
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 06 February 2020
Sec. Forensic Psychiatry
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.01027
Introduction: Core psychopathy is characterized by grandiosity, callousness, manipulativeness, and lack of remorse, empathy, and guilt. It is often comorbid with conduct disorder and antisocial personality disorder (ASPD). Psychopathy is present in forensic as well as prison and general populations. In recent years, an increasing amount of neuroimaging studies has been conducted in order to elucidate the obscure neurobiological etiology of psychopathy. The studies have yielded heterogenous results, and no consensus has been reached.
Aims: This study systematically reviewed and qualitatively summarized functional and structural neuroimaging studies conducted on individuals with psychopathic traits. Furthermore, this study aimed to evaluate whether the findings from different MRI modalities could be reconciled from a neuroanatomical perspective.
Materials and Methods: After the search and auditing processes, 118 neuroimaging studies were included in this systematic literature review. The studies consisted of structural, functional, and diffusion tensor MRI studies.
Results: Psychopathy was associated with numerous neuroanatomical abnormalities. Structurally, gray matter anomalies were seen in frontotemporal, cerebellar, limbic, and paralimbic regions. Associated gray matter volume (GMV) reductions were most pronounced particularly in most of the prefrontal cortex, and temporal gyri including the fusiform gyrus. Also decreased GMV of the amygdalae and hippocampi as well the cingulate and insular cortices were associated with psychopathy, as well as abnormal morphology of the hippocampi, amygdala, and nucleus accumbens. Functionally, psychopathy was associated with dysfunction of the default mode network, which was also linked to poor moral judgment as well as deficient metacognitive and introspective abilities. Second, reduced white matter integrity in the uncinate fasciculus and dorsal cingulum were associated with core psychopathy. Third, emotional detachment was associated with dysfunction of the posterior cerebellum, the human mirror neuron system and the Theory of Mind denoting lack of empathy and persistent failure in integrating affective information into cognition.
Conclusions: Structural and functional aberrancies involving the limbic and paralimbic systems including reduced integrity of the uncinate fasciculus appear to be associated with core psychopathic features. Furthermore, this review points towards the idea that ASPD and psychopathy might stem from divergent biological processes.
Psychopathy is linked to biological processes in the brain, and is a highly heritable disorder (1). Structural and functional magnetic resonance imaging (MRI) have provided means to investigate these processes, but both the results and the definition of psychopathy have been heterogenic (2–4). Features and behaviors, such as lack of empathy, remorse, and guilt as well as manipulativeness, callousness, and grandiosity comprise the core psychopathic traits. Antisocial conduct is often comorbid with these core traits, which together are referred as to psychopathy (5–7).
The display of psychopathic behaviors is a reliable predictor for poor academic achievement, criminality, behavioral problems, and for adverse psychosocial consequences and mental health (8, 9). The prevalence of psychopathy is approximately 1% in the general population (10, 11), 3% in forensic population (12), 4% amongst corporate managers (13), and 20% in prison population (14). Furthermore, conduct disorder (CD) is often present amongst the majority of offenders with clinical psychopathy before the age of fifteen, and antisocial personality disorder (ASPD) after the age of eighteen (15). The PCL-R superordinate interpersonal-affective factor of psychopathy is not a prerequisite for CD and ASPD, but they are, however, often comorbid (16). Moreover, psychopaths having successfully avoided criminal conviction are sometimes referred to as successful psychopaths (17). However, in this context, the word “successful” does not imply success in other aspects of life (17).
Psychopathy is believed to have a neurobiological origin (18), and, in the past years, various neuroimaging studies have tried to resolve the perplexing etiology behind psychopathy (2, 4, 19). The structure, connectivity, and white matter tracts of brains of individuals displaying psychopathic traits have been visualized with numerous methods including conventional MRI, functional MRI, diffusion tensor MRI (DTI) voxel-based morphometry (VBM), (19), single photon emission computed tomography (SPECT), positron emission tomography (PET), and electroencephalogram (EEG) (20).
Despite an increase in neuroimaging studies in this field, there is no systematic review summarizing structural MRI, functional MRI, and DTI findings to date. Previous reviews have yielded inconsistent results [see e.g. (2, 3, 18)]. Diversity in sample demographics and characteristics as well as variation in task designs and imaging techniques make the interpretation and generalization of neuroimaging results difficult (3). Put differently, the functions, structures, and interconnections of brain regions associated with psychopathy remain unclear. A qualitative summary covering the three radiological submodalities might facilitate our understanding of psychopathy, and give insight to its neurobiological correlates and obscure neurobiological etiology.
The aim of this study was to conduct a systematic literature review on MRI neuroimaging of psychopathic traits, to summarize findings from different MRI modalities that cover different aspects of neural function and structure, and to examine whether these aspects were consistent.
This study is a systematic literature review on MRI neuroimaging of psychopathic traits, conducted per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (21).
For inclusion in the study, the record must have been published in a peer-reviewed journal in English, Finnish or Swedish. Because psychopathy is prevalent in various populations and both genders, we also included community samples in addition to prison and forensic populations. Consequently, both genders were included in our study. Furthermore, most records use PCL-R (5–7) as the measure of psychopathy, but records with PCL-R derived instruments were also included.
If the sample mean age was less than 17.50 years, the record was excluded. This criterion applied to both affected subjects and control groups. This criterion resulted in exclusion of early adolescence studies, but allowed room for late adolescence studies.
The following databases were accessed to acquire records for study: PubMed (NCBI), Medline (Ovid), PsycINFO (Ovid), PsycARTICLES (Ovid), Embase, and Criminal Justice Abstracts (EBSCO). The search was executed on the 4th of February 2019. Apart from categorical psychopathy, search strings, such as callous-unemotional traits, conduct disorder, and antisocial behavior, were used in order to encapsulate the dimensional continuum of psychopathy. The search strings and methods are available in Supplementary Materials.
The screened records (n = 526) were rated for either inclusion or exclusion by three independent assessors at the Niuvanniemi Hospital (BM Mika Johanson and forensic psychiatrics MD Olli Vaurio, and MD, PhD Markku Lähteenvuo). The initial interrater reliability for inclusion and exclusion was estimated with Fleiss' Kappa, reaching a Kappa value of 0.942 and an initial agreement percent across the raters of 97.34%. All articles with initial disagreement were re-rated within the group, and a decision for either inclusion or exclusion was made in consensus. As a result, a total of 118 records were included in the study and 408 excluded (Figure 1). The characteristics and key findings of each included study are summarized in the Review Matrix (Table S1). Excluded records with reasons for exclusion are available in Table S2.
Data from the included records (n = 118) were extracted and coded to form the review matrix (Table S1). The coded data included author and year of the record, type, and design of the study, sample characteristics, exclusion criteria, covariates, behavioral measures, MRI modality and method, and key findings. Every record was assigned with a unique and corresponding number. Type and design of the study included also the mean psychopathy score for the sample. Sample characteristics included sample size, mean age, and percentage of females. Based on the data in the review, matrix, functional, structural, and diffusion tensor MRI findings that correlated with psychopathy dimensionally or categorically, were compiled to Table 1. Findings that correlated with core psychopathy only were compiled to Table 2.
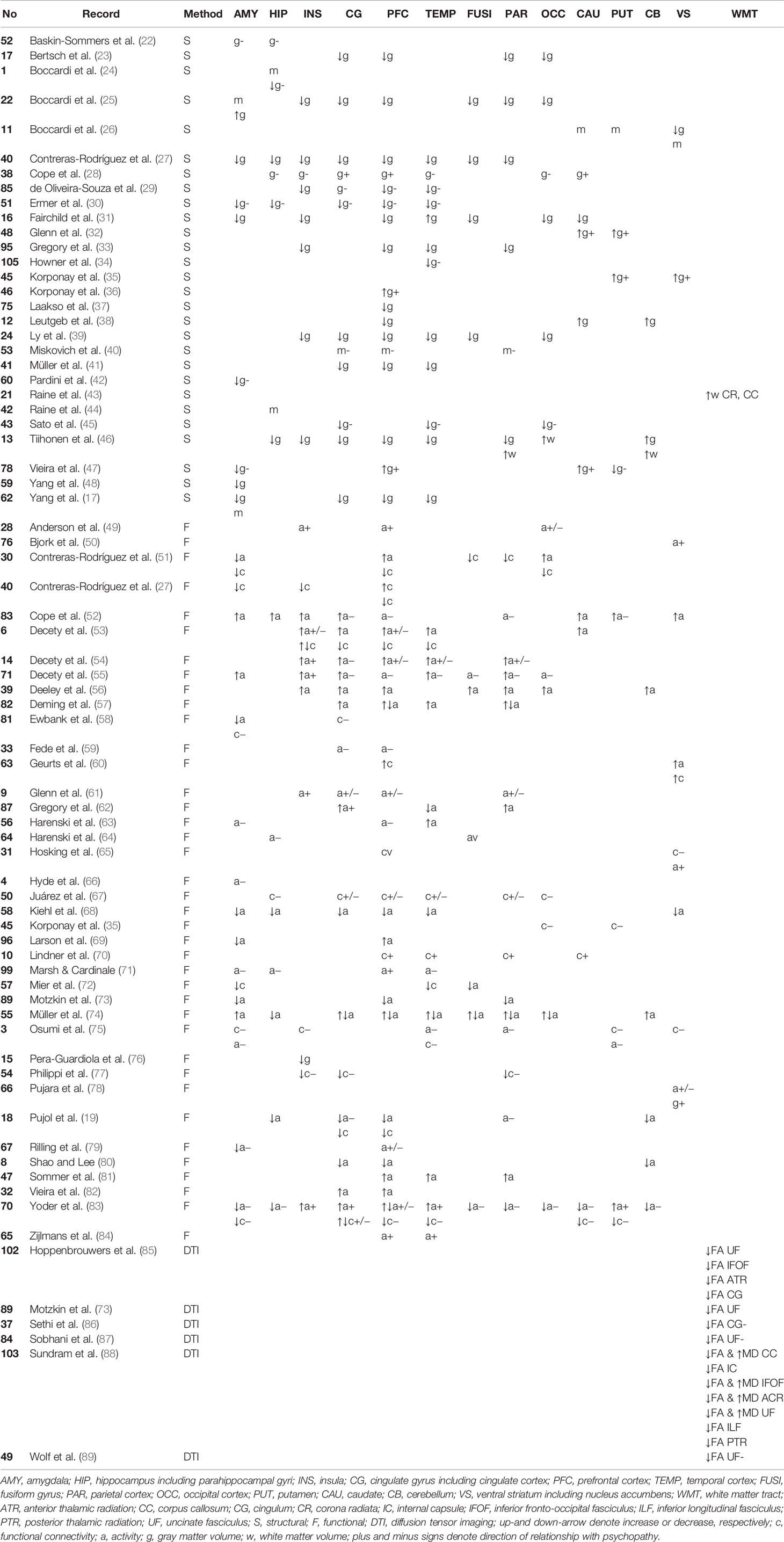
Table 1 Key neuroanatomical areas affected in psychopathy categorically and dimensionally. The records are grouped by method. Dimensional correlations in terms of total psychopathy score are shown.
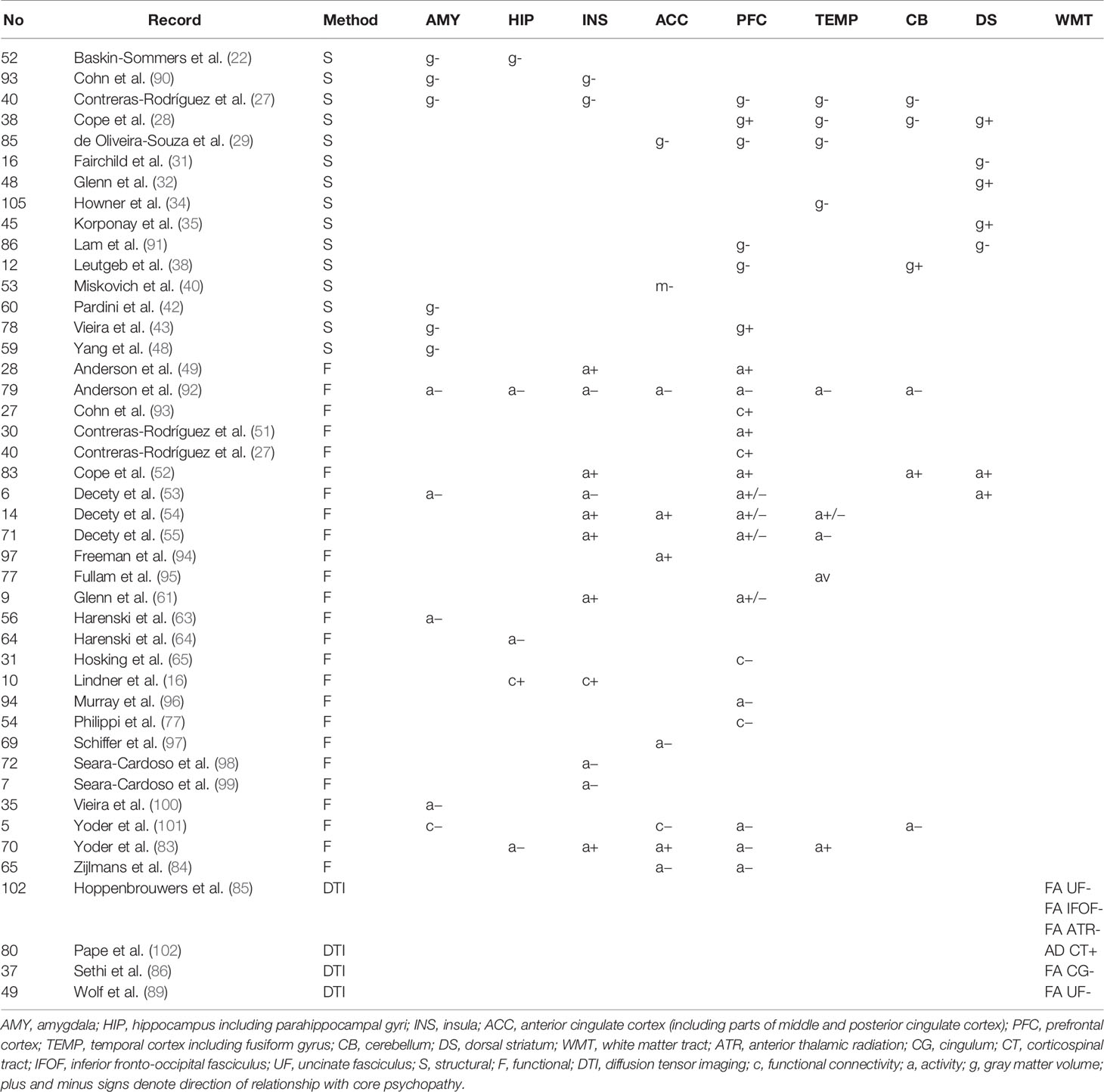
Table 2 Key neuroanatomical regions and their correlation to interpersonal-affective dimensions of psychopathy only.
Apart from the review matrix, the records were divided into three groups based on whether they aimed to investigate the neural correlates of (i) psychopathy or psychopathic traits, (ii) ASPD, or (iii) CD. The included studies were further divided into structural, functional, and diffusion tensor MRI studies (Figure 2). Due to the great number of functional neuroimaging studies of psychopathy, these studies were grouped according to task or setting into six groups (Table S3), in order to simplify the summarization process. The six groups were (i) fairness, (ii) moral issue, (iii) viewing affective content, (iv) reward, (v) lying and deception, and (vi) default mode network. The default mode network refers to interconnected areas in the brain, the activity of which reduces in goal-oriented tasks. The areas comprise of ventro- and dorsomedial prefrontal cortex, posterior cingulate cortex, precuneus, and lateral parietal cortex (103). Normal function of the default mode network is associated with self-referential (104), affective (105), and moral cognitive abilities (106, 107).
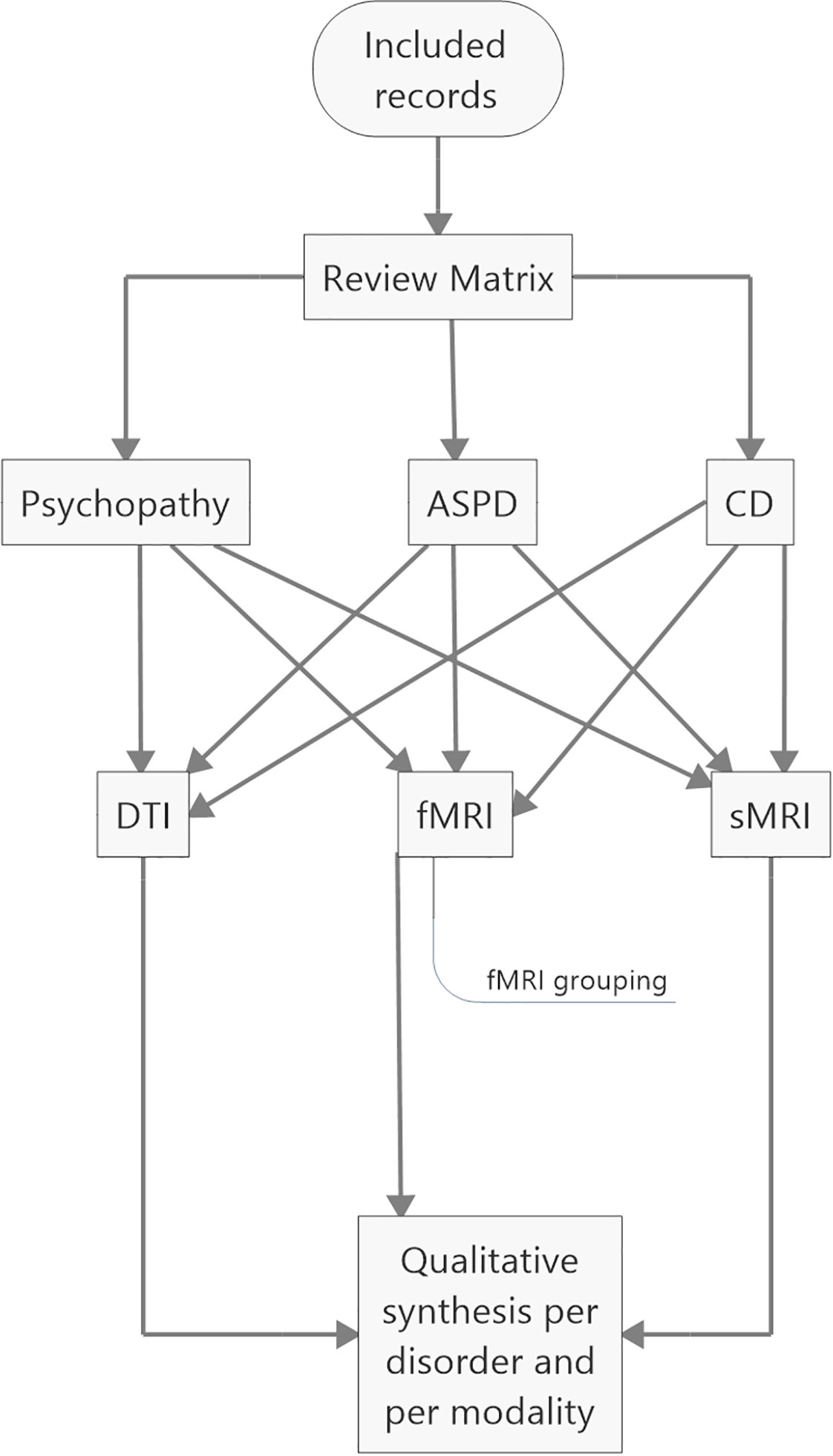
Figure 2 Coding and analyzing processes. ASPD, antisocial personality disorder; CD, conduct disorders; DTI, diffusion tensor MRI; fMRI, functional MRI; sMRI, structural MRI.
Several aberrancies were reported in the psychopathic brain in structural, functional, and diffusion tensor imaging studies. The neuroanatomical regions with most reported aberrancies in individuals with psychopathic traits categorically or dimensionally as a function of total psychopathy score are summarized in Table 1. Further, findings correlating with core psychopathy, i.e. interpersonal-affective dimensions only, are summarized in Table 2. These areas comprised to great extent of frontotemporal and limbic regions. These areas are also illustrated in Figure 3. The prefrontal correlates marked in Table 2 are divided into functional and anatomical subregions in Table S4.
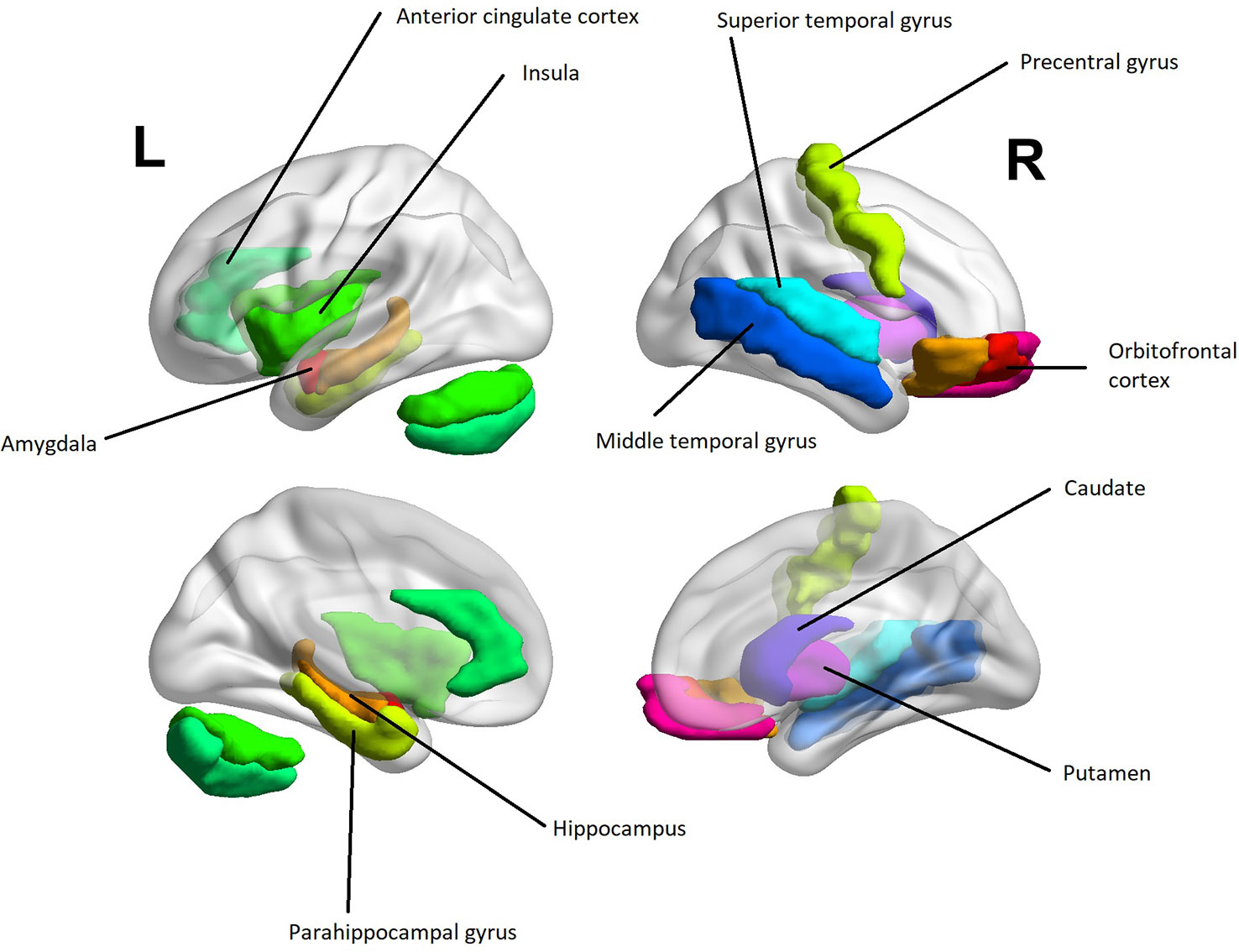
Figure 3 A heuristic anatomical map of brain regions correlating with interpersonal-affective dimensions of psychopathy with lateral and medial views (Table 2). The purpose of the figure is to provide an insight into anatomical localizations. To preserve readability, some of the regions are omitted or are present only on the other hemisphere. The visualization was done with the BrainNet Viewer [(107) http://www.nitrc.org/projects/bnv/]. The regions of interest were obtained from the Automated Anatomic Labeling Atlas (108).
Findings regarding psychopathy are presented first in order of modality. Thereafter, findings related to ASPD and CD are presented and compared to those of psychopathy.
Structurally, aberrancies were described mostly in terms of gray matter volume (GMV) reductions. For a brief summary of implicated brain regions, please see Table S5. Moreover and intriguingly, “successful psychopaths” did not show any significant GMV loss compared to healthy controls, whereas their “unsuccessful” counterparts showed prominent losses (17).
Decreased GMV was reported in several areas of the prefrontal cortex: orbitofrontal cortex (17, 25, 29, 30, 37, 46), dorsomedial prefrontal cortex (23, 33, 38, 39), frontal gyri (25, 33, 37, 39, 41), frontopolar cortex (25, 29, 46), precentral gyri supplementary motor area, sensory motor cortex (25), ventromedial prefrontal cortex, lateral prefrontal cortex (27), and dorsolateral prefrontal cortex (37). However, a few studies reported a positive association between orbitofrontal cortex GMV and degree of psychopathy (28, 36, 47).
Decreases in GMV were seen in the temporal regions (17, 110). Most prominent areas of decreased GMV were the superior temporal gyrus (39, 41), middle temporal gyrus (27, 46), superior temporal sulcus (29, 45), fusiform gyrus (25, 27), and the temporal poles (30, 33, 39).
A decrease in GMV in the parietal cortices were reported in two notable areas: the precuneus (23, 25, 27) and the postcentral gyrus (23, 33, 46). Moreover, increased white matter volume (WMV) was observed in the occipital and parietal lobes as well as in the left cerebellum (46).
The reported GMV reductions in the occipital cortex appeared to be of general nature (23, 39). Areas that were specified include the cuneus (25) and peristriate cortex (45) of the visual processing areas.
Several regions of the limbic system, the orbitofrontal cortex included (111), showed decreased GMV or abnormal morphology in psychopathy. In particular, the PCL-R superordinate psychopathy was related with decreased GMV across the paralimbic and limbic regions (22).
The amygdalae showed decreased GMV in psychopathy (17, 30, 42, 47, 48, 51). Somewhat contradictory to these findings, Boccardi and colleagues (25) reported larger global amygdalar volumes in a group of psychopathic subjects compared to healthy control group. Further, the amygdalae of psychopathic subjects showed aberrant morphology in the basolateral nuclei (17, 25).
In addition to the amygdalae, the hippocampi (24, 27, 30, 44) and the parahippocampal gyri (30, 46) showed reduced GMV. Further, two studies reported abnormal morphologies in the hippocampi. First, Boccardi and colleagues (24) found that the hippocampi of psychopathic individuals had a double convex morphology in comparison to the normal single convex form. Secondly, Raine et al. (44) found that unsuccessful psychopathic individuals had a volumetric asymmetry in the anterior hippocampi with the right side being larger than the left compared to both successful psychopathic individuals and healthy controls.
Decreased GMV was reported in the subdivisions of the cingulate cortex including the anterior cingulate cortex (25, 39), middle cingulate cortex (41) and posterior cingulate cortex (23, 27, 30, 45, 46). Moreover, psychopathy was associated with abnormal gyrification of the middle cingulate cortex extending into the dorsomedial prefrontal cortex and right parietal cortex in a study by Miskovich and colleagues (40). With respect to the anterior cingulate cortex, divergent results were reported by Glenn, Yang, Raine, and Colletti (112) who did not find differences in volumes between psychopathic individuals and controls. Of note, the control group in this study had a PCL-R mean score of 11.5. Furthermore, a positive correlation between the anterior cingulate cortex volume and psychopathic traits was reported by Cope and colleagues (28).
Also, the insular cortex showed reduced GMV in psychopathy (27, 29, 33, 39, 46, 76).
Psychopathy may be accompanied by increased total striatum volume (32, 35). Glenn, Raine, Yaralian, and Yang (32) noted an increase in GMV bilaterally in the globus pallidus, putamen, and in the right caudate body. Similarly, Leutgeb and colleagues (38) showed increased GMV in the left globus pallidus and caudate. The enlarged striatum has also been attributed to bilateral nucleus accumbens and putamen (35). Converging evidence was provided by a positive correlation between GMV in the nucleus accumbens (78), putamen, and caudate (28), and the degree of psychopathy. There are, however, also contradictory results. Firstly, Vieira et al. (47) found increased GMV in the left caudate, but decreased GMV in the left putamen. Secondly, Boccardi et al. (26) did not find any differences in putamen and caudate volumes in psychopathy, albeit the structures manifested aberrant morphology. Moreover, the nucleus accumbens showed a considerable 13% GMV reduction and abnormal morphology (26).
Increased GMV (38, 46) and a positive association between lifestyle-antisocial dimensions of the PCL-R (27) were reported with respect to the cerebellum (38, 46). However, negative associations between cerebellar GMV and interpersonal traits (28) and interpersonal-affective (27) dimensions were also found. Furthermore, decreased cerebellar WMV correlated with psychopathy (113), providing contradictory results to findings mentioned above.
In functional MRI studies with game-related tasks, psychopathic subjects exhibited reduced amygdalar activity in unfair versus fair conditions (75, 79, 82). Psychopathic subjects showed decreased amygdalar activity when rejecting an unfair offer, and decreased connectivity between amygdala and the limbic regions (75). Osumi and colleagues (75) argue further that amygdalar hypoactivity is indicative of attenuated reactive aggression, allowing the psychopathic subjects to adapt their behavior in order to pursuit personal gain. Furthermore, Viera and colleagues (82) noted that psychopathic subjects showed increased activity in the ventromedial prefrontal cortex and right rostral anterior cingulate cortex in response to unfair offers, whereas the control group showed increased activity in the left dorsolateral prefrontal cortex, which according to Viera et al. (82) implies divergent neural circuitries in decision making.
Several studies implicated dysfunction of the limbic system in psychopathy in the context of moral evaluations (59, 63, 99, 101, 114). Activity in the anterior insular cortex, which modulates anticipated guilt, was attenuated in psychopathic subjects, and the activity negatively correlated with interpersonal psychopathic traits (98). Psychopathic subjects also showed diminished functional connectivity in regions associated with empathetic and emotional processing, specifically between the anterior insular cortex and right temporoparietal junction as well as between the ventromedial prefrontal cortex and amygdala (101). Moreover, hypoactivity in the dorsolateral prefrontal cortex was shown (100). In a similar vein, Pujol et al. (114) found attenuated functional connectivity within the default mode network, particularly between the posterior cingulate cortex and nearby visual areas and medial prefrontal cortex, extending to ventrolateral prefrontal cortex and dorsolateral prefrontal cortex. Psychopathic subjects also demonstrated decreased activity in the hippocampi, posterior cingulate cortex, and medial prefrontal cortex (114). Consistently, Marsh and Cardinale (71) found decreased activity in the right amygdala, parahippocampal gyrus, and precunei.
Moral severity ratings were correlated with increased activity in the right posterior temporal cortex in psychopathic subjects, whereas in the control group ratings were associated with increased activity in the amygdala (63). Furthermore, the control group showed increased activity in the ventromedial prefrontal cortex and anterior temporal cortex during neutral and moral versus non-moral picture recognition (63). This setting was replicated in a female sample, and the results were mostly in line with those of the male sample with temporoparietal hypoactivity being more pronounced in female psychopathic individuals (64). Further, attenuated activity in the posterior cingulate cortex and temporoparietal junction were also seen in psychopathic subjects when judging traits of self and others (57).
In a great number of studies, the focus lay on investigating connectivity changes of the default mode network. The studies argued further that dysfunction of the default mode network is a key element in psychopathy (27, 67, 70, 73, 77, 92–94, 115). Firstly, decreased functional connectivity was shown between medial-dorsal frontal cortices and limbic regions including the amygdala (27, 73), posterior cingulate cortex (67, 73, 77), insula, and hypothalamus (27). Further supporting limbic and paralimbic dysfunction, Anderson, Maurer, Steele, and Kiehl (92) discovered that core psychopathy was associated with reduced activity in the dorsal anterior cingulate cortex (dACC), posterior cingulate cortex, amygdalae, temporoparietal junction, insula, and parahippocampal gyri, thus also indicative of a dysfunctional salience network. Furthermore, psychopathy was associated with decreased connectivity between the posterior cingulate cortex and parietal cortex (77). Secondly, the medial prefrontal cortex, a subregion of the default mode network, failed to attenuate below baseline in psychopathic subjects at task (94). Somewhat contrariwise, a positive correlation between psychopathic traits and mPFC attenuation was found by Sheng and colleagues (115) in a non-categorial community sample. The researchers did not, however, report the mean or total psychopathy score for the non-categorical sample, leaving the interpretation of the result difficult. Providing further evidence with respect to prefrontal connectivity bias in psychopathy, psychopathic traits were associated with increased functional connectivity between the dorsolateral prefrontal cortex and the medial-dorsal frontal cortices (27), increased connectivity in the frontopolar cortex within the default mode network (93) and more generally in the PFC (36). Thirdly, the correlation between dysfunctional default mode network and psychopathic traits was recently reported in females alike (70).
Psychopathic subjects showed increased performance in deception and lying (61, 80, 95). Particularly, lying related reductions in activity were seen in the dorsolateral prefrontal cortex, suggestive of prior cognitive training (61, 80). Fullam, McKie, and Dolan (95) did not find activity changes in the dorsolateral prefrontal cortex, but rather increased activity in the ventrolateral prefrontal cortex in all groups. The researchers did, however, conclude similarly that deception is prominent in psychopathy, and it engages more executive cognitive regions of the brain (95).
Psychopathic subjects showed decreased ability to recognize and process emotions (51, 72, 74, 76). From a structural viewpoint, emotion recognition was ascribed to the dorsomedial prefrontal cortex, orbitofrontal cortex, anterior insular cortex, and posterior cerebellum in psychopathic subjects, whereas this was attributed to the temporal cortex and amygdala in the control group (76). Functionally, increased activity in the medial prefrontal cortex and visual cortices were seen in psychopathic subjects in emotion recognition, whereas increased amygdalar activity was seen in healthy controls (51). In a similar vein, Volman et al. (116) found decreased functional connectivity between the prefrontal cortex and amygdala in psychopathic subjects in a facial emotion recognition task. Moreover, psychopathic subjects exhibited decreased functional connectivity between bilateral visual prefrontal cortices and the left amygdala, indicative of persistent failure in incorporating emotion into cognition (51).
Somewhat divergent from these findings, firstly, PCL-R score positively correlated with success rate in identifying certain emotions in a study by Decety, Chen, Harenski, and Kiehl (117). Secondly, Anderson et al. (49) found that interpersonal-affective traits correlated with decreased activity in visual cortices. Nonetheless, the researchers did concur with respect to increased activity in the medial prefrontal cortex. Thirdly, no groupwise differences regarding amygdalar activity was found by Deeley and colleagues (56), but they discovered reduced activity in the fusiform gyrus in facial processing in psychopathic subjects. Decety and colleagues (55) discovered similar findings with respect to activity in the amygdala and fusiform gyrus, but noted additionally decreased activity in other areas associated with facial processing, that is, in the superior temporal sulcus, orbitofrontal cortex, inferior occipital gyrus, inferior frontal gyrus (IFG), and ventromedial prefrontal cortex across all emotion ranges. Furthermore, psychopathic subjects showed an increase in activity in the anterior insular cortex in this setting (55). Thirdly, Müller et al. (74) discovered an increase in activity in the right amygdala, anterior cingulate cortex, and left superior temporal gyrus in psychopathic subjects that were exposed to emotional images with negative valence. However, Zijlmans et al. (84) could not find evidence of amygdalar involvement, but showed that callous-emotional (CU) traits positively correlated with activity in the left superior temporal gyrus and cingulate cortex. Of note, the healthy control group had a greater total psychopathy score than the multi-problem group they were compared to in this study.
The role of amygdala in emotion processing in psychopathy appears inconsistent. Community samples showed that amygdalar hypoactivity was associated with CU traits in processing both positive and negative emotions (100, 118). In contrast, Sadeh and colleagues (119) discovered that impulsive-antisocial dimension of psychopathy positively correlated with amygdalar activity. Moreover, Larson and colleagues (69) found that amygdalar activity did not differ between psychopathic subjects and control group when explicitly attending to a threat. However, psychopathic subjects exhibited decreased fear potentiated startle in terms of reduced amygdalar activity and concomitant increase in activity in the ventrolateral prefrontal cortex and dorsolateral prefrontal cortex when the subjects were engaged in an attentive task prior to presenting the threat (69).
Mier and colleagues (72) investigated the recognition of affective mental states, and found a prominent and widespread hypoactivity in the mirror neuron system of psychopathic subjects, more specifically in the amygdala, inferior prefrontal gyrus, and superior temporal sulcus. Furthermore, psychopathic subjects lacked connectivity between the superior temporal sulcus and amygdala (72). Consistent with a dysfunctional mirror neuron system, Sommer and colleagues (81) discovered that psychopathic subjects exhibited increased activity in attention- and outcome-related areas, including the orbitofrontal cortex, temporoparietal junction, and medial prefrontal cortex, whereas the control group exhibited increased activity in areas associated with empathy and the mirror neuron system including the superior frontal gyrus and supramarginal gyrus. Converging results were found by Reniers and colleagues (120) showing that higher degree of psychopathic traits entailed similar activity changes in areas involved in empathy and moral decision making including the inferior parietal lobule, supramarginal gyrus, precuneus, dorsolateral prefrontal cortex, and medial prefrontal cortex.
In response to pain depicting scenarios, psychopathic subjects showed attenuated activation of also other empathy-related regions including ventromedial prefrontal cortex, periaqueductal gray matter (PAG), posterior superior temporal sulcus (pSTS), and lateral orbitofrontal cortex (54). However, they showed increased activation of mentalizing-related regions including anterior insular cortex, dorsomedial prefrontal cortex, and dorsal striatum (54). Decety, Chen, Harenski, and Kiehl (53) discovered that in psychopathic subjects empathy-eliciting circuits such as the anterior middle cingulate cortex, anterior insular cortex, supplementary motor area, IFG, amygdala, and somatosensory cortex were activated when imaging oneself in pain. However, these circuits were not activated during a third person perspective, i.e. when imagining others in pain. Furthermore, in the third person perspective, psychopathic subjects exhibited an increase in activity in the ventral striatum (53). In fact, activity in the ventral striatum correlated with core psychopathic traits in a similar setting (117). Moreover, Seara-Cardoso, Viding, Lickley, and Sebastian (98) showed that neural responses to imagining others in pain depended on the dimension of psychopathy. More specifically, interpersonal-affective traits negatively correlated with activity in the bilateral anterior insular cortex, IFG, and middle cingulate cortex, whereas antisocial lifestyle traits positively correlated with activity in these areas (98). Molenberghs and colleagues (120) discovered somewhat convergent neural correlates in a punishment setting by showing that a higher degree of psychopathic traits correlated with less activity in brain areas involved in perceiving others in pain, including the anterior insular cortex, orbitofrontal cortex, and dACC. Sitaram and colleagues (122) conducted a pilot study on volitional regulation of the anterior insular cortex by employing negative emotional imageries in conjunction with contingent feedback. They found that one of the four psychopathic subjects learned to regulate the anterior insular cortex.
A number of studies reported aberrancies in the reward circuitry in psychopathy. Hosking and colleagues (65) showed that increased subjective value-related activity in the right nucleus accumbens was associated with psychopathy. Furthermore, psychopathic subjects exhibited decreased functional connectivity between ventromedial prefrontal cortex and nucleus accumbens, and this functional connectivity was inversely associated with the frequency of criminal convictions. These findings were ascribed to the interpersonal-affective dimension of psychopathy in particular (65). In contrast, Korponay and colleagues (35) discovered several resting-state functional connectivity aberrancies driven by the lifestyle-antisocial dimension including striato-midbrain, striatostriatal, and corticostriatal connectivities with the latter including increased connectivity between the dorsolateral prefrontal cortex and nucleus accumbens. In a similar vein, Geurts and colleagues (60) found that psychopathic subjects showed increased reward expectancy related activity in the ventral striatum, attributed to impulsive-antisocial traits. Psychopathic subjects also exhibited decreased reward expectancy related activity in the PAG, and increased functional connectivity between the dorsomedial prefrontal cortex and ventral striatum (60). Pujara and colleagues (78) discovered, however, that all dimensions of psychopathy were associated with increased activity in the ventral striatum in a gain versus loss condition. Convergently, reward anticipation in psychopathy correlated with activity in the nucleus accumbens and anterior mesofrontal cortex (50). In a similar setting, Buckholtz and colleagues (123) attributed the increased activity in the nucleus accumbens to antisocial and impulsive traits. As an alternative to monetary rewards, Cope and colleagues (52) approached the setting from a different angle. They presented imprisoned substance-dependent psychopathic subjects drug-related cues and discovered that psychopathy was associated with decreased activity in the anterior cingulate cortex, posterior cingulate cortex, amygdala, hippocampus, globus pallidus, caudate, and frontal gyri.
The integrity of white matter structures appeared to play a pivotal role in psychopathy. Several studies showed reduced fractional anisotropy (FA) in the uncinate fasciculus on the right side (73, 87, 89) and bilaterally (16, 85, 88). These findings were in general attributed to the interpersonal-affective dimensions (85, 89, 124), but also to a lesser extent to lifestyle-antisocial dimensions (85). Moreover, increased radial diffusivity (RD) in the uncinate fasciculus correlated with the interpersonal dimension of psychopathy (16).
In addition to the uncinate fasciculus, aberrancies in other various white matter structures were reported. Sethi and colleagues (86) found that psychopathic subjects exhibited decreased FA in the left dorsal cingulum, indicative of emotional detachment and dysfunction of the default mode network. In turn, Hoppenbrouwers and colleagues (85) discovered bilaterally decreased FA in the uncinate fasciculus, anterior thalamic radiation, and inferior fronto-occipital fasciculus. Furthermore, Yoder, Porges, and Decety (83) conducted a tractography of the amygdalar subnuclei and found that CU traits negatively correlated with functional connectivity between dACC and the central amygdalar subnucleus (83).
Similar to psychopathy, ASPD was associated with gray matter aberrancies in the limbic and cortical areas. Decreased GMV was noted in anterior cingulate cortex (125–127) superior temporal sulcus, superior temporal gyrus, frontal gyri (125), orbitofrontal cortex (125, 127, 128), sensory motor area, frontopolar cortex (127), medial prefrontal cortex (127, 128), and rectal gyrus (128). In a study by Kolla et al. (110), psychopathic and ASPD groups were compared to each other, and psychopathic individuals had a more pronounced decrease in GMV in temporal and cerebellar regions. Further, contrary to findings in psychopathy, several of the regions with gray matter reductions were accompanied by increased surface area, most notably in the superior temporal gyrus, superior frontal gyrus, superior temporal sulcus, supramarginal gyrus, orbitofrontal cortex, insula, and parahippocampal gyrus (125). Furthermore, ASPD subjects had lower right thalamic volume compared to healthy controls (129). Both the volume of the anterior cingulate cortex and that of the right thalamus negatively correlated with psychosocial deprivation (126, 129). Moreover, increased GMV and WMV were found in ASPD subjects in the inferior parietal lobule and precuneus, respectively (130). However, a study by Howner et al. (34) showed that ASPD individuals had a decreased global brain volume compared to healthy controls.
Tang et al. (131) investigated resting-state neural activity in ASPD and found that ASPD subjects showed decreased activity in the posterior cerebellum and middle frontal gyrus (MFG). Contrariwise, ASPD subjects showed increased activity in the middle occipital gyrus, inferior temporal gyrus, and inferior occipital gyrus (130). Similarly, Liu, Liao, Jiang, and Wang (132) found decreased activity in the posterior cerebellum, but also in temporal areas and in the orbitofrontal cortex. Recently, Kolla and colleagues (133, 134) noted that monoamine oxidase A (MAOA) genotype was associated with ASPD. High activity MAOA subjects showed increased resting-state functional connectivity between caudate, frontopolar cortex, and anterior cingulate cortex compared to low activity MAOA subjects and healthy controls. The researchers also found that instrumental aggression and functional connectivity from the ventral striatum to the precuneus had an inverse correlation in the low activity MAOA subjects, and a positive correlation to the angular gyrus (134). Increased corticostriatal resting-state connectivity was also described in psychopathic individuals (35).
Aberrant neural correlates were also found at task. Firstly, Kumari et al. (135) found that ASPD individuals showed decreased activity in the left frontal gyrus, anterior cingulate cortex, and precuneus in an n-back setting. Secondly, decreased activity in the thalamus was noted in a NoGo condition suggesting impaired control inhibition (136). In a similar vein, Schiffer et al. (97) discovered in a Stroop color naming task that response times and activity in the dorsolateral prefrontal cortex correlated with impulsivity. Furthermore, the ASPD group showed decreased activity most prominently in the left dACC and Wernicke's area compared to healthy controls. Importantly, the decreased activity in the dACC was associated with interpersonal-affective dimensions of psychopathy (97). However, Gregory et al. (62) found more divergent neural activity in ASPD and psychopathy in a reversal learning setting. The researchers found that psychopathic individuals responded to punishment with increased activation of the posterior cingulate cortex and insula, whereas ASPD subjects showed decreased activity in these areas (62). Further evidence for divergent neural correlates was provided by Murray, Shaw, Forbes, and Hyde (96) who showed that antisocial behavior, but not CU traits, was associated with decreased activity in the ventral striatum and dorsolateral prefrontal cortex during reward anticipation.
In a facial emotion processing condition, Hyde, Votruba-Drzal, Hariri, and Manuck (66) found that ASPD traits positively correlated with amygdalar activity, whereas with psychopathic traits the correlation was negative. Indeed, amygdalar hyperreactivity was linked especially to reactive aggression (137). Furthermore, ASPD traits positively and psychopathic traits negatively correlated with tendency to feel unpleasant emotional states (66). In recognizing emotional states based on eyes only, Schiffer and colleagues (138) found no group differences in performance in ASPD versus healthy controls. Contrariwise, psychopathy was associated with diminished ability to recognize emotions. However, ASPD subjects did exhibit decreased activity in the amygdala and increased activity in the left medial prefrontal cortex, ventrolateral prefrontal cortex, pSTS, temporoparietal junction, fusiform gyrus, and precuneus (138).
Akin to psychopathy, decreased FA was seen in the uncinate fasciculus, inferior fronto-occipital fasciculus, and anterior thalamic radiation in ASPD (88, 139). Of note, axial diffusivity (AD) and radial diffusivity (RD) revealed additional regions not detected with FA alone, implying abnormal axonal structure and demyelination in ASPD, respectively. Importantly, impulsivity negatively correlated with AD in the corpus callosum, posterior corona radiata, and posterior thalamic radiation, whereas risky behavior positively correlated with RD in the superior longitudinal fasciculus and inferior fronto-occipital fasciculus (139). Moreover, the antisocial lifestyle dimensions of psychopathy were associated with decreased FA and increased mean diffusivity (MD) in the frontal lobe (88).
CD was associated partly with similar GMV reductions as were seen in psychopathy including amygdala, insula, dorsomedial prefrontal cortex, orbitofrontal cortex, fusiform gyrus, and inferior and superior occipital cortex (31). Dissimilar to psychopathy, a decrease in caudate GMV and an increase in frontal operculum and inferior temporal gyrus GMV was seen. However, the researchers also noted that CU traits positively correlated with the caudate nucleus and ventral striatum consistent with findings of increased striatal volumes in psychopathy. Furthermore, compared to healthy controls, the GMV changes in CD were similar irrespective of childhood- or adolescence on-set with the exception of adolescence on-set group showing GMV reductions in the orbitofrontal cortex. (31). In turn, Budhiraja et al. (140) investigated the brain structure of young women with prior CD diagnosis. They noted an increase in GMV in the superior temporal gyrus and a decrease in GMV in the anterior cingulate cortex, hippocampus, and lingual gyrus, which were attributed mainly to substance use disorder (SUD), anxiety, and depression symptoms (140). In comparison, decreased GMV in both the anterior cingulate cortex and the superior temporal gyrus were reported in psychopathy. Moreover, the findings of Budhiraja et al. (140) may also imply gender specific changes in CD as the sample in Fairchild et al. (31) only included males. Indeed, Lindner et al. (124) emphasized that CD in males and females differed in terms of genotype and phenotype. However, a study by Cohn et al. (90) found reduced gray matter concentration in the insula and amygdala irrespective of gender. CU traits negatively correlated with these findings (90), which are in line with findings in psychopathy. Similarly, a longitudinal cohort study by Pardini, Raine, Erickson, and Loeber (42) showed that decreased bilateral amygdalar volume was associated with higher levels of psychopathy from childhood to adulthood. Furthermore, the amygdalar volumes successfully predicted increased psychopathic features and committing violent acts in a 3-year follow up. The researchers underscored the possibility of amygdalar lateralization by showing that the left amygdalar volume negatively correlated with the lifestyle dimension of psychopathy, while the right amygdalar volume negatively correlated with interpersonal-affective dimensions (42). Moreover, prior CD diagnoses were shown to predict decreased amygdalar activity, higher CU traits and increased aggression as well as impulsivity in adulthood (141).
The few functional neuroimaging studies of CD included in this review yielded results quite similar to those in psychopathy. Firstly, resting-state functional connectivity analysis revealed aberrancies in the default mode and salience networks, and also in the frontoparietal network. CU traits were associated with increased connectivity in the left frontopolar cortex within the default mode network. In turn, impulsivity was associated with increased connectivity in the left IFG within the frontoparietal network as well as the left amygdala within the salience network (93). Secondly, Ewbank and colleagues (58) investigated facial emotion processing in CD and found that CD subjects showed decreased amygdalar activity compared to healthy controls. Moreover, psychopathic traits were associated with reduced connectivity between the ventral anterior cingulate cortex and the left amygdala (58).
In a study by Lindner et al. (124), young women with a prior CD diagnosis exhibited reduced AD in the forceps minor and the genu and the body of corpus callosum compared to comorbidity matched controls without CD. Furthermore, the researchers could not ascertain abnormal FA in the uncinate fasciculus (124), as was seen in psychopathy on the contrary. However, Pape and colleagues (102) found a positive correlation between FA and with grandiose-manipulative traits in the uncinate fasciculus, corpus callosum, inferior fronto-occipital fasciculus, corticospinal tract, forceps minor, and anterior thalamic radiation in a mixed sample, albeit a non-categorical one. The direction of correlation was negative for RD in the same tracts and a number of other WM tracts. Further, they found that CU traits positively correlated with AD in the corticospinal tract (102). In a similar vein, Passamonti et al. (142) discovered that CD subjects had increased FA, increased AD and decreased RD bilaterally in the external capsule and uncinate fasciculus compared to healthy controls. As this was a male sample, the findings of Lindner et al. (124) may imply gender differences in CD.
The aim of this study was to conduct a systematic literature review on MRI neuroimaging of psychopathic traits, to summarize findings from different MRI modalities that cover different aspects of neural function and structure, and to examine whether these aspects were consistent. A total of 118 records were included in the study. The records consisted mainly of neuroimaging of clinical psychopathy, but also of non-clinical psychopathic traits, antisocial personality disorder, and conduct disorder. Both structurally and functionally, most aberrancies were described in frontotemporal regions as well as in limbic and paralimbic structures.
Psychopathic individuals exhibited decreased GMV in frontotemporal, limbic, paralimbic, and cerebellar structures. Although findings indicated both reduced GMV and abnormal morphology of the hippocampus, evidence for enlargement of the temporal horns in psychopathy was not found nor was it investigated in particular. The temporal horns of the lateral ventricles lie adjacent to the hippocampi. Thus, decreased volumes in hippocampi can inversely correlate with that of temporal horns (143). Temporal horn enlargement has been implicated in some psychiatric diagnoses including Alzheimer disease (144) and schizophrenia (145). Moreover, global GMV of psychopathic individuals does not appear to significantly differ from that of general population (19).
Dysfunction of the default mode network was found. This was anticipated as the default mode network consists of areas overlapping the limbic and paralimbic regions including the temporoparietal junction, posterior cingulate cortex, precuneus, and medial prefrontal cortex (92, 146). Certainly, these regions exhibited decreased GMV, activity, and functional connectivity in psychopathic subjects. The dysfunctional default mode network could, to a degree, relate to the aberrant behavior displayed in psychopathy as the normal function of the default mode network is associated with reflective self-awareness (104), emotional reflection (105), moral judgment (106, 107), and the ability to relive past experiences and construct possible futures (147). However, ASPD was also associated with dysfunction in several networks including default mode (134), attention, cerebellar (131), and frontoparietal control networks (148).
Furthermore, findings from DTI studies corroborate the aforesaid notions. The uncinate fasciculus was the white matter tract with most anomalies in terms of decreased FA. The uncinate fasciculus connects the amygdala to ventromedial prefrontal cortex and orbitofrontal cortex and is ostensibly responsible for several cognitive and affective functions that are erring in psychopathy including moral judgment, empathy, value representation, and stimulus-reinforced learning (14, 149, 150). However, reduced FA in the uncinate fasciculus cannot be considered strictly specific to psychopathy as similar findings were reported in ASPD and have previously been reported in patients with generalized anxiety disorder (151) and major depression disorder (152). Notwithstanding, reduced FA seems to be a viable marker for affective and social disorders. Another white matter tract implicated in psychopathy was the dorsal cingulum that connects posterior cingulate cortex to medial prefrontal cortex and is associated with social and emotional cognition (153). Decreased FA in this tract was associated with interpersonal-affective dimensions of psychopathy and emotional detachment. As similar findings have been reported in other psychiatric conditions such as post-traumatic stress disorder (154) and schizophrenia (155), reduced FA in the dorsal cingulum is also not specific for psychopathy. Moreover, Hoppenbrouwers and colleagues (85) suggest that a dysfunctional striato-thalamo-frontal network and mesolimbic reward system is present in psychopathy. Yoder, Porges, and Decety (101) postulate further in their tractography study that different psychopathic traits may arise from different parts of the highly specialized amygdala.
These findings are in accordance with the recently proposed Impaired Integration Theory (IIT) (156). The IIT attempts to integrate psychopathic manifestations, such as emotional detachment and impaired ability to incorporate perceived information into operant and contextual learning, with brain abnormalities inherent to psychopathy (156).
Interestingly, empathy-related regions in the brain were active in psychopathic subjects when imagining oneself in pain (53). However, when imagining others in pain, these areas were not active. This being said, psychopathic individuals appear not to lack the apparatus for empathy, yet they are evidently unable to simulate and understand the internal states of others. Moreover, the activation of ventral striatum in imagining others in pain might indicate that psychopathic individuals take pleasure in observing others in pain (53). Furthermore, the recognition of the affective mental states of others in psychopathy was attributed to decreased activity in the mirror neuron system (MNS) and increased activity in outcome-related regions (72, 81). A similar compensation mechanism for deficient empathy by engaging more cognitive areas of the brain was also seen in ASPD (138). Concisely, the MNS represents a mechanism by which the motor processes and representations of one individual displaying a motor function can be induced in another individual by merely observing the first individual (157). However, such a mimicry is likely insufficient to understand the emotions or actions of others (157), which is a complex cognitive process involving the Theory of Mind (ToM) comprising areas significantly overlapping with the default mode network (158). Dysfunction of the MNS has also been reported in autism spectrum disorders (159).
Psychopathic individuals display lack of empathy and affective cognition, and they might even be unconquerable by love. The mesolimbic reward system, together with limbic and paralimbic system, contribute to the feeling of romantic love (160). All these three systems were dysfunctional in psychopathy. In addition, according to a recent qualitative study of former spouses to psychopathic individuals per the PCL-R, the former spouses were repeatedly subjected to coercion, conning, and manipulation (161). We speculate that psychopathic individuals might not be capable of romantic love, based on the notion that love and desire are two neuroanatomically and fundamentally separate entities (160). Data on this topic are scarce, and the topic opens up interesting opportunities for future studies.
Also, intriguingly, aberrant cerebellar function and structure were reported in psychopathy. Beyond the cerebellum's traditional role in motor functions, an increasing amount of evidence indicates that the cerebellum has functions pertaining to emotional and cognitive control as well as morality (162–164). Schmahmann (165) posits that the cognitive and limbic functions of the cerebellum lie in the posterior lobe, in line with the findings in this review. Firstly, the posterior cerebellar lobe exhibited reduced activity in a moral judgment task in psychopathic subjects (114). Secondly, emotion recognition was associated with increased GMV in the posterior cerebellar lobe (76). Thirdly, reduced resting-state activity in the posterior cerebellum was found in ASPD subjects (131, 132). Moreover, lesion studies have shown that damage to the posterior cerebellar lobe can lead to deficient cognitive and affective information processing (166). We suggest that a deeper investigation into the role cerebellum in psychopathy is warranted and might result in new insights.
Only one of the imaging studies focused on a specific genotype and its relationship to ASPD (134). Twin studies suggest that heredity play a pivotal role in psychopathic traits across childhood (167–170), adolescence (171–175), and adulthood (172, 177, 178). Up to 70% of the variance in psychopathic traits may be attributable to genetics according to recent studies (177–180). However, the involved genes remain to be identified (180). One noteworthy candidate is the human serotonin transporter gene (SLC6A4) (181, 182). SLC6A4 manifests in two forms, and carriers of the short allele are predisposed to negative mental health aspects including anxiety, depression, substance use disorder, and suicide (181), whereas homozygosity of the long allele is associated with emotional detachment and psychopathic traits (182). Another candidate is the X-linked monoamine oxidase A (MAOA) gene and its high (MAOA-H) and low activity alleles (MAOA-L) (183). Individuals with absent or low acting MAOA are more prone to aggressive and impulsive behavior and exhibit higher psychopathic traits (184). Furthermore, identifying genes may reveal viable biomarkers for psychopathy. Recently psychopathy was also associated with upregulation of Ribosomal protein L10 Pseudogene 9 (RPL10P9), Zinc finger protein 132 (ZNF132), and downregulation of Cadherin-5 (CDH5) and Opioid receptor Delta 1 (OPRD1) genes, which explained 30% to 92% of the variance in psychopathic symptoms in a stem cell derived study by Tiihonen et al. (185). Identifying more genes and examining their relationship to brain structure and function might provide useful information of the neurobiological etiology of psychopathy. Some of the variance seen in genetic or proteomic studies might also be visualizable with modern or upcoming imaging techniques.
Discovering viable biomarkers for psychopathy is challenging. The results in this review suggest that psychopathy and ASPD might stem from dissimilar biological processes and show divergent neural correlates, yet antisociality and core features of psychopathy are clumped into one disorder. The hypothesis of divergent neural correlates explains not only why some heterogeneity was seen in neuroimaging results of psychopathy, but also why there were many similar anomalies in ASPD and psychopathy (Table 3). For example, Sato and colleagues (45) managed to discriminate psychopathic subjects from healthy controls based on gray matter changes, but the psychopathic subjects all had comorbid ASPD. Further, even though Sadeh et al. (119) did not find a correlation between core psychopathy and amygdalar hypoactivity, the researchers emphasize, however, that this finding is not in direct contradiction with the theory of amygdalar hypoactivity in psychopathy, but rather that it provides evidence of divergent neural correlates with respect to more general antisociality and core psychopathy. It has, however, also been suggested that ASPD is a subtype of psychopathy (186). Furthermore, these two conceptually dissimilar notions are occasionally used arbitrarily (187). Emphasizing both the inconsistent use of the terms and the dissimilarities between antisociality and psychopathy, ASPD has aptly been described as “a euphemism par excellence” [(188) p. 301]. Taking the aforesaid into consideration, it is difficult to reach high specificity for potential biomarkers for core psychopathy unless interpersonal-affective and lifestyle-antisocial dimensions are considered separately. To play with the thought and try to ensnare core psychopathy, the following combination of tests could be attempted. Firstly, a DTI showing reduced FA in the uncinate fasciculus, possibly accompanied by increased RD. Secondly, an fMRI with an affective mental state recognition or empathy-related task showing both an increase in activity in outcome and attention-related areas and a concomitant decrease in activity in the MNS and ToM.
There has been increasing interest to understand and discover the neural correlates of psychopathy during the past years. Although certain noteworthy patterns and neural correlates have frequently transpired, the neurobiological etiology of psychopathy remains obscure. Furthermore, the findings suggest that “successful” psychopathic individuals may not show similar structural gray matter changes as their “unsuccessful” counterparts. Consequently, if the single thing separating “successful” psychopathy from “unsuccessful” psychopathy is a criminal conviction, then a vast amount of neuroimaging data is yet to be obtained. The majority of the neuroimaging studies are conducted in forensic or prison-related settings, and these unsuccessful psychopathic individuals “represent only the tip of a very large iceberg” [(189) p. 115]. Therefore, focusing on non-clinical and community settings could facilitate the unraveling of the etiology of psychopathy.
This review has several strengths. Firstly, three MRI submodalities were included in this study. Secondly, neuroimaging studies of psychopathic traits in community and clinical settings were included in addition to forensic and prison populations. Thirdly, we included studies with both genders in this review. Fourthly, we strived to include a number of adolescence studies as well, as psychopathic traits manifest as a continuum from childhood to adulthood.
The qualitative synthesis was not without challenges. Firstly, a plethora of different tasks were seen in functional neuroimaging. These tasks needed to be grouped to be able to provide a coherent written summary. Furthermore, some compromise between the readability and high level of details needed to be made, although the Review Matrix contains findings in a more detailed level. Secondly, psychopathy and psychopathic traits have both various definitions and instruments to measure them. Including other instruments apart from the PCL-R can be seen both as a limitation and strength. On the one hand, this can hinder the generalizability of the results. On the other hand, more studies in various settings met the inclusion criteria due to this decision. Further, several of the non-PCL-R instruments are cross-validated with the PCL-R.
Perhaps the most challenging aspect of this review was taking into consideration the high comorbidity of the trait continuums of ASPD, CD, and psychopathy. These heritable disorders reflect independent structural and functional aberrancies in the brain, but also seem to manifest convergent biological processes to some extent. For example, both CD and ASPD are related to dysfunction of the default mode network (93, 130) and to decreased GMV in limbic and cortical regions (31, 128). Further, the said disorders are not mutually exclusive nor are they biologically dichotomic constructs. These confounders and the arbitrary use the notions of psychopathy and ASPD call for coherence and attentiveness in future research. Another comorbidity of note is substance abuse disorder, which damages the integrity of white matter (190, 191), and induces volumetric gray matter reductions (192, 193).
Another limitation of note is that this review focused on MRI submodalities. A review on PET, SPECT, and EEG could shed light on the abstruse neurobiological etiology of psychopathy, and even add support to our findings. The age criterion applied in this review comprises a limitation as it led to the exclusion of several studies. As such, psychopathic traits in childhood and adolescence may require a systematic literature review of their own. Moreover, notwithstanding the inclusion of females in this review, the majority of the studies were conducted on males. This warrants caution in generalizing the results and more research on female psychopathy. Further, it is paramount to mention that “the lack of longitudinal neuroimaging means that persistence of neural abnormalities can only be inferred, not investigated” as aptly put by Linder [(194) p. 68].
This systematic review sums that structural and functional aberrancies involving the limbic and paralimbic systems including reduced integrity of the uncinate fasciculus appear to be associated with core psychopathic features. A deeper investigation into the role of the cerebellum in psychopathy is also warranted and might result in new insights. Furthermore, the evidence suggests that ASPD and psychopathy stem from divergent biological processes. Still, more neuroimaging studies are warranted particularly with respect to female and community psychopathy.
All datasets generated for this study are included in the article/Supplementary Material.
MJ, ML, and JT conceived the presented idea. MJ wrote the draft of the manuscript and constructed the tables and figures. ML and JT provided critical feedback and helped shape the manuscript. ML and OV assisted MJ in reviewing the records as specified in the Materials and Methods section. MJ and ML calculated the Cohen's kappa.
This study was funded by the Finnish Ministry of Social Affairs and Health through a developmental fund for Niuvanniemi Hospital.
ML is a major share holder and board member at Genomi Solutions Ltd., a Finnish bioinformatics company. He has also received grants or honoraria from Sunovion Ltd. and Orion Pharma Ltd. and research scholarships from the Finnish Cultural Foundation and Finnish Medical Foundation. JT has received personal fees from the Finnish Medicines Agency (Fimea), AstraZeneca, Bristol-Meyers Squibb, Eli Lilly, F Hoffman-La Roche, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Novartis, Organon, Otsuka, and Pfizer; and has received grants from the Stanley Foundation, Sigrid Jusélius Foundation, Eli Lilly, and Janssen-Cilag.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We wish to thank Ms. Aija Räsänen for secretarial assistance and PhD Philip Lindner for his comments and insight.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.01027/full#supplementary-material
1. Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Psychopathic personality traits: heritability and genetic overlap with internalizing and externalizing psychopathology. Psychol Med (2005) 35(5):637–48. doi: 10.1017/S0033291704004180
2. Griffiths SY, Jalava JV. A comprehensive neuroimaging review of PCL-R defined psychopathy. Aggress Violent Behav (2017) 36:60–75. doi: 10.1016/j.avb.2017.07.002
3. Koenigs M, Baskin-Sommers A, Zeier J, Newman JP. Investigating the neural correlates of psychopathy: a critical review. Mol Psychiatry (2011) 16(8):792–9. doi: 10.1038/mp.2010.124
4. Santana EJ. The Brain of the Psychopath: A Systematic Review of Structural Neuroimaging Studies. Psychol Neurosci (2016) 9(4):420–43. doi: 10.1037/pne0000069
5. Hare R. The Hare Psychopathy Checklist-Revised. 2nd ed. Ontario, Toronto: Multi-Health Systems (2003).
6. Hare R. Psychopathy: A Clinical and Forensic Overview. Psychiatr Clinics North America (2006) 29(3):709–24. doi: 10.1016/j.psc.2006.04.007
7. Hare R, Neumann CS. Psychopathy: Assessment and Forensic Implications. Can J Psychiatry (2009) 54(12):791–802. doi: 10.1177/070674370905401202
8. Hemphälä M, Kosson D, Westerman J, Hodgins S. Stability and predictors of psychopathic traits from mid-adolescence through early adulthood. Scand J Psychol (2015) 56(1):649–58. doi: 10.1111/sjop.12257
9. Waller R, Dishion T, Shaw D, Gardner F, Wilson M, Hyde L. Does early childhood callous-unemotional behavior uniquely predict behavior problems or callous-unemotional behavior in late childhood? Dev Psychol (2016) 52(1):1805–19. doi: 10.1037/dev0000165
10. Coid J, Yang M, Ullrich S, Roberts A, Hare RD. Prevalence and correlates of psychopathic traits in the household population of Great Britain. Int J Law Psychiatry (2009) 32(2):65–73. doi: 10.1016/j.ijlp.2009.01.002
11. Neumann C, Hare R. Psychopathic traits in a large community sample: Links to violence, alcohol use, and intelligence. J Consult Clin Psychol (2008) 76(5):893–9. doi: 10.1037/0022-006X.76.5.893
12. Werner KB, Few LR, Bucholz KK. Epidemiology, Comorbidity, and Behavioral Genetics of Antisocial Personality Disorder and Psychopathy. Psychiatr Ann (2015) 45(4):195–9. doi: 10.3928/00485713-20150401-08
13. Babiak P, Neumann C, Hare R. Corporate psychopathy: Talking the walk. Behav Sci Law (2010) 28(1):174–93. doi: 10.1002/bsl.925
14. Koenigs M. The role of prefrontal cortex in psychopathy. Rev In Neurosci (2012) 23(3):253–62. doi: 10.1515/revneuro-2012-0036
15. Ogloff J. Psychopathy/antisocial personality disorder conundrum. Aust New Z J Psychiatry (2006) 40(1):519–28. doi: 10.1080/j.1440-1614.2006.01834.x
16. Lindner P, Budhiraja M, Westerman J, Savic I, Jokinen J, Tiihonen J, et al. White matter correlates of psychopathic traits in a female community sample. Soc Cogn Affect Neurosci (2017) 12(9):1500–10. doi: 10.1093/scan/nsx070
17. Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. J Abnormal Psychol (2010) 119(3):546–54. doi: 10.1037/a0019611
18. Poeppl TB, Donges MR, Mokros A, Rupprecht R, Fox PT, Laird AR, et al. A view behind the mask of sanity: meta-analysis of aberrant brain activity in psychopaths. Mol Psychiatry (2019) 24(3):463–70. doi: 10.1038/s41380-018-0122-5
19. Pujol J, Harrison BJ, Contreras-Rodriguez O, Cardoner N. The contribution of brain imaging to the understanding of psychopathy. Psychol Med (2019) 49(1):20–31. doi: 10.1017/S0033291718002507
20. Pridmore S, Chambers A, McArthur M. Neuroimaging in psychopathy. Aust New Z J Psychiatry (2005) 39(10):856–65. doi: 10.1111/j.1440-1614.2005.01679.x
21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
22. Baskin-Sommers AR, Neumann CS, Cope LM, Kiehl KA. Latent-variable modeling of brain gray-matter volume and psychopathy in incarcerated offenders. J Abnormal Psychol (2016) 125(6):811–7. doi: 10.1037/abn0000175
23. Bertsch K, Grothe M, Prehn K, Vohs K, Berger C, Hauenstein K. Brain volumes differ between diagnostic groups of violent criminal offenders. Eur Arch Psychiatry Clin Neurosci (2013) 263(7):593–606. doi: 10.1007/s00406-013-0391-6
24. Boccardi M, Ganzola R, Rossi R, Sabattoli F, Laakso MP, Repo-Tiihonen E, et al. Abnormal hippocampal shape in offenders with psychopathy. Hum Brain Mapp (2010) 31(3):438–47. doi: 10.1002/hbm.20877
25. Boccardi M, Frisoni GB, Hare RD, Cavedo E, Najt P, Pievani M, et al. Cortex and amygdala morphology in psychopathy. Psychiatry Res: Neuroimaging (2011) 193(2):85–92. doi: 10.1016/j.pscychresns.2010.12.013
26. Boccardi M, Bocchetta M, Aronen HJ, Repo-Tiihonen E, Vaurio O, Thompson PM, et al. Atypical nucleus accumbens morphology in psychopathy: Another limbic piece in the puzzle. Int J Law Psychiatry (2013) 36(2):157–67. doi: 10.1016/j.ijlp.2013.01.008
27. Contreras-Rodríguez O, Pujol J, Batalla I, Harrison BJ, Soriano-Mas C, Deus J, et al. Functional Connectivity Bias in the Prefrontal Cortex of Psychopaths. Biol Psychiatry (2015) 78(9):647–55. doi: 10.1016/j.biopsych.2014.03.007
28. Cope LM, Shane MS, Segall JM, Nyalakanti PK, Stevens MC, Pearlson GD, et al. Examining the effect of psychopathic traits on gray matter volume in a community substance abuse sample. Psychiatry Res: Neuroimaging (2012) 204(2–3):91–100. doi: 10.1016/j.pscychresns.2012.10.004
29. de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Azevedo Ignácio F, Tovar-Moll F, et al. Psychopathy as a disorder of the moral brain: Fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. NeuroImage (2008) 40(3):1202–13. doi: 10.1016/j.neuroimage.2007.12.054
30. Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. J Abnormal Psychol (2012) 121(3):649–58. doi: 10.1037/a0026371
31. Fairchild G, Passamonti L, Hurford G, Hagan CC, Von Dem Hagen EAH, Van Goozen SHM, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am J Psychiatry (2011) 168(6):624–33. doi: 10.1176/appi.ajp.2010.10081184
32. Glenn AL, Raine A, Yaralian PS, Yang Y. Increased Volume of the Striatum in Psychopathic Individuals. Biol Psychiatry (2010) 67(1):52–8. doi: 10.1016/j.biopsych.2009.06.018
33. Gregory S, Ffytche D, Simmons A, Kumari V, Howard M, Hodgins S, et al. The Antisocial Brain: Psychopathy Matters. Arch Gen Psychiatry (2012) 69(9):962. doi: 10.1001/archgenpsychiatry.2012.222
34. Howner K, Eskildsen SF, Fischer H, Dierks T, Wahlund L-O, Jonsson T, et al. Thinner cortex in the frontal lobes in mentally disordered offenders. Psychiatry Res: Neuroimaging (2012) 203(2–3):126–31. doi: 10.1016/j.pscychresns.2011.12.011
35. Korponay C, Pujara M, Deming P, Philippi C, Decety J, Kosson DS, et al. Impulsive-Antisocial Dimension of Psychopathy Linked to Enlargement and Abnormal Functional Connectivity of the Striatum. Biol Psychiatry: Cogn Neurosci Neuroimaging (2017a) 2(2):149–57. doi: 10.1016/j.bpsc.2016.07.004
36. Korponay C, Pujara M, Deming P, Philippi C, Decety J, Kosson DS, et al. Impulsive-antisocial psychopathic traits linked to increased volume and functional connectivity within prefrontal cortex. Soc Cogn Affect Neurosci (2017b) 12(7):1169–78. doi: 10.1093/scan/nsx042
37. Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatry Res: Neuroimaging (2002) 114(2):95–102. doi: 10.1016/S0925-4927(02)00005-7
38. Leutgeb V, Leitner M, Wabnegger A, Klug D, Scharmüller W, Zussner T, et al. Brain abnormalities in high-risk violent offenders and their association with psychopathic traits and criminal recidivism. Neuroscience (2015) 308:194–201. doi: 10.1016/j.neuroscience.2015.09.011
39. Ly M, Motzkin JC, Philippi CL, Kirk GR, Newman JP, Kiehl KA, et al. Cortical Thinning in Psychopathy. Am J Psychiatry (2012) 169(7):743–9. doi: 10.1176/appi.ajp.2012.11111627
40. Miskovich TA, Anderson NE, Harenski CL, Harenski KA, Baskin-Sommers AR, Larson CL, et al. Abnormal cortical gyrification in criminal psychopathy. NeuroImage: Clin (2018) 19:876–82. doi: 10.1016/j.nicl.2018.06.007
41. Müller JL, Gänßbauer S, Sommer M, Döhnel K, Weber T, Schmidt-Wilcke T, et al. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evid Voxel-Based Morphometry Psychiatry Res: Neuroimaging (2008) 163(3):213–22. doi: 10.1016/j.pscychresns.2007.08.010
42. Pardini DA, Raine A, Erickson K, Loeber R. Lower Amygdala Volume in Men is Associated with Childhood Aggression, Early Psychopathic Traits, and Future Violence. Biol Psychiatry (2014) 75(1):73–80. doi: 10.1016/j.biopsych.2013.04.003
43. Raine LT, Taylor K, Hellige JB, Bihrle S, Lacasse L, Colletti P. Corpus callosum abnormalities in psychopathic antisocial individuals. Arch Gen Psychiatry (2003) 60(11):1134–42. doi: 10.1001/archpsyc.60.11.1134
44. Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, et al. Hippocampal structural asymmetry in unsuccessful psychopaths. Biol Psychiatry (2004) 55(2):185–91. doi: 10.1016/S0006-3223(03)00727-3
45. Sato JR, de Oliveira-Souza R, Thomaz CE, Basílio R, Bramati IE, Amaro E, et al. Identification of psychopathic individuals using pattern classification of MRI images. Soc Neurosci (2011) 6(5–6):627–39. doi: 10.1080/17470919.2011.562687
46. Tiihonen J, Rossi R, Laakso MP, Hodgins S, Testa C, Perez J, et al. Brain anatomy of persistent violent offenders: More rather than less. Psychiatry Res - Neuroimaging (2008) 163(3):201–12. doi: 10.1016/j.pscychresns.2007.08.012
47. Vieira JB, Ferreira-Santos F, Almeida PR, Barbosa F, Marques-Teixeira J, Marsh AA. Psychopathic traits are associated with cortical and subcortical volume alterations in healthy individuals. Soc Cogn Affect Neurosci (2015) 10(12):1693–704. doi: 10.1093/scan/nsv062
48. Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of Deformations Within the Amygdala in Individuals With Psychopathy. Arch Gen Psychiatry (2009) 66(9):986. doi: 10.1001/archgenpsychiatry.2009.110
49. Anderson NE, Steele VR, Maurer JM, Rao V, Koenigs MR, Decety J, et al. Differentiating emotional processing and attention in psychopathy with functional neuroimaging. Cogn Affect Behav Neurosci (2017) 17(3):491–515. doi: 10.3758/s13415-016-0493-5
50. Bjork JM, Chen G, Hommer DW. Psychopathic tendencies and mesolimbic recruitment by cues for instrumental and passively obtained rewards. Biol Psychol (2012) 89(2):408–15. doi: 10.1016/j.biopsycho.2011.12.003
51. Contreras-Rodríguez O, Pujol J, Batalla I, Harrison BJ, Bosque J, Ibern-Regàs I, et al. Disrupted neural processing of emotional faces in psychopathy. Soc Cogn Affect Neurosci (2014) 9(4):505–12. doi: 10.1093/scan/nst014
52. Cope LM, Vincent GM, Jobelius JL, Nyalakanti PK, Calhoun VD, Kiehl KA. Psychopathic traits modulate brain responses to drug cues in incarcerated offenders. Front In Hum Neurosci (2014) 8:1–16. doi: 10.3389/fnhum.2014.00087
53. Decety J, Chen C, Harenski C, Kiehl KA. An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Front In Hum Neurosci (2013) 7:1–12. doi: 10.3389/fnhum.2013.00489
54. Decety J, Skelly LR, Kiehl KA. Brain Response to Empathy-Eliciting Scenarios Involving Pain in Incarcerated Individuals With Psychopathy. JAMA Psychiatry (2013) 70(6):638. doi: 10.1001/jamapsychiatry.2013.27
55. Decety J, Skelly L, Yoder KJ, Kiehl KA. Neural processing of dynamic emotional facial expressions in psychopaths. Soc Neurosci (2014) 9(1):36–49. doi: 10.1080/17470919.2013.866905
56. Deeley Q, Daly E, Surguladze S, Tunstall N, Mezey G, Beer D, et al. Facial emotion processing in criminal psychopathy. Br J Psychiatry (2006) 189(06):533–9. doi: 10.1192/bjp.bp.106.021410
57. Deming P, Philippi CL, Wolf RC, Dargis M, Kiehl KA, Koenigs M. Psychopathic traits linked to alterations in neural activity during personality judgments of self and others. NeuroImage: Clin (2018) 18:575–81. doi: 10.1016/j.nicl.2018.02.029
58. Ewbank MP, Passamonti L, Hagan CC, Goodyer IM, Calder AJ, Fairchild G. Psychopathic traits influence amygdala–anterior cingulate cortex connectivity during facial emotion processing. Soc Cogn Affect Neurosci (2018) 13(5):525–34. doi: 10.1093/scan/nsy019
59. Fede SJ, Borg JS, Nyalakanti PK, Harenski CL, Cope LM, Sinnott-Armstrong W, et al. Distinct neuronal patterns of positive and negative moral processing in psychopathy. Cogn Affect Behav Neurosci (2016) 16(6):1074–85. doi: 10.3758/s13415-016-0454-z
60. Geurts DEM, von Borries K, Volman I, Bulten BH, Cools R, Verkes R-J. Neural connectivity during reward expectation dissociates psychopathic criminals from non-criminal individuals with high impulsive/antisocial psychopathic traits. Soc Cogn Affect Neurosci (2016) 11(8):1326–34. doi: 10.1093/scan/nsw040
61. Glenn AL, Han H, Yang Y, Raine A, Schug RA. Associations between psychopathic traits and brain activity during instructed false responding. Psychiatry Res: Neuroimaging (2017) 266:123–37. ((Glenn A.L.,YWxnbGVubjFAdWEuZWR1) Center for the Prevention of Youth Behavior Problems, Department of Psychology, University of Alabama, Tuscaloosa, AL, United States). doi: 10.1016/j.pscychresns.2017.06.008
62. Gregory S, Blair RJ, Ffytche D, Simmons A, Kumari V, Hodgins S, et al. Punishment and psychopathy: a case-control functional MRI investigation of reinforcement learning in violent antisocial personality disordered men. Lancet Psychiatry (2015) 2(2):153–60. doi: 10.1016/S2215-0366(14)00071-6
63. Harenski K, Shane M, Kiehl K. Aberrant neural processing of moral violations in criminal psychopaths. J Abnormal Psychol (2010) 119(4):863–74. doi: 10.1037/a0020979
64. Harenski C, Edwards B, Harenski K, Kiehl K. Neural correlates of moral and non-moral emotion in female psychopathy. Front In Hum Neurosci (2014) 8:741. doi: 10.3389/fnhum.2014.00741
65. Hosking JG, Kastman EK, Dorfman HM, Samanez-Larkin GR, Baskin-Sommers A, Kiehl KA, et al. Disrupted Prefrontal Regulation of Striatal Subjective Value Signals in Psychopathy. Neuron (2017) 95(1):221–231.e4. doi: 10.1016/j.neuron.2017.06.030
66. Hyde LW, Byrd AL, Votruba-Drzal E, Hariri AR, Manuck SB. Amygdala reactivity and negative emotionality: Divergent correlates of antisocial personality and psychopathy traits in a community sample. J Abnormal Psychol (2014) 123(1):214–24. doi: 10.1037/a0035467
67. Juárez M, Kiehl KA, Calhoun VD. Intrinsic limbic and paralimbic networks are associated with criminal psychopathy. Hum Brain Mapp (2013) 34(8):1921–30. doi: 10.1002/hbm.22037
68. Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry (2001) 50(9):677–684. doi: 10.1016/S0006-3223(01)01222-7
69. Larson CL, Baskin-Sommers AR, Stout DM, Balderston NL, Curtin JJ, Schultz DH, et al. The interplay of attention and emotion: top-down attention modulates amygdala activation in psychopathy. Cogn Affect Behav Neurosci (2013) 13(4):757–70. doi: 10.3758/s13415-013-0172-8
70. Lindner P, Flodin P, Budhiraja M, Savic I, Jokinen J, Tiihonen J, et al. Associations of Psychopathic Traits With Local and Global Brain Network Topology in Young Adult Women. Biol Psychiatry: Cogn Neurosci Neuroimaging (2018) 3(12):1003–12. doi: 10.1016/j.bpsc.2018.04.010
71. Marsh AA, Cardinale EM. When psychopathy impairs moral judgments: neural responses during judgments about causing fear. Soc Cogn Affect Neurosci (2014) 9(1):3–11. doi: 10.1093/scan/nss097
72. Mier D, Haddad L, Diers K, Dressing H, Meyer-Lindenberg A, Kirsch P. Reduced embodied simulation in psychopathy. World J Biol Psychiatry (2014) 15(6):479–87. doi: 10.3109/15622975.2014.902541
73. Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced Prefrontal Connectivity in Psychopathy. J Neurosci (2011) 31(48):17348–57. doi: 10.1523/JNEUROSCI.4215-11.2011
74. Müller JL, Sommer M, Wagner V, Lange K, Taschler H, Röder CH, et al. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths. Biol Psychiatry (2003) 54(2):152–62. doi: 10.1016/S0006-3223(02)01749-3
75. Osumi T, Nakao T, Kasuya Y, Shinoda J, Yamada J, Ohira H. Amygdala dysfunction attenuates frustration-induced aggression in psychopathic individuals in a non-criminal population. J Affect Disord (2012) 142(1–3):331–8. doi: 10.1016/j.jad.2012.05.012
76. Pera-Guardiola V, Contreras-Rodríguez O, Batalla I, Kosson D, Menchón JM, Pifarré J, et al. Brain structural correlates of emotion recognition in psychopaths. PloS One (2016) 11(5):e0149807. doi: 10.1371/journal.pone.0149807
77. Philippi CL, Pujara MS, Motzkin JC, Newman J, Kiehl KA, Koenigs M. Altered Resting-State Functional Connectivity in Cortical Networks in Psychopathy. J Neurosci (2015) 35(15):6068–78. doi: 10.1523/JNEUROSCI.5010-14.2015
78. Pujara M, Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Neural correlates of reward and loss sensitivity in psychopathy. Soc Cogn Affect Neurosci (2014) 9(6):794–801. doi: 10.1093/scan/nst054
79. Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, et al. Neural Correlates of Social Cooperation and Non-Cooperation as a Function of Psychopathy. Biol Psychiatry (2007) 61(11):1260–71. doi: 10.1016/j.biopsych.2006.07.021
80. Shao R, Lee TMC. Are individuals with higher psychopathic traits better learners at lying? Behavioural and neural evidence. Trans Psychiatry (2017) 7(7):e1175. doi: 10.1038/tp.2017.147
81. Sommer M, Sodian B, Döhnel K, Schwerdtner J, Meinhardt J, Hajak G. In psychopathic patients emotion attribution modulates activity in outcome-related brain areas. Psychiatry Res: Neuroimaging (2010) 182(2):88–95. doi: 10.1016/j.pscychresns.2010.01.007
82. Vieira JB, Almeida PR, Ferreira-Santos F, Barbosa F, Marques-Teixeira J, Marsh AA. Distinct neural activation patterns underlie economic decisions in high and low psychopathy scorers. Soc Cogn Affect Neurosci (2014) 9(8):1099–107. doi: 10.1093/scan/nst093
83. Yoder K, Porges E, Decety J. Amygdala subnuclei connectivity in response to violence reveals unique influences of individual differences in psychopathic traits in a nonforensic sample. Hum Brain Mapp (2015) 36(4):1417–28. doi: 10.1002/hbm.22712
84. Zijlmans J, Marhe R, Bevaart F, Luijks M-JA, van Duin L, Tiemeier H, et al. Neural Correlates of Moral Evaluation and Psychopathic Traits in Male Multi-Problem Young Adults. Front In Psychiatry (2018) 9:248. doi: 10.3389/fpsyt.2018.00248
85. Hoppenbrouwers SS, Nazeri A, de Jesus DR, Stirpe T, Felsky D, Schutter DJLG, et al. White Matter Deficits in Psychopathic Offenders and Correlation with Factor Structure. PloS One (2013) 8(8):e72375. doi: 10.1371/journal.pone.0072375
86. Sethi A, Gregory S, Dell'Acqua F, Periche Thomas E, Simmons A, Murphy DGM, et al. Emotional detachment in psychopathy: Involvement of dorsal default-mode connections. Cortex (2015) 62:11–9. doi: 10.1016/j.cortex.2014.07.018
87. Sobhani M, Baker L, Martins B, Tuvblad C, Aziz-Zadeh L. Psychopathic traits modulate microstructural integrity of right uncinate fasciculus in a community population. NeuroImage: Clin (2015) 8:32–8. doi: 10.1016/j.nicl.2015.03.012
88. Sundram F, Deeley Q, Sarkar S, Daly E, Latham R, Craig M, et al. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex (2012) 48(2):216–29. doi: 10.1016/j.cortex.2011.06.005
89. Wolf RC, Pujara MS, Motzkin JC, Newman JP, Kiehl KA, Decety J, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp (2015) 36(10):4202–9. doi: 10.1002/hbm.22911
90. Cohn MD, Viding E, McCrory E, Pape L, van den Brink W, Doreleijers TAH, et al. Regional grey matter volume and concentration in at-risk adolescents: Untangling associations with callous-unemotional traits and conduct disorder symptoms. Psychiatry Res: Neuroimaging (2016) 254:180–7. doi: 10.1016/j.pscychresns.2016.07.003
91. Lam BHY, Yang Y, Schug RA, Han C, Liu J, Lee TMC. Psychopathy moderates the relationship between orbitofrontal and striatal alterations and violence: the investigation of Individuals accused of homicide. Front Hum Neurosci (2017) 579(11):1–11. doi: 10.3389/fnhum.2017.00579
92. Anderson NE, Maurer JM, Steele VR, Kiehl KA. Psychopathic traits associated with abnormal hemodynamic activity in salience and default mode networks during auditory oddball task. Cogn Affect Behav Neurosci (2018) 18(3):564–80. doi: 10.3758/s13415-018-0588-2
93. Cohn MD, Pape LE, Schmaal L, van den Brink W, van Wingen G, Vermeiren RRJM, et al. Differential relations between juvenile psychopathic traits and resting state network connectivity. Hum Brain Mapp (2015) 36(6):2396–405. doi: 10.1002/hbm.22779
94. Freeman SM, Clewett DV, Bennett CM, Kiehl KA, Gazzaniga MS, Miller MB. The posteromedial region of the default mode network shows attenuated task-induced deactivation in psychopathic prisoners. Neuropsychology (2015) 29(3):493–500. doi: 10.1037/neu0000118
95. Fullam RS, McKie S, Dolan MC. Psychopathic traits and deception: functional magnetic resonance imaging study. Br J Psychiatry (2009) 194(03):229–35. doi: 10.1192/bjp.bp.108.053199
96. Murray L, Shaw DS, Forbes EE, Hyde LW. Reward-Related Neural Correlates of Antisocial Behavior and Callous–Unemotional Traits in Young Men. Biol Psychiatry: Cogn Neurosci Neuroimaging (2017) 2(4):346–54. doi: 10.1016/j.bpsc.2017.01.009
97. Schiffer B, Pawliczek C, Müller B, Forsting M, Gizewski E, Leygraf N, et al. Neural mechanisms underlying cognitive control of men with lifelong antisocial behavior. Psychiatry Res: Neuroimaging (2014) 222(1–2):43–51. doi: 10.1016/j.pscychresns.2014.01.008
98. Seara-Cardoso A, Viding E, Lickley RA, Sebastian CL. Neural responses to others' pain vary with psychopathic traits in healthy adult males. Cogn Affect Behav Neurosci (2015) 15(3):578–88. doi: 10.3758/s13415-015-0346-7
99. Seara-Cardoso A, Sebastian CL, McCrory E, Foulkes L, Buon M, Roiser JP, et al. Anticipation of guilt for everyday moral transgressions: The role of the anterior insula and the influence of interpersonal psychopathic traits. Sci Rep (2016) 6:1–10. doi: 10.1038/srep36273
100. Vieira JB, Tavares TP, Marsh AA, Mitchell DGV. Emotion and personal space: neural correlates of approach-avoidance tendencies to different facial expressions as a function of coldhearted psychopathic traits. Hum Brain Mapp (2017) 38(3):1492–506. doi: 10.1002/hbm.23467
101. Yoder K, Harenski C, Kiehl K, Decety J. Neural networks underlying implicit and explicit moral evaluations in psychopathy. Trans Psychiatry (2015) 5(8):e625–5. doi: 10.1038/tp.2015.117
102. Pape LE, Cohn MD, Caan MWA, van Wingen G, van den Brink W, Veltman DJ, et al. Psychopathic traits in adolescents are associated with higher structural connectivity. Psychiatry Res: Neuroimaging (2015) 233(3):474–80. doi: 10.1016/j.pscychresns.2015.07.023
103. Raichle ME. The Brain's Default Mode Network. Annu Rev Neurosci (2015) 38(1):433–47. doi: 10.1146/annurev-neuro-071013-014030
104. Kjaer TW, Nowak M, Lou HC. Reflective Self-Awareness and Conscious States: PET Evidence for a Common Midline Parietofrontal Core. NeuroImage (2002) 17(2):1080–6. doi: 10.1006/nimg.2002.1230
105. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci (2001) 98(7):4259–64. doi: 10.1073/pnas.071043098
106. Greene J, Haidt J. How (and where) does moral judgment work? Trends In Cogn Sci (2002) 6(12):517–23. doi: 10.1016/S1364-6613(02)02011-9
107. Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, et al. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci (2008) 105(28):9781–6. doi: 10.1073/pnas.0711791105
108. Xia M, Wang J, He Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PloS One (2013) 8(7):e68910. doi: 10.1371/journal.pone.0068910
109. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage (2002) 15(1):273–89. doi: 10.1006/nimg.2001.0978
110. Kolla NJ, Gregory S, Attard S, Blackwood N, Hodgins S. Disentangling possible effects of childhood physical abuse on gray matter changes in violent offenders with psychopathy. Psychiatry Res: Neuroimaging (2014) 221(2):123–6. doi: 10.1016/j.pscychresns.2013.11.008
111. Rolls ET. Limbic systems for emotion and for memory, but no single limbic system. Cortex (2015) 62:119–57. doi: 10.1016/j.cortex.2013.12.005
112. Glenn AL, Yang Y, Raine A, Colletti P. No volumetric differences in the anterior cingulate of psychopathic individuals. Psychiatry Res: Neuroimaging (2010) 183(2):140–3. doi: 10.1016/j.pscychresns.2010.05.009
113. Beckwith TJ, Dietrich KN, Wright JP, Altaye M, Cecil KM. Reduced regional volumes associated with total psychopathy scores in an adult population with childhood lead exposure. NeuroToxicology (2018) 67:1–26. doi: 10.1016/j.neuro.2018.04.004
114. Pujol J, Batalla I, Contreras-Rodríguez O, Harrison BJ, Pera V, Hernández-Ribas R, et al. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Soc Cogn Affect Neurosci (2012) 7(8):917–23. doi: 10.1093/scan/nsr075
115. Sheng T, Gheytanchi A, Aziz-Zadeh L. Default network deactivations are correlated with psychopathic personality traits. PloS One (2010) 5(9):1–7. doi: 10.1371/journal.pone.0012611
116. Volman I, Katinka Louise von Borries A, Hendrik Bulten B, Jan Verkes R, Toni I, Roelofs K. (2016). Testosterone Modulates Altered Prefrontal Control of Emotional Actions in Psychopathic Offenders. ENeuro 3(1). doi: 10.1523/ENEURO.0107-15.2016
117. Decety J, Chen C, Harenski CL, Kiehl KA. Socioemotional processing of morally-laden behavior and their consequences on others in forensic psychopaths. Hum Brain Mapp (2015) 36(6):2015–26. doi: 10.1002/hbm.22752
118. Han T, Alders GL, Greening SG, Neufeld RWJ, Mitchell DGV. Do fearful eyes activate empathy-related brain regions in individuals with callous traits? Soc Cogn Affect Neurosci (2012) 7(8):958–68. doi: 10.1093/scan/nsr068
119. Sadeh N, Spielberg JM, Heller W, Herrington JD, Engels AS, Warren SL, et al. Emotion disrupts neural activity during selective attention in psychopathy. Soc Cogn Affect Neurosci (2013) 8(3):235–46. doi: 10.1093/scan/nsr092
120. Reniers RLEP, Corcoran R, Völlm BA, Mashru A, Howard R, Liddle PF. Moral decision-making, ToM, empathy and the default mode network. Biol Psychol (2012) 90(3):202–10. doi: 10.1016/j.biopsycho.2012.03.009
121. Molenberghs P, Bosworth R, Nott Z, Louis WR, Smith JR, Amiot CE, et al. The influence of group membership and individual differences in psychopathy and perspective taking on neural responses when punishing and rewarding others. Hum Brain Mapp (2014) 35(10):4989–99. doi: 10.1002/hbm.22527
122. Sitaram R, Caria A, Veit R, Gaber T, Ruiz S, Birbaumer N. Volitional control of the anterior insula in criminal psychopaths using real-time fMRI neurofeedback: a pilot study. Front In Behav Neurosci (2014) 8:344. doi: 10.3389/fnbeh.2014.00344
123. Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci (2010) 13(4):419–21. doi: 10.1038/nn.2510
124. Lindner P, Savic I, Ritnikov R, Budhiraja M, Liu Y, Jokinen J, et al. Conduct disorder in females is associated with reduced corpus callosum structural integrity independent of comorbid disorders and exposure to maltreatment. Trans Psychiatry (2016) 6(1):e714–4. doi: 10.1038/tp.2015.216
125. Jiang W, Li G, Liu H, Shi F, Wang T, Shen C, et al. Reduced cortical thickness and increased surface area in antisocial personality disorder. Neuroscience (2016) 337:143–52. ((Jiang W.; Liu H.; Wang W.,Y2pyLndhbmd3ZWlAdmlwLjE2My5jb20=) Department of Radiology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China). doi: 10.1016/j.neuroscience.2016.08.052
126. Kumari V, Uddin S, Premkumar P, Young S, Gudjonsson GH, Raghuvanshi S, et al. Lower anterior cingulate volume in seriously violent men with antisocial personality disorder or schizophrenia and a history of childhood abuse. Aust New Z J Psychiatry (2014) 48(2):153–61. doi: 10.1177/0004867413512690
127. Narayan VM, Narr KL, Kumari V, Woods RP, Thompson PM, Toga AW, et al. Regional Cortical Thinning in Subjects With Violent Antisocial Personality Disorder or Schizophrenia. Am J Psychiatry (2007) 164(9):1418–27. doi: 10.1176/appi.ajp.2007.06101631
128. Raine A, Yang Y, Narr K. Sex differences in orbitofrontal gray as a partial explanation for sex differences in antisocial personality. Mol Psychiatry (2011) 16:227–236. doi: 10.1038/mp.2009.136
129. Kumari V, Gudjonsson G, Rahguvanshi S, Barkataki I, Taylor P, Sumich A, et al. Reduced thalamic volume in men with antisocial personality disorder or schizophrenia and a history of serious violence and childhood abuse. Eur Psychiatry (2013) 28(4):225–34. doi: 10.1016/j.eurpsy.2012.03.002
130. Tang Y, Liu W, Chen J, Liao J, Hu D, Wang W. Altered spontaneous activity in antisocial personality disorder revealed by regional homogeneity. NeuroReport (2013) 24(11):590–5. doi: 10.1097/WNR.0b013e3283627993
131. Tang Y, Jiang W, Liao J, Wang W, Luo A. Identifying Individuals with Antisocial Personality Disorder Using Resting-State fMRI. PloS One (2013) 8(4):e60652. doi: 10.1371/journal.pone.0060652
132. Liu H, Liao J, Jiang W, Wang W. Changes in low-frequency fluctuations in patients with antisocial personality disorder revealed by resting-state functional MRI. PloS One (2014) 9(3):1–6. doi: 10.1371/journal.pone.0089790
133. Kolla NJ, Dunlop K, Downar J, Links P, Michael Bagby R, Wilson AA, et al. Association of ventral striatum monoamine oxidase-A binding and functional connectivity in antisocial personality disorder with high impulsivity: A positron emission tomography and functional magnetic resonance imaging study. Eur Neuropsychopharmacol (2016) 26(4):777–86. doi: 10.1016/j.euroneuro.2015.12.030
134. Kolla NJ, Dunlop K, Meyer JH, Downar J. Corticostriatal Connectivity in Antisocial Personality Disorder by MAO-A Genotype and Its Relationship to Aggressive Behavior. Int J Neuropsychopharmacol (2018) 21(8):725–33. doi: 10.1093/ijnp/pyy035
135. Kumari V, Aasen I, Taylor P, Ffytche DH, Das M, Barkataki I, et al. Neural dysfunction and violence in schizophrenia: An fMRI investigation. Schizophr Res (2006) 84(1):144–64. doi: 10.1016/j.schres.2006.02.017
136. Barkataki I, Kumari V, Das M, Sumich A, Taylor P, Sharma T. Neural correlates of deficient response inhibition in mentally disordered violent individuals. Behav Sci Law (2008) 26(1):51–64. doi: 10.1002/bsl.787
137. Bobes MA, Ostrosky F, Diaz K, Romero C, Borja K, Santos Y, et al. Linkage of functional and structural anomalies in the left amygdala of reactive-aggressive men. Soc Cogn Affect Neurosci (2013) 8(8):928–36. doi: 10.1093/scan/nss101
138. Schiffer B, Pawliczek C, Müller BW, Wiltfang J, Brüne M, Forsting M, et al. Neural Mechanisms Underlying Affective Theory of Mind in Violent Antisocial Personality Disorder and/or Schizophrenia. Schizophr Bull (2017) 43(6):1229–39. doi: 10.1093/schbul/sbx012
139. Jiang W, Shi F, Liu H, Li G, Ding Z, Shen H, et al. Reduced White Matter Integrity in Antisocial Personality Disorder: A Diffusion Tensor Imaging Study. Sci Rep (2017) 7:43002. doi: 10.1038/srep43002
140. Budhiraja M, Savic I, Lindner P, Jokinen J, Tiihonen J, Hodgins S. Brain structure abnormalities in young women who presented conduct disorder in childhood/adolescence. Cogn Affect Behav Neurosci (2017) 17(4):869–85. doi: 10.3758/s13415-017-0519-7
141. Holz NE, Boecker-Schlier R, Buchmann AF, Blomeyer D, Jennen-Steinmetz C, Baumeister S, et al. Ventral striatum and amygdala activity as convergence sites for early adversity and conduct disorder. Soc Cogn Affect Neurosci (2017) 12(2):261–72. doi: 10.1093/scan/nsw120
142. Passamonti L, Fairchild G, Fornito A, Goodyer IM, Nimmo-Smith I, Hagan CC, et al. Abnormal Anatomical Connectivity between the Amygdala and Orbitofrontal Cortex in Conduct Disorder. PloS One (2012) 7(11):e48789. doi: 10.1371/journal.pone.0048789
143. Giesel FL, Thomann PA, Hahn HK, Politi M, Stieltjes B, Weber M-A, et al. Comparison of manual direct and automated indirect measurement of hippocampus using magnetic resonance imaging. Eur J Radiol (2008) 66(2):268–73. doi: 10.1016/j.ejrad.2007.06.009
144. Macdonald KE, Bartlett JW, Leung KK, Ourselin S, Barnes J. The Value of Hippocampal and Temporal Horn Volumes and Rates of Change in Predicting Future Conversion to AD. Alzheimer Dis Assoc Disord (2013) 27(2):168–73. doi: 10.1097/WAD.0b013e318260a79a
145. Chance SA, Esiri MM, Crow TJ. Ventricular enlargement in schizophrenia: a primary change in the temporal lobe? Schizophr Res (2003) 62(1–2):123–31. doi: 10.1016/S0920-9964(02)00344-4
146. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci (2003) 100(1):253–8. doi: 10.1073/pnas.0135058100
147. Ostby Y, Walhovd KB, Tamnes CK, Grydeland H, Westlye LT, Fjell AM. Mental time travel and default-mode network functional connectivity in the developing brain. Proc Natl Acad Sci (2012) 109(42):16800–4. doi: 10.1073/pnas.1210627109
148. Jiang W, Shi F, Liao J, Liu H, Wang T, Shen C, et al. Disrupted functional connectome in antisocial personality disorder. Brain Imaging Behav (2017) 11(4):1071–84. doi: 10.1007/s11682-016-9572-z
149. Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc B: Biol Sci (2008) 363(1503):2557–65. doi: 10.1098/rstb.2008.0027
150. Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends In Cogn Sci (2007) 11(9):387–92. doi: 10.1016/j.tics.2007.07.003
151. Tromp DPM, Grupe DW, Oathes DJ, McFarlin DR, Hernandez PJ, Kral TRA, et al. Reduced Structural Connectivity of a Major Frontolimbic Pathway in Generalized Anxiety Disorder. Arch Gen Psychiatry (2012) 69(9):925. doi: 10.1001/archgenpsychiatry.2011.2178
152. de Kwaasteniet B, Ruhe E, Caan M, Rive M, Olabarriaga S, Groefsema M, et al. Relation Between Structural and Functional Connectivity in Major Depressive Disorder. Biol Psychiatry (2013) 74(1):40–7. doi: 10.1016/j.biopsych.2012.12.024
153. Catani M, Thiebault de Schotten M. Atlas of human brain connections. Oxford: Oxford University Press (2012). doi: 10.1093/med/9780199541164.001.0001
154. Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: A diffusion tensor imaging study. Psychiatry Res: Neuroimaging (2013) 214(3):260–8. doi: 10.1016/j.pscychresns.2013.09.002
155. Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, et al. Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophr Res (2009) 114(1–3):119–27. doi: 10.1016/j.schres.2009.05.012
156. Hamilton RKB, Hiatt Racer K, Newman JP. Impaired integration in psychopathy: A unified theory of psychopathic dysfunction. psychol Rev (2015) 122(4):770–91. doi: 10.1037/a0039703
157. Rizzolatti G, Sinigaglia C. The mirror mechanism: a basic principle of brain function. Nat Rev Neurosci (2016) 17(12):757–65. doi: 10.1038/nrn.2016.135
158. de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary Systems for Understanding Action Intentions. Curr Biol (2008) 18(6):454–7. doi: 10.1016/j.cub.2008.02.057
159. Baird AD, Scheffer IE, Wilson SJ. Mirror neuron system involvement in empathy: A critical look at the evidence. Soc Neurosci (2011) 6(4):327–35. doi: 10.1080/17470919.2010.547085
160. Diamond LM, Dickenson JA. The neuroimaging of love and desire: review and future directions. Clin Neuropsychiatry (2012) 9(1):39–46.
161. Leedom LJ, Geislin E, Hartoonian Almas L. “Did he ever love me?” A qualitative study of life with a psychopathic husband. Family Intimate Partner Violence Q (2012) 5(2):103–35.
162. Balsters JH, Whelan CD, Robertson IH, Ramnani N. Cerebellum and Cognition: Evidence for the Encoding of Higher Order Rules. Cereb Cortex (2013) 23(6):1433–43. doi: 10.1093/cercor/bhs127
163. Demirtas-Tatlidede A, Schmahmann JD. Morality: incomplete without the cerebellum? Brain (2013) 136(8):e244–4. doi: 10.1093/brain/awt070
164. Schutter DJLG, van Honk J. The Cerebellum in Emotion Regulation: A Repetitive Transcranial Magnetic Stimulation Study. Cerebellum (2009) 8(1):28–34. doi: 10.1007/s12311-008-0056-6
165. Schmahmann JD. The Role of the Cerebellum in Cognition and Emotion : Personal Reflections Since 1982 on the Dysmetria of Thought Hypothesis, and … The Role of the Cerebellum in Cognition and Emotion : Personal Reflections Since 1982 on the Dysmetria of Thought Hypoth. Neuropsychol Rev (2014) 20(3):236–60. doi: 10.1007/s11065-010-9142-x
166. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex (2010) 46(7):831–44. doi: 10.1016/j.cortex.2009.11.008
167. Bezdjian S, Raine A, Baker LA, Lynam DR. Psychopathic personality in children: genetic and environmental contributions. psychol Med (2011) 41(03):589–600. doi: 10.1017/S0033291710000966
168. Bezdjian S, Tuvblad C, Raine A, Baker L. The Genetic and Environmental Covariation Among Psychopathic Personality Traits, and Reactive and Proactive Aggression in Childhood. Child Dev (2011) 82(4):1267–81. doi: 10.1111/j.1467-8624.2011.01598.x
169. Fontaine NMG, Rijsdijk FV, McCrory EJP, Viding E. Etiology of Different Developmental Trajectories of Callous-Unemotional Traits. J Am Acad Child Adolesc Psychiatry (2010) 49(7):656–64. doi: 10.1016/j.jaac.2010.03.014
170. Viding E, Blair RJR, Moffitt TE, Plomin R. Evidence for substantial genetic risk for psychopathy in 7-year-olds. J Child Psychol Psychiatry (2005) 46(6):592–7. doi: 10.1111/j.1469-7610.2004.00393.x
171. Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Continuity and change in psychopathic traits as measured via normal-range personality: A longitudinal-biometric study. J Abnormal Psychol (2006) 115(1):85–95. doi: 10.1037/0021-843X.115.1.85
172. Forsman M, Lichtenstein P, Andershed H, Larsson H. Genetic effects explain the stability of psychopathic personality from mid- to late adolescence. J Abnormal Psychol (2008) 117(3):606–17. doi: 10.1037/0021-843X.117.3.606
173. Larsson H, Andershed H, Lichtenstein P. A genetic factor explains most of the variation in the psychopathic personality. J Abnormal Psychol (2006) 115(2):221–30. doi: 10.1037/0021-843X.115.2.221
174. Larsson H, Tuvblad C, Rijsdijk FV, Andershed H, Grann M, Lichtenstein P. A common genetic factor explains the association between psychopathic personality and antisocial behavior. psychol Med (2007) 37(01):15. doi: 10.1017/S003329170600907X
175. Taylor J, Loney BR, Bobadilla L, Iacono WG, McGue M. Genetic and Environmental Influences on Psychopathy Trait Dimensions in a Community Sample of Male Twins. J Abnormal Child Psychol (2003) 31(6):633–45. doi: 10.1023/A:1026262207449
176. Brook M, Panizzon MS, Kosson DS, Sullivan EA, Lyons MJ, Franz CE, et al. Psychopathic Personality Traits in Middle-Aged Male Twins: A Behavior Genetic Investigation. J Pers Disord (2010) 24(4):473–86. doi: 10.1521/pedi.2010.24.4.473
177. Tuvblad C, Wang P, Bezdjian S, Raine A, Baker LA. Psychopathic personality development from ages 9 to 18: Genes and environment. Dev Psychopathol (2016) 28(01):27–44. doi: 10.1017/S0954579415000267
178. Tuvblad C, Bezdjian S, Raine A, Baker LA. The heritability of psychopathic personality in 14- to 15-year-old twins: A multirater, multimeasure approach. psychol Assess (2014) 26(3):704–16. doi: 10.1037/a0036711
179. Viding E, McCrory EJ. Genetic and neurocognitive contributions to the development of psychopathy. Dev Psychopathol (2012) 24(03):969–83. doi: 10.1017/S095457941200048X
180. Viding E, Price TS, Jaffee SR, Trzaskowski M, Davis OSP, Meaburn EL, et al. Genetics of Callous-Unemotional Behavior in Children. PloS One (2013) 8(7):e65789. doi: 10.1371/journal.pone.0065789
181. Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic Sensitivity to the Environment: The Case of the Serotonin Transporter Gene and Its Implications for Studying Complex Diseases and Traits. Am J Psychiatry (2010) 167(5):509–27. doi: 10.1176/appi.ajp.2010.09101452
182. Glenn AL. The other allele: Exploring the long allele of the serotonin transporter gene as a potential risk factor for psychopathy: A review of the parallels in findings. Neurosci Biobehav Rev (2011) 35(3):612–20. doi: 10.1016/j.neubiorev.2010.07.005
183. Kolla NJ, Vinette SA. Monoamine Oxidase A in Antisocial Personality Disorder and Borderline Personality Disorder. Curr Behav Neurosci Rep (2017) 4(1):41–8. doi: 10.1007/s40473-017-0102-0
184. Kolla NJ, Matthews B, Wilson AA, Houle S, Michael Bagby R, Links P, et al. Lower Monoamine Oxidase-A Total Distribution Volume in Impulsive and Violent Male Offenders with Antisocial Personality Disorder and High Psychopathic Traits: An [11C] Harmine Positron Emission Tomography Study. Neuropsychopharmacology (2015) 40(11):2596–603. doi: 10.1038/npp.2015.106
185. Tiihonen J, Koskuvi M, Lähteenvuo M, Virtanen PLJ, Ojansuu I, Vaurio O, et al. Neurobiological roots of psychopathy. Mol Psychiatry (2019). doi: 10.1038/s41380-019-0488-z
186. Coid J, Ullrich S. Antisocial personality disorder is on a continuum with psychopathy. Compr Psychiatry (2010) 51(4):426–33. doi: 10.1016/j.comppsych.2009.09.006
187. Del Casale A, Kotzalidis GD, Rapinesi C, Di Pietro S, Alessi MC, Di Cesare G, et al. Functional Neuroimaging in Psychopathy. Neuropsychobiology (2015) 72(2):97–117. doi: 10.1159/000441189
188. Cowan L. The Psychopath: What's Love Got to Do with It? psychol Perspect (2014) 57(3):291–311. doi: 10.1080/00332925.2014.936241
189. Hare R. Without conscience: The disturbing world of the psychopaths among us. New York, NY: Guilford Press (1993).
190. Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal Changes in White Matter Integrity Among Adolescent Substance Users. Alcohol: Clin Exp Res (2013) 37:E181–9. doi: 10.1111/j.1530-0277.2012.01920.x
191. Konrad A, Vucurevic G, Lorscheider M, Bernow N, Thümmel M, Chai C, et al. Broad Disruption of Brain White Matter Microstructure and Relationship with Neuropsychological Performance in Male Patients with Severe Alcohol Dependence. Alcohol Alcohol (2012) 47(2):118–26. doi: 10.1093/alcalc/agr157
192. Fein G, Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical Gray Matter Loss in Treatment-Naive Alcohol Dependent Individuals. Alcohol: Clin Exp Res (2002) 26(4):558–64. doi: 10.1111/j.1530-0277.2002.tb02574.x
193. Mon A, Durazzo TC, Abe C, Gazdzinski S, Pennington D, Schmidt T, et al. Structural brain differences in alcohol-dependent individuals with and without comorbid substance dependence. Drug Alcohol Depend (2014) 144:170–7. doi: 10.1016/j.drugalcdep.2014.09.010
Keywords: psychopathy, neuroimaging, review, antisocial, callous-unemotional, emotional detachment
Citation: Johanson M, Vaurio O, Tiihonen J and Lähteenvuo M (2020) A Systematic Literature Review of Neuroimaging of Psychopathic Traits. Front. Psychiatry 10:1027. doi: 10.3389/fpsyt.2019.01027
Received: 12 September 2019; Accepted: 30 December 2019;
Published: 06 February 2020.
Edited by:
Pietro Pietrini, IMT School for Advanced Studies Lucca, ItalyReviewed by:
Stefano Ferracuti, Sapienza University of Rome, ItalyCopyright © 2020 Johanson, Vaurio, Tiihonen and Lähteenvuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mika Johanson, bWlrYS5qb2hhbnNvbkBzdHVkLmtpLnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.