
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 02 November 2020
Sec. Psychological Therapy and Psychosomatics
Volume 11 - 2020 | https://doi.org/10.3389/fpsyt.2020.571636
 Eva M. J. Peters1,2*
Eva M. J. Peters1,2* Melanie Neusetzer1
Melanie Neusetzer1 Secil Akinci3
Secil Akinci3 Aysenur Murat1
Aysenur Murat1 Sabine Treuherz3
Sabine Treuherz3 Matthias Rose2
Matthias Rose2 Frank Leweke3
Frank Leweke3 Falk Leichsenring3
Falk Leichsenring3 Melanie L. Conrad2,4†
Melanie L. Conrad2,4† Johannes Kruse3†
Johannes Kruse3†Objective: In experimental settings, systemically elevated inflammation markers interfere with major depression treatment. In German healthcare, compulsory national health insurance covers treatment of a wide variety of depressive disorders, if it follows evidence-based medicine guidelines combining recommended therapies. To date, little is known about the relevance of immune system cytokine production with regard to real-world clinical care for patients with moderate depression.
Methods: Seventy three patients with moderate depression subjected to multimodal psychotherapeutic inpatient therapy (mPT) following a psychodynamic concept at a German university hospital were included. As a primary outcome, mPT success, evidenced by delta HADS “depression,” was analyzed according to tumor necrosis factor alpha (TNFα) production by peripheral blood mononuclear cells (PBMC) after phytohemagglutinin (PHA) challenge at baseline. Secondary outcomes addressed the inflammatory response and mental health comparing high and low TNFα-producers.
Results: First, higher PBMC TNFα production at baseline predicted a better mPT-outcome (R2 0.162, p = 0.014). Second, patients with high TNFα (hTNF) at baseline produced significantly more acute inflammatory cytokines [interleukin (IL)1β, IL6), TH1/TH2 cytokines [interferon gamma (IFNγ), IL4] as well as eotaxin and IL2 compared to low TNFα producers (lTNF) (Cohen's ds between −0.532 and −1.013). Demographic data, diagnosis subtype-distribution, medication, systemic inflammation markers [C-reactive protein (CRP), high mobility group box 1 (HMGB1), leptin], anxiety and depression (HADS) did not differ. From baseline to mPT-discharge, HADS “depression” decreased in both hTNF (11.31 to 5.47, p = 0.001, d = 1.184) and lTNF patients (11.50–7.92, p = 0.001, d = −0.765), while PBMC cytokine production decreased significantly in hTNF (Cohen's ds between −0.304 and −0.345) with a significant group by time interaction for TH1/TH2 ratio. At the end of therapy, comparison of TNF groups revealed significantly lower depression-scores in hTNF compared to lTNF patients (5.47 compared to 7.92, p = 0.035, d = 0.504).
Conclusions: Our study demonstrates successful treatment of depression in a clinical care setting using multimodal psychotherapy based on a psychodynamic concept following guideline recommendation. The greatest improvement in patient depression was linked to the highest production of TNFα by PBMCs at baseline. Our study contributes to the definition of patient subpopulations with differing cytokine responses that are related to succesful treatment of depression.
The interaction between depression and inflammation is an intensely debated topic (1–8). Evidence is accumulating that stress-exaggerated inflammation can contribute to the development of depression and at the same time promote infections and non-communicable diseases such as auto-immune, metabolic or cardio-vascular disease (9–14). Such maladaptive inflammatory activity is therefore held partly responsible for the frequent co-morbidities that accompany depressive disorders and with the increased medical care required for respective patients. Since the growing number of co-morbid patients is a costly burden for health care systems worldwide, there is an urgent need to further clarify if inflammation interferes with treatment and if successful treatment of depression improves maladaptive inflammatory responses.
Present concepts of the role of inflammation in depression are based on randomized controlled trials employing mostly patients suffering from major depressive disorder (15). In these studies, depression is associated at it's peak with high levels of general inflammation markers such as C-reactive protein (CRP) as well as high levels of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interleukin 6 (IL6). In a number of pharmacologic and behavioral studies, the successful treatment of depression resulted in simultaneous normalization of depression and cytokine levels (16–22). However, there is considerable variation in reports linking depression and inflammation (22) and little is known about the effects in patients with moderate depression scores or the response to other treatment concepts. Due to the observation that patients with major depression and high levels of inflammation markers appear to be more resistant to pharmacologic or behavioral treatment (11, 20, 22–26), this topic warrants further investigation of the role of inflammation in different therapeutic approaches and patient subpopulations.
Some of the variation observed between studies may be accounted for by the types of inflammatory molecules measured and the sampling material employed. For instance, many studies employed CRP measurement in serum or plasma. Increased CRP levels in depression is a rather late event and marks a persistent pro-inflammatory state. Prior to the development of a pro-inflammatory state the examination of peripheral mononuclear cell (PBMC) cytokine production is an excellent method to measure pro-inflammatory immune responses. These cells can be extracted from patient blood samples and challenged ex vivo by immune activators such as phytohaemagglutinin (PHA) to study, if the immune response to challenge is hyper-reactive. Among the cytokines released by PBMCs, several studies have revealed the importance of TNFα as a possible early biomarker for immune system hyper-reactivity in depressed patients. TNFα was shown in a meta-analysis to interfere with depression treatment success more frequently than other cytokines (20). TNFα was also shown to participate both in innate and adaptive immune responses that are disrupted in depressed patients with non-communicable disease. In addition, this cytokine can interact with the hypothalamus pituitary adrenal stress axis to modulate cortisol release with consequences for the immune response (27). Finally, biologics that neutralize TNFα activity were among the first anti-inflammatory medications established, and are now respected for their effectiveness, relative safety and anti-depressant effect (21, 28). TNFα may therefore be a useful lead marker for the study of depression treatment effects on immune system excitability.
Randomized controlled trials are best praxis to study treatment success (29). In mental health research, they commonly follow a single therapeutic concept and are tailored to treat a specific mental condition in a selected outpatient population. This approach is highly efficient to prove the efficacy of the therapeutic concept in question for a defined aspect of mental health. In reality, however, patient's health issues are often multi-dimensional. In routine clinical care settings this issue is met by the combination of therapeutic approaches recommended by evidence-based medicine guidelines. Within the framework of the compulsory national health insurance of the German healthcare system for example, psychotherapy is covered by insurance companies if the hospital adheres to consensus recommendations as well as a defined treatment schedule (30–34). The resulting multimodal psychotherapeutic inpatient therapy (mPT) can be based on the behavioral orientation as well as the psychodynamic orientation. It is mandatory that it comprises at least three individual and group psychotherapy sessions per week in combination with at least one session of physiotherapy, art therapy and music therapy. In addition, it is mandatory that patients receive 24 h medical care, interact with specialized nurses at least three times a day, and interact with a multi-professional supervised team of doctors and psychotherapists at least once a day. In psychosomatic medicine, mPT is often given to patients with moderate depression for which sufficient ambulatory care is not available, or depression that can be expected to improve in a psychotherapeutic setting after removal from a patients home environment. There is, however, a distinct lack of studies that address the effects of mPT and it's interaction with inflammation (14, 16, 35).
Taken together, the interaction between moderate depression and inflammation in a routine clinical care setting focussing on the psychodynamic concept has yet to be studied (14, 16, 35–37). In the present naturalistic study, PBMC cytokine production after PHA challenge was therefore analyzed in 73 patients with the primary diagnosis ICD10 F32.1/2 or F33.1/2 that received mPT at a university hospital with psychodynamic orientation. Prediction of mPT outcome by baseline PBMC cytokine production was studied in order to learn if a potential hyper-reactivity of PBMC to PHA challenge can interfere with the treatment. In addition, patients were categorized at baseline into high TNFα-producers (hTNF) or low TNFα-producers (lTNF) to illustrate treatment effects, and three analyses were performed using this design: baseline differences between hTNF and lTNF groups, changes over time in response to therapy within each TNFα group, and differences in mPT outcome between hTNF and lTNF patients.
A naturalistic study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the ethics committee of the Justus-Liebig University, Gießen, Germany. Recruitment and assessment of study volunteers followed standardized procedures. Briefly, all patients successively admitted to the department of psychosomatic medicine at the Justus-Liebig-University in Gießen, Germany for mPT between December 2011 and February 2014 were asked permission to take a blood sample and to self-report stress and mental health. All enrolled patients provided written informed consent. We analyzed patients with depression according to ICD-10 diagnostic criteria, defined by F32.1/2 depressive episode and F33.1/2 recurrent depressive disorder. None of the patients had additional diagnosis of anxiety disorders or post traumatic stress disorder. Patients in inpatient treatment were allowed to continue with their pre-admission medication and tea, coffee or nicotine consumption. Patients with alcohol and drug issues were only assigned to psychosomatic inpatient treatment if they had stopped consumption for a considerable time prior to hospital admission. Current substance dependency was a criterion for exclusion. Of the 250 patients treated during that time, patients with other diagnosis (N = 112), mPT <3 weeks or incomplete questionnaires (N = 49), and patients with CRP higher than 14.93 mg/dl (indication of acute infection, mean+3× sd all depressives T1, N = 6) were excluded from the analysis. A remaining total of 73 patients were included in the analysis.
The Department of Psychosomatics and Psychotherapy at the Justus Liebig University in Giessen is a German university hospital that has an inpatient unit, a day care center, and an outpatient clinic (including special outpatient clinics and a consultation and liaison service) treating approximately 400, 300, and 1,400 patients per year, respectively. The clinic follows a psychodynamic concept, complemented by targeted elements of behavioral therapy following a fixed weekly schedule. The therapeutic approach connects psychodynamic psychotherapy-based individual and group therapy, psychoeducation, music therapy, art therapy, movement therapy including elements of qigong, learning theory-based behavioral training for active stress management, autogenic training, progressive muscle relaxation according to Jacobson and functional relaxation, social work, and medical treatment according to consensus guideline recommendations. The clinical and scientific activities of the clinic focus on the therapy of people with various psychosomatic disorders, functional physical complaints, pain, primarily physical illnesses with accompanying mental health problems (e.g., cancer, diabetes, skin diseases), life crises, depression, anxiety disorders, eating disorders and post-traumatic stress disorder. Patients' admission for psychotherapeutic inpatient treatment does not depend on the severity of their disease but is initiated when it is believed that sufficient disease control can only be achieved in the inpatient setting (32). Besides the symptoms, the conditions that lead to symptom development, co-morbidities, treatment goals, motivation for treatment, structure of patient personality, professional, social and family situation, and the patient's treatment wishes are taken into account. In German psychosomatic medicine, these criteria often apply to patients with moderate depression and high psycho-social stress while patients with severe depression and suicidal ideation are mostly treated in psychiatric clinics.
Patients were asked to refrain from coffee, tea, nicotine or physical activity on the morning of blood collection. Blood samples were obtained between 8 and 9 o'clock in the morning on the first and last day of the hospital stay and immediately processed. Serum was obtained and stored at −80°C for future analysis. PBMC were harvested by Ficoll separation, which allows for the isolation of all nucleated cells in the blood sample. 1.25 × 106 PBMC per patient and timepoint were stimulated with phytohaemagglutinin (PHA, Sigma-Aldrich, St. Louis, MO, USA) in Aim V medium at 37°C/5% CO2. After 24 h of stimulation, supernatants were collected and stored at −80°C for future analysis. PHA was chosen because it is a lectin from phaeseolus vulgaris, which is widely used in immunological studies to promote proliferation and provoke cytokine release from PBMC for example in depressive patients (38, 39). PHA was also chosen, because it acts on a wide variety of cells of the immune system. Since distribution of PBMC subpopulations can differ greatly between individuals but all cell populations respond to a non-specific challenge, all PBMC can be expected to contribute to a potential hyper-inflammatory response. For this reason, measurement of overall cytokine production by challenged PBMC illustrates the overall reactivity of a patient's immune cells in the circulation to such a challenge.
Cytokines in cell culture supernatant of all patients in the study were measured using a bio-plex pro human cytokine 11-plex assay (IL1β, IL2, IL4, IL5, IL6, IL10, IL12p70, IL17A, eotaxin, interferon gamma [IFNγ] and TNFα (Biorad Laboratories, Munich, Germany) optimized according to routine standard procedures by testing duplicates. To ensure optimal sample comparison, each 96 well plate for cytokine analysis contained samples from both patient groups and care was taken to include all time points from an individual on the same 96 well plate. The lower limit of detection (in pg/ml) and inter-assay coefficient of variation were as follows: IL1β: 0.6/8, IL2: 1.6/9, IL4: 0.7/8, IL5: 0.6/10, IL6: 2.6/11, IL10: 0.7/6, IL12p70: 3.5/6, IL17: 3.3/6, Eotaxin: 2.5/11, IFNγ: 6.4/9, TNFα: 6/6. The reliability of Biorad multiplex cytokine assays has been validated in the following publication (40). A Bio-Plex 200 System was used as recommended by the manufacturer (Bio-Rad) and concentrations were calculated using Bio-Plex Manager Software 6.
Enzyme-linked immunosorbent assay (ELISA) was used to detect serum levels of CRP, HMBG1, leptin. HMGB1 and leptin (IBL International GMBH, Hamburg, Germany) as recommended by the respective manufacturers. The intra-assay coefficients of variation for all reported ELISAs were <3% and all results measured were within the detection range.
The subscales “depression” and “anxiety” of the Hospital Anxiety and Depression Scale (HADS) were used to assess symptom load and delta HADS “depression” subtracting T2 from T1 levels to assess treatment success. Test quality criteria of the employed questionnaire are described in the following citation (41).
Power analysis for the main hypothesis was performed with G*Power 3.1. We calculated the numbers of subjects that are needed to calculate a regression with a power of 0.80 at an a-level of 0.05 assuming a small to medium effect size of 0.1 as commonly found in quasi-experimental naturalistic studies in psychotherapy research. This resulted in a required power of N = 73.
Statistics for Windows software (SPSS), version 24 (Armonk, NY, United States) was used for the statistical evaluation. The nominal item sex was converted into the dummy variables male = 1, female = 2. Missing cytokine values were replaced with the half of the lowest values when cytokines were detectable but below the quantification limit as recommended by the manufacturer (<10% of all measurements). For psychometric assessments, ordinal items were converted to a scale ranging from 0 to 100 points where applicable. The category “does not apply” and item non-response were coded as missing values. Logarithmic transformations to achieve approximately normal data distribution were performed where applicable.
To investigate the relationship between TNFα baseline production and mPT outcome defined as delta HADS “depression” (T1–T2), multiple linear regression models were used, controlling for potential confounders (e.g., age and sex) (42). This allowed us to reject the null hypothesis that baseline TNFα levels are irrelevant for treatment outcome (see results section Ethical considerations and recruitment of participants).
In addition to the regression analysis, the Median-Split-Method was used to separate the participants into two categories according to PBMC TNFα production at baseline (T1) with the aim to illustrate characteristics of patients with high TNFα production and to learn what measurements besides the main outcome HADS ‘depression’ showed improvement after treatment in high TNFα producers. Ranking was hence done by a separate variable than the main outcome and based on data assessed prior to treatment rather than dividing the group in treatment responders and non-responders post-hoc. Participants with TNFα values ≥29.04 pg/ml were grouped as high TNFα producers (hTNF) and all others as low TNFα producers (lTNF). All metric values were calculated as mean and standard deviation (sd) per group and time point. According to group assignment, baseline analysis of differences in socio-demographic and clinical data between the lTNF and hTNF groups was done by Chi-X2-Test for ordinal data and by Mann–Whitney U-test for metric data. For hTNF and lTNF, group comparisons of laboratory and self-report data at baseline and after mPT, Students t-tests for independent samples were used. To compare mean values of laboratory and self-report data at baseline and after therapy within the groups, paired t-tests with repeated measures were used. As TNFα was not the main outcome and a Median and not a Mean Split was performed, regression to the mean was not considered an issue with respect to the main outcome HADS “depression” and all other measures besides TNFα. Because of the small number of participating patients, a bootstrapping technique was used to test for the significance of all t-tests. This treats a given sample as the population using intensive computer resampling (1,000 sampling) (43, 44). P < 0.05 were considered significant. Effect sizes were computed using Cohen's d.
To analyse potential interactions between developments over time in the two subgroups, two way ANOVAs were calculated.
We found that baseline TNFα production of PBMC in response to PHA challenge predicted mPT outcomes, and higher logTNFα at T1 (baseline) predicted higher improvement of HADS “depression” (delta HADS “depression” = T2 (discharge) scores—T1 (admission) scores) in the total patient sample. This analysis proved to be robust with respect to various confounders included in three different models (for details on models see legend to Table 1). However, the R2 was relatively low in all models (Table 1). High IFNγ and IL10 also predicted better HADS “depression” outcome, albeit with lower R2 than TNFα in the confounder controlled model (not shown). Other cytokines and general inflammation markers (CRP, HMGB1, leptin) did not predict outcome (not shown) whereby the levels of general inflammation markers were generally low.
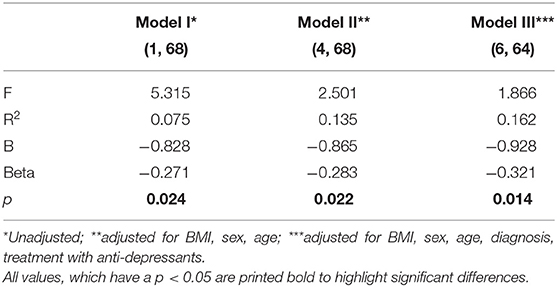
Table 1. Prediction of ΔHADS “depression” by Log TNFα. Uni- and multivariate regression analysis in confounder controlled models.
Further analysis was conducted after splitting the sample into hTNF and lTNF patients. The question was, if hTNF showed e.g., higher BMI or lower socio-educational status as reported in other studies reporting a pro-inflammatory status in depression. Concerning baseline data at admission, no differences between the groups were detected with respect to diagnosis subtype distribution (ICD10 F32.1/2, F33.1/2), weeks of mPT, age, sex, BMI, family situation, or education. Also, no difference was found with respect to medication, which was generally low in both groups (e.g., only 10/37 and 15/36 took anti-depressants) (Table 2). Hence, clinically hTNF and lTNF groups were not discernable prior to therapy.
The first question here was, if hTNF patients showed a general hyper-responsiveness to PHA challenge, or if certain aspects of the immune response such as acute inflammatory markers were selectively upregulated, as suggested by a number of previous studies on pro-inflammatory cytokines in depression. Depressive patients in the hTNF group showed significantly higher PBMC cytokine production than the lTNF group. Significantly increased cytokine levels in hTNF patients with medium to high effect sizes included IL1β and IL6 (acute inflammatory cytokines), IFNγ [T-helper cell type (TH) 1 cytokine], IL17A (TH17 cytokine), IL4 (TH2 cytokine), eotaxin (eosinophilic inflammation), and IL2 (regulation of inflammation) (Table 3A). However, with respect to general markers of pro-inflammatory status (serum CRP, HMGB1, and leptin), no significant difference was seen between hTNF and lTNF groups and CRP levels were generally low and in the non-pathologic range though a tendency toward a slightly higher CRP was present in hTNF (Table 3B).
Second, we wanted to know, if hTNF patients were suffering from more depressive symptoms. Using a representative self-report instrument to assess mental health in the dimensions anxiety and depression (HADS), no significant differences were found between hTNF and lTNF patients with respect to disease severity at baseline and the mean in both groups corresponded to moderate depression (45, 46) (Table 3C).
Here we were interested to learn about improved outcomes with respect to PBMC cytokine production and mental health. From the beginning to the end of therapy, hTNF patients showed a decrease in all measured PBMC cytokines, with significant reductions in IFNγ, IL4 and eotaxin (Table 4A). In lTNF patients we observed significant differences in the TH1/TH2 ratio. With respect to broad markers of inflammation (CRP, HMGB1, and leptin), no significant changes were seen in the total group or in either hTNF or lTNF patients from the beginning to the end of therapy (Table 4B). Finally, symptoms of anxiety and depression (HADS) improved highly significantly in both hTNF and lTNF groups from the beginning to the end of therapy, whereby larger effect sizes were observed in the hTNF group (d = 1.1–1.3) than the lTNF group (d = 0.6–0.9) (Table 4C). Additionally we performed a two way ANOVA analysis of key immune measures. This revealed a significant time by group interaction for TNFα and TH1/TH2 ratios (Figure 1).
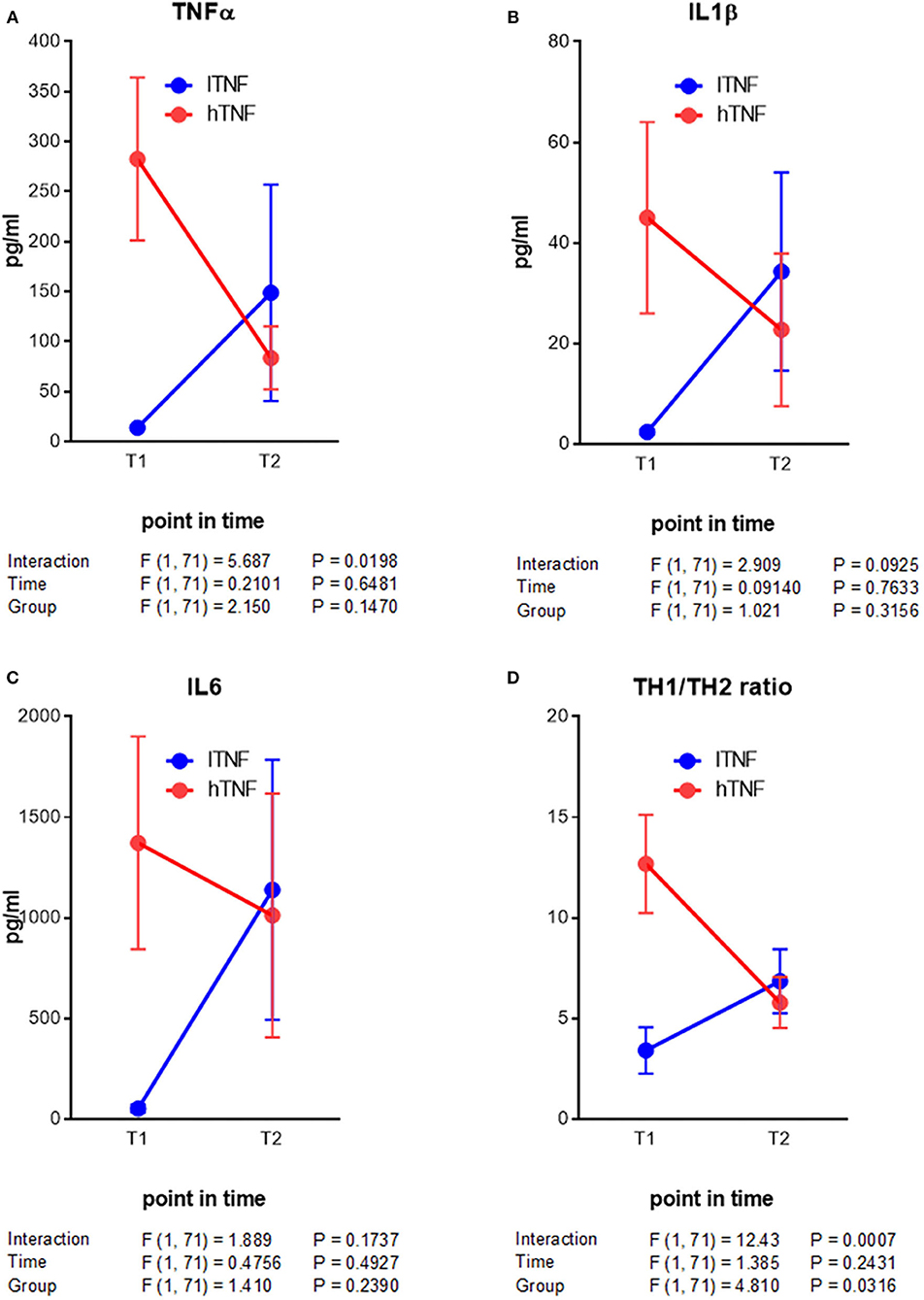
Figure 1. Analysis of interaction of contrarious cytokine level developments in depressive patients after mPT. (A–C) Acute inflammatory response cytokines. (D) TH1/TH2 ratio. Results of statistical analysis are given below the graphs.
Finally, we wanted to know the extent to which inflammation markers and mental health improved in hTNF and lTNF. After therapy, differences between hTNF and lTNF groups could no longer be detected with respect to cytokines (Table 5A), while broad markers of inflammation remained without difference (Table 5B). By contrast, the HADS “depression” score was significantly lower in hTNF patients compared to lTNF patients, which remained close to moderate depression cut-off (Table 5C).
We report a significant response to multimodal psychotherapeutic inpatient therapy (mPT) following a psychodynamic concept in patients with moderate depression and high production of TNFα by PBMC at the time of hospital admission. In addition, when the patients were assigned to a high TNFα producing subgroup (hTNF) and compared to a low TNFα producing subgroup (lTNF), the hTNF exhibited a distinct PBMC cytokine production profile in response to PHA challenge, but did not differ significantly in clinical data, general inflammation markers or mental health at baseline. mPT improved mood and mental health in both subgroups. Importantly however, hTNF displayed significantly higher mood improvement after therapy in comparison to lTNF. To our knowledge, this is the first time that improved cytokine production and mental health in moderate depression is reported together in a routine clinical care setting with psychodynamic orientation.
This outcome contrasts a number of studies reporting treatment resistance in major depressive patients with high TNFα levels, which requires careful discussion (3, 11, 22, 47, 50–60). In our study, we measured cytokine production by peripheral blood mononuclear cells (PBMC) stimulated ex vivo by a PHA challenge in a sample of patients with relatively normal general inflammation markers. Our results suggest that in the absence of pathologically increased general inflammation markers, hyper-reactivity of PBMC to an inflammatory challenge identified patients on the verge of developing a pro-inflammatory state at a point in time, where that process was well-reversible. In most previous studies addressing inflammation markers in depression, plasma or serum samples were used for assessment. In these bodily fluids, the presence of a cocktail of high concentrations of cytokines indicates in vivo activation of an inflammatory cascade. If cytokine levels in serum or plasma are increased, this usually correlates with raised levels of general markers of inflammation and tissue damage such as CRP. Cytokines are thus measurable in plasma or serum, when stress-associated biomolecular processes have taken place that caused cytokines to spill-over from damaged tissue into the circulation. This consideration also provides an explanation why there is considerable variation in serum or plasma cytokine levels reported in depressive patients as patients in different phases of stress-induced inflammatory damage may have been included (22).
In this study, TNFα production by PBMCs was successfully employed as an indicator cytokine for the prediction of treatment outcome and for the description of patient subpopulations. Analysis of cytokine levels produced by PBMC may therefore be a robust method to determine a patient's inflammatory hyper-reactivity to challenge prior to damage development. It is promising that hTNF patients are especially responsive to mPT and encourages longitudinal studies investigating the risk of long-term co-morbid disease development after treatment in these patients. The results also argue for the necessity of intervention in patients with moderate depression and that these patients may profit from additional anti-inflammatory treatment. At the same time, it can be hypothesized that patients that show little responsiveness of their PBMC to challenge require intensified treatment.
In support of the concept that subpopulations of depression patients can be defined by inflammatory activity, many studies report higher baseline cytokine levels in depressives prior to treatment when compared with healthy controls. Furthermore, these studies reveal that after treatment, depressives and healthy controls no longer differ in their cytokine levels (3, 22, 53, 60–65). Interestingly, the reported levels of cytokines in these studies prior to treatment were similar to the levels observed in our hTNF patients prior to mPT. Also, studies in which baseline cytokine levels in depressives did not differ from healthy controls, reported an increase in pro-inflammatory cytokines after treatment similar to our observation in lTNF (66, 67). It is therefore feasible to argue that there are at least two subpopulations of patients with depression with regard to their immune responsiveness, one that is hyper-reactive to challenge and well-treatable and another that is immunologically innate, not challengeable and more resistant to therapy.
Due to the naturalistic hospital setting, the lack of healthy controls or untreated control patients, the unavailability of a randomized controlled design, and successive recruitment are clear limitations of our study. Also, the possibility of a regression to the mean has to be considered, when looking at dichotomized data. In an experimental set up providing data that may depend on many variables, chance can be involved and it is to be ruled out that extreme outcomes observed at one point in time are followed by more moderate ones at another by chance and not due to a real improvement. If regression to the mean were the case, the measures observed in our patients prior to mPT would not differ from measures previously reported in healthy populations and the standard deviations in all analyzed subpopulations would be larger at T2 compared to T1 (68). However, our here analyzed data set did not fulfill these criteria for regression to the mean and allowed the comparison of hTNF and lTNF patients under real life conditions in the light of published data on healthy cytokine production by PBMC.
Also to be considered, one study reported that patients responsive to treatment displayed higher baseline cytokine levels than patients non-responsive to treatment (69). Hence, alternative to the approach employed in our study, response to treatment could have been used to subdivide study populations and analyze characteristics of patients with unfavorable treatment outcome. However, treatment response can only be determined post-hoc, while assessment of responsiveness to immune challenge can distinguish subgroups prior to treatment. Of course, the technical requirements for PBMC isolation and ex vivo challenge with PHA are rather high and may require a university hospital setting with a fully equipped immunology laboratory at hand. Though compared to flow cytometry for the identification of PBMC subpopulations, the determination of cytokines in cell culture supernatants can be done with comparably smaller effort. Future studies in this vein could establish this more complex but highly instructive analysis side by side with more easily applicable protocols to determine hyper-reactivity of the immune system to challenge in the clinic, for example by using skin tests (70).
Another consideration is that it has been known for some time that exposure to traumatizing experiences as well as substantial challenges to the immune system during early childhood may contribute not only to posttraumatic stress disorder and depression but also to lifelong changes in neuroendocrine and inflammatory responsiveness (71, 72). Recent research has found that, being traumatized reduced the adaptive capacity of the neuroendocrine and immune systems to interact efficiently in response to a new challenge (73–75). However, the changes resulting from traumatization tend to be lasting and can be expected to increase systemic inflammation markers (76). As we did not observe this, and our patient population showed impact of event scale scores below the cut-off of 26 (data not shown), this consideration may not play a role in our patient sample.
In summary, analysis of PBMC cytokine production provides a robust view of immune system reactivity that links high TNFα production in moderately depressive patients at baseline with improved mPT treatment outcome. We report normalization of cytokine production in response to challenge in hTNF after mPT, demonstrating that depression-associated changes in immune system function are reversible in this subpopulation of patients in a clinical psychosomatic inpatient care setting employing multimodal psychotherapeutic inpatient therapy focusing on a psychodynamic concept. This suggests that mPT is effective both on the mental and the somatic level, which contributes to the understanding of psychoimmune circuits involved in depression. Our findings in patients subjected to mPT suggest that the capacity of cells of the immune system to produce inflammatory cytokines can indicate greater adaptive responsiveness of the affected depressive patients to therapy and suggest a more complex relationship between inflammation and depression than previously hypothesized.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics committee of the Justus-Liebig University, Gießen, Germany. The patients/participants provided their written informed consent to participate in this study.
EP conceptualized and designed the study. MN and AM recruited patients and collected the biological samples under the supervision of EP, SA, FLei, and MC. MN generated the cytokine data with support from AM and under the supervision of MC. MN, AM, and ST collected all other data. EP, MN, and ST performed data processing and statistical analysis. MR and JK provided general support. EP, MC, and JK provided funding and supervised all stages of the study. MR, SA, FLew, and JK provided clinical supervision for the study. EP, MN, and ST interpreted the data. EP drafted the manuscript and coordinated the manuscript writing. All authors read, revised critically, and approved the final manuscript.
The conduct of the research was partially supported by funds from the Von Behring-Röntgen-Foundation (Von Behring-Röntgen-Stiftung).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We gratefully acknowledge the professional language editing services of Annette Smith, Toronto, Canada and the technical support of Susanne Tumala Psychoneuroimmunology Laboratory, Giessen, Germany.
1. Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett. (2008) 29:287–91.
2. Dantzer R. Depression and inflammation: an intricate relationship. Biol Psychiatry. (2012) 71:4–5. doi: 10.1016/j.biopsych.2011.10.025
3. Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. (2014) 45:77–86. doi: 10.1016/j.psyneuen.2014.03.019
4. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. (2015) 172:1075–91. doi: 10.1176/appi.ajp.2015.15020152
5. Grosse L, Hoogenboezem T, Ambree O, Bellingrath S, Jorgens S, de Wit HJ, et al. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav Immun. (2016) 54:38–44. doi: 10.1016/j.bbi.2015.12.003
6. Haapakoski R, Ebmeier KP, Alenius H, Kivimaki M. Innate and adaptive immunity in the development of depression: an update on current knowledge and technological advances. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 66:63–72. doi: 10.1016/j.pnpbp.2015.11.012
7. Farooq RK, Asghar K, Kanwal S, Zulqernain A. Role of inflammatory cytokines in depression: focus on interleukin-1beta. Biomed Rep. (2017) 6:15–20. doi: 10.3892/br.2016.807
8. Strawbridge R, Young AH, Cleare AJ. Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatr Dis Treat. (2017) 13:1245–62. doi: 10.2147/NDT.S114542
9. Maes M, Lambrechts J, Bosmans E, Jacobs J, Suy E, Vandervorst C, et al. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol Med. (1992) 22:45–53. doi: 10.1017/S0033291700032712
10. Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. (2009) 1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x
11. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
12. Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. (2011) 11:625–32. doi: 10.1038/nri3042
13. Fleshner M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav Immun. (2013) 27:1–7. doi: 10.1016/j.bbi.2012.08.012
14. Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci. (2013) 53:52–62. doi: 10.1016/j.mcn.2012.10.002
15. Carvalho AF, Solmi M, Sanches M, Machado MO, Stubbs B, Ajnakina O, et al. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl Psychiatry. (2020) 10:152. doi: 10.1038/s41398-020-0835-5
16. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
17. DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev. (2010) 34:130–43. doi: 10.1016/j.neubiorev.2009.07.014
18. Rohleder N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems −2011 curt richter award winner. Psychoneuroendocrinology. (2012) 37:307–16. doi: 10.1016/j.psyneuen.2011.12.015
19. Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. (2014) 76:181–9. doi: 10.1097/PSY.0000000000000049
20. Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur Neuropsychopharmacol. (2015) 25:1532–43. doi: 10.1016/j.euroneuro.2015.06.007
21. Bhattacharya A, Drevets WC. Role of neuro-immunological factors in the pathophysiology of mood disorders: implications for novel therapeutics for treatment resistant depression. Curr Top Behav Neurosci. (2017) 31:339–56. doi: 10.1007/7854_2016_43
22. Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. (2017) 135:373–87. doi: 10.1111/acps.12698
23. Rojas PS, Fritsch R, Rojas RA, Jara P, Fiedler JL. Serum brain-derived neurotrophic factor and glucocorticoid receptor levels in lymphocytes as markers of antidepressant response in major depressive patients: a pilot study. Psychiatry Res. (2011) 189:239–45. doi: 10.1016/j.psychres.2011.04.032
24. Eyre HA, Stuart MJ, Baune BT. A phase-specific neuroimmune model of clinical depression. Prog Neuropsychopharmacol Biol Psychiatry. (2014) 54:265–74. doi: 10.1016/j.pnpbp.2014.06.011
25. Fischer S, Strawbridge R, Vives AH, Cleare AJ. Cortisol as a predictor of psychological therapy response in depressive disorders: systematic review and meta-analysis. Br J Psychiatry. (2017) 210:105–9. doi: 10.1192/bjp.bp.115.180653
26. Arteaga-Henriquez G, Simon MS, Burger B, Weidinger E, Wijkhuijs A, Arolt V, et al. Low-grade inflammation as a predictor of antidepressant and anti-inflammatory therapy response in MDD patients: a systematic review of the literature in combination with an analysis of experimental data collected in the EU-MOODINFLAME consortium. Front Psychiatry. (2019) 10:458. doi: 10.3389/fpsyt.2019.00458
27. Papargyri P, Zapanti E, Salakos N, Papargyris L, Bargiota A, Mastorakos G. Links between HPA axis and adipokines: clinical implications in paradigms of stress-related disorders. Expert Rev Endocrinol Metab. (2018) 13:317–32. doi: 10.1080/17446651.2018.1543585
28. Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. (2016) 23:335–43. doi: 10.1038/mp.2016.167
29. Leichsenring F, Ruger U. [Psychotherapy and evidence-based medicine (EBM)–randomized controlled vs. naturalistic studies: is there only one gold standard?]. Z Psychosom Med Psychother. (2004) 50:203–17. doi: 10.13109/zptm.2004.50.2.203
30. Liebherz S, Rabung S. Do patients' symptoms and interpersonal problems improve in psychotherapeutic hospital treatment in Germany? a systematic review and meta-analysis. PLoS ONE. (2014) 9:e105329. doi: 10.1371/journal.pone.0105329
31. DGPPN B., KBV, AWMF (eds.) for the Guideline Group Unipolar Depression., (2015). S3-Leitlinie/Nationale Versorgungsleitlinie Unipolare Depression – Langfassung. Available online at: www.depression.versorgungsleitlinien.de
32. Nosper M. Krankenhausbehandlung und psychosomatische Rehabilitation richtig verordnen. Psychotherapeutenjournal. (2017) 4:331–8.
33. Harter M, Jansen A, Berger M, Baumeister H, Bschor T, Harfst T, et al. [Psychotherapy of depressive disorders: evidence in chronic depression and comorbidities]. Nervenarzt. (2018) 89:252–62. doi: 10.1007/s00115-018-0485-5
34. Meister R, Jansen A, Berger M, Baumeister H, Bschor T, Harfst T, et al. [Psychotherapy of depressive disorders : procedures, evidence and perspectives]. Nervenarzt. (2018) 89:241–51. doi: 10.1007/s00115-018-0484-6
35. Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. (2014) 140:774–815. doi: 10.1037/a0035302
36. Hiles SA, Baker AL, de Malmanche T, Attia J. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychol Med. (2012) 42:2015–26. doi: 10.1017/S0033291712000128
37. Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. (2016) 80:23–32. doi: 10.1016/j.biopsych.2015.05.017
38. Lopes RP, Grassi-Oliveira R, de Almeida LR, Stein LM, Luz C, Teixeira AL, et al. Neuroimmunoendocrine interactions in patients with recurrent major depression, increased early life stress and long-standing posttraumatic stress disorder symptoms. Neuroimmunomodulation. (2012) 19:33–42. doi: 10.1159/000327352
39. Lin P, Ding B, Wu Y, Dong K, Li Q. Mitogen-stimulated cell proliferation and cytokine production in major depressive disorder patients. BMC Psychiatry. (2018) 18:330. doi: 10.1186/s12888-018-1906-5
40. Surenaud M, Manier C, Richert L, Thiebaut R, Levy Y, Hue S, et al. Optimization and evaluation of Luminex performance with supernatants of antigen-stimulated peripheral blood mononuclear cells. BMC Immunol. (2016) 17:44. doi: 10.1186/s12865-016-0182-8
41. Herrmann C. International experiences with the hospital anxiety and depression scale–a review of validation data and clinical results. J Psychosom Res. (1997) 42:17–41. doi: 10.1016/S0022-3999(96)00216-4
42. Wosu AC, Valdimarsdottir U, Shields AE, Williams DR, Williams MA. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Ann Epidemiol. (2013) 23:797–811.e92. doi: 10.1016/j.annepidem.2013.09.006
43. Mooney CZ, Duval RD. Bootstrapping: A Nonparametric approach to Statistical Inference. Newbury Park, CA: Sage Publications, Inc (1993).
44. Good PI. Permutation, Parametric and Bootstrap Tests of Hypotheses. New York, NY: Springer (2005).
45. Walker J, Postma K, McHugh GS, Rush R, Coyle B, Strong V, et al. Performance of the hospital anxiety and depression scale as a screening tool for major depressive disorder in cancer patients. J Psychosom Res. (2007) 63:83–91. doi: 10.1016/j.jpsychores.2007.01.009
46. Stern AF. The hospital anxiety and depression scale. Occup Med. (2014) 64:393–4. doi: 10.1093/occmed/kqu024
47. Peacock BN, Scheiderer DJ, Kellermann GH. Biomolecular aspects of depression: a retrospective analysis. Compr Psychiatry. (2017) 73:168–80. doi: 10.1016/j.comppsych.2016.11.002
48. Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol. (1994) 98:71–7. doi: 10.1111/j.1365-2249.1994.tb06609.x
49. Kim OY, Chae JS, Paik JK, Seo HS, Jang Y, Cavaillon JM, et al. Effects of aging and menopause on serum interleukin-6 levels and peripheral blood mononuclear cell cytokine production in healthy nonobese women. Age. (2012) 34:415–25. doi: 10.1007/s11357-011-9244-2
50. Weizman R, Laor N, Podliszewski E, Notti I, Djaldetti M, Bessler H. Cytokine production in major depressed patients before and after clomipramine treatment. Biol Psychiatry. (1994) 35:42–7. doi: 10.1016/0006-3223(94)91166-5
51. Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H. Cytokine production and serum proteins in depression. Scand J Immunol. (1995) 41:534–8. doi: 10.1111/j.1365-3083.1995.tb03604.x
52. Myint AM, Leonard BE, Steinbusch HW, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J Affect Disord. (2005) 88:167–73. doi: 10.1016/j.jad.2005.07.008
53. Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, et al. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. (2007) 2007:76396. doi: 10.1155/2007/76396
54. Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. (2008) 18:230–3. doi: 10.1016/j.euroneuro.2007.06.004
55. Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. (2012) 139:230–9. doi: 10.1016/j.jad.2011.08.003
56. Amitai M, Taler M, Carmel M, Michaelovsky E, Eilat T, Yablonski M, et al. The relationship between plasma cytokine levels and response to selective serotonin reuptake inhibitor treatment in children and adolescents with depression and/or anxiety disorders. J Child Adolesc Psychopharmacol. (2016) 26:727–32. doi: 10.1089/cap.2015.0147
57. Davami MH, Baharlou R, Ahmadi Vasmehjani A, Ghanizadeh A, Keshtkar M, Dezhkam I, et al. Elevated IL-17 and TGF-beta serum levels: a positive correlation between T-helper 17 cell-related pro-inflammatory responses with major depressive disorder. Basic Clin Neurosci. (2016) 7:137–42. doi: 10.15412/J.BCN.03070207
58. Kim YK, Na KS, Myint AM, Leonard BE. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 64:277–84. doi: 10.1016/j.pnpbp.2015.06.008
59. Schmidt FM, Schroder T, Kirkby KC, Sander C, Suslow T, Holdt LM, et al. Pro- and anti-inflammatory cytokines, but not CRP, are inversely correlated with severity and symptoms of major depression. Psychiatry Res. (2016) 239:85–91. doi: 10.1016/j.psychres.2016.02.052
60. Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. (2017) 76:197–205. doi: 10.1016/j.psyneuen.2016.11.031
61. Kim YK, Suh IB, Kim H, Han CS, Lim CS, Choi SH, et al. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol Psychiatry. (2002) 7:1107–14. doi: 10.1038/sj.mp.4001084
62. Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology. (2003) 170:429–33. doi: 10.1007/s00213-003-1566-z
63. Gazal M, Souza LD, Fucolo BA, Wiener CD, Silva RA, Pinheiro RT, et al. The impact of cognitive behavioral therapy on IL-6 levels in unmedicated women experiencing the first episode of depression: a pilot study. Psychiatry Res. (2013) 209:742–5. doi: 10.1016/j.psychres.2013.03.002
64. Dahl J, Ormstad H, Aass HC, Sandvik L, Malt UF, Andreassen OA. Recovery from major depressive disorder episode after non-pharmacological treatment is associated with normalized cytokine levels. Acta Psychiatr Scand. (2016) 134:40–7. doi: 10.1111/acps.12576
65. Moreira FP, Cardoso Tde A, Mondin TC, Souza LD, Silva R, Jansen K, et al. The effect of proinflammatory cytokines in cognitive behavioral therapy. J Neuroimmunol. (2015) 285:143–6. doi: 10.1016/j.jneuroim.2015.06.004
66. Kagaya A, Kugaya A, Takebayashi M, Fukue-Saeki M, Saeki T, Yamawaki S, et al. Plasma concentrations of interleukin-1beta, interleukin-6, soluble interleukin-2 receptor and tumor necrosis factor alpha of depressed patients in Japan. Neuropsychobiology. (2001) 43:59–62. doi: 10.1159/000054867
67. Marques-Deak AH, Neto FL, Dominguez WV, Solis AC, Kurcgant D, Sato F, et al. Cytokine profiles in women with different subtypes of major depressive disorder. J Psychiatr Res. (2007) 41:152–9. doi: 10.1016/j.jpsychires.2005.11.003
68. Zwingmann C, Wirtz M. [Regression to the mean]. Rehabilitation. (2005) 44:244–51. doi: 10.1055/s-2005-866924
69. Schmidt FM, Kirkby KC, Lichtblau N. Inflammation and immune regulation as potential drug targets in antidepressant treatment. Curr Neuropharmacol. (2016) 14:674–87. doi: 10.2174/1570159X14666160115130414
70. Schakel L, Veldhuijzen DS, Crompvoets PI, Bosch JA, Cohen S, van Middendorp H, et al. Effectiveness of stress-reducing interventions on the response to challenges to the immune system: a meta-analytic review. Psychother Psychosom. (2019) 88:274–86. doi: 10.1159/000501645
71. Bauer ME, Wieck A, Lopes RP, Teixeira AL, Grassi-Oliveira R. Interplay between neuroimmunoendocrine systems during post-traumatic stress disorder: a minireview. Neuroimmunomodulation. (2010) 17:192–5. doi: 10.1159/000258721
72. Marco EM, Macri S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotox Res. (2011) 19:286–307. doi: 10.1007/s12640-010-9205-z
73. Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. (2016) 89:892–909. doi: 10.1016/j.neuron.2016.01.019
74. Watson IP, Brune M, Bradley AJ. The evolution of the molecular response to stress and its relevance to trauma and stressor-related disorders. Neurosci Biobehav Rev. (2016) 68:134–47. doi: 10.1016/j.neubiorev.2016.05.010
75. Hori H, Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci. (2019) 73:143–53. doi: 10.1111/pcn.12820
Keywords: multimodal psychotherapeutic inpatient therapy, stress, depression, tumor necrosis factor alpha (TNFα), inflammation
Citation: Peters EMJ, Neusetzer M, Akinci S, Murat A, Treuherz S, Rose M, Leweke F, Leichsenring F, Conrad ML and Kruse J (2020) Multimodal Psychotherapeutic Inpatient Therapy of Depression Is Successful in Patients With High Cytokine Production. Front. Psychiatry 11:571636. doi: 10.3389/fpsyt.2020.571636
Received: 11 June 2020; Accepted: 05 October 2020;
Published: 02 November 2020.
Edited by:
Kai G. Kahl, Hannover Medical School, GermanyReviewed by:
Karl Bechter, University of Ulm, GermanyCopyright © 2020 Peters, Neusetzer, Akinci, Murat, Treuherz, Rose, Leweke, Leichsenring, Conrad and Kruse. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eva M. J. Peters, ZXZhLnBldGVyc0BldmEtcGV0ZXJzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.