- 1Mental Health Research Institute, Tomsk National Research Medical Center of the Russian Academy of Sciences, Tomsk, Russia
- 2Department of Psychiatry, Donders Institute for Brain, Cognition, and Behavior, Radboudumc, Nijmegen, Netherlands
- 3Unit of PharmacoTherapy, Epidemiology, & Economics, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, Netherlands
- 4Nijmegen Institute for Scientist-Practitioners in Addiction (NISPA), Radboud University Nijmegen, Nijmegen, Netherlands
Background: Alcohol Use Disorder (AUD) and depressive disorder often co-exist and have a shared heritability. This study aimed to investigate Brain-Derived Neurotrophic Factor (BDNF) and three Cell Adhesion Molecules (CAMs) as transdiagnostic biomarkers in AUD and depression co-morbidity.
Methods: In a cross-sectional study, patients with AUD (n=22), AUD and depression (n=19), and healthy controls (n=20) were examined. Depression and anxiety severity were assessed using the Hamilton Depression Rating Scale and the Hamilton Anxiety Rating Scale. Anhedonia, alcohol use and dependence, craving, and social adaptation were assessed through self-report questionnaires. BDNF and CAM concentrations in peripheral serum were measured after overnight fasting using a Luminex assay. After controlling for age and gender, biomarker levels were compared across groups. The association between biomarker concentrations and symptom severity scales were explored using correlation and multiple regression analyses.
Results: BDNF and Neuronal CAM were lower in patients with AUD with and without depression compared to healthy controls. No differences were observed for Vascular CAM-1 and Interstitial CAM-1. BDNF correlated negatively with anhedonia levels. BDNF, age and gender together explained 21% of variability in anhedonia levels.
Conclusion: This pilot study suggests that peripheral levels of BDNF and NCAM might be reduced in AUD with and without comorbid mood disorder. Since low BDNF levels were associated with self- reported anhedonia across these conditions, BDNF and anhedonia might reflect transdiagnostic aspects involved in AUD and depression.
Introduction
Psychiatric disorders, including addictive and depressive disorders, are widespread in the world and pose a significant public health burden. Alcohol use disorder (AUD) and depressive disorder are among the most prevalent psychiatric conditions. The 12-month prevalence of alcohol use disorder in the Russian Federation is as high as 20.9%, compared to 13.9% in the USA. The prevalence rates of depressive disorders in these countries are 5.9% and 5.5%, respectively (1, 2). Moreover, AUD and depression often co-occur, with the presence of either disorder doubling the risks of the other (3). Importantly, this common co-morbidity aggravates clinical symptoms, worsens prognosis, and limits therapeutic response of both conditions (4).
Several authors suggest shared etiological mechanisms mediating frequent co-morbidity of psychiatric disorders, including AUD and depression. Indeed, genetic studies indicate significant shared heritability of AUD and depression (5). In line with the Research Domain Criteria (RDoC) several transdiagnostic symptom domains are shared in AUD and depression (6). These RDoC domains include symptoms of positive valence (e.g. reward processing and anhedonia), negative valence (e.g. anxiety), social functioning (e.g. social communication and perception), cognition, and arousal (7). In order to further our understanding of potential transdiagnostic mechanisms contributing to co-occurrence of AUD and depression, insight in shared biological abnormalities is indispensable.
Peripheral biomarkers might shed light on shared biological abnormalities in AUD and depression. For instance, brain-derived neurotrophic factor (BDNF) has been proposed to play a major role in the pathophysiology of depression, and has also been related to AUD (8, 9). According to the neurotrophin hypothesis, stress might decrease BDNF levels, which could lead to decreased neuronal plasticity, resulting in depression (10). Several meta-analyses have confirmed that serum BDNF concentrations are decreased in untreated depressed patients and normalized by antidepressant treatment (11–14). However, contradictory results regarding the relationship between BDNF, BDNF genotype polymorphisms and depression exist (15).
Though BDNF in AUD has received less attention, a meta-analysis also found lower serum BDNF levels in active alcohol users compared to controls (16). This difference was most pronounced after detoxification (16). Yet, genetic studies did not support the role of BDNF in AUD (17). It is unclear to what extent co-morbid conditions, such as depressive disorder affect the relationship between BDNF and AUD. Therefore, a more transdiagnostic approach is required, studying the role of BDNF in single and co-morbid cases of depressive disorder and AUD.
Several other peripheral biomarkers are of interest in the context of depressive disorder and AUD co-morbidity. For instance, genome wide association studies on substance use disorders (SUD) have implicated genetic variants in cell adhesion molecule (CAM) genes in SUD (18). Moreover, addiction has been linked to decreased polysialylated (PSA-)NCAM levels in multiple animal studies (19–22). Variation within CAM genotypes has also been associated with treatment response in patients with depressive disorders (23). Furthermore, post-mortem studies revealed increased levels of CAM gene expression in prefrontal cortex in patients with depressive disorder, and increased CAM levels have been observed in the cerebrospinal fluid of patients with depressive disorder (24–26). CAMs are among the most abundant proteins in the nervous system, and play essential roles in synaptic plasticity and functioning (27). Interestingly, diverse CAMs seem to interact with BDNF. For example, BDNF was shown to restore long-term potentiation in the hippocampus of polysialylated (PSA-) NCAM deficient mice (28). Depression is associated with both decreased levels of BDNF and PSA-NCAM levels (29). In contrast, vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1) might have opposing effects to BDNF. VCAM-1 was inversely correlated with serum BDNF in healthy men, and BDNF inhibited expression of ICAM-1 in interleukin-1b-treated human endothelial cells in vitro (30, 31). In patients suffering from depression, soluble forms of ICAM-1 and VCAM-1 were increased, as opposed to reduced levels of BDNF (32).
Little is known on levels of BDNF and CAMs in patients with co-occurrence of AUD and depressive disorder. Therefore, in this pilot study we measured levels of both BDNF and CAMs in patients with AUD with and without depressive disorder, and controls. We combined these data with measures of severity of both disorders. We hypothesized that 1) BDNF and NCAM levels were decreased, and that VCAM-1 and ICAM-1 levels were increased in patients with AUD (with and without co-morbid depression); and 2) that BDNF and NCAM levels were decreased, and VCAM-1 and ICAM-1 levels were increased in patients with AUD with depression co-morbidity as compared to AUD only. Furthermore, we expected biomarkers to correlate with symptom severity levels. Finally, we explored the relationship of BDNF and CAM biomarkers with transdiagnostic symptom domains anhedonia, anxiety, and social disfunction.
Materials and Methods
Design
In a cross-sectional transdiagnostic case-control study the relationship between phenotype measures of depression and AUD were compared across two diagnostic groups (AUD with and without depressive disorder), and healthy controls. The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki 1975, revised in Fortaleza, Brazil, 2013), and approved by the Institutional Medical Review Board (Protocol of the local ethics committee at the Research Institute of Mental Health № 101 from 13 June 2017). All participants provided written informed consent.
Participants
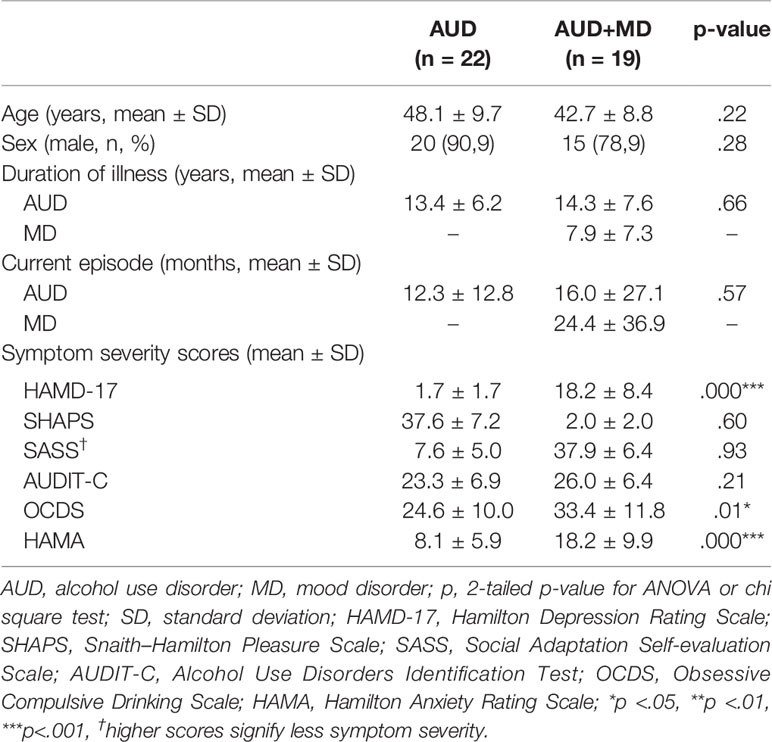
Participants with AUD with depressive disorder (n=19) and without depressive disorder (n=22), were recruited from the departments of affective and addictive disorders of Mental Health Research Institute of the Tomsk National Research Medical Center. Inclusion criteria were: a diagnosis of AUD (F10.2) with or without depressive episode/dysthymia (F31, F32, F33, F34.1), according to ICD-10; ages 18–60 years. We excluded patients with other comorbid mental disorders, for instance schizophrenia, intellectual disability, and alcoholic psychoses, and patients with acute physical diseases. The screening for relevant pathology for in/exclusion of subjects was performed through clinical assessment by three trained psychiatrists (OR, GS, and NB) on the first day of admission. Alcohol history and withdrawal severity were assessed correspondingly. Patients in the state of withdrawal received benzodiazepine therapy to alleviate withdrawal symptoms. The duration of alcohol withdrawal as estimated by the treating psychiatrist was on average 2–4 days after admission.
The control group consisted of 20 healthy volunteers recruited through local advertisements at the MHRI and Tomsk University. Healthy individuals were screened using a self-report questionnaire. The questionnaire screens for both physical and mental pathology, e.g. endocrine, neurological, gynecological and psychiatric disorders.
Measurements
Phenotype Measures
Depressive symptoms were assessed using the 17 item Hamilton Depression Rating Scale (HAMD-17), the most widely used clinician-rated measure of depression severity, with scores ranging from 0 to 52 (33). Higher scores indicate higher severity levels of depression. The HAMD-17 is part of an official Russian translation of the SIGH-SAD by K.V. Danilenko and N.K. Danilenko (34). The HAMD is a reliable tool for the assessment of depression severity, applied across the globe, though psychometric properties of the official Russian version have not been published (35). Anxiety symptoms were measured using the Hamilton Anxiety Rating Scale (HAM-A), a semi-structured interview. The HAM-A has a score range from 0 to 56 (36). Higher scores indicate higher severity levels of anxiety. The remaining transdiagnostic symptom domains were assessed using Russian translations of commonly applied self-report questionnaires. Anhedonia, a core level of depression, was assessed using the Snaith–Hamilton Pleasure Scale (SHAPS) (37). This is a 14-item questionnaire, with a total score range from 0 to 14. Alcohol use and dependency were measured using the Alcohol Use Disorders Identification Test–Consumption (AUDIT-C) (38). The total score on this 10 item questionnaire ranges from 0 to 40. Craving was assessed using the Obsessive Compulsive Drinking Scale (OCDS), which consist of 7 items related to drinking compulsions (behavior) and 7 items related to drinking obsessions (thoughts), total score 0 to 40 (39). The Social Adaptation Self-evaluation Scale (SASS) was used to measure the level of social adaptation (40). The SASS consists of 21 items, and has a total score ranging from 0 to 60. For all questionnaires except the SASS, higher scores indicate higher symptom severity. For the SASS, higher scores signify better social adjustment, i.e. less severe psychopathology. All self-report questionnaires were common Russian translation of the originals (verified by back-translation into English). The psychometric tests were performed during the first week of admission, after remission of withdrawal or intoxication symptoms.
Biomarkers
Peripheral venous blood was collected from each subject at 8.00–9.00 a.m. on the morning after hospital admission, after eight hours of overnight fasting before intake of any food or medication. Blood was sampled in BD Vacutainer tubes with coagulation activator and centrifuged at 2000 rcf at 4 ° C for 20 min. Serum samples were stored at -80oC until they could be analyzed.
Concentrations of analytes were determined on the MAGPIX multiplex analyzer (Luminex, USA) using xMAP® Technology. Panel HNDG3MAG-36K by MILLIPLEX® MAP (Merck, Darmstadt, Germany) was used to determine the levels of the markers BDNF, sICAM-1, sVCAM-1, and NCAM. Further details are described in the Supplementary Material. The detected information is processed by special Luminex xPONENT® software, with subsequent export of data to the MILLIPLEX® Analyst 5.1 program.
Data Analysis
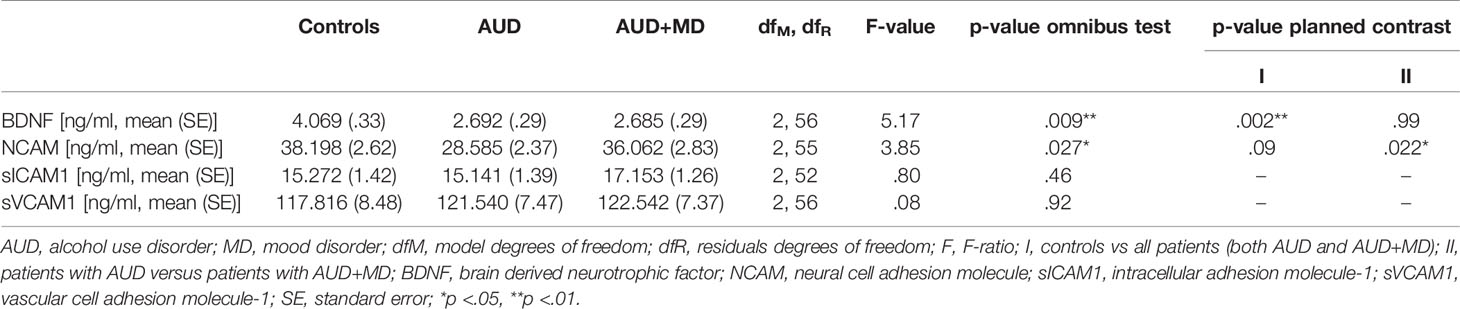
Mean serum biomarker levels were compared among the groups using analysis of covariance (ANCOVA), controlling for the confounding variables age and gender. Planned contrast analysis was used to make individual comparisons between groups. Partial correlations were conducted between serum biomarker levels and the symptom severity scales, controlling for age and gender. Biomarkers and symptom severity measures which displayed significant correlations were selected to be further examined by regression analysis. To investigate the extent to which these biomarkers and the variables age and gender contribute to symptom severity, multiple regression analyses was performed. All analyses were carried out using SPSS version 25 Windows. The significance level for all analyses was set at 2-sided P<0.05.
Results
The demographic and clinical characteristic across the different patient groups are shown in Table 1. The control group was younger (37.2 versus 45.6 years, p<.001) and more often female (75.0% versus 14.6%, p<.001) as compared to the patient groups. In the group patients with AUD and a comorbid mood disorder, four had a unipolar depressive disorder (21.1%), four a recurrent depressive disorder (21.1%), eight dysthymia (42.1%), and three a bipolar depressive disorder (15.8%). Though patients with AUD and comorbid mood disorder displayed higher levels of depression and anxiety severity as compared to the patients with only AUD (mean HAMD-17 18.2 ± 8.4 versus 7.6 ± 5.0 respectively, p<.001; mean HAMA 18.2 ± 9.9 versus 8.1 ± 5.9 respectively, p<.001), they showed similar severity levels across other symptom domains (e.g., mean SHAPS scores 1.7 ± 1.7 and 2.0 ± 2.0 respectively; p = .60).
The mean serum BDNF level was lower in patients with AUD with and without comorbidity in comparison to healthy controls (mean concentration 2.685 ± .29 and 2.692 ± .29 versus 4.069 ± .33 ng/ml respectively, p = .009). There were no differences between the two patient samples. Mean serum NCAM levels were lower in patients with AUD with and without comorbid mood disorder compared to healthy controls (mean concentration 36.062 ± 2.83 and 28.585 ± 2.37 versus 38.198 ± 2.62 ng/ml respectively, p = .027). This effect was mainly driven by lower levels of NCAM in patients with AUD only, as compared to patients with AUD and a mood disorder. The adjusted mean levels of sICAM1 and sVCAM1 did not significantly differ between the diagnostic groups. Mean biomarker levels across the different study groups, controlling for age and gender are reported in Table 2.
A significant negative correlation (controlling for age and gender) was found between BDNF and SHAPS scores, indicating the lower the BDNF level, the more severe the anhedonia (see Supplementary Table 1). There were no other significant correlations observed.
Multiple regression analysis confirmed the association between BDNF and anhedonia, indicating BDNF, age and gender together explained 21% of variability in anhedonia levels (see Table 3). The β-value coefficient indicated that a unit fall in BDNF level equals an average rise of 0.44 units in SHAPS score (p = .008). Furthermore, also female gender was significantly associated with higher anhedonia levels (β = .12, p = .026).
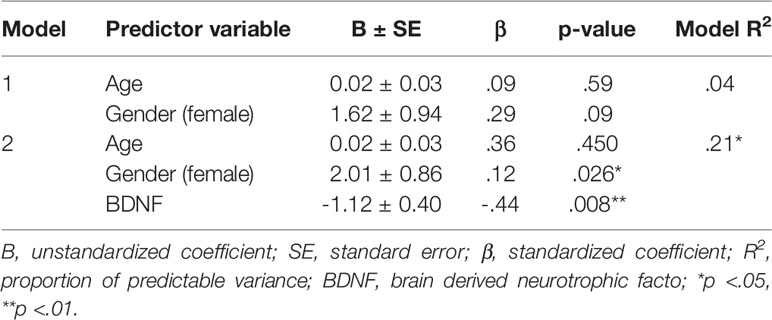
Table 3 Results of multiple regression analysis to assess the nature of the independent relationship between BDNF level and anhedonia severity (Snaith–Hamilton Pleasure Scale).
Discussion
This pilot study investigated BDNF and CAM levels transdiagnostically in patients with AUD with, and without depression, and healthy controls. The results suggest lower BDNF and NCAM levels in patients with AUD with and without comorbid depression, compared to healthy controls. Lower BDNF levels were associated with higher levels of anhedonia. There were no other relationships observed between biomarker and symptom levels.
Our findings on reduced BDNF levels in AUD and mood disorders are in line with our hypothesis and several human studies indicating that both disorders are associated with decreased levels of BDNF (11–13, 16). However, contradictory findings have been observed regarding serum BDNF and depression, and the evidence for reduced BDNF in patients with AUD remains limited (41, 42).
We observed a negative correlation of BDNF levels with anhedonia, but not with the severity of depressive symptoms in general. This might be explained by the high levels of dysthymia patients in the comorbid group. It has previously been shown that BDNF levels are indeed decreased in patients with major depressive disorder, but not in patients with dysthymia. This might have contributed to a lack of association between BDNF levels and general depression symptom severity levels (43). Furthermore, it has been suggested that dopaminergic depletion, as commonly observed in addicted patients, is associated with anhedonia (44). Depressive co-morbidity in patients with AUD may thus be characterized by more pronounced levels of anhedonia, as compared to other symptom domains of depression (e.g. anxiety or negative mood). The association between BDNF and anhedonia levels in absence of an association between BDNF and general depression severity could indicate that in our comorbid sample anhedonia might indeed have been the most affected symptom domain of depression.
Though our findings should be considered preliminary due to limited sample size and the cross-sectional nature of our pilot study impedes any causal inferences, it is tempting to speculate about potential mechanisms involved in our findings. Several animal studies show that BDNF deficiency reduces sucrose preference, a loss of sensitivity to reward which has been suggested to model anhedonia (45–48). Anhedonia is a core symptom of depression, and also plays an essential role in addictive disorders (49, 50). In our sample, peripheral BDNF levels were indeed specifically related with self-reported anhedonia levels in the combined sample. This suggests a potential transdiagnostic mechanism of low BDNF-related anhedonia in AUD depression co-morbidity.
Low levels of BDNF might be associated with the transdiagnostic symptom of anhedonia through alterations in ventral extrapyramidal circuits regulating motivation to reward-seeking (success leads to pleasure, hedonia) and distress-avoiding (success leads to happiness, euphoria) activities in both types of disorders (51). The activity of these circuits is regulated by ascending monoaminergic neurons originating within the midbrain, which are in turn controlled by an evolutionary well conserved system of the habenuloid complex (51). Glutamatergic lateral habenula-projecting globus pallidus neurons are involved in the evaluation of the results of reward-seeking activities and inhibit the motivation to continue them via ascending dopaminergic pathways to the ventral striatum (51). Previous findings using deep brain stimulation of the lateral habenula suggest that these glutamatergic neurons are sensitive to BDNF-induced neuroplastic changes (52).
The decreased serum NCAM levels in patients with AUD with and without comorbid depression, is in contrast with a clinical study which found increased PSA-NCAM1 expression in lethally intoxicated patients with opioid use disorder (53). Similar results were obtained by Cirielli and colleagues (54). However, it has been demonstrated in mice that decreased expression of PSA-NCAM is related to individual risk for alcohol-related behaviors (55). Moreover, several other animal studies suggest that both substance use disorder and depression are associated with lower NCAM levels (19–22, 29). This might suggest differential mechanisms involved in addiction to different substances. Furthermore, it has to be acknowledged that our study measured NCAM levels shortly after detoxification, while in the study of Weber et al. and Cirielli et al. patients were lethally intoxicated with illicit drugs (53, 54). It is unknown to what extent intoxication, withdrawal and abstinence affect peripheral biomarker levels, including NCAM (53, 56).
In contrast to our hypothesis, we did not find any differences in sICAM-1 and sVCAM-1 levels across the different groups. Since one clinical study did find elevated levels of both s-ICAM-1 and sVCAM-1 in 33 elderly patients with depression, this might be related to insufficient power in our study or less contribution of ischemia-induced inflammation in our patients with AUD and depression (32). Insufficient power could also explain the absence of any differences in BNDF and NCAM levels in patients with AUD and comorbid depression, as compared to AUD only.
The current pilot study should be considered in the light of several limitations. First, our pilot study included only about 20 individuals per group, and the control group was younger and had more females in comparison with the two patient groups. Despite controlling for age and gender statistically, any confounding effects of age and gender cannot be fully ruled out. The current findings thus await confirmation in substantially large and well-matched samples. Secondly, our design did not include a group of patients with mood disorders only. It would be of great interest to investigate BDNF, CAM, and anhedonia levels in a sample of patients with only mood disorders in further research. Moreover, we cannot be certain that the reliability and validity of the Russian translated questionnaires is comparable with their characteristics within USA citizens. Future studies should test the psychometric characteristics of these translated instruments in Russian populations in comparison to those of other nations. Another limitation of the current study is its cross-sectional nature, impeding causal inferences about the relationship between biomarkers and psychopathology. Future studies using multi-level biomarkers, including genomic, transcriptomic and metabolomic markers might shed further light on the observed relationships. More experimental designs, including animal studies, and application of neuro-imaging techniques, are needed to gain better insight in causal mechanisms involved. Furthermore, the relationship between peripheral biomarker levels and brain tissue levels has been suggested in an animal study, but awaits confirmation in future studies (57).
In conclusion, this pilot study suggests that peripheral levels of BDNF and NCAM might be reduced in AUD with and without comorbid mood disorder. Since low BDNF levels were associated with self-reported anhedonia across conditions, future studies might further explore anhedonia as a transdiagnostic symptom in AUD and depression. Furthermore, future studies should further investigate potential mechanisms involved in the association between BDNF, NCAM, and AUD and depression.
Data Availability Statement
The datasets generated for this study are available on every reasonable request to SI (aXZhbm92YW5paXB6QGdtYWlsLmNvbQ==), following approval of the Board of Directors of the MHRI, in line with local guidelines and regulations.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Medical Review Board (Protocol of the local ethics committee at the Research Institute of Mental Health № 101 from 13 June 2017). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AL, AS, and SI instigated and designed the study. SI coordinated and supervised the study. EMM designed and performed the statistical analysis. LL, OR, GS, and SI wrote the study protocol. LL and OR monitored the study. OR, IL and GS collected clinical data. EVM and EE collected blood samples. AB measured the biomarkers in serum. LL and OR recorded all data in an Excel database. NB supervised the clinical work. SI supervised the technical work. LL, OR, EMM, AL, AS, and SI wrote the manuscript. EMM and AL reviewed and adapted the manuscript. All authors read the paper and agree with its content.
Funding
The study was funded by a grant from the Russian Science Foundation (project No. 19-15-00023). The number of affiliations of the Russian contributors is required to be limited to a single (main) institution. This grant is applied to pay for reagents and salaries of Russian (co-)authors, as well as the publication fee.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00296/full#supplementary-material
References
1. World Health Organization. Depression and other common mental disorders. In: Global Health Estimates. WHO: Geneva (2017).
3. Boden JM, Fergusson DM. Alcohol and depression. Addiction (2011) 106(5):906–14. doi: 10.1111/j.1360-0443.2010.03351.x
4. Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med (2005) 118(4):330–41. doi: 10.1016/j.amjmed.2005.01.007
5. Foo JC, Streit F, Treutlein J, Ripke S, Witt SH, Strohmaier J, et al. Shared genetic etiology between alcohol dependence and major depressive disorder. Psychiatr Genet (2018) 28(4):66–70. doi: 10.1097/ypg.0000000000000201
6. Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry (2010) 167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379
7. Carcone D, Ruocco AC. Six Years of Research on the National Institute of Mental Health’s Research Domain Criteria (RDoC) Initiative: A Systematic Review. Front Cell Neurosci (2017) 11:46. doi: 10.3389/fncel.2017.00046
8. Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature (2008) 455(7215):894–902. doi: 10.1038/nature07455
9. Sharma S, Graham R, Rohde R, Ceballos NA. Stress-induced change in serum BDNF is related to quantitative family history of alcohol use disorder and age at first alcohol use. Pharmacol Biochem Behav (2017) 153:12–7. doi: 10.1016/j.pbb.2016.12.002
10. Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry (1997) 54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002
11. Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol (2008) 11(8):1169–80. doi: 10.1017/s1461145708009309
12. Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry (2008) 64(6):527–32. doi: 10.1016/j.biopsych.2008.05.005
13. Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A, et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry (2010) 11(6):763–73. doi: 10.3109/15622971003611319
14. Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry (2014) 19(7):791–800. doi: 10.1038/mp.2013.105
15. Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry (2007) 12(12):1079–88. doi: 10.1038/sj.mp.4002075
16. Ornell F, Hansen F, Schuch FB, Pezzini Rebelatto F, Tavares AL, Scherer JN, et al. Brain-derived neurotrophic factor in substance use disorders: A systematic review and meta-analysis. Drug Alcohol Depend (2018) 193:91–103. doi: 10.1016/j.drugalcdep.2018.08.036
17. Forero DA, Lopez-Leon S, Shin HD, Park BL, Kim DJ. Meta-analysis of six genes (BDNF, DRD1, DRD3, DRD4, GRIN2B and MAOA) involved in neuroplasticity and the risk for alcohol dependence. Drug Alcohol Depend (2015) 149:259–63. doi: 10.1016/j.drugalcdep.2015.01.017
18. Zhong X, Drgonova J, Li CY, Uhl GR. Human cell adhesion molecules: annotated functional subtypes and overrepresentation of addiction-associated genes. Ann New York Acad Sci (2015) 1349:83–95. doi: 10.1111/nyas.12776
19. Mackowiak M, Chocyk A, Dudys D, Wedzony K. Activation of CB1 cannabinoid receptors impairs memory consolidation and hippocampal polysialylated neural cell adhesion molecule expression in contextual fear conditioning. Neuroscience (2009) 158(4):1708–16. doi: 10.1016/j.neuroscience.2008.11.037
20. Heidmets LT, Kalda A, Zharkovsky A. Acute amphetamine treatment decreases the expression of 180-200 kDa isoform of polysialic acid linked neural cell adhesion molecule in mouse hippocampus. Brain Res (2007) 1165:89–97. doi: 10.1016/j.brainres.2007.06.033
21. Mackowiak M, Grzegorzewska M, Budziszewska B, Chocyk A, Hess G, Wedzony K. Cocaine decreases the expression of PSA-NCAM protein and attenuates long-term potentiation via glucocorticoid receptors in the rat dentate gyrus. Eur J Neurosci (2008) 27(11):2928–37. doi: 10.1111/j.1460-9568.2008.06255.x
22. Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, et al. Nicotine self-administration impairs hippocampal plasticity. J Neurosci (2002) 22(9):3656–62. doi: 10.1523/JNEUROSCI.22-09-03656.2002
23. Fabbri C, Crisafulli C, Gurwitz D, Stingl J, Calati R, Albani D, et al. Neuronal cell adhesion genes and antidepressant response in three independent samples. Pharmacogenomics J (2015) 15(6):538–48. doi: 10.1038/tpj.2015.15
24. Laifenfeld D, Karry R, Klein E, Ben-Shachar D. Alterations in cell adhesion molecule L1 and functionally related genes in major depression: a postmortem study. Biol Psychiatry (2005) 57(7):716–25. doi: 10.1016/j.biopsych.2004.12.016
25. Thomas AJ, Ferrier IN, Kalaria RN, Davis S, O’Brien JT. Cell adhesion molecule expression in the dorsolateral prefrontal cortex and anterior cingulate cortex in major depression in the elderly. Br J Psychiatry (2002) 181:129–34. doi: 10.1017/s0007125000161847
26. Poltorak M, Frye MA, Wright R, Hemperly JJ, George MS, Pazzaglia PJ, et al. Increased neural cell adhesion molecule in the CSF of patients with mood disorder. J Neurochem (1996) 66(4):1532–8. doi: 10.1046/j.1471-4159.1996.66041532.x
27. Sytnyk V, Leshchyns’ka I, Schachner M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci (2017) 40(5):295–308. doi: 10.1016/j.tins.2017.03.003
28. Muller D, Djebbara-Hannas Z, Jourdain P, Vutskits L, Durbec P, Rougon G, et al. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule- deficient hippocampus. Proc Natl Acad Sci United States America (2000) 97(8):4315–20. doi: 10.1073/pnas.070022697
29. Serra MP, Poddighe L, Boi M, Sanna F, Piludu MA, Sanna F, et al. Effect of Acute Stress on the Expression of BDNF, trkB, and PSA-NCAM in the Hippocampus of the Roman Rats: A Genetic Model of Vulnerability/Resistance to Stress-Induced Depression. Int J Mol Sci (2018) 19(12):e3745. doi: 10.3390/ijms19123745
30. Takeda K, Obinata Y, Konishi A, Kajiya M, Matsuda S, Mizuno N, et al. Brain-Derived Neurotrophic Factor Inhibits Intercellular Adhesion Molecule-1 Expression in Interleukin-1beta- Treated Endothelial Cells. Cell Biochem Biophys (2016) 74(3):399–406. doi: 10.1007/s12013-016-0749-2
31. Lee IT, Lee WJ, Tsai IC, Liang KW, Lin SY, Wan CJ, et al. Brain-derived neurotrophic factor not associated with metabolic syndrome but inversely correlated with vascular cell adhesion molecule-1 in men without diabetes. Clin Chim Acta (2012) 413(9-10):944–8. doi: 10.1016/j.cca.2012.02.013
32. Dimopoulos N, Piperi C, Salonicioti A, Mitsonis C, Liappas I, Lea RW, et al. Elevation of plasma concentration of adhesion molecules in late-life depression. Int J Geriatr Psychiatry (2006) 21(10):965–71. doi: 10.1002/gps.1592
33. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
34. Williams JBW, Link MJ, Rosenthal NE, Amira L, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale—Seasonal Affective Disorder Version (SIGH-SAD). New York, NY: New York State Psychiatric Institute (1992).
35. Worboys M. The Hamilton Rating Scale for Depression: The making of a “gold standard” and the unmaking of a chronic illness, 1960-1980. Chronic Illn (2013) 9(3):202–19. doi: 10.1177/1742395312467658
36. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol (1959) 32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
37. Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry (1995) 167(1):99–103. doi: 10.1192/bjp.167.1.99
38. Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care World Health Organization (WHO Publication No. 01.6a). WHO: Geneva (2001).
39. Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res (1995) 19(1):92–9. doi: 10.1111/j.1530-0277.1995.tb01475.x
40. Bosc M, Dubini A, Polin V. Development and validation of a social functioning scale, the Social Adaptation Self-evaluation Scale. Eur Neuropsychopharmacol (1997) 7(Suppl 1):S57–70; discussion S1- 3. doi: 10.1016/s0924-977x(97)00420-3
41. Papakostas GI, Shelton RC, Kinrys G, Henry ME, Bakow BR, Lipkin SH, et al. Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: a pilot and replication study. Mol Psychiatry (2013) 18(3):332–9. doi: 10.1038/mp.2011.166
42. Elfving B, Buttenschon HN, Foldager L, Poulsen PH, Andersen JH, Grynderup MB, et al. Depression, the Val66Met polymorphism, age, and gender influence the serum BDNF level. J Psychiatr Res (2012) 46(9):1118–25. doi: 10.1016/j.jpsychires.2012.05.003
43. Aydemir O, Deveci A, Taskin OE, Taneli F, Esen-Danaci A. Serum brain-derived neurotrophic factor level in dysthymia: a comparative study with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31(5):1023–6. doi: 10.1016/j.pnpbp.2007.02.013
44. Gold MS, Blum K, Febo M, Baron D, Modestino EJ, Elman I, et al. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti- reward systems. Front Biosci (Schol Ed) (2018) 10:309–25. doi: 10.2741/s518
45. Dong BE, Xue Y, Sakata K. The effect of enriched environment across ages: A study of anhedonia and BDNF gene induction. Genes Brain Behav (2018) 17(8):e12485. doi: 10.1111/gbb.12485
46. Jha S, Dong B, Sakata K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl Psychiatry (2011) 1:e40. doi: 10.1038/tp.2011.33
47. Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry (2007) 61(2):187–97. doi: 10.1016/j.biopsych.2006.03.021
48. Sakata K, Jin L, Jha S. Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav (2010) 9(7):712–21. doi: 10.1111/j.1601-183X.2010.00605.x
49. Loonen AJ, Ivanova SA. Circuits regulating pleasure and happiness in major depression. Med Hypotheses (2016) 87:14–21. doi: 10.1016/j.mehy.2015.12.013
50. Destoop M, Morrens M, Coppens V, Dom G. Addiction, Anhedonia, and Comorbid Mood Disorder. A Narrative Review. Front Psychiatry (2019) 10:311. doi: 10.3389/fpsyt.2019.00311
51. Batalla A, Homberg JR, Lipina TV, Sescousse G, Luijten M, Ivanova SA, et al. The role of the habenula in the transition from reward to misery in substance use and mood disorders. Neurosci Biobehav Rev (2017) 80:276–85. doi: 10.1016/j.neubiorev.2017.03.019
52. Hoyer C, Kranaster L, Sartorius A, Hellweg R, Gass P. Long-term course of brain-derived neurotrophic factor serum levels in a patient treated with deep brain stimulation of the lateral habenula. Neuropsychobiology (2012) 65(3):147–52. doi: 10.1159/000335243
53. Weber M, Modemann S, Schipper P, Trauer H, Franke H, Illes P, et al. Increased polysialic acid neural cell adhesion molecule expression in human hippocampus of heroin addicts. Neuroscience (2006) 138(4):1215–23. doi: 10.1016/j.neuroscience.2005.11.059
54. Cirielli V, Cima L, Chindemi C, Danzi O, Ghimenton C, Eccher A, et al. Cortical Expression of the Polysialylated Isoform of the Neural Cell Adhesion Molecule on Brain Tissue to Recognize Drug- Related Death: An Exploratory Analysis. Am J Forensic Med Pathol (2018) 39(1):8–13. doi: 10.1097/paf.0000000000000366
55. Barker JM, Torregrossa MM, Taylor JR. Low prefrontal PSA-NCAM confers risk for alcoholism- related behavior. Nat Neurosci (2012) 15(10):1356–8. doi: 10.1038/nn.3194
56. Hidese S, Hattori K, Sasayama D, Miyakawa T, Matsumura R, Yokota Y, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry (2017) 76:12–8. doi: 10.1016/j.pnpbp.2017.02.016
Keywords: biomarker, transdiagnostic, brain-derived neurotrophic factor, cell adhesion molecule, depressive disorder, addiction, alcohol use disorder, anhedonia
Citation: Levchuk LA, Meeder EMG, Roschina OV, Loonen AJM, Boiko AS, Michalitskaya EV, Epimakhova EV, Losenkov IS, Simutkin GG, Bokhan NA, Schellekens AFA and Ivanova SA (2020) Exploring Brain Derived Neurotrophic Factor and Cell Adhesion Molecules as Biomarkers for the Transdiagnostic Symptom Anhedonia in Alcohol Use Disorder and Comorbid Depression. Front. Psychiatry 11:296. doi: 10.3389/fpsyt.2020.00296
Received: 01 January 2020; Accepted: 25 March 2020;
Published: 20 April 2020.
Edited by:
Carlos Roncero, University of Salamanca, SpainReviewed by:
Margarida Corominas-Roso, Vall d’Hebron University Hospital, SpainMauro Ceccanti, Sapienza University of Rome, Italy
Copyright © 2020 Levchuk, Meeder, Roschina, Loonen, Boiko, Michalitskaya, Epimakhova, Losenkov, Simutkin, Bokhan, Schellekens and Ivanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anton J. M. Loonen, YS5qLm0ubG9vbmVuQHJ1Zy5ubA==
 Lyudmila A. Levchuk
Lyudmila A. Levchuk Elise M. G. Meeder
Elise M. G. Meeder Olga V. Roschina1
Olga V. Roschina1 Anton J. M. Loonen
Anton J. M. Loonen Anastasiia S. Boiko
Anastasiia S. Boiko Ekaterina V. Michalitskaya
Ekaterina V. Michalitskaya Innokentiy S. Losenkov
Innokentiy S. Losenkov German G. Simutkin
German G. Simutkin Nikolay A. Bokhan
Nikolay A. Bokhan Svetlana A. Ivanova
Svetlana A. Ivanova