
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry, 26 August 2020
Sec. Psychopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fpsyt.2020.00859
This article is part of the Research TopicGone to Pot: Examining the Association Between Cannabis Use and Medical/Psychiatric DisordersView all 8 articles
Cannabis use during the critical neurodevelopmental period of adolescence, may lead to brain structural, functional, and histological alterations that may underpin some of the longer-term behavioral and psychological harms associated with it. The endocannabinoid system performs a key regulatory and homeostatic role, that undergoes developmental changes during adolescence making it potentially more susceptible to the effects of exposure to cannabis during adolescence. Here, we synthesize evidence from human studies of adolescent cannabis users showing alterations in cognitive performance as well as in brain structure and function with relevant preclinical evidence to summarize the current state of knowledge. We also focus on the limited evidence that speaks to the hypothesis that cannabis use during adolescence, may pose a greater risk than use during adulthood, identify gaps in current evidence and suggest directions for new research. Existing literature is consistent with the association of cannabis use during adolescence and neurological changes. Adolescence cannabis users show altered functional connectivity within known functional circuits, that may underlie inefficient recruitment of brain regions, as largely increased functional activation has been observed compared to controls. This disruption in some cases may contribute to the development of adverse mental health conditions; increasing the chances or accelerating the onset, of their development. Preclinical evidence, further supports disruption from cannabis use being specific to the developmental period. Future studies are required to better investigate adolescent cannabis use with more accuracy using better defined groups or longitudinal studies and examine the permanency of these changes following caseation of use. Furthermore, research is required to identify heritable risk factors to cannabis use. There is a need for caution when considering the therapeutic potential of cannabis for adolescence and particularly in public discourse leading to potential trivialization of possible harm from cannabis use in adolescence. Current evidence indicates that adolescence is a sensitive period during which cannabis use may result in adverse neurocognitive effects that appear to show a level of permanency into adulthood.
Cannabis is the most used illicit drug worldwide, with those who go on to become habitual users most commonly beginning use during adolescence (1, 2). Attitudes are changing globally toward cannabis, with a trend toward legalization and a push for exploration into its medicinal potential. Therefore, consideration of possible harms associated with cannabis use, particularly during the vulnerable period of adolescence (3, 4), should be a key part of the discourse. Potentially greater vulnerability to harm from cannabis use during adolescence and their subsequent persistence has long been an area of scholarly focus, with numerous reviews aiming to tease apart the effects of cannabis use in adolescence compared to adulthood on cognition (5–9), neuro-structure (6, 10, 11), and neuro-functioning (6, 7, 10).
Adolescence is a critical period of neural development (12) and a later stage opportunity to sculpt the brain before a person reaches adulthood (13). Coupled with this, adolescence is also characterized by behavioral changes such as increased risk taking behavior (14) which may increase the likelihood of experimentation with drug taking (15). During infancy and childhood, brain growth focuses on increasing volume, producing more gray matter and neuronal synapses (16). During adolescence this shifts to creating more robust neuronal pathways. Useful neurons, dendrites, and synapses are selected for preservation, while others are pruned and eliminated, with increases in whole brain white matter occurring up until a person reaches their early twenties (17). During adolescent brain development, a decrease in grey matter can be observed in overall brain volume (18) as a result of pruning and eventual elimination of neurons in a ‘fine tuning’ of the brain, with cognitive maturation paralleling this elimination phase (13, 19, 20). Because of these reorganizational processes, the adolescent brain is highly sensitive to exogenous assault, such as from psychotropic substances, thereby posing a window of vulnerability to the emergence of developmental disturbances resulting from such exposure. This is particularly true of substances that target the endocannabinoid system, which, along with its other functions, plays a vital role in adolescent neuronal maturation (21).
The endocannabinoid system is a homeostatic regulatory system for various physiological processes (22, 23), playing a particularly important role during critical periods of developmental change (24, 25), through its retrograde inhibitory effect in an individual synapse-specific manner (26) acting via cannabinoid 1 (CB1) receptors, its main central receptor (27, 28). Exogenous assaults on this system, such as through cannabis use, may disrupt performance and cause desensitization or down regulation of receptors (29, 30). Adolescence represents a period of increased susceptibility to excitotoxicity from glutamate signaling (31, 32), which cannabis may further exacerbate through the mechanism of inhibiting GABAergic inhibitory action on glutaminergic neurons (33). Preclinical evidence suggests that during adolescence, the CB1 receptor shows a steadily increasing cortical expression, until stabilization in adulthood (34–36). An opposite pattern of expression is observed in the striatum, implying a role for CB1 receptor signaling in changes in the regulation of cortico-striatal transmission occurring during neuronal development (37, 38). A similar opposing pattern of changes are also seen with cortical and striatal dopamine synthesis levels, with levels going up in frontal regions and down in the nucleus accumbens and striatum during adolescence (39, 40). In light of the cross-talk between these signaling systems (41), dysregulation of both these neurotransmitter systems may result from exposure to exogenous cannabinoids during adolescence (42).
Patterns of cannabis use have been shown to be associated with greater harm in general when used regularly as opposed to infrequently; daily as opposed to once in a while; and when used in greater amount as opposed to smaller amounts (4, 43–46). Recent changes in cannabinoid consumption may have further increased the potential of harm in adolescent users. Potency of THC in herbal cannabis, the most common form of cannabis currently being consumed (47, 48), doubled in the 10 years up to 2005 in the UK (49) with similar patterns in Europe and the USA (50), and new preparations of cannabis such as resin oils may have up to a 75% THC content (51). Synthetic cannabinoids, which bind to the cannabinoid receptor 1 often with higher affinity making them potentially more harmful, are also being consumed increasingly nowadays (52, 53). Following legalization in some jurisdictions, commercialization of cannabis for use in vaporizing pens (54), drinks, sweets, and lollypops (55), may have made cannabis use more appealing to younger users and minimized the perceived harms of the drug (56).
A number of previous reviews have summarized evidence from animal and human studies focusing on the effects of cannabis use in adolescence (6–8, 57, 58). Comparing these alterations to those seen in adults (5, 9, 10), it has been suggested that adolescence may be a period of greater sensitivity to cannabinoid exposure. Here, we attempt to synthesize evidence from human studies of adolescent cannabis users showing alterations in cognitive performance as well as in brain structure and function with relevant preclinical evidence to summarize the current state of knowledge. We aim to also focus on studies that investigate the hypothesis that cannabis use during adolescence, a critical period of neurodevelopment, may pose a greater risk than use during adulthood, identify gaps in current evidence and suggest directions for new research.
Human studies investigating cognition, brain structure and function following adolescent cannabis exposure, and animal studies directly comparing the cannabinoid exposure effects between adolescent and adult animals, were identified through a bibliography search of previous systematic and narrative reviews (5, 6, 10, 59, 60). To capture papers that have been published since the previous reviews, a search was carried out using the PUBMED database for relevant studies using the search terms “cannabis” or “marijuana” or “cannabinoid”, and “adolescence” or “young adult” or ‘early-onset”, which was completed on the 6/1/2020. These additional papers were screened initially through a search of titles and abstracts and finally a full article review. For the purposes of this review, we included studies that have specifically investigated alterations associated with adolescent cannabis use, compared early-onset cannabis users with later onset users, or used longitudinal design with a focus on effects of adolescent-onset use. Direct comparison of adolescent exposure with comparable extent of exposure during adulthood only in humans is the key piece of evidence that speaks to the central question addressed in this review (“Is the adolescent brain at greater vulnerability to the effects of cannabis as evident from structural, functional, and cognitive performance alterations?”). However, this is something that is difficult to directly address in human studies and has never been systematically investigated in humans to the best of our knowledge. To address this limitation in extant human evidence, we have summarized relevant preclinical evidence addressing this specific gap. We specifically focused on animal studies that directly compared cannabinoid exposure effects between adolescent and adult exposed groups as well as histological studies following adolescent cannabinoid exposure to address the gap in human evidence. Such controlled experimental evidence is lacking as far as human studies are concerned for obvious practical reasons. To further clarify, we have not systematically summarized the larger body of animal research related to the effects of cannabinoids, but only that relevant to our narrower focus on comparative studies using adolescent versus adult design.
Narrative synthesis of the different strands of evidence (human cognitive, structural imaging, and functional imaging evidence as well as preclinical evidence) relevant to the central issue under examination here are summarized under different sub-sections below and also presented in Tables 1–4, respectively.
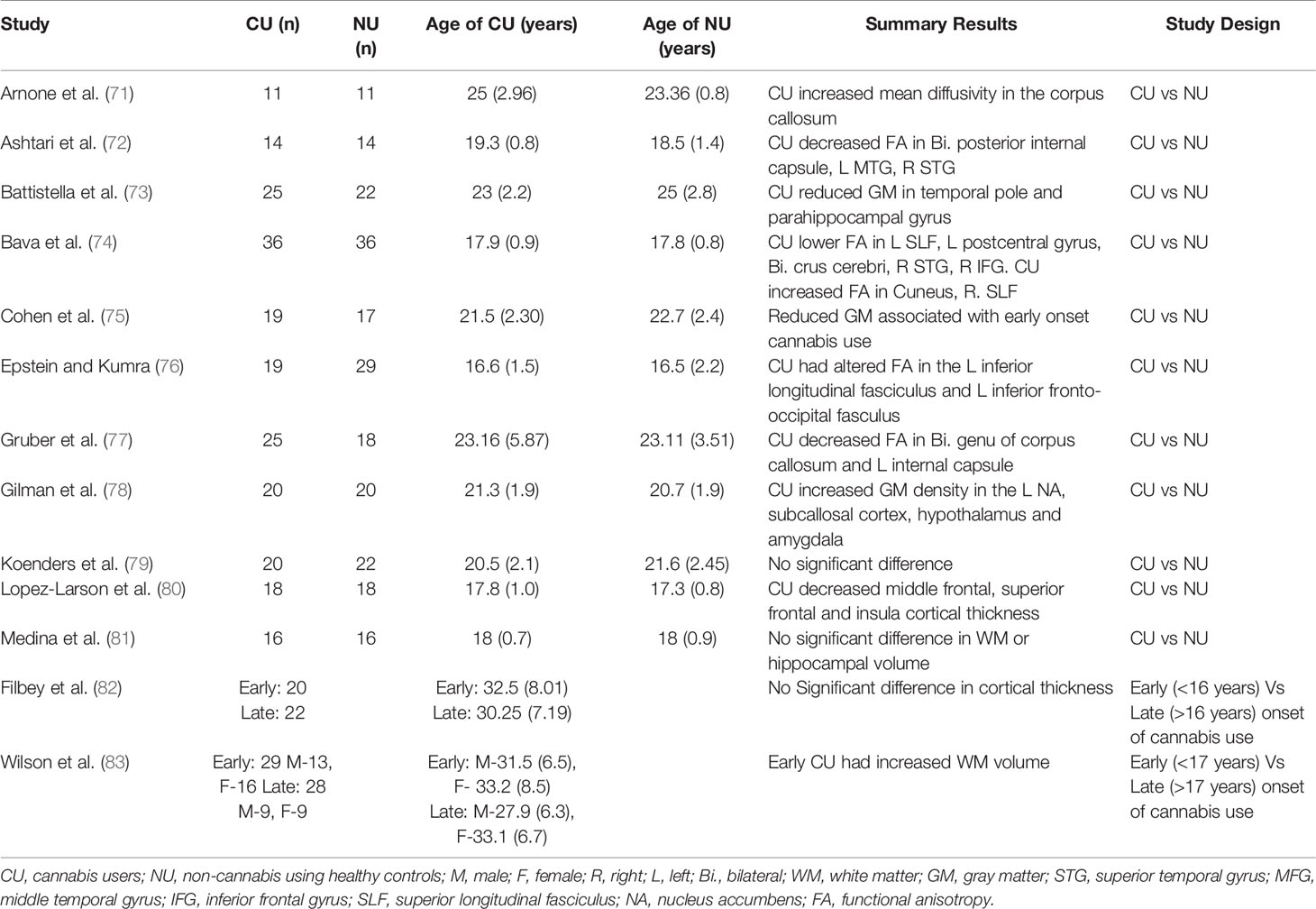
Table 2 Studies investigating brain structural alterations using neuroimaging in adolescent cannabis users.
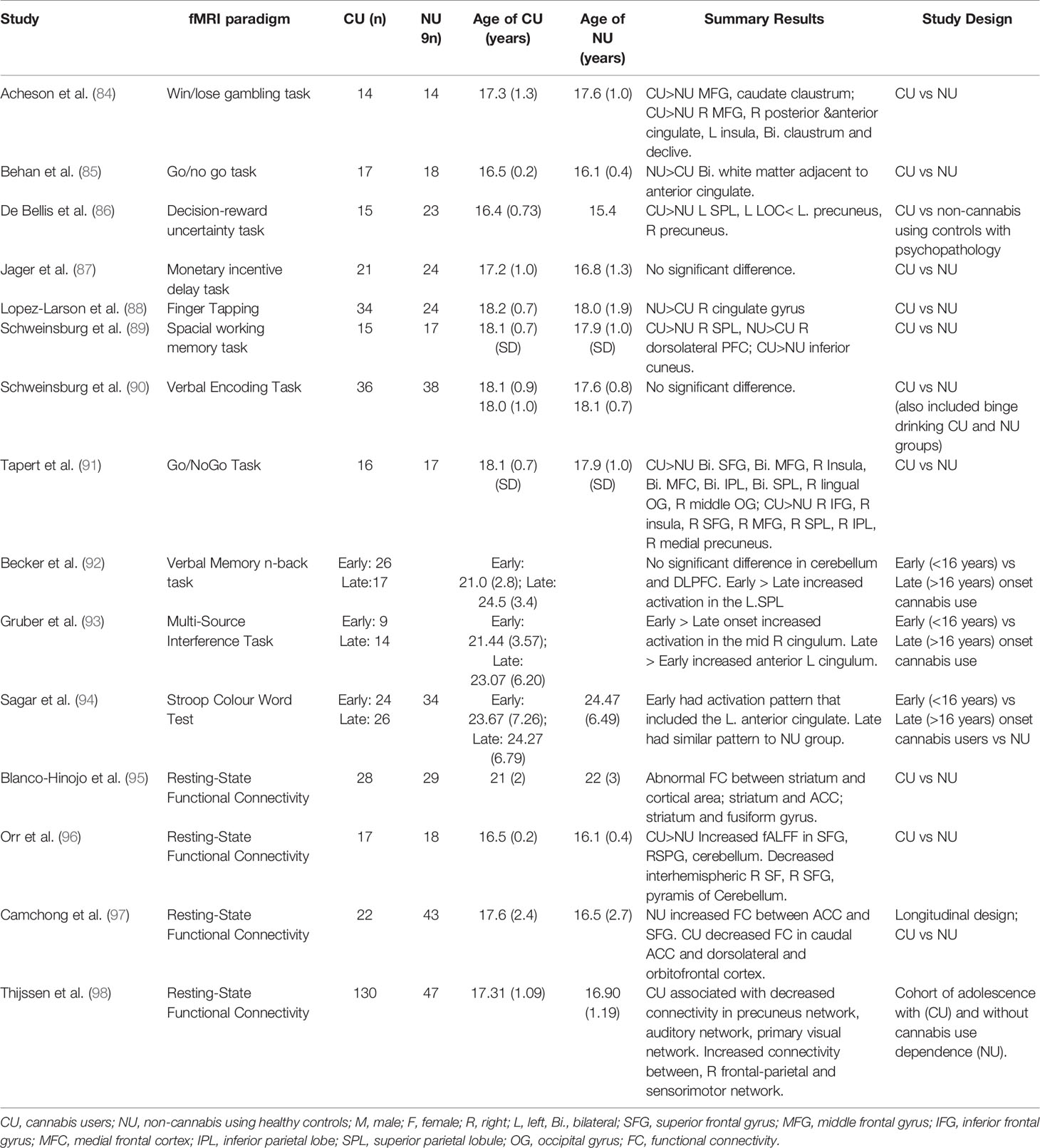
Table 3 Studies investigating brain functional alterations using functional magnetic resonance imaging in adolescent cannabis users.
Human studies (please also see Table 1 for a list of studies and summary results) have reported impaired cognition across a number of domains (executive functioning, processing speed, attention, and memory) in adolescent cannabis users when compared to controls of the same age (8, 116, 117), with some suggestion that the magnitude of this impairment may be greater than in adult cannabis users particularly in the domains of learning and memory (89). Cognitive performance deficits spanning a range of executive function domains have been found in cannabis users to be associated with age of onset of cannabis use, such that earlier onset was related to worse performance (61–68, 118). In a longitudinal cohort study following participants up to age 38, adolescent-onset cannabis users were found to have greater decline in IQ than adult-onset users when correcting for pre-use educational scores (70). Another study found a bi-directional relationship between cannabis use and cognitive performance such that poorer short-term memory and working memory performance at age 13 (prior to initiation of cannabis use) was associated with earlier age of onset of cannabis use, and earlier onset and more frequent cannabis use during adolescence in turn was associated with decline in verbal IQ, executive function domains of trial, and error learning and reward processing by age 20 (69). It is worth noting that a meta-analysis of cross-sectional studies investigating the association between cognitive performance and cannabis use in adolescents and young adults reported only a modest overall effect which was no longer significant when considering studies of abstinent users, and also did not find any association with either age of cannabis user or age of onset of cannabis use (119). However, meta-analysis of existing studies that included participants with a wide range of age at recruitment and age of onset of cannabis use, as well as variability in the measurement of cannabis use, are inherently limited by heterogeneity in the data that was pooled to estimate the effect of interest, making any interpretation challenging.
A number of studies have employed magnetic resonance imaging (MRI) (please also see Table 2 for a list of studies and summary results)to investigate alterations in brain structure associated with adolescent-onset of cannabis use and reported greater reduction in grey matter (73, 75, 83), hippocampal (81), and white matter (74, 77) volumes in adolescent-onset cannabis users compared to age-matched non-users. However, reduction in grey matter (82) and hippocampal (78) volumes have not always been consistently seen. Interestingly, earlier age of onset of cannabis use has also been associated with increase in white matter volume (83), orbitofrontal connectivity (120), and cortical thickness of the superior frontal gyrus (80). Deficits in white matter structural integrity have also been found in the prefrontal corpus callosum of adult cannabis users with onset of use in early adolescence compared to non-users (71). Further, frontotemporal structural connectivity has been found to be reduced in adolescent cannabis users compared to non-users (72), and similar alterations have been reported in the inferior longitudinal and inferior fronto-occipital fasciculi (white matter tracts connecting the occipital and temporal-occipital areas with the anterior-temporal regions (121) and the frontal lobe with temporal and occipital regions (122), respectively), with decreasing values at follow up as a function of the amount of cannabis used (76).
Consistent with this, a longitudinal study indicated that grey matter alterations found in the hippocampus, amygdala, and superior temporal gyrus of adolescent onset cannabis users do not change further following use during adulthood, suggesting that structural changes may be predominantly occurring in adolescent users (79), potentially as a result of altered pruning during adolescence.
Functional magnetic resonance imaging (fMRI) (please also see Table 3 for a list of studies and summary results)while performing cognitive activation tasks or at rest has been used to compare adolescent cannabis users to non-using adolescent control groups (84–90, 91, 96). Increased brain activity has been observed in cannabis-using adolescents compared to age-matched controls (i) during reward processing, in the middle frontal gyrus, parietal lobe, occipital gyrus, precuneus, caudate, cingulate, insula, and claustrum (84, 86); (ii) during inhibition, in the frontal and occipital gyri, parietal lobe, precuneus, and cingulate (85, 91); (iii) during memory performance, in the superior parietal lobe and cuneus (123), (iv) and during resting-state (96). The dorsolateral prefrontal cortex was found to have decreased activation in adolescent cannabis users compared to age-matched controls during memory performance (123). However, compared to non-using adolescent controls no altered activation was reported in adolescent cannabis users during reward (87) or inhibition (90) processing. Further, region of interest (ROI) analysis specific to task found hyperactivation in prefrontal regions during memory processing (124) and in the amygdala while fear processing (125) compared to controls. This hyperactivation may be as a result of increased effort to maintain task performance or reflect reduced cortical efficiency (117).
Using a whole-brain analysis approach, a meta-analysis of studies specifically investigating alteration in brain activation associated with cannabis use in adolescence, reported significantly greater activation in cannabis users compared to controls over a range of tasks in the right inferior parietal gyrus (extending to the superior parietal gyrus and angular gyrus) and right putamen (extending to the striatum and insula) (126). These regions are part of the salience and default mode networks (127, 128), potentially suggesting an impairment in the functioning of brain regions involved in the control and switching between states of carrying out a mental task and rest; necessary for the efficient allocation of attentional resources while performing mental tasks. Even following a period of abstinence long enough to allow cannabis metabolites to have left the body, these networks in adolescent cannabis users persisted to show altered activation (129). Interestingly, in contrast to the meta-analysis of adolescent cannabis user studies showing greater activation in cannabis users across a number of brain regions (126) a meta-analysis of adult only studies found brain functional alterations in both directions (i.e. hyperactivation and hypoactivation) (126). While not directly comparable, these differences may reflect a range of differences between study populations in the two meta-analyses, including differences in the extent of exposure to cannabis as well as other drugs. A subsequent study performed in adult cannabis users with a narrow range of age of onset of cannabis use during adolescence found evidence of inefficient medial temporal and midbrain function underlying slower verbal learning (130).
Despite being limited, functional imaging studies have also investigated whether early and late-onset cannabis users differed in their brain activity (92–94) while performing cognitive tasks. While performing memory tasks, early onset cannabis users had increased activation in the superior parietal lobe, the inferior and superior frontal and superior temporal gyri, insula, and precuneus (92), compared to late-onset users. While performing inhibitory processing tasks, early onset cannabis users had altered functioning where they activated different regions of the anterior cingulate cortex (ACC) compared to controls, while late onset users showed activation patterns that were similar to a control group of non-users (93, 94).
Other studies have investigated alteration in the functional connectivity between different regions of the brain in the context of cannabis use to complement and help better understand the differences in brain activation seen in cannabis users (126). A longitudinal study of resting state functional connectivity comparing adolescence cannabis users and non-users found decreased connectivity between the ACC and the dorsolateral and orbital frontal cortices in adolescent cannabis users across 18 months of cannabis use, while connectivity between the ACC and the superior frontal gyrus increased over time in healthy controls (97). Examination of resting state connectivity between the central executive network, default mode network and sensory networks in a cohort of adolescents found decreased connectivity in all networks as a function of longer duration of cannabis use (98). Similar alterations were seen in connectivity between the striatum and frontal–limbic circuit during a comparison of adolescent cannabis users with non-users, in addition to attenuation of the negatively correlated functional connectivity between the striatum and the fusiform gyrus, a region that serves a critical role in the recognition of significant visual features; it is important to note that some of these observations appeared to normalize after abstinence (95). Reduced interhemispheric connectivity in adolescent cannabis users compared to non-users has also been observed, associated with dependence levels (96).
Direct comparison of adolescent exposure with comparable exposure during adulthood is challenging to address in human studies. Preclinical studies have further advanced current understanding of the effects of cannabinoids in adolescence, with experimental designs allowing for systematic investigation and better control of potential confounding factors (please see Table 4 for a list of studies and summary results). Behavioral studies of animals exposed to CB1 receptor agonists, including THC (99, 100, 102, 103), during adolescence compared to those exposed in adulthood found the former to have more significant cognitive impairments when compared to age-matched controls, while the latter had minimal impairment (101–104, 107, 108, 131). However, other studies did not find any difference in impairments comparing the adolescent exposed group to the adult exposed group (105) and some others did not find any deficit following chronic cannabinoid exposure in either the adolescent exposed group or the adult exposed group compared to controls (99, 100).
Histological investigations in preclinical studies suggest neurobiological changes in animals exposed to cannabinoids during adolescence. Adolescent THC-treated rats maintained increased expression of the CB1 gene compared to adult THC treated rats (103). Furthermore, adolescent animals had a reduction in compensatory downregulation of CB1 receptors following acute exposure to synthetic cannabinoids, compared to adults exposed animals (109). Protein expression in adolescence has also been found to be altered in prefrontal regions and the hippocampus (106, 132), possibly due to altered CB1 receptor-mediated regulation of downstream signaling proteins. Further, depression in GABA and glutamate receptors, leading to a disruption of the excitatory-inhibitory balance, has been observed in the hippocampus following adolescent cannabinoid exposure (110, 112) compared to animals treated with a vehicle, with an opposite effect on GABA receptors seen in adult treated rats (114). Similar findings in the prefrontal cortex (113, 115) and hyperactivity of mesocorticolimbic dopaminergic systems have been found in adolescent treated animals compared to animals treated with a vehicle (111).
As evident from our summary of current research, existing literature is generally consistent with the idea that cannabis use in adolescence is associated with neurocognitive changes. Meta-analytic evidence suggests greater functional activation in adolescent cannabis users compared to controls, whereas adult users show a combination of hyper- and hypo- activation in a number of brain regions. Functional connectivity between brain regions and within known functional circuits is altered in those with adolescent cannabis use and may underlie the observed differences in brain activation, perhaps from inefficient recruitment of regions required for task performance. Such disordered organization of brain circuitry during adolescence may underlie greater functional deficits in adolescent cannabis users than those starting use as adults. Preclinical evidence further supports the idea that the detrimental effects of cannabis use may be greater specifically as result of exposure during the developmental period.
Adolescence seems to be a period of vulnerability to change, with brain structural alterations associating with cannabis use (79). However, structural changes in cortical and subcortical regions do not show great consistency in terms of direction of change (133). Functional alteration in cannabis users, do show some consistency towards increased activation. However, evidence of the regional pattern of this change has been less consistent, possibly due to differences between studies in the cognitive tasks employed, although brain regions that are part of the large functional networks, such as the salience and default mode networks (127, 128) show more robust evidence for functional alteration in adolescent cannabis users (126, 134). Observed functional network alterations could stem from altered pruning consistent with evidence of alterations in white matter volume (74, 77), integrity (71) and connectivity (72, 76, 121, 122). Brain regions that are key components of these large scale networks that have been found to be affected in adolescent cannabis users, have also been shown to be acutely modulated by THC, the key psychoactive ingredient in cannabis, in experimental studies (135–137), indicating that the alterations noted in adolescent cannabis users are likely related to cannabis use as opposed to being linked to potential confounding factors that are challenging to control for in observational studies.
Early onset of cannabis use during adolescence has been associated with poorer performance in cognitive tasks. However, the extent to which these deficits persist following an adequate period of abstinence remains unclear (119, 129). Whether adaptive changes occur in terms of functioning, particularly after a period of abstinence, bringing cognitive performance of abstinent cannabis users up to the level of non-users despite residual differences in brain functional activation (129) remains to be tested.
Altered functioning of salience and reward networks as well as altered inhibitory control from early cannabis use has been suggested to increase susceptibility to risky behaviors, addiction, and dependence (138). Also, the acute psychoactive effects of cannabis have been found to differ between adults and adolescents (139, 140), with adolescents perceiving them less (139), which may potentially underlie early onset users often becoming more persistent users than those with a later age of onset (70). However, whether these persistent brain functional alterations underlie short-term or longer-term risks of mental, social, and behavioral disturbances (4, 141–145) in young people remains to be tested.
It is possible that altered cognitive function and alteration in the neural substrates underlying those cognitive processes as summarized above, may underlie poorer educational outcomes and increased school drop-out rate (68, 69, 145–151) in early-onset cannabis users. However, it is worth noting the lack of evidence in this regard. Further, other factors such as pre-existing characteristics (139, 149), lower IQ and poorer executive functioning predating cannabis use (152–155), and decreased time spent in schooling (156) may also increase the likelihood of poor educational outcomes independently. Poor school performance in turn has been associated with increased likelihood of development of a psychiatric disorder (157).
Another important question relates to whether evidence summarized above helps us understand potential mechanisms through which cannabis use during adolescence may increase the risk of onset for psychiatric disorders such as schizophrenia (3, 158, 159), depression (4, 160, 161) and substance use disorders (68, 162) in adolescent-onset users compared to those with onset of use later on in life. The precise neurobiological mechanisms underlying the association between cannabis use and increased risk of development of psychosis, especially what underlies greater risk in those with onset of use during adolescence remains unclear, though there are several potential candidate explanatory mechanisms. Impaired functioning following cannabis use in adolescence may alter the functioning of components of the salience network, such as the insula, involved in the switching between large scale brain networks (125) that is critical to the efficient allocation of cognitive resources. Involvement of components of this switching process in conjunction with altered functioning of cross-modal hubs such as the angular gyrus, involved in the integration and retrieval of multi-modal information (163) and in the allocation of attentional resources and attribution of meaning and salience in association with components of the salience network, may result in altered attribution of salience in the context of cannabis use (164), that is also thought to underlie symptoms of psychosis (165). Functional connectivity alterations between the default mode network and salience network as summarized here may underlie the psychopathology of psychosis (166) indicating a potential convergent mechanistic substrate. Alterations following adolescent-onset cannabis exposure in the efficiency of medial temporal cortex (130) also converge with medial temporal alterations seen in psychosis (167–169). Using cannabis during the critical developmental period of adolescence may also lead to alterations in two neurotransmitter systems, such as altered glutamate (170) and dopamine (171) signaling, both of which have been implicated in the etiology of psychosis (172). Investigation of adolescent-onset cannabis users suggest that glial function may also be altered following cannabis use (173) indicating another point of convergence between alterations noted in the context of cannabis use and in schizophrenia (174) suggesting a potential mechanistic explanation.
Research into the adverse effects of psychoactive drug use during adolescence has numerous challenges (175), particularly in terms of systematic quantification of extent of cannabis use (176), as frequency and duration of cannabis use along with participant age may play a key role in influencing any observed alterations in brain and behavior (5). Currently, there is a lack of adequately powered systematic studies with well-defined groups of early-onset and late-onset cannabis users matched for potential confounding factors such as levels of cannabis exposure, which may help begin to address the question whether cannabis use during adolescence is associated greater brain structural and functional alterations than later onset use. Similarly, three-way analysis of early-onset users, late-onset users, and age-matched controls would help identify areas of functional and structural alteration common to all cannabis users as well as alterations that are specifically associated with adolescent onset use. Neurochemical changes are also yet to be investigated in a way that adequately addresses difference between adolescent or adult-onset cannabis users and should be a consideration for future studies. In terms of its remit, this current review has been limited to the brain alterations associated with adolescent cannabis use, as indexed by evidence of structural, neurophysiological, and cognitive performance alterations. There is also a wider body of evidence regarding the association between adolescent cannabis use and an increased risk of development of emotional disturbances, psychosis or addiction, which were not reviewed here. Future efforts should also attempt to systematically summarize the longer-term mental health consequences of adolescent cannabis use and integrate them with biological evidence that may underpin them.
Important potential confounding factors that have not always been considered adequately but are worth consideration include abstinence (119, 129) and tolerance (177, 178) to the effects of cannabis. Future studies need to take these factors into consideration at the design stage. Another important consideration in this context relates to the issue of cause and effect relationship. In light of the cross-sectional design of the majority of studies, much of the currently available human evidence is unable to disentangle the nature of the relationship between adolescent cannabis use and neurocognitive alterations, i.e., whether adolescent cannabis use is a cause or consequence of these alterations or whether their association is linked to an underlying predisposition that increases the likelihood of both adolescent cannabis use and certain neurocognitive outcomes. More systematic longitudinal studies, which have been limited thus far, are necessary to start addressing some of these questions. Other approaches such as sibling/twin study designs (179, 180) or analytic approaches (4, 179, 181) as have been employed in the context of cannabis use and other adverse outcomes may also offer alternative methods of addressing these questions even in the absence of experimental data that constitute the gold standard evidence for establishing causal relationships. Future studies in large samples are also necessary to disentangle whether different sub-groups of adolescent cannabis users have differential vulnerability to the effects of cannabis, potentially mediated by genetic differences (182–185). Future studies also need to investigate whether the emergence of new cannabis preparations and increasing use of synthetic cannabis may have altered the usage patterns in adolescence and resulted in greater harm from adolescent use.
While there is growing interest in the therapeutic potential of cannabis (186–188) and evidence of benefit only for certain cannabinoids such as cannabidiol for certain childhood epilepsies (189, 190), or potential for benefit for neurodevelopmental disorders such as schizophrenia (191–195) that typically have an onset in late adolescence and early adulthood, evidence summarized above indicate the need for caution. This is a particular concern as specific cannabinoids (such as cannabidiol) with therapeutic potential are often conflated with cannabis/medicinal cannabis in the public discourse leading to potential trivialization of possible harm from cannabis use in adolescent users and reinforcement of the narrative that cannabis use is a harmless recreational activity in young people. Collectively, despite the obvious limitations outlined above, current evidence indicates that adolescence is a sensitive period during which cannabis use may result in adverse neurocognitive effects that appear to show a level of permanency into adulthood.
SB and GB-H designed the study. GB-H performed the literature searches for the studies included and composed the initial draft, with substantial contributions for content and presentation from MC, VG, and SB.
GB-H was supported by funding from the Society for the Study of Addictions for postdoctoral work with SB that included this review. SB is supported by grant from the National Institute of Health Research (NIHR) Efficacy and Mechanism Evaluation scheme (UK).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Clark TT, Doyle O, Clincy A. Age of first cigarette, alcohol, and marijuana use among U.S. biracial/ethnic youth: a population-based study. Addict Behav (2013) 38(9):2450–4. doi: 10.1016/j.addbeh.2013.04.005
2. Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA, et al. Youth risk behavior surveillance–United States, 2013. MMWR Suppl (2014) 63(4):1–168.
3. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ (2002) 325(7374):1212–3. doi: 10.1136/bmj.325.7374.1212
4. Schoeler T, Theobald D, Pingault JB, Farrington DP, Coid JW, Bhattacharyya S. Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: findings from 40 years of follow-up. psychol Med (2018) 48(13):1–8. doi: 10.1017/S0033291717003658
5. Ganzer F, Bröning S, Kraft S, Sack PM, Thomasius R. Weighing the Evidence: A Systematic Review on Long-Term Neurocognitive Effects of Cannabis Use in Abstinent Adolescents and Adults. Neuropsychol Rev (2016) 26(2):186–222. doi: 10.1007/s11065-016-9316-2
6. Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: an overview of animal and human research. Curr Drug Abuse Rev (2008) 1(2):114–23. doi: 10.2174/1874473710801020114
7. James A, James C, Thwaites T. The brain effects of cannabis in healthy adolescents and in adolescents with schizophrenia: a systematic review. Psychiatry Res (2013) 214(3):181–9. doi: 10.1016/j.pscychresns.2013.07.012
8. Levine A, Clemenza K, Rynn M, Lieberman J. Evidence for the Risks and Consequences of Adolescent Cannabis Exposure. J Am Acad Child Adolesc Psychiatry (2017) 56(3):214–25. doi: 10.1016/j.jaac.2016.12.014
9. Gorey C, Kuhns L, Smaragdi E, Kroon E, Cousijn J. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci (2019) 269(1):37–58. doi: 10.1007/s00406-019-00981-7
10. Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PloS One (2013) 8(2):e55821. doi: 10.1371/journal.pone.0055821
11. Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr Pharm Des (2014) 20(13):2138–67. doi: 10.2174/13816128113199990435
12. Sturman DA, Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev (2011) 35(8):1704–12. doi: 10.1016/j.neubiorev.2011.04.003
13. Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev (2003) 27(1-2):3–18. doi: 10.1016/S0149-7634(03)00005-8
14. Dayan J, Bernard A, Olliac B, Mailhes AS, Kermarrec S. Adolescent brain development, risk-taking and vulnerability to addiction. J Physiol Paris (2010) 104(5):279–86. doi: 10.1016/j.jphysparis.2010.08.007
15. Walker DM, Bell MR, Flores C, Gulley JM, Willing J, Paul MJ. Adolescence and Reward: Making Sense of Neural and Behavioral Changes Amid the Chaos. J Neurosci (2017) 37(45):10855–66. doi: 10.1523/JNEUROSCI.1834-17.2017
16. Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci (1999) 2(10):861–3. doi: 10.1038/13158
17. Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, et al. Changes in white matter microstructure during adolescence. Neuroimage (2008) 39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043
18. Blakemore SJ. Development of the social brain during adolescence. Q J Exp Psychol (Hove) (2008) 61(1):40–9. doi: 10.1080/17470210701508715
19. Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol (2000) 54(1-3):241–57. doi: 10.1016/S0301-0511(00)00058-2
20. Choudhury S, Blakemore SJ, Charman T. Social cognitive development during adolescence. Soc Cognit Affect Neurosci (2006) 1(3):165–74. doi: 10.1093/scan/nsl024
21. Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol (2008) 18(11):826–34. doi: 10.1016/j.euroneuro.2008.06.009
22. Di Marzo V, Piscitelli F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics (2015) 12(4):692–8. doi: 10.1007/s13311-015-0374-6
23. Zou S, Kumar U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int J Mol Sci (2018) 19(3). doi: 10.3390/ijms19030833
24. Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci (2003) 6(10):1048–57. doi: 10.1038/nn1126
25. Sarne Y, Mechoulam R. Cannabinoids: between neuroprotection and neurotoxicity. Curr Drug Targets CNS Neurol Disord (2005) 4(6):677–84. doi: 10.2174/156800705774933005
26. Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science (2003) 302(5642):84–8. doi: 10.1126/science.1088208
27. Herkenham M. Characterization and localization of cannabinoid receptors in brain: an in vitro technique using slide-mounted tissue sections. NIDA Res Monogr (1991) 112:129–45. doi: 10.1037/e496242006-009
28. Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) (2006) 30(Suppl 1):S13–8. doi: 10.1038/sj.ijo.0803272
29. Martin BR. Role of lipids and lipid signaling in the development of cannabinoid tolerance. Life Sci (2005) 77(14):1543–58. doi: 10.1016/j.lfs.2005.05.005
30. Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxaz inyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther (2002) 303(1):36–44. doi: 10.1124/jpet.102.035618
31. Olney JW, Farber NB, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Environmental agents that have the potential to trigger massive apoptotic neurodegeneration in the developing brain. Environ Health Perspect (2000) 108(Suppl 3):383–8. doi: 10.1289/ehp.00108s3383
32. Olney JW. Excitotoxicity, apoptosis and neuropsychiatric disorders. Curr Opin Pharmacol (2003) 3(1):101–9. doi: 10.1016/S1471-4892(02)00002-4
33. Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol (2010) 92(3):370–85. doi: 10.1016/j.pneurobio.2010.06.010
34. Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol (1995) 17(1):25–30. doi: 10.1016/0892-0362(94)00053-G
35. Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci (2003) 17(9):1747–54. doi: 10.1046/j.1460-9568.2003.02599.x
36. Verdurand M, Nguyen V, Stark D, Zahra D, Gregoire MC, Greguric I, et al. Comparison of Cannabinoid CB(1) Receptor Binding in Adolescent and Adult Rats: A Positron Emission Tomography Study Using [F]MK-9470. Int J Mol Imaging (2011) 2011:548123. doi: 10.1155/2011/548123
37. Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse (2011) 65(4):278–86. doi: 10.1002/syn.20844
38. Van Waes V, Beverley JA, Siman H, Tseng KY, Steiner H. CB1 Cannabinoid Receptor Expression in the Striatum: Association with Corticostriatal Circuits and Developmental Regulation. Front Pharmacol (2012) 3:21. doi: 10.3389/fphar.2012.00021
39. Teicher MH, Barber NI, Gelbard HA, Gallitano AL, Campbell A, Marsh E, et al. Developmental differences in acute nigrostriatal and mesocorticolimbic system response to haloperidol. Neuropsychopharmacology (1993) 9(2):147–56. doi: 10.1038/npp.1993.53
40. Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/-)-7-OH-DPAT. Naunyn Schmiedebergs Arch Pharmacol (1997) 356(2):173–81. doi: 10.1007/PL00005038
41. Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, et al. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci (2012) 32(21):7316–24. doi: 10.1523/JNEUROSCI.4284-11.2012
42. Rubino T, Parolaro D. The Impact of Exposure to Cannabinoids in Adolescence: Insights From Animal Models. Biol Psychiatry (2016) 79(7):578–85. doi: 10.1016/j.biopsych.2015.07.024
43. Quattrone D, Ferraro L, Tripoli G, La Cascia C, Quigley H, Quattrone A, et al. Daily use of high-potency cannabis is associated with more positive symptoms in first-episode psychosis patients: the EU-GEI case-control study. psychol Med (2020), 1–9. doi: 10.1017/S0033291720000082
44. Schoeler T, Petros N, Di Forti M, Klamerus E, Foglia E, Ajnakina O, et al. Effects of continuation, frequency, and type of cannabis use on relapse in the first 2 years after onset of psychosis: an observational study. Lancet Psychiatry (2016) 3(10):947–53. doi: 10.1016/S2215-0366(16)30188-2
45. Schoeler T, Monk A, Sami MB, Klamerus E, Foglia E, Brown R, et al. Continued versus discontinued cannabis use in patients with psychosis: a systematic review and meta-analysis. Lancet Psychiatry (2016) 3(3):215–25. doi: 10.1016/S2215-0366(15)00363-6
46. Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr Bull (2016) 42(5):1262–9. doi: 10.1093/schbul/sbw003
47. Potter DJ, Hammond K, Tuffnell S, Walker C, Di Forti M. Potency of Delta(9) -tetrahydrocannabinol and other cannabinoids in cannabis in England in 2016: Implications for public health and pharmacology. Drug Test Anal (2018) 10(4):628–35. doi: 10.1002/dta.2368
48. Hargreaves J SK. Home Office National Statistics, Seizures of Drugs in England and Wales, Financial Year Ending 31 March 2016. (2016). Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/564412/seizures-drugs-hosb1316.pdf.
49. Potter DJ, Clark P, Brown MB. Potency of delta 9-THC and other cannabinoids in cannabis in England in 2005: implications for psychoactivity and pharmacology. J Forensic Sci (2008) 53(1):90–4. doi: 10.1111/j.1556-4029.2007.00603.x
50. Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci (2019) 269(1):5–15. doi: 10.1007/s00406-019-00983-5
51. Raber JC, Elzinga S, Kaplan C. Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. J Toxicol Sci (2015) 40(6):797–803. doi: 10.2131/jts.40.797
52. Martinotti G, Santacroce R, Papanti D, Elgharably Y, Prilutskaya M, Corazza O. Synthetic Cannabinoids: Psychopharmacology, Clinical Aspects, Psychotic Onset. CNS Neurol Disord Drug Targets (2017) 16(5):567–75. doi: 10.2174/1871527316666170413101839
53. Cohen K, Weinstein AM. Synthetic and Non-synthetic Cannabinoid Drugs and Their Adverse Effects-A Review From Public Health Prospective. Front Public Health (2018) 6:162. doi: 10.3389/fpubh.2018.00162
54. Morean ME, Lipshie N, Josephson M, Foster D. Predictors of Adult E-Cigarette Users Vaporizing Cannabis Using E-Cigarettes and Vape-Pens. Subst Use Misuse (2017) 52(8):974–81. doi: 10.1080/10826084.2016.1268162
55. Ciolino LA, Ranieri TL, Taylor AM. Commercial cannabis consumer products part 1: GC-MS qualitative analysis of cannabis cannabinoids. Forensic Sci Int (2018) 289:429–37. doi: 10.1016/j.forsciint.2018.05.032
56. Daniulaityte R, Lamy FR, Barratt M, Nahhas RW, Martins SS, Boyer EW, et al. Characterizing marijuana concentrate users: A web-based survey. Drug Alcohol Depend (2017) 178:399–407. doi: 10.1016/j.drugalcdep.2017.05.034
57. Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, Shollenbarger S. Considering Cannabis: The Effects of Regular Cannabis Use on Neurocognition in Adolescents and Young Adults. Curr Addict Rep (2014) 1(2):144–56. doi: 10.1007/s40429-014-0019-6
58. Renard J, Rushlow WJ, Laviolette SR. What Can Rats Tell Us about Adolescent Cannabis Exposure? Insights from Preclinical Research. Can J Psychiatry (2016) 61(6):328–34. doi: 10.1177/0706743716645288
59. Bloomfield MAP, Hindocha C, Green SF, Wall MB, Lees R, Petrilli K, et al. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol Ther (2019) 195:132–61. doi: 10.1016/j.pharmthera.2018.10.006
60. Cohen K, Weinstein A. The Effects of Cannabinoids on Executive Functions: Evidence from Cannabis and Synthetic Cannabinoids-A Systematic Review. Brain Sci (2018) 8(3). doi: 10.3390/brainsci8030040
61. Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacol (Berl) (2010) 212(4):613–24. doi: 10.1007/s00213-010-1988-3
62. Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol Addict Behav (2012) 26(3):496–506. doi: 10.1037/a0026269
63. Levar N, Francis AN, Smith MJ, Ho WC, Gilman JM. Verbal Memory Performance and Reduced Cortical Thickness of Brain Regions Along the Uncinate Fasciculus in Young Adult Cannabis Users. Cannabis Cannabinoid Res (2018) 3(1):56–65. doi: 10.1089/can.2017.0030
64. Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacol (Berl) (2011) 216(1):131–44. doi: 10.1007/s00213-011-2203-x
65. Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Reflection impulsivity in adolescent cannabis users: a comparison with alcohol-using and non-substance-using adolescents. Psychopharmacol (Berl) (2012) 219(2):575–86. doi: 10.1007/s00213-011-2486-y
66. Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacol (Berl) (1999) 142(3):295–301. doi: 10.1007/s002130050892
67. Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, et al. Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry (2011) 198(6):442–7. doi: 10.1192/bjp.bp.110.077479
68. Pope HG Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend (2003) 69(3):303–10. doi: 10.1016/S0376-8716(02)00334-4
69. Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: A longitudinal study from early adolescence to young adulthood. Dev Psychopathol (2017) 29(4):1253–66. doi: 10.1017/S0954579416001280
70. Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U.S.A. (2012) 109(40):E2657–64. doi: 10.1073/pnas.1206820109
71. Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage (2008) 41(3):1067–74. doi: 10.1016/j.neuroimage.2008.02.064
72. Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res (2009) 43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002
73. Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology (2014) 39(9):2041–8. doi: 10.1038/npp.2014.67
74. Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res (2009) 173(3):228–37. doi: 10.1016/j.pscychresns.2009.04.005
75. Cohen M, Rasser PE, Peck G, Carr VJ, Ward PB, Thompson PM, et al. Cerebellar grey-matter deficits, cannabis use and first-episode schizophrenia in adolescents and young adults. Int J Neuropsychopharmacol (2012) 15(3):297–307. doi: 10.1017/S146114571100068X
76. Epstein KA, Kumra S. White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: A naturalistic diffusion tensor imaging study. Psychiatry Res (2015) 232(1):34–41. doi: 10.1016/j.pscychresns.2014.10.010
77. Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Lukas SE. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacol (Berl) (2014) 231(8):1455–65. doi: 10.1007/s00213-013-3326-z
78. Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci (2014) 34(16):5529–38. doi: 10.1523/JNEUROSCI.4745-13.2014
79. Koenders L, Cousijn J, Vingerhoets WA, van den Brink W, Wiers RW, Meijer CJ, et al. Grey Matter Changes Associated with Heavy Cannabis Use: A Longitudinal sMRI Study. PloS One (2016) 11(5):e0152482. doi: 10.1371/journal.pone.0152482
80. Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, et al. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res (2011) 220(1):164–72. doi: 10.1016/j.bbr.2011.02.001
81. Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry (2007) 48(6):592–600. doi: 10.1111/j.1469-7610.2007.01728.x
82. Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cognit Neurosci (2015) 16:16–22. doi: 10.1016/j.dcn.2015.10.001
83. Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis (2000) 19(1):1–22. doi: 10.1300/J069v19n01_01
84. Acheson A, Ray KL, Hines CS, Li K, Dawes MA, Mathias CW, et al. Functional activation and effective connectivity differences in adolescent marijuana users performing a simulated gambling task. J Addict (2015) 2015:783106. doi: 10.1155/2015/783106
85. Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, et al. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology (2014) 84:131–7. doi: 10.1016/j.neuropharm.2013.05.027
86. De Bellis MD, Wang L, Bergman SR, Yaxley RH, Hooper SR, Huettel SA. Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend (2013) 133(1):134–45. doi: 10.1016/j.drugalcdep.2013.05.020
87. Jager G, Block RI, Luijten M, Ramsey NF. Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: a cross-sectional multicenter fMRI study. J Psychoactive Drugs (2013) 45(2):156–67. doi: 10.1080/02791072.2013.785837
88. Lopez-Larson MP, Rogowska J, Bogorodzki P, Bueler CE, McGlade EC, Yurgelun-Todd DA. Cortico-cerebellar abnormalities in adolescents with heavy marijuana use. Psychiatry Res (2012) 202(3):224–32. doi: 10.1016/j.pscychresns.2011.11.005
89. Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev (2008) 1(1):99–111. doi: 10.2174/1874473710801010099
90. Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction (2011) 106(3):564–73. doi: 10.1111/j.1360-0443.2010.03197.x
91. Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacol (Berl) (2007) 194(2):173–83. doi: 10.1007/s00213-007-0823-y
92. Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog Neuropsychopharmacol Biol Psychiatry (2010) 34(6):837–45. doi: 10.1016/j.pnpbp.2010.03.032
93. Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett (2012) 511(2):89–94. doi: 10.1016/j.neulet.2012.01.039
94. Sagar KA, Dahlgren MK, Gönenç A, Racine MT, Dreman MW, Gruber SA. The impact of initiation: Early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Dev Cognit Neurosci (2015) 16:84–92. doi: 10.1016/j.dcn.2015.03.003
95. Blanco-Hinojo L, Pujol J, Harrison BJ, Macia D, Batalla A, Nogue S, et al. Attenuated frontal and sensory inputs to the basal ganglia in cannabis users. Addict Biol (2017) 22(4):1036–47. doi: 10.1111/adb.12370
96. Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, et al. Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse (2013) 39(6):372–81. doi: 10.3109/00952990.2013.848213
97. Camchong J, Lim KO, Kumra S. Adverse Effects of Cannabis on Adolescent Brain Development: A Longitudinal Study. Cereb Cortex (2017) 27(3):1922–30. doi: 10.1093/cercor/bhw015
98. Thijssen S, Rashid B, Gopal S, Nyalakanti P, Calhoun VD, Kiehl KA. Regular cannabis and alcohol use is associated with resting-state time course power spectra in incarcerated adolescents. Drug Alcohol Depend (2017) 178:492–500. doi: 10.1016/j.drugalcdep.2017.05.045
99. Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav (2006) 83(3):448–55. doi: 10.1016/j.pbb.2006.03.006
100. Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of delta9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol (2007) 18(5-6):563–9. doi: 10.1097/FBP.0b013e3282ee7b7e
101. Gleason KA, Birnbaum SG, Shukla A, Ghose S. Susceptibility of the adolescent brain to cannabinoids: long-term hippocampal effects and relevance to schizophrenia. Transl Psychiatry (2012) 2:e199. doi: 10.1038/tp.2012.122
102. Harte LC, Dow-Edwards D. Sexually dimorphic alterations in locomotion and reversal learning after adolescent tetrahydrocannabinol exposure in the rat. Neurotoxicol Teratol (2010) 32(5):515–24. doi: 10.1016/j.ntt.2010.05.001
103. Kasten CR, Zhang Y, Boehm SL,2. Acute and long-term effects of Delta9-tetrahydrocannabinol on object recognition and anxiety-like activity are age- and strain-dependent in mice. Pharmacol Biochem Behav (2017) 163:9–19. doi: 10.1016/j.pbb.2017.10.012
104. O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol (2004) 18(4):502–8. doi: 10.1177/0269881104047277
105. O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol (2006) 20(5):611–21. doi: 10.1177/0269881106065188
106. Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, et al. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology (2008) 33(5):1113–26. doi: 10.1038/sj.npp.1301475
107. Renard J, Krebs MO, Jay TM, Le Pen G. Long-term cognitive impairments induced by chronic cannabinoid exposure during adolescence in rats: a strain comparison. Psychopharmacol (Berl) (2013) 225(4):781–90. doi: 10.1007/s00213-012-2865-z
108. Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology (2003) 28(10):1760–9. doi: 10.1038/sj.npp.1300225
109. Dalton VS, Zavitsanou K. Cannabinoid effects on CB1 receptor density in the adolescent brain: an autoradiographic study using the synthetic cannabinoid HU210. Synapse (2010) 64(11):845–54. doi: 10.1002/syn.20801
110. Higuera-Matas A, Miguens M, Coria SM, Assis MA, Borcel E, del Olmo N, et al. Sex-specific disturbances of the glutamate/GABA balance in the hippocampus of adult rats subjected to adolescent cannabinoid exposure. Neuropharmacology (2012) 62(5-6):1975–84. doi: 10.1016/j.neuropharm.2011.12.028
111. Renard J, Rosen LG, Loureiro M, De Oliveira C, Schmid S, Rushlow WJ, et al. Adolescent Cannabinoid Exposure Induces a Persistent Sub-Cortical Hyper-Dopaminergic State and Associated Molecular Adaptations in the Prefrontal Cortex. Cereb Cortex (2017) 27(2):1297–310. doi: 10.1093/cercor/bhv335
112. Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus (2009) 19(8):763–72. doi: 10.1002/hipo.20554
113. Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis (2015) 73:60–9. doi: 10.1016/j.nbd.2014.09.015
114. Verdurand M, Dalton VS, Zavitsanou K. GABA(A) receptor density is altered by cannabinoid treatment in the hippocampus of adult but not adolescent rats. Brain Res (2010) 1351:238–45. doi: 10.1016/j.brainres.2010.06.032
115. Zamberletti E, Beggiato S, Steardo L Jr., Prini P, Antonelli T, Ferraro L, et al. Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol Dis (2014) 63:35–47. doi: 10.1016/j.nbd.2013.10.028
116. Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Curr Pharm Des (2014) 20(13):2186–93. doi: 10.2174/13816128113199990426
117. Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav (2009) 92(4):559–65. doi: 10.1016/j.pbb.2009.04.001
118. Jones JD, Calkins ME, Scott JC, Bach EC, Gur RE. Cannabis Use, Polysubstance Use, and Psychosis Spectrum Symptoms in a Community-Based Sample of U.S. Youth J Adolesc Health (2017) 60(6):653–9. doi: 10.1016/j.jadohealth.2017.01.006
119. Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, Gur RC. Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry (2018) 75(6):585–95. doi: 10.1001/jamapsychiatry.2018.0335
120. Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U.S.A. (2014) 111(47):16913–8. doi: 10.1073/pnas.1415297111
121. Herbet G, Zemmoura I, Duffau H. Functional Anatomy of the Inferior Longitudinal Fasciculus: From Historical Reports to Current Hypotheses. Front Neuroanat (2018) 12:77. doi: 10.3389/fnana.2018.00077
122. Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex (2010) 46(5):691–9. doi: 10.1016/j.cortex.2009.07.015
123. Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res (2008) 163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018
124. Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry (2010) 49(6):561–72, 72.e1-3. doi: 10.1016/j.jaac.2010.02.001
125. Spechler PA, Orr CA, Chaarani B, Kan KJ, Mackey S, Morton A, et al. Cannabis use in early adolescence: Evidence of amygdala hypersensitivity to signals of threat. Dev Cognit Neurosci (2015) 16:63–70. doi: 10.1016/j.dcn.2015.08.007
126. Blest-Hopley G, Giampietro V, Bhattacharyya S. Residual effects of cannabis use in adolescent and adult brains - A meta-analysis of fMRI studies. Neurosci Biobehav Rev (2018) 88:26–41. doi: 10.1016/j.neubiorev.2018.03.008
127. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci (2011) 15(10):483–506. doi: 10.1016/j.tics.2011.08.003
128. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A (2008) 105(34):12569–74. doi: 10.1073/pnas.0800005105
129. Blest-Hopley G, Giampietro V, Bhattacharyya S. Regular cannabis use is associated with altered activation of central executive and default mode networks even after prolonged abstinence in adolescent users: Results from a complementary meta-analysis. Neurosci Biobehav Rev (2019) 96:45–55. doi: 10.1016/j.neubiorev.2018.10.026
130. Blest-Hopley G, O’Neill A, Wilson R, Giampietro V, Bhattacharyya S. Disrupted parahippocampal and midbrain function underlie slower verbal learning in adolescent-onset regular cannabis use. Psychopharmacol (Berl) (2019). doi: 10.1007/s00213-019-05407-9
131. Schneider M, Drews E, Koch M. Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behav Pharmacol (2005) 16(5-6):447–54. doi: 10.1097/00008877-200509000-00018
132. Renard J, Szkudlarek HJ, Kramar CP, Jobson CEL, Moura K, Rushlow WJ, et al. Adolescent THC Exposure Causes Enduring Prefrontal Cortical Disruption of GABAergic Inhibition and Dysregulation of Sub-Cortical Dopamine Function. Sci Rep (2017) 7(1):11420. doi: 10.1038/s41598-017-11645-8
133. Silveri MM, Dager AD, Cohen-Gilbert JE, Sneider JT. Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neurosci Biobehav Rev (2016) 70:244–59. doi: 10.1016/j.neubiorev.2016.06.042
134. Blest-Hopley G, Giampietro V, Bhattacharyya S. Regular cannabis use is associated with altered activation of central executive and default mode networks even after prolonged abstinence in adolescent users: Results from a complementary meta-analysis. Neurosci Biobehav Rev (2018) 96:45–55. doi: 10.1016/j.neubiorev.2018.10.026
135. Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O’Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry (2009) 66(4):442–51. doi: 10.1001/archgenpsychiatry.2009.17
136. Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, et al. Induction of psychosis by Delta9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry (2012) 69(1):27–36. doi: 10.1001/archgenpsychiatry.2011.161
137. Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Malhi S, et al. Impairment of inhibitory control processing related to acute psychotomimetic effects of cannabis. Eur Neuropsychopharmacol (2015) 25(1):26–37. doi: 10.1016/j.euroneuro.2014.11.018
138. Leyton M, Vezina P. Striatal ups and downs: their roles in vulnerability to addictions in humans. Neurosci Biobehav Rev (2013) 37(9 Pt A):1999–2014. doi: 10.1016/j.neubiorev.2013.01.018
139. Mokrysz C, Freeman TP, Korkki S, Griffiths K, Curran HV. Are adolescents more vulnerable to the harmful effects of cannabis than adults? A placebo-controlled study in human males. Transl Psychiatry (2016) 6(11):e961. doi: 10.1038/tp.2016.225
140. Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacol (Berl) (2009) 206(1):1–21. doi: 10.1007/s00213-009-1585-5
141. Boden J. M., Lee J. O., Hordwood L. J., Grest C. V., McLeod G. F., et al. Modelling possible causality in the associations between unemployment, cannabis use, and alcohol misuse. Soc Sci Med (2017) 175:127–34. doi: 10.1016/j.socscimed.2017.01.001
142. Fergusson D. M., Boden J. M., Hordwood L. J. Psychosocial sequelae of cannabis use and implications for policy: findings from the Christchurch Health and Development Study. Soc Psychiatry Psychiatr Epidemiol (2015) 50:(9), 1317–1326. doi: 10.1007/s00127-015-1070-x.
143. Sami M. B., Bhattacharyya S. Are cannabis-using and non-using patients different groups? Towards understanding the neurobiology of cannabis use in psychotic disorders. J Psychopharmacol. (2018) 32:(8), 825–849. doi: 10.1177/0269881118760662.
144. Schoeler T., Theobald D., Pingault JB., et al. Continuity of cannabis use and violent offending over the life course. Psychol Med. (2016) 46:(8), 1663–1677. doi: 10.1017/S0033291715003001
145. Silins E, Fergusson DM, Patton GC, Horwood LJ, Olsson CA, Hutchinson DM, et al. Adolescent substance use and educational attainment: An integrative data analysis comparing cannabis and alcohol from three Australasian cohorts. Drug Alcohol Depend (2015) 156:90–6. doi: 10.1016/j.drugalcdep.2015.08.034
146. Coffey C, Patton GC. Cannabis Use in Adolescence and Young Adulthood: A Review of Findings from the Victorian Adolescent Health Cohort Study. Can J Psychiatry (2016) 61(6):318–27. doi: 10.1177/0706743716645289
147. Fergusson DM, Horwood LJ, Beautrais AL. Cannabis and educational achievement. Addiction (2003) 98(12):1681–92. doi: 10.1111/j.1360-0443.2003.00573.x
148. Lynskey MT, Coffey C, Degenhardt L, Carlin JB, Patton G. A longitudinal study of the effects of adolescent cannabis use on high school completion. Addiction (2003) 98(5):685–92. doi: 10.1046/j.1360-0443.2003.00356.x
149. McCaffrey DF, Pacula RL, Han B, Ellickson P. Marijuana use and high school dropout: the influence of unobservables. Health Econ (2010) 19(11):1281–99. doi: 10.1002/hec.1561
150. Stiby AI, Hickman M, Munafo MR, Heron J, Yip VL, Macleod J. Adolescent cannabis and tobacco use and educational outcomes at age 16: birth cohort study. Addiction (2015) 110(4):658–68. doi: 10.1111/add.12827
151. Windle M, Wiesner M. Trajectories of marijuana use from adolescence to young adulthood: predictors and outcomes. Dev Psychopathol (2004) 16(4):1007–27. doi: 10.1017/S0954579404040118
152. David AS, Malmberg A, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. psychol Med (1997) 27(6):1311–23. doi: 10.1017/S0033291797005680
153. Giancola PR, Tarter RE. Executive Cognitive Functioning and Risk for Substance Abuse. psychol Sci (1999) 10(3):203–5. doi: 10.1111/1467-9280.00135
154. Pechtel P, Woodman A, Lyons-Ruth K. Early Maternal Withdrawal and Nonverbal Childhood IQ as Precursors for Substance Use Disorder in Young Adulthood: Results of a 20-Year Prospective Study. Int J Cognit Ther (2012) 5(3):316–29. doi: 10.1521/ijct.2012.5.3.316
155. Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, et al. A longitudinal study of premorbid IQ Score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry (2004) 61(4):354–60. doi: 10.1001/archpsyc.61.4.354
156. Brinch CN, Galloway TA. Schooling in adolescence raises IQ scores. Proc Natl Acad Sci U S A (2012) 109(2):425–30. doi: 10.1073/pnas.1106077109
157. Ullman VZ, Hornik-Lurie T, Reichenberg A. A population-based study of premorbid scholastic achievement among patients with psychiatric disorders. Psychiatry Res (2017) 253:281–6. doi: 10.1016/j.psychres.2017.04.017
158. Mustonen A, Niemela S, Nordstrom T, Murray GK, Maki P, Jaaskelainen E, et al. Adolescent cannabis use, baseline prodromal symptoms and the risk of psychosis. Br J Psychiatry (2018) 212(4):227–33. doi: 10.1192/bjp.2017.52
159. Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction (2004) 99(10):1333–41. doi: 10.1111/j.1360-0443.2004.00806.x
160. Leadbeater BJ, Ames ME, Linden-Carmichael AN. Age-varying effects of cannabis use frequency and disorder on symptoms of psychosis, depression and anxiety in adolescents and adults. Addiction (2019) 114(2):278–93. doi: 10.1111/add.14459
161. Gobbi G, Atkin T, Zytynski T, Wang S, Askari S, Boruff J, et al. Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry (2019) 76(4):426–34. doi: 10.1001/jamapsychiatry.2018.4500
162. Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA (2003) 289(4):427–33. doi: 10.1001/jama.289.4.427
163. Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist (2013) 19(1):43–61. doi: 10.1177/1073858412440596
164. Wijayendran SB, O’Neill A, Bhattacharyya S. The effects of cannabis use on salience attribution: a systematic review. Acta Neuropsychiatrica (2018) 30(1):43–57. doi: 10.1017/neu.2016.58
165. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry (2003) 160(1):13–23. doi: 10.1176/appi.ajp.160.1.13
166. O’Neill A, Mechelli A, Bhattacharyya S. Dysconnectivity of Large-Scale Functional Networks in Early Psychosis: A Meta-analysis. Schizophr Bull (2018). 45:579–90. doi: 10.1093/schbul/sby094
167. Allen P, Azis M, Modinos G, Bossong MG, Bonoldi I, Samson C, et al. Increased Resting Hippocampal and Basal Ganglia Perfusion in People at Ultra High Risk for Psychosis: Replication in a Second Cohort. Schizophr Bull (2017). 44:1323–31. doi: 10.1093/schbul/sbx169
168. Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, et al. Resting Hyperperfusion of the Hippocampus, Midbrain, and Basal Ganglia in People at High Risk for Psychosis. Am J Psychiatry (2016) 173(4):392–9. doi: 10.1176/appi.ajp.2015.15040485
169. Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus (2001) 11(5):520–8. doi: 10.1002/hipo.1068
170. Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: A systematic review of human and animal evidence. Neurosci Biobehav Rev (2016) 64:359–81. doi: 10.1016/j.neubiorev.2016.03.010
171. Sami MB, Rabiner EA, Bhattacharyya S. Does cannabis affect dopaminergic signaling in the human brain? A systematic review of evidence to date. Eur Neuropsychopharmacol (2015) 25(8):1201–24. doi: 10.1016/j.euroneuro.2015.03.011
172. Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol (2015) 29(2):97–115. doi: 10.1177/0269881114563634
173. Blest-Hopley G, O’Neill A, Wilson R, Giampietro V, Lythgoe D, Egerton A, et al. Adolescent-onset heavy cannabis use associated with significantly reduced glial but not neuronal markers and glutamate levels in the hippocampus. Addict Biol (2019), e12827. doi: 10.1111/adb.12827
174. Singh S, Khushu S, Kumar P, Goyal S, Bhatia T, Deshpande SN. Evidence for regional hippocampal damage in patients with schizophrenia. Neuroradiology (2018) 60(2):199–205. doi: 10.1007/s00234-017-1954-4
175. Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol Teratol (2007) 29(1):1–9. doi: 10.1016/j.ntt.2006.11.006
176. Freeman TP, Lorenzetti V. ‘Standard THC units’: a proposal to standardize dose across all cannabis products and methods of administration. Addiction (2019) 115:1207–16. doi: 10.1111/add.14842
177. Colizzi M, Bhattacharyya S. Cannabis Use and the Development of Tolerance: A Systematic Review of Human Evidence. Neurosci Biobehav Rev (2018) 93:1–25. doi: 10.1016/j.neubiorev.2018.07.014
178. Colizzi M, McGuire P, Giampietro V, Williams S, Brammer M, Bhattacharyya S. Modulation of acute effects of delta-9-tetrahydrocannabinol on psychotomimetic effects, cognition and brain function by previous cannabis exposure. Eur Neuropsychopharmacol (2018) 28(7):850–62. doi: 10.1016/j.euroneuro.2018.04.003
179. McGrath J, Welham J, Scott J, Varghese D, Degenhardt L, Hayatbakhsh MR, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry (2010) 67(5):440–7. doi: 10.1001/archgenpsychiatry.2010.6
180. Paul S, Bhattacharyya S. Does thinner right entorhinal cortex underlie genetic liability to cannabis use? psychol Med (2018) 48(16):1–10. doi: 10.1017/S0033291718000417
181. Fergusson DM, Horwood LJ, Ridder EM. Tests of causal linkages between cannabis use and psychotic symptoms. Addiction (2005) 100(3):354–66. doi: 10.1111/j.1360-0443.2005.01001.x
182. Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Prata D, et al. Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of δ-9-tetrahydrocannabinol on midbrain and striatal function. Mol Psychiatry (2012) 17(12):1152–5. doi: 10.1038/mp.2011.187
183. Bhattacharyya S, Iyegbe C, Atakan Z, Martin-Santos R, Crippa JA, Xu X, et al. (AKT1) genotype mediates sensitivity to cannabis-induced impairments in psychomotor control. psychol Med (2014) 44(15):3315–28. doi: 10.1017/S0033291714000920
184. Decoster J, van Os J, Myin-Germeys I, De Hert M, van Winkel R. Genetic variation underlying psychosis-inducing effects of cannabis: critical review and future directions. Curr Pharm Des (2012) 18(32):5015–23. doi: 10.2174/138161212802884591
185. Morgan CJ, Freeman TP, Powell J, Curran HV. AKT1 genotype moderates the acute psychotomimetic effects of naturalistically smoked cannabis in young cannabis smokers. Transl Psychiatry (2016) 6:e738. doi: 10.1038/tp.2015.219
186. Wong SS, Wilens TE. Medical Cannabinoids in Children and Adolescents: A Systematic Review. Pediatrics (2017) 140(5). doi: 10.1542/peds.2017-1818
187. Doherty M, Power L, Attala M, Vadeboncoeur C. Use of oral cannabis extracts in the pediatric palliative care setting: A retrospective chart review. Palliative Med (2020) 34(3):435–7. doi: 10.1177/0269216320904315
188. Gherzi M, Milano G, Fucile C, Calevo MG, Mancardi MM, Nobili L, et al. Safety and pharmacokinetics of medical cannabis preparation in a monocentric series of young patients with drug resistant epilepsy. Complement Ther Med (2020) 51:102402. doi: 10.1016/j.ctim.2020.102402
189. Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N Engl J Med (2018) 378(20):1888–97. doi: 10.1056/NEJMoa1714631
190. Devinsky O, Cross JH, Wright S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med (2017) 377(7):699–700. doi: 10.1056/NEJMc1708349
191. McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am J Psychiatry (2018) 175(3):225–31. doi: 10.1176/appi.ajp.2017.17030325
192. Schoevers J, Leweke JE, Leweke FM. Cannabidiol as a treatment option for schizophrenia: recent evidence and current studies. Curr Opin Psychiatry (2020) 33(3):185–91. doi: 10.1097/YCO.0000000000000596
193. Wilson R, Bossong MG, Appiah-Kusi E, Petros N, Brammer M, Perez J, et al. Cannabidiol attenuates insular dysfunction during motivational salience processing in subjects at clinical high risk for psychosis. Transl Psychiatry (2019) 9(1):203. doi: 10.1038/s41398-019-0534-2
194. Bhattacharyya S, Wilson R, Appiah-Kusi E, O’Neill A, Brammer M, Perez J, et al. Effect of Cannabidiol on Medial Temporal, Midbrain, and Striatal Dysfunction in People at Clinical High Risk of Psychosis: A Randomized Clinical Trial. JAMA Psychiatry (2018) 75(11):1107–17. doi: 10.1001/jamapsychiatry.2018.2309
Keywords: cannabis, marijuana, adolescence, neurodevelopment, neurofuctioning
Citation: Blest-Hopley G, Colizzi M, Giampietro V and Bhattacharyya S (2020) Is the Adolescent Brain at Greater Vulnerability to the Effects of Cannabis? A Narrative Review of the Evidence. Front. Psychiatry 11:859. doi: 10.3389/fpsyt.2020.00859
Received: 27 April 2020; Accepted: 06 August 2020;
Published: 26 August 2020.
Edited by:
Rajiv Radhakrishnan, Yale University, United StatesReviewed by:
Jorge Manzanares, Miguel Hernández University of Elche, SpainCopyright © 2020 Blest-Hopley, Colizzi, Giampietro and Bhattacharyya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sagnik Bhattacharyya, c2FnbmlrLjIuYmhhdHRhY2hhcnl5YUBrY2wuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.