
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 06 June 2019
Sec. Social Psychiatry and Psychiatric Rehabilitation
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.00366
 Claudia Carmassi1
Claudia Carmassi1 Laura Palagini1*
Laura Palagini1* Danila Caruso1
Danila Caruso1 Isabella Masci1
Isabella Masci1 Lino Nobili2,3
Lino Nobili2,3 Antonio Vita4
Antonio Vita4 Liliana Dell’Osso1
Liliana Dell’Osso1Background: A compelling number of studies, conducted in both children and adults, have reported an association between sleep disturbances/circadian sleep alterations and autism spectrum disorder (ASD); however, the data are sparse and the nature of this link is still unclear. The present review aimed to systematically collect the literature data relevant on sleep disturbances and circadian sleep dysrhythmicity related to ASD across all ages and to provide an integrative theoretical framework of their association.
Methods: A systematic review of the MEDLINE, PubMed, and Cochrane databases was conducted from November 2018 to February 2019. The search strategies used were MeSH headings and keywords for “sleep–wake circadian rhythms” OR “circadian sleep disorders” OR “sleep–wake pattern” OR “sleep disorders” OR “melatonin” AND “autism spectrum disorder” OR “autism”.
Results: One hundred and three studies were identified, 15 regarded circadian sleep dysrhythmicity, 74 regarded sleep disturbances, and 17 regarded melatonin alterations in children and adults with ASD. Our findings suggested that autistic subjects frequently present sleep disturbances in particular short sleep duration, low sleep quality/efficiency, and circadian sleep desynchronization such as delayed phases and/or eveningness. Sleep disturbances and circadian sleep alterations have been related to the severity of autistic symptoms. Genetic studies have shown polymorphisms in circadian CLOCK genes and in genes involved in melatonin pathways in subjects with ASD.
Conclusions: Sleep disturbances and circadian sleep alterations are frequent in subjects with autistic symptoms. These subjects have shown polymorphisms in clock genes expression and in genes involved in melatonin production. The impairment of circadian sleep regulation may increase the individual’s vulnerability to develop symptoms of ASD by altering the sleep regulation in toto, which plays a key role in normal brain development. Even though controversies and “research gaps” are present in literature at this point, we may hypothesize a bidirectional relation between circadian sleep dysfunction and ASD. In particular, circadian sleep dysrhythmicity may predispose to develop ASD symptoms and vice versa within a self-reinforcing feedback loop. By targeting sleep disturbances and circadian sleep dysrhythmicity, we may improve treatment strategies for both children and adults with ASD.
● Children and adults with autistic symptoms often experience sleep disturbances and alterations in circadian sleep rhythmicity.
● Children and adults with autistic symptoms have shown mutations and polymorphisms in clock gene expression related to irregular and delayed sleep phases and to sleep problems.
● Abnormalities in the expression of genes regulating melatonin pathways may be responsible for low melatonin levels and for circadian sleep disturbances in subjects with autistic symptoms.
● Genetic abnormalities in the circadian system may impair the sleep system in toto with negative consequences on brain development contributing to autistic symptoms.
● The impairment of circadian sleep rhythmicity may increase the individual’s vulnerability to develop symptoms of autism spectrum disorder.
● Vice versa, alteration in brain development related to autism spectrum disorder autistic symptoms may contribute to sleep disturbances within a self-reinforcing loop.
● The evaluation and treatment of circadian sleep disorders in autism spectrum disorders may be useful to improve the trajectories of subjects with autistic symptoms.
Autism spectrum disorder (ASD) is an early-onset neurodevelopmental disorder whose core features have been defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (1). It is characterized as persistent difficulties in social interaction and communication, presence of stereotypic behaviors, restricted interests, and atypical sensory reactivity. ASD, in fact, encompasses a set of clinical phenotypes that includes previously described autistic disorder (AD) and Asperger syndrome (AS), from severe to mild variants as endpoints of a continuum upon a spectrum model approach (2, 3). Intellectual disability is observed in more than half of ASD cases (4, 5) and ASD autistic symptoms also affect social language skills and emotion regulation. In addition, ASD symptoms are often related to coexisting mental disorders and to other developmental disorders (6).
Within the last decades, the diagnosis rate of autism has increased dramatically, and it has been reported that cases of ASD have a rate of 0.6–0.8% in preschool children, 1.0% in school children and young adults, and around 1.0% in adults (7, 8). In the last few years, an increased interest has been developed for mild forms of ASD, which often remain undiagnosed or misdiagnosed until adulthood. Although ASD is defined as a developmental disorder because symptoms appear within the first 2 years of life, it is generally considered a lifelong disorder with negative consequences on scholastic, working, social, and economic performances and quality of life. Some studies have shown that mild forms of autism are related to high rates of psychiatric comorbidity in adulthood such as anxiety, mood disorders, psychosis, stress-related disorders, and suicidal behaviors (9, 10).
In this framework, understanding the mechanisms involved in the development of ASD should be a priority for identifying early markers that could help improve early diagnosis with a significant impact on lifelong prognosis (3). The mechanisms underlying the development of ASD may be the result of the interaction between multiple gene arrangement and the environment that may lead to the alteration of brain structures and functions (11, 12). This interaction may determine epigenetic alterations disrupting the regulation of gene expression with a negative impact on biological pathways relevant for brain development (13). Abnormalities during brain development in autistic subjects go beyond the “social brain” encompassing sensory processing and attentional control. In fact, current evidence strongly supports a model of brain-wide abnormalities during the early development of autistic children (12). Indeed, it remains an open question if a single mechanism or several independent factors can lead to the emergence of autism.
In the last few years, a new hypothesis has emerged, suggesting the role of circadian system desynchronization in the development of ASD (11, 14). Human physiological and biochemical processes as well as behavioral patterns have a circadian rhythmicity orchestrated by the master biological clock of the hypothalamus: the suprachiasmatic nuclei (SCN). The expression of many genes changes rhythmically over 24 h and the specific circadian genes are responsible for the main SCN clock-working machinery as well as that of subsidiary clocks at the peripheral level [among them: circadian locomotor output cycles kaput (CLOCK), brain and muscle ARNT-like protein 1 (BMAL1), cryptochromes CRY1-2, and band period homolog (PER)] [(15, 16); for an overview see Ref. (17)]. The suprachiasmatic nuclei is daily synchronized by environmental signals such as light, food intake, activities, or social cues and exposure to stress/trauma (18–20), and while driving, secretion of the melatonin hormone regulates peripheral clock within feed-forward mechanism. Rhythmic clock gene expression regulates multiple monoaminergic brain regions that control mood and motivational behaviors, stress and inflammatory systems, reward circuits, arousal, and sleep by interacting with the homeostatic regulation of sleep and wake [for an overview, see Refs. (17, 21)]. The circadian system is critical for the synchronization with the environment and allows a correct functioning of various internal physiological processes essential for the optimization of responses to environmental fluctuations and for the strengthening of homeostatic control mechanisms (21). Abnormalities in the maturation of the circadian system principally lead to alterations in the sleep–wake pattern (22), which may interest around 50–80% of subjects with ADs (23). Wimpory and colleagues (24) have hypothesized that timing and social timing deficits were relevant in subjects with AD symptoms and were related to pathological variations in the structure/function of clock/clock-related genes: the authors hypothesized a key role for circadian sleep dysregulation in autistic symptoms. This hypothesis has been confirmed by later studies, which have shown circadian-relevant gene abnormalities in ADs (25, 26). Mutations in Clock, Bmal1, Cry1, and Cry2 genes determine an alteration in the circadian system regulation as well as in sleep fragmentation (27, 28). Sleep is increasingly recognized as a key process in neurodevelopment and in brain optimization processes [for an overview, see Ref. (29)]. Both humans and animals’ data have shown that sleep is essential for maturation of fundamental brain functions, and epidemiological findings increasingly indicate that children with early sleep disturbances suffer from later cognitive, attentional, and psychiatric problems. Indeed, from birth throughout infancy and early childhood, sleep patterns undergo dramatic changes that include the gradual consolidation of sleep and waking cycles, the intensification of deep NREM sleep slow-wave activity (EEG power in the 1–4.5 Hz frequency range), and a progressive decrease of REM sleep proportion. It has been suggested that REM sleep is an inducer of brain development and of early myelination in the sensory processing areas in the fetus and the newborns and that it follows the maturational trajectory of the brain (29–34). Animal studies have shown that REM sleep has multifaceted functions in brain development, including learning and memory consolidation by selectively eliminating and maintaining newly formed synapses (35). On the other hand, slow-wave sleep and sleep spindles (10–14 Hz) seem to be even involved in synaptic remodeling, being important for synaptic strength and synchronized neuronal firing, and it has been shown that while following the trajectory of brain maturation, they may orchestrate synaptic plasticity and pruning during brain development (30, 36–40). Particularly, Ringli and Huber hypothesized that slow-wave sleep may contribute to cortical maturation by playing a role in the balance of brain synaptic strengthening/formation weakening/elimination that is tilted during development (39). Sleep promotes myelination and oligodendrocyte precursor cell proliferation (41), enhances transcription of genes involved in synthesis and maintenance of membranes and myelin too (42), and modulates the neuronal membrane homeostasis (43). Since adequate sleep has been proposed to be fundamental for brain development (31, 35, 39), sleep has received considerable research attention, as it appears to be important in the study of neurodevelopmental psychopathology (31, 39, 44–46). Hence, if sleep is fundamental for brain development, we may hypothesize that sleep disturbances, via alterations in brain development, may contribute to autistic symptoms. This idea has been previously developed for other psychiatric disorders. In fact, extensive data have shown that poor sleep during childhood and adolescence is related to alterations in brain development (39, 44, 47–53) to problems in cognitive, attentional, emotional, and behavioral areas, including risk-taking and aggression; and to psychiatric conditions such as attention deficit hyperactivity disorder and mood disorders (54). Indeed, we may also hypothesize that alteration in brain development related to ASD may contribute to sleep disturbances within a self-reinforcing loop.
On this basis, this review was aimed to systematically collect the literature data relevant on sleep disturbances and circadian sleep dysrhythmicity related to ASD across all ages; a comprehensive review that has been conducted recently was limited to youth (55). In particular, we aimed to construct an integrative theoretical framework of their association: a comprehensive framework would be quite useful from a clinical and therapeutic point of view for identifying elements to evaluate and target in the clinical practice.
The hypothesis of this review was that abnormal maturation of the circadian sleep system may lead to the disruption of the sleep system in toto that, by impairing brain neurodevelopment and melatonin production, may have a key role in ASD. The investigation of such elements may be helpful to a better understanding of the neurodevelopmental pathways of ASD. Hence, the aim of this review was to systematically collect the literature data on sleep and circadian disturbances in children and adults with ASD.
We performed a systematic review, based on Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guidelines, of articles published up till February 2019, and indexed in the following databases: MEDLINE, PubMed, and Cochrane Library. The search strategies used MeSH headings and keywords for “sleep–wake circadian rhythms” OR “circadian sleep disorders” OR “sleep–wake pattern” OR “sleep disorders” OR “melatonin” AND “autism spectrum disorder” OR “autism.”
Inclusion criteria: The searches were limited to the English language studies conducted on human populations, including longitudinal, cross-sectional, or case–control studies, analyzing the relationship between sleep disturbances and/or circadian sleep disorders and ASD.
Exclusion criteria: Studies that did not have as principal focus the evaluation of sleep disturbances and/or circadian sleep rhythms related to autism were excluded. We also excluded studies that investigated subjects with other neurodevelopmental disorders or intellectual disabilities.
The abstracts located from the search strategy were entered into EndNote. The searches were limited to the English language studies conducted on human populations, including longitudinal, cross-sectional, or case–control studies, analyzing the relationship between sleep disturbances and/or circadian sleep disorders and ASD. Title and abstracts of all non-duplicated papers were independently screened by two of the authors (DC and IM). Potential pertinent papers were retained and assessed for eligibility by screening the full text. Two senior authors (LP and CC) acted as arbitrators when there is disagreement in any screening stage. All the authors independently evaluated the quality of the included studies. Disagreements between authors were resolved by consensus. Initial literature search returned 1,443 records (among which 7 studies were searched manually from other sources), 652 after exclusion of duplicates. Following preliminary screening of the titles and the exclusion of reviews and case reports, 492 of the retrieved articles were excluded; further 57 records were excluded after reading abstracts and full texts based on the relevance of data for the aim of the present study (Figure 1).
Meta-analysis was not conducted due to heterogeneity in definition and measurement of outcomes.
The systematic review of literature returned 103 articles focused on the sleep–wake pattern in neurodevelopmental disorders on children and an adult population according to the evolution of nosography; these studies include both previously defined diagnoses (56), such as AD, AS, childhood disintegrative disorder (CDD), pervasive developmental disorder (PDD), and not otherwise specified (NAS) (2), and the most recent diagnosis of ASD (1).
Seven studies have evaluated the alterations of circadian rhythms in children with ASD (Table 1). The first study (57) reported no tendency for irregular sleep–wake pattern, delayed sleep phase syndrome, or advanced sleep phase syndrome in children with ASD. Similar results were found in another study and in addition, no circadian phase preference was detected (61). On the other hand, several studies have shown that children with ASD may show sleeplessness with two specific circadian problems: phase delay of sleep periods and an irregular sleep–wake pattern. Some recent studies have investigated the genetic patterns of circadian rhythmicity in autistic subjects. First, Nicholas et al. (25) screened 110 individuals with ASD and their parents, analyzing the single-nucleotide polymorphisms (SNPs) in 11 clock/clock-related genes. A significant allelic association was detected for Period Circadian Regulator 1 (PER1) and Neuronal PAS domain protein 2 (NPAS2) and ASD. A later study (59) described the circadian deficits in del 2q231 of lymphoblastoid cell lines (LCLs) in 19 ASD children with high prevalence of sleep disturbances. They found that circadian gene mRNA levels of NR1D2 (nuclear receptor subfamily 1 group D member 1), PER1, PER2, and PER3 were altered in del 2q23.1. Yang et al. (26) sequenced the coding regions of 18 canonical clock genes and clock-controlled genes in 28 ASD patients and 23 controls. They detected several mutations/SNPs in circadian-relevant genes affecting gene function in the ASD patients. Mutations in NR1D1, CLOCK, and ARNTL2 were detected only in individuals with ASD with sleep disorder. The most recent work (60) tested mutations in all exons of NR1D1 in 198 autistic subjects. They detected single-base changes with an amino acid substitution in the coding region of NR1D1 in individuals with ASD. In summary, children with ASD show circadian and sleep rhythms alterations such as irregular and delayed sleep phase and several mutations in the expression of clock genes.
Sixty-five studies have been evaluated on the sleep pattern of children with ASD (Table 2), and some of them reported a prevalence rate of sleep disturbances between 64% and 93% (65, 71, 95, 108, 109, 114, 125) underlining a great prevalence of sleep disturbances during early life (62, 63, 65, 86, 109, 125) with no gender differences (62, 89). In particular, total sleep time has shown to be reduced starting from 30 months of age toward adolescence (98, 104, 107).
Researches based on parental reports and objective measurements (actigraphy and polysomnography) have suggested that autistic children are more likely to have sleep difficulties than children with other neurodevelopment disorder (69, 70, 74, 75, 78, 80, 83, 90, 107) and children with normal development (62, 64, 69, 80, 85, 90, 91, 120). Among autistic subjects, some studies have reported that children with intellectual disabilities and/or with low levels of functioning may experience higher frequency of sleep disturbances (58, 62), while other studies have reported that high-functioning autistics may experience more sleep problems (shorter sleep duration, early night awakenings, and longer sleep latencies) than low-functioning children and control groups (63, 64). Some studies conducted in adolescents have also reported that those with high-functioning autism were three times more likely to have sleep problems than their peers with normal development (102). The most frequent sleep pattern observed across all the studies included the following: difficulties falling asleep (63–65, 72, 78, 89, 91, 109), restless sleep (64, 72), difficulties falling asleep in own beds with late bedtime (58, 104, 119), frequent nighttime awakenings (58, 63, 65, 70, 72, 78, 85, 91, 104, 107, 119), early morning awakenings (63, 65, 72), low sleep efficiency (85, 91), short time duration (58, 63, 72, 109, 104, 119), and daytime sleeplessness (71, 109). Nightmares, morning headaches, crying during sleep, sleep apnea (72), and sleepwalking (64, 72) were also frequent. Although sleep problems were significant across all ages, subjects during adolescence tend to show a shorter sleep duration than toddlers who tend to present more frequent bedtime resistance, sleep anxiety, parasomnias, and night awakenings (98, 115). Some of the studies reviewed have analyzed sleep disturbances by means of the polysomnographic registrations in autistic subjects. Studies have shown a significant reduction of REM sleep proportion and a significant presence of REM activity in non-REM sleep compared to subjects with normal development (66, 69). One study (66) on 10 autistic children and 8 children with Down Syndrome reported sleep continuity was disturbed by an increased number of night awakenings with a consequent reduction of sleep efficiency. In a later study (68), patients with AS showed a decrease in sleep time in the first two-thirds of the night and an increase in number of shifts into REM sleep from night awakenings. As reported in other studies, subjects with ASD may experience prolonged sleep latency, decreased sleep efficiency, and decreased sleep duration on the first night of polysomnographic registration (80, 93, 120). Studies based on actigraphic registrations confirmed the data recorded using logs and/or sleep diaries observing frequent early night arousals, a delay of sleep period, insomnia, and less total sleep time within the 24 h in autistic children (67, 86, 87, 90, 126). As reported in several studies, insomnia and sleepiness were correlated to the severity and prevalence of typical autism behavioral symptoms (69, 71, 73, 76, 77, 83, 84, 95, 99, 100, 112, 113). In particular, a study of Schreck et al. (73) found a correlation between insomnia and the prevalence of communication problems due to an increased sensitivity to stimuli in the sleeping environment. Further studies (101, 111) found a correlation between typical autistic behaviors and chronic insomnia. Children with ASD and poor sleep were characterized by a major representation of symptoms of autism such as restricted/repetitive behaviors (91, 113), language impairment, problems with reciprocal social interactions, low overall intelligence (80, 100, 103, 109), and even physical aggression, irritability, and oppositional defiant disorder (83, 84, 116, 122). Affective disturbances, particularly depression (80) and anxiety-related problems (71, 95, 103, 105, 109), have even been described in ASD with poor sleep. Some research groups (97, 100, 106) have analyzed the relation between insomnia, psychopathology, and daytime functioning in both children and adolescents with autism and reported a severity of depressed mood, anxiety, and daytime functioning in relation to sleep disturbances. Rzepecka and colleagues (95) reported a significant correlation between sleep problems, challenging behavior, and anxiety in children with intellectual disability and/or ASD. This study indicated that the 41.9% of the variance of challenging behavior was predicted by sleep problems and anxiety. The first study (110) that has analyzed the relationship between anxiety, sensory problems, and sleep disturbances in autism showed that children with anxiety and sensory overresponsivity may be particularly predisposed to develop sleep problems. A recent study of Veatch et al. (123) has demonstrated that shorter sleep duration was associated not only with depressive and obsessive–compulsive disorders but also with social impairment such as a failure to develop peer relationships and with maladaptive behaviors in ASD. Children who reported 7 h of sleep per night showed a severe impairment in social/communication compared to ASD children who tended to sleep 10–11 h per night (123). Anxiety, autism symptom severity, sensory sensitivities, snoring, obstructive sleep apneas, pain, and gastrointestinal problems were also associated with sleep disturbances (103, 108, 112, 118). In summary, sleep disturbances are frequent in children and adolescents with ASD. Insomnia and sleepiness were the most commonly reported symptoms together with long sleep latency, long time spent awake after sleep onset, early time awakening, and short sleep time. As shown in several studies, sleep disturbances have been related to the severity of ASD symptoms such as difficulties in social interaction and communication, presence of stereotypic behaviors, as well as anxiety and depressive symptoms.
Five studies analyzed the circadian rhythmicity in adults with ASD (Table 3). The most frequent circadian sleep disorders was the delayed sleep phase (126, 128–130). The most recent study (130) has investigated two groups (41 adults with ASD and intellectual disability versus 51 normal development) by means of ambulatory circadian monitoring, recording temperature, motor activity, body position, sleep, and light intensity; the circadian phase advanced was more common in the ASD group compared to controls. In summary, despite the fact that a few studies have investigated circadian sleep dysrhythmicity in adults with ASD, all the results showed that a delayed sleep phase is frequent in adults with ASD.
To date, only nine studies have described the sleep pattern on adults with ASD (Table 4). Three studies conducted from the same group (131–133) that compared AS to controls have shown similar polysomnographic sleep patterns. On the other hand, some further studies have suggested that 80% of adolescents and young adults with autism and AS may experience sleep problems (68, 106, 135). Sleep disturbances described in adults with ASD were similar to those described in children and were characterized by low sleep efficiency, short sleep duration, long sleep latency, frequent nighttime awakenings, and daytime sleepiness (135, 137). High-functioning adults with ASD reported insomnia and/or a significant phase advancement showing longer sleep latency, more frequent nocturnal awakenings, lower sleep efficiency, increased duration of NREM stage 1, and decreased non-REM slow-wave sleep compared to the healthy control group (134). Further, low total sleep time was correlated with social and communication impairments in autistic subjects (106, 134). Some authors have also reported that the presence of poor sleep in adults with high-functioning autism correlated with various aspects of motor output on nonverbal performance tasks, suggesting that sleep disturbances in ASD might affect attention and/or memory components in the nonverbal modality (136). In summary, the main sleep problems in adults with ASD were insomnia, low sleep efficiency, short sleep duration, long sleep latency, frequent nighttime awakenings, and daytime sleepiness. As shown in some studies, sleep disturbances were related to difficulties in social interaction, communication, and cognitive performance.
According to our research methods, we found 17 studies on the role of melatonin in circadian rhythm dysregulation in ASD (Table 5). Two different studies (138, 139) measured melatonin serum levels every 4 h for 24 h in autistic subjects compared to healthy controls. They found an abnormal melatonin circadian rhythm, low blood concentration during the night related to sleep disturbances, and a tendency to have high daytime melatonin levels in autistic subjects. A later study by Tordjman and colleagues (140) analyzes the overnight urinary excretion of the predominant melatonin metabolite, 6-sulfatoxymelatonin (6-SM). Sixty-three percent of post-pubertal subjects with ASD showed low 6-SM, most marked in males and prepubertal children; this finding was confirmed in a later study (145). Another research group (146) confirmed a reduction in urinary secretion of 6-SM in subjects with ASD. The previous results were confirmed in another study, and low nocturnal excretion of 6-SM was associated with a greater severity of autistic symptoms (verbal language and repetitive behaviors). In a study of Pagan et al., (150), patients with ASD showed higher serotonin and lower melatonin levels than healthy controls. Patients with melatonin deficit reported more frequent sleep-onset and sleep-maintenance insomnia than patients with normal melatonin levels. The same research group investigated, for the first time, melatonin synthesis in the pineal gland and in the gut of patients with ASD, reinforcing the hypothesis that the nocturnal increase in circulating melatonin was reduced in those subjects and caused by enzymatic disruption of both aralkylamine N-acetyltransferase (AANAT) and acetylserotonin O-methyltransferase (ASMT) involved in melatonin synthesis confirming a melatonin reduction (153). An Afghanistan study (152) confirmed a reduction of melatonin serum level that was related to the severity of ASD symptoms. Recently, melatonin salivary concentrations have been studied in ASD subjects with and without anxiety and/or depression compared to controls (154). The timing of the dim light melatonin onset (DLMO) did not differ between the two groups, but advances and delays of the melatonin rhythms were the circadian sleep disorder more frequently observed in ASD subjects. Regarding the genetic pathways of melatonin, several studies focused on the variations of genes that regulate the synthesis, metabolism, and mechanism of action of melatonin. A multicenter study, the Paris Autism Research International (Sib-pair study) (141), sequenced all ASMT exons and promoters in 250 individuals with ASD. The ASD group showed non-conservative variations of the protein sequence of ASMT, and only two ASD families presented a splicing mutation. Forty-three individuals with ASD revealed a highly significant decrease of ASMT activity and melatonin level compared to 48 controls. Another subsequent research from the Sib-pair study (142) analyzed 941 individuals: 295 patients with ASD, 362 controls, and 284 individuals from different ethnic backgrounds. They sequenced MTNR1A and MTNR1B (melatonin receptor 1A and 1B) genes coding for melatonin 1 (MT1) and melatonin 2 (MT2) receptors, and G protein-coupled receptor 50 (GPR50) gene, coding for the orphan melatonin-related receptor GPR50. Authors found MTNR1A and MTNR1B non-synonymous mutations altering the functional properties of the human melatonin receptors. Regarding GPR50, they detected a significant association between ASD and two variations in affected males. In the same year, Sweden researchers (143) screened the genetic mutations of AANAT, ASMT, MTNR1A, MTNR1B, and GPR50, encoding both synthesis enzymes and the three main receptors of melatonin, in 109 patients with ASD and 188 controls. In this study, several rare variants were found in melatonin-related genes in patients with ASD, including the mutation in ASMT. A Chinese study (147) investigated all ASMT exons and the neighboring region in 398 individuals with ASD and 437 healthy controls. They detected new rare coding mutations of ASMT affecting the protein sequence only in six individuals of the ASD group, but the authors did not find significant differences of genotypic distribution and allele frequencies of the common SNPs in ASMT between groups. Only one study (148) investigated a large sample of 1,747 subjects by means of the analysis of the SNPs and the duplication of exons 2–8 in ASMT, in order to identify genes involved in autism psychopathology. The authors identified the association between one SNP in the last intron of ASMT and social interaction impairments in females, but they did not detect any relation with language impairment or restricted and repetitive behaviors. According to previous research, Veatch et al. (151) studied 15 ASD children with sleep disturbances, of whom 11 were treated with melatonin, and found that an association between sleep onset delay and dysfunctional variation in genes related to the melatonin pathway, especially with regard to cytochrome (CYP) 1A2. They also, observed a strong correlation between genotypes in ASMT and in CYP1A2, particularly in the subset of children who responded to treatment with supplemental melatonin. In summary, most of the studies that focused on autistic children have found abnormal melatonin circadian secretion and low blood and salivary melatonin concentrations during the night with a tendency to have high daytime melatonin levels. Regarding the genetic pathways of melatonin, several studies focused on the variations of genes that regulate the synthesis, metabolism, and mechanism of action of melatonin (AANAT, ASMT, MTNR1A, MTNR1B, and GPR50) showing several rare genetic variants of them. These findings have been related to sleep disturbances in ASD.
This systematic review aimed to provide the current status of knowledge about sleep disturbances and circadian sleep disorders in ASD across the lifespan. The current data have shown a number of several striking findings with regard to circadian sleep rhythmicity and ASD, but the nature of their link remains unclear. The literature on adult population is scant compared to studies on children. Although few studies have investigated this topic in adult subjects, similar results have been found compared to children. As shown in our results, studies have reported a high frequency of sleep problems and alterations of circadian sleep rhythmicity in ASD across all ages (55, 65, 71, 95, 108, 109, 114, 125). Difficulties in falling asleep, frequent nighttime awakenings, and short sleep duration were the sleep disturbances most frequently described in both children and adults with ASD (72, 78, 79, 124, 130). Accordingly, these results were also confirmed by means of actigraphic and polysomnographic registration, displaying specific irregular sleep–wake cycles, low sleep efficiency, long sleep latency, insomnia, daytime sleepiness (68, 91, 97, 106, 119, 120, 132, 133, 134, 136, 137), delayed circadian phases, and evening preference as chronotype associated with ASD. According to our searches, polymorphisms in CLOCK genes that regulate sleep may be associated with ASD (25, 26, 59, 60). Evidence is not strong although there are four separate studies implicating the alteration in CLOCK gene expression in the dysregulation of circadian sleep rhythmicity in ASD (25, 26, 59, 60). Melatonin dysregulation, which includes delay in melatonin peak, reduction in amplitude, and alteration in melatonin gene expression, may also contribute to circadian sleep desynchronization in ASD [for an overview, see Ref. (155)]. In some studies, blood, salivary, or urinary levels of melatonin or of its metabolites have been shown to be reduced in autistic subjects (138–140, 145, 150) and to be correlated directly or indirectly with severe autistic behaviors such as verbal communication as well as repetitive behaviors and daytime sleepiness (146, 152). According to our results, abnormalities in melatonin-related genes (enzymes involved in melatonin synthesis, metabolism and⁄or melatonin receptor function) may be related to low melatonin levels in ASD or may lead to an altered response to melatonin in a proportion of individuals with ASD (141–143, 148, 153).
Several studies pointed out the potential correlation between sleep disturbances and the severity of autistic symptoms above all repetitive behaviors and deficits in verbal communication and/or in social reciprocity (73, 100, 103, 109, 113, 156, 157). In summary the current data have shown several striking findings with regard to sleep/circadian sleep function and ASD, but the nature of the link remains unclear. We may hypothesize that polymorphisms in clock genes and alterations in melatonin pathways may contribute to the dysregulation of the circadian sleep rhythmicity and consequently to the dysregulation of sleep system in toto. According to the theories about the functions of sleep during development (30, 36–40), we may hypothesize that sleep disturbances may negatively influence brain maturation, contributing to autism symptoms. It has already been hypothesized that even a modest and initial impairment of circadian sleep rhythmicity may increase the individual’s vulnerability to ASD [for an overview, see Ref. (11)]. Indeed, on the other hand, autistic symptoms may reinforce sleep disturbances, creating a self-reinforcing feedback loop (Figure 2). This framework should be useful in order to identify elements to evaluate and target in the clinical practice. In particular, sleep disturbances and circadian sleep alterations may represent a novel therapeutic target in ASD. According to this model, by treating sleep and circadian sleep disorders in ASD, we should contribute to an improvement in ASD symptoms. There is strong evidence about the use of melatonin in ASD, indicating its beneficial effects on sleep and autistic symptoms. As suggested by some studies (158–165), melatonin may improve sleep difficulties in ASD subjects even when administered in association with concurrent psychotropic medications (160) and/or cognitive behavior therapy (164). Treatment with melatonin seems to improve sleep disturbances in the majority of children and adults with autistic symptoms (166), showing effectiveness on sleep duration, sleep latency, and nocturnal and early morning awakenings (158, 159, 161–165, 167, 168). Most importantly, by treating sleep and circadian sleep disorders with melatonin, an improvement in typical autistic behaviors has been shown in some studies (158, 163, 168). Data in children with neurodevelopmental disorders failed to point to serious adverse events associated with the use of melatonin (160, 162, 153, 165, 169) except for morning sleepiness (165), headache, and fatigue (167). These findings shed light on a rising hypothesis of an integrative model that may explain the interference of circadian rhythms and its variables during critical periods of brain development in ASD (Figure 2). Evaluating and targeting sleep disturbances and circadian sleep disorders in ASD should be useful to improve the trajectory of ASD. Indeed, the current data have shown a “research gap” about this topic. Future research should focus on the systematic study of circadian sleep dysrhythmicity in children and adults with ASD in relation to clock gene expression and to melatonin production.
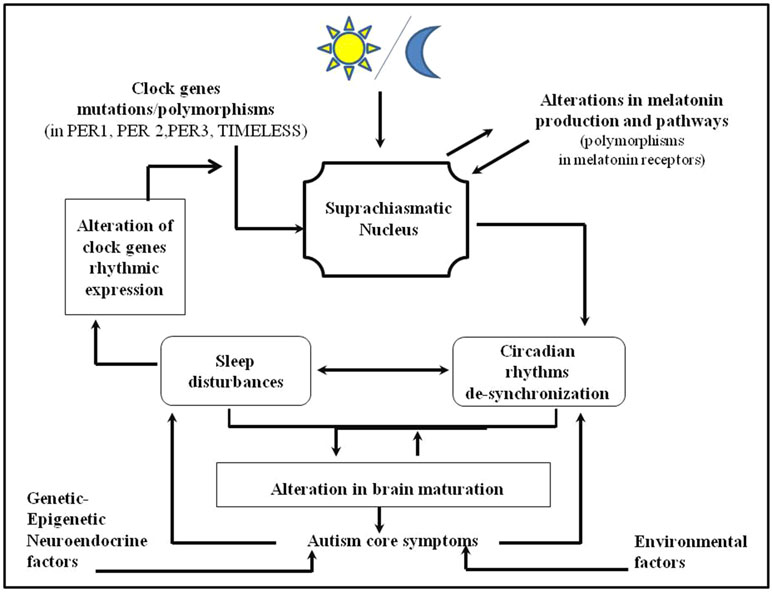
Figure 2 Integrative model of the relationship between autism spectrum disorders, circadian and sleep dysrhythmicity. Polymorphism in clock genes and alterations in melatonin pathways may contribute to alterations in circadian sleep rhythms and consequently in the sleep regulation in toto. Altered sleep may negatively influence the brain maturation contributing to the autism core symptomatology. Vice versa, autism symptomatology may reinforce sleep disturbances creating a self-reinforcing loop between them.
Our findings should be considered in the light of several limitations. Primarily, although this review aimed to summarize the most relevant studies on the relation between circadian sleep disorders and ASD, the inclusion/exclusion of specific studies may reflect our individual point of view or expertise and training. Moreover, some studies included may have been underpowered (some had only small sample sizes and small numbers of subjects enrolled) and/or some of them did not include control groups. Another limitation was the heterogeneity of the studies regarding the characteristics of the sample such as age, scholarity, study design, diagnostic criteria and measures of assessment, severity of sleep, and autistic symptoms that are potentially very important and should be considered routinely in future studies. Since the studies included in our research cover a wide temporal range (1984–2019), it is necessary to consider the nosography evolution of neurodevelopmental disorders over time, which could be a further study limitation. Psychiatric comorbidity and/or the use of psychopharmacological drugs may interfere with the duration and severity of both autistic symptoms and sleep disturbances. In addition, the potential roles of inert brain anomalies, the potential impact of brain injury during childbirth, and the potential influence of adverse environmental stressors in the early development of the child need to be addressed in further studies as being potential reasons for an apparent rise in the prevalence of ASD symptoms with later developmental stages.
Overall, results from this systematic review highlight the idea that sleep and circadian sleep disturbances are frequent in subjects with autistic symptoms who have shown polymorphisms in clock gene expression and in genes involved in melatonin production. The impairment of circadian sleep regulation may increase the individual’s vulnerability to develop symptoms of ASD by impairing the sleep regulation in toto, which instead plays a key role in normal brain development. Even though controversies and “research gaps” are present in literature at this point, we may hypothesize a bidirectional relation between circadian sleep dysfunction and ASD. In particular, circadian sleep dysrhythmicity may predispose to develop ASD symptoms and vice versa within a self-reinforcing loop. An early identification and assessment of circadian sleep dysrhythmicity could be useful for improving treatment strategies in both children and adults with ASD.
LP, CC, LD’O, DC, and IM designed the study and finalized the article. IM and DC managed the literature search. IM and DC elaborated the PubMed results and developed the first draft of the manuscript. LP and CC reviewed the manuscript. LP, CC, AV, LN, and LD’O revised the final version. All authors contributed to and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders 5th edition, DSM-5. Washington, DC (2013). doi: 10.1176/appi.books.9780890425596
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. IV edition-text revision, DSM-IV-TR. Washington, DC (2000).
3. Fernell E, Landgren M, Lindstrom K, Johnson M, Gillberg C. Children and young people with neurodevelopmental problems: support and efforts must be given even if not all diagnostic criteria are met. Lakartidningen (2013) 110(38):1674.
4. Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychol Med (2011) 41(3):619–27. doi: 10.1017/S0033291710000991
5. Klin A, Klaiman C, Jones W. Reducing age of autism diagnosis: developmental social neuroscience meets public health challenge. Rev Neurol (2015) 60 Suppl 1:S3–11.
6. Belardinelli C, Raza M, Taneli T. Comorbid behavioral problems and psychiatric disorders in autism spectrum disorders. J Child Dev Disord (2016) 2:11. doi: 10.4172/2472-1786.100019
7. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years—autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ (2018) 67(6):1–23. doi: 10.15585/mmwr.ss6706a1
8. Brugha T, Spiers N, Bankart J, Cooper S, McManus S, Scott F, et al. Epidemiology of autism in adults across age groups and ability levels. Br J Psychiatry (2016) 209(6):498–503. doi: 10.1192/bjp.bp.115.174649
9. Dell’Osso L, Abelli M, Pini S, Carpita B, Carlini M, Mengali F, et al. The influence of gender on social anxiety spectrum symptoms in a sample of university students. Riv Psichiatr (2015) 50(6):295–301. doi: 10.1708/2098.22688
10. Dell’Osso L, Carpita B, Cremone IM, Muti D, Diadema E, Barberi FM, et al. The mediating effect of trauma and stressor related symptoms and ruminations on the relationship between autistic traits and mood spectrum. Psychiatry Res (2018). doi: 10.1016/j.psychres.2018.10.040
11. Geoffray MM, Nicolas A, Speranza M, Georgieff N. Are circadian rhythms new pathways to understand Autism Spectrum Disorder? J Physiol Paris (2016) 110(4 Pt B):434–8. doi: 10.1016/j.jphysparis.2017.06.002
12. Gliga T, Jones EJ, Bedford R, Charman T, Johnson MH. From early markers to neuro-developmental mechanisms of autism. Dev Rev (2014) 34(3):189–207. doi: 10.1016/j.dr.2014.05.003
13. Siu MT, Weksberg R. Epigenetics of autism spectrum disorder. Adv Exp Med Biol (2017) 978:63–90. doi: 10.1007/978-3-319-53889-1_4
14. Tordjman S, Anderson GM, Kermarrec S, Bonnot O, Geoffray MM, Brailly-Tabard S, et al. Altered circadian patterns of salivary cortisol in low-functioning children and adolescents with autism. Psychoneuroendocrinology (2014) 50:227–45. doi: 10.1016/j.psyneuen.2014.08.010
15. McClung CA. Mind your rhythms: an important role for circadian genes in neuroprotection. J Clin Invest (2013) 123(12):4994–6. doi: 10.1172/JCI73059
16. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature (2002) 418(6901):935–41. doi: 10.1038/nature00965
17. Bellivier F, Geoffroy PA, Etain B, Scott J. Sleep- and circadian rhythm-associated pathways as therapeutic targets in bipolar disorder. Expert Opin Ther Targets (2015) 19(6):747–63. doi: 10.1517/14728222.2015.1018822
18. Aas M, Kauppi K, Brandt CL, Tesli M, Kaufmann T, Steen NE, et al. Childhood trauma is associated with increased brain responses to emotionally negative as compared with positive faces in patients with psychotic disorders. Psychol Med (2017) 47(4):669–79. doi: 10.1017/S0033291716002762
19. Foster RG, Kreitzman L. The rhythms of life: what your body clock means to you! Exp Physiol (2014) 99(4):599–606. doi: 10.1113/expphysiol.2012.071118
20. Roenneberg T, Merrow M. Entrainment of the human circadian clock. Cold Spring Harb Symp Quant Biol (2007) 72:293–9. doi: 10.1101/sqb.2007.72.043
21. Kalsbeek A, Merrow M, Roenneberg T, Foster RG. Neurobiology of circadian timing. Preface Prog Brain Res (2012) 199:11–2. doi: 10.1016/B978-0-444-59427-3.00031-9
22. Zee PC, Vitiello MV. Circadian rhythm sleep disorder: irregular sleep wake rhythm type. Sleep Med Clin (2009) 4(2):213–8. doi: 10.1016/j.jsmc.2009.01.009
23. Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, and possible biopsychosocial aetiologies. Sleep Med Rev (2009) 13(6):403–11. doi: 10.1016/j.smrv.2009.02.003
24. Wimpory D, Nicholas B, Nash S. Social timing, clock genes and autism: a new hypothesis. J Intellect Disabil Res (2002) 46(Pt 4):352–8. doi: 10.1046/j.1365-2788.2002.00423.x
25. Nicholas B, Rudrasingham V, Nash S, Kirov G, Owen MJ, Wimpory DC. Association of Per1 and Npas2 with autistic disorder: support for the clock genes/social timing hypothesis. Mol Psychiatry (2007) 12(6):581–92. doi: 10.1038/sj.mp.4001953
26. Yang Z, Matsumoto A, Nakayama K, Jimbo EF, Kojima K, Nagata K, et al. Circadian-relevant genes are highly polymorphic in autism spectrum disorder patients. Brain Dev (2016) 38(1):91–9. doi: 10.1016/j.braindev.2015.04.006
27. Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep (2005) 28(4):395–409. doi: 10.1093/sleep/28.4.395
28. Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, et al. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci (2000) 20(21):8138–43. doi: 10.1523/JNEUROSCI.20-21-08138.2000
29. Frank MG. Sleep and developmental plasticity not just for kids. Prog Brain Res (2011) 193:221–32. doi: 10.1016/B978-0-444-53839-0.00014-4
30. Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am J Physiol Regul Integr Comp Physiol (2013) 304(4):R296–303. doi: 10.1152/ajpregu.00422.2012
31. Kurth S, Olini N, Huber R, LeBourgeois M. Sleep and early cortical development. Curr Sleep Med Rep (2015) 1(1):64–73. doi: 10.1007/s40675-014-0002-8
32. Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav Brain Res (1995) 69(1–2):1–11. doi: 10.1016/0166-4328(95)00018-O
33. Mirmiran M, Van Someren E. Symposium: normal and abnormal REM sleep regulation: the importance of REM sleep for brain maturation. J Sleep Res (1993) 2(4):188–92. doi: 10.1111/j.1365-2869.1993.tb00088.x
34. Peirano PD, Algarin CR. Sleep in brain development. Biol Res (2007) 40(4):471–8. doi: 10.4067/S0716-97602007000500008
35. Li W, Ma L, Yang G, Gan WB. REM sleep selectively prunes and maintains new synapses in development and learning. Nature neuroscience (2017) 20(3):427–37. doi: 10.1038/nn.4479
36. Lindemann C, Ahlbeck J, Bitzenhofer SH, Hanganu-Opatz IL. Spindle activity orchestrates plasticity during development and sleep. Neural Plast (2016) 2016:5787423. doi: 10.1155/2016/5787423
37. Cirelli C, Tononi G. Sleep and synaptic homeostasis. Sleep (2015) 38(1):161–2. doi: 10.5665/sleep.4348
38. Feinberg I, Davis NM, de Bie E, Grimm KJ, Campbell IG. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school-night and extended sleep. Am J Physiol Regul Integr Comp Physiol (2012) 302(5):R533–40. doi: 10.1152/ajpregu.00532.2011
39. Ringli M, Huber R. Developmental aspects of sleep slow waves: linking sleep, brain maturation and behavior. Prog Brain Res (2011) 193:63–82. doi: 10.1016/B978-0-444-53839-0.00005-3
40. Buchmann A, Ringli M, Kurth S, Schaerer M, Geiger A, Jenni OG, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex (2011) 21(3):607–15. doi: 10.1093/cercor/bhq129
41. Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci (2013) 33(36):14288–300. doi: 10.1523/JNEUROSCI.5102-12.2013
42. Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron (2004) 41(1):35–43. doi: 10.1016/S0896-6273(03)00814-6
43. Hinard V, Mikhail C, Pradervand S, Curie T, Houtkooper RH, Auwerx J, et al. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci (2012) 32(36):12506–17. doi: 10.1523/JNEUROSCI.2306-12.2012
44. Gregory AM, Sadeh A. Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Med Rev (2012) 16(2):129–36. doi: 10.1016/j.smrv.2011.03.007
45. Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci (2008) 9(12):947–57. doi: 10.1038/nrn2513
46. Sadeh A, Tikotzky L, Kahn M. Sleep in infancy and childhood: implications for emotional and behavioral difficulties in adolescence and beyond. Curr Opin Psychiatry (2014) 27(6):453–9. doi: 10.1097/YCO.0000000000000109
47. Gregory AM, Caspi A, Eley TC, Moffitt TE, Oconnor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol (2005) 33(2):157–63. doi: 10.1007/s10802-005-1824-0
48. Sadeh A, Flint-Ofir E, Tirosh T, Tikotzky L. Infant sleep and parental sleep-related cognitions. J Fam Psychol (2007) 21(1):74–87. doi: 10.1037/0893-3200.21.1.74
49. Tesler N, Gerstenberg M, Huber R. Developmental changes in sleep and their relationships to psychiatric illnesses. Curr Opin Psychiatry (2013) 26(6):572–9. doi: 10.1097/YCO.0b013e328365a335
50. Simola P, Liukkonen K, Pitkaranta A, Pirinen T, Aronen ET. Psychosocial and somatic outcomes of sleep problems in children: a 4-year follow-up study. Child Care Health Dev (2014) 40(1):60–7. doi: 10.1111/j.1365-2214.2012.01412.x
51. Touchette E, Petit D, Seguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep (2007) 30(9):1213–9. doi: 10.1093/sleep/30.9.1213
52. Volk C, Huber R. Sleep to grow smart? Arch Ital Biol (2015) 153(2–3):99–109. doi: 10.12871/000398292015235
53. Palagini L, Domschke K, Benedetti F, Foster RG, Wulff K, Riemann D. Developmental pathways towards mood disorders in adult life: is there a role for sleep disturbances? J Affect Disord (2019) 243:121–32. doi: 10.1016/j.jad.2018.09.011
54. Geiger A, Achermann P, Jenni OG. Sleep, intelligence and cognition in a developmental context: differentiation between traits and state-dependent aspects. Progress in Brain Research (2010) 167–79. doi: 10.1016/b978-0-444-53702-7.00010-5
55. Díaz-Román A, Zhang J, Delorme R, Beggiato A, Cortese S. Sleep in youth with autism spectrum disorders: systematic review and meta-analysis of subjective and objective studies. Evid Based Mental Health (2018) 21(4):146–54. doi: 10.1136/ebmental-2018-300037
56. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. IV edition, DSM-IV. Washington, DC (1994).
57. Takase M, Taira M, Sasaki H. Sleep–wake rhythm of autistic children. Psychiatry Clin Neurosci (1998) 52(2):181–2. doi: 10.1111/j.1440-1819.1998.tb01017.x
58. Giannotti F, Cortesi F, Cerquiglini A, Miraglia D, Vagnoni C, Sebastiani T, et al. An investigation of sleep characteristics, EEG abnormalities and epilepsy in developmentally regressed and non-regressed children with autism. J Autism Dev Disord (2008) 38(10):1888–97. doi: 10.1007/s10803-008-0584-4
59. Mullegama SV, Alaimo JT, Chen L, Elsea SH. Phenotypic and molecular convergence of 2q23.1 deletion syndrome with other neurodevelopmental syndromes associated with autism spectrum disorder. Int J Mol Sci (2015) 16(4):7627–43. doi: 10.3390/ijms16047627
60. Goto M, Mizuno M, Matsumoto A, Yang Z, Jimbo EF, Tabata H, et al. Role of a circadian-relevant gene NR1D1 in brain development: possible involvement in the pathophysiology of autism spectrum disorders. Sci Rep (2017) 7:43945. doi: 10.1038/srep43945
61. van der Heijden KB, Stoffelsen RJ, Popma A, Swaab H. Sleep, chronotype, and sleep hygiene in children with attention-deficit/hyperactivity disorder, autism spectrum disorder, and controls. Eur Child Adolesc Psychiatry (2018) 27(1):99–111. doi: 10.1007/s00787-017-1025-8
62. Hoshino Y, Watanabe H, Yashima Y, Kaneko M, Kumashiro H. An investigation on sleep disturbance of autistic children. Folia Psychiatr Neurol Jpn (1984) 38(1):45–51. doi: 10.1111/j.1440-1819.1984.tb00353.x
63. Richdale AL, Prior MR. The sleep/wake rhythm in children with autism. Eur Child Adolesc Psychiatry (1995) 4(3):175–86. doi: 10.1007/BF01980456
64. Patzold LM, Richdale AL, Tonge BJ. An investigation into sleep characteristics of children with autism and Asperger’s disorder. J Paediatr Child Health (1998) 34(6):528–33. doi: 10.1046/j.1440-1754.1998.00291.x
65. Taira M, Takase M, Sasaki H. Sleep disorder in children with autism. Psychiatry Clin Neurosci (1998) 52(2):182–3. doi: 10.1111/j.1440-1819.1998.tb01018.x
66. Diomedi M, Curatolo P, Scalise A, Placidi F, Caretto F, Gigli GL. Sleep abnormalities in mentally retarded autistic subjects: Down’s syndrome with mental retardation and normal subjects. Brain Dev (1999) 21(8):548–53. doi: 10.1016/S0387-7604(99)00077-7
67. Hering E, Epstein R, Elroy S, Iancu DR, Zelnik N. Sleep patterns in autistic children. J Autism Dev Disord (1999) 29(2):143–7. doi: 10.1023/A:1023092627223
68. Godbout R, Bergeron C, Limoges E, Stip E, Mottron L. A laboratory study of sleep in Asperger’s syndrome. Neuroreport (2000) 11(1):127–30. doi: 10.1097/00001756-200001170-00025
69. Elia M, Ferri R, Musumeci SA, Del Gracco S, Bottitta M, Scuderi C, et al. Sleep in subjects with autistic disorder: a neurophysiological and psychological study. Brain Dev (2000) 22(2):88–92. doi: 10.1016/S0387-7604(99)00119-9
70. Honomichl RD, Goodlin-Jones BL, Burnham M, Gaylor E, Anders TF. Sleep patterns of children with pervasive developmental disorders. J Autism Dev Disord (2002) 32(6):553–61. doi: 10.1023/A:1021254914276
71. Wiggs L, Stores G. Sleep patterns and sleep disorders in children with autistic spectrum disorders: insights using parent report and actigraphy. Dev Med Child Neurol (2004) 46(6):372–80. doi: 10.1017/S0012162204000611
72. Gail Williams P, Sears LL, Allard A. Sleep problems in children with autism. J Sleep Res (2004) 13(3):265–8. doi: 10.1111/j.1365-2869.2004.00405.x
73. Schreck KA, Mulick JA, Smith AF. Sleep problems as possible predictors of intensified symptoms of autism. Res Dev Disabil (2004) 25(1):57–66. doi: 10.1016/j.ridd.2003.04.007
74. Polimeni MA, Richdale AL, Francis AJ. A survey of sleep problems in autism, Asperger’s disorder and typically developing children. J Intellect Disabil Res (2005) 49(Pt 4):260–8. doi: 10.1111/j.1365-2788.2005.00642.x
75. Cotton S, Richdale A. Brief report: parental descriptions of sleep problems in children with autism, Down syndrome, and Prader–Willi syndrome. Res Dev Disabil (2006) 27(2):151–61. doi: 10.1016/j.ridd.2004.12.003
76. Allik H, Larsson JO, Smedje H. Sleep patterns of school-age children with Asperger syndrome or high-functioning autism. J Autism Dev Disord (2006) 36(5):585–95. doi: 10.1007/s10803-006-0099-9
77. Allik H, Larsson JO, Smedje H. Insomnia in school-age children with Asperger syndrome or high-functioning autism. BMC Psychiatry (2006) 6:18. doi: 10.1186/1471-244X-6-18
78. Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, Hansen RL. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. J Sleep Res (2008) 17(2):197–206. doi: 10.1111/j.1365-2869.2008.00650.x
79. Liu X, Hubbard JA, Fabes RA, Adam JB. Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry Hum Dev (2006) 37(2):179–91. doi: 10.1007/s10578-006-0028-3
80. Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep (2006) 29(12):1563–71. doi: 10.1093/sleep/29.12.1563
81. Miano S, Bruni O, Elia M, Trovato A, Smerieri A, Verrillo E, et al. Sleep in children with autistic spectrum disorder: a questionnaire and polysomnographic study. Sleep Med (2007) 9(1):64–70. doi: 10.1016/j.sleep.2007.01.014
82. Bruni O, Ferri R, Vittori E, Novelli L, Vignati M, Porfirio MC, et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep (2007) 30(11):1577–85. doi: 10.1093/sleep/30.11.1577
83. DeVincent CJ, Gadow KD, Delosh D, Geller L. Sleep disturbance and its relation to DSM-IV psychiatric symptoms in preschool-age children with pervasive developmental disorder and community controls. J Child Neurol (2007) 22(2):161–9. doi: 10.1177/0883073807300310
84. Dominick KC, Davis NO, Lainhart J, Tager-Flusberg H, Folstein S. Atypical behaviors in children with autism and children with a history of language impairment. Res Dev Disabil (2007) 28(2):145–62. doi: 10.1016/j.ridd.2006.02.003
85. Allik H, Larsson JO, Smedje H. Sleep patterns in school-age children with Asperger syndrome or high-functioning autism: a follow-up study. J Autism Dev Disord (2008) 38(9):1625–33. doi: 10.1007/s10803-008-0543-0
86. Goodlin-Jones BL, Tang K, Liu J, Anders TF. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. J Am Acad Child Adolesc Psychiatry (2008) 47(8):930–8. doi: 10.1097/CHI.ObO13e3181799f7c
87. Goodlin-Jones B, Schwichtenberg AJ, Iosif AM, Tang K, Liu J, Anders TF. Six-month persistence of sleep problems in young children with autism, developmental delay, and typical development. J Am Acad Child Adolesc Psychiatry (2009) 48(8):847–54. doi: 10.1097/CHI.0b013e3181a8135a
88. Goodlin-Jones B, Tang K, Liu J, Anders TF. Sleep problems, sleepiness and daytime behavior in preschool-age children. J Child Psychol Psychiatry (2009) 50(12):1532–40. doi: 10.1111/j.1469-7610.2009.02110.x
89. Paavonen EJ, Vehkalahti K, Vanhala R, von Wendt L, Nieminen-von Wendt T, Aronen ET. Sleep in children with Asperger syndrome. J Autism Dev Disord (2008) 38(1):41–51. doi: 10.1007/s10803-007-0360-x
90. Souders MC, Mason TB, Valladares O, Bucan M, Levy SE, Mandell DS, et al. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep (2009) 32(12):1566–78. doi: 10.1093/sleep/32.12.1566
91. Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, Malow BA. Defining the sleep phenotype in children with autism. Dev Neuropsychol (2009) 34(5):560–73. doi: 10.1080/87565640903133509
92. Buckley AW, Rodriguez AJ, Jennison K, Buckley J, Thurm A, Sato S, et al. Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Arch Pediatr Adolesc Med (2010) 164(11):1032–7. doi: 10.1001/archpediatrics.2010.202
93. Giannotti F, Cortesi F, Cerquiglini A, Vagnoni C, Valente D. Sleep in children with autism with and without autistic regression. J Sleep Res (2011) 20(2):338–47. doi: 10.1111/j.1365-2869.2010.00882.x
94. Anders TF, Iosif AM, Schwichtenberg AJ, Tang K, Goodlin-Jones BL. Six-month sleep-wake organization and stability in preschool-age children with autism, developmental delay, and typical development. Behav Sleep Med (2011) 9(2):92–106. doi: 10.1080/15402002.2011.557991
95. Rzepecka H, McKenzie K, McClure I, Murphy S. Sleep, anxiety and challenging behaviour in children with intellectual disability and/or autism spectrum disorder. Res Dev Disabil (2011) 32(6):2758–66. doi: 10.1016/j.ridd.2011.05.034
96. Schwichtenberg AJ, Iosif AM, Goodlin-Jones B, Tang K, Anders T. Daytime sleep patterns in preschool children with autism, developmental delay, and typical development. Am J Intellect Dev Disabil (2011) 116(2):142–52. doi: 10.1352/1944-7558-116.2.142
97. Anders T, Iosif AM, Schwichtenberg AJ, Tang K, Goodlin-Jones B. Sleep and daytime functioning: a short-term longitudinal study of three preschool-age comparison groups. Am J Intellect Dev Disabil (2012) 117(4):275–90. doi: 10.1352/1944-7558-117.4.275
98. Goldman SE, Richdale AL, Clemons T, Malow BA. Parental sleep concerns in autism spectrum disorders: variations from childhood to adolescence. J Autism Dev Disord (2012) 42(4):531–8. doi: 10.1007/s10803-011-1270-5
99. Sikora DM, Johnson K, Clemons T, Katz T. The relationship between sleep problems and daytime behavior in children of different ages with autism spectrum disorders. Pediatrics (2012) 130 Suppl 2:S83–90. doi: 10.1542/peds.2012-0900F
100. Taylor MA, Schreck KA, Mulick JA. Sleep disruption as a correlate to cognitive and adaptive behavior problems in autism spectrum disorders. Res Dev Disabil (2012) 33(5):1408–17. doi: 10.1016/j.ridd.2012.03.013
101. Sivertsen B, Posserud MB, Gillberg C, Lundervold AJ, Hysing M. Sleep problems in children with autism spectrum problems: a longitudinal population-based study. Autism (2012) 16(2):139–50. doi: 10.1177/1362361311404255
102. Baker E, Richdale A, Short M, Gradisar M. An investigation of sleep patterns in adolescents with high-functioning autism spectrum disorder compared with typically developing adolescents. Dev Neurorehabil (2013) 16(3):155–65. doi: 10.3109/17518423.2013.765518
103. Hollway JA, Aman MG, Butter E. Correlates and risk markers for sleep disturbance in participants of the Autism Treatment Network. J Autism Dev Disord (2013) 43(12):2830–43. doi: 10.1007/s10803-013-1830-y
104. Humphreys JS, Gringras P, Blair PS, Scott N, Henderson J, Fleming PJ, et al. Sleep patterns in children with autistic spectrum disorders: a prospective cohort study. Arch Dis Child (2014) 99(2):114–8. doi: 10.1136/archdischild-2013-304083
105. Nadeau JM, Arnold EB, Keene AC, Collier AB, Lewin AB, Murphy TK. Frequency and clinical correlates of sleep-related problems among anxious youth with autism spectrum disorders. Child Psychiatry Hum Dev (2015) 46(4):558–66. doi: 10.1007/s10578-014-0496-9
106. Richdale AL, Baker E, Short M, Gradisar M. The role of insomnia, pre-sleep arousal and psychopathology symptoms in daytime impairment in adolescents with high-functioning autism spectrum disorder. Sleep Med (2014) 15(9):1082–8. doi: 10.1016/j.sleep.2014.05.005
107. Hodge D, Carollo TM, Lewin M, Hoffman CD, Sweeney DP. Sleep patterns in children with and without autism spectrum disorders: developmental comparisons. Res Dev Disabil (2014) 35(7):1631–8. doi: 10.1016/j.ridd.2014.03.037
108. Tudor ME, Walsh CE, Mulder EC, Lerner MD. Pain as a predictor of sleep problems in youth with autism spectrum disorders. Autism (2015) 19(3):292–300. doi: 10.1177/1362361313518994
109. May T, Cornish K, Conduit R, Rajaratnam SM, Rinehart NJ. Sleep in high-functioning children with autism: longitudinal developmental change and associations with behavior problems. Behav Sleep Med (2015) 13(1):2–18. doi: 10.1080/15402002.2013.829064
110. Mazurek MO, Petroski GF. Sleep problems in children with autism spectrum disorder: examining the contributions of sensory over-responsivity and anxiety. Sleep Med (2015) 16(2):270–9. doi: 10.1016/j.sleep.2014.11.006
111. Wang G, Liu Z, Xu G, Jiang F, Lu N, Baylor A, Owens J. Sleep disturbances and associated factors in Chinese children with autism spectrum disorder: a retrospective and cross-sectional study. Child Psychiatry Hum Dev (2016) 47(2):248–58. doi: 10.1007/s10578-015-0561-z
112. Hirata I, Mohri I, Kato-Nishimura K, Tachibana M, Kuwada A, Kagitani-Shimono K, et al. Sleep problems are more frequent and associated with problematic behaviors in preschoolers with autism spectrum disorder. Res Dev Disabil (2016) 49-50:86–99. doi: 10.1016/j.ridd.2015.11.002
113. Hundley RJ, Shui A, Malow BA. Relationship between subtypes of restricted and repetitive behaviors and sleep disturbance in autism spectrum disorder. J Autism Dev Disord (2016) 46(11):3448–57. doi: 10.1007/s10803-016-2884-4
114. Irwanto N, Rehatta M, Hartini S, Takada S. Sleep problem of children with autistic spectrum disorder assessed by Children Sleep Habits Questionnaire-Abbreviated in Indonesia and Japan. Kobe J Med Sci (2016) 62(2):E22–6.
115. Fletcher FE, Foster-Owens MD, Conduit R, Rinehart NJ, Riby DM, Cornish KM. The developmental trajectory of parent-report and objective sleep profiles in autism spectrum disorder: associations with anxiety and bedtime routines. Autism (2017) 21(4):493–503. doi: 10.1177/1362361316653365
116. Mazurek MO, Sohl K. Sleep and behavioral problems in children with autism spectrum disorder. J Autism Dev Disord (2016) 46(6):1906–15. doi: 10.1007/s10803-016-2723-7
117. Kheirouri S, Kalejahi P, Noorazar SG. Plasma levels of serotonin, gastrointestinal symptoms, and sleep problems in children with autism. Turk J Med Sci (2016) 46(6):1765–72. doi: 10.3906/sag-1507-68
118. Mutluer T, Karakoc Demirkaya S, Abali O. Assessment of sleep problems and related risk factors observed in Turkish children with Autism spectrum disorders. Autism Res (2016) 9(5):536–42. doi: 10.1002/aur.1542
119. Veatch OJ, Reynolds A, Katz T, Weiss SK, Loh A, Wang L, et al. Sleep in children with autism spectrum disorders: how are measures of parent report and actigraphy related and affected by sleep education? Behav Sleep Med (2016) 14(6):665–76. doi: 10.1080/15402002.2015.1065408
120. Aathira R, Gulati S, Tripathi M, Shukla G, Chakrabarty B, Sapra S, et al. Prevalence of sleep abnormalities in Indian children with autism spectrum disorder: a cross-sectional study. Pediatr Neurol (2017) 74:62–7. doi: 10.1016/j.pediatrneurol.2017.05.019
121. Kose S, Yilmaz H, Ocakoglu FT, Ozbaran NB. Sleep problems in children with autism spectrum disorder and intellectual disability without autism spectrum disorder. Sleep Med (2017) 40:69–77. doi: 10.1016/j.sleep.2017.09.021
122. Sannar EM, Palka T, Beresford C, Peura C, Kaplan D, Verdi M, Grados M. Sleep problems and their relationship to maladaptive behavior severity in psychiatrically hospitalized children with autism spectrum disorder (ASD). J Autism Dev Disord (2018) 48(11):3720–6. doi: 10.1007/s10803-017-3362-3
123. Veatch OJ, Sutcliffe JS, Warren ZE, Keenan BT, Potter MH, Malow BA. Shorter sleep duration is associated with social impairment and comorbidities in ASD. Autism Res (2017) 10(7):1221–38. doi: 10.1002/aur.1765
124. Verhoeff ME, Blanken LME, Kocevska D, Mileva-Seitz VR, Jaddoe VWV, White T, et al. The bidirectional association between sleep problems and autism spectrum disorder: a population-based cohort study. Mol Autism (2018) 9:8. doi: 10.1186/s13229-018-0194-8
125. Malow BA, Katz T, Reynolds AM, Shui A, Carno M, Connolly HV, et al. Sleep difficulties and medications in children with autism spectrum disorders: a registry study. Pediatrics (2016) 137 Suppl;2:98–104. doi: 10.1542/peds.2015-2851H
126. Hare DJ, Jones S, Evershed K. A comparative study of circadian rhythm functioning and sleep in people with Asperger syndrome. Autism (2006a) 10(6):565–75. doi: 10.1177/1362361306068509
127. Hare DJ, Jones S, Evershed K. Objective investigation of the sleep–wake cycle in adults with intellectual disabilities and autistic spectrum disorders. J Intellect Disabil Res (2006b) 50(Pt 10):701–10. doi: 10.1111/j.1365-2788.2006.00830.x
128. Baker EK, Richdale AL. Examining the behavioural sleep–wake rhythm in adults with autism spectrum disorder and no comorbid intellectual disability. J Autism Dev Disord (2017) 47(4):1207–22. doi: 10.1007/s10803-017-3042-3
129. Goldman SE, Alder ML, Burgess HJ, Corbett BA, Hundley R, Wofford D, et al. Characterizing sleep in adolescents and adults with autism spectrum disorders. J Autism Dev Disord (2017) 47(6):1682–95. doi: 10.1007/s10803-017-3089-1
130. Ballester P, Martinez MJ, Javaloyes A, Inda MD, Fernandez N, Gazquez P, et al. Sleep problems in adults with autism spectrum disorder and intellectual disability. Autism Res (2019) 12(1):66–79. doi: 10.1002/aur.2000
131. Tani P, Lindberg N, Nieminen-von Wendt T, von Wendt L, Alanko L, Appelberg B, Porkka-Heiskanen T. Insomnia is a frequent finding in adults with Asperger syndrome. BMC Psychiatry (2003) 3:12. doi: 10.1186/1471-244x-3-12
132. Tani P, Lindberg N, Nieminen-von Wendt T, von Wendt L, Virkkala J, Appelberg B, Porkka-Heiskanen T. Sleep in young adults with Asperger syndrome. Neuropsychobiology (2004) 50(2):147–52. doi: 10.1159/000079106
133. Tani P, Lindberg N, Nieminen-von Wendt T, von Wendt L, Alanko L, Appelberg B, Porkka-Heiskanen T. Actigraphic assessment of sleep in young adults with Asperger syndrome. Psychiatry Clin Neurosci (2005) 59(2):206–8. doi: 10.1111/j.1440-1819.2005.01359.x
134. Limoges E, Mottron L, Bolduc C, Berthiaume C, Godbout R. Atypical sleep architecture and the autism phenotype. Brain (2005) 128(Pt 5):1049–61. doi: 10.1093/brain/awh425
135. Oyane NM, Bjorvatn B. Sleep disturbances in adolescents and young adults with autism and Asperger syndrome. Autism (2005) 9(1):83–94. doi: 10.1177/1362361305049031
136. Limoges E, Bolduc C, Berthiaume C, Mottron L, Godbout R. Relationship between poor sleep and daytime cognitive performance in young adults with autism. Res Dev Disabil (2013) 34(4):1322–35. doi: 10.1016/j.ridd.2013.01.013
137. Baker EK, Richdale AL. Sleep patterns in adults with a diagnosis of high-functioning autism spectrum disorder. Sleep (2015) 38(11):1765–74. doi: 10.5665/sleep.5160
138. Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Brief report: circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J Autism Dev Disord (1995) 25(6):641–54. doi: 10.1007/BF02178193
139. Kulman G, Lissoni P, Rovelli F, Roselli MG, Brivio F, Sequeri P. Evidence of pineal endocrine hypofunction in autistic children. Neuro Endocrinol Lett (2000) 21(1):31–4.
140. Tordjman S, Anderson GM, Pichard N, Charbuy H, Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol Psychiatry (2005) 57(2):134–8. doi: 10.1016/j.biopsych.2004.11.003
141. Melke J, Goubran Botros H, Chaste P, Betancur C, Nygren G, Anckarsater H, et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol Psychiatry (2008) 13(1):90–8. doi: 10.1038/sj.mp.4002016
142. Chaste P, Clement N, Mercati O, Guillaume JL, Delorme R, Botros HG, et al. Identification of pathway-biased and deleterious melatonin receptor mutants in autism spectrum disorders and in the general population. PLoS One (2010) 5(7):e11495. doi: 10.1371/journal.pone.0011495
143. Jonsson L, Ljunggren E, Bremer A, Pedersen C, Landen M, Thuresson K, et al. Mutation screening of melatonin-related genes in patients with autism spectrum disorders. BMC Med Genomics (2010) 3:10. doi: 10.1186/1755-8794-3-10
144. Mulder EJ, Anderson GM, Kemperman RF, Oosterloo-Duinkerken A, Minderaa RB, Kema IP. Urinary excretion of 5-hydroxyindoleacetic acid, serotonin and 6-sulphatoxymelatonin in normoserotonemic and hyperserotonemic autistic individuals. Neuropsychobiology (2010) 61(1):27–32. doi: 10.1159/000258640
145. Leu RM, Beyderman L, Botzolakis EJ, Surdyka K, Wang L, Malow BA. Relation of melatonin to sleep architecture in children with autism. J Autism Dev Disord (2011) 41(4):427–33. doi: 10.1007/s10803-010-1072-1
146. Tordjman S, Anderson GM, Bellissant E, Botbol M, Charbuy H, Camus F, et al. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology (2012) 37(12):1990–7. doi: 10.1016/j.psyneuen.2012.04.013
147. Wang L, Li J, Ruan Y, Lu T, Liu C, Jia M, et al. Sequencing ASMT identifies rare mutations in Chinese Han patients with autism. PLoS One (2013) 8(1):e53727. doi: 10.1371/journal.pone.0053727
148. Jonsson L, Anckarsater H, Zettergren A, Westberg L, Walum H, Lundstrom S, et al. Association between ASMT and autistic-like traits in children from a Swedish nationwide cohort. Psychiatr Genet (2014) 24(1):21–7. doi: 10.1097/YPG.0000000000000010
149. Goldman SE, Adkins KW, Calcutt MW, Carter MD, Goodpaster RL, Wang L, et al. Melatonin in children with autism spectrum disorders: endogenous and pharmacokinetic profiles in relation to sleep. J Autism Dev Disord (2014) 44(10):2525–35. doi: 10.1007/s10803-014-2123-9
150. Pagan C, Delorme R, Callebert J, Goubran-Botros H, Amsellem F, Drouot X, et al. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Transl Psychiatry (2014) 4:e479. doi: 10.1038/tp.2014.120
151. Veatch OJ, Pendergast JS, Allen MJ, Leu RM, Johnson CH, Elsea SH, et al. Genetic variation in melatonin pathway enzymes in children with autism spectrum disorder and comorbid sleep onset delay. J Autism Dev Disord (2015) 45(1):100–10. doi: 10.1007/s10803-014-2197-4
152. Abdulamir HA, Abdul-Rasheed OF, Abdulghani EA. Low oxytocin and melatonin levels and their possible role in the diagnosis and prognosis in Iraqi autistic children. Saudi Med J (2016) 37(1):29–36. doi: 10.15537/smj.2016.1.13183
153. Pagan C, Goubran-Botros H, Delorme R, Benabou M, Lemiere N, Murray K, et al. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci Rep (2017) 7(1):2096. doi: 10.1038/s41598-017-02152-x
154. Baker EK, Richdale AL, Hazi A, Prendergast LA. Assessing the dim light melatonin onset in adults with autism spectrum disorder and no comorbid intellectual disability. J Autism Dev Disord (2017) 47(7):2120–37. doi: 10.1007/s10803-017-3122-4
155. Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol (2011) 53(9):783–92. doi: 10.1111/j.1469-8749.2011.03980.x
156. Tordjman S, Najjar I, Bellissant E, Anderson GM, Barburoth M, Cohen D, et al. Advances in the research of melatonin in autism spectrum disorders: literature review and new perspectives. Int J Mol Sci (2013) 14(10):20508–42. doi: 10.3390/ijms141020508
157. Tordjman S, Davlantis KS, Georgieff N, Geoffray MM, Speranza M, Anderson GM, et al. Autism as a disorder of biological and behavioral rhythms: toward new therapeutic perspectives. Front Pediatr (2015) 3:1. doi: 10.3389/fped.2015.00001
158. Garstang J, Wallis M. Randomized controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child Care Health Dev (2006) 32(5):585–9. doi: 10.1111/j.1365-2214.2006.00616.x
159. Wasdell MB, Jan JE, Bomben MM, Freeman RD, Rietveld WJ, Tai J, et al. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J Pineal Res (2008) 44(1):57–64. doi: 10.1111/j.1600-079X.2007.00528.x
160. Andersen IM, Kaczmarska J, McGrew SG, Malow BA. Melatonin for insomnia in children with autism spectrum disorders. J Child Neurol (2008) 23(5):482–5. doi: 10.1177/0883073807309783
161. Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med (2009) 5(2):145–50.
162. Wright B, Sims D, Smart S, Alwazeer A, Alderson-Day B, Allgar V, et al. Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: a randomised controlled crossover trial. J Autism Dev Disord (2011) 41(2):175–84. doi: 10.1007/s10803-010-1036-5
163. Malow B, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes D, et al. Melatonin for sleep in children with autism: a controlled trial examining dose, tolerability, and outcomes. J Autism Dev Disord (2012) 42(8):1729–37. doi: 10.1007/s10803-011-1418-3
164. Cortesi F, Giannotti F, Sebastiani T, Panunzi S, Valente D. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res (2012) 21(6):700–9. doi: 10.1111/j.1365-2869.2012.01021.x
165. Ayyash HF, Preece P, Morton R, Cortese S. Melatonin for sleep disturbance in children with neurodevelopmental disorders: prospective observational naturalistic study. Expert Rev Neurother (2015) 15(6):711–7. doi: 10.1586/14737175.2015.1041511
166. Galli-Carminati G, Deriaz N, Bertschy G. Melatonin in treatment of chronic sleep disorders in adults with autism: a retrospective study. Swiss Med Wkly (2009) 139(19–20):293–6. doi: smw-12342
167. Gringras P, Nir T, Breddy J, Frydman-Marom A, Findling RL. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry (2017) 56(11):948–57.e944. doi: 10.1016/j.jaac.2017.09.414
168. Paavonen EJ, Nieminen-von Wendt T, Vanhala R, Aronen ET, von Wendt L. Effectiveness of melatonin in the treatment of sleep disturbances in children with Asperger disorder. J Child Adolesc Psychopharmacol (2003) 13(1):83–95. doi: 10.1089/104454603321666225
Keywords: autism spectrum disorder, circadian rhythms, sleep disturbances, neurodevelopmental, melatonin, clock genes
Citation: Carmassi C, Palagini L, Caruso D, Masci I, Nobili L, Vita A and Dell’Osso L (2019) Systematic Review of Sleep Disturbances and Circadian Sleep Desynchronization in Autism Spectrum Disorder: Toward an Integrative Model of a Self-Reinforcing Loop. Front. Psychiatry 10:366. doi: 10.3389/fpsyt.2019.00366
Received: 25 February 2019; Accepted: 13 May 2019;
Published: 06 June 2019.
Edited by:
Armida Mucci, Università degli Studi della Campania Luigi Vanvitelli Caserta, ItalyReviewed by:
Emilio Baliki Liociri Ovuga, Gulu University, UgandaCopyright © 2019 Carmassi, Palagini, Caruso, Masci, Nobili, Vita and Dell’Osso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Palagini, bHBhbGFnaW5pQHRpc2NhbGkuaXQ=; bC5wYWxhZ2luaUB0aXNjYWxpLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.