- 1Discipline of Addiction Medicine, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
- 2Psychological Medicine, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
- 3Drug Health Services, Royal Prince Alfred Hospital, Camperdown, NSW, Australia
- 4Department of Biochemistry, University of Otago, Dunedin, New Zealand
- 5Department of Psychology, University of Otago, Dunedin, New Zealand
Background: Risk behaviors for young adults such as alcohol use are associated with increased risk of morbidity and mortality. Patterns of risk behavior may be genetically determined and vary between genders. Previous studies in both young adults and heavy drinking adult samples have demonstrated that some genotypes, such as OPRM1 A118G, COMT Val158Met and DRD2 Taq1A and DRD4 C52IT, may predict addictive behaviors including alcohol consumption and impulsivity, although results have been mixed.
Methods: This study aimed to investigate the predictive relationship of these four single nucleotide polymorphisms (SNPs) prospectively on student patterns of drinking using a micro-longitudinal daily diary design in a sample of 628 young adults ages 18–25 of predominantly of European ethnicity. Linear mixed models were used to examine the effect of SNPs on the number of drinks per drinking session with gender as a moderating variable.
Results: There were no main effects for genotype on alcohol consumption, nor for gender × genotype for any of the SNPs. There was a trend for an effect of the DRD2 Taq1A on the number of drinks per drinking day and for the interaction of gender and DRD2 Taq1A on the number of drinks per drinking day.
Conclusion: These findings suggest that the DRD2 Taq1A, OPRM1 A118G, DRD4 C521T, or COMT Val158Met polymorphisms, are not associated with alcohol consumption in young adults, although there may be a relationship between DRD2 Taq1A and alcohol consumption in young adult males.
Introduction
Risk behaviors such as regular and heavy alcohol use are often initiated during the teenage years and continue on into early adulthood (1). Regular alcohol use and binge drinking are risk behaviors of particular concern and are associated with increased risk of morbidity and mortality (2). In recent years, certain genes and single nucleotide polymorphisms (SNPs) have become of interest due to their possible roles in the development of addiction and risk behaviors. Some of these genes and their SNPs include DRD2 Taq1A, DRD4 C521T, COMT Val158Met, and OPRM1 A118G.
The OPRM1 gene codes for the μ-opioid receptor and is associated with pain modulation (3). The G allele of the A118G has been widely studied with some studies reporting an association with cravings for alcohol in adult heavy drinkers (4) and alcohol misuse in adolescents (5). One study observed appetitive behaviors for alcohol were associated with the G allele in a sample of heavy drinking young males (6). However, an ethnic-specific meta-analysis by Chen et al. (7) demonstrated that the A118G SNP is associated with alcohol dependence in Asian adults but not among Caucasian adults. Although potentially underpowered, one recent study in a sample of 106 social drinkers with European ancestry demonstrated no association between OPRM1 rs1799971 genotype and alcohol consumption or sensitivity (8).
DRD2 and DRD4 are the genes that code for the D2 and D4 dopamine receptors, respectively. Two single nucleotide polymorphisms (SNP) associated with dopamine receptors DRD2 (Taq1A) and DRD4 (521CT) have been studied in relation to addictive behaviors. The A1 allele of the DRD2 (Taq1A) polymorphism has been associated with reduced expression of the D2 dopamine receptor (9). Meta-analyses have demonstrated an association between the Taq1A polymorphism and alcohol dependence (10, 11) albeit with a moderate effect size and potential for some publication bias influenced by racial ancestry (11). The DRD4 C521T SNP has been suggested to be related to novelty-seeking behavior (12–14) although there have been no studies to date examining any relationship with alcohol. Another polymorphism in the variable number of tandem repeat (VNTR) polymorphism of DRD4 has been observed to moderate the effect of alcohol consumption on social bonding in young adult social drinkers (15). There have been no studies examining the DRD4 C521T SNP.
The COMT gene codes for the catechol-o-methyltransferase enzyme that degrades neurotransmitters (16). The Met (A) allele of the Val158Met SNP is associated with reduced enzymatic activity of catechol-o-methyltransferase, which has been suggested to have possible implications in neuropsychiatric disorders (17) in addition to both gambling and drinking problems in non-clinical samples (18) and impulsive behavior in young adults (19). Studies examining the association of the Val158Met SNP with alcohol problems in adults have reported conflicting results. For example, one study has associated the COMT Met/Met genotype with greater alcohol use in social drinkers (20) while others have found no association with alcohol dependence (21). Additionally, one study found sex-specific associations for high-activity COMT and alcohol dependence, seen in male alcohol dependent participants only (22). It is possible that the effect of the Met/Met genotype on drinking behavior is more easily observed in social drinkers rather than in association with a clinical diagnosis, and/or is associated with risky decision making.
Taken together, the literature regarding the above candidate genotypes and alcohol consumption is somewhat inconsistent, which may be due to differences across studies including varying ethnicity, clinical heterogeneity, and sample selection such as studies of young social drinkers vs. adult dependent drinkers. This study thus aims to extend this literature by investigating the relationship of the above-mentioned candidate genotypes on prospective alcohol consumption across a 2 week period in a sample of university students.
Materials and Methods
Participants and Procedure
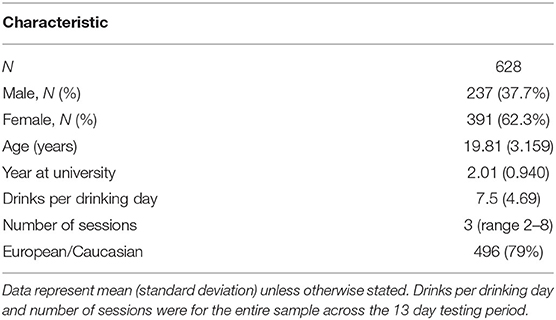
Participants were 628 young adults aged between 18 and 25 years of age studying at the University of Otago, New Zealand (see Table 1 for demographics). The data were collected in 2011 and 2012 as part of the Daily Life Study, and carried out in accordance with the recommendations and approval of the University of Otago Human Ethics Committee (10/777). All participants gave written informed consent in accordance with the Declaration of Helsinki by the 18th WMA General Assembly. Students were recruited predominantly from psychology courses and additional students were recruited through student job search, flyers, and social networking sites. Psychology students were reimbursed with class credit and were reimbursed for costs involved. Those recruited elsewhere were given renumeration. All renumeration was scaled based on their participation within the experiment.
Participants completed a baseline survey with demographic questions and trait measures in private cubicles, completed an Internet daily diary survey each day between 3 and 8 pm for 13 consecutive days, gave a non-fasting 22 ml venous blood sample at an on-campus clinic (4 people gave a saliva sample), and completed a final survey. Participants were asked in the daily diary how many standard drinks they had consumed the night before and were given a guide to estimate it.
Genotyping
Genotypes for the OPRM1 (A118G), DRD2_taq1A (ANKK1-Taq1a variant), DRD4_521(C521T), COMT (Val158Met) were obtained from blood (99%) or saliva samples. DNA was extracted from peripheral white blood cells using a guanidine-HCl-based procedure with chloroform extraction (23) and genotyped for the rs1799971, rs1800497, rs1800955, rs4680 genotypes using a Life Technologies TaqMan 5 nuclease assay (probe id C_3290335_10) and performed according to manufacturer's instructions. Fourteen percent of samples were repeated with 100% concordance in genotype. Hardy Weinberg Equilibriums (HWEs) were calculated using the Court lab–HW calculator.
Data Analysis
We aimed to determine the association between each genotype and the number of drinks per drinking day over the 13 days when participants were surveyed. A drinking day or session was counted as >0 standard drinks in the preceding day. We included data only for the participants that reported at least one drinking session and those that had completed at least 50% of the surveys.
Multilevel modeling was conducted in SPSS for Windows, version 25, employing linear mixed effects modeling. A random intercept-only model was fitted with participant as the random factor and dependent variable of number of drinks per drinking day. Separate models were conducted per genotype, which were added as fixed effects, whereby the major-allele homozygote was the reference genotype. As the sample was predominantly female (62.3%), gender was included as a control fixed factor main effect and a moderator with genotype. Ethnicity in this sample was also heavily skewed toward European Caucasian (79%) and was likewise added a control fixed factor (European Caucasian or Non-European Caucasian). The p-value was set to <0.0125 using a Bonferroni correction (0.05 divided by 4 genotypes) to adjust for multiple hypothesis testing. Based on an estimated sample prevalence of G- for OPRM1 for AUD vs. non AUD in young social drinkers (51.9 and 16.3%) with a medium magnitude effect size, estimated from (5), with a final sample of 628, we had more than sufficient power to detect a medium size difference between the homozygotes at α = 0. 0125.
Results
Sample Demographics and Drinking Measures
Table 1 summarizes the demographic and clinical characteristics of the sample. We sampled N = 628 university students who were predominantly female (62.3%, N = 391) and predominantly of European ethnicity (79%, N = 496; Asian = 9.9%, N = 62; Pacific Islander = 3.3%, N = 21; Indian = 3%, N = 19; Middle Eastern = 1.1%, N = 7, Mixed = 1.6%, N = 10, Other = 2.1%, N = 13). The average number of surveys completed was 11.41 (SD = 1.945) out of 13, which was an 88% response rate. Participants consumed an average 7.5 standard drinks (SD = 4.69) per drinking day, with a overall median of 3 drinking sessions (range: 2–8).
Effects of Polymorphism Variants on Drinking
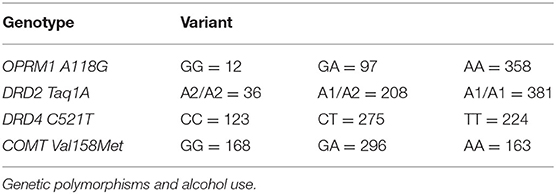
Genotype distributions per polymorphism are depicted in Table 2 and mixed model fixed effects tests are displayed in Table 3 and full models are displayed in the Supplementary Tables 1–4.
OPRM1 A118G rs1799971
Genotype distributions of the OPRM1 A118G polymorphism were GG (N = 12), GA (N = 97), AA (N = 358), which were in HWE (χ 2= 2.92, p = 0.09). Fixed effects tests revealed males consumed significantly more drinks per day than females (p <0.001), but there were no significant effects of gene variants, ethnicity, or genotype and gender interaction (p's > 0.218).
DRD2 Taq1A rs1800497
DRD2 Taq1A polymorphism distributions were A1/A1 (N = 381), A1/A2 (N =208), A2/A2 (N =36), which were in HWE (χ2 = 1.140, p = 0.286). Fixed effects tests demonstrated males consumed significantly more drinks per drinking day than females (p <0.001). There were trends for DRD2 Taq1A variant differences (p = 0.089), and stronger trend toward an interaction of polymorphism variant and gender interaction (p = 0.059), with fixed effects estimates showing a significant interaction with gender and comparison between homozygous variants A1/A1 vs. A2/A2 (p = 0.048). Sidak-adjusted pairwise comparisons of these variants stratified by gender revealed males with the A2/A2 variant (estimated mean = 13.7) drank significantly more per day than males with A1/A1 variant (estimated mean = 8.58) (p = 0.021), with no effect seen for females or other comparisons (p's > 0.113). No effect for ethnicity was seen (p = 0.216).
DRD4 C521T rs1800955
DRD4 C521T polymorphism distributions were CC (N = 123), CT (N = 275), TT (N = 224), which were did not deviate from HWE (χ2 = 5.243, p = 0.022, p = 0.09 corrected). Fixed effects tests again demonstrated males consumed significantly more drinks per drinking day (p <0.001). However, no main effects of ethnicity, gene variant, or variant by interaction effects were observed related to number of drinks per drinking day (p's > 0.365).
COMT Val158Met rs4680
Genotype distributions of the COMT Val158Met polymorphism were GG (N = 168), GA (N = 296), AA (N = 163), which were in HWE (χ2 = 1.9495, p = 0.163). A significant gender effect was seen with males consuming more drinks per drinking day (p <0.001), but no significant main effects for ethnicity, variant, or variant and gender interaction upon number of drinks per drinking day were seen (p's > 0.348).
Discussion
No evidence of main effects of gene variants upon consumption per drinking day were seen in this sample of young adult social drinkers. There were also no significant gene x gender interactions found for OPRM1 A118G, COMT Val158Met and DRD4 C521T SNPs. There was a trend for an effect of the DRD2 Taq1A on the number of drinks per drinking day and for the interaction of gender and DRD2 Taq1A on the number of drinks per drinking day.
An association of DRD2 Taq1A on alcohol consumption and presence of alcohol dependence has been previously reported in the literature. One meta-analysis demonstrated that positivity of the A1 allele was associated with a greater odds of alcohol dependence (10). In the current study we observed two trends regarding DRD2 Taq1A rs1800497 on alcohol consumption worth noting including a main effect for DRD2 Taq1A and also for an interaction between gender and the DRD2 Taq1A genotype. Our results are somewhat inconsistent with previous literature given that we demonstrated that a trend for an association between A1/A1 and lower levels of alcohol consumption in males relative to those with A2/A2, whereas there were no differences observed among females. Gender differences have also previously been reported in relation to the DRD2 Taq1A rs1800497 SNP, however in these studies, alcohol dependent males demonstrated a higher proportion of A1 allele than females, and healthy controls (24, 25). It is possible that the predictive role of DRD2 Taq1A on drinking over time in a sample of young adult social drinkers may differ to an association study examining presence vs. absence of alcohol dependence whereby heavy drinking patterns have already been established. This may be due to different proximal factors for drinking behavior such as varying drinking motives that influence the gene and environment relationship. For example, important drinking motives for college students include social (drinking to improve parties or gatherings), and conformity (drinking due to social pressure or a need to fit in) that may not be present for older adults (26).
The null findings for the OPRM1 A118G genotype on alcohol consumption are worth noting. Although the OPRM1 A118G genotype has been widely studied with many studies reporting an association with alcohol dependence, meta-analyses concluded no association of the genotype with a risk of alcohol dependence in Caucasian samples (7, 27). A more recent study reported no association of the OPRM1 A118G genotype on alcohol consumption or sensitivity in social drinkers of European ancestry in a human laboratory testing paradigm (8). In the current study, we employed multi-level linear mixed modeling to examine the relationship between OPRM1 A118G genotype and alcohol consumption, our results are consistent with emerging literature suggesting the association of the OPRM1 A118G genotype with heavy drinking may not be strong in Caucasian samples. Moreover, similar to the above, our sample consists of young adult social drinkers such that the results, while consistent with the latter described human laboratory study (8), may not extend to studies examining association with older adults with alcohol dependence.
Limitations
While the goal of the current study was to investigate the relationship of the above-mentioned candidate genotypes on prospective alcohol consumption in young adults, there was no assessment and identification of alcohol use disorder in this population. This limited our capacity to determine the specific role of these genotypes on drinking behavior associated with moderate to severe alcohol use disorder. Furthermore, it is possible that we did not have sufficient power to detect small effect sizes regarding associations with DRD4 C521T, DRD2 Taq1A, COMT Val158Met and OPRM1 A118G and thus we encourage replication in larger samples.
Conclusion
Our findings suggest that the DRD2 Taq1A, OPRM1 A118G, DRD4 C521T, or COMT Val158Met polymorphisms, are not associated with alcohol consumption in young adults, although there may be a relationship between DRD2 Taq1A and alcohol consumption in young adult males. Further longitudinal research is required to understand the possible interactions between gender and DRD2 Taq1A on alcohol consumption in young adults.
Data Availability Statement
The dataset presented in this article is not readily available because participants in the Daily Life Study (run between 2011 and 2014) did not consent for their data to be publically available. Requests to access the dataset should be directed to TC at dGFtbGluLmNvbm5lckBvdGFnby5hYy5ueg==.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Otago Human Ethics Committee (10/777). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TC conceived the Daily Life Study and led collection of the behavioral data. TM led the genetics analysis. KM, WL, PC, and BR conceived the paper idea. WL performed the statistical analysis with assistance from PC and BR. PC and KM wrote the article with assistance from WL. All authors read and reviewed the final manuscript.
Funding
This research was supported by a University of Otago Research grant to TC and a Health Research Council of New Zealand Grant (12/709) to TC (PI) and TM (AI).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank members of the 2011 and 2012 Daily Experiences Lab for help with data collection and Hadyn Youens for assistance with programming.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.549429/full#supplementary-material
References
1. Alex Mason W, Hitch JE, Kosterman R, McCarty CA, Herrenkohl TI, David Hawkins J. Growth in adolescent delinquency and alcohol use in relation to young adult crime, alcohol use disorders, and risky sex: a comparison of youth from low-versus middle-income backgrounds. J Child Psychol Psychiatr. (2010) 51:1377–85. doi: 10.1111/j.1469-7610.2010.02292.x
2. Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2018) 392:1015–35. doi: 10.1016/S0140-6736(18)31310-2
3. Zadina JE, Hackler L, Ge L-J, Kastin AJ. A potent and selective endogenous agonist for the μ-opiate receptor. Nature. (1997) 386:499. doi: 10.1038/386499a0
4. Van Den Wildenberg E, Wiers RW, Dessers J, Janssen RG, Lambrichs EH, Smeets HJ, et al. A functional polymorphism of the μ-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcoholism. (2007) 31:1–10. doi: 10.1111/j.1530-0277.2006.00258.x
5. Miranda R, Ray L, Justus A, Meyerson LA, Knopik VS, McGeary J, et al. Initial evidence of an association between OPRM1 and adolescent alcohol misuse. Alcoholism. (2010) 34:112–22. doi: 10.1111/j.1530-0277.2009.01073.x
6. Wiers R, Rinck M, Dictus M, Van den Wildenberg E. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes Brain Behav. (2009) 8:101–6. doi: 10.1111/j.1601-183X.2008.00454.x
7. Chen D, Liu L, Xiao Y, Peng Y, Yang C, Wang Z. Ethnic-specific meta-analyses of association between the OPRM1 A118G polymorphism and alcohol dependence among Asians and Caucasians. Drug Alcohol Depend. (2012) 123:1–6. doi: 10.1016/j.drugalcdep.2011.10.012
8. Sloan M, Klepp T, Gowin J, Swan J, Sun H, Stangl B, et al. The OPRM1 A118G polymorphism: converging evidence against associations with alcohol sensitivity and consumption. Neuropsychopharmacology. (2018) 43:1530–8. doi: 10.1038/s41386-017-0002-8
9. Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23. 1. Human Mutat. (2004) 23:540–5. doi: 10.1111/j.0020-2754.1998.00540.x
10. Munafo M, Matheson I, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case–control studies and evidence of publication bias. Mol Psychiatr. (2007) 12:454. doi: 10.1038/sj.mp.4001938
11. Wang F, Simen A, Arias A, Lu Q-W, Zhang H. A large-scale meta-analysis of the association between the ANKK1/DRD2 Taq1A polymorphism and alcohol dependence. Human Genet. (2013) 132:347–58. doi: 10.1007/2Fs00439-012-1251-6
12. Bailey JN, Breidenthal SE, Jorgensen MJ, McCracken JT, Fairbanks LA. The association of DRD4 and novelty seeking is found in a nonhuman primate model. Psychiatr Genet. (2007) 17:23–7. doi: 10.1097/YPG.0b013e32801140f2
13. Ronai Z, Szekely A, Nemoda Z, Lakatos K, Gervai J, Staub M, et al. Association between novelty seeking and the– 521 C/T polymorphism in the promoter region of the DRD4 gene. Mol Psychiatr. (2001) 6:35. doi: 10.1038/sj.mp.4000832
14. Schinka J, Letsch E, Crawford F. DRD4 and novelty seeking: results of meta-analyses. Am J Med Genet. (2002) 114:643–8. doi: 10.1002/ajmg.10649
15. Creswell KG, Sayette MA, Manuck SB, Ferrell RE, Hill SY, Dimoff JD. DRD4 polymorphism moderates the effect of alcohol consumption on social bonding. PLoS ONE. (2012) 7:e28914. doi: 10.1371/journal.pone.0028914
16. Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. (1958) 233:702–5.
17. Lachman HM, Papolos DF, Saito T, Yu Y-M, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. (1996) 6:243–50. doi: 10.1097/00008571-199606000-00007
18. Guillot CR, Fanning JR, Liang T, Berman ME. COMT associations with disordered gambling and drinking measures. J Gambling Stud. (2015) 31:513–24. doi: 10.1007/s10899-013-9434-1
19. Soeiro-De-Souza MG, Stanford MS, Bio DS, Machado-Vieira R, Moreno RA. Association of the COMT Met158 allele with trait impulsivity in healthy young adults. Mol Med Rep. (2013) 7:1067–72. doi: 10.3892/mmr.2013.1336
20. Kauhanen J, Hallikainen T, Tuomainen TP, Koulu M, Karvonen MK, Salonen JT, et al. Association between the functional polymorphism of catechol-O-methyltransferase gene and alcohol consumption among social drinkers. Alcoholism. (2000) 24:135–9. doi: 10.1111/j.1530-0277.2000.tb04582.x
21. Foroud T, Wetherill LF, Dick DM, Hesselbrock V, Nurnberger J, John I, Kramer J, et al. Lack of association of alcohol dependence and habitual smoking with catechol-O-methyltransferase. Alcoholism. (2007) 31:1773–9. doi: 10.1111/j.1530-0277.2007.00505.x
22. Šerý O, Didden W, Mikeš V, Pitelová R, Znojil V, Zvolský P. The association between highactivity COMT allele and alcoholism. Neuroendocrinol Lett. (2006) 27:231–5.
23. McKinney C, Fanciulli M, Merriman ME, Phipps-Green A, Alizadeh BZ, Koeleman BP, et al. Association of variation in Fcγ receptor 3B gene copy number with rheumatoid arthritis in Caucasian samples. Annals Rheumat Dis. (2010) 69:1711–6. doi: 10.1136/ard.2009.123588
24. López-Castromán J, Vaquero-Lorenzo C, Perez-Rodriguez MM, Diaz-Hernandez M, Fernandez-Piqueras J, Saiz-Ruiz J, et al. Gender effect on association between DRD2 polymorphism and substance dependence in a Spanish sample. Drug Alcohol Depend. (2009) 101:210–2. doi: 10.1016/j.drugalcdep.2008.12.011
25. Prasad P, Ambekar A, Vaswani M. Dopamine D2 receptor polymorphisms and susceptibility to alcohol dependence in Indian males: a preliminary study. BMC Med Genet. (2010) 11:24. doi: 10.1186/1471-2350-11-24
26. O'Hara R, Armeli S, Tennen H. College students' drinking motives and social-contextual factors: comparing associations across levels of analysis. Psychol Addict Behav. (2015) 29:420–429. doi: 10.1037/adb0000046
Keywords: alcohol consumption, genetics, risky (problem) drinking, emerging adult, OPRM1 A118G, DRD2
Citation: Chung P, Logge WB, Riordan BC, Haber PS, Merriman ME, Phipps-Green A, Topless RK, Merriman TR, Conner T and Morley KC (2020) Genetic Polymorphisms on OPRM1, DRD2, DRD4, and COMT in Young Adults: Lack of Association With Alcohol Consumption. Front. Psychiatry 11:549429. doi: 10.3389/fpsyt.2020.549429
Received: 06 April 2020; Accepted: 17 November 2020;
Published: 07 December 2020.
Edited by:
Amine Benyamina, Assistance Publique Hopitaux De Paris, FranceReviewed by:
Mauro Ceccanti, Sapienza University of Rome, ItalyGeorgios Demetrios Kotzalidis, Sapienza University of Rome, Italy
Copyright © 2020 Chung, Logge, Riordan, Haber, Merriman, Phipps-Green, Topless, Merriman, Conner and Morley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsten C. Morley, a2lyc3Rlbi5tb3JsZXlAc3lkbmV5LmVkdS5hdQ==
†These authors have contributed equally to this work
 Patrick Chung
Patrick Chung Warren B. Logge
Warren B. Logge Benjamin C. Riordan
Benjamin C. Riordan Paul S. Haber
Paul S. Haber Marilyn E. Merriman4
Marilyn E. Merriman4 Ruth K. Topless
Ruth K. Topless Tony R. Merriman
Tony R. Merriman Tamlin Conner
Tamlin Conner Kirsten C. Morley
Kirsten C. Morley