
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 25 November 2020
Sec. Mood Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fpsyt.2020.585201
 Xiuli Qiu1
Xiuli Qiu1 Jinfeng Miao1
Jinfeng Miao1 Yan Lan1
Yan Lan1 Wenzhe Sun1
Wenzhe Sun1 Yuxi Chen2
Yuxi Chen2 Ziqin Cao3
Ziqin Cao3 Guo Li1
Guo Li1 Xin Zhao1
Xin Zhao1 Zhou Zhu1*†
Zhou Zhu1*† Suiqiang Zhu1*†
Suiqiang Zhu1*†Background: Post-stroke depression (PSD) is one of the most common complications after stroke, which seriously affects patients' recovery outcome. Although vascular depression has been extensively studied, the relationship between cerebral artery stenosis and PSD has not been clarified so far.
Methods: Two hundred ninety-eight patients with ischemic stroke (72 women, 226 men) with computed tomography angiography (CTA) or magnetic resonance angiography (MRA) were included in this study. Cerebral artery stenosis ≥50% was used as the cut-off value. The DSM-V diagnostic criteria of PSD was met and the 17-item Hamilton Rating Scale for Depression (HAMD-17) score over 7 at discharge and 3 months after stroke onset was regarded as the primary outcome. The χ2-test, Mann-Whitney U-test, and t-test were used to check for statistical significance.
Results: At discharge, Barthel index (p < 0.001), left middle cerebral artery stenosis (p = 0.019), drinking history (p = 0.048), basilar artery stenosis (p = 0.037) were significantly associated with PSD. At 3 months after ischemic stroke onset, Barthel index (p = 0.011), left middle cerebral artery stenosis (p = 0.012), female gender (p = 0.001) were significantly associated with PSD.
Conclusions: The findings demonstrated that left middle cerebral artery and basilar artery stenosis are associated with PSD. It was suggested that cerebral artery stenosis was a risk factor of PSD and should be recognized and intervened early.
Registration Number: ChiCTR-ROC-17013993.
Post-stroke depression (PSD), characterized by obvious reduction in mood and physical vitality (1–3), is a common psychiatric comorbidity after stroke. In the first few months after stroke, the incidence of PSD fluctuates around 33–40% among stroke survivors (4, 5). PSD negatively impacts motor skill recuperation, aggravates cognitive deficits, and increases the risk of stroke recurrence and death. At the same time, it also exerts a negative effect on the quality of life of survivors and brings heavy burden to caregivers. Therefore, early detection of PSD high-risk groups as well as targeted preventive and treatment measures are particularly important to improve the prognosis of PSD patients.
So far, the pathophysiological mechanism of PSD is unclear (6). Evidence from neuropsychology, genetics, and epidemiology suggests that cerebrovascular changes may cause PSD (7, 8). PSD was also found to be associated with stroke lesions in the left frontal lobe (9, 10). Moreover, previous studies have shown that severe internal carotid artery stenosis can cause depression (11), and treatment with internal carotid artery stenting can improve depressive symptoms (12). Studies have also shown that the hemodynamics changes of the middle cerebral artery are related to the onset of depression in the elderly (13). In addition, animal studies have shown that depression-like behavior in rats was related to cerebral hypoperfusion (14). It has been well-established that artery stenosis can cause hemodynamic disorders and hypoperfusion. The patient with cerebral artery stenosis may have experienced corresponding cerebral hypoperfusion for a long time before the onset of ischemic stroke. However, it has not been studied whether depression after ischemic stroke was related to cerebral artery stenosis.
The main purpose of this study was to evaluate whether cerebral artery stenosis in patients with ischemic stroke measured by computed tomography angiography (CTA) or magnetic resonance angiography (MRA) was related to PSD at discharge and 3 months after the attack. Specifically, nomogram based on multiple logistic regression analysis or Cox regression model was widely used as a convenient clinical tool for risk assessment of clinical events of interest (15, 16). Therefore, the study intends to construct a convenient nomogram screening tool that integrates the predictors of clinical characteristics and location of cerebral artery stenosis to predict PSD at discharge and 3-month after ischemic stroke onset.
This was a prospective cohort study (Registration number: ChiCTR-ROC-17013993) in the department of neurology, Tongji Hospital, Huazhong University of Science and Technology. The study recruited 298 patients with consecutive acute ischemic stroke (AIS) admitted to the department of neurology of Tongji hospital from August 2018 to May 2019. The inclusion criteria were: (1) ages 18 years and over; (2) admitted to hospital within 7 days after ischemic stroke onset; (3) AIS patients confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) scans. Exclusion criteria were: (1) brain tumors, metastatic encephaloma, brain trauma, and other non-vascular causes resulted in brain disorder; (2) the history of depression, antidepressants, dementia, and other mental illness before ischemic stroke; (3) aphasia, deafness, blindness, and cognitive dysfunction (a Mini-Mental State Examination score <17 points in people with less than primary school education); (4) failure to complete follow-up; (5) transient ischemic attack (TIA) and subarachnoid hemorrhage. This study was approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology (Approved No. of ethic committee: TJ-IRB20171108). According to the Declaration of Helsinki, all subjects signed an informed consent form.
Upon admission, a standardized questionnaire was used to collect detailed information about each patient's demographic and medical history, including age, gender, education level, smoking history, drinking history, hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, history of stroke, and treatment of stenosis (thrombolysis/thrombectomy/stents implantation). In our study, drinking history and smoking history were binary variables, no-smoker, and no drinking were regard as no smoking and drinking history, others were considered to have smoking and drinking history (17, 18). The National Institutes of Health Stroke Scale (NIHSS), Barthel index (BI), Modified Rankin scale (MRS), Social Support Rating Scale (SSRS), and the 17-item Hamilton Rating Scale for Depression (HAMD-17) were evaluated by two qualified and formally trained doctors (X.S. and W.S) at discharge and 3 months after ischemic stroke onset. MRA or CTA was completed during hospitalization.
PSD was diagnosed by a psychiatrist who was blinded to the study at discharge and 3 months after the onset of ischemic stroke. HAMD-17 was used to measure the severity of depressive symptoms. The DSM-V PSD diagnostic criteria (depression caused by other medical conditions) was met and the HAMD-17 score over 7 (19–22) at discharge and 3 months after ischemic stroke onset was used as the primary outcome.
A total of 298 patients completed CTA or MRA, of which 84 patients were scanned on a GE 3.0T MR scanner (Discovery MR 750 System, GE Healthcare, Milwaukee, WI, USA) with an 8-channel head coil. 3D Time-of-flight MRA was obtained using TR/TE = 19/3.4 ms, FOV = 31.4 × 22 cm, thickness = 1.2 mm, matrix = 256 × 256, and NEX = 1, number of slices = 160. Two hundred fourteen patients underwent CTA scanning from the aortic arch to vertex with the Discovery CT750 HD scanner (HDCT, GE Healthcare, Milwaukee, WI, USA). In brief, non-enhanced CT brain scan was first performed, followed by contrast enhanced CTA. The CTA images were obtained when contrast agent (Iopromide 370, Bayer Schering Pharma AG) with a dose of 1.0 ml/kg was injected at a rate of 5 ml/s, followed by 40 ml of saline chase injected intravenously at a rate of 5 ml/s. Standard 3-dimensional CT angiography scanning parameters were used. The slice thickness was 0.63 mm, section interval was 0.3 mm, the helical pitch was 1, 26.8 cm × 25 cm DFOV (display field of view), 80 kVp and 400 mAs, rotation time of 0.5 s.
The degree of stenosis was estimated on source image or maximum intensity projection (MIP) images of CTA by the diameter stenosis. The measurement method refers to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) (23). Two professional neurologists (X.Q. and J.M) independently and quantitatively measured diameters on coronal, sagittal, or horizontal. In order to maintain consistency in the selection of vessel wall locations, the narrowest vessel was selected on both CT angiographic and source images. The cerebral arterial system evaluated included bilateral middle cerebral artery M1 segment, bilateral internal carotid artery C1 segment and basilar artery. An internationally standardized equation was used to calculate the degree of stenosis. Percent stenosis = [1 – (D stenosis/D normal)*100] (D Stenosis: the diameter of the most severe stenosis; D normal: the diameter of the proximal normal artery). Artery stenosis was defined as the diameter of arterial lumen below 50%. In patients who underwent MR angiography (MRA), the degree of stenosis was measured by MRA. Measurements were also taken by the two professional neurologists (X.Q. and J.M). The measurement, calculation and classification method were the same for CTA. Cerebral atrophy was evaluated by Ventricle-to-brain ratio (VBR) (24), VBR = [(width of anterior horns of lateral ventricle/corresponding brain width at the same level) + (biventricular width at the level of the body of caudate nucleus/corresponding brain width at the same level) + (width of occipital horns of lateral ventricle/corresponding brain width at the same level)]/3. CT, MRI, or DWI (Diffusion Weighted Image) confirmed the location of lesion and counted all lesions when the patient had multi-site cerebral infarctions.
Data analysis were performed using the Statistical Program for Social Sciences (SPSS) statistical software (version 25, Chicago, IL, USA). Intraclass correlation coefficient (ICC) was used to determine the interobserver consistency for the measurements of the degree of artery stenosis and the HAMD-17 score. Continuous data were described by the median and IQR (25th−75th percentile) or mean with standard deviation and compared using t-test or Mann–Whitney U-test (when continuous variables had skewed distributions). The dichotomous data were described by the number and percentage of pages and compared using χ2-test. Every variable was analyzed by univariate analysis to cover all potentially important predictors. Variables with P ≤ 0.10 in univariate analysis were included in multivariable logistic regression analysis and with p < 0.05 considered statistically significant.
Based on the relevant factors, a nomogram to predict PSD was established at discharge and 3 months after ischemic stroke onset. The nomogram was based on the results of multivariate logistic regression analysis and the R package “rms” in R software version 3.5.1 (http://www.r-project.org/). The nomogram was based on the ratio of converting each regression coefficient in multiple logistic regression to 0–100-point scale. The effect of the variable with the highest β coefficient (absolute value) was assigned 100 points. The predictive performance of the nomogram was measured by the concordance index (C index) equivalent to area under the curve value (AUC), and calibrated with 200 bootstrap samples. A higher C-index indicates better ability to separate PSD patients with different depression risk. The calibration curves are used to compare the predicted and observed probabilities in the study. If the model was calibrated correctly, the dots on the calibration plot should be close to a 45° diagonal line.
A total of 511 patients participated in this study, and 298 patients who met the inclusion criteria formed the study sample (Figure 1). The age of the enrolled patients was 57.42 ± 10.15 (mean ± SD), 75.84% of patients were male and 24.16% of patients were female, 40.27% patients had smoking history, 16.44% patients had drinking history, 54.36% patients had a history of hypertension, and 24.83% patients had a history of diabetes mellitus. The proportion of patients with low, medium, and high education level were 33.22, 53.69, and 13.09%. All patients were admitted 4.24 ± 2.23 days after onset of ischemic stroke, and the time of hospitalization was 12.53 ± 6.43 days. In addition, two patients were on antiepileptics for post-stroke epilepsy, 28 were on beta blockers for hypertension and the proportion of PSD patients receiving antidepressant treatment at discharge and 3 months was 28.2% (37/131 = 0.282) and 30.0% (40/133 = 0.300), respectively. In our study, the percentage of patients receiving neurorehabilitative treatment after discharge was 23.5% (70/298 = 0.235).
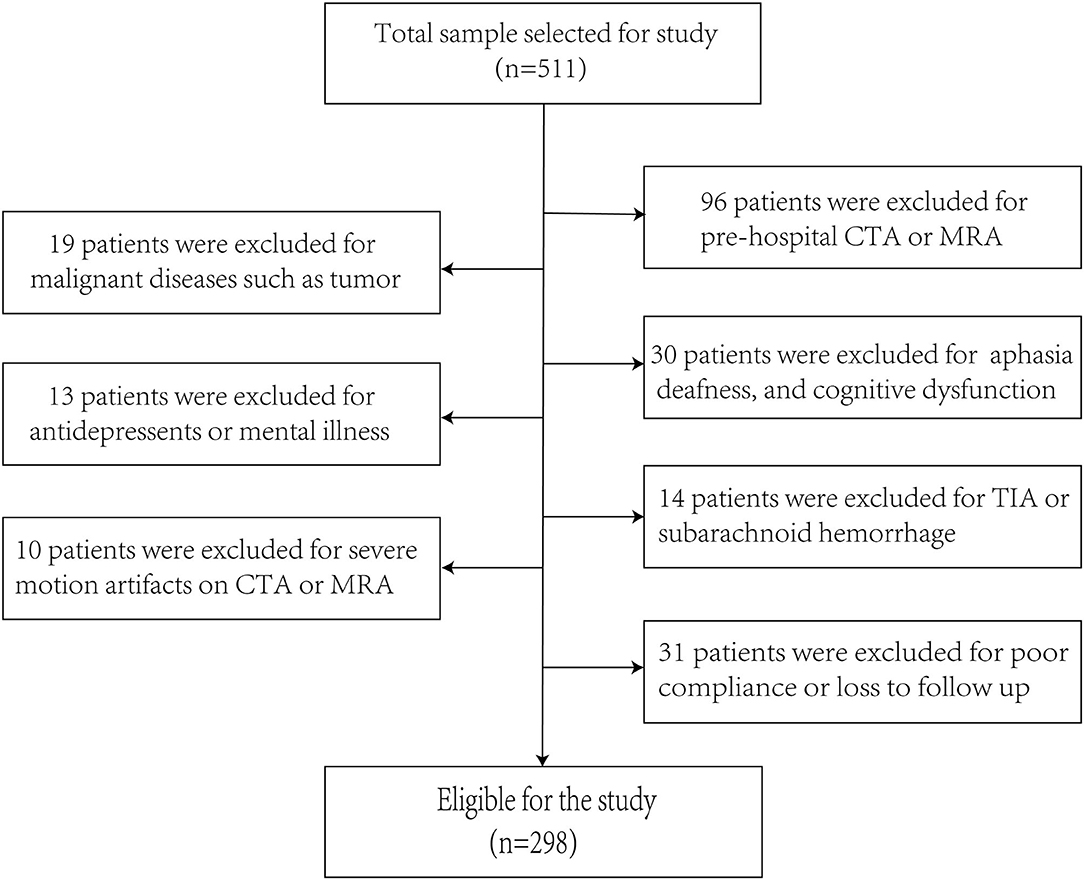
Figure 1. The enrollment flow chart of this study. A flowchart drawn according to inclusion and exclusion criteria.
Table 1 shows a comparison of baseline information between the PSD and non-PSD groups at discharge and 3 months after ischemic stroke onset. At discharge, the PSD group had a lower proportion of drinking history (p = 0.036), higher NIHSS score (p < 0.001), lower BI score (p < 0.001), higher MRS score (p < 0.001), higher ratio of left middle cerebral artery stenosis (p = 0.008), and basilar artery stenosis (p = 0.019). At 3 months after the onset of ischemic stroke, compared with non-PSD patients, the PSD patients had higher proportion of female (p < 0.001), higher NIHSS score (p = 0.001), lower BI score (p < 0.001), higher MRS score (p < 0.001), and higher proportion of left middle cerebral artery stenosis (p = 0.011). Table 2 shows the relationship between lesion location and PSD in 298 patients, among which 83 patients had multi-site cerebral infarction, and each lesion location was counted for these patients. No statistical significance was found (p > 0.05). The measurements of CTA (ICC = 0.905; 95% CI: 0.661–0.976), MRA (ICC = 0.885; 95% CI: 0.602–0.970) and HAMD-17 score (ICC = 0.917; 95% CI: 0.790–0.967) had high interobserver consistency.
Multivariable logistic regression analysis was performed to find the BI [odds ratio (OR) = 0.974, 95% confidence interval (CI) 0.965–0.983, p = 0.000], left middle cerebral artery stenosis (OR = 2.118, 95% CI: 1.130–3.968, p = 0.019), drinking history (OR = 0.489, 95% CI: 0.241–0.994, p = 0.048), basilar artery stenosis (OR = 3.773, 95% CI: 1.080–13.178, p = 0.037) was independent and significantly correlated with PSD at discharge (Table 3). BI (OR = 0.989, 95% CI: 0.980–0.997, p = 0.011), left middle cerebral artery stenosis (OR = 2.161, 95% CI: 1.181–3.956, p = 0.012), female (OR = 2.779, 95% CI: 1.561–4.948, p = 0.001) were independently and significantly related with PSD at 3 months after the onset of ischemic stroke (Table 4).
Based on the results of the multiple logistic regression analysis, all independent prognostic factors for PSD was brought into the construction of the nomograms as in Figure 2. Each variable was projected up to the value of the small ruler to obtain the score for each parameter. The points were added across independent variables to derive total point, which were converted to predicted probabilities. The nomogram C-index at discharge was 0.736 (95% CI: 0.709–0.763), and the nomogram C-index was 0.689 (95% CI: 0.661–0.717) for 3 months after ischemic stroke onset. In addition, the optimal calibration curve demonstrating the agreements between prediction and actual observation on the presence of PSD at discharge and 3 months after ischemic stroke onset.
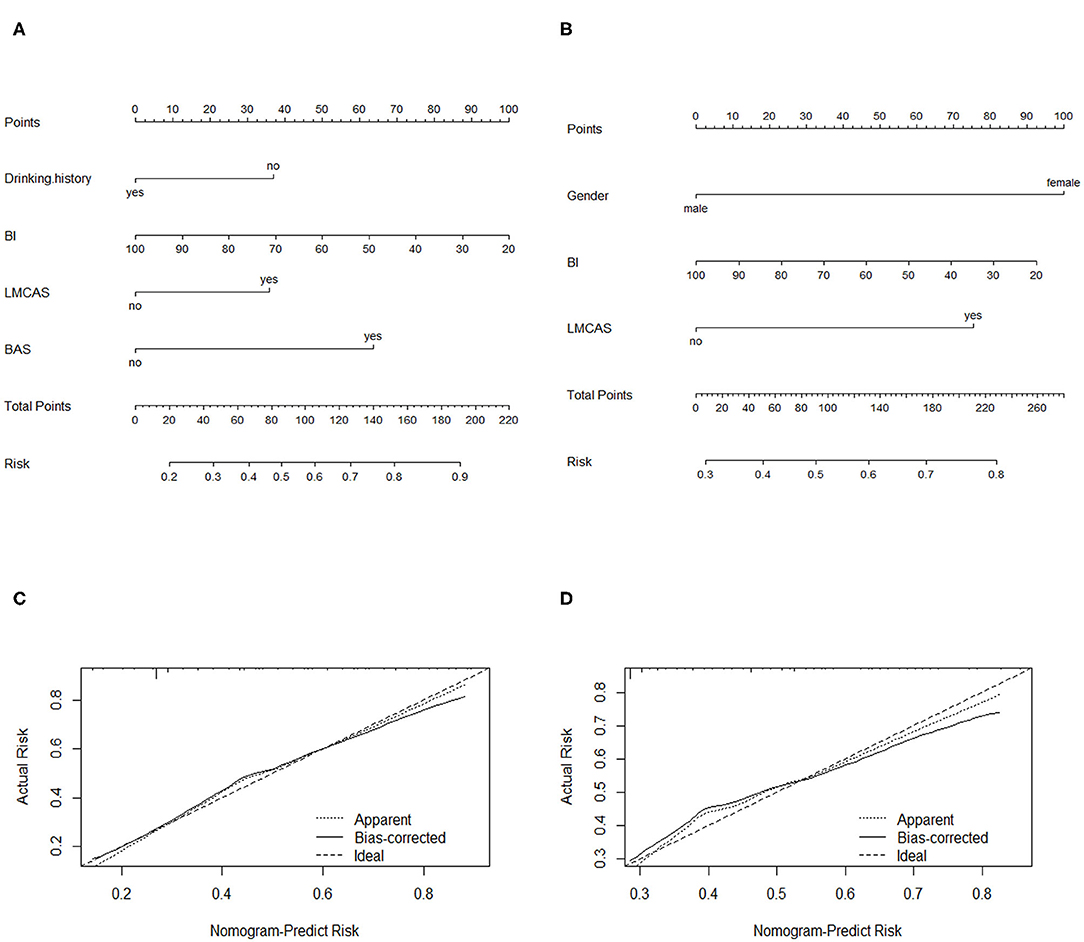
Figure 2. Nomograms of predicting PSD (A) at discharge and (C) at 3 months after ischemic stroke onset. BI, Barthel index; LMCAS, left middle cerebral artery stenosis; BAS: basilar artery stenosis Calibration plots of the nomograms for PSD prediction of the (B) at discharge and (D) at 3 months after ischemic stroke onset. X-axis represents the nomogram-predicted probability of depression; Y-axis represents the actual depression probability. A perfectly accurate nomogram prediction model would result in a plot in which the observed and predicted probability for given groups fall along 45-degree line.
This prospective cohort study showed that left middle cerebral artery and basilar artery stenosis were risk factors of PSD. As shown in Table 1, the ratio of left middle cerebral artery and basilar artery stenosis at discharge was much higher than that in non-PSD (26.7 vs. 14.4%, 8.4 vs. 2.4%), and the ratio of left middle cerebral artery stenosis 3 months after ischemic stroke onset in PSD was higher than non-PSD (26.3 vs. 14.5%). This study suggests that cerebral artery stenosis should be considered a potential etiology of PSD.
Previous studies have found that high-grade internal carotid artery stenosis was associated with depressive symptoms (25). This study showed that left middle cerebral artery stenosis was associated with PSD at discharge and 3 months after ischemic stroke onset. It is well-known that the middle cerebral artery supplies blood flow to the frontal, temporal, parietal, caudate nucleus, and the frontal cortex is responsible for emotional regulation. When middle cerebral artery stenosis reaches 50%, hemodynamic disorder and hypoperfusion of specific brain region begin to occur (26). Moreover, clinical studies found that decreased blood flow in the frontal, temporal and parietal lobes was associated with depressive symptoms in the elderly (13, 27, 28). When the patient has chronic cerebral artery stenosis but no ischemic stroke, the hypoperfusion caused by artery stenosis may be compensated by collateral circulation, while decompensation occurs when acute ischemic stroke occurs due to rapid artery occlusion. Due to the brain's higher energy requirements and lower energy reserves, when hypoperfusion occurs, the supply of glucose and oxygen became insufficient, resulting in metabolic toxicity (29, 30). Neuronal dysfunction of sympathetic nerve fibers may lead to an imbalance of serotonergic or noradrenergic neurotransmitter associated with depression.
The findings of the study indicate that the left middle cerebral artery stenosis increased the risk of PSD might associate with the dominant hemisphere of the left hemisphere. The left frontal lobe is associated with positive emotions and well-being, while the right frontal lobe is associated with negative emotion regulation (31–33). As a result, hypoperfusion in the left frontal lobe caused by left middle cerebral artery stenosis was more likely to cause depressive symptoms.
In this study, internal carotid artery stenosis was not related to PSD. However, previous studies have shown that internal carotid artery stenosis was associated with depression, with artery stenosis above 80% as the cut-off value (12). Previous studies had shown that when the internal carotid artery stenosis was >70%, the blood flow decreased significantly with the increase of stenosis degree (34). According to 2003 Radiological Society of North America (RSNA) Annual Meeting's carotid artery stenosis diagnostic criteria issued by the ultrasound division, hemodynamic disorders occur when the internal carotid artery stenosis was above 50%, and obvious hemodynamic disorders appear when internal carotid artery stenosis was above 70%. Given that the internal carotid artery was larger in diameter, no obvious hemodynamic disorder and hypoperfusion occurred when 50% was used as the cut-off value, which might be the reason why there was no association found between internal carotid artery stenosis and depression.
Interestingly, in this study, the basilar artery was associated with PSD at discharge. When basilar artery stenosis occurs, chronic ischemia occurs in the cerebellum and brainstem. Previous literatures found that the cerebellum plays an important role in emotion regulation (35–37). Jiang et al. (38) reported that patients with depression experience abnormal connections in the prefrontal-thalamic-cerebellar circuits. The “remote effects” of cerebellar or brainstem damage may be related to the occurrence of PSD, with supratentorial damage in one cerebral hemisphere leading to decreased metabolism in the contralateral cerebellar hemisphere. Therefore, damage to one side of the cerebellar hemisphere can also lead to a decline in the metabolism of the frontal cortex, and the dysfunction of the frontal cortex may also lead to depression (39). The disappearance of depression 3 months after stroke may be related to the shorter duration of low metabolism (40), and the normal metabolism of the frontal lobe can be restored by compensating of the surrounding brain tissue (41).
Previous studies have found that the location of lesion was related to PSD (42–44), such as prefrontal, temporal lobe, etc. However, in this study the association between the lesion location and the onset of depression was not found, may be caused by including patients with lesion volume varied from lacunar infarction to large cerebral infarction, and there was a correlation between lesion volume and severity of depression (45, 46). This study showed that patients with higher BI scores had lower rates of depression, which indicated that better recovery of limb function had lower rates of depression. Previous studies have found that the degree of limb function recovery was related to the occurrence of PSD (47–49). The BI scores also measures the ability of daily living after stroke. Higher BI score indicated better recovery of limb function (50).
In addition, PSD patients at discharge in this study had lower rates of drinking history than non-PSD patients (11.5 vs. 20.5%), indicating that moderate alcohol consumption was a protective factor for PSD (51–53). The mental effects of alcohol were mainly due to GABA (gamma-aminobutyric acid) receptor-mediated sedation, disinhibition, relaxation and negative thoughts suppression, the opiate-mediated reward neurotransmitter systems that lead to positive feedback of alcohol consumption (54, 55). However, the effects of alcohol disappeared 3 months after stroke, probably due to a stoppage of drinking for most patients after stroke. In this study, female had a higher risk of depression than male at 3 months after stroke. Epidemiological studies showed that the risk of depression was 2.5 times higher in female than male (56). To make matters worse, since the average age of female in this study was 57.90 ± 9.89, female patients also faced an increased risk of postmenopausal depression. According to the study of menopausal depression symptoms among Chinese women, postmenopausal (56.6 ± 4.9) depression rates were higher than premenopausal (45.3 ± 3.2) and menopausal (48.6 ± 3.4) depression (57).
This was a prospective cohort study and the first to show that stenosis of the left middle cerebral artery and basilar artery were associated with PSD. In the future treatment of cerebrovascular diseases and mood disorders, the potential impact of artery stenosis on mood should be considered. While focusing on stroke recurrence in future studies, the sample size of endovascular therapy should be further increased to observe the potential effect of endovascular therapy on mood. Normogram is widely used in surgical risk assessment, tumor prognosis and survival analysis (58–61). As far as the current prediction models are concerned, the nomogram that are easy for clinicians to use has good accuracy and discrimination power for the prediction results, which is helpful for clinical decision-making. We established two normograms with good predictive effect, which could accurately predict the PSD risk at discharge and 3 months, contributing to the early identification of high-risk PSD population and timely adoption of preventive or therapeutic measures to promote the recovery of patients after stroke.
As for advantages of this study. Firstly, being the first to show association between the stenosis of the left middle cerebral artery and basilar artery with PSD, this prospective cohort study was able to investigate the causal relationship between arterial stenosis and PSD. Also, this study included social demographics, risk factors of cerebrovascular disease and locations of ischemic strokes to rule out the influence of confounding factors.
As for some limitations that should be considered. Firstly, there may be errors in the manual measurement of artery stenosis ratio, and software measurement based on computer technology can improve the accuracy in the future. Secondly, the sample size was small, patients who did not complete CTA or MRA, aphasia, or deafness were not included in the study, which might increase selection bias. Thirdly, we did not include the lesions size and apraxia into the analysis, which are important factors affecting PSD (62). In our future research, we will combine artificial intelligence, brain atlas and voxel-based analysis to analyze the influence of the lesion location and lesions size on PSD. Lastly, other cerebral arteries were not included in the evaluation due to measurement difficulties.
An association was found between left middle cerebral artery stenosis and basilar artery stenosis, BI score, gender and drinking history and PSD. For the patients with stroke, early rehabilitation was particularly important. PSD significantly affects the rehabilitation and quality of life of the patients. Therefore, early identification of PSD and appropriate preventive measures are particularly important. This finding may be conducive to the detection of high-risk patients and early intervention to promote full recovery. Importantly, this study suggested that the potential impact of artery stenosis on mood should be considered, which may be a potential new approach for PSD treatment. In the future, studies with the larger sample size of endovascular treatment should be conducted to observe the potential effect of endovascular treatment on PSD
The datasets presented in this article are not readily available because further data mining is ongoing. Requests to access the datasets should be directed to MTU3Mzg4NjIzNTdAMTYzLmNvbQ==.
The studies involving human participants were reviewed and approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
SZ and ZZ led the study. XQ performed the data analysis and implemented the methodology. XS, WS, JM, YL, GL, and XZ collected the data. ZZ and XQ prepared the original draft. SZ reviewed and edited the final manuscript. YC touched the language of this article. All authors contributed to the article and approved the submitted version.
This work was financially supported by the National Key R&D Program of China [grant number 2017YFC1310000], the Fundamental Research Funds for the Central Universities [grant number 2018KFYXMPT015], and Hubei Technological Innovation Special Fund [grant number 2019ACA132]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. ZZ and SZ had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to acknowledge all participants of this project and investigators for collecting data. We would like to thank Xiaoyan Song for collecting the data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.585201/full#supplementary-material
1. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. (2002) 52:253–64. doi: 10.1016/S0006-3223(02)01424-5
2. Tateno A, Kimura M, Robinson RG. Phenomenological characteristics of poststroke depression: early- versus late-onset. Am J Geriatr Psychiatry. (2002) 10:575–82. doi: 10.1097/00019442-200209000-00011
3. Paradiso S, Vaidya J, Tranel D, Kosier T, Robinson RG. Nondysphoric depression following stroke. J Neuropsychiatry Clin Neurosci. (2008) 20:52–61. doi: 10.1176/jnp.2008.20.1.52
4. Hackett ML, Kohler S, O'Brien JT, Mead GE. Neuropsychiatric outcomes of stroke. Lancet Neurol. (2014) 13:525–34. doi: 10.1016/S1474-4422(14)70016-X
5. Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:14–21. doi: 10.1192/bjp.bp.111.107664
6. Huang G, Chen H, Wang Q, Hong X, Hu P, Xiao M, et al. High platelet-to-lymphocyte ratio are associated with post-stroke depression. J Affect Disord. (2019) 246:105–11. doi: 10.1016/j.jad.2018.12.012
7. Baldwin RC, Tomenson B. Depression in later life. A comparison of symptoms and risk factors in early and late onset cases. Br J Psychiatry. (1995) 167:649–52. doi: 10.1192/bjp.167.5.649
8. Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. Am J Psychiatry. (1997) 154:562–5. doi: 10.1176/ajp.154.4.562
9. Brodaty H, Sachdev PS, Withall A, Altendorf A, Valenzuela MJ, Lorentz L. Frequency and clinical, neuropsychological and neuroimaging correlates of apathy following stroke - the Sydney stroke study. Psychol Med. (2005) 35:1707–16. doi: 10.1017/S0033291705006173
10. Hama S, Yamashita H, Shigenobu M, Watanabe A, Kurisu K, Yamawaki S, et al. Post-stroke affective or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. Eur Arch Psychiatry Clin Neurosci. (2007) 257:149–52. doi: 10.1007/s00406-006-0698-7
11. Bernstein J, Friedman RA. Lithium-associated hyperthyroidism treated with lithium withdrawal: a case report. Am J Psychiatry. (2011) 168:438–9. doi: 10.1176/appi.ajp.2010.10101521
12. Mlekusch W, Mlekusch I, Minar E, Haumer M, Kopp CW, Ahmadi R, et al. Is there improvement of “vascular depression” after carotid artery stent placement? Radiology. (2006) 240:508–14. doi: 10.1148/radiol.2402051043
13. Tiemeier H, Bakker SL, Hofman A, Koudstaal PJ, Breteler MM. Cerebral haemodynamics and depression in the elderly. J Neurol Neurosurg Psychiatry. (2002) 73:34–9. doi: 10.1136/jnnp.73.1.34
14. Lee SR, Choi B, Paul S, Seo JH, Back DB, Han JS, et al. Depressive-like behaviors in a rat model of chronic cerebral hypoperfusion. Transl Stroke Res. (2015) 6:207–14. doi: 10.1007/s12975-014-0385-3
15. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/S1470-2045(14)71116-7
16. Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. (2015) 33:8619. doi: 10.1200/JCO.2014.56.6661
17. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. (2017) 357:j2099. doi: 10.1136/bmj.j2099
18. Kim JW, Byun MS, Yi D, Lee JH, Ko K, Jeon SY, et al. Association of moderate alcohol intake with in vivo amyloid-beta deposition in human brain: a cross-sectional study. PLoS Med. (2020) 17:e1003022. doi: 10.1371/journal.pmed.1003022
19. Tu WJ, Qiu HC, Liu Q, Li XM, Zhao JZ, Zeng XW. Decreased level of irisin, a skeletal muscle cell-derived myokine, is associated with post-stroke depression in the ischemic stroke population. J Neuroinflamm. (2018) 15:133. doi: 10.1186/s12974-018-1177-6
20. Liang JF, Yue YY, Jiang HT, Geng DQ, Wang J, Lu JX, et al. Genetic variations in the p11/tPA/BDNF pathway are associated with post stroke depression. J Affect Disord. (2018) 226:313–25. doi: 10.1016/j.jad.2017.09.055
21. Zhao JY, Ren WW, Lv DZ, Zhu ZY, Wang QZ, He JC. Low triiodothyronine syndrome is a predictor of post-stroke depression. Int J Geriatr Psych. (2017) 32:352–3. doi: 10.1002/gps.4639
22. Wang GL, Zhou YT, Bu XQ, Peng H, Xu T, Wang AL, et al. Antiphospholipid antibodies predict post-stroke depression after acute ischemic stroke. J Affect Disord. (2019) 257:160–5. doi: 10.1016/j.jad.2019.07.013
23. North American Symptomatic Carotid Endarterectomy Trial C, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. (1991) 325:445–53. doi: 10.1056/NEJM199108153250701
24. He JR, Zhang Y, Lu WJ, Liang HB, Tu XQ, Ma FY, et al. Age-related frontal periventricular white matter hyperintensities and mir-92a-3P are associated with early-onset post-stroke depression. Front Aging Neurosci. (2017) 9:328. doi: 10.3389/fnagi.2017.00328
25. Rao R, Jackson S, Howard R. Depression in older people with mild stroke, carotid stenosis and peripheral vascular disease: a comparison with healthy controls. Int J Geriatr Psych. (2001) 16:175–83. doi: 10.1002/1099-1166(200102)16:2<175::AID-GPS298>3.0.CO;2-0
26. Naritomi H, Sawada T, Kuriyama Y, Kinugawa H, Kaneko T, Takamiya M. Effect of chronic middle cerebral artery stenosis on the local cerebral hemodynamics. Stroke. (1985) 16:214–9. doi: 10.1161/01.STR.16.2.214
27. Lemke H, de Castro AG, Schlattmann P, Heuser I, Neu P. Cerebrovascular reactivity over time-course - from major depressive episode to remission. J Psychiatr Res. (2010) 44:132–6. doi: 10.1016/j.jpsychires.2009.06.010
28. Toma S, MacIntosh BJ, Swardfager W, Goldstein BI. Cerebral blood flow in bipolar disorder: a systematic review. J Affect Disord. (2018) 241:505–13. doi: 10.1016/j.jad.2018.08.040
29. Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pelligrino D. Cerebral blood flow response to functional activation. J Cerebr Blood F Met. (2010) 30:2–14. doi: 10.1038/jcbfm.2009.188
30. de la Torre JC, Olmo AD, Valles S. Can mild cognitive impairment be stabilized by showering brain mitochondria with laser photons? Neuropharmacology. (2019) 171:107841. doi: 10.1016/j.neuropharm.2019.107841
31. Urry HL, Nitschke JB, Dolski I, Jackson DC, Dalton KM, Mueller CJ, et al. Making a life worth living: neural correlates of well-being. Psychol Sci. (2004) 15:367–72. doi: 10.1111/j.0956-7976.2004.00686.x
32. Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. (2007) 27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007
33. Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. (2000) 126:890–909. doi: 10.1037/0033-2909.126.6.890
34. Shakur SF, Hrbac T, Alaraj A, Du X, Aletich VA, Charbel FT, et al. Effects of extracranial carotid stenosis on intracranial blood flow. Stroke. (2014) 45:3427–9. doi: 10.1161/STROKEAHA.114.006622
35. Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. (2005) 4:290–4. doi: 10.1080/14734220500348584
37. Sacchetti B, Scelfo B, Strata P. Cerebellum and emotional behavior. Neuroscience. (2009) 162:756–62. doi: 10.1016/j.neuroscience.2009.01.064
38. Jiang YC, Duan MJ, Chen X, Zhang XX, Gong JN, Dong DB, et al. Aberrant prefrontal-thalamic-cerebellar circuit in schizophrenia and depression: evidence from a possible causal connectivity. Int J Neural Syst. (2019) 29:1850032. doi: 10.1142/S0129065718500326
39. Srinivasan A, Miller W, Stys P, Goyal M. Crossed cerebellar diaschisis in stroke. Neurology. (2004) 62:2130. doi: 10.1212/01.WNL.0000123088.81455.F2
40. Pappata S, Tran Dinh S, Baron JC, Cambon H, Syrota A. Remote metabolic effects of cerebrovascular lesions: magnetic resonance and positron tomography imaging. Neuroradiology. (1987) 29:1–6. doi: 10.1007/BF00341027
41. Liebeskind DS. Collateral circulation. Stroke. (2003) 34:2279–84. doi: 10.1161/01.STR.0000086465.41263.06
42. Metoki N, Sugawara N, Hagii J, Saito S, Shiroto H, Tomita T, et al. Relationship between the lesion location of acute ischemic stroke and early depressive symptoms in Japanese patients. Ann Gen Psychiatry. (2016) 15:12. doi: 10.1186/s12991-016-0099-x
43. Wichowicz H, Gasecki D, Landowski J, Lass P, Swierkocka M, Wiśniewski G, et al. Clinical utility of chosen factors in predicting post-stroke depression: a one year follow-up. Psychiatr Pol. (2015) 49:683–96. doi: 10.12740/PP/38439
44. Zhang T, Jing X, Zhao X, Wang C, Liu Z, Zhou Y, et al. A prospective cohort study of lesion location and its relation to post-stroke depression among Chinese patients. J Affect Disord. (2012) 136:e83–e7. doi: 10.1016/j.jad.2011.06.014
45. Sharpe M, Hawton K, Seagroatt V, Bamford J, House A, Molyneux A, et al. Depressive disorders in long-term survivors of stroke. Associations with demographic and social factors, functional status, and brain lesion volume. Br J Psychiatry. (1994) 164:380–6. doi: 10.1192/bjp.164.3.380
46. Terroni L, Amaro E, Iosifescu DV, Tinone G, Sato JR, Leite CC, et al. Stroke lesion in cortical neural circuits and post-stroke incidence of major depressive episode: a 4-month prospective study. World J Biol Psychiatry. (2011) 12:539–48. doi: 10.3109/15622975.2011.562242
47. van de Weg FB, Kuik DJ, Lankhorst GJ. Post-stroke depression and functional outcome: a cohort study investigating the influence of depression on functional recovery from stroke. Clin Rehabil. (1999) 13:268–72. doi: 10.1191/026921599672495022
48. Pohjasvaara T, Vataja R, Leppavuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol. (2001) 8:315–9. doi: 10.1046/j.1468-1331.2001.00182.x
49. Zhang N, Wang CX, Wang AX, Bai Y, Zhou Y, Wang YL, et al. Time course of depression and one-year prognosis of patients with stroke in mainland China. CNS Neurosci Ther. (2012) 18:475–81. doi: 10.1111/j.1755-5949.2012.00312.x
50. Brown C, Hasson H, Thyselius V, Almborg AH. Post-stroke depression and functional independence: a conundrum. Acta Neurol Scand. (2012) 126:45–51. doi: 10.1111/j.1600-0404.2011.01595.x
51. Gea A, Martinez-Gonzalez MA, Toledo E, Sanchez-Villegas A, Bes-Rastrollo M, Nunez-Cordoba JM, et al. A longitudinal assessment of alcohol intake and incident depression: the SUN project. BMC Public Health. (2012) 12:954. doi: 10.1186/1471-2458-12-954
52. Gea A, Beunza JJ, Estruch R, Sanchez-Villegas A, Salas-Salvado J, Buil-Cosiales P, et al. Alcohol intake, wine consumption and the development of depression: the predimed study. BMC Med. (2013) 11:192. doi: 10.1186/1741-7015-11-192
53. Paulson D, Shah M, Herring D, Scott R, Herrera M, Brush D, et al. The relationship between moderate alcohol consumption, depressive symptomatology, and C-reactive protein: the health and retirement study. Int J Geriatr Psych. (2018) 33:316–24. doi: 10.1002/gps.4746
54. Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. (2012) 62:42–53. doi: 10.1016/j.neuropharm.2011.08.040
55. Bellos S, Skapinakis P, Rai D, Zitko P, Araya R, Lewis G, et al. Longitudinal association between different levels of alcohol consumption and a new onset of depression and generalized anxiety disorder: results from an international study in primary care. Psychiat Res. (2016) 243:30–4. doi: 10.1016/j.psychres.2016.05.049
56. Weissman MM, Leaf PJ, Holzer CE 3rd, Myers JK, Tischler GL. The epidemiology of depression. An update on sex differences in rates. J Affect Disord. (1984) 7:179–88. doi: 10.1016/0165-0327(84)90039-9
57. Tang R, Luo M, Li J, Peng Y, Wang Y, Liu B, et al. Symptoms of anxiety and depression among Chinese women transitioning through menopause: findings from a prospective community-based cohort study. Fertil Steril. (2019) 112:1160–71. doi: 10.1016/j.fertnstert.2019.08.005
58. Ma XH, Wei JW, Gu DS, Zhu YJ, Feng B, Liang M, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. (2019) 29:3595–605. doi: 10.1007/s00330-018-5985-y
59. Lei ZQ, Li J, Wu D, Xia Y, Wang Q, Si AF, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis b virus-related hepatocellular carcinoma within the milan criteria. JAMA Surg. (2016) 151:356–63. doi: 10.1001/jamasurg.2015.4257
60. He CB, Zhang Y, Cai ZY, Duan FT, Lin XJ, Li SP. Nomogram to predict cancer-specific survival in patients with pancreatic acinar cell carcinoma: a competing risk analysis. J Cancer. (2018) 9:4117–27. doi: 10.7150/jca.26936
61. Pan F, Cui SH, Wang WM, Gu AQ, Jiang LY. Survival analysis for lung adenosquamous carcinoma patients with brain metastasis. J Cancer. (2018) 9:3707–12. doi: 10.7150/jca.27441
Keywords: post-stroke depression, cerebral artery diseases, stroke, nomogram, prediction
Citation: Qiu X, Miao J, Lan Y, Sun W, Chen Y, Cao Z, Li G, Zhao X, Zhu Z and Zhu S (2020) Association of Cerebral Artery Stenosis With Post-stroke Depression at Discharge and 3 Months After Ischemic Stroke Onset. Front. Psychiatry 11:585201. doi: 10.3389/fpsyt.2020.585201
Received: 20 July 2020; Accepted: 28 October 2020;
Published: 25 November 2020.
Edited by:
Agorastos Agorastos, Aristotle University of Thessaloniki, GreeceCopyright © 2020 Qiu, Miao, Lan, Sun, Chen, Cao, Li, Zhao, Zhu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhou Zhu, emhvdXpodUBodXN0LmVkdS5jbg==; Suiqiang Zhu, emh1c3VpcWlhbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.