
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 14 June 2019
Sec. Behavioral and Psychiatric Genetics
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.00394
 Nora Eszlari1,2*
Nora Eszlari1,2* Peter Petschner1,3
Peter Petschner1,3 Xenia Gonda2,3,4
Xenia Gonda2,3,4 Daniel Baksa1,5
Daniel Baksa1,5 Rebecca Elliott6,7
Rebecca Elliott6,7 Ian Muir Anderson6,7
Ian Muir Anderson6,7 John Francis William Deakin6,7,8
John Francis William Deakin6,7,8 Gyorgy Bagdy1,2,3
Gyorgy Bagdy1,2,3 Gabriella Juhasz1,5,6
Gabriella Juhasz1,5,6The serotonin system has been suggested to moderate the association between childhood maltreatment and rumination, with the latter in its turn reported to be a mediator in the depressogenic effect of childhood maltreatment. Therefore, we investigated whether the associations of two epigenetic regulatory polymorphisms in the HTR2A serotonin receptor gene with Ruminative Responses Scale rumination and its two subtypes, brooding and reflection, are moderated by childhood adversity (derived from the Childhood Trauma Questionnaire) among 1,501 European white adults. We tested post hoc whether the significant associations are due to depression. We also tested the replicability of the significant results within the two subsamples of Budapest and Manchester. We revealed two significant models: both the association of methylation site rs6311 with rumination and that of miRNA binding site rs3125 (supposed to bind miR-1270, miR-1304, miR-202, miR-539 and miR-620) with brooding were a function of childhood adversity, and both interaction findings were significantly present both in the never-depressed and in the ever-depressed group. Moreover, the association of rs3125 with brooding could be replicated across the separate subsamples, and remained significant even when controlling for lifetime depression and the Brief Symptom Inventory depression score. These findings indicate the crucial importance of involving stress factors when considering endophenotypes and suggest that brooding is a more promising endophenotype than a broader measure of rumination. Transdiagnostic relevance of the brooding endophenotype and the potential of targeting epigenetic regulatory polymorphisms of HTR2A in primary and secondary prevention of depression and possibly of other disorders are also discussed.
Ruminative response style (rumination) is a passive and repetitive way of responding to distress and depressed mood, and it predicts future depression (1). It has two subtypes: the more maladaptive brooding and the less maladaptive reflection (2). Brooding means passive comparisons with unachieved standards, whereas reflection denotes purposeful problem-solving strategies (2).
It has been suggested that the depressogenic effect of childhood maltreatment is partly mediated by rumination (3). However, both retrospective studies (4, 5) and also a longitudinal one (6) indicates that this mediatory effect is carried by the brooding but not the reflection subtype of rumination, although one study found no evidence on the mediating effect of brooding between childhood maltreatment and adolescent internalizing or externalizing psychopathology (7). The relationship between childhood maltreatment and reflection is contradictory in itself, since two studies suggest their positive association (4, 6), but another one suggests no association between them (5). In contrast, brooding level is consistently predicted by childhood maltreatment (4, 6) and its all forms except for physical neglect (5).
The moderating role of the 5-HTTLPR length polymorphism of the serotonin transporter gene in the effect of childhood maltreatment on rumination (8) indicates that the serotonin system seems to play a relevant role in rumination. In addition, tryptophan depletion in humans provokes cognitive inflexibility and a deficit in inhibitory control (9), both of which have been associated with rumination (10, 11), further supporting the involvement of serotonin function in rumination.
Among elements of the serotonin system, the neocortical serotonin 2A (5-HT2A) receptor appears to be a promising additional candidate with regard to rumination, since it mediates the effect of tryptophan depletion on response inhibition (12), and it has also shown alterations in radioligand binding and functional regulation by messenger RNA levels and by protein kinase A activity in the prefrontal cortex in several psychiatric disorders (13). Moreover, frontal 5-HT2A binding has been associated with dysfunctional attitudes among patients in a major depressive episode (14, 15), and 5-HT2A binding in the left dorsal prefrontal cortex has shown a positive correlation with the anticipatory worry subscale of harm avoidance (16). Dysfunctional attitudes and worry are both related to rumination (1, 10), consistent with a link between 5-HT2A and rumination.
Early life stress can affect gene expression through epigenetic mechanisms including DNA methylation and microRNA (miRNA) expression (17). Therefore, in investigating the link between rumination-related phenotypes, childhood maltreatment and functioning of 5-HT2A, single nucleotide polymorphisms (SNPs) facilitating epigenetic regulation of the HTR2A gene encoding the receptor appear to be promising targets. Such regulatory SNPs have extensively been investigated in the background of rumination-related phenotypes. Increased HTR2A gene expression has been inconsistently linked to either the T allele of the methylation site T102C (rs6313) (18) SNP of exon-1 in the cortex (15), or to its C allele in the frontal lobe (19). Nevertheless, TC heterozygotes perform worse on the Wisconsin Card Sorting Task (WCST) than both homozygote groups (20, 21), and WCST performance correlates negatively with rumination (1). Rs6313 is in high linkage disequilibrium (LD) with another methylable SNP in the promoter region of the gene, rs6311 (-1438 A/G) (19, 22–24). Rs6313 T allele corresponds to rs6311 T allele (this latter one is measured on the positive strand of the DNA double helix, https://genome.ucsc.edu/) (25). Rs6311 also entails controversial phenotypic associations. Its T allele is related to an increased promoter function (15, 26–28) and mood disorders in one study (26) but unrelated to either major depression or bipolar depression according to meta-analyses (29, 30); nevertheless, its CC genotype (defined also on the positive strand) is the one that confers a risk for higher neuroticism, depression, and emotion-based coping strategies (31), all of which have been associated with rumination (1).
In our present study, we investigated the associations of rumination with two regulatory SNPs from the two ends of the HTR2A gene. In addition to the promoter methylation site rs6311 (25), we chose the miRNA binding site rs3125 (https://snpinfo.niehs.nih.gov/snpinfo/snpfunc.html) residing in the 3′ UTR (untranslated region), the other regulatory side of the gene, according to hg19 database of the UCSC Genome Browser (https://genome.ucsc.edu/). Rs3125 is supposed to bind miR-1270, miR-1304, miR-202, miR-539, and miR-620 (https://snpinfo.niehs.nih.gov/cgi-bin/snpinfo/mirna.cgi?2_rs3125). It can be a tag SNP, representing its haploblock that consists of two SNPs (Figure 1), and it has been associated with depression among cardiac patients (32). We tested the main effect of these two SNPs, and their interactions with childhood adversity, on rumination and its two subtypes: brooding and reflection. In case of finding a significant effect, we tested whether or not it is mediated or moderated by depression. We hypothesize to reveal both main effects and interaction effects of the SNPs, based on the abundant main effect findings of HTR2A and the interaction effect literature of 5-HTTLPR. As supplementary analyses, we ran the same models with 5-HTTLPR.
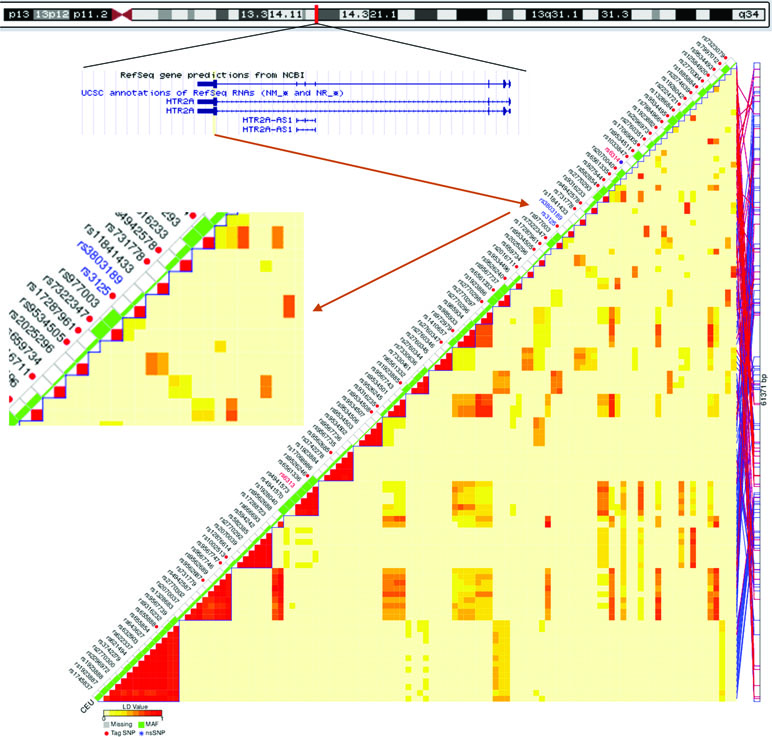
Figure 1 Position of rs3125 within the HTR2A gene on chromosome 13 (https://genome.ucsc.edu/, build hg19), and among tag single nucleotide polymorphisms (SNPs) of the gene in the HapMap CEU population (https://snpinfo.niehs.nih.gov/snpinfo/snptag.html). CEU = a population with Northern and Western European ancestry (https://www.sanger.ac.uk/resources/downloads/human/hapmap3.html); LD = linkage disequilibrium between each pair of SNPs, measured with r2; MAF = minor allele frequency.
Our study, carried out in accordance with the Declaration of Helsinki, and being part of the NewMood (New Molecules in Mood Disorders) study funded by the European Union (Sixth Framework Program of the EU, LSHM-CT-2004-503474), was approved by the Scientific and Research Ethics Committee of the Medical Research Council, Budapest, Hungary, and by the North Manchester Local Research Ethics Committee, Manchester, United Kingdom.
Subjects provided written informed consent, and they were recruited through advertisements and general practices from Budapest, Hungary, and through advertisements, general practices, and a website from Greater Manchester, UK. They received nothing for participation.
There were 1,501 adults (18–60 years old) from Budapest (N = 470) and Manchester (N = 1,031) that provided self-report questionnaire data about sex, age, and rumination, which reported to be of European white ethnic origin and were successfully genotyped for rs3125. None of them reported having had any relative participating in the study. Among these included participants, rs6311 was successfully genotyped in 469 subjects from Budapest and 1,017 from Manchester, and childhood adversity measure was provided by 468 subjects from Budapest and 1,030 from Manchester. Data on both rs6311 and childhood adversity were available in 467 subjects from Budapest, and 1,016 from Manchester. Supplementary Figure 1 displays a flowchart on inclusion criteria and data availability.
We measured rumination and its two subscales, brooding and reflection, with the 10-item Ruminative Responses Scale (RRS) (2). Five items of RRS belong to the brooding subscale, and five items to the reflection subscale, and scores on the two subscales add up to the rumination score. Brooding and reflection scores have a Pearson correlation of r = 0.487 (N = 1,501; p < 0.00001) in the combined sample, r = 0.308 (N = 470; p < 0.00001) in the Budapest subsample, and r = 0.521 (N = 1,031; p < 0.00001) in the Manchester subsample.
We used depression items plus the additional items from the Brief Symptom Inventory (BSI) (33) to measure current depressive symptoms. Each of the rumination scores and the BSI depression score was calculated as a weighted score: the sum of item scores divided by the number of completed items. Lifetime depression was measured by a question in the background questionnaire, and had been validated previously with face-to-face diagnostic interviews within a subpopulation, yielding a 91.7% sensitivity and 89.8% specificity (34).
Our childhood adversity measure (provided as Supplementary Data) was based on the Childhood Trauma Questionnaire (CTQ) (35), but is different from that in the specific items. Childhood adversity score means the sum score of four items regarding emotional and physical abuse and neglect, and two items about parental loss. We had validated this short childhood adversity measure previously with the 28-item CTQ within a subpopulation, yielding a significant Pearson r = 0.75 (p < 0.001) (34).
By a genetic saliva sampling kit, subjects provided buccal mucosa cells personally or by post, from which genomic DNA was extracted according to Ref. (36). Rs3125 and rs6311 were genotyped with the Sequenom MassARRAY technology (www.sequenom.com, Sequenom, San Diego, CA, USA). Genotyping of 5-HTTLPR has been detailed in one of our previous papers (37). All laboratory works were performed under the ISO 9001:2000 quality management requirements, and it was blinded with regard to phenotype.
We used Plink v1.07 (http://zzz.bwh.harvard.edu/plink/) to calculate Hardy–Weinberg equilibrium and allele frequencies, and, with the aid of individually written scripts in R (38), to run regression models. SPSS25 was used to perform χ2 or t tests, as appropriate, for descriptive statistics, and univariate general linear models for visualization purposes in the figures. In these general linear models, childhood adversity score was grouped into three categories: low (0–3); medium (4–6); and high (7 or more). Possible childhood adversity score ranges from 0 to 18, and in our study from 0 to 16. The same grouping to low, medium, and high scores had been applied in our previous study (39). In the general linear models run to visualize interaction effects, covariates were main effects of the respective SNP and childhood adversity, and the outcome variable had been controlled for the covariates detailed below, in a previous general linear model.
In our Plink linear regression models population, sex and age were covariates in all the analyses, and in case of each rumination subscale as the outcome, the other subscale was also a covariate. When testing an SNP × childhood adversity interaction effect, main effects of both the SNP and childhood adversity were included as covariates in the model.
As primary analyses, 36 regression equations were run in the Budapest + Manchester combined sample: with either of the two SNPs as predictors, on rumination, brooding and reflection as the outcome variable, testing either a main effect or an interaction effect of the SNP, in additive, dominant, and recessive models.
QVALUE v1.0 (40) was used to calculate q-values of false discovery rate (FDR) for the p-values of these 36 tests, without robust method, with a 0 to 0.99 range of λ (by 0.05), and a bootstrap method to estimate the overall proportion of true null hypotheses, π0. We consider results with a q-value of ≤0.05 as significant after correction for multiple testing.
For these primary tests, power calculation was carried out with Quanto v1.2 (http://biostats.usc.edu/Quanto.html), at a type I error rate of 0.05, assuming an RG2 = 1% for the SNP in the main effect models, and, in case of interaction models, assuming an RGE2 = 0.5% for the gene–by–environment (GxE) interaction, RG2 = 0% for the genetic main effect, and based on coefficients of determination with childhood adversity, an RE2 = 0.066 for rumination, 0.0756 for brooding and 0.0276 or 0.0282 (as appropriate for the respective sample size) for reflection.
For significant findings among the 36 primary tests, we tested possible mediating effects in secondary (post hoc) analyses as follows. First, we built the same model as that of the significant finding, with logistic regression on lifetime depression and linear regression on BSI depression as the outcome. Second, we ran the same model again, on the rumination variable as the outcome and including both depression variables as additional covariates. Third, we ran the model on both depression variables separately, including the rumination variable as an additional covariate. To control for the overfitting of these models due to the several covariates, 100,000 permutations were run on the relevant term in each mediation model. To speed up analyses, permutation was run in Plink v1.90b6.9 (4th March, 2019; https://www.cog-genomics.org/plink/1.9/). A label-swapping, max(T) permutation procedure was applied, yielding an empirical p-value for the regression term.
Besides mediating, moderating effects of depression were also tested post hoc for the significant findings, by running the same model separately in those who reported lifetime depression (ever-depressed) and in those who did not (never-depressed).
Significant findings were post hoc tested whether holding true in the separate Budapest and Manchester subsamples, by the same models described above but without population as a covariate.
To test effects of the 5-HTTLPR length polymorphism residing within the promoter region of the serotonin transporter gene, its main effects and interaction effects with childhood adversity were tested within the combined sample in a manner similar to HTR2A testing, as supplementary analyses.
In all post hoc analyses, and also in results of descriptive statistics and supplementary analyses, the threshold for significance was p ≤ 0.05, and for trend, it was p ≤ 0.10.
For frequencies or means of the variables, see Table 1 and Supplementary Table 1. We can see that the two subsamples significantly differ from each other in all variables except for rs6311 and 5-HTTLPR genotype frequencies.
Both HTR2A SNPs are in Hardy–Weinberg equilibrium in the combined sample and in the Manchester and Budapest subsamples (rs3125 has a p = 0.909 in the combined sample, p = 0.616 in Budapest, and p = 0.607 in Manchester; and rs6311 yields a p = 0.163 in the combined sample, p = 0.704 in Budapest, and p = 0.051 in Manchester). For rs3125, C is the minor allele, with an allele frequency of 0.1289. For rs6311, T is the minor allele, yielding an allele frequency of 0.4078.
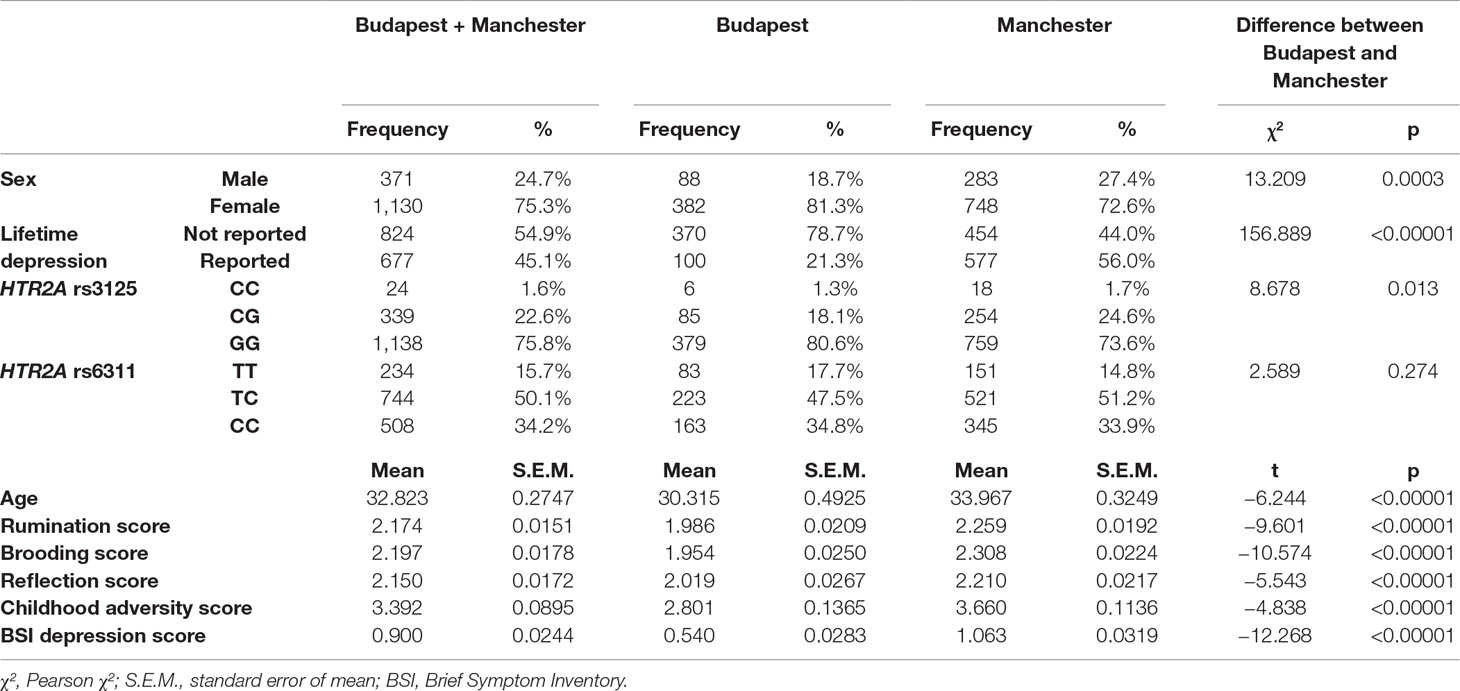
Table 1 Descriptive statistics for the combined Budapest + Manchester sample and the two subsamples.
Table 2 demonstrates that there is a significant gene-environment correlation in case of rs3125 and childhood adversity score in the combined sample and in Manchester.
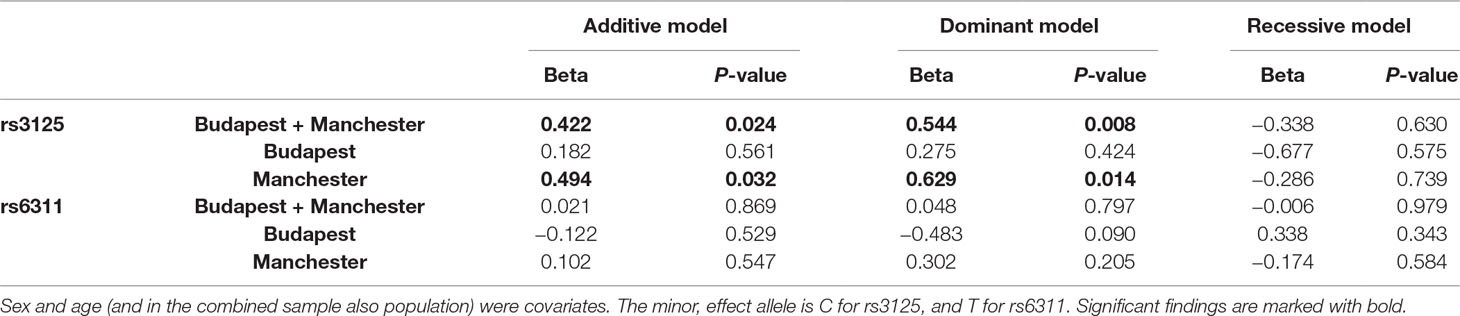
Table 2 Effect of each HTR2A single nucleotide polymorphism (SNP) as predictor, for childhood adversity as outcome, in linear regression models.
Among the 36 primary analysis models, those that test only the main effect of the SNP, without interaction term, have a 97.16% and a 97.28% power to detect it in case of rs6311 and rs3125, respectively. In case of testing the interaction effect with childhood adversity, rs6311 has 80.55% power for rumination, 80.95% for brooding, and 78.98% for reflection. Rs3125, in interaction with childhood adversity, has 80.94% power for rumination, 81.34% for brooding, and 79.36% for reflection. These findings indicate sufficient power to detect the tested genetic effects.
Among the 36 equations (Table 3), only the rs6311 × childhood adversity interaction on rumination in an additive model (Figure 2), and the rs3125 × childhood adversity interaction on brooding in both an additive and a dominant (Figure 3) model, proves to be significant after correction for multiple testing.
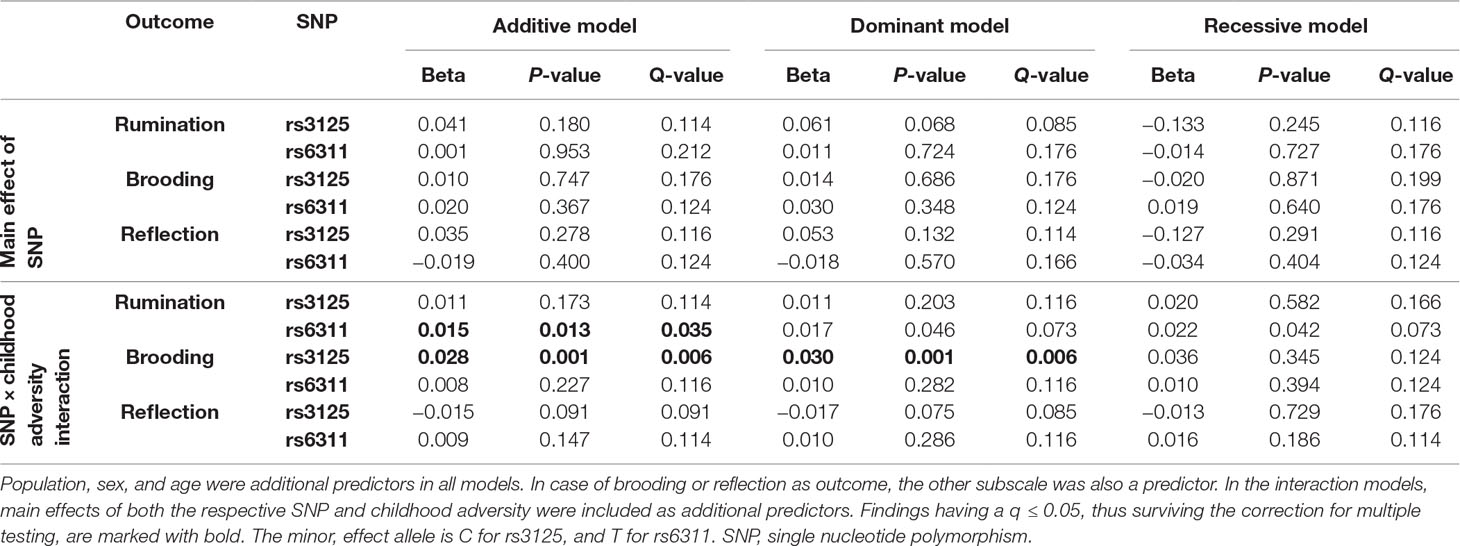
Table 3 Effect of each HTR2A SNP as predictor, for each rumination variable as outcome, in linear regression models.
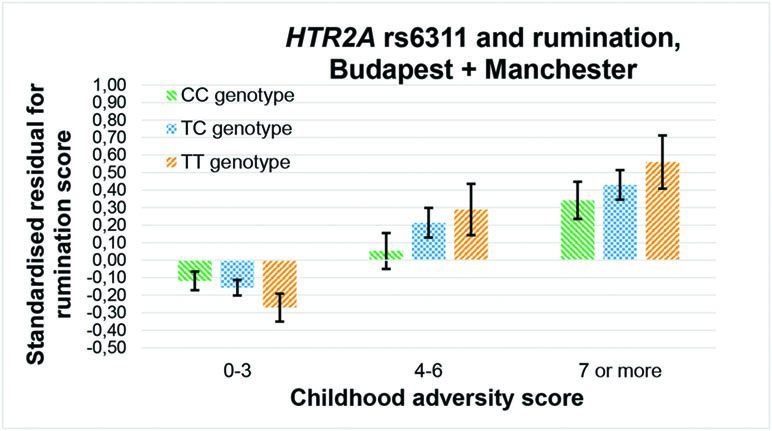
Figure 2 Interaction effect of childhood adversity and HTR2A rs6311 genotype on rumination score in a general linear model performed with visualization purposes.
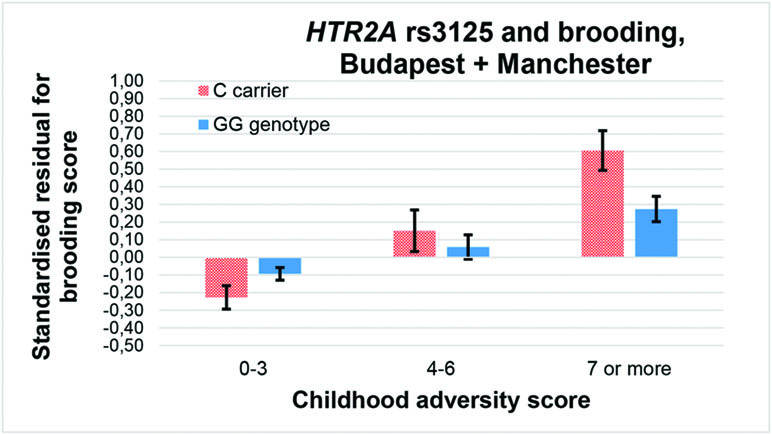
Figure 3 Interaction effect of childhood adversity and HTR2A rs3125 genotype on brooding score in a general linear model performed with visualization purposes.
The rs6311 × childhood adversity interaction on rumination in an additive model remains significant both in ever-depressed (N = 670; β = 0.015; p = 0.045) and in never-depressed (N = 813; β = 0.019; p = 0.028) participants, indicating that depression history does not moderate this effect.
Similarly, the rs3125 × childhood adversity interaction on brooding remains significant in both ever-depressed (N = 676; additive β = 0.026; p = 0.022; dominant β = 0.029; p = 0.023) and never-depressed (N = 822; additive β = 0.032; p = 0.034; dominant β = 0.033; p = 0.033) participants.
To test the possible mediating role of depression in the rs6311 × childhood adversity interaction effect on rumination and, vice versa, the possible mediating role of rumination in the same genetic effect on depression, the following prerequisites have to be met. Depression phenotypes have to show a significant positive correlation with rumination, and have to be associated with the rs6311 × childhood adversity interaction term in the same direction as rumination does. Rumination indeed shows a positive association with lifetime depression (N = 1,483; t = −18.304; p < 0.00001; with rumination means of 1.944 in the never-depressed and 2.451 in the ever-depressed group) and with BSI depression (N = 1,482; Pearson r = 0.570; p < 0.00001). However, the rs6311 x childhood adversity additive model does not yield a significant effect on either lifetime depression (N = 1,483; odds ratio (OR) = 1.022; p = 0.419), or BSI depression (N = 1,482; β = −0.001; p = 0.894), therefore we cannot test the possible mediating role of depression in this genetic effect on rumination. Nevertheless, including both depression phenotypes as covariates, the rs6311 × childhood adversity interaction on rumination in an additive model becomes more significant but with almost the same effect size (N = 1,482; β = 0.014; p = 0.005; empirical p = 0.005) as can be seen without controlling for depression (Table 3). This strengthening of significance may be due to the confounding effect of depression in the common variance of rumination and rs6311 × childhood adversity.
Prerequisites of testing the mediating role of depression in the significant rs3125 × childhood adversity effect on brooding are met. Brooding has a significant positive association with both lifetime depression (N = 1,498; t = −18.896; p < 0.00001; with means of 1.920 in the never-depressed and 2.534 in the ever-depressed group) and BSI depression (N = 1,497; Pearson r = 0.618; p < 0.00001). The rs3125 × childhood adversity interaction effect that has been found significant on brooding, is a trend on both lifetime depression (N = 1,498; additive OR = 1.081; p = 0.070; dominant OR = 1.087; p = 0.064) and BSI depression (N = 1,497; additive β = 0.024; p = 0.067; dominant β = 0.026; p = 0.060). All these associations enabled us to test mediating effects. Including both depression phenotypes as additional covariates, the rs3125 × childhood adversity interaction effect on brooding remains significant at a nominal p ≤ 0.05 level in both additive (β = 0.018; p = 0.015; empirical p = 0.015) and dominant (β = 0.019; p = 0.017; empirical p = 0.017) models. However, including brooding as an additional covariate, the effect of the rs3125 × childhood adversity interaction term considerably weakens on both lifetime depression (additive OR = 1.035; p = 0.449; empirical p = 0.453; dominant OR = 1.038; p = 0.430; empirical p = 0.431) and BSI depression (additive β = 0.005; p = 0.660; empirical p = 0.659; dominant β = 0.006; p = 0.600; empirical p = 0.600).
The rs6311 × childhood adversity additive model on rumination is not replicable, since it is not significant in Budapest (β = 0.009; p = 0.309), but it is significant in Manchester (β = 0.015; p = 0.050) (Figure 4).
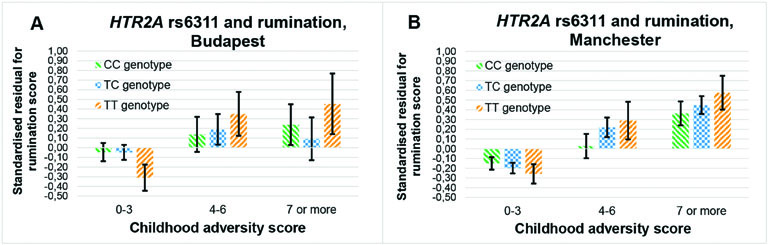
Figure 4 Interaction effect of childhood adversity and HTR2A rs6311 genotype on rumination score in a general linear model performed with visualization purposes in the Budapest (A) and Manchester (B) subsamples.
The rs3125 × childhood adversity effect on brooding can be replicated, because it is significant in both Budapest (additive β = 0.042; p = 0.026; dominant β = 0.042; p = 0.029) and Manchester (additive β = 0.024; p = 0.016; dominant β = 0.026; p = 0.016) (Figure 5).
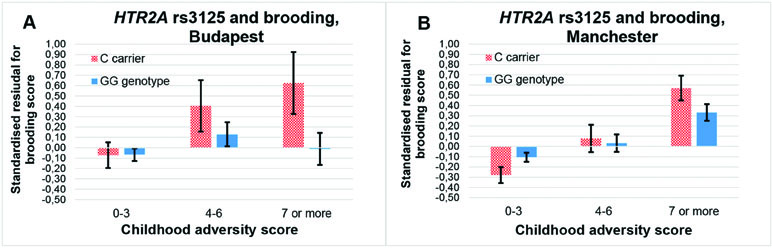
Figure 5 Interaction effect of childhood adversity and HTR2A rs3125 genotype on brooding score in a general linear model performed with visualization purposes in the Budapest (A) and Manchester (B) subsamples.
Supplementary Table 2 demonstrates that 5-HTTLPR is not associated with childhood adversity, entailing that there is no gene-environment correlation in its case.
Supplementary Table 3 shows that 5-HTTLPR is not associated with rumination, brooding or reflection, either in main effect or in interaction with childhood adversity.
We have demonstrated that both of two HTR2A polymorphisms related to two distinct epigenetic regulatory mechanisms exert a significant effect on current adult rumination as a function of childhood stress, independently of current and lifetime depression status. Both the methylation site rs6311 and the miRNA binding site rs3125 appear to contribute to the endophenotypic manifestation of rumination, and these findings also support the need to consider the role of childhood stress when considering endophenotypes. Moreover, the impact of the rs3125 × childhood adversity interaction on brooding could be replicated in Budapest and Manchester, remained significant when controlling for depression, and also fully mediated the weak effect of the same interaction term on depression. Thus, rs3125 and brooding show a stronger contribution to the endophenotypic manifestation of rumination phenotypes than rs6311 and a broader measure of rumination that encompasses the reflection subtype in addition to the brooding subtype. Rs3125 and childhood adversity may also convey a transdiagnostic relevance to the brooding endophenotype.
Contrary to former findings concerning HTR2A and rumination-related phenotypes (20, 21, 31), in our study, we found no main effect of any SNP in itself on rumination, but only an interaction with childhood adversity proved to be significant, similarly to the 5-HTTLPR × childhood maltreatment interaction effect on rumination reported by Antypa and Van der Does (8). In line with this, our former results with 5-HTTLPR and childhood adversity have also demonstrated the role of early tuning of the serotonin system in adulthood stress reactivity leading to depression (39). Although our present study did not find any significant association between 5-HTTLPR and rumination itself, a possible mediating link between the detrimental impact of childhood maltreatment and serotonin transporter gene function may be regulation by epigenetic mechanisms. Epigenetic alterations, which are heritable and environmentally modifiable, denote reversible modifications to the genome, and they engender alterations in gene expression patterns in a cell-type specific manner (41). Early life stress has been proposed to have long-term consequences on brain structure and mental health outcomes via epigenetic mechanisms including methylation and miRNA expression, which were found to influence mental health phenotypes and serotonin transporter expression, respectively (17). An exposure during development may impact epigenetic states more broadly than later exposure (42), and especially miRNAs can totally switch expression during development but fine-tune it in adult tissues (43). In addition to former (but not present) findings with 5-HTTLPR, our present results with HTR2A also underline the relevance of the serotonin system in the long-lasting effects of childhood stress that may be transmitted by epigenetic mechanisms.
In the promoter region of HTR2A, the cytosine at position -1439 can only be methylated in case of a G allele (or C allele if measured on the complementary, positive strand of DNA, as in our study) at the adjacent -1438 A/G (rs6311) polymorphism (19, 25, 44). Although methylation level of the promoter region of HTR2A was inversely related to the transient phenotype of antipsychotic use in the frontal lobe of schizophrenic and bipolar patients (19), its methylation in the placenta can entail long-term impacts on psychiatric phenotypes (44, 45). Previous contradictory results with rs6311 (26, 29–31) can be resolved by considering the moderating role of stress level, but recent stress has been suggested as much important as early stress in these terms. TT genotype denoted a risk for depression in case of a high childhood adversity level (46), and for a reduced heart rate variability only in case of a high level of recent stress (47). Heart rate variability has an inverse association with brooding (48), so our present results (see Figure 2) corroborate these former findings with another type of stress. As the T allele of rs6311 enhances expression (15, 26–28), and in accordance with that tryptophan depletion impairs response inhibition via an increased 5-HT2A density within the right inferior frontal gyrus (12), we can hypothesize that a genetically heightened expression of HTR2A may make the level of rumination more dependent on 5-HT2A-mediated serotonin transmission, thus more sensitive to environmental impacts affecting the serotonin system, such as tryptophan depletion or stress.
Similarly, former results with the miRNA binding site rs3125 were inconclusive on bipolar disorder when stress level was not taken into account (49). On the other hand, the C allele appeared to increase risk of depression in cardiac patients (32) which can be considered a stressed group, corroborating our results also with the C allele as a risk variant for brooding only in case of high childhood stress (Figures 3 and 5). Rs3125 can bind to five different miRNAs (https://snpinfo.niehs.nih.gov/cgi-bin/snpinfo/mirna.cgi?2_rs3125) (50). Among them, miR-539 bound by the G allele of rs3125 showed a reduced expression in anterior cingulate cortex (ACC) in an animal model of chronic neuropathic pain (51). Although ACC has demonstrated a negative association in volume and resting state activity with rumination (52), and 5-HT2A binding in ACC has been negatively related to treatment resistance in major depression (53), the exact role of rs3125 in HTR2A expression and the role of miRNA-regulated HTR2A expression in brooding have to be elucidated in the future. It should also be clarified whether childhood or recent timing of stress matters in the effect of rs3125 on brooding.
Both HTR2A × childhood adversity interaction effects were replicable separately in the ever-depressed and the never-depressed group, contributing to the endophenotypic manifestation of rumination. Namely, an endophenotype, by definition, should reside on the causal pathway between genetics and the disorder (54, 55), and rumination is not only augmented by a depressive episode even after recovery (56, 57), but it also predicts future onset of depression in never-depressed individuals (58). Our results point to the role of genetics in these associations and thus complete the picture with endophenotypic features, highlighting that HTR2A affects rumination not only in or after a depressive episode, but equally in those who have not yet developed or will never develop the disorder. Since high rumination constitutes a risk for depression, screening never-depressed people based on HTR2A rs3125 and rs6311 genotypes and levels of childhood adversity and rumination may once be part of a comprehensive picture in the primary prevention of depression. Similar screening in ever-depressed people could similarly aid relapse prevention as well as decision on pharmacotherapeutic and psychotherapeutic intervention.
Although rumination score has shown endophenotypic features in our study, in that its association with rs6311 can be replicated both in the ever-depressed and the never-depressed group, it also seems multifaceted and can be further deconstructed to even simpler endophenotypes (59). Brooding may be its more useful subtype in primary and secondary depression prevention than the broader measure of rumination that includes also reflection. Indeed, reflection has not shown any association with any of the HTR2A polymorphisms in our present results.
Brooding may be a more useful endophenotype first because the rs3125 × childhood adversity interaction on brooding is replicable in Budapest and Manchester, while the rs6311 × childhood adversity interaction on rumination is significant only in Manchester but not in Budapest. Robustness of GxE (gene–by–environment) results on brooding is remarkable also because participants from Manchester have higher scores on childhood adversity and brooding, have a lower frequency of rs3125 GG genotype, and are more depressed than participants from Budapest (Table 1). Since our subsamples are not at all representative for the two populations in the variables of interest, further investigation is needed regarding the relative cross-population robustness of these genetic associations with brooding and the broader measure of rumination compared to each other.
Second, because the effect of HTR2A on brooding seems more relevant in depression than its effect on more broadly measured rumination. While the rs6311 × childhood adversity interaction term is not associated with depression at all, the rs3125 × childhood adversity interaction term has a trend effect on depression, which disappears if controlling for brooding. Brooding, however, remains associated with rs3125 × childhood adversity even if controlling for depression. All of these results point to a stronger association of rs3125 with brooding than with depression and suggest that rs3125 contributes to brooding as an endophenotype (59) that confers a risk for depression.
The investigated HTR2A genotypes exert an effect only on the broad measure of rumination and its brooding subtype but not on reflection. Brooding, being a more maladaptive subtype of rumination than reflection (2), may be a closer construct to those phenotypes previously having shown an association with 5-HT2A or HTR2A: response inhibition deficit (12), perseverative errors on WCST (21), worry (16), dysfunctional attitudes (14, 15), neuroticism and emotion-based coping strategies (31). Moreover, our genetic findings with HTR2A × childhood adversity on rumination, brooding, and depression underline and expand previous results that only brooding but not reflection is important in the depressogenic effect of childhood maltreatment (4–6).
Further research is needed to clarify the origin of difference between miRNA binding and methylation of HTR2A in contributing to the endophenotypic manifestation of either brooding or the broader measure of rumination. Although considerable evidence underscores the interconnectedness of miRNA function and DNA methylation in gene regulation (60), also pointing to the precedence of miRNA functioning over DNA methylation in neuronal differentiation (61) and possibly in haloperidol effects (62), the exact relationship of these two types of epigenetic regulation regarding particularly HTR2A expression needs to be clarified.
Nevertheless, we can suggest that rs3125 can be a more useful biomarker in primary and secondary depression prevention than rs6311.
The fact that the rs3125 × childhood adversity interaction effect on brooding explains the same interaction effect on depression but is not explained by depression, implies that it goes beyond depression and may play a role in the potential transdiagnostic relevance of the brooding endophenotype. It may be part of the endophenotype conveying a risk for the disorders having been linked to both rumination and HTR2A, such as alcohol abuse (63–65), binge eating (66, 67) and obsessive-compulsive disorder (68–70). Transdiagnostic relevance of specifically the brooding subtype of rumination has already been suggested in obsessive-compulsive disorder and generalized anxiety disorder among unipolar depressed patients (68). Usefulness of HTR2A rs3125, childhood adversity and brooding in the primary and secondary prevention of all these disorders should be revealed in future studies.
Our study has several limitations which must be stated. Our statements about the effect of childhood adversity on adulthood rumination and the moderating role of genetic variants in this effect could be proven only in case of a longitudinal study design. However, our design being cross-sectional, we can draw conclusions only about associations between childhood stress and adulthood phenotype. A longitudinal design could similarly aid in clarifying the causal roles of rumination and depression in each other.
Furthermore, childhood stress was assessed retrospectively, and only by self-report of our subjects, but not ascertained by other informants. Thus assessment of childhood adversity is subject to memory, recall bias and also state-dependent recall, and could be biased by voluntary distortions related to psychiatric and personality disorders. Similarly, lifetime depression assessment was based on self-report but not corroborated by actual previous disease history.
Since we found a gene-environment correlation between rs3125 and childhood adversity both in the combined sample and in Manchester, it was crucial to replicate our rs3125 interaction findings in Budapest, and to include main effects of gene and environment as covariates in these interaction models. Our rs3125 × childhood adversity finding on brooding could be replicated also in Budapest, underlining that it was not, or not only due to the gene-environment correlations.
In the present study, we did not consider the behavior of individual items with respect to the applied scale scores, but treated them as equivalently weighted in adding up to the respective score. In the future, item-response analyses should argue for or against this equivalent ponderation of the individual items.
Another limitation of our study is the low number of SNPs within the investigated gene. Tagging HTR2A with more SNPs, or broadening our scope to rare variants, structural variants, copy number variants or other length polymorphisms, besides variants with a more diverse spectrum of annotation types (such as amino acid change as an additional type of consequence on gene functioning), will be able to provide a deeper insight into the role of HTR2A gene in the association of childhood stress and rumination. The same concept can then be applied in hypothesis-free genome-wide investigations, going beyond candidate genes.
Rumination was measured only by RRS, a self-report questionnaire that asks people to rate themselves related to when they feel depressed. Other rumination measurements would also be worth investigations with regard to HTR2A.
Stress was defined only as childhood stress. A more detailed picture would be gained by targeting the possible role of other types of stress, such as recent stress or medical conditions.
Our study population is limited to European white participants. To strengthen clinical relevance of our present results, future studies should confirm the same associations in other ethnicities.
Future studies should measure actual epigenetic markers that are supposed to mediate between the revealed GxE interactions and rumination phenotypes.
Both of the investigated genetic variations involved in transmitting two distinct epigenetic regulatory mechanisms, promoter DNA methylation and miRNA binding in the 3′ UTR, acting on HTR2A gene, contribute to the endophenotypic manifestation of rumination, in that their effects can be detectable not only in depression but before or without the emergence of depression. Potentials of targeting HTR2A genetics in depression prevention deserve further studies. Our results on action of HTR2A in rumination phenotypes also underscore the need to include childhood adversity assessment in these possible prevention strategies, and, more broadly, to include stress or other environmental factors in considering endophenotypes, especially in case of polymorphisms conveying epigenetic impacts. Brooding seems a more promising candidate endophenotype in mediating vulnerability to depression than rumination measured more broadly, since its association with HTR2A can be replicated across two different European populations, and mediates the same genetic association with depression. The effect of rs3125 on brooding, depending on childhood adversity level, may have a wider transdiagnostic relevance for other disorders in which brooding may be important such as obsessive-compulsive disorder.
The datasets generated for this study are available on request to the corresponding author.
Our study, carried out in accordance with the Declaration of Helsinki, was approved by the Scientific and Research Ethics Committee of the Medical Research Council, Budapest, Hungary, and by the North Manchester Local Research Ethics Committee, Manchester, United Kingdom. Subjects provided written informed consent.
JD, GB, IA, GJ, RE, and XG designed the study. GJ and XG performed data collection. NE, PP, and DB undertook statistical analyses. NE managed the literature search and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study, as part of NewMood, was supported by the Sixth Framework Program of the European Union (LSHM-CT-2004-503474). Moreover, it was supported by the Hungarian Academy of Sciences (MTA-SE Neuropsychopharmacology and Neurochemistry Research Group), by the Hungarian Brain Research Program (grants KTIA_13_NAPA-II/14 and 2017-1.2.1-NKP-2017-00002, and with the Hungarian National Development Agency, the Hungarian Academy of Sciences and Semmelweis University, grant KTIA_NAP_13-2-2015-0001, MTA-SE-NAP B Genetic Brain Imaging Migraine Research Group), by the National Development Agency (KTIA_NAP_13-1-2013-0001), by the New National Excellence Program of The Ministry of Human Capacities (grants ÚNKP-16-3; ÚNKP-17-3-III-SE-2; ÚNKP-17-4-I-SE-8 and ÚNKP-18-4-SE-33), by TAMOP-4.2.1.B-09/1/KMR-2010-0001, and by the National Institute for Health Research Manchester Biomedical Research Centre. XG is recipient of the Janos Bolyai Research Fellowship of the Hungarian Academy of Sciences. None of the sponsors had any role in study design, data collection, analysis or interpretation, writing the report, or in the decision to submit the paper for publication.
Preliminary results of this work were presented by Nora Eszlari on the ECNP Workshop for Junior Scientists in Europe, 17–20 March 2016, Nice, France. It was published as an abstract in European Neuropsychopharmacology 26, S77–S78, with the title “Brooding subtype of rumination is modulated by the interplay between serotonin receptor 2A gene and childhood adversity” (72).
Results of this work appeared first and only in Nora Eszlari’s PhD dissertation (71). It is in line with the policy of Semmelweis University, and the dissertation can be accessed online (http://semmelweis.hu/wp-content/phd/phd_live/vedes/export/eszlarinora.d.pdf).
RE has received consultancy fees from P1vital and Cambridge Cognition. IA has received grant support from AstraZeneca and Servier, honorarium for speaking from Lundbeck, and consultancy fees from Alkermes, Janssen, Lundbeck/Otsuka, and Servier. JD has performed speaking engagements, consultancy, and research for P1vital, Autifony, Bristol-Myers Squibb, AstraZeneca, Eli Lilly, Janssen-Cilag, Servier, and Schering Plough, with all fees paid to the University of Manchester. He also has share options in P1vital. The firms declared above have not influenced study design, data collection, analysis, interpretation, manuscript preparation, or decision to submit the paper for publication, in any manner.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Cheadle and the Heaton Mersey Medical Practices for recruitment. We further thank Diana Chase, Darragh Downey, Kathryn Lloyd-Williams, Emma J. Thomas, and Zoltan G. Toth for recruitment and data acquisition. We thank Krisztina Mekli and Hazel Platt for genotyping. We also thank Fanni Bákonyi for her help in figure preparation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00394/full#supplementary-material
1. Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci (2008) 3:400–24. doi: 10.1111/j.1745-6924.2008.00088.x
2. Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cogn Ther Res (2003) 27:247–59. doi: 10.1023/A:1023910315561
3. Spasojević J, Alloy LB. Who becomes a depressive ruminator? Developmental antecedents of ruminative response style. J Cogn Psychother (2002) 16:405–19. doi: 10.1891/jcop.16.4.405.52529
4. Raes F, Hermans D. On the mediating role of subtypes of rumination in the relationship between childhood emotional abuse and depressed mood: brooding versus reflection. Depress Anxiety (2008) 25:1067–70. doi: 10.1002/da.20447
5. O’mahen HA, Karl A, Moberly N, Fedock G. The association between childhood maltreatment and emotion regulation: two different mechanisms contributing to depression? J Affect Disord (2015) 174:287–95. doi: 10.1016/j.jad.2014.11.028
6. Padilla Paredes P, Calvete E. Cognitive vulnerabilities as mediators between emotional abuse and depressive symptoms. J Abnorm Child Psychol (2014) 42:743–53. doi: 10.1007/s10802-013-9828-7
7. Heleniak C, Jenness JL, Stoep AV, Mccauley E, Mclaughlin KA. Childhood maltreatment exposure and disruptions in emotion regulation: a transdiagnostic pathway to adolescent internalizing and externalizing psychopathology. Cogn Ther Res (2016) 40:394–415. doi: 10.1007/s10608-015-9735-z
8. Antypa N, Van Der Does AJ. Serotonin transporter gene, childhood emotional abuse and cognitive vulnerability to depression. Genes Brain Behav (2010) 9:615–20. doi: 10.1111/j.1601-183X.2010.00593.x
9. Logue SF, Gould TJ. The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav (2014) 123:45–54. doi: 10.1016/j.pbb.2013.08.007
10. Larsen BA, Christenfeld NJ. Cardiovascular disease and psychiatric comorbidity: the potential role of perseverative cognition. Cardiovasc Psychiatry Neurology (2009) 2009:791017. doi: 10.1155/2009/791017
11. Whitmer AJ, Gotlib IH. An attentional scope model of rumination. Psychol Bull (2013) 139:1036–61. doi: 10.1037/a0030923
12. Macoveanu J, Hornboll B, Elliott R, Erritzoe D, Paulson OB, Siebner H, et al. Serotonin 2A receptors, citalopram and tryptophan-depletion: a multimodal imaging study of their interactions during response inhibition. Neuropsychopharmacology (2013) 38:996–1005. doi: 10.1038/npp.2012.264
13. Aznar S, Klein AB. Regulating prefrontal cortex activation: an emerging role for the 5-HT(2)A serotonin receptor in the modulation of emotion-based actions? Mole Neurobiol (2013) 48:841–53. doi: 10.1007/s12035-013-8472-0
14. Meyer JH, Mcmain S, Kennedy SH, Korman L, Brown GM, Dasilva JN, et al. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry (2003) 160:90–9. doi: 10.1176/appi.ajp.160.1.90
15. Stein DJ, Hemmings S, Moolman-Smook H, Audenaert K. 5-HT2A: its role in frontally mediated executive function and related psychopathology. CNS Spectr (2007) 12:512–6. doi: 10.1017/S1092852900021246
16. Baeken C, Bossuyt A, De Raedt R. Dorsal prefrontal cortical serotonin 2A receptor binding indices are differentially related to individual scores on harm avoidance. Psychiatry Res Neuroimaging (2014) 221:162–8. doi: 10.1016/j.pscychresns.2013.12.005
17. Lopizzo N, Bocchio Chiavetto L, Cattane N, Plazzotta G, Tarazi FI, Pariante CM, et al. Gene–environment interaction in major depression: focus on experience-dependent biological systems. Front Psychiatry (2015) 6:68. doi: 10.3389/fpsyt.2015.00068
18. Bani-Fatemi A, Howe AS, Matmari M, Koga A, Zai C, Strauss J, et al. Interaction between methylation and CpG single-nucleotide polymorphisms in the HTR2A gene: association analysis with suicide attempt in schizophrenia. Neuropsychobiology (2016) 73:10–5. doi: 10.1159/000441191
19. Abdolmaleky HM, Yaqubi S, Papageorgis P, Lambert AW, Ozturk S, Sivaraman V, et al. Epigenetic dysregulation of HTR2A in the brain of patients with schizophrenia and bipolar disorder. Schizophr Res (2011) 129:183–90. doi: 10.1016/j.schres.2011.04.007
20. Ucok A, Alpsan H, Cakir S, Saruhan-Direskeneli G. Association of a serotonin receptor 2A gene polymorphism with cognitive functions in patients with schizophrenia. Am J Med Genet B Neuropsychiatr Genet (2007) 144B:704–7. doi: 10.1002/ajmg.b.30463
21. Lane HY, Liu YC, Huang CL, Hsieh CL, Chang YL, Chang L, et al. Prefrontal executive function and D1, D3, 5-HT2A and 5-HT6 receptor gene variations in healthy adults. J Psychiatry Neurosci (2008) 33:47–53.
22. Saiz PA, Garcia-Portilla MP, Paredes B, Arango C, Morales B, Alvarez V, et al. Association between the A-1438G polymorphism of the serotonin 2A receptor gene and nonimpulsive suicide attempts. Psychiatry Genet (2008) 18:213–8. doi: 10.1097/YPG.0b013e3283050ada
23. Banlaki Z, Elek Z, Nanasi T, Szekely A, Nemoda Z, Sasvari-Szekely M, et al. Polymorphism in the serotonin receptor 2a (HTR2A) gene as possible predisposal factor for aggressive traits. PLoS One (2015) 10:e0117792. doi: 10.1371/journal.pone.0117792
24. Dong ZQ, Li XR, He L, He G, Yu T, Sun XL. 5-HTR1A and 5-HTR2A genetic polymorphisms and SSRI antidepressant response in depressive Chinese patients. Neuropsychiatr Dis Treat (2016) 12:1623–29. doi: 10.2147/NDT.S93562
25. Ghadirivasfi M, Nohesara S, Ahmadkhaniha HR, Eskandari MR, Mostafavi S, Thiagalingam S, et al. Hypomethylation of the serotonin receptor type-2A Gene (HTR2A) at T102C polymorphic site in DNA derived from the saliva of patients with schizophrenia and bipolar disorder. Am J Med Genet B Neuropsychiatr Genet (2011) 156B:536–45. doi: 10.1002/ajmg.b.31192
26. Bonnier B, Gorwood P, Hamon M, Sarfati Y, Boni C, Hardy-Bayle MC. Association of 5-HT(2A) receptor gene polymorphism with major affective disorders: the case of a subgroup of bipolar disorder with low suicide risk. Biol Psychiatry (2002) 51:762–5. doi: 10.1016/S0006-3223(01)01228-8
27. Parsons MJ, D’souza UM, Arranz MJ, Kerwin RW, Makoff AJ. The -1438A/G polymorphism in the 5-hydroxytryptamine type 2A receptor gene affects promoter activity. Biol Psychiatry (2004) 56:406–10. doi: 10.1016/j.biopsych.2004.06.020
28. Myers RL, Airey DC, Manier DH, Shelton RC, Sanders-Bush E. Polymorphisms in the regulatory region of the human serotonin 5-HT2A receptor gene (HTR2A) influence gene expression. Biol Psychiatry (2007) 61:167–73. doi: 10.1016/j.biopsych.2005.12.018
29. Gu L, Long J, Yan Y, Chen Q, Pan R, Xie X, et al. HTR2A-1438A/G polymorphism influences the risk of schizophrenia but not bipolar disorder or major depressive disorder: a meta-analysis. J Neurosci Res (2013) 91:623–33. doi: 10.1002/jnr.23180
30. Jin C, Xu W, Yuan J, Wang G, Cheng Z. Meta-analysis of association between the -1438a/g (rs6311) polymorphism of the serotonin 2A receptor gene and major depressive disorder. Neurol Res (2013) 35:7–14. doi: 10.1179/1743132812Y.0000000111
31. Fiocco AJ, Joober R, Poirier J, Lupien S. Polymorphism of the 5-HT2A receptor gene: association with stress-related indices in healthy middle-aged adults. Front Behav Neurosci (2007) 1:3. doi: 10.3389/neuro.08.003.2007
32. Mccaffery JM, Duan QL, Frasure-Smith N, Barhdadi A, Lesperance F, Theroux P, et al. Genetic predictors of depressive symptoms in cardiac patients. Am J Med Genet B Neuropsychiatr Genet (2009) 150B:381–8. doi: 10.1002/ajmg.b.30824
33. Derogatis LR. BSI: Brief Symptom Inventory: Administration, Scoring, and Procedures Manual. Minneapolis: National Computer Systems Pearson, Inc. (1993).
34. Juhasz G, Dunham JS, Mckie S, Thomas E, Downey D, Chase D, et al. The CREB1-BDNF-NTRK2 pathway in depression: multiple gene–cognition–environment interactions. Biol Psychiatry (2011) 69:762–71. doi: 10.1016/j.biopsych.2010.11.019
35. Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry (1994) 151:1132–6. doi: 10.1176/ajp.151.8.1132
36. Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet (2003) 33:67–72. doi: 10.1023/A:1021055617738
37. Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Juhasz G, et al. New evidence for the association of the serotonin transporter gene (SLC6A4) haplotypes, threatening life events, and depressive phenotype. Biol Psychiatry (2008) 64:498–504. doi: 10.1016/j.biopsych.2008.03.030
38. Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2013). Available: http://www.R-project.org/ [Accessed].
39. Juhasz G, Gonda X, Hullam G, Eszlari N, Kovacs D, Lazary J, et al. Variability in the effect of 5-HTTLPR on depression in a large European population: the role of age, symptom profile, type and intensity of life stressors. PloS One (2015) 10:e0116316. doi: 10.1371/journal.pone.0116316
40. Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation, and simultaneous conservative consistency of false discovery rates: a unified approach. J R Stat Soc Series B (2004) 66:187–205. doi: 10.1111/j.1467-9868.2004.00439.x
41. Tammen SA, Friso S, Choi SW. Epigenetics: the link between nature and nurture. Mol Aspects Med (2013) 34:753–64. doi: 10.1016/j.mam.2012.07.018
42. Nestler EJ, Pena CJ, Kundakovic M, Mitchell A, Akbarian S. Epigenetic basis of mental illness. Neuroscientist (2016) 22:447–63. doi: 10.1177/1073858415608147
43. Issler O, Chen A. Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci (2015) 16:201–12. doi: 10.1038/nrn3879
44. Paquette AG, Lesseur C, Armstrong DA, Koestler DC, Appleton AA, Lester BM, et al. Placental HTR2A methylation is associated with infant neurobehavioral outcomes. Epigenetics (2013) 8:796–801. doi: 10.4161/epi.25358
45. Paquette AG, Marsit CJ. The developmental basis of epigenetic regulation of HTR2A and psychiatric outcomes. J Cell Biochem (2014) 115:2065–72. doi: 10.1002/jcb.24883
46. Dressler WW, Balieiro MC, Ferreira De Araujo L, Silva WA Jr., Ernesto Dos Santos J. Culture as a mediator of gene–environment interaction: cultural consonance, childhood adversity, a 2A serotonin receptor polymorphism, and depression in urban Brazil. Soc Sci Med (2016) 161:109–17. doi: 10.1016/j.socscimed.2016.05.033
47. Chang CC, Fang WH, Chang HA, Chang TC, Shyu JF, Huang SY. Serotonin 2A receptor (5-HT2A) gene promoter variant interacts with chronic perceived stress to modulate resting parasympathetic activity in humans. Psychoneuroendocrinology (2017) 76:119–26. doi: 10.1016/j.psyneuen.2016.11.015
48. Woody ML, Mcgeary JE, Gibb BE. Brooding rumination and heart rate variability in women at high and low risk for depression: group differences and moderation by COMT genotype. J Abnorm Psychol (2014) 123:61–7. doi: 10.1037/a0035450
49. Mcauley EZ, Fullerton JM, Blair IP, Donald JA, Mitchell PB, Schofield PR. Association between the serotonin 2A receptor gene and bipolar affective disorder in an Australian cohort. Psychiatr Genet (2009) 19:244–52. doi: 10.1097/YPG.0b013e32832ceea9
50. Peterson SM, Thompson JA, Ufkin ML, Sathyanarayana P, Liaw L, Congdon CB. Common features of microRNA target prediction tools. Front Gene (2014) 5:23. doi: 10.3389/fgene.2014.00023
51. Ding M, Shen W, Hu Y. The Role of miR-539 in the anterior cingulate cortex in chronic neuropathic pain. Pain Med (2017) 18:2433–42. doi: 10.1093/pm/pnx004
52. Kuhn S, Vanderhasselt MA, De Raedt R, Gallinat J. Why ruminators won’t stop: the structural and resting state correlates of rumination and its relation to depression. J Affect Disord (2012) 141:352–60. doi: 10.1016/j.jad.2012.03.024
53. Baeken C, De Raedt R, Bossuyt A. Is treatment-resistance in unipolar melancholic depression characterized by decreased serotonin2A receptors in the dorsal prefrontal – anterior cingulate cortex? Neuropharmacology (2012) 62:340–6. doi: 10.1016/j.neuropharm.2011.07.043
54. Walters JT, Owen MJ. Endophenotypes in psychiatric genetics. Mol Psychiatry (2007) 12(10):886–90. doi: 10.1038/sj.mp.4002068
55. Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med (2007) 37:163–80. doi: 10.1017/S0033291706008750
56. Bagby RM, Rector NA, Bacchiochi JR, Mcbride C. The stability of the response styles questionnaire rumination scale in a sample of patients with major depression. Cogn Ther Res (2004) 28:527–38. doi: 10.1023/B:COTR.0000045562.17228.29
57. Gibb BE, Grassia M, Stone LB, Uhrlass DJ, Mcgeary JE. Brooding rumination and risk for depressive disorders in children of depressed mothers. J Abnorm Child Psychol (2012) 40:317–26. doi: 10.1007/s10802-011-9554-y
58. Wilkinson PO, Croudace TJ, Goodyer IM. Rumination, anxiety, depressive symptoms and subsequent depression in adolescents at risk for psychopathology: a longitudinal cohort study. BMC Psychiatry (2013) 13:250. doi: 10.1186/1471-244X-13-250
59. Gottesman Ii, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry (2003) 160:636–45. doi: 10.1176/appi.ajp.160.4.636
60. Poddar S, Kesharwani D, Datta M. Interplay between the miRNome and the epigenetic machinery: implications in health and disease. J Cell Physiology (2017) 232:2938–45. doi: 10.1002/jcp.25819
61. Abernathy DG, Kim WK, Mccoy MJ, Lake AM, Ouwenga R, Lee SW, et al. MicroRNAs induce a permissive chromatin environment that enables neuronal subtype-specific reprogramming of adult human fibroblasts. Cell Stem Cell (2017) 21:332–48. doi: 10.1016/j.stem.2017.08.002
62. Swathy B, Banerjee M. Haloperidol induces pharmacoepigenetic response by modulating miRNA expression, global DNA methylation and expression profiles of methylation maintenance genes and genes involved in neurotransmission in neuronal cells. PLoS One (2017) 12:e0184209. doi: 10.1371/journal.pone.0184209
63. Nolen-Hoeksema S, Harrell ZA. Rumination, depression, and alcohol use: tests of gender differences. J Cogn Psychother (2002) 16:391–403. doi: 10.1891/jcop.16.4.391.52526
64. Caselli G, Ferretti C, Leoni M, Rebecchi D, Rovetto F, Spada MM. Rumination as a predictor of drinking behaviour in alcohol abusers: a prospective study. Addiction (2010) 105:1041–8. doi: 10.1111/j.1360-0443.2010.02912.x
65. Cao J, Liu X, Han S, Zhang CK, Liu Z, Li D. Association of the HTR2A gene with alcohol and heroin abuse. Hum Genet (2014) 133:357–65. doi: 10.1007/s00439-013-1388-y
66. Nolen-Hoeksema S, Stice E, Wade E, Bohon C. Reciprocal relations between rumination and bulimic, substance abuse, and depressive symptoms in female adolescents. J Abnorm Psychol (2007) 116:198–207. doi: 10.1037/0021-843X.116.1.198
67. Koren R, Duncan AE, Munn-Chernoff MA, Bucholz KK, Lynskey MT, Heath AC, et al. Preliminary evidence for the role of HTR2A variants in binge eating in young women. Psychiatr Genet (2014) 24:28–33. doi: 10.1097/YPG.0000000000000014
68. Watkins ER. Depressive rumination and co-morbidity: evidence for brooding as a transdiagnostic process. J Ration Emot Cogn Behav Ther (2009) 27:160–75. doi: 10.1007/s10942-009-0098-9
69. Taylor S. Molecular genetics of obsessive-compulsive disorder: a comprehensive meta-analysis of genetic association studies. Mol Psychiatry (2013) 18:799–805. doi: 10.1038/mp.2012.76
70. Taylor S. Disorder-specific genetic factors in obsessive-compulsive disorder: a comprehensive meta-analysis. Am J Med Genet B Neuropsychiatr Genet (2016) 3:325–32. doi: 10.1002/ajmg.b.32407
71. Eszlari N. Biological mechanisms in the background of ruminative response style. [dissertation]. Budapest, Hungary: Semmelweis University (2018).
Keywords: childhood stress, rumination, brooding, serotonin system, perseverative thought
Citation: Eszlari N, Petschner P, Gonda X, Baksa D, Elliott R, Anderson IM, Deakin JFW, Bagdy G and Juhasz G (2019) Childhood Adversity Moderates the Effects of HTR2A Epigenetic Regulatory Polymorphisms on Rumination. Front. Psychiatry 10:394. doi: 10.3389/fpsyt.2019.00394
Received: 04 March 2019; Accepted: 17 May 2019;
Published: 14 June 2019.
Edited by:
Divya Mehta, Queensland University of Technology, AustraliaReviewed by:
Ludwig Stenz, Université de Genève, SwitzerlandCopyright © 2019 Eszlari, Petschner, Gonda, Baksa, Elliott, Anderson, Deakin, Bagdy and Juhasz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nora Eszlari, ZXN6bGFyaS5ub3JhQHBoYXJtYS5zZW1tZWx3ZWlzLXVuaXYuaHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.