
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 16 November 2020
Sec. Public Mental Health
Volume 11 - 2020 | https://doi.org/10.3389/fpsyt.2020.492006
This article is part of the Research TopicEcological Disaster NeuropsychiatryView all 8 articles
 Claudia Carmassi1
Claudia Carmassi1 Valerio Dell'Oste1,2*
Valerio Dell'Oste1,2* Carlo Antonio Bertelloni1
Carlo Antonio Bertelloni1 Claudia Foghi1
Claudia Foghi1 Elisa Diadema1,2
Elisa Diadema1,2 Federico Mucci1,2
Federico Mucci1,2 Gabriele Massimetti1
Gabriele Massimetti1 Alessandro Rossi3
Alessandro Rossi3 Liliana Dell'Osso1
Liliana Dell'Osso1Background: Increasing evidence indicates that survivors to traumatic events may show disruption of sleep pattern, eating and sexual behaviors, and somatic symptoms suggestive of alterations of biorhythmicity and vegetative functions. Therefore, the aim of this study was to investigate these possible alterations in a sample of survivors in the aftermath of earthquake exposure, with particular attention to gender differences and impact of post-traumatic stress disorder (PTSD).
Methods: High school senior students, who had been exposed to the 2009 L'Aquila earthquake, were enrolled 21 months after the traumatic event and evaluated by the Trauma and Loss Spectrum Self-Report to investigate PTSD rates and by a domain of the Mood Spectrum Self-Report–Lifetime Version (MOODS-SR), to explore alterations in circadian/seasonal rhythms and vegetative functions.
Results: The rates of endorsement of MOODS-SR rhythmicity and vegetative functions domain and subdomain scores were significantly higher in survivors with PTSD with respect to those without it. Among all earthquake survivors, women reported higher scores than men on the rhythmicity and vegetative functions domain and subdomain scores, except for the rhythmicity and sexual functions ones. Female survivors without PTSD showed significantly higher scores than men in the rhythmicity and vegetative functions total scores and the sleep and weight and appetite subdomains. Potentially traumatic events burden predicted rhythmicity and vegetative functions impairment, with a moderation effect of re-experiencing symptoms.
Conclusions: We report impairments in rhythmicity, sleep, eating, and sexual and somatic health in survivors to a massive earthquake, particularly among subjects with PTSD and higher re-experiencing symptoms, with specific gender-related differences. Evaluating symptoms of impaired rhythmicity and vegetative functions seems essential for a more accurate assessment and clinical management of survivors to a mass trauma.
Post-traumatic stress disorder (PTSD) is a complex syndrome that may occur after exposure to traumatic events, characterized by severe and often chronic psychological, physiological, and cognitive symptoms. Neurobiological dysfunctions and hormonal alterations have been reported to contribute to the manifestations of the disorder, particularly to the impairment in emotional regulation, memory, and learning. Generally, these symptoms have been explored through the investigation of endocrine rhythmicity and temporal synchrony in brain activity (1). Although most of the research focused on sleep organization, studies on endocrine rhythmicity revealed that some abnormalities of both cortisol and sympathetic nervous system activity are frequently, albeit not constantly, observed in PTSD patients (2, 3). The most typical changes are a flattening of the diurnal secretion of cortisol and the hyperactivity of the sympathetic nervous system; these alterations have also been linked to an impairment in cognitive functioning, in particular in consolidation of emotional memories, attention, learning, vigilance, and arousal (4, 5).
Although there is evidence of a relationship between trauma exposure, PTSD, and sleep alterations, scant data are still available about the onset of other types of alterations in vegetative functions in survivors to traumatic events, such as eating behaviors or sexual habits. Possible gender differences in such symptomatology have been even reported to a lesser extent. Current literature recognizes that women may have a two-fold risk for PTSD, with respect to men exposed to the same traumatic event (6–9). Again, increased evidence suggests the former to report more severe symptoms than the latter, with the only exception of reckless and self-destructive behaviors (10–15), although a few studies have specifically focused on neurovegetative alterations in PTSD.
Some greater short- and long-term sleep impairments have been reported in PTSD patients in the aftermath of both natural or war disasters, compared with the general population (16). These include difficulty falling asleep, engaging in atypical sleep disruptive behaviors, frequent awakenings, and nightmares (17–19). Women have also been reported to present a higher prevalence of event-related insomnia and nightmares than men (20–22).
The presence of PTSD has also been recently associated with impaired eating behaviors, such as night eating, food addiction, binge eating, maladaptive eating as a coping strategy, weight change, and overweight/obesity over time, up to the onset of full-blown eating disorders (23–25). Moreover, in samples exposed to a traumatic event, significantly higher rates of eating disorders in women than in men were described (26–28).
To a lesser extent, somatic symptoms and sexual dysfunctions have also been associated with PTSD (29–32). About 50–80% of PTSD patients complain of chronic physical symptoms (33, 34). A first study carried out in sample of 142 civilian war survivors showed women to report significantly higher levels of somatic symptoms than men, whereas levels of PTSD symptoms were similar in the two sexes (35). More recently, sexual dysfunctions, such as lack of sexual desire/pleasure or pain/problems during intercourse, have been reported in subjects exposed to trauma (36–39), but limited data are available for PTSD civilian patients. In a literature revision, Yehuda et al. (36) highlighted the comorbidity between sexual dysfunctions and PTSD in war veterans and proposed some biological and psychological underpinnings of this phenomenon. Another review including 11 articles on sexual dysfunctions in PTSD veterans showed that all but one study reported a significantly increased prevalence of sexual dysfunctions, especially erectile ones and decreased sexual desire (37). Finally, a recent study on 300 veterans with PTSD showed that sexual dysfunction was predicted by the severity of the D cluster of PTSD symptoms (39).
Italy is among the most seismically active countries in Europe, although deadly earthquakes are not very common. On April 6, 2009, an earthquake of Richter Magnitude 6.3 struck L'Aquila, Italy, a town with a population of 72,000 residents and a health district of 105,000 residents. The toll of the earthquake included 309 deaths, more than 1,600 individuals injured, and 66,000 displaced.
A great part of central Italy was involved by the seismic event, and large parts of the town of L'Aquila were destroyed. The final evaluation of the damages showed this as the fifth more devastating earthquake in Italy in the last century.
Given the paucity of available information, the present study was aimed at investigating alterations in rhythmicity and neurovegetative functions, with a particular attention to the gender differences and the impact of PTSD symptoms, among survivors of the 2009 L'Aquila earthquake in Italy.
The target population included high school senior students living in the town of L'Aquila, who had been exposed to the 2009 earthquake, enrolled 21 months after the catastrophic event. The total sample included 512 subjects (280 men and 232 women). Other details on sociodemographic characteristics and clinical of the study sample were reported elsewhere (8, 40). The Ethics Committee of the University of L'Aquila and the school councils approved all recruitment and assessment procedures. Subjects provided written informed consent after receiving a complete description of the study, in accordance with the Declaration of Helsinki.
The assessment instruments used in the present study included the modified versions of the Trauma and Loss Spectrum Self-Report (TALS-SR) (41) and the Mood Spectrum Self-Report–Lifetime Version (MOODS-SR) (42) to evaluate symptoms that occurred in the aftermath of the earthquake exposure. These instruments were developed in the framework of the international collaborative research project named Spectrum Project, aimed at developing and validating tools to diagnose the spectrum of clinical manifestations of the Diagnostic and Statistical Manual of Mental Disorders (DSM) disorders, and they showed a good validity and reliability (41, 42).
The TALS-SR includes 116 items exploring the lifetime experience of a range of loss and/or traumatic events and lifetime symptoms, behaviors, and personal characteristics that might represent manifestations and/or risk factors for the development of a stress response syndrome. It is composed by nine domains, namely, loss events (I), grief reactions (II), potentially traumatic events (III), reactions to losses or upsetting events (IV), re-experiencing (V), Avoidance and numbing (VI), maladaptive coping (VII), arousal (VIII), and personal characteristics/risk factors (IX). According to previous studies also including populations of young adults (40, 43, 44), DSM-5 PTSD diagnosis was assessed utilizing the following matching between symptom criteria and TALS-SR items: criterion B (B1 = 80, B2 = 77, B3 = 79, B4 = 78, and B5 = 81), criterion C (C1 = 86, C2 = 87 and/or 88 and/or 89), criterion D (D1 = 90, D2 = 95, D3 = 85, D4 = 96, D5 = 91, D6 = 93, and D7 = 92), and criterion E (E1 = 108, E2 = 99 and/or 100 and/or 102 and/or 103 and/or 104, E3 = 106, E4 = 107, E5 = 105, and E6 = 109). Because of the sample characteristics, criterion A was considered satisfied.
Trauma and Loss Spectrum Self-Report presented good intraclass correlation coefficients (from 0.934 to 0.994) with SCI-TALS, the interview version used for assessing post-traumatic stress symptomatology. SCI-TALS, similarly, showed a good internal consistency (Kuder–Richardson coefficient exceeding the minimum standard of 0.50 for each domain) (41).
The MOODS-SR is a 140-item questionnaire exploring mood spectrum symptoms, coded dichotomously, as present or absent, for one or more periods of at least 3–5 days. According to previous researches and to the aims of the present study (11, 26, 43), we adopted a modified version of the instrument assessing symptoms developed in the aftermath of earthquake. Items are organized into three manic and three depressive domains, exploring “mood,” “energy,” and “cognition,” besides a rhythmicity and vegetative functions domain. This latter explores alterations in the circadian rhythms and vegetative functions, including changes in energy; physical well-being; mental and physical efficiency related to the weather and season; and changes in appetite, sleep, and sexual activities across 29 items (26, 43).
The rhythmicity subdomain consists of six items investigating the changes in mood, energy, interests and efficiency during the course of the year or even during the day, according to the weather, the season, and the phase of menstrual cycle or in case of disruption of circadian rhythms. The vegetative functions subdomains are sleep (12 items, investigating insomnia, sleepiness, reduced need for sleep, changes in sleep related to external stimuli, season, jet-lag syndrome, and menstrual cycle), weight and appetite (4 items, concerning changes in taste, changes in appetite and weight, craving for carbohydrates), sexual functions (5 items, examining the reduction of sexual interest, difficulties with sexual stimulation and orgasm, increased sexual interest, and tendency to promiscuity), and physical symptoms (5 items, regarding headache, xerostomia, constipation, nausea, and other gastrointestinal problems).
Mood Spectrum Self-Report–Lifetime Version, the self-report version of SCI-MOODS, presented good intraclass correlation coefficients (from 0.88 to 0.97) with the interview format (SCI-MOODS).
SCI-MOODS, the interview version for assessing mood symptomatology, had a good internal consistency (Cronbach α ranged between 0.72 and 0.92) (42).
The Mann–Whitney U tests were computed in order to compare MOODS-SR domain scores and vegetative functions subdomain scores in men vs. women, and PTSD vs. non-PTSD survivors.
Furthermore, to study the possible interaction effects of gender and PTSD on each MOODS-SR domain and rhythmicity and vegetative functions subdomain score, we performed the gender comparison within the subsample of patient with and without PTSD.
A multiple linear regression was used to study the relationships between the TALS-SR domains and the MOODS-SR rhythmicity and vegetative functions domain and to identify the strongest predictors. Subsequently, taking into account the TALS-SR domains that showed a significant association with the MOODS-SR rhythmicity and vegetative functions domain, a moderation analysis (with predictor and moderator centered before the analysis) was conducted. The Hayes's PROCESS tool was utilized.
All statistical analyses were carried out using the Statistical Package for Social Science (SPSS Inc., Chicago, IL, 2018), version 25.0.
Full data were available for 450 subjects, of whom 197 (47.8%) were women and 253 (56.2%) were men (mean age ± SD, 17.64 ± 0.78 years). A PTSD diagnosis, according to the DSM-5 criteria, was present in 162 (36.0%) subjects, specifically in 61 men (24.1%) and 101 women (51.3%).
The rates of endorsement of the MOODS-SR rhythmicity and vegetative functions domain were significantly higher (p < 0.001) among survivors with PTSD (2.81 ± 3.67) with respect to those without it (0.98 ± 2.67). Statistically significantly higher total domain scores were present in the total sample in female (1.95 ± 2.93) than in male survivors (1.40 ± 2.99) (p < 0.001). Again, women without PTSD (1.18 ± 2.06) showed higher scores than men (0.88 ± 2.33) (p = 0.008).
All the MOODS-SR rhythmicity and vegetative functions subdomain scores were significantly higher in survivors with PTSD than in those without it (Table 1).
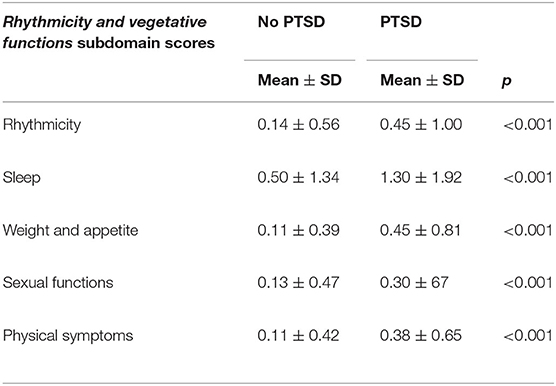
Table 1. Comparison of MOODS-SR rhythmicity and vegetative functions subdomain scores among L'Aquila earthquake survivors with (n = 162) and without (n = 288) PTSD.
Among all earthquake survivors, statistically significantly higher mean scores of all the rhythmicity and vegetative functions subdomain scores, with the only exception of the rhythmicity and sexual functions subdomains, were detected in women than in men. In the subgroup of survivors without PTSD, female survivors showed significantly higher scores in the sleep and weight and appetite subdomains with respect with male ones (Table 2).
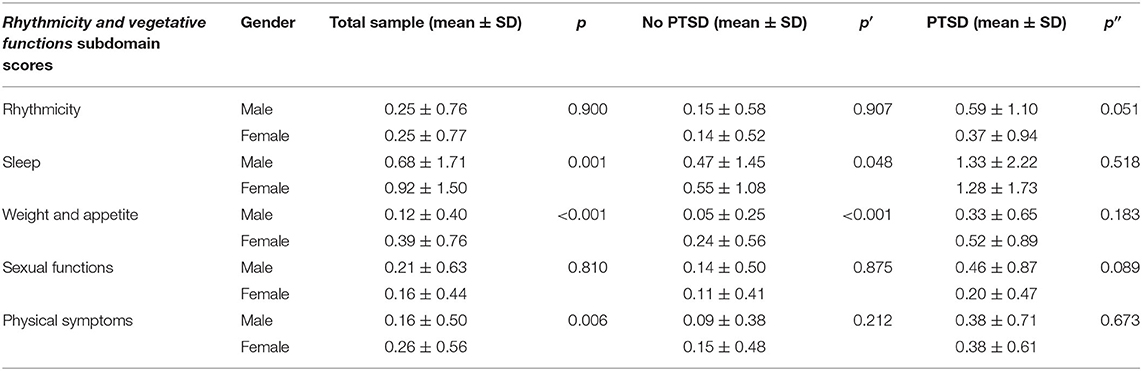
Table 2. Gender differences in MOODS-SR Rhythmicity and vegetative functions subdomain scores in the total sample (n = 450) and in L'Aquila earthquake survivors with (n = 162) or without (n = 288) PTSD.
The multiple linear regression model, which provided the TALS-SR domain scores as independent variables and the MOODS-SR rhythmicity and vegetative functions domain score as a dependent variable, identified TALS-SR potentially traumatic events (III), re-experiencing (V), and maladaptive coping (VII) domain scores as significant predictors (Table 3).
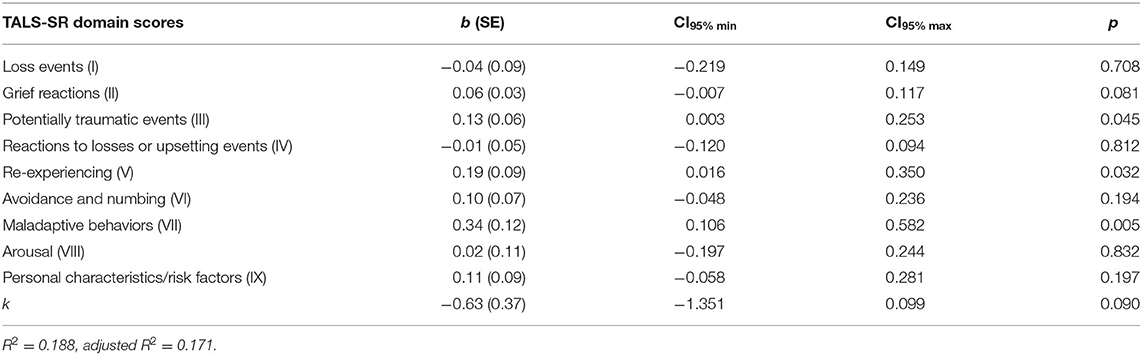
Table 3. Multiple linear regression: TALS-SR domain scores as predictive variables and MOODS-SR rhythmicity and vegetative functions domain score as dependent variable.
Investigating on the possible interactions of TALS-SR potentially traumatic events with re-experiencing and/or with maladaptive coping domains, a significant moderation effect of re-experiencing was found. The interaction was highly significant, b = 0.10, CI95% (0.052, 0.140), p < 0.001, indicating that the relationship between TALS-SR potentially traumatic events domain and the MOODS-SR rhythmicity and vegetative functions domain was moderated by the TALS-SR re-experiencing domain (Table 4A). Particularly, observing the conditional effect of the TALS-SR potentially traumatic events on MOODS-SR rhythmicity and vegetative functions domain at the values of the moderator TALS-SR re-experiencing domain, the relationship between potentially traumatic events and rhythmicity and vegetative functions domains emerged in subjects with medium–high levels of re-experiencing only (Table 4B).
To the best of our knowledge, this is the first study to specifically explore alterations in rhythmicity and vegetative functions in young adults surviving a massive earthquake, with a particular attention to the association with gender and PTSD diagnosis.
As previously reported in a related study (8), females reported double rates of PTSD diagnosis with respect to males. This result is in line with previous findings from other researches, in the aftermath of a disaster, in which females were twice as likely to develop a full PTSD rather than males, with mean rates up to more than 50% (45–47).
Our data highlighted the presence of a sort of disruption in rhythmicity and vegetative functions, with statistically significantly higher rates in survivors with than in those without PTSD. Even if we have no follow-up data on the same cohort of subjects, we hypothesize that the PTSD symptomatology, emerged in our sample, had a chronic course, with a stability of symptoms over time, as shown by previous studies on similar samples in the aftermath of a catastrophic traumatic event (8, 10, 26, 40, 48, 49). So, we may argue that alterations in rhythmicity and vegetative functions, specifically emerged in our research in the aftermath of the earthquake, were related to the PTSD symptomatology.
Despite female survivors showed significantly more sleep alterations, impairment in eating behaviors, and somatic symptoms than male ones, a trend toward a similar symptom severity across gender in survivors with PTSD can be observed. In particular, comparing survivors with and without PTSD, we found significant differences in the rhythmicity subdomain. Previous studies described how alterations in the rhythmicity of endocrine secretion, sleep patterns, or temporal synchrony of brain activity could be related to PTSD severity (1). Noteworthy, no gender differences emerged in rhythmicity symptoms in our sample, while suggesting a similar pattern of dysregulation across genders that should be further investigated in larger samples. It is interesting to highlight that alterations were actually slightly higher in men with PTSD. We may argue that these disturbances in circadian rhythms could be related to a mood spectrum diathesis in men already found to be associated with a higher incidence of maladaptive behaviors (10–13).
According to the current diagnostic criteria, sleep alterations are considered a core symptom of PTSD (50, 51). Approximately 70% of PTSD individuals report co-occurring sleep problems, such as greater trouble initiating and maintaining sleep (19), which might contribute to the development and maintenance of the disorder (52–54). Indeed, sleep problems prior to any traumatic event may increase the likelihood of developing PTSD (55), and they often do not remit after PTSD-focused interventions (56). Recently, sleep alterations were also identified as potential risk factors for suicidal ideation and attempts in PTSD (57, 58). Our results corroborated the higher prevalence of these symptoms in women exposed to a traumatic event, even without the onset of a full-blown post-traumatic symptomatology (20–22). Kobayashi et al. (21), in a sample of 45 (17 women and 28 men) non-amnesic injured patients admitted to a trauma center, reported women to present more nightmares and sleep-interfering disruptive nocturnal behaviors, especially hot flashes and memories/nightmares of trauma soon after their injuries, compared with men. Ansara and Hindin (20) collected data from a sample of 676 female and 455 male victims of interpersonal violence and highlighted a greater presence of sleep problems in the former than in the latter exposed to similar traumatic experiences. More recently, after examining the prevalence of insomnia and nightmares within a national sample of 2,647 adults who had been exposed to one or more potentially traumatic event, women were more likely to endorse experience of insomnia and nightmares compared to men, while individuals suffering from PTSD showed no gender-related differences (22).
Association between traumatic life events and dysfunctional eating pattern has also been widely studied (59–62). Findings suggest that emotional eating is common in veterans reporting PTSD symptoms that, even of any degree of severity, seem to be associated with more emotional eating (63). Moreover, a correlation was reported between PTSD symptomatology and both underweight and obese conditions in a sample of young people who experienced different traumas (64). Post-traumatic stress disorder patients suffer from more obesity and higher waist-to-hip ratio than subjects without such disorder (65, 66). Indeed, the association between PTSD and eating disorders or diet dysregulation in the female gender has been stressed. Post-traumatic stress disorder symptoms were associated with an increased frequency of binge eating, as well as unhealthy dieting behaviors (67). In a sample of 103 women with eating disorders, 23.1% of anorexia nervosa and 25.5% of bulimia nervosa patients reported a current diagnosis of PTSD, with more severe eating symptomatology in those with cumulative traumatization (68). Mason et al. (24) reported an association between PTSD symptoms and obesity only in women with respect to men in a sample of 7,438 subjects.
Recent studies highlighted the relationship between PTSD and physical symptoms (43, 69–72). Subjects with both PTSD and chronic physical symptoms report a greater severity of the clinical picture (73, 74), a worse prognosis (73, 75), and a greater disability (76–78) than those with PTSD or physical symptoms only. McAndrew et al. (79) suggested that there could be a bidirectional relationship; that is, increases in PTSD symptoms would predict later increases in physical symptoms, or increases in physical symptoms would predict later increases in PTSD symptoms. In our sample, the physical symptoms resulted to be more compromised in women than in men, but in subjects who develop PTSD, the gender difference seemed to disappear. In this regard, contrasting data have been reported in the literature. The pathway from trauma exposure to finally reduced or impaired somatic health, mediated by PTSD, seems to be more pronounced in men than in women (80). A systematic review confirmed that individuals who reported exposure to trauma, especially men, showed an increased prevalence of functional somatic syndromes and how the magnitude of the association with PTSD was significantly stronger than with trauma exposure alone (81). Conversely, in a recent study, women showed higher levels of somatization symptoms than men, whereas levels of PTSD symptoms were similar in the two sexes (35).
This study also reported sexual dysfunctions in the aftermath of an earthquake. It has also been reported in the literature that sexual dysfunctions are greater in trauma-exposed individuals with PTSD, compared with similarly exposed survivors without it, regardless of the nature of the trauma (82–86). Our data showed sexual dysfunctions mainly in men, in both the total sample of survivors and in the subsamples with or without PTSD, although these findings were not statistically significant. Scant data are available about gender differences in sexual dysfunctions in PTSD patients and mainly concern veterans. Studies on male combat-exposed veterans found that erectile dysfunction was present in 85% of veterans with PTSD vs. 22% of veterans without it, with a three-fold increased risk of sexual dysfunction induced by PTSD (83). Furthermore, 25.1% of male veterans and 12.7% of female ones presented a sexual dysfunction diagnosis and/or prescription treatment for sexual dysfunction, especially if they were suffering from PTSD (87). Male veterans with PTSD had similar levels of sexual activity, compared with those without PTSD, whereas women with PTSD were less likely to be sexually active compared to women without PTSD. Veterans with PTSD were also less likely to report sex-life satisfaction, as compared with those without PTSD (87). In other studies on war veterans, women reported more sexual problems and less sexual satisfaction than men post-deployment (88), and female veterans who had PTSD and/or depression were more likely to report painful sex and were less likely to say sex is important and less likely to be emotionally satisfied in their relationship (89).
Our results also showed the number of potentially traumatic events, related to the earthquake, as predictors of the MOODS-SR rhythmicity and vegetative functions impairment, positively moderated by re-experiencing symptoms. There is evidence that traumatic experiences, including disasters, lead to changes in diurnal cortisol patterns (90–92) and that a cumulative trauma exposure may progressively disrupt circadian rhythms and other vegetative functions, including sleep, eating behaviors, or somatic complaints (70, 93–99). Our results showed the relationship between the traumatic events burden and rhythmicity and vegetative functions impairment in subjects with medium–high levels of re-experiencing symptoms only. A possible interpretation is that re-experiencing symptoms may lead to chronic sleep disruption, which may further pre-dispose the patient to severe distress and impairment (100, 101), and traumatic event-related thoughts may represent conditioned stimuli that elicit a conditioned waking response (102). Further, daytime re-experiencing symptoms are theorized to be the result of a failure to fully elaborate, integrate, and process traumatic event–related stimuli and subsequent information (103–105), leading to prolonged rumination on the trauma and its consequences. Interestingly, a moderate, positive relationship between rumination and PTSD symptoms in trauma-exposed adults was previously reported (106), with a stronger association between rumination and intrusive re-experiencing than avoidance or hyperarousal. In this perspective, emerging literature suggests the possible role of re-experiencing and trauma's rumination in fully mediating the relationship between distress and somatic complaints (43, 94, 107).
While interpreting the results of the present study, some important limitations should be taken into account. First is the lack of information about Axis I psychiatric comorbidities, such as mood disorders, and any related treatment, which could impact rhythmicity and vegetative functions. Second is the use of self-report instruments that could be considered less accurate than the rating of the clinician. Third is the homogeneity of the study sample that included only non-clinical high school students. Fourth is the use of a domain of a self-report questionnaire not specifically validated as independent questionnaire for assessing the daily and monthly changes in rhythmicity and vegetative functions. Finally is the lack of evaluation of the impact of chronotype on the PTSD symptomatology.
In conclusion, our results highlight impairments in rhythmicity, sleep, eating, and sexual and somatic health in survivors to a massive earthquake, particularly among subjects with PTSD, with differences between the two genders. Potentially traumatic events burden predicts rhythmicity and vegetative functions impairment, with a moderation effect of re-experiencing symptoms. Evaluating symptoms of impaired rhythmicity and vegetative functions seems essential for a more accurate assessment and clinical management of survivors to mass traumas, such as earthquakes or other catastrophic events.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of the University of L'Aquila. The patients/participants provided their written informed consent to participate in this study.
CC, AR, and LD participated to the conception and design of the study. CC and VD participated to the interpretation of the data, the draft and critical revision of this article. VD and GM undertook the statistical analysis. CC, VD, GM, AR, CF, ED, and FM participated to the critical revision of the manuscript. All authors agreed to be cited as co-authors, accepting the order of authorship, and approved the final version of manuscript and the manuscript submission to Frontiers in Psychiatry.
The University funding of the Universities of Pisa and L'Aquila supported the present study; no other economic source was interested.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Dayan J, Rauchs G, Guillery-Girard B. Rhythms dysregulation: a new perspective for understanding PTSD? J Physiol Paris. (2016)110:453–60. doi: 10.1016/j.jphysparis.2017.01.004
2. Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. (2012) 37:1039–47. doi: 10.1016/j.psyneuen.2011.12.001
3. Nicholson EL, Bryant RA, Felmingham KL. Interaction of noradrenaline and cortisol predicts negative intrusive memories in posttraumatic stress disorder. Neurobiol Learn Mem. (2014) 112:204–11. doi: 10.1016/j.nlm.2013.11.018
4. Bremner JD, Vermetten E. The hippocampus and post-traumatic disorders. In: Bartsch T, editor. The Clinical Neurobiology of the Hippocampus: An Integrative View. Oxford: Oxford University Press (2012). p. 262–72.
5. Barsegyan A, McGaugh JL, Roozendaal B. Noradrenergic activation of the basolateral amygdala modulates the consolidation of object-in-context recognition memory. Front Behav Neurosci. (2014) 8:160. doi: 10.3389/fnbeh.2014.00160
6. Christiansen DM, Elklit A. Risk factors predict post-traumatic stress disorder differently in men and women. Ann Gen Psychiatry. (2008) 7:24. doi: 10.1186/1744-859X-7-24
7. Ditlevsen DN, Elklit A. Gender, trauma type, and PTSD prevalence: a re-analysis of 18 nordic convenience samples. Ann Gen Psychiatry. (2012) 11:26. doi: 10.1186/1744-859X-11-26
8. Dell'Osso L, Carmassi C, Massimetti G, Daneluzzo E, Di Tommaso S, Rossi A. Full and partial PTSD among young adult survivors 10 months after the L'Aquila 2009 earthquake:gender differences. J Affect Disord. (2011) 131:79–83. doi: 10.1016/j.jad.2010.11.023
9. Carmassi C, Foghi C, Dell'Oste V, Bertelloni CA, Fiorillo A, Dell'Osso L. Risk and protective factors for PTSD in caregivers of adult patients with severe medical illnesses: a systematic review. Int J Environ Res Public Health. (2020) 17:5888. doi: 10.3390/ijerph17165888
10. Dell'Osso L, Carmassi C, Stratta P, Massimetti G, Akiskal KK, Akiskal HS, et al. Gender differences in the relationship between maladaptive behaviors and post-traumatic stress disorder. A study on 900 L'Aquila 2009 earthquake survivors. Front Psychiatry. (2013) 3:111. doi: 10.3389/fpsyt.2012.00111
11. Dell'Osso L, Stratta P, Conversano C, Massimetti E, Akiskal KK, Akiskal HS, et al. Lifetime mania is related to post-traumatic stress symptoms in high school students exposed to the 2009 L'Aquila earthquake. Compr Psychiatry. (2014) 55:357–62. doi: 10.1016/j.comppsych.2013.08.017
12. Carmassi C, Stratta P, Massimetti G, Bertelloni CA, Conversano C, Cremone IM, et al. New DSM-5 maladaptive symptoms in PTSD: gender differences and correlations with mood spectrum symptoms in a sample of high school students following survival of an earthquake. Ann Gen Psychiatry. (2014) 13:28. doi: 10.1186/s12991-014-0028-9
13. Carmassi C, Bertelloni CA, Gesi C, Conversano C, Stratta P, Massimetti G, et al. New DSM-5 PTSD guilt and shame symptoms among Italian earthquake survivors: impact on maladaptive behaviors. Psychiatry Res. (2017) 251:142–7. doi: 10.1016/j.psychres.2016.11.026
14. Carmassi C, Bertelloni CA, Salarpi G, Diadema E, Avella MT, Dell'Oste V, et al. Is there a major role for undetected autism spectrum disorder with childhood trauma in a patient with a diagnosis of bipolar disorder, self-injuring, and multiple comorbidities? Case Rep Psychiatry. (2019) 2019:4703795. doi: 10.1155/2019/4703795
15. Carpita B, Muti D, Muscarella A, Dell'Oste V, Diadema E, Massimetti G, et al. Sex differences in the relationship between PTSD spectrum symptoms and autistic traits in a sample of university students. Clin Pract Epidemiol Ment Health. (2019) 15:110–9. doi: 10.2174/1745017901915010110
16. Sinha SS. Trauma-induced insomnia: a novel model for trauma and sleep research. Sleep Med Rev. (2016) 25:74–83. doi: 10.1016/j.smrv.2015.01.008
17. Calhoun PS, Wiley M, Dennis MF, Means MK, Edinger JD, Beckham JC. Objective evidence of sleep disturbance in women with posttraumatic stress disorder. J Trauma Stress. (2007) 20:1009–18. doi: 10.1002/jts.20255
18. Mysliwiec V, Brock MS, Creamer JL, O'Reilly BM, Germaine A, Roth B. Trauma associated sleep disorder: a parasomnia induced by trauma. Sleep Med Rev. (2018) 37:94–104. doi: 10.1016/j.smrv.2017.01.004
19. Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. (2000) 41:469–78. doi: 10.1053/comp.2000.16568
20. Ansara DL, Hindin MJ. Psychosocial consequences of intimate partner violence for women and men in Canada. J Interpers Violence. (2011) 26:1628–45. doi: 10.1177/0886260510370600
21. Kobayashi I, Delahanty DL. Gender differences in subjective sleep after trauma and the development of posttraumatic stress disorder symptoms: a pilot study. J Trauma Stress. (2013) 26:467–74. doi: 10.1002/jts.21828
22. Milanak ME, Zuromski KL, Cero I, Wilkerson AK, Resnick HS, Kilpatrick DG. Traumatic event exposure, posttraumatic stress disorder, and sleep disturbances in a national sample of U.S. adults. J Trauma Stress. (2019) 32:14–22. doi: 10.1002/jts.22360
23. Mitchell KS, Wolf EJ. PTSD, food addiction, and disordered eating in a sample of primarily older veterans: the mediating role of emotion regulation. Psychiatry Res. (2016) 243:23–9. doi: 10.1016/j.psychres.2016.06.013
24. Mason SM, Frazier PA, Austin SB, Harlow BL, Jackson B, Raymond NC, et al. Posttraumatic stress disorder symptoms and problematic overeating behaviors in young men and women. Ann Behav Med. (2017) 51:822–32. doi: 10.1007/s12160-017-9905-1
25. Dorflinger LM, Ruser CB, Masheb RM. Night eating among veterans with obesity. Appetite. (2017) 117:330–4. doi: 10.1016/j.appet.2017.07.011
26. Carmassi C, Bertelloni CA, Massimetti G, Miniati M, Stratta P, Rossi A, et al. Impact of DSM-5 PTSD and gender on impaired eating behaviors in 512 Italian earthquake survivors. Psychiatry Res. (2015) 225:64–9. doi: 10.1016/j.psychres.2014.10.008
27. Breland JY, Phibbs CS, Hoggatt KJ, Washington DL, Lee J, Haskell S, et al. The obesity epidemic in the veterans health administration: prevalence among key populations of women and men veterans. J Gen Intern Med. (2017) 32(Suppl. 1):11–7. doi: 10.1007/s11606-016-3962-1
28. Mitchell KS, Mazzeo SE, Schlesinger MR, Brewerton TD, Smith BN. Comorbidity of partial and subthreshold ptsd among men and women with eating disorders in the national comorbidity survey-replication study. Int J Eat Disord. (2012) 45:307–15. doi: 10.1002/eat.20965
29. Kotler M, Cohen H, Aizenberg D, Matar M, Loewenthal U, Kaplan Z, et al. Sexual dysfunction in male posttraumatic stress disorder patients. Psychother Psychosom. (2000) 69:309–15. doi: 10.1159/000012413
30. Westermeyer JJ, Campbell R, Lien R, Spring M, Johnson DR, Butcher J, et al. HADStress: a somatic symptom screen for posttraumatic stress among Somali refugees. Psychiatr Serv. (2010) 61:1132–7. doi: 10.1176/ps.2010.61.11.1132
31. Gupta MA. Review of somatic symptoms in post-traumatic stress disorder. Int Rev Psychiatry. (2013) 25:86–99. doi: 10.3109/09540261.2012.736367
32. Noel M, Wilson AC, Holley AL, Durkin L, Patton M, Palermo TM. Posttraumatic stress disorder symptoms in youth with vs without chronic pain. Pain. (2016) 157:2277–84. doi: 10.1097/j.pain.0000000000000642
33. Amital D, Fostick L, Polliack ML, Segev S, Zohar J, Rubinow A, et al. Posttraumatic stress disorder, tenderness, and fibromyalgia syndrome: are they different entities? J Psychosom Res. (2006) 61:663–9. doi: 10.1016/j.jpsychores.2006.07.003
34. Beckham JC, Crawford AL, Feldman ME, Kirby AC, Hertzberg MA, Davidson JR, et al. Chronic posttraumatic stress disorder and chronic pain in Vietnam combat veterans. J Psychosom Res. (1997) 43:379–89. doi: 10.1016/S0022-3999(97)00129-3
35. Morina N, Schnyder U, Klaghofer R, Müller J, Martin-Soelch C. Trauma exposure and the mediating role of posttraumatic stress on somatic symptoms in civilian war victims. BMC Psychiatry. (2018) 18:92. doi: 10.1186/s12888-018-1680-4
36. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. (2015) 12:1107–19. doi: 10.1111/jsm.12856
37. Bentsen IL, Giraldi AG, Kristensen E, Andersen HS. Systematic review of sexual dysfunction among veterans with post-traumatic stress disorder. Sex Med Rev. (2015) 3:78–87. doi: 10.1002/smrj.47
38. Richardson JD, Ketcheson F, King L, Forchuk CA, Hunt R, St Cyr K, et al. Sexual dysfunction in male Canadian armed forces members and veterans seeking mental health treatment. Mil Med. (2019) 185:68–74. doi: 10.1093/milmed/usz163
39. Letica-Crepulja M, Stevanović A, Protuder M, Popović B, Salopek-Žiha D, Vondraček S. Predictors of sexual dysfunction in veterans with post-traumatic stress disorder. J Clin Med. (2019) 8:432. doi: 10.3390/jcm8040432
40. Carmassi C, Akiskal HS, Yong SS, Stratta P, Calderani E, Massimetti E, et al. Post-traumatic stress disorder in DSM-5: estimates of prevalence and criteria comparison versus DSM-IV-TR in a non-clinical sample of earthquake survivors. J Affect Disord. (2013) 151:843–8. doi: 10.1016/j.jad.2013.07.020
41. Dell'Osso L, Carmassi C, Rucci P, Conversano C, Shear MK, Calugi S, et al. A multidimensional spectrum approach to post-traumatic stress disorder: comparison between the structured clinical interview for trauma and loss spectrum (SCI-TALS) and the self-report instrument (TALS-SR). Compr Psychiatry. (2009) 50:485–90. doi: 10.1016/j.comppsych.2008.11.006
42. Dell'Osso L, Armani A, Rucci P, Frank E, Fagiolini A, Corretti G, et al. Measuring mood spectrum: comparison of interview (SCI-MOODS) and self-report (MOODS-SR) instruments. Compr Psychiatry. (2002) 43:69–73. doi: 10.1053/comp.2002.29852
43. Carmassi C, Dell'Oste V, Barberi FM, Pedrinelli V, Cordone A, Cappelli A, et al. Do somatic symptoms relate to PTSD and gender after earthquake exposure? A cross-sectional study on young adult survivors in Italy. CNS Spectr. (2020) 1–7. doi: 10.1017/S1092852920000097. [Epub ahead of print].
44. Carmassi C, Bertelloni CA, Dell'Oste V, Foghi C, Diadema E, Cordone A, et al. Post-traumatic stress burden in a sample of hospitalized patients with bipolar disorder: which impact on clinical correlates and suicidal risk?. J Affect Disord. (2020) 262:267–72. doi: 10.1016/j.jad.2019.10.044
45. Bowler RM, Han H, Gocheva V, Nakagawa S, Alper H, DiGrande L, et al. Gender differences in probable posttraumatic stress disorder among police responders to the 2001 World Trade Center terrorist attack. Am J Ind Med. (2010) 53:1186–96. doi: 10.1002/ajim.20876
46. Marthoenis M, Nirwana A, Fathiariani L. Prevalence and determinants of posttraumatic stress in adolescents following an earthquake. Indian J Psychiatry. (2019) 61:526–8. doi: 10.4103/psychiatry.IndianJPsychiatry_35_19
47. Lai TJ, Chang CM, Connor KM, Lee LC, Davidson JR. Full andpartial PTSD among earthquake survivors in rural Taiwan. J Psychiatr Res. (2004) 38:313–22. doi: 10.1016/j.jpsychires.2003.08.005
48. Buselli R, Corsi M, Baldanzi S, Chiumiento M, Del Lupo E, Dell'Oste V, et al. Professional quality of life and mental health outcomes among health care workers exposed to Sars-Cov-2 (Covid-19). Int J Environ Res Public Health. (2020) 17:6180. doi: 10.3390/ijerph17176180
49. Carmassi C, Foghi C, Dell'Oste V, Cordone A, Bertelloni CA, Bui E, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. (2020) 292:113312. doi: 10.1016/j.psychres.2020.113312
50. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, UT: American Psychiatric Publishing (2013).
51. Carmassi C, Bertelloni CA, Cordone A, Cappelli A, Massimetti E, Dell'Oste V, Dell'Osso L. Exploring mood symptoms overlap in PTSD diagnosis: ICD-11 and DSM-5 criteria compared in a sample of subjects with Bipolar Disorder. J Affect Disord. (2020) 276:205–11. doi: 10.1016/j.jad.2020.06.056
52. Mellman TA, Hipolito MM. Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr. (2006) 11:611–5. doi: 10.1017/S1092852900013663
53. Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. (2008) 12:185–95. doi: 10.1016/j.smrv.2007.09.003
54. Brownlow JA, Harb GC, Ross RJ. Treatment of sleep disturbances in post-traumatic stress disorder: a review of the literature. Curr Psychiatry Rep. (2015) 17:41. doi: 10.1007/s11920-015-0587-8
55. Bryant RA, Creamer M, O'Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. (2010) 33:69–74. doi: 10.1093/sleep/33.1.69
56. Nappi CM, Drummond SP, Hall JM. Treating nightmares and insomnia in posttraumatic stress disorder: a review of current evidence. Neuropharmacology. (2012) 62:576–85. doi: 10.1016/j.neuropharm.2011.02.029
57. Colvonen PJ, Straus LD, Stepnowsky C, McCarthy MJ, Goldstein LA, Norman SB. Recent advancements in treating sleep disorders in co-occurring PTSD. Curr Psychiatry Rep. (2018) 20:48. doi: 10.1007/s11920-018-0916-9
58. Dell'Osso L, Massimetti G, Conversano C, Bertelloni CA, Carta MG, Ricca V, et al. Alterations in circadian/seasonal rhythms and vegetative functions are related to suicidality in DSM-5 PTSD. BMC Psychiatry. (2014) 14:352. doi: 10.1186/s12888-014-0352-2
59. Micali N, Martini MG, Thomas JJ, Eddy KT, Kothari R, Russell E, et al. Lifetime and 12-month prevalence of eating disorders amongst women in mid-life: a population-based study of diagnoses and risk factors. BMC Med. (2017) 15:12. doi: 10.1186/s12916-016-0766-4
60. Sachs-Ericsson N, Keel PK, Holland L, Selby EA, Verona E, Cougle JR, et al. Parental disorders, childhood abuse, and binge eating in a large community sample. Int J Eat Disord. (2012) 45:316–25. doi: 10.1002/eat.20938
61. Bartlett BA, Iverson KM, Mitchell KS. Intimate partner violence and disordered eating among male and female veterans. Psychiatry Res. (2018) 260:98–104. doi: 10.1016/j.psychres.2017.11.056
62. Afifi TO, Sareen J, Fortier J, Taillieu T, Turner S, Cheung K, et al. Child maltreatment and eating disorders among men and women in adulthood: results from a nationally representative United States sample. Int J Eat Disord. (2017) 50:1281–96. doi: 10.1002/eat.22783
63. Dorflinger LM, Masheb RM. PTSD is associated with emotional eating among veterans seeking treatment for overweight/obesity. Eat Behav. (2018) 31:8–11. doi: 10.1016/j.eatbeh.2018.07.005
64. Roenholt S, Beck NN, Karsberg SH, Elklit A. Post-traumatic stress symptoms and childhood abuse categories in a national representative sample for a specific age group: associations to body mass index. Eur J Psychotraumatol. (2012) 3:17188. doi: 10.3402/ejpt.v3i0.17188
65. Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. (2010) 39:61–78. doi: 10.1007/s12160-010-9165-9
66. Pagoto S, Schneider KL, Bodenlos JS, Appelhans BM, Whited MC, Ma Y, et al. Association of post-traumatic stress disorder and obesity in a nationally representative sample. Obesity. (2012) 20:200–5. doi: 10.1038/oby.2011.318
67. Buchholz LJ, King PR, Wray LO. Rates and correlates of disordered eating among women veterans in primary care. Eat Behav. (2018) 30:28–34. doi: 10.1016/j.eatbeh.2018.05.002
68. Tagay S, Schlottbohm E, Reyes-Rodriguez ML, Repic N, Senf W. Eating disorders, trauma, PTSD, and psychosocial resources. Eat Disord. (2014) 22:33–49. doi: 10.1080/10640266.2014.857517
69. Kapfhammer HP. Acute and long-term mental and physical sequelae in the aftermath of traumatic exposure—some remarks on “the body keeps the score”. Psychiatr Danub. (2018) 30:254–72. doi: 10.24869/psyd.2018.254
70. Conversano C, Carmassi C, Bertelloni CA, Marchi L, Micheloni T, Carbone MG, et al. Potentially traumatic events, post-traumatic stress disorder and post-traumatic stress spectrum in patients with fibromyalgia. Clin Exp Rheumatol. (2019) 37(Suppl. 116):39–43.
71. Carmassi C, Dell'Oste V, Ceresoli D, Moscardini S, Bianchi E, Landi R, et al. Frequent attenders in general medical practice in Italy: a preliminary report on clinical variables related to low functioning. Neuropsychiatr Dis Treat. (2018) 15:115–25. doi: 10.2147/NDT.S179013
72. Carmassi C, DellOste V, Cordone A, Pedrinelli V, Cappelli A, Ceresoli D, et al. Relationships between somatic symptom and panic-agoraphobic spectrum among frequent attenders of the general practice in Italy. J Nerv Ment Dis. (2020) 208:540–8. doi: 10.1097/NMD.0000000000001163
73. Morasco BJ, Lovejoy TI, Lu M, Turk DC, Lewis L, Dobscha SK. The relationship between PTSD and chronic pain: mediating role of coping strategies and depression. Pain. (2013) 154:609–16. doi: 10.1016/j.pain.2013.01.001
74. Vaegter HB, Andersen TE, Harvold M, Andersen PG, Graven-Nielsen T. Increased pain sensitivity in accident-related chronic pain patients with comorbid posttraumatic stress. Clin J Pain. (2018) 34:313–21. doi: 10.1097/AJP.0000000000000543
75. Rosenbloom BN, Khan S, McCartney C, Katz J. Systematic review of persistent pain and psychological outcomes following traumatic musculoskeletal injury. J Pain Res. (2013) 6:39–51. doi: 10.2147/JPR.S38878
76. Martin AL, Halket E, Asmundson GJ, Flora DB, Katz J. Posttraumatic stress symptoms and the diathesis-stress model of chronic pain and disability in patients undergoing major surgery. Clin J Pain. (2010) 26:518–27. doi: 10.1097/AJP.0b013e3181e15b98
77. Outcalt SD, Kroenke K, Krebs EE, Chumbler NR, Wu J, Yu Z, et al. Chronic pain and comorbid mental health conditions: independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. J Behav Med. (2015) 38:535–43. doi: 10.1007/s10865-015-9628-3
78. Akerblom S, Perrin S, Rivano Fischer M, McCracken LM. The impact of PTSD on functioning in patients seeking treatment for chronic pain and validation of the posttraumatic diagnostic scale. Int J Behav Med. (2017) 24:249–59. doi: 10.1007/s12529-017-9641-8
79. McAndrew LM, Lu SE, Phillips LA, Maestro K, Quigley KS. Mutual maintenance of PTSD and physical symptoms for veterans returning from deployment. Eur J Psychotraumatol. (2019) 10:1608717. doi: 10.1080/20008198.2019.1608717
80. Schnurr PP, Wachen JS, Green BL, Kaltman S. Trauma exposure, PTSD, and physical health. In: Friedman MJ, Keane TM, Resick TA, editors. Handbook of PTSD. Practice and Science. 2nd ed. New York, NY: Guilford Press (2014). p. 502–21.
81. Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, et al. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. (2014) 76:2–11. doi: 10.1097/PSY.0000000000000010
82. Badour CL, Gros DF, Szafranski DD, Acierno R. Problems in sexual functioning among male OEF/OIF veterans seeking treatment for posttraumatic stress. Compr Psychiatry. (2015) 58:74–81. doi: 10.1016/j.comppsych.2014.12.012
83. Cosgrove DJ, Gordon Z, Bernie JE, Hami S, Montoya D, Stein MB, et al. Sexual dysfunction in combat veterans with post-traumatic stress disorder. Urology. (2002) 60:881–4. doi: 10.1016/S0090-4295(02)01899-X
84. Dekel R, Solomon Z. Marital relations among former prisoners of war: contribution of posttraumatic stress disorder, aggression, and sexual satisfaction. J Fam Psychol. (2006) 20:709–12. doi: 10.1037/0893-3200.20.4.709
85. Letourneau EJ, Resnick HS, Kilpatrick DG, Saunders BE, Best CL. Comorbidity of sexual problems and posttraumatic stress disorder in female crime victims. Behav Ther. (1996) 27:321–36. doi: 10.1016/S0005-7894(96)80020-7
86. Carmassi C, Dell'Oste V, Pedrinelli V, Barberi FM, Rossi R, Bertelloni CA, et al. Is sexual dysfunction in young adult survivors to the L'Aquila earthquake related to post-traumatic stress disorder? A gender perspective. J Sex Med. (2020) 17:1770–8. doi: 10.1016/j.jsxm.2020.05.016
87. Breyer BN, Fang SC, Seal KH, Ranganathan G, Marx BP, Keane TM, et al. Sexual health in male and female Iraq and Afghanistan U.S. war veterans with and without PTSD: findings from the VALOR cohort. J Trauma Stress. (2016) 29:229–36. doi: 10.1002/jts.22097
88. Suvak MK, Brogan LA, Shipherd JC. Predictors of sexual functioning in a sample of U.S. marines: an 11-year follow-up study. Int J Sex Health. (2012) 24:26–44. doi: 10.1080/19317611.2011.640387
89. Sadler AG, Mengeling MA, Fraley SS, Torner JC, Booth BM. Correlates of aexual functioning in women veterans: mental health, gynecologic health, health status, and sexual assault history. Int J Sex Health. (2012) 24:60–77. doi: 10.1080/19317611.2011.640388
90. Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, et al. Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry. (1996) 153:929–34. doi: 10.1176/ajp.153.7.929
91. Weems CF. Biological correlates of child and adolescent responses to disaster exposure: a bio-ecological model. Curr Psychiatry Rep. (2015) 17:51. doi: 10.1007/s11920-015-0588-7
92. Nicolson NA, Ponnamperuma T. Gender moderates diurnal cortisol in relation to trauma and PTSD symptoms: a study in Sri Lankan adolescents. Psychoneuroendocrinology. (2019) 104:122–31. doi: 10.1016/j.psyneuen.2019.02.012
93. Bevans K, Cerbone A, Overstreet S. Relations between recurrent trauma exposure and recent life stress and salivary cortisol among children. Dev Psychopathol. (2008) 20:257–72. doi: 10.1017/S0954579408000126
94. Garnefski N, van Rood Y, de Roos C, Kraaij V. Relationships Between traumatic life events, cognitive emotion regulation strategies, and somatic complaints. J Clin Psychol Med Settings. (2017) 24:144–51. doi: 10.1007/s10880-017-9494-y
95. Carmassi C, Barberi FM, Cordone A, Maglio A, Dell'Oste V, Dell'Osso L. Trauma, PTSD and post-traumatic stress spectrum: 15 years' experience on a multidimensional approach to trauma related psychopathology. J Psychopathol. (2020) 26:4–11. doi: 10.36148/2284-0249-376
96. Carmassi C, Rossi A, Pedrinelli V, Cremone IM, Dell'Oste V, Stratta P, et al. PTSD in the aftermath of a natural disaster: what we learned from the Pisa-L'Aquila collaboration project. J Psychopathol. (2020) 26:99–106. doi: 10.36148/2284-0249-377
97. Echeverri-Alvarado B, Pickett S, Gildner D. A model of post-traumatic stress symptoms on binge eating through emotion regulation difficulties and emotional eating. Appetite. (2020) 150:104659. doi: 10.1016/j.appet.2020.104659
98. Ge F, Li Y, Yuan M, Zhang J, Zhang W. Identifying predictors of probable posttraumatic stress disorder in children and adolescents with earthquake exposure: a longitudinal study using a machine learning approach. J Affect Disord. (2020) 264:483–93. doi: 10.1016/j.jad.2019.11.079
99. Carmassi C, Shear KM, Corsi M, Bertelloni CA, Dell'Oste V, Dell'Osso L. Mania following bereavement: state of the art and clinical evidence. Front Psychiatry. (2020) 11:366. doi: 10.3389/fpsyt.2020.00366
100. Kobayashi I, Sledjeski EM, Spoonster E, Fallon WF Jr, Delahanty DL. Effects of early nightmares on the development of sleep disturbances in motor vehicle accident victims. J Trauma Stress. (2008) 21:548–55. doi: 10.1002/jts.20368
101. Babson K, Feldner M, Badour C, Trainor C, Blumenthal H, Sachs-Ericsson N, et al. Posttraumatic stress and sleep: differential relations across types of symptoms and sleep problems. J Anxiety Disord. (2011) 25:706–13. doi: 10.1016/j.janxdis.2011.03.007
102. Krakow B, Hollifield M, Johnston L, Koss M, Schrader R, Warner TD, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA. (2001) 286:537–45. doi: 10.1001/jama.286.5.537
103. Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behav Res Ther. (2000) 38:319–45. doi: 10.1016/S0005-7967(99)00123-0
104. Nielsen T, Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev. (2007) 11:295–310. doi: 10.1016/j.smrv.2007.03.004
105. Dell'Osso L, Cremone IM, Carpita B, Dell'Oste V, Muti D, Massimetti G, et al. Rumination, posttraumatic stress disorder, and mood symptoms in borderline personality disorder. Neuropsychiatr Dis Treat. (2019) 15:1231–8. doi: 10.2147/NDT.S198616
106. Szabo YZ, Warnecke AJ, Newton TL, Valentine JC. Rumination and posttraumatic stress symptoms in trauma-exposed adults: a systematic review and meta-analysis. Anxiety Stress Coping. (2017) 30:396–414. doi: 10.1080/10615806.2017.1313835
Keywords: rhythmicity, post-traumatic stress disorder (PTSD), eating behavior, sexual function, somatic symptoms, sleep, natural disaster, vegetative functions
Citation: Carmassi C, Dell'Oste V, Bertelloni CA, Foghi C, Diadema E, Mucci F, Massimetti G, Rossi A and Dell'Osso L (2020) Disrupted Rhythmicity and Vegetative Functions Relate to PTSD and Gender in Earthquake Survivors. Front. Psychiatry 11:492006. doi: 10.3389/fpsyt.2020.492006
Received: 16 August 2019; Accepted: 14 September 2020;
Published: 16 November 2020.
Edited by:
Konstantin Loganovsky, National Academy of Medical Science of Ukraine, UkraineReviewed by:
Pinar Guzel Ozdemir, Yüzüncü Yil University, TurkeyCopyright © 2020 Carmassi, Dell'Oste, Bertelloni, Foghi, Diadema, Mucci, Massimetti, Rossi and Dell'Osso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerio Dell'Oste, dmFsZXJpby5kZWxsb3N0ZUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.