 ± 9.5). When the patients were classified on the basis of their average PANSS P subscale scores, there was a trend toward lower IL-2R levels in the patients with high P subscale scores (n = 54) than in those with low P subscale scores (n = 65) (393.9 ± 150.8 vs. 333.6 ± 132.8 U/ml, respectively; z = −2.4, p = 0.017). IL-2R levels were lower in the patients with high G subscale scores (n = 47) than in those with low G subscale scores (n = 72) (399.7 ± 148.5 vs. 335.7 ± 135.9 U/ml, respectively; z = −2.4, p = 0.017).
To corroborate the correlations between IL levels and cognitive dysfunction, patients were placed in one of two subgroups based on the mean MOCA visuospatial/executive subscores (3.4 ± 1.3), naming subscores (2.9 ± 0.3), attention subscores (1.3 ± 0.6), language subscores (1.2 ± 0.6), abstraction subscores (1.4 ± 0.5), delayed recall subscores (2.2 ± 1.5), and orientation subscores (5.9 ± 0.5). Higher IL-8 levels were observed only in the patients with low delayed recall subscores (n = 70) compared with those with high delayed recall subscores (n = 49) (462.4 ± 656.1 vs. 165.2 ± 185.7 pg/ml, respectively; z = −3.4, p = 0.001).
Discussion
This is the first study to evaluate the relationships between the severity of psychotic symptoms, cognitive abilities and serum levels of cytokines in MAP patients. The results of this study indicated that (1) serum levels of IL-6 and IL-8 were increased in MAP patients; (2) IL-2R levels had a negative correlation with the intensity of positive psychotic symptoms, but IL-6 and IL-8 levels did not; (3) cognitive dysfunction was present in the MAP patients (MOCA total scores of 26 and higher are generally considered normal); and (4) the severity of different types of cognitive impairment was positively correlated with IL-6, IL-8, and IL-10 levels. These results agree with the aforementioned findings indicating altered immune response activity in MAP patients (14–18).
The serum IL-2R levels in MAP patients were higher than normal in our study. Few studies have reported the relationship between IL-2R and MAP, but several studies have found that in vivo and in vitro exposure to METH resulted in changes in IL-2 levels. IL-2 plays a critical role in the immune response as a T-cell growth factor. Most studies have reported that serum IL-2 levels were reduced following METH exposure (18–20), which might result from decreased spleen, thymus, and peripheral T-lymphocyte cellularities (21). In METH-treated mice, there were decreases in the ratios of spleen and thymus weight to body weight, which indicated that METH induced immunosuppression and decreased IL-2 production by splenocytes (18). Moreover, IL-2 levels were lower in splenocytes from mice treated with concanavalin A (Con A) and METH than in splenocytes from mice treated with only Con A, which might be because in vitro METH exposure suppresses the induction of specific cytotoxic T-lymphocytes and alters the function of lymphocytes and NK cells (18, 19). Further study showed that exposure to METH induced mitochondrial dysfunction in the form of marked decreases in mitochondrial membrane potential, increased mitochondrial mass, enhanced protein nitrosylation and diminished protein levels of complexes I, III, and IV of the electron transport chain. These changes paralleled the reduction in IL-2 secretion and T cell proliferative responses after METH treatment (22). However, the finding of lower IL-2R levels in our present study was in conflict with the results of other studies (23, 24). This inconsistency may be due to the clinical status of the patients; for example, the participants in the Loftis study were adults in early recovery from METH dependence; in contrast, our participants were MAP inpatients, and their duration and frequency of METH use were different (23). In addition, the samples in some studies were brain tissue from mice, while our study used samples of peripheral serum in humans, which might have caused differences.
Another finding in this study was the significant negative relationship between IL-2R levels and PANSS positive subscale scores. These results suggest a possible correlation between IL-2R and the psychotic symptoms of MAP patients. There are few studies in this area, but the results of our present study agree with the findings in schizophrenia patients (25, 26). Studies have demonstrated that IL-2 increases dopamine (DA) turnover in the prefrontal cortex (27) and stimulates the release of DA in rat striatal cells (28). Higher CSF levels of IL-2 in neuroleptic-free schizophrenia patients could result in elevated DA neurotransmission and may contribute to psychotic symptoms (29). Moreover, IL-2 dose-dependently modulated the release of endogenous DA in a biphasic pattern, increasing release from striatal neurons at lower concentrations and inhibiting release at high concentrations, suggesting that the different IL-2 levels may play different roles in the pathophysiological process underlying psychotic symptoms (30).
We found that IL-6 and IL-8 levels were increased in MAP patients. Some studies have found that METH-activated microglia increase IL-6 and IL-8 expression in astrocytes (17, 31). mGluR5 receptor activity may be altered by METH, which activates the Akt/PI3K cascade. This may further activate the phosphorylation of IκB-α via the kinase activity of IKK, releasing the free form of heterodimeric NF-κB. Finally, active NF-κB translocates from the cytoplasm to the nucleus and promotes the transcription of IL-6 and IL-8 (17, 31). Another study showed that the PI3-Akt pathway and ERK1/2 mediated the induction of IL-6 and IL-8 by METH (15, 16). These findings were based on brain samples; however, METH has been shown to cause blood-brain barrier damage (32–34) and monocyte infiltration into the central nervous system (CNS) (35), and this, along with elevated levels of inflammatory markers, might indicate that peripheral cytokine levels are similar to CNS cytokine levels.
In our study, the interesting result was the consistent link between IL-6 and IL-8 levels and specific domains of cognitive performance. We found that increased IL-6 could be associated with naming impairments and that IL-8 could be associated with memory. Many studies have shown that abnormal IL-6 and IL-8 levels may be associated with cognitive dysfunction. Blood levels of IL-6 are significantly elevated in individuals with Alzheimer's disease (36, 37), and polymorphisms in the IL-6 gene that decrease plasma IL-6 levels may be associated with a lower risk of developing Alzheimer's disease (38). Increased serum concentrations of IL-6 and IL-8 have been associated with poor performance in memory, speed and motor function domains and predict increased risks of cognitive decline in an elderly general population (39). As levels of IL-8 increased, African Americans demonstrated slower reaction times on a delayed memory task (40). In addition to their roles in neurodegenerative processes, apoptosis and excitotoxicity, cytokines are capable of influencing neurotransmitter (41) and neuroendocrine responses (42) that subserve cognition and can directly modulate neuronal and glial cell function (43). These results indicate that the levels of peripheral IL-6 and IL-8 might be important biological indicators associated with the severity of cognitive dysfunction. It might be helpful to evaluate the severity of psychosis and develop a therapeutic strategy for MAP.
There were some limitations in this study. The elevated cytokine levels in MAP could easily be attributed to the psychotic condition instead of the MUD. We will design 3 groups, MAP, MUD without psychosis and healthy, in the next study to address this problem. There are few articles about serum cytokine levels in MAP, and in some articles about patients with schizophrenia, the sample size may have been insufficient, which raises the risk of spurious findings when making multiple comparisons. Moreover, the ability to extrapolate to larger human populations may be important given differences in drug use patterns and aspects of immune function across different ethnic populations.
Conclusions
This study showed that IL-6 and IL-2R levels were elevated in MAP patients. Positive psychotic symptoms were found to negatively correlate with IL-2R, while the frequency of METH use and cognitive function were correlated with IL-6 levels. Such results suggest that immune disturbance may be involved in MAP and may have a relationship with more severe psychopathology in MAP.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Wuhan Mental Health Center Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GW, QD, XY, and HZ were responsible for the study concept and design. XY and XL contributed to the acquisition of patient' clinical data. XZ and HZ performed the serum cytokine analysis. QX, HZ, and GW assisted with data analysis and interpretation of findings. XY drafted the manuscript. GW and QD provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Funding
This study was conducted under funding from National Key R&D Program of China (2017YFC1310400), Wuhan medical research program (WX19Y22 and WX19Z31).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the Wuhan Government HIV prevention program.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.594766/full#supplementary-material
References
2. Chinese National Narcotics Control Commission. Annual Report on Drug Control in China. Zurich (2018).
3. Rawson RA, Condon TP. Why do we need an addiction supplement focused on methamphetamine? Addiction. (2007) 102(Suppl.1):1–4. doi: 10.1111/j.1360-0443.2006.01781.x
4. Forray A, Sofuoglu M. Future pharmacological treatments for substance use disorders. Br J Clin Pharmacol. (2014) 77:382–400. doi: 10.1111/j.1365-2125.2012.04474.x
5. Hsieh JH, Stein DJ, Howells FM. The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci. (2014) 8:537. doi: 10.3389/fnhum.2014.00537
6. Potvin S, Pelletier J, Grot S, Hebert C, Barr AM, Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: a meta-analysis. Addict Behav. (2018) 80:154–160. doi: 10.1016/j.addbeh.2018.01.021
7. Beardsley PM, Hauser KF. Glial modulators as potential treatments of psychostimulant abuse. Adv Pharmacol. (2014) 69:1–69. doi: 10.1016/B978-0-12-420118-7.00001-9
8. Papageorgiou M, Raza A, Fraser S, Nurgali K, Apostolopoulos V. Methamphetamine and its immune-modulating effects. Maturitas. (2019) 121:13–21. doi: 10.1016/j.maturitas.2018.12.003
9. Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. (1995) 2:241–8. doi: 10.1159/000097202
10. Dahan S, Bragazzi NL, Yogev A, Bar-Gad M, Barak V, Amital V, et al. The relationship between serum cytokine levels and degree of psychosis in patients with schizophrenia. Psychiatry Res. (2018) 268:467–72. doi: 10.1016/j.psychres.2018.07.041
11. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. (2016) 21:1696–709. doi: 10.1038/mp.2016.3
12. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76.
13. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
14. Karimi-Haghighi S, Dargahi L, Haghparast A. Cannabidiol modulates the expression of neuroinflammatory factors in stress- and drug-induced reinstatement of methamphetamine in extinguished rats. Addict Biol. (2019) 25:e12740. doi: 10.1111/adb.12740
15. Shah A, Silverstein PS, Singh DP, Kumar A. Involvement of metabotropic glutamate receptor 5, AKT/PI3K signaling and NF-κB pathway in methamphetamine-mediated increase in IL-6 and IL-8 expression in astrocytes. J Neuroinflammation. (2012) 9:52. doi: 10.1186/1742-2094-9-52
16. Liu X, Silverstein PS, Singh V, Shah A, Qureshi N, Kumar A. Methamphetamine increases LPS-mediated expression of IL-8, TNF-α and IL-1β in human macrophages through common signaling pathways. PLoS ONE. (2012) 7:e33822. doi: 10.1371/journal.pone.0033822
17. Du SH, Zhang W, Yue X, Luo XQ, Tan XH, Liu C, et al. Role of CXCR1 and Interleukin-8 in methamphetamine-induced neuronal apoptosis. Front Cell Neurosci. (2018) 12:230. doi: 10.3389/fncel.2018.00230
18. In SW, Son EW, Rhee DK, Pyo S. Methamphetamine administration produces immunomodulation in mice. J Toxicol Environ Health A. (2005) 68:2133–45. doi: 10.1080/15287390500177156
19. Hadden JW. Immunopharmacology and immunotoxicology. Adv Exp Med Biol. (1991) 288:1–11. doi: 10.1007/978-1-4684-5925-8_1
20. Sriram U, Haldar B, Cenna JM, Gofman L, Potula R. Methamphetamine mediates immune dysregulation in a murine model of chronic viral infection. Front Microbiol. (2015) 6:793. doi: 10.3389/fmicb.2015.00793
21. Freire-Garabal M, Balboa JL, Nfiiiez MJ, Castaño MT, Llovo JB, Fernández-Rial JC, et al. Effects of amphetamine on T-cell immune responses in mice. Life Sci. (1991) 49:107. doi: 10.1016/0024-3205(91)90570-2
22. Potula R, Hawkins BJ, Cenna JM, Fan S, Dykstra H, Ramirez SH, et al. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J Immunol. (2010) 185:2867–76. doi: 10.4049/jimmunol.0903691
23. Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. (2011) 20:59–68. doi: 10.1007/s12640-010-9223-x
24. Wu XL, Li X, Li Y, Kong LP, Fang JL, Zhou XS, et al. The overexpression of Thioredoxin-1 suppressing inflammation induced by methamphetamine in spleen. Drug Alcohol Depend. (2016) 159:66–71. doi: 10.1016/j.drugalcdep.2015.11.021
25. Zhang XY, Zhou DF, Zhang PY, Wu GY, Cao LY, Shen YC. Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: association with psychopathology. Schizophr Res. (2002) 57:247–58. doi: 10.1016/S0920-9964(01)00296-1
26. An HM, Tan YL, Shi J, Wang ZR, Soars JC, Wu JQ, et al. Altered IL-2, IL-6 and IL-8 serum levels in schizophrenia patients with tardive dyskinesia. Schizophr Res. (2015) 162:261–8. doi: 10.1016/j.schres.2014.12.037
27. Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, Anisman H, et al. Cytokine-specific central monoamine alterations induced by interleukin-1, 2, and 6. Brain Res. (1994) 643:40–9. doi: 10.1016/0006-8993(94)90006-X
28. Lapchak PA. A role of interleukin-2 in the regulation of striatal dopamingergic function. NeuroReport. (1992) 3:165–8. doi: 10.1097/00001756-199202000-00011
29. Licinio J, Seibyl JP, Altemus M, Charney DS, Krystal JH. Elevated CSF levels of interleukin-2 in neuroleptic-free schizophrenic patients. Am J Psychiatry. (1993) 150:1408–10. doi: 10.1176/ajp.150.9.1408
30. Petitto JM, McCarthy DB, Rinker CM, Huang Z, Getty T. Modulation of behavioral and neurochemical measures of forebrain dopamine function in mice by species-specific interleukin-2. J Neuroimmunol. (1997) 73:183–90. doi: 10.1016/S0165-5728(96)00196-8
31. Wang B, Chen T, Wang J. Methamphetamine modulates the production of interleukin-6 and tumor necrosis factor-alpha via the cAMP/PKA/CREB signaling pathway in lipopolysaccharide-activated microglia. Int Immunopharmacol. (2018) 56:168–78. doi: 10.1016/j.intimp.2018.01.024
32. Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol. (2008) 3:203–17. doi: 10.1007/s11481-008-9121-7
33. Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. (2006) 1:223–36. doi: 10.1007/s11481-006-9025-3
34. Silva AP, Martins T, Baptista S, Goncalves J, Agasse F, Malva JO. Brain injury associated with widely abused amphetamines: neuroinflammation, neurogenesis and blood-brain barrier. Curr Drug Abuse Rev. (2010) 3:239–54. doi: 10.2174/1874473711003040239
35. Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse. (2006) 60:521–32. doi: 10.1002/syn.20324
36. Singh VK, Guthikonda P. Circulating cytokines in Alzheimer's disease. J Psychiatr Res. (1997) 31:657–60. doi: 10.1016/S0022-3956(97)00023-X
37. Galimberti D, Schoonenboom N, Scarpini E, Scheltens P. Chemokines in serum and cerebrospinal fluid of Alzheimer's disease patients. Ann Neurol. (2003) 53:547–8. doi: 10.1002/ana.10531
38. Papassotiropoulos A, Bagli M, Jessen F, Bayer TA, Maier W, Rao ML, et al. A genetic variation of the inflammatory cytokine interleukin-6 delays the initial onset and reduces the risk for sporadic Alzheimer's disease. Ann Neurol. (1999) 45:666–8. doi: 10.1002/1531-8249(199905)45:5<666::AID-ANA18>3.0.CO;2-3
39. Baune BT, Ponath G, Golledge J, Varga G, Arolt V, Rothermundt M, et al. Association between IL-8 cytokine and cognitive performance in an elderly general population—The MEMO-Study. Neurobiol Aging. (2008) 29:937–44. doi: 10.1016/j.neurobiolaging.2006.12.003
40. Goldstein FC, Zhao L, Steenland K, Levey AI. Inflammation and cognitive functioning in African Americans and Caucasians. Int J Geriatr Psychiatry. (2015) 30:934–41. doi: 10.1002/gps.4238
41. Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. (1999) 461:117–27. doi: 10.1007/978-0-585-37970-8_8
42. McCann SM, Kimura M, Karanth S, Yu WH, Mastronardi CA, Rettori V. The mechanism of action of cytokines to control the release of hypothalamic and pituitary hormones in infection. Ann N Y Acad Sci. (2000) 917:4–18. doi: 10.1111/j.1749-6632.2000.tb05368.x
Keywords: cognitive, methamphetamine, psychosis, serum cytokine, addiction
Citation: Yang X, Zhao H, Liu X, Xie Q, Zhou X, Deng Q and Wang G (2020) The Relationship Between Serum Cytokine Levels and the Degree of Psychosis and Cognitive Impairment in Patients With Methamphetamine-Associated Psychosis in Chinese Patients. Front. Psychiatry 11:594766. doi: 10.3389/fpsyt.2020.594766
Received: 07 September 2020; Accepted: 23 November 2020;
Published: 11 December 2020.
Edited by:
Jianhua Chen, Shanghai Jiao Tong University, ChinaReviewed by:
Eline Borger Rognli, Oslo University Hospital, NorwayPatrick Arthur Randall, Pennsylvania State University, United States
Copyright © 2020 Yang, Zhao, Liu, Xie, Zhou, Deng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qijian Deng, ZGVuZ3FpamlhbkBjc3UuZWR1LmNu; Gang Wang, d2FuZ2dhbmdzY2lAMTYzLmNvbQ== orcid.org/0000-0001-9755-3370
†These authors have contributed equally to this work
 Xue Yang1†
Xue Yang1† Xuebing Liu
Xuebing Liu Qijian Deng
Qijian Deng Gang Wang
Gang Wang