- 1Department of Psychiatry and Psychotherapy, University Hospital, Ludwig-Maximilians University Munich, Munich, Germany
- 2Department of Psychiatry and Psychotherapy, Universitätsklinikum Jena, Jena, Germany
- 3Laboratory of Neuroscience (LIM27), Institute of Psychiatry, University of São Paulo, São Paulo, Brazil
Background: The beneficial effects of exercise training on depressive symptoms are well-established. In the past years, more research attention has been drawn to the specific effects of exercise training on depressive symptoms in somatically ill patients. This reviews aims at providing a comprehensive overview of the current findings and evidence of exercise interventions in somatic disorders to improve depressive symptoms.
Methods: We systematically searched PubMed and Cochrane databases and extracted meta-analyses from somatically ill patients that underwent exercise interventions and provided information about the outcome of depressive symptoms.
Results: Of the 4123 detected publications, 39 were selected for final analysis. Various diseases were included (breast-cancer, prostate cancer, mixed-cancer, cardiovascular disease, coronary heart disease, hemodialysis, fibromyalgia syndrome, acute leukemia, other hematological malignancies, heart failure, HIV, multiple sclerosis, mixed neurological disorders, Parkinson's disease, stroke, ankylosing spondylitis, traumatic brain injury, lupus erythematodes). Most meta-analyses (33/39) found beneficial effects on depressive symptoms, but quality of the included studies as well as duration, intensity, frequency, and type of exercise varied widely.
Conclusion: Exercise training has the potential to improve depressive symptoms in patients with somatic disorders. For specific training recommendations, more high quality studies with structured exercise programs and better comparability are needed.
Introduction
Depression alone and comorbid with other chronic somatic diseases accounts for significant disease burden worldwide (1, 2). By 2030, depression is in addition to HIV/AIDS and ischemic heart disease assumed to be one of the three leading causes of burden of disease (3). Exercise therapy in depressive patients, as an independent intervention and as an adjunct intervention to antidepressant medication or psychotherapy, has been discussed to improve clinical outcomes in somatic diseases (4, 5). Compared to pharmacological treatment, the beneficial effects of exercise seem to be similar and are present at short-term and during follow-up periods of up to one year (6, 7). Especially for somatically unstable patient, this offers new treatment perspectives without the common side-effects of psychopharmacological medication. Usually both the depressive symptoms and the underlying somatic condition improve as a consequence of exercise therapy.
In a large World Health Survey of 245 404 participants from 60 countries, an average between 9.3 and 23.0% of participants with one or more chronic physical diseases had comorbid depressive symptoms (2). For numerous somatic diseases, an association with depression is well-established. For example, patients with multiple sclerosis (8), stroke (9), parkinson's disease (10), diabetes mellitus (11), breast cancer (12) or heart failure (13) have an elevated risk for developing a major depressive disorder (MDD). From a psychiatric perspective, it is important to note that a recently published study with 1,237,194 participants showed that individuals who exercised (various types of disciplines) had 1.49 (43.2%) fewer days of poor mental health in the past month than non-exercising individuals (14).
As the comorbid physical diseases often complicate the pharmacological treatment of depressive symptoms, further research approaches with a focus on alternative therapy options are urgently needed. Based on the beneficial effects of physical exercise in MDD (15), prior studies investigated the effects of exercise in patients with somatic disorders and comorbid depressive symptoms. A meta-analysis of different chronic illnesses including stroke, ischemic heart attack, fibromyalgia, dementia, and other psychiatric diseases, revealed a significant overall reduction of depressive symptoms. The majority of effects were derived from cardiovascular diseases, chronic pain and fibromyalgia (16). The results lead to the conclusion that physical exercise seems to have positive effects on depressive symptoms in patients with various somatic diseases without subdividing the different diseases with specific recommendations. However, a comprehensive overview of the available evidence of how exercise may improve depressive symptoms in patients with somatic diseases is lacking.
In this systematic meta-review, we will provide a detailed overview of recent meta-analyses evaluating different somatic diseases with comorbid depressive symptoms. The efficacy and the types and durations of exercise will be analyzed. Up to date, meta-analyses of specific diseases or disease groups (e.g., cardiovascular diseases) have been published as well as meta-analyses comprising different somatic diseases with an emphasis on cardiovascular diseases, fibromyalgia and pain (16). With our review, we aim to close this gap and point to limitations of prior studies as well as possible future research strategies.
Methods
We systematically searched PubMed and Cochrane databases with all combinations of the following search terms: depressive disorder OR depression AND exercise, depressive disorder OR depression AND physical activity, depressive disorder OR depression AND endurance training, depressive disorder OR depression AND training, depressive disorder OR depression AND resistance training, depressive disorder OR depression AND aerobic, depression OR depressive disorder AND somatic AND exercise, depression OR depressive disorder AND physical illness. The database search was last updated on 31th July 2018. All citations were screened for relevance by title in a first step, by abstract in a second step and by full-text in a last step. In all included meta-analyses, the citations were screened manually for further relevant meta-analyses that may have not been detected by the systematic search. The systematic literature search and selection was performed by AR, the selection was afterwards reviewed independently by SK.
Both AR and SK retrieved the relevant information and the results were compared. In case of disagreement, a third author (AH) was consulted. Inclusion criteria were: meta-analysis investigating the effects of exercise on depressive symptoms in patients with comorbid physical illness based on interventional trials, published within the past 10 years. Exclusion criteria contained meta-analyses published earlier than within the past 10 years, systematic reviews without meta-analyses, meta-analyses in non-English language, meta-analyses only investigating depressive patients with no somatic comorbidities, lack of data post-intervention. No limitations were defined for the type or duration of exercise and the type of somatic illness.
Results
Study Selection
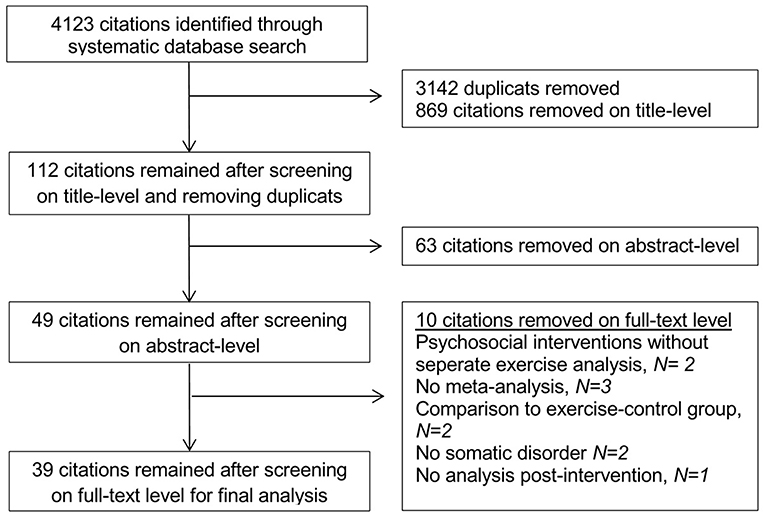
The initial search without further restrictions resulted in 103,468 citations. After limiting the citations for the past 10 years, 60,430 citations remained. When only considering the meta-analyses, 4,123 citations remained and after eliminating duplicates, 981 citations were included for further analysis. The screening on title-level eliminated 869 citations (112 remaining), the screening on abstract-level another 63 citations (49 remaining). After full-text screening, 39 citations remained for final analysis (see Figure 1).
Study Characteristics
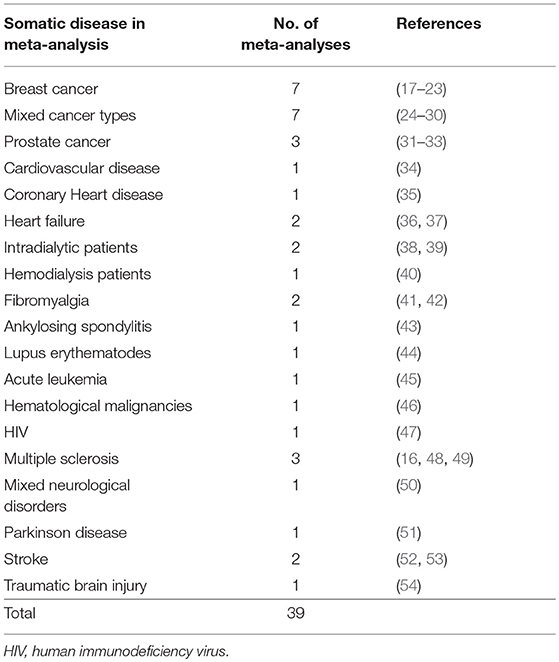
Table 1 provides an overview of the included somatic diseases. The duration of the single studies varied between three (46) to 52 (21) weeks, the intensity between one time (51) and seven times (37) per week and the duration of the sessions between 30 and 122 min (16). Adherence rate was solely reported in two meta-analyses (21, 22) and intensity of the training with heart rate controlled exertion in one meta-analysis (37). The interventions consisted of aerobic exercise, resistance training, balance training, yoga, moderate cycling, walking, nordic walking, running, swimming, tai chi chuan, qigong, Bobath exercises, jogging, calisthenics. Across all trials, the mean sample size was 658 with a minimum of 39 (28) and a maximum of 3.425 (36). Depressive symptoms were rated with different questionnaires (observer or self-assessment): BDI (Beck Depression Inventory), CDI (Children Depression Inventory), CES-D (Center for Epidemiological Studies Depression Scale), DASS (Depression Anxiety Stress Scales), GDS (Geriatric Depression Scale), HAD (Hospital Anxiety and Depression Scale), HAM-D (Hamilton Rating Scale for Depression), SCL-90 (Symptom Checklist-90), TAS (Toronto Attitude Scale), POMS (Profile of Mood States), SAS (Symptom Assessment Scale), CSDD (Cornell Scale for Depression in Dementia), IDS-SR (Inventory of Depressive Symptomatology Self Report), LPD (Levine-Pilowsky Depression Questionnaire), MADRS (Montgomery-Asberg Depression Rating Scale), MDI (Major Depression Inventory), POMS (Profile of Mood States), QOL (Quality of Life), DASS (Depression Anxiety Stress Scales), TAS (Toronto Attitude Scale), MAACL (Multiple Affect Adjective Checklist), HDCDS (Hare-Davis Cardiac Depression Scale), SDS (Self-rating Depression Scale), BSI-18 (Brief Symptom Inventory). Thus, in the different meta-analyses, various different questionnaires were used, most often self-rating questionnaires: the BDI I/II in 26, the HADS in 18 and the CES-D in 17 meta-analyses. The majority of meta-analyses included more than three different questionnaires. As observer-rating scales, HAMD was used in four and MADRS in three meta-analyses.
Risk of Bias Within and Across Studies
Most meta-analyses (51) reported about different cancer-type patients followed by neurological disorders (7). For other diseases, such as HIV, only one meta-analysis could be identified. The variety of exercise modalities and the different durations of interventions lead to a reduced general transferability of the conclusions. Recommendations regarding effects of specific interventions on depressive symptoms in specific somatic conditions can hardly be drawn. None of the trials included in the respective meta-analyses could be declared double-blind as this is challenging to fulfill with exercise interventions. The application of various different rating-scales is another potential risk of bias. Moreover, most of the included studies in the respective meta-analyses did not define depressive symptoms as primary outcome parameter.
Results of Individual Studies
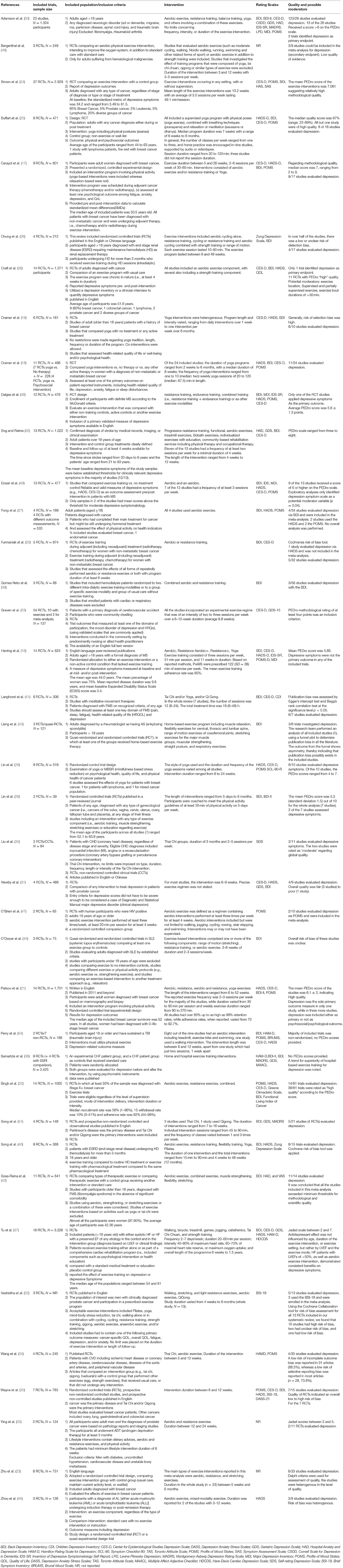
A detailed summary of the included studies with inclusion/exclusion criteria, study population, intervention summary and outcomes is provided in Tables 2, 3.
Exercise Interventions in Cancer Patients
Exercise interventions in breast cancer patients
Our search resulted in seven meta-analyses that met the inclusion criteria. The majority found positive effects of exercise on depressive symptoms in these cohorts (17, 21–23, 55). Different types of interventions were summarized (aerobic, resistance, aerobic and resistance, yoga exercises), with one meta-analysis providing more detailed information about differences between the exercise types: aerobic interventions yielded a large and significant effect on depression at the last follow-up measurement compared with the non-exercising control group (21). Another meta-analysis pointed toward the beneficial effects of an intervention duration of longer than 12 weeks (22).
One meta-analysis only showed little improvement in depression after exercise intervention (20). The two remaining meta-analyses with overlapping populations of Cramer et al. (18, 19) were heterogeneous. In the 2012 analysis, large short-term effects were shown (19), in the 2017 analysis these results could not be reproduced (18).
Exercise interventions in mixed cancer-samples
Most of these meta-analyses included a majority of breast-cancer patients.
Yoga intervention Buffart et al. showed significant large reductions in depressive symptoms of yoga interventions, one of the included RCTs included lymphoma patients, the other 6 trials breast cancer (25). Lin et al. (29) summarized 6/8 RCTs with breast cancer and different forms of yoga interventions and showed that depressive symptoms improved significantly in the intervention groups (29).
In the cohort of Wayne et al. breast cancer was also the main disease. The overall effect size favored Thai Chi and QiGong interventions on depressive symptoms (30).
Aerobic or mixed intervention Brown et al. (24) (65% of patients with breast cancer) found a small overall reduction in depressive symptoms of aerobic and yoga interventions compared to standard care among all types of cancer. Subgroup analysis by cancer type revealed significant reductions in depressive symptoms among breast cancer survivors, but no significant difference in depressive symptoms among prostate, leukemia, lymphoma, and colorectal cancer survivors.
Another meta-analysis by Craft et al. revealed modest beneficial effects of exercise (26). Fong et al. evaluated 75% breast cancer patients and showed different outcomes with regard to different depression scales: measured by the BDI, physical activity (aerobic) was associated with reduced depression. Subgroups of four RCTs used the HAMD and POMS scales, the outcomes in this cohort were not significant (27).
In another meta-analysis by Lin et al. (28) with various gynecological cancer-types and various types of activity (i.e., aerobic training, muscle strengthening, stretching exercises or education regarding exercise) no statistically significant improvement could be shown. None of the included RCTs appeared in both analyses (28).
Exercise interventions in Patients with Prostate Cancer
Three meta-analyses were found eligible for this review with regard to prostate cancer. All of them included various types of exercise and one meta-analysis included lifestyle interventions consisting of exercise and other interventions (e.g., dietary advice) (33). The largest meta-analysis with four RCTs found significantly reduced depressive symptoms, while the two smaller ones with 3 RCTs and 2 RCTs found no statistically significant differences (31–33).
Exercise Interventions in Patients With Cardiovascular Disease and Coronary Heart Disease
For cardiovascular diseases, one meta-analysis could be included in our meta- review (34). 4 RCTs summarizing patients with ischemic heart disease or coronary artery disease, cerebrovascular disease, diseases of the aorta and arteries, and peripheral vascular disease compared Thai Chi, qigong, baduanjin interventions to control interventions (e.g., strength training or no intervention). Both outcome measures (HAMD and POMS) showed significant improvements in the intervention groups. One meta-analysis with 2 RCTs/CCTs analyzed the effects of 3-months of Thai Chi Interventions and found positive effects in the intervention group concerning the depressive symptoms (35).
Exercise Interventions in Patients With Heart Failure
The two included meta-analyses showed an improvement in depressive symptoms compared to standard care in large cohorts (36, 37).
Intradialytic Exercise Interventions and Exercise Interventions in Hemodialysis Patients
We could include two meta-analyses for the cohort of intradialytic exercise (38, 39). Exercise interventions included aerobic training, resistance training, or a combination with strength training or range of motion. Both publications showed significant improvements of depressive symptoms (38, 39).
One meta-analysis included intradialytic exercise patients and exercise interventions in hemodialysis patients on non-dialytic days (40). Beneficial effects of exercise were reported.
Exercise Interventions in Patients With Fibromyalgia, Ankylosing Spondylitis and Lupus Erythematodes (LE)
The two cited meta-analyses for fibromyalgia (41, 42) were able to present improved depressive symptoms. The interventions were heterogeneous with Tai Chi and/or Yoga, and/or Qi Gong (41) and aerobic exercise, combined exercise, muscle strengthening, flexibility, stretching (42).
Our search resulted in one meta-analysis that revealed a statistically significant beneficial effect of home-based stretching exercise in reducing depressive symptoms in patients with ankylosing spondylitis (43).
In a meta-analysis of patients with LE, significantly lower depression scores were found in the exercise groups (stretching or aerobic exercise) compared to controls (44).
Exercise Interventions in Patients With Acute Leukemia and Other Hematological Malignancies
Our systematic search resulted in two meta-analyses, one including solely acute leukemia (45) and one including various hematological malignancies (46). Zhou et al. found no significant differences between the exercise (aerobic exercise, mixed-modality exercise) and control groups, whereas Bergenthal showed a statistically significant benefit for the exercise group with aerobic exercise or combined aerobic/strength exercise in a larger cohort.
Exercise Interventions in Patients With HIV
We were able to identify one meta-analysis with a small sample size and various forms of exercise interventions (e.g., walking, jogging, cycling, rowing, stair stepping, and swimming). A significant improvement could be displayed in the depression-dejection sub scale of the POMS d (47).
Exercise Interventions in Patients With Neurological Diseases
Three meta-analyses revealed small but significantly positive effects of exercise on depressive symptoms in multiple sclerosis (16, 48, 49). Different forms of exercise were allowed (Aerobic, Resistance Aerobic+, Yoga).
In Parkinson's disease, exercise interventions (Tai Chi and/or Qigong) led to significantly improved depressive symptoms (51).
Overall, physical exercise resulted in less depressive symptoms in the two suitable meta-analyses of stroke patients (52, 53). After 10 weeks of follow-up, this beneficial effect could not be maintained (52).
One meta-analysis included various neurological disorders and various exercise interventions. Depressive symptoms were significantly reduced. The effect of exercise on depression was slightly larger in the MS studies compared with the other studies. In addition to the above mentioned neurological disorders (with overlapping single studies), other diseases like Alzheimer's disease, were included (50).
Exercise Interventions in Patients With Traumatic Brain Injury
In one meta-analysis, two RCTs and seven non-RCTs were included and found statistically significant, positive effects of exercise on depressive symptoms (54).
Discussion
We were able to include 39 meta-analyses confirming that the application of exercise as an add-on treatment in patients with somatic disorders is a frequently studied intervention. As stated above, different reasons for this observation can be discussed: the positive effects in depression alone (15), the principle lack of side-effects and the overall improvement of fitness and mortality. In our meta-review, most meta-analyses showed these (expected) beneficial effects of exercise on depressive symptoms (33/39). The six meta-analyses without these effects evaluated different exercise modalities in different types of diseases (18, 20, 28, 32, 33, 45). One of these meta-analyses provided data of breast cancer patients receiving yoga therapy and found no significant effect on depressive symptoms of yoga when comparing to no therapy, but did find significant improvements of depressive symptoms when comparing yoga to psychosocial intervention (7 and 4 included trials) (18). Another meta-analysis with breast-cancer patients found no significant results (20). The smallest of the included meta-analyses with 39 participants (2 trials) also evaluated depressive symptoms in any type of gynecological cancer and found no significant effects (28). Neither of the two meta-analyses with prostate cancer patients nor the meta-analyses with acute lymphoblastic leukemia (ALL) patients found significant reductions in depressive symptoms (32, 33, 45).
Five meta-analyses reported long-term effects with follow-up periods from 3 to 9 months of the exercise interventions (19, 21, 37, 41, 52). The results were heterogeneous with two meta-analyses showing no significant effects in a 3 to 9 months period (19, 52). One meta-analysis only found positive long-term effects during 3 to 6 months for one of the possible exercise interventions in subgroup analyses (41). The two remaining meta-analyses pointed to positive long-term effects in a 3–6 months period (21, 37). In all of these studies except one (37) the follow-up cohort was much smaller compared to the overall sample size. The small sample sizes on the one hand and the small number of meta-analyses in total restrict the generalizability of these results. Future studies should include a follow-up period of at least 3 months (preferably three and 3 months assessments) to accumulate data for meaningful statements.
Following the American College of Sports Medicine (ACSM) guidelines, healthy adults should engage in moderate aerobic exercise training for ≥30 min per day on ≥5 days per week, vigorous aerobic exercise training for ≥20 min per day on ≥3 days per week, or a combination of both moderate and vigorous aerobic training. On another 2–3 days, healthy adults should perform resistance exercises for each of the major muscle groups and flexibility exercises for each major muscle-tendon groups on ≥2 days per week (56). Future studies should lean on these recommendations to help homogenize the results.
In literature, many different rating scales (self- and observer-rating) have been introduced to assess depressive symptoms, reflecting the variety of questionnaires used in the original trials and the consecutive meta-analyses. For example, the practice guidelines for the treatment of depression in Germany (57) list nine possible questionnaires as valid tools. In specific somatic disorders, validation studies of the most often used scales were performed: HAMD for multiple sclerosis patient (58) and for stroke patients (59). BDI for cancer patients (60) and heart failure patients (61). In a mixed cancer population, a review recommended the CES-D as most precise instrument if depression is the sole focus (62). It has been stated that discrepancies between BDI and HAMD scores (self- and observer rating scales) could be due to different personality traits (e.g., high neuroticism is associated with higher BDI scores) and that therefore both should be regarded separately (63). To date, valid recommendations for all included somatic disorders or general recommendations for specific questionnaires to access depressive symptoms could not be identified. For future studies, similar outcome measured should be implemented to ensure comparability and both self- and observer rating scales should be combined.
The possible reasons for the varying observations regarding the different intervention types and the measuring time points could be the same reasons that complicate meta-analyses of the existing exercise trials. As stated above, in none of the meta-analyses, the included trials had matching exercise protocols regarding type, duration and frequency of the intervention. Most often no data was provided about the attendance rate of the participants and about the aerobic capacity before/after the intervention as a controlling variable. The quality of the trials ranged from very low to good quality (also with regard to the exercise-specific difficulty of blinding the intervention). In none of the meta-analyses the depressive symptoms after the exercise intervention were the primary endpoint in all of the included trials, so moreover the validity of the conclusions has to be discussed cautiously.
In summary, we were able to identify a large interest in this field of research for the above demonstrated reasons. In most of the meta-analyses, regardless of the underlying somatic disease, beneficial effects of exercise on depressive symptoms could be observed—keeping in mind that depressive symptoms were mainly secondary outcomes in the included studies. Some of the somatic diseases, especially cancer and in this field breast cancer, were overrepresented compared to others like heart failure.
More standardized trials with better comparability are required to draw specific conclusions about the recommended type and duration of exercise in different diseases. The current findings point to beneficial effects of various forms of exercise, but the implementation in guidelines and for example in therapy strategies that are supported more extensively is still difficult because of the lack of clarity of specific outcomes. Especially the diversity of outcome parameters impedes the comparability of interventions within and between somatic disorders. Moreover, most meta-analyses did not report types of other antidepressant interventions like medication or psychotherapy. Thus, it is difficult to answer the question whether exercise therapy is efficient as an add-on treatment to an ongoing pharmaco- or psychotherapy or whether it should be offered as a single intervention in patients with somatic disorders and depression. Finally, information is sparse regarding whether patients continue to exercise after the intervention (e.g., in sport clubs) or whether their overall activity level (e.g., measured by pedometers or more accurately by accelerometers) increases and how this is related to outcome.
Conclusion
In this meta-review, we provide an overview of existing evidence for the effects of exercise on depressive symptoms in various somatic disorders. The results are promising, but meaningful recommendations are lacking because of heterogeneous study protocols. For better comparability, we would recommend to implement the following standards in future studies: homogeneous outcome measures, one self-rating scale (e.g., BDI) and one observer-rating scale (e.g., HAMD or MADRS). Moreover, specific information of other antidepressant treatments (especially medication and psychotherapy) should be consistently reported. State and duration of the somatic disease should be provided. Future studies should lean on recommendation of the ACSM regarding type and intensity of the intervention (e.g., moderate aerobic exercise training for ≥30 min per day on ≥5 days per week or vigorous aerobic exercise training for ≥20 min per day on ≥3 days per week; on another 2–3 days, resistance exercises for each of the major muscle groups and flexibility exercises for each major muscle-tendon groups on ≥2 days per week) (56). For aerobic training, bicycle ergometers are a widely-used and practical possibility (also for e.g., patients with arthrosis). The duration of the intervention should last at least 12 weeks. Attendance rates, setting (e.g., group activity, with/without supervision) and training intensity should be monitored, preferably with spiroergometric examinations at the start and the end of the study. Follow-up examinations of 3 and 6 months should be performed, and also in this period, information about antidepressant medication should be provided. On these occasions, information about the overall fitness level (e.g., via pedometer/accelerometer measurements over at least 3 days or via activity questionnaires like IPAQ) should be documented.
Author Contributions
AR and AH conceived the study. AR and SK performed the qualitative analyses. AR, SK, IM, and AH wrote the first draft. AS and PF supervised the project. BM provided methodological advice; and all authors were involved in the reviewing the manuscript and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Luppa M, Heinrich S, Angermeyer MC, König H-H, Riedel-Heller SG. Cost-of-illness studies of depression: a systematic review. J Affect Disord. (2007) 98:29–43. doi: 10.1016/j.jad.2006.07.017
2. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: Results from the World Health Surveys. Lancet. (2007) 370:851–8. doi: 10.1016/S0140-6736(07)61415-9
3. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
4. Kvam S, Kleppe CL, Nordhus IH, Hovland A. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. (2016) 202:67–86. doi: 10.1016/j.jad.2016.03.063
5. Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: A meta-analysis of randomized trials. Sports Med. (2009) 39:491–511. doi: 10.2165/00007256-200939060-00004
6. Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. (2007) 69:587–96. doi: 10.1097/PSY.0b013e318148c19a
7. Hoffman BM, Babyak MA, Craighead WE, Sherwood A, Doraiswamy PM, Coons MJ, et al. Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom Med. (2011) 73:127–33. doi: 10.1097/PSY.0b013e31820433a5
8. Solaro C, Gamberini G, Masuccio FG. Depression in multiple sclerosis: epidemiology, aetiology, diagnosis and treatment. CNS Drugs. (2018) 32:117–33. doi: 10.1007/s40263-018-0489-5
9. Arwert HJ, Meesters JJL, Boiten J, Balk F, Wolterbeek R, Vliet Vlieland TPM. Post stroke depression, a long term problem for stroke survivors. Am J Phys Med Rehabil. (2018) 97:565–71. doi: 10.1097/PHM.0000000000000918
10. Camargo CHF, Serpa RA, Jobbins VA, Berbetz FA, Sabatini JS. Differentiating between apathy and depression in patients with parkinson disease dementia. Am J Alzheimer's Dis Other Dement. (2018) 33:30–4. doi: 10.1177/1533317517728333
11. Eker S. Prevalence of depression symptoms in diabetes mellitus. Open Access Maced J Med Sci. (2018) 6:340–3. doi: 10.3889/oamjms.2018.085
12. Park EM, Gelber S, Rosenberg SM, Seah DSE, Schapira L, Come SE, et al. Anxiety and depression in young women with metastatic breast cancer: a cross-sectional study. (2018). Psychosomatics 59:251–8. doi: 10.1016/j.psym.2018.01.007
13. Gagin R, HaGani N, Shinan-Altman S, Roguin A. Coping with depression and anxiety among heart failure patients. Harefuah. (2018) 157:81–4.
14. Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH, et al. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry. (2018) 5:739–46. doi: 10.1016/S2215-0366(18)30227-X
15. Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. (2016) 77:42–51. doi: 10.1016/j.jpsychires.2016.02.023
16. Herring MP, Puetz TW, O'Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. (2012) 172:101–11. doi: 10.1001/archinternmed.2011.696
17. Carayol M, Bernard P, Boiche J, Riou F, Mercier B, Cousson-Gelie F, et al. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: what is the optimal dose needed? Ann Oncol. (2013) 24:291–300. doi: 10.1093/annonc/mds342
18. Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Coch Database Syst Rev. (2017) 1:CD010802. doi: 10.1002/14651858.CD010802.pub2
19. Cramer H, Lange S, Klose P, Paul A, Dobos G. Yoga for breast cancer patients and survivors: a systematic review and meta-analysis. BMC Cancer. (2012) 12:412. doi: 10.1186/1471-2407-12-412
20. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochr Database Syst Rev. (2016) 9:CD005001. doi: 10.1002/14651858.CD005001.pub3
21. Patsou ED, Alexias GD, Anagnostopoulos FG, Karamouzis MV. Effects of physical activity on depressive symptoms during breast cancer survivorship: a meta-analysis of randomised control trials. ESMO Open. (2017) 2:e000271. doi: 10.1136/esmoopen-2017-000271
22. Singh B, Spence RR, Steele ML, Sandler CX, Peake JM, Hayes SC. A systematic review and meta-analysis of the safety, feasibility and effect of exercise in women with stage II+ breast cancer. Arch Phys Med Rehabil. (2018) 99:2621–36. doi: 10.1016/j.apmr.2018.03.026
23. Zhu G, Zhang X, Wang Y, Xiong H, Zhao Y, Sun F. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trails. OncoTargets Ther. (2016) 9:2153–68. doi: 10.2147/OTT.S97864
24. Brown JC, Huedo-Medina TB, Pescatello LS, Ryan SM, Pescatello SM, Moker E, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS ONE. (2012) 7:e30955. doi: 10.1371/journal.pone.0030955
25. Buffart L, van Uffelen M, Jannique GZ, Riphagen II, Brug J, van Mechelen W, Brown WJ, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. (2012) 12:559. doi: 10.1186/1471-2407-12-559
26. Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. (2012) 21:3–19. doi: 10.1158/1055-9965.EPI-11-0634
27. Fong DYT, Ho JWC, Hui BPH, Lee AM, Macfarlane DJ, Leung SSK, et al. Physical activity for cancer survivors: Meta-analysis of randomised controlled trials. BMJ. (2012) 344:e70. doi: 10.1136/bmj.e70
28. Lin K-Y, Frawley HC, Denehy L, Feil D, Granger CL. Exercise interventions for patients with gynaecological cancer: a systematic review and meta-analysis. Physiotherapy. (2016) 102:309–19. doi: 10.1016/j.physio.2016.02.006
29. Lin K-Y, Hu Y-T, Chang K-J, Lin H-F, Tsauo J-Y. Effects of yoga on psychological health, quality of life, and physical health of patients with cancer: a meta-analysis. Evid Based Compl Altern Med. (2011) 2011:659876. doi: 10.1155/2011/659876
30. Wayne PM, Lee MS, Novakowski J, Osypiuk K, Ligibel J, Carlson LE, et al. Tai Chi and Qigong for cancer-related symptoms and quality of life: a systematic review and meta-analysis. J Cancer Surviv. (2017). doi: 10.1007/s11764-017-0665-5
31. Newby TA, Graff JN, Ganzini LK, McDonagh MS. Interventions that may reduce depressive symptoms among prostate cancer patients: a systematic review and meta-analysis. Psychooncology. (2015) 24:1686–93. doi: 10.1002/pon.3781
32. Vashistha V, Singh B, Kaur S, Prokop LJ, Kaushik D. The effects of exercise on fatigue, quality of life, and psychological function for men with prostate cancer: systematic review and meta-analyses. Eur Urol Focus. (2016) 2:284–95. doi: 10.1016/j.euf.2016.02.011
33. Ying M, Zhao R, Jiang D, Gu S, Li M. Lifestyle interventions to alleviate side effects on prostate cancer patients receiving androgen deprivation therapy: a meta-analysis. Jpn J Clin Oncol. (2018) 48:827–34. doi: 10.1093/jjco/hyy101
34. Wang X-Q, Pi Y-L, Chen P-J, Liu Y, Wang R, Li X, et al. Traditional chinese exercise for cardiovascular diseases: systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. (2016) 5:e002562. doi: 10.1161/JAHA.115.002562
35. Liu T, Chan AW, Liu YH, Taylor-Piliae RE. Effects of Tai Chi-based cardiac rehabilitation on aerobic endurance, psychosocial well-being, and cardiovascular risk reduction among patients with coronary heart disease: a systematic review and meta-analysis. Eur J Cardiovasc Nurs. (2017). 17:368-383 doi: 10.1177/1474515117749592
36. Samartzis L, Dimopoulos S, Tziongourou M, Koroboki E, Kyprianou T, Nanas S. SSRIs versus exercise training for depression in chronic heart failure: a meta-analysis of randomized controlled trials Int J Cardiol. (2013) 168:4956–8. doi: 10.1016/j.ijcard.2013.07.143.
37. Tu R-H, Zeng Z-Y, Zhong G-Q, Wu W-F, Lu Y-J, Bo Z-D, et al. Effects of exercise training on depression in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Eur J Heart Fail. (2014) 16:749–57. doi: 10.1002/ejhf.101
38. Chung Y-C, Yeh M-L, Liu Y-M. Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Nurs. (2017) 26:1801–13. doi: 10.1111/jocn.13514
39. Gomes Neto M, Lacerda FFR, de Lopes AA, Martinez BP, Saquetto MB. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: Systematic review and meta-analysis. Clin Rehabil. (2018) 32:1189–202. doi: 10.1177/0269215518760380
40. Song Y-Y, Hu R-J, Diao Y-S, Chen L, Jiang X-L. Effects of exercise training on restless legs syndrome, depression, sleep quality, and fatigue among hemodialysis patients: a systematic review and meta-analysis. J Pain Symptom Manage. (2018) 55:1184–95. doi: 10.1016/j.jpainsymman.2017.12.472
41. Langhorst J, Klose P, Dobos GJ, Bernardy K, Hauser W. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int. (2013) 33:193–207. doi: 10.1007/s00296-012-2360-1
42. Sosa-Reina MD, Nunez-Nagy S, Gallego-Izquierdo T, Pecos-Martin D, Monserrat J, Alvarez-Mon M. Effectiveness of therapeutic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomized clinical trials. Biomed Res Int. (2017) 2017:2356346. doi: 10.1155/2017/2356346
43. Liang H, Zhang H, Ji H, Wang C. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. (2015) 34:1737–44. doi: 10.1007/s10067-015-2913-2
44. O'Dwyer T, Durcan L, Wilson F. Exercise and physical activity in systemic lupus erythematosus: a systematic review with meta-analyses. Semin Arthritis Rheum. (2017) 47:204–15. doi: 10.1016/j.semarthrit.2017.04.003
45. Zhou Y, Zhu J, Gu Z, Yin X. Efficacy of exercise interventions in patients with acute leukemia: a meta-analysis. PLoS ONE. (2016) 11:e0159966. doi: 10.1371/journal.pone.0159966
46. Bergenthal N, Will A, Streckmann F, Wolkewitz K-D, Monsef I, Engert A, et al. Aerobic physical exercise for adult patients with haematological malignancies. Cochr Database Syst Rev. (2014) 11:CD009075. doi: 10.1002/14651858.CD009075.pub2
47. O'Brien KK, Tynan A-M, Nixon SA, Glazier RH. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis. (2016) 16:182. doi: 10.1186/s12879-016-1478-2
48. Dalgas U, Stenager E, Sloth M. The effect of exercise on depressive symptoms in multiple sclerosis based on a meta-analysis and critical review of the literature. Eur J Neurol. (2015) 22:443. doi: 10.1111/ene.12576
49. Ensari I, Motl RW, Pilutti LA. Exercise training improves depressive symptoms in people with multiple sclerosis: results of a meta-analysis. J Psychosom Res. (2014) 76:465–71. doi: 10.1016/j.jpsychores.2014.03.014
50. Adamson BC, Ensari I, Motl RW. Effect of exercise on depressive symptoms in adults with neurologic disorders: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2015) 96:1329–38. doi: 10.1016/j.apmr.2015.01.005
51. Song R, Grabowska W, Park M, Osypiuk K, Vergara-Diaz GP, Bonato P, et al. The impact of Tai Chi and Qigong mind-body exercises on motor and non-motor function and quality of life in Parkinson's disease: A systematic review and meta-analysis. Parkinsonism Relat Disord. (2017) 41:3–13. doi: 10.1016/j.parkreldis.2017.05.019
52. Eng JJ, Reime B. Exercise for depressive symptoms in stroke patients: a systematic review and meta-analysis. Clin Rehabil. (2014) 28:731–9. doi: 10.1177/0269215514523631
53. Graven C, Brock K, Hill K, Joubert L. Are rehabilitation and/or care co-ordination interventions delivered in the community effective in reducing depression, facilitating participation and improving quality of life after stroke? Disabil Rehabil. (2011) 33:1501–20. doi: 10.3109/09638288.2010.542874
54. Perry DC, Sturm VE, Peterson MJ, Pieper CF, Bullock T, Boeve BF, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg. (2016) 124:511–26. doi: 10.3171/2015.2.JNS14503
55. Bae S-C, Gun SC, Mok CC, Khandker R, Nab HW, Koenig AS, et al. Improved health outcomes with etanercept versus usual DMARD therapy in an Asian population with established rheumatoid arthritis. BMC Muscul Disord. (2013) 14:13. doi: 10.1186/1471-2474-14-13
56. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, et al. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
57. AWMF. S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression: Langfassung. 2nd ed. Berlin (2015).
58. Raimo S, Trojano L, Spitaleri D, Petretta V, Grossi D, Santangelo G. Psychometric properties of the Hamilton Depression Rating Scale in multiple sclerosis. Qual Life Res. (2015) 24:1973–80. doi: 10.1007/s11136-015-0940-8
59. Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics. (2002) 43:386–93. doi: 10.1176/appi.psy.43.5.386
60. Mystakidou K, Tsilika E, Parpa E, Smyrniotis V, Galanos A, Vlahos L. Beck depression inventory: exploring its psychometric properties in a palliative care population of advanced cancer patients. Eur J Cancer Care. (2007) 16:244–50. doi: 10.1111/j.1365-2354.2006.00728.x
61. Lahlou-Laforêt K, Ledru F, Niarra R, Consoli SM. Validity of beck depression inventory for the assessment of depressive mood in chronic heart failure patients. J Affect Disord. (2015) 184:256–60. doi: 10.1016/j.jad.2015.05.056
62. Luckett T, Butow PN, King MT, Oguchi M, Heading G, Hackl NA, et al. A review and recommendations for optimal outcome measures of anxiety, depression and general distress in studies evaluating psychosocial interventions for English-speaking adults with heterogeneous cancer diagnoses. Supportive Care Cancer. (2010) 18:1241–62. doi: 10.1007/s00520-010-0932-8
63. Schneibel R, Brakemeier E-L, Wilbertz G, Dykierek P, Zobel I, Schramm E. Sensitivity to detect change and the correlation of clinical factors with the hamilton depression rating scale and the beck depression inventory in depressed inpatients. Psychiatry Res. (2012) 198:62–7. doi: 10.1016/j.psychres.2011.11.014
Keywords: depressive symptoms, training, aerobic, somatic disease, comorbidity
Citation: Roeh A, Kirchner SK, Malchow B, Maurus I, Schmitt A, Falkai P and Hasan A (2019) Depression in Somatic Disorders: Is There a Beneficial Effect of Exercise? Front. Psychiatry 10:141. doi: 10.3389/fpsyt.2019.00141
Received: 30 September 2018; Accepted: 26 February 2019;
Published: 20 March 2019.
Edited by:
Joseph Firth, Western Sydney University, AustraliaReviewed by:
Mirko Manchia, Università Degli Studi di Cagliari, ItalyGarcia Ashdown-Franks, University of Toronto, Canada
Copyright © 2019 Roeh, Kirchner, Malchow, Maurus, Schmitt, Falkai and Hasan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Astrid Roeh, YXN0cmlkLnJvZWhAbWVkLnVuaS1tdWVuY2hlbi5kZQ==
 Astrid Roeh
Astrid Roeh Sophie K. Kirchner1
Sophie K. Kirchner1 Berend Malchow
Berend Malchow Isabel Maurus
Isabel Maurus Andrea Schmitt
Andrea Schmitt Peter Falkai
Peter Falkai Alkomiet Hasan
Alkomiet Hasan