- 1Centre for Forensic Family and Youth Care Studies, Leiden University, Leiden, Netherlands
- 2Leiden Institute for Brain and Cognition (LIBC), Leiden University, Leiden, Netherlands
- 3Institute of Medical Psychology, Charité Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health (BIH), Berlin, Germany
- 4Faculty of Behavioural and Movement Sciences, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 5Institute of Psychology, Clinical Psychology Unit, Leiden University, Leiden, Netherlands
- 6Primary Care Unit, School of Clinical Medicine, University of Cambridge, Cambridge, United Kingdom
- 7Department of Psychology, Education and Child Studies, Erasmus University Rotterdam, Rotterdam, Netherlands
Background: Experiencing maltreatment during childhood exerts substantial stress on the child and increases the risk for overweight and obesity later in life. The current study tests whether hair cortisol—a measure of chronic stress—and its metabolite cortisone mediate the relation between abuse and neglect on the one hand, and body mass index (BMI) on the other.
Method: The sample consisted of 249 participants aged 8 to 87 years (M = 36.13, SD = 19.33). We collected data on child abuse and neglect using questionnaires, measured cortisol and cortisone concentrations in hair, and BMI. In a structural model, the effects of abuse and neglect on hair cortisol, hair cortisone, and BMI were tested, as well as the covariance between hair cortisol and BMI, and hair cortisone and BMI.
Results: Within the sample, 23% were overweight but not obese and 14% were obese. Higher levels of experienced abuse were related to higher cortisone concentrations in hair (β = 0.24, p < .001) and higher BMI (β = 0.17, p =.04). Neglect was not related to hair cortisol, hair cortisone, or BMI. Hair cortisol and cortisone did not mediate the association between maltreatment, and BMI. Sensitivity analyses demonstrate the same pattern of results in a subsample of adult participants currently not living with their parents. However, in younger participants who were still living with their parents, the associations between abuse and cortisone (β = 0.14, p =.35) and abuse and BMI (β = 0.02, p =.92) were no longer significant.
Conclusion: These findings confirm that experiencing abuse is related to higher BMI but suggest that hair cortisol and cortisone are not the mechanism underlying the association between child maltreatment and BMI. This is the first study to show abuse may be associated to elevated concentrations of hair cortisone—evidence of long-term alterations in chronic stress levels. Future research may benefit from exploring the effects of maltreatment on weight gain in longitudinal designs, including measures of other potential mediators such as eating as a coping mechanism, and more direct indicators of metabolic health.
Introduction
Abuse and neglect are adverse childhood experiences that exert substantial stress on the child, and violate expectations of a safe and stable environment (1, 2). Experiencing abuse or neglect during childhood increases the risk for a number of negative mental (3–5) and physical health outcomes (6–8)—among them an increased risk for overweight and obesity later in life (9–11). The relation between childhood maltreatment and overweight has been confirmed by two meta-analyses including 190,285 and 112,708 participants, respectively (12, 13). Longitudinal studies with several assessments have shown that maltreatment is related to accelerated weight gain (14–17), but report conflicting results with regard to which type of maltreatment drives this effect.
Overweight and obesity present a global health challenge with rising prevalence (18) and with increased risk for diabetes, cardiovascular disease, cancer, and overall mortality (19–24). Unfortunately, it has proven difficult to develop effective interventions to prevent obesity (25), but targeting the mechanisms that underlie the relation between maltreatment and weight gain, such as stress, might offer new effective strategies (26). It has been proposed that mental health mediates the association between maltreatment and BMI. One study investigated posttraumatic stress disorder as a potential mechanism but only found a weak mediation effect (27). Another study found that depression fully mediated the association between physical abuse and BMI in girls but not in boys and did not play a role for other types of maltreatment (17). An alternative approach would be to focus on biological factors such as stress physiology. It has been suggested that maltreatment experiences and increases in BMI could be connected through chronically elevated levels of cortisol (9, 12). Cortisol has been implicated both as a consequence of maltreatment and as a factor involved in weight gain. Therefore, the current study tests the mediational role of hair cortisol (and its metabolite cortisone) in the association between abuse and neglect on the one hand, and BMI on the other hand.
If abuse and neglect are encountered during developmentally sensitive periods, they have the potential to not only elicit an acute stress response but also to reprogram one of the central human stress response systems: the hypothalamic–pituitary–adrenal axis [HPA axis; (28)]. When confronted with a stressor the HPA axis is activated and after a number of intermediary steps, cortisol is released from the adrenal gland and prepares the body to respond to danger (29). Therefore, HPA axis activity is often indexed by measuring cortisol levels. One meta-analysis found evidence of blunted wake-up cortisol levels in maltreated children and adults, with stronger effect sizes in agency-referred samples, but no effect of maltreatment on the cortisol awakening response and the diurnal cortisol pattern (30). Another meta-analysis focused on studies measuring the cortisol response following a social stressor (31). There was evidence of blunted cortisol reactivity in maltreated individuals and this effect was stronger in adults than in children. This can be explained from a theoretical perspective: Both the allostatic load model and the attenuation hypothesis argue that stress will initially elicit an increased stress response in the form of heightened cortisol levels, but over a prolonged period, will result in a blunted stress response (32, 33).
The majority of the above described research has investigated HPA-axis functioning by measuring circulating cortisol in saliva, which offers insight into acute cortisol levels across the day and in response to specific stressors. In recent years, measures have been complemented by assessing cortisol concentrations in hair samples, which represent a more chronic measure of cortisol (34, 35). Cortisol circulating in the bloodstream is incorporated by the hair through passive diffusion.
Several studies have found associations between experienced maltreatment and hair cortisol levels later in life (e.g., 36, 37). A meta-analysis found evidence of elevated cortisol concentrations in hair following trauma sometimes years later (including maltreatment)—albeit with small effect sizes (38). Interestingly, this study revealed two classes of effects. The first class consisted of studies that showed hypocortisolism with a moderate effect size whereas the second class consisted of studies that showed hypercortisolism with a small effect size. While the first class of studies had larger effect sizes, it contained fewer studies: four compared to 24 in the second class. All four studies in the first class investigated child maltreatment. The second class covered a broader spectrum of traumatic experiences but also included 16 studies on child maltreatment. The entire meta-analysis included studies with populations of different ages. However, age did not moderate the effect of trauma on hair cortisol levels. Ultimately, it remained unclear which factors determine whether hair cortisol concentration is blunted or elevated following child maltreatment. In the current study we investigated the unique effects of abuse and neglect on hair cortisol levels in a sample with a wide age range.
In addition to cortisol levels, cortisone levels may be relevant in the context of maltreatment and BMI. Cortisol can be metabolized into the inactive cortisone by 11β-hydroxysteroid dehydrogenase (11B-HSD) type 2 (39). Essentially no research has been conducted to investigate the effects of maltreatment on cortisone. Therefore, the present study also includes a measure of hair cortisone.
Stress and cortisol also play a central role in obesity. Early evidence of the causal role cortisol plays in obesity comes from studies observing weight gain in patients who are administered glucocorticoids (40–42). Additional research suggests that cortisol might be involved in fat accumulation and weight gain (43) by inducing greater food intake (44–46) and disrupting the regulation of fat storage (47). Generally, higher levels of cortisol have been linked to higher BMI (48) but there are also examples of hypocortisolism and higher BMI (49). To date, only a few studies have explicitly explored the relationship between cortisol measured in hair and obesity and most of these studies have relatively small sample sizes. Overall, these studies generally find elevated cortisol concentrations in obese children (50) and adults (51, 52) but not all studies find a relationship (53). Also, it has been suggested that the impact of cortisol on weight not only depends on how much cortisol is secreted but also how it is metabolized. The release of more cortisol may be triggered if more cortisol is transformed to cortisone (54).
The current study adds to the existing research by testing the mediating role of hair cortisol—as an objective measure of chronic stress and HPA-axis functioning—in the relation between retrospectively measured maltreatment and the BMI of children and adults. To our knowledge, only one study has investigated cortisol dysregulation as a mediator between maltreatment and BMI (55). This study found that a flatter cortisol awakening response partially mediated the effect of early adversity on BMI. Since the cortisol awakening response is not correlated to cumulative cortisol secretion measured by hair cortisol it is still unknown whether overall cortisol production measured in hair mediates the relation between maltreatment and BMI. In addition, the current study focused on the unique effects of abuse and neglect on cortisol levels and BMI in line with the theoretical proposition that they represent different types of stressors (56). It has been suggested that cortisone is an alternative, parallel measure of cortisol (35). For a better understanding of HPA axis functioning and corticosteroids in the body (57), we investigated the concentration of both the active cortisol and the inactive cortisone in hair.
We expected that abuse and neglect experienced in childhood would be associated with higher BMI at the time of the study. Further, we expected that abuse and neglect would be related to hair cortisol and cortisone concentrations and that these would in turn be related to BMI. We hypothesize that the effect of maltreatment on BMI would be mediated by elevated hair cortisol and cortisone concentrations. Given mixed results in previous research, we explored whether abuse and neglect had differential effects.
It has been argued that the initial response to experiencing maltreatment is an increased stress reaction, but that over time chronic stress results in down-regulation (30, 32, 58). In this study, we included children and adults. As a result, for some participants experiences of abuse and neglect were potentially acute if they still lived with their parents at the time of the study while it was in the past for the participants who had moved out of their parental home—in some cases decades ago. These two groups might display different HPA axis activity. Therefore, we explored whether the same associations would be observed when performing the analyses for these two groups separately. Following the allostatic load model, elevated cortisol levels would be expected in younger participants who had experienced maltreatment and blunted cortisol levels in adult participants who had experienced maltreatment. Moreover, an association between maltreatment and BMI may only arise in adults.
Method
Sample
The sample of the current study was drawn from the Dutch 3G Parenting Study that investigates intergenerational transmission of parenting styles, stress and emotion regulation using a multi-generational design (for details see 59, 60).
For this investigation, we included participants if they agreed to provide a hair sample and had sufficient hair growth resulting in a sample of N = 280. Of these participants, n = 31 were excluded because of corticosteroid use in the previous three months. The final sample of 249 participants came from 60 families with an average of 4.15 family members per family (range: 1 to 18), aged 8 to 87 years (M = 36.13, SD = 19.33).
Procedure
Participants attended one or two 7-hour lab visits at the Leiden University Medical Center with their nuclear families. During these lab visits, participants completed questionnaires, computer tasks, interviews, and family interaction tasks. In addition, samples of hair, saliva, and buccal cells were collected. All participants signed informed consent. Parents cosigned informed consent if the participant was under the age of 18 years. Also, maltreatment questionnaires of underage participants were inspected after each lab visit. Ethical approval was obtained from the Ethics Committee of the Leiden University Medical Centre (reference number: P11.134).
Instruments
Child Maltreatment
We measured child maltreatment experiences with a combination of two self-report questionnaires: the Parent-Child Conflict Tactics Scales (CTSPC, 61) and the Childhood Trauma Questionnaire (CTQ, 62, 63). Four maltreatment subtypes were assessed: 1) physical abuse (13 items; CTSPC), 2) emotional abuse (five items; CTSPC), 3) physical neglect (four items; CTSPC), and 4) emotional neglect (six items; CTSPC and CTQ). The CTSPC and the CTQ use different rating scales to assess frequencies. In order to be consistent across items, a 5-point scale ranging from (1) never to (5) (almost) always was used for all items. The emotional neglect items from the CTQ were reverse coded.
To measure maltreatment, we used a multi-informant approach in which we combined child- and parent-reports. All participants reported on whether they had experienced maltreatment (i.e., child-report). For the child-report score, participants reported on maternal and paternal behavior separately. Per subtype, we first calculated averages for maltreatment perpetrated by mother and maltreatment perpetrated by father. Then, per subtype, the higher score of mother or father was included in the child-report score. In addition, if a participant participated with a parent, that parent also reported on whether they had perpetrated maltreatment towards that particular participant (i.e., parent-report). The same approach was used to calculate the parent-report score. At least one parent score was available for 61% of the participants. Child-report and parent-report were averaged. Lastly, abuse and neglect scores were calculated by averaging the average for physical and the average for emotional abuse (r(247) =.64, p < .001) and the average for physical and the average for emotional neglect (r(247) =.38, p < .001), respectively. Cronbach's αs show acceptable internal consistency for child-report (abuse: αmother =.92, αfather =.91; neglect: αmother =.86, αfather =.86) and parent-report (abuse: αmother =.78, αfather =.84; neglect: αmother =.76, αfather =.67).
It should be noted that the procedure for younger participants was slightly adjusted. For participants under 12 years of age, experienced maltreatment was assessed orally and questions about very severe physical abuse were omitted. Participants who were 12 years or older and living with their parents at the time of the study indicated whether they had experienced maltreatment within the last year or in the years before. Per item, the higher score of these two was included. For the full questionnaire and further details, see Data Sheet 1.
Hair Cortisol and Cortisone
During the lab visit, a research assistant cut approximately 100 hairs closely from the scalp at the back of the head. In most cases, the most proximal 3 cm were analyzed. For six participants, the available hair was shorter than 3 cm: for one participant 1 cm of hair was available, for two participants 2 cm of hair, and for three participants 2.5 cm of hair. Hair samples were prepared by weighing them, cutting them in smaller pieces with surgical scissors, and washing them with 1.0 ml of LC—MS grade isopropanol for 2 min. The hair samples were incubated over night with 1.4 ml LC—MS grade methanol and in presence of 100 µl internal standard (cortisol-d4) for 18 h at 25°C while gently shaking to extract cortisol and cortisone. In line with previous research (e.g., 34, 57), we log-transformed to reduce skewness.
At a growth-rate of approximately one centimeter per month, one centimeter of hair represents the cumulative cortisol secretion of the past month (64). It is possible to reliably assess cortisol in the 3 cm of hair most proximal to the scalp, i.e., representing a retrospective measure of the last three months (35). Hair cortisol has been shown to be related to average salivary cortisol levels from repeated measures across several days but is not associated to the cortisol awakening response or the diurnal cortisol pattern (65–67). Moreover, test-retest correlations indicate that hair cortisol is more stable over time than cortisol measured in saliva or urine (65, 68).
BMI
Participants either self-reported on height and weight or, if they did not know, their height and weight were measured by a research assistant. BMI was calculated from these height and weight measures (kilograms/meters2). BMI and age were correlated [r(233) =.56, p < .001]. When assessing BMI in children and adolescents, it has been recommended to use age and gender specific scores based on external reference values (69). Therefore, for participants younger than 21 years, raw BMI scores were transformed into standard deviation scores (SDS) based on a representative Dutch sample (70) applying the LMS method (71) in the software package childsds (72) implemented in R (73). For individuals of 21 years and older, no SDS were available. In order to combine scores from both age groups, adult BMI scores were transformed by regressing out age and sex, and standardizing the residuals. This combined score will be denoted as BMI-z in the remainder of the manuscript.
Hair Related Variables
We assessed several factors that might affect hair cortisol and cortisone levels using a questionnaire. We used open questions to ask participants about their ethnicity, medication use, and hair color. Answers were coded to match previous studies on determinants of hair cortisol and cortisone (34, 57). Ethnicity was recoded into Northern European and other, and medication use into yes or no. Hair color was recoded into black, brown, blond, red, and grey. Further, participants reported whether or not they had dyed, bleached, or permed their hair in the last 3 months, whether they washed their hair more or less than three times a week, whether they had washed their hair more or less than 24 h before taking the hair sample, and whether they had used any hair styling products on the day of sampling. Lastly, the astronomical season of the lab visit was included.
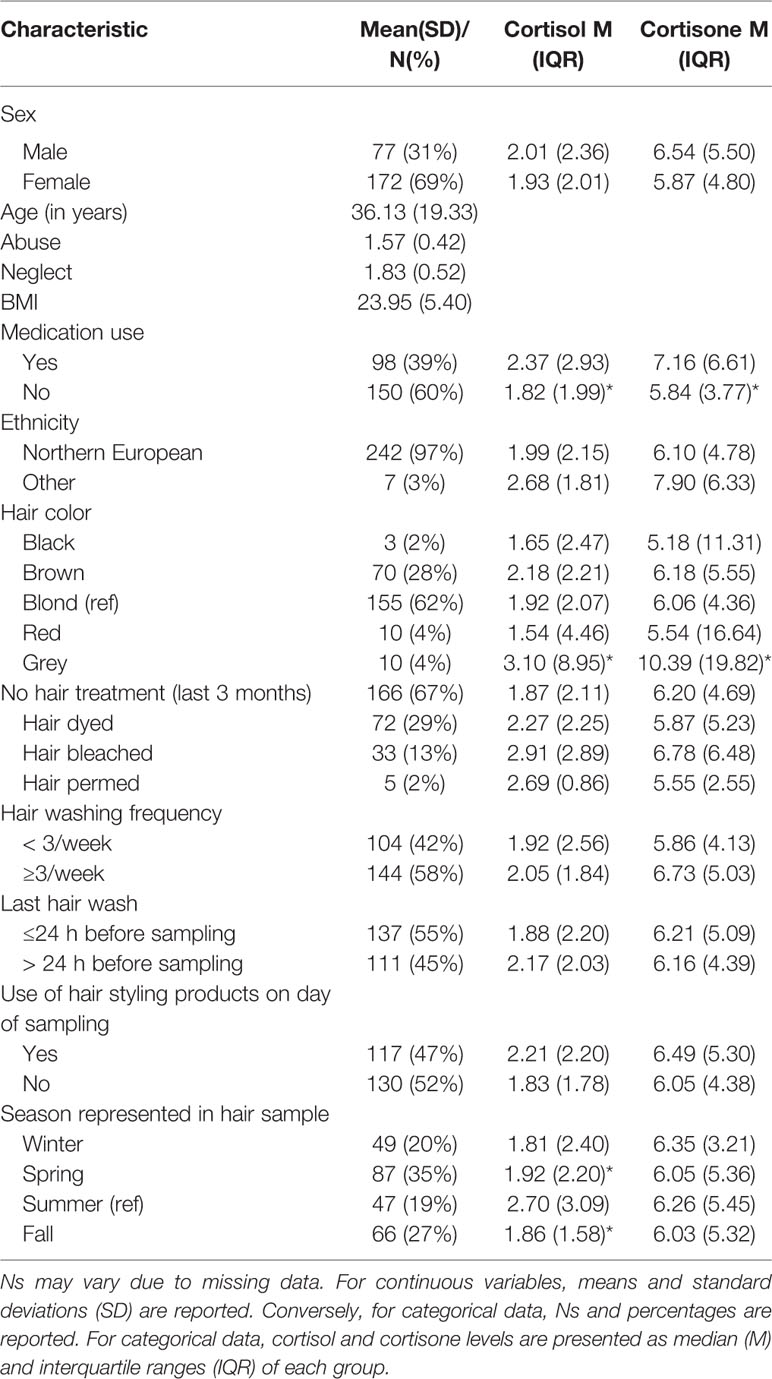
Since most of these confounders, with the exception of season, hair color, and medication, were not related to either hair cortisol or cortisone (see Table 1) and adding them increases the number of free parameters, we did not include them in the main analysis but we explored relevant confounders in a sensitivity analysis. All variables were dummy coded. Season was dichotomized to differentiate participants who had participated in summer compared to any other season. Information on hair dying, bleaching, and perming was summarized to indicate whether a participant had undergone any hair treatment in the past three months or not. Adding these confounders as independent variables to be regressed on hair cortisol and cortisone resulted in poor model fit (X2 = 142.75 (p =.00, df = 47), RMSEA = 0.90, and CFI = 0.78). Of all the confounding variables, only season (summer vs. other season) was significantly related to cortisol (β = -0.13, p =.03) and medication use was related to cortisone (β =.16, p =.02). This is in line with the preliminary analyses (Table 1). Contrary to the preliminary analyses, grey hair was neither related to hair cortisol nor to cortisone—likely because the effect was explained by age. Therefore, a sensitivity analysis was performed only including season and medication use as potential confounders.
Demographic Information
Age and sex were included as demographic variables as well as household socioeconomic status (SES). To assess SES we asked participant of 18 years and older about their household income and highest completed education. Yearly household income was measured on a 7-point scale ranging from (1) less than € 15,000 to (7) more than € 65,000. Due to changes in the Dutch educational system, first and second generation participants rated education on a 7-point scale and third generation participants rated education on a 10-point scale. Both scales were rescaled to a 4-point scale. Based on standardized household income and standardized completed educational level a composite household SES score was calculated. If data of two partners living in the same household was available their scores were averaged for the household SES score. Children living with their parents shared their parents' household SES score.
Analysis
Outliers
Outliers (i.e., z > ± 3.29) were winsorized (74) by adding or subtracting the difference between the last two acceptable raw values to the last acceptable value. We winsorized two outliers for abuse, one for neglect, four for hair cortisol, seven for hair cortisone, and one for BMI.
Missingness
The missing information were as follows: 2.4% hair cortisol, 1.2% hair cortisone, 5.6% BMI, 1.2% hair dyed, 1.2% hair bleached, 0.8% hair permed, 0.4% hair washing frequency, 0.4% on last hair wash, and 0.8% on hair product use. Little's missing completely at random (MCAR) test was not significant (χ2 = 27.20, df = 22, p = 0.20) indicating that data was MCAR. Therefore, we imputed missing values using full information maximum likelihood (FIML).
Structural Model
The following analyses were implemented in the R package lavaan 0.6-5 (75). A structural model was used to simultaneously test the effects of abuse and neglect on hair cortisol, hair cortisone, BMI-z, as well as the covariance between hair cortisol and BMI-z, and hair cortisone and BMI-z. Theoretically, we expect that glucocorticoids would affect BMI but methodologically we cannot infer causality in our study. By measuring cortisol and cortisone in hair we get an estimate covering the past 3 months, while BMI-z was measured during the test day. A model in which glucocorticoids and BMI-z mutually reinforce each other is plausible (76, 77). Thus, given the temporal proximity of these measures and the possibility of BMI-z affecting HPA-axis functioning, we tested a bidirectional relation rather than a directional effect between cortisol and cortisone on the one hand, and BMI-z on the other hand. SES was always included as a covariate. We controlled for age and sex in the prediction of hair cortisol and cortisone but not BMI-z because this score was already corrected for age and sex effects. We allowed abuse and neglect to covary with each other and cortisol and cortisone to covary with each other. Based on bivariate correlations, we also included covariation between age and abuse and neglect, as well as SES and neglect.
The model parameters are determined using a maximum likelihood estimator. Standard errors were bootstrapped (1,000 samples) because some of the variables had a non-normal distribution. To assess fit, we inspected comparative fit index (CFI) and the root mean square error of approximation (RMSEA). A CFI above .90 and an RMSEA below .06 was considered to describe a good fit between the model and the observed data (78).
Results
Descriptive Statistics
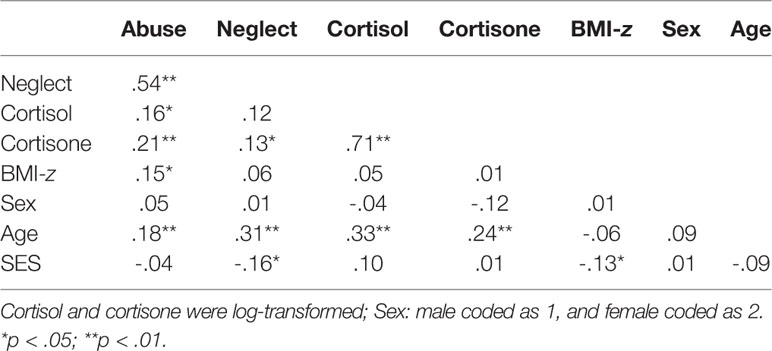
Correlations and descriptive statistics are reported in Tables 1 and 2. No differences between men and women were observed but older participants had higher levels of abuse, neglect, cortisol, and cortisone (ps < .01). SES was negatively related to neglect and BMI-z (ps < .05). Abuse and neglect were correlated (p < .01) as were cortisol and cortisone (p < .01). Lastly, abuse was correlated to cortisol, cortisone, and BMI-z (p < .05) whereas neglect was only related to cortisone (p < .05). For participants who were younger than 21 years, a BMI SDS of 1 or higher was considered overweight and of 2 or higher was considered obese (79). For participants who were 21 and older, the cut-offs were raw BMI scores of 25 and 30 for overweight and obese, respectively (80). Fifty-seven participants (23%) were overweight but not obese and 35 were obese (14%). Note that we use BMI categories only for descriptive purposes here but work with continuous, standardized scores (BMI-z) in the analyses for optimal power (81).
Structural Model
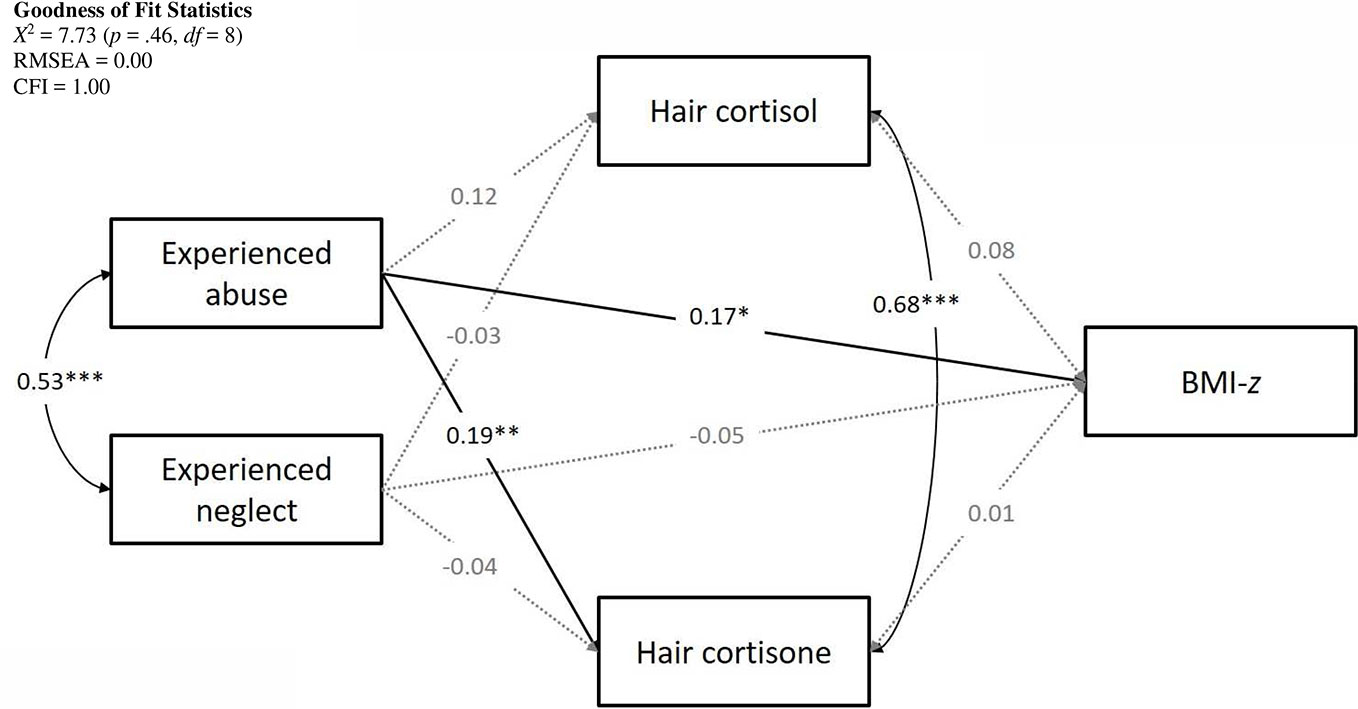
The hypothesized model fit the observed data well with X2 = 7.73 (p =.46, df = 8), RMSEA = 0.00, and CFI = 1.00. The complete model with standardized path coefficients is presented in Figure 1.
Pathways to BMI-z
Lower SES was associated with higher BMI-z scores (β = -0.13, p =.02). Age and sex were not included as covariates as BMI-z was already corrected for age and sex. There was a significant positive association between abuse and BMI-z (β = 0.17, p =.04) with higher levels of experienced abuse predicting higher BMI-z. The effect of experienced neglect on BMI-z was not significant (β = -0.05, p =.57). Hair cortisol and hair cortisone were not associated with BMI-z (cortisol: β = 0.08, p =.22; cortisone: β = 0.01, p =.85).
Pathways to Hair Cortisol
Hair cortisol concentrations were higher in older participants (β = 0.35, p < .001) and participants with a higher SES (β = 0.13, p =.05) but were not signif ica ntly associated with sex (β = -0.07, p =.23). Taking into account these demographic variables, there were no significant relations with abuse (β = 0.12, p =.06) or neglect (β = -0.03, p =.61).
Pathways to Hair Cortisone
We observed higher hair cortisone levels for older participants (β = 0.24, p < .001) and men compared to women (β = -.13, p =.02). There was no association with SES (β = 0.04, p =.16). Controlling for age, sex, and SES, experiencing abuse was significantly related to hair cortisone. Higher levels of experienced abuse were related to greater cortisone concentrations (β = 0.19, p < .01). Conversely, the effect of experienced neglect was not significant (β = -0.04, p =.56).
Indirect Effects
An indirect effect can be significant even if not all of the direct paths are significant. For completeness, we tested whether any of the indirect effects were significant, however, this was not the case (ps > .38).
Sensitivity and Exploratory Analyses
A number of additional analyses were conducted to exclude alternative explanations for the findings. For one, trauma may affect individuals in a different way depending on whether it is ongoing or not. Therefore, we ran the analyses separately for participants who were still sharing a household with their parents and those who were not. Of the total sample, 173 participants were not living with their parents at the time of the data collection. Running the model in this subsample, we found good model fit [X2 = 7.61 (p =.47, df = 8), RMSEA = 0.00, and CFI = 1.00]. We found the same pattern of significant results and similar strength and direction of path coefficients. There were 76 participants who were living with their parents at the time of the data collection. The model fit for this subsample was acceptable [X2 = 5.79 (p =.67, df = 8), RMSEA = 0.00, and CFI = 1.00]. However, running the path model for this subgroup led to a different pattern of results with the previously significant effects of abuse on hair cortisone concentration (β = 0.14, p =.35) and BMI-z (β = 0.02, p =.92) disappearing. This might suggest an age effect but could also be the result of a lack of power in the last analysis as this group was smaller than the group of participants who were not living with their parents.
We initially tested the unique effects of cortisol and cortisone. Cortisol and cortisone were highly correlated. In two sensitivity analyses we tested whether cortisone suppressed the effects of cortisol and vice versa by running the analyses for cortisol and cortisone separately. In both cases the estimates of model fit were satisfactory [cortisol only: X2 = 7.71 (p =.46, df = 8), RMSEA = 0.00, and CFI = 1.00; cortisone only: X2 = 7.62 (p =.47, df = 8), RMSEA = 0.00, and CFI = 1.00] and the same pattern of significant results emerged, with similar path coefficients, suggesting that there was no suppression effect. It has also been suggested that the cortisol:cortisone ratio and the sum of cortisol and cortisone may be meaningful indicators of HPA-axis activity (57). Therefore, we ran two additional models for the ratio and the sum. Both models had good model fit [ratio: X2 = 7.71 (p =.46, df = 8), RMSEA = 0.00, and CFI = 1.00; sum: X2 = 7.64 (p =.47, df = 8), RMSEA = 0.00, and CFI = 1.00]. Ratio was predicted by age (β =.24, p =.001) and SES (β =.14, p =.03) but not abuse or neglect. Ratio and BMI-z were also not related (β =.10, p =.10). The sum of cortisol and cortisone was related to age (β =.32, p < .001) and abuse (β =.16, p =.02) but not BMI-z (β =.03, p =.64).
Physical and emotional maltreatment might have differential effects. Therefore, we explored physical and emotional abuse, and emotional neglect in separate models. We omitted physical neglect from these analyses because on its own, physical neglect did not have sufficient variance (M = 1.19, SD = 0.28) or internal reliability (α =.24–.64). For physical abuse, model fit was good [X2 = 2.16 (p =.34, df = 2), RMSEA = 0.02, and CFI = 0.99]. Physical abuse showed similar associations with the variables in the model as overall abuse did. There was a significant association with BMI-z (β =.15, p =.02), cortisol (β =.12, p =.05) and cortisone (β =.16, p =.01). Results were also similar for emotional abuse [model fit: X2 = 2.15 (p =.34, df = 2), RMSEA = 0.02, and CFI = 0.99]. Emotional abuse was associated with BMI-z (β =.16, p =.02), cortisol (β =.11, p =.05), and cortisone (β =.16, p =.01). The pattern of results for emotional neglect was also similar to overall neglect. Model fit was adequate [X2 = 1.91 (p =.39, df = 2), RMSEA = 0.00, and CFI = 1.00]. Emotional neglect was not associated with any variables of interest (BMI-z: β =.02, p =.74; cortisol: β =.03, p =.58, cortisone: β =.07, p =.07).
We also ran the model again with season and medication use as control variables for hair cortisol and cortisone, respectively. This resulted in a model that fit the data adequately [X2 = 37.86 (p =.01, df = 20), RMSEA = 0.60, and CFI = 0.95] and did not affect the path coefficients substantially.
Discussion
This study investigated the effect of experiencing child maltreatment on BMI and the mediating role of chronic stress, indexed by hair cortisol and cortisone. Confirming previous research (12, 13), we found a relation between reported experiences of abuse and higher BMI-z. Moreover, we observed higher concentrations of hair cortisone in individuals who had experienced abuse. However, contrary to our expectations, neither hair cortisol nor hair cortisone mediated the association between abuse and BMI-z. Neither hair cortisol nor hair cortisone had a direct effect on BMI. Moreover, neglect did not affect hair cortisol, hair cortisone, or BMI-z.
In line with most previous research (12, 13), abuse was related to higher BMI. This finding indicates that early experiences can initiate a trajectory that results in weight gain later on and implicates psycho-emotional risk factors in the etiology of overweight. Accordingly, recent models of obesity put greater emphasis on affective aspects of weight gain (76).
As expected, abuse was related to increases in hair cortisone (and trend-level increases in cortisol) suggesting chronic levels of stress in individuals with abuse experiences – in some cases decades after the abuse occurred. This is also in line with the majority of research on maltreatment and hair cortisol which suggest elevated cortisol secretion (38). It is interesting to contrast these findings with evidence of hypocortisolism in maltreated individuals. For instance, meta-analytic evidence suggests that in response to a social stressor, cortisol levels are blunted rather than increased in maltreated individuals. Since hair cortisol represents a cumulative measure of cortisol secretion, this might suggest that abused individuals encounter or perceive more stressful events than non-maltreated individuals—even if their stress response to the particular event is blunted. Alternatively, basal cortisol and cortisone levels may be higher in individuals who experienced childhood abuse.
Evidence of hypocortisolism has generally been interpreted in the context of the allostatic load model: over time the stress response is downregulated to maintain homeostasis. The current study found evidence of elevated cortisone levels in abused individuals—in particular in adults. However, this is not necessarily in contradiction to the allostatic load model since intra-individual cortisone levels may have been downregulated over time, but the cross-sectional nature of the study does not allow us to test this hypothesis. Collectively, these findings show that experiences of abuse get embedded in the body's physiology by reprogramming HPA-axis and higher BMI but that these might be separate processes.
From a methodologic perspective, this finding also supports the measurement of hair cortisone in addition to hair cortisol. At the moment, the majority of studies focus on hair cortisol and do not include a hair cortisone measures. Cortisone levels are generally higher than cortisol levels. It has been suggested that this might make cortisone more readily detectable and a more powerful indicator of chronic stress (57). Effects of abuse on cortisol seem to parallel the effects on cortisone but might require a larger sample size than cortisone. Given that it can be difficult to achieve large sample sizes when studying specific populations, measuring hair cortisone could hold a lot of promise. Moving forward, it will be important to gain a better understanding of the relation between cortisol and cortisone in general and in hair specifically. The correlation between cortisol and cortisone is indicative of a strong relation but also suggests that they are not entirely interchangeable. Functionally, cortisone is less active than cortisol but it is nevertheless an important indicator of HPA axis activity since cortisone and cortisol are closely linked through the 11B-HSD enzymes. Cortisol can be metabolized into cortisone by 11B-HSD type 2 and cortisone can be transformed back into cortisol by 11B-HSD type 1. Therefore, it has been suggested that including measures of 11B-HSD type 1 and 2 activity could provide a completer picture of HPA axis functioning (82). On an endocrine level, 11B-HSD may be involved in the HPA axis feedback loop and contribute to the daily production of cortisol (83).
Our results suggest that the association between childhood abuse and later BMI is not mediated by hair cortisol and cortisone. This can be attributed to the fact that, contrary to our expectations, hair cortisol and cortisone were not associated with BMI-z. Previous research has suggested a relation between HPA-axis dysregulation and obesity but results were inconsistent (84). More sensitive measures to assess the relation between HPA-axis functioning and obesity might be site-specific cortisol activity such as intra-adipocyte cortisol concentrations and abdominal adiposity measures to assess body fat distribution (48, 85). Also, differences in hair cortisol may only arise at higher levels of BMI. For instance, one study found that obese participants had higher hair cortisol levels compared to overweight and normal weight participants but no difference between overweight and normal weight participants (52). In the current study, 14% of the sample was obese whereas the majority (63%) was normal weight.
Stress may affect men and women differently. While there is meta-analytic evidence suggesting that the effect of maltreatment on obesity is stronger in women, another meta-analysis suggested that psychosocial stress encountered in adulthood has a stronger effect on BMI in men (86). The same study found stronger effects of psychosocial stress on obesity if the follow-up period was longer. In the current study, two-thirds of the participants were women. Hair cortisol and cortisone measures cover a period of three months whereas years or decades may have elapsed since experiencing abuse. Taken together, these details might explain why we observed an association between abuse and BMI-z but no effect of cortisol and cortisone on BMI-z because abuse would affect women more strongly and has a longer follow-up period.
Addressing the affective component of obesity could have profound consequences because traditional weight loss interventions primarily focus on non-affective causes by modifying the energy balance by decreasing energy intake and increasing energy expenditure. And while interventions focusing on diet and exercise have been shown to reliably reduce weight, their effects on weight loss are small (87–89). Making matters worse, participants struggle to maintain weight loss and often drop out without completing the intervention (90, 91). For individuals with childhood abuse experiences, these traditional approaches to weight loss may not be sufficient because they do not fully address the underlying problem.
An important issue to consider concerns the timing of interventions. It appears to be generally true that early prevention is more effective than later intervention because smaller changes in behavior are necessary to maintain than to lose weight (92). In the present study, there was no association between abuse and BMI in the younger subgroup who were still living with their parents and are therefore still at risk of parent-to-child abuse. This result should be interpreted with some caution since this subgroup was small. It is interesting to note that in the younger subgroup the association between abuse and cortisone remained similar in strength even though it was not significant anymore. However, the association between abuse and BMI essentially dropped to zero—possibly a sign that this is not solely a power issue. This is in line with meta-analytic evidence showing no effect of maltreatment on BMI in children and adolescents (12). Taken together, this indicates that childhood abuse might be an early risk factor for obesity but the effects may only become apparent later on. Early identification of groups who are at heightened risk for developing overweight is a crucial component of effective prevention.
It may also be relevant to explore other mediating factors such as eating as coping behavior. Research shows that abused individuals are more likely to a develop binge eating disorder (93). This may be an attempt to regulate negative emotions through emotional eating (10, 94). In line with this assumption, symptoms of depression and post-traumatic stress have been found to mediate the relation between maltreatment and obesity—but the evidence is tenuous (17, 27). It has also been argued that unhealthy eating patterns as a result of maltreatment may be associated with decreases in prefrontal cortex volume and less inhibitory control (12). One study has found that sexual abuse resulted in higher BMI in impulsive individuals only. However, the moderating effect of impulsivity was not found for other types of maltreatment (95). More research is needed to explore the mechanisms underlying the effect of maltreatment on BMI.
In the current study, neglect was not related to BMI. The type of maltreatment might affect how the HPA-axis is reprogrammed. It has been argued that abuse and neglect can be differentiated along two dimensions of adverse childhood experiences: abuse representing threat and neglect representing deprivation (96, 97). Threat may not elicit the same stress response as deprivation. For instance, one study found that adolescents who had experienced abuse had chronically elevated stress levels, whereas adolescents who had experienced neglect had blunted stress levels (cited in 98), possibly because threat requires activation of the stress system whereas deprivation does not. Another explanation for this finding might be that participants reported little physical neglect. Therefore, the neglect score primarily represents emotional neglect. Previous evidence suggests that emotional neglect might not be related to higher BMI and obesity (12). It would be interesting to explore how emotional neglect, specifically, differs from the other types of maltreatment. Importantly, these results do not suggest that emotional neglect is not a relevant adverse childhood experience. Rather, these findings suggest that it is time to move away from a purely cumulative model which ignores the type of experience (56). Different types of maltreatment and adverse childhood experiences might affect different developmental pathways in different ways. For instance, emotional neglect might not disrupt stress pathways such as HPA-axis development but it may be detrimental to learning pathways such as reward learning because it deprives the child of positive and sensitive emotional input (56, 99).
Limitations
The current study had several shortcomings. We did not assess during which developmental period maltreatment occurred. It has been suggested that neglect during the first two to three years of life is particularly crucial in programming HPA-axis functioning (37, 98). Our assessment of maltreatment does not allow us to discern between neglect starting early in the development and neglect that occurred later in the development. Moreover, at least in child reports, any maltreatment that occurred in the first three to four years is unlikely to be remembered and hence, unlikely to be reported (100). Another consequence of not knowing the exact timing of maltreatment experiences is that we could not include the exact time that had elapsed since maltreatment as a potential moderator.
Moreover, the cross-sectional nature of this study means that we cannot draw any conclusion on the causality of the associations. Child maltreatment was assessed retrospectively and thus current stress levels may have affected participants' responses. Generally, agreement between prospective and retrospective measures of child maltreatment tends to be low (101). Baldwin and colleagues (101) argue that prospective and retrospective measures are not interchangeable, but both are valuable. The advantage of retrospective reports is that they may capture more true cases as prospective reports often rely on official records which often underestimate incidence of maltreatment and only capture the most extreme cases. Moreover, cortisone levels are unlikely to account for the association between abuse and cortisone entirely—especially since we used multi-informant scores of maltreatment for most of the participants. What is more, if maltreatment scores were driven by current cortisone levels, we would expect this to affect neglect scores as much as abuse scores which was not the case. Hair cortisol and cortisone levels represent the cumulative secretion of glucocorticoids over the three months prior to measuring BMI. This close temporal proximity makes it difficult to draw strong conclusions with regards to the causal effect of cortisol and cortisone on BMI. For this reason, we tested a bidirectional effect. Since this association was not significant we can conclude that in the present study there was no relation between cortisol and cortisone on the one hand, and BMI on the other hand.
Lastly, BMI is the most commonly used metabolic outcome and is intended to, indirectly, assess body fat percentage (102). However, it is a crude measure and there might be better ways to assess overweight. One disadvantage of BMI is that it is confounded by physical fitness (103, 104). Due to the ease of assessing BMI compared to more direct measures of body fat percentage, it is unlikely to disappear soon. It also allows the comparison with population norms and other studies. For future research, it might be interesting to incorporate other indicators of metabolic health such as leptin deficiency (105) in addition to BMI. It should also be noted, that in some cases, BMI was based on self-report which may introduce some imprecision.
Conclusion and Implications
Experiencing physical and emotional abuse during childhood can have negative consequences which in some cases are still present decades later. The current study found that experienced abuse was related to elevated levels of hair cortisone and higher BMI. Both elevated cortisone, as a measure of chronic stress, and higher BMI may be related to further negative outcomes and risks such as hypertension, cardiovascular disease, and inflammation (106–110). These findings emphasize that interventions for weight-loss can benefit from integrating psychological components that address stress specifically—especially in individuals with trauma history. Alternatively, finding ways to increase (low to moderate intensity) physical activity might be an accessible way to address both imbalance in the HPA-axis (111) and reduce health risks associated with overweight and obesity (112). The current study found no effect of neglect on cortisol or cortisone or BMI. More and more research is showing differential effects of abuse and neglect (113). This suggests that treatment should be tailored to the specific type of maltreatment individuals have experienced, and we that should not expect that one size fits all.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Leiden University Medical Centre. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
The 3G Parenting Study was conceptualized by MI, BE, and MB-K. KP, LB, RB, and LC-B performed the data collection. KP and LA developed the research question. KP performed the statistical analyses and wrote the manuscript with input from all the authors. All authors approved the final manuscript.
Funding
The study was supported by the Netherlands Organization for Scientific Research (MB-K: VICI grant [no. 453-09-003]; LA: VIDI grant [no. 016.145.360]; MI: NWO SPINOZA prize) and grants of Leiden University to initiate and support the Research Profile Area Health, Prevention and the Human Life Cycle awarded to MI, P. Assendelft, and B. van Hemert.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to all the families that have invested their time by participating in this study and to the students whose contribution to the data collection was invaluable. We thank Rudi Westendorp for his contribution to the conception of the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00387/full#supplementary-material.
Data Sheet 1 | Child Maltreatment Instrument.
References
1. McLaughlin KA, Sheridan MA, Nelson CA. Neglect as a Violation of Species-Expectant Experience: Neurodevelopmental Consequences. Biol Psychiatry (2017) 82:462–71. doi: 10.1016/j.biopsych.2017.02.1096
2. Jaffee SR, Christian CW. The Biological Embedding of Child Abuse and Neglect Implications for Policy and Practice. Soc Res Child Dev (2014) 28:1–19. doi: 10.1002/j.2379-3988.2014.tb00078.x
3. Cecil CAM, Viding E, Fearon P, Glaser D, McCrory EJ. Disentangling the mental health impact of childhood abuse and neglect. Child Abuse Negl (2017) 63:106–19. doi: 10.1016/j.chiabu.2016.11.024
4. Vachon DD, Krueger RF, Rogosch FA, Cicchetti D. Assessment of the harmful psychiatric and behavioral effects of different forms of child maltreatment. JAMA Psychiatry (2015) 72:1135–42. doi: 10.1001/jamapsychiatry.2015.1792
5. Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PloS Med (2012) 9:e1001349. doi: 10.1371/journal.pmed.1001349
6. Chen E, Turiano NA, Mroczek DK, Miller GE. Association of reports of childhood abuse and all-cause mortality rates in women. JAMA Psychiatry (2016) 73:920. doi: 10.1001/jamapsychiatry.2016.1786
7. Basu A, McLaughlin K a., Misra S, Koenen KC. Childhood Maltreatment and Health Impact: The Examples of Cardiovascular Disease and Type 2 Diabetes Mellitus in Adults. Clin Psychol Sci Pract (2017) 24:125–39. doi: 10.1111/cpsp.12191
8. Fuller-Thomson E, Brennenstuhl S. Making a link between childhood physical abuse and cancer: Results from a regional representative survey. Cancer (2009) 115:3341–50. doi: 10.1002/cncr.24372
9. Bentley T. Widom CS. A 30-year follow-up of the effects of child abuse and neglect on obesity in adulthood. Obesity (2009) 17:1900–5. doi: 10.1038/oby.2009.160
10. Greenfield EA, Marks NF. Violence from parents in childhood and obesity in adulthood: Using food in response to stress as a mediator of risk. Soc Sci Med (2009) 68:791–8. doi: 10.1016/j.socscimed.2008.12.004
11. Mason SM, Santaularia NJ, Berge JM, Larson N, Neumark-Sztainer D. Is the childhood home food environment a confounder of the association between child maltreatment exposure and adult body mass index? Prev Med (Baltim) (2018) 110:86–92. doi: 10.1016/j.ypmed.2018.02.016
12. Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry (2014) 19:544–54. doi: 10.1038/mp.2013.54
13. Hemmingsson E, Johansson K, Reynisdottir S. Effects of childhood abuse on adult obesity: A systematic review and meta-analysis. Obes Rev (2014) 15:882–93. doi: 10.1111/obr.12216
14. Power C, Pinto Pereira SM, Li L. Childhood Maltreatment and BMI Trajectories to Mid-Adult Life: Follow-Up to Age 50y in a British Birth Cohort. PloS One (2015) 10:e0119985. doi: 10.1371/journal.pone.0119985
15. Shin SH, Miller DP. A longitudinal examination of childhood maltreatment and adolescent obesity: Results from the National Longitudinal Study of Adolescent Health (AddHealth) Study. Child Abus Negl (2012) 36:84–94. doi: 10.1016/j.chiabu.2011.08.007
16. Sokol RL, Gottfredson NC, Shanahan ME, Halpern CT. Relationship between child maltreatment and adolescent body mass index trajectories. Child Youth Serv Rev (2018) 93:196–202. doi: 10.1016/j.childyouth.2018.07.024
17. Sacks RM, Takemoto E, Andrea S, Dieckmann NF, Bauer KW, Boone-Heinonen J. Childhood Maltreatment and BMI Trajectory: The Mediating Role of Depression. Am J Prev Med (2017) 53:625–33. doi: 10.1016/j.amepre.2017.07.007
18. Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet (2011) 377:557–67. doi: 10.1016/S0140
19. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res Clin Pract (2010) 89:309–19. doi: 10.1016/j.diabres.2010.04.012
20. Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet (2014) 383:970–83. doi: 10.1016/S0140-6736(13)61836-X
21. Flegal KM, Kit BK, Orpana H. Association of All-Cause Mortality With Overweight and Obesity Using Standard BodyMass Index Categories A Systematic Review andMeta-analysis. JAMA (2013) 309:71–82. doi: 10.1001/jama.2012.113905
22. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health (2009) 9. doi: 10.1186/1471-2458-9-88
23. Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int J Obes (2011) 35:891–8. doi: 10.1038/ijo.2010.222
24. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (2008) 371:596–78. doi: 10.1016/S0140-6736(08)60269-X
25. Roberto CA, Swinburn B, Hawkes C, Huang TTK, Costa SA, Ashe M, et al. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet (2015) 385:2400–9. doi: 10.1016/S0140-6736(14)61744-X
26. Mason SM, Austin SB, Bakalar JL, Boynton-Jarrett R, Field AE, Gooding HC, et al. Child Maltreatment's Heavy Toll: The Need for Trauma-Informed Obesity Prevention. Am J Prev Med (2016) 50:646–9. doi: 10.1016/j.amepre.2015.11.004
27. Duncan AE, Sartor CE, Jonson-Reid M, Munn-Chernoff MA, Eschenbacher MA, Diemer EW, et al. Associations between body mass index, post-traumatic stress disorder, and child maltreatment in young women. Child Abus Negl (2015) 45:154–62. doi: 10.1016/j.chiabu.2015.02.007
28. Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav (2006) 50:632–9. doi: 10.1016/j.yhbeh.2006.06.010
29. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res (2002) 53:865–71. doi: 10.1016/S0022-3999(02)00429-4
30. Bernard K, Frost A, Bennett CB, Lindhiem O. Maltreatment and diurnal cortisol regulation: A meta-analysis. Psychoneuroendocrinology (2017) 78:57–67. doi: 10.1016/j.psyneuen.2017.01.005
31. Bunea IM, Szentágotai-Tătar A, Miu AC. Early-life adversity and cortisol response to social stress: a meta-analysis. Transl Psychiatry (2017) 7:1274. doi: 10.1038/s41398-017-0032-3
32. McEwen BS. Protective and damaging effects of stress mediatiors. N Engl J Med (1998) 338:171–9. doi: 10.1056/NEJM199801153380307
33. Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev (2006) 30:376–89. doi: 10.1016/j.neubiorev.2005.08.002
34. Rippe RCA, Noppe G, Windhorst DA, Tiemeier H, van Rossum EFC, Jaddoe VWV, et al. Splitting hair for cortisol? Associations of socio-economic status, ethnicity, hair color, gender and other child characteristics with hair cortisol and cortisone. Psychoneuroendocrinology (2016) 66:56–64. doi: 10.1016/j.psyneuen.2015.12.016
35. Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology (2017) 77:261–74. doi: 10.1016/j.psyneuen.2016.12.017
36. Hinkelmann K, Muhtz C, Dettenborn L, Agorastos A, Wingenfeld K, Spitzer C, et al. Association Between Childhood Trauma and Low Hair Cortisol in Depressed Patients and Healthy Control Subjects. Biol Psychiatry (2013) 74:e15–7. doi: 10.1016/j.biopsych.2013.04.021
37. Schalinski I, Teicher MH, Rockstroh B. Early neglect is a key determinant of adult hair cortisol concentration and is associated with increased vulnerability to trauma in a transdiagnostic sample. Psychoneuroendocrinology (2019) 108:35–42. doi: 10.1016/j.psyneuen.2019.06.007
38. Khoury JE, Bosquet Enlow M, Plamondon A, Lyons-Ruth K. The association between adversity and hair cortisol levels in humans: A meta-analysis. Psychoneuroendocrinology (2019) 103:104–17. doi: 10.1016/j.psyneuen.2019.01.009
39. Krozowski Z, Li KXZ, Koyama K, Smith RE, Obeyesekere VR, Stein-Oakley A, et al. The type I and type II 11β-hydroxysteroid dehydrogenase enzymes. J Steroid Biochem Mol Biol. (1999) 69:391–401. doi: 10.1016/S0960-0760(99)00074-6
40. McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol (2008) 20:131–7. doi: 10.1097/BOR.0b013e3282f51031
41. Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Care Res (2006) 55:420–6. doi: 10.1002/art.21984
42. Tataranni PA, Enette Larson D, Snitker W, Young JB, Flatt JP, Rawssin E, et al. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol Metab (1996) 271:E317–25. doi: 10.1152/ajpendo.1996.271.2.E317
43. Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism (2011) 60:1500–10. doi: 10.1016/j.metabol.2011.06.012
44. Ferreira de Sá DS, Schulz A, Streit FE, Turner JD, Oitzl MS, Blumenthal TD, et al. Cortisol, but not intranasal insulin, affects the central processing of visual food cues. Psychoneuroendocrinology (2014) 50:311–20. doi: 10.1016/j.psyneuen.2014.09.006
45. Vicennati V, Pasqui F, Cavazza C, Garelli S, Casadio E, di Dalmazi G, et al. Cortisol, energy intake, and food frequency in overweight/obese women. Nutrition (2011) 27:677–80. doi: 10.1016/j.nut.2010.07.016
46. George SA, Khan S, Briggs H, Abelson JL. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology (2010) 35:607–12. doi: 10.1016/j.psyneuen.2009.09.017
47. Spencer SJ, Tilbrook A. The glucocorticoid contribution to obesity. Stress (2011) 14:233–46. doi: 10.3109/10253890.2010.534831
48. Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology (2015) 62:301–18. doi: 10.1016/j.psyneuen.2015.08.014
49. Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, et al. Diurnal salivary cortisol is associated with body mass index and waist circumference: The multiethnic study of atherosclerosis. Obesity (2013) 21:E56–63. doi: 10.1002/oby.20047
50. Veldhorst MAB, Noppe G, Jongejan MHTM, Kok CBM, Mekic S, Koper JW, et al. Increased Scalp Hair Cortisol Concentrations in Obese Children. J Clin Endocrinol Metab (2014) 99:285–90. doi: 10.1210/jc.2013-2924
51. Stalder T, Steudte S, Alexander N, Miller R, Gao W, Dettenborn L, et al. Cortisol in hair, body mass index and stress-related measures. Biol Psychol (2012) 90:218–23. doi: 10.1016/j.biopsycho.2012.03.010
52. Wester VL, Staufenbiel SM, Veldhorst M a B, Visser J a., Manenschijn L, Koper JW, et al. Long-term cortisol levels measured in scalp hair of obese patients. Obesity (2014) 22:1956–8. doi: 10.1002/oby.20795
53. Olstad DL, Ball K, Wright C, Abbott G, Brown E, Turner AI. Hair cortisol levels, perceived stress and body mass index in women and children living in socioeconomically disadvantaged neighborhoods: The READI study. Stress (2016) 19:158–67. doi: 10.3109/10253890.2016.1160282
54. Björntorp P, Rosmond R. Obesity and cortisol. Nutrition (2000) 10:924–36. doi: 10.1016/S0899-9007(00)00422-6
55. Miller KF, Arbel R, Shapiro LS, Han SC, Margolin G. Brief report: Does the cortisol awakening response link childhood adversity to adult BMI? Heal Psychol (2018) 37:526–9. doi: 10.1037/hea0000601
56. Mclaughlin KA, Sheridan MA. Beyond Cumulative Risk: A Dimensional Approach to Childhood Adversity. Curr Dir Psychol Sci (2016) 25:239–45. doi: 10.1177/0963721416655883
57. Staufenbiel SM, Penninx BWJH, de Rijke YB, van den Akker ELT, van Rossum EFC. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology (2015) 60:182–94. doi: 10.1016/j.psyneuen.2015.06.011
58. Guilliams TG, Edwards L. The Stress Response System Chronic Stress and the HPA Axis: Clinical Assessment and Therapeutic Considerations. Stand (2010) 9:1–12.
59. Pittner K, Van IJzendoorn MH, Alink LRA, Buisman RSM, Compier-de Block LHCGC, van den Berg LJM, et al. The Genetic and Environmental Etiology of Child Maltreatment in a Parent-Based Extended Family Design. Dev Psychopathol (2019) 31:157–72. doi: 10.1017/S0954579418001608
60. Buisman RSM, Pittner K, Tollenaar MS, Lindenberg J, van den Berg LJM Compier-deBlock LHCG, et al. Intergenerational transmissionof child maltreatment using a multi-informant multi-generation family design. PLoS ONE (2020) 15(3):e0225839. doi: 10.1371/journal.pone.0225839
61. Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the parent-child Conflict Tactics Scales: development and psychometric data for a national sample of American parents. Child Abus Negl (1998) 22:249–70. doi: 10.1016/S0145-2134(97)00174-9
62. Bernstein DP, Fink L, Handeisman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry (1994) 151:1132–6. doi: 10.1176/ajp.151.8.1132
63. Thombs BD, Bernstein DP, Lobbestael J, Arntz A. A validation study of the Dutch Childhood Trauma Questionnaire-Short Form: Factor structure, reliability, and known-groups validity. Child Abus Negl (2009) 33:518–23. doi: 10.1016/j.chiabu.2009.03.001
64. Stalder T, Kirschbaum C. Analysis of cortisol in hair - State of the art and future directions. Brain Behav Immun (2012) 26:1019–29. doi: 10.1016/j.bbi.2012.02.002
65. Short SJ, Stalder T, Marceau K, Entringer S, Moog NK, Shirtcliff EA, et al. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology (2016) 71:12–8. doi: 10.1016/j.psyneuen.2016.05.007
66. Xie Q, Gao W, Li J, Qiao T, Jin J, Deng H, et al. Correlation of cortisol in 1-cm hair segment with salivary cortisol in human: hair cortisol as an endogenous biomarker. Clin Chem Lab Med (2011) 49:2013–9. doi: 10.1515/CCLM.2011.706
67. Zhang Q, Chen Z, Chen S, Yu T, Wang J, Wang W, et al. Correlations of hair level with salivary level in cortisol and cortisone. Life Sci (2018) 193:57–63. doi: 10.1016/j.lfs.2017.11.037
68. Zhang Q, Chen Z, Chen S, Xu Y, Deng H. Intraindividual stability of cortisol and cortisone and the ratio of cortisol to cortisone in saliva, urine and hair. Steroids (2017) 118:61–7. doi: 10.1016/j.steroids.2016.12.008
69. Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes (2006) 30:590–4. doi: 10.1038/sj.ijo.0803300
70. Fredriks AM, Van Buuren S, Wit JM, Verloove-Vanhorick SP. Body index measurements in 1996-7 compared with 1980. Arch Dis Child (2000) 82:107–12. doi: 10.1136/adc.82.2.107
71. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr (1990) 44:45–60.
72. Vogel M. (2019). Data and Methods Around Reference Values in Pediatrics R Package Version 0.7.4.
73. R Development Core Team. (2019). R: The R Project for Statistical Computing. , Available at: https://www.r-project.org/.
75. Rosseel Y. lavaan: An R Package for Structural Equation Modeling. J Stat Softw May (2012) 48:1–35. doi: 10.18637/jss.v048.i02
76. Hemmingsson E. A new model of the role of psychological and emotional distress in promoting obesity: Conceptual review with implications for treatment and prevention. Obes Rev (2014) 15:769–79. doi: 10.1111/obr.12197
77. Jackson SE, Kirschbaum C, Steptoe A. Perceived weight discrimination and chronic biochemical stress: A population-based study using cortisol in scalp hair. Obesity (2016) 24:2515–21. doi: 10.1002/oby.21657
78. Hu L-T, Bentler PM. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria Versus New Alternatives. Struct Equ Model (1999) 6:1–55. doi: 10.1080/10705519909540118
79. De Onis M, Lobstein T. Defining obesity risk status in the general childhood population: Which cut-offs should we use? Int J Pediatr Obes (2010) 5:458–60. doi: 10.3109/17477161003615583
80. World Health Organization. Obesity: Preventing and Managing the Global Epidemic. In: . Report on a WHO Consultation on Obesity. Geneva: World Health Organization (1998).
81. Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat Med (2006) 25:127–41. doi: 10.1002/sim.2331
82. Scharlau F, Pietzner D, Vogel M, Gaudl A, Ceglarek U, Thiery J, et al. Evaluation of hair cortisol and cortisone change during pregnancy and the association with self-reported depression, somatization, and stress symptoms. Stress (2018) 21:43–50. doi: 10.1080/10253890.2017.1392507
83. Chapman K, Holmes M, Seckl J. 11-Hydroxysteroid Dehydrogenases: Intracellular Gate-Keepers of Tissue Glucocorticoid Action. Physiol Rev (2013) 93:1139–206. doi: 10.1152/physrev.00020.2012.-Glucocorticoid
84. Rodriguez CM, Tucker MC. Predicting Maternal Physical Child Abuse Risk Beyond Distress and Social Support: Additive Role of Cognitive Processes. J Child Fam Stud (2015) 24:1780–90. doi: 10.1007/s10826-014-9981-9
85. Dallman MF, La FSE, NC P, Gomez F, Houshyar H, Akana SF. Minireview: Glucocorticoids-Food Intake, Abdominal Obesity, and Wealthy Nations in 2004. Endocrinology (2004) 145:2633–8. doi: 10.1210/en.2004-0037
86. Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and adiposity: A meta-analysis of longitudinal studies. Obesity (2011) 19:771–8. doi: 10.1038/oby.2010.241
87. Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, Summerbell CD. Effect of reducing total fat intake on body weight: systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ (2012) 345:e7666. doi: 10.1136/bmj.e7666
88. Moyer VA. Screening for and Management of Obesity in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med (2012) 157:373–8. doi: 10.7326/0003-4819-157-5-201209040-00475
89. Ekelund U, Besson H, Luan A, May AM, Sharp SJ, Brage S, et al. Physical activity and gain in abdominal adiposity and body weight: prospective cohort study in 288,498 men and women. Am J Clin Nutr (2011) 93:826–35. doi: 10.3945/ajcn.110.006593
90. Lemstra M, Bird Y, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence (2016) 10:1547–59. doi: 10.2147/PPA.S103649
91. Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. J Am Diet Assoc (2007) 107:1755–67. doi: 10.1016/j.jada.2007.07.017
92. Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation (2012) 126:126–32. doi: 10.1161/CIRCULATIONAHA.111.087213
93. Caslini M, Bartoli F, Crocamo C, Dakanalis A, Clerici M, Carrà G. Disentangling the association between child abuse and eating disorders: A systematic review and meta-analysis. Psychosom Med (2016) 78:79–90. doi: 10.1097/PSY.0000000000000233
94. Michopoulos V, Powers A, Moore C, Villarreal S, Ressler KJ, Bradley B. The mediating role of emotion dysregulation and depression on the relationship between childhood trauma exposure and emotional eating. Appetite (2015). 91:129–36 doi: 10.1016/j.appet.2015.03.036
95. Brown S, Mitchell TB, Fite PJ, Bortolato M. Impulsivity as a moderator of the associations between child maltreatment types and body mass index. Child Abus Negl (2017) 67:137–46. doi: 10.1016/j.chiabu.2017.02.029
96. Mclaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev (2014) 47:578–91. doi: 10.1016/j.neubiorev.2014.10.012
97. Sheridan MA, Mclaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends Cognit Sci (2014) 18:580–5. doi: 10.1016/j.tics.2014.09.001
98. Reilly EB, Gunnar MR. Neglect, HPA axis reactivity, and development. Int J Dev Neurosci (2019) 78:100–8. doi: 10.1016/j.ijdevneu.2019.07.010
99. King LS, Humphreys KL, Gotlib IH. The neglect–enrichment continuum: Characterizing variation in early caregiving environments. Dev Rev (2019) 51:109–22. doi: 10.1016/j.dr.2019.01.001
100. Hayne H. Infant memory development: Implications for childhood amnesia. Dev Rev (2004) 24:33–73. doi: 10.1016/j.dr.2003.09.007
101. Baldwin JR, Reuben A, Newbury JB, Danese A. Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiatry (2019) 76:584–93. doi: 10.1001/jamapsychiatry.2019.0097
102. Meeuwsen S, Horgan GW, Elia M. The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clin Nutr (2010). 29:560–6 29:560–6doi: 10.1016/j.clnu.2009.12.011
103. Ode JJ, Pivarnik JM, Reeves MJ, Knous JL. Body mass index as a predictor of percent fat in college athletes and nonathletes. Med Sci Sports Exerc (2007) 39:403–9. doi: 10.1249/01.mss.0000247008.19127.3e
104. Kruschitz R, Wallner-Liebmann SJ, Hamlin MJ, Moser M, Ludvik B. Detecting Body Fat-A Weighty Problem BMI versus Subcutaneous Fat Patterns in Athletes and Non-Athletes. PloS One (2013) 8:72002. doi: 10.1371/journal.pone.0072002
105. Danese A, Dove R, Belsky DW, Henchy J, Williams B, Ambler A, et al. Leptin deficiency in maltreated children. Transl Psychiatry (2014) 4:e446. doi: 10.1038/tp.2014.79
106. Kim C-S, Park H-S, Kawada T, Kim J-H, Lim D, Hubbard NE, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (2006) 30:1347–55. doi: 10.1038/sj.ijo.0803259
107. Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci (2012) 102:5995–9. doi: 10.1073/pnas.1118355109
108. Hall JE, Hildebrandt DA, Kuo J. Obesity Hypertension: Role of Leptin and Sympathetic Nervous System Essential Hypertension Is Closely Related to Excess Weight Gain. Am J Hypertens (2001) 14:103S–15S. doi: 10.1016/S0895-7061(01)02077-5
109. Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, et al. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress Int J Biol Stress (2010) 14:73–81. doi: 10.3109/10253890.2010.511352
110. Claire Wang Y, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lance (2011) 378:815–25. doi: 10.1016/S0140-6736(11)60814-3
111. Vitale JA, Lombardi G, Weydahl A, Banfi G. Biological rhythms, chronodisruption and chrono-enhancement: The role of physical activity as synchronizer in correcting steroids circadian rhythm in metabolic dysfunctions and cancer. Chronobiol Int J Biol Med Rhythm Res (2018). doi: 10.1080/07420528.2018.1475395
112. Gaesser GA, Angadi SS, Sawyer BJ. Exercise and Diet, Independent of Weight Loss, Improve Cardiometabolic Risk Profile in Overweight and Obese Individuals. Phys Sportsmed (2011) 39:87–97. doi: 10.3810/psm.2011.05.1898
113. Buisman RSM, Bakermans-Kranenburg MJ, Pittner K, Compier-de Block LHCG, van den Berg LJM, van IJzendoorn MH, et al. Parents' experiences of childhood abuse and neglect are differentially associated with behavioral and autonomic responses to their offspring. Dev Psychobiol (2019) 61:dev.21822. doi: 10.1002/dev.21822
Keywords: child maltreatment, abuse, neglect, hair cortisol, hair cortisone, hypothalamic–pituitary–adrenal axis, body mass index
Citation: Pittner K, Buisman RSM, van den Berg LJM, Compier-de Block LHCG, Tollenaar MS, Bakermans-Kranenburg MJ, van IJzendoorn MH, Elzinga BM and Alink LRA (2020) Not the Root of the Problem—Hair Cortisol and Cortisone Do Not Mediate the Effect of Child Maltreatment on Body Mass Index. Front. Psychiatry 11:387. doi: 10.3389/fpsyt.2020.00387
Received: 01 February 2020; Accepted: 17 April 2020;
Published: 08 May 2020.
Edited by:
Andreas Menke, University Hospital Wuerzburg, GermanyReviewed by:
Kurt Leroy Hoffman, Autonomous University of Tlaxcala, MexicoNadia Cattane, Centro San Giovanni di Dio Fatebenefratelli (IRCCS), Italy
Copyright © 2020 Pittner, Buisman, van den Berg, Compier-de Block, Tollenaar, Bakermans-Kranenburg, van IJzendoorn, Elzinga and Alink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharina Pittner, cGl0dG5lci5rYXRoYXJpbmFAZ21haWwuY29t
 Katharina Pittner
Katharina Pittner Renate S. M. Buisman1,2,4
Renate S. M. Buisman1,2,4 Marieke S. Tollenaar
Marieke S. Tollenaar Bernet M. Elzinga
Bernet M. Elzinga