- 1Department of Geriatric Psychiatry, Suzhou Mental Health Center, Suzhou Guangji Hospital, the Affiliated Guangji Hospital of Soochow University, Suzhou, China
- 2Department of Psychiatry, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 3Department of Psychiatry, the Second Xiangya Hospital of Central South University, China National Clinical Research Center on Mental Health Disorders, China National Technology Institute on Mental Disorders, Hunan Technology Institute of Psychiatry, Hunan Key Laboratory of Psychiatry and Mental Health, Mental Health Institute of Central South University, Changsha, China
Aim: Accumulated evidence indicates that neurotrophin deregulations, oxidative stress injury, and mitochondrial dysfunction have been involved in bipolar disorder (BD); however, their real roles in BD are unclear. Investing the possible interaction between three systems is worthwhile understanding this complex process.
Methods: We measured plasma brain-derived neurotrophic factor (BDNF) level, leukocytes mitochondrial DNA copy number (mtDNAcn), and activities of antioxidant enzymes in BD patients (n = 97) and healthy controls (n = 31). Analysis of variance and linear regression analyses were performed to explore the interaction between mtDNAcn, antioxidant enzymes, and BDNF.
Results: Compared with healthy controls, there were significant decreases of glutathione peroxidase activity, BDNF levels, and mtDNA content, significant increases of manganese superoxide dismutase (MnSOD) activity among BD patients (all p < 0.05). Regression analysis showed MnSOD activity had a moderate effect on BDNF (beta = 0.23, t = 8.5, p = 0.001). Copper zinc SOD and total SOD activity were significantly correlated with Hamilton Depression Scale scores in depressive patients (r = −0.38, p = 0.013; r = −0.35, p = 0.022). Unexpectedly, we observed no significant correlation between mtDNA content and BDNF in BD patients (p > 0.05).
Conclusion: The findings coincide with our hypothesis that abnormal antioxidant enzymes, mtDNAcn, and peripheral BDNF may be involved in the course of BD. There were significant correlations between peripheral BDNF, antioxidant enzyme activities and mtDNAcn, suggesting that oxidative stress, mitochondrial function, and BDNF may influence each other in BD.
Introduction
Bipolar disorder (BD) is a debilitating and progressive psychiatric disorder, characterized by recurrent episodes of mania and depression, affecting more than 1% of the population worldwide (1). BD is considered one of the leading causes of disability among adults (2), and causes a heavy burden on patients, their families, and society (3). Despite the clinical importance of BD, the neurobiological underpinning of BD remains unclear. It is known that BD has been supposed to be not only a psychiatric disease, but rather a multi-system disorder (4), involving multiple factors, such as genetic, environmental, and social background; neurotrophin deregulations, oxidative stress injury, and mitochondrial dysfunction (5, 6). These potential biomarkers are very important to understand the pathophysiology of BD, and develop new treatment strategies.
Brain-derived neurotropic factor (BDNF), an important member of neurotrophins, plays a crucial role in neurodevelopment, neuronal plasticity, and survival. Meta-analysis has shown that peripheral BDNF levels are significantly decreased in manic and depressive states of BD (6). Furthermore, BDNF levels could be restored after psychiatric treatment (7), indicating that BDNF may be a promising biomarker for disease activity in BD (6, 8, 9). Intact BDNF as a small-secreted protein, could cross the blood-brain barrier (10), so BDNF levels in the blood may reflect similar levels of BDNF in the brain (11). Although BDNF has been reported to be involved in the pathology of BD, the role of BDNF in the genesis and development of BD is still not well understood.
Mitochondria are key cellular organelles that are responsible for generating energy through oxidative phosphorylation, and related to cellular growth, resilience, death, neuroplasticity, and neuronal activity (12, 13). Mitochondrial dysfunction and oxidative stress have been shown to be involved in BD (14). Mitochondrial DNA (mtDNA) is vulnerable to oxidative or genotoxic injury because of a lack of protection by histones, causing large DNA mutations and functional impairments. Genetic, molecular, and postmortem studies have confirmed that the impairments of neural systems in BD can be caused by mitochondrial deregulations (14). Oxidative stress involves an oxidant-antioxidant imbalance, which may also play important roles in BD pathophysiology. Convergent evidence indicates systemic changes of oxidative stress agents as well as antioxidant agents during the development of BD (15). For example, superoxide dismutase (SOD) and glutathione peroxidase (GPx), two key enzymes of the antioxidant system, were reported to be related to mood episodes. Although several clinical studies found abnormal levels of SOD and GPx in manic and depressive patients, when compared with healthy controls, inconsistent or even contradictory data has been frequently reported (15–17).
Growing evidence from animal and in vitro studies has suggested that oxidative stress, mitochondrial function, and BDNF have a complex and reciprocal relationship. Mitochondrial complex I is responsible for oxidative phosphorylation and generating ATP in mitochondria, and is related to the levels of intra and extracellular BDNF (18, 19). BDNF has also been reported to improve mitochondrial respiratory coupling by providing neuroprotection after its interaction with ATPase (19, 20). Several studies have shown increased oxidative stress and decreased BDNF levels, indicating that BDNF may play a protective role against oxidative damage in neurons (21, 22). He et al. showed that BDNF increased the antioxidant capacity of cell by selectively enhancing the expression levels of MnSOD (22). Although abnormal BDNF levels, enzymes activities, and mtDNA content (a biomarker, which reflects the mitochondrial function) were found in BD, there have been few studies on the relationship between BDNF, oxidative stress, and mtDNA in patients with BD. Only one study has reported that serum BDNF levels are related with the levels of antioxidant enzymes including SOD, and GPx in BD (23). Two studies reported that, patients with BD exhibited a negative association between serum BDNF levels and lipid peroxidation (24, 25). To our best knowledge, no study has been performed to examine the relationship between mtDNA content and BDNF in individuals with BD. Despite the inconsistencies, the above findings indicated that oxidative stress damage and mitochondrial dysfunction may be linked to BDNF levels to disrupt cellular resilience in BD.
Clarifying the relationship of mitochondrial biology, oxidative stress, and BDNF in mood disorders will provide us more clues to the mechanism of BD. In this study, we measured the leukocyte mtDNA content, plasma antioxidant enzymes including manganese SOD (MnSOD), copper zinc SOD (CuZnSOD), total SOD, and GPx, and BDNF levels to explore i) whether these biomarkers were altered in acute patients with BD; ii) whether there was a correlation among BDNF levels, SOD enzymes activities, mtDNA content, and clinical symptoms. We hypothesized that abnormal antioxidant enzymes activities, mtDNAcn, and peripheral BDNF levels occurred simultaneously in BD patients, and changes of antioxidant enzymes and mtDNA content were associated with the levels of BDNF.
Materials and Methods
Subjects and Assessments
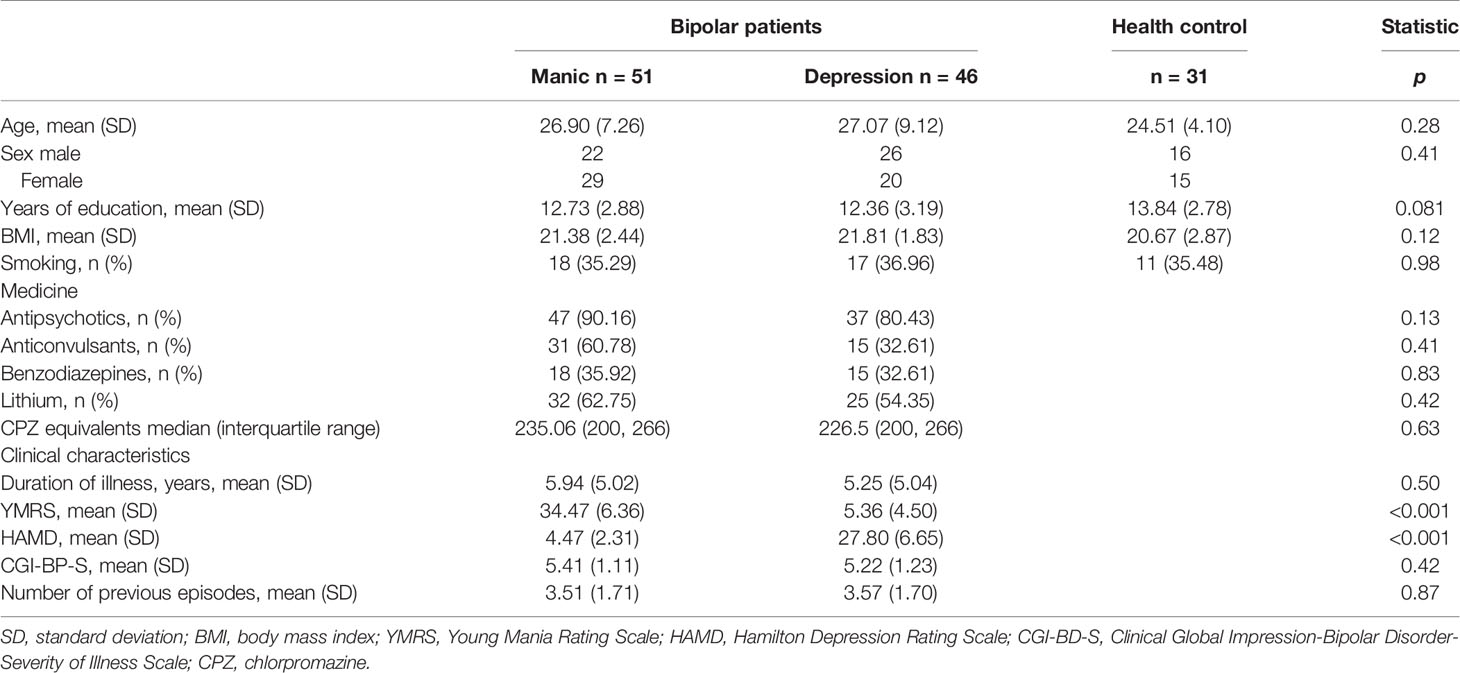
Patients with BD I type, including 51 manic and 46 depressive state individuals were recruited from the Suzhou Guangji Hospital (Jiangsu Province, China) during the period from July 2017 to May 2018. Inclusion criteria for BD patients were adults aged 18–65 years and defined by the occurrence of no less than two mood episodes of BD. All BD-I patients were diagnosed by more than two psychiatrists with expertise according to the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV). Types and doses of medication were recorded, but information on the duration of treatment was lacking. The affective symptoms were assessed using the Young Mania Rating Scale (YMRS), Hamilton Depression Scale (HAMD), and Clinical Global Impression-Bipolar Disorder-Severity of Illness Scale (CGI-BP-S). Thirty-one healthy participants were enrolled by advertisement. The volunteers were screened for psychiatric disorders using SCID-IV, non-patients edition (SCID-NP). Inclusion criteria for healthy control subjects were adults aged 18–65 years, with no history of mental disorders in the volunteers and their first-degree relatives. Exclusion criteria of all subjects were as follows: other mental illness, neurological diseases, substance abuse, several medical conditions including diabetes, common metabolic diseases, and cardiovascular diseases. All participants and/or their guardians read and signed the informed consent form. The study was approved by the ethics committee of Suzhou Guangji Hospital.
Biomarker Assay
Six milliliters of blood was withdrawn into ethylenediamine tetraacetic acid tubes from each fasted participant before taking medicine, between 7:00 and 9:00 am. For plasma isolation, 3 ml of blood was centrifuged at 3,500 g for 10 min. Three milliliters of venous blood and isolated plasma were kept at −80°C for further analyses. BDNF levels were evaluated with a duplicate enzyme immunoassay according to the manufacturer’s instructions (Mlbio, Shanghai, China). The minimal limit of detection of the assay was 0.1 ng/ml. The inter-assay coefficient of variation (CV) was < 7% and the intra-assay CV was < 6%. Plasma MnSOD, CuZnSOD, total SOD (TSOD), and GPx activities were measured in triplicate by the spectrophotometric method (26, 27) using commercially available assay kits (Mlbio, Shanghai, China). The lower limit of detection of SOD (MnSOD, CuZnSOD, TSOD) and GPx activities were 5.0 and 10.0 U/ml, respectively. The inter-assay and intra-assay CV ranged from 2.7 to 6.1%. All measurements were performed by the same technician. (See details in the Supplementary Methods).
Mitochondrial DNA Content Measurement
The detailed measurement of mtDNA copy number (mtDNAcn) was described in our previous research (28, 29). In brief, genomic DNA was extracted following the protocol using the QIAmp DNA Mini Kit (Qiagen, Hilden, Germany). The relative mtDNAcn was determined by the fluorescence-based quantitative polymerase chain reaction (qPCR). The primers for the mitochondrial gene (NADH dehydrogenase 1 gene, ND1) were as follows: F (5’-CCCTAAAACCCGCCACATCT-3’) and R (5’-GAGCGATGGTGAGAGCTA-AGGT-3’). The primers for the reference nuclear gene (human hemoglobin beta gene, HBB) were F (5’-GTGCACCTGACTCCTGAGGAGA-3’) and R (5’-CCTTGATACCAACCTGCCCAG-3’). All experiments were conducted in triplicates. The amplification of qPCR was performed using the standard curve assay. Efficiencies of the amplifications were between 93.4 and 108.2%. The intra-assay coefficient of variation (CV) was < 3.7%, and the inter-assay CV was < 3.3%. (See details in the Supplementary Methods).
Statistical Analysis
The Kolmogorov-Smirnov test was used to test the normality of continuous variables. The variables with normality are shown as the mean ± SD; whereas the skewed distributed variables are presented as the median and interquartile ranges. The data with non-normality were log-transformed into a normal distribution. Differences of values among multiple groups were analyzed using chi-square tests for categorical variables, Student’s t tests, and one-way analysis of variance for continuous variables. Bonferroni corrections were performed to correct for multiple comparisons. Pearson’s correlations and partial correlations were conducted to examine the relationship between variables. A multiple stepwise linear regression analysis was performed with the BDNF as the dependent variable. Variables with a significant association with BDNF were imputed as independent. Based on a recent meta-analysis of peripheral BDNF in BD, the duration of illness, symptom severity (CGI-BP-S scores), and age were added as adjustment variables (3). All the data were analyzed using SPSS statistical software for Windows, version 22.0 (SPSS, Chicago, IL, USA). Two-side tests and adjusted p < 0.05 were considered significant.
Results
Table 1 shows the demographic, clinical, and pharmacologic data of individuals. All participants were young, with mean ages of 26.90 ± 7.26 (manic group), 27.07 ± 9.12 (depressive group), and 24.51 ± 4.10 (healthy controls). No significant differences in the three groups were found in terms of age, gender, smoking, and body mass index (BMI). The education grade of the healthy group was slightly higher than patients groups; however it was not statistically significant (p = 0.081). The manic group displayed serious manic symptoms, whereas the depressed group showed noticeable depressive symptoms. Other clinical characteristics were similar between the two groups (manic and depressive groups).
Analysis of variance showed that the patient groups presented higher MnSOD activity than healthy volunteers (p < 0.05). After adjustments for age, gender, education, and BMI, the differences were still significant (p = 0.004) (Table 2). Post hoc test showed that there were significantly increased MnSOD levels in manic and depressive patients compared with controls (manic group vs. controls, adjusted p < 0.001, depressive group vs. controls, adjusted p = 0.01, Figure 1B), whereas there was no significant difference between the manic and depressed groups (p > 0.05, Figure 1B). The patient groups did not differ in their CuZnSOD activities when compared with controls (p = 0.26). Differences of TSOD (MnSOD plus CuZnSOD) activity among the three groups also did not reach statistical significance (p = 0.091), after adjusting for covariates. GPx activity was reduced in BD patients. When controlling for covariates, the differences of GPx levels were still significant (p = 0.008). Post hoc test showed that only depressive group had a decreased GPx activity (p < 0.05, Figure 1C). The activity of GPx did not alter when comparing manic group and healthy controls (p > 0.05, Figure 1C).
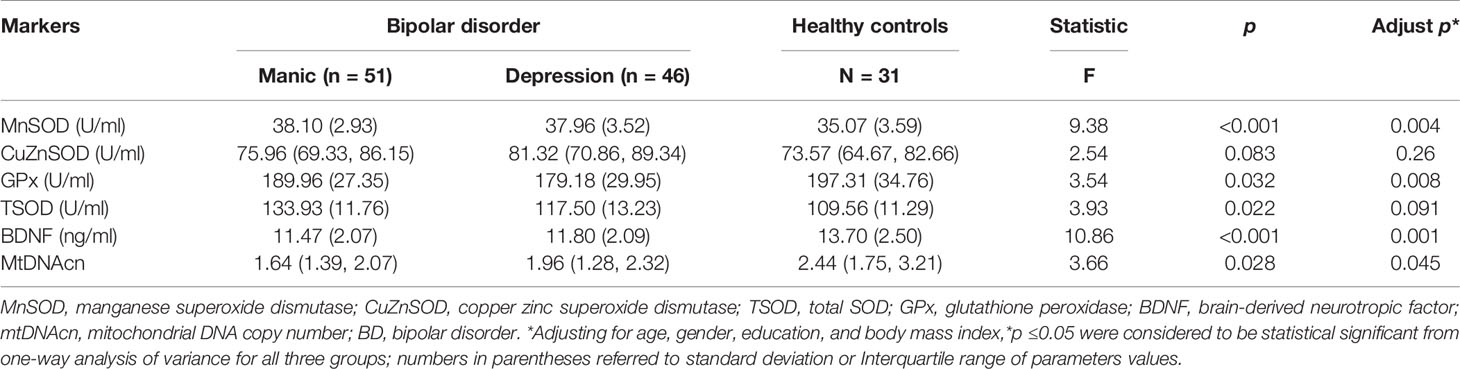
Table 2 Antioxidant enzymes activities, brain-derived neurotropic factor (BDNF) levels, and mitochondrial DNA copy number (mtDNAcn) in bipolar disorder (BD) patients and healthy controls.
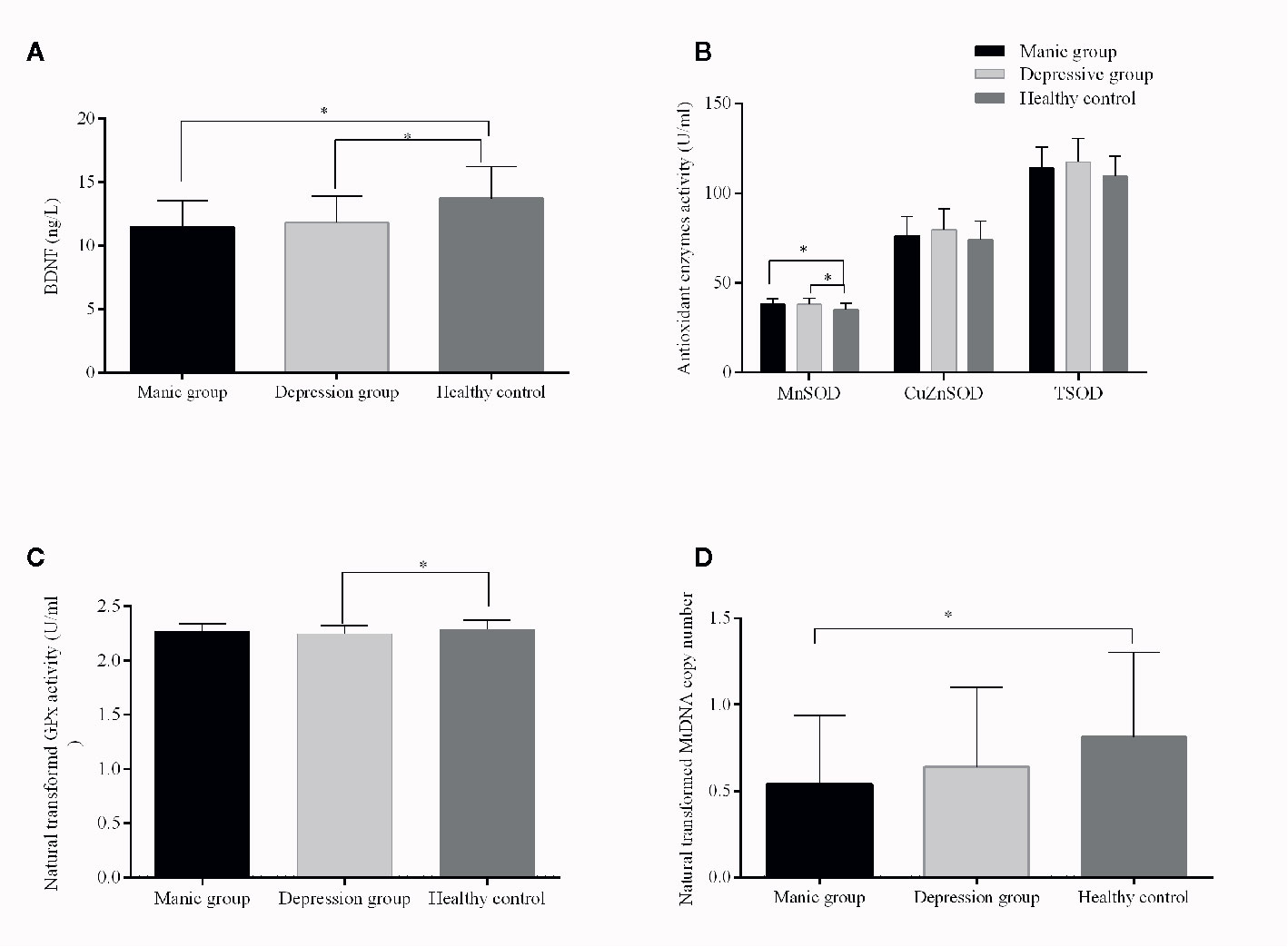
Figure 1 Brain-derived neurotrophic factor (BDNF) levels and TSOD, MnSOD, CuZnSOD, GPx enzyme activities, and mitochondrial DNA (mtDNA) copy number from patients with bipolar disorder and from healthy controls. The results are expressed as the mean+SD, * p ≤ 0.05 (controlled for age, gender, education, and BMI). Compared with controls, (A): lower levels of BDNF in manic and depressive patients; (B): increased MnSOD activities in manic and depressive patients; (C): decreased GPx activity in depressive patients; (D): lower mtDNAcn in manic patients.
The two patient groups had lower levels of BDNF than health controls (manic vs. controls, p < 0.001; depressive vs. controls, p = 0.005, Figure 1A). Besides, no significant differences in BDNF levels were observed in manic and depressive groups (p > 0.05, Figure 1A). MtDNA content of BD patients was significantly decreased compared with healthy controls (p = 0.028). When the covariates were included, significant differences remained (p = 0.045). Paired comparison indicated, only the mtDNAcn of manic group had a significant decrease when compared with healthy controls (adjusted p = 0.03, Figure 1D), whereas negative results were found for other comparisons (manic vs. depressive, adjusted p = 0.56; depressive vs. controls, adjusted p = 0.27, Figure 1D).
For the depressed group, CuZnSOD and TSOD activities were significantly correlated with HAMD scores (r = −0.35, p = 0.017; r = −0.329, p = 0.026). To further identify the relationship between these antioxidant enzyme activities and clinical characteristics, partial correlation analyses were performed (adjusted for age, sex, BMI, and smoking). The results also indicated that there were negative correlations between CuZnSOD activity, TSOD activity, and HAMD scores (r = −0.38, p = 0.013; r = −0.35, p = 0.022, respectively) (Figures 2A, B). There were no significant associations between HAMD scores and MnSOD activity (r = −0.078, p = 0.61), GPx activity (r = −0.03, p = 0.98), MtDNAcn (r = −0.23, p = 0.13), or BDNF (r = 0.04, p = 0.98). Regarding the manic group, we also did not find any significant correlations between YMRS scores and CuZnSOD activity (r = −0.061, p = 0.67), MnSOD activity (r = 0.14, p = 0.34), GPx activity (r = 0.013, p = 0.93) as well as BDNF (r = 0.012, p = 0.93). (All correlations for the biomarkers and clinical symptom scores were listed as Supplementary Results).
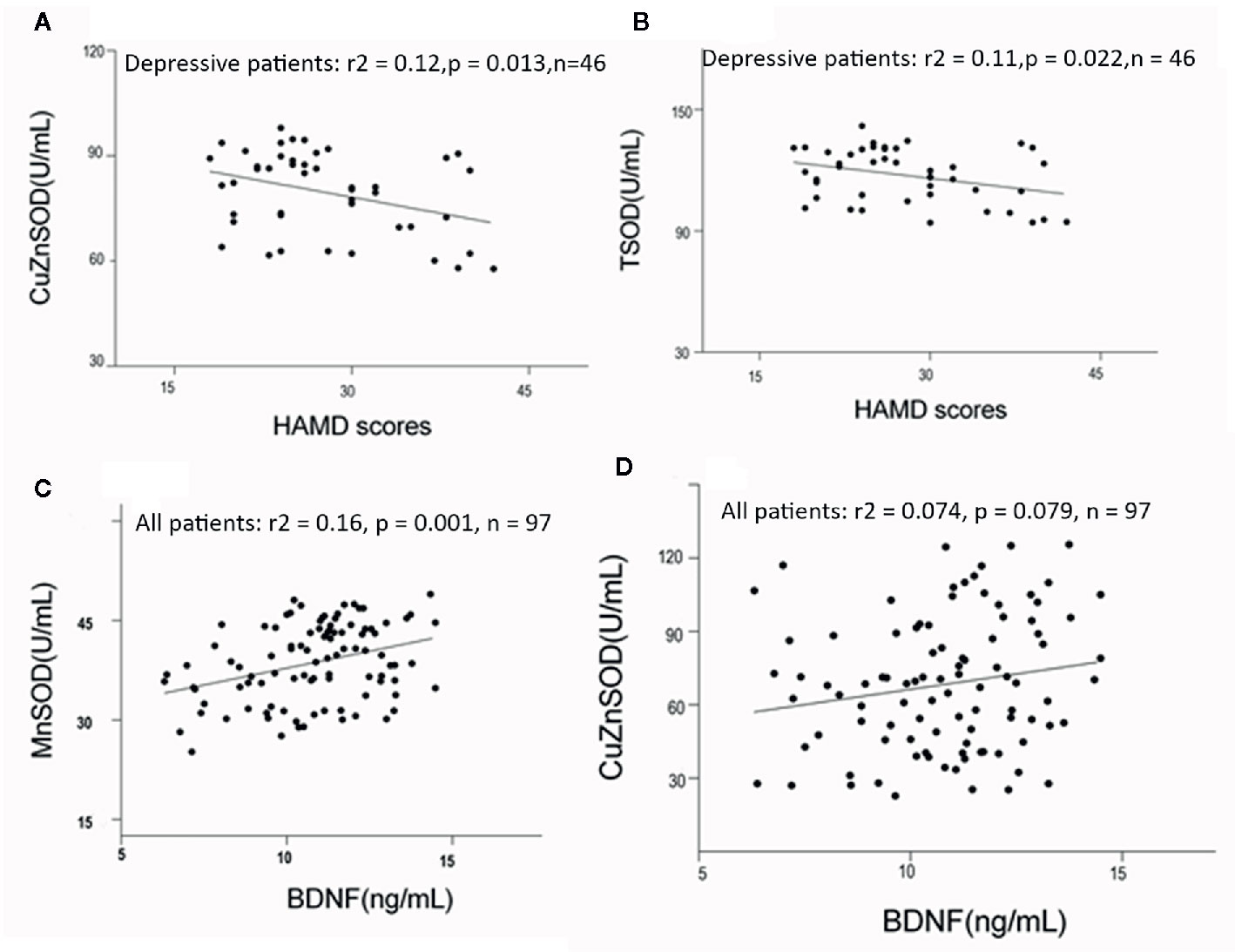
Figure 2 (A, B) Correlations among HAMD scores and antioxidant enzymes activities in bipolar disorder patients (adjusted for age, sex, BMI, and smoking, using partial correlation test). (C, D) Correlation between BDNF levels and antioxidant enzymes in bipolar disorder (adjusted for duration of illness, symptom severity, and age, using linear regression analyses). HAMD, Hamilton Depression Scale; BMI, body mass index.
No significant correlations were found between mtDNA content and BDNF (r = −0.062, p = 0.54), or between GPx activity and BDNF (r = −0.063, p = 0.53) in all acute patients. Circulating BDNF was positively associated with MnSOD and CuZnSOD activities (r = 0.41, p < 0.001, r = 0.24, p = 0.005), (Figures 2C, D). Linear regression analyses were conducted to identify influencing factors for BDNF levels. The results showed MnSOD remained significant with a moderate effect on BDNF, (beta = 0.23, t = 8.5, p = 0.001). CuZnSOD activity, duration of illness, age, and symptom severity were not found to be important factors influencing BDNF levels in this regression model (all p > 0.05). In addition, we analyzed possible correlations between BDNF and other biomarkers in the healthy control group. There were no significant correlations between BDNF and CuZnSOD (r = 0.096, p = 0.613), MnSOD (r = −0.12, p = 0.52), GPx (r = 0.23, p = 0.22), or mtDNAcn (r = −0.12, p = 0.11) in healthy controls.
Discussion
In the present study, we have three principal findings: a) compared to healthy controls, there was a significant decrease in plasma GPx activity and BDNF levels, a significant increase in plasma MnSOD activity, but no significant increase in TSOD in acute BD patients. b) A significant positive correlation between BDNF levels and MnSOD activity was firstly identified in BD patients. c) There was no significant correlation between mtDNA content and BDNF in BD patients.
We found abnormal GPx and MnSOD activities in manic and depressive patients, indicating oxidative stress damage in acute episodes. SOD and GPx are regarded as the first line of antioxidant defense (30). Elevated MnSOD activity and an increasing trend of CuZnSOD or TSOD activity is considered as response of the organism to high levels of superoxide, while lowered activity of GPx could induce accumulation of reactive oxygen species (ROS) that could cause protein, lipid, and DNA damages (31). Total SOD and GPx activities did not show a similar trend of changes, indicating the complexity of antioxidant defenses in manic and depressive episodes. Consistent with these findings, several previous studies have demonstrated changes of antioxidant enzymes. TSOD activity was measured rather than MnSOD in previous studies. Some reports showed higher TSOD activities during depressive and manic stages, but not in euthymia (17, 32, 33). Importantly, Machado-Vieira et al. reported increased levels of TSOD activity in unmedicated patients (17). In contrast, other studies showed decreased or normal TSOD activity (34, 35). Two meta-analyses suggested, these antioxidant enzymes formed an intricate relationship, and showed no overall significant differences compared with health controls (15, 36). Despite discrepancies among these researches, most evidence pointed to a weak antioxidant capacity and oxidative imbalance in BD.
This data showed lower BDNF levels and increased MnSOD activity in acute patients. Furthermore, BDNF levels were positively correlated with abnormal MnSOD activity after adjusting for covariates, consistent with the results of positive correlations between BDNF and TSOD in BD (23), BDNF, and CuZnSOD in schizophrenia (37). It seems contradictory that there were reduced BDNF levels, increased MnSOD activities, but a positive association between BDNF levels and CuZnSOD, MnSOD activities. Actually, all BD patients were not first-episode in this study. Recurring episodes of patients influence the outcome of BD by increasing a patient’s vulnerability to subsequent episodes (38, 39). Episodic nature of bipolar disorder may be especially detrimental to maintaining systemic homeostasis, with a repeated need for adaptation and re-setting of parameters (39, 40), which may lead to high levels of oxidative stress (41, 42), and decreased BDNF levels (42, 43). Abnormal SOD activities (34, 35, 44) and BDNF levels (43, 45, 46) were proved in euthymic patients with BD. We currently could not provide a good mechanism to explain the positive association. More longitudinal clinical studies are needed to resolve this problem.
SOD is the major enzyme to scavenge ROS and reduce oxidative stress (30). MnSOD is one such antioxidant enzyme found exclusively in the matrix of mitochondria, where it has a protective effect on mitochondrial function (47). Several studies have also confirmed the neuroprotection by MnSOD overexpression (48–51). Animal studies indicated that BDNF administration could increase the expression or production of antioxidant enzymes including MnSOD, and it may work through activating transcription factors such as NFκB, specificity protein 1, and activating protein 1 that could upregulate MnSOD expression (22, 52–55). Katusic suggested that BDNF might selectively unregulate MnSOD expression (22). Some studies also described significant relationships between oxidative stress parameters and BDNF in individuals with BD and schizophrenia (23, 37). Despite the lack of consistency, these results all suggested an interrelationship between neurotrophic and antioxidant processes in BD. More research using preclinical and clinical studies is needed to verify and clarify the relationships, especially in BD.
This data showed no correlation between BDNF and mtDNA content, an important parameter reflecting the activity of mitochondrial function and biogenesis. Mitochondrial dysfunction has been demonstrated to play important roles in BD, and may be a central feature of BD. A significant decreased mtDNAcn levels in manic patients was found in this study. The change of mtDNAcn was discussed in our previous report (28) and confirmed in later studies (56, 57). To the best of our knowledge, this is the first study to explore the relationship between BDNF and mtDNAcn in BD. Contrary to our expectations, BDNF was not associated with mtDNAcn levels. Emerging studies have shown that the effects of BDNF on mitochondria function were the same molecular pathway of its neuroprotective effect, mediated by the classic trkB-MEK and Bcl-2/Bcl-xL pathways, resulting in increased brain mitochondrial respiratory coupling at complex I (19). MnSOD is vital for mitochondrial integrity and function, and may interact with BDNF, hinting that BDNF may affect mitochondrial function. The link between BDNF and mitochondrial function may be independent of the changes of mtDNAcn, but this warrants further investigation.
In addition, we found that CuZnSOD and TSOD activities were significantly correlated with HAMD scores in depressive group. After controlling for confounding factors, the negative association between CuZnSOD, TSOD, and depressive symptom scores remained significant. Similar results were also found in previous studies (58, 59). Meta-analysis pointed out that antioxidant levels were lower in patients with major depressive disorder. Furthermore, the antioxidant levels were increased when depressive symptoms were relieved after antidepressant medication (59), suggesting that changes of antioxidant levels could be associated with the severity of depressive symptoms. Accumulating evidence revealed that using antioxidants as a therapeutic strategy for BD was beneficial in the treatment of depressed BD patients (60–62). The mechanism by which antioxidant enzymes were correlated with depression may be related to the hyperactivity of the HPA axis, which has been well noticed in depression (63–65). A recent meta-analysis of HPA axis activity confirmed that BD was associated with abnormalities of stress-related pathways in the brain (66). Activation of HPA axis activity is related to imbalance of redox system and produces deleterious reactive oxygen species (67). The burden of aggravating free radicals may induce an increase in antioxidant enzymes activity, which may be involved in the pathophysiology of this disease.
There were several limitations to be mentioned in this study. First, some important factors regarding physical exercise, metabolic parameters (i.e., impaired glucose metabolism), as well as lifestyle (i.e., smoking and alcohol consumption) were not available in the present study. Second, age is an important factor that may have an impact on these biological parameters. Oxidative stress, BDNF, and mtDNAcn were reported to be associated with age in patients with BD (68–71). The biomarkers were only measured in young subjects with BD and healthy controls in this study, rather than comparisons in all age groups. Therefore, the influence of age on these results in this study can not be excluded. Third, the impact of these different kinds of medications on biochemical parameters could not be examined in this study, and no definite conclusion was made in previous studies. Yi et al. reported that SOD activities were not altered after 6 weeks of lithium treatment (72), but Selek et al. found opposite results in a similar study (34), suggesting that more research is needed in future. Similar inconsistent conclusions have also been reported in mtDNA copy number (70, 73). A meta-analysis showed that there were small increases in peripheral BDNF levels after pharmacological treatment for manic episodes, and no significant changes before and after treatment for depressive episodes (8). Based on the available evidence, the effect of drug treatment on the biomarkers can not be ignored. Fourth, this was a cross-sectional study and our sample size was small, so this study could not draw any conclusions on causality. Further prospective investigations with large samples are required to further determine the interrelationship between oxidative stress parameters, mitochondrial function, and BDNF in BD.
In summary, oxidative stress, abnormal mtDNAcn, and decreased BDNF levels may be involved in the complicated pathophysiology of BD. We observed an independent positive correlation between MnSOD activity and BDNF levels. CuZnSOD and TSOD activities were also significantly associated with HAMD scores in depressive BD patients. This study supported our hypothesis that oxidative stress, mitochondrial function, and BDNF might influence each other, indicating diverse biological systems could be involved in the pathophysiology of BD. The exact biological pathways and the role of our findings remain largely unknown in BD. Better controlled longitudinal clinical studies and fundamental research need to be conducted in future.
Data Availability Statement
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to d2QxOTg1MjlAMTYzLmNvbQ==.
Ethics Statement
The studies involving human participants were reviewed and approved by ethics committee of Suzhou Guangji Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DW and HL wrote the first draft of this manuscript, and HL edited the subsequent versions. HL, XD, and HR are responsible for the data collection and analysis. JZ and LY gave critical revision for the manuscript. XC and DW were responsible for the designing the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the social development program of Wuxi (CSE31N1724), the Key Diagnosis and treatment Program of Suzhou (LCZX201615), National Natural Science Foundation of China (81801325).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are deeply grateful to all participants for participating in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.514658/full#supplementary-material
References
1. Gardner HH, Kleinman NL, Brook RA, Rajagopalan K, Smeeding JE. The Economic Impact of Bipolar Disorder in an Employed Population From an Employer Perspective. J Clin Psychiatry (2006) 67(8):1209–18. doi: 10.4088/JCP.v67n0806
2. Alonso J, Petukhova M, Vilagut G, Chatterji S, Heeringa S, Ustun TB, et al. Days out of role due to common physical and mental conditions: results from the WHO World Mental Health surveys. Mol Psychiatry (2011) 16(12):1234–46. doi: 10.1038/mp.2010.101
3. Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry (2011) 68(3):241–51. doi: 10.1001/archgenpsychiatry.2011.12
4. Leboyer M, Soreca I, Scott J, Frye M, Henry C, Tamouza R, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord (2012) 141(1):1. doi: 10.1016/j.jad.2011.12.049
5. Sigitova E, Fisar Z, Hroudova J, Cikankova T, Raboch J. Biological hypotheses and biomarkers of bipolar disorder. Psychiatry Clin Neurosci (2017) 71(2):77–103. doi: 10.1111/pcn.12476
6. Munkholm K, Vinberg M, Kessing LV. Peripheral blood brain-derived neurotrophic factor in bipolar disorder: a comprehensive systematic review and meta-analysis. Mol Psychiatry (2016) 21(2):216–28. doi: 10.1038/mp.2015.54
7. Fernandes BS, Gama CS, Ceresér KM, Yatham LN, Fries GR, Colpo G, et al. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: A systematic review and meta-regression analysis. J Psychiatr Res (2011) 45(8):995–1004. doi: 10.1186/s12916-015-0529-7
8. Fernandes BS, Molendijk ML, Kohler CA, Soares JC, Leite CMGS, Machado-Vieira R, et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med (2015) 13:289. doi: 10.1186/s12916-015-0529-7
9. Lin PY. State-dependent decrease in levels of brain-derived neurotrophic factor in bipolar disorder: a meta-analytic study. Neurosci Lett (2009) 466(3):139–43. doi: 10.1016/j.neulet.2009.09.044
10. Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology (1998) 37(12):1553–61. doi: 10.1016/s0028-3908(98)00141-5
11. Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett (2002) 328(3):0–264. doi: 10.1016/s0304-3940(02)00529-3
12. Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, et al. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta (2010) 1802(1):92–9. doi: 10.1016/j.bbadis.2009.09.002
13. Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell (2004) 119(6):873–87. doi: 10.1016/j.cell.2004.11.003
14. Scaini G, Rezin GT, Carvalho AF, Streck EL, Berk M, Quevedo J. Mitochondrial dysfunction in bipolar disorder: Evidence, pathophysiology and translational implications. Neurosci Biobehav Rev (2016) 68:694–713. doi: 10.1016/j.neubiorev.2016.06.040
15. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res (2014) 218(1-2):61–8. doi: 10.1016/j.psychres.2014.04.005
16. Khairova R, Pawar R, Salvadore G, Juruena MF, de Sousa RT, Soeiro-de-Souza MG, et al. Effects of lithium on oxidative stress parameters in healthy subjects. Mol Med Rep (2012) 5(3):680–2. doi: 10.3892/mmr.2011.732
17. Machado-Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser V Jr., da Silva Vargas R, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci Lett (2007) 421(1):33–6. doi: 10.1016/j.neulet.2007.05.016
18. Kim HK, Mendonca KM, Howson PA, Brotchie JM, Andreazza AC. The link between mitochondrial complex I and brain-derived neurotrophic factor in SH-SY5Y cells - The potential of JNX1001 as a therapeutic agent. Eur J Pharmacol (2015) 764:379–84. doi: 10.1016/j.ejphar.2015.07.013
19. Markham A, Cameron I, Bains R, Franklin P, Kiss JP, Schwendimann L, et al. Brain-derived neurotrophic factor-mediated effects on mitochondrial respiratory coupling and neuroprotection share the same molecular signalling pathways. Eur J Neurosci (2012) 35(3):366–74. doi: 10.1111/j.1460-9568.2011.07965.x
20. Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci (2004) 20(5):1189–96. doi: 10.1111/j.1460-9568.2004.03578.x
21. Valvassori SS, Arent CO, Steckert AV, Varela RB, Jornada LK, Tonin PT, et al. Intracerebral Administration of BDNF Protects Rat Brain Against Oxidative Stress Induced by Ouabain in an Animal Model of Mania. Mol Neurobiol (2015) 52(1):353–62. doi: 10.1007/s12035-014-8873-8
22. He T, Katusic ZS. Brain-derived neurotrophic factor increases expression of MnSOD in human circulating angiogenic cells. Microvascular Res (2012) 83(3):366–71. doi: 10.1016/j.mvr.2012.01.001
23. Mansur RB, Santos CM, Rizzo LB, Cunha GR, Asevedo E, Noto MN, et al. Inter-relation between brain-derived neurotrophic factor and antioxidant enzymes in bipolar disorder. Bipolar Disord (2016) 18(5):433–9. doi: 10.1111/bdi.12418
24. Newton DF, Naiberg MR, Andreazza AC, Scola G, Dickstein DP, Goldstein BI. Association of Lipid Peroxidation and Brain-Derived Neurotrophic Factor with Executive Function in Adolescent Bipolar Disorder. Psychopharmacology (2017) 234(4):647–56. doi: 10.1007/s00213-016-4500-x
25. Tsai MC, Huang TL. Thiobarbituric acid reactive substances (TBARS) is a state biomarker of oxidative stress in bipolar patients in a manic phase. J Affect Disord (2015) 173:22–6. doi: 10.1016/j.jad.2014.10.045
26. Floyd RA. Methods in Molecular Biology, Volume 108, Free Radical and Antioxidant Protocols. Edited by Donald Armstrong. Humana Press, Totowa, New Jersey, 1998. 455 pp. Analyt Biochem (1999) 270(2):342–0. doi: 10.1006/abio.1999.4101
27. Paglia DE. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med (1967) 70(1):158–69. doi: 10.1080/15389588.2012.749466
28. Dong W, Li Z, Liu W, Zhou J, Ma X, Tang J, et al. Differential mitochondrial DNA copy number in three mood states of bipolar disorder. BMC Psychiatry (2018) 18(1):149. doi: 10.1186/s12888-018-1717-8
29. Li Z, Hu M, Zong X, He Y, Wang D, Dai L, et al. Association of telomere length and mitochondrial DNA copy number with risperidone treatment response in first-episode antipsychotic-naïve schizophrenia. Sci Rep (2016) 5(1):18553. doi: 10.1038/srep18553
30. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature (2000) 408(6809):239–47. doi: 10.1038/35041687
31. Steckert AV, Valvassori SS, Moretti M, Dal-Pizzol F, Quevedo J. Role of oxidative stress in the pathophysiology of bipolar disorder. Neurochem Res (2010) 35(9):1295–301. doi: 10.1007/s11064-010-0195-2
32. Andreazza AC, Cassini C, Rosa AR, Leite MC, Almeida LMVD, Nardin PC, et al. Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res (2007) 41(6):523–9. doi: 10.1016/j.jpsychires.2006.07.013
33. Kunz M, Gama CS, Andreazza AC, Salvador M, Ceresér KM, Gomes FA, et al. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2008) 32(7):1677–81. doi: 10.1016/j.pnpbp.2008.07.001
34. Selek S, Savas HA, Gergerlioglu HS, Bulbul F, Uz E, Yumru M. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J Affect Disord (2008) 107(1-3):89–94. doi: 10.1016/j.jad.2007.08.006
35. Gergerlioglu HS, Savas HA, Bulbul F, Selek S, Uz E, Yumru M. Changes in nitric oxide level and superoxide dismutase activity during antimanic treatment. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31(3):697–702. doi: 10.1016/j.jad.2007.08.006
36. Andreazza AC, Kauer-Sant’Anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: A meta-analysis. J Affect Disord (2008) 111(2):135–44. doi: 10.1016/j.jad.2008.04.013
37. Xiu MH, Li Z, Chen DC, Chen S, Curbo ME, Wu HE, et al. Interrelationships Between BDNF, Superoxide Dismutase, and Cognitive Impairment in Drug-Naive First-Episode Patients With Schizophrenia. Schizophr Bull (2020). doi: 10.1093/schbul/sbaa062
38. Ketter TA, Houston JP, Adams DH, Risser RC, Tohen M. Differential Efficacy of Olanzapine and Lithium in Preventing Manic or Mixed Recurrence in Patients With Bipolar I Disorder Based on Number of Previous Manic or Mixed Episodes. J Clin Psychiatry (2006) 67(1):95–101. doi: 10.4088/jcp.v67n0113
39. Gama CS, Kunz M, Magalhães PV, Kapczinski F. Staging and neuroprogression in bipolar disorder: a systematic review of the literature. Rev Bras Psiquiatr (2013) 35(1):70–4. doi: 10.1016/j.rbp.2012.09.001
40. Mcewen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metab Clin Exp (2003) 52(supp-S2):0–16. doi: 10.1016/s0026-0495(03)00295-6
41. Munkholm K, Poulsen HE, Kessing LV, Vinberg M. Elevated levels of urinary markers of oxidatively generated DNA and RNA damage in bipolar disorder. Bipolar Disord (2015) 17(3):257–68. doi: 10.1111/bdi.12245
42. Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev (2011) 35(3):804–17. doi: 10.1016/j.neubiorev.2010.10.001
43. Kauer-Sant’Anna M, Kapczinski F, Andreazza AC, Bond DJ, Lam RW, Young LT, et al. Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int J Neuropsychopharmacol Off Sci J Collegium Internat Neuropsychopharmacologicum (CINP) (2009) 12(4):447–58. doi: 10.1017/s1461145708009310
44. Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res (2003) 121(2):0–122. doi: 10.1016/s0165-1781(03)00220-8
45. Suwalska A, Sobieska M, Rybakowski JK. Serum Brain-Derived Neurotrophic Factor in Euthymic Bipolar Patients on Prophylactic Lithium Therapy. Neuropsychobiology (2010) 62(4):229–34. doi: 10.1159/000319949
46. Södersten K, Pålsson E, Ishima T, Funa K, Landén M, Hashimoto K, et al. Abnormality in serum levels of mature brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in mood-stabilized patients with bipolar disorder: A study of two independent cohorts. J Affect Disord (2014) 160:1–9. doi: 10.1016/j.jad.2014.01.009
47. Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med (2002) 33(3):337–49. doi: 10.1016/s0891-5849(02)00905-x
48. Hai-Feng H, Fei G, Yuan-Zhao C, Wen S, Qing X. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci Ther (2012) 18(10):811–8. doi: 10.1111/j.1755-5949.2012.00380.x
49. Wu H, Zhang Y, Shi X, Zhang J, Ma E. Overexpression of Mn-superoxide dismutase in Oxya chinensis mediates increased malathion tolerance. Chemosphere (2017) 181:352. doi: 10.1016/s0891-5849(02)00905-x
50. Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer’s disease. FASEB J (2009) 23(8):2459–66. doi: 10.1096/fj.09-132928
51. Hsu CC, Hines JN, Oberley TD, Clair DKS, Kasarskis EJ. Overexpression of MnSOD serves a neuroprotective role in transgenic mice expressing mutant SOD1. Free Radical Biol Med (1999) 27(99):S140. doi: 10.1016/s0891-5849(99)90963-2
52. Yoo JM, Lee BD, Sok DE, Ma JY, Kim MR. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol (2017) 11(C):592–9. doi: 10.1016/j.redox.2016.12.034
53. Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discovery (2011) 10(3):209–19. doi: 10.1038/nrd3366
54. Tuvikene J, Pruunsild P, Orav E, Esvald EE, Timmusk T. AP-1 Transcription Factors Mediate BDNF-Positive Feedback Loop in Cortical Neurons. J Neurosci Off J Soc Neurosci (2016) 36(4):1290. doi: 10.1523/JNEUROSCI.3360-15.2016
55. Donovan MJ, Lin MI, Wiegn P, Ringstedt T., Kraemer R., Hahn R., et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development (2000) 127(21):4531–40. doi: 10.1023/A:1008187310534
56. Tsujii N, Otsuka I, Okazaki S, Yanagi M, Numata S, Yamaki N, et al. Mitochondrial DNA Copy Number Raises the Potential of Left Frontopolar Hemodynamic Response as a Diagnostic Marker for Distinguishing Bipolar Disorder From Major Depressive Disorder. Front Psychiatry (2019) 10:312. doi: 10.3389/fpsyt.2019.00312
57. Yamaki N, Otsuka I, Numata S, Yanagi M, Mouri K, Okazaki S, et al. Mitochondrial DNA copy number of peripheral blood in bipolar disorder: The present study and a meta-analysis. Psychiatry Res (2018) 269:115–7. doi: 10.1016/j.psychres.2018.08.014
58. Russo AJ. Increased serum Cu/Zn SOD in individuals with clinical depression normalizes after zinc and anti-oxidant therapy. Nutr Metab Insights,2010,3(2010-06-17) (2010) 2010(3):37–42. doi: 10.4137/nmi.s5044
59. Jiménez-Fernández S, Gurpegui M, Díaz-Atienza F, Pérez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J Clin Psychiatry (2015) 76(12):1658–67. doi: 10.4088/jcp.14r09179
60. Aboul-Fotouh S. Coenzyme Q10 displays antidepressant-like activity with reduction of hippocampal oxidative/nitrosative DNA damage in chronically stressed rats. Pharmacol Biochem Behav (2013) 104: (1):105–12. doi: 10.1016/j.pbb.2012.12.027
61. Amr M, El-Mogy A, Shams T, Vieira K, Lakhan SE. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. Nutr J 12,1(2013-03-09) (2013) 12(1):1–8. doi: 10.1186/1475-2891-12-31
62. El-Shamy KA, Koriem KMM, Fadl NN, El-Azma MHA, Arbid MSS, Morsy FA, et al. Oral supplementation with geranium oil or anise oil ameliorates depressed rat-related symptoms through oils antioxidant effects. J complement Integr Med (2019) 17(2). doi: 10.1515/jcim-2019-0028
63. Belvederi Murri M, Pariante C, Mondelli V, Masotti M, Atti AR, Mellacqua Z, et al. HPA axis and aging in depression: Systematic review and meta-analysis. Psychoneuroendocrinology (2014) 41:46–62. doi: 10.1016/j.psyneuen.2013.12.004
64. Yang SJ, Yu HY, Kang DY, Ma ZQ, Qu R, Fu Q, et al. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol Biochem Behav (2014) 124:451–7. doi: 10.1016/j.pbb.2014.07.015
65. Zhang JQ, Wu XH, Feng Y, Xie XF, Fan YH, Yan S, et al. Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: involvement of the neuroinflammatory pathway. Acta Pharmacol Sin (2016) 37(9):1141–53. doi: 10.1038/aps.2016.63
66. Belvederi MM, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology (2016) 63:327–42. doi: 10.1016/j.psyneuen.2015.10.014
67. van Bodegom M, Homberg JR, Henckens M. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front Cell Neurosci (2017) 11:87. doi: 10.3389/fncel.2017.00087
68. Yatham LN, Kapczinski F, Andreazza AC, Young LT, Lam RW, Kauer-Sant’Anna M. Accelerated age-related decrease in brain-derived neurotrophic factor levels in bipolar disorder. Int J Neuropsychopharmacol (2009) 12(1):137–9. doi: 10.1017/s1461145708009449
69. Andreazza AC, Kapczinski F, Kauer-Sant’Anna M, Walz JC, Bond DJ, Gonçalves CA, et al. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci JPN (2009) 34(4):263–71. doi: 10.1016/j.jpsychires.2009.01.008
70. Kumar P, Efstathopoulos P, Millischer V. Mitochondrial DNA copy number is associated with psychosis severity and anti-psychotic treatment. Sci Rep (2018) 8(1):12743. doi: 10.1038/s41598-018-31122-0
71. Chang CC, Jou SH, Lin TT, Liu CS. Mitochondrial DNA variation and increased oxidative damage in euthymic patients with bipolar disorder. Psychiatry Clin Neurosci (2014) 68(7):551–7. doi: 10.1111/pcn.12163
72. Lv Q, Guo Y, Zhu M, Geng R, Yi Z. Predicting individual responses to lithium with oxidative stress markers in drug-free bipolar disorder. World J Biol Psychiatry (2019) 20(10):778–89. doi: 10.1080/15622975.2019.1663929
Keywords: bipolar disorder, brain-derived neurotrophic factor, mitochondrial DNA, oxidative stress, manganese superoxide dismutase
Citation: Wang D, Li H, Du X, Zhou J, Yuan L, Ren H, Yang X, Zhang G and Chen X (2020) Circulating Brain-Derived Neurotrophic Factor, Antioxidant Enzymes Activities, and Mitochondrial DNA in Bipolar Disorder: An Exploratory Report. Front. Psychiatry 11:514658. doi: 10.3389/fpsyt.2020.514658
Received: 25 November 2019; Accepted: 17 August 2020;
Published: 11 September 2020.
Edited by:
Alfredo B. Cuellar-Barboza, Autonomous University of Nuevo León, MexicoCopyright © 2020 Wang, Li, Du, Zhou, Yuan, Ren, Yang, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Wang, d2QxOTg1MjlAMTYzLmNvbQ==
 Dong Wang
Dong Wang Hong Li2
Hong Li2 Guangya Zhang
Guangya Zhang