
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 31 March 2020
Sec. Schizophrenia
Volume 11 - 2020 | https://doi.org/10.3389/fpsyt.2020.00121
This article is part of the Research TopicThe Role of Inflammation in the Etiology and Treatment of SchizophreniaView all 12 articles
Schizophrenia is a severe brain disorder that is associated with neurodevelopmental insults, such as prenatal inflammation, that introduce redox-immune-inflammatory alterations and risk for psychotic symptoms later in life. Nutraceuticals may offer useful adjunctive benefits. The aim of this study was to examine the therapeutic effects of Garcinia mangostana Linn (GML) and one of its active constituents, α-mangostin (AM), alone and as adjunctive treatment with haloperidol (HAL) on schizophrenia related bio-behavioral alterations in a maternal immune-activation (MIA) model. Sprague–Dawley dams were exposed to lipopolysaccharide (LPS) (n = 18) or vehicle (n = 3) on gestational days 15 and 16. Male offspring (n = 72) were treated from PND 52–66 with either vehicle, HAL (2 mg/kg), GML (50 mg/kg), HAL + GML, AM (20 mg/kg), or HAL + AM. Control dams and control offspring were treated with vehicle. In order to cover the mood–psychosis continuum, prepulse inhibition (PPI) of startle, open field test (locomotor activity), and the forced swim test (depressive-like behavior) were assessed on PND's 64–65, followed by assay of frontal–cortical lipid peroxidation and plasma pro-inflammatory cytokines, viz. interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α). MIA-induced deficits in sensorimotor gating were reversed by HAL and HAL + GML, but not GML and AM alone. MIA-induced depressive-like behavior was reversed by AM and GML alone and both in combination with HAL, with the combinations more effective than HAL. MIA-induced cortical lipid peroxidation was reversed by HAL and AM, with elevated IL-6 levels restored by GML, AM, HAL, and HAL + GML. Elevated TNF-α was only reversed by GML and HAL + GML. Concluding, prenatal LPS-induced psychotic- and depressive-like bio-behavioral alterations in offspring are variably responsive to HAL, GML, and AM, with depressive (but not psychosis-like) manifestations responding to GML, AM, and combinations with HAL. AM may be a more effective antioxidant than GML in vivo, although this does not imply an improved therapeutic response, for which trials are required.
Schizophrenia is a severe psychiatric disorder with a chronic course, affecting ~1% of the global population (1). This debilitating disease manifests in early adulthood (2), presenting with positive (hallucinations and delusions), negative (social withdrawal, apathy, and anhedonia), and cognitive symptoms (working memory deficits, attention disorders, and altered information processing) (3). However, the underlying etiological mechanisms remain elusive (4). Similarly, the treatment outcome for schizophrenia remains suboptimal (5), especially with regard to negative and cognitive symptoms (6, 7).
The neurodevelopmental hypothesis has provided a valuable framework for establishing the relationship between pathologic processes during early brain development and the development of schizophrenia later in life (8, 9). This hypothesis suggests an interaction between genetic predisposition and early life environmental vulnerability factors such as malnutrition, substance abuse, obstetric complications, season of birth and infection, and exacerbation by later stresses such as substance abuse, social defeat, and trauma (10–13).
Viral or bacterial maternal infection during pregnancy has been linked to increased risk for developing schizophrenia in the offspring (14, 15), with immune activation rather than the infectious agent, itself, being deemed causal (16, 17). Indeed, trauma is associated with immune activation and increased risk of psychosis (18). Immune adjuvants such as lipopolysaccharide (LPS) (19–21), polyinosinic:polycytidylic acid (poly I:C) (22–24), human influenza virus (25), and cytokines (26, 27) induce diverse biological and behavioral abnormalities in rodents following prenatal maternal exposure. LPS, an endotoxin derived from the cell wall of Gram-negative bacteria, mimics an infection by activating the synthesis and release of pro-inflammatory cytokines, including interleukin-1β (IL-1b), IL-6, and tumor necrosis factor-α (TNF-α) (28–30) and engenders various schizophrenia-like behavioral, neurochemical, and inflammatory changes (31–33).
Oxidative stress underscores various psychiatric conditions (34, 35), in particular, schizophrenia (36). Increased reactive oxygen species (ROS) and reduced antioxidants observed in schizophrenia may contribute to the neuroprogression of the disorder (37) and to the development of cognitive dysfunction (38, 39). Indeed, the antioxidant, N-acetyl cysteine (NAC), has therapeutic benefits in various clinical domains of schizophrenia, but especially negative symptoms (40, 41) and cognition (42), while having also demonstrated efficacy in preclinical animal models (43–45).
There is an increased drive to integrate nutraceuticals and psychotropic herbal medicines into conventional medical practice (46). Co-prescription of certain herbal medicines with traditional pharmaceuticals may display complementary pharmacodynamic actions and so provide a beneficial synergistic effect (46, 47). However, little study has occurred that has directly explored such augmentation effects (46). With raw herbal extracts containing a vast array of potentially bioactive ingredients, the question remains whether the observed pharmacological effect is ingredient specific or a sum effect of the total extract.
The anti-inflammatory and antioxidant activities of herbal bioactive compounds have been widely observed, particularly in a group of polyphenols referred to as xanthones (48). Garcinia mangostana Linn (GML) is a fruit native to Southeast Asia known to contain constituents including xanthones, flavonoids, triterpenoids, and benzophenones (49). Extracts of the fruit have exhibited antioxidant (50, 51), anti-inflammatory (52, 53), antibacterial (54), and antidepressant effects (55). In particular, α-mangostin (AM), a primary component of GML, presents with substantial pharmacological properties (56, 57), including antioxidant activity (58), as well as having moderate inhibitory effects on 5HT2A receptors and cyclic adenosine monophosphate (cAMP) phosphodiesterase (PDE) (49), actions that hint at possible clinical utility as a pharmacological intervention in psychiatric disorders.
The aim of this study was to establish whether maternal immune activation (MIA) induced schizophrenia-like behavior and redox-inflammatory alterations in offspring can be reversed with the typical antipsychotic, haloperidol (HAL), GML, and AM separately. Second, since the most common use for nutraceuticals in clinical psychiatry is as an adjunctive treatment (46), we investigated whether adjunctive treatment with GML or AM is able to augment the response to HAL. The inclusion of AM is 2-fold; to investigate whether any observed pharmacological effects of GML may be specific for one of the known bio-active constituents of the extract, i.e., AM, or whether these actions underscore a sum effect of the total extract and, second, to link any effects to a known psychotropic property of AM and/or GML. In order to cover the mood-psychosis continuum, behavioral analyses focused on positive (sensorimotor gating; locomotor hyperactivity) and negative (depression) related symptoms. Moreover, by measuring associated changes in plasma and brain redox-inflammatory markers, it explores possible activity within a key neuropathological feature of the illness, viz. immune-inflammatory dysfunction (35). This study has importance to the field in that a plant extract and one of its known bioactive constituents are compared to a reference control pharmaceutical agent across a range of behavioral and biological parameters of relevance to schizophrenia.
In order to determine the authenticity and constituents of GML, separation of prenylated xanthones found in GML was achieved utilizing reversed-phase high-performance liquid chromatography (HPLC) with diode-array detection (DAD) [see Oberholzer et al., (55)].
Pregnant female Sprague–Dawley (SD) dams were used during the prenatal phase of the study. Male pups were weaned (PND 21) and used for the remainder of the study. Since this and our earlier paper (55) represent the first bio-behavioral studies evaluating the possible psychotropic benefits of GML in translational rodent models of neuropsychiatric illness, and that the hormone cycle of female rats is well known to influence the outcome of behavioral and pharmacological studies, e.g., Regenass et al. (59) and Harvey et al., (60), only male rats were used in the study.
In order to remove experimental bias, animals were randomly allocated by an experienced animal technologist blind to the study (61) to 12 rats per group (62). The number of rats per group was as directed by a statistical power analysis. Animals were bred, supplied, and housed at the Vivarium (South African Veterinary Council reg. no. FR15/13458; South African National Accreditation System good laboratory practice compliance no. G0019) of the Pre-Clinical Drug Development Platform of the North-West University (NWU) in identical cages containing corncob, under conditions of constant temperature (22 ± 1°C) and humidity (50 ± 10%) with a 12:12-h light/dark cycle (lights on 06:00 to 18:00). Food and water were provided ad libitum in the home cage, with corncob changed at least once a week. All experiments were approved by the AnimCare animal research ethics committee (National Health Research Ethics Council reg. no. AREC-130913-015) of the NWU. Animals were maintained, and all procedures performed in accordance with the code of ethics in research, training, and testing of drugs in South Africa and complied with national legislation (Ethical approval numbers: NWU-00376-16-A5 and NWU-00147-14-A5). The study design and procedures were according to the Animal Research: Reporting in vivo Experiments (ARRIVE) Guidelines (61).
The exposure and treatment layout of the MIA model is presented in Figure 1. Treated dams (n = 18) received LPS from gestational days 15–16 with control dams (n = 3) receiving saline from gestational days 15–16. These GDs were chosen on the grounds of a previous study showing decreased fetal demise at this stage, as well as the correlation of this period with second trimester human pregnancy, suspected to be a critical period for the development of schizophrenia (63). Male offspring (±4 per dam) was used in the remainder of the study. A previous study did not demonstrate protective effects of cross-fostering in a MIA model (64). Off-spring was therefore not cross-fostered with healthy dams.
A total number of 72 male offspring from LPS-exposed dams (n = 72) were randomly divided into six treatment groups, each comprising 12 rats/group (65). These groups received oral dosing of the following: vehicle (saline, 1 ml/kg), HAL (2 mg/kg po) (66–69); GML (50 mg/kg po) (55), HAL + GML (HAL + GML) (at the previously mentioned doses), AM (20 mg/kg po) (70) and haloperidol + α-mangostin (HAL + AM) (at the previously mentioned doses) (Figure 1). Male offspring from the control dams (n = 8) received oral dosing of vehicle. The respective drug treatments continued for 16 days from PND 51–66 (55). During the last 2 days of treatment, all groups were subject to behavioral testing as follows: (1) prepulse inhibition (PPI) of startle on day 13 of treatment (PND 63), (2) the open field test (OFT) on day 14 of treatment (PND 64), and the forced swim test (FST) on day 14 of treatment (PND 64). The animals were euthanized 36 h later by decapitation with trunk blood and brain tissue collected and stored at −80°C for later neurochemical analysis.
LPS (100 μg/kg) from Escherichia coli (E. coli) (Sigma-Aldrich, Johannesburg, South Africa) was dissolved in saline and administered subcutaneously (SC) to pregnant dams on GD 15–16 (30, 63). HAL (2 mg/kg/day; Sigma-Aldrich, Johannesburg, South Africa) was dissolved in a minimum volume of glacial acetic acid, then further diluted with distilled water and the pH adjusted using 10 N NaOH to 6–6.25 and administered by oral gavage (66). The dose of HAL was selected for oral dosing specifically and in line with an earlier study (71). The ground dried pericarp of GML fruit (Industrial Analytical, Kyalami, South Africa) was mixed in a 0.1% xanthan gum solution to aid suspension and administered by oral gavage, at a dose of 50 mg/kg/day (55). AM (Sigma-Aldrich, Castle Hill, Australia) was dissolved in polyethylene glycol (PEG) 400 vehicle (PEG 400:water ratio = 6:4, v/v) (72) and administered orally (20 mg/kg/day) (70).
In order to assess whether the applied drug treatments are equally effective with respect to mood vs. psychosis-related manifestations of schizophrenia, PPI of startle (psychosis like), locomotor activity, and despair in the FST (depressive like) behaviors were assessed on PND's 64–65. Indeed, an earlier study found GML to be an effective antidepressant vs. imipramine using a genetic rodent model of depression (55). The current study design would not only re-affirm the earlier noted observation but do so in another translational model, while it would also possibly extend GML's scope of application to psychotic disorders like schizophrenia. This approach has validity since MIA not only evokes psychosis-like behavior in rat offspring (31–33) but also depressive-like manifestations (21), thereby presenting its suitability for studying broad pharmacological responses of relevance to schizophrenia.
PPI is used to determine deficits in sensorimotor gating, well described in schizophrenia (73) and representative of cognitive fragmentation (74). PPI was assessed in illuminated and ventilated sound-attenuated startle chambers (SR-LAB, San Diego Instruments, San Diego, USA), as described previously (75). Startle amplitudes were defined as the average of 100 × 1-ms stabilimeter readings collected at stimulus onset. The stabilimeter was calibrated before each session.
Briefly, the startle session began with a 5-min acclimatization period, during which a 68-dB background noise level was maintained throughout the session; the basal startle response was then measured with 10 trials of a single 40-ms 120-dB white noise as a startle stimulus; after this, 80 trials of randomly delivered pulses, including 20 trials of 120 dB PULSE-ALONE trials, 50 PREPULSE trials (with intensities of 72, 76, 80, or 84 dB) and 10 trials with no pulse was delivered. A final 10 trials of single 40-ms 120-dB PULSE-ALONE startle stimuli was then supplied. After the testing session, the percentage PPI (%PPI) for the four pre-pulse intensities was calculated as %PPI = [100–(startle response for PREPULSE + PULSE trial)/(startle response for PULSE ALONE trial) × 100].
The OFT was used to exclude any confounding locomotor effects of treatment in the FST (76). Moreover, motor activity in the OFT may be indicative of underlying neurotransmitter alterations, especially subcortical dopaminergic hyperactivity that has relevance to schizophrenia (77). Rats were tested individually in an open field arena (1 × 1 m), with total distance moved (cm) scored for 5 min using EthoVision XT® software (Noldus Information Technology, Wageningen, Netherlands).
The FST was used to screen for antidepressant-like properties following prenatal LPS exposure and drug treatment (78, 79). Negative symptoms of schizophrenia are closely related to depressive behavior (80), while schizophrenia is often co-morbid with major depression (81). The FST was performed as described previously (82), except the final swim was over a period of 7 min with the first and last minute discarded during analysis (55). Immobility time was scored as floating behavior with the rat maintaining only the necessary movements to keep its head above the water vs. escape-directed swimming (horizontal movements throughout the cylinder) and struggling or climbing (upward-directed movements in cylinder) behavior (76). The latter are noted for representing serotonergic and noradrenergic-mediated escape-directed behaviors, respectively (82). These behavioral components were recorded and scored on video by investigators blind to treatment, expressed in units of time (s). Behavior was scored using manual continuous timer software (FST Scoreboard 2.0 software; Academic Support Services: Information Technology in Education, NWU), previously validated against the traditional 5-s time-sampling technique (83).
Thirty-six hours after the final behavioral analysis, rats were euthanized by decapitation, after which trunk blood was collected into pre-chilled, 4-ml vacutainer tubes (SGVac) containing dipotassium ethylenediaminetetraacetic acid (K2EDTA) solution as anticoagulant. Frontal cortex and striatum were dissected out on an ice-cooled glass slab as described previously (62, 84). Liquid nitrogen was used to fix the above brain regions and stored at −80°C until the day of analysis. The tissue was pre-split into aliquots for use in the different assays to avoid freeze–thaw–freeze changes and possible deactivation of components. On the day of assay, the tissue was weighed and allowed to thaw on ice. A 10% tissue homogenate was then prepared in a phosphate-buffered saline (PBS) using a Teflon homogenizer (84).
Thiobarbituric acid reactive substance (TBARS) is a by-product of lipid peroxidation. The Parameter™ TBARS assay from R&D Systems (Minneapolis, USA; catalog number KGE013) was used to analyze lipid peroxidation in brain tissue (45), according to the manufacturer's instructions. Absorbance was read at 532 nm using a Bio-Tek FL600 Microplate Fluorescence Reader (Bio-Tek, Instruments, Inc., 381 Highland Park, Winooski, VT, USA).
Plasma TNF-α was measured using the Rat TNF-α ELISA MAX™ Deluxe Set (catalog number 438204) from Bio Legend (San Diego, USA). IL-6 was measured using the Rat IL-6 ELISA Kit (catalog number E-EL-R0015) from Elabscience® (Wuhan, China). Both were performed in accordance with the manufacturer's instructions. Absorbance was read at 450 nm using the above noted instrument.
One-way factorial analysis of variance (ANOVA) and Bonferroni post hoc tests were used for the statistical analyses of FST scores, brain lipid peroxidation levels, and plasma cytokine analyses. For analysis of %PPI data, two-way ANOVA with repeated measures was used with Bonferroni post hoc tests. However, in order to compare the MIA model with the control group, an unpaired Student's t-test was used to analyze each parameter. To ensure there is complete equality of the variances of the differences between all variations of related groups, assumption of sphericity was conducted with Mauchly's test. If the assumption of sphericity was not met, the Greenhouse–Geisser correction was used. Normal distribution of the variables was assessed with a Q–Q plot and histogram for all variables in each treatment group. All data were normally distributed and expressed as the mean ± standard error of the mean (SEM), with a value of p < 0.05 considered statistically significant. Where additional detail was deemed useful, for example, when statistical significance was narrowly missed, a Cohen's d calculation was performed to establish effect size and practical significance: medium effect (0.5 ≥ d < 0.8), large effect (0.8 ≥ d < 1.3), and very large effect (d ≥ 1.3) sizes. Only large-to-very large effect sizes are presented in the figures and text. All data were analyzed and graphics prepared using GraphPad Prism 7, San Diego California, USA.
MIA model validation, i.e., LPS vs. saline control, was analyzed separately using T-tests and presented in Figures 2–6. Thereafter, untreated LPS (MIA model) were compared to LPS plus the various drug treatments and analyzed separately using the appropriate ANOVA followed by post hoc Bonferroni analysis. The latter are also presented in Figures 2–6.
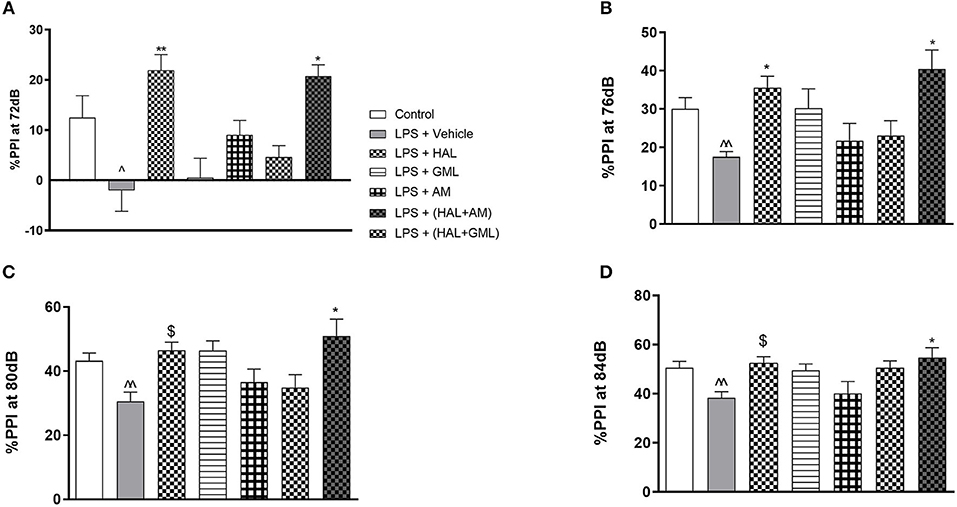
Figure 2. Sensorimotor gating with regard to percent prepulse inhibition (%PPI) of startle at (A) 72 dB, (B) 76 dB, (C) 80 dB, and (D) 84 dB in rats exposed to saline and treated with vehicle (Control) as well as rats exposed to LPS receiving vehicle and the various drug treatments as indicated [unpaired Student's t-test for Control vs. LPS + vehicle; ∧p < 0.05, ∧∧p < 0.01; two-way ANOVA with repeated measures for the different dB intensities, Bonferroni post hoc test; *p < 0.05, **p < 0.01 vs. LPS + Vehicle. $d ≥ 1.3 vs. LPS + Vehicle (Cohen's d value)].
A chromatogram of GML used in this study, and analyzed using reversed-phase HPLC with DAD, was found to contain predominantly α-mangostin (11.7%) and γ-mangostin (1.1%) (55).
When considering the LPS model alone compared to the vehicle control group, unpaired Student's t-tests revealed no significant differences between the groups at the respective startle blocks (data not shown).
Regarding %PPI and comparing the LPS-exposed group to the vehicle group using unpaired Student's t-tests, the LPS-exposed control group (LPS + vehicle) presented with significant deficits in %PPI at 72 dB (p = 0.0248), 76 dB (p = 0.003), 80 dB (p = 0.007), and 84 dB (p = 0.007) when compared to the control group (saline + vehicle) (Figures 2A–D).
Unpaired Student's t-test revealed a significant increase in locomotor activity in the LPS exposed group (LPS + vehicle) compared to the saline control group (saline + vehicle) (p = 0.039) (Figure 3).
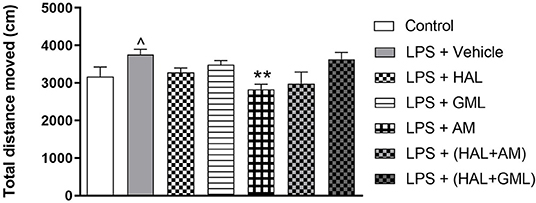
Figure 3. Locomotor activity (total distance moved in cm), analyzed in the OFT in rats exposed to saline and treated with vehicle (Control) as well as rats exposed to LPS receiving vehicle and the various drug treatments as indicated (unpaired Student's t-test for Control vs. LPS + vehicle; ∧p < 0.05; one-way ANOVA, Bonferroni post hoc test; **p < 0.01 vs. LPS + Vehicle).
Unpaired Student's t-test revealed a significant increase in immobility in the LPS-exposed rats (LPS + vehicle) when compared to the control group (saline + vehicle) (p < 0.0001) (Figure 4A). A significant decrease in both swimming (p = 0.0002) (Figure 4C) and struggling (p < 0.0001) (Figure 4B) behaviors was also observed in the LPS-exposed group (LPS + vehicle) compared to the control group (saline + vehicle).
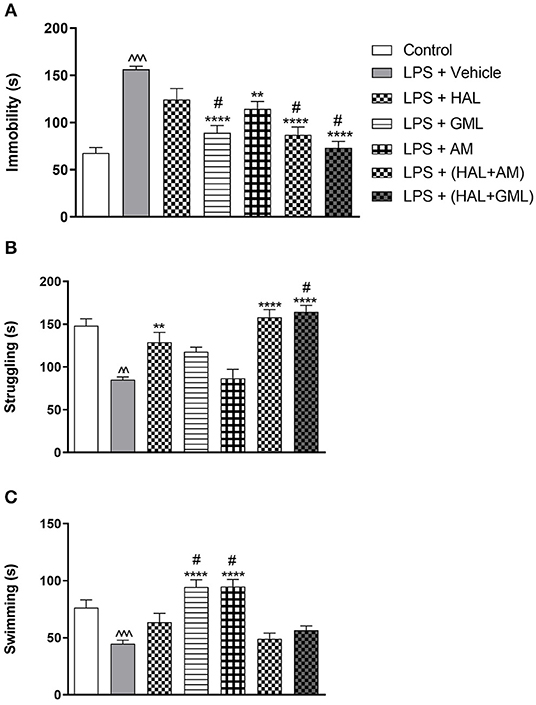
Figure 4. The forced swim test (FST) with regard to (A) immobility, (B) struggling, and (C) swimming behavior in rats exposed to saline and treated with vehicle (Control) as well as rats exposed to LPS receiving vehicle and the various drug treatments as indicated (unpaired Student's t-test for Control vs. LPS + vehicle; ∧∧p < 0.01, ∧∧∧p < 0.001; one-way ANOVA, Bonferroni post hoc test; **p < 0.01, ****p < 0.0001 vs. LPS + Vehicle; #p < 0.05 vs. LPS + HAL).
Using unpaired Student's t-tests, frontal cortical malondialdehyde (MDA) levels were significantly increased in the LPS-exposed rats (LPS + vehicle) (p = 0.030), compared to the saline control group (saline + vehicle) (Figure 5A). In the striatum, significantly elevated levels of MDA were also observed in the LPS-exposed rats (LPS + vehicle) (p < 0.0001) in comparison with the saline control group (saline + vehicle) (Figure 5B).
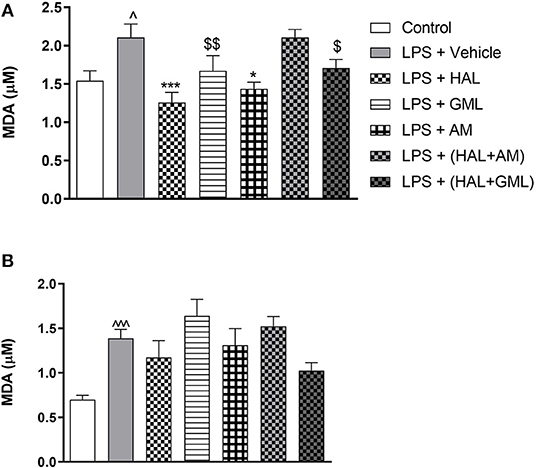
Figure 5. Lipid peroxidation as quantified by malondialdehyde (MDA) accumulation in (A) frontal cortex and (B) striatum in rats exposed to saline and treated with vehicle (Control) as well as rats exposed to LPS receiving vehicle and the various drug treatments as indicated [unpaired Student's t-test for Control vs. LPS + vehicle; ∧p < 0.05, ∧∧∧p < 0.0001; one-way ANOVA, Bonferroni post hoc test; *p < 0.05, ***p < 0.001 vs. LPS + Vehicle. $d = 0.5 ≥ d < 0.8, $$d = 0.8 ≥ d < 1.3 vs. LPS + Vehicle (Cohen's d value)].
Unpaired Student's t-test revealed that plasma IL-6 levels were significantly elevated in the LPS-exposed group (LPS + vehicle) when compared to the saline control group (saline + vehicle) (p = 0.0005) (Figure 6A).
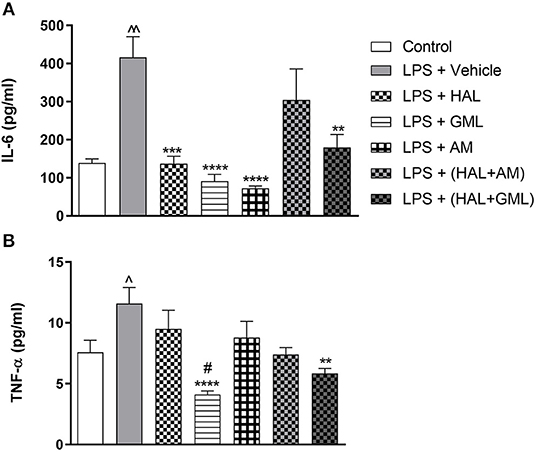
Figure 6. Plasma cytokine levels of (A) IL-6 and (B) TNF-α in rats exposed to saline and treated with vehicle (Control) as well as rats exposed to LPS receiving vehicle and the various drug treatments as indicated (unpaired Student's t-test for Control vs. LPS + vehicle; ∧p < 0.05, ∧∧p < 0.001; one-way ANOVA, Bonferroni post hoc test; **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. LPS + Vehicle; #p < 0.05 vs. LPS + HAL).
Unpaired Student's t-test displayed significantly elevated plasma TNF-α levels in the LPS-exposed group (LPS + vehicle) when compared to their saline control group (saline + vehicle) (p = 0.041) (Figure 6B).
Two-way ANOVA with repeated measures for each startle block in all the groups receiving the respective treatment or vehicle indicated a significant treatment × startle block interaction [F(3, 73) = 67.69, p < 0.0001] but no significant main effect of startle block [F(3, 73) = 1.62, p = 0.128] or treatment [F(3, 73) = 1.023, p = 0.417] on startle amplitude (data not shown). Bonferroni post hoc testing on the startle amplitude in all the LPS-exposed groups receiving the respective treatments or vehicle indicated that all the groups had a significant decrease (p < 0.05) in startle amplitude from blocks 1 to 4 (data not shown). Bonferroni post hoc testing also revealed no significant differences between any of the exposed and treatment groups at the respective startle blocks.
When considering drug treatment in the LPS model (Figure 2), two-way ANOVA revealed a significant interaction between treatment and PPI intensity [F(6, 77) = 295.66, p < 0.0001] as well as a significant main effect of treatment [F(6, 77) = 5.63, p < 0.0001] and PPI intensity [F(6, 77) = 1.83, p = 0.04] on %PPI in all the groups receiving the respective treatments or vehicle. Bonferroni post hoc testing demonstrated that HAL significantly reversed %PPI deficits in the LPS-exposed rats at 72 dB (p = 0.009) (Figure 2A) and 76 dB (p = 0.032) (Figure 2B). However, a very large effect size was observed in the LPS-exposed HAL-treated rats compared to their LPS vehicle-treated controls at 80 dB (d = 1.7) and 84 dB (d = 1.6) (Figures 2C,D, respectively). The combination treatment of GML + HAL successfully reversed %PPI deficits at all four of the prepulse intensities: 72 dB (p = 0.017), 76 dB (p = 0.002), 80 dB (p = 0.003), and 84 dB (p = 0.014) vs. the LPS-exposed control group (Figures 2A–D, respectively). However, no significant differences were observed in the LPS-exposed rats treated with GML alone, AM alone, or the combination of HAL + AM vs. the LPS + vehicle-exposed group (Figures 2A–D).
A one-way ANOVA of the OFT data in all the groups receiving the respective treatments or vehicle revealed a significant main effect of treatment on total distance moved [F(6, 75) = 14.43, p < 0.0001]. When considering drug treatment in the LPS model, Bonferroni post hoc tests revealed no significant differences in the total distance moved in the LPS-exposed treatment groups receiving HAL, GML, HAL + GML, and HAL + AM when compared to the LPS-exposed control group (LPS + vehicle) (Figure 3). However, a significant decrease in locomotor activity was observed in the LPS-exposed group treated with AM (p = 0.006) compared to the control group (LPS + vehicle) (Figure 3).
A one-way ANOVA of all the groups revealed a significant main effect of treatment on immobility [F(6, 77) = 16.02, p < 0.0001], struggling [F(6, 77) = 15.44, p < 0.0001], and swimming [F(6, 77) = 12.85, p < 0.0001]. When considering drug treatment in the LPS model (Figure 4), Bonferroni post hoc testing indicated a significant decrease in immobility in all the LPS-exposed treatment groups receiving GML (p < 0.0001), AM (p = 0.004), HAL + GML (p < 0.0001), and HAL + AM (p < 0.0001) compared to the LPS-exposed control group (LPS + vehicle) (Figure 4A). The effect of HAL alone did not reach significance (Figure 4A). However, treatment with GML alone (p = 0.034), HAL + GML (p < 0.0001), and HAL + AM (p = 0.03) showed a significantly greater decrease in immobility when compared to the HAL-treated LPS-exposed group (Figure 4A).
With regard to struggling behavior in the LPS-exposed rats, HAL (p = 0.005), HAL + GML (p < 0.0001), and HAL + AM (p < 0.0001) displayed a significant increase in struggling compared to the LPS-exposed control group (LPS + vehicle) (Figure 4B). The combination treatment of HAL + GML in the LPS rats displayed a significantly greater increase in struggling behavior (p = 0.049) when compared to HAL treatment alone in the LPS rats (Figure 4B).
Swimming behavior was significantly increased in the LPS groups receiving GML (p < 0.0001) and AM (p < 0.0001) treatment compared to the LPS-exposed control group (Figure 4C). A significant increase in swimming behavior was observed between the LPS-exposed group receiving HAL alone vs. both the LPS-exposed groups receiving GML (p = 0.005) or AM (p = 0.004), respectively (Figure 4C).
One-way ANOVA showed a significant main effect of treatment on lipid peroxidation in the frontal cortex [F(6, 78) = 5.234, p < 0.0001] and the striatum [F(6, 77) = 3.956, p = 0.002]. When considering drug treatment in the LPS model (Figure 5), Bonferroni post hoc analysis revealed that treatment with HAL (p = 0.001) and AM (p = 0.02) significantly reduced frontal cortical MDA levels in LPS-exposed rats, compared to the LPS-exposed control group (LPS + vehicle), but was unaffected by any of the other LPS-exposed treatment groups (LPS + GML, LPS + HAL + GML, and LPS + HAL + AM) vs. the LPS-exposed control group (Figure 5A). GML and GML + HAL showed a trend toward reducing MDA levels, with a large (d = 1.0) and medium (d = 0.7) effect size observed in the LPS-exposed rats treated with GML and GML + HAL, respectively, compared to their vehicle-treated controls (Figure 5A). Finally, none of the respective treatments showed a significant reduction in striatal MDA levels in the LPS-exposed rats compared to the LPS-exposed control group (Figure 5B).
One-way ANOVA revealed a significant main effect of treatment on IL-6 [F(6, 76) = 5.93, p < 0.0001] in the LPS and vehicle-exposed and treated groups. When considering drug treatment in the LPS model (Figure 6A), Bonferroni post hoc analyses showed that treatment with HAL (p = 0.021), GML (p = 0.002), AM (p < 0.0001), and HAL + GML (p = 0.01) significantly reversed elevated levels of IL-6 in the LPS-exposed groups. However, HAL + AM had no significant effect on IL-6 plasma levels in the LPS-exposed animals compared to the vehicle control (Figure 6A).
One-way ANOVA revealed a significant main effect of treatment on TNF-α levels [F(6, 77) = 5.96, p < 0.0001] in the LPS and vehicle-exposed groups receiving the respective treatments (Figure 6B). When considering drug treatment in the LPS model (Figure 6B), Bonferroni post hoc testing displayed that GML treatment successfully reversed elevated TNF-α levels in the LPS-exposed rats when compared to the LPS-exposed control group (LPS + vehicle) (p < 0.0001) (Figure 6B). In addition, GML was significantly more effective in decreasing plasma TNF-α levels in LPS-exposed animals than the HAL-treated LPS-exposed group (LPS + HAL) (p = 0.0081) (Figure 6B). Treatment with HAL + GML also significantly reduced TNF-α plasma levels in LPS-exposed animals in comparison to the LPS-exposed control group (p = 0.004) (Figure 6B). The remaining three treatment groups viz. HAL, AM, and HAL + AM showed no significant reduction in plasma levels of TNF-α when compared to the LPS-exposed control group (LPS + vehicle) (Figure 6B).
MIA induced sensorimotor gating deficits and depressive-like behavior concurrent with elevated cortico-striatal lipid peroxidation and elevated plasma pro-inflammatory cytokines in offspring. HAL reversed the changes in PPI, cortical (not striatal) lipid peroxidation, and elevated plasma IL-6, but failed to reverse depressive manifestations. GML + HAL effectively reversed PPI, while GML, AM, and HAL + AM did not. Conversely, GML and AM alone reversed depressive-like behaviors, more so than HAL, while the GML + HAL combination also reversed depressive-like symptoms. AM reversed both cortical (not striatal) lipid peroxidation and elevated plasma IL-6. GML and HAL + GML reversed elevations in IL-6 and TNF-α. These data present evidence for GML and its active constituent, AM, as being effective as adjunctive treatments but differently active with respect to psychotic and mood-related behaviors.
In line with previous findings (22, 31, 45, 63, 85), prenatal LPS exposure significantly compromised PPI in late adolescent offspring (Figure 2). HAL significantly reversed PPI deficits at 72 and 76 dB, with a similar trend and very large effect sizes at 80 and 84 dB (Figure 2), consistent with data from previous chronic (86) and acute (87–89) treatment studies in animals. Overactive dopaminergic processes are suggested to underlie the reduction in PPI (90), which explains the ability of HAL (D2 antagonist) to reverse said deficits (91). Although GML + HAL was effective in reversing MIA-induced PPI deficits across all four startle responses, this was not the case for GML or AM separately or for AM + HAL (Figure 2). The latter suggests that AM may abrogate the antipsychotic-like response to HAL, a particular interesting finding. In fact, AM presents with known antioxidant (58), 5HT2A, and a cAMP-PDE inhibitory (49) activity that to varying degrees may underlie these effects. Certainly, 5HT2A receptors play a prominent role in psychotic-like behavior (35) as well as in atypical antipsychotic drug design (35), and hence may play a role in the results described here. On the other hand, HAL is a potent pro-oxidant (92) and pro-inflammatory agent (93). Depending on the stage of disease progression, these actions, together with potent D2 inhibition, may mediate HAL's useful antipsychotic effects. However, these same pro-oxidative actions are purported to cause striatal toxicity and to cause late-onset treatment-related complications (92, 93). Although speculative, could these actions of HAL be countered by the antioxidant and cAMP-PDE inhibitory actions of AM, and possibly even reverse its antipsychotic effects? This opens up new ideas on how antipsychotics work and warrants further study.
Locomotor hyperactivity represents positive symptom schizophrenia, especially psychotic agitation (94). Prenatal LPS-exposed offspring demonstrated increased locomotor activity in the OFT (Figure 3), a behavioral response that has been ascribed to hyperdopaminergia (95). HAL lowered LPS-induced locomotor hyperactivity, albeit not significantly, in line with its antidopaminergic/antipsychotic actions. Only AM effectively reduced hyperlocomotion (Figure 3), yet it failed to alter PPI deficits (noted above). AM is a selective, competitive histamine antagonist (96) with sedative properties (97) that may adversely affect startle response in the PPI test. That said, the study design did not allow us to assess the effects of drug treatments on startle response in healthy controls, although rats exposed to LPS + AM did not differ significantly to rats exposed to LPS + vehicle. This and the above-noted HAL + AM findings prompt further research into the putative “antipsychotic-like” effects of AM. No other treatment had any noteworthy effects on locomotor activity (Figure 3), although the locomotor effects described for AM may complicate interpretation of swimming behavior in the FST (see below).
The negative symptoms of schizophrenia comprise affective flattening, alogia, anhedonia, asociality, and avolition (lack of motivation) (98), congruent with the basic symptoms of depression (99). Although the FST assesses behavioral despair, it has been suggested to signify an absence of motivational behavior which is commonly seen in schizophrenia (100). Indeed, LPS-exposed offspring presented with significant depressive-like behaviors (increased immobility) (Figure 4A) and reduced active coping (swimming and climbing) (Figures 4B,C), in agreement with earlier studies (101, 102). Especially, atypical antipsychotics may present with antidepressant activity (103, 104), while clinical (105) and preclinical (106) studies have described the pro-depressant effects of HAL (105, 106). In the present study, however, HAL showed a small, albeit negligible, effect to reverse MIA-associated immobility in the FST and reduced swimming (Figures 4A,C), although significantly increased struggling/climbing behavior (Figure 4B). Importantly, GML significantly decreased immobility and increased swimming behaviors (Figures 4A,C), antidepressant effects congruent with an earlier study in Flinders Sensitive Line (FSL) rats (55). Interestingly, the latter study described GML's prominent serotonergic actions and therapeutic equivalence with imipramine, which is shown here, too, but less emphatically (elevated swimming) (Figure 4C). Moreover, GML is a superior antidepressant to HAL (Figures 4A,C). Similarly, AM also exhibited significant antidepressant-like properties regarding its effects on immobility and swimming behavior, although not as marked as GML alone (immobility) (Figure 4A). Of note, GML and AM augmented the actions of HAL on immobility (Figure 4A) with GML bolstering the HAL effect on struggling (Figure 4B), suggesting a bolstering of HAL's action via mechanisms other than D2 receptor blockade. Despite AM suppressing locomotor activity, as noted above, this action did not affect its ability to reduce immobility and to increase swimming in the FST, thus highlighting a psychogenic action to bolster escape-driven behavior that is not related to, or mediated by, an increase in locomotor activity. Given the noted antioxidant actions of GML and its constituents, other antioxidants like NAC (107) are also antidepressant in the FST. Interestingly, NAC seems to have specific benefit in especially negative-symptom schizophrenia (40, 41), thus highlighting that antioxidants may have preferential psychopharmacological actions as antidepressants, which is borne out in this study as well.
Oxidative damage is implicated in the pathophysiology and neuroprogression of schizophrenia (35–37). Schizophrenia patients present with increased plasma lipid peroxidation (108–110), possibly correlated with certain clinical features (111). Adjunctive treatment with antioxidants improve symptoms in animal models (43, 112) as well as patients with schizophrenia (40). Prenatal LPS exposure significantly increased cortical and striatal lipid peroxidation in offspring (Figure 5), in agreement with previous findings (45, 113). Mouse models of oxidative stress are associated with cognitive and motivational deficits, as well as dysfunction of the prefrontal cortex (114). Schizophrenia is a hyperdopaminergic state (35) where dopamine metabolism contributes to oxidative stress by lowering glutathione (GSH) levels, which in turn is abrogated by D1/D2 receptor antagonists (36, 115). Importantly, HAL treatment significantly reduced lipid peroxidation in the frontal cortex (Figure 5A), while not having any marked effect in the striatum (Figure 5B). However, total striatum was analyzed here, whereas it is predominantly the ventral striatum encompassing the ventral tegmentum that is more relevant in rodents for an association with schizophrenia (116). The fact that HAL did not significantly reduce striatal lipid peroxidation may also be explained by HAL's known pro-oxidant actions in the striatum following chronic treatment (117) and which is associated with its long-term locomotor side effects. This action may otherwise obscure any possible antioxidant abilities in reducing MDA levels as was evident in the frontal cortex. This differential pro-oxidant action for HAL in these two brain regions is not new. In fact, Martins et al. (118) found that chronic HAL treatment increases oxidative stress in the striatum but decreases such levels in the cerebral cortex. Importantly, HAL still reversed LPS-induced PPI deficits (Figure 2), reiterating the importance of the frontal cortex in antipsychotic action (119). Moreover, here, we also show a frontal cortical role for its redox modulatory actions and how this may affect behavior. The frontal cortex is involved in cognitive processes such as working memory, behavioral flexibility, and attention (120). With regard to the antidepressant-like effects of GML and AM, the frontal cortex is also implicated in the development of depression and, hence, in antidepressant response (121, 122).
Although GML possesses antioxidant activity in vitro (123–126), GML had no effect on striatal lipid peroxidation, although it prompted a large effect size reduction in the frontal cortex (Figure 5A), thus qualitatively similar to that observed with HAL. Earlier, we found that chronic GML reversed elevated hippocampal lipid peroxidation in FSL rats (55), although the discrepancy between these two studies may be due to the different translational models used and the brain region assayed. AM also presents with antioxidant activity (58), here, significantly and again selectively reducing LPS-induced lipid peroxidation in the frontal cortex (Figure 5A). The absence of obvious antioxidant actions for GML and AM in the striatum is noteworthy, but may be related to assaying the whole striatum, as noted for HAL earlier. The diverse antioxidant actions of AM, viz. modulating GSH levels (127), free radical scavenging (128), inhibiting low-density lipoprotein oxidation (129), may afford it a more prominent antioxidant action than raw GML in vivo. As AM is the dominant bioactive xanthone in GML pericarp (55), it probably provides the dominant antioxidant activity observed with the raw extract. However, the more pronounced antioxidant action of AM does not translate into an improved behavioral outcome for AM over GML (Figures 2, 3), while GML and AM only offered small (GML) to negligible (AM) benefits in combination with HAL with regard to redox markers (Figure 5A). This suggests that GML may be offering beneficial effects through mechanisms other than antioxidant activity alone.
Immune-inflammatory dysfunction has been extensively reported in schizophrenia (35), specifically elevated levels of pro-inflammatory cytokines (130), while being a protagonist for oxidative stress (131). Pro-inflammatory cytokines have a developmental role in the brain (132, 133) and are implicated in the pathogenesis of neurodevelopmental disorders such as schizophrenia (134, 135). IL-6 and TNF-α levels are elevated in schizophrenia (136) and animal models (45, 137). Likewise, IL-6 and TNF-α were elevated in MIA offspring (Figures 6A,B) together with increased cortico-striatal lipid peroxidation (Figure 5). HAL-associated reversal of elevated IL-6 levels (Figure 6A) is consistent with clinical findings (138, 139), although it did not alter elevated plasma TNF-α levels (Figure 6B). Here, both GML and AM treatment reduced elevated plasma IL-6 levels (Figure 6A), with GML, but not AM, also reducing TNF-α levels (Figure 6B). This suggests a broader immunosuppressant action for GML vs. HAL or AM. AM has been shown to decrease inflammatory cytokines following LPS induction (140), to inhibit IL-2 release (141) and to suppress IL-6 expression (142). HAL + GML, but not HAL + AM, successfully reversed elevated IL-6 and TNF-α levels, although not more so than HAL alone (Figures 6A,B). In fact, HAL has immunosuppressive effects (143) as does GML have anti-inflammatory properties (125, 144). However, that neither GML nor AM bolstered the antioxidant effects of HAL again asserts that any beneficial effects offered by adjunctive GML treatment may involve mechanisms over and above inflammatory-redox processes. This warrants further study.
These findings have significance as a catalyst for future pre-clinical and clinical studies. Considering the important role of inflammation in the progression of mood and psychotic disorders, there is a growing interest in nutraceuticals with anti-inflammatory/antioxidant activity in psychiatry. That GML and AM have evinced therapeutic efficacy in the MIA model, as well as possess anti-inflammatory and antioxidant properties, suggests potential as a novel adjunctive treatment for these disorders (145). However, there appears to be distinct differential effects with respect to the mood–psychosis continuum, with a bias in favor of a depressed mood component. This prompts further investigation into GML's clinical benefits as an antidepressant vs. an antipsychotic. These aspects need deeper consideration in further animal studies but also in controlled clinical trials (146).
Certain limitations to this study are worth noting. Given the less-than-adequate antipsychotic-like effects for GML and AM, it would have been informative to include another schizophrenia-like behavioral assessment in the protocol to confirm these findings, e.g., memory, social interaction. Moreover, a dose titration analysis for GML and AM may have revealed a dose-dependent association in their behavioral effects, especially since the dose used for GML was based on a prior antidepressant study in another animal model (55). We also did not explore synergistic effects with atypical agents, which could differ to HAL. Biological analysis could have benefitted from regional striatal analysis, i.e., ventral, rostral, as opposed to assay of the whole striatum, as was done here. The study design did not allow for the assessment of drug treatments in healthy controls, which may have allowed for more in-depth explanation of treatment effects in LPS-exposed animals. Finally, having the same number of animals in both the saline-treated and LPS-exposed groups could have been an added benefit.
Schizophrenia is plagued by poor treatment outcomes and the limited efficacy of currently available antipsychotics (147–149). Supplementary treatment with nutraceutical anti-inflammatory agents and antioxidants such as GML may offer distinct therapeutic benefits (46). Plant extracts invariably contain a rich mixture of various bioactive constituents, yet little is known whether the pharmacological properties of a given extract are the sum of one or a group of inherent constituents or the result of the unique mix that the raw extract offers. This study has attempted to highlight this important question. Unlike the reference antipsychotic, HAL, chronic treatment with GML or AM failed to impact on sensorimotor gating deficits in the MIA model. However, both GML and AM not only displayed significant antidepressant-like properties but also bolstered the anti-immobility response to HAL. This is noteworthy as unlike atypical antipsychotics, HAL is not a recognized antidepressant, while here, it only marginally reduced immobility in the FST. GML and AM were both anti-inflammatory in the model, which may underlie their antidepressant effects. AM and, to a lesser degree, GML abrogated frontal cortical oxidative stress. This study confirms the antidepressant-like effects of GML described in another translational model of depression, the FSL rat (55). Having performed this study in a MIA model supports the use of GML and AM to address depressive symptoms in schizophrenia. However, their ability to address broader psychotic manifestations of the illness, e.g., PPI deficits, requires further study. Whether GML or AM is able to confer therapeutic benefits remains to be confirmed in dose–response and other clinical studies (150).
The datasets generated for this study are available on request to the corresponding author.
The animal study was reviewed and approved by AnimCare animal research ethics committee (National Health Research Ethics Council reg. no. AREC-130913-015) of the North-West University (Ethical approval numbers: NWU-00376-16-A5 and NWU-00147-14-A5).
JL: all laboratory work, data collection and formal analysis, validation, and first draft of the manuscript. MM: all figures, supervised behavioral methods and analysis, and statistical analysis. MB and OD: study design, data interpretation, and manuscript review. BH: conceptualization, methodology and statistics, writing—review and editing, supervision of JL, project administration, and funding acquisition.
This work was funded by the National Research Foundation (NRF; BH; grant number 77323) and Deakin University, Geelong, Australia (BH). The grant-holder acknowledges that opinions, findings, and conclusions or recommendations expressed in any publication generated by NRF-supported research are those of the authors and that the NRF accepts no liability whatsoever in this regard. MB is supported by an NHMRC Senior Principal Research Fellowship (APP1059660 and APP1156072). OD is an R.D. Wright Biomedical Research Fellow and has received grant support from the Brain and Behavior Foundation, Simons Autism Foundation, Stanley Medical Research Institute, Deakin University, Lilly, NHMRC, and Australasian Society for Bipolar and Depressive Disorders (ASBDD)/Servier.
Over the past 3 years, BH has participated in advisory boards, received honoraria from Servier, and received research funding from Servier, Lundbeck, Deakin University, Cannabis Science Inc., and HG&H Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors wish to thank Kobus Venter and Antoinette Fick (Vivarium, North-West University) for their input into the breeding and housing of the animals. We also acknowledge Walter Dreyer for his assistance during the ELISA analyses. We also acknowledge Mr. Brendan Holland, Deakin University, Australia, for performing the chromatographic fingerprint of raw GML extract. OD was supported by an R.D. Wright NHMRC Biomedical Research Fellowship (APP1145634).
AM, α-mangostin; ANOVA, analysis of variance; ARRIVE, Animal Research: Reporting in vivo Experiments; cAMP, cyclic adenosine monophosphate; DAD, diode-array detection; FST, forced swim test; GML, Garcinia mangostana Linn; HAL, haloperidol; HPLC, high-pressure liquids chromatography; IL, interleukin; LPS, lipopolysaccharide; MDA, malondialdehyde; MIA, maternal immune activation; NAC, N-acetyl cysteine; NWU, North-West University; OFT, open field test; PBS, phosphate-buffered saline; PDE, phosphodiesterase; PEG, polyethylene glycol; PND, post-natal day; Po, per os; Poly I:C, polyinosinic, polycytidylic acid; PPI, prepulse inhibition; SD, Sprague–Dawley; TBARS, thiobarbituric acid reactive substance; TNF, tumor necrosis factor.
1. Harris LW, Guest PC, Wayland MT, Umrania Y, Krishnamurthy D, Rahmoune H, et al. Schizophrenia: metabolic aspects of aetiology, diagnosis and future treatment strategies. Psychoneuroendocrinology. (2013) 38:752–66. doi: 10.1016/j.psyneuen.2012.09.009
2. Owen MJ, O'Donovan MC. Schizophrenia and the neurodevelopmental continuum: evidence from genomics. World Psychiatr. (2017) 16:227–35. doi: 10.1002/wps.20440
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Publishing (2013).
4. Meyer U. Developmental neuroinflammation and schizophrenia. Prog NeuropsychopharmacolBiol Psychiatr. (2013) 42:20–34. doi: 10.1016/j.pnpbp.2011.11.003
5. Lindenmayer J-P, Liu-Seifert H, Kulkarni PM, Kinon BJ, Stauffer V, Edwards SE, et al. Medication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior response. J Clin Psychiatr. (2009) 70:990–6. doi: 10.4088/JCP.08m04221
6. Chue P, Lalonde JK. Addressing the unmet needs of patients with persistent negative symptoms of schizophrenia: emerging pharmacological treatment options. Neuropsychiatr Dis Treat. (2014) 10:777. doi: 10.2147/NDT.S43404
7. Buckley PF, Stahl S. Pharmacological treatment of negative symptoms of schizophrenia: therapeutic opportunity or Culdesac? Acta Psychiatr Scand. (2007) 115:93–100. doi: 10.1111/j.1600-0447.2007.00992.x
8. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. (1987) 44:660–9.
9. Owen MJ, O'Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatr. (2011) 198:173–5. doi: 10.1192/bjp.bp.110.084384
10. Giezendanner S, Walther S, Razavi N, Van Swam C, Fisler MS, Soravia LM, et al. Alterations of white matter integrity related to the season of birth in schizophrenia: a DTI study. PloS ONE. (2013) 8:e75508. doi: 10.1371/journal.pone.0075508
11. Brown AS. The environment and susceptibility to schizophrenia. Progr Neurobiol. (2011) 93:23–58. doi: 10.1016/j.pneurobio.2010.09.003
12. Kirkbride JB, Susser E, Kundakovic M, Kresovich JK, Davey Smith G, Relton CL. Prenatal nutrition, epigenetics and schizophrenia risk: can we test causal effects? Epigenomics. (2012) 4:303–15. doi: 10.2217/epi.12.20
13. Davis J, Eyre H, Jacka FN, Dodd S, Dean O, McEwen S, et al. A review of vulnerability and risks for schizophrenia: beyond the two hit hypothesis. Neurosci Biobehav Rev. (2016) 65:185–94. doi: 10.1016/j.neubiorev.2016.03.017
14. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatr. (2009) 167:261–80. doi: 10.1176/appi.ajp.2009.09030361
15. Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. (2012) 72:1272–6. doi: 10.1002/dneu.22024
16. Penner JD, Brown AS. Prenatal infectious and nutritional factors and risk of adult schizophrenia. Exp Rev Neurother. (2007) 7:797–805. doi: 10.1586/14737175.7.7.797
17. Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. (2009) 204:313–21. doi: 10.1016/j.bbr.2008.12.016
18. Chase KA, Melbourne JK, Rosen C, McCarthy-Jones S, Jones N, Feiner BM, et al. Traumagenics: At the intersect of childhood trauma, immunity and psychosis. Psychiatr Res. (2018) 273:369–77. doi: 10.1016/j.psychres.2018.12.097
19. Kirsten TB, Taricano M, Florio JC, Palermo-Neto J, Bernardi MM. Prenatal lipopolysaccharide reduces motor activity after an immune challenge in adult male offspring. Behav Brain Res. (2010) 211:77–82. doi: 10.1016/j.bbr.2010.03.009
20. Lin YL, Lin SY, Wang S. Prenatal lipopolysaccharide exposure increases anxiety-like behaviors and enhances stress-induced corticosterone responses in adult rats. Brain Behav Immunity. (2012) 26:459–68. doi: 10.1016/j.bbi.2011.12.003
21. Lin YL, Wang S. Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behav Brain Res. (2014) 259:24–34. doi: 10.1016/j.bbr.2013.10.034
22. Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatr. (2006) 59:546–54. doi: 10.1016/j.biopsych.2005.07.031
23. Wolff AR, Bilkey DK. The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav Brain Res. (2010) 213:323–7. doi: 10.1016/j.bbr.2010.05.008
24. Van den Eynde K, Missault S, Fransen E, Raeymaekers L, Willems R, Drinkenburg W, et al. Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav Brain Res. (2014) 258:179–86. doi: 10.1016/j.bbr.2013.10.005
25. Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. (2003) 23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003
26. Tohmi M, Tsuda N, Watanabe Y, Kakita A, Nawa H. Perinatal inflammatory cytokine challenge results in distinct neurobehavioral alterations in rats: implication in psychiatric disorders of developmental origin. Neurosci Res. (2004) 50:67–75. doi: 10.1016/j.neures.2004.05.010
27. Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. (2007) 27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007
28. Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. (2005) 29:913–47. doi: 10.1016/j.neubiorev.2004.10.012
29. Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immunity. (2010) 24:881–97. doi: 10.1016/j.bbi.2010.03.005
30. Baharnoori M, Bhardwaj SK, Srivastava LK. Neonatal behavioral changes in rats with gestational exposure to lipopolysaccharide: a prenatal infection model for developmental neuropsychiatric disorders. Schizophrenia Bull. (2012) 38:444–56. doi: 10.1093/schbul/sbq098
31. Borrell J, Vela JM, Arévalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats: implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. (2002) 26:204–15. doi: 10.1016/S0893-133X(01)00360-8
32. Basta-Kaim A, Budziszewska B, Leśkiewicz M, Fijał K, Regulska M, Kubera M, et al. Hyperactivity of the hypothalamus-pituitary-adrenal axis in lipopolysaccharide-induced neurodevelopmental model of schizophrenia in rats: effects of antipsychotic drugs. Eur J Pharmacol. (2011) 650:586–95. doi: 10.1016/j.ejphar.2010.09.083
33. Zhu F, Zhang L, Ding Y-Q, Zhao J, Zheng Y. Neonatal intrahippocampal injection of lipopolysaccharide induces deficits in social behavior and prepulse inhibition and microglial activation in rats: implication for a new schizophrenia animal model. Brain Behav Immunity. (2014) 38:166–74. doi: 10.1016/j.bbi.2014.01.017
34. Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. (2008) 11:851–76. doi: 10.1017/S1461145707008401
35. Brand SJ, Möller M, Harvey BH. A review of biomarkers in mood and psychotic disorders: a dissection of clinical vs. preclinical correlates. Curr Neuropharmacol. (2015) 13:324–68. doi: 10.2174/1570159X13666150307004545
36. Bitanihirwe BK, Woo TU. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev. (2011) 35:878–93. doi: 10.1016/j.neubiorev.2010.10.008
37. Davis J, Moylan S, Harvey BH, Maes M, Berk M. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust New Zealand J Psychiatr. (2014) 48:512–29. doi: 10.1177/0004867414533012
38. Iguchi Y, Kosugi S, Nishikawa H, Lin Z, Minabe Y, Toda S. Repeated exposure of adult rats to transient oxidative stress induces various long-lasting alterations in cognitive and behavioralfunctions. PLoS ONE. (2014) 9:e114024. doi: 10.1371/journal.pone.0114024
39. Bas A, Gultekin G, Incir S, Bas TO, Emul M, Duran A. Level of serum thioredoxin and correlation with neurocognitive functions in patients with schizophrenia using clozapine and other atypical antipsychotics. Psychiatry Res. (2017) 247:84–89. doi: 10.1016/j.psychres.2016.11.021
40. Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia-a double-blind, randomized, placebo-controlled trial. Biol Psychiatr. (2008) 64:361–8. doi: 10.1016/j.biopsych.2008.03.004
41. Carmeli C, Knyazeva MG, Cuénod M, Do KQ. Glutathione precursor N-acetyl-cysteine modulates EEG synchronization in schizophrenia patients: a double-blind, randomized, placebo-controlled trial. PLoS ONE. (2012) 7:e29341. doi: 10.1371/journal.pone.0029341
42. Rapado-Castro M, Dodd S, Bush AI, Malhi GS, Skvarc DR, On ZX, et al. Cognitive effects of adjunctive N-acetyl cysteine in psychosis. Psychol Med. (2017) 47:866–876. doi: 10.1017/S0033291716002932
43. Möller M, Du Preez JL, Viljoen FP, Berk M, Emsley R, Harvey BH. Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain Behav Immun. (2013) 30:156–67. doi: 10.1016/j.bbi.2012.12.011
44. Cabungcal JH, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. (2014) 83:1073–84. doi: 10.1016/j.neuron.2014.07.028
45. Swanepoel T, Möller M, Harvey BH. N-acetyl cysteine reverses bio-behavioural changes induced by prenatal inflammation, adolescent methamphetamine exposure and combined challenges. Psychopharmacology. (2018) 235:351–68. doi: 10.1007/s00213-017-4776-5
46. Sarris J. Herbal medicines in the treatment of psychiatric disorders: a 10 year updated review. Phytother Res. (2018) 32:1147–62. doi: 10.1002/ptr.6055
47. Marx W, Moseley G, Berk M, Jacka F. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. (2017) 76:427–36. doi: 10.1017/S0029665117002026
48. Gutierrez-Orozco F, Failla ML. Biological activities and bioavailability of mangosteen xanthones: a critical review of the current evidence. Nutrients. (2013) 5:3163–83. doi: 10.3390/nu5083163
49. Chin Y-W, Kinghorn AD. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini Rev Org Chem. (2008) 5:355–64. doi: 10.2174/157019308786242223
50. Yoshikawa M, Harada E, Miki A, Tsukamoto K, Liang S, Yamahara J, et al. Antioxidant constituents from the fruit hulls of mangosteen (Garcinia mangostana L.) originating in Vietnam. YakugakuZasshi J Pharm Soc Japan. (1994) 114:129–33. doi: 10.1248/yakushi1947.114.2_129
51. Jung H-A, Su B-N, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. (2006) 54:2077–82. doi: 10.1021/jf052649z
52. Chairungsrilerd N, Furukawa K. I., Ohta T, Nozoe S, Ohizumi Y. Histaminergic and serotonergic receptor blocking substances from the medicinal plant Garcinia mangostana. Planta Med. (1996) 62:471–2. doi: 10.1055/s-2006-957943
53. Chen L-G, Yang L-L, Wang C-C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. (2008) 46:688–93. doi: 10.1016/j.fct.2007.09.096
54. Chomnawang MT, Surassmo S, Wongsariya K, Bunyapraphatsara N. Antibacterial activity of Thai medicinal plants against methicillin-resistant Staphylococcus aureus. Fitoterapia. (2009) 80:102–4. doi: 10.1016/j.fitote.2008.10.007
55. Oberholzer I, Möller M, Holland B, Dean O, Berk M, Harvey B. Garcinia mangostana Linn displays antidepressant-like and pro-cognitive effects in a genetic animal model of depression: a bio-behavioral study in the flinders sensitive line rat. Metab Brain Dis. (2018) 33:467–80. doi: 10.1007/s11011-017-0144-8
56. Sakagami Y, Iinuma M, Piyasena K, Dharmaratne H. Antibacterial activity of α-mangostin against vancomycin resistant Enterococci (VRE) and synergism with antibiotics. Phytomedicine. (2005) 12:203–8. doi: 10.1016/j.phymed.2003.09.012
57. Nakagawa Y, Iinuma M, Naoe T, Nozawa Y, Akao Y. Characterized mechanism of α-mangostin-induced cell death: caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg Med Chem. (2007) 15:5620–8. doi: 10.1016/j.bmc.2007.04.071
58. Fang Y, Su T, Qiu X, Mao P, Xu Y, Hu Z, et al. Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death. Sci Rep. (2016) 6:21018. doi: 10.1038/srep21018
59. Regenass W, Möller M, Harvey BH. Studies into the anxiolytic actions of agomelatine in social isolation reared rats: role of corticosterone and sex. J Psychopharmacol. (2018) 32:134–45. doi: 10.1177/0269881117735769
60. Harvey BH, Regenass W, Dreyer W, Möller M. Social isolation rearing-induced anxiety and response to agomelatine in male and female rats: role of corticosterone, oxytocin, and vasopressin. J Psychopharmacol. (2019) 33:640–6. doi: 10.1177/0269881119826783
61. Kilkenny C, Browne W, Cuthill IC, et al. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. (2010) 160:1577–9. doi: 10.1111/j.1476-5381.2010.00872.x
62. Toua C, Brand L, Möller M, Emsley R, Harvey B. The effects of sub-chronic clozapine and haloperidol administration on isolation rearing induced changes in frontal cortical N-methyl-d-aspartate and D 1 receptor binding in rats. Neuroscience. (2010) 165:492–9. doi: 10.1016/j.neuroscience.2009.10.039
63. Fortier M-E, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. (2007) 181:270–7. doi: 10.1016/j.bbr.2007.04.016
64. Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res. (2006) 173:243–57. doi: 10.1007/s00221-006-0419-5
65. Iturria SJ. A method for obtaining randomized block designs in preclinical studies with multiple quantitative blocking variables. Pharm Stat. (2011) 10:169–74. doi: 10.1002/pst.445
66. Gao X, Hashimoto T, Cooper T, Tamminga C. The dose-response characteristics of rat oral dyskinesias with chronic haloperidol or clozapine administration. J Neural Trans. (1997) 104:97–104. doi: 10.1007/BF01271298
67. Schmitt U, Dahmen N, Fischer V, Weigmann H, Rao ML, Reuss, et al. Chronic oral haloperidol and clozapine in rats: a behavioral evaluation. Neuropsychobiology. (1999) 39:86–91. doi: 10.1159/000026566
68. Schleimer SB, Johnston GA, Henderson JM. Novel oral drug administration in an animal model of neuroleptic therapy. J Neurosci Methods. (2005) 146:159–64. doi: 10.1016/j.jneumeth.2005.02.004
69. Terry AV, Gearhart DA, Warner S, Hohnadel EJ, Middlemore M-L, Zhang G, et al. Protracted effects of chronic oral haloperidol and risperidone on nerve growth factor, cholinergic neurons, and spatial reference learning in rats. Neuroscience. (2007) 150:413–24. doi: 10.1016/j.neuroscience.2007.09.014
70. Li L, Brunner I, Han AR, Hamburger M, Kinghorn AD, Frye R, et al. Pharmacokinetics of α-mangostin in rats after intravenous and oral application. Mol Nutr Food Res. (2011) 55:S67–S74. doi: 10.1002/mnfr.201000511
71. Nel A, Harvey BH. Haloperidol-induced dyskinesia is associated with striatal NO synthase suppression: reversal with olanzapine. Behav Pharmacol. (2003) 14:251–5. doi: 10.1097/00008877-200305000-00010
72. Han SY, You BH, Kim YC, Chin Y-W, Choi YH. Dose-independent ADME properties and tentative identification of metabolites of α-mangostin from Garcinia mangostana in mice by automated microsampling and UPLC-MS/MS methods. PLoS ONE. (2015) 10:e0131587. doi: 10.1371/journal.pone.0131587
73. Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. (2001) 156:234–8. doi: 10.1007/s002130100810
74. Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatr. (2006) 63:1325–35. doi: 10.1001/archpsyc.63.12.1325
75. Möller M, Du Preez JL, Emsley R, Harvey BH. Isolation rearing-induced deficits in sensorimotor gating and social interaction in rats are related to cortico-striatal oxidative stress, and reversed by sub-chronic clozapine administration. Eur Neuropsychopharmacol. (2011) 21:471–83. doi: 10.1016/j.euroneuro.2010.09.006
76. Liebenberg N, Harvey BH, Brand L, Brink CB. Antidepressant-like properties of phosphodiesterase type 5 inhibitors and cholinergic dependency in a genetic rat model of depression. Behav Pharmacol. (2010) 21:540–7. doi: 10.1097/FBP.0b013e32833befe5
77. van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophrenia Bull. (2009) 36:246–70. doi: 10.1093/schbul/sbp132
78. Porsolt RD, Brossard G, Hautbois C, Roux S. Models of affective illness: forced swimming and tail suspension tests in rodents. Curr Protoc Pharmacol. (2000) 10:5.8.1–9. doi: 10.1002/0471141755.ph0508s10
79. Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. (1997) 8:523–32. doi: 10.1097/00008877-199711000-00010
80. Pokorski M, Warzecha A. Depression and religiosity in older age. Eur J Med Res. (2011) 16:401. doi: 10.1186/2047-783X-16-9-401
81. Gozdzik-Zelazny A, Borecki L, Pokorski M. Depressive symptoms in schizophrenic patients. Eur J Med Res. (2011) 16:549. doi: 10.1186/2047-783X-16-12-549
82. Harvey BH, Duvenhage I, Viljoen F, Scheepers N, Malan SF, Wegener G, et al. Role of monoamine oxidase, nitric oxide synthase and regional brain monoamines in the antidepressant-like effects of methylene blue and selected structural analogues. Biochem Pharmacol. (2010) 80:1580–91. doi: 10.1016/j.bcp.2010.07.037
83. Steyn SF, Harvey BH, Brink CB. Immediate and long-term antidepressive-like effects of pre-pubertal escitalopram and omega-3 supplementation combination in young adult stress-sensitive rats. Behav Brain Res. (2018) 351:49–62. doi: 10.1016/j.bbr.2018.05.021
84. Möller M, Du Preez JL, Viljoen FP, Berk M, Harvey BH. N-acetyl cysteine reverses social isolation rearing induced changes in cortico-striatal monoamines in rats. Metab Brain Dis. (2013) 28:687–96. doi: 10.1007/s11011-013-9433-z
85. Basta-Kaim A, Fijał K, Budziszewska B, Regulska M, Leśkiewicz M, Kubera M, et al. Prenatal lipopolysaccharide treatment enhances MK-801-induced psychotomimetic effects in rats. Pharmacol Biochem Behav. (2011) 98:241–9. doi: 10.1016/j.pbb.2010.12.026
86. Romero E, Ali C, Molina-Holgado E, Castellano B, Guaza C, Borrell J. Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology. (2007) 32:1791–804. doi: 10.1038/sj.npp.1301292
87. Binder EB, Kinkead B, Owens MJ, Kilts CD, Nemeroff CB. Enhanced neurotensin neurotransmission is involved in the clinically relevant behavioral effects of antipsychotic drugs: evidence from animal models of sensorimotor gating. J Neurosci. (2001) 21:601–8. doi: 10.1523/JNEUROSCI.21-02-00601.2001
88. Kusljic S, Brosda J, Van Den Buuse M. Effects of haloperidol and clozapine on sensorimotor gating deficits induced by 5-hydroxytryptamine depletion in the brain. Br J Pharmacol. (2006) 147:800–7. doi: 10.1038/sj.bjp.0706641
89. Hadamitzky M, Harich S, Koch M, Schwabe K. Deficient prepulse inhibition induced by selective breeding of rats can be restored by the dopamine D2 antagonist haloperidol. Behav Brain Res. (2007) 177:364–7. doi: 10.1016/j.bbr.2006.11.037
90. Fabricius K, Helboe L, Fink-Jensen A, Wörtwein G, Steiniger-Brach B, Sotty F. Increased dopaminergic activity in socially isolated rats: an electrophysiological study. Neurosci Lett. (2010) 482:117–22. doi: 10.1016/j.neulet.2010.07.014
91. Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatr. (2010) 15:372–83. doi: 10.1038/mp.2008.44
92. Harvey BH, Joubert C, du Preez JL, Berk M. Effect of chronic N-acetyl cysteine administration on oxidative status in the presence and absence of induced oxidative stress in rat striatum. Neurochem Res. (2008) 33:508–17. doi: 10.1007/s11064-007-9466-y
93. Baumeister D, Ciufolini S, Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology. (2016) 233:1575–89. doi: 10.1007/s00213-015-4044-5
94. Powell SB, Zhou X, Geyer MA. Prepulse inhibition and genetic mouse models of schizophrenia. Behav Brain Res. (2009) 204:282–94. doi: 10.1016/j.bbr.2009.04.021
95. Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. J Affect Disord. (2010) 120:200–6. doi: 10.1016/j.jad.2009.04.018
96. Chairungsrilerd N, Furukawa K. I., Ohta T, Nozoe S, Ohizumi Y. Pharmacological properties of α-mangostin, a novel histamine H 1 receptor antagonist. Eur J Pharmacol. (1996) 314:351–6. doi: 10.1016/S0014-2999(96)00562-6
97. Shankaranarayan D, Gopalakrishnan Ct, Kameswaran L. Pharmacological profile of mangostin and its derivatives. Arch Int Pharm Ther. (1979) 239:257–69.
98. Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, et al. Definition and description of schizophrenia in 69 the DSM-5. Schizophrenia Res. (2013) 150:3–10. doi: 10.1016/j.schres.2013.05.028
99. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psychiatr. (2013) 170:165–72. doi: 10.1176/appi.ajp.2012.12010109
100. Chatterjee M, Jaiswal M, Palit G. Comparative evaluation of forced swim test and tail suspension test as models of negative symptom of schizophrenia in rodents. ISRN Psychiatr. (2012) 595141. doi: 10.5402/2012/595141
101. Enayati M, Solati J, Hosseini M-H, Shahi H-R, Saki G, Salari A-A. Maternal infection during late pregnancy increases anxiety-and depression-like behaviors with increasing age in male offspring. Brain Res Bull. (2012) 87:295–302. doi: 10.1016/j.brainresbull.2011.08.015
102. O'connor J, Lawson M, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2, 3-dioxygenase activation in mice. Mol Psychiatr. (2009) 14:511–22. doi: 10.1038/sj.mp.4002148
103. Han C, Wang S-M, Kato M, Lee S-J, Patkar AA, Masand PS, et al. Second-generation antipsychotics in the treatment of major depressive disorder: current evidence. Exp Rev Neurother. (2013) 13:851–70. doi: 10.1586/14737175.2013.811901
104. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pacific Psychiatr. (2016) 8:179–88. doi: 10.1111/appy.12186
105. Heinz A, Knable MB, Coppola R, Gorey JG, Jones DW, Lee K-S, et al. Psychomotor slowing, negative symptoms and dopamine receptor availability-an IBZM SPECT study in neuroleptic-treated and drug-free schizophrenic patients. Schizophrenia Res. (1998) 31:19–26. doi: 10.1016/S0920-9964(98)00003-6
106. Weiner I, Schiller D, Gaisler-Salomon I, Green A, Joel D. A comparison of drug effects in latent inhibition and the forced swim test differentiates between the typical antipsychotic haloperidol, the atypical antipsychotics clozapine and olanzapine, and the antidepressants imipramine and paroxetine. Behav Pharmacol. (2003) 14:215–22. doi: 10.1097/00008877-200305000-00005
107. Ferreira FR, Biojone C, Joca SR, Guimaraes FS. Antidepressant-like effects of N-acetyl-L-cysteine in rats. Behav Pharmacol. (2008) 19:747–50. doi: 10.1097/FBP.0b013e3283123c98
108. Akyol Ö, Herken H, Uz E, Fadillioglu E, Ünal S, Sögüt S, et al. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients: the possible role of oxidant/antioxidant imbalance. Progr Neuro Psychopharmacol Biol Psychiatr. (2002) 26:995–1005. doi: 10.1016/S0278-5846(02)00220-8
109. Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophrenia Res. (2002) 58:1–10. doi: 10.1016/S0920-9964(01)00334-6
110. Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, et al. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatr. (2003) 53:56–64. doi: 10.1016/S0006-3223(02)01443-9
111. Gama CS, Salvador M, Andreazza AC, Lobato MI, Berk M, Kapczinski F, et al. Elevated serum thiobarbituric acid reactive substances in clinically symptomatic schizophrenic males. Neurosci Lett. (2008) 433:270–3. doi: 10.1016/j.neulet.2008.01.018
112. Phensy A, Duzdabanian HE, Brewer S, Panjabi A, Driskill C, Berz A, et al. Antioxidant treatment with N-acetyl cysteine prevents the development of cognitive and social behavioral deficits that result from Perinatal Ketamine treatment. Front Behav Neurosci. (2017) 11:106. doi: 10.3389/fnbeh.2017.00106
113. Zhu Y, Carvey PM, Ling Z. Altered glutathione homeostasis in animals prenatally exposed to lipopolysaccharide. Neurochem Int. (2007) 50:671–80. doi: 10.1016/j.neuint.2006.12.013
114. Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D, et al. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci USA. (2013) 110:12462–7. doi: 10.1073/pnas.1307925110
115. Grima G, Benz B, Parpura V, Cuénod M, Do KQ. Dopamine-induced oxidative stress in neurons with glutathione deficit: implication for schizophrenia. Schizophrenia Res. (2003) 62:213–24. doi: 10.1016/S0920-9964(02)00405-X
116. McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. (2019) 42:205–20. doi: 10.1016/j.tins.2018.12.004
117. Martins MR, Petronilho FC, Gomes KM, Dal-Pizzol F, Streck EL, Quevedo J. Antipsychotic-induced oxidative stress in rat brain. Neurotoxic Res. (2008) 13:63–9. doi: 10.1007/BF03033368
118. Artigas F. The prefrontal cortex: a target for antipsychotic drugs. Acta Psychiatr Scand. (2010) 121:11–21. doi: 10.1111/j.1600-0447.2009.01455.x
119. Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatr. (2010) 67:199–207. doi: 10.1016/j.biopsych.2009.08.026
120. Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Ann Rev Psychol. (2002) 53:545–74. doi: 10.1146/annurev.psych.53.100901.135148
121. Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. (2008) 213:93–118. doi: 10.1007/s00429-008-0189-x
122. Puripattanavong J, Khajorndetkun W, Chansathirapanich W. Improved isolation of α-mangostin from the fruit hull of Garcinia mangostana and its antioxidant and antifungal activity. Planta Med. (2006) 72:328. doi: 10.1055/s-2006-950128
123. Weecharangsan W, Opanasopit P, Sukma M, Ngawhirunpat T, Sotanaphun U, Siripong P. Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn.). Med Principles Pract. (2006) 15:281–287. doi: 10.1159/000092991
124. Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia. (2007) 78:401–8. doi: 10.1016/j.fitote.2007.02.019
125. Zhao Y, Liu J-P, Lu D, Li P-Y, Zhang L-X. A new antioxidant xanthone from the pericarp of Garcinia mangostana Linn. Nat Product Res. (2010) 24:1664–70. doi: 10.1080/14786419.2010.499539
126. Márquez-Valadez B, Maldonado PD, Galván-Arzate S, Méndez-Cuesta LA, Pérez-De La Cruz V, Pedraza-Chaverrí J, et al. Alpha-mangostin induces changes in glutathione levels associated with glutathione peroxidase activity in rat brain synaptosomes. Nutr Neurosci. (2012) 15:13–9. doi: 10.1179/147683012X13327575416400
127. Williams P, Ongsakul M, Proudfoot J, Croft K, Beilin L. Mangostin inhibits the oxidative modification of human low density lipoprotein. Free Rad Res. (1995) 23:175–84. doi: 10.3109/10715769509064030
128. Mahabusarakam W, Proudfoot J, Taylor W, Croft K. Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin. Free Radic Res. (2000) 33:643–59. doi: 10.1080/10715760000301161
129. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatr. (2011) 70:663–71. doi: 10.1016/j.biopsych.2011.04.013
130. Möller M, Swanepoel T, Harvey BH. Neurodevelopmental animal models reveal the convergent role of neurotransmitter systems, inflammation, and oxidative stress as biomarkers of Schizophrenia: implications for novel drug development. ACS Chem Neurosci. (2015) 6:987–1016. doi: 10.1021/cn5003368
131. Zhao B. Schwartz JP. Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J Neurosci Res. (1998) 52:7–16. doi: 10.1002/(SICI)1097-4547(19980401)52:1<7::AID-JNR2>3.0.CO;2-I
132. Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. (2009) 64:61–78. doi: 10.1016/j.neuron.2009.09.002
133. Manu P, Correll CU, Wampers M, Mitchell AJ, Probst M, Vancampfort D, et al. Markers of inflammation in schizophrenia: association vs. causation. World Psychiatr. (2014) 13:189–92. doi: 10.1002/wps.20117
134. Na K-S, Jung H-Y, Kim Y-K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Progr Neuro Psychopharmacol Biol Psychiatr. (2014) 48:277–86. doi: 10.1016/j.pnpbp.2012.10.022
135. Erbagci AB, Herken H, Köylüoglu O, Yilmaz N, Tarakçioglu M. Serum IL-1β, sIL-2R, IL-6, IL-8 and TNF-α in schizophrenic patients, relation with symptomatology and responsiveness to risperidone treatment. Mediat Inflamm. (2001) 10:109–15. doi: 10.1080/09629350123895
136. Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophrenia Res. (2001) 47:27–36. doi: 10.1016/S0920-9964(00)00032-3
137. Maes M, Bosmans E, Calabrese J, Smith R, Meltzer HY. Interleukin-2 and interleukin-6 in schizophrenia and mania: effects of neuroleptics and mood stabilizers. J Psychiatr Res. (1995) 29:141–52. doi: 10.1016/0022-3956(94)00049-W
138. Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC. Changes in serum interleukin-2,-6, and-8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. J Clin Psychiatry. (2004) 65:940–7. doi: 10.4088/jcp.v65n0710
139. Maes M, Bosmans E, Kenis G, De Jong R, Smith RS, Meltzer HY. In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophrenia Res. (1997) 26:221–5. doi: 10.1016/S0920-9964(97)00057-1
140. Liu S-H, Lee L-T, Hu N-Y, Huange K-K, Shih Y-C, Munekazu I, et al. Effects of alpha-mangostin on the expression of anti-inflammatory genes in U937 cells. Chinese Med. (2012) 7:19. doi: 10.1186/1749-8546-7-19
141. Kasemwattanaroj P, Moongkarndi P, Pattanapanyasat K, Mangmool S, Rodpai E, Samer J, et al. Immunomodulatory activities of alpha-mangostin on peripheral blood mononuclear cells. Nat Prod Commun. (2013) 8:1257–60. doi: 10.1177/1934578X1300800919
142. Yiemwattana I, Kaomongkolgit R. Alpha-mangostin suppresses IL-6 and IL-8 expression in P. gingivalis LPS-stimulated human gingival fibroblasts. Odontology. (2015) 103:348–55. doi: 10.1007/s10266-014-0160-7
143. Song C, Lin A. h., Kenis G, Bosmans E, Maes M. Immunosuppressive effects of clozapine and haloperidol: enhanced production of the interleukin-1 receptor antagonist. Schizophrenia Res. (2000) 42:157–64. doi: 10.1016/S0920-9964(99)00116-4
144. Nakatani K, Atsumi M, Arakawa T, Oosawa K, Shimura S, Nakahata, et al. Inhibitions of histamine release and prostaglandin E2 synthesis by mangosteen, a Thai medicinal plant. Biol Pharm Bull. (2002) 25:1137–41. doi: 10.1248/bpb.25.1137
145. Ashton MM, Dean OM, Walker AJ, Bortolasci CC, Ng CH, Hopwood M, et al. The therapeutic potential of mangosteen pericarp as an adjunctive therapy for bipolar disorder and Schizophrenia. Front Psychiatr. (2019) 10:115. doi: 10.3389/fpsyt.2019.00115
146. Ashton MM, Berk M, Ng CH, Hopwood M, Dodd S, Turner A, et al. Efficacy of adjunctive Garcinia mangostana Linn (mangosteen) pericarp for bipolar depression: study protocol for a proof-of-concept trial. Braz J Psychiatry. (2019) 41:245–53. doi: 10.1590/1516-4446-2018-0114
147. Novick D, Haro JM, Suarez D, Perez V, Dittmann RW, Haddad PM. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. (2010) 176:109–13. doi: 10.1016/j.psychres.2009.05.004
148. Keefe RSE, Harvey PD. Cognitive impairment in schizophrenia. In: Geyer M, Gross G, editors. Novel Antischizophrenia Treatments. Handbook of Experimental Pharmacology, Vol. 213. Berlin, Heidelberg: Springer (2012).
149. Haddad P, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. (2014) 5:43–62. doi: 10.2147/PROM.S42735
Keywords: antidepressant, oxidative stress, immunity, antipsychotic, complimentary medicine, maternal inflammation, mangosteen, adjunctive treatment
Citation: Lotter J, Möller M, Dean O, Berk M and Harvey BH (2020) Studies on Haloperidol and Adjunctive α-Mangostin or Raw Garcinia mangostana Linn Pericarp on Bio-Behavioral Markers in an Immune-Inflammatory Model of Schizophrenia in Male Rats. Front. Psychiatry 11:121. doi: 10.3389/fpsyt.2020.00121
Received: 28 October 2019; Accepted: 12 February 2020;
Published: 31 March 2020.
Edited by:
Norbert Müller, Ludwig Maximilian University of Munich, GermanyReviewed by:
Bartlomiej Stanczykiewicz, Wroclaw Medical University, PolandCopyright © 2020 Lotter, Möller, Dean, Berk and Harvey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian H. Harvey, YnJpYW4uaGFydmV5QG53dS5hYy56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.