
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry, 26 March 2020
Sec. Psychological Therapy and Psychosomatics
Volume 11 - 2020 | https://doi.org/10.3389/fpsyt.2020.00222
This article is part of the Research Topic"No Words for Feelings, yet!". Exploring Alexithymia, Disorder of Affect Regulation and “Mind-Body” connection.View all 15 articles
 Indre Bileviciute-Ljungar1*
Indre Bileviciute-Ljungar1* Danielle Friberg2
Danielle Friberg2Introduction: Unrefreshing sleep is one of the diagnostic criteria in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), which could be explained by sleep disorders, for example obstructive sleep apnea, reported in our previous study with polysomnography. Our previous findings also indicate difficulties in emotional regulation when measuring alexithymia by TAS-20 (Toronto Alexithymia Scale) and level of emotional awareness by LEAS (Level of Emotional Awareness Scale) in ME/CFS patients. However, the reasons for this are unknown. The purpose of this study was to investigate correlations between data from subjective emotional regulation and polysomnography.
Methods: Twenty-three ME/CFS patients (5 men and 18 women) of mean age 43, and 30 matched healthy controls (9 males and 21 women) of mean age 45, filled in TAS-20, LEAS, and Hospital Depression and Anxiety Scale (HADS). A polysomnography was performed on patients but not on healthy controls. Thus, values of normal population were used for sleep evaluation in ME/CFS patients.
Result: There were significant differences between patients and controls in several aspects of emotional regulation, for example LEAS-self and LEAS-total. Seventy percent of the patients had increased numbers of awakenings (shifts from any sleep stage to awake), 22% had obstructive sleep apneas, and 27% had periodic limb movements. Correlation analysis showed that number of awakenings significantly correlated with LEAS-self and LEAS-total, p < 0.01, respectively. There were no other significant correlations.
Conclusion: This pilot study demonstrated significant correlations between reduced emotional awareness and number of awakenings in polysomnography. Future studies with larger cohorts need to be conducted.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is characterized by pathological fatigue and fatigability and a number of symptoms, presented over a 6 months period. Patients also report pain, gastrointestinal symptoms, and cognitive difficulties (1). They also complain about the feeling of malaise and fever, both worsening with effort, as well as sore throat and/or lymph nodes (1). The prevalence varies from 1.2%, when the stricter Canadian criteria are applied, to 2.6%, when the broad Oxford criteria are used (2, 3). Sleep disturbances is another important criterion of ME/CFS. However, the exact definition of sleep disturbances in case definition guidelines for ME/CFS is not clear (4). Subjectively reported “non-restorative sleep” or “waking non-rested in the morning” is good enough to classify the presence of sleep disturbances. Our recent study of a total of 381 patients referred for ME/CFS showed that 20.5% (n = 78) of patients fulfilled the criteria for further investigation with full-night polysomnography (5). Thus, patients had increased excessive daytime sleepiness and/or tiredness. Among them, 31 (40.3%) patients were diagnosed with obstructive sleep apnea (OSA), 7 (8.9%) patients with periodic limb movement disorder, and 32 (41.0%) patients with restless legs syndrome. Taken together, among those reporting pathological sleep symptoms, 69.3% of patients had one or more sleep disorders. Further, a majority (84%) had an increased “number of arousals” per sleep hour (mean 8.7, SD 5.1). However, these arousals were actually a shift from any sleep stage to waking, and are in the present study called “number of awakenings”. A majority of these awakenings were probably caused by the sleep disorders. Other authors have speculated that sleep disorders in general contribute not only to fatigue (6) and cognition failure (7), the key symptoms in ME/CFS, but also to emotional regulation (8). Since emotional regulation starts with identification of emotions and situations related to these emotions (8), the emotional awareness seems to be crucial in the process of emotional regulation. Our recent study indicated that ME/CFS patients had increased alexithymia by scoring in Toronto Alexithymia Scale (TAS-20) and lower levels of emotional awareness by scoring in Level of Emotional Awareness Scale (LEAS), as compared to healthy controls (9).
Alexithymia can be described as a reduced capacity to conceptualize emotional information, related to both verbal and non-verbal stimuli (10). This leads to disturbances of affect regulation (11). For example, alexithymic individuals have difficulties identifying and describing their own or others' feelings (10) and have an externally-oriented thinking style with a scarcity of fantasy life (12, 13). Alexithymia might be considered as an original personality trait (14) or may develop as a result of a stressful situation as well as medical/psychiatric illness (15). Emotional awareness is another construct related to alexithymia but does not include externally-oriented thinking (16). Both measures show a significant but weak negative correlation (17).
Sleep disorders such as obstructive sleep apnoea (OSA) and periodic limb movement disease are well-known causes of sleep fragmentation, with awakenings and arousals, noted during polysomnography (18).
The aim of this study was to investigate correlations between the alexithymia (TAS-20) and the level of emotional awareness (LEAS), as well as depression and anxiety [Hospital Anxiety and Depression Scale (HADS)], with objective sleep parameters from polysomnography in patients with ME/CFS.
Twenty of the 23 ME/CFS patients were included from our recently published cohorts (5, 9). Three additional patients who underwent polysomnography, but whose results from questionnaires had not previously been analyzed, were also included. The inclusion criteria for the present study was diagnosis of ME/CFS given by a multidisciplinary team, completed TAS-20 and LEAS, and a full-night polysomnography. Our criteria for performing polysomnography (PSG) were excessive daytime sleepiness, evaluated by Epworth Sleepiness Scale (ESS) ≥ 10, and/or often or always tired during mornings and/or daytime. A full-night polysomnography was performed at the Department of Otorhinolaryngology (ORL), Karolinska University Hospital. Afterwards, the results of the manual polysomnography-scorings were analyzed by a specialist in sleep medicine (Danielle Friberg).
All 23 patients had entered a clinical ME/CFS project at Danderyd University Hospital, Stockholm during the period from 2011 to 2013. During 2011–2016, the unit at the Danderyd University Hospital served as a public healthcare tertiary clinic for patients with chronic fatigue. The majority of patients were referred from primary healthcare clinics, psychiatry, and neurology departments within Stockholm County. The majority of patients were already thoroughly investigated and had a long history of fatigue. Their fatigue was not explained by other primary diseases and they were refereed to ME/CFS clinic for further investigation.
The diagnosis of ME/CFS was made in accordance with the Centers for Disease Control (19) and/or Canadian criteria (1), summarized in Table 1. During 2011–2012, patients were evaluated by a team: a clinician, who was a specialist in rehabilitation medicine or neurology; a psychologist, who performed a standardized psychiatric interview and neuropsychological testing; a physiotherapist, an ergotherapist, a social worker, and a nurse. The laboratory tests were performed for all patients in order to exclude other pathological conditions, since ME/CFS is considered to be an exclusionary diagnosis. The majority of patients were also referred to brain magnetic resonance imaging to exclude brain disorders. Due to administrative changes, the complete team-based evaluation was terminated 2013. Thus, the physician and psychologist performed clinical evaluation during a 90-minutes visit.
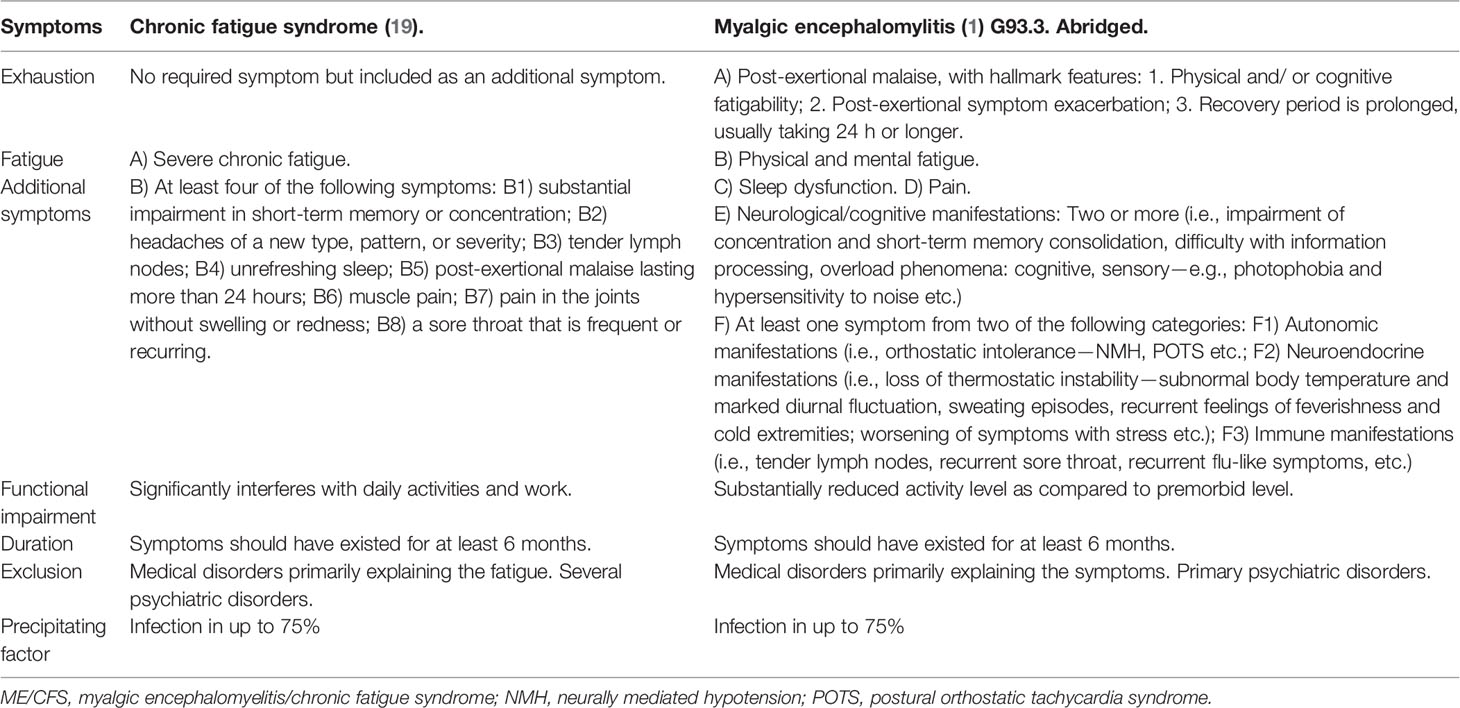
Table 1 Comparison of set of criteria for ME/CFS according to CDC/Fukuda (19) and Canada (1), both coded as G93.3 in ICD-10.
The group of healthy controls (n = 30) had previously participated in studies on emotional awareness (5) and health-related life quality (20). The healthy control group was recruited by using a convenience sample of family, friends, and co-workers. Healthy controls should not have had current or previous diagnosis of ME/CFS and other comorbidities such as psychiatric disorders, chronic pain, allergies/asthma, sleep disorders, hypothyreosis, heart disorders, or be on the sick-leave. They also scored high for health-related quality of life (20).
● The ESS—a self-administered questionnaire measures daytime sleepiness, the maximum score is 24 points (21). The patients also responded to a non-validated questionnaire, confirming the clinical symptoms for further indication to PSG.
● The HADS screens the levels of anxiety and depression, the maximum score is 21 point for each measure (22).
● The TAS-20 is a self-reported questionnaire measuring alexithymic traits. Twenty items are rated using a 5-point Likert scale. The items are divided into three subscales: 1) Difficulty Identifying Feelings (DIF); 2) Difficulty Describing Feelings (DDF); and 3) Externally Oriented Thinking (EOT). The TAS-20 has demonstrated good internal consistency with test–retest reliability in the Swedish population (23). In the present study, the Cronbach´s alpha coefficient for TAS-20 subscales was 0.8.
LEAS is an observer-rated measure of emotional awareness (24). The shortened 10-item version of the LEAS (LEAS-A) was applied. The test consists of 10 descriptions of emotion-provoking interactions between two people. Participants were asked to describe how they would feel as a protagonist in each scene and how “the other person” would feel. Answers were quantified according a manual of scoring rules. Scores from 0–5 were assigned for the categories “self,” “other,” and “total,” with lower scores reflecting a lower level of emotional awareness. Maximum “total” scores of 50 and “self” or “other” scores of 40 were possible. A score of 0 was given when the subject either gave no answer or did not describe thoughts (i.e., I feel that it is expensive.). A score of 1 was given when bodily sensations were described (i.e., I would feel fatigued.). A score of 2 was given to an action tendency or an undifferentiated affect state (i.e., I would like to run away. My friend would feel good.). A score of 3 was given when a single emotion was described (i.e., I would feel happy.). A score of 4 was given to blends of emotions (i.e., I would feel angry and a bit of sadness.). A score of 5 was given to multiple blends of emotions (i.e., I would feel disappointed. But if someone else won, I would be happy that it was my friend. My friend will be proud and happy, but also concerned about me.). The Cronbach´s alpha coefficient for LEAS subscales was 0.94.
A full-night polysomnography was performed using the Embla technology (Flaga Medical; Reykjavik, Iceland) in a sleep laboratory. PSG recordings were interpreted manually by registered technicians, later on also checked by a specialist in sleep medicine (Danielle Friberg). Due to the fact that a sleep laboratory was used in a day-care unit, the patients were woken at 6 am. Totally, 16 channels were recorded: electroencephalography (EEG) (sensors C3-A2, O1-A2, O2-A1, C4-A1), electrooculography (EOG) (left and right), electromyography (EMG) chin and tibialis (left and right), oronasal flowmetry, respiratory movements (abdomen and thorax), snoring, electrocardiography (ECG), pulse, and body position. PSG parameters of American Academy of Sleep Medicine 2007 for normal values were used for comparisons (25).
The use of clinical data for scientific analysis was considered by the regional ethical review board in Stockholm (Ref. no. 2014/300-31) and approved by Danderyd University Hospital (DS2014-0447).
Descriptive statistics were presented by percentage, medium (minimum-maximum), and by mean with standard deviation as well as 95% confidence interval, when appropriated due to the qualitative vs. quantitative parameters measured. Nonparametric tests (Mann-Whitney U) were used for group comparisons of ordinal data from questionnaires, and parametric independent two-tailed t-tests were used for age. A p-value <0.05 was considered significant for these tests. A non-parametric Spearman correlation test was used to analyze correlations between emotional questionnaires (TAS-20, LEAS, and HADS) and sleep parameters, respectively. Due to multiple analysis, a stricter criterion for statistical significance was used, a p-value less than 0.01 was considered a significant correlation, and p-value less than 0.05 was considered a weak correlation. Cronbach´s alpha was used for internal consistency of TAS-20 and LEAS.
The statistical package SPSS 25.0 was used for coding and analyzing the data.
Twenty-three patients (5 men and 18 women) with a mean age of 43 (SD 12) years, and a mean body mass index (BMI) of 24 (SD 3) were included in the study. Their mean ESS score was 7.6 points and there were 7 (30%) patients with a pathological ESS ≥ 10.
Healthy controls (9 males and 21 women) were 45 years old (SD 12) and there was no difference between the groups concerning age and gender (BMI of controls not measured). Comparisons between ME/CFS patients and healthy controls showed that ME/CFS patients had significantly higher scores in TAS-DIF and total TAS-20, as well as significantly lower values in LEAS-self and LEAS total (Table 2).
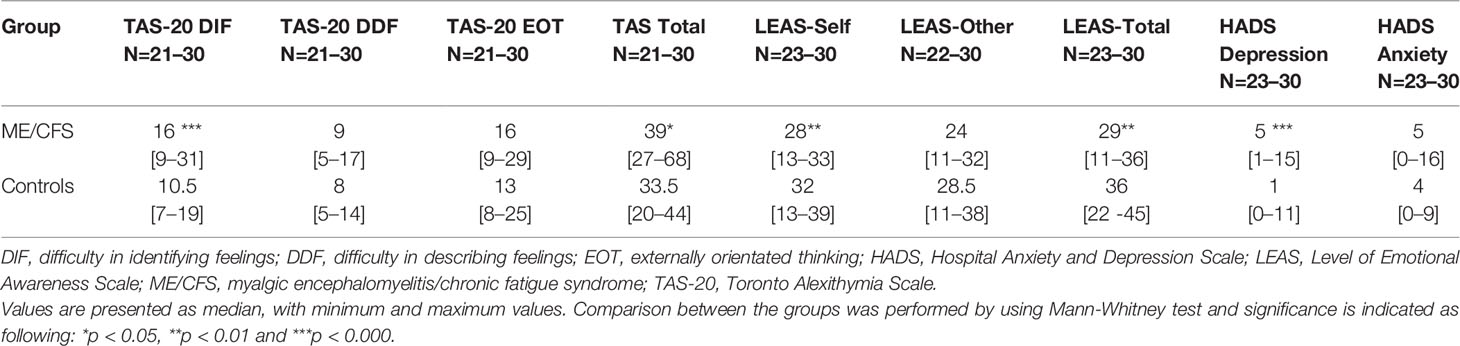
Table 2 Results from three questionnaires concerning alexithymia (TAS-20), emotional awareness (LEAS) and mood (HADS) from ME/CFS patients and healthy controls.
The clinical emotional status was also scored using the HADS (Table 2). Six (26%) patients scored ≥ 10 in HADS depression and 4 (17%) ≥ 10 in HADS anxiety. Among the controls, only 1 (3%) participant scored 11 in HADS depression and no-one scored ≥ 10 in HADS anxiety. The HADS depression index was also significantly higher in ME/CFS patients as compared to controls (Table 2).
HADS depression correlated significantly with TAS-20 (r = 0.47, p = 0.000; r = 0.37, p = 0.008 and r = 0.49, p = 0.000; DIF, DDF, and TAS-total, respectively) and LEAS (r = −0.44, p = 0.001; r = −0.36, p = 0.008, and r = −0.51, p = 0.000; LEAS-self, LEAS-other, and LEAS-total, respectively). HADS anxiety had a weak correlation with DDF (r = 0.3, p = 0.02) but with no other TAS/LEAS parameters.
Table 3 summarizes sleep measures from the polysomnography. The majority of patients had reduced total sleep (100%) and increased sleep latency (78%). For more than half of the patients, the percentage of N1 stage was higher (61%) and REM was lower (57%). The number of awakenings was higher in 16 patients (70%), though the total arousal index was impaired only in 5 (22%) of patients. Their mean apnoea-hypopnoea index (AHI) was 2.9 (SD 3.2), and 22% had an AHI > 5, which is the definition of OSA, and 27% had a periodic limb movements index (PLMI) above 5, but only one had a PLM arousal index above 5.
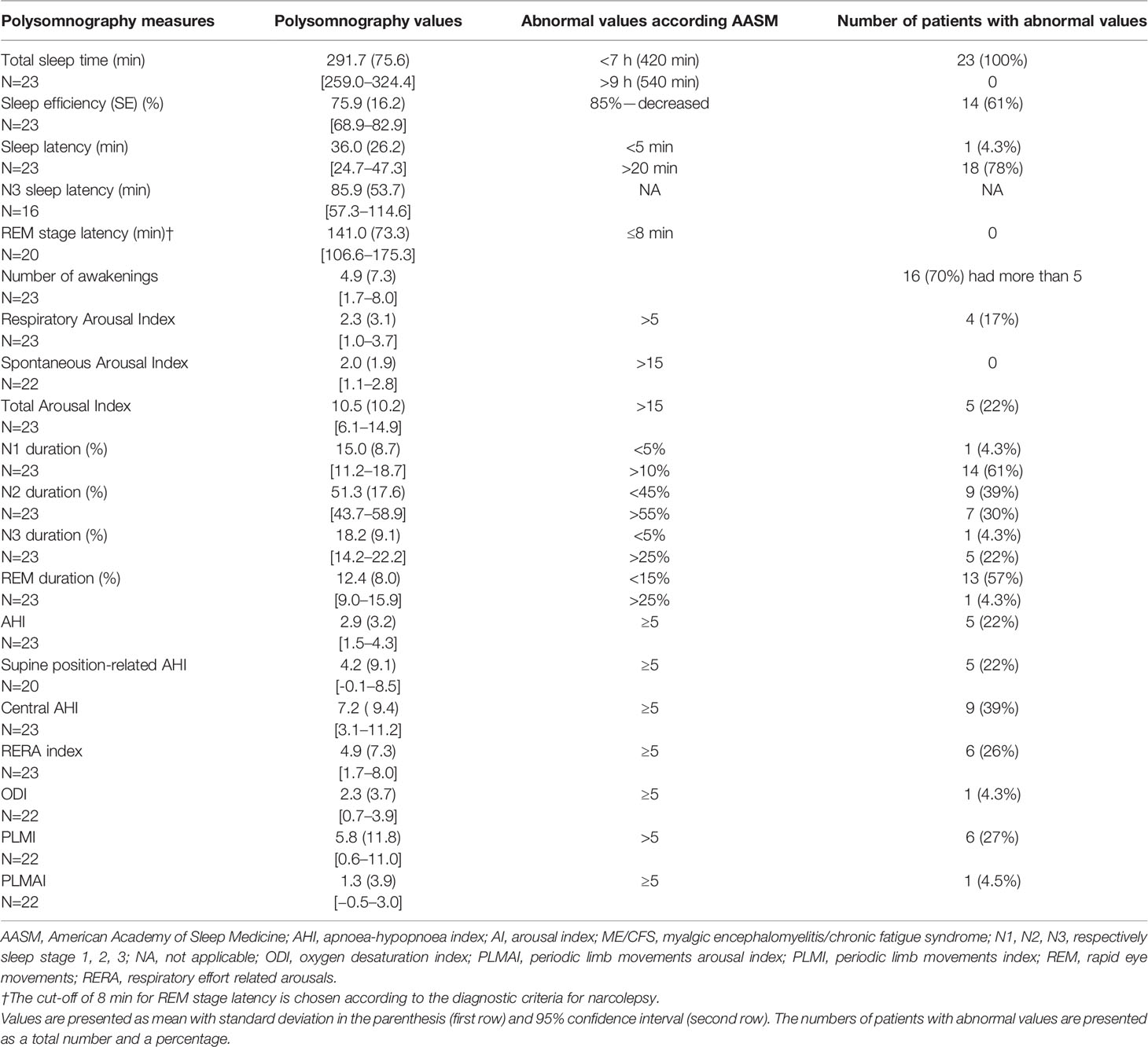
Table 3 The results from polysomnography of ME/CFS patients and compared to normal values according to the AASM 2007 (25).
Table 4 summarizes correlation analysis between polysomnography and emotional parameters TAS-20, LEAS, and HADS. A negative significant correlation was found between LEAS-self and LEAS total with number of awakenings registered by polysomnography (r = −0.6, p = 0.005–0.001). Weak correlations (p < 0.05) were found between DIF TAS-20 and total TAS-20 with percentage of deep sleep (r = −05, p = −0.02) as well as between LEAS-self and LEAS-total with percentage of N1 (r = −0.5, p = 0.02–0.03), respectively.
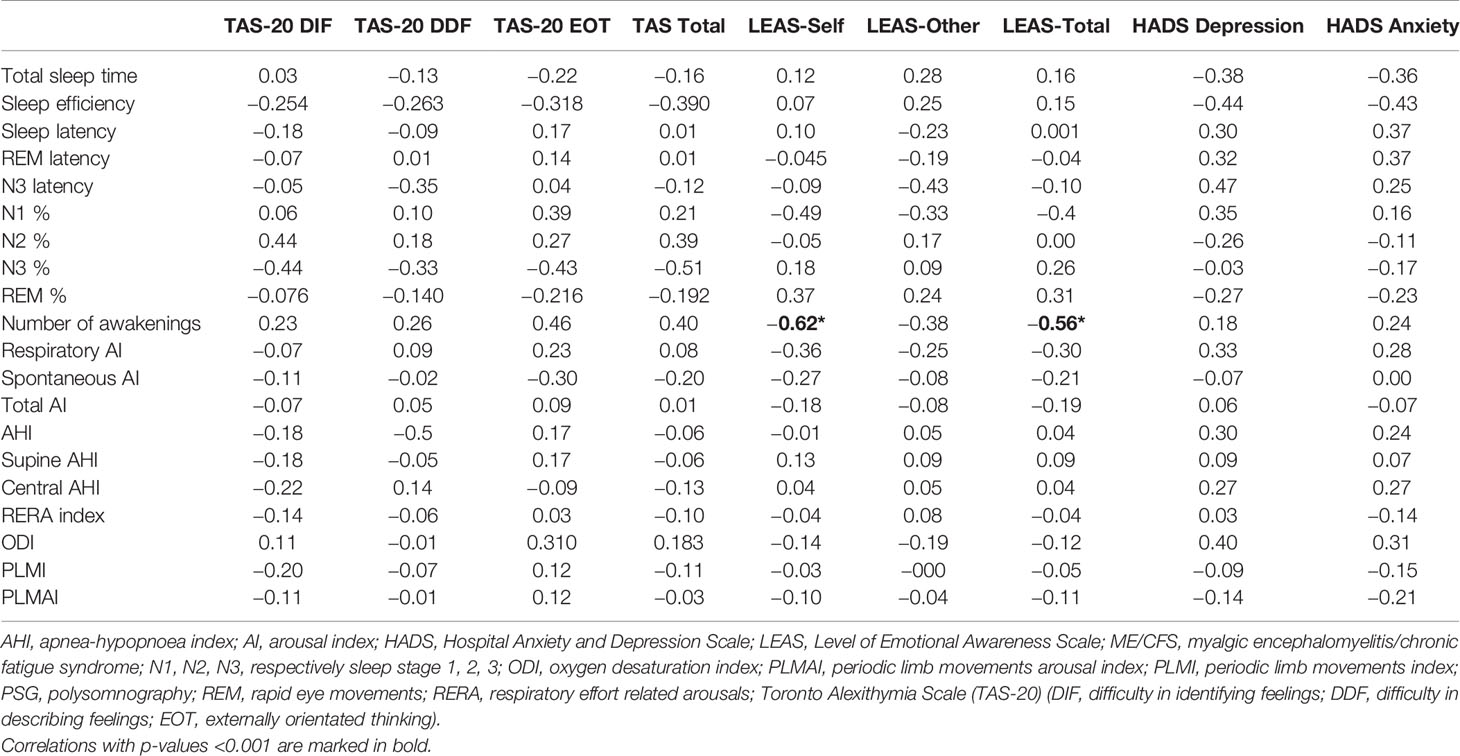
Table 4 Results from Spearman correlation analysis with r-values between sleep parameters from polysomnography and questionnaires TAS-20, LEAS and HADS, respectively, in ME/CFS patients.
There were two weak negative correlations between sleep efficiency and HADS depression (r = −0.44, p = 0.04) and anxiety (r = −0.43, p = 0.04), respectively, Table 4.
The focus of this study was the correlation analysis between parameters of sleep from polysomnograhy and emotional regulation evaluated with validated questionnaires TAS-20, LEAS, and HADS, respectively. The main results were the significant negative correlations between LEAS-self and LEAS-total and number of awakenings in polysomnography. This indicate that the awakenings and sleep fragmentation might be an important sleep disturbance, affecting emotional awareness. The awakenings could to some extent be explained by the fact that several patients had milder forms of other sleep disorders, for example OSA, periodic limb movement, and or restless legs. Interestingly, another study has shown that the severity of the OSA does not directly correlate with the severity of mood symptoms. Using the becks depression inventory (BDI) and State-Trait Anxiety Inventory Lee and colleagues investigated anxiety and depression in association with OSA severity (26). Interestingly, anxiety and depressive symptoms were more prevalent in patients with mild OSA than those with severe OSA. However, there were no other significant correlations between TAS-20 or HADS and polysomnography parameters, but this is only a pilot study with a small study sample, therefore no conclusions can be drawn. In a previous study, alexithymia measured by TAS-20 was associated with shorter pre-REM sleep stages (N1 and N3) in normal healthy participants (27). At the same time an increase in REM episodes but not in REM duration was associated with alexithymia (27).
In the present study, the different correlation results between polysomnography measures and TAS-20 vs. LEAS, indicate that alexithymia and emotional awareness could have different associations with sleep, and probably are regulated in different ways, though overlapping each other. This is also in line with our previously published meta-analytic review on correlation patterns between TAS-20 and LEAS in different psychiatric, psychosomatic, and somatic disorders (17). It is known that arousals and awakenings contribute to daytime sleepiness (18). However, the research on the role of arousals and awakenings is not so easy to perform since most of the studies are carried out on sleep deprivation in healthy subjects or animals, which is not the same as pathological arousals due to long-term disease status (28). The experiments with sleep deprivation in healthy subjects have demonstrated negative effects on mainly facial expression (29). As suggested, sleep deprivation amplifies amygdala reactivity in response to negative emotional stimuli, triggers emotional sensitivity, increases central sympathetic tonus, and disrupts peripheral autonomic nervous system feedback to visceral body information (29). Sleep is crucial in normalizing central adrenergic signaling, which usually occurs during REM sleep (30). In other words, there is a connection between long-term sleep disturbances, impaired emotional regulation and psychosomatic symptoms through several regulatory mechanisms. This connection might be in part applicable to ME/CFS patients, since clinically there is a broad panorama of both mental and somatic symptoms as well as increased tonus in the sympathetic nervous system.
In general, emotional regulation is described by identification of an emotion to be regulated; selection of emotion regulation strategy and implementation of this strategy (31). Emotional awareness and alexithymia are, therefore, crucial for identification of an emotion as well as placing it in a conscious context before applying the regulatory strategies. Research on sleep disorders and emotional awareness is scarce, but one study has indeed reported that mindfulness promotes better emotional regulation and improves sleep (32). Evaluation of healthy controls is also important since both insomnia (33) and alexithymia (34) are found in the normal population.
Limitations of this study are the absence of sleep recordings in healthy controls, and a small study sample with no previous power analysis. The correlation analysis with a strict limit for significant p values might result in false negative results, and also multiple correlations might create positive false results randomly.
There are also several strengths: namely, the use of gold standard polysomnography, validated questionnaires, well-defined and thoroughly investigated ME/CFS patients.
Our pilot study could indicate a biological mechanism such as sleep fragmentation, affecting emotional awareness. Further studies on emotional regulation should include both healthy participants and patient populations, even those with other psychosomatic and neurological conditions known to have symptomatology of sleep disorders. Furthermore, there is a need of more objective evaluation of emotional regulation, since TAS-20 and LEAS carry a risk of learning moment when applied several times. Altogether, future research is important to explore our preliminary results, including treatment strategies on both sleep disorders and emotional regulation in order to understand the connection between sleep and emotions.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by the regional ethical review board in Stockholm (Ref. no. 2014/300-31) and approved by Danderyd University Hospital (DS2014-0447). The patients/participants provided their written informed consent to participate in this study.
IB-L has put together and analyzed the results of sleep parameters and emotional regulation. She wrote the main draft of the manuscript and created the tables. DF performed the major job in analyzing the sleep protocols and reviewing the manuscript with tables.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We appreciate the contribution of Daniel Maroti in the recruitment of healthy controls and evaluation of the TAS-20 and LEAS, and Paul Murphy RPSGT, Certified Expert Somnologist-Technologist for scoring the polysomnographies.
1. Carruthers BM, Jain AK, De Meirleir KL, Peterson DI, Klimas NG, Lerner AM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment guidelines: a consensus document. J Chronic Fatigue Syndrome (2003) 11:7–115. doi: 10.1300/J092v11n01_02
2. Nacul LC, Lacerda EM, Pheby D, Campion P, Molokhia M, Fayyaz S, et al. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: a repeated cross-sectional study in primary care. BMC Med (2011) 9:91. doi: 10.1186/1741-7015-9-91
3. Reeves WC, Wagner D, Nisenbaum R, Jones JF, Gurbaxani B, Solomon L, et al. Chronic fatigue syndrome–a clinically empirical approach to its definition and study. BMC Med (2005) 3:19. doi: 10.1186/1741-7015-3-19
4. Mariman A, Vogelaers D, Tobback E, Delesie L, Hanoulle I, Pevernagie D. Sleep in the chronic fatigue syndrome. Sleep Med Rev (2013) 17(3):193–9. doi: 10.1016/j.smrv.2012.06.003
5. Pajediene E, Bileviciute-Ljungar I, Friberg D. Sleep patterns among patients with chronic fatigue: a polysomnography-based study. Clin Respiratory J (2018) 12(4):1389–97. doi: 10.1111/crj.12667
6. Geiger-Brown J, Rogers VE, Trinkoff AM, Kane RL, Bausell RB, Scharf SM. Sleep, sleepiness, fatigue, and performance of 12-hour-shift nurses. Chronobiol Int (2012) 29(2):211–9. doi: 10.3109/07420528.2011.645752
7. Tarokh L, Saletin JM, Carskadon MA. Sleep in adolescence: Physiology, cognition and mental health. Neurosci Biobehav Rev (2016) 70:182–8. doi: 10.1016/j.neubiorev.2016.08.008
8. Palmer CA, Alfano CA. Sleep and emotion regulation: An organizing, integrative review. Sleep Med Rev (2017) 31:6–16. doi: 10.1016/j.smrv.2015.12.006
9. Maroti D, Molander P, Bileviciute-Ljungar I. Differences in alexithymia and emotional awareness in exhaustion syndrome and chronic fatigue syndrome. Scand J Psychol (2017) 58(1):52–61. doi: 10.1111/sjop.12332
10. Lane RD, Sechrest L, Reidel R, Weldon V, Kaszniak A, Schwartz GE. Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosom Med (1996) 58(3):203–10. doi: 10.1097/00006842-199605000-00002
11. Waller E, Scheidt CE. Somatoform disorders as disorders of affect regulation: a development perspective. Int Rev Psychiatry (2006) 18(1):13–24. doi: 10.1080/09540260500466774
12. Taylor GJ, Bagby RM. New trends in alexithymia research. Psychother Psychosom (2004) 73(2):68–77. doi: 10.1159/000075537
13. Taylor GJ, Bagby RM, Parker JD. The alexithymia construct. A potential paradigm for psychosomatic medicine. Psychosomatics (1991) 32(2):153–64. doi: 10.1016/S0033-3182(91)72086-0
14. Taylor GJ. Alexithymia: concept, measurement, and implications for treatment. Am J Psychiatry (1984) 141(6):725–32. doi: 10.1176/ajp.141.6.725
15. de Vente W, Kamphuis JH, Emmelkamp PM. Alexithymia, risk factor or consequence of work-related stress? Psychother Psychosom (2006) 75(5):304–11. doi: 10.1159/000093953
16. Lane RD, Schwartz GE. Levels of emotional awareness: a cognitive-developmental theory and its application to psychopathology. Am J Psychiatry (1987) 144(2):133–43. doi: 10.1176/ajp.144.2.133
17. Maroti D, Lilliengren P, Bileviciute-Ljungar I. The Relationship Between Alexithymia and Emotional Awareness: A Meta-Analytic Review of the Correlation Between TAS-20 and LEAS. Front Psychol (2018) 9:453. doi: 10.3389/fpsyg.2018.00453
19. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med (1994) 121(12):953–9. doi: 10.7326/0003-4819-121-12-199412150-00009
20. Maroti D, Bileviciute-Ljungar I. Similarities and differencies between health-related quality of life in patients with exhaustion syndrome and chronic fatigue syndrome. Fatigue: Biomedicine Health Behav (2018) 6(4):208–19. doi: 10.1080/21641846.2018.1515583
21. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep (1991) 14(6):540–5. doi: 10.1093/sleep/14.6.540
22. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand (1983) 67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
23. Simonsson-Sarnecki M, Lundh LG, Torestad B, Bagby RM, Taylor GJ, Parker JD. A Swedish translation of the 20-item Toronto Alexithymia Scale: cross-validation of the factor structure. Scand J Psychol (2000) 41(1):25–30. doi: 10.1111/1467-9450.00167
24. Lane RD, Quinlan DM, Schwartz GE, Walker PA, Zeitlin SB. The Levels of Emotional Awareness Scale: a cognitive-developmental measure of emotion. J Pers Assess (1990) 55(1-2):124–34. doi: 10.1080/00223891.1990.9674052
25. Iber C A-IS, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester NY, USA: American Academy of Sleep Medicine (2007).
26. Lee SA, Yoon H, Kim HW. Is severe obstructive sleep apnoea associated with less depressive symptoms? J Psychosom Res (2019) 122:6–12. doi: 10.1016/j.jpsychores.2019.04.017
27. Bazydlo R, Lumley MA, Roehrs T. Alexithymia and polysomnographic measures of sleep in healthy adults. Psychosom Med (2001) 63(1):56–61. doi: 10.1097/00006842-200101000-00007
28. Saper CB, Fuller PM. Wake-sleep circuitry: an overview. Curr Opin Neurobiol (2017) 44:186–92. doi: 10.1016/j.conb.2017.03.021
29. Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, et al. The sleep-deprived human brain. Nat Rev Neurosci (2017) 18(7):404–18. doi: 10.1038/nrn.2017.55
30. Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol (2014) 10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716
31. Gross JJ. Emotion Regulation: Current Status and Future Prospects. Psychol Inq (2015) 26(1):1–26. doi: 10.1080/1047840X.2014.940781
32. Howell AJ, Digdon ,NL, Buro K, Sheptycki AR. Relations among mindfulness, well-being, and sleep. Pers Individ Differences. (2008) 45(8):773–7. doi: 10.1016/j.paid.2008.08.005
33. Kay-Stacey M, Attarian H. Advances in the management of chronic insomnia. BMJ (2016) 354:i2123. doi: 10.1136/bmj.i2123
Keywords: myalgic encephalomyelitis/chronic fatigue syndrome, sleep, awakenings, alexithymia, emotional awareness
Citation: Bileviciute-Ljungar I and Friberg D (2020) Emotional Awareness Correlated With Number of Awakenings From Polysomnography in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—A Pilot Study. Front. Psychiatry 11:222. doi: 10.3389/fpsyt.2020.00222
Received: 20 November 2019; Accepted: 06 March 2020;
Published: 26 March 2020.
Edited by:
Domenico De Berardis, Azienda Usl Teramo, ItalyCopyright © 2020 Bileviciute-Ljungar and Friberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Indre Bileviciute-Ljungar, aW5kcmUubGp1bmdhckBraS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.