
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry, 09 April 2019
Sec. Child and Adolescent Psychiatry
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.00194
This article is part of the Research TopicComorbidity and Autism Spectrum DisorderView all 33 articles
Background: Many individuals with autism spectrum disorder (ASD) have co-occurring gastrointestinal (GI) symptoms, but the etiology is poorly understood. These GI symptoms often coincide with problem behaviors and internalizing symptoms, which reduces the quality of life for these individuals.
Methods: This study examined the relationships among GI problems, problem behaviors, and internalizing symptoms in a sample of 340 children and adolescents with ASD who are patients at the University of Missouri Thompson Center for Autism & Neurodevelopmental Disorders.
Results: The majority of patients experienced constipation (65%), about half experienced stomachaches or stomach pain (47.9%), and others experienced nausea (23.2%) or diarrhea (29.7%). Young children with aggressive problem behaviors were 11.2% more likely to have co-occurring nausea; whereas, older children showed more complex relationships between internalizing symptoms and GI symptoms. Older children with greater anxiety symptoms were 11% more likely to experience constipation, but 9% less likely to experience stomachaches. Older children with greater withdrawn behavior were 10.9% more likely to experience stomachaches, but 8.7% less likely to experience constipation. Older children with greater somatic complaints were 11.4% more likely to experience nausea and 11.5% more likely to experience stomachaches.
Conclusions: Results suggest that the presentation of externalizing problem behavior and internalizing symptoms associated with GI problems differs between young children and older children with ASD. Therefore, behavior may have different relationships with GI symptoms at different ages, which may have implications for the treatment of and clinical approach to GI disturbances in ASD.
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction across multiple contexts, as well as restricted and repetitive patterns of behavior, interests, and activities that occur early in life and cause clinically significant impairment (1). A variety of gastrointestinal (GI) issues commonly occur in ASD, including lower GI symptoms (i.e., constipation, diarrhea) and upper GI symptoms (i.e., nausea and vomiting, stomach aches and pains) (2–10), but the etiology is poorly understood.
Children with ASD have been shown to experience a range of GI symptoms, with the prevalence shown to be anywhere from 9 to 91% (2), which is likely due differences in assessment and context. However, it appears that many individuals with ASD suffer from constipation (2, 3, 11, 12). One association with GI issues in ASD may be the response to stress, since some individuals with ASD show an altered stress response (13) and recent research has shown connections between lower GI symptoms, sensory over-responsivity, and anxiety (14), as well as altered psychophysiological (11) and endocrine (12) responses to stress-inducing stimuli. These associations suggest that activation of the sympathetic nervous system and the hypothalamic-pituitary axis may be associated with GI disorders in ASD.
Consistent with the theory that stress is linked to GI symptoms in ASD, co-occurring internalizing symptoms, such as anxiety and depression, are common in ASD (15) and associated with GI symptoms as well. Heightened stress and anxiety, rigid-compulsive behavior, and sleep problems, have been shown to be associated with GI symptoms in ASD, especially constipation (6, 11, 12, 16–19). Children with ASD who experience GI symptoms also have co-occurring externalizing problems, as studies have shown that children with ASD with co-occurring GI symptoms had increased irritability when compared to those with ASD and no GI symptoms (5, 6).
The present study aimed to examine relationships among GI symptoms, externalizing problem behavior, and internalizing symptoms in a large sample of young children and older children and adolescents with ASD. We expected that the associations between GI symptoms and co-occurring conditions would be different across age groups. This study also aimed to determine which internalizing or externalizing problem behaviors would be associated with which GI symptoms. We hypothesized that regardless of age, anxiety would be associated with more constipation and diarrhea but less stomachaches and nausea due to the heightened stress responses association with lower GI symptoms.
This study included 340 children and adolescents with ASD ranging in age from 2 to 18 years old (M = 5.56, SD = 3.67) that are clinic patients at University of Missouri Thompson Center for Autism & Neurodevelopmental Disorders in Columbia, Missouri. All participants provided written informed consent in accordance with the Declaration of Helsinki, and the study was approved and carried out in accordance with the recommendations of the University of Missouri Health Sciences Institutional Review Board. Written informed consent was obtained from the parents/caregivers for all participants under the age of 18. Diagnosis of ASD was confirmed using the Autism Diagnostic Observation Schedule (20) or the Autism Diagnostic Interview—Revised (21). The sample included only participants from the database who had at least one GI symptom reported at the most recent clinic visit.
The sample was parsed into two age groups based on which version of the Child Behavior Checklist (CBCL) (22) the caregivers had completed for their child: younger (completed the CBCL for ages 2–5) or older (completed the CBCL for ages 6–18). The younger group consisted of 200 children (80% male), ranging in age from 2 to 5 (M = 3.03; SD = 1.07). The older group consisted of 140 children (77.1% male), ranging in age from 6 to 18 (M = 9.19; SD = 2.94).
At each clinic visit, caregivers completed questionnaires about their child's developmental history and milestones. The responses to the questionnaires from the most recent clinic visit were obtained from the Thompson Center database, and the following variables were extracted and analyzed: dietary problems, nutrition problems, GI symptoms, and internalizing and externalizing symptoms.
Dietary problems were examined with the sum of 12 dichotomous caregiver-endorsed symptoms, with a total range from 0 to 12. The individual items included whether or not the child experienced the following: feeding issues in infancy, current feeding issues, picky eating, milk aversion, nonfood item cravings, food group aversion, food reactions, special diet, difficulty with solids, difficulty with liquids, lethargy, or dehydration.
Nutrition problems were determined by the caregiver's response to the question, “Is the child's nutrition adequate?.” This variable was dummy coded, such that 0 = adequate nutrition and 1 = the child's nutrition was not adequate.
Caregivers completed a questionnaire about their concerns now or in the past about the following GI symptoms in their child: constipation, diarrhea, nausea or vomiting, and stomachaches or stomach pain. These were dichotomous variables, dummy coded such that 0 = no concerns, and 1 = concern. A total GI symptoms score was created by summing the four types of concerns, for a range of 1–4. In addition to the score for total GI problems, each individual GI symptom was considered separately in a subsequent analysis.
Measures of internalizing and externalizing symptoms were derived from caregiver responses on the CBCL (22). The CBCL is a parent-report measure assessing behavioral and emotional symptoms in children. Items are rated on a 3-point Likert scale (0 = not true, 1 = somewhat or sometimes true, and 2 = very true or often true). Two versions of the CBCL are available based on the child's age. The CBCL has strong psychometric properties, including test-retest reliability, inter-rater agreement, and internal consistency (23–25).
Demographic covariates included age, gender, and household income, which were provided by caregivers at the time of assessment. Intelligence was assessed using a range of different assessments, each normalized to a mean of 100 and standard deviation of 15 (See Table 1).
First, a simple bivariate correlation matrix among our variables was produced to determine which demographic or descriptive child and family covariates to include in our main analyses. Then, to evaluate the relationship between internalizing symptoms and externalizing problem behaviors for children with certain GI symptoms, we performed separate logistic regressions with the four GI symptoms as outcome variables for each age group. The primary predictors of interest were the internalizing and externalizing symptom subscales on the CBCL.
The majority of the sample experienced constipation (65%). About half of the children experienced stomachaches or stomach pain (47.9%), and others experienced nausea (23.2%) or diarrhea (29.7%). The average number of total GI symptoms was 1.66 (SD = 0.880), with a range from 1 to 4. The vast majority of the sample was not taking medications for GI symptoms (92.9%). However, over half of the children (53.2%) were taking at least one medication for other reasons (e.g., ADHD, aggression, anxiety symptoms, seizures, or sleep problems).
The two age groups did not differ significantly in gender composition [χ2(1) = 0.403, p = 0.308], nonverbal IQ [t(182) = 0.578, p = 0.564], verbal IQ [t(153) = −0.989, p = 0.324], or full scale IQ [t(168) = 0.869, p = 0.386]. Families with younger children did have less household income [χ2(4) = 9.985, p = 0.041]. Younger and older children took similar amounts of GI medications [χ2(1) = 0.830, p = 0.242] and had similar rates of the four types of GI symptoms (p's > 0.05). Older children were taking more total medications than younger children [t(178.05) = −4.401, p < 0.001]. Younger children had significant more dietary problems [t(224) = 3.182, p = 0.002], but similarly adequate nutrition as compared to older children [χ2 (1) = 0.007, p = 0.524]. See Table 1 for descriptive statistics by age group.
Covariates were determined by identifying any significant bivariate correlations for each age group. Therefore, in the younger age group, we controlled for dietary problems, total number of medications, GI medications, and nutrition problems. In the older age group, we controlled for gender and dietary problems.
In younger children, aggressive problem behavior was a significant predictor of nausea, (B = 0.106, SE = 0.052, p < 0.05). Children with more aggression were 11.2% more likely to experience nausea problems.
In older children, several internalizing and externalizing symptoms were predictive of different GI problems. Older children with greater anxiety were 11% more likely to experience constipation problems (B = 0.094, SE = 0.048, p < 0.05), but 9% less likely to experience stomachaches (B = −0.099, SE = 0.050, p < 0.01.) Older children with greater withdrawn behavior were 10.9% more likely to experience stomachaches (B = 0.086, SE = 0.042, p < 0.05), but 8.7% less likely to experience constipation (B = −0.131, SE = 0.046, p < 0.01). Finally, older children with greater somatic complaints were 11.4% more likely to experience nausea (B = 0.130, SE = 0.050, p < 0.01) and 11.5% more likely to experience stomachaches (B = 0.138, SE = 0.049, p < 0.01). See Table 2 for logistic regression results.
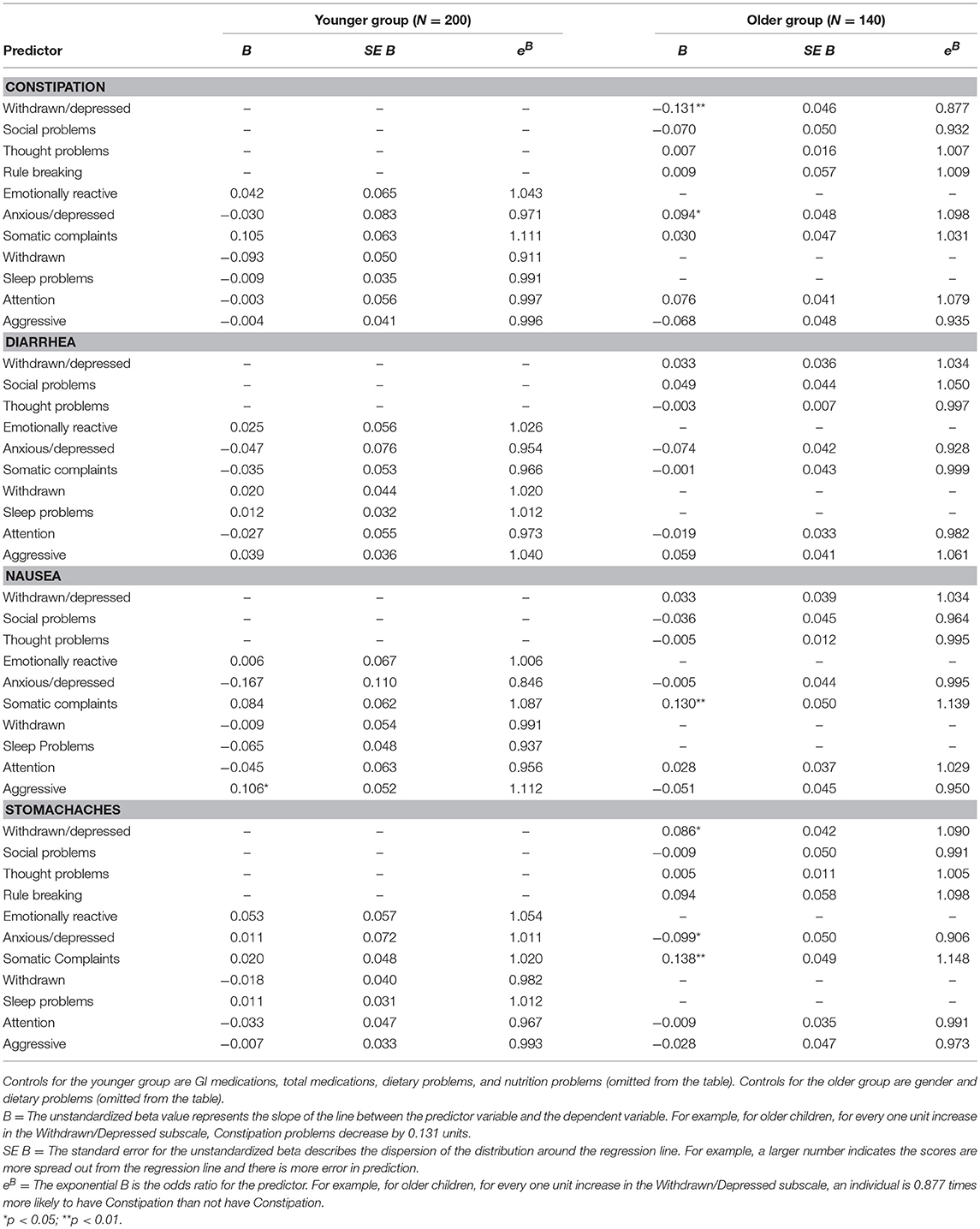
Table 2. Logistic regression results for internalizing and externalizing symptoms predicting GI symptoms in younger (aged 2–5) and older (aged 6–18) children with ASD.
In the current study, constipation accounted for 65% of the GI symptoms in a sample of 340 children and adolescents with ASD. This finding corroborates previous reports in the literature showing that constipation accounts for a significant amount of GI complaints in ASD (2, 11, 12). Regarding externalizing problem behavior, presence of aggressive behavior was associated with nausea in children aged 2–5, suggesting that aggression may be an indicator of nausea in young children in this this population. However, no other GI problems were significantly associated with problem behavior and internalizing symptoms in young children with ASD. One explanation for this relationship may be that young children with ASD who are non-verbal use aggression as a means of communicating somatic complaints, such as internal abdominal pain and GI discomfort (2).
Children and adolescents aged 6–18 revealed associations between internalizing symptoms but not externalizing problem behaviors, and GI symptoms. In the older group, presence of anxiety conferred an 11% increase in the presence of constipation symptoms, which corroborates recent reports on an association between anxiety and lower GI tract symptoms (11, 12). Given these findings, future research may wish to examine the effects of behavioral and/or pharmacological stress and anxiety reductions on GI symptoms in ASD. Interestingly, withdrawn and depressed behavior was associated with an 11% increase in stomachaches, but a 9% decrease in constipation. Previous research has also shown a relationship between upper GI tract symptoms, including stomachaches, and depression in ASD, suggesting that depression and anxiety may place an individual with ASD at heightened risk for GI disturbance, but in different manners. Taken together, it is possible that GI disorders and behavioral problems are related in ASD as a means of communicating their discomfort given the core language deficits in those with ASD. Further research is needed to disentangle the relationship between GI symptoms, anxiety, and depression to determine how these disorders cluster with upper or lower GI tract disorders in ASD.
This study has a number of limitations that should be considered alongside of the results. First, the sample is largely male and Caucasian in both age groups, making it difficult to generalize the findings to females and non-Caucasian individuals with ASD. Second, the GI symptoms were from caregiver reports and were not further assessed with a gastroenterological evaluation. Future studies of GI disorders in ASD should aim to utilize standardized measurements to assess GI symptoms, especially those which are ASD-specific (33, 34). Last, participants included in this study were selected based on having at least one GI symptom, so the results may not be representative of the general population of those with ASD. Furthermore, the sample of participants was from one clinic in the Midwestern United States, and so data from other clinics in other regions are needed in order to generalize these findings to the larger ASD population. In sum, this study provides further evidence of the relationship between co-occurring conditions and GI symptoms in ASD, and highlights the need to evaluate age-related developmental differences in the associations among these symptoms, which may prove to be important when developing GI treatments for those with ASD across the lifespan.
This study was carried out in accordance with the recommendations of the Health Sciences Institutional Review Board at the University of Missouri with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Health Sciences Institutional Review Board at the University of Missouri.
BF and DB conceived the aforementioned studies conducted at the University of Missouri Thompson Center for Autism & Neurodevelopmental Disorders. DB provided expertise on ASD and GI disorders in ASD. KD provided the statistical analysis for the study. NT maintained the database from which the data for this study were obtained. All authors contributed to the development of the manuscript and approved the final version that was submitted for review.
This study was funded by general funds provided to the University of Missouri Thompson Center for Autism & Neurodevelopmental Disorders in Columbia, Missouri.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the families and their children for participating in this important research study. We also thank the numerous staff members at the Thompson Center for their hard work on maintaining the clinic database to make studies such as this possible.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association (2013).
2. Buie T, Campbell DB, Fuchs GJ III, Furuta GT, Levy J, van de Water J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. (2010) 125S1:S1–18. doi: 10.1542/peds.2009-1878C
3. McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal 86 symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. (2014) 133:872–83. doi: 10.1542/peds.2013-3995
4. Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics. (2014) 133:e54–63. doi: 10.1542/peds.2013-0819
5. Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Develop Disord. (2014) 44:1117–27. doi: 10.1007/s10803-013-1973-x
6. Fulceri F, Morelli M, Santocchi E, Cena H, Del Bianco T, Narzisi A, et al. Gastrointestinal symptoms and behavioral problems in preschoolers with autism spectrum disorder. Digest Liver Dis. (2016) 48:248–54. doi: 10.1016/j.dld.2015.11.026
7. Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. (2012) 5:101–8. doi: 10.1002/aur.237
8. Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics. (2010) 7:320–7. doi: 10.1016/j.nurt.2010.06.001
9. Mouridsen SE, Rich B, Isager T. A longitudinal study of gastrointestinal diseases in individuals diagnosed with infantile autism as children. Child Care Health Develop. (2010) 36:437–43. doi: 10.1111/j.1365-2214.2009.01021.x
10. Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: a populationbased study. Pediatrics. (2009) 124:680–6. doi: 10.1542/peds.2008-2933
11. Ferguson BJ, Marler S, Altstein LL, Lee EB, Akers J, Sohl K, et al. Psychophysiological associations with gastrointestinal symptomatology in autism spectrum disorder. Autism Res. (2017) 10:276–88. doi: 10.1002/aur.1646
12. Ferguson BJ, Marler S, Altstein LL, Lee EB, Mazurek MO, McLaughlin A, et al. Associations between cytokines, endocrine stress response, and gastrointestinal symptoms in autism spectrum disorder. Brain Behav Immun. (2016) 58:57–62. doi: 10.1016/j.bbi.2016.05.009
13. Lopata C, Volker MA, Putnam SK, Thomeer ML, Nida RE. Effect of social familiarity on salivary cortisol and self-reports of social anxiety and stress in children with high functioning autism spectrum disorders. J Autism Develop Disord. (2008) 38:1866–77. doi: 10.1007/s10803-008-0575-5
14. Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A, et al. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. J Abnor Child Psychol. (2013) 41:165–76. doi: 10.1007/s10802-012-9668-x
15. Hovarth K, Perman JA. Autism and gastrointestinal symptoms. Curr Gastroenterol Rep. (2002) 4:251–8. doi: 10.1007/s11894-002-0071-6
16. Coury DL, Ashwood P, Fasano A, Fuchs G, Geraghty M, Kaul A, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. (2012) 130(Suppl. 2):S160–8. doi: 10.1542/peds.2012-0900N
17. Bishop-Fitzpatrick L, Mazefsky CA, Minshew NJ, Eack SM. The relationship between stress and social functioning in adults with autism spectrum disorder and without intellectual disability. Autism Res. (2015) 8:164–73. doi: 10.1002/aur.1433
18. Nikolov RN, Bearss KE, Lettinga J, Erickson C, Rodowski M, Aman MG, et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Develop Disord. (2009) 39:405–13. doi: 10.1007/s10803-008-0637-8
19. Peters B, Williams KC, Gorrindo P, Rosenberg D, Lee EB, Levitt P, et al. Rigid-compulsive behaviors are associated with mixed bowel symptoms in autism spectrum disorder. J Autism Develop Disord. (2014) 44:1425–32. doi: 10.1007/s10803-013-2009-2
20. Lord C, DiLavorne PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services (2002).
21. Lord C, Rutter M, le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Develop Disord. (1994) 24:659–85. doi: 10.1007/BF02172145
22. Achenbach TM, Rescorla L. Manual for the ASEBA School-Age Forms & Profiles: An Integrated System of Multi-informant Assessment. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families. (2001).
23. Achenbach TM, Dumenci L, Rescorla LA. DSM-Oriented and empirically based approaches to constructing scales from the same item pools. J Clin Child Adoles Psychol. (2003) 32:328–40. doi: 10.1207/S15374424JCCP3203_02
24. Nakamura BJ, Ebesutani C, Bernstein A, Chorpita BF. (2009). A psychometric analysis of the child behavior checklist DSM-oriented scales. J. Psychopathol. Behav. Assess. 31, 178–189. doi: 10.1007/s10862-008-9119-8
25. Frizzo GB, Pedrini JR, de Souza DS, Bandeira DR, Borsa JC. Reliability of child behavior checklist and teacher's report form in a sample of brazilian children. Universitas Psychologica. (2014) 14:149–56. doi: 10.11144/Javeriana.upsy14-1.rcbc
26. Elliot CD. Differntial Ability Scales. 2nd ed. San Antonio, TX: PsychCorp Harcourt Assessment Inc (2007).
27. Wechsler D. Wechsler Abbreviated Scale of Intelligence. 2nd ed (wasi-ii). San Antonio, TX: NCS Pearson (2011).
28. Wechsler D. Wechsler Intelligence Scale for Children 4th ed (wisc-iv). San Antonio, TX: NCS Pearson (2003).
29. Wechsler D. Wechsler Intelligence Scale for Children-fifth edition. Bloomington, MN: Pearson (2014).
30. Wechsler D. The Wechsler Preschool and Primary Scale Of Intelligence. 3rd ed. San Antonio, TX: The Psychological Corporation (2002).
31. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 4th ed. Bloomington, MN: Pearson (2012).
32. Roid GH, Miller LJ, Pomplun M, Koch C. Leiter International Performance. 3rd ed (leiter-3). Wood Dale. IL: Stoelting (2013).
33. Walker LS, Caplan-Dover A, Rasquin-Weber A. Rome III Diagnostic Questionnaire for the Pediatric Functional GI Disorders. (2006).
Keywords: autism spectrum disorder, gastrointestinal disorders, problem behavior, internalizing symptoms, anxiety, depression
Citation: Ferguson BJ, Dovgan K, Takahashi N and Beversdorf DQ (2019) The Relationship Among Gastrointestinal Symptoms, Problem Behaviors, and Internalizing Symptoms in Children and Adolescents With Autism Spectrum Disorder. Front. Psychiatry 10:194. doi: 10.3389/fpsyt.2019.00194
Received: 21 December 2018; Accepted: 18 March 2019;
Published: 09 April 2019.
Edited by:
Manuel Fernando Casanova, University of South Carolina, United StatesReviewed by:
Robert Hendren, University of California, San Francisco, United StatesCopyright © 2019 Ferguson, Dovgan, Takahashi and Beversdorf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bradley J. Ferguson, ZmVyZ3Vzb25iakBoZWFsdGgubWlzc291cmkuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.